Abstract
Humans exhibit major age-related shifts in social relationships along with changes in social and emotional psychological processes that underpin these behavioural shifts. Does social ageing in non-human primates follow similar patterns, and if so, what are the ultimate evolutionary consequences of these social shifts? Here we synthesize empirical evidence for shifts in social behaviour and underlying psychological processes across species. Focusing on three elements of social behaviour and cognition that are important for humans—propensities to engage with others, the positive versus negative valence of these interactions, and capabilities to influence others, we find evidence for wide variation in the trajectories of these characteristics across primates. Based on this, we identify potential modulators of the primate social ageing process, including social organization, sex and dominance status. Finally, we discuss how comparative research can contextualize human social ageing.
This article is part of the theme issue ‘Evolution of the primate ageing process’.
Keywords: ageing, lifespan, primates, social behaviour, social cognition
1. Introduction
Humans are living into old age more than ever before, making it critical to understand the processes of ageing. It is increasingly recognized that successful ageing is not just a matter of physical health, but also depends on social functioning: people with strong social support experience enhanced wellbeing, health and longevity [1–3]. Yet people also show complex patterns of decline, preservation and even improvement in their social behaviour and cognition during ageing. For example, older adults tend to exhibit smaller social networks, yet have more emotionally satisfying interactions, and exhibit greater skillfulness in solving social conflicts, compared with younger adults [4–8]. Variation across these different facets of sociality can therefore shape the dynamics and outcomes of successful ageing.
One way to understand the consequences of these different ageing trajectories is to contextualize humans in a broader comparative framework accounting for ageing patterns across species. Other primates have provided crucial insights into the physiological ageing process [9,10], and can also be a valuable comparative model for social ageing owing to their close genetic relatedness with humans, shared social complexity, and relatively long lifespans. Moreover, the observational techniques commonly used with animals allow unparalleled records of natural social interactions, as well as opportunities for lifespan longitudinal studies that may be challenging to carry out in humans. Yet while recent work on primates has examined how variation in primate sociality impacts lifetime biological outcomes like fitness, health and longevity [11–14], there has little systematic examination of how patterns of sociality themselves shift during the ageing process. Rather, most work on primate social ageing has focused on descriptive changes of different components of sociality, typically within a single species or sex studied in isolation.
In order to understand the processes of primate social ageing, here we take a ‘bottom-up’ approach to synthesize current data from non-human primates. Our aim is to characterize patterns of social behaviour and underlying proximate mechanisms across diverse primate species. First, we review age-related changes across different primate species in order to characterize variation in social ageing trajectories. We then synthesize current data to examine the potential modulators of the primate ageing process by identifying commonalities and divergences in patterns across species, between males and females, and according to dominance status—and further identify key gaps in current knowledge that are crucial areas for future research. Finally, we discuss how understanding primate social ageing across different populations and individuals can inform human social ageing.
2. Social ageing patterns across primates
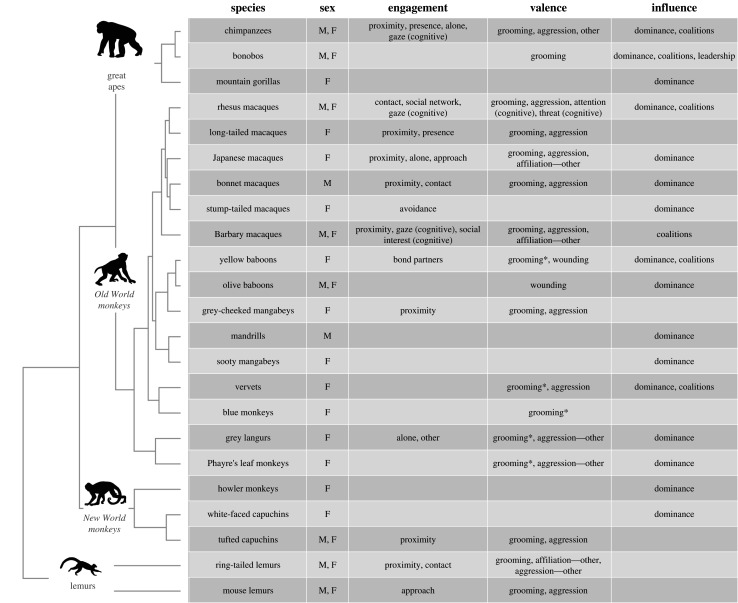
We examine studies tracking age-related changes in primates across three domains: (1) social engagement, or how socially active or reclusive an individual is, as a crucial pre-requisite for other social interactions; (2) the valence of social interactions, or the positive versus negative quality of their initiated behaviours; and (3) social influence, or an individual's ability to flexibly impact others' behaviour. While these distinctions are not necessarily typically used to categorize social behaviour in traditional approaches in primatology, they are important domains for understanding human ageing, and conceptually capture different ways that individuals can adjust their social behaviour during the ageing process. Across these domains, we focus primarily on changes within adult age-classes, not just between juvenility and adulthood. Importantly, different studies of different populations often use different metrics (see figure 1 for a summary of different relevant metrics we clustered under these domains, and electronic supplementary material, tables S1–S3 for details about all metrics and studies). Given this variability in metrics, we focus on comparing ageing trajectories within a study (increases, decreases or no change with age) rather than comparisons of absolute behavioural rates across species, sexes or studies.
Figure 1.
Social ageing metrics across species. Social metrics and phylogenetic distribution of current social ageing data. Sex indicates if there ever are any data for both sexes (see electronic supplementary material, for detailed breakdown by metric). Social engagement metrics comprised time spent alone, avoidance of others, approach towards others, spatial proximity, physical contact, presence in a party or group, number of bond partners, social network size, gaze following, and social interest in photographs and vocalizations. Social valence primarily comprised grooming given (*denotes a modified grooming index; affiliation—other indicates an alternative measure of affiliation described in the electronic supplementary material) and aggression given (*denotes a modified aggression index; aggression—other indicates an alternative measure of aggression described in the electronic supplementary material). Other metrics include rate of wounding, relative attention to positive versus negative stimuli, and threat response to emotional signals. Social influence metrics comprised dominance rank, coalitions (coalitionary aggression) and leadership of group movements. Primate phylogeny extracted from [15].
(a). Social engagement
During ageing, humans exhibit reductions in their social activity and interest. Older adults often report smaller social networks than younger adults [5,16], and show declines in their general attention to others' social cues like gaze direction that track this general pattern of increasing social disengagement with age in experiments [17]. In non-humans, these kinds of shifts have been assessed both in cognitive experiments and in natural behaviours (figure 1). Psychological traits can be measured by examining changes in primates' responses to and interest in social stimuli like conspecific photographs or vocalizations [18], as well as their propensity to respond to social cues in gaze-following tasks [19–21]. Observational metrics such as time spent alone, spatial proximity or approaching others in natural behaviour [22–24] can also capture an individual's willingness to socially engage with others by initiating interactions or allowing interactions to occur—because being around others is a pre-requisite for many other kinds of interactions.
Current data indicate that many primates become markedly less social with advancing age. This phenomenon has been best-studied among female-philopatric Old World monkeys, where females remain in their natal group and males disperse. For example, older rhesus macaques (Macaca mulatta) [25], stump-tailed macaques (Macaca arctoides) [26], Japanese macaques (Macaca fuscata) [26–30], long-tailed macaques (sometimes called crab-eating macaques; Macaca fascicularis) [31,32] and Barbary macaques (Macaca sylvanus) [18,23] all exhibit reduced social engagement in terms of spatial distance to others, time spent alone, approaching others or total network size. While there has been less work examining psychological mechanisms, older rhesus macaques also show a declining propensity to follow other's gaze [19,20]. Several other catarrhines, platyrrhines and strepsirrhines share this general pattern of increasing reclusiveness. For example, older grey langurs (sometimes called Hanuman langurs; Semnopithecus entellus; previously called Presbytis entellus) [33,34], grey-cheeked mangabeys (Lophocebus albigena, previously called Cercocebus albigena) [35], tufted capuchins (Sapajus apella) [22], ring-tailed lemurs (Lemur catta) [36], and grey mouse lemurs (Microcebus murinus) [37] are less likely to be in close proximity to others, or engage in fewer overall social behaviours.
Yet such social withdrawal is not universal, as some groups show steady rates of social engagement during ageing. For example, male bonnet macaques (Macaca radiata) exhibit no major age-related changes in social contact [38], and female yellow baboons (Papio cynocephalus) exhibit no changes in number of strong bond partners [39] (although they may reduce the number of weak bond partners). Notably, while older Barbary macaques show some declines in social contact, they nonetheless maintain steady profiles of interest in conspecific social stimuli such as photographs or vocalizations [18], and continue to follow other's gaze like younger monkeys [20]. Finally, older male chimpanzees (Pan troglodytes) are more likely to be alone, but exhibit increased sociality when they do engage: they are more likely to be in larger parties and in proximity to others compared with younger adults [40]. By contrast, older female chimpanzees are less likely to be present in parties [41] (although note this study consisted of one old male and two old females), and show declines in gaze following [21]. More generally, there may be important sex differences in social engagement: almost all of the studies of female-philopatric Old World monkeys to date focus on females, but males can show a different pattern. For example, whereas older female rhesus monkeys show declining social contact time and smaller networks, older males show steady or increasing social engagement [25,42]. The reverse is true in ring-tailed lemurs: older females increase physical contact, whereas males show no changes [36].
The pattern of age-related change in current data indicates that there may not be intrinsic constraints on older individuals' ability to socially engage with others. While some primates show declines in engagement in line with the costs imposed by senescence—as physical declines may make it more difficult for older individuals to keep up with the rest of the group [43]—others may not change or even increase their social engagement. One possibility is that socioecological context impacts these patterns, as the socially dominant sex often has more preserved social functioning in old age when data exist from both sexes within a species. In particular, males are the dominant sex in both chimpanzees and macaques, and show greater social engagement during ageing compared with females. In contrast, lemurs are one of the few primate taxa exhibiting female dominance [44], and female ring-tailed lemurs maintain or increase their sociality with age (note that mouse lemurs show a dispersed social system without comparable dominance patterns). Yet it is important to note that there is often only or more data from one sex (for example, females are much better studied among Old World primates; figure 1), so this could also reflect an overall difference in ageing trajectories across species. To test this, it will be important to have more systematic comparisons across the sexes within-species, as well as to compare closely related taxa with variation in sexual dominance systems, such as Eulemur [44].
(b). Valence of social interactions
Humans show age-related changes in not just the quantity but also the nature or quality of their social interactions. In particular, older adults show a well-documented bias in attention and memory for positive social information (e.g. looking longer at and showing better recall for happy facial expressions compared with negative ones), a shift from the ‘negativity’ bias more commonly seen in younger adults [5,7]. Importantly, this psychological shift has concordant behavioural manifestations: older adults exhibit a greater prioritization of positive social interactions, and show decreases in interpersonal conflicts [5,45]. That is to say, older adults' social relationships get ‘better’ with age [46]. In animals, parallel shifts in the valence of behavioural interactions can therefore be assessed by examining the relative frequency of negative social interactions (such as aggression given) compared with positive interactions (such as grooming given). Finally, some work has experimentally tested animals' attention or responses to emotionally valanced information [47,48], more closely mirroring human experimental work looking at attentional biases.
Current evidence suggests that, unlike in humans, an increasing negativity bias in behaviour may be common across other primates. For example, multiple macaque species show a pattern of declining rates of grooming given, but relatively consistent engagement in aggression during ageing. Older rhesus macaques show these changes in giving grooming and aggression even though older individuals continue to receive grooming from others at high rates, and are less likely themselves to be attacked [13,25,42,49]. Similarly, older Barbary macaques [18,23], long-tailed macaques [32], bonnet macaques [38] and Japanese macaques [24,27,29,30] also show declines in grooming given but initiate aggressive behaviours like younger adults. Macaques show similar shifts in their production of and responses to negative emotional signals or contexts: the production of negative emotional signals such as yawning and scratching increases in older adults [32]; production of conspecific threat signals generally stays consistent or increases in adult animals [42,50]; and older macaques can show exacerbated responses to external threats in controlled experiments [47]. This aligns with converging experimental evidence looking at cognitive biases, showing that older macaques attend relatively more to negative socioemotional stimuli (photographs of conspecific threat faces) compared with neutral or positive expressions [48], similar to human research measuring looking responses to affective stimuli using similar paradigms [51,52].
A general pattern of increasing behavioural negativity also appears in several other Old World monkey species. For example, olive baboons (Papio anubis) [53], vervet monkeys (Chlorocebus pygerythrus) [54], grey langurs [34], mangabeys [35] and Phayre's leaf monkeys (Trachypithecus phayrei) [55] show steady initiation of aggression or risk of wounding from aggression across adulthood. Yet grooming declines in yellow baboons [56], blue monkeys (Cercopithecus mitis) [57], mangabeys [35], Phayre's leaf monkeys [33] and potentially grey langurs (although this is inconsistent [33]). The only New World monkey with comparable data is the tufted capuchin, where aggression rates stay fairly consistent and grooming declines, especially in females [22]. Along the same lines, old mouse lemurs show declines in grooming and affiliation as well as increases in initiating aggression [37,58], although ring-tailed lemurs do not show changes [36].
Overall, current evidence suggests that a relative negativity bias in behaviour may be a widely conserved pattern across primates: multiple Old World monkeys (including species with different social organizations) as well as at least one New World monkey and one strepsirrhine species show a general pattern of steady aggression but declining grooming, and none shows a relative positivity bias in behaviour. Thus, the positivity bias seen in humans does not seem to be an intrinsic consequence of ageing in primates. A clear exception to this predominant pattern is the valence of chimpanzee interactions. For example, old chimpanzees give less aggression than younger adults, but maintain affiliative social behaviours like grooming [40,40,59]. Similarly, agreeable personality traits increase in older chimpanzees, as in humans [60,61]. While there are not comparable studies of ageing in bonobos (Pan paniscus), adult male and female bonobos show age-related increases in grooming compared with subadults [62] (see also [63] for grooming networks in older adults), so there is a clear need for additional studies of bonobos.
Why might humans and chimpanzees exhibit increasing positivity while many other primates show increasing negativity (figure 2b)? One possibility is that this stems from the flexible fission–fusion social organization seen in both humans and chimpanzees [64]. This social system means that male chimpanzees often have more flexibility in choice of whom they spend time with than primates that exhibit low fission–fusion dynamics such as macaques [65,66]. Moreover, bonds among chimpanzees are not as strongly patterned by kinship compared with many female-philopatric Old World monkeys [64,67]. Relationships with non-kin may require more time and effort to maintain than is the case for kin, given that kinship bonds intrinsically provide indirect fitness benefits and are important for many species [67], resulting in different patterns of affiliation and aggression during ageing. Another relevant factor may be life history: chimpanzees are among the most long-lived non-human mammals in terms of total lifespan, and can live more than 60 years in the wild [68]. This means that chimpanzees, like humans, spend many years—even decades—as older individuals. During this period they can continue to obtain fitness benefits through affiliative strategies that require maintaining bonds with non-kin allies [69], a strategy that might be less relevant for other species. To test these possibilities, it is crucial to have data from other fission–fusion species (such as spider monkeys [70]), as well as from bonobos, who share life history and basic social organization with chimpanzees, and gorillas, who have reduced lifespans compared with chimpanzees [71].
Figure 2.
Comparative patterns of social ageing. (a) Current data on social engagement indicate that the socially dominant sex (which depends on social organization) can show preserved functioning during ageing, whereas the subordinate sex shows greater withdrawal. (b) The relative valence of social interactions in many primates shows increasing behavioural negativity, whereas chimpanzees (and humans) show relative positivity. (c) There are diverse trajectories for social influence such as dominance status, with major differences according to sex, social organization and philopatry.
(c). Social influence
A final important change in human social relationships during ageing concerns older adults' abilities to flexibly impact others' behaviour. Older adults can exhibit general deficits in social cognition such as theory of mind skills, yet also can also show greater ‘wisdom’ and thus increasing skillfulness at addressing complex social conflicts [72–74]. Relevant changes have been assessed in non-humans by examining strategic social behaviour, such as acquisition of high dominance status, propensity to form coalitions, and leadership of group movements. Other relevant metrics such as third-party interventions like consolation [75], or experimental cognitive studies of strategic deception or other aspects of theory of mind [76], and partner choice in cooperation [77], have not been studied in the context of ageing to our knowledge (see also figure 1).
Current evidence indicates that age-related changes in dominance rank, the best-studied metric to date, are highly variable across species. In many male primates, dominance exhibits an inverted-U-shape with age, such that the youngest and oldest adults are lowest ranking, while prime-aged adults achieve the highest status. This pattern is evident in male chimpanzees [78,79] and many male Old World monkeys, including mandrills (Mandrillus sphinx) [80], olive baboons [81], yellow baboons [81–84] and some populations of Japanese macaques [85,86]. While male rhesus macaques sometimes show this pattern [85], rank is also sometimes achieved through queueing, such that individuals with the longest tenure length have the highest rank [87–89] and rank correlates better with tenure than age [90]. In other cases, age had no effect on male rhesus dominance [85,91]. Queueing is likely the result of synchrony in female reproductive cycles in this species, which reduces reproductive skew and thus the reproductive benefits of rank [92].
In females, in contrast, dominance often shows more linear changes. For example, rank declines with age in Phayre's leaf monkeys [33], grey langurs [34,93] and howler monkeys (Alouatta palliata) [94]. But some species exhibit no overall change or even increases with age. For example, rank remains stable in female olive baboons [81], female stump-tail macaques [26], female vervet monkeys [54] and female sooty mangabeys (Cercocebus atys, previously called Cercocebus torquatus) [95]. An important consideration here is that matrilineal social organizations can create complex age effects. For example, among female baboons and macaques, daughters' dominance rank is inherited from their mother in inverse birth order, such that youngest daughter has the highest rank [96,97]. Thus, rank within the matriline generally decreases with age as younger sisters reach maturity. An exception is the oldest female of each matriline, where age is positively related to status within the matriline [98,99]. Lifespan patterns for these females are even further complicated when looking at overall rank within the whole group: if a female from the highest ranking matriline enters the group's adult hierarchy, members of all other matrilines accordingly drop in rank. Female white-faced capuchins (Cebus capucinus) also exhibit matrilineal rank, although the pattern of matrilineal inheritance may be more relaxed [100,101]. Finally, some female apes obtain rank via queuing: both female chimpanzees [102], and female bonobos exhibit increases in rank with age [103–105]. However, female mountain gorillas (Gorilla beringei beringei) exhibit the inverted-U pattern seen in many males [106].
There have been fewer studies looking at other aspects of social influence, but current evidence suggests that at least some other relevant behaviours also show these variable age patterns. For example, coalitionary aggression in male chimpanzees shows a U-shaped pattern, such that a greater proportion of aggression is coalitionary in younger and older individuals [107] (but see [79] for evidence of declines in an extremely large community). Thus overall lower-ranking age classes show more coalitions, even though these coalitions may have different consequences for rank and fitness for these different cohorts [108]. In other species, participation in coalitions does not shift much with age. For example, older female vervet monkeys maintain high rates of coalitionary aggression specifically in support of their daughters [54]. While this may be a common pattern for species with matrilineal dominance inheritance, where coalitionary interventions support close maternal kin [54,109], this pattern is not consistent as coalitionary aggression actually increases with age in female rhesus macaques [110]. Coalitionary aggression also increases for male Barbary macaques [111] and male baboons [112,113] (although see [83]), which may be because coalitions help individuals maintain rank and access mating opportunities as they age, or because successful coalitions require social experience acquired with age [83,113,114]. The role of social experience could also explain why older female bonobos are more likely to lead group movements and form coalitions [63,115].
Current data therefore suggest that social influence shows a complex pattern that varies across species and sexes (figure 2c). This may stem in part from how dominance affects an individual's access to resources like mates. In species with high despotism and reproductive skew, young adults entering the adult hierarchy will start to compete for high rank (and thus mating access) immediately. This competition is typically physical in nature for males, so age-related change in dominance follows an inverted-U: both younger and older males have less fighting ability than prime-aged males, because they are either still growing or losing muscle mass. Thus, if rank strongly predicts reproductive success, a decline in rank with age (either linear or an inverted-U-shape) is likely due to a decline in physical status. This also intersects with philopatry: in male-philopatric species like chimpanzees, males start competing for rank as adolescents so peak rank is obtained later [78,79,107,116], whereas males who immigrate into a group when they are already fully grown can sometimes occupy their highest rank shortly after dispersal in female-philopatric species like macaques and baboons [117]. Finally, among species or age-sex classes with low despotism and reproductive skew, such that rank is less important for fitness, individuals often obtain rank through queuing rather than competition [118]. Consequently, older individuals are more dominant, as in female chimpanzees and bonobos [63,102,104,105].
3. Comparative social ageing: state of the art and future directions
There are many ways to be an old primate: social ageing in primates is a complex phenomenon with great variation in how socially engaged older animals are, in the relative valence of their social interactions, and in the forms of social influence they can exert over others. While primate social ageing is still a nascent field of study, here we synthesize some key findings from current data looking at changes across different social domains. Then, we identify limitations of current work and consequent key areas for future study.
(a). Modulators of the primate social ageing process
Current data suggest some key themes concerning the pace and pattern of primate social ageing across species (figure 2). First, social context can modulate the primate ageing process: current data indicate that species differing in their social organization and philopatry can exhibit different ageing trajectories. For example, older female macaques often exhibit declines in social engagement—as indexed by proximity or social network size—and an increasing negativity bias—with declines in giving grooming but consistent levels of aggression [13,97]. This may be related to their female-philopatric social organization: females in such species form bonds primarily with kin, so social networks may shrink as their ageing kin die, yet older females can obtain fitness benefits by supporting their daughters in agonistic contexts [54]. Yet different species may show different patterns. For example, older male chimpanzees exhibit some age-related increases in social engagement, as well as an increasing positivity bias with increasing grooming. This might stem in part from the fact that chimpanzees exhibit fission–fusion groups with a high degree of social choice, and further that males may be more likely to form flexible, long-term bonds with both kin and non-kin [64], as discussed previously.
A related theme is that sex matters: males and females can have different ageing trajectories. For example, while older primates generally withdraw from social interactions, the socially dominant sex may sometimes exhibit preserved social behaviour, as is seen in representative species of apes, Old World monkeys and strepsirrhines (e.g. male chimpanzees, male rhesus macaques and female ring-tailed lemurs). This may be due in part to the differential importance of social bonds across sexes (although this would not necessarily explain declines in female macaques, given their strong female–female bonds [13]). Importantly, some of these apparent sex differences may also be due to differences in sex-specific mortality, with one sex outliving the other by several years [119] and therefore revealing different patterns of change in behaviour in old age. Yet given that current work often focuses on only one sex in isolation—especially in Old World monkeys where much work to date has focused on females (figure 1)—more direct comparisons across sexes are crucial to assess how and why sex modulates social ageing.
Finally, social status both changes with age, and can impact other aspects of sociality. High rank is generally correlated with reproductive success [69,108,120–122], so age-related declines in social status presents a biological problem. As such, older animals may sometimes shift their social behaviour to accommodate falling rank, such as how older chimpanzees with declining physical stature may increasingly exploit social bonds and coalitionary aggression to obtain fitness [69,107,123]. A corollary to this point is that it is important to assess dominance status in studies of social ageing, as many primate studies have not directly accounted for rank effects on other aspects of sociality. For example, while young and old female Japanese macaques appear to have similar patterns of behaviour at first glance, dominance and age have opposing effects: older low-ranking females have larger social networks but spend less time socializing, whereas older high-ranking females have smaller social networks and devote more time to social interactions [124].
(b). Gaps in current data
To understand why and how different patterns of social ageing emerge, there is a critical need to examine a phylogenetically diverse set of species. As such, an important limitation of primate social ageing data to date concerns their phylogenetic distribution: there has been a much greater focus on work on female-philopatric Old World monkeys than other taxonomic groups (figure 1). For example, platyrrhines and strepsirrhines are under-represented in current work, yet exhibit social features that are uncommon in Old World primates, including female dominance in lemurs [44], and cooperative breeding in callitrichids. The lesser apes (gibbons and siamangs) are important examples of monogamy, and the great apes also show great diversity in social organization. While some of these limitations reflect the current state of primate research effort as a whole, some species like marmosets are well-studied models for physiological ageing [125] yet are nonetheless not represented in current social ageing work.
A second gap concerns within-species variation across sexes and populations. While both sexes of some species have been well-studied, in other groups like Old World monkeys there are often data from only one sex (figure 1 and electronic supplementary material, tables S1–S3), which is an important caveat for interpreting potential sex differences. In addition, there have been few studies comparing across populations of the same species, which is another crucial test of how socioecological conditions shape ageing. For example, among rhesus macaques and chimpanzees, males living in large groups can show different age-related patterns in dominance rank [85,117] and coalitionary aggression [79,107] compared with males in smaller groups. This may be because group size impacts reproductive skew: younger male macaques acquire top ranks in smaller groups with higher reproductive skew, whereas rank is acquired through queueing in larger groups [85,117], likely because male rank has less of an impact on reproductive success when many males are present [92]. Along the same lines, changes in social ageing patterns may vary across captive versus wild populations, for example, owing to differences in competition. For example, a fair amount of research on macaque species has focused on provisioned populations [e.g. 13,18,19,23–29,48,111,122,124,126,127], which may show different ageing patterns from the wild owing to socioecological effects.
A final gap concerns how social ageing impacts different kinds of social domains and metrics. We differentiated between patterns of overall social engagement (being around or near others) and the emotional valence of social interactions (the positive versus negative quality of those interactions, as indexed by grooming versus agonism), in contrast to many approaches in primatology that consider both proximity and grooming as examples of affiliation. It is, therefore, an important empirical question whether these behaviours are unified or dissociated during ageing. Current data indicate that there can be differences in a given species' ageing trajectories for engagement versus valence (figure 2), which suggests that these metrics may indeed reflect divergent processes. Furthermore, a key aspect of human social ageing concerns changes in social selectivity: older adults have smaller social networks but focus more on close, important social relationships [5,16,128]. While much recent high-profile work on primate behaviour has focused on the fitness consequences of strong social bonds [11,12,38], its notable that most work to date on primate social ageing has not accounted for the specific identity of the partner. Indeed, we could find only one study that specifically addressed strong social bonds during ageing [39]. As such, a more nuanced approach differentiating between social bonds by the value and persistence over time is crucial.
4. Conclusion and implications for human ageing
Social ageing in non-human primates can provide novel insights into the human ageing process because both social context and cognitive status are associated with differences in physical health and mortality in humans [4,6,11]. Current data indicate that primates show high levels of variation in ageing trajectories across different sexes and social organizations, which can help pinpoint the kinds of social contexts that promote successful ageing. For example, variation across primate social organization and philopatry can serve as a referential model for the impact of social support networks and the presence of family members in human ageing [4]. Similarly, the biological effects of sex on primate ageing can help disentangle the effects of sex from culturally mediated effects of gender in humans [19]. Finally, dominance status in primates [129] can provide a model for understanding how disparities due to socioeconomic status shape ageing in humans.
A major challenge for human ageing research is that humans have extremely slow life histories, so the processes of ageing are difficult to study. As such, many common animal models have faster life histories that are more tractable for ageing research [9,125]. Yet a long lifespan may itself change the dynamics of ageing, which highlights the crucial importance of understanding ageing in other long-lived primates. For example, an important aspect of human social ageing is that older adults show increasing social positivity bias towards positive socioemotional interactions [5,7], yet current work suggests few other primate species show this kind of shift, besides potentially long-lived chimpanzees (figure 2). More generally, comparative work on long-lived non-humans can address conceptual gaps in human work. For example, changes in human social functioning occur gradually over decades and thus are often studied with cross-sectional designs that introduce generational confounds like educational attainment and sociocultural experiences. By contrast, longitudinal approaches are a fundamental aspect of long-term primate field sites.
Finally, future studies of non-human primate ageing can address open questions in the study of human ageing concerning the links between causal mechanisms and real-world social behaviour. For example, studies of socioemotional cognition are a key line of evidence in human social ageing, yet there have been relatively few studies examining social cognition in ageing non-humans [18–21,47,48]. Yet promoting healthy social ageing requires an understanding of both how ageing plays out in natural social contexts, and the underlying mechanisms that might be amenable to targeted interventions. Recent studies of free-ranging primates combining both experiments and observations show how these approaches can be synthesized [18]. As such, non-human primates can provide a pathway for understanding changes in real-world social behaviour during ageing, as well as inferring and intervening on causal mechanisms shaping these patterns.
Supplementary Material
Acknowledgements
We thank Dr Noah Snyder-Mackler and two anonymous reviewers for their suggestions and comments.
Data accessibility
This article has no additional data.
Authors' contributions
Z.P.M. and A.G.R. both conceived of this manuscript, reviewed the literature and drafted the manuscript.
Competing interests
We declare we have no competing interests.
Funding
This work was supported by National Institute on Aging grant no. R01AG04395 National Science Foundation grant nos 1926653 and 1926737, and Sloan Foundation Fellowship grant no. 1944881.
References
- 1.Holt-Lunstad J, Smith TB, Layton JB. 2010. Social relationships and mortality risk: a meta-analytic review. PLoS Med. 7, e1000316 ( 10.1371/journal.pmed.1000316) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang YC, Boen C, Gerken K, Li T, Schropp K, Harris KM. 2016. Social relationships and physiological determinants of longevity across the human life span. Proc. Natl. Acad. Sci. USA 113, 578–583. ( 10.1073/pnas.1511085112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rafnsson SB, Orrell M, d'Orsi E, Hogervorst E, Steptoe A. 2020. Loneliness, social integration, and incident dementia over 6 years: prospective findings from the English Longitudinal Study of Ageing. Gerontology 75, 114–124. ( 10.1093/geronb/gbx087) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Antonucci TC, Ajrouch KJ, Webster NJ, Zahodne LB. 2019. Social relations across the life span: scientific advances, emerging issues, and future challenges. Annu. Rev. Dev. Psychol. 1, 313–336. ( 10.1146/annurev-devpsych-121318-085212) [DOI] [Google Scholar]
- 5.Charles ST, Carstensen LL. 2009. Social and emotional aging. Annu. Rev. Psychol. 61, 383–409. ( 10.1146/annurev.psych.093008.100448) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rook KS. 2015. Social networks in later life: weighing positive and negative benefits on health and wellbeing. Curr. Dir. Psychol. Sci. 24, 45–51. ( 10.1177/0963721414551364) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carstensen LL, DeLiema M. 2018. The positivity effect: a negativity bias in youth fades with age. Curr. Opin. Behav. Sci. 19, 7–12. ( 10.1016/j.cobeha.2017.07.009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baltes PB, Smith J. 2008. The fascination of wisdom: its nature, ontogeny, and function. Perspect. Psychol. Sci. 3, 56–64. ( 10.1111/j.1745-6916.2008.00062.x) [DOI] [PubMed] [Google Scholar]
- 9.Lane MA. 2000. Nonhuman primate models in biogerontology. Exp. Gerontol. 35, 533–541. ( 10.1016/S0531-5565(00)00102-9) [DOI] [PubMed] [Google Scholar]
- 10.Lowenstine LJ, McManamon R, Terio KA. 2016. Comparative pathology of aging great apes: bonobos, chimpanzees, gorillas, and orangutans. Vet. Pathol. 53, 250–276. ( 10.1177/0300985815612154) [DOI] [PubMed] [Google Scholar]
- 11.Snyder-Mackler N, et al. 2020. Social determinants of health and survival in humans and other animals. Science 368, eaax9553 ( 10.1126/science.aax9553) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Archie EA, Tung J, Clark M, Altmann J, Alberts SC. 2014. Social affiliation matters: both same-sex and opposite-sex relationships predict survival in wild female baboons. Proc. R. Soc. B 281, 20141261 ( 10.1098/rspb.2014.1261) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brent LJN, Ruiz-Lambides A, Platt ML. 2017. Family network size and survival across the lifespan of female macaques. Proc. R. Soc. B 284, 20170515 ( 10.1098/rspb.2017.0515) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tung J, Archie EA, Altmann J, Alberts SC. 2016. Cumulative early life adversity predicts longevity in wild baboons. Nat. Commun. 7, 11181 ( 10.1038/ncomms11181) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arnold C, Matthews LJ, Nunn CL. 2020. The 10kTrees website: a new online resource for primate phylogeny. Evol. Anthropol. 19, 114–118. ( 10.1002/evan.20251) [DOI] [Google Scholar]
- 16.Carstensen LL. 1992. Social and emotional patterns in adulthood: support for socioemotional selectivity theory. Psychol. Aging 7, 331–338. ( 10.1037/0882-7974.7.3.331) [DOI] [PubMed] [Google Scholar]
- 17.Slessor G, Phillips LH, Bull R. 2008. Age-related declines in basic social perception: evidence from tasks assessing eye-gaze processing. Psychol. Aging 23, 812–822. ( 10.1037/a0014348) [DOI] [PubMed] [Google Scholar]
- 18.Almeling L, Hammerschmidt K, Senn-Reulen H, Freund AM, Fischer J. 2016. Motivational shifts in aging monkeys and the origins of social selectivity. Curr. Biol. 26, 1744–1749. ( 10.1016/j.cub.2016.04.066) [DOI] [PubMed] [Google Scholar]
- 19.Rosati AG, Arre AM, Platt ML, Santos LR. 2016. Rhesus monkeys show human-like changes in gaze following across the lifespan. Proc. R. Soc. B 283, 20160376 ( 10.1098/rspb.2016.0376) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rosati AG, Santos LR. 2017. Tolerant Barbary macaques maintain juvenile levels of social attention in old age, but despotic rhesus macaques do not. Anim. Behav. 130, 199–207. ( 10.1016/j.anbehav.2017.06.019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lacreuse A, Russell JL, Hopkins W, Herndon JG. 2014. Cognitive and motor aging in female chimpanzees. Neurobiol. Aging 35, 623–632. ( 10.1016/j.neurobiolaging.2013.08.036) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schino G, Pinzaglia M. 2018. Age-related changes in the social behavior of tufted capuchin monkeys. Am. J. Primatol. 80, e22746 ( 10.1002/ajp.22746) [DOI] [PubMed] [Google Scholar]
- 23.Almeling L, Senn-Reulen H, Hammerschmidt K, Freund AM, Fischer J. 2017. Social interactions and activity patterns of old Barbary macaques: further insights into the foundations of social selectivity. Am. J. Primatol. 79, e22711 ( 10.1002/ajp.22711) [DOI] [PubMed] [Google Scholar]
- 24.Nakamichi M. 2003. Age-related differences in social grooming among adult female Japanese monkeys (Macaca fuscata). Primates 44, 239–246. ( 10.1007/s10329-003-0036-x) [DOI] [PubMed] [Google Scholar]
- 25.Corr JA. 2003. Social behavior in aged rhesus macaques. Coll. Antropol. 27, 87–94. [PubMed] [Google Scholar]
- 26.Hauser MD, Tyrrell G. 1984. Old age and its behavioral manifestations: a study on two species of macaque. Folia Primatol. 43, 24–35. ( 10.1159/000156168) [DOI] [PubMed] [Google Scholar]
- 27.Nakamichi M. 1984. Behavioral characteristics of old female Japanese monkeys in a free-ranging group. Primates 25, 192–203. ( 10.1007/BF02382391) [DOI] [Google Scholar]
- 28.Kato E. 1999. Effects of age, dominance, and seasonal changes on proximity relationships in female Japanese macaques (Macaca fuscata) in a free-ranging group at Katsuyama. Primates 40, 291–300. ( 10.1007/BF02557553) [DOI] [Google Scholar]
- 29.Nakamichi M. 1991. Behavior of old females: comparison of Japanese monkeys in the Arashiyama East and West groups. In The monkeys of Arashiyama: 35 years of research in Japan and the West (eds Fedigan LM, Asquith PJ), pp. 175–193. Albany, NY: State University of New York Press. [Google Scholar]
- 30.McDonald Pavelka MS. 1990. Do old female monkeys have a specific social role? Primates 31, 363–373. ( 10.1007/BF02381107) [DOI] [Google Scholar]
- 31.van Noordwijk MA, van Schaik CP. 1987. Competition among female long-tailed macaques, Macaca fascicularis. Anim. Behav. 35, 577–589. ( 10.1016/S0003-3472(87)80284-1) [DOI] [Google Scholar]
- 32.Veenema HC, Spruihit BM, Gispen WH, van Hooff JARAM. 1997. Aging, dominance history, and social behavior in Java-monkeys (Macaca fascicularis). Neurobiol. Aging 18, 509–515. ( 10.1016/S0197-4580(97)00107-3) [DOI] [PubMed] [Google Scholar]
- 33.Borries C, Koenig A. 2008. Reproductive and behavioral characteristics of aging in female Asian colobines. In Primate reproductive aging (eds Atsalis A, Margulis SW, Hof P), pp. 80–102. Basel, Switzerland: Karger. [DOI] [PubMed] [Google Scholar]
- 34.Hrdy SB, Hrdy DB. 1976. Hierarchical relations among female Hanuman langurs (Primates: Colobinae, Presbytis entellus). Science 193, 913–915. ( 10.1126/science.193.4256.913) [DOI] [PubMed] [Google Scholar]
- 35.Wasser PM. 1978. Postreproductive survival and behavior in a free-ranging female mangabey. Folia Primatol. 29, 142–160. ( 10.1159/000155836) [DOI] [PubMed] [Google Scholar]
- 36.McGuire KM. 2017. The social behavior and dynamics of old ring-tailed lemurs (Lemur catta) at the Duke Lemur Center. Master's thesis, University of Colorado at Boulder. [Google Scholar]
- 37.Picq JL. 1992. Aging and social behavior in captivity in Microcebus murinus. Folia Primatol. 59, 217–220. ( 10.1159/000156664) [DOI] [PubMed] [Google Scholar]
- 38.Silk JB. 1994. Social relationships of male bonnet macaques: male bonding in a matrilineal society. Behaviour 130, 271–291. ( 10.1163/156853994X00569) [DOI] [Google Scholar]
- 39.Silk JB, Altmann J, Alberts SC. 2006. Social relationships among adult female baboons (Papio cynocephalus) I. Variation in the strength of social bonds. Behav. Ecol. Sociobiol. 61, 183–195. ( 10.1007/s00265-006-0249-2) [DOI] [Google Scholar]
- 40.Machanda ZP, Rosati AG, Hagberg L, Otali E, Emery Thomson M, Muller M, Wrangam R. 2019. Changing social relationships among aging male chimpanzees. Am. J. Phys. Anthropol. 168, 149. [Google Scholar]
- 41.Huffman MA. 1990. Some socio-behavioral manifestations of old age. In Chimpanzees of the Mahale Mountains: sexual and life history strategies (ed. Nishida T.), pp. 237–255. Tokyo, Japan: University of Tokyo Press. [Google Scholar]
- 42.Suomi SJ, Novack MA, Well A. 1996. Aging in rhesus monkeys: different windows on behavioral continuity and change. Dev. Psychol. 32, 1116–1128. ( 10.1037/0012-1649.32.6.1116) [DOI] [Google Scholar]
- 43.Veenema HC, van Hooff JARAM, Gipsen WH, Spruihit BM. 2001. Increased rigidity with age in social behavior of Java-monkeys (Macaca fasicularis). Neurobiol. Aging 22, 273–281. ( 10.1016/S0197-4580(00)00204-9) [DOI] [PubMed] [Google Scholar]
- 44.Petty JMA, Drea CM. 2015. Female rule in lemurs is ancestral and hormonally mediated. Scient. Rep. 5, 9631 ( 10.1038/srep09631) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Charles ST, Piazza JR, Luong G, Almeida DM. 2009. Now you see it, now you don't: age differences in affective reactivity to social tensions. Psychol. Aging 24, 645–653. ( 10.1037/a0016673) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Luong G, Charles ST, Fingerman KL. 2011. Better with age: social relationships across adulthood. J. Soc. Pers. Relat. 28, 9–23. ( 10.1177/0265407510391362) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bliss-Moreau E, Baxter MG. 2018. Estradiol treatment in a nonhuman primate model of menopause preserves affective reactivity. Behav. Neurosci. 132, 224–229. ( 10.1037/bne0000253) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rosati AG, Arre AM, Platt ML, Santos LR. 2018. Developmental shifts in social cognition: socioemotional biases across the lifespan in rhesus monkeys. Behav. Ecol. Sociobiol. 72, 163 ( 10.1007/s00265-018-2573-8) [DOI] [Google Scholar]
- 49.Bernstein IS, Ehardt CL. 1985. Age-sex differences in the expression of agonistic behavior in rhesus monkey (Macaca mulatta) groups. J. Comp. Psychol. 99, 115–132. ( 10.1037/0735-7036.99.2.115) [DOI] [PubMed] [Google Scholar]
- 50.Bernstein IS, Ehardt CL. 1986. The influence of kinship and socialization on aggressive behaviour in rhesus monkeys (Macaca mulatta). Anim. Behav. 34, 739–747. ( 10.1016/S0003-3472(86)80057-4) [DOI] [Google Scholar]
- 51.Isaacowitz DM, Wadlinger HA, Goren D, Wilson HR. 2006. Is there an age-related positivity effect in visual attention? A comparison of two methodologies. Emotion 6, 511–516. ( 10.1037/1528-3542.6.3.511) [DOI] [PubMed] [Google Scholar]
- 52.Isaacowitz DM, Wadlinger HA, Goren D, Wilson HR. 2006. Selective preference in visual fixation away from negative images in old age? An eye-tracking study. Psychol. Aging 21, 40–48. ( 10.1037/0882-7974.21.1.40) [DOI] [PubMed] [Google Scholar]
- 53.MacCormick HA, MacNulty DR, Bosaker AL, Lehman C, Bailey A, Collins DA, Packer C. 2012. Male and female aggression: lessons from sex, rank, age, and injury in olive baboons. Behav. Ecol. 23, 684–691. ( 10.1093/beheco/ars021) [DOI] [Google Scholar]
- 54.Fairbanks LA, McGuire MT. 1986. Age, reproductive value, and dominance-related behavior in vervet monkey females: cross-generational influences on social relationships and reproduction. Anim. Behav. 34, 1710–1721. ( 10.1016/S0003-3472(86)80258-5) [DOI] [Google Scholar]
- 55.Lu A, Borries C, Gustison ML, Larney E, Koenig A. 2016. Age and reproductive status influence dominance in wild female Phayre's leaf monkeys. Anim. Behav. 117, 145–153. ( 10.1016/j.anbehav.2016.04.020) [DOI] [Google Scholar]
- 56.Archie EA, Altmann J, Alberts SC. 2014. Costs of reproduction in a long-lived female primate: injury risk and wound healing. Behav. Ecol. Sociobiol. 68, 1183–1193. ( 10.1007/s00265-014-1729-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rowell TE, Wilson C, Cords M. 1991. Reciprocity and partner preference in grooming of female blue monkeys. Int. J. Primatol. 12, 319–336. ( 10.1007/BF02547615) [DOI] [Google Scholar]
- 58.Aujard F, Perret M. 1998. Age-related effects on reproductive function and sexual competition in the male prosimian primate, Microcebus murinus. Physiol. Behav. 64, 513–519. ( 10.1016/S0031-9384(98)00087-0) [DOI] [PubMed] [Google Scholar]
- 59.Baker KC. 2000. Advanced age influences chimpanzee behavior in small social groups. Zoo Biol. 19, 111–119. () [DOI] [Google Scholar]
- 60.Altshul DM, Hopkins WD, Herrelko ES, Inoue-Murayama M, Matsuzawa T, King JE, Ross SR, Weiss A. 2018. Personality links with lifespan in chimpanzees. eLife 7, e33781 ( 10.7554/eLife.33781) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.King JM, Weiss A, Sisco MW. 2008. Aping humans: age and sex effects.in chimpanzee (Pan troglodytes) and human (Homo sapiens) personality. J. Comp. Psychlogy 122, 418–427. ( 10.1037/a0013125) [DOI] [PubMed] [Google Scholar]
- 62.Franz C. 1999. Allogrooming behavior and grooming site preferences in captive bonobos (Pan paniscus): association with female dominance. Int. J. Primatol. 20, 525–546. ( 10.1023/A:1020338706800) [DOI] [Google Scholar]
- 63.Tokuyama N, Furuichi T. 2017. Leadership of old females in collective departures in wild bonobos (Pan paniscus) at Wamba. Behav. Ecol. Sociobiol. 71, 55 ( 10.1007/s00265-017-2277-5) [DOI] [Google Scholar]
- 64.Langergraber KE, Mitani JC, Vigilant L. 2007. The limited impact of kinship on cooperation in wild chimpanzees. Proc. Natl Acad. Sci. USA 104, 7786–7790. ( 10.1073/pnas.0611449104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Aureli F, et al. 2008. Fission-fusion dynamics: new research frameworks. Curr. Anthropol. 49, 627–654. ( 10.1086/586708) [DOI] [Google Scholar]
- 66.Gilby IC, Wrangham RW. 2008. Association patterns among wild chimpanzees (Pan troglodytes schweinfurthii) reflect sex differences in cooperation. Behav. Ecol. Sociobiol. 62, 1831–1842. ( 10.1007/s00265-008-0612-6) [DOI] [Google Scholar]
- 67.Silk JB. 2009. Nepotistic cooperation in non-human primate groups. Phil. Trans. R. Soc. B 364, 3243–3254. ( 10.1098/rstb.2009.0118) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Emery Thompson M, Sabbi K. 2019 Evolutionary demography of the great apes. In Human evolutionary demography (eds Sear R, Burger O), pp. 1–75. Cambridge, UK: Open Book Publishers; See https://osf.io/p59eu/. [Google Scholar]
- 69.Muller MN, et al. 2020. Sexual dimorphism in chimpanzee (Pan troglodytes schweinfurthii) and human age-specific fertility. J. Hum. Evol. 144, 102795 ( 10.1016/j.jhevol.2020.102795) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.McFarland Symington M. 1990. Fission-fusion social organization in Ateles and Pan. Int. J. Primatol. 11, 47–61. ( 10.1007/BF02193695) [DOI] [Google Scholar]
- 71.Bronikowski AM, et al. 2011. Aging in the natural world: comparative data reveal similar mortality patterns across primates. Science 331, 1325–1328. ( 10.1126/science.1201571) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Henry JD, Ruffman T, Phillips LH, Bailey PE. 2012. A meta-analytic review of age differences in theory of mind. Pyschol. Aging 28, 826–839. ( 10.1037/a0030677) [DOI] [PubMed] [Google Scholar]
- 73.Grossman I, Na J, Varnum MEW, Park DC, Kitayama S, Nisbett RE. 2010. Reasoning about social conflicts improves into old age. Proc. Natl. Acad. Sci. USA 107, 7246–7250. ( 10.1073/pnas.1001715107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Happe FG, Winner E, Brownell H. 1998. The getting of wisdom: theory of mind in old age. Dev. Psychol. 34, 358–362. ( 10.1037/0012-1649.34.2.358) [DOI] [PubMed] [Google Scholar]
- 75.Romero T, Castellnos MA, de Waal FBM. 2010. Consolation as possible expression of sympathetic concern among chimpanzees. Proc. Natl Acad. Sci. USA 107, 12 110–12 115. ( 10.1073/pnas.1006991107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Call J, Tomasello M. 2008. Does the chimpanzee have a theory of mind? 30 years later. Trends Cogn. Sci. 12, 187–192. ( 10.1016/j.tics.2008.02.010) [DOI] [PubMed] [Google Scholar]
- 77.Melis AP, Warneken F. 2016. The psychology of cooperation: insights from chimpanzees and children. Evol. Anthropol. 25, 297–305. ( 10.1002/evan.21507) [DOI] [PubMed] [Google Scholar]
- 78.Hasegawa M, Kutsukake N. 2015. Bayesian competitiveness estimation predicts dominance turnover among wild male chimpanzees. Behav. Ecol. Sociobiol. 69, 89–99. ( 10.1007/s00265-014-1821-9) [DOI] [Google Scholar]
- 79.Watts DP. 2018. Male dominance relationships in an extremely large chimpanzee community at Ngogo, Kibale National Park, Uganda. Behaviour 115, 969–1009. ( 10.1163/1568539X-00003517) [DOI] [Google Scholar]
- 80.Setchell JM, Wickings EJ, Knapp LA. 2005. Life history in male mandrills (Mandrillus sphinx): physical development, dominance rank, and group association. Am. J. Phys. Anthropol. 131, 498–510. ( 10.1002/ajpa.20478) [DOI] [PubMed] [Google Scholar]
- 81.Packer C, Collins DA, Eberly LE. 2000. Problems with primate sex ratios. Phil. Trans. R. Soc. Lond. B 355, 1627–1635. ( 10.1098/rstb.2000.0725) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Altmann J, et al. 1996. Behavior predicts genetic structure in a wild primate group. Proc. Natl Acad. Sci. USA 93, 5797–5801. ( 10.1073/pnas.93.12.5797) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Noë R, Sluijter AA. 1993. Which adult male savanna baboons form coalitions? Int. J. Primatol. 16, 77–105. ( 10.1007/BF02700154) [DOI] [Google Scholar]
- 84.Alberts SC, Watts HE, Altmann JE. 2003. Queuing and queue-jumping: long-term patterns of reproductive skew in male savannah baboons, Papio cynocephalus. Anim. Behav. 65, 821–840. ( 10.1006/anbe.2003.2106) [DOI] [Google Scholar]
- 85.Sprague DS. 1998. Age, dominance rank, natal status, and tenure among male macaques. Am. J. Phys. Anthropol. 105, 511–521. () [DOI] [PubMed] [Google Scholar]
- 86.Takahashi H. 2002. Changes of dominance rank, age, and tenure of wild Japanese macaque males in the Kinkazan A troop during seven years. Primates 43, 133–138. ( 10.1007/BF02629673) [DOI] [PubMed] [Google Scholar]
- 87.Berard J. 1999. A four-year study of the association between male dominance rank, residency status, and reproductive activity in rhesus macaques (Macaca mulatta). Primates 40, 159–175. ( 10.1007/BF02557708) [DOI] [PubMed] [Google Scholar]
- 88.Manson JH. 1995. Do female rhesus macaques choose novel males? Am. J. Primatol. 37, 285–296. ( 10.1002/ajp.1350370403) [DOI] [PubMed] [Google Scholar]
- 89.Bercovitch FB. 1997. Reproductive strategies of rhesus macaques. Primates 38, 247–263. ( 10.1007/BF02381613) [DOI] [Google Scholar]
- 90.Vessey SH, Meikle DB. 1987. Factors affecting social behavior and reproductive success of male rhesus monkeys. Int. J. Primatol. 8, 281–292. ( 10.1007/BF02735177) [DOI] [Google Scholar]
- 91.Higham JP, Heistermann M, Maestripieri D. 2011. The energetics of male–male endurance rivalry in free-ranging rhesus macaques, Macaca mulatta. Anim. Behav. 81, 1001–1007. ( 10.1016/j.anbehav.2011.02.001) [DOI] [Google Scholar]
- 92.Higham JP, Maestripieri D. 2014. The costs of reproductive success in male rhesus macaques (Macaca mulatta) on Cayo Santiago. Int. J. Primatol. 35, 661–676. ( 10.1007/s10764-014-9789-x) [DOI] [Google Scholar]
- 93.Borries C, Sommer V, Srivastava A. 1991. Dominance, age, and reproductive success in free-ranging female Hanuman langurs (Presbytis entellus). Int. J. Primatol. 12, 231–257. ( 10.1007/BF02547586) [DOI] [Google Scholar]
- 94.Clarke MR, Glander KE. 1984. Female reproductive success in a group of free-ranging howling monkeys (Alouatta palliata) in Costa Rica. In Female primate: studies by women primatologists (ed. Small M.), pp. 111–126. New York, NY: Alan R. Liss. [Google Scholar]
- 95.Gust DA, Gordon TP. 1994. The absence of a matrilineally based dominance system in sooty mangabeys, Cercocebus torquatus atys. Anim. Behav. 47, 589–594. ( 10.1006/anbe.1994.1082) [DOI] [Google Scholar]
- 96.Chapais B, Schulman SR. 1980. An evolutionary model of female dominance relations in primates. J. Theor. Biol. 82, 47–89. ( 10.1016/0022-5193(80)90090-9) [DOI] [PubMed] [Google Scholar]
- 97.Chikazawa D, Gordon TP. 1979. Mother-daughter dominance reversals in rhesus monkeys (Macaca mulatta). Primates 20, 301–305. ( 10.1007/BF02373382) [DOI] [Google Scholar]
- 98.Silk JB, Samuels A, Rodman PS. 1981. Hierarchical organization of female Macaca radiata in captivity. Primates 22, 84–95. ( 10.1007/BF02382559) [DOI] [Google Scholar]
- 99.Hausfater G, Altmann J, Altmann S. 1982. Long-term consistency of dominance relations among female baboons (Papio cynocephalus). Science 217, 752–755. ( 10.1126/science.217.4561.752) [DOI] [PubMed] [Google Scholar]
- 100.Perry S, Manson JH, Muniz L, Gros-Louis J, Vigilant L. 2008. Kin-biased social behaviour in wild adult female white-faced capuchins, Cebus capucinus. Anim. Behav. 76, 187–199. ( 10.1016/j.anbehav.2008.01.020) [DOI] [Google Scholar]
- 101.Bergstrom ML, Fedigan LM. 2010. Dominance among female white-faced capuchin monkeys (Cebus capucinus): hierarchical linearity, nepotism, strength and stability. Behaviour 147, 899–931. ( 10.1163/000579510X497283) [DOI] [Google Scholar]
- 102.Foerster S, Franz M, Murray CM, Gilby IC, Feldblum JT, Walker KK, Pusey AE. 2016. Chimpanzee females queue but males compete for social status. Scient. Rep. 6, 35404 ( 10.1038/srep35404) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ihobe H. 1992. Male-male relationships among wild bonobos (Pan paniscus) at Wamba, Republic of Zaire. Primates 33, 163–179. ( 10.1007/BF02382747) [DOI] [Google Scholar]
- 104.Furuichi T. 1997. Agonistic interactions and matrifocal dominance rank of wild bonobos (Pan paniscus) at Wamba. Int. J. Primatol. 18, 855–887. ( 10.1023/A:1026327627943) [DOI] [Google Scholar]
- 105.Stevens JMG, Vervaecke H, van Elsacker L. 2008. The bonobo's adaptive potential: social relations under captive conditions. In The bonobos: behavior, ecology, and conservation (eds Furuichi T, Thompson J). Berlin, Germany: Springer. [Google Scholar]
- 106.Robbins MM, Robbins AM, Gerald-Steklis N, Steklis HD. 2005. Long-term dominance relationships in female mountain gorillas: strength, stability, and determine of rank. Behaviour 142, 779–809. ( 10.1163/1568539054729123) [DOI] [Google Scholar]
- 107.Muller MN, Emery Thomson M, Enigk DK, Hagberg L, Machanda ZP, Sabbi K, Wrangam RW. 2019. Aggresson, coalition formation, and aging in wild chimpanzees. Am. J. Phys. Anthropol. 168, 171. [Google Scholar]
- 108.Gilby IC, Brent LJN, Wroblewski EE, Rudicell RS, Hahn BH, Goodall J, Pusey AE. 2013. Fitness benefits of coalitionary aggression in male chimpanzees. Behav. Ecol. Sociobiol. 67, 373–381. ( 10.1007/s00265-012-1457-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.van Noordwijk MA, van Schaik CP. 1999. The effects of dominance rank and group size on female lifetime reproductive success in wild long-tailed macaques, Macaca fascicularis. Primates 40, 105–130. ( 10.1007/BF02557705) [DOI] [PubMed] [Google Scholar]
- 110.Bernstein IS, Ehardt CL. 1985. Agonistic aiding: kinship, rank, age, and sex influences. Am. J. Primatol. 8, 37–52. ( 10.1002/ajp.1350080105) [DOI] [PubMed] [Google Scholar]
- 111.Rathke EM, Berghänel A, Bissonnette A, Ostner J, Schülke O. 2017. Age-dependent change of coalitionary strategy in male Barbary macaques. Primate Biol. 4, 1–7. ( 10.5194/pb-4-1-2017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bercovitch FB. 1988. Coalitions, cooperation, and reproductive tactices among adult male baboons. Anim. Behav. 36, 1198–1209. ( 10.1016/S0003-3472(88)80079-4) [DOI] [Google Scholar]
- 113.Noe R. 1992. Alliance formation among male baboons: shopping for profitable partners. In Coalitions and human alliances and other animals (eds Harcourt AH, de Waal FBM), pp. 1–19. Oxford, UK: Oxford University Press. [Google Scholar]
- 114.Smuts BB. 1987. Sexual competition and mate choice. In Primate societies (eds Smuts BB, Cheney DL, Seyfarth RM, Wrangam R, Struhsaker TT), pp. 385–399. Chicago, IL: Chicago University Press. [Google Scholar]
- 115.Tokuyama N, Furuichi T. 2016. Do friends help each other? Patterns of female coalition formation in wild bonobos at Wamba. Anim. Behav. 119, 27–35. ( 10.1016/j.anbehav.2016.06.021) [DOI] [Google Scholar]
- 116.Muller MN, Wrangham RW. 2004. Dominance, aggression and testosterone in wild chimpanzees: a test of the ‘challenge hypothesis’. Anim. Behav. 67, 113–123. ( 10.1016/j.anbehav.2003.03.013) [DOI] [Google Scholar]
- 117.van Noordwijk MA, van Schaik CP. 2004. Sexual selection and the careers of primate males: paternity concentration, dominance-acquisition tactics and transfer decisions. In Sexual selection in primates: new and comparative perspectives (eds Kappeler PM, Schaik CPV), pp. 208–229. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 118.van Schaik CP, Pandit SA, Vogel ER. 2006. Toward a general model for male-male coalitions in primate groups. In Cooperation in primates and humans: mechanisms and evolution (eds Kappeler PM, van Schaik CP), pp. 151–172. Berlin, Germany: Springer. [Google Scholar]
- 119.Wich SA, Steenbeek R, Sterck EH, Korstjens AH, Willems EP, van Schaik CP. 2007. Demography and life history of Thomas langurs (Presbytis thomasi). Am. J. Primatol. 69, 641–651. ( 10.1002/ajp.20386) [DOI] [PubMed] [Google Scholar]
- 120.Newton-Fisher NE, Thompson ME, Reynolds V, Boesch C, Vigilant L. 2010. Paternity and social rank in wild chimpanzees (Pan troglodytes) from the Budongo Forest, Uganda. Am. J. Phys. Anthropol. 142, 417–428. ( 10.1002/ajpa.21241) [DOI] [PubMed] [Google Scholar]
- 121.Ellis L. 1995. Dominance and reproductive success among nonhuman animals: a cross-species comparison. Ethol. Sociobiol. 16, 257–333. ( 10.1016/0162-3095(95)00050-U) [DOI] [Google Scholar]
- 122.Wroblewski EE, Murray CM, Keele BF, Schumacher-Stankey JC, Hahn BH, Pusey AE. 2009. Male dominance rank and reproductive success in chimpanzees, Pan troglodytes schweinfurthii. Anim. Behav. 77, 873–885. ( 10.1016/j.anbehav.2008.12.014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Bray J, Pusey AE, Gilby IC. 2016. Incomplete control and concessions explain mating skew in male chimpanzees. Proc. R. Soc. B 283, 20162071 ( 10.1098/rspb.2016.2071) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Pavelka MSM, Gillespie MW, Griffin L. 1991. The interacting effect of age and rank on the sociability of adult female Japanese monkeys. In The monkeys of Arashiyama: thirty-five years of research in Japan and the West (eds Fedigan LM, Asquith PJ), pp. 194–208. Albany, NY: State University of New York Press. [Google Scholar]
- 125.Tardiff SD, Mamsfield KG, Ratnam R, Ross CN, Ziegler TE. 2011. The marmoset as a model of aging and age-related diseases. ILAR J. 52, 54–65. ( 10.1093/ilar.52.1.54) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Corr JA. 2000. The effects of aging on social behavior in male and female rhesus macaques of Cayo Santiago. PhD thesis, Ohio State University. [Google Scholar]
- 127.Nakamichi M. 2016. Primate social behavior: understanding the social relationships of Japanese macaques. In Cognitive neuroscience robotics B (eds Kasaki M, Ishiguro H, Asada M, Osaka M, Fujikado T), pp. 59–100. Tokyo, Japan: Springer. [Google Scholar]
- 128.Carstensen LL. 2006. The influence of a sense of time on human development. Science 312, 1913–1915. ( 10.1126/science.1127488) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lea AJ, Akinyi MY, Nyakundi R, Mareri P, Nyundo F, Kariuki K, Alberts AC, Archie EA, Tung J. 2018. Dominance rank-associated gene expression is widespread, sex-specific, and a precursor to high social status in wild male baboons. Proc. Natl Acad. Sci. USA 115, E12163–E12171. ( 10.1073/pnas.1811967115) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This article has no additional data.