Abstract
Executive function (EF) is a complex construct that reflects multiple higher-order cognitive processes such as planning, updating, inhibiting and set-shifting. Decline in these functions is a hallmark of cognitive ageing in humans, and age differences and changes in EF correlate with age-related differences and changes in association cortices, particularly the prefrontal areas. Here, we review evidence for age-related decline in EF and associated neurobiological changes in prosimians, New World and Old World monkeys, apes and humans. While EF declines with age in all primate species studied, the relationship of this decline with age-related alterations in the prefrontal cortex remains unclear, owing to the scarcity of neurobiological studies focusing on the ageing brain in most primate species. In addition, the influence of sex, vascular and metabolic risk, and hormonal status has rarely been considered. We outline several methodological limitations and challenges with the goal of producing a comprehensive integration of cognitive and neurobiological data across species and elucidating how ageing shapes neurocognitive trajectories in primates with different life histories, lifespans and brain architectures. Such comparative investigations are critical for fostering translational research and understanding healthy and pathological ageing in our own species.
This article is part of the theme issue ‘Evolution of the primate ageing process’.
Keywords: chimpanzee, cognition, grey mouse lemur, macaque, marmoset, prefrontal cortex
1. Introduction
Living organisms adapt to their environment through processing information [1,2]. Nuanced control over information processing operations plays an important role in fostering efficient adaptation of individuals and species. In primates that must adapt to a wide variety of environments, such nuanced and precise control over cognition may be crucial for success. Executive function (EF), a construct that encompasses multiple aspects of cognitive control, exhibits significant age-related differences and may constitute a fundamental feature of human cognitive ageing. It remains unclear, however, whether other primates show age-related declines in EF. Comparative studies can illuminate features of cognitive and brain ageing that are unique to the human lineage versus those shared with other primates. Considering EF and its neural substrates, this comparative approach is particularly important. Primate species occupy distinct ecological niches and have diverse social structures. They may, therefore, have evolved qualitatively different EF abilities [3], along with differential patterns of cortical organization. Yet, the patterns of brain and cognitive ageing may be conserved across primate orders, suggesting that general principles underlie the diversity of brains and cognitive abilities and their temporal dynamics. Below we describe patterns of age-related decline in EF and associated brain structures in humans and non-human primate (NHP) models of human cognitive ageing.
2. Humans
The importance of cognitive control for effective information processing is widely acknowledged [4,5]. The term ‘executive functions’ was introduced into the discussion of purposeful control of behaviour in the 1970s [6], with the prefrontal cortex (PFC) conceptualized as the prime brain substrate of EF. The idea of frontal cortex supremacy in cognition dates at least to the early nineteenth century, when Gratiolet crowned the frontal cortex as the most important region of the human brain signifying its supreme power (…la plus importante région du cerveau humain le signe de la puissance souveraine… [7, p. 64]).
EF is a multifactorial construct encompassing many cognitive skills and abilities, such as inhibition of irrelevant information, storing and simultaneously manipulating items in short-term memory, updating information during action, planning future actions, adjusting behaviour in response to changes in incentives, and shifting attention across tasks—to name a few [8]. Attempts at validating the EF construct via confirmatory factor analysis (CFA) revealed multiple EF sub-constructs—shifting, monitoring and updating of streaming information, and inhibition of prepotent responses—that were ‘moderately [inter]correlated…but…clearly separable’ [9, p. 49]. Such findings led to a proposed pattern of ‘unity and diversity,’ in which EF components are related but distinct [10].
Therefore, classic neuropsychological tests of EF, such as the Wisconsin Card Sorting Test (WCST)1, Stroop task2, Tower of Hanoi3 or N-back task4, assess inter-related cognitive skills and cannot be viewed as mere indicators of prefrontal integrity and functionality [11]. Nonetheless, EF indicators have been associated with PFC integrity in lesion studies [12,13] and with PFC volume in structural neuroimaging investigations [14], suggesting that ‘bigger is better’. Meta-analyses of functional MRI studies also revealed reliable activation of this region during the WCST [15], Stroop [16] and working memory (WM) tasks [17].
Negative associations between age and EF in healthy adults are well established. Older adults perform worse than their younger counterparts on EF tasks such as WM, WCST, Stroop and dual-task management [18,19]. Age-related declines in EF are also evident in longitudinal studies [20,21], and have been related to changes in the integrity of prefrontal areas. The lateral PFC shrinks at a rate of about 5% per decade [22]; the adjacent prefrontal white matter loses volume too [23,24]. Many studies found moderate associations between regional volumes of the PFC and EF performance [25]. Functional neuroimaging studies have also linked poor performance on EF indicators (e.g. WCST) and reduced activation within the PFC in older people [26,27].
Some authors have argued that EF dysfunction is a fundamental feature of human cognitive ageing (e.g. [28]). However, it is important to determine the extent to which other primates also exhibit age-related declines in EF and associated neural substrates. Cognitive ageing investigations have used several NHP species, based on close phylogenetic proximity to humans (chimpanzees, macaques), unique characteristics such as a short lifespan (grey mouse lemurs, marmosets), or likelihood of specific brain pathologies (chimpanzees, grey mouse lemurs). A comparative perspective, which contrasts patterns of neurocognitive ageing in primates with different brain organizations, life histories and lifespans, may foster identifying biological factors associated with cognitive ageing and revealing potential targets for therapeutic intervention against age-related cognitive decline in our own species.
3. Non-human primate studies: rationale and methods
NHPs share many neuroanatomical, physiological and behavioural characteristics with humans that make them invaluable for translational research [29,30]. NHPs provide further advantages for cognitive research because they perform in settings that closely match human testing conditions. Various test batteries adapted to NHPs capture the cognitive domains traditionally assessed in human neuropsychological studies, such as WM, set-shifting, and other EFs. These tests can be administered by a human experimenter who manually presents physical stimuli that the monkey may displace in order to receive a reward, most often in the context of a Wisconsin general testing apparatus (WGTA, [31]). More controlled automated systems, in which monkeys obtain a reward by responding via joystick or touch to stimuli displayed on a computer screen have replaced the WGTA over time. Studies of humans with focal brain damage [32] and NHPs with brain lesions [33] have allowed the mapping of cognitive processes onto specific neural structures and systems. As early as 1936, Jacobsen [33] reported that rhesus monkeys with bilateral prefrontal lesions exhibited severe impairment in delayed-response (DR) performance, a task in which they had to remember the position of a reward after various delays. The neurophysiological studies that followed demonstrated that neurons in the dorsolateral PFC (dlPFC) were active during the delays and encoded the location of the cue or the direction of the response, establishing the DR as a benchmark task for assessing WM in NHPs [34]. These seminal studies greatly influenced the field of cognitive ageing, which adopted the framework that age-related decline in a particular cognitive domain reflects compromised integrity of the underlying brain tissues [35–37]. Today, multiple neuroscience tools, such as in vivo neuroimaging, electrophysiology and postmortem histological studies can be combined with cognitive assessments to uncover the neural bases of age-related cognitive decline. Most recent technological breakthroughs, which allow the monitoring and manipulation of neural activity in behaving animals, have considerable potential for advancing cognitive ageing research, but are still at various stages of development in NHPs [30].
4. The rhesus macaque
The majority of studies conducted in ageing NHPs have relied on the genus Macaca, primarily rhesus (M. mulatta), with some studies conducted in cynomolgus (M. fascicularis) or bonnet macaques (M. radiata). Another useful Old World primate model for ageing and Alzheimer's disease (AD) [38] is the vervet monkey (Chlorocebus aethiops). However, since data on age-related cognitive decline are not yet available in this species, the following section focuses on macaque data.
Rhesus monkeys can live up to 40 years, but have a median lifespan of 27 years and are considered old by the age of 20. The extant literature reveals close parallels between macaques and humans in cognitive and brain ageing [39–43]. Compared with younger animals, aged rhesus monkeys show reliable delay-dependent decrements in WM assessed by performance on the DR task [44,45]. Aged rhesus monkeys also show impaired cognitive flexibility, traditionally assessed via reversal learning or set-shifting tasks (e.g. WCST). In reversal learning, monkeys learn which of two stimuli is associated with a reward (discrimination), followed by a reversal, in which the reward becomes associated with the previously incorrect stimulus. Adjustment to reversals takes more trials in aged monkeys than in younger animals [39,44,46,47]. The former perseverate, i.e. continue following an established prepotent response despite changing task requirements. Perseverative errors are also observed in a modified version of the WCST [48], in which old rhesus monkeys (24–30 years) require more time than young adults (5–10 years) to learn the shifts. Importantly, these age differences are already observed in middle-age animals (12–19 years), suggesting that decline in EF is one of the earliest cognitive changes in normal primate ageing [49].
Area 46 of the dlPFC plays a critical role in EF and has been extensively studied in the rhesus monkey. As the dlPFC shows no neuronal loss [50], the thinning of grey matter (13%) in this area [51] may reflect loss of neuropil—dendrites and dendritic spines [52]. In addition, the dlPFC exhibits substantial age-related alterations in myelin [53] and white matter [54]. Alterations of the gross PFC structure in aged rhesus monkeys do not correlate with age-related declines in WM, as assessed by the DR [55]. Rather, impaired DR performance with age has been associated with reduced persistent firing of delay neurons in area 46 of the dlPFC [56] and with spine loss in the dlPFC [57]. Although one study reported that WCST scores in aged rhesus monkeys correlated with decrements in grey matter volume, including the PFC [58], more subtle neurobiological changes are likely to contribute to EF declines with age. For example, WCST performance correlated with decreases in α-1 and α-2 adrenergic receptor binding in the PFC in another study [59]. Notably, factors such as sex [60], hormonal status [61] and arterial hypertension [62] all influence EF in rhesus monkeys but are often overlooked in neurobiological studies.
In sum, macaques display age-related declines in EF starting at middle age. Alterations in the composition of the PFC neuropil rather than loss of neurons probably drive these age-related declines.
5. The grey mouse lemur
The grey mouse lemur (Microcebus murinus), endemic to Madagascar, is one of the smallest primates. Its average lifespan is around 3 years in the wild and 5–7 years in captivity, with maximum age of up to 14 years [63]. Following discoveries of age-related proteopathies, including β-amyloid [64] and tau [64,65], as well as cerebral atrophy [64] and behavioural and cognitive changes [66] in the mouse lemur, the histochemical, structural and cognitive/behavioural aspects of brain ageing in this species received great attention [67–69].
Using a test battery and a corridor apparatus designed for mouse lemurs, studies of age-related changes in EF demonstrated that old individuals (7–11 years) performed worse than young animals (2–4 years) in extra-dimensional set-shifting and in pairwise spatial discrimination reversal learning, with no age differences in a go/no go successive visual discrimination task [70]. The age-related deficits in extradimensional set-shifting and spatial reversal learning were later replicated [71], along with an impairment of pairwise visual discrimination reversal learning [71] also found in a touchscreen-based version of the task [72]. Age-related WM deficits are also apparent in this species. A small study comparing young (2–4 years) and old (9–10 years) animals on a DR task, showed more drastic performance decrements with increasing delay in aged individuals [73]. A linear age-related deficit in spontaneous alternation behaviour during exploration of a four-armed maze was interpreted as an age-related decline in spatial WM [74]. Notably, mouse lemurs show substantial individual differences in cognitive ageing. The aged cohorts, similar to those of humans, include individuals that age healthily, along with animals with age-related cerebral and cognitive pathologies [71,72].
While a possible linkage of EF decline and structural/cytochemical alterations of prefrontal cerebral structures in aged mouse lemurs has been postulated [70–72], there is little empirical evidence for such a link. Lesion studies or pharmacological intervention experiments targeting prefrontal function and cognitive performance are absent. In vivo MRI studies revealed differential patterns of structural brain ageing in lemurs. These investigations showed age-related ventricular expansions and atrophy in temporo-parietal areas in individuals older than 3.5 years [75]. As in humans, the pathological process progresses gradually, often starting in frontal brain regions of middle-aged individuals (3–6 years) and spreading to temporo-parietal and finally occipital areas [76]. Preliminary analysis from the same study also suggests concomitant intracellular amyloid accumulation [76]. As in humans, regional volumes and cortical thickness of the temporal and hippocampal regions showed the strongest association with age. However, in contrast to humans, significantly reduced volume and thickness were noted in occipital and cingulate regions, whereas the fronto-parietal areas appeared age-invariant (1–11 years, n = 34; [71]). The age differences in the cingulate, temporal and occipital regions were replicated in subsequent studies using manual delineation of brain areas [77], and automated voxel-based [78], or 3D-MRI atlas-based morphometry [79]. In addition, in line with human findings, marked age-related atrophy of the caudate nucleus was consistently reported [71,77–79]. However, age-related alterations of the premotor cortex and the superior parietal lobule were also described [77,79]. Finally, automated methods revealed age-related atrophy in an area that corresponds to Brodmann areas 13–16, believed to be functionally equivalent to prefrontal regions in humans [78,79]. Interestingly, the study by Picq and collaborators [71] also provided EF data for a subset of individuals (2–8 years, n = 16). A composite measurement of set-shifting and reversal learning correlated with the volume of the septal region, the caudate nucleus, and the splenium of the corpus callosum, as well as with the thickness of the anterior and posterior cingulate cortices and the associative visual cortical areas, suggesting overlap in EF circuitry between mouse lemurs and humans.
In summary, extradimensional set-shifting and reversal learning are consistently impaired with age in the mouse lemur, with moderate evidence for age-related deficits in WM. Additional studies are needed to clarify the neural basis of these changes.
6. The common marmoset
The common marmoset (Callithrix jacchus) is a small (300–500 g) New World monkey from the Callitrichinae subfamily and is endemic to Brazil. Its average lifespan in captivity is about 10 years, with a maximum of 21 years [80]. Marmosets are considered old by the age of 8, when first signs of ageing such as cortical β-amyloid deposition [81], reduced neurogenesis in the dentate gyrus [82], and presbycusis [83] are observed [84]. Owing to their small size and short lifespan, marmosets make an excellent model for ageing research [84–86]. Yet, marmosets have only recently been included in cognitive ageing investigations. A small study comparing reversal learning performance of four aged marmosets (10–14 years) with historical records from a younger cohort (1.7–3.5 years) reported more errors in aged animal for the initial discrimination and reversal but noted that this difference disappeared with further testing [87]. A larger study of 35 marmosets (2–14 years) found that aged monkeys exhibited deficits in reversal learning and delayed matching-to-position [88], with two critical periods identified for the emergence of deficits: a reduced ability to update WM information across trials was observed around 4–4.5 years of age, while difficulty with inhibitory control emerged around the 6–7th year.
To date, studies of ageing in the marmoset, like most investigations of ageing in all species, have been cross-sectional and therefore unable to determine whether neural or physiological changes precede or follow the decline in cognitive function. However, the short lifespan of the marmoset makes feasible longitudinal investigations of changes in individual animals undergoing the ageing process. In an ongoing study [89,90], 28 marmosets were followed longitudinally as they aged from 4–5 to 8–9 years. The animals performed reversal learning for 4 years using different stimuli each year. Reliable deficits emerged by year 4 in both the discrimination and the reversal portions of the task. In addition, sex differences in ageing trajectories were revealed, with females showing greater decline than males. Finally, prominent individual differences indicated pathological ageing in some individuals. These preliminary data suggest EF declines with age in marmosets and add longitudinal findings to the extant literature. In addition, they highlight a major influence of sex on age-related decline in EF that was overlooked in most NHP studies.
Being amenable to gene editing [91] bolsters marmosets' importance as a model for neuroscience research [92,93], and their use in neuroimaging, neurophysiological and neurobiological studies has increased in recent years. Yet, studies focusing on brain ageing in this species are still in their infancy. The marmoset brain exhibits increased β-amyloid deposition [81,94] and abnormally phosphorylated tau [94] with age. Decreased neurogenesis is also observed in the dentate gyrus of older animals [82]. Liu et al. [95] reported age-dependent reductions in whole brain and cerebral white and grey matter volumes in the marmoset brain, without providing region-specific analyses. A small study [96] investigating myelin composition in brain tissues of four aged and two young marmosets reported reduced myelin thickness, density and fraction in older age. Age-related myelin changes in the genu of the corpus callosum, which contains fibres connecting homologous PFC regions of the hemispheres, correlated with performance on the detour reaching task, a task of PFC-dependent inhibitory control.
Collectively, the extant data suggest age-related EF impairment in the marmoset, with more prominent declines observed in females. More research is needed to characterize brain ageing in this species and fully grasp the relationships between age-related neural changes and declining EF performance in each sex.
7. The chimpanzee
In captivity, the chimpanzee (Pan troglodytes) has a life expectancy of 28.3 years at birth, with an estimated maximum lifespan of 74 years [97]. Among NHPs, the chimpanzee has the longest lifespan, the largest brain and PFC [98], and cognitive abilities closest to those of humans [99]. Very little is known about cognitive ageing in chimpanzees. An early study in eight young (11–19 years) and eight old (28–40 years) chimpanzees of unspecified sex found no age differences in a series of object discriminations and a wheel-rotating task [100]. Later investigation in 19 chimpanzees (7–41 years) of unspecified sex revealed no age-related deficits in discrimination tasks but identified an age-related impairment at the shortest retention delays (less than 5 s) in the DR and in an oddity task [101]. After a pause of nearly half a century, research on chimpanzee cognitive ageing resumed with three studies providing evidence for age-related decline in the chimpanzee, especially in the EF domain. In the first study, 38 female chimpanzees (10–52 years) were tested over a period of 3 years on the primate cognition test battery (PCTB) [102], a battery originally designed to compare social and physical cognition in apes and humans [103]. Age-related performance decrements were observed in two tasks of social cognition (attention-getting behaviour and gaze following), with minimal age effects found for tasks of physical cognition (tasks of causality, quantity and spatial processing). However, the task of spatial WM (DR with minimal delay) showed performance decline in the older females (greater than 50 years) over the course of the study, suggesting that such deficits may develop at oldest ages. More recently, the PCTB was administered to a large sample of chimpanzees at the National Center for Chimpanzee Care (MD Anderson Cancer Center). When these data were combined with previously published data from the Yerkes Primate Center [104], DR scores were available in 223 individuals (9–54 years). No significant linear association was found between age and DR performance, but a significant quadratic trend was observed, with younger (9–20 years) and older individuals (≥40 years) performing worse than middle-aged chimpanzees (21–39 years). Similar findings were reported in a study that used a motor task to assess EF in five species of apes, including chimpanzees [105]. The participants slid a horizontal door to the left or to the right in order to obtain a reward. Following acquisition of the task, the response requirement was reversed. Both young and old apes made more errors in the reversal phase than the middle-aged ones, suggesting a developmental trajectory and an age-related decline in reversal learning performance. Finally, a recent study used the WCST to evaluate cognitive flexibility in 30 female chimpanzees (12–56 years old; [106]). The number of trials required for each dimension increased with age and the number of perseverative errors was significantly higher in middle-aged and older chimpanzees than in younger ones.
In contrast to the behavioural results, in vivo and ex vivo MRI scans of chimpanzee brains reveal modest age-related changes. Sherwood and colleagues [107] measured total grey and white matter volume, as well as frontal lobe grey and white matter volume in a sample of 99 chimpanzees and 87 humans. Linear and cubic associations between age and grey and white matter volume for the entire neocortex and the frontal lobe were observed in humans. By contrast, no significant associations were found between age and any of the measures of total neocortical or frontal lobe grey and white matter volume in the chimpanzee. In a subsequent study of female humans (n = 178), chimpanzees (n = 32) and rhesus monkeys (n = 20), age was associated with smaller total grey matter volume in all three species [108], but no decreases were observed in white matter volume of the chimpanzees or macaques. In these studies, the chimpanzee samples included relatively few old animals (age ≥40 years). A later MRI study on 219 chimpanzees that included 38 older individuals [109] found little evidence for age-related differences in total grey and white matter volume, overall gyrification, and grey matter thickness. However, the brains of old chimpanzees were less gyrified than those of sub-adults and young adults, and white matter volume increased earlier in life but decreased in later years.
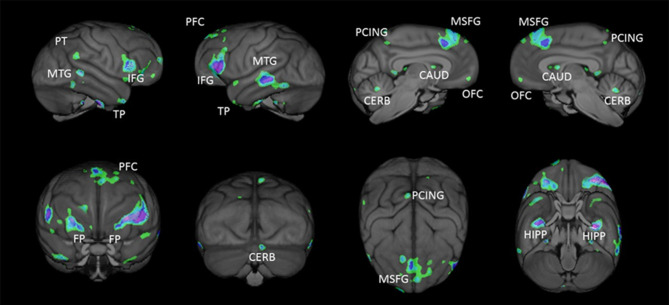
Voxel-based morphometry (VBM) of age-related changes in grey matter volume of 216 chimpanzees (9–54 years) revealed negative associations with age in some regions, with the strongest correlations found in the inferior frontal gyrus, left dlPFC, bilateral superior medial cortex, middle temporal gyrus, orbitofrontal cortex, right frontal pole and right inferior parietal cortex (figure 1). In another study, cortical thickness, surface area and local gyrification in 34 brain regions were estimated using Freesurfer [110] after the chimpanzee brain scans (n = 77) were warped in the human template. Only few associations between age and surface area were found; however, a number of negative associations between age and cortical thickness and gyrification were revealed, the most prominent within the frontal lobe. Thus, VBM analysis may be more likely to reveal age-related differences in the chimpanzee brain than gross volumetric measures.
Figure 1.
VBM analysis of age-related decline in grey matter volume (p < 0.001, uncorrected) in the chimpanzee brain (n = 216). The coloured regions reflect those brain areas that have reductions in grey matter volume with increasing age. PFC, prefrontal cortex; PT, planum temporale; FP, frontal pole; MSFG, medial superior frontal gyrus; MTG, middle temporal gyrus; TP, temporal lobe; IFG, inferior frontal gyrus; HIPP, hippocampus; PCING posterior cingulate; CAUD, caudate; CERB, cerebellum; OFC, orbitofrontal cortex. W. D. Hopkins, unpublished data.
In summary, three recent studies have provided evidence for age-related decline in EF in the chimpanzee, with some of the deficits emerging at middle age. However, the neural substrates underlying these age differences remain unclear, as imaging studies have revealed only modest age-related differences in the chimpanzee brain.
8. Conclusion
Prominent EF declines that characterize ageing in humans have been documented in all other primates studied so far. The ubiquity of these changes across primate orders, in species with widely different life histories, lifespans and brain architectures, suggests that age-related declines in EF are a fundamental feature of primate cognitive ageing. Furthermore, several observations suggest that EF decline represents one of the earliest age-related cognitive changes in humans, rhesus macaques and chimpanzees. Thus, EF is a useful construct and a potential early marker of age-related cognitive decline across primate species. More research is needed to precisely identify the neural bases of such cognitive changes. Age-related atrophy of the PFC, which is associated with age-related EF decrements in humans, appears to be absent in other NHPs, including chimpanzees. Instead, age-related changes in white matter and more subtle changes at the level of neurotransmitters, synaptic density and neuronal firing may underlie age-related EF declines in NHPs. As the majority of these neurobiological data come from macaques, it remains unclear whether species differences in primate brain ageing reflect differences in longevity, ecological niche or other factors.
Limitations that characterize NHP studies suggest caution in interpretation of the findings. Mitigating these limitations is crucial for advancing the understanding of primate cognitive ageing. First, there are few studies integrating cognitive and brain ageing assessments in NHPs. Second, small sample sizes, lack of sex comparisons and study of selective age ranges may lead to inaccurate or incomplete results. Low statistical power, typical in NHP research, precludes discovery of moderate or small changes and differences, which may be theoretically meaningful. Therefore, larger datasets are needed for a better understanding of the mechanisms of neurocognitive ageing in NHPs and in humans. Study of primate species with a shorter natural life expectancy (e.g. marmosets or mouse lemurs) may promote the creation of larger datasets with longitudinal measurements in both sexes. Third, noninvasive neuroimaging studies of NHPs, particularly great apes, are necessary for interpreting human studies in an evolutionary context. Such studies should employ methodologies similar to those used in humans. Fourth, given the prominent role of vascular and metabolic risk factors in human brain and cognitive ageing [111], more attention should be paid to these factors in NHP investigations. Specifically, publications should include detailed descriptions of diet, food restriction, activity opportunities and adiposity. Fifth, studies of NHPs should rely less upon single tasks to evaluate EF, and include multi-indicator, construct-based approaches. Such strategy would capitalize upon the rich findings that have emerged from studies of human cognitive and neural ageing.
Acknowledgements
The authors acknowledge support from NIH grants R01AG046266 (A.L.), R01AG011230 (N.R.), P01AG02642 (J.G.H.), and NS42867 and U42OD011197 (W.D.H.), National Center for Research Resources P51RR000165 and the Office of Research Infrastructure Programs P51OD011132.
Endnotes
Wisconsin Card Sorting Test: a test of set-shifting and inhibition of prepotent responses where participants have to sort cards according to changing rules according to colour, number or shape.
Stroop task: a task in which participants view colour names written in congruent or incongruent colour and report the colour of the word, not the word itself. The task is considered a test of inhibition and response selection.
Tower of Hanoi: a task of planning. Participants have to solve a puzzle in the shortest time while using a minimal number of moves. The planning of moves involves thinking ahead and using recursive approach.
N-back task: a task of working memory. Participants view a sequence of stimuli, one-by-one. For each stimulus, they have to determine if the current stimulus is the same as the one presented N trials beforehand.
Ethics
This article provides data collected in chimpanzees. The animals were cared for in accordance with the guidelines of the US National Research Council's Guide for the Care and Use of Laboratory Animals, the US Public Health Service's Policy on Humane Care and Use of Laboratory Animals, and the Guide for the Care and Use of Laboratory Animals (2011), 8th edition. All procedures used with the chimpanzees were approved by the local institutional animal care and use committees.
Data accessibility
The VBM analysis revealing the age-related changes in grey matter volume were performed on magnetic resonance images (MRI) available in the National Chimpanzee Brain Resource (www.chimpanzeebrain.org).
Authors' contributions
All authors have contributed equally to the manuscript.
Competing interests
We declare we have no competing interests.
References
- 1.Trewavas A. 2009. What is plant behaviour? Plant Cell Environ. 32, 606–616. ( 10.1111/j.1365-3040.2009.01929.x) [DOI] [PubMed] [Google Scholar]
- 2.Verschure PF, Pennartz CM, Pezzulo G. 2014. The why, what, where, when and how of goal-directed choice: neuronal and computational principles. Phil. Trans. R. Soc. B 369, 20130483 ( 10.1098/rstb.2013.0483) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rosati AG. 2017. The evolution of primate executive function: from response control to strategic decision-making. In Evolution of nervous systems, 2nd edn (eds Kaas J, Krubitzer L), pp. 423–437. Amsterdam, The Netherlands: Elsevier. [Google Scholar]
- 4.Broadbent DE. 1954. The role of auditory localization in attention and memory span. J. Exp. Psychol. 47, 191 ( 10.1037/h0054182) [DOI] [PubMed] [Google Scholar]
- 5.Goldstein S, Nagliari JA. 2013. Handbook of executive functioning. New York, NY: Springer. [Google Scholar]
- 6.Pribram KH. 1973. The primate frontal cortex: executive of the brain. In Psychophysiology of the frontal lobes (eds KH Pribram, AR Luria), pp. 293–314. New York, NY: Academic Press. [Google Scholar]
- 7.Gratiolet P. 1854. Mémoire sur les plis cérébraux de l’homme et des primates [Thesis on cerebral folds of man and primates]. Paris, France: Arthus Bertrand. [In French.] [Google Scholar]
- 8.Diamond A. 2013. Executive functions. Annu. Rev. Psychol. 64, 135–168. ( 10.1146/annurev-psych-113011-143750) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A, Wager TD. 2000. The unity and diversity of executive functions and their contributions to complex ‘frontal lobe’ tasks: a latent variable analysis. Cogn. Psychol. 41, 49–100. ( 10.1006/cogp.1999.0734) [DOI] [PubMed] [Google Scholar]
- 10.Friedman NP, Miyake A. 2017. Unity and diversity of executive functions: individual differences as a window on cognitive structure. Cortex 86, 186–204. ( 10.1016/j.cortex.2016.04.023) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burgess PW, Stuss DT. 2017. Fifty years of prefrontal cortex research: impact on assessment. J. Int. Neuropsychol. Soc. 23, 755–767. ( 10.1017/S1355617717000704) [DOI] [PubMed] [Google Scholar]
- 12.Luria AR. 1966. Higher cortical functions in man. Oxford, UK: Basic Books. [Google Scholar]
- 13.Teuber HL. 1972. Unity and diversity of frontal lobe functions. Acta Neurobiol. Exp. 32, 615–656. [PubMed] [Google Scholar]
- 14.Yuan P, Raz N. 2014. Prefrontal cortex and executive functions in healthy adults: a meta-analysis of structural neuroimaging studies. Neurosci. Biobehav. Rev. 42, 180–192. ( 10.1016/j.neubiorev.2014.02.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buchsbaum BR, Greer S, Chang WL, Berman KF. 2005. Meta-analysis of neuroimaging studies of the Wisconsin Card-Sorting task and component processes. Hum. Brain Mapp. 25, 35–45. ( 10.1002/hbm.20128) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Laird AR, McMillan KM, Lancaster JL, Kochunov P, Turkeltaub PE, Pardo JV, Fox PT. 2005. A comparison of label-based review and ALE meta-analysis in the Stroop task. Hum. Brain Mapp. 25, 6–21. ( 10.1002/hbm.20129) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rottschy C, Langner R, Dogan I, Reetz K, Laird AR, Schulz JB, Fox PT, Eickhoff SB. 2012. Modelling neural correlates of working memory: a coordinate-based meta-analysis. Neuroimage 60, 830–846. ( 10.1016/j.neuroimage.2011.11.050) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rhodes MG. 2004. Age-related differences in performance on the Wisconsin Card Sorting Test: a meta-analytic review. Psychol. Aging 19, 482 ( 10.1037/0882-7974.19.3.482) [DOI] [PubMed] [Google Scholar]
- 19.Brockmole JR, Logie RH. 2013. Age-related change in visual working memory: a study of 55,753 participants aged 8–75. Front. Psychol. 4, 12 ( 10.3389/fpsyg.2013.00012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hultsch DF, Hertzog C, Small BJ, McDonald-Miszczak L, Dixon RA. 1992. Short-term longitudinal change in cognitive performance in later life. Psychol. Aging 7, 571 ( 10.1037/0882-7974.7.4.571) [DOI] [PubMed] [Google Scholar]
- 21.Sapkota S, Bäckman L, Dixon RA. 2017. Executive function performance and change in aging is predicted by apolipoprotein E, intensified by catechol-O-methyltransferase and brain-derived neurotrophic factor, and moderated by age and lifestyle. Neurobiol. Aging 52, 81–89. ( 10.1016/j.neurobiolaging.2016.12.022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Raz N, Lindenberger U, Rodrigue KM, Kennedy KM, Head D, Williamson A, Dahle C, Gerstorf D, Acker JD. 2005. Regional brain changes in aging healthy adults: general trends, individual differences and modifiers. Cereb. Cortex 15, 1676–1689. ( 10.1093/cercor/bhi044) [DOI] [PubMed] [Google Scholar]
- 23.Salat DH, Kaye JA, Janowsky JS. 1999. Prefrontal gray and white matter volumes in healthy aging and Alzheimer disease. Arch. Neurol. 56, 338–344. ( 10.1001/archneur.56.3.338) [DOI] [PubMed] [Google Scholar]
- 24.O'Sullivan M, Jones DK, Summers PE, Morris RG, Williams SCR, Markus HS. 2001. Evidence for cortical ‘disconnection’ as a mechanism of age-related cognitive decline. Neurology 57, 632–638. ( 10.1212/WNL.57.4.632) [DOI] [PubMed] [Google Scholar]
- 25.Head D, Kennedy KM, Rodrigue KM, Raz N. 2009. Age differences in perseveration: cognitive and neuroanatomical mediators of performance on the Wisconsin Card Sorting Test. Neuropsychologia 47, 1200–1203. ( 10.1016/j.neuropsychologia.2009.01.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nagahama Y, Fukuyama H, Yamauchi H, Katsumi Y, Magata Y, Shibasaki H, Kimura J. 1997. Age-related changes in cerebral blood flow activation during a card sorting test. Exp. Brain Res. 114, 571–577. ( 10.1007/PL00005665) [DOI] [PubMed] [Google Scholar]
- 27.Esposito G, Kirkby BS, Van Horn JD, Ellmore TM, Berman KF. 1999. Context-dependent, neural system-specific neurophysiological concomitants of ageing: mapping PET correlates during cognitive activation. Brain 122, 963–979. ( 10.1093/brain/122.5.963) [DOI] [PubMed] [Google Scholar]
- 28.West RL. 1996. An application of prefrontal cortex function theory to cognitive aging. Psychol. Bull. 120, 272 ( 10.1037/0033-2909.120.2.272) [DOI] [PubMed] [Google Scholar]
- 29.Phillips KA, et al. 2014. Why primate models matter. Am. J. Primatol. 6, 801–827. ( 10.1002/ajp.22281) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Buffalo EA, Movshon JA, Wurtz RH. 2019. From basic brain research to treating human brain disorders. Proc. Natl Acad. Sci. USA 116, 26 167–26 172. ( 10.1073/pnas.1919895116) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harlow H, Bromer JA. 1938. A test-apparatus for monkeys. Psychol. Rec. 19, 434–438. ( 10.1007/BF03393227) [DOI] [Google Scholar]
- 32.Milner B. 1963. Effects of different brain lesions on card sorting: the role of the frontal lobes. Arch. Neurol. 9, 90–100. ( 10.1001/archneur.1963.00460070100010) [DOI] [Google Scholar]
- 33.Jacobsen CF. 1936. Studies of cerebral function in primates. I. The functions of the frontal association areas in monkeys. Comp. Psychol. Monogr. 13, 1–60. [Google Scholar]
- 34.Funahashi S. 2017. Working memory in the prefrontal cortex. Brain Sci. 7, 49 ( 10.3390/brainsci7050049) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Herndon JG, Lacreuse A. 2002. The rhesus monkey model as a heuristic resource in cognitive aging research. In Aging in nonhuman primates. Interdisciplinary topics in gerontology (eds Erwin JM, Hof PR), pp. 178–195. Basel, Switzerland: Karger. [Google Scholar]
- 36.Lacreuse A, Herndon JG. 2009. Nonhuman primate models of cognitive aging. In Animal models of human cognitive aging. Aging medicine (eds Bizon J, Woods A), pp. 29–58. Totowa, NJ: Humana Press. [Google Scholar]
- 37.Baxter MG. 2001. Cognitive aging in nonhuman primates. In Functional neurobiology of aging (eds Hof PR, Mobbs CV), pp. 407–420. San Diego, CA: Academic Press. [Google Scholar]
- 38.Latimer CS, et al. 2019. A nonhuman primate model of early Alzheimer's disease pathologic change: implications for disease pathogenesis. Alzheimer's Dementia 15, 93–105. ( 10.1016/j.jalz.2018.06.3057) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Herndon JG, Moss MB, Rosene DL, Killiany RJ. 1997. Patterns of cognitive decline in aged rhesus monkeys. Behav. Brain Res. 87, 25–34. ( 10.1016/S0166-4328(96)02256-5) [DOI] [PubMed] [Google Scholar]
- 40.Rapp PR. 1993. Neuropsychological analysis of learning and memory in the aged nonhuman primate. Neurobiol. Aging 14, 627–629. ( 10.1016/0197-4580(93)90050-L) [DOI] [PubMed] [Google Scholar]
- 41.Hara Y, Rapp P, Morrison J. 2012. Neuronal and morphological bases of cognitive decline in aged rhesus monkeys. AGE 34, 1051–1073. ( 10.1007/s11357-011-9278-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Voytko ML. 1993. Cognitive changes during normal aging in monkeys assessed with an automated test apparatus. Neurobiol. Aging 14, 643–644. ( 10.1016/0197-4580(93)90055-G) [DOI] [PubMed] [Google Scholar]
- 43.Ottinger MA, Mattison JA, Zelinski EM, Wu JM, Kohama SG, Roth GS, Lane MA, Ingram DK, Conn PM. 2006. The rhesus macaque as a model of human aging and age-related disease. In Handbook of models for human aging (ed. Conn PM.), pp. 457–484. San Diego, CA: Academic Press. [Google Scholar]
- 44.Bartus JM, Fleming D, Johnson HR. 1978. Aging in the rhesus monkey: debilitating effects on short-term memory. J. Gerontol. 34, 209–219. ( 10.1093/geronj/34.2.209) [DOI] [PubMed] [Google Scholar]
- 45.Rapp PR, Amaral DG. 1989. Evidence for task-dependent memory dysfunction in the aged monkey. J. Neurosci. 9, 3568–3576. ( 10.1523/JNEUROSCI.09-10-03568.1989) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lai ZC, Moss MB, Killiany RJ, Rosene DL, Herndon JG. 1995. Executive system dysfunction in the aged monkey: spatial and object reversal learning. Neurobiol. Aging 16, 947–954. ( 10.1016/0197-4580(95)02014-4) [DOI] [PubMed] [Google Scholar]
- 47.Voytko ML. 1999. Impairments in acquisition and reversals of two-choice discriminations by aged rhesus monkeys. Neurobiol. Aging 20, 617–627. ( 10.1016/S0197-4580(99)00097-4) [DOI] [PubMed] [Google Scholar]
- 48.Moore TL, Killiany RJ, Herndon JG, Rosene DL, Moss MB. 2003. Impairment in abstraction and set shifting in aged rhesus monkeys. Neurobiol. Aging 24, 125–134. ( 10.1016/S0197-4580(02)00054-4) [DOI] [PubMed] [Google Scholar]
- 49.Moore TL, Killiany RJ, Herndon JG, Rosene DL, Moss MB. 2006. Executive system dysfunction occurs as early as middle-age in the rhesus monkey. Neurobiol. Aging 27, 1484–1493. ( 10.1016/j.neurobiolaging.2005.08.004) [DOI] [PubMed] [Google Scholar]
- 50.Peters A, Morrison JH, Rosene DL, Hyman BT. 1998. Are neurons lost from the primate cerebral cortex during normal aging? Cereb. Cortex 8, 295–300. ( 10.1093/cercor/8.4.295) [DOI] [PubMed] [Google Scholar]
- 51.Alexander GE, et al. 2008. Age-related regional network of magnetic resonance imaging gray matter in the rhesus macaque. J. Neurosci. 28, 2710–2718. ( 10.1523/JNEUROSCI.1852-07.2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dickstein DL, Weaver CM, Luebke JI, Hof PR. 2012. Dendritic spine changes associated with normal aging. Neuroscience 251, 21–32. ( 10.1016/j.neuroscience.2012.09.077) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Peters A, Sethares C. 2002. Aging and the myelinated fibers in prefrontal cortex and corpus callosum of the monkey. J. Comp. Neurol. 442, 277–291. ( 10.1002/cne.10099) [DOI] [PubMed] [Google Scholar]
- 54.Wisco JJ, Killiany RJ, Guttmann CRG, Warfield SK, Moss MB, Rosene DL. 2008. An MRI study of age-related white and gray matter volume changes in the rhesus monkey. Neurobiol. Aging 29, 1563–1575. ( 10.1016/j.neurobiolaging.2007.03.022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.O'Donnell KA, Rapp PR, Hof PR. 1999. Preservation of prefrontal cortical volume in behaviorally characterized aged macaque monkeys. Exp. Neurol. 160, 300–310. ( 10.1006/exnr.1999.7192) [DOI] [PubMed] [Google Scholar]
- 56.Wang M, Gamo NJ, Yang Y, Jin LE, Wang X-J, Laubach M, Mazer JA, Lee D, Arnsten AFT. 2011. Neuronal basis of age-related working memory decline. Nature 476, 210–213. ( 10.1038/nature10243) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Young ME, Ohm DT, Dumitriu D, Rapp PR, Morrison JH. 2014. Differential effects of aging on dendritic spines in visual cortex and prefrontal cortex of the rhesus monkey. Neuroscience 274, 33–43. ( 10.1016/j.neuroscience.2014.05.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sridharan A, et al. 2012. Brain volumetric and microstructural correlates of executive and motor performance in aged rhesus monkeys. Front. Aging Neurosci. 4, 31 ( 10.3389/fnagi.2012.00031) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Moore TL, Schettler SP, Killiany RJ, Herndon JG, Luebke JI, Moss MB, Rosene DL. 2005. Cognitive impairment in aged rhesus monkeys associated with monoamine receptors in the prefrontal cortex. Behav. Brain Res. 160, 208–221. ( 10.1016/j.bbr.2004.12.003) [DOI] [PubMed] [Google Scholar]
- 60.Lacreuse A, Kim CB, Rosene DL, Killiany RJ, Moss MB, Moore TL, Chennareddi L, Herndon JG. 2005. Sex, age, and training modulate spatial memory in the rhesus monkey (Macaca mulatta). Behav. Neurosci. 119, 118–126. ( 10.1037/0735-7044.119.1.118) [DOI] [PubMed] [Google Scholar]
- 61.Lacreuse A, Mong JA, Hara Y. 2015. Neurocognitive effects of estrogens across the adult lifespan in nonhuman primates: state of knowledge and new perspectives. Horm. Behav. 74, 157–166. ( 10.1016/j.yhbeh.2015.03.001) [DOI] [PubMed] [Google Scholar]
- 62.Moore TL, Killiany RJ, Rosene DL, Prusty S, Hollander W, Moss MB. 2002. Impairment of executive function induced by hypertension in the rhesus monkey (Macaca mulatta). Behav. Neurosci. 116, 387 ( 10.1037/0735-7044.116.3.387) [DOI] [PubMed] [Google Scholar]
- 63.Hämäläinen A, Dammhahn M, Aujard F, Eberle M, Hardy I, Kappeler PM, Perret M, Schliehe-Diecks S, Kraus C. 2014. Senescence or selective disappearance? Age trajectories of body mass in wild and captive populations of a small-bodied primate. Proc. R. Soc. B 281, 20140830 ( 10.1098/rspb.2014.0830) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bons N, Mestre N, Petter A. 1992. Senile plaques and neurofibrillary changes in the brain of an aged lemurian primate, Microcebus murinus. Neurobiol. Aging 13, 99–105. ( 10.1016/0197-4580(92)90016-Q) [DOI] [PubMed] [Google Scholar]
- 65.Delacourte A, Sautiere PE, Wattez A, Mourton-Gilles C, Petter A, Bons N. 1995. Biochemical characterization of Tau proteins during cerebral aging of the lemurian primate Microcebus murinus. C. R. Acad. Sci. Ser. III Sci. Vie 318, 85–89. [PubMed] [Google Scholar]
- 66.Picq J-L. 1993. Radial maze performance in young and aged grey mouse lemurs (Microcebus murinus). Primates 34, 223–226. ( 10.1007/BF02381394) [DOI] [Google Scholar]
- 67.Bons N, Rieger F, Prudhomme D, Fisher A, Krause KH. 2006. Microcebus murinus: a useful primate model for human cerebral aging and Alzheimer's disease? Genes Brain Behav. 5, 120–130. ( 10.1111/j.1601-183x.2005.00149.x) [DOI] [PubMed] [Google Scholar]
- 68.Nadkarni NA, Bougacha S, Garin C, Dhenain M, Picq J-L. 2018. Digital templates and brain atlas dataset for the mouse lemur primate. Data Brief 21, 1178–1185. ( 10.1016/j.dib.2018.10.067) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Picq JL. 2016. The gray mouse lemur (Microcebus murinus): a novel cognitive primate brain aging model. In The dwarf and mouse lemurs of Madagascar: biology, behavior and conservation biogeography of the Cheirogaleidae (eds S Lehman, U Radespiel, E Zimmermann), pp. 381–404. Cambridge, UK: Cambridge University Press ( 10.1017/CBO9781139871822.021) [DOI]
- 70.Picq J-L. 2007. Aging affects executive functions and memory in mouse lemur primates. Exp. Gerontol. 42, 223–232. ( 10.1016/j.exger.2006.09.013) [DOI] [PubMed] [Google Scholar]
- 71.Picq J-L, Aujard F, Volk A, Dhenain M. 2012. Age-related cerebral atrophy in nonhuman primates predicts cognitive impairments. Neurobiol. Aging 33, 1096–1109. ( 10.1016/j.neurobiolaging.2010.09.009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Joly M, Ammersdorfer S, Schmidtke D, Zimmermann E. 2014. Touchscreen-based cognitive tasks reveal age-related impairment in a primate aging model, the grey mouse lemur (Microcebus murinus). PLoS ONE 9, e109393 ( 10.1371/journal.pone.0109393) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Picq JL. 1995. Effects of aging upon recent memory in Microcebus murinus. Aging 7, 17–22. [DOI] [PubMed] [Google Scholar]
- 74.Languille S, et al. 2015. Deficits of psychomotor and mnesic functions across aging in mouse lemur primates. Front. Behav. Neurosci. 8, 446 ( 10.3389/fnbeh.2014.00446) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dhenain M, Michot JL, Privat N, Picq JL, Boller F, Duyckaerts C, Volk A. 2000. MRI description of cerebral atrophy in mouse lemur primates. Neurobiol. Aging 21, 81–88. ( 10.1016/S0197-4580(00)00098-1) [DOI] [PubMed] [Google Scholar]
- 76.Kraska A, et al. 2011. Age-associated cerebral atrophy in mouse lemur primates. Neurobiol. Aging 32, 894–906. ( 10.1016/j.neurobiolaging.2009.05.018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fritz RG, Zimmermann E, Picq J-L, Lautier C, Meier M, Kästner S, Schmidtke D. 2020. Sex-specific patterns of age-related cerebral atrophy in a nonhuman primate Microcebus murinus. Neurobiol. Aging 91, 148–159. ( 10.1016/j.neurobiolaging.2020.02.027) [DOI] [PubMed] [Google Scholar]
- 78.Sawiak SJ, Picq J-L, Dhenain M. 2014. Voxel-based morphometry analyses of in vivo MRI in the aging mouse lemur primate. Front. Aging Neurosci. 6, 82 ( 10.3389/fnagi.2014.00082) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nadkarni NA, Bougacha S, Garin C, Dhenain M, Picq J-L. 2019. A 3D population-based brain atlas of the mouse lemur primate with examples of applications in aging studies and comparative anatomy. Neuroimage 185, 85–95. ( 10.1016/j.neuroimage.2018.10.010) [DOI] [PubMed] [Google Scholar]
- 80.Nishijima K, Saitoh R, Tanaka S, Ohsato-Suzuki M, Ohno T, Kitajima S. 2012. Life span of common marmoset (Callithrix jacchus) at CLEA Japan breeding colony. Biogerontology 13, 439–443. ( 10.1007/s10522-012-9388-1) [DOI] [PubMed] [Google Scholar]
- 81.Geula C, Nagykery N, Wu CK. 2002. Amyloid-β deposits in the cerebral cortex of the aged common marmoset (Callithrix jacchus): incidence and chemical composition. Acta Neuropathol. 103, 48–58. ( 10.1007/s004010100429) [DOI] [PubMed] [Google Scholar]
- 82.Leuner B, Kozorovitskiy Y, Gross CG, Gould E. 2007. Diminished adult neurogenesis in the marmoset brain precedes old age. Proc. Natl Acad. Sci. USA 104, 17 169–17 173. ( 10.1073/pnas.0708228104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Harada T, Tokuriki M, Tanioka Y. 1999. Age-related changes in the brainstem auditory evoked potentials of the marmoset. Hear. Res. 128, 119–124. ( 10.1016/S0378-5955(98)00201-9) [DOI] [PubMed] [Google Scholar]
- 84.Tardif SD, Mansfield KG, Ratnam R, Ross CN, Ziegler TE. 2011. The marmoset as a model of aging and age-related diseases. ILAR J. 52, 54–65. ( 10.1093/ilar.52.1.54) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ross CN. 2019. Marmosets in aging research. In The common marmoset in captivity and biomedical research (eds Fox JG, Marini RP, Wachtman LM, Tardif SD, Mansfield K), pp. 355–376. New York, NY: Academic Press. [Google Scholar]
- 86.Austad SN. 1997. Small nonhuman primates as potential models of human aging. ILAR J. 38, 142–147. ( 10.1093/ilar.38.3.142) [DOI] [PubMed] [Google Scholar]
- 87.Munger EL, Takemoto A, Raghanti MA, Nakamura K. 2017. Visual discrimination and reversal learning in aged common marmosets (Callithrix jacchus). Neurosci. Res. 124, 57–62. ( 10.1016/j.neures.2017.06.002) [DOI] [PubMed] [Google Scholar]
- 88.Sadoun A, Rosito M, Fonta C, Girard P. 2018. Key periods of cognitive decline in a nonhuman primate model of cognitive aging, the common marmoset (Callithrix jacchus). Neurobiol. Aging 74, 1–14. ( 10.1016/j.neurobiolaging.2018.10.003) [DOI] [PubMed] [Google Scholar]
- 89.Workman KP, Healey B, Carlotto A, Lacreuse A. 2019. One-year change in cognitive flexibility and fine motor function in middle-aged male and female marmosets (Callithrix jacchus). Am. J. Primatol. 81, e22924 ( 10.1002/ajp.22924) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.LaClair M, et al. 2019. Sex differences in cognitive flexibility and resting brain networks in middle-aged marmosets. Eneuro 6, ENEURO.0154–19.2019 ( 10.1523/ENEURO.0154-19.2019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sasaki E, et al. 2009. Generation of transgenic non-human primates with germline transmission. Nature 459, 523–527. ( 10.1038/nature08090) [DOI] [PubMed] [Google Scholar]
- 92.Okano H, Sasaki E, Yamamori T, Iriki A, Shimogori T, Yamaguchi Y, Kasai K, Miyawaki A. 2016. Brain/MINDS: a Japanese national brain project for marmoset neuroscience. Neuron 92, 582–590. ( 10.1016/j.neuron.2016.10.018) [DOI] [PubMed] [Google Scholar]
- 93.Miller CT. 2017. Why marmosets? Dev. Neurobiol. 77, 237–243. ( 10.1002/dneu.22483) [DOI] [PubMed] [Google Scholar]
- 94.Rodriguez-Callejas JD, Fuchs E, Perez-Cruz C. 2016. Evidence of Tau hyperphosphorylation and dystrophic microglia in the common marmoset. Front. Aging Neurosci. 8, 315 ( 10.3389/fnagi.2016.00315) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Liu JV, Bock NA, Silva AC. 2011. Rapid high-resolution three-dimensional mapping of T1 and age-dependent variations in the non-human primate brain using magnetization-prepared rapid gradient-echo (MPRAGE) sequence. Neuroimage 56, 1154–1163. ( 10.1016/j.neuroimage.2011.02.075) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Phillips KA, Watson CM, Bearman A, Knippenberg AR, Adams J, Ross C, Tardif SD. 2019. Age-related changes in myelin of axons of the corpus callosum and cognitive decline in common marmosets. Am. J. Primatol. 81, e22949 ( 10.1002/ajp.22949) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Havercamp K, Watanuki K, Tomonaga M, Matsuzawa T, Hirata S. 2019. Longevity and mortality of captive chimpanzees in Japan from 1921 to 2018. Primates 60, 525–535. ( 10.1007/s10329-019-00755-8) [DOI] [PubMed] [Google Scholar]
- 98.Rilling JK. 2014. Comparative primate neuroimaging: insights into human brain evolution. Trends Cogn. Sci. 18, 46–55. ( 10.1016/j.tics.2013.09.013) [DOI] [PubMed] [Google Scholar]
- 99.Tomasello M, Call J. 1997. Primate cognition. New York, NY: Oxford University Press. [Google Scholar]
- 100.Bernstein IS. 1961. Response variability and rigidity in the adult chimpanzee. J. Gerontol. 16, 381–386. ( 10.1093/geronj/16.4.381) [DOI] [PubMed] [Google Scholar]
- 101.Riopelle AJ, Rogers CM. 1965. Age changes in chimpanzees. In Behavior of nonhuman primates: modern research trends (eds Schrier AM, et al.), pp. 449–462. New York, NY: Academic Press. [Google Scholar]
- 102.Lacreuse A, Russell JL, Hopkins WD, Herndon JG. 2014. Cognitive and motor aging in female chimpanzees. Neurobiol. Aging 35, 623–632. ( 10.1016/j.neurobiolaging.2013.08.036) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Herrmann E, Call J, Hernandez-Lloreda MV, Hare B, Tomasello M. 2007. Humans have evolved specialized skills of social cognition: the cultural intelligence hypothesis. Science 317, 1360–1366. ( 10.1126/science.1146282) [DOI] [PubMed] [Google Scholar]
- 104.Hopkins WD, Russell JL, Schaeffer J. 2014. Chimpanzee intelligence is heritable. Curr. Biol. 24, 1649–1652. ( 10.1016/j.cub.2014.05.076) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Manrique HM, Call J. 2015. Age-dependent cognitive inflexibility in great apes. Anim. Behav. 102, 1–6. ( 10.1016/j.anbehav.2015.01.002) [DOI] [Google Scholar]
- 106.Lacreuse A, Parr L, Chennareddi L, Herndon JG. 2018. Age-related decline in cognitive flexibility in female chimpanzees. Neurobiol. Aging 72, 83–88. ( 10.1016/j.neurobiolaging.2018.08.018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sherwood CC, Gordon AD, Allen JS, Phillips KA, Erwin JM, Hof PR, Hopkins WD. 2011. Aging of the cerebral cortex differs between humans and chimpanzees. Proc. Natl Acad. Sci. USA 108, 13 029–13 034. ( 10.1073/pnas.1016709108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chen X, et al. 2013. Brain aging in humans, chimpanzees (Pan troglodytes), and rhesus macaques (Macaca mulatta): magnetic resonance imaging studies of macro- and microstructural changes. Neurobiol. Aging 34, 2248–2260. ( 10.1016/j.neurobiolaging.2013.03.028) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Autrey MM, Reamer LA, Mareno MC, Sherwood CC, Herndon JG, Preuss T, Schapiro SJ, Hopkins WD. 2014. Age-related effects in the neocortical organization of chimpanzees: gray and white matter volume, cortical thickness, and gyrification. Neuroimage 101, 59–67. ( 10.1016/j.neuroimage.2014.06.053) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Desikan RS, et al. 2006. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 31, 968–980. ( 10.1016/j.neuroimage.2006.01.021) [DOI] [PubMed] [Google Scholar]
- 111.Dahle CL, Jacobs BS, Raz N. 2009. Aging, vascular risk, and cognition: blood glucose, pulse pressure, and cognitive performance in healthy adults. Psychol. Aging 24, 154–162. ( 10.1037/a0014283) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The VBM analysis revealing the age-related changes in grey matter volume were performed on magnetic resonance images (MRI) available in the National Chimpanzee Brain Resource (www.chimpanzeebrain.org).