Abstract
Cell Cycle and Apoptosis Regulator 1 (CCAR1) and Cell Cycle and Apoptosis Regulator 2 (CCAR2) have emerged as key players in physiology and pathophysiology, with critical roles in the DNA damage response, nuclear receptor function, and Wnt signaling, among other activities. Contradictory reports exist on the functional duality of CCAR1 and CCAR2 as either tumor promoters or suppressors, suggesting that CCAR1 and CCAR2 have the hallmarks of gene chameleons. We review herein the mechanistic, preclinical, and human translational findings for CCAR1 and CCAR2, based on available RNA and protein expression data from human studies, The Cancer Genome Atlas (TCGA) data mining, gene knockout mouse models, and cell‐based assays. Multiple factors contribute to the divergent activities of CCAR1 and CCAR2, including tissue type, mutation/genetic background, protein‐protein interactions, dynamic regulation via posttranslational modifications, and alternative RNA splicing. An array of protein partners interact with CCAR1 and CCAR2 in the context of tumor promotion and suppression, including β‐catenin, androgen receptor, p21Cip1/Waf1, tumor protein p53 (p53), sirtuin 1, and histone deacetylase 3. Genetic changes frequently found in cancer, such as TP53 mutation, also serve as critical determinants of survival outcomes in cancer patients. This review seeks to provide the impetus for further investigation into CCAR1 and CCAR2 as potential master regulators of metabolism, aging, and cancer.
Keywords: apoptosis, CCAR1, CCAR2, cell cycle, DBC1
Cell cycle and apoptosis regulator 1 (CCAR1) and CCAR2 have emerged as key players in physiology and pathophysiology, with important roles in Wnt signaling, nuclear receptor function, adipogenesis, and the DNA damage response. Due to the diverse array of protein partners, including beta‐catenin, androgen receptor, p21, p53, sirtuin 1, and histone deacetylase 3, literature reports exist on the functional duality of CCAR1 and CCAR2 as either tumor promoters or suppressors. Genetic changes frequently found in cancer, such as TP53 mutation, also serve as critical determinants of patient survival outcomes and impact the roles of CCAR1 and CCAR2 as master regulators of metabolism, aging, and cancer.

Abbreviations
- APC‐2
Anaphase Promoting Complex 2
- AR
androgen receptor
- ATM
ataxia telangiectasia mutated
- ATR
ataxia telangiectasia and Rad 3‐related
- BET
bromodomain and extraterminal domain
- BRD9
bromodomain‐containing protein 9
- CCAR1
Cell Cycle and Apoptosis Regulator 1
- CCAR2
Cell Cycle and Apoptosis Regulator 2
- CCNB1
Cyclin B1
- CRC
colorectal cancer
- DBC1
Deleted in Breast Cancer 1
- DBC2
Deleted in Breast Cancer 2
- DBIRD
DBC1‐ and ZIRD‐containing
- ERα
estrogen receptor α
- FOXP3
Forkhead box P3
- GATA2
GATA Binding Protein 2
- HCC
hepatocellular carcinoma
- hMOF
human MOF
- hnRNP1A
heterogeneous nuclear ribonucleoprotein A1
- IHC
immunohistochemistry
- LST‐3
Lateral Signaling Target‐3
- MCC
Mutated in Colorectal Cancer
- MYC
Myelocytomatosis
- Notch3
Neurogenic locus notch homolog protein 3
- OS
overall patient survival
- p21
p21Cip1/Waf1
- p53
tumor protein p53
- Par‐4
prostate apoptosis response‐4
- PTMs
posttranslational modifications
- RFS
recurrence‐free survival
- SIRT1
Sirtuin 1
- SWI/SNF
Switch/Sucrose Nonfermentable
- TCGA
The Cancer Genome Atlas
- THAP1
THAP‐Domain‐Containing Protein 1
- ZIRD
ZNF‐protein interacting with nuclear mRNPs and DBC1
1. INTRODUCTION
Cell Cycle and Apoptosis Regulator 1 (CCAR1) and Cell Cycle and Apoptosis Regulator 2 (CCAR2) evolved from the common ancestor Lateral Signaling Target‐3 in Caenorhabditis elegans 1 , 2 (Figure 1). These paralog proteins have emerged as key players in physiology and pathophysiology, with roles in Wnt signaling, nuclear receptor function, adipogenesis, apoptosis, and the DNA damage response. 3 , 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 Such wide‐ranging activities derive, in large part, from the diverse array of protein partners implicated in CCAR1 and CCAR2 function (Figure 2A,B).
Figure 1.
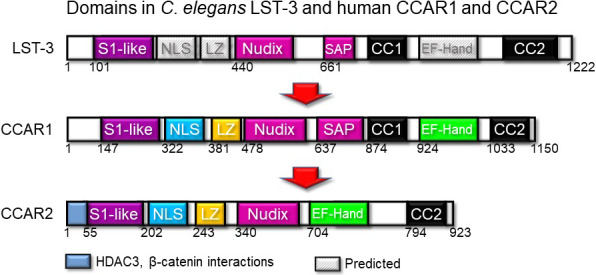
Comparison of protein domains in Cell Cycle and Apoptosis Regulator 1 (CCAR1) and Cell Cycle and Apoptosis Regulator 2 (CCAR2) with the common ancestor Lateral Signaling Target‐3 (LST‐3) in Caenorhabditis elegans. CC, protein‐protein interaction domain; LZ, leucine zipper; NLS, nuclear localization signal; S1‐like, homology to an RNA interaction domain; SAP, homology to DNA‐binding motif for chromosomal organization
Figure 2.
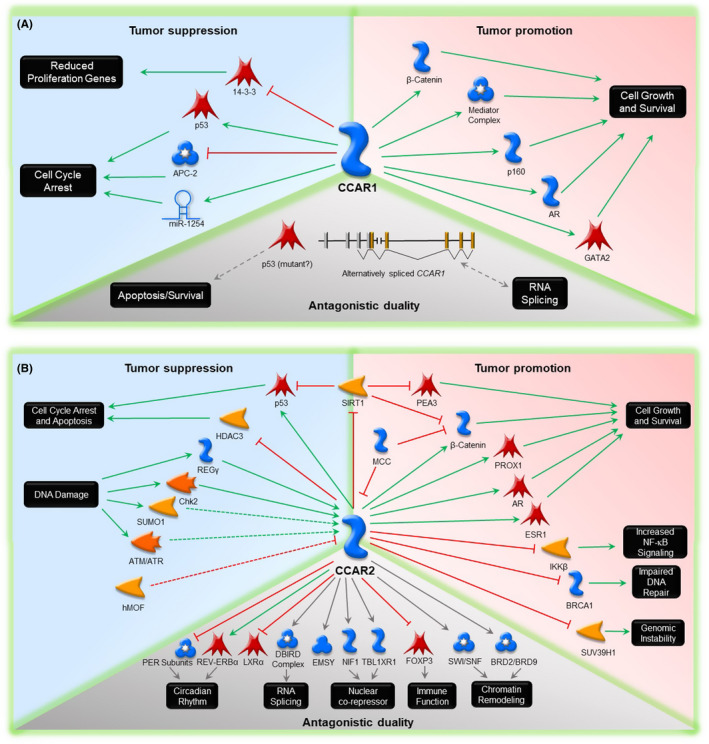
A, Cell Cycle and Apoptosis Regulator 1 (CCAR1) and B, Cell Cycle and Apoptosis Regulator 2 (CCAR2) protein partners leading to tumor suppression (cyan), promotion (pink), or potential antagonistic duality (grey). Green arrows = activation; red lines = inhibition; grey lines = not fully elucidated; dashed lines = predicted interactions. For all other abbreviations, refer to the Abbreviations section
Cell Cycle and Apoptosis Regulator 1 was first discovered as a regulator of apoptosis signaling in breast cancer cells and was named Cell Cycle and Apoptosis Regulator Protein‐1. 10 Subsequently, CCAR2 gained attention as a modulator of tumor protein p53 (p53) activity in response to DNA damage signaling, inhibiting the activity of Sirtuin 1 (SIRT1) and histone deacetylase 3 (HDAC3) via protein‐protein interactions. 1 , 6 , 14 , 15 The name originally ascribed to CCAR2, Deleted in Breast Cancer 1 (DBC1), is regarded as a misnomer because the protein can be overexpressed in mammary cancer and other malignancies. 16
Conflicting reports exist on the roles of CCAR1 and CCAR2 in cancer etiology. For example, CCAR2 facilitates tumor suppressor functions of p53 6 , 14 , 17 or serves as an oncogenic driver of Wnt/β‐catenin signaling, 4 thereby exhibiting “antagonistic duality”. 18 So‐called “gene chameleons” are becoming better understood in the context of their nuanced roles in the regulation of gene expression. 17 , 18 , 19 , 20 , 21 , 22 This review summarizes current clinical, preclinical, and molecular findings on CCAR family members in cancer etiology.
2. CCAR1: TUMOR PROMOTION VS SUPPRESSION
Divergent actions of CCAR1 arise from changes in cell cycle, proliferation, growth, and survival, with phenotypic outcomes involving altered β‐catenin/Wnt signaling, 3 nuclear receptor activity, 8 , 12 adipogenesis, 9 and apoptosis. 10 , 11 , 12 , 13 For example, in T‐cell acute lymphoblastic leukemia cells, tumor promoter or suppressor outcomes depend on alternative splice variants of CCAR1, 13 giving rise to antagonistic duality (Figure 2A).
Immunohistochemistry (IHC) data for CCAR1 were reported in a handful of human translational studies. In hepatocellular carcinoma (HCC), CCAR1 levels were correlated with unfavorable overall survival (OS) and recurrence‐free survival (RFS). 23 In colorectal cancer (CRC), CCAR1 interacted with and activated β‐catenin. 3 Depletion of CCAR1 in colon cancer cells inhibited β‐catenin‐dependent target gene expression and suppressed anchorage‐independent growth. Furthermore, CCAR1 regulated nuclear receptor signaling by recruiting the Mediator complex to affect key proliferation genes 12 and stabilized androgen receptor (AR) and GATA Binding Protein 2 (GATA2). 8 In accordance with the reported IHC findings for HCC, 23 CCAR1 mRNA levels were associated with significantly reduced OS (Figure 3A), as was the case for renal cancer (Figure 3B), whereas the reverse scenario was detected for ovarian cancer (Figure 3C), with CCAR1 protein immunolocalized to the nuclear compartment in tissue microarrays (Figure 3D,E).
Figure 3.
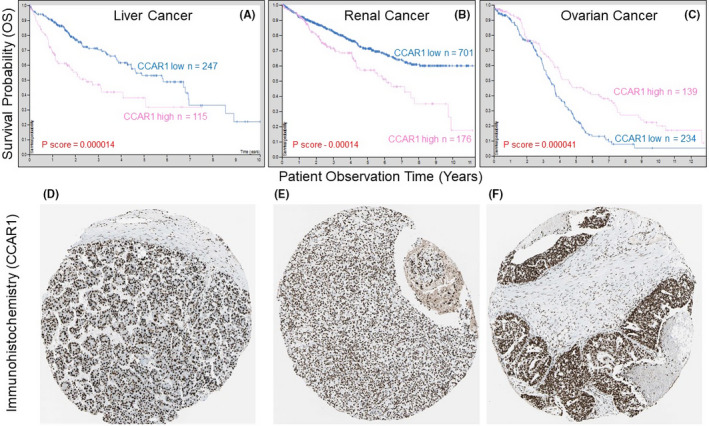
A‐C, Overall survival (OS) in liver, renal, and ovarian cancer patients with high vs low CCAR1 mRNA expression in tumors. D‐F, immunodetection of nuclear Cell Cycle and Apoptosis Regulator 1 (CCAR1) for the corresponding tumor types shown in A‐C; immunohistochemistry (IHC) images were obtained from the Human Protein Atlas (https://www.proteinatlas.org/)
In breast cancer cells, CCAR1 was reported to induce apoptosis. 10 Overexpression of CCAR1 caused elevated levels of cyclin‐dependent kinase inhibitor p21Cip 1 /Waf1 (p21) and reduced the transcriptional activity of proliferative genes, such as Myelocytomatosis (MYC) and Cyclin B1 (CCNB1). 10 CCAR1 was also shown to activate p53, but the mechanisms were not elucidated, 12 especially in the context of p53 mutation status (see below).
Because of the low CCAR1 expression in some breast cancer patients, attempts were made to induce CCAR1 levels and/or alter its function. 24 , 25 , 26 CCAR1 “functional mimics” duplicated CCAR1 binding to Anaphase Promoting Complex 2 (APC‐2) and halted the cell cycle to enhance apoptosis. 24 Recently, the 5′ UTR sequence of CCAR1 was shown to stabilize and increase the activity of microRNA miR‐1254 that is highly downregulated in breast cancer. 25 Withaferin A, a bioactive compound from the medicinal plant Withania somnifera, upregulated CCAR1 in mesothelioma, resulting in the inhibition of proteasome activity and the induction of apoptosis. 26
3. CCAR2: TUMOR PROMOTION VS SUPPRESSION
A genetic screen was undertaken for potential tumor suppressor genes located in a region of human chromosome 8 that was deleted in breast cancer. 16 Candidate genes included DBC1 and Deleted in Breast Cancer 2 (DBC2). The former gene designation proved to be a case of erroneous “guilt by association,” given the typical expression patterns in mammary cancer; hence the preferred naming as CCAR2. This contrasts to DBC2 in the same genetic locus, which is as a bona fide tumor suppressor gene. 16
Subsequently, multiple studies examined CCAR2 expression in various human cancers and the associated clinical outcomes, 27 , 28 , 29 , 30 , 31 which supported an oncogenic role in certain instances and a tumor suppressor role in others (Table 1). Molecular analyses implicated a diverse array of protein partners (Figure 2B), as discussed below.
Table 1.
Tumor suppressor vs promoter roles for CCAR2 in various cancer types
| Cancer type | Sample type | Method | Outcome | Reference |
|---|---|---|---|---|
| Tumor suppressor role | ||||
| Colorectal cancer | 51 sporadic CRCs | SNP Array | LOH on 8p (containing CCAR2) in 40 microsatellite stable sporadic colon cancer patients | [32] |
| Gall bladder carcinoma | 104 gallbladder carcinomas | IHC | CCAR2 is associated with better OS and clinicopathologic variables | [34] |
| Gastric cancer | 452 gastric cancers | IHC | CCAR2 is associated with lower stage, lesser lymphatic invasion, and better OS | [30] |
| 557 cohort gastric cancers | IHC | CCAR2 is related to lower stage, lymph node metastasis, and better prognosis | [31] | |
| Laryngeal and hypopharyngeal carcinoma | 120 LSCC or HSCC | IHC | CCAR2 is correlated to lower lymph node metastasis and tumor differentiation | [35] |
| 41 HNSCC Cell Lines | SNP Array | LOH on 8p22‐p21.3 (containing CCAR) (88.7%) | [33] | |
| Pancreatic ductal adenocarcinoma | 104 stage I and II PDAC | IHC | CCAR2 is associated with better survival and differentiated tumors | [36] |
| Tumor promoter role | ||||
| Breast cancer | 48 breast core‐needle biopsies | IHC | CCAR2 is associated with tumor nuclear grade | [72] |
| 122 breast core‐needle biopsies | IHC | CCAR2 is associated with distant metastatic relapse, increased tumor stage, poor OS & RFS | [54] | |
|
202 ER‐negative breast cancers 128 ER‐negative/HER2‐positive |
IHC | CCAR2 is related to lower RFS in ER(−) and ER(−)/HER2(+) cancers | [51] | |
| Clear cell renal carcinoma | 200 CRCC | IHC | CCAR2 expression correlates with shorter OS, RFS, and CSS | [36] |
| Colorectal cancer | 186 CRC | IHC | CCAR2 is overexpressed in tumor compared with adjacent normal, and is associated with tumor grade, TNM stage, metastatic status, and poor OS | [59] |
| 200 CRC | IHC | CCAR2 expression correlates with lower RFS | [4] | |
| Diffuse large B‐cell lymphoma | 101 DLBCL | IHC | CCAR2 is associated with high clinical stage, elevated serum lactate dehydrogenase levels, prognostic index score, shorter OS & RFS | [57] |
| Esophageal squamous cell carcinoma | 165 ESCC & 34 normal | IHC | CCAR2 is overexpressed in ESCC and associated with poor prognosis | [53] |
| Gastric cancer | 142 gastric adenocarcinomas | IHC | CCAR2 is overexpressed in tumors, associated with stage, lymph node metastasis, and lower OS | [29] |
| 187 gastric carcinomas | IHC | Phosphorylated CCAR2 is associated with higher tumor grade, poor OS & RFS | [27] | |
| 177 gastric cancers | IHC | CCAR2 is associated with stage, lymph node metastasis, tumor invasion, shorter OS & RFS | [28] | |
| Hepatocellular carcinoma | 158 HCC | IHC | CCAR2 is associated with poor OS & RFS | [55] |
| 55 matched HCC and normal | IHC | CCAR2 is overexpressed in HCC and is associated with tumor size, stage, and differentiation | ||
| Osteosarcoma | 35 Osteosarcoma | IHC | CCAR2 is associated with shorter OS, RFS, and higher clinical stage | [58] |
| Ovarian carcinoma | 104 Ovarian carcinomas | IHC | CCAR2 is overexpressed in tumors and associated with stage, metastasis, platinum resistance, histological grade, poor OS & RFS | [60] |
| Soft tissue sarcoma | 104 Soft tissue sarcomas | IHC | CCAR2 is associated with stage, grade, mitotic counts, distant metastasis, lower OS & RFS | [52] |
Abbreviations: CCAR2, Cell Cycle and Apoptosis Regulator 2; CRC, colorectal cancer; CRCC, clear cell renal carcinoma; DLBCL, diffue large B‐cell lymphoma; ER, estrogen receptor; ESCC, esophageal squamous cell carcinoma; HCC, hepatocellular carcinoma; HER2, human epidermal growth factor receptor 2; HNSCC, head and neck squamous cell carcinoma; HSCC, hypopharyngeal squamous cell carcinoma; IHC, immunohistochemistry; LOH, loss of heterozygosity; LSCC, laryngeal squamous cell carcinoma; OS, overall patient survival; PDAC, pancreatic ductal adenocarcinoma; RFS, recurrence‐free survival; SNP, single nucleotide polymorphism.
Based on loss of heterozygosity, a tumor suppressor role for CCAR2 was proposed in CRC and in head and neck malignancies. 32 , 33 In gastric cancer, however, high CCAR2 expression was associated with lower disease stage, attenuated lymph node invasion/metastasis, and better overall prognosis and survival. 30 , 31 Elevated CCAR2 also predicted better clinicopathological variables and OS in gall bladder carcinoma patients. 34 Likewise, CCAR2 was associated with favorable clinical outcomes, such as reduced lymph node metastasis and tumor differentiation, in laryngeal and hypopharyngeal carcinoma. 35 In pancreatic ductal adenocarcinoma, the majority of tumors showed high CCAR2 expression; however, this was associated with better OS, and the tumors expressing less CCAR2 tended to be poorly differentiated. 36
As noted for CCAR1 (Figure 3), CCAR2 can be a “friend or foe” depending on the cancer type (Figure 4). For example, CCAR2 predicted poor OS in liver cancer, but improved prognosis in ovarian and renal cancer (Figure 4A‐C), with the protein immunolocalized to the nuclear compartment in tissue microarrays (Figure 4D‐F).
Figure 4.
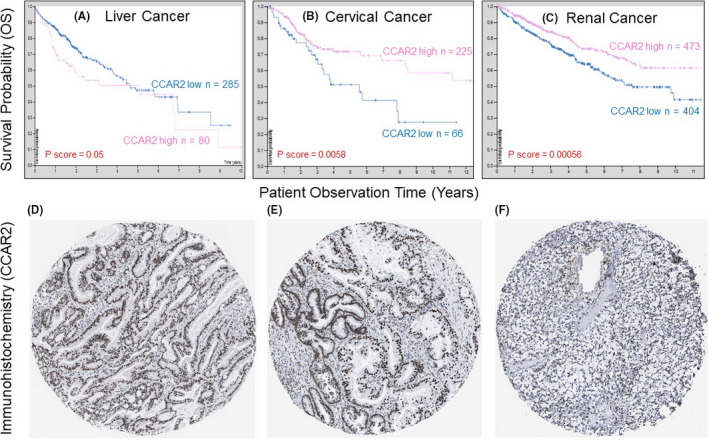
A‐C, OS in liver, cervical, and renal cancer patients with high vs low CCAR2 mRNA expression. D‐F, immunodetection of nuclear Cell Cycle and Apoptosis Regulator 2 (CCAR2) for the tumor types shown in A‐C; IHC images were obtained from the Human Protein Atlas, see Figure 3 legend
Data from The Cancer Genome Atlas (TCGA) indicated that CCAR2 “high” expression was associated with improved OS for cancers of the breast (P = .008) and colon (P = .037), and with higher RFS in prostate cancer (P = .04) (Figure 5A‐C). Opposite trends for CCAR2 (Figure 5D‐F) are discussed below.
Figure 5.
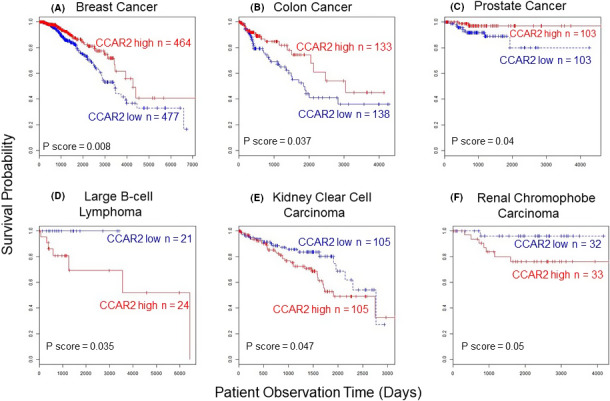
High CCAR2 expression (red lines) predicted favorable survival outcomes in A, breast, B, colon, and C, prostate cancer, but poor survival in D, large B‐cell lymphoma, E, kidney clear cell carcinoma, and F, renal chromophobe carcinoma. Results from The Cancer Genome Atlas (TCGA) database indicating overall patient survival (OS), except for recurrence‐free survival (RFS) in panel C
Genetic knockout of Ccar2 led to spontaneous lymphomas, liver tumors, lung tumors, and teratomas as well as poor OS compared with C57BL/6 wild‐type mice. 17 A tumor suppressor role of Ccar2 was attributed to Ccar2‐mediated regulation of p53 stability. However, Ccar2 null status in the 129/JxC57BL/6J background did not enhance tumorigenesis, 37 indicating discordant outcomes according to mouse strain/genetics. 38
In cell‐based assays, depletion of CCAR2 decreased apoptosis in response to DNA‐damaging agents, such as etoposide or radiation. 6 , 14 , 39 , 40 Overexpression of CCAR2 led to increased sensitivity upon exposure to DNA‐damaging agents, 40 , 41 , 42 via a direct role of CCAR2 in DNA damage signaling 43 , 44 or indirectly through its ability to activate p53. 6 , 14 , 17 Tumor suppressor functions of CCAR2 highlighted a key role for CCAR2/SIRT1 protein‐protein interactions. 6 Inhibition of SIRT1 by CCAR2 allowed p53 to be acetylated and activated, triggering apoptosis, 6 , 14 whereas in cells that lacked endogenous SIRT1/CCAR2 interactions, no apoptosis occurred. 45 Similarly, ataxia telangiectasia mutated (ATM)/ataxia telangiectasia and Rad 3‐related (ATR) proteins phosphorylated CCAR2 at Thr454 following DNA damage, which increased SIRT1 binding. 39 , 42 Recent studies implicated other protein partners, posttranslational modifications (PTMs), and long noncoding RNAs. 41 , 44 , 46 , 47 , 48 For example, CCAR2 was acetylated by human MOF (hMOF), also known as Lysine Acetyltransferase 8, and these acetylation sites disrupted CCAR2/SIRT1 binding and increased SIRT1 activity. 40 , 49 Interestingly, the N‐terminus of CCAR2 binds to HDAC3 (Figure 1), inhibiting deacetylase activity and altering the subcellular distribution. 50 Thus, CCAR2 serves as a potential regulator of class I and class III deacetylases associated with oncogenesis.
Although high CCAR2 expression in TCGA data predicted improved survival in breast, colon, and prostate cancer patients (Figure 5A‐C), the reverse scenario was observed for large B‐cell lymphoma, kidney clear cell carcinoma, and renal chromophobe carcinoma (Figure 5D‐F). Reports also have noted that high CCAR2 expression was associated with reduced OS and RFS in osteosarcoma, soft tissue sarcoma, clear cell renal carcinoma, diffuse large B‐cell lymphoma, breast cancer, esophageal squamous cell carcinoma, CRC, and HCC. 4 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 Tumor stage/grade, lymph node metastasis, and distant metastasis were associated with high CCAR2 expression in many cancer types. 28 , 55 , 57 , 58 , 59 , 60
Interestingly, duality for CCAR2 has been noted in CRC and gastric cancer according to p53 mutation status. Wild‐type and mutant forms of p53 typically exert opposing effects during tumorigenesis, and both forms can be stabilized by CCAR2 through SIRT1 inhibition and p53 acetylation. 17 Because CCAR2 can interact with both wild‐type and mutant p53, these protein‐protein interactions can dictate whether CCAR2 functions as a tumor suppressor or promoter. For instance, in low‐grade glioma, TCGA data indicated RFS was independent of CCAR2 expression (Figure 6A) and p53 mutation status (Figure 6B). However, taking the TP53 WT (green line) and TP53 mutant status (black line) into Figure 6C,D, respectively, highly significant differences in RFS were observed with CCAR2 as a covariate. Specifically, with TP53 WT, low CCAR2 expression was associated with poor prognosis (Figure 6C, blue vs red lines, P < .002), whereas in the TP53 mutant background, the reverse was true, with low CCAR2 predicting better survival (Figure 6D, blue vs red lines, P < .005).
Figure 6.
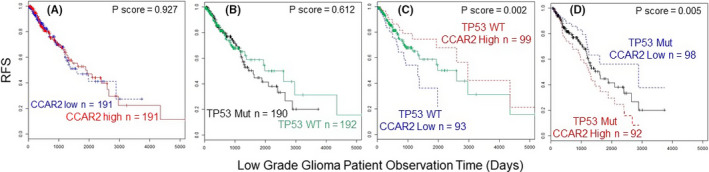
Kaplan‐Meier curves for CCAR2 and TP53 mutation status in glioma. Results from The Cancer Genome Atlas (TCGA) database indicating recurrence‐free survival (RFS), all panels. A, Comparison of CCAR2 high vs CCAR2 low expression; B, comparison of TP53 wild type vs TP53 mutant status; C, wild type TP53 with CCAR2 high vs CCAR2 low expression as a covariate; D, mutant TP53 with CCAR2 high vs CCAR2 low as a covariate. Mut, mutant TP53; WT, wild‐type TP53
4. POSTTRANSCRIPTIONAL MODIFICATIONS AFFECTING CCAR1 AND CCAR2 DUALITY
The functional duality of CCAR2 also is influenced by PTMs, including phosphorylation and acetylation. 61 , 62 , 63 , 64 , 65 , 66 , 67 For example, in gastric cancer, phosphorylated CCAR2 but not unphosphorylated protein was associated with poor OS, RFS, and higher tumor grade. 27 Phosphorylation of CCAR2 by casein kinase‐2α upregulated epithelial‐mesenchymal transition‐related genes, such as matrix metalloproteinases and N‐cadherin 2. 27
As noted above, CCAR2 serves as a regulator of class I and class III deacetylases and is subject to reversible acetylation. CCAR2 acetylation by hMOF at lysines K112 and K215 disrupted CCAR2/SIRT1 binding, leading to increased SIRT1 activity. 40 , 49 Recently, 68 CCAR2 was identified as an early target for acetylation by sulforaphane, a dietary preventive agent that caused inhibition and turnover of HDAC3 in colon cancer cells. 69 N‐terminal acetylation of CCAR2 at K54 and K96 sites diminished its interactions with β‐catenin, interfering with Wnt coactivator functions of CCAR2, whereas a C‐terminal K916 acetylation site provided a bromodomain and extraterminal domain (BET)/bromodomain‐containing protein 9 (BRD9) “acetyl switch” that was linked mechanistically to the suppression of adenomatous colon polyps in a preclinical model of colorectal cancer. 68 Under the same conditions, acetylation of CCAR1 was not observed, 68 indicating that CCAR1 and CCAR2 can undergo differential regulation via PTMs, depending on the circumstances involved.
CCAR2 also influences PTMs on other key cellular proteins. For example, CCAR2 activated β‐catenin in the colon by binding to and promoting Lys49 acetylation of β‐catenin via SIRT1 inhibition. 4 , 67 Mutated in Colorectal Cancer (MCC), a gene that is commonly mutated and inactivated in CRC, keeps β‐catenin under check by sequestering the CCAR2/β‐catenin complex in the cytosol and maintaining β‐catenin in the deacetylated form. However, when MCC is mutated, the protein product (R506Q) is unable to relocate CCAR2 to the cytosol, and the brake on β‐catenin is released, thereby promoting oncogenesis. 67
5. CONCLUSIONS
Although CCAR2 associations have been corroborated experimentally for relatively few proteins, recent proteomic analyses 15 identified hundreds of candidates in the CCAR2 interactome, some of which are illustrated in Figure 2B. However, mechanistic leads have yet to be pursued in many cases. For example, CCAR2 interactions are noteworthy in the case of Switch/Sucrose Nonfermentable (SWI/SNF) chromatin remodeling factors that are commonly mutated in cancer. Mutation status was highlighted for p53, with divergent survival outcomes for low‐grade glioma patients at high vs low CCAR2 expression levels (Figure 5C,D). Antagonistic duality due to p53 mutation also likely affects CCAR1 (Figure 2A), for which even less is known in terms of the interacting partners.
Another noteworthy example is provided by Forkhead box P3 (FOXP3) (Figure 2B). Interaction with CCAR2 destabilizes FOXP3, a master regulator of regulatory T cells, diminishing immunosuppressive functions, 70 with implications for cancer immune surveillance and autoimmune diseases. In the case of BRD2/BRD9 interactions with CCAR2 (Figure 2B), competition among the “readers” of acetylated histone and nonhistone proteins provided a mechanistic explanation for the synergy observed due to combined deacetylase and bromodomain inhibition in CRC prevention. 68
Alternative splicing mechanisms also warrant further investigation. For example, CCAR2 regulates alternative splicing mechanisms via associations with ZNF‐protein interacting with nuclear mRNPs and DBC1 (ZIRD) and heterogeneous nuclear ribonucleoprotein A1 (hnRNP1A) in the DBC1‐ and ZIRD‐containing (DBIRD) complex. 71 Competitive interactions of Prostate apoptosis response‐4 (Par‐4)/THAP‐Domain‐Containing Protein 1 (THAP1) and Neurogenic locus notch homolog protein 3 (Notch3) on the CCAR1 promoter resulted in alternative CCAR1 pre‐mRNA splicing (Figure 2A), and transcripts with opposing activities on cell survival in leukemia. 13
A fundamentally important question concerns the extent to which new therapeutic avenues might be realized in the clinical setting, given that CCAR1 and CCAR2 have the hallmarks of gene chameleons. 18 Based on recent findings, 68 CRC patients might be stratified according to high vs low CCAR2/β‐catenin expression before using combined deacetylase plus bromodomain inhibition for precision medicine. A similar approach might be considered in the context of p53 mutation and high CCAR2 expression for glioma patients (Figure 6D).
In summary, this review provided a direct comparison of CCAR1 and CCAR2 as dynamically regulated proteins with diverse roles in tumor promotion and suppression (Table 1). Elucidating the functions of CCAR1 and CCAR2 during cancer development will require a better understanding of the diverse array of interacting partners (Figure 2A,B), many of which have established roles in malignancy, such as p21, p53, β‐catenin, SIRT1, and HDAC3. Despite the available preclinical, human translational, and mechanistic information, large gaps exist in the scientific literature. This review seeks to provide the impetus for further investigation into CCAR1 and CCAR2 as potential master regulators of metabolism, aging, and cancer. 1
DISCLOSURE
Authors declare no competing interests.
AVAILABILITY OF SUPPORTING DATA
All information is available in the public domain, including PubMed, TCGA, and the Human Protein Atlas (https://pubmed.ncbi.nlm.nih.gov/tcga; https://portal.gdc.cancer.gov/repository; https://www.proteinatlas.org/).
AUTHORS’ INFORMATION
Each author approved of the submitted version and agreed to be personally accountable for their own contributions and to ensure that questions related to the accuracy and integrity of the work are appropriately investigated, resolved, and documented. Author affiliations are provided on page 1.
ACKNOWLEDGEMENTS
Authors’ original research was supported in part by grants CA090890 and CA122959 from the US National Cancer Institute (NCI), NCI PREVENT contract No. HHSN261201500018I (Task Orders HHSN26100004, 75N91019D00021), the John S. Dunn Foundation, and a Texas A&M Chancellor’s Research Initiative. Laboratory members who contributed to the original work on CCAR2, β‐catenin, and HDAC inhibition 68 , 69 are gratefully acknowledged.
Johnson GS, Rajendran P, Dashwood RH. CCAR1 and CCAR2 as gene chameleons with antagonistic duality: Preclinical, human translational, and mechanistic basis. Cancer Sci. 2020;111:3416–3425. 10.1111/cas.14579
Contributor Information
Praveen Rajendran, Email: prajendran@tamu.edu.
Roderick H. Dashwood, Email: rdashwood@tamu.edu.
REFERENCES
- 1. Chini EN, Chini CC, Nin V, Escande C. Deleted in breast cancer‐1 (DBC‐1) in the interface between metabolism, aging and cancer. Biosci Rep. 2013;33:637‐643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Brunquell J, Yuan J, Erwin A, Westerheide SD, Xue B. DBC1/CCAR2 and CCAR1 are largely disordered proteins that have evolved from one common ancestor. Biomed Res Int. 2014;2014:418458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ou CY, Kim JH, Yang CK, Stallcup MR. Requirement of cell cycle and apoptosis regulator 1 for target gene activation by Wnt and beta‐catenin and for anchorage‐independent growth of human colon carcinoma cells. J Biol Chem. 2009;284:20629‐20637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yu EJ, Kim SH, Kim HJ, et al. Positive regulation of β‐catenin‐PROX1 signaling axis by DBC1 in colon cancer progression. Oncogene. 2016;35:3410‐3418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Trauernicht AM, Kim SJ, Kim NH, Boyer TG. Modulation of estrogen receptor alpha protein level and survival function by DBC‐1. Mol Endocrinol. 2007;21:1526‐1536. [DOI] [PubMed] [Google Scholar]
- 6. Kim JE, Chen J, Lou Z. DBC1 is a negative regulator of SIRT1. Nature. 2008;451:583‐586. [DOI] [PubMed] [Google Scholar]
- 7. Koyama S, Wada‐Hiraike O, Nakagawa S, et al. Repression of estrogen receptor beta function by putative tumor suppressor DBC1. Biochem Biophys Res Commun. 2010;392:357‐362. [DOI] [PubMed] [Google Scholar]
- 8. Seo WY, Jeong BC, Yu EJ, et al. CCAR1 promotes chromatin loading of androgen receptor (AR) transcription complex by stabilizing the association between AR and GATA2. Nucleic Acids Res. 2013;41:8526‐8536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ou CY, Chen TC, Lee JV, Wang JC, Stallcup MR. Coregulator cell cycle and apoptosis regulator 1 (CCAR1) positively regulates adipocyte differentiation through the glucocorticoid signaling pathway. J Biol Chem. 2014;289:17078‐17086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rishi AK, Zhang L, Boyanapalli M, et al. Identification and characterization of a cell cycle and apoptosis regulatory protein‐1 as a novel mediator of apoptosis signaling by retinoid CD437. J Biol Chem. 2003;278:33422‐33435. [DOI] [PubMed] [Google Scholar]
- 11. Zhang L, Levi E, Majumder P, et al. Transactivator of transcription‐tagged cell cycle and apoptosis regulatory protein‐1 peptides suppress the growth of human breast cancer cells in vitro and in vivo. Mol Cancer Ther. 2007;6:1661‐1672. [DOI] [PubMed] [Google Scholar]
- 12. Kim JH, Yang CK, Heo K, Roeder RG, An W, Stallcup MR. CCAR1, a key regulator of mediator complex recruitment to nuclear receptor transcription complexes. Mol Cell. 2008;31:510‐519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lu C, Li JY, Ge Z, Zhang L, Zhou GP. Par‐4/THAP1 complex and Notch3 competitively regulated pre‐mRNA splicing of CCAR1 and affected inversely the survival of T‐cell acute lymphoblastic leukemia cells. Oncogene. 2013;32:5602‐5613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhao W, Kruse JP, Tang Y, Jung SY, Qin J, Gu W. Negative regulation of the deacetylase SIRT1 by DBC1. Nature. 2008;451:587‐590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Giguère SS, Guise AJ, Jean Beltran PM, et al. The proteomic profile of deleted in breast cancer 1 (DBC1) interactions points to a multifaceted regulation of gene expression. Mol Cell Proteomics. 2016;15:791‐809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hamaguchi M, Meth JL, von Klitzing C, et al. DBC2, a candidate for a tumor suppressor gene involved in breast cancer. Proc. Natl Acad Sci USA. 2002;99:13647‐13652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Qin B, Minter‐Dykhouse K, Yu J, et al. DBC1 functions as a tumor suppressor by regulating p53 stability. Cell Rep. 2015;10:1324‐1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Stepanenko AA, Vassetzky YS, Kavsan VM. Antagonistic functional duality of cancer genes. Gene. 2013;529:199‐207. [DOI] [PubMed] [Google Scholar]
- 19. Kensler TW, Wakabayashi N. Nrf2: friend or foe for chemoprevention? Carcinogenesis. 2010;31:90‐99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Di Marcotullio L, Canettieri G, Infante P, Greco A, Gulino A. Protected from the inside: endogenous histone deacetylase inhibitors and the road to cancer. Biochim Biophys Acta. 2011;1815:241‐252. [DOI] [PubMed] [Google Scholar]
- 21. Kim JE, Chen J, Lou Z. p30 DBC is a potential regulator of tumorigenesis. Cell Cycle. 2009;8:2932‐2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Muthu M, Cheriyan VT, Rishi AK. CARP‐1/CCAR1: a biphasic regulator of cancer cell growth and apoptosis. Oncotarget. 2015;6:6499‐6510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ha SY, Kim JH, Yang JW, Kim J, Kim B, Park CK. The overexpression of CCAR1 in hepatocellular carcinoma associates with poor prognosis. Cancer Res Treat. 2016;48:1065‐1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Puliyappadamba VT, Wu W, Bevis D, et al. Antagonists of anaphase‐promoting complex (APC)‐2‐cell cycle and apoptosis regulatory protein (CARP)‐1 interaction are novel regulators of cell growth and apoptosis. J Biol Chem. 2011;286:38000‐38017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Li G, Wu X, Qian W, Cai H, Sun X, Zhang W. CCAR1 5’ UTR as a natural miRancer of miR‐1254 overrides tamoxifen resistance. Cell Res. 2016;26:655‐673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yang H, Wang Y, Cheryan VT, et al. Withaferin A inhibits the proteasome activity in mesothelioma in vitro and in vivo. PLoS One. 2012;7:e41214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bae JS, Park SH, Kim KM, et al. CK2α phosphorylates DBC1 and is involved in the progression of gastric carcinoma and predicts poor survival of gastric carcinoma patients. Int J Cancer. 2015;136:797‐809. [DOI] [PubMed] [Google Scholar]
- 28. Cha EJ, Noh SJ, Kwon KS, et al. Expression of DBC1 and SIRT1 is associated with poor prognosis of gastric carcinoma. Clin Cancer Res. 2009;15:4453‐4459. [DOI] [PubMed] [Google Scholar]
- 29. Huan Y, Wu D, Zhou D, Sun B, Li G. DBC1 promotes anoikis resistance of gastric cancer cells by regulating NF‐κB activity. Oncol Rep. 2015;34:843‐849. [DOI] [PubMed] [Google Scholar]
- 30. Kang Y, Jung WY, Lee H, Lee E, Kim A, Kim BH. Expression of SIRT1 and DBC1 in gastric adenocarcinoma. Korean J Pathol. 2012;46:523‐531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Noguchi A, Kikuchi K, Zheng H, et al. SIRT1 expression is associated with a poor prognosis, whereas DBC1 is associated with favorable outcomes in gastric cancer. Cancer Med. 2014;3:1553‐1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Reid JF, Gariboldi M, Sokolova V, et al. Integrative approach for prioritizing cancer genes in sporadic colon cancer. Genes Chromosomes Cancer. 2009;48:953‐962. [DOI] [PubMed] [Google Scholar]
- 33. Ye H, Pungpravat N, Huang BL, et al. Genomic assessments of the frequent loss of heterozygosity region on 8p21.3‐p22 in head and neck squamous cell carcinoma. Cancer Genet Cytogenet. 2007;176:100‐106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Won KY, Cho H, Kim GY, et al. High DBC1 (CCAR2) expression in gallbladder carcinoma is associated with favorable clinicopathological factors. Int J Clin Exp Pathol. 2015;8:11440‐11445. [PMC free article] [PubMed] [Google Scholar]
- 35. Yu XM, Liu Y, Jin T, et al. The expression of SIRT1 and DBC1 in laryngeal and hypopharyngeal carcinomas. PLoS One. 2013;8:e66975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pinho AV, Mawson A, Gill A, et al. Sirtuin 1 stimulates the proliferation and the expression of glycolysis genes in pancreatic neoplastic lesions. Oncotarget. 2016;7:74768‐74778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Qiang L, Kon N, Zhao W, et al. Hepatic SirT1‐dependent gain of function of stearoyl‐CoA desaturase‐1 conveys dysmetabolic and tumor progression functions. Cell Rep. 2015;11:1797‐1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ward JM, Mahler JF, Maronpot RR, Sundberg JP. Pathology of Genetically Engineered Mice. Ames, IA: Iowa State University Press; 2000:394. [Google Scholar]
- 39. Yuan J, Luo K, Liu T, Lou Z. Regulation of SIRT1 activity by genotoxic stress. Genes Dev. 2012;26:791‐796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zheng H, Yang L, Peng L, et al. hMOF acetylation of DBC1/CCAR2 prevents binding and inhibition of SirT1. Mol. Cell. Biol. 2013;33:4960‐4970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Magni M, Ruscica V, Buscemi G, et al. Chk2 and REGγ‐dependent DBC1 regulation in DNA damage induced apoptosis. Nucleic Acids Res. 2014;42:13150‐13160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zannini L, Buscemi G, Kim JE, Fontanella E, Delia D. DBC1 phosphorylation by ATM/ATR inhibits SIRT1 deacetylase in response to DNA damage. J Mol Cell Biol. 2012;4:294‐303. [DOI] [PubMed] [Google Scholar]
- 43. López‐Saavedra A, Gómez‐Cabello D, Domínguez‐Sánchez MS, et al. A genome‐wide screening uncovers the role of CCAR2 as an antagonist of DNA end resection. Nat Commun. 2016;7:12364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Magni M, Ruscica V, Restelli M, Fontanella E, Buscemi G, Zannini L. CCAR2/DBC1 is required for Chk2‐dependent KAP1 phosphorylation and repair of DNA damage. Oncotarget. 2015;6:17817‐17831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kim W, Kim JE. Deleted in breast cancer 1 (DBC1) deficiency results in apoptosis of breast cancer cells through impaired responses to UV‐induced DNA damage. Cancer Lett. 2013;333:180‐186. [DOI] [PubMed] [Google Scholar]
- 46. Chen R, Liu Y, Zhuang H, et al. Quantitative proteomics reveals that long non‐coding RNA MALAT1 interacts with DBC1 to regulate p53 acetylation. Nucleic Acids Res. 2017;45:9947‐9959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lee J, Adelmant G, Marto JA, Lee DH. Dephosphorylation of DBC1 by protein phosphatase 4 is important for p53‐mediated cellular functions. Mol Cells. 2015;38:697‐704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Park JH, Lee SW, Yang SW, et al. Modification of DBC1 by SUMO2/3 is crucial for p53‐mediated apoptosis in response to DNA damage. Nat. Commun. 2014;5:5483. [DOI] [PubMed] [Google Scholar]
- 49. Hubbard BP, Loh C, Gomes AP, et al. Carboxamide SIRT1 inhibitors block DBC1 binding via an acetylation‐independent mechanism. Cell Cycle. 2013;12:2233‐2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Chini CC, Escande C, Nin V, Chini EN. HDAC3 is negatively regulated by the nuclear protein DBC1. J Biol Chem. 2010;285:40830‐40837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kim HJ, Kim SH, Yu EJ, Seo WY, Kim JH. A positive role of DBC1 in PEA3‐mediated progression of estrogen receptor‐negative breast cancer. Oncogene. 2015;34:4500‐4508. [DOI] [PubMed] [Google Scholar]
- 52. Kim JR, Moon YJ, Kwon KS, et al. Expression of SIRT1 and DBC1 is associated with poor prognosis of soft tissue sarcomas. PLoS One. 2013;8:e74738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kim SH, Kim JH, Yu EJ, Lee KW, Park CK. The overexpression of DBC1 in esophageal squamous cell carcinoma correlates with poor prognosis. Histol Histopathol. 2012;27:49‐58. [DOI] [PubMed] [Google Scholar]
- 54. Lee H, Kim KR, Noh SJ, et al. Expression of DBC1 and SIRT1 is associated with poor prognosis for breast carcinoma. Hum Pathol. 2011;42:204‐213. [DOI] [PubMed] [Google Scholar]
- 55. Li C, Liao J, Wu S, Fan J, Peng Z, Wang Z. Overexpression of DBC1, correlated with poor prognosis, is a potential therapeutic target for hepatocellular carcinoma. Biochem Biophys Res Commun. 2017;494:511‐517. [DOI] [PubMed] [Google Scholar]
- 56. Noh SJ, Kang MJ, Kim KM, et al. Acetylation status of P53 and the expression of DBC1, SIRT1, and androgen receptor are associated with survival in clear cell renal cell carcinoma patients. Pathology. 2013;45:574‐580. [DOI] [PubMed] [Google Scholar]
- 57. Park HS, Bae JS, Noh SJ, et al. Expression of DBC1 and androgen receptor predict poor prognosis in diffuse large B cell lymphoma. Transl Oncol. 2013;6:370‐381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Wagle S, Park SH, Kim KM, et al. DBC1/CCAR2 is involved in the stabilization of androgen receptor and the progression of osteosarcoma. Sci Rep. 2015;5:13144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Zhang Y, Gu Y, Sha S, et al. DBC1 is over‐expressed and associated with poor prognosis in colorectal cancer. Int J Clin Oncol. 2014;19:106‐112. [DOI] [PubMed] [Google Scholar]
- 60. Cho D, Park H, Park SH, et al. The expression of DBC1/CCAR2 is associated with poor prognosis of ovarian carcinoma. J Ovarian Res. 2015;8:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Hornbeck PV, Zhang B, Murray B, Kornhauser JM, Latham V, Skrzypek E. PhosphoSitePlus, 2014: mutations, PTMs and recalibrations. Nucleic Acids Res. 2015;43:D512‐520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Best SA, Nwaobasi AN, Schmults CD, Ramsey MR. CCAR2 is required for proliferation and tumor maintenance in human squamous cell carcinoma. J Invest Dermatol. 2017;137:506‐512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Park SH, Riley P 4th, Frisch SM. Regulation of anoikis by deleted in breast cancer‐1 (DBC1) through NF‐κB. Apoptosis. 2013;18:949‐962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Restelli M, Magni M, Ruscica V, et al. A novel crosstalk between CCAR2 and AKT pathway in the regulation of cancer cell proliferation. Cell Death Dis. 2016;7:e2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Trauernicht AM, Kim SJ, Kim NH, Clarke R, Boyer TG. DBC‐1 mediates endocrine resistant breast cancer cell survival. Cell Cycle. 2010;9:1218‐1219. [DOI] [PubMed] [Google Scholar]
- 66. Kim W, Jeong JW, Kim JE. CCAR2 deficiency augments genotoxic stress‐induced apoptosis in the presence of melatonin in non‐small cell lung cancer cells. Tumour Biol. 2014;35:10919‐10929. [DOI] [PubMed] [Google Scholar]
- 67. Pangon L, Mladenova D, Watkins L, et al. MCC inhibits beta‐catenin transcriptional activity by sequestering DBC1 in the cytoplasm. Int J Cancer. 2015;136:55‐64. [DOI] [PubMed] [Google Scholar]
- 68. Rajendran P, Johnson GS, Li L, et al. CCAR2 acetylation establishes a BET/BRD9 acetyl switch in response to combined deacetylase and bromodomain inhibition. Cancer Res. 2019;79:918‐927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Rajendran P, Delage B, Dashwood WM, et al. Histone deacetylase turnover and recovery in sulforaphane‐treated colon cancer cells: competing actions of 14‐3‐3 and Pin1 in HDAC3/SMRT corepressor complex dissociation/reassembly. Mol Cancer. 2011;10:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Gao Y, Tang J, Chen W, et al. Inflammation negatively regulates FOXP3 and regulatory T‐cell function via DBC1. Proc Natl Acad Sci USA. 2015;112:E3246‐3254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Close P, East P, Dirac‐Svejstrup AB, et al. DBIRD complex integrates alternative mRNA splicing with RNA polymerase II transcript elongation. Nature. 2012;484:386‐389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Hiraike H, Wada‐Hiraike O, Nakagawa S, et al. Expression of DBC1 is associated with nuclear grade and HER2 expression in breast cancer. Exp Ther Med. 2011;2:1105‐1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All information is available in the public domain, including PubMed, TCGA, and the Human Protein Atlas (https://pubmed.ncbi.nlm.nih.gov/tcga; https://portal.gdc.cancer.gov/repository; https://www.proteinatlas.org/).


