Abstract
The efficiency of upfront consolidation with high‐dose chemotherapy/autologous stem‐cell transplantation (HDCT/ASCT) for newly diagnosed high‐risk diffuse large B‐cell lymphoma (DLBCL) may be influenced by induction chemotherapy. To select better induction chemotherapy regimens for HDCT/ASCT, a randomized phase II study was conducted in high‐risk DLBCL patients having an age‐adjusted International Prognostic Index (aaIPI) score of 2 or 3. As induction chemotherapy, 6 cycles of R‐CHOP‐14 (arm A) or 3 cycles of R‐CHOP‐14 followed by 3 cycles of CHASER (arm B) were planned, and patients who responded proceeded to HDCT with LEED and ASCT. The primary endpoint was 2‐y progression‐free survival (PFS), and the main secondary endpoints included overall survival, overall response rate, and adverse events (AEs). In total, 71 patients were enrolled. With a median follow‐up of 40.3 mo, 2‐y PFS in arms A and B were 68.6% (95% confidence interval [CI], 50.5%‐81.2%) and 66.7% (95% CI: 48.8%‐79.5%), respectively. Overall survival at 2 y in arms A and B was 74.3% (95% CI: 56.4%‐85.7%) and 83.3% (95% CI: 66.6%‐92.1%). Overall response rates were 82.9% in arm A and 69.4% in arm B. During induction chemotherapy, 45.7% and 75.0% of patients in arms A and B, respectively, had grade ≥ 3 non‐hematologic toxicities. One patient in arm A and 6 in arm B discontinued induction chemotherapy due to AEs. In conclusion, R‐CHOP‐14 showed higher 2‐y PFS and less toxicity compared with R‐CHOP‐14/CHASER in patients with high‐risk DLBCL, suggesting the former to be a more promising induction regimen for further investigations (UMIN‐CTR, UMIN000003823).
Keywords: autologous stem‐cell transplantation, diffuse large B‐cell lymphoma, high‐dose chemotherapy, induction chemotherapy, JCOG‐LSG
The efficiency of upfront consolidation with high‐dose chemotherapy/autologous stem‐cell transplantation (HDCT/ASCT) for newly diagnosed high‐risk diffuse large B‐cell lymphoma (DLBCL) may be influenced by induction chemotherapy. To select better induction chemotherapy regimens for HDCT/ASCT, a randomized phase II study was conducted in patients with high‐risk DLBCL who had an age‐adjusted International Prognostic Index (aaIPI) score of 2 or 3. R‐CHOP‐14 showed higher 2‐y PFS and less toxicity compared with R‐CHOP‐14/CHASER in patients with high‐risk DLBCL, suggesting the former to be a more promising induction regimen for further investigations.
1. INTRODUCTION
R‐CHOP is the standard therapy in patients with diffuse large B‐cell lymphoma (DLBCL), irrespective of the risk assessed using the International Prognostic Index (IPI), but approximately 40%‐50% of patients in higher risk groups are not cured. 1 , 2 The role of high‐dose chemotherapy (HDCT) and autologous stem‐cell transplantation (ASCT) has been investigated because HDCT followed by ASCT (HDCT/ASCT) was established by the PARMA trial as the standard salvage treatment strategy in relapsed and refractory DLBCL. 3 Therefore, HDCT in an upfront setting was investigated to improve the clinical outcome of newly diagnosed DLBCL. In the rituximab era, although 2 prospective randomized trials showed improvement in progression‐free survival (PFS), 4 , 5 improvement in overall survival (OS) could not be achieved in all trials including these 2 trials. 4 , 5 , 6 , 7 These results suggested that HDCT/ASCT is effective in the first progression of R‐CHOP therapy, although HDCT/ASCT as salvage setting may be applicable for approximately half of patient that have relapsed. An upfront HDCT/ASCT strategy has been expected to improve the prognosis of poor‐risk DLBCL, nevertheless its refinement remains necessary.
Two randomized trials in the pre‐rituximab era showed that a short course of an induction regimen followed by HDCT/ASCT failed to improve outcomes, suggesting that induction therapy plays a crucial role. 8 , 9 For the optimization of induction chemotherapy before HDCT/ASCT, we hypothesized that increased dose intensity (DI) of R‐CHOP or addition of non‐cross‐resistant chemotherapy might be effective. Among the several candidate regimens, we adopted a 2‐wk interval R‐CHOP therapy (R‐CHOP‐14) as DI regimens for standard R‐CHOP based on the results of a Japanese randomized phase II study of CHOP‐14 and dose‐escalated CHOP in aggressive non‐Hodgkin lymphoma (JCOG 9505), which concluded that CHOP‐14 was superior to dose‐escalated CHOP for PFS. 10 Moreover, in the JCOG 9809 study, which was a phase III trial comparing CHOP‐14 with CHOP‐21 subgroup analysis, indicated that the efficacy of CHOP‐14 was slightly greater than that of CHOP‐21 in terms of OS and PFS in younger patients although the difference of efficacy was not statistically significant. 11 The other regimen, CHASER, was adopted as a non‐cross‐resistant regimen to R‐CHOP, which was originally developed as the salvage regimen containing etoposide and cytarabine, and was effective in refractory and relapsed DLBCL. 12 , 13 We prepared these 2 induction regimens, R‐CHOP‐14 and R‐CHOP‐14, followed by CHASER to clarify their efficacy in combining with HDCT/ASCT.
To select a better induction regimen for HDCT/ASCT, the Lymphoma Study Group of Japan Clinical Oncology Group (JCOG‐LSG) conducted a randomized phase II selection design study, JCOG0908, in previously untreated patients with high‐intermediate (HI)‐risk or high (H)‐risk DLBCL on age‐adjusted International Prognostic Index (aaIPI). This study was registered with UMIN‐CTR, UMIN000003823.
2. MATERIALS AND METHODS
2.1. Trial information
The study protocol was approved by the Protocol Review Committee of JCOG and by the respective institutional review boards. Written informed consent was obtained from each patient before enrolment in accordance with the Declaration of Helsinki.
2.2. Eligibility criteria
The major eligibility criteria were as follows: previously untreated CD20‐positive DLBCL or primary mediastinal large B‐cell lymphoma based on World Health Organization (WHO) classification (2008) 14 of measurable lymphoma lesion(s); aaIPI HI or H 15 ; age 20‐65 y; Eastern Cooperative Oncology Group performance status (PS) of 0‐2; and Ann Arbor stage II bulky, III, IV according to the American Joint Committee on Cancer Manual 6th edition. 16
2.3. Randomization and masking
Patients were randomly assigned in a 1:1 ratio to the R‐CHOP‐14 arm (arm A) or R‐CHOP‐14/CHASER arm (arm B) at the JCOG Data Center, using a minimization method with biased‐coin assignment balancing on institute and aaIPI (HI vs H).
2.4. Treatment
Arm A consisted of 6 cycles of R‐CHOP‐14 (rituximab 375 mg/m2, cyclophosphamide 750 mg/m2, doxorubicin 50 mg/m2, vincristine 1.4 mg/m2 [maximum 2 mg/body], on day 1, and prednisone 100 mg on days 1‐5, granulocyte‐colony stimulating factor (G‐CSF) on days 8‐13, every 2 wk), and arm B had 3 cycles of R‐CHOP‐14 followed by 3 cycles of CHASER (rituximab 375 mg/m2 on day 1, cyclophosphamide 1200 mg/m2 on day 2, cytarabine 2000 mg/m2 on days 3‐4, dexamethasone 40 mg/body on days 2‐4 and etoposide 100 mg/m2 on days 2‐4, G‐CSF from day 8, every 3 wk). 13 , 17 From the 4th cycle, peripheral blood stem cells (PBSCs) were harvested over 2 × 106 CD34‐positive cells/kg. After induction therapy, patients with complete response (CR) or partial response (PR) proceeded to HDCT with LEED (cyclophosphamide 60 mg/kg on days −4 and −3, etoposide 500 mg/m2 on days −4 to −2, melphalan 130 mg/m2 on day −1 and dexamethasone 40 mg/body on days −4 to −1) and ASCT on day 0. Radiation therapy was performed on a solitary mass after ASCT.
2.5. Response assessment and endpoint
Responses were assessed by restaging at the end of the induction therapy, HDCT, and radiotherapy, if applied, according to the Revised Response Criteria for Malignant Lymphoma 2007. 18 AEs were graded according to the Common Terminology Criteria for Adverse Events version 3.0. Pathological diagnosis was centrally reviewed by 3 hematopathologists.
The primary endpoint was 2‐y PFS which defined time from registration until the following events: disease progression, relapse, or death from any cause. It was censored at the final confirmation date of PFS. The secondary endpoints were 5‐y PFS; 2‐ and 5‐y OS calculated from the date of registration until death from any cause or censored at the last follow‐up date; CR rate, overall response rate (ORR), the proportion of AEs, and incidence of secondary neoplasms.
2.6. Statistical consideration
The sample size was determined as 70, which had at least 80% probability of selecting the better arm with an expected 2‐y PFS of 65% in the worse arm and 75% in the better arm (Simon's selection design). 19
3. RESULTS
3.1. Patients characteristics
From June 2010 to February 2015, 71 patients were enrolled from 25 institutes and all randomly assigned as follows: 35 to arm A and 36 to arm B (Figure 1). The patients characteristics are summarized in Table 1, and all factors were balanced between both arms. On the Central Pathological Review, 3 patients with follicular lymphoma (FLG3A), mantle cell lymphoma in arm A, or B‐cell lymphoblastic lymphoma (B‐LBL) in arm B were deemed ineligible. These patients were included in the following analyses.
FIGURE 1.
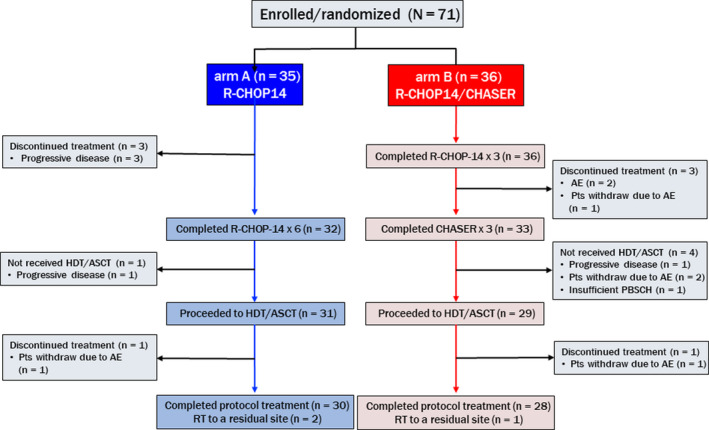
Flow diagram of randomized patients in the JCOG0908 study comparing R‐CHOP‐14 (arm A) with R‐CHOP‐14/CHASER (arm B). AE, adverse event; ASCT, autologous stem‐cell transplantation; CHASER, cyclophosphamide, cytarabine, etoposide, dexamethasone, and rituximab; HDCT, high‐dose chemotherapy; Pts, patients; R‐CHOP‐14, rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone with 14‐d interval; RT, radiation therapy
TABLE 1.
Patients characteristics
| Arm A (n = 35) | Arm B (n = 36) | |
|---|---|---|
| Median age (range) | 57 (23‐64) | 55.5 (30‐65) |
| Male/female | 18/17 | 18/18 |
| aaIPI | ||
| H‐I | 25 (71.4%) | 28 (77.8%) |
| H | 10 (28.6%) | 8 (22.2%) |
| ECOG PS | ||
| 0/1 | 24 (68.6%) | 25 (69.4%) |
| ≧2 | 11 (31.4%) | 11 (30.6%) |
| LDH > normal range | 35 (100%) | 34 (94.4%) |
| Ann Arbor stage | ||
| I | 0 | 0 |
| II | 1 (2.9%) | 1 (2.8%) |
| III | 8 (22.9%) | 14 (38.9%) |
| IV | 26 (74.3%) | 21 (58.3%) |
| Age | ||
| <61 | 24 (68.6%) | 31 (86.1%) |
| ≧61 | 11 (31.4%) | 5 (13.9%) |
| Number of extranodal sites | ||
| 0‐1 | 17 (48.6%) | 20 (55.6%) |
| 2 or more | 18 (51.4%) | 16 (44.4%) |
| B‐symptom(+) | 17 (48.6%) | 13 (36.1%) |
| Tumor mass | ||
| <5 cm | 7 (20.0%) | 10 (27.8%) |
| ≧5 cm | 17 (48.6%) | 16 (44.4%) |
| ≧10 cm | 11 (31.4%) | 10 (27.8%) |
| Mediastinal mass ≧1/3 thorax | 6 (17.1%) | 1 (2.8%) |
Abbreviations: aaIPI, age‐adjusted International Prognostic Index; ECOG PS, Eastern Cooperative Oncology Group performance status; LDH, lactate dehydrogenase.
3.2. Treatment courses
During the induction chemotherapy, treatment was discontinued in 4 out of 35 patients in arm A because of disease progression (Figure 1). In arm B, a total of 7 out of 36 patients could not proceed to HDCT due to disease progression in 1 patient, AEs in 2 patients, patient refusal due to AE in 3 patients and insufficient stem‐cell harvest in 1 patient. In total, 31 patients in arm A and 29 in arm B proceeded to HDCT/ASCT. During HDCT, 1 patient in each arm discontinued treatment because of withdrawal due to AEs. Finally, 30 patients in arm A and 28 in arm B completed the protocol treatments. Radiation therapy to a residual site was given to 2 patients in arm A and 1 patient in arm B after HDCT/ASCT.
PBSC harvest was carried out for 33 out of 35 patients in arm A and all 36 patients in arm B. The median numbers of harvested PBSCs were 5.4 (2.10‐25.70) × 106/kg cells in arm A and 10.3 (0.58‐78.00) × 106/kg cells in arm B. All patients in arm A had successful PBSC collections, but 2 out of 36 patients in arm B failed to obtain 2 × 106/kg or more CD34‐positive PBSCs. (Table 2).
TABLE 2.
Collection of CD34‐positive cells
| Number of CD34‐positive cells | Arm A (n = 35) | Arm B (n = 36) |
|---|---|---|
| Median (range) (×106/kg) | 5.4 (2.1‐25.7) | 10.3 (0.6‐78.0) |
| ≧2 × 106/kg | 33 (94.3%) | 34 (94.4%) |
| <2 × 106/kg | 0 | 2 (5.6%) |
| Not performed | 2 (5.7%) | 0 |
3.3. Efficacy
With a median follow‐up of 40.3 mo (range: 1.0‐75.9) among all registered patients, 2‐y PFS as the primary endpoint in arms A and B were estimated to be 68.6% (95% confidence interval [CI]: 50.5%‐81.2%) and 66.7% (95% CI: 48.8%‐79.5%), respectively (Figure 2). PFS at 5‐y was 68.6% (95% CI: 50.5%‐81.2%) in arm A and 62.7% (95% CI: 44.3%‐76.6%) in arm B.
FIGURE 2.
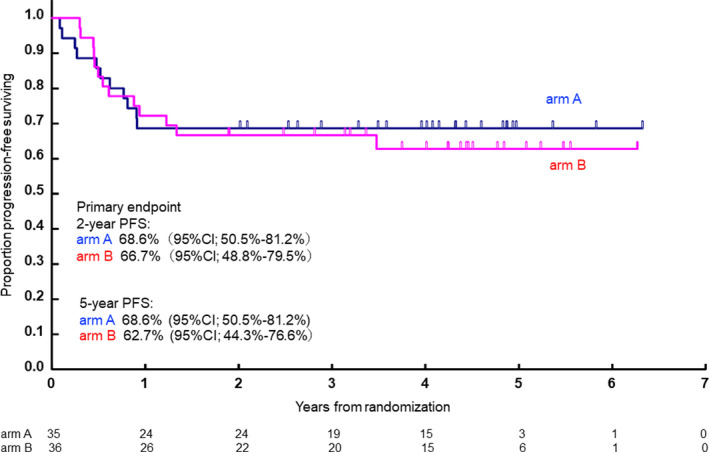
Progression‐free survival of R‐CHOP‐14 (arm A) and R‐CHOP‐14/CHASER (arm B)
The 2‐y OS was estimated to be 74.3% (95% CI: 56.4%‐85.7%) in arm A and 83.3% (95% CI: 66.6%‐92.1%) in arm B. OS at 5 y was 66.9% (95% CI: 44.3%‐82.0%) in arm A and 79.5% (95% CI: 61.5%‐89.8%) in arm B (Figure 3).
FIGURE 3.
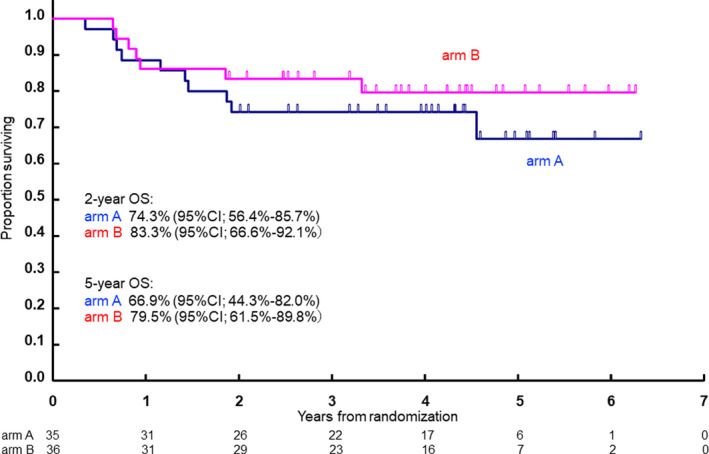
Overall survival of R‐CHOP‐14 (arm A) and R‐CHOP‐14/CHASER (arm B)
CR rate/ORR after induction therapy of all registered patients of arms A and B were 62.9%/88.6% and 61.1%/94.4%, respectively. After HDCT and radiation therapy, CR rate/ORR in arm A reached 68.6%/82.9%, whereas those of arm B decreased to 63.9%/69.4% (Table 3).
TABLE 3.
Response by treatment group
| Arm A (n = 35) | Arm B (n = 36) | |
|---|---|---|
| After induction | ||
| OR, n (%, 95% CI) | 31 (88.6, 73.3‐96.8) | 34 (94.4, 81.3‐99.3) |
| CR, n (%, 95% CI) | 22 (62.9, 44.9‐78.5) | 22 (61.1, 43.5‐76.9) |
| After HDCT or RT | ||
| OR, n (%, 95% CI) | 29 (82.9, 66.4‐93.4) | 25 (69.4, 51.9‐83.7) |
| CR, n (%, 95% CI) | 24 (68.6, 50.7‐83.2) | 23 (63.9, 46.2‐79.2) |
Abbreviations: CI, confidence interval; CR, complete response; HDCT, high‐dose chemotherapy; OR, overall response; RT, radiation therapy.
3.4. Exploratory analysis
In a subgroup analysis based on each aaIPI risk group, there was no significant difference in 2‐y PFS and 2‐y OS between the study arms (Figure 4).
FIGURE 4.
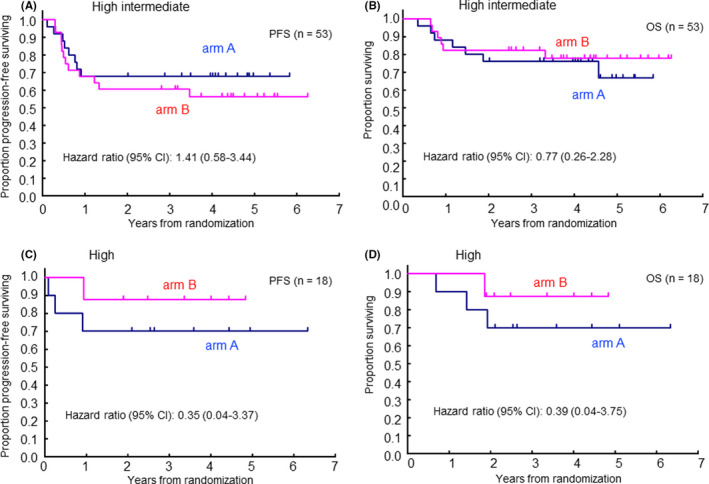
Progression‐free survival (PFS) and overall survival (OS) by each aa‐IPI risk group (A) PFS in high‐intermediate risk, (B) OS in high‐intermediate risk, (C) PFS in high risk, (D) OS in high risk
Univariate analysis revealed no significant difference in gender, B‐symptom, PS, or tumor size of ≤10 cm. Regarding the clinical stage, arm B showed favorable OS in patients with stage II to III disease (PFS, HR 0.37:95% CI 0.10‐1.37; OS, HR 0.10:95% CI 0.01‐0.84), but no superiority of arm B in stage IV. In tumor size, arm B showed a favorable tendency in PFS and OS in patients with greater than 10 cm (PFS, HR 0.27:95% CI 0.05‐1.35; OS, HR 0.20:95% CI 0.02‐1.71). (Table S1).
3.5. Safety
In the induction treatments, arm B showed higher myelosuppression: 100% of grade 4 neutropenia, 94% of grade 3‐4 anemia, and 100% of grade 3‐4 thrombocytopenia. Febrile neutropenia was also more frequent in arm B (55.6%) than arm A (17.1%). Overall grade 3‐4 non‐hematological toxicity was not frequent in either arm. Grade 4 AEs were observed in 3 patients: hypokalemia and hyperamylasemia in patients in arm A and aspartate aminotransferase (AST) elevation due to cholecystitis in a patient in arm B. (Table 4) However, in arm B, 6 out of 36 patients discontinued the protocol therapy related to AEs, including myelosuppression, cystitis, pneumonia, hemolysis, retinopathy, and eosinophilia of unknown cause, and all events occurred during the CHASER regimen.
TABLE 4.
Grade 3 and 4 adverse events a by treatment arm
| Induction therapy | Arm A (n = 35) | Arm B (n = 36) | ||
|---|---|---|---|---|
| Toxicity | Grade 3 | Grade 4 | Grade 3 | Grade 4 |
| Leukopenia | 10 (28.6%) | 14 (40%) | 0 | 36 (100%) |
| Anemia | 8 (22.9%) | 0 | 23 (63.9%) | 11 (30.6%) |
| Thrombopenia | 0 | 0 | 5 (13.9%) | 31 (86.1%) |
| Neutropenia | 8 (22.9%) | 15 (42.9%) | 0 | 36 (100%) |
| Hypoalbuminemia | 1 (2.9%) | — | 0 | — |
| AST | 1 (2.9%) | 0 | 2 (5.6%) | 1 (2.8%) |
| ALT | 4 (11.4%) | 0 | 6 (16.7%) | 0 |
| GGT | 4 (11.4%) | 0 | 5 (13.9%) | 0 |
| Hyponatremia | 2 (5.7%) | 0 | 2 (5.6%) | 0 |
| Hyperkalemia | 1 (2.9%) | 0 | 0 | 0 |
| Hypokalemia | 3 (8.6%) | 1 (2.9%) | 6 (16.7%) | 0 |
| Hyperglycemia | 0 | 0 | 1 (2.8%) | 0 |
| Amylase | 0 | 1 (2.9%) | 0 | 0 |
| Febrile neutropenia | 6 (17.1%) | 0 | 20 (55.6%) | 0 |
| HDCT | Arm A (n = 31) | Arm B (n = 29) | ||
|---|---|---|---|---|
| Toxicity | Grade 3 | Grade 4 | Grade 3 | Grade 4 |
| Leukopenia | 0 | 31 (100%) | 0 | 29 (100%) |
| Anemia | 10 (32.3%) | 0 | 21 (72.4%) | 0 |
| Thrombopenia | 2 (6.5%) | 29 (93.5%) | 2 (6.9%) | 27 (93.1%) |
| Neutropenia | 3 (9.7%) | 26 (83.9%) | 5 (17.2%) | 23 (79.3%) |
| AST | 0 | 0 | 1 (3.4%) | 0 |
| ALT | 2 (6.5%) | 0 | 5 (17.2%) | 0 |
| GGT | 2 (6.5%) | 0 | 2 (6.9%) | 0 |
| Hyponatremia | 5 (16.1%) | 0 | 3 (10.3%) | 0 |
| Hyperkalemia | 0 | 0 | 0 | 0 |
| Hypokalemia | 4 (12.9%) | 0 | 2 (6.9%) | 0 |
| Left ventricular systolic dysfunction | 1 (3.2%) | 0 | 0 | 0 |
| Febrile neutropenia | 20 (64.5%) | 0 | 13 (44.8%) | 0 |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; GGT, γ‐glutamyl‐transferase; HDCT, high‐dose chemotherapy
Adverse events were categorized and graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE), version 3.0.
In HDCT/ASCT, no grade 4 non‐hematological toxicity was observed, and grade 3 gastrointestinal toxicities were rare. One patient in arm A discontinued the HDCT because of grade 3 heart failure. (Table 4) There were no deaths related to the protocol treatment during the study period. Secondary neoplasms were observed in 3 patients including 1 patient who had prostatic cancer and 1 patient with rectal cancer in arm A, and 1 patient with lung cancer in arm B.
4. DISCUSSION
In this study, we conducted a randomized phase II trial to select a better induction regimen for HDCT/ASCT in patients with previously untreated DLBCL with the HI or H risk of aaIPI. Although both arms showed almost comparable PFS, ORR, CR rate, and OS, arm B showed higher toxicities, especially hematological AEs. From its higher 2‐y PFS and lower toxicity, R‐CHOP‐14 was considered to be a more promising induction regimen in patients with previously untreated DLBCL with the HI or H risk of aaIPI.
Comparing the results of 4 prospective randomized trials in the rituximab era, which demonstrated 69%‐75% 2‐y PFS in patients with newly diagnosed DLBCL who received HDCT/ASCT. 4 , 5 , 6 , 7 , 20 the present study showed comparable PFS in both arms. Considering that R‐CHOP gives 50% 4‐y PFS in patients with high risk in the revised R‐IPI, which is comparable with HI and H risk in the aaIPI, 1 upfront HDCT/ASCT would be expected to improve PFS compared with R‐CHOP alone. However, only 2 of the 4 trials showed superiority to no HDCT/ASCT. 4 , 5 , 6 , 7 Furthermore, OS, 2‐y OS, or 3‐y OS in the upfront HDCT/ASCT setting in the rituximab era were 74%‐82%, none of which showed superiority to rituximab plus CHOP compared with chemotherapy. 4 , 5 , 6 , 7 The S9704 study, whose subjects had aggressive lymphoma, compared 6 cycles of (R‐)CHOP with HDCT/ASCT to 8 cycles of (R‐)CHOP, excluding patients below PR with induction therapy. The HDCT/ASCT group was superior to the (R‐)CHOP group for 2‐y PFS (69% vs 55% [HR, 0.58; 95% CI: 0.40‐0.85]) and equivalent for 2‐y OS (74% vs 71% [HR, 0.79; 95% CI: 0.52‐1.22]). 4 In subgroup analysis based on aaIPI, the high‐risk patients showed favorable 2‐y OS in the HDCT/ASCT group. 4 High‐risk patients in our study also showed a tendency for favorable 2‐y PFS and 2‐y OS in arm B that may be an effect of CHASER: non‐cross‐resistant induction chemotherapy.
An Italian group compared 2 different dose level of induction immune‐chemotherapy, R‐CHOP‐14 and more intensive R‐MegaCHOP‐14, with or without HDCT/ASCT in their DLCL04 study. 5 The study resulted in superior 2‐y failure‐free survival and equivalent OS in HDCT/ASCT and no difference in efficacy was demonstrated between these 2 induction regimens plus HDCT/ASCT. These results indicated the efficacy of R‐CHOP‐14 as an induction therapy of HDCT/ASCT. In our study, arm A (6 cycles of R‐CHOP‐14) showed higher PFS than arm B although the difference was marginal. Arm A showed 14% higher ORR at the end of entire therapy course than arm B, nevertheless ORR after induction in both arms were almost same. We assumed that lower ORR of arm B was due to 19.4% of patients being non‐evaluable for the response because they did not receive HDCT/ASCT.
To proceed to HDCT/ASCT, it is important to achieve CR or PR using induction chemotherapy prior to HDCT/ASCT. In this study, 88.6% (31/35) of patients in arm A and 80.5% (29/36) of patients in arm B received HDCT/ASCT. In particular, high ORR of 94.4% in the R‐CHOP‐14/CHASER arm B induction was notable. Four patients in arm A did not proceed to HDCT/ASCT due to inadequate responses whereas, in arm B, the reasons were 1 patient with progressive disease, 5 patients with toxicities, and 1 patient with insufficient stem‐cell collection. Thus, R‐CHOP‐14/CHASER seemed to be a more potent regimen in terms of efficacy but was more toxic.
In the exploratory subgroup analyses of IPI, HI risk patients had a tendency for better PFS in arm A and identical OS, but H risk patients revealed tendencies for better PFS and OS in arm B. Among the univariate analyses of various parameters, only a bulky tumor above 10 cm diameter had a favorable tendency in arm B. This finding suggested that a more intensive induction regimen might be needed to control poor‐prognosis patients. In addition, the subgroup analysis of stage II to III patients in OS showed statistically favorable in arm B, which may contribute to a tendency of superiority of OS curve in arm B in spite of not statistical significance.
In terms of toxicities, both induction regimens showed manageable profiles. More patients with grade 3 and 4 hematologic toxicities, especially neutropenia (arm A vs arm B; 65.7% vs 100%), thrombocytopenia (0% vs 100%), and febrile neutropenia (17.1% vs 55.6%) were observed in arm B. These differences would come from higher doses of cyclophosphamide and high‐dose cytarabine, which the CHASER regimen contains. Secondary neoplasms were observed in 3 patients: 1 with prostatic cancer and 1 with rectal cancer in arm A and 1 with lung cancer in the arm B. The frequency of secondary neoplasms in previous studies including HDCT/ASCT in the rituximab era was 1%‐3.6%. 5 , 6 , 7 The proportion in the present study was similar and further observation is necessary.
In this study, R‐CHOP‐14 showed higher 2‐y PFS than R‐CHOP‐14/CHASER, however it was not confirmed that R‐CHOP‐14 induction regimen was superior to R‐CHOP‐14/CHASER because this study was not a phase III trial. Although in this randomized phase II study, the assumption for sample size calculation was a 10% difference between the better and worse arms (Simon's selection design), the observed PFS difference was only 1.9% (68.6% vs 66.7%). HDCT/ASCT upfront consolidation may be beneficial for patients with DLBCL with high‐risk disease. The 4‐y PFS in such patients ranged from 64% to 78% after treatment with a rituximab‐based induction regimen, 4 , 21 , 22 , 23 , 24 , 25 , 26 comparing favorably with the 50% PFS after treatment with R‐CHOP alone. 1 , 27 Although this finding needs to be confirmed prospectively, carrying out such a trial will be difficult because of the small fraction of patients with DLBCL who presented with high risk.
In conclusion, both R‐CHOP‐14 and R‐CHOP‐14/CHASER regimens as an induction prior to consolidative HDCT with LEED and ASCT in patients aged 65 y or less with aaIPI HI or H newly diagnosed DLBCL demonstrated reasonable response rates with durable PFS and OS. From the higher 2‐y PFS and less toxicity, R‐CHOP‐14 may be a more promising induction regimen for further investigations especially in patients with high‐risk DLBCL.
ETHICAL CONSIDERATIONS
This study protocol was approved by constituted Ethics Committee of all the participating institutions. Participating institutions were as the following: Aichi Cancer Center, Nagoya, Japan; National Cancer Center Hospital, Tokyo, Japan; Nagasaki University Hospital, Nagasaki, Japan; Japanese Red Cross Nagoya Daini Hospital, Nagoya, Japan; Nagoya University Graduate School of Medicine, Nagoya, Japan; Sapporo Hokuyu Hospital, Sapporo, Japan; Tohoku University Hospital, Sendai, Japan; Saitama Cancer Center, Ina‐cho, Japan; National Cancer Center East, Kashiwa, Japan; Chiba Cancer Center, Chiba, Japan; NTT Medical Center, Tokyo, Japan; Hyogo Cancer Center, Akashi, Japan; National Hospital Organization Kyushu Cancer Center, Fukuoka, Japan; National Hospital Organization Nagasaki Medical Center, Ohmura, Japan; National Hospital Organization Kumamoto Medical Center, Kumamoto, Japan; National Hospital Organization Hokkaido Cancer Center, Sapporo, Japan; Saitama Medical Center, Saitama Medical University, Kawagoe, Japan; National Hospital Organization Nagoya Medical Center, Nagoya, Japan; Nagoya City University Graduate School of Medical Sciences, Nagoya, Japan; Kyoto Prefectural University of Medicine, Kyoto, Japan; Ehime University Hospital, Toon, Japan; Fukuoka University Hospital, Fukuoka, Japan; Saga University, Saga, Japan; Kumamoto University School of Medicine, Kumamoto Japan; University of the Ryukyus Hospital, Nishihara, Japan
DISCLOSURE
Yoshitoyo Kagami has nothing to disclose; Kazuhito Yamamoto reports grants from the Ministry of Health, Labor and Welfare, grants from the National Cancer Center, during the conduct of the study; grants from AbbVie, grants from AstraZeneca, grants from Bayer, grants from Celgene, grants and personal fees from Chugai, grants and personal fees from Eisai, grants from Incyte/IQVIA, grants and personal fees from Mundipharma, grants from Nippon Shinyaku, grants from Novartis, grants from Solasia Pharma, grants from SymBio, grants from Yakult, grants from Zenyaku, personal fees from HUYA/IQVIA, personal fees from Takeda; Taro Shibata has nothing to disclose; Kensei Tobinai reports personal fees from Eisai, grants and personal fees from Takeda, personal and grants from Mundipharma, personal fees from HUYA Bioscience International, personal and grants from Kyowa Kirin, personal and grants from Celgene, personal and grants from Chugai, personal and grants from Ono, personal fees from Yakult, personal fees from Daiichi Sankyo, personal fees from Bristol‐Myers Squibb, personal fees from Meiji Seika Kaisha, personal fees from Solasia, personal fees from Verastem, personal fees from Zenyaku Kogyo, grants from Janssen, grants from Eisai, grants from Takeda, grants from AbbVie, outside the submitted work; Yoshitaka Imaizumi has nothing to disclose; Toshiki Uchida reports personal fees from Pfizer outside the submitted work; Kazuyuki Shimada reports grants from Celgene, grants from Otsuka, grants from MSD, grants from Kyowa Kirin outside the submitted work; Koichiro Minauchi has nothing to disclose; Noriko Fukuhara reports personal fees and grants from Chugai, personal fees from Kyowa Kirin, grants from Eisai, grants from Ono, grants from AbbVie, grants from Bayer, grants from Soleisia, grants from Gilead sciences, grants from Incyte corporation outside the submitted work; Hirofumi Kobayashi has nothing to disclose; Nobuhiko Yamauchi reports personal fees from Takeda, grants from Amgen, grants from Daiichi Sankyo, grants from Celgene outside the submitted work; Hideki Tsujimura has nothing to disclose; Akira Hangaishi has nothing to disclose; Ryo Tominaga has nothing to disclose; Youko Suehiro reports grants from Chugai, grants from Novartis, grants from Bayer, grants from Eisai, grants from Ono, grants from Otsuka, grants from Pfizer, grants from Amgen, grants from Daiichi Sankyo, grants from Biopharma, grants from Solasia, grants from Celgene, grants from Takeda, outside the submitted work; Shinichiro Yoshida has nothing to disclose; Yoshiko Inoue has nothing to disclose; Sachiko Suzuki has nothing to disclose; Michihide Tokuhira has nothing to disclose; Shigeru Kusumoto reports grants and personal fees from Chugai; Junya Kuroda reports grants from Kyowa Kirin, grants from Chugai, outside the submitted work; Yoshihiro Yakushijin has nothing to disclose; Yasushi Takamatsu reports personal fee and annual scholarship endowment from Celgene, personal fee and annual scholarship endowment from Takeda, personal fee and annual scholarship endowment from Ono, personal fees from Janssen, annual scholarship endowment from Chugai, annual scholarship endowment from TAIHO, annual scholarship endowment from Astellas, annual scholarship endowment from Kyowa Hakko Kirin, endowed chair or accepted a researcher from Takeda, Ono, Beckman Coulter, outside the submitted work; Yasushi Kubota has nothing to disclose; Kisato Nosaka reports personal fees from Celgene outside the submitted work; Satoko Morishima has nothing to disclose; Shigeo Nakamura has nothing to disclose; Michinori Ogura reports personal fees from Meiji Seika outside the submitted work; Dai Maruyama reports personal fees and grants from Takeda, personal fees and grants from Celgene, personal fees and grants from Mundipharma, personal fees from Eisai, personal fees and grants from Janssen, personal fees from Chugai, personal fees from Kyowa Kirin, grants from Ono, grants from MSD, grants from Novartis, outside the submitted work; Tomomitsu Hotta scientific advisor from SymBio, outside the submitted work; Yasuo Morishima has nothing to disclose; Kunihiro Tsukasaki reports annual scholarship endowment from Chugai, outside the submitted work; Hirokazu Nagai reports grants from Bayer, grants from AstlaZeneca, grants from Zenyaku, personal fees and grants from Takeda, grants from Mundipharma, grants from SymBio, personal fees and grants from Celgene, personal fees, grants and annual scholarship endowment from, personal fees from Eisai, outside the submitted work.
Supporting information
Table S1
ACKNOWLEDGMENTS
The authors are grateful to pathologists (Dr. Yoshihiro Matsuno, Dr. Kengo Takeuchi) for their support in the Central Pathological Review and the members of the JCOG Data Center and JCOG Operations Office (Dr. Tomonori Mizutani, Dr. Tomoko Kataoka, Mr. Gakuto Ogawa Dr. Tomohiro Kadota, Dr. Yuya Sato, Mr. Junki Mizusawa, and Dr. Kenichi Miyamoto), data management (Ms Yuko Watanabe), and oversight of the study management (Dr. Haruhiko Fukuda).
Kagami Y, Yamamoto K, Shibata T, et al. R‐CHOP‐14 versus R‐CHOP‐14/CHASER for upfront autologous transplantation in diffuse large B‐cell lymphoma: JCOG0908 study. Cancer Sci. 2020;111:3770–3779. 10.1111/cas.14604
Yoshitoyo Kagami and Kazuhito Yamamoto contributed equally to this work.
This study was presented in part at the 59th Annual Meeting and Exposition of the American Society of Hematology on December 11, 2017, in Atlanta, GA, USA.
Funding information
This work was supported in part by the National Cancer Center Research and Development Fund (grant numbers 23‐A‐16, 23‐A‐17, 26‐A‐4, 29‐A‐39); and by the Ministry of Health, Labor and Welfare Grants‐in‐Aid for Cancer Research (grant numbers 16‐6, 20S‐1, 20S‐6, 23‐A‐16, 23‐A‐17), and Health and Labor Sciences Research Grants for Clinical Cancer Research (grant numbers 19‐27 and 22‐29).
REFERENCES
- 1. Sehn LH, Berry B, Chhanabhai M, et al. The revised International Prognostic Index (R‐IPI) is a better predictor of outcome than the standard IPI for patients with diffuse large B‐cell lymphoma treated with R‐CHOP. Blood. 2007;109:1857 1861. [DOI] [PubMed] [Google Scholar]
- 2. Sehn LH, Donaldson J, Chhanabhai M, et al. Introduction of combined CHOP plus rituximab therapy dramatically improved outcome of diffuse large B‐cell lymphoma in British Columbia. J Clin Oncol. 2005;23:5027‐5033. [DOI] [PubMed] [Google Scholar]
- 3. Philip T, Guglielmi C, Hagenbeek A, et al. Autologous bone marrow transplantation as compared with salvage chemotherapy in relapses of chemotherapy‐sensitive non‐Hodgkin's lymphoma. N Engl J Med. 1995;333:1540‐1545. [DOI] [PubMed] [Google Scholar]
- 4. Stiff PJ, Unger JM, Cook JR, et al. Autologous transplantation as consolidation for aggressive non‐Hodgkin's lymphoma. N Engl J Med. 2013;369:1681‐1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chiappella A, Martelli M, Angelucci E, et al. Rituximab‐dose‐dense chemotherapy with or without high‐dose chemotherapy plus autologous stem‐cell transplantation in high‐risk diffuse large B‐cell lymphoma (DLCL04): final results of a multicentre, open‐label, randomised, controlled, phase 3 study. Lancet Oncol. 2017;18:1076‐1088. [DOI] [PubMed] [Google Scholar]
- 6. Cortelazzo S, Tarella C, Gianni AM, et al. Randomized trial comparing R‐CHOP versus high‐dose sequential chemotherapy in high‐risk patients with diffuse large B‐Cell lymphomas. J Clin Oncol. 2016;34:4015‐4022. [DOI] [PubMed] [Google Scholar]
- 7. Schmitz N, Nickelsen M, Ziepert M, et al. Conventional chemotherapy (CHOEP‐14) with rituximab or high‐dose chemotherapy (MegaCHOEP) with rituximab for young, high‐risk patients with aggressive B‐cell lymphoma: an open‐label, randomised, phase 3 trial (DSHNHL 2002–1). Lancet Oncol. 2012;13:1250‐1259. [DOI] [PubMed] [Google Scholar]
- 8. Gisselbrecht C, Lepage E, Molina T, et al. Shortened first‐line high‐dose chemotherapy for patients with poor‐prognosis aggressive lymphoma. J Clin Oncol. 2002;20:2472‐2479. [DOI] [PubMed] [Google Scholar]
- 9. Verdonck LF, van Putten WLJ, Hagenbeek A, et al. Comparison of CHOP chemotherapy with autologous bone marrow transplantation for slowly responding patients with aggressive non‐Hodgkin's lymphoma. N Engl J Med. 1995;332:1045‐1051. [DOI] [PubMed] [Google Scholar]
- 10. Itoh K, Ohtsu T, Fukuda H, et al. Randomized phase II study of biweekly CHOP and dose‐escalated CHOP with prophylactic use of lenograstim (glycosylated G‐CSF) in aggressive non‐Hodgkin's lymphoma: Japan Clinical Oncology Group Study 9505. Ann Oncol. 2002;13:1347‐1355. [DOI] [PubMed] [Google Scholar]
- 11. Ohmachi K, Tobinai K, Kobayashi Y, et al. Phase III trial of CHOP‐21 versus CHOP‐14 for aggressive non‐Hodgkin's lymphoma: final results of the Japan Clinical Oncology Group Study, JCOG 9809. Ann Oncol. 2011;22:1382‐1391. [DOI] [PubMed] [Google Scholar]
- 12. Ogura M, Kagami Y, Taji H, et al. Pilot phase I/II study of new salvage therapy (CHASE) for refractory or relapsed malignant lymphoma. Int J Hematol. 2003;77:503‐511. [DOI] [PubMed] [Google Scholar]
- 13. Oki Y, Ogura M, Kato H, et al. Phase II study of a salvage regimen using cyclophosphamide, high‐dose cytarabine, dexamethasone, etoposide, and rituximab in patients with relapsed or refractory B‐cell non‐Hodgkin's lymphoma. Cancer Sci. 2008;99:179‐184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Stein H, Warnke RA, Chan WC, et al. Diffuse large B‐cell lymphoma, not otherwise specified In: Swerdlow SH, Campo E, Harris NL, eds. WHO classification of tumours of haematopoietic and lymphoid tissues. Lyon, France: IARC Press; 2008:233‐237. [Google Scholar]
- 15. Project IN‐HsLPF . A predictive model for aggressive non‐Hodgkin's lymphoma. N Engl J Med. 1993;329:987‐994. [DOI] [PubMed] [Google Scholar]
- 16. Armitage JO, Cabanillas F, Evans I, et al. Lymphoid Neoplasms In: Greene FL, Page DL, Fleming ID, et al., AJCC Cancer Staging Manual, Sixth Edition. 6th ed. Chicago, IL: Springer; 2002;391‐406. [Google Scholar]
- 17. Ogura M, Yamamoto K, Morishima Y, et al. R‐High‐CHOP/CHASER/LEED with autologous stem cell transplantation in newly diagnosed mantle cell lymphoma: JCOG0406 STUDY. Cancer Sci. 2018;109:2830‐2840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cheson BD, Pfistner B, Juweid ME, et al. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25:579‐586. [DOI] [PubMed] [Google Scholar]
- 19. Simon R, Wittes RE, Ellenberg SS. Randomized phase II clinical trials. Cancer Treat Rep. 1985;69:1375‐1381. [PubMed] [Google Scholar]
- 20. Pfreundschuh M, German High‐Grade Non‐Hodgkin Lymphoma Study G . High‐dose chemotherapy and autologous stem‐cell transplantation for DLBCL in the rituximab era. Lancet Oncol. 2017;18:989‐991. [DOI] [PubMed] [Google Scholar]
- 21. Dilhuydy M‐S, Lamy T, Foussard C, et al. Front‐line high‐dose chemotherapy with rituximab showed excellent long‐term survival in adults with aggressive large B‐cell lymphoma: final results of a Phase II GOELAMS Study. Biol Blood Marrow Transplant. 2010;16:672‐677. [DOI] [PubMed] [Google Scholar]
- 22. Fitoussi O, Belhadj K, Mounier N, et al. Survival impact of rituximab combined with ACVBP and upfront consolidation autotransplantation in high‐risk diffuse large B‐cell lymphoma for GELA. Haematologica. 2011;96:1136‐1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Glass B, Ziepert M, Reiser M, et al. High‐dose therapy followed by autologous stem‐cell transplantation with and without rituximab for primary treatment of high‐risk diffuse large B‐cell lymphoma. Ann Oncol. 2010;21:2255‐2261. [DOI] [PubMed] [Google Scholar]
- 24. Greb A, Bohlius J, Schiefer D, Schwarzer G, Schulz H, Engert A. High‐dose chemotherapy with autologous stem cell transplantation in the first line treatment of aggressive non‐Hodgkin lymphoma (NHL) in adults. Cochrane Database Syst Rev. 2008:CD004024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tarella C, Zanni M, Di Nicola M, et al. Prolonged survival in poor‐risk diffuse large B‐cell lymphoma following front‐line treatment with rituximab‐supplemented, early‐intensified chemotherapy with multiple autologous hematopoietic stem cell support: a multicenter study by GITIL (Gruppo Italiano Terapie Innovative nei Linfomi). Leukemia. 2007;21:1802‐1811. [DOI] [PubMed] [Google Scholar]
- 26. Vitolo U, Chiappella A, Angelucci E, et al. Dose‐dense and high‐dose chemotherapy plus rituximab with autologous stem cell transplantation for primary treatment of diffuse large B‐cell lymphoma with a poor prognosis: a phase II multicenter study. Haematologica. 2009;94:1250‐1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ziepert M, Hasenclever D, Kuhnt E, et al. Standard International prognostic index remains a valid predictor of outcome for patients with aggressive CD20+ B‐cell lymphoma in the rituximab era. J Clin Oncol. 2010;28:2373‐2380. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1


