Abstract
Chimpanzees (Pan troglodytes) are a crucial model for understanding the evolution of human health and longevity. Cardiovascular disease is a major source of mortality during ageing in humans and therefore a key issue for comparative research. Current data indicate that compared to humans, chimpanzees have proatherogenic blood lipid profiles, an important risk factor for cardiovascular disease in humans. However, most work to date on chimpanzee lipids come from laboratory-living populations where lifestyles diverge from a wild context. Here, we examined cardiovascular profiles in chimpanzees living in African sanctuaries, who range semi-free in large forested enclosures, consume a naturalistic diet, and generally experience conditions more similar to a wild chimpanzee lifestyle. We measured blood lipids, body weight and body fat in 75 sanctuary chimpanzees and compared them to publicly available data from laboratory-living chimpanzees from the Primate Aging Database. We found that semi-free-ranging chimpanzees exhibited lower body weight and lower levels of lipids that are risk factors for human cardiovascular disease, and that some of these disparities increased with age. Our findings support the hypothesis that lifestyle can shape health indices in chimpanzees, similar to effects observed across human populations, and contribute to an emerging understanding of human cardiovascular health in an evolutionary context.
This article is part of the theme issue ‘Evolution of the primate ageing process’.
Keywords: primates, health, ageing, lifestyle, human evolution
1. Introduction
Cardiovascular disease, including disorders of the heart and blood vessels, is a major cause of human mortality, especially for adults over 65 years [1]. Yet increasing evidence indicates that high rates of cardiovascular disease, and particularly ischaemic heart disease, may be a relatively recent phenomenon in human evolution associated with the transition to a sedentary lifestyle [2,3]. Within small-scale subsistence societies, even the elderly can maintain relatively healthy cardiovascular profiles [4,5], suggesting cardiovascular disease may stem in part from human bodies being poorly adapted to novel industrialized environments [6,7]. Here, we test the hypothesis that the links between lifestyle, ageing patterns and cardiovascular disease risk are conserved with other primates by examining one of our closest living relatives, chimpanzees (Pan troglodytes). In particular, we examined biomarkers of cardiovascular health across the lifespan in chimpanzees living in African sanctuaries with species-typical diets and ranging opportunities. We then compared these to publicly available data on chimpanzees living in laboratories characterized by a more processed diet and limited access to physical activity.
In humans, cardiovascular disease typically involves atherosclerosis, in which arteries become narrowed by the build-up of plaque, restricting oxygenated blood flow to the heart and brain and increasing the likelihood of a heart attack or stroke [8]. Risk of plaque formation, and hence cardiovascular disease, is increased by high levels of proatherogenic blood lipids including cholesterol, triglycerides and especially low-density lipoproteins (LDLs), but decreased by high concentrations of circulating high-density lipoproteins (HDLs) [9,10]. High levels of proatherogenic blood lipids are often comorbid with obesity, which can have independent negative effects on cardiovascular health [11].
Cardiovascular disease is most prevalent among the elderly, and as such, has been described as a ‘disease of ageing’ [12]. Indeed, humans are characterized by an extremely slow life-history relative to other animals [13], which suggests that high rates of cardiovascular disease in later life might be explained in part by our species' extended lifespan. Yet research in small-scale subsistence societies has shown that humans can maintain excellent cardiovascular health into old age. For example, healthy blood lipid profiles have been reported across the lifespan in hunter–gatherer groups such as the Hadza [5], as well as in forager–horticulturalists like the Tsimane [4] and the Shuar [14]; cardiovascular disease is also rare or absent in these populations. These patterns of cardiovascular health have been attributed to high levels of physical activity and healthy diets—key features of subsistence lifestyles dependent on hunting, foraging or small-scale horticulture that characterize modern small-scale societies and also typify a majority of human evolutionary history [15]. That individuals in these groups can often reach ages exceeding 70 years without developing severe atherosclerosis [16] suggests that the high risk of cardiovascular disease observed in post-industrialized populations reflects an evolutionary mismatch rather than an inevitable artefact of increased human longevity [6,7].
Other apes, such as chimpanzees, provide a valuable comparative model for understanding the evolutionary history of human health and ageing. Given that human risk for cardiovascular disease may involve physiological pathways that are conserved from our last common ancestor with chimpanzees [17], chimpanzees provide a crucial comparative test of whether the sensitivity of blood lipids to lifestyle risk factors is a shared trait. In addition to their close genetic similarity to humans, chimpanzees are also very long-lived, with some individuals surviving into their 50s or 60s in the wild [18,19]. These shared life-history characteristics mean that chimpanzees can elucidate whether age-related changes in cardiovascular health are present in other long-lived species. Chimpanzees are also an important model for understanding cardiovascular health in particular because high concentrations of proatherogenic blood lipids are evidently not as life-threatening among chimpanzees as they are among humans. Although heart failure is the leading cause of death in captive apes, it typically results from interstitial myocardial fibrosis [17,20], which involves the accumulation of collagen in the myocardium, rather than from atherosclerosis [21]. Elevated proatherogenic blood lipids do not appear to increase risk of fibrosis or atherosclerosis in other apes, nor does this risk increase appreciably with age [20] (though fibrotic disease may be linked to obesity and ageing in gorillas [22]). Therefore, chimpanzee data can illuminate age-related changes in biomarkers of cardiac health independent of confounding survivorship effects that can occur in humans [23].
Prior work indicates that captive apes exhibit very high levels of proatherogenic blood lipids [24,25], in some cases even higher than those seen in post-industrial human populations [26]. However, most data come from laboratory or zoo populations that are often limited in their access to ranging space and consume a diet consisting mainly of primate chow [27] that is low in fat and simple carbohydrates, but contains highly processed grains absent from a wild diet [28]. Accordingly, captive chimpanzees typically have greater body weight than wild conspecifics and many are obese [29,30]. Thus, the lipid profiles of captive chimpanzees may not be typical of the species but could rather reflect changes in lifestyle analogous to those experienced by post-industrial human populations. Indeed, dietary cholesterol and physical activity levels are known to influence lipid profiles in humans [31,32] and other primate species [33–35]. Furthermore, little work to date has examined age-related change in these health biomarkers in apes, so it is currently unclear if ageing patterns seen in humans are shared with chimpanzees. Thus, studying the trajectory of cardiovascular health across the lifespan in apes living in more natural contexts can provide an integrative understanding of the evolution of human cardiovascular disease, as well as inform captive welfare strategies in these species [36–38].
In the current work, we assessed age-related trajectories of cardiovascular health markers in wild-born chimpanzees living in two African sanctuaries, and compared them to previous data from laboratory-living chimpanzees. In contrast to laboratory populations, chimpanzees living in African sanctuaries experience diets, activity levels and social contexts that are more similar to wild chimpanzee lifestyles [39]. African sanctuaries meet the ‘top 10’ suggestions for the care of captive chimpanzees in terms of space, environmental complexity and social groupings based on this species' wild behaviour [27], and sanctuary-living chimpanzees exhibit species-typical patterns of behaviour, cognition and physiology [40–42]. Yet, unlike in wild populations, blood collection is possible during routine health exams. We used these data to test whether (i) age-related changes and (ii) lifestyle effects on body weight and blood lipid concentrations are conserved across humans and chimpanzees. If so, we predicted healthier cardiovascular profiles in sanctuary-living than laboratory chimpanzees, especially during ageing.
2. Methods
(a). Sanctuary chimpanzee data collection
We measured body weight and skinfold thickness and collected blood samples from 75 chimpanzees during routine health checks at Tchimpounga Chimpanzee Sanctuary in the Republic of Congo (n = 25 chimpanzees) and Ngamba Island Chimpanzee Sanctuary in Uganda (n = 48 chimpanzees for all measures; two additional individuals were assessed only for body weight). Chimpanzees at Tchimpounga were assessed once, and all but one chimpanzee at Ngamba were assessed annually across two consecutive years. Our final sample for blood lipid measures consisted of 37 males and 36 females ranging in age from 1 to 33 years (mean = 20.2 years). In addition, we collated up to 10 years of historical body weight measurements from Ngamba.
All sanctuary chimpanzees at both sites were socially housed, and the majority had semi-free-ranging access to large tracts of tropical forest enclosures (15–40 hectares). In addition to the wild foods they could eat in the forests, individuals were supplemented with a variety of species-appropriate food, including fruits and vegetables, porridge and milk (primarily for infants), but not the high-calorie chow characteristic of laboratory populations. Sanctuary apes are primarily wild-born orphans mother-reared in the wild for 1–3 years, although some individuals may have arrived later after previously living in another sanctuary or a zoo setting, and four individuals were born at the sanctuaries (contraception failures). Upon arrival individuals are rapidly integrated into conspecific groups at the sanctuary. We used age estimates made by sanctuary veterinarians at arrival, validated by measurements of body weight and dental emergence when available, following prior work [43,44]. Many chimpanzees at Ngamba likely comprise subspecies Pan troglodytes schweinfurthii, and many at Tchimpounga may comprise P. t. troglodytes, but detailed subspecies information is unknown, and animals are not necessarily confiscated in their place of origin.
All blood sample collection and morphometric measurements occurred during routine health examinations performed by sanctuary veterinarians. Body weight was measured using a scale, and skinfold thickness was assessed using a Lange caliper [45] at four locations (biceps, triceps, inguinal and subscapular). Skinfold thickness is an index of subcutaneous fat, which correlates with lipid levels and cardiovascular disease risk in humans [46]. This approach provides a more direct measure of adiposity than body weight alone and is feasible with sanctuary populations (unlike more invasive dual energy X-ray absorptiometry). We repeated all skinfold measurements for a subset of eight chimpanzees, and intra-observer reliability was high (r = 0.9). We conducted rapid on-site diagnostics of total cholesterol, triglycerides, HDLs, and LDLs using a portable Alere Cholestech LDX System, which measures lipid concentrations in a small sample (40 µl) using enzymatic methodology (see electronic supplementary material for assay details).
(b). Laboratory chimpanzee comparison dataset
We then extracted publicly available data on laboratory-living chimpanzees from the Primate Aging Database (accessed September 2019) [47]. This repository contains data from healthy, non-experimental captive primates [30,48,49] including three US laboratory chimpanzee populations: Alamogordo Primate Facility, M.D. Anderson Cancer Center, and Yerkes National Primate Research Center. We extracted data on age, sex, body weight, total cholesterol, triglycerides, HDLs and LDLs as available. The total dataset comprised 205 males and 266 females ranging in age from 0 to 59.25 years (mean = 16.3 years); note that each timepoint did not necessarily include all relevant measurements. The database categorized sites by housing status (indoors; outdoors; or some outdoor access) and diet (only primate chow; or chow supplemented with fruit and vegetables) but does not provide detailed information on individual diets, reproductive status, parity or cause of death. We expect laboratory chimpanzees to be primarily subspecies P. t. verus but again did not have specific individual data. Individuals in this sample approach 60 years, so it is unlikely that this older cohort are second-generation laboratory chimpanzees; any differences from the sanctuary sample are therefore unlikely to be due entirely to maternal effects.
(c). Statistical analyses and data availability
We analysed data with linear mixed models using the lmer function in R v. 3.6.1. Our general approach was to first construct a base model with random effects of subject identity (to control for unbalanced repeated measurements) and sex, and then test the significance of fixed effects of subject's age, facility (laboratory or sanctuary), and an age X facility interaction in subsequent models using likelihood ratio tests. Post hoc tests were then conducted using the emtrends and emmeans functions to assess age-related trends and pairwise comparisons. Additional analyses examined variation between sites to test for more nuanced lifestyle effects (see electronic supplementary material). We used age as a continuous predictor but some figures depict age cohorts for ease of interpretation (juveniles less than 15 years, adults 15–30 years, and older adults over 30 [50]).
3. Results
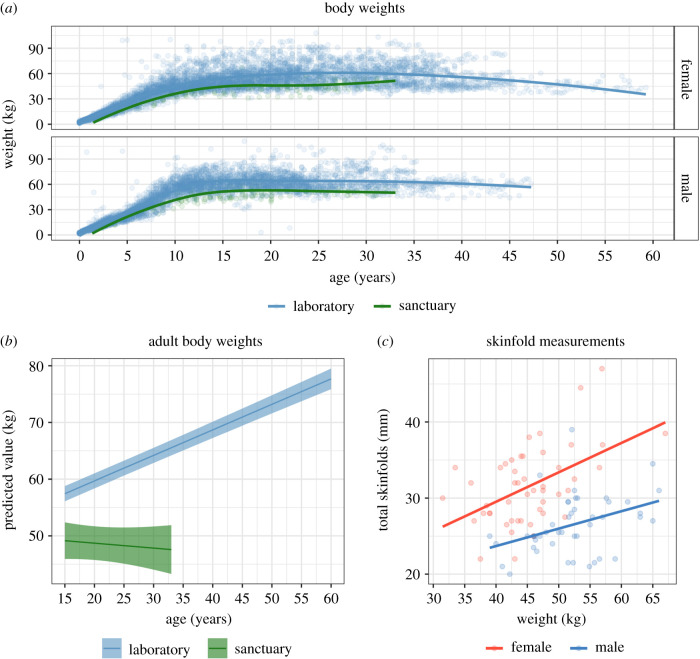
We first addressed whether sanctuary-living and laboratory-living chimpanzees differed in body weights (see figure 1a; n = 465 measurements from 75 sanctuary chimpanzees, 8652 measurements from 462 laboratory chimpanzees). As initial inspection of the data suggested nonlinear age effects, we added both age and age2 in the second model, which improved model fit [χ2 = 12 294.00, d.f. = 2, p < 0.0001]; post hoc comparisons showed that weight increased with age. We then added facility (laboratory or sanctuary) in the third model, which further improved fit [χ2 = 77.11, d.f. = 1, p < 0.0001]: sanctuary chimpanzees had lower weight overall than laboratory chimpanzees. We finally added the age X facility interaction, which further improved fit [χ2 = 101.08, d.f. = 2, p < 0.0001]; comparison of age trends revealed that weight increased more rapidly with age in laboratory compared to sanctuary chimpanzees [p < 0.001]. In the full model, sex (males heavier than females), facility, age and age X facility were all significant predictors (see electronic supplementary material, table S1 for parameters). We also ran the same basic analysis on the subset of data including only adults, and again found that weight increased more with age in laboratory chimpanzees (see figure 1b for plot of estimated effects, and electronic supplementary material for details).
Figure 1.
Body weight and adiposity. (a) Scatterplot of body weight, panelled by sex. Line indicates loess fit. (b) Estimates of age-related change in body weight in adults. Ribbons indicate 95% confidence interval estimates from models accounting for individual identity, sex, age and facility, and are truncated at the age range for each site. (c) Scatterplot of body weight by skinfold summary score in adult sanctuary chimpanzees, split by sex. Line indicates linear fit. (Online version in colour.)
We then confirmed that total body weight was a reasonable proxy for adiposity using skinfold measurements from sanctuary chimpanzees. We examined a summary skinfold score (combining subscapular, inguinal, biceps and triceps measurements; figure 1c), limiting analysis to 61 adult sanctuary chimpanzees with complete data. The base model controlled for subject, sex and age. Model comparisons showed that individuals with heavier body weights had greater total skinfold thickness [χ2 = 21.55, d.f. = 1, p < 0.0001]. Females also had thicker skinfold measurements than males when accounting for age and weight (see electronic supplementary material, table S2). We found similar effects for each skinfold measurement when analysed individually (see electronic supplementary material). This indicates that lower weights in the sanctuary populations likely reflect lower adiposity, not just differences in body size across populations or subspecies.
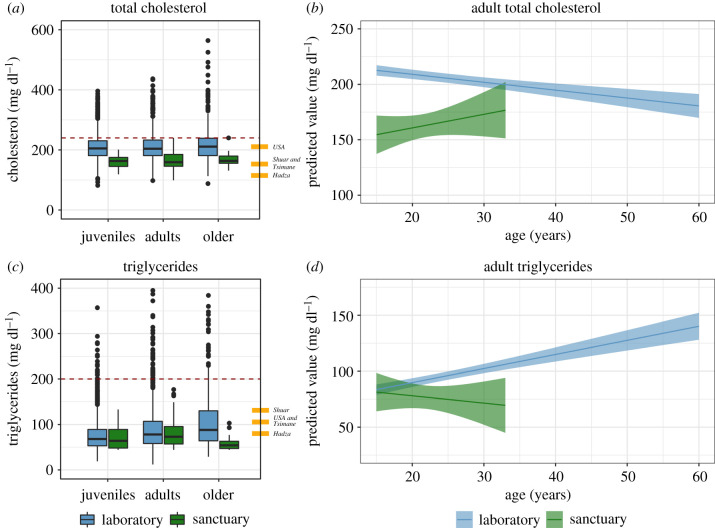
We next addressed whether sanctuary-living and laboratory-living chimpanzees differed in their total cholesterol (figure 2a; n = 120 measurements from 73 sanctuary chimpanzees, 5466 measurements from 462 laboratory chimpanzees). In total, 1116 measurements (20.4% of the total) from laboratory chimpanzees were above the adult human cardiovascular disease risk threshold of 240 mg dl−1 [51], whereas only 2 (1.6%) from sanctuary chimpanzees were. Total cholesterol surprisingly decreased with age in this sample [χ2 = 397.93, d.f. = 1, p < 0.0001], and sanctuary chimpanzees had overall lower cholesterol [χ2 = 52.81, d.f. = 1, p < 0.0001]. Including age X facility also further improved fit [χ2 = 7.43, d.f. = 1, p < 0.01]: whereas sanctuaries did not show major age-related change, laboratories showed a decline with age [p < 0.01]. In the full model, sex (males lower than females), age, facility and age X facility were significant predictors (see electronic supplementary material, table S3). As this pattern could be due to higher dietary cholesterol in infants and juveniles, we conducted the same analysis on only adults and found largely similar results (see figure 2b and electronic supplementary material). We also examined whether body weight differences between sanctuary and laboratory populations could account for this pattern by controlling for body weight (note that body weights were available for only 55% of laboratory cholesterol measurements), and again found largely similar results (see electronic supplementary material).
Figure 2.
Total cholesterol and triglycerides. (a) Boxplots of total cholesterol in chimpanzees (adult human reference values: Hadza, 115 mg dl−1; Tsimane, 153 mg dl−1; Shuar, 153 mg dl−1; USA, 211 mg dl−1; dashed line indicates human cardiac risk threshold: over 240 mg dl−1). (b) Estimates of age-related changes in adult cholesterol. (c) Boxplots of triglycerides in chimpanzees (human reference values: Hadza, 80 mg dl−1; Tsimane, 106 mg dl−1; Shuar, 131 mg dl−1; USA, 105 mg dl−1; dashed line indicates human cardiac risk threshold: over 200 mg dl−1). (d) Estimates of age-related changes in adult triglycerides. Boxplots cluster data by age cohort and facility; line indicates group median; whiskers reflect inter-quartile range; dots indicate outliers. In estimate plots, ribbons indicate 95% confidence interval estimates from model accounting for individual identity, sex, age and facility and are truncated at the age range represented for each site. Human values reported in ref. [3] Hadza, [5]; Tsimane, [4]; Shuar, [14]; USA, [32]. Risk thresholds are from the National Cholesterol Education Program [51], following Raichlen et al. [5]. (Online version in colour.)
We next examined triglycerides (see figure 2c; n = 120 measurements from 73 sanctuary chimpanzees, 5240 measurements from 462 laboratory chimpanzees). One hundred and two measurements (1.9% of the total) from laboratory chimpanzees were above the adult human cardiovascular disease risk threshold of 200 mg dl−1 [51], whereas none from sanctuary chimpanzees were. Triglycerides increased with age [χ2 = 353.52, d.f. = 1, p < 0.0001]; sanctuary chimpanzees had overall lower triglycerides [χ2 = 12.11, d.f. = 1, p < 0.001]; and including age X facility improved fit [χ2 = 6.90, d.f. = 1, p < 0.01]: triglycerides increased more with age in the laboratory populations [p < 0.01; see electronic supplementary material, table S4]. Data checks controlling for body weight and examining only adults indicated largely similar results with two main differences: (i) males had lower triglycerides only when controlling for body weight and when limiting analyses to adults; and (ii) body weight predicted higher triglycerides, but the inclusion of the age X facility interaction did not improve fit when controlling for body weight, suggesting that population differences in triglycerides may be due to differences in body weights (see figure 2d and electronic supplementary material).
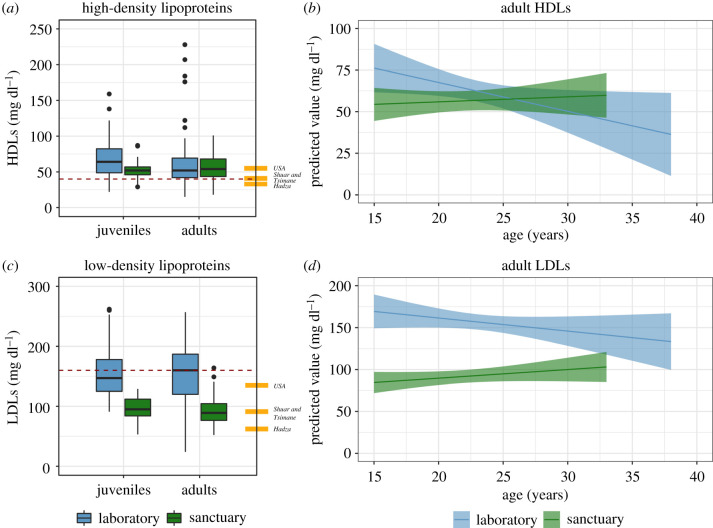
We then examined HDLs and LDLs. While we had estimates for both lipids for all the sanctuary chimpanzees in our dataset (120 measurements from 73 chimpanzees), there were fewer observations from laboratory chimpanzees and all were from a single site with a restricted age range (216 measurements from 85 chimpanzees for HDLs; 206 from 85 chimpanzees for LDLs; maximum age of 37 years). An initial comparison of HDLs (which are cardioprotective in humans) showed that 34 measurements (15.7%) from laboratory chimpanzees were below the healthy threshold indicative of risk in adult humans of 40 mg dl−1 [51], and 16 (13.3%) were in sanctuary chimpanzees. HDLs decreased with age [χ2 = 9.44, d.f. = 1, p < 0.01]; sanctuaries tended to have lower levels overall than laboratories [χ2 = 3.57, d.f. = 1, p = 0.06]; and inclusion of age X facility improved fit [χ2 = 7.10, d.f. = 2, p < 0.05] with greater declines in laboratories. There were similar findings from the adult and weight-controlled samples (see figure 3a,b and electronic supplementary material, table S5). Additionally, total cholesterol to HDLs ratios, high values of which are used as an index of cardiovascular disease risk in humans [10], were lower in sanctuary chimpanzees (see electronic supplementary material). High LDL is also a major cardiovascular disease risk factor in humans, and 93 laboratory measurements (45.1%) were above the adult human cardiovascular risk threshold of 150 mg dl−1 [51], whereas only 1 (0.01%) was in sanctuary chimpanzees. LDL levels decreased with age [χ2 = 15.98, d.f. = 1, p < 0.0001]; and sanctuaries had lower levels than laboratories [χ2 = 100.13, d.f. = 1, p < 0.0001]; but there was no improvement by including age X lifestyle [χ2 = 1.14, d.f. = 1, p = 0.29]. These findings generally held with additional checks described above (see figure 3c,d and electronic supplementary material, table S6). This indicates that there were greater differences in LDLs than in HDLs across the populations, with lower LDL levels in sanctuaries.
Figure 3.
High-density and low-density lipoproteins. (a) Boxplots of HDLs in chimpanzees (adult human reference values: Hadza, 34 mg dl−1; Tsimane, 39 mg dl−1; Shuar, 41 mg dl−1; USA, 55 mg dl−1; dashed line indicates human cardiac risk threshold: under 40 mg dl−1). (b) Estimates of age-related changes in adult HDLs. (c) Boxplots of LDLs in chimpanzees (human reference values: Hadza, 62 mg dl−1; Tsimane, 93 mg dl−1; Shuar, 89 mg dl−1; USA, 135 mg dl−1; dashed line indicates human cardiac risk threshold: over 160 mg dl−1). (d) Estimates of age-related changes in adult LDLs. Boxplots cluster data by age cohort and facility; line indicates group median; whiskers reflect inter-quartile range; dots indicate outliers. In estimate plots, ribbons indicate 95% confidence interval estimates from model accounting for individual identity, sex, age and facility and are truncated at the age range represented for each site. Human values reported in ref. [3]; Hadza, [5]; Tsimane, [4]; Shuar, [14]; USA, [32]. Risk thresholds are from the National Cholesterol Education Program [51], following Raichlen et al. [5]. (Online version in colour.)
Our final set of analyses used information on diet and housing context to examine how more nuanced lifestyle characteristics impact body weights and blood lipids. In addition to sanctuary chimpanzees categorized as semi-free-ranging with plant-based diets, information provided by the laboratories to the Primate Aging Database indicated that the laboratory sample included three distinct lifestyle categories: (i) only indoor access with chow diet; (ii) mixed indoor/outdoor access with chow diet; and (iii) outdoor access with chow diet supplemented by fruits and vegetables. We examined how these lifestyle categories impacted body weight, total cholesterol and triglycerides in adults (note that all the HDL and LDL data came from the outdoors/mixed diet laboratory population so we could not assess those here). These analyses first confirmed that the sanctuaries generally exhibited lower lipid levels compared to the laboratory populations, even when accounting for lifestyle variation among laboratories. Second, laboratory chimpanzees living indoors had lower overall body weights and lower slopes for age-related change than other laboratories, which may reflect reduced muscle mass. Third, only the outdoors/mixed diet laboratory population exhibited age-related decreases in total cholesterol, likely driving the counterintuitive finding that cholesterol declined with age. Finally, there were greater age-related increases in triglycerides in the two laboratory populations eating chow than in the laboratory population with outdoor access and a mixed diet (see electronic supplementary material, table S7). A final analysis comparing body weights, total cholesterol and triglycerides across all five sites supported our general approach of collapsing the two sanctuary sites into a single lifestyle category, as we found that the two sanctuary sites with similar lifestyles exhibited similar cardiovascular profiles despite likely comprising different subspecies, whereas laboratory chimpanzees, who are likely the same subspecies, exhibited differential profiles based on lifestyle (see electronic supplementary material, table S8).
4. Discussion
We assessed the impact of old age and lifestyle on chimpanzee cardiovascular profiles to understand evolutionary processes shaping human and nonhuman ape cardiovascular health. We found that wild-born, semi-free-ranging chimpanzees living in African sanctuaries had consistently lower body weights as well as lower total cholesterol, triglycerides, and LDL levels compared to laboratory populations. This indicates that, rather than necessarily having higher levels of proatherogenic blood lipids compared to humans, cardiovascular profiles in chimpanzees are influenced by lifestyle. Chimpanzees with a plant-based diet and substantial opportunity for physical activity generally fell in healthy human ranges, while laboratory chimpanzees more commonly fell in the range indicative of cardiovascular disease risk for humans, similar to sedentary people [24–26]. While veterinary care and controlled diets experienced by the study populations make direct comparisons challenging, our findings support the hypothesis that lifestyle effects on cardiovascular health are evolutionarily conserved across chimpanzees and humans and thus were likely present in our last common ancestor. More fine-grained data across lifestyle contexts may reveal how these biomarkers are related to future health and longevity in apes [17,22].
Unlike in humans, where biomarkers of cardiovascular disease risk increase during ageing, we found mixed evidence for age-related change in chimpanzees. Body weight and triglycerides increased during ageing in the laboratory populations, but total cholesterol, HDLs and LDLs all unexpectedly decreased. This decline may be related to a management intervention in the laboratories, such as a change in diet, within the lifetime of the oldest cohort. This would be consistent with the finding that the decrease in cholesterol was driven by one site with the lowest levels of triglycerides (reflecting relatively lower dietary fat) among the laboratories. Unfortunately, detailed care and feeding information was not included in the Primate Aging Database, so we are not able to directly assess this. However, because no other site actually showed significant increases in cholesterol with age, our data are most consistent with the conclusion that age-related increases in total cholesterol are specific to post-industrial human populations [3].
We further found that male chimpanzees exhibited lower proatherogenic blood lipid levels and lower adiposity than females across the lifespan. Body fat is also higher in human women compared with men cross-culturally [3], as is prevalence of obesity in the post-industrial world [52]. This similarity is likely due to the adaptive value of fat stores in unpredictable environments [53] and the high energetic demands of female reproduction [54]. By contrast, blood lipids are typically lower in women, although lipid levels tend to increase after menopause [55] with the loss of estrogen [56]. However, because menopause is a derived human trait that rarely if ever occurs in other primates [57], this cannot explain the sex differences observed in the current study. Alternatively, this finding may reflect an example of the male–female health-survival paradox [58], which appears to have deep evolutionary roots [59]. In chimpanzees, females are reported to live longer [18,19] and may be more prone to some illnesses, although heart disease is more prevalent in males [20].
A major outstanding question from this work is why similar levels of adiposity and blood lipids result in differential cardiovascular disease risk in chimpanzees and humans. One possibility is that humans are more sensitive to or experience more extreme evolutionary mismatches [6,7], particularly regarding diet and physical activity. Hunter–gatherer diets are characterized by high-quality but difficult to obtain foods such as fatty meat and carbohydrate-rich honey [60], and as a result humans appear to have evolved to fatten in times of food abundance [53]. Thus, high-calorie post-industrial human diets often lead to obesity, especially when paired with high rates of physical inactivity [61,62]. By contrast, even captive chimpanzees with nutrient-dense diets exhibit low body fat relative to subsistence human populations [63]. Humans may be further predisposed to atherosclerosis due to genetic adaptations to increased consumption of red meat in the recent past [64]. Finally, while chimpanzees are adapted to short bursts of resistance activity such as climbing or aggression [65], humans are thought to be adapted to consistent moderate activity like walking to facilitate wider ranging during foraging [66]. Human hearts therefore maximize cardiac output during bipedal endurance activity and have been proposed to show a greater response to sedentism compared with other apes [65].
A final important consideration is the role of chronic low-grade inflammation [67]. In post-industrialized human populations, inflammation is often associated with obesity and other risk factors of cardiovascular disease and is additionally proposed to be directly involved in the pathogenesis of atherosclerosis [68]. This may again be linked to a mismatch between an adaptive pro-inflammatory response and reduced pathogen exposure [69,70]. That some small-scale societies exhibit extremely low rates of cardiovascular disease despite having high inflammatory biomarkers supports the hypothesis that a high parasite load may protect against atherosclerosis in combination with healthy lipid profiles [70]. Current data suggest inflammation may not be associated with ischaemic heart disease in other great apes [20], but systematic, longitudinal studies are lacking. Additional inflammatory biomarker data from naturalistic chimpanzee populations living under greater pathogen stress will help tease apart the links between ageing, lifestyle and cardiac health across humans and other great apes.
Supplementary Material
Acknowledgements
We thank Rosemary Bettle, Alex Tumukunde and Titus Mukungu for assistance with data collection, and Emily Otali and Audrey Salvy for assistance with research permissions. Melissa Emery Thompson, Tony Goldberg, Herman Pontzer, David Raichlen and Ian Wallace provided helpful advice and comments about methods or the manuscript. We thank the animal caretakers at both Ngamba Island Chimpanzee Sanctuary and Tchimpounga Chimpanzee Sanctuary, as well as the Uganda Wildlife Authority and the Congolese Ministry of Scientific Research for supporting our research.
Ethics
Research at Ngamba Island Chimpanzee Sanctuary was approved by the Uganda Wildlife Authority and the Uganda National Council for Science and Technology, and research at Tchimpounga Chimpanzee Sanctuary was approved by the Ministry of Scientific Research and Technological Innovation in Congo and the Jane Goodall Institute. Work at both sites was also approved by the Institutional Animal Care and Use Committee at Harvard University (#14-07-206-1) and at the University of Michigan (#8102). Research practices and animal care procedures at both sites complied with the Pan-African Sanctuary Alliance standards.
Data accessibility
Data collected for this project is available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.4xgxd2571 [71]. The comparison dataset is available from the Primate Aging Database at https://primatedatabase.org/.
Authors' contributions
M.F.C. and A.G.R. designed the study; M.F.C., A.C., J.R., L.A., S.F.N., R.A. and A.G.R. collected the sanctuary data; M.F.C. and A.G.R. extracted the comparison data from the Primate Aging Database; M.F.C. and A.G.R. analysed the data; M.F.C. and A.G.R. wrote the manuscript with input from all authors.
Competing interests
We declare we have no competing interests.
Funding
This study was funded by the National Institute on Aging and the Office for Research on Women's Health of the National Institutes of Health (Award R01AG049395) and a Sloan Foundation Fellowship to A.G.R.
References
- 1.Xu J, Murphy SL, Kochanek KD, Arias E. 2016. Mortality in the United States, 2015. NCHS Data Brief 267, 1–8. [PubMed] [Google Scholar]
- 2.Allam AH, et al. 2011. Atherosclerosis in ancient Egyptian mummies: the Horus study. JACC Cardiovasc. Imaging 4, 315–327. ( 10.1016/j.jcmg.2011.02.002) [DOI] [PubMed] [Google Scholar]
- 3.Pontzer H, Wood BM, Raichlen DA. 2018. Hunter-gatherers as models in public health. Obes. Rev. 19, 24–35. ( 10.1111/obr.12785) [DOI] [PubMed] [Google Scholar]
- 4.Kaplan H, et al. 2017. Coronary atherosclerosis in indigenous South American Tsimane: a cross-sectional cohort study. Lancet 389, 1730–1739. ( 10.1016/S0140-6736(17)30752-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Raichlen DA, et al. 2017. Physical activity patterns and biomarkers of cardiovascular disease risk in hunter-gatherers. Am. J. Hum. Biol. 29, e22919 ( 10.1002/ajhb.22919) [DOI] [PubMed] [Google Scholar]
- 6.Eaton SB, Konner M, Shostak M. 1988. Stone Agers in the fast lane: chronic degenerative diseases in evolutionary perspective. Am. J. Med. 84, 739–749. ( 10.1016/0002-9343(88)90113-1) [DOI] [PubMed] [Google Scholar]
- 7.Lieberman DE. 2013. The story of the human body: evolution, health, and disease. New York, NY: Pantheon Books. [PubMed] [Google Scholar]
- 8.Falk E. 2006. Pathogenesis of atherosclerosis. J. Am. Coll. Cardiol. 47, C7–C12. ( 10.1016/j.jacc.2005.09.068) [DOI] [PubMed] [Google Scholar]
- 9.Castelli W. 1984. Epidemiology of coronary heart disease: the Framingham study. Am. J. Med. 76, 4–12. ( 10.1016/0002-9343(84)90952-5) [DOI] [PubMed] [Google Scholar]
- 10.Linton MF, Yancey PG, Davies SS, Jerome WG, Linton EF, Song WL, Doran AC, Vickers KC. 2000. The role of lipids and lipoproteins in atherosclerosis. South Dartmouth, MA: Endotext. [Google Scholar]
- 11.Lavie CJ, Milani RV, Ventura HO. 2009. Obesity and cardiovascular disease. J. Am. Coll. Cardiol. 53, 1925–1932. ( 10.1016/j.jacc.2008.12.068) [DOI] [PubMed] [Google Scholar]
- 12.Chiao YA, Lakatta E, Ungvari Z, Dai D-F, Rabinovitch P. 2016. Cardiovascular disease and aging. In Advances in geroscience (eds Sierra F, Kohanski R), pp. 121–160. Cham, Switzerland: Springer International. [Google Scholar]
- 13.Gurven MD, Gomes CM. 2017. Mortality, senescence, and life span. In Chimpanzees and human evolution (eds Muller MN, Wrangham RW, Pilbeam DR), pp. 181–216. Cambridge, MA: Belknap Press of Harvard University Press. [Google Scholar]
- 14.Liebert MA, Snodgrass JJ, Madimenos FC, Cepon TJ, Blackwell AD, Sugiyama LS. 2013. Implications of market integration for cardiovascular and metabolic health among an indigenous Amazonian Ecuadorian population. Ann. Hum. Biol. 40, 228–242. ( 10.3109/03014460.2012.759621) [DOI] [PubMed] [Google Scholar]
- 15.Marlowe FW. 2005. Hunter-gatherers and human evolution. Evol. Anthropol. 14, 54–67. ( 10.1002/evan.20046) [DOI] [PubMed] [Google Scholar]
- 16.Gurven M, Kaplan H. 2007. Longevity among hunter-gatherers: a cross-cultural examination. Popul. Dev. Rev. 33, 321–365. ( 10.1111/j.1728-4457.2007.00171.x) [DOI] [Google Scholar]
- 17.Varki N, et al. 2009. Heart disease is common in humans and chimpanzees, but is caused by different pathological processes. Evol. Appl. 2, 101–112. ( 10.1111/j.1752-4571.2008.00064.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Muller MN, Wrangham RW. 2014. Mortality rates among Kanyawara chimpanzees. J. Hum. Evol. 66, 107–114. ( 10.1016/j.jhevol.2013.10.004) [DOI] [PubMed] [Google Scholar]
- 19.Wood BM, Watts DP, Mitani JC, Langergraber KE. 2017. Favorable ecological circumstances promote life expectancy in chimpanzees similar to that of human hunter-gatherers. J. Hum. Evol. 105, 41–56. ( 10.1016/j.jhevol.2017.01.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lowenstine LJ, McManamon R, Terio KA. 2016. Comparative pathology of aging great apes: bonobos, chimpanzees, gorillas, and orangutans. Vet. Pathol. 53, 250–276. ( 10.1177/0300985815612154) [DOI] [PubMed] [Google Scholar]
- 21.Kong P, Christia P, Frangogiannis NG. 2014. The pathogenesis of cardiac fibrosis. Cell. Mol. Life Sci. 71, 549–574. ( 10.1007/s00018-013-1349-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dennis PM, et al. 2019. Cardiac disease is linked to adiposity in male gorillas (Gorilla gorilla gorilla). PLoS ONE 14, 1–8. ( 10.1371/journal.pone.0218763) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kleinbaum DG, Morgenstern H, Kupper LL. 1981. Selection bias in epidemiologic studies. Am. J. Epidemiol. 113, 452–463. ( 10.1093/oxfordjournals.aje.a113113) [DOI] [PubMed] [Google Scholar]
- 24.Herndon JG, Tigges J. 2001. Hematologic and blood biochemical variables of captive chimpanzees: cross-sectional and longitudinal analyses. Comp. Med. 51, 60–69. [PubMed] [Google Scholar]
- 25.Ihrig M, Tassinary LG, Bernacky B, Keeling ME. 2001. Hematologic and serum biochemical reference intervals for the chimpanzee (Pan troglodytes) categorized by age and sex. Comp. Med. 51, 30–37. [PubMed] [Google Scholar]
- 26.Howell S, Hoffman K, Bartel L, Schwandt M, Morris J, Fritz J. 2003. Normal hematologic and serum clinical chemistry values for captive chimpanzees (Pan troglodytes). Comp. Med. 53, 413–423. [PubMed] [Google Scholar]
- 27.Pruetz J, McGrew WC. 2001. What does a chimpanzee need? Using natural behavior to guide the care and management of captive populations. Spec. Top. Primatol. 2, 16–37. [Google Scholar]
- 28.Wilson ME, Fisher J, Fischer A, Lee V, Harris RB, Bartness TJ. 2008. Quantifying food intake in socially housed monkeys: social status effects on caloric consumption. Physiol. Behav. 94, 586–594. ( 10.1016/j.physbeh.2008.03.019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pusey AE, Oehlert GW, Williams JM, Goodall J. 2005. Influence of ecological and social factors on body mass of wild chimpanzees. Int. J. Primatol. 26, 3–31. ( 10.1007/s10764-005-0721-2) [DOI] [Google Scholar]
- 30.Videan EN, Fritz J, Murphy J. 2007. Development of guidelines for assessing obesity in captive chimpanzees (Pan troglodytes). Zoo Biol. 26, 93–104. ( 10.1002/zoo.20122) [DOI] [PubMed] [Google Scholar]
- 31.Hu FB, Willett WC. 2002. Optimal diets for prevention of coronary heart disease. J. Am. Med. Assoc. 288, 2569 ( 10.1001/jama.288.20.2569) [DOI] [PubMed] [Google Scholar]
- 32.Van Der Velde J, Savelberg H, Schaper N, Koster A. 2015. Moderate activity and fitness, not sedentary time, are independently associated with cardio-metabolic risk in U.S. adults aged 18–49. Int. J. Environ. Res. Public Health 12, 2330–2343. ( 10.3390/ijerph120302330) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mattison JA, et al. 2012. Impact of caloric restriction on health and survival in rhesus monkeys from the NIA study. Nature 489, 318–321. ( 10.1038/nature11432) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kleinert M, et al. 2018. Animal models of obesity and diabetes mellitus. Nat. Rev. Endocrinol. 14, 140–162. ( 10.1038/nrendo.2017.161) [DOI] [PubMed] [Google Scholar]
- 35.Getz GS, Reardon CA. 2012. Animal models of atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 32, 1104–1115. ( 10.1161/ATVBAHA.111.237693) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schmidt DA, Ellersieck MR, Cranfield MR, Karesh WB. 2006. Cholesterol values in free-ranging gorillas (Gorilla gorilla gorilla and Gorilla beringei) and Bornean orangutans (Pongo pygmaeus). J. Zoo Wildl. Med. 37, 292–300. ( 10.1638/05-040.1) [DOI] [PubMed] [Google Scholar]
- 37.Atencia R, Revuelta L, Somauroo JD, Shave RE. 2015. Electrocardiogram reference intervals for clinically normal wild-born chimpanzees (Pan troglodytes). Am. J. Vet. Res. 76, 688–693. ( 10.2460/ajvr.76.8.688) [DOI] [PubMed] [Google Scholar]
- 38.Drane AL, et al. 2019. Cardiac structure and function characterized across age groups and between sexes in healthy wild-born captive chimpanzees (Pan troglodytes) living in sanctuaries. Am. J. Vet. Res. 80, 547–557. ( 10.2460/ajvr.80.6.547) [DOI] [PubMed] [Google Scholar]
- 39.Stokes R, Tully G, Rosati AG. 2018. Pan African Sanctuary Alliance: securing a future for the African great apes. Int. Zoo Yearbook 52, 173–181. ( 10.1111/izy.12174) [DOI] [Google Scholar]
- 40.Wobber V, Hare B. 2011. Psychological health of orphan bonobos and chimpanzees in African sanctuaries. PLoS ONE 6, e17147 ( 10.1371/journal.pone.0017147) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rosati AG, Herrmann E, Kaminski J, Krupenye C, Melis AP, Schroepfer K, Tan J, Warneken F, Wobber V. 2013. Assessing the psychological health of captive and wild apes: a response to Ferdowsian et al. (2011). J. Comp. Psychol. 127, 329–336. ( 10.1037/a0029144) [DOI] [PubMed] [Google Scholar]
- 42.Wobber V, Herrmann E, Hare B, Wrangham R, Tomasello M. 2014. Differences in the early cognitive development of children and great apes. Dev. Psychobiol. 56, 547–573. ( 10.1002/dev.21125) [DOI] [PubMed] [Google Scholar]
- 43.Wobber V, Wrangham R, Hare B. 2010. Bonobos exhibit delayed development of social behavior and cognition relative to chimpanzees. Curr. Biol. 20, 226–230. ( 10.1016/j.cub.2009.11.070) [DOI] [PubMed] [Google Scholar]
- 44.Rosati AG. 2019. Heterochrony in chimpanzee and bonobo spatial memory development. Am. J. Phys. Anthropol. 169, 302–321. ( 10.1002/ajpa.23833) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.CDC/NCHS. 2007. National Health and Nutrition Examination Survey (NHANES) anthropometry procedures manual. Hyattsville, MD: US Department of Health and Human Services, Centers for Disease Control and Prevention. https://wwwn.cdc.gov/nchs/data/nhanes/2007-2008/manuals/manual_an.pdf
- 46.Hariri AA, Oliver NS, Johnston DG, Stevenson JC, Godsland IF. 2013. Adiposity measurements by BMI, skinfolds and dual energy X-ray absorptiometry in relation to risk markers for cardiovascular disease and diabetes in adult males. Dis. Markers 35, 753–764. ( 10.1155/2013/763907) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.2019. Primate Aging Database. See primatedatabase.org.
- 48.Kemnitz JW. 2019. Database for indices of aging in nonhuman primates. Innov. Aging 3, S957 ( 10.1093/geroni/igz038.3472) [DOI] [Google Scholar]
- 49.Dansereau G, Wey TW, Legault V, Brunet MA, Kemnitz JW, Ferrucci L, Cohen AA. 2019. Conservation of physiological dysregulation signatures of aging across primates. Aging Cell 18, e12925 ( 10.1111/acel.12925) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hill K, Boesch C, Goodall J, Pusey A, Williams J, Wrangham R. 2001. Mortality rates among wild chimpanzees. J. Hum. Evol. 40, 437–450. ( 10.1006/jhev.2001.0469) [DOI] [PubMed] [Google Scholar]
- 51.Expert Panel on Detection, Evaluation and Treatment of High Blood Cholesterol in Adults. 2001. Executive summary of the third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III). J. Am. Med. Assoc. 285, 2486–2497. ( 10.1001/jama.285.19.2486) [DOI] [PubMed] [Google Scholar]
- 52.Kanter R, Caballero B. 2012. Global gender disparities in obesity: a review. Adv. Nutr. 3, 491–498. ( 10.3945/an.112.002063) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Neel J. 1962. Diabetes mellitus: a ‘thrifty’ genotype rendered detrimental by ‘progress’? Am. J. Hum. Genet. 77, 694–703. [PMC free article] [PubMed] [Google Scholar]
- 54.Ellison. 2003. On fertile ground: a natural history of human reproduction. Cambridge, MA: Harvard University Press. [Google Scholar]
- 55.Mosca L, Barrett-Connor E, Kass Wenger N. 2011. Sex/gender differences in cardiovascular disease prevention. Circulation 124, 2145–2154. ( 10.1161/CIRCULATIONAHA.110.968792) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Moolman JA. 2006. Unravelling the cardioprotective mechanism of action of estrogens. Cardiovasc. Res. 69, 777–780. ( 10.1016/j.cardiores.2006.01.001) [DOI] [PubMed] [Google Scholar]
- 57.Emery Thompson M, et al. 2007. Aging and fertility patterns in wild chimpanzees provide insights into the evolution of menopause. Curr. Biol. 17, 2150–2156. ( 10.1016/j.cub.2007.11.033) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lindahl-Jacobsen R, Hanson HA, Oksuzyan A, Mineau GP, Christensen K, Smith KR. 2013. The male-female health-survival paradox and sex differences in cohort life expectancy in Utah, Denmark, and Sweden 1850–1910. Ann. Epidemiol. 23, 161–166. ( 10.1016/j.annepidem.2013.02.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Alberts SC, Archie EA, Gesquiere LR, Altmann J, Vaupel JW, Christensen K. 2014. The male-female health-survival paradox: a comparative perspective on sex differences in aging and mortality. In Sociality, hierarchy, health: comparative biodemography: a collection of papers (eds Weinstein M, Lane MA), pp. 339–364. Washington, DC: National Academies Press. [PubMed] [Google Scholar]
- 60.Crittenden AN, Schnorr SL. 2017. Current views on hunter-gatherer nutrition and the evolution of the human diet. Am. J. Phys. Anthropol. 162, 84–109. ( 10.1002/ajpa.23148) [DOI] [PubMed] [Google Scholar]
- 61.Barquera S, Pedroza-Tobías A, Medina C, Hernández-Barrera L, Bibbins-Domingo K, Lozano R, Moran AE. 2015. Global overview of the epidemiology of atherosclerotic cardiovascular disease. Arch. Med. Res. 46, 328–338. ( 10.1016/j.arcmed.2015.06.006) [DOI] [PubMed] [Google Scholar]
- 62.Healy GN, Matthews CE, Dunstan DW, Winkler EAH, Owen N. 2011. Sedentary time and cardio-metabolic biomarkers in US adults: NHANES 2003–06. Eur. Heart J. 32, 590–597. ( 10.1093/eurheartj/ehq451) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pontzer H, et al. 2016. Constrained total energy expenditure and metabolic adaptation to physical activity in adult humans. Curr. Biol. 26, 410–417. ( 10.1016/j.cub.2015.12.046) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kawanishi K, Dhar C, Do R, Varki N, Gordts PLSM, Varki A. 2019. Human species-specific loss of CMP-N-acetylneuraminic acid hydroxylase enhances atherosclerosis via intrinsic and extrinsic mechanisms. Proc. Natl Acad. Sci. USA 116, 16 036–16 045. ( 10.1073/pnas.1902902116) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shave RE, et al. 2019. Selection of endurance capabilities and the trade-off between pressure and volume in the evolution of the human heart. Proc. Natl Acad. Sci. USA 116, 19 905–19 910. ( 10.1073/pnas.1906902116) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bramble DM, Lieberman DE. 2004. Endurance running and the evolution of Homo. Nature 432, 345–352. ( 10.1038/nature03052) [DOI] [PubMed] [Google Scholar]
- 67.Finch CE. 2012. Evolution of the human lifespan, past, present, and future: phases in the evolution of human life expectancy in relation to the inflammatory load. Proc. Am. Philos. Soc. 156, 9–44. [PubMed] [Google Scholar]
- 68.Willerson JT. 2004. Inflammation as a cardiovascular risk factor. Circulation 109, II-2–II-10. ( 10.1161/01.CIR.0000129535.04194.38) [DOI] [PubMed] [Google Scholar]
- 69.Rubio-Ruiz ME, Peredo-Escárcega AE, Cano-Martínez A, Guarner-Lans V. 2015. An evolutionary perspective of nutrition and inflammation as mechanisms of cardiovascular disease. Int. J. Evol. Biol. 2015, 1–10. ( 10.1155/2015/179791) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gurven MD, Trumble BC, Stieglitz J, Blackwell AD, Michalik DE, Finch CE, Kaplan HS. 2016. Cardiovascular disease and type 2 diabetes in evolutionary perspective: a critical role for helminths? Evol. Med. Public Health 2016, 338–357. ( 10.1093/emph/eow028) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cole MF, Cantwell A, Rukundo J, Ajarova L, Fernandez-Navarro S, Atencia R, Rosati AG. 2020. Data from: Healthy cardiovascular biomarkers across the lifespan in wild-born chimpanzees (Pan troglodytes) Dryad Digital Repository. ( 10.5061/dryad.4xgxd2571) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Cole MF, Cantwell A, Rukundo J, Ajarova L, Fernandez-Navarro S, Atencia R, Rosati AG. 2020. Data from: Healthy cardiovascular biomarkers across the lifespan in wild-born chimpanzees (Pan troglodytes) Dryad Digital Repository. ( 10.5061/dryad.4xgxd2571) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Data collected for this project is available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.4xgxd2571 [71]. The comparison dataset is available from the Primate Aging Database at https://primatedatabase.org/.