Abstract
Energy investment in reproduction is predicted to trade off against other necessary physiological functions like immunity, but it is unclear to what extent this impacts fitness in long-lived species. Among mammals, female primates, and especially apes, exhibit extensive periods of investment in each offspring. During this time, energy diverted to gestation and lactation is hypothesized to incur short and long-term deficits in maternal immunity and lead to accelerated ageing. We examined the relationship between reproduction and immunity, as measured by faecal parasite counts, in wild female chimpanzees (Pan troglodytes schweinfurthii) of Kibale National Park, Uganda. While we observed higher parasite shedding (counts of eggs, cysts and larvae) in pregnant chimpanzees relative to cycling females, parasites rapidly decreased during early lactation, the most energetically taxing phase of the reproductive cycle. Additionally, while our results indicate that parasite shedding increases with age, females with higher fertility for their age had lower faecal parasite counts. Such findings support the hypothesis that the relatively conservative rate of female reproduction in chimpanzees may be protective against the negative effects of reproductive effort on health.
This article is part of the theme issue ‘Evolution of the primate ageing process’.
Keywords: life history, trade-offs, reproduction, immunity, ageing, infection
1. Introduction
Life-history theory predicts that energetic investments in reproduction come at the expense of survival-enhancing functions, such as cellular repair and immunity [1,2]. Ideal conditions, like high resource availability and low extrinsic mortality, may allow for a more balanced allocation of resources across the lifespan, but these conditions are not typical of wild species. Selection should then favour early life investment in reproduction over survival to old age, causing cumulative deficits in energy allocated to immune function that lead to degenerative ageing (‘the disposable soma theory’: [3]). Individuals unable to recover deficits in nutrition and energy allocated to immunity during single reproductive bouts may incur cumulative effects on morbidity with increased fertility [4]. This accumulated burden of reproduction may be particularly challenging for female apes who invest heavily in singleton offspring requiring years of care, and whose reproductive success is dependent on survival to old age [5]. Alternatively, the selection of females may compensate for slow reproductive rates by increasing investments in immune defence that promote survival and future reproduction [6–8]. Despite the straightforward predictions, the extent to which cumulative reproductive effort impacts variation in health and ageing within and among wild ape species is unknown.
Immunosenescence, an integral component of the ageing process, features prominently in the life history of long-lived species, with potential to impact both survival and fertility [9,10]. The ageing immune system has been defined by declines in adaptive immunity, through exhaustion of naïve CD4 T cells with antigenic exposure, and increases in systemic inflammation [11–13]. Assessing ageing and immunity in wild species remains a difficult task, particularly for primates where non-invasive sampling is a necessity. Nevertheless, a handful of studies of wild vertebrate populations have described declines in markers of adaptive immunity and/or increases in inflammation with age, including in Soay sheep (Ovis aries) [14], roe deer (Capreolus capreolus) [15] and collared flycatchers (Ficedula albicollis) [16]. In species where collecting invasive samples is not possible, measures of ecto- and endoparasite burdens have been used as indirect proxies for pathogen resistance, which in part involves immune function. Parasitic infection is a nutritional and energetic stressor on hosts, eliciting costly defence mechanisms related to both resistance and tolerance of infection [10,17,18]. For example, high-pathogen burden and elevated leucocytes are associated with an increase in resting metabolic rate, a measure related to the energetic cost of homeostasis, of 10–15% in a natural subsistence human population [19]. While not a direct measure of host immunity, higher parasite burden reflects a reduced ability to control, eliminate and/or tolerate parasite infection.
A few studies have demonstrated proximate trade-offs between female reproductive effort and health-related outcomes. For example, relative to the non-pregnant/non-lactating condition, pregnancy and/or lactation is associated with higher mortality in rhesus macaques [20], slower wound healing in baboons [21] and higher parasite burden in several mammals [22–27]. Across vertebrates, a number of studies also broadly support a trade-off between high fertility and reduced longevity [28,29]. Among female primates, including humans, results are inconsistent; some suggest that high fertility may be associated with negative health outcomes only in resource poor environments (reviewed in [27,30,31–34]). All primates share unusually high parental investment and long lifespans [5]. A slow pace of reproduction necessitates a prolonged reproductive life, but allows females to reduce their daily costs of pregnancy and lactation. High reproductive investment and longevity are further exaggerated in human females, who exhibit an accelerated pace of reproduction, often care for multiple dependent offspring, and whose lifespan extends well past reproductive senescence. The human reproductive pattern may be dependent on the availability of cooperative provisioning of offspring and mothers [35–37], which is absent in our living great ape relatives. Currently, however, we lack comparative data evaluating costs of reproduction on health in wild ape species. Such data offer the potential for resolving the relative contributions of physiology, ecology and behaviour to derived features of human life history.
Here, we examine whether reproductive status or cumulative reproductive effort in female eastern chimpanzees (Pan troglodytes schweinfurthii) results in demonstrable effects on immunity, as measured by shedding of parasite eggs, cysts and larvae. We predict that female chimpanzees in high effort phases of the reproductive cycle (pregnancy and early lactation) will exhibit higher prevalence, intensity and richness of parasites compared with females in low effort phases (cycling and late lactational amenorrhoea) (prediction 1). We additionally predict that female chimpanzees with higher lifetime reproductive effort have higher parasite counts for their age (prediction 2). We sampled female chimpanzees multiple times over different reproductive states to increase our ability to isolate the effects of reproduction from individual variation. We focused our efforts on two chimpanzee communities, Kanyawara and Ngogo, from the same population within Kibale National Park, Uganda. Kanyawara and Ngogo chimpanzees, while close in proximity, have previously been shown to experience differences in habitat quality and food availability, which may be related to observed differences in community size, density and activity patterns at these two sites [38]. Thus, we additionally tested the prediction that chimpanzees in the area of higher energy availability would have lower overall parasitism and experience reduced costs of reproduction (prediction 3).
2. Methods
(a). Field sites
Wild female chimpanzees were studied in Kibale National Park, Uganda, between July 2015 and December 2017. Kibale is located in southwestern Uganda and comprises 795 km2 of tropical and sub-tropical rainforest [39]. The Kanyawara community of chimpanzees within Kibale was habituated for long-term study in 1987 and numbered 49–55 chimpanzees during the study period. Our sample included four subadult (aged 10–13) females, one of whom experienced a pregnancy, and 17 adult (aged 14 to 56) females from the Kanyawara community. The Ngogo community, habituated for study in 1995, averages 190–200 chimpanzees and provided samples from 60 adult females (aged 14 to 68) during this study.
Although Kanyawara and Ngogo are separated by only approximately 10 km and dispersal between them is common [40], the Ngogo chimpanzees have access to significantly higher densities of food resources [38], have a higher energy balance than Kanyawara chimpanzees [41], and have reported the highest survival rates of any known wild chimpanzee community [42]. However, estimated weaning ages are similar [43,44]. Thus, the comparison between Kanyawara and Ngogo allows us to examine whether resource access moderates the effects of reproductive effort on parasite shedding.
(b). Reproductive status and cumulative reproductive effort
Reproductive status was categorized into four levels: cycling, pregnant, early lactation and late lactation. Pregnancy was determined by back-calculating 228 days from the birth date of the most recently born offspring [45]. Although chimpanzee infants are often not fully weaned until four to six years of age [43,44,46], nursing intensity and maternal energy costs decline precipitously by about two years postpartum [47,48]. Thus, we defined early lactation as the first two years postpartum with a living infant. Females with infants older than two years, or whose infants had died, were categorized as either cycling or in late lactation, depending on whether they had exhibited maximally tumescent sexual swellings postpartum or remained in lactational amenorrhoea, respectively. The cycling category also included nulliparous females after they exhibited their first maximally tumescent sexual swellings.
The long-term reproductive effort is ordinarily quantified by examining parity. For our question, this measure was not ideal because some females experienced many early infant deaths, thus their parity was high but they had invested very little in lactation. To better address long-term reproductive effort, we calculated a measure of cumulative reproductive effort which captured the amount of time that females had spent in pregnancy and the more costly phase of early lactation. This included the sum of all months in which a female was pregnant or had a living infant under 2 years of age. In some cases (n = 20 of 81 females), females were already mothers at first identification at the onset of the long-term study, thus, it was necessary to estimate their early fertility. We used the known relationship between cumulative pregnancy and lactation months and age for those females of known parity (n = 61 females) to estimate the missing years of reproductive effort (electronic supplementary material, figure S1). The positive linear relationship between cumulative pregnancy and lactation months and age represented a yearly gain of 1.79 pregnancy and 4.76 lactation months, respectively, for the average female aged 10 to 38 years. These slopes were multiplied by the number of years the female was not observed and added to known pregnancy and lactation months for the female in question. We defined the total number of pregnancy and lactation months, observed and estimated, as cumulative reproductive effort (CRE). Among the 20 females for which an estimation of CRE was required, three of the oldest females from Ngogo had only one observed birth between them and would have required calculation for the majority, if not all, of their reproductive effort. For this reason, they were removed from analyses. Of the remaining 17 females that required estimation of a component of their CRE, a mean of approximately 7.75 years (range 2–18 years) required estimation, and their average age during the study was 43 years (range: 25–56 years).
(c). Faecal sample collection and parasite microscopy
Faecal samples were preserved in the field in 10% formalin, transported to the University of Wisconsin-Madison, Madison, WI, USA, and stored at room temperature until processing. One gram of preserved faecal material was first removed from each sample and resuspended in 10% formalin. Gastrointestinal parasite eggs, cysts and larvae were sedimented using a protocol adapted from Ash & Orihel [49]. Briefly, one gram of faeces was drained of excess formalin and the sediment was washed through two sheets of grade 10 cheesecloth with 0.08% sodium chloride solution into a reservoir. The slurry was resuspended in 0.08% sodium chloride in a 15 ml tube filled to capacity and spun on a centrifuge at 500 rcf for one minute to form a pellet of eggs, cysts and larvae. The sodium chloride was then decanted, and the process repeated twice. After three washes with sodium chloride, the pellet was suspended in 10 ml of 10% formalin and 3 ml of ethyl-acetate, shaken vigorously for 30 s and then centrifuged at 500 rcf for 2 min. The resulting pellet was viewed under 10× magnification using compound light microscopes for the identification of helminth eggs and larvae. All measurements and photographs were taken under 40× objective, and final measurements were recorded in millimeters. One-half slide from each sample was processed at 40× objective for the identification of protozoan cysts and their enumeration by species as many (greater than three views where more than two parasites were visible in the field of view), moderate (at least three views where more than two parasites were present in the field of view), few (at least three views where more than one parasite was present in the field of view), rare (one to five parasites seen on entire slide) or absent (no parasite observed).
(d). Dataset construction and analyses
Four response variables from parasitological analyses were calculated. First, we quantified parasite richness as the number of different parasite eggs, cysts and larval morphotypes identified in each faecal sample (including both helminths and protozoans). Next, we examined prevalence (i.e. presence/absence) and intensity (i.e. total egg count) of nodule worms from the genus Oesophagostomum. Nematodes of this genus are known pathogens of humans and non-human primates and can cause weakness, diarrhoea, abdominal pain, weight loss and internal lesions to the body cavity and organs in chimpanzees [50]. They are also known to occur at high prevalence in Kibale chimpanzees [51–57]. Similarly, we assessed the prevalence of Iodamoeba sp. infection, as measured by the presence or absence of cysts. Iodamoeba are protozoans that are generally considered non-pathogenic symbionts [58]. Oesophagostomum and Iodamoeba were the most prevalent helminths and protozoans, respectively, observed in our study (see Results), making them amenable to quantitative analysis to examine the influence of reproduction on relatively pathogenic versus non-pathogenic parasites.
The final dataset for analysis included 78 female chimpanzees (n = 412 observations) aged 10 to 56 years old, including 21 females (n = 150 faecal samples) from Kanyawara and 57 females (n = 262 faecal samples) from Ngogo (electronic supplementary material, figure S2). Six females in the 10- to 15-year-old age class were new immigrants to the communities, which can be stressful for young females [59]. However, the new immigrants did not differ in measures of parasitism from the broader sample based on preliminary analyses, so we did not include immigration status as a fixed effect in our models. A table of descriptive statistics for fixed effects and response variables is provided in the electronic supplementary material, table S3. All analyses were conducted in R [60] with the faecal sample as the unit of analysis, and chimpanzee identity and year and month of sample collection included in all models as random effects. As measures of parasite eggs, cysts and larvae are often overdispersed among hosts, and count data often include many zeros (i.e. no infection observed), several distributions were considered. The following distributions were selected as they represented the best fit to each type of response based on plots of distributions and residuals: Poisson for parasite richness, binomial for both prevalence models and negative binomial for Oesophagostomum intensity. To examine associations between reproduction and parasite shedding, we examined our four parasite response variables in generalized linear or logistic mixed models using the lme4 [61] and glmmTMB [62] packages. All models included reproductive status (prediction 1), CRE (prediction 2), community, age in years (continuous) and season (i.e. wet and dry). Because CRE and age were intrinsically related and led to multicollinearity in our models, they could not be used in the same model in their raw forms. After conducting exploratory analyses which revealed that models with age alone, as assessed by a likelihood-ratio test, produced better fits than those with CRE alone, we transformed the CRE measure by calculating the residuals of a simple linear regression of CRE on age [63]. Using the residual CRE in the analysis allowed us to assess the influence of CRE on parasite shedding, independent from the influence of age. Residual CRE and age were both mean-centred for modelling. We also considered interaction terms between the community and reproductive status and between community and CRE in all models, addressing the prediction that habitat quality might moderate trade-offs between reproduction and parasite shedding (prediction 3). All two-way interactions were explored and eliminated from the model if they were non-significant. Significance of fixed effects from regression models was determined by Wald Chi-square tests. If a fixed effect was a factor with more than two levels, a Tukey test was employed to determine significant differences between pairs of means.
3. Results
Six genera of gastrointestinal helminths were identified, including two taxa of Oesophagostomum (nodule worm) eggs, two taxa of Strongyloides eggs, Trichostrongylus, Trichuris (whipworm), Enterobius (pinworm) and Mammomonogamus. Hookworm was also diagnosed, but the genus of the hookworm present (i.e. Ancylostoma or Necator) could not be determined via microscopy such that both genera were combined for analyses. An additional six strongylid nematode larval morphotypes were included in analyses, but were not identified to genus. Six genera of protozoan parasites were also identified, including Iodamoeba, Giardia, three species of Entamoeba, Blastocystis, Chilomastix and Endolimax (for prevalence, table 1). Of the 13 genera identified, five genera are known to contain species pathogenic to chimpanzees, including Oesophagostomum, Strongyloides, Mammomonogamus, Entamoeba, and Giardia [56,64–69]. Additionally, hookworm, whether Necator or Ancylostoma, would also be considered a pathogenic infection [65]. It is worth noting, however, that even infections that are normally asymptomatic could cause morbidity, or mortality, when the host is in poor health [55].
Table 1.
Number of faecal samples positive, and per cent positive, for each parasite genus found in female chimpanzees (n = 81 females and 432 faecal samples) of Kibale National Park, Uganda, between July 2015 and December 2017. (Samples include females aged 10 to 68 years old. An asterisk denotes taxonomic groups known to contain at least one species pathogenic in chimpanzees [56,64–69].)
| genus | positive | % positive |
|---|---|---|
| helminths | ||
| Oesophagostomum* | 315 | 77 |
| hookworm* | 84 | 20.5 |
| Trichostrongylus | 74 | 18.1 |
| Strongyloides* | 41 | 10 |
| Trichuris | 2 | 0.5 |
| Enterobius | 1 | 0.2 |
| Mammomonogamus* | 1 | 0.2 |
| protozoans | ||
| Iodamoeba | 199 | 48.7 |
| Entamoeba* | 116 | 28.4 |
| Blastocystis | 34 | 8.3 |
| Giardia* | 33 | 8.1 |
| Chilomastix | 3 | 0.7 |
| Endolimax | 1 | 0.2 |
(a). Effects of reproductive status
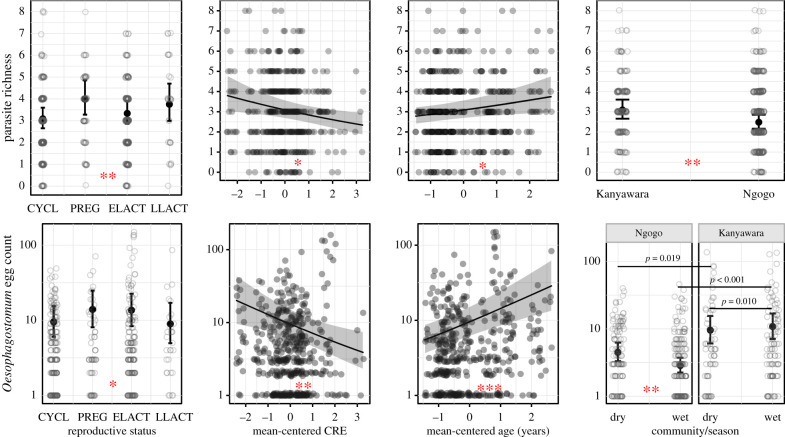
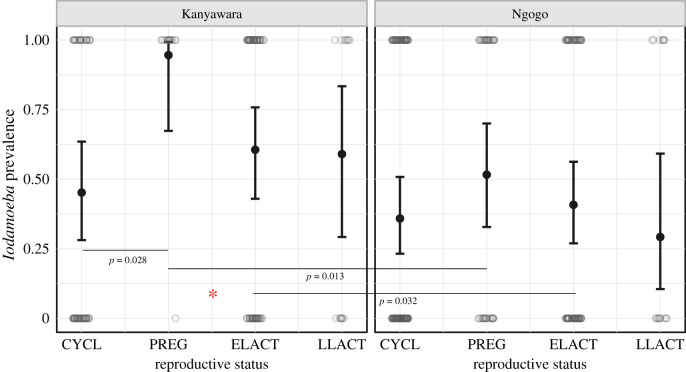
Contrary to our prediction, parasite richness and Iodamoeba prevalence were not significantly higher in female chimpanzees in the first two years of lactation versus those in the cycling condition (table 2; figures 1 and 2, and the electronic supplementary material, figure S4). While Oesophagostomum prevalence and intensity were significantly higher in early lactation than the cycling condition in the regression models, early lactation did not remain significantly different from females in cycling status in post hoc, pairwise comparisons (for prevalence: βELACT – CYCL = 0.677 ± 0.324, p = 0.156; for intensity: βELACT – CYCL = 0.389 ± 0.189, p = 0.168). Owing to the importance of this parasite on chimpanzee health (i.e. high prevalence, high variation in intensity among females and pathogenicity) at Kanyawara and Ngogo, we further examined how Oesophagostomum egg counts varied continuously over the full course of the reproductive cycle (figure 3). Egg counts increased dramatically from the beginning to end of pregnancy, peaking approximately 3–4 months postpartum before declining. Thus, while Oesophagostomum egg counts are not elevated on average during pregnancy and early lactation as we defined them, they do appear to be elevated during the last months of gestation and the first months of lactation.
Table 2.
Model summary from regression analyses examining the influence of reproductive status (cycling, pregnant, early lactation and late lactation), cumulative reproductive effort (months), age (years), community (Kanyawara and Ngogo) and season (wet and dry), on parasite shedding in wild female chimpanzees. (Four models were considered, each with a different response variable, including parasite richness, Oesophagostomum prevalence and intensity, and Iodamoeba prevalence. Faecal parasites were measured as counts of eggs, cysts and larvae. Both CRE (residual) and age were mean-centred for analysis. Significant p-values are italicized.)
| fixed effects | parasite richness |
Oesophagostomum intensity |
Oesophagostomum prevalence |
Iodamoeba prevalence |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| estimate | std. error | p-value | estimate | std. error | p-value | estimate | std. error | p-value | estimate | std. error | p-value | |
| (intercept) | 1.128 | 0.078 | <0.001 | 2.146 | 0.267 | <0.001 | 1.664 | 0.428 | <0.001 | −0.193 | 0.381 | 0.613 |
| reproductive status | ||||||||||||
| pregnant | 0.254 | 0.088 | 0.004 | 0.417 | 0.219 | 0.057 | 0.455 | 0.403 | 0.259 | 3.052 | 1.097 | 0.005 |
| early lactation | 0.077 | 0.071 | 0.281 | 0.389 | 0.189 | 0.039 | 0.677 | 0.324 | 0.036 | 0.623 | 0.413 | 0.132 |
| late lactation | 0.194 | 0.113 | 0.085 | −0.071 | 0.303 | 0.814 | 0.424 | 0.629 | 0.501 | 0.558 | 0.660 | 0.398 |
| CRE (residual) | −0.085 | 0.034 | 0.011 | −0.332 | 0.115 | 0.004 | −0.574 | 0.172 | <0.001 | −0.329 | 0.131 | 0.012 |
| community | ||||||||||||
| Ngogo | −0.220 | 0.071 | 0.002 | −0.895 | 0.306 | 0.003 | −0.628 | 0.484 | 0.194 | −0.388 | 0.402 | 0.334 |
| age | 0.073 | 0.031 | 0.019 | 0.445 | 0.108 | <0.001 | 0.502 | 0.161 | 0.002 | 0.159 | 0.123 | 0.195 |
| season | ||||||||||||
| wet | 0.018 | 0.067 | 0.786 | 0.138 | 0.237 | 0.561 | 0.620 | 0.503 | 0.218 | 0.110 | 0.319 | 0.729 |
| community * season | ||||||||||||
| Ngogo:wet | — | — | — | −0.774 | 0.285 | 0.007 | −1.347 | 0.586 | 0.022 | — | — | — |
| reproductive status * community | ||||||||||||
| pregnant:Ngogo | — | — | — | — | — | — | — | — | — | −2.405 | 1.168 | 0.039 |
| early lactation:Ngogo | — | — | — | — | — | — | — | — | — | −0.414 | 0.513 | 0.420 |
| late lactation:Ngogo | — | — | — | — | — | — | — | — | — | −0.861 | 0.877 | 0.327 |
| random effects | variance | std. dev. | variance | std. dev. | variance | std. dev. | variance | std. dev. | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| year and month | 0.006 | 0.078 | 0.060 | 0.245 | 0.147 | 0.384 | 0.326 | 0.571 | ||||
| female chimpanzee | 0.000 | 0.000 | 0.485 | 0.697 | 0.196 | 0.443 | 0.000 | 0.000 |
Figure 1.
Model predicted (lines) and raw (points) data describing the relationship between parasite richness and Oesophagostomum egg counts and model predictors. Asterisks denote level of statistical significance from the global models: 0.05(*), 0.01(**) and 0.001(***). Bars with p-values source to post hoc pairwise comparisons of interactions between fixed effects. Reproductive status is denoted by cycling (CYCL), pregnant (PREG), early lactation (ELACT) and late lactation (LLACT). Cumulative reproductive effort represents the residual variation from a regression of age and CRE, as measured by the number of months a female had been pregnant or in early lactation in her lifetime. (Online version in colour.)
Figure 2.
Model predicted (bars) and raw (points) data for the interaction between reproductive status and community by Iodamoeba prevalence. Asterisks denote the level of statistical significance from the global model: 0.05(*), 0.01(**) and 0.001(***). Bars with p-values source to post hoc pairwise comparisons of interactions between fixed effects. Reproductive status is denoted by cycling (CYCL), pregnant (PREG), early lactation (ELACT) and late lactation (LLACT). (Online version in colour.)
Figure 3.
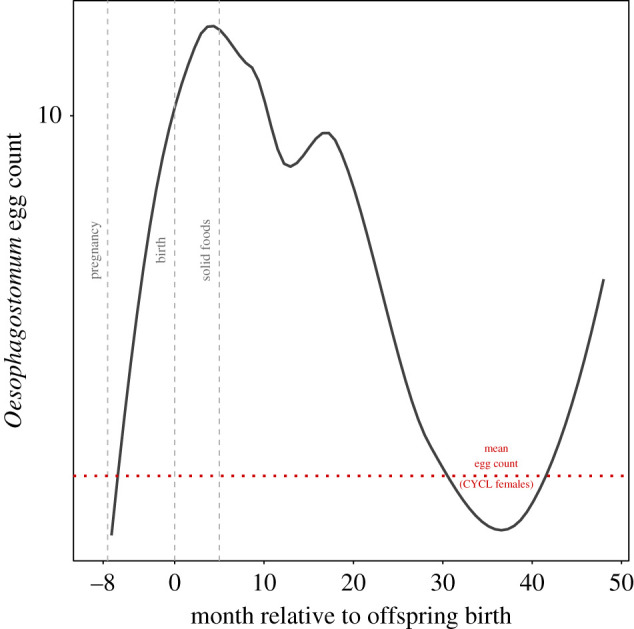
Loess curve of the relationship between Oesophagostomum egg count and month relative to offspring birth averaged over both communities, and in relation to average egg counts (mean = 5.98 eggs sample−1) for cycling females (CYCL). Vertical lines approximate different stages in chimpanzee infant development. (Online version in colour.)
Relative to cycling, pregnancy did yield higher parasite richness (βPREG – CYCL = 0.254 ± 0.088, p = 0.004). Pregnancy was also associated with higher Iodamoeba prevalence, the non-pathogenic parasite, at Kanyawara (βPREG – CYCL = 3.052 ± 1.097, p = 0.028), but not at Ngogo (βPREG – CYCL = 0.647 ± 0.392, p = 0.351).
(b). Effects of age and cumulative reproductive effort
Age was a strong positive predictor of parasite richness (χ21 = 5.504, p = 0.019) and Oesophagostomum prevalence (χ21 = 9.769, p = 0.002) and intensity (χ21 = 17.060, p < 0.001), but not Iodamoeba prevalence (χ21 = 1.679, p = 0.195). Age did not interact with reproductive status, cumulative reproductive effort, or with any other fixed effects in our models (table 2; figure 1 and the electronic supplementary material, figure S4).
Cumulative reproductive effort (corrected for age) was a significant predictor of parasite shedding in all models, but not in the direction predicted. Female chimpanzees with higher CRE than predicted by their age experienced lower parasite richness (χ21 = 6.447, p = 0.011; βCRE = −0.085 ± 0.034, p = 0.011), Oesophagostomum prevalence (χ21 = 11.192, p < 0.001; βCRE = −0.574 ± 0.172, p < 0.001) and intensity (χ21 = 8.280, p = 0.004; βCRE = −0.332 ± 0.115, p = 0.004), and Iodamoeba prevalence (χ21 = 6.356, p = 0.012; βCRE = −0.329 ± 0.131, p = 0.012).
(c). Effects of community
Chimpanzee community was a significant predictor of parasite shedding in every model, with a lower number of parasite eggs, cysts and larvae observed at Ngogo in all cases (table 2; figures 1 and 2, and the electronic supplementary material, figure S4). We also observed an interaction between the community and reproductive status for Iodamoeba prevalence (βPREG:Ngogo = −2.405 ± 1.168, p = 0.039), but not for any other measures of parasite burden. Specifically, Iodamoeba prevalence was significantly different between female chimpanzees at Kanyawara and Ngogo in both pregnancy (βKanyawara – Ngogo = 2.794 ± 1.118, p = 0.013) and early lactation (βKanyawara – Ngogo = 0.802 ± 0.373, p = 0.032). The effects of CRE did not differ between communities.
4. Discussion
In this study, we assayed faecal parasite counts to test the hypothesis that reproduction imposes energetic trade-offs that may accelerate the ageing process in female chimpanzees. We found a robust increase in parasite shedding with age. However, we did not find substantial evidence to support our predictions that the energetic costs of reproduction compromise females' ability to resist gastrointestinal parasites. While pregnancy was associated with increased parasitism, early lactation generally was not. One highly prevalent, pathogenic taxa remained elevated during the first few months of lactation, but notably began declining at a time when maternal energetic costs should have been the most intense, meaning that egg counts returned to levels statistically indistinguishable from those of cycling females several years before the typical age of weaning. Higher reproductive effort across the life course was also associated with lower, rather than higher, parasite shedding. Although there is every reason to believe that the energy devoted to reproduction imposes constraints on the immune system, our data suggest that this trade-off is not sufficient to impact parasite shedding in female chimpanzees.
Our study does provide evidence to suggest that energy availability can influence parasite shedding. We found significantly fewer parasites in Ngogo, the community of chimpanzees that enjoys relatively high resource availability, than in Kanyawara. This may suggest that under increased energy limitations, the collateral costs of reproduction might be more obvious. Such conditions were not met in our study, as even Kanyawara did not experience the predicted increase in parasites with early lactation or residual CRE. Notably, mortality rates of both communities are much lower than have been reported from other study populations, suggesting that our study population as whole experiences relatively healthy conditions [42,70]. While beyond the scope of the current analysis, in the future, we aim to investigate the role of individual energy balance and physiological stress on faecal parasite loads.
Lactation is the most energy-intense phase of the female mammalian reproductive cycle [71,72]. This is owing not only to the increased mass of the infant that must be fed and carried but to the collateral costs of energy conversion needed to produce breastmilk [73]. If the direct cost of reproduction is the key constraint on the immune system, then we would have expected the strongest evidence of elevated parasite counts during the first 2 years of lactation, when nursing intensity is highest and the infant's brain is growing [47,48,74]. In our examination of Oesophagostomum counts across the reproductive cycle, egg counts that had increased throughout pregnancy remained high for the first few months postpartum, suggesting that there may be some temporary costs to immune function associated with the period of exclusive nursing. However, faecal parasite counts began to decline precipitously even before six months, the approximate age when infant chimpanzees first sample solid foods [43,44]. Given that infants are growing and their needs increasing, mother's costs should remain high or even increase for many months past this point. However, prior evidence from Kanyawara indicates that mothers are able to slowly increase their energy balance at this time [48]. Taken together, these data suggest that energetic constraints on maternal health, when they occur, are likely to be transient relative to the typical 4–6 year period that chimpanzee mothers nurse their infants [43,44,46]. These findings conform with recent evidence from Kanyawara that lactation does not significantly increase rates of respiratory illness [75] or concentrations of urinary cortisol [76]. It is possible that female chimpanzees in early lactation are mitigating costs by reducing activity and risk associated with feeding competition during this time. For example, lactating females at Kanyawara, the community with lower food availability, spend more time resting and less time feeding than lactating females at Ngogo, though cycling females in the two communities do not differ [38]. Offsetting energetic stress through changes in behaviour is one strategy that could reduce impact on the immune system.
We observed an increase in Iodamoeba prevalence and parasite richness associated with pregnancy. Furthermore, post hoc examinations of Oesophagostomum intensity support an increase in faecal egg count throughout pregnancy. Pregnancy has previously been associated with higher parasitism in a natural fertility human population [32,77] and a few non-human primate species (reviewed in [27]). The higher parasite burden observed during pregnancy in our study could be the result of energy deficits to immunity created by the growing fetus. However, this explanation is insufficient, as parasite shedding did not continue to increase during early lactation. Instead, it is more likely that gestating females are at increased susceptibility to parasites owing to repatterning of the maternal immune system that helps prevent rejection of fetal tissues [78,79]. This idea is further supported when we acknowledge that, while Iodamoeba and Oesophagostomum both increase during pregnancy, a pathogenic helminth like Oesophagostomum may take the immune system longer to control or eliminate owing to the parasite's size and maturation time. This may explain the short period of a continued increase in Oesophagostomum egg count postpartum.
Our results are congruent with other studies supporting senescence as a ubiquitous feature of mammalian biology, even among wild animals subject to high-pathogen environments and extrinsic mortality [15,80,81]. While we did not measure immune function directly, other studies have demonstrated that age changes in gastrointestinal parasites correspond with changes in immunological parameters [15]. Age-related changes in parasite burden have been inconsistent in studies across primate taxa [82], possibly owing to the often small and cross-sectional sample designs. Alternatively, mortality selection may be obscuring the ability to detect age-related changes in parasitism if only the most fit individuals survive to old age [83]. However, Hämäläinen et al. [82] propose intriguing functional explanations that might be addressed with further accumulation of comparative data, including differences in pathogenicity of parasites, changes in behaviour patterns of old individuals that mitigate the risk of infection, and acquired immunity over the life course mitigating late-life infections.
It is important to note that the age increase we observed in female chimpanzees may itself reflect the cumulative costs of reproduction, as follows from theory. What we could not observe was variation among same-aged individuals that could be attributed to different reproductive histories. The absence of evidence of higher parasite shedding during early lactation in individual reproductive bouts, the decline in Oesophagostomum egg counts observed in early months postpartum and the lower parasite shedding observed in female chimpanzees with higher fertility all suggest that chimpanzee females are usually able to negotiate costs of reproduction without demonstrable impacts on health-related outcomes. This pattern contrasts with that for wild baboons, where female condition declines throughout lactation [84] and lactating females show a reduced ability to heal wounds [21]. Our results probably indicate phenotypic correlations, such that better quality individuals or those with more resources can afford to reproduce at a faster rate while maintaining a healthy soma [85]. Female chimpanzees show great variability in reproductive rates, and metabolic profiles indicate that this timing is linked not to infant development or milk intake, but to the ability of mothers to maintain a positive energy balance before conceiving another infant [48,86]. In other words, the pace of reproduction is tailored to the ability of females to afford its costs, a strategy that is shared with humans [30,87]. Such findings support the hypothesis that the relatively conservative rate of female reproduction in chimpanzees may be protective against the negative effects of reproductive effort on health.
Chimpanzee community was a significant predictor of parasite shedding in this study, with Kanyawara females exhibiting higher parasitism across all measures. This difference is unlikely owing to differences in genetics or demographics. High levels of gene flow have been demonstrated between the two communities [40], and the larger community size and density of chimpanzees at Ngogo should predict higher parasite burden [88]. Dietary quality is an important determinant of immune function [10,17] and has previously been associated with parasite burden and markers of immunity in other wild mammals [15]. Chimpanzees at Ngogo experience a higher density of ripe fruit trees [38], greater access to high-quality food items [89,90], and higher overall energy balance [41]. Therefore, we suspect the lower parasite burden observed at Ngogo traces to increased ability to invest in immune defence afforded by greater or more reliable access to high-value foods. Given that our study only considered two chimpanzee communities, we cannot rule out the influence of other ecological differences between communities, such as altitude, faunal communities and/or human-sourced alteration of habitat.
In summary, our results suggest that the energetic trade-offs between reproduction and immune function do not exert substantial impacts on parasite shedding in wild female chimpanzees. However, we found considerable variation among individuals and between communities in levels of faecal parasite shedding, and strong evidence that ageing is accompanied by parasite-related outcomes that may be reflective of immunosenescence. These conclusions suggest a future priority to address drivers of this variation, such as genetics, sociality and direct measures of energy availability. Finally, our study could only examine one non-invasive proxy of immunity, and we look forward to future studies that permit a more complete understanding of the complex interactions between chimpanzee hosts, their immune system and the community of microorganisms they support, including insights into how parasites influence host survival and reproduction.
Supplementary Material
Acknowledgements
We thank the Makerere Biological Field Station, Uganda Wildlife Authority, and the Uganda National Council for Science and Technology for providing local support and permissions. This project would not have been possible without the dedicated field teams at Kanyawara and Ngogo, and an equally dedicated team of undergraduate and veterinary student project assistants at the University of Wisconsin – Madison, including: Kayla Bonack, Kate Burkart, Cristina Diaz, Richie Dulli, Kylie Grady and Gaby Shay.
Ethics
This project was permitted through Institutional Animal Care and Use Committees (Approval 15-200302-MC) at the University of New Mexico, Uganda Wildlife Authority and Uganda National Council for Science and Technology. Sample export permits were obtained on an as-needed basis from Centers for Disease Control and Prevention for shipment of faecal samples to the U.S.
Data accessibility
Data for regression analyses are available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.m0cfxpp0n [91].
Authors' contributions
S.R.P. and M.E.T. designed the study and drafted the manuscript. S.R.P., M.E.T., T.L.G., S.F., J.C., J.C.M., K.L., M.N.M., Z.P.M., R.W.W. and E.O. contributed to data collection and analysis. All authors contributed to the interpretation of results and editing of the manuscript.
Competing interests
We declare we have no competing interests.
Funding
This research was supported by funding from the NIH (National Institute on Aging and Office for Research on Women's Health R01-AG049395, to M.E.T., T.L.G., R.W.W., J.C.M.), the National Science Foundation (BCS-1613185 to S.R.P. and M.E.T.), the Leakey Foundation (to S.R.P.), as well as through long-term funding by the NSF (BSC-1355014), the Leakey Foundation, the Wenner Gren Foundation, University of New Mexico, and Harvard University.
References
- 1.Gadgil M, Bossert WH. 1970. Life historical consequences of natural selection. Am. Nat. 104, 1–24. ( 10.1086/282637) [DOI] [Google Scholar]
- 2.Stearns S. 1992. The evolution of life histories. London, UK: Oxford University Press. [Google Scholar]
- 3.Kirkwood TBL, Rose MR. 1991. Evolution of senescence: late survival sacrificed for reproduction. Phil. Trans. R. Soc. B 332, 15–24. ( 10.1098/rstb.1991.0028) [DOI] [PubMed] [Google Scholar]
- 4.Jelliffe DB, Maddocks I. 1964. Notes on ecologic malnutrition in the New Guinea Highlands. Clin. Pediatr. 3, 432–438. ( 10.1177/000992286400300710) [DOI] [PubMed] [Google Scholar]
- 5.Charnov EL, Berrigan D. 1993. Why do female primates have such long lifespans and so few babies? or life in the slow lane. Evol. Anthropol. 1, 191–194. ( 10.1002/evan.1360010604) [DOI] [Google Scholar]
- 6.Rolff J. 2002. Batean's principle and immunity. Proc. R. Soc. Lond. B 269, 867–872. ( 10.1098/rspb.2002.1959) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zuk M, Stoehr AM. 2002. Immune defense and host life history. Am. Nat. 160, S9–22. ( 10.1086/342131) [DOI] [PubMed] [Google Scholar]
- 8.King AM, Kirkwood TBL, Shanley DP. 2017. Explaining sex differences in lifespan in terms of optimal energy allocation in the baboon: sex role specialization and lifespan. Evolution 71, 2280–2297. ( 10.1111/evo.13316) [DOI] [PubMed] [Google Scholar]
- 9.Sheldon BC, Verhulst S. 1996. Ecological immunology: costly parasite defences and trade-offs in evolutionary ecology. Trends Ecol. Evol. 11, 317–321. ( 10.1016/0169-5347(96)10039-2) [DOI] [PubMed] [Google Scholar]
- 10.Lochmiller RL, Deerenberg C. 2000. Trade-offs in evolutionary immunology: just what is the cost of immunity? Oikos 88, 87–98. ( 10.1034/j.1600-0706.2000.880110.x) [DOI] [Google Scholar]
- 11.Linton PJ, Dorshkind K. 2004. Age-related changes in lymphocyte development and function. Nat. Immunol. 5, 133–139. ( 10.1038/ni1033) [DOI] [PubMed] [Google Scholar]
- 12.Finch CE. 2010. Evolution of the human lifespan and diseases of aging: roles of infection, inflammation, and nutrition. Proc. Natl Acad. Sci. USA 107(suppl_1), 1718–1724. ( 10.1073/pnas.0909606106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Singh T, Newman AB. 2011. Inflammatory markers in population studies of aging. Ageing Res. Rev. 10, 319–329. ( 10.1016/j.arr.2010.11.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nussey DH, Watt K, Pilkington JG, Zamoyska R, McNeilly TN. 2012. Age-related variation in immunity in a wild mammal population: immune aging in wild sheep. Aging Cell 11, 178–180. ( 10.1111/j.1474-9726.2011.00771.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheynel L, et al. 2017. Immunosenescence patterns differ between populations but not between sexes in a long-lived mammal. Sci. Rep. 7, 13700 ( 10.1038/s41598-017-13686-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cichon M, Sendecka J, Gustafsson L. 2003. Age-related decline in humoral immune function in collared flycatchers. J. Evol. Biol. 16, 1205–1210. ( 10.1046/j.1420-9101.2003.00611.x) [DOI] [PubMed] [Google Scholar]
- 17.McDade TW. 2003. Life history theory and the immune system: steps toward a human ecological immunology. Am. J. Phys. Anthropol. 122(S37), 100–125. ( 10.1002/ajpa.10398) [DOI] [PubMed] [Google Scholar]
- 18.Soares MP, Teixeira L, Moita LF. 2017. Disease tolerance and immunity in host protection against infection. Nat. Rev. Immunol. 17, 83–96. ( 10.1038/nri.2016.136) [DOI] [PubMed] [Google Scholar]
- 19.Gurven MD, Trumble BC, Stieglitz J, Yetish G, Cummings D, Blackwell AD, Beheim B, Kaplan HS, Pontzer H. 2016. High resting metabolic rate among Amazonian forager-horticulturalists experiencing high pathogen burden. Am. J. Phys. Anthropol. 161, 414–425. ( 10.1002/ajpa.23040) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoffman CL, Ruiz-Lambides AV, Davila E, Maldonado E, Gerald MS, Maestripieri D. 2008. Sex differences in survival costs of reproduction in a promiscuous primate. Behav. Ecol. Sociobiol. 62, 1711–1718. ( 10.1007/s00265-008-0599-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Archie EA, Altmann J, Alberts SC. 2014. Costs of reproduction in a long-lived female primate: injury risk and wound healing. Behav. Ecol. Sociobiol. 68, 1183–1193. ( 10.1007/s00265-014-1729-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Festa-Bianchet M. 1989. Individual differences, parasites, and the costs of reproduction for bighorn ewes (Ovis canadensis). J. Anim. Ecol. 58, 785 ( 10.2307/5124) [DOI] [Google Scholar]
- 23.Christe P, Arlettaz R, Vogel P. 2000. Variation in intensity of a parasitic mite (Spinturnix myoti) in relation to the reproductive cycle and immunocompetence of its bat host (Myotis myotis). Ecol. Lett. 3, 207–212. ( 10.1046/j.1461-0248.2000.00142.x) [DOI] [Google Scholar]
- 24.Cattadori IM, Boag B, Bjørnstad ON, Cornell SJ, Hudson PJ. 2005. Peak shift and epidemiology in a seasonal host–nematode system. Proc. R. Soc. B 272, 1163–1169. ( 10.1098/rspb.2004.3050) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.East ML, Otto E, Helms J, Thierer D, Cable J, Hofer H. 2015. Does lactation lead to resource allocation trade-offs in the spotted hyaena? Behav. Ecol. Sociobiol. 69, 805–814. ( 10.1007/s00265-015-1897-x) [DOI] [Google Scholar]
- 26.De Nys HM, Calvignac-Spencer S, Boesch C, Dorny P, Wittig RM, Mundry R, Leendertz FH. 2014. Malaria parasite detection increases during pregnancy in wild chimpanzees. Malar. J. 13, 413 ( 10.1186/1475-2875-13-413) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martínez-Mota R, Garber PA, Palme R, Gillespie TR. 2017. The relative effects of reproductive condition, stress, and seasonality on patterns of parasitism in wild female black howler monkeys (Alouatta pigra). Am. J. Primatol. 79, e22669 ( 10.1002/ajp.22669) [DOI] [PubMed] [Google Scholar]
- 28.Hayward AD, Mar KU, Lahdenperä M, Lummaa V. 2014. Early reproductive investment, senescence and lifetime reproductive success in female Asian elephants. J. Evol. Biol. 27, 772–783. ( 10.1111/jeb.12350) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lemaître J-F, Berger V, Bonenfant C, Douhard M, Gamelon M, Plard F, Gaillard JM. 2015. Early-late life trade-offs and the evolution of ageing in the wild. Proc. R. Soc. B 282, 20150209 ( 10.1098/rspb.2015.0209) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gurven M, Costa M, Trumble B, Stieglitz J, Beheim B, Eid Rodriguez D, Hooper PL, Kaplan H. 2016. Health costs of reproduction are minimal despite high fertility, mortality and subsistence lifestyle. Sci. Rep. 6, 30056 ( 10.1038/srep30056) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Akinyi MY, Jansen D, Habig B, Gesquiere LR, Alberts SC, Archie EA. 2019. Costs and drivers of helminth parasite infection in wild female baboons. J. Anim. Ecol. 88, 1029–1043. ( 10.1111/1365-2656.12994) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Anderson AS, Trumble BC, Hové C, Kraft TS, Kaplan H, Gurven M, Blackwell AD. 2020. Old friends and friendly fire: pregnancy, hookworm infection, and anemia among tropical horticulturalists. Am. J. Hum. Biol. 32, e23337 ( 10.1002/ajhb.23337) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ziomkiewicz A, Sancilio A, Galbarczyk A, Klimek M, Jasienska G, Bribiescas RG. 2016. Evidence for the cost of reproduction in humans: high lifetime reproductive effort is associated with greater oxidative stress in post-menopausal women. PLoS ONE 11, e0145753 ( 10.1371/journal.pone.0145753) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ziomkiewicz A, Frumkin A, Zhang Y, Sancilio A, Bribiescas RG. 2018. The cost of reproduction in women: reproductive effort and oxidative stress in premenopausal and postmenopausal American women. Am. J. Hum. Biol. 30, e23069 ( 10.1002/ajhb.23069) [DOI] [PubMed] [Google Scholar]
- 35.Kaplan H, Hill K, Lancaster J, Hurtado AM. 2000. A theory of human life history evolution: diet, intelligence, and longevity. Evol. Anthropol. 9, 156–185. () [DOI] [Google Scholar]
- 36.Hawkes K, O'Connell JF, Jones NGB, Alvarez H, Charnov EL. 1998. Grandmothering, menopause, and the evolution of human life histories. Proc. Natl Acad. Sci. USA 95, 1336–1339. ( 10.1073/pnas.95.3.1336) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reiches MW, Ellison PT, Lipson SF, Sharrock KC, Gardiner E, Duncan LG. 2009. Pooled energy budget and human life history. Am. J. Hum. Biol. 21, 421–429. ( 10.1002/ajhb.20906) [DOI] [PubMed] [Google Scholar]
- 38.Potts KB, Watts DP, Wrangham RW. 2011. Comparative feeding ecology of two communities of chimpanzees (Pan troglodytes) in Kibale National Park, Uganda. Int. J. Primatol. 32, 669–690. ( 10.1007/s10764-011-9494-y) [DOI] [Google Scholar]
- 39.Chapman CA, Struhsaker TT, Lambert JE. 2005. Thirty years of research in Kibale National Park, Uganda, reveals a complex picture for conservation. Int. J. Primatol. 26, 539–555. ( 10.1007/s10764-005-4365-z) [DOI] [Google Scholar]
- 40.Langergraber KE, et al. 2011. Genetic and ‘cultural’ similarity in wild chimpanzees. Proc. R. Soc. B 278, 408–416. ( 10.1098/rspb.2010.1112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Emery Thompson M, Muller MN, Wrangham RW, Lwanga JS, Potts KB. 2009. Urinary C-peptide tracks seasonal and individual variation in energy balance in wild chimpanzees. Horm. Behav. 55, 299–305. ( 10.1016/j.yhbeh.2008.11.005) [DOI] [PubMed] [Google Scholar]
- 42.Wood BM, Watts DP, Mitani JC, Langergraber KE. 2017. Favorable ecological circumstances promote life expectancy in chimpanzees similar to that of human hunter-gatherers. J. Hum. Evol. 105, 41–56. ( 10.1016/j.jhevol.2017.01.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bădescu I, Katzenberg MA, Watts DP, Sellen DW. 2017. A novel fecal stable isotope approach to determine the timing of age-related feeding transitions in wild infant chimpanzees. Am. J. Phys. Anthropol. 162, 285–299. ( 10.1002/ajpa.23116) [DOI] [PubMed] [Google Scholar]
- 44.Bray J, Emery Thompson M, Muller MN, Wrangham RW, Machanda ZP. 2018. The development of feeding behavior in wild chimpanzees (Pan troglodytes schweinfurthii). Am. J. Phys. Anthropol. 165, 34–46. ( 10.1002/ajpa.23325) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wallis J. 1997. A survey of reproductive parameters in the free-ranging chimpanzees of Gombe National Park. Reproduction 109, 297–307. ( 10.1530/jrf.0.1090297) [DOI] [PubMed] [Google Scholar]
- 46.Lonsdorf EV, Stanton MA, Pusey AE, Murray CM. 2020. Sources of variation in weaned age among wild chimpanzees in Gombe National Park, Tanzania. Am. J. Phys. Anthropol. 171, 419–429. ( 10.1002/ajpa.23986) [DOI] [PubMed] [Google Scholar]
- 47.Clark CB. 1977. A preliminary report on weaning among chimpanzees of the Gombe National Park, Tanzania. In Primate bio-social development (eds Chevalier-Skolnikoff S, Poirier F), pp. 235–250. New York, NY: Garland Press. [Google Scholar]
- 48.Thompson ME, Muller MN, Wrangham RW. 2012. The energetics of lactation and the return to fecundity in wild chimpanzees. Behav. Ecol. 23, 1234–1241. ( 10.1093/beheco/ars107) [DOI] [Google Scholar]
- 49.Ash LR, Orihel TC. 1991. Parasites: A guide to laboratory procedures and identification. Chicago, IL: American Society of Clinical Pathologists. [Google Scholar]
- 50.Krief S, Jamart A, Mahé S, Leendertz FH, Mätz-Rensing K, Crespeau F, Bain O, Guillot J. 2008. Clinical and pathologic manifestation of oesophagostomosis in African great apes: does self-medication in wild apes influence disease progression? J. Med. Primatol. 37, 188–195. ( 10.1111/j.1600-0684.2008.00285.x) [DOI] [PubMed] [Google Scholar]
- 51.Ashford RW, Reid GDF. 2000. Intestinal parasites of the chimpanzee Pan troglodytes in Kibale Forest, Uganda. Ann. Trop. Med. Parasitol. 94, 173–179. ( 10.1080/00034983.2000.11813526) [DOI] [PubMed] [Google Scholar]
- 52.Krief S, Huffman MA, Sévenet T, Guillot J, Bories C, Hladik CM, Wrangham RW. 2005. Noninvasive monitoring of the health of Pan troglodytes schweinfurthii in the Kibale National Park, Uganda. Int. J. Primatol. 26, 467–490. ( 10.1007/s10764-005-2934-9) [DOI] [Google Scholar]
- 53.Krief S, Vermeulen B, Lafosse S, Kasenene JM, Nieguitsila A, Berthelemy M, L'Hostis M, Bain O, Guillot J. 2010. Nodular worm infection in wild chimpanzees in western Uganda: a risk for human health? PLoS Negl. Trop. Dis. 4, e630 ( 10.1371/journal.pntd.0000630) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Muehlenbein MP. 2005. Parasitological analyses of the male chimpanzees (Pan troglodytes schweinfurthii) at Ngogo, Kibale National Park, Uganda. Am. J. Primatol. 65, 167–179. ( 10.1002/ajp.20106) [DOI] [PubMed] [Google Scholar]
- 55.Masi S, Chauffour S, Bain O, Todd A, Guillot J, Krief S. 2012. Seasonal effects on great ape health: a case study of wild chimpanzees and western gorillas. PLoS ONE 7, e49805 ( 10.1371/journal.pone.0049805) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ghai RR, Chapman CA, Omeja PA, Davies TJ, Goldberg TL. 2014. Nodule worm infection in humans and wild primates in uganda: cryptic species in a newly identified region of human transmission. PLoS Negl. Trop. Dis. 8, e2641 ( 10.1371/journal.pntd.0002641) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cibot M, Guillot J, Lafosse S, Bon C, Seguya A, Krief S. 2015. Nodular worm infections in wild non-human primates and humans living in the Sebitoli area (Kibale National Park, Uganda): do high spatial proximity favor zoonotic transmission? PLoS Negl. Trop. Dis. 9, e0004133 ( 10.1371/journal.pntd.0004133) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Roberts LS, Janovy JR. 2000. Foundations of parasitology, 6th edn Boston, MA: McGraw-Hill. [Google Scholar]
- 59.Kahlenberg SM, Thompson ME, Muller MN, Wrangham RW. 2008. Immigration costs for female chimpanzees and male protection as an immigrant counterstrategy to intrasexual aggression. Anim. Behav. 76, 1497–1509. ( 10.1016//j.anbehav.2008.05.029) [DOI] [Google Scholar]
- 60.R Core Team. 2018. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 61.Bates D, Maechler M, Bolker B, Walker S. 2015. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48. ( 10.18637/jss.v067.i01) [DOI] [Google Scholar]
- 62.Brooks ME, Kristensen K, van Benthem KJ, Magnusson A, Berg CW, Nielsen A, Skaug HJ, Machler M, Bolker BM. 2017. glmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. The R Journal 9, 378 ( 10.32614/RJ-2017-066) [DOI] [Google Scholar]
- 63.Graham MH. 2003. Confronting multicollinearity in ecological multiple regression. Ecology 84, 2809–2815. ( 10.1890/02-3114) [DOI] [Google Scholar]
- 64.Petrzelkova KJ, Hasegawa H, Moscovice LR, Kaur T, Issa M, Huffman MA. 2006. Parasitic nematodes in the chimpanzee population on Rubondo Island, Tanzania. Int. J. Primatol. 27, 767–776. ( 10.1007/s10764-006-9043-2) [DOI] [Google Scholar]
- 65.Gillespie TR, et al. 2010. Demographic and ecological effects on patterns of parasitism in eastern chimpanzees (Pan troglodytes schweinfurthii) in Gombe National Park, Tanzania. Am. J. Phys. Anthropol. 143, 534–544. ( 10.1002/ajpa.21348) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Howells ME, Pruetz J, Gillespie TR. 2010. Patterns of gastro-intestinal parasites and commensals as an index of population and ecosystem health: the case of sympatric western chimpanzees (Pan troglodytes verus) and Guinea baboons (Papio hamadryas papio) at Fongoli, Senegal. Am. J. Phys. Anthropol. 71, 1–7. [DOI] [PubMed] [Google Scholar]
- 67.Tokiwa T, Modry D, Ito A, Pomajbikova K, Peterzeljova KJ, Imai S. 2010. A new entodiniomorphid ciliate. Troglocorys cava n. g., n. sp., from the wild eastern chimpanzee (Pan troglodytes schweinfurthii) from Uganda. . J Eukaryot Microbiol. 57, 1–6. ( 10.1111/j.1550-7408.2009.00456.x) [DOI] [PubMed] [Google Scholar]
- 68.Ryan U, Cacciò SM. 2013. Zoonotic potential of Giardia. Int. J. Parasitol. 43, 943–956. ( 10.1016/j.ijpara.2013.06.001) [DOI] [PubMed] [Google Scholar]
- 69.Drakulovski P, Bertout S, Locatelli S, Butel C, Pion S, Mpoudi-Ngole E, Delaporte E, Peeters M, Mallié M. 2014. Assessment of gastrointestinal parasites in wild chimpanzees (Pan troglodytes troglodytes) in southeast Cameroon. Parasitol. Res. 113, 2541–2550. ( 10.1007/s00436-014-3904-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Muller MN, Wrangham RW. 2014. Mortality rates among Kanyawara chimpanzees. J. Hum. Evol. 66, 107–114. ( 10.1016/j.jhevol.2013.10.004) [DOI] [PubMed] [Google Scholar]
- 71.Gittleman JL, Thompson SD. 1988. Energy allocation in mammalian reproduction. Am. Zool. 28, 863–875. ( 10.1093/icb/28.3.863) [DOI] [Google Scholar]
- 72.Dufour DL, Sauther ML. 2002. Comparative and evolutionary dimensions of the energetics of human pregnancy and lactation. Am. J. Hum. Biol. 14, 584–602. ( 10.1002/ajhb.10071) [DOI] [PubMed] [Google Scholar]
- 73.Prentice AM, Prentice A. 1988. Energy costs of lactation. Ann. Rev. Nutr. 8, 63–79. ( 10.1146/annurev.nu.08.070188.000431) [DOI] [PubMed] [Google Scholar]
- 74.Leigh S. 2004. Brain growth, life history, and cognition in primate and human evolution. Am. J. Primatol. 62, 139–164. ( 10.1002/ajp.20012) [DOI] [PubMed] [Google Scholar]
- 75.Emery TM, Machanda ZP, Scully EJ, Enigk DK, Otali E, Muller MN, Goldberg TL, Chapman CA, Wrangham RW. 2018. Risk factors for respiratory illness in a community of wild chimpanzees (Pan troglodytes schweinfurthii). R. Soc. Open Sci. 5, 180840 ( 10.1098/rsos.180840) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Thompson ME, et al. 2020. Wild chimpanzees exhibit human-like aging of glucocorticoids regulation. Proc. Natl Acad. Sci. USA 117, 8424–8430. ( 10.1073/pnas.1920593117) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Blackwell AD, Tamayo MA, Beheim B, Trumble BC, Stieglitz J, Hooper PL, Martin M, Kaplan H, Gurven M. 2015. Helminth infection, fecundity, and age of first pregnancy in women. Science 350, 970–972. ( 10.1126/science.aac7902) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mor G, Cardenas I. 2010. The immune system in pregnancy: a unique complexity. Am. J. Reprod. Immunol. 63, 425–433. ( 10.1111/j.1600-0897.2010.00836.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mor G, Cardenas I, Abrahams V, Guller S. 2011. Inflammation and pregnancy: the role of the immune system at the implantation site. Ann. NY Acad. Sci. 1221, 80–87. ( 10.1111/j.1749-6632.2010.05938.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nussey DH, Froy H, Lemaitre J-F, Gaillard J-M, Austad SN. 2013. Senescence in natural populations of animals: widespread evidence and its implications for bio-gerontology. Ageing Res. Rev. 12, 214–225. ( 10.1016/j.arr.2012.07.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jones OR, et al. 2014. Diversity of ageing across the tree of life. Nature 505, 169–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hämäläinen A, Raharivololona B, Ravoniarimbinina P, Kraus C. 2015. Host sex and age influence endoparasite burdens in the gray mouse lemur. Front. Zool. 12, 25 ( 10.1186/s12983-015-0118-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Vaupel JW, Manton KG, Stallard E. 1979. The impact of heterogeneity in individual frailty on the dynamics of mortality. Demography 16, 439–454. ( 10.2307/2061224) [DOI] [PubMed] [Google Scholar]
- 84.Gesquiere LR, Altmann J, Archie EA, Alberts SC. 2018. Interbirth intervals in wild baboons: environmental predictors and hormonal correlates. Am. J. Phys. Anthropol. 166, 107–126. ( 10.1002/ajpa.23407) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Van Noordwijk AJ, de Jong G. 1986. Acquisition and allocation of resources: their influence on variation in life history tactics. Am. Nat. 128, 137–142. ( 10.1086/284547) [DOI] [Google Scholar]
- 86.Emery Thompson M, Muller MN, Sabbi K, Machanda ZP, Otali E, Wrangham RW. 2016. Faster reproductive rates trade off against offspring growth in wild chimpanzees. Proc. Natl Acad. Sci. USA 113, 7780–7785. ( 10.1073/pnas.1522168113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ellison PT. 2003. Energetics and reproductive effort. Am. J. Hum. Biol. 15, 342–351. ( 10.1002/ajhb.10152) [DOI] [PubMed] [Google Scholar]
- 88.Altizer S, et al. 2003. Social organization and parasite risk in mammals: integrating theory and empirical studies. Annu. Rev. Ecol. Evol. Syst. 34, 517–547. ( 10.1146/annurev.ecolsys.34.030102.151725) [DOI] [Google Scholar]
- 89.Balcomb SR, Chapman CA, Wrangham RW. 2000. Relationship between chimpanzee (Pan troglodytes) density and large, fleshy-fruit tree density: conservation implications. Am. J. Primatol. 51, 197–203. () [DOI] [PubMed] [Google Scholar]
- 90.Emery Thompson M, Wrangham RW. 2008. Diet and reproductive function in wild female chimpanzees (Pan troglodytes schweinfurthii) at Kibale National Park, Uganda. Am. J. Phys. Anthropol. 135, 171–181. ( 10.1002/ajpa.20718) [DOI] [PubMed] [Google Scholar]
- 91.Phillips SR, et al. 2020. Data from: Faecal parasites increase with age but not reproductive effort in wild female chimpanzees. Dryad Digital Repository. ( 10.5061/dryad.m0cfxpp0n) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Phillips SR, et al. 2020. Data from: Faecal parasites increase with age but not reproductive effort in wild female chimpanzees. Dryad Digital Repository. ( 10.5061/dryad.m0cfxpp0n) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Data for regression analyses are available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.m0cfxpp0n [91].