Abstract
While declining physical performance is an expected consequence of ageing, human clinical research has placed increasing emphasis on physical frailty as a predictor of death and disability in the elderly. We examined non-invasive measures approximating frailty in a richly sampled longitudinal dataset on wild chimpanzees. Using urinary creatinine to assess lean body mass, we found moderate but significant declines in physical condition with age in both sexes. While older chimpanzees spent less of their day in the trees and feeding, they did not alter activity budgets with respect to travel or resting. There was little evidence that declining lean body mass had negative consequences independent of age. Old chimpanzees with poor lean body mass rested more often but did not otherwise differ in activity. Males, but not females, in poor condition were more likely to exhibit respiratory illness. Poor muscle mass was associated acutely with death in males, but it did not predict future mortality in either sex. While there may be some reasons to suspect biological differences in the susceptibility to frailty in chimpanzees versus humans, our data are consistent with recent reports from humans that lean, physically active individuals can successfully combat frailty.
This article is part of the theme issue ‘Evolution of the primate ageing process’.
Keywords: sarcopenia, ageing, primates, evolution, activity, health
1. Introduction
In this study, we investigate how physical condition and performance are affected by ageing in wild chimpanzees. Declining physical performance is a product of the degenerative processes of ageing, owing to the accumulated consequences of illness and injuries and decreased investment in somatic repair. Rather than just a side effect of these other processes, physical ‘frailty’ has itself emerged as a clinically significant phenomenon in humans, predicting a variety of poor health outcomes, including death [1,2]. Frailty has been defined as a loss of functional homeostasis, or ‘the ability of an individual to withstand illness without loss of function’ [3]. With advancing age, individuals experience a progressive loss of physiological reserves necessary for routine somatic function and repair [4]. Once this process is sufficiently advanced, frail individuals experience deficits in performing the activities of daily living [5]. Diagnostic criteria vary, but the frailty syndrome is typically defined by some combination of weight loss, muscle weakness, reduced physical activity, slowness and/or exhaustion [6,7]. Sarcopenia, or loss of muscle mass and function [8], is often a contributor to frailty but is also an independent predictor of death and disability [9].
While frailty is a significant health burden among the elderly, it is far from ubiquitous, affecting approximately 10% of people aged 65 years and over [10]. Risk factors include low socio-economic status [11], inadequate physical activity [12,13] and obesity [14]. Physical frailty is also strongly correlated with inflammation [15]. Thus, it is not clear whether physical frailty is a common feature of ageing in long-lived species or a unique challenge of ageing in humans associated with a greatly extended lifespan and/or unique lifestyle factors.
In closely related non-human primate species, there is mixed evidence that physical frailty accumulates with age. Among captive cercopithecines, age was not associated with changes in body mass or activity, though older individuals walked slower and climbed less [16]. However, ageing wild baboons exhibited small but significant declines in body mass index [17]. For chimpanzees, which are most closely related to humans and have maximum lifespans exceeding 60 years [18], evidence is indirect. Wild chimpanzee skeletons exhibit limited evidence of degenerative joint disease or arthritis, usually mild or secondary to trauma, along with osteoporosis, skeletal trauma, and tooth wear, all of which might contribute to frailty [19–22]. One study of wild chimpanzees found moderate age-related declines in male, but not female, body mass [23]. Unlike humans, non-human primates do not receive substantial support from group-mates if their physical condition compromises foraging. To meet their nutritional needs, primates often need to travel over long distances and climb high into the forest canopy. Poor physical performance potentially compromises feeding competition and increases vulnerability to attacks by predators or conspecifics. Thus, it is plausible that long-term survival in a frail condition is not possible in the wild. Remarkably little is known about the natural causes of death in wild primates, often because the dead are not able to be recovered for necropsy. However, longitudinal data on the living can reveal how degenerative processes like frailty contribute to or predict mortality.
Here, we examine long-term data on wild chimpanzees to determine how physical condition and function change with age and whether this physical decline can be understood as an increase in frailty, i.e. if declining physical condition has negative effects on health, survival and activity. To examine changes in condition, we analysed 21 years of longitudinal data on urinary creatinine, a validated marker of lean body mass that has been used to assess sarcopenia in humans [24,25]. Mortality rates in this population accelerate between the ages of 30 and 35 years [26]; thus we predicted that estimated lean body mass (ELBM) would decline at approximately the same ages. To examine age-related changes in physical performance equivalent to components of human frailty indices (e.g. slowness, weakness, reduced activity), we analysed 8.5 years of individual activity budgets, predicting that older individuals would climb less (i.e. spend less of the day in trees versus on the ground), spend more time resting, and spend less time travelling and feeding. Finally, to assess whether declining physical condition contributes to a frailty-like syndrome, we tested the prediction that individuals with low ELBM would be less active, experience higher rates of respiratory illness, and have higher mortality. In Kanyawara, respiratory signs are the most readily and frequently observed indicators of morbidity, increase in frequency with age, and are the most common known cause of adult death [27]. In humans, frailty increases both the likelihood of respiratory illness and the likelihood of recovery in elderly populations [28,29].
2. Methods
Data were collected from the Kanyawara community of wild chimpanzees in the Kibale National Park, Uganda. The Kanyawara chimpanzees were first studied by Gilbert Isabirye-Basuta from 1983 to 1985, and the long-term continuous study was established by Richard Wrangham in 1987. Prior to 2009, all data were collected during full-day group follows, comprising 15 min scan samples of subgroup composition, chimpanzee diet and ad libitum behavioural data. From August 2009, observers switched to daily follows of focal animals, recording the same group-level data as before, in addition to scan samples of focal activity state every minute. Urine sample collections began in 1997. Clinical signs were recorded on a daily basis. Our study focused on individuals aged 15 and over (sample size information, electronic supplementary material, table S1). Few individuals lived past age 55 (two females, one male), so for all but the survival analyses, we capped ages at 55 years to avoid overfitting. We excluded one individual who had lost both feet as a result of wire snare injuries, as his ELBM was unusually low, and his mobility was compromised.
Chimpanzees born into Kanyawara since 1983 have ages known to at least the nearest year. Chimpanzees that were immature at first identification could also be aged with a low margin of error (2–3 years), as could immigrant females, owing to a narrow age range for natal dispersal [18]. Individuals that were adults at the beginning of long-term observations were assigned ages based on their physical features and the age/numbers of dependent offspring [26]. This applied to 15 individuals in the urine dataset and four for the focal activity budgets. Because their exact ages are uncertain, we verified our analyses in a restricted dataset excluding them (maximum age = 45). This yielded minor differences in some statistical outcomes (see electronic supplementary material), but confirmed the major findings of the full dataset.
(a). Lean body mass
ELBM was derived from a previously validated approach to modifying the 24 h creatinine method [30] for use with spot urine samples [31,32] (see electronic supplementary material). Creatinine was assessed using the colorimetric Jaffe reaction, while specific gravity was assessed using a hand-held refractometer (Atago PAL-10S). These measures are highly correlated estimates of urinary water content, but only creatinine is dependent on muscle mass. Thus, the residual variation in creatinine that is not explained by specific gravity provides a relative approximation of ELBM. Because overly dilute samples yield exceedingly high residuals, we omitted 463 samples with specific gravity less than 1.003. To obtain creatinine residuals for use in the analyses, we calculated a global fit of creatinine against specific gravity (minus 1) and its squared transformation (R2 = 0.832, p < 0.00001). Our final sample comprised 9802 urine samples from 30 adult females and 10 566 samples from 19 adult males.
(b). Activity
Activity budgets were collected by trained Ugandan field staff during focal follows between August 2009 and December 2017 (N = 2758 focals of 35 chimpanzees, mean ± s.d. duration = 9.8 ± 2.7 h per focal). A focal chimpanzee was usually selected at the nesting site (i.e. upon waking), and if possible, followed by a pair of observers for the entire day until it nested again. If a focal was lost, another was selected as soon as possible. While observers attempted to distribute focal observations as evenly as possible over time, some subjects were located more frequently than others. When possible, multiple observers followed multiple focal individuals. Activity states and substrate (tree or ground) were recorded to the nearest minute. We divided time in each follow according to the following activity states: (a) feeding: active selection or ingestion; (b) travelling: walking, running or climbing; (c) resting: no active behaviour; (d) other (not analysed): including socializing and miscellaneous rare behaviours; (e) out of view. Minutes of feeding, travelling and resting, as well as time spent in trees (versus on the ground) were analysed per follow and were corrected for the total minutes in view of observers using an offset term.
To control for the effects of temporal changes in diet on activity and ELBM, we calculated a measure of ripe fruit consumption from 15 min group-level scan observations. This was defined as the proportion of scans in which chimpanzees were observed eating ripe fruit out of all observations of feeding and was calculated for the two weeks prior to and including the date of observation (i.e. urine sample or focal follow).
(c). Data analysis
To characterize age trends in ELBM and activity, we constructed generalized additive mixed models (GAMM) using the mgcv::gam package in R. Outcome measures were rescaled to positive, non-zero values, as necessary, and fitted using the gamma distribution and log link function. We ran an initial GAMM to determine the overall fit, controlling for sex, ripe fruit consumption and random effects for chimpanzee identity and unique study month. Next, we reran the models with separate smooths to age by sex and, by re-specifying sex as an ordered factor, estimated the difference between the smooths for males and females (i.e. age by sex interaction). We constrained fits to a relatively small k-value (5) to avoid overfitting and used gam.check to verify normality and an unbiased error distribution. Descriptive statistics are provided for activity budgets as means of individual means for chimpanzee with greater than or equal to 100 h of observation.
To evaluate whether deteriorating physical condition affects chimpanzees' health and daily activities, we examined whether low ELBM predicted three types of response (activity, respiratory signs and death). We calculated mean ELBM for each individual in 1-year intervals (chimpanzee-years) to ensure thorough sampling of individuals in each interval. Chimpanzee-years with fewer than 10 urine samples were omitted. Studies of humans frequently define sarcopenia using a threshold of muscle mass ≥ 2 s.d. below the average for healthy, young adults of the same sex [33]. Accordingly, we derived normative and threshold values based on the distribution of individual means between the ages 15.00 and 29.99 years. The distribution of creatinine residuals was too narrow for the 2 s.d. threshold to be useful; females, in particular, rarely dipped below this threshold (electronic supplementary material, figure S1). Therefore, we applied a threshold of 1.5 s.d. to capture a reasonable subset of data at the low end of the distribution (6.5% of female chimpanzee-years, 20.5% of male chimpanzee-years), consistent with rates of sarcopenia defined in human studies [33]. Sarcopenia was coded as present or absent (1/0), and because younger individuals sometimes met the criteria, we examined interactions between sarcopenia and age to determine whether poor body condition was specifically detrimental for older chimpanzees. Full model specifications are provided in the electronic supplementary material.
3. Results
(a). Lean body mass
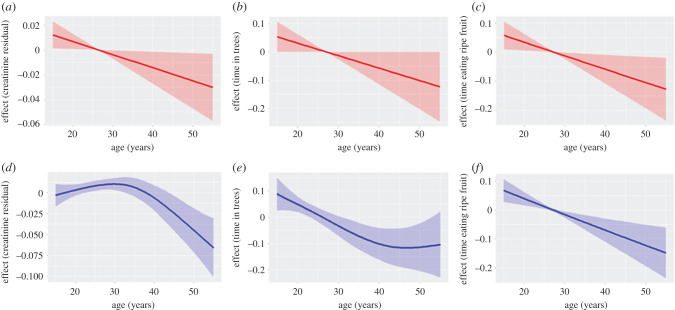
As chimpanzees aged, their ELBM, as estimated by residual creatinine excretion, declined significantly (table 1). Age smooths were significantly different between the sexes (figure 1a,d). In females, the effect of ageing was approximately linear. In males, there was a small increase with age through the early 30s, after which ELBM sharply declined. By age 40, the average male had lower ELBM than at any previous adult age. Marginal effects indicate a rate of decline in males approximately twice that of females. While this analysis, which considers individual random effects, showed clear and strong influences of ageing within individuals, such effects were less apparent in cross-section (figure 2). Males aged 15–40 had similar ELBM estimates, while the few males remaining over age 50 had markedly lower values, in the range typical of females. While poor ELBM was evident in some females in their 30s, the few longer-lived individuals often registered in robust condition. Taken together, these results indicate robust age changes within longitudinal data on individuals that were masked in the cross-sectional data.
Table 1.
Results of GAMM models for estimated lean body mass (ELBM) and activity budgets. The model was estimated controlling for sex (upper section), then respecified to determine whether smooth functions differed by sex (lower section). edf, estimated degrees of freedom.
| variable | ELBM | in trees | travel | feed | ripe fruit | rest | |
|---|---|---|---|---|---|---|---|
| global fit | adjusted R2 | 0.318 | 0.569 | 0.347 | 0.589 | 0.374 | 0.543 |
| SexM | estimate t p |
0.141 12.6 <0.0001 |
−0.424 −12.1 <0.0001 |
0.140 2.7 0.007 |
−0.061 −2.6 0.008 |
−0.086 −3.1 0.002 |
0.007 0.2 0.823 |
| age (smooth) | edf F p |
2.785 5.5 0.0006 |
1.004 11.1 0.0008 |
2.322 1.1 0.480 |
1.003 6.4 0.011 |
1.239 12.2 <0.0001 |
1.228 0.3 0.744 |
| diet (smooth) | edf F p |
3.280 6.3 0.0002 |
1.425 1.7 0.12 |
1.005 13.0 0.0003 |
1.746 9.6 <0.0001 |
2.099 56.6 <0.0001 |
2.821 4.3 0.008 |
| chimpanzee (random) | edf F p |
34.424 33.1 <0.0001 |
23.062 6.4 <0.0001 |
22.574 5.3 <0.0001 |
19.589 2.6 <0.0001 |
12.738 1.9 <0.0001 |
20.946 3.7 <0.0001 |
| study month (random) | edf F p |
219.499 33.1 <0.0001 |
57.831 2.2 <0.0001 |
68.411 2.5 <0.0001 |
75.787 4.7 <0.0001 |
34.944 1.5 <0.0001 |
71.838 3.8 <0.0001 |
| fits by sex | |||||||
| females (smooth) | edf F p |
1.000 4.8 0.028 |
1.001 4.1 0.044 |
2.666 1.9 0.134 |
1.005 2.7 0.100 |
1.011 5.4 0.019 |
1.075 0.1 0.885 |
| males (smooth) | edf F p |
2.981 6.2 0.0002 |
1.535 5.0 0.021 |
1.002 0.1 0.732 |
1.015 3.5 0.062 |
1.027 11.2 0.0008 |
1.005 0.2 0.670 |
| age × SexM (smooth) | edf F p |
4.172 4.3 0.0005 |
1.538 0.2 0.733 |
1.002 1.0 0.324 |
1.011 0.01 0.938 |
1.008 0.1 0.700 |
1.003 0.01 0.912 |
Figure 1.
Results of GAMMs for (a,d) ELBM (urinary creatinine residuals), (b,e) time spent in trees, and (c,f) time spent eating ripe fruit, showing marginal effects (plus 95% confidence interval) for age, controlling for diet and random effects of sample month and individual identity. Females: red, top row; males: blue, bottom row. For non-significant effects, see electronic supplementary material, figure S2. (Online version in colour.)
Figure 2.
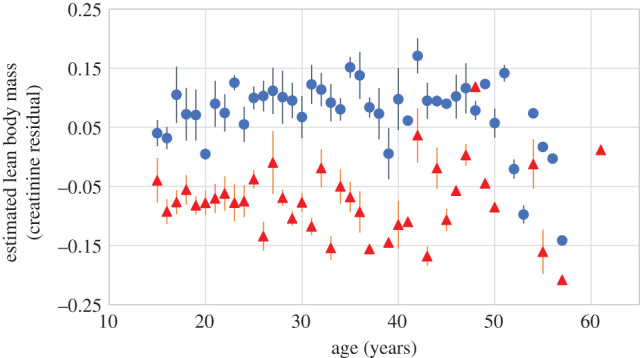
Cross-sectional association between age and ELBM. Shown are means ± s.e. across individual means for each age for males (blue circles, 190 male-years) and females (red triangles, 186 female-years). Individuals with fewer than 10 data points per age were omitted. (Online version in colour.)
(b). Activity
As chimpanzees aged, they spent less time in trees (figure 1b,e and table 1). Males spent significantly less time in trees than females overall; thus while the effect for females was approximately linear across adulthood, smooths for males reached a minimum asymptote by age 40. However, there was no significant difference in the effect of ageing between males and females. Females over age 30 years spent 67.3% (±9.2 s.d.) of their time in the trees, compared with 70.9% (±8.5) for younger females, a difference equating to 26 min in a 12 h day. Males over age 30 years spent 41.4% (±4.9) of their time in trees compared with 47.2% (±4.0) for younger males, equating to a 42 min difference in a 12 h day.
Given that most chimpanzee foods are in the canopy, spending less time in the trees may be expected to reduce foraging. Indeed, when both sexes were considered together, older chimpanzees spent significantly less time feeding overall than younger chimpanzees (table 1). However, neither the age smooth for males nor that for females reached significance (electronic supplementary material, figure S2). The effect of age was stronger for time spent feeding on chimpanzees' preferred resource, ripe fruit, which is almost exclusively located in the canopy (figure 1c,f). Feeding on ripe fruit declined moderately but significantly with age in both females (29.0 ± 4.9% under 30 years versus 27.0 ± 3.0% over 30 years) and males (27.5 ± 2.1% under 30 years versus 24.0 ± 3.8% over 30 years), with no significant difference in the age effect between the sexes.
Contrary to the prediction that older chimpanzees are less physically active, age had no effect on the amount of time that chimpanzees spent travelling or resting (table 1; electronic supplementary material, figure S2).
(c). Does declining physical condition have detrimental effects?
Since ageing was associated with simultaneous declines in ELBM and some measures of activity, it would be reasonable to predict a causal link between poor condition, impaired mobility and shifting activity budgets. To evaluate this possibility, we calculated annual averages for each chimpanzee on each measure and used linear mixed models (LMMs) to examine whether sarcopenia, defined as ELBM ≥1.5 s.d. below the mean of young adults, predicted activity measures independently of age (electronic supplementary material, table S2). Sarcopenia did not predict time spent travelling, time feeding on ripe fruit, or time in trees. Resting was predicted by an interaction between sarcopenia and age (LMM, estimate = 0.208, t = 2.6, p = 0.011). Those in poor condition when young tended to rest less than others, while those in poor condition when old rested more than others (electronic supplementary material, figure S3). The only other marginally significant effect was for time feeding, but this was in the direction opposite to that predicted: individuals with sarcopenia tended to feed more often than others.
Next, we used generalized linear mixed models (GLMMs) to assess whether monthly incidence of respiratory signs was higher among chimpanzees whose ELBM fell below the sarcopenia threshold in the previous year (model extended from [27]). Our ELBM calculation did not include the month in which respiratory signs were assessed to avoid confounding effects of illness on condition. In males, sarcopenia led to a 65% increased likelihood of exhibiting respiratory signs (GLMM, χ2 = 5.8, p = 0.016, electronic supplementary material, table S3). This relationship was not age-dependent (age × sarcopenia interaction, z = −1.2, p = 0.23). On the other hand, sarcopenia did not significantly explain respiratory illness in females, and the trend was in the direction opposite to that predicted (estimate = −1.215, χ2 = 3.7, p = 0.054).
Finally, we used time-varying Cox proportional hazards models to assess whether sarcopenia predicted mortality in the current year or 1 year in the future. Using the 1.5 s.d. threshold, sarcopenia did not predict mortality in the current or following year, whether assessed for the combined sample or for each sex separately (p > 0.10, electronic supplementary material, table S4). A post hoc sensitivity analysis of alternative sarcopenia thresholds revealed that for males, mortality in the current year was predicted only by ELBM ≥ 2 s.d. below the healthy mean (electronic supplementary material, figure S4, hazard ratio (HR) = 2.5, p = 0.036). Even with this stricter threshold, sarcopenia did not predict mortality in the following year. No threshold we examined could predict mortality in females.
4. Discussion
Our study found a significant decline in physical condition (ELBM) with ageing in wild chimpanzees, accompanied by moderate changes in physical activity. These findings, like those from baboons [17], suggest that, despite shorter lifespans than humans, deteriorating physical function is a common challenge of old age in wild primates. However, these effects were small, and we found only limited evidence that chimpanzees experience physical frailty that is equivalent to the human clinical syndrome. We predicted that older chimpanzees would be less active. These predictions were partially borne out. Chimpanzees spent less time in trees and fed less as they aged, though these effects were not specifically predicted by low ELBM. Chimpanzees rested more with age, but only when they exhibited sarcopenia. Ageing did not affect travel time. Sarcopenia increased the likelihood of respiratory illness, but only among males. This effect was not specific to older individuals, but as the prevalence of both respiratory signs and sarcopenia increases with age, the association still indicates higher risk as individuals age. Finally, sarcopenia did not predict likelihood of dying 1 year in the future, though unusually low ELBM characterized males in the year that they died. The latter likely indicates that physical condition is affected acutely by illness, but we have no evidence to support the hypothesis that declining body condition might contribute independently to mortality.
As the frailty syndrome in humans is defined by its ability to predict adverse health outcomes, our data suggest that an equivalent may not exist in wild chimpanzees. Our sarcopenia threshold captured both young and old individuals, and few individuals were chronically below this threshold. Instead, chimpanzees experienced substantial fluctuations in ELBM throughout life, perhaps owing to periods of illness and poor food availability, but they appear to be resilient to these insults over the long term. That being said, our data clearly indicate that ageing is associated with increasing physical constraints. These constraints are likely to require accommodations to behavioural strategies and may contribute in small measure to health and fitness in ways other than those assessed here.
Older chimpanzees were less likely to be in trees versus on the ground and spent less time eating ripe fruit. Ageing might affect weakness or fatigue in such a way that limits climbing behaviour. This is suggested by our finding that older chimpanzees with poor ELBM rested more than others. Chimpanzees consume both arboreal resources (e.g. ripe fruit, 68% of diet) and terrestrial resources (e.g. piths, 20% [34]). The effect of ageing on ripe fruit feeding was stronger than that for overall feeding, supporting the idea that climbing may have been the key limitation on feeding. If so, this effect was independent of physical condition, as the individuals with the lowest ELBM did not climb or feed less often for their age. On the other hand, even in human populations with abundant food, intake declines with age because caloric requirements change, as do digestion and the mechanisms that regulate satiety [35]. Thus, it is alternatively possible that chimpanzees have less desire to feed as they age, and this might affect how much time they spend in trees. Tooth wear and missing teeth are common in older chimpanzees, so this may also constrain feeding [21]. Since our data concern the time devoted to feeding, rather than rate of intake, we can only infer that older chimpanzees took in fewer calories than younger ones. Similarly, while we observed no age differences in time spent travelling, our results leave open the possibility that older chimpanzees might travel less far and at a slower speed.
A number of interesting sex differences emerged from our analysis, suggesting that females are less vulnerable to physical ageing than males. On the one hand, age declines in ELBM and activity (when they occurred) began at an early age in females and were approximately linear across adulthood, while ELBM did not begin to deteriorate in males until their mid-30s. On the other hand, the magnitude of the decline was greater in males, and males were more likely to meet threshold criteria for sarcopenia. This finding is similar to analyses of body mass in chimpanzees at Gombe, where males showed a significant decline with age, while females did not [23]. Notably, the decline in body mass among Gombe males began about 10 years before the decline in ELBM among Kanyawara chimpanzees. Such a difference in the rate of ageing may be expected, as males at Gombe also experience a 10 year reduction in adult life expectancy compared with Kanyawara chimpanzees [26,36]. Additionally, we found that only males showed an increase in respiratory signs when in poor condition, and only males showed any association, however weak, between condition and mortality. Studies in humans tend to find higher rates of frailty among women versus men, but sarcopenia is more frequent in men, and both diagnoses more strongly predict mortality in men [33,37,38]. Both chimpanzees and humans exhibit sexual dimorphism in body size and muscle mass during young adulthood. Increased risk of sarcopenia and frailty in males may reflect the challenges of maintaining a costly phenotype. Testosterone correlates positively with ELBM for Kanyawara males [32]. Thus, frailty and sarcopenia may be influenced by the declining testosterone in ageing males [39,40]. Given the importance of aggressive physical competition in chimpanzee males, declining muscle mass and physical performance probably have broader repercussions than those assessed here, likely contributing to age-associated loss of status and mating success [41,42].
Differences in the risk and impact of physical frailty in chimpanzees and humans may arise from several sources. One possibility is that bipedalism and its attendant adaptations have increased the stress on the musculoskeletal system in humans [43]. Alternatively, it is possible that chimpanzees simply do not live to an age at which their physical condition has depleted enough to constrain mobility and survival. Healthy humans do not begin to exhibit clinically recognized frailty until their 60s [10], an age reached by only a tiny fraction of wild chimpanzees [18]. Indeed, it is possible that impaired physical condition is so detrimental to chimpanzees, who receive little in the way of direct support from others, that frail individuals die too quickly to be detectable in our data. The flatter age profile in our cross-sectional models versus mixed-effects models for ELBM suggests the influence of mortality selection [44], such that those alive at the latest ages were those who were able to maintain robust physical condition. However, our data do not support the conclusion that the progressive losses of function and condition in normal ageing should be sufficient to produce these selection effects. Instead, this heterogeneity more plausibly results from broader variation in health, which allows individuals to resist the long-term impacts of injuries and disease on physical condition. It should also be noted that wild chimpanzees can survive with severe physical impairments. One male in our study community has survived for more than a decade after the loss of both feet to wire snares.
The explanations above presume a species difference in biology. However, there are reasons to suspect that humans may not have been as vulnerable to frailty in our recent evolutionary past. Like other aspects of human ageing, physical frailty has been examined almost exclusively within industrialized populations, in which high rates of obesity and sedentism are associated with chronic inflammation, a known predictor of frailty [15]. These conditions differ starkly from those experienced during most of human history, and evidence is mounting that common diseases of ageing—coronary artery disease [45], osteoarthritis [46,47], diabetes [48] and Alzheimer's disease [49,50]—have only very recently increased in prevalence. Similarly, studies of small-scale societies practising subsistence lifestyles report little evidence of sarcopenia or a frailty syndrome, despite long adult lifespans and relatively high burdens of nutritional stress and infectious disease [51,52]. For example, Hadza foragers and Pokot pastoralists exhibit minor age-related changes in body mass and physical activity, but elders maintain physical activity levels above those of even young adults in developed nations [51,53,54]. These anthropological data corroborate recent clinical literature in suggesting that habitual physical activity and avoidance of obesity are protective against frailty even at late ages [12–14]. Our data on wild chimpanzees lend further support to the idea that ageing of physical condition and performance may occur without necessarily having pathological consequences.
Our study was limited by several challenges of studying an endangered species in the wild, including a relatively small sample of individuals, reliance on age estimates for older subjects, substitution of non-invasive measures to approximate muscle mass and frailty, and the inability to obtain measures on demand. This constrains our ability to make direct comparisons with humans that employ standardized frailty instruments, though even these vary considerably between studies. Owing to the long lifespan of chimpanzees relative to our study period, we also cannot rule out the possibility that cohort effects on ELBM or activity may have influenced the observed age patterns. On the other hand, our study benefits from its longitudinal nature, which offers unusually dense, long-term sampling of individuals compared with typical cross-sectional or cohort studies. Additionally, like studies of humans in small-scale societies, studies of primates in the wild are advantaged by revealing how individuals age in the face of the natural subsistence habits and ecological stressors that would have shaped the ageing process over evolutionary history. While it is likely that severely depleted physical condition or performance is far more lethal in chimpanzees than in socially supported humans, our data suggest that this is not a natural or inevitable consequence of ageing.
Supplementary Material
Acknowledgements
We sincerely thank the Kibale Chimpanzee Project field staff and project managers for daily data collection. For laboratory and database assistance, we thank Lindsey Hagberg, Megan Cole, Heather Moles, Dipti Kandlikar, Jayda Patterson, Sarah Schmidt, Lara Saipe Durgavitch, Sarah Phillips, Kamden Cornell and Jillian Rutherford. Thank you to Alexandra Rosati and two anonymous reviewers for productive feedback.
Ethics
This study was authorized by the Makerere University Biological Field Station, the Uganda National Council for Science and Technology and the Uganda Wildlife Authority. All research protocols were non-invasive and were approved by the University of New Mexico and Harvard University Institutional Animal Care and Use Committees.
Data accessibility
All datasets and code are available in Dryad: https://dx.doi.org/10.5061/dryad.rn8pk0p5z [55].
Authors' contributions
All authors contributed to acquisition or analysis of data. M.E.T. drafted the initial manuscript, and all co-authors collaborated in editing and approving the final version.
Competing interests
We have no competing interests.
Funding
Long-term research has been supported by an active grant from the National Institutes of Health (grant no. NIA/ORWH R01-AG049395) and past grants from the U.S. National Science Foundation (grant nos BCS-0849380, BCS-1355014 and IOS-LTREB-0416125), National Institutes of Health (AI058715), Leakey Foundation and Wenner-Gren Foundation.
References
- 1.Chang S-F, Lin P-L. 2015. Frail phenotype and mortality prediction: a systematic review and meta-analysis of prospective cohort studies. Int. J. Nursing Stud. 52, 1362–1374. ( 10.1016/j.ijnurstu.2015.04.005) [DOI] [PubMed] [Google Scholar]
- 2.Fried LP, et al. 1998. Risk factors for 5-year mortality in older adults: the cardiovascular health study. JAMA 279, 585–592. ( 10.1001/jama.279.8.585) [DOI] [PubMed] [Google Scholar]
- 3.Wells JL, Seabrook JA, Stolee P, Borrie MJ, Knoefel F. 2003. State of the art in geriatric rehabilitation. Part I: review of frailty and comprehensive geriatric assessment. Arch. Phys. Med. Rehab. 84, 890–897. ( 10.1016/S0003-9993(02)04929-8) [DOI] [PubMed] [Google Scholar]
- 4.Lang PO, Michel JP, Zekry D. 2009. Frailty syndrome: a transitional state in a dynamic process. Gerontology 55, 539–549. ( 10.1159/000211949) [DOI] [PubMed] [Google Scholar]
- 5.Vermeulen J, Neyens JCL, van Rossum E, Spreeuwenberg MD, de Witte LP. 2011. Predicting ADL disability in community-dwelling elderly people using physical frailty indicators: a systematic review. BMC Geriatr. 11, 33 ( 10.1186/1471-2318-11-33) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fried LP, Ferrucci L, Darer J, Williamson JD, Anderson G. 2004. Untangling the concepts of disability, frailty, and comorbidity: implications for improved targeting and care. J. Gerontol. A 59, M255–M263. ( 10.1093/gerona/59.3.M255) [DOI] [PubMed] [Google Scholar]
- 7.Buckinx F, Rolland Y, Reginster J-Y, Ricour C, Petermans J, Bruyère O. 2015. Burden of frailty in the elderly population: perspectives for a public health challenge. Arch. Publ. Health 73, 19 ( 10.1186/s13690-015-0068-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosenberg IH. 1997. Sarcopenia: origins and clinical relevance. J. Nutr. 127, 990S–991S. ( 10.1093/jn/127.5.990S) [DOI] [PubMed] [Google Scholar]
- 9.Cesari M, Landi F, Vellas B, Bernabei R, Marzetti E. 2014. Sarcopenia and physical frailty: two sides of the same coin. Front. Aging Neurosci. 6, 192 ( 10.3389/fnagi.2014.00192) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Collard RM, Boter H, Schoevers RA, Oude Voshaar RC. 2012. Prevalence of frailty in community-dwelling older persons: a systematic review. J. Am. Geriatr. Soc. 60, 1487–1492. ( 10.1111/j.1532-5415.2012.04054.x) [DOI] [PubMed] [Google Scholar]
- 11.Szanton SL, Seplaki CL, Thorpe RJ, Allen JK, Fried LP. 2010. Socioeconomic status is associated with frailty: the women's health and aging studies. J. Epidemiol. Commun. Health 64, 63–67. ( 10.1136/jech.2008.078428) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Landi F, Abbatecola AM, Provinciali M, Corsonello A, Bustacchini S, Manigrasso L, Cherubini A, Bernabei R, Lattanzio F. 2010. Moving against frailty: does physical activity matter? Biogerontology 11, 537–545. ( 10.1007/s10522-010-9296-1) [DOI] [PubMed] [Google Scholar]
- 13.Peterson MJ, et al. 2009. Physical activity as a preventative factor for frailty: the Health, Aging, and Body Composition study. J. Gerontol. A 64A, 61–68. ( 10.1093/gerona/gln001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Porter SKN, McDonald SR, Bales CW. 2014. Obesity and physical frailty in older adults: a scoping review of lifestyle intervention trials. J. Am. Med. Direct. Assoc. 15, 240–250. ( 10.1016/j.jamda.2013.11.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Soysal P, et al. 2016. Inflammation and frailty in the elderly: a systematic review and meta-analysis. Age Res. Rev. 31, 1–8. ( 10.1016/j.arr.2016.08.006) [DOI] [PubMed] [Google Scholar]
- 16.Shively CA, et al. 2012. Aging and physical mobility in group-housed Old World monkeys. Age 34, 1123–1131. ( 10.1007/s11357-011-9350-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alberts SC, Archie EA, Gesquiere LR, Altmann J, Vaupel JW, Christensen K. 2014. The male–female health-survival paradox: a comparative perspective on sex differences in aging and mortality. Sociality, hierarchy, health: comparative biodemography: a collection of papers. Washington, DC: National Academies Press. [PubMed] [Google Scholar]
- 18.Emery Thompson M, Sabbi KH. In press Evolutionary demography of the great apes. In Human evolutionary demography (eds Sear R, Burger O, Lee R). Cambridge, UK: Open Book Publishers. [Google Scholar]
- 19.Jurmain R. 1989. Trauma, degenerative disease, and other pathologies among the Gombe chimpanzees. am. J. Phys. Anthropol. 80, 229–237. ( 10.1002/ajpa.1330800211) [DOI] [PubMed] [Google Scholar]
- 20.Sumner DR, Morbeck ME, Lobbick JJ. 1989. Apparent age-related bone loss among adult female Gombe chimpanzees. Am. J. Phys. Anthropol. 79, 225–234. ( 10.1002/ajpa.1330790210) [DOI] [PubMed] [Google Scholar]
- 21.Morbeck M, Galloway A, Sumner DR. 2002. Getting old at Gombe: skeletal aging in wild-ranging chimpanzees. Interdisc. Top. Gerontol. 31, 48–62. ( 10.1159/000061458) [DOI] [Google Scholar]
- 22.Carter ML, Pontzer H, Wrangham RW, Kerbis-Peterhans J. 2008. Skeletal pathology in Pan troglodytes schweinfurthii in Kibale National Park. Am. J. Phys. Anthropol. 135, 389–403. ( 10.1002/ajpa.20758) [DOI] [PubMed] [Google Scholar]
- 23.Pusey AE, Oehlert GW, Williams JM, Goodall J. 2005. Influence of ecological and social factors on body mass of wild chimpanzees. Int. J. Primatol. 26, 3–31. ( 10.1007/s10764-005-0721-2) [DOI] [Google Scholar]
- 24.Frontera WR, Hughes VA, Lutz KJ, Evans WJ. 1991. A cross-sectional study of muscle strength and mass in 45- to 78-yr-old men and women. J. Appl. Physiol. 71, 644–650. ( 10.1152/jappl.1991.71.2.644) [DOI] [PubMed] [Google Scholar]
- 25.Mancini DM, Walter D, Reichek N, Lenkinski R, McCully KK, Mullen JL, Wilson JR. 1992. Contribution of skeletal muscle atrophy to exercise intolerance and altered muscle metabolism in heart failure. Circulation 85, 1364–1373. ( 10.1161/01.CIR.85.4.1364) [DOI] [PubMed] [Google Scholar]
- 26.Muller MN, Wrangham RW. 2014. Mortality rates among Kanyawara chimpanzees. J. Hum. Evol. 66, 107–114. ( 10.1016/j.jhevol.2013.10.004) [DOI] [PubMed] [Google Scholar]
- 27.Emery Thompson M, Machanda ZP, Scully EJ, Enigk DK, Otali E, Muller MN, Goldberg TL, Chapman CA, Wrangham RW. 2018. Risk factors for respiratory illness in a community of wild chimpanzees (Pan troglodytes schweinfurthii). R. Soc. Open Sci. 5, 180840 ( 10.1098/rsos.180840) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kundi H, Wadhera RK, Strom JB, Valsdottir LR, Shen C, Kazi DS, Yeh RW. 2019. Association of frailty with 30-day outcomes for acute myocardial infarction, heart failure, and pneumonia among elderly adults. JAMA Cardiol. 4, 1084–1091. ( 10.1001/jamacardio.2019.3511) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ferrucci L, Guralnik JM, Pahor M, Corti MC, Havlik RJ. 1997. Hospital diagnoses, Medicare charges, and nursing home admissions in the year when older persons become severely disabled. JAMA 277, 728–734. ( 10.1001/jama.1997.03540330050034) [DOI] [PubMed] [Google Scholar]
- 30.Forbes G, Bruining G. 1976. Urinary creatinine excretion and lean body mass. Am. J. Clin. Nutr. 29, 1359–1366. ( 10.1093/ajcn/29.12.1359) [DOI] [PubMed] [Google Scholar]
- 31.Emery Thompson M, Muller MN, Sabbi K, Machanda ZP, Otali E, Wrangham RW. 2016. Faster reproductive rates trade off against offspring growth in wild chimpanzees. Proc. Natl Acad. Sci. USA 113, 7780–7785. ( 10.1073/pnas.1522168113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Emery Thompson M, Muller MN, Wrangham RW. 2012. Technical note: variation in muscle mass in wild chimpanzees: application of a modified urinary creatinine method. Am. J. Phys. Anthropol. 149, 622–627. ( 10.1002/ajpa.22157) [DOI] [PubMed] [Google Scholar]
- 33.Melton LJ, Khosla S, Crowson CS, O'Connor MK, O'Fallon WM, Riggs BL. 2000. Epidemiology of sarcopenia. J. Am. Geriatr. Soc. 48, 625–630. ( 10.1111/j.1532-5415.2000.tb02658.x) [DOI] [PubMed] [Google Scholar]
- 34.Emery Thompson M, Wrangham RW. 2008. Diet and reproductive function in wild female chimpanzees (Pan troglodytes schweinfurthii) at Kibale National Park, Uganda. Am. J. Phys. Anthropol. 135, 171–181. ( 10.1002/ajpa.20718) [DOI] [PubMed] [Google Scholar]
- 35.Morley JE. 2001. Decreased food intake with aging. J. Gerontol. A 56, 81–88. ( 10.1093/gerona/56.suppl_2.81) [DOI] [PubMed] [Google Scholar]
- 36.Bronikowski AM, et al. 2016. Female and male life tables for seven wild primate species. Scient. Data 3, 160006 ( 10.1038/sdata.2016.6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gordon EH, Peel NM, Samanta M, Theou O, Howlett SE, Hubbard RE. 2017. Sex differences in frailty: a systematic review and meta-analysis. Exp. Gerontol. 89, 30–40. ( 10.1016/j.exger.2016.12.021) [DOI] [PubMed] [Google Scholar]
- 38.Landi F, Liperoti R, Fusco D, Mastropaolo S, Quattrociocchi D, Proia A, Tosato M, Bernabei R, Onder G. 2012. Sarcopenia and mortality among older nursing home residents. J. Am. Med. Direct. Assoc. 13, 121–126. ( 10.1016/j.jamda.2011.07.004) [DOI] [PubMed] [Google Scholar]
- 39.Muller MN. 2017. Testosterone and reproductive effort in male primates. Horm. Behav. 91, 36–51. ( 10.1016/j.yhbeh.2016.09.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hyde Z, Flicker L, Almeida OP, Hankey GJ, McCaul KA, Chubb SAP, Yeap BB. 2010. Low free testosterone predicts frailty in older men: the Health in Men Study. J. Clin. Endocrinol. Metabol. 95, 3165–3172. ( 10.1210/jc.2009-2754) [DOI] [PubMed] [Google Scholar]
- 41.Muller MN, et al. 2020. Sexual dimorphism in chimpanzee (Pan troglodytes schweinfurhii) age-specific fertility. J. Hum. Evol. 144, 102795 ( 10.1016/j.jhevol.2020.102795) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Watts DP. 2018. Male dominance relationships in an extremely large chimpanzee community at Ngogo, Kibale National Park, Uganda. Behaviour 155, 969–1009. ( 10.1163/1568539X-00003517) [DOI] [Google Scholar]
- 43.Jurmain R. 2000. Degenerative joint disease in African great apes: an evolutionary perspective. J. Hum. Evol. 39, 185–203. ( 10.1006/jhev.2000.0413) [DOI] [PubMed] [Google Scholar]
- 44.Vaupel JW, Manton KG, Stallard E. 1979. The impact of heterogeneity in individual frailty on the dynamics of mortality. Demography 16, 439–454. ( 10.2307/2061224) [DOI] [PubMed] [Google Scholar]
- 45.Kaplan H, et al. 2017. Coronary atherosclerosis in indigenous South American Tsimane: a cross-sectional cohort study. Lancet 389, 1730–1739. ( 10.1016/S0140-6736(17)30752-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Berenbaum F, Wallace IJ, Lieberman DE, Felson DT. 2018. Modern-day environmental factors in the pathogenesis of osteoarthritis. Nat. Rev. Rheumatol. 14, 674–681. ( 10.1038/s41584-018-0073-x) [DOI] [PubMed] [Google Scholar]
- 47.Wallace IJ, Worthington S, Felson DT, Jurmain RD, Wren KT, Maijanen H, Woods RJ, Lieberman DE. 2017. Knee osteoarthritis has doubled in prevalence since the mid-20th century. Proc. Natl Acad. Sci. USA 114, 9332–9336. ( 10.1073/pnas.1703856114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pontzer H. 2012. Lessons from the Hadza: poor diets wreck efforts to prevent obesity and diabetes. Diabetes Voice 57, 26–29. [Google Scholar]
- 49.Fox M. 2018. ‘Evolutionary medicine’ perspectives on Alzheimer's disease: review and new directions. Age Res. Rev. 47, 140–148. ( 10.1016/j.arr.2018.07.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Trumble BC, Stieglitz J, Blackwell AD, Allayee H, Beheim B, Finch CE, Gurven M, Kaplan H. 2016. Apolipoprotein E4 is associated with improved cognitive function in Amazonian forager-horticulturalists with a high parasite burden. FASEB 31, 1508–1515. ( 10.1096/fj.201601084R) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pontzer H, Wood BM, Raichlen DA. 2018. Hunter-gatherers as models in public health. Obes. Rev. 19, 24–35. ( 10.1111/obr.12785) [DOI] [PubMed] [Google Scholar]
- 52.Gurven M, Costa M, Ben T, Stieglitz J, Beheim B, Eid Rodriguez D, Hooper PL, Kaplan H. 2016. Health costs of reproduction are minimal despite high fertility, mortality and subsistence lifestyle. Scient. Rep. 6, 30056 ( 10.1038/srep30056) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Raichlen DA, et al. 2017. Physical activity patterns and biomarkers of cardiovascular disease risk in hunter-gatherers. Am. J. Hum. Biol. 29, e22919 ( 10.1002/ajhb.22919) [DOI] [PubMed] [Google Scholar]
- 54.Sayre MK, Pike IL, Raichlen DA. 2019. High levels of objectively measured physical activity across adolescence and adulthood among the Pokot pastoralists of Kenya. Am. J. Hum. Biol. 31, e23205 ( 10.1002/ajhb.23205) [DOI] [PubMed] [Google Scholar]
- 55.Emery Thompson M, Machanda ZP, Fox SA, Sabbi KH, Otali E, Thompson González N, Muller MN, Wrangham RW. 2020. Data from: Evaluating the impact of physical frailty during ageing in wild chimpanzees (Pan troglodytes schweinfurthii) Dryad Digital Repository. ( 10.5061/dryad.rn8pk0p5z) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Emery Thompson M, Machanda ZP, Fox SA, Sabbi KH, Otali E, Thompson González N, Muller MN, Wrangham RW. 2020. Data from: Evaluating the impact of physical frailty during ageing in wild chimpanzees (Pan troglodytes schweinfurthii) Dryad Digital Repository. ( 10.5061/dryad.rn8pk0p5z) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
All datasets and code are available in Dryad: https://dx.doi.org/10.5061/dryad.rn8pk0p5z [55].