Abstract
Evolutionary theories of ageing point to reproduction as a significant factor to consider when asking why ageing occurs and why there is inter-individual variation in its progression. Reproduction in human females is costly, in terms of energy, nutrients and metabolic adjustments. Thus, it is expected that women who experienced high reproductive effort resulting from multiple reproductive events will age faster. However, the evidence for long-term negative effects of reproduction is not conclusive. The lack of understanding of whether there are trade-offs between reproduction and ageing in women is partly due to methodological challenges. The costs of reproduction are often calculated based only on parity, while other elements contributing to these costs (e.g. breastfeeding, timing of reproduction) are neglected, which may significantly underestimate the total costs and obscure the all-important inter-individual variation in such costs. Costs must be evaluated in relation to individual characteristics, including developmental conditions, nutritional status and social support that a mother receives during reproduction. Furthermore, ageing and health must be assessed based on comprehensive markers rather than arbitrarily assembled variables. Finally, longitudinal rather than cross-sectional studies and new statistical approaches are needed to reveal how much of a decline in health and progressing ageing can actually be attributed to past reproductive processes.
This article is part of the theme issue ‘Evolution of the primate ageing process'.
Keywords: ageing, evolutionary trade-offs, parity, pregnancy, reproduction, women's health
1. Introduction
Reproduction in humans, especially for females, is costly in terms of expended energy, allocated nutrients, and implementation of the necessary metabolic adjustments and of the committed time [1]. In humans, just like in any other species, natural selection has favoured traits that are beneficial for successful reproduction, even though they may not be beneficial for health, especially in older age. Thus, it is hypothesized that women with a high reproductive effort will age faster than those with a lower investment in reproduction.
The aim of this article is to provide an overview of those features of reproduction in human females that are relevant to our understanding of reproduction as having long-term costs, especially in the context of ageing. This review provides selected examples that suggest that evolutionary-based trade-offs imposed by reproduction contribute to ageing in women. Rather than providing a comprehensive review, the primary goal here is to understand why the results of studies on reproduction and health are so often contradictory. Despite abundant theoretical predictions, there is a lack of concordance between the results of empirical studies that were aimed to explore if intense reproduction did indeed have a long-term negative impact on maternal health. These contradictory findings are partly due to various methodological oversights: a lack of comprehensive data on the costs of reproduction, numerous confounding factors, a lack of longitudinal studies on changes in the health status of older women, and focusing only on overly simplistic indices of health rather than on more comprehensive indices of ageing.
Since the focus of this review is on reproduction in relation to ageing, I will not discuss studies investigating whether costs of reproduction are related to lifespan [2]. In this review, I specifically ask whether high reproductive effort contributes to faster ageing for women.
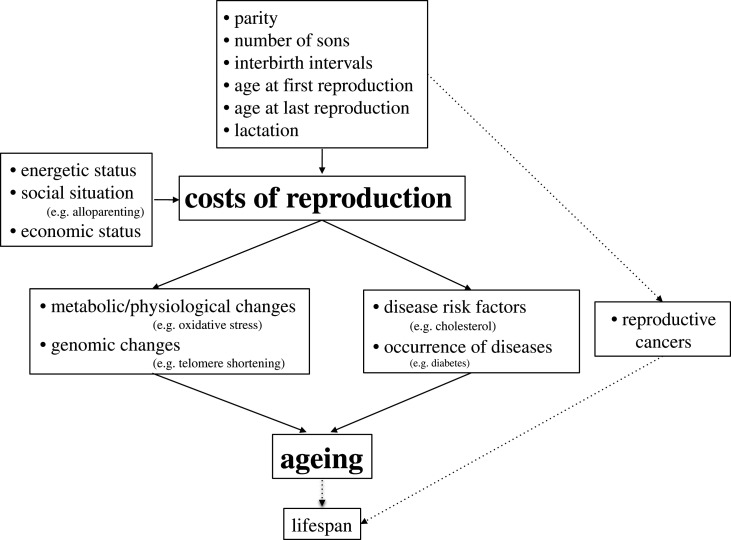
The first part of this article describes the costs of reproduction, including pregnancy, lactation, and childcare that often lasts until an older age owing to grandmothering (figure 1). It is not only the number of pregnancies, or the duration of milk production, but the timing of reproduction, its beginning, ending and spacing during the reproductive span are also important to consider when evaluating the costs of reproduction. In addition, reproduction may have a different impact on the maternal organism depending on its overall condition, most importantly its nutritional status. The characteristics of the socio-economic environment are, therefore, potential confounders and may explain why some women are more susceptible than others to the negative impact of reproductive processes.
Figure 1.
Costs of reproduction and ageing in human females.
The evidence pointing to the long-term impact of reproduction, which is the impact that it has on ageing, could be organized into two different, although connected, types. First, epidemiological research shows that the incidence of many diseases increases with progressing age, and I will point out studies that show that the risk of these diseases is higher in women with a history of intense reproduction, in comparison with women who had lower costs. Second, many changes occur in physiology and metabolism (e.g. telomere shortening, accumulation of oxidative stress, DNA methylation) with advancing age. These changes often lead to the occurrence of age-related diseases or to an overall decline in fitness and health. Thus, these changes can also be understood as mechanisms of ageing and age-related diseases.
2. Costs of reproduction
(a). Mother–offspring interests are in conflict
Resources are limited. This fact creates the necessities of allocating resources among competing processes, of which reproduction is the most evolutionarily relevant. The concept of mother–offspring conflict explains why the mother and developing fetus do not share the same ‘view’ on the partitioning of limited resources during pregnancy. During gestation, genes with paternal and maternal origin may differently impact the maternal physiology. Father's genes favour increased fetal growth, while mother's genes favour lower growth of the fetus. Larger size at birth positively influences infant survival. However, meeting higher demands from the fetus is costly and may reduce the chances of mother's future reproduction. The fetus's genes, especially those of paternal origin, can influence the signals being sent to the maternal organism and, in consequence, can increase the flow of nutrients to the fetoplacental unit. Nutrient flow to the fetus is regulated by placental hormones and proteins (e.g. α-fetoprotein). A high concentration of these substances in maternal blood is related to higher risks of developing hypertension and diabetes during pregnancy [3]. Higher levels of α-fetoprotein correlate with a higher risk of developing preeclampsia (i.e. elevated blood pressure during pregnancy). This suggests that the fetus has physiological mechanisms to manipulate the maternal metabolism in order to increase the flow of nutrients, even at the expense of maternal health.
Genetic differences between the mother and the fetus also lead to challenges faced by the maternal immune system, which has to tolerate the immunologically foreign fetus and placenta. The maternal immune system must suppress its activity in order not to attack the fetus but, at the same time, it must provide protection against pathogens for both the maternal and fetal organisms. It is well established that the fetus influences the maternal immune system and that multiple mechanisms reduce the chance of immune rejection of the fetus [4].
(b). Costs of pregnancy and lactation
The weight gain for a 40-week pregnancy for a woman with body mass index (BMI) within a normal range before gestation is between 11.5 and 16 kg [5]. Weight gain comprises water, protein and fat in the fetus, placenta, uterus, amniotic fluid, maternal blood volume, mammary gland and maternal adipose tissue. To achieve this increase in body weight, women should increase their energy intake by approximately 200, 300 and 400 kcal d−1 in the first, second and third trimesters respectively. The total energy cost of a 40-week pregnancy is about 77 000 kcal [6].
The number of pregnancies or births, while important, only accounts for part of the overall, total costs of reproduction. Breastfeeding is more energetically costly than pregnancy, with a daily energy requirement of 630 kcal d−1 for exclusive breastfeeding and about 460 kcal d−1 for partial breastfeeding [6]. Assuming exclusive breastfeeding during months 1–6 post-partum and partial breastfeeding for months 7–12, 1 year of breastfeeding requires about 196 000 additional kilocalories.
Pregnant and lactating women meet the energetic costs of reproduction through increased food intake and/or reduction in physical activity. However, women are able to support pregnancy and milk production on a much lower energy intake than required. Energy-sparing physiological mechanisms can be employed in pregnancy. In well-nourished women, the basal metabolic rate (BMR) usually rises in pregnancy, soon after conception and until delivery. In women having poor nutrition, the BMR begins to grow only in the second half of pregnancy. However, in undernourished women, as documented by studies in the rural Gambia, the BMR actually declines even until the third trimester. This means that the average BMR in undernourished women can be lower in pregnancy than in the non-pregnant state. Poor nutritional status is often accompanied by intense work that demands high energy expenditures [7].
Pregnancy and delivery are challenging for the cardiovascular system. Pregnant women have hypercoagulability, a 40% increase in plasma volume and a 50% increase in cardiac output [8]. Physiological adaptations during pregnancy include marked insulin resistance, atherogenic dyslipidaemia, fat accumulation and inflammation [8]. These changes occur in healthy pregnancies and are necessary to meet the metabolic demands of the mother and fetus. While most of these changes reverse after parturition, others, such as, for example, lower high-density lipoprotein cholesterol levels, permanent weight gain and greater abdominal obesity, may persist for many years after birth [9].
(c). Costs of having daughters versus sons
Differences between the intrauterine development of male and female fetuses are evident. Much less research attention, however, is given to understanding whether developing male and female fetuses also differently impact the organisms of their mothers. The birth weight of boys is, on the average, about 100 g higher than that of girls, and maternal energy intake is about 10% higher during male pregnancy, suggesting that male fetuses have higher energy requirements [10]. Women carrying boys have higher blood pressure and have different cortisol levels and diurnal cortisol patterns [11]. They also have higher levels of pro-inflammatory cytokines and angiogenic factors, while those with female fetuses have higher levels of regulatory cytokines [12]. Male pregnancies more often have complications, including gestational diabetes, placenta praevia and preeclampsia [13] and may also have long-term detrimental effects on the mother [14]. Women with preeclampsia have higher blood pressure several years after birth [15], and those with gestational diabetes are more likely to develop insulin resistance, and about 60% of them will have type 2 diabetes in later life [16].
The immune function of women pregnant with boys generates response against male-specific minor histocompatibility (HY) antigens [17]. Maternal immune response against HY has been suggested as a potential explanation for a positive correlation between the number of sons and C-reactive protein concentrations in 90-year-old women [17]. Observing elevated levels of pro-inflammatory cytokines in women having more sons many years after births suggests that reproduction, especially when having boys, may contribute to ‘inflammageing’, a sign of the degradation of immune defence.
(d). Childcare
Humans are unique in having several dependents of different ages at the same time. In traditional societies, mothers often increase physical activity to provide food for children after weaning and to carry infants, which requires additional energy [18]. Women are more energetically efficient than men when carrying hip loads, and using a sling while carrying a baby decreases the cost by about 16% [18]; however, this still adds significant expense to the overall maternal energy budget.
Considerable variation exists across different societies and individuals, depending on the mode of subsistence, family wealth and structure, and help available from other family and community members (alloparenting). In general, alloparenting has been a widespread phenomenon in human societies; however, mothers usually have a much higher share of childcare than the alloparents. Alloparenting is important when considering maternal costs of reproduction because it contributes to reducing energy expenditure for mothers. In some small-scale societies, for every 10% increase in allocare, maternal engagement in childcare decreases by 25%, which saves her about 165 kcal d−1 [19].
Alloparenting is associated with reversibility of roles, and when women become grandmothers, they are often involved in taking care of grandchildren. This means that the costs associated with reproduction persist beyond the time of raising their own children. While caregiving has some benefits for wellbeing of grandmothers, it is also associated with physical work, the sharing of limited resources with grandchildren, and psychological stress, and as such can negatively affect women's health in old age [20].
3. The timing of reproduction in relation to its costs
(a). Early reproduction
In adult reproducing females, resources are divided between maternal maintenance and the needs of the developing fetus. In adolescent mothers, resources are still being used for maternal growth, which creates an additional challenge for the allocation of energy and nutrients. This suggests that for women who reproduce early (i.e. when they are still growing), reproduction may be more detrimental than in women who conceive as adults with finished growth.
In Italian women, giving birth before the age of 20 was related to poorer self-rated health [21]. In a national sample of women in the USA, giving birth during and shortly after puberty was associated with a higher risk of having health problems. The longer the first birth was delayed, up to about the age 34, the lower was the risk of poor health [22].
(b). Inter-birth intervals
When a particular bout of pregnancy and lactation has been completed, the maternal organism does not face allocation decisions any more and the organism recuperates. The more time elapses until the next pregnancy, the more resources can be used for maternal maintenance and repair [23]. This implies that inter-birth intervals are important to consider when researching the costs of reproduction. It could be predicted that women of the same parity and cumulative duration of breastfeeding will differ in the severity of long-term physiological damage to their organisms because of the differences in the mean length of spacing between their consecutive pregnancies. It is expected that women with shorter spacing will experience faster ageing.
Since short intervals are associated with adverse pregnancy outcomes, clinical and public health guidelines recommend interpregnancy intervals (i.e. intervals between delivery and conception of the subsequent pregnancy) of at least 18–24 months [24]. In a population-based cohort study in Canada, short interpregnancy intervals were related to increased maternal and fetal risks for women of all ages [24]. In England and Wales, mothers of twins and those with an inter-birth interval of less than 18 months had increased mortality risk at ages 50–89 [25]. Short interpregnancy intervals were also associated with an increased risk of gestational diabetes and of beginning the subsequent pregnancy obese [26], which can have a long-term detrimental impact on a woman's health.
(c). Age at last reproduction
Older mothers, especially those with high parity, have increased risks of complications during pregnancy and during delivery. Data from 29 countries documented higher frequency for obstetric complications, including anaemia, post-partum haemorrhage, fetal malpresentation and a higher risk of maternal death in older mothers [27]. Older mothers also more frequently have gestational diabetes, ante-partum haemorrhage and placenta praevia [28]. Women giving birth past 34 years of age had a strongly increased risk of health problems [22].
It may be assumed that having children in older age would be more detrimental to the maternal organism. At the same time, however, the ability to conceive and sustain a pregnancy that results in a live birth could be treated as a signal of a woman's good health condition. The chance of conception declines with age [29], as does the chance of having a healthy pregnancy. Thus, women with poor health should be less likely to have children with progressing age. It is, therefore, possible that a positive relationship between age at last reproduction and the lifespan of women, as documented by some studies [30], could be explained by their better health as a factor permitting late reproduction, rather than late reproduction leading to better health, and thus longer life. It is also possible that overall ageing is correlated with the ageing of the reproductive system. Women that can reproduce late have ‘younger’ reproductive systems and live longer because they, in general, age more slowly.
4. Does reproduction have a long-term negative impact? Health and age-related diseases
Reproduction in women engenders high energetic and physiological costs, but the question remains whether there is any physiological damage that persists after reproduction has been completed. If so, do reproduction-related changes to the organism contribute to faster ageing?
Although the organism is equipped with many mechanisms that evolved to repair damage, there is an ongoing discussion on their efficacy. Are they capable of repairing damage so well that women who vary in reproductive costs end up not differing in health status in their old age? Repair mechanisms themselves are energetically costly [31] and the evidence for the effectiveness of damage-repairing processes is mixed. Thus, it can be hypothesized that the organism of a woman with high reproductive effort is not able to effectively compensate for all the physiological damage caused by having children. This section briefly reviews the known relationships between the costs of reproduction and the risks of several of the most commonly occurring older-age diseases.
(a). Reproduction and osteoporosis
During reproduction, calcium required by the developing skeleton of the baby comes entirely from maternal bones. During pregnancy, maternal bone mineral density decreases by 5% (such loss increases the risk of fracture by 50%) and during the three to five months of lactation by 3–6% [32]. Thus, each reproductive event depletes maternal bones of calcium and decreases their mineral density, which should lead to osteoporosis, especially if pregnancy and breastfeeding occur frequently. In post-menopausal Korean women, for instance, higher parity and a longer duration of breastfeeding was indeed associated with a higher risk of osteoporosis [33]. By contrast, a meta-analysis based mostly on white women not only did not detect increased risks of osteoporosis, but actually showed a decreased osteoporotic fracture risk associated with increasing parity of up to five live births [34]. While bone mineral density does indeed decrease during pregnancy and lactation (owing to increased calcium demands), an increased load on the bones as a result of higher body weight in pregnancy, carrying the infant, and higher physical activity during childrearing do, in fact, stimulate an increase in bone density. Thus, long-term effects, such as the risk of osteoporosis, depend on many factors, including diet during and after reproduction, and type, duration and intensity of physical activity [35].
(b). Reproduction and cardiovascular diseases
The impact of reproduction on the cardiovascular system is not unidirectional. While pregnancy is related to many potentially negative changes to the cardiovascular system [36] and to diseases such as hypertension, high levels of oestrogens during pregnancy are in fact beneficial for cardiovascular health. Oestrogens contribute to a reduction of oxidative stress [37], and protect against telomere shortening and epigenetic ageing [38,39]. However, during the post-partum period and when breastfeeding occurs with a high daily frequency over a long period of time, the levels of oestrogens are very low, in particular in mothers with poor nutritional status.
A meta-analysis based on cohort studies showed a positive association between the number of pregnancies and an increased risk of cardiovascular disease; however, the increase in risk due to parity was low [40]. Another meta-analysis showed the women with parity of at least 5 had higher cardiovascular disease mortality than women with parity 2–4 [41]. The biomarkers of cardiovascular disease are also higher owing to parity. A gradual increase in low-grade albuminuria (protein in urine, a marker of an increased risk of cardiovascular health problems) with parity was observed in women from China [42]. Giving birth early (at less than 20 years of age) also contributes to a greater overall cardiovascular risk in older age [43].
(c). Reproduction and diabetes
Insulin resistance is a normal feature of a healthy pregnancy, but gestational diabetes which is a serious health problem develops in about 7% of all pregnancies [8]. Women with gestational diabetes are more likely to have type 2 diabetes in later life. There is also a relationship between parity and the risk of diabetes: this risk rose with the number of children in British women [44] and having five or more births increased the risk of diabetes by 27% in a large prospective cohort study of US women [45]. In more than 14 000 Chinese women, those who had at least two children had up to a 59% higher risk of diabetes, compared with women with one live birth [46]; a meta-analysis also confirmed a positive relation between parity and type 2 diabetes in China [47].
(d). Reproduction and metabolic syndrome
The so-called metabolic syndrome is a combination of risk factors, including obesity, glucose intolerance, insulin resistance, dyslipidaemia and hypertension, which predispose individuals to highly increased risks of cardiovascular disease and type 2 diabetes. In rural Bangladesh, women with parity 4 or higher had 1.65 times higher odds of having the metabolic syndrome compared with those with parity 0 to 1 [48]. In the USA, a significant increase in the occurrence of the metabolic syndrome was observed in women who had two or more children [49]. In Chinese women aged 50–93, each birth increased the odds of having the metabolic syndrome by 16% [50]. In British women, parity was inversely associated with high-density lipoprotein cholesterol (‘good’ cholesterol) and was positively associated with triglycerides and diabetes [44].
Parity is related to increased body weight. In China, women with high parity had an increased BMI, waist circumference, systolic blood pressure, fasting plasma glucose and insulin, and decreased high-density lipoprotein cholesterol [42]. In British women, the number of children was positively associated with a BMI and waist–hip ratio [44]. Women with higher parity have higher blood pressure, which is partly explained by higher BMI; however, even among women with a low BMI, those having five or more children had higher systolic blood pressure than those with one to three children [51].
(e). Reproduction and general health status
Self-rated health is a good indicator of morbidity and mortality. Chinese women with four children or more were more likely to have poorer self-rated health than those with one to three children [52]. In Italy, women having four or more children were more likely to report poor self-rated health and life activity limitations [21]. In rural Poland, self-rated health was also related to parity, but only to the number of sons [14].
A study on Tsimane women did not observe a significant impact of their extremely high total fertility rate of 9.1 on long-term costs of reproduction [53]. However, even though this study employed a more comprehensive measure of health status than previous research, it is possible that simple anthropometric and blood (haemoglobin, erythrocyte sedimentation rate and white blood cell count) characteristics used to assess health status may not be the appropriate and/or sufficiently sensitive biomarkers to assess ageing in relation to reproductive costs.
5. Does reproduction have a long-term negative impact? The mechanisms behind reproductive costs and ageing
(a). Reproduction and oxidative stress
It is assumed that the production of reactive oxygen species, such as free radicals in the mitochondria, is more intense in individuals that allocate more energy than others to reproduction. Individuals with intense reproductive effort are also more prone to oxidative damage because diverting resources to reproduction may reduce the ability to allocate energy into maintenance mechanisms. Polish women with four or more pregnancies had significantly elevated levels of a biomarker of DNA oxidative damage and of an antioxidative defence enzyme [54]. However, in women from the USA, no differences in oxidative damage were observed in relation to parity [55].
(b). Reproduction and inflammageing
Ageing is associated with a gradual deterioration of immune function [56]. A common feature of ageing in humans is inflammageing, i.e. an increase in the levels of markers of inflammation in the blood. It is well established that inflammation increases in pregnancy, but there is also a positive relation between parity and levels of C-reactive protein in advanced age [17,57], suggesting a low-grade chronic inflammation as a cost of reproduction. Chronic inflammation is a risk factor for the development and progression of many diseases, including breast cancer.
(c). Reproduction and telomere length
Telomere shortening is a common phenomenon in cellular ageing. Studies on non-human species suggest that intense reproductive effort leads to faster telomere shortening [58]. In human females, evidence for changes in telomere length in relation to reproduction is still limited. Among reproductive-age women in the USA, leucocyte telomeres were shorter in parous compared with nulliparous women [59]. In young Filipino women, those with higher numbers of pregnancies had shorter telomeres [60], similarly to post-menopausal women in the USA [61] and to women participating in the Sister Study [62]. Inconsistent with the hypothesis that high costs of reproduction lead to shorter telomeres are findings in Mayan women where a higher number of surviving children was related to longer telomeres [63].
(d). Reproduction and epigenetic age
Epigenetic clocks, based on testing the methylation of DNA, are new tools for estimating biological age [64]. Pregnancy in women is related to changes in DNA methylation. It is unclear, however, if these methylation changes are reversible, returning to pre-pregnancy values after birth [65], or if they have long-lasting effects on DNA methylation levels. In young Filipino women, epigenetic age increased with the number of pregnancies [60] and in US women, parity was associated with small increases in epigenetic age [66].
(e). Reproduction and microchimerism
Microchimerism could also play a role in explaining the long-term impact of reproduction on the maternal organism [67]. During pregnancy, fetal cells can migrate into the mother's body. They can act as stem cells, developing into specialized cells, including epithelial, heart, liver and neural cells. Fetal cells have the ability to manipulate the maternal organism by redirecting its resources to the fetus and can persist in the maternal body long after the pregnancy, and thus may have a long-term impact on maternal health.
Autoimmune diseases, which are much more frequent in women than in men, have been proposed as a harmful example of microchimerism [67]. On the other hand, a protective effect of microchimerism has been noted for breast cancer, based on the observation that male DNA, assumed to be of fetal origin, was more frequently present in the breast tissue of healthy women than in women with breast cancer [68]. At present, the role of microchimerism as a mechanism inducing long-term health problems as a consequence of reproduction is a promising, but still underdeveloped, area of research.
6. Confounding factors
(a). Good socio-economic status (quality of life) makes the costs of reproduction easier to bear
Trade-offs between reproduction and ageing result partly from the fact that resources are limited and that these insufficient resources must be divided between competing metabolic processes. Well-off women with higher socio-economic status often do not experience any nutritional shortages, in terms of either calories or nutrients. By contrast, women with lower status may face nutritional shortages and have higher levels of energy-demanding physical activity. They may not be able to increase the nutritional value of food or reduce physical activity when pregnant or lactating.
(b). Conditions during early development influence reproductive strategy and health in older age
An individual's biomedical status is influenced by the conditions experienced during their early (i.e. fetal and childhood) development, and these are, at least partially, due to the maternal environment [69]. Such intergenerational effects have been shown to be important predictors of health in adulthood. Past biological and social experiences can be important to consider when attempting to understand why some women are more susceptible than others to reproduction-related health damage. For example, poor conditions during fetal development are related to lower potential fertility in women and to high sensitivity of reproductive physiology to energetic stress during adulthood [70].
Conditions during childhood are also important. For example, it was hypothesized that women who received low parental investment follow a life-history strategy that involves prioritizing reproduction and immediate survival at the expense of their own growth and maintenance [71]. Such a strategy would lead to early and intense reproduction (consecutive pregnancies followed by short inter-birth intervals). Allocation to reproduction and early survival would, however, lead to trade-offs resulting in poor health in older age.
Mechanisms that would allow women with poor investment to reproduce early are not well understood [72]. Evidence that poor social or nutritional environmental conditions lead to early menarche [73] or that poor conditions lead to higher fecundity are not convincing and are often contradictory [74]. Nevertheless, given the growing evidence of the importance of the impact of early environment on health, adding this dimension to studies on trade-offs between reproduction and ageing may lead to a better understanding of inter-individual variation.
7. Some methodological challenges in the studies on costs of reproduction and health
Analyses of the trade-offs between reproduction and health in humans are often based on data from studies that were not originally designed for this purpose. This results in the use of arbitrarily selected indices of health, because they are not necessarily those that are based on validated biomarkers of ageing processes. Additionally, these studies are most often cross-sectional rather than longitudinal, which means that, especially for health aspects that could change in both directions, it is difficult to determine if reproduction indeed causes faster ageing.
Analyses based on population data are not able to calculate total costs of reproduction because some information (e.g. breastfeeding) is not available. This may lead not only to a vast underestimation of lifetime reproductive costs but also to neglecting inter-individual variation among women. To cope with imperfection in the way that researchers build estimates of lifetime reproductive effort, structural equation modelling has been suggested [75], but this still requires that appropriate data are available.
Furthermore, when phenotypic trade-offs are detected, but the cultural and socio-economic context has not been addressed, one's ability to distinguish the causes of faster ageing disappears. The poor health of women with large families may result from their socio-economic status and not from physiological damage to the organism by reproductive processes. Women from low socio-economic backgrounds often have high parity. Therefore, a comparison of relationships between parity and health for women and also for their partners would help to distinguish between socio-economic versus reproductive determinants of health status. Another selection bias in studies on trade-offs is exclusion of women who died before menopause [75], which may lead to focusing on the most robust individuals.
Reproduction is related to an increased risk of some diseases, but also to a reduced risk of others. The risks of breast, uterine and ovarian cancers vary in relation to reproduction. Early age at first birth and high parity, especially when accompanied by long breastfeeding, reduce the risks of these cancers. Thus, the health and ageing of women with high reproductive effort may, on the one hand, be under the influence of increased risks of cardiovascular diseases and diabetes, and, on the other hand, influenced by reduced risks of reproductive cancers. By contrast, the health of women with low reproductive effort may be a result of a lower risk of cardiovascular disease and diabetes but an increased risk of reproductive cancers. As a result of these conflicting forces, the overall health, ageing and mortality risk may not differ among women with high and low reproductive efforts.
8. Conclusion
When attempting to understand human health and ageing, it is helpful to paraphrase Theodosius Dobzhansky's famous sentence, noting that nothing in public health and medicine ‘makes sense except in the light of evolution’ [76]. Consequently, many arguments presented in this review originate in evolutionary thinking.
When intense reproduction requires the allocation of resources at the expense of other metabolic functions, the individual's health suffers. In human females, reproduction is costly; thus, as expected, many studies have documented that women with intense reproduction have a higher risk of age-related diseases. There is also accumulating evidence pointing to plausible mechanisms underlying the relationship between reproduction and ageing. Yet, the evidence of trade-offs between reproduction and health and ageing is not conclusive, as many studies have not found any significant associations between these, or have even suggested better health in women with higher costs of reproduction.
These contradictory results are partly due to methodological shortcomings. Research on trade-offs between reproduction and ageing requires longitudinal studies that would at the same time account for inter-individual variation. The variation among women in their sensitivity to potential damage by reproduction may be due to differences in genetics, early life experience, socio-economic status and social support.
Estimates of the costs of reproduction should be made not just based on parity, but also including other factors that either raise these costs, such as long breastfeeding, or alleviate them, such as a high socio-economic status. Ageing and health status must be evaluated based on a comprehensive assessment and not just on an array of incidental variables that are available because they happen to have been collected for some other purpose.
Understanding whether reproduction has long-term costs for the maternal organism is important for testing predictions of life-history theory and enriching evolutionary perspectives on health [77], but it can also provide guidance for evidence-based medical care and for creating public health programmes that focus on ageing women who have experienced high costs of having children.
Acknowledgements
I thank Melissa Emery Thompson, Alexandra Rosati and Noah Snyder-Mackler for organizing this theme issue.
Data accessibility
This article has no additional data.
Competing interests
I declare I have no competing interests.
Funding
This study was supported by National Science Centre Grant UMO-2017/25/B/NZ7/01509, NN404 273440.
References
- 1.Jasienska G. 2009. Reproduction and lifespan: trade-offs, overall energy budgets, intergenerational costs, and costs neglected by research. Am. J. Hum. Biol. 21, 524–532. ( 10.1002/ajhb.20931) [DOI] [PubMed] [Google Scholar]
- 2.Gagnon A. 2015. Natural fertility and longevity. Fertil. Steril. 103, 1109–1116. ( 10.1016/j.fertnstert.2015.03.030) [DOI] [PubMed] [Google Scholar]
- 3.Petry CJ, Ong KK, Dunger DB. 2007. Does the fetal genotype affect maternal physiology during pregnancy? Trends Mol. Med. 13, 414–421. ( 10.1016/j.molmed.2007.07.007) [DOI] [PubMed] [Google Scholar]
- 4.Schjenken JE, Tolosa JM, Paul JW, Clifton WL, Smith R. 2012. Mechanisms of maternal immune tolerance during pregnancy. In Recent advances in research on the human placenta (ed. Zheng J.), pp. 211–242. IntechOpen; ( 10.5772/33541) [DOI] [Google Scholar]
- 5.Kominiarek MA, Peaceman AM. 2017. Gestational weight gain. Am. J. Obstet. Gynecol. 217, 642–651. ( 10.1016/j.ajog.2017.05.040) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Butte NF, King JC. 2005. Energy requirements during pregnancy and lactation. Pub. Health Nutr. 8, 1010–1027. ( 10.1079/PHN2005793) [DOI] [PubMed] [Google Scholar]
- 7.Lindsay CA, Huston L, Amini SB, Catalano PM. 1997. Longitudinal changes in the relationship between body mass index and percent body fat in pregnancy. Obstet. Gynecol. 89, 377–382. ( 10.1016/s0029-7844(96)00517-0) [DOI] [PubMed] [Google Scholar]
- 8.Kovacs CS, Deal CL. 2020. Maternal-fetal and neonatal endocrinology. Physiology, pathophysiology, and clinical management. New York, NY: Academic Press. [Google Scholar]
- 9.Gunderson EP, Murtaugh MA, Lewis CE, Quesenberry CP, West DS, Sidney S. 2004. Excess gains in weight and waist circumference associated with childbearing: the coronary artery risk development in young adults study (CARDIA). Int. J. Obes. 28, 525–535. ( 10.1038/sj.ijo.0802551) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tamimi RM, Lagiou P, Mucci LA, Hsieh CC, Adami HO, Trichopoulos D. 2003. Average energy intake among pregnant women carrying a boy compared with a girl. Br. Med. J. 326, 1245–1246. ( 10.1136/bmj.326.7401.1245) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown ZA, Schalekamp-Timmermans S, Hofman A, Jaddoe V, Steegers E. 2015. Fetal sex specific differences in maternal vascular adaptation to pregnancy. Pregnancy Hypertens. 5, 31–32. ( 10.1016/j.preghy.2014.10.064) [DOI] [Google Scholar]
- 12.Enninga EAL, Nevala WK, Creedon DJ, Markovic SN, Holtan SG. 2015. Fetal sex-based differences in maternal hormones, angiogenic factors, and immune mediators during pregnancy and the postpartum period. Am. J. Reprod. Immunol. 73, 251–262. ( 10.1111/aji.12303) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aibar L, Puertas A, Valverde M, Carrillo MP, Montoya F. 2012. Fetal sex and perinatal outcomes. J. Perinat. Med. 40, 271–276. ( 10.1515/jpm-2011-0137) [DOI] [PubMed] [Google Scholar]
- 14.Galbarczyk A, Klimek M, Nenko I, Jasienska G. 2019. Sons may be bad for maternal health at older age: new evidence for costs of reproduction in humans. J. Gerontol. A Biol. Sci. Med. Sci. 74, 648–651. ( 10.1093/gerona/gly190) [DOI] [PubMed] [Google Scholar]
- 15.Pouta A, Hartikainen AL, Sovio U, Gissler M, Laitinen J, McCarthy MI, Ruokonen A, Elliott P, Jarvelin MR. 2004. Manifestations of metabolic syndrome after hypertensive pregnancy. Hypertension 43, 825–831. ( 10.1161/01.Hyp.0000120122.39231.88) [DOI] [PubMed] [Google Scholar]
- 16.Noctor E, Dunne FP. 2015. Type 2 diabetes after gestational diabetes: the influence of changing diagnostic criteria. World J. Diabetes 6, 234–244. ( 10.4239/wjd.v6.i2.234) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marttila S, Nevalainen T, Kananen L, Jylhava J, Jylha M, Hervonen A, Ilonen J, Hurme M. 2015. Number of sons contributes to ageing-associated inflammation. Scient. Rep. 5, 8631 ( 10.1038/srep08631) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wall-Scheffler CM, Myers MJ. 2017. The biomechanical and energetic advantages of a mediolaterally wide pelvis in women. Anat. Rec. 300, 764–775. ( 10.1002/ar.23553) [DOI] [PubMed] [Google Scholar]
- 19.Kramer KL, Veile A. 2018. Infant allocare in traditional societies. Physiol. Behav. 193, 117–126. ( 10.1016/j.physbeh.2018.02.054) [DOI] [PubMed] [Google Scholar]
- 20.Coall DA, Hertwig R. 2010. Toward an integrative framework of grandparental investment. Behav. Brain Sci. 33, 40–59. ( 10.1017/s0140525X10000014) [DOI] [PubMed] [Google Scholar]
- 21.Tomassini C, Di Gessa G, Egidi V. 2018. Fertility histories and health in later life in Italy. In A demographic perspective on gender, family and health in Europe (eds Doblhammer G, Gumà J), pp. 263–281. Cham, Switzerland: Springer. [Google Scholar]
- 22.Mirowsky J. 2005. Age at first birth, health, and mortality. J. Health Soc. Behav. 46, 32–50. ( 10.1177/002214650504600104) [DOI] [PubMed] [Google Scholar]
- 23.Ellison PT. 2003. On fertile ground. Cambridge, MA: Harvard University Press. [Google Scholar]
- 24.Schummers L, Hutcheon JA, Hernandez-Diaz S, Williams PL, Hacker MR, VanderWeele J, Norman WV. 2018. Association of short interpregnancy interval with pregnancy outcomes according to maternal age. JAMA Intern. Med. 178, 1661–1670. ( 10.1001/jamainternmed.2018.4696) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grundy E, Tomassini C. 2005. Fertility history and health in later life: a record linkage study in England and Wales. Soc. Sci. Med. 61, 217–228. ( 10.1016/j.socscimed.2004.11.046) [DOI] [PubMed] [Google Scholar]
- 26.Hanley GE, Hutcheson JA, Kinniburgh BA, Lee L. 2017. Interpregnancy interval and adverse pregnancy outcomes: an analysis of successive pregnancies. Obstet. Gynecol. 129, 408–415. ( 10.1097/AOG.0000000000001891) [DOI] [PubMed] [Google Scholar]
- 27.Laopaiboon M, et al. 2014. Advanced maternal age and pregnancy outcomes: a multicountry assessment. BJOG 121, 49–56. ( 10.1111/1471-0528.12659) [DOI] [PubMed] [Google Scholar]
- 28.Carolan MC, Davey MA, Biro M, Kealy M. 2013. Very advanced maternal age and morbidity in Victoria, Australia: a population based study. BMC Pregnancy Childb. 13, 80 ( 10.1186/1471-2393-13-80) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lipson SF, Ellison PT. 1996. Comparison of salivary steroid profiles in naturally occurring conception and non-conception cycles. Hum. Reprod. 11, 2090–2096. ( 10.1093/oxfordjournals.humrep.a019055) [DOI] [PubMed] [Google Scholar]
- 30.Sun FG, Sebastiani P, Schupf N, Bae H, Andersen SL, McIntosh A, Abel H, Elo IT, Perls TT. 2015. Extended maternal age at birth of last child and women's longevity in the Long Life Family Study. Menopause 22, 26–31. ( 10.1097/gme.0000000000000276) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kirkwood TBL, Austad SN. 2000. Why do we age? Nature 408, 233–238. ( 10.1038/35041682) [DOI] [PubMed] [Google Scholar]
- 32.Karlsson MK, Ahlborg HG, Karlsson C. 2005. Female reproductive history and the skeleton—a review. BJOG 112, 851–856. ( 10.1111/j.1471-0528.2005.00571.x) [DOI] [PubMed] [Google Scholar]
- 33.Lee EN, Choe SY, Choi EH, Lee MJ. 2019. Effects of parity and breast feeding duration on the risk of osteoporosis in postmenopausal Korean women: a systematic review and meta-analysis. J. Menopausal Med. 25, 100–107. ( 10.6118/jmm.19197) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang Q, Huang Q, Zeng Y, Liang JJ, Liu SY, Gu X, Liu JA. 2016. Parity and osteoporotic fracture risk in postmenopausal women: a dose-response meta-analysis of prospective studies. Osteoporos. Int. 27, 319–330. ( 10.1007/s00198-015-3351-3) [DOI] [PubMed] [Google Scholar]
- 35.Stieglitz J, Beheim BA, Trumble BC, Madimenos FC, Kaplan H, Gurven M. 2015. Low mineral density of a weight-bearing bone among adult women in a high fertility population. Am. J. Phys. Anthropol. 156, 637–648. ( 10.1002/ajpa.22681) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Loerup L, Pullon RM, Birks J, Fleming S, Mackillop LH, Gerry S, Watkinson PJ. 2019. Trends of blood pressure and heart rate in normal pregnancies: a systematic review and meta-analysis. BMC Med. 17, 167 ( 10.1186/s12916-019-1399-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Behl C, Skutella T, Lezoualch F, Post A, Widmann M, Newton CJ, Holsboer F. 1997. Neuroprotection against oxidative stress by estrogens: structure-activity relationship. Mol. Pharmacol. 51, 535–541. ( 10.1124/mol.51.4.535) [DOI] [PubMed] [Google Scholar]
- 38.Shin YA, Lee KY. 2016. Low estrogen levels and obesity are associated with shorter telomere lengths in pre- and postmenopausal women. J. Exerc. Rehabil. 12, 238–246. ( 10.12965/jer.1632584.292) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Levine ME, et al. 2016. Menopause accelerates biological aging. Proc. Natl Acad. Sci. USA 113, 9327–9332. ( 10.1073/pnas.1604558113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li WZ, Ruan WY, Lu ZX, Wang DM. 2019. Parity and risk of maternal cardiovascular disease: a dose–response meta-analysis of cohort studies. Eur. J. Prevent. Cardiol. 26, 592–602. ( 10.1177/2047487318818265) [DOI] [PubMed] [Google Scholar]
- 41.Lv HC, Wu HY, Yin JS, Qian JY, Ge JB. 2015. Parity and cardiovascular disease mortality: a dose-response meta-analysis of cohort studies. Scient. Rep. 5, 13411 ( 10.1038/srep13411) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sun K, Lin DZ, Feng QL, Li F, Qi YQ, Feng WT, Ren M, Yan L, Liu D. 2019. Number of parity is associated with low-grade albuminuria in middle-aged and elderly Chinese women. BMC Women's Health 19, 117 ( 10.1186/s12905-019-0814-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rosendaal NTA, Alvarado B, Wu YY, Velez MP, da Camara SMA, Pirkle CM. 2017. Adolescent childbirth is associated with greater Framingham risk scores for cardiovascular disease among participants of the IMIAS. J. Am. Heart Assoc. 6, e007058 ( 10.1161/jaha.117.007058) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lawlor DA, Emberson JR, Ebrahim S, Whincup PH, Wannamethee SG, Walker M, Smith GD. 2003. Is the association between parity and coronary heart disease due to biological effects of pregnancy or adverse lifestyle risk factors associated with child-rearing? Findings from the British Women's Heart and Health Study and the British Regional Heart Study. Circulation 107, 1260–1264. ( 10.1161/01.CIR.0000053441.43495.1A) [DOI] [PubMed] [Google Scholar]
- 45.Nicholson WK, Asao K, Brancati F, Coresh J, Pankow JS, Powe NR. 2006. Parity and risk of type 2 diabetes—the Atherosclerosis Risk in Communities study. Diabetes Care 29, 2349–2354. ( 10.2337/dc06-0825) [DOI] [PubMed] [Google Scholar]
- 46.Tian YH, Shen LJ, Wu J, Chen WH, Yuan J, Yang HD, Wang YJ, Liang Y, Wu TC. 2014. Parity and the risk of diabetes mellitus among Chinese women: a cross-sectional evidence from the Tongji-Gongfeng Cohort Study. PLoS ONE 9, e0104810 ( 10.1371/journal.pone.0104810) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li PY, Shan ZL, Zhou L, Xie ML, Bao W, Zhang Y, Rong Y, Yang W, Liu LG. 2016. Parity and risk of type 2 diabetes: a systematic review and dose-response meta-analysis. Eur. J. Endocrinol. 175, R231–R245. ( 10.1530/eje-16-0321) [DOI] [PubMed] [Google Scholar]
- 48.Akter S, Jesmin S, Rahman MM, Islam MM, Khatun MT, Yamaguchi N, Akashi H, Mizutani T. 2013. Higher gravidity and parity are associated with increased prevalence of metabolic syndrome among rural Bangladeshi women. PLoS ONE 8, e0068319 ( 10.1371/journal.pone.0068319) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gunderson EP, Jacobs DR, Chiang V, Lewis CE, Tsai A, Quesenberry CP, Sidney S. 2009. Childbearing is associated with higher incidence of the metabolic syndrome among women of reproductive age controlling for measurements before pregnancy: the CARDIA study. Am. J. Obstet. Gynecol. 201, 31 ( 10.1016/j.ajog.2009.03.031) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lao XQ, et al. 2006. Parity and the metabolic syndrome in older Chinese women: the Guangzhou Biobank Cohort Study. Clin. Endocrinol. 65, 460–469. ( 10.1111/j.1365-2265.2006.02615.x) [DOI] [PubMed] [Google Scholar]
- 51.Taylor JY, Sampson DA, Anderson CM, Caldwell D, Taylor AD. 2012. Effects of parity on blood pressure among West African Dogon women. Ethn. Dis. 22, 360–366. [PMC free article] [PubMed] [Google Scholar]
- 52.Li XM, Jiang QB, Li SZ, Feldman MW. 2018. Female fertility history and mid-late-life health: findings from China. J. Women Aging 30, 62–74. ( 10.1080/08952841.2016.1259445) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gurven M, Costa M, Trumble B, Stieglitz J, Beheim B, Rodriguez DE, Hooper PL, Kaplan H. 2016. Health costs of reproduction are minimal despite high fertility, mortality and subsistence lifestyle. Scient. Rep. 6, 30056 ( 10.1038/srep30056) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ziomkiewicz A, Sancilio A, Galbarczyk A, Klimek M, Jasienska G, Bribiescas RG. 2016. Evidence for the cost of reproduction in humans: high lifetime reproductive effort is associated with greater oxidative stress in post-menopausal women. PLoS ONE 11, e0145753 ( 10.1371/journal.pone.0145753) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ziomkiewicz A, Frumkin A, Zhang YW, Sancilio A, Bribiescas RG. 2018. The cost of reproduction in women: reproductive effort and oxidative stress in premenopausal and postmenopausal American women. Am. J. Hum. Biol. 30, e23069 ( 10.1002/ajhb.23069) [DOI] [PubMed] [Google Scholar]
- 56.Pawelec G, Larbi A, Derhovanessian E. 2010. Senescence of the human immune system. J. Comp. Pathol. 141, S39–S44. ( 10.1016/j.jcpa.2009.09.005) [DOI] [PubMed] [Google Scholar]
- 57.Rosenberg N, Daviglus ML, DeVon HA, Park CG, Eldeirawi K. 2017. The association between parity and inflammation among Mexican-American women of reproductive age varies by acculturation level: results of the National Health and Nutrition Examination Survey (1999–2006). Women's Health Iss. 27, 485–492. ( 10.1016/j.whi.2017.03.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sudyka J. 2019. Does reproduction shorten telomeres? Towards integrating individual quality with life-history strategies in telomere biology. Bioessays 41, 1900095 ( 10.1002/bies.201900095) [DOI] [PubMed] [Google Scholar]
- 59.Pollack AZ, Rivers K, Ahrens KA. 2018. Parity associated with telomere length among US reproductive age women. Hum. Reprod. 33, 736–744. ( 10.1093/humrep/dey024) [DOI] [PubMed] [Google Scholar]
- 60.Ryan CP, Hayes MG, Lee NR, McDade TW, Jones MJ, Kobor MS, Kuzawa CW, Eisenberg DTA. 2018. Reproduction predicts shorter telomeres and epigenetic age acceleration among young adult women. Scient. Rep. 8, 11100 ( 10.1038/s41598-018-29486-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gray KE, Schiff MA, Fitzpatrick AL, Kimura M, Aviv A, Starr JR. 2014. Leukocyte telomere length and age at menopause. Epidemiology 25, 139–146. ( 10.1097/ede.0000000000000017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kresovich JK, Parks CG, Sandler DP, Taylor JA. 2018. Reproductive history and blood cell telomere length. Aging 10, 2383–2393. ( 10.18632/aging.101558) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Barha CK, Hanna CW, Salvante KG, Wilson SL, Robinson WP, Altman RM, Nepomnaschy PA. 2016. Number of children and telomere length in women: a prospective, longitudinal evaluation. PLoS ONE 11, e0146424 ( 10.1371/journal.pone.0146424) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lu AT, et al. 2019. DNA methylation GrimAge strongly predicts lifespan and healthspan. Aging 11, 303–327. ( 10.18632/aging.101684) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.White WM, Brost BC, Sun Z, Rose C, Craici I, Turner S, Garovic VD. 2012. Normal early pregnancy. A transient state of epigenetic change favoring hypomethylation. Epigenetics 1, 729–734. ( 10.4161/epi.20388) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kresovich AK, Harmon QE, Xu ZL, Nichols HB, Sandler DR, Taylor JA. 2019. Reproduction, DNA methylation and biological age. Hum. Reprod. 34, 1965–1973. ( 10.1093/humrep/dez149) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Boddy AM, Fortunato A, Sayres MW, Aktipis A. 2015. Fetal microchimerism and maternal health: a review and evolutionary analysis of cooperation and conflict beyond the womb. Bioessays 37, 1106–1118. ( 10.1002/bies.201500059) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gadi VK. 2010. Fetal microchimerism in breast from women with and without breast cancer. Breast Cancer Res. Treat. 121, 241–244. ( 10.1007/s10549-009-0548-1) [DOI] [PubMed] [Google Scholar]
- 69.Bateson P, et al. 2004. Developmental plasticity and human health. Nature 430, 419–421. ( 10.1038/nature02725) [DOI] [PubMed] [Google Scholar]
- 70.Jasienska G, Thune I, Ellison PT. 2006. Fatness at birth predicts adult susceptibility to ovarian suppression: an empirical test of the predictive adaptive response hypothesis. Proc. Natl Acad. Sci. USA 103, 12 759–12 762. ( 10.1073/pnas.0605488103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wells JCK, et al. 2019. Low maternal capital predicts life history trade-offs in daughters: why adverse outcomes cluster in individuals. Front. Public Health 7, 206 ( 10.3389/fpubh.2019.00206) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Coall DA, Tickner M, McAllister LS, Sheppard P. 2016. Developmental influences on fertility decisions by women: an evolutionary perspective. Phil. Trans. R. Soc. B 371, 20150146 ( 10.1098/rstb.2015.0146) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sear R, Sheppard P, Coall DA. 2019. Cross-cultural evidence does not support universal acceleration of puberty in father-absent households. Phil. Trans. R. Soc. B 374, 20180124 ( 10.1098/rstb.2018.0124) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Klimek M, Galbarczyk A, Colleran H, Thune I, Ellison PT, Ziomkiewicz A, Jasienska G. 2015. Digit ratio (2D:4D) does not correlate with daily 17β-estradiol and progesterone concentrations in healthy women of reproductive age. Am. J. Hum. Biol. 27, 667–673. ( 10.1002/ajhb.22717) [DOI] [PubMed] [Google Scholar]
- 75.Helle S. 2018. Accounting for measurement error in human life history trade-offs using structural equation modeling. Am. J. Hum. Biol. 30, e23075 ( 10.1002/ajhb.23075) [DOI] [PubMed] [Google Scholar]
- 76.Dobzhansky T. 1973. Nothing in biology makes sense except in the light of evolution. Am. Biol. Teacher 35, 125–129. ( 10.1002/ajhb.23075) [DOI] [Google Scholar]
- 77.Jasienska G, Bribiescas RG, Furberg AS, Helle S, Nunez-de la Mora A. 2017. Human reproduction and health: an evolutionary perspective. Lancet 390, 510–520. ( 10.1016/s0140-6736(17)30573-1) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.