Abstract
Given that oropharyngeal squamous cell carcinoma (OPSCC) have now surpassed cervical cancer as the most common human papillomavirus (HPV)‐driven cancer, there is an interest in developing non‐invasive predictive biomarkers to early detect HPV‐driven OPSCC. In total, 665 cancer‐free individuals were recruited from Queensland, Australia. Oral HPV16 DNA positivity in those individuals was determined by our in‐house developed sensitive PCR method. Individuals with (n = 9) or without (n = 12) oral HPV16 infections at baseline were followed for a median duration of 24 mo. Individuals with persistent oral HPV16 infection (≥ 30 mo) were invited for clinical examination of their oral cavity and oropharynx by an otolaryngologist. Oral HPV16 DNA was detected in 12 out of 650 cancer‐free individuals (1.8%; 95% confidence interval [CI]: 1.0‐3.2). Of the 3 individuals with persistent oral HPV16 infection, the first individual showed no clinical evidence of pathology. The second individual was diagnosed with a 2 mm invasive squamous cell carcinoma (T1N0M0) positive for both p16INK4a expression and HPV16 DNA. The third individual was found to have a mildly dysplastic lesion in the tonsillar region that was negative for p16INK4a expression and HPV16 DNA and she continues to have HPV16 DNA in her saliva. Taken together, our data support the value of using an oral HPV16 DNA assay as a potential screening tool for the detection of microscopic HPV‐driven OPSCC. Larger multicenter studies across various geographic regions recruiting populations at a higher risk of developing HPV‐driven OPSCC are warranted to extend and confirm the results of the current investigation.
Keywords: biomarkers, human papillomavirus, oropharyngeal squamous cell carcinoma, saliva, screening tools
1. This study gives unique insights into the use of serial saliva sampling to detect human papillomavirus as a screening tool.2. The serial measurements of HPV16 viral load in oral rinse samples can identify individuals at risk of developing oropharyngeal lesions.
1. INTRODUCTION
Oropharyngeal squamous cell carcinoma (OPSCC) (which includes the tonsillar area, the base of tongue, the soft palate and the oropharynx) is one of the most common subsites of head and neck squamous cell carcinomas (HNSCC), accounting for approximately 97 000 deaths annually worldwide. 1 In addition to excessive tobacco and alcohol consumption, human papillomavirus (HPV) (predominately HPV16) infection is regarded as an important causative agent for OPSCC. 2 Over the past 2 decades, the incidence rate of HPV‐driven OPSCC is escalating when compared with non‐HPV‐driven HNSCC in the developed world, including Australia. 3 , 4 For instance, an increase in HPV‐driven oropharyngeal carcinoma (OPC) cases from 20.2% to 63.5% has been reported in Australia over the last 2 decades. 3
Current literature suggests that individuals with a greater number of lifetime sexual partners and high‐risk sexual behavior are associated with an increased risk of developing HPV‐driven OPSCC. 5 , 6 , 7 A recent study reported that the prevalence of HPV16 infection was higher in men who currently smoked and had more than 5 lifetime oral sexual partners. 8 In concordance with previous studies, we found a positive association between poor oral hygiene/health and oral HPV16 infection. 9 Importantly, chronic periodontitis was found to be associated with HPV status of HNSCC. 10 In addition, it is well established that immunocompromised people (including organ transplant patients) are at a higher risk of HPV‐driven cancer including OPSCC when compared with the general population. 11
Accumulating evidence indicates that persistent high‐risk HPV infections are strongly associated with the development of HPV‐driven malignancies. 12 , 13 This is supported by Agalliu et al who prospectively demonstrated a positive association between HPV infection and oropharyngeal malignancies. 14 Moreover, Kreimer et al reported the presence of HPV‐specific antibodies well before the development of OPSCC, further supporting the role of persistent infections in the development of oropharyngeal malignancies. 15 Previous studies have reported that the HPV DNA in both saliva and tumor tissue samples was positively correlated. 16 , 17 Meanwhile, oncogenic persistent oral HPV infection has recently been shown to be a precursor to malignancy, 18 and as such could potentially be used as a biomarker to diagnose, monitor disease progression, and tumor recurrence in OPSCC patients. 19
While oral HPV infection is the primary risk factor for OPSCC, it is still under debate whether oral HPV DNA measurements could be applied as a screening tool to identify individuals at a higher risk of developing OPSCC. The first aim of this study was to determine any associations between oral HPV16 infections, lifestyle factors, and oral health parameters in cancer‐free individuals. The second aim was to understand the natural history of oral HPV16 infection in cancer‐free individuals. The third aim was to identify whether cancer‐free individuals with persistent oral HPV16 infection exhibit or progress to malignancy.
2. METHODS AND MATERIALS
2.1. Study recruitment
Between May 2016 and October 2017, 665 participants were recruited from individuals visiting The Royal Brisbane and Women's Hospital (RBWH), The University of Queensland School of Dentistry (UQDS), The Queensland University of Technology Health Clinics (QUTHC) and Logan Hospital as shown in Figure 1.
FIGURE 1.
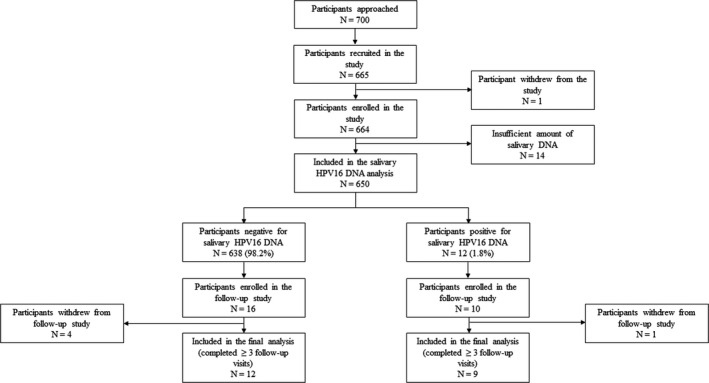
Flow chart of study recruitment
Participants were recruited into 3 groups. Group 1 included 202 participants recruited from the general public as community controls. Those individuals who had previously been vaccinated against any high‐risk HPV, those on antiretroviral drug therapy, and those having any previous history of any cancer or radiation to head and neck region were excluded from Group 1.
Groups 2 and 3 included 141 and 322 participants recruited from the UQDS as healthy individuals with good and poor oral hygiene, respectively. Inclusion criteria for both groups were identical, namely age over 18 y, and at least 20 teeth remaining in the mouth. The exclusion criteria for Group 2 and 3 were (a) prior HPV vaccination, (b) a history of any cancer or irradiation to the head and neck region, (c) diabetes, autoimmune disease, cardiovascular disease, blood disorders, hepatic dysfunction, dry mouth, aphthous ulcers and any other systemic disease that could affect oral health, and (d) use of medications such as lipid‐lowering drugs, hormonal replacement therapy, or supplements other than oral contraceptives.
Twelve participants within Groups 1, 2 and 3 who tested positive for oral HPV16 at baseline as well as 16 randomly selected participants (from Groups 2 and 3) who tested negative at baseline were recruited for the follow‐up study. Of those, 9 and 12 HPV16‐positive and HPV16‐negative participants, respectively, were followed up at 3‐6 monthly intervals for a median duration of 24 mo in the entire follow‐up study as shown in the Figure 1. The others were either lost to follow‐up or withdrew from the follow‐up study.
This study protocol was approved by the QUT research ethics committee (1400000641), UQ research ethics committee (2014000862) and RBWH human research ethics committee (HREC/16/QRBW/447). All individuals provided written informed consent prior to participation.
2.2. Sample collection procedure
Participants swished 200 mL of water around their mouth and then swallowed it. This step cleansed the mouth of food debris. Following this, a drool saliva (or unstimulated saliva) sample was collected, followed by 2 oral rinse samples, as previously described. 20 , 21
2.3. Procedures specific to each group following sample collection
All participants in Groups 2 and 3 underwent an oral examination. The following clinical variables were recorded: (a) presence or absence of bleeding on probing (BoP) (recorded as absent or mild–severe), (b) Plaque index, 22 (c) Calculus index, 23 (d) Decayed, missing, and filled teeth (DMFT) index 24 and (e) Periodontal Screening and Recording (PSR). 25 BoP was recorded 30 s following probing the gingival sulcus all around the teeth. Participants were categorized as poor oral hygiene had plaque scores >1 and/or calculus scores >0.7.
PSR was used to determine the presence or absence of periodontal (gum) disease in subjects in Groups 2 and 3. The dentition was divided into 6 sextants and each sextant received a score. If any of the 6 scores were >3 or there was any clinical evidence of periodontal disease (recession, mobility or furcation involvement), the participant was classified as having periodontal disease. 26
2.4. DNA isolation
Total DNA was isolated from unstimulated saliva, oral rinse and tissues samples using the QIAmp DNA Mini Kit (Qiagen) in accordance with the manufacturer's instructions. Briefly, 200 μL of lysis buffer was added to saliva and tissues samples with Proteinase K and incubated at 56°C until the pellets were completely lysed. For tissue samples, an additional incubation step (70°C for 10 min) was performed with another 200 μL of lysis buffer. Then, 200 μL of 100% ethanol was added to the mixture and subsequently transferred to columns as per the manufacturer's protocol.
2.5. HPV16 DNA nested PCR
To improve the sensitivity and specificity of the PCR assay, a HPV16 DNA nested PCR was developed as previously described. 21 Two pairs of primers flanking the HPV16 E6 opening reading frame (ORF) region were designed (1F: GTTTCAGGACCCACAGGAGC; 1R: GTCATATACCTCACGTCGCAGT; 2F: CAGGAGCGACCCAGAAAGTT, 2R:ACTGTTGCTTGCAGTACACAC). Human β‐globin was used as an internal control (Forward: CAACTTCCACGGTTCACC; Reverse: GAAGAGCCAAGGACAGGTAC). In addition, HPV16 DNA positivity in the saliva samples was further confirmed using Sanger sequencing.
2.6. HPV16 E6/7 DNA qPCR analysis
For HPV16 viral load calculation, HPV16 E6/E7 DNA standard calibration curve was generated using qPCR assay with the QuantStudio™ 7 Flex Real‐Time PCR System (Applied Biosystems, Foster City, CA, USA) as previously described. 20 Forward (ACCGGTCGATGTATGTCTTGTTG) and reverse (GATCAGTTGTCTCTGGTTGCAAATC) primers targeted against the region of E6/7 ORF were used. HPV16 standard curve was designed from threshold cycle plotted vs the logarithm of the copy number of 8‐fold serially diluted (1 × 101 to 1 × 108 copies) of HPV16 plasmid (ATCC® 45113™). To confirm the results, all oral HPV16 negative and positive samples were run at least in duplicate and triplicate, respectively, to confirm the results.
2.7. Immunohistochemistry
To determine the cellular and tissue structure, hematoxylin and eosin (H&E) staining on formalin‐fixed paraffin‐embedded (FFPE) slides was performed. HPV status was evaluated by Queensland pathologists using the CINtec® p16INK4a Histology Kit (E6H4 clone) (Roche MTM Laboratories, Heidelberg, Germany) according to manufacturer's instructions. p16INK4a was regarded as positive when the majority (>70%) of tumor cells had a strong, diffuse nuclear and cytoplasmic staining pattern.
2.8. High‐risk HPV in situ hybridization
In situ hybridization (ISH) was performed on FFPE section using the Ventana INFORM HPV III Family 16 probe (B) kit (Ventana Medical System, Inc), according to the manufacturers’ protocol. HPV was regarded as positive when blue staining was co‐localized with the nuclei of tumor cells.
2.9. Statistical analysis
Fisher's exact test was used to measure the significance of difference in oral HPV16 infection and lifestyle and oral health parameters between HPV16‐positive and HPB16‐negative individuals. All statistical tests were two‐sided and P‐values less than .05 were considered significant. All statistical analysis was performed using GraphPad Prism 8 software version 8.2.1 (GraphPad Software Inc).
3. RESULTS
3.1. Characteristics of the study population
The demographic of clinically cancer‐free individuals (n = 650) with sufficient amounts of DNA is summarized in Table S1. Participants had a median age of 52 y (range 18‐89 y). More than half of the study population was female (56.9%), ever‐smokers (55.4%), never‐drinkers (53.1%) and the majority were Caucasian (84.3%); 454 (69.8%) participants underwent oral examination. The presence of BoP (89.2%), supra‐gingival plaque (59%) and calculus (69.6%) were common in this study cohort. Only 211 out of 454 participants (46.5%) had a high score of DMFT. Most of them presented with poor oral hygiene (69.8%) and periodontal conditions (68.1%). Overall, the oral HPV16 prevalence at baseline was 1.8% (12/650) (95% confidence interval [CI]: 1.0‐3.2) in this study population. Participant lifestyle and oral health‐related parameters in relation to oral HPV16 infection are detailed in Table 1. No significant difference in both parameters was observed between HPV16‐negative and HPV16‐positive groups.
TABLE 1.
Participant lifestyle and oral health parameters in relation to oral HPV16 infection
| Variables | HPV16 positive | HPV16 negative | P‐value | ||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| Age (years) | |||||
| <55 | 4 | 33.3 | 351 | 55 | .153 |
| ≥55 | 8 | 66.7 | 287 | 45 | |
| Sex | |||||
| Male | 6 | 50 | 278 | 43.6 | .772 |
| Female | 6 | 50 | 360 | 56.4 | |
| Race/ethnicity | |||||
| Caucasian | 10 | 83.3 | 538 | 84.3 | >.999 |
| Other | 2 | 16.7 | 100 | 15.7 | |
| Smoking status | |||||
| Never | 6 | 50 | 354 | 55.5 | .774 |
| Ever | 6 | 50 | 284 | 44.5 | |
| Alcohol consumption | |||||
| Never | 7 | 58.3 | 338 | 53 | .776 |
| Ever | 5 | 41.7 | 300 | 47 | |
| BoP | |||||
| Absent | 0 | 0 | 49 | 11 | >.999 |
| Mild–Severe | 7 | 100 | 398 | 89 | |
| Plaque index | |||||
| <1 | 4 | 57.1 | 182 | 40.7 | .452 |
| >1 | 3 | 42.9 | 265 | 59.3 | |
| Calculus index | |||||
| <0.7 | 3 | 42.9 | 135 | 30.2 | .439 |
| >0.7 | 4 | 57.1 | 312 | 69.8 | |
| Oral hygiene | |||||
| Good | 3 | 42.9 | 134 | 30 | .436 |
| Poor | 4 | 57.1 | 313 | 70 | |
| DMFT | |||||
| 0‐14 | 2 | 28.6 | 241 | 53.9 | .258 |
| 15‐28 | 5 | 71.4 | 206 | 46.1 | |
| Periodontitis | |||||
| Absent | 1 | 14.3 | 144 | 32.2 | .439 |
| Present | 6 | 85.7 | 303 | 67.8 | |
3.2. Natural history of oral HPV16 infection in cancer‐free individuals
Nine HPV16‐positive individuals were followed for a median of 24 mo (range 9‐42 mo) and completed ≥ 3 follow‐up visits, while 12 HPV16‐negative individuals who were randomly selected were followed for a median of 24 mo (range 18‐24 mo) and completed ≥ 4 follow‐up visits as shown in Table S2. Among the 9 HPV16‐positive individuals, 4 (44.4%) were found to persist ≥ 3 mo and 3 (33.3%) persisted ≥ 30 mo (indicated as Individuals 1, 4, and 9). Most individuals (66.6%) were cleared within 6 mo of infections. None of the 12 initially HPV16 negative individuals developed an incident oral HPV16 infection at any follow‐up visits. As shown in Figure 2, Individual 1 had a low HPV16 viral load at baseline (11.37 viral copies/50 ng) and remained unchanged throughout the follow‐up period. Individual 4 had increasing levels of oral HPV16 DNA (from 3.43 to 1281.69 viral copies/50 ng) over the course of the study, 18 while Individual 9 with oral squamous papilloma of the soft palate had the highest HPV16 viral load at baseline (940.83 viral copies/50 ng) that remained high during follow‐up.
FIGURE 2.
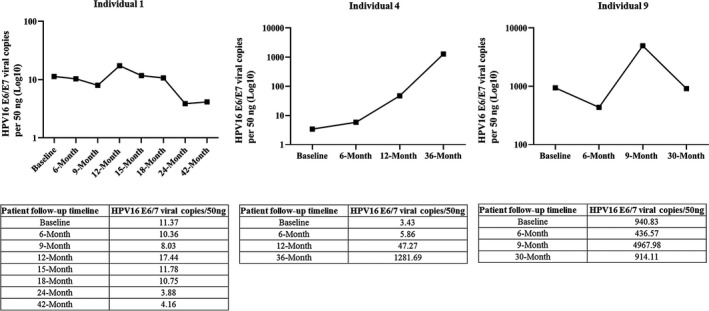
Salivary HPV16 viral load in Individuals 1, 4, and 9 throughout the follow‐up period. Individual 1 presented with no clinical abnormalities; Individual 4 was diagnosed with p16INK4a positive tonsillar squamous cell carcinoma (T1N0M0) and Individual 9 diagnosed with a p16INK4a negative mild dysplastic lesion in the tonsillar region
3.3. Clinical examination and imaging studies of the oropharynx in individuals with persistent oral HPV16 infection
The 3 individuals with persistent oral HPV16 infection (≥30 mo) were invited for full clinical examination of their oral cavity and oropharynx using both white light and narrow‐band imaging by an otolaryngologist.
Individual 1 demonstrated no clinical abnormalities and no radiological imaging was performed.
Individual 4 18 presented with tonsillar asymmetry and a large number of tonsillar mucous retention cysts as well as some subtle narrow‐band imaging (NBI) changes in the left gloss‐tonsillar sulcus. Magnetic resonance imaging (MRI) scan did not demonstrate any abnormalities (as shown in Figure S1). The patient was offered surveillance or examination under anesthesia (NBI) with NBI‐guided tongue base biopsies and bilateral tonsillectomy. The patient elected to undergo surgery and all specimens were sent for histopathology.
Individual 9 presented with an area of NBI change in the right anterior tonsillar pillar and a small raised lesion on the left base of the tongue (BOT) as well as generalized left BOT hypertrophy. MRI scanning suggested left BOT hypertrophy with no distinct lesions. The patient was offered surveillance or examination under anesthesia (EUA)‐ and NBI‐guided biopsies. The patient elected to undergo surgical biopsies. The decision to offer surgery in both cases was undertaken in conjunction with the Head and Neck Multidisciplinary Team at the RBWH.
3.4. Histologically confirmed diagnosis of oropharyngeal lesions in individuals with persistent oral HPV16 infection
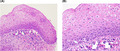
Based on the H&E staining, Individuals 4 and 9 were diagnosed with a 2 mm microinvasive squamous cell carcinoma (T1N0M0) 18 and a mild dysplastic lesion in the tonsillar region (Figure 3A,B), respectively. A diffuse and strong p16INK4a immunohistochemistry staining (≥70% of tumor cells) as well as HPV16 DNA positivity in the tumor tissues samples were only detected in Individual 4 as previously described. 18 Further confirmation of high‐risk HPV was achieved by performing HPV family 16 probe‐ISH test on FFPE section. Positive nuclear signals of HPV family 16 were only detected in tumor cells that exactly corresponded to the region of occult carcinoma and p16INK4a‐positive staining (Figure 4). Individual 9 was negative for both p16INK4a expression and HPV on ISH. Interestingly, HPV16 DNA in the saliva of Individual 4 reduced to undetectable levels 2 wk after tonsillectomy. In contrast, oral HPV16 viral load in Individual 9 remained detectable 2 wk after surgery.
FIGURE 3.
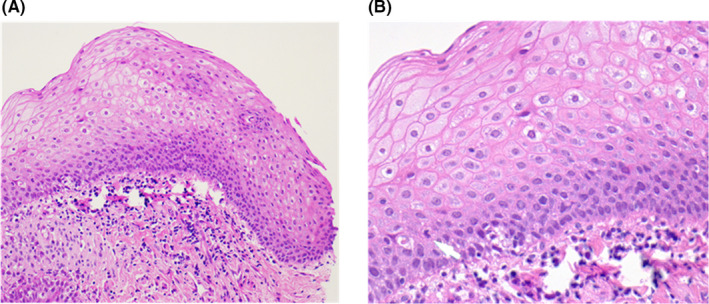
Individual 9 was histologically diagnosed with a p16INK4a negative low grade (mild) squamous dysplasia. A, Hematoxylin‐eosin (H&E; ×200 magnification). A mild squamous dysplasia presented as squamous atypia in the lower third of epithelial thickness and was found in the tonsillar region. B, H&E ×400. A mitosis was found in the suprabasal epithelium (white arrow). Note that, there is no full thickness dysplasia and no invasive carcinoma
FIGURE 4.
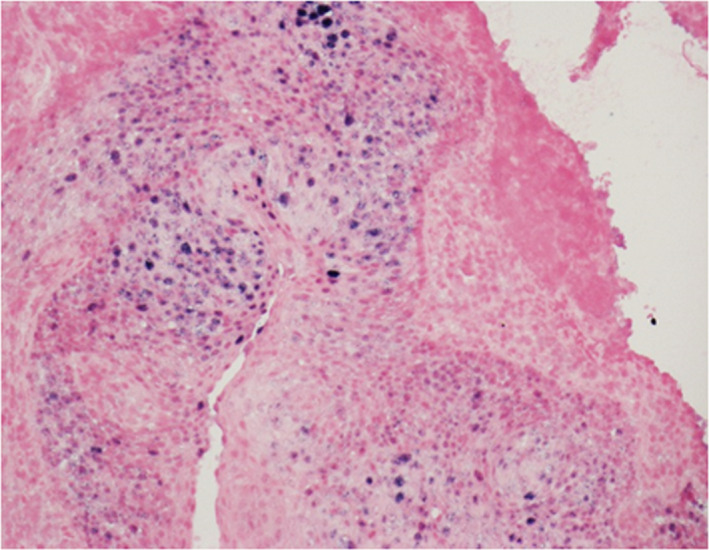
Individual 4 was diagnosed with p16INK4a positive tonsillar squamous cell carcinoma and was positive for HPV on in situ hybridization. High‐risk HPV in situ hybridization (ISH; ×200 magnification). Positive HPV16 family ISH nuclear signals in blue in the region of occult carcinoma
4. DISCUSSION
HPV‐driven OPSCC incidence particularly in the male population is rising rapidly in high‐income countries, including Australia. OPSCC has overtaken cervical cancer as the most common HPV‐driven cancers in the UK and the USA. 27 Therefore, it is pivotal to understand how to identify individuals at risk of developing HPV‐driven OPSCC. Unlike cervical cancer, at present there is no clinically validated screening test for HPV‐driven OPSCC. Screening for HPV‐driven OPSCC has long been a topic of debate as no‐one has ever successfully detected an occult lesion either through a salivary or serological test. In this study, we report on 2 asymptomatic, cancer‐free individuals with oropharyngeal lesions that were identified based on repeat measurement of oral HPV16 screening.
There is growing scientific evidence indicating that the current prophylactic HPV vaccines (nine‐valent HPV vaccine; Gardasil 9) could prevent nearly 100% of infections with the HPV types targeted by the vaccines, but do not help those already infected with HPV. Cross‐sectional studies have shown that HPV vaccination is associated with a reduction in vaccine‐type oral HPV prevalence 28 but, to our knowledge, there is lack of formal prospective studies aiming to determine vaccine efficacy against oral HPV infection or HPV‐driven OPSCC within clinical settings. Given that oral HPV infection is associated with a 22‐fold increased risk of developing HPV‐driven OPSCC, 14 there is great interest in the epidemiology of oral HPV infection in the general population over the past 2 decades. A recent meta‐analyses of 63 studies (sample size = 56 600) indicated that the prevalence of oral infection with any HPV strains and only HPV16 in the general population was 7.7% (95% CI, 6.8‐8.6) and 1.4% (95% CI, 1.0‐1.9), respectively. 29 In concordance with the findings of previous studies from other western countries, our reported oral HPV16 prevalence rate in a group of healthy Australians was 1.8%; 95% CI: 1.0‐3.2.
Increasing evidence supports the notion that epithelial wounds in the oral cavity mediated by chronic inflammation may serve as a portal of entry for the HPV. 30 , 31 For instance, periodontitis has been associated with HPV‐positive HNSCC, particularly in OPSCC. 10 A recent study by Dalla Torre et al reported a statistically significant association with respect to oral HPV infections for individuals with tooth loss and a higher number of extracted teeth. 32 Moreover, they also demonstrated a positive association between poor oral hygiene practices (with high levels of dental plaque and gingivitis) and oral HPV infection. Surprisingly, in the present study we found no statistically significant difference in both lifestyle (ie smoking and tobacco consumption) and oral health‐related parameters between oral HPV16‐negative and HPV16‐positive groups. Interestingly, an overwhelming majority of oral HPV16‐positive individuals presented with BoP (100%), a higher score for DMFT (71%) and for periodontal conditions (86%), further supporting the notion that chronic inflammation in the oral cavity, which occurs in periodontitis, may facilitate oral HPV infection.
The clinical utility of detecting persistent HPV DNA in saliva as a means of identifying individuals at risk of developing OPSCC or as a possible screening method for active malignancy has been a matter of significant debate. 33 , 34 It is obvious that population screening based upon oncogenic oral HPV detection would be challenging due to the lack of visible precancerous lesions for HPV‐driven OPSCC. Moreover, the origin of HPV in oral rinse/saliva samples remains unknown, and further investigations are warranted to reveal the clinical significance of an oral HPV test as a screening tool in the general population. When planning such studies, a key question will be how many samples are necessary from one patient over a given time interval. In our laboratory, we have performed multiple sample collections (at least 5 times over a period of 2 wk) on 5 cancer‐free individuals (2 with low HPV16 viral loads (>20 copies per 50 ng) and 3 with HPV16 DNA negative). The agreement for HPV detection within the samples was 100%. This consistency suggests that variation from day to day is not significant. There is limited knowledge on the natural history of oral HPV infections in the general population. The reported duration of clearance for oral HPV16 infection varies from 3.5 to 20.7 mo 29 ; while oral HPV16 persistent infection was only reported in a few studies and varies widely from 33.3% to 80.0% within 24 mo in those studies. 35 , 36 , 37 , 38 This large variation may be explained by several factors, including different race or ethnicity, sampling variability, as well as the use of different detection methods. In agreement with previously published data, the clearance and persistence rates of oral HPV16 infections in our study cohort were 66.6% within 6 mo and 33.3% within 30 mo, respectively.
The majority of cancer‐free individuals with oral HPV infection appears to have either transient infections or fail to progress to malignancy. We have successfully found 2 oropharyngeal lesions through the serial measurements of HPV16 DNA from saliva samples. Of the 3 individuals with persistent oral HPV16 infections, 2 with a higher oral HPV16 DNA viral load were found to have oropharyngeal lesions and one of these demonstrated a malignancy. Our findings are in keeping with previous studies showing that high HPV viral load serves as a predictor for long‐term persistent infection, as well as the risk of developing HPV‐driven cancer. 39 , 40 In addition, retrospective studies suggest the potential clinical utility of oral HPV as a biomarker for treatment surveillance in HPV‐positive HNSCC. 41 , 42 Consistent with previous studies, HPV viral loads in the saliva sample from the patient with tonsillar carcinoma decreased to an undetectable level at 2 wk following tonsillectomy.
However, there was no detectable level of tumor HPV16 DNA or p16INK4a in an individual with an oral mild dysplastic lesion. This may be due to the size of oropharyngeal lesions at an earlier stage, which are usually microscopic and inconspicuous, and therefore hampering an accurate clinical diagnosis. Indeed, a MRI examination of the oropharynx and neck failed to detect an occult lesion at the tonsillar region of the other individual, further supporting the potential clinical utility of an oral HPV test as a screening tool for HPV‐driven OPSCC. The limitations of this study are as follows: the relatively small sample size and a single‐site recruitment (only restricted in Queensland). Larger multicenter studies from diverse geographic regions with at‐risk populations (particularly in males) may overcome these shortcomings. In summary, this study demonstrates the potential for screening for early HPV‐driven OPSCC based upon persistent oral HPV16 detection in saliva samples.
DISCLOSURE
Gert C. Scheper is an employee of Janssen Vaccines & Prevention BV.
Supporting information
Supplementary Material
Figure S1
Table S1
Table S2
ACKNOWLEDGMENTS
We would like to thank the members of Saliva & Liquid Biopsy Translational Research Team for their assistance in sample collection and processing. We thank the staff from Herston Oral Health Centre as well as Associate Professor Bernard Whitfield, Director ENT at Logan Hospital, Australia. In addition, we thank volunteers who took part in this study. This work was supported by the Johnson & Johnson Family of Companies grant to CP.
Tang KD, Vasani S, Menezes L, et al. Oral HPV16 DNA as a screening tool to detect early oropharyngeal squamous cell carcinoma. Cancer Sci. 2020;111:3854–3861. 10.1111/cas.14585
REFERENCES
- 1. Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359‐386. [DOI] [PubMed] [Google Scholar]
- 2. Chi AC, Day TA, Neville BW. Oral cavity and oropharyngeal squamous cell carcinoma–an update. CA Cancer J Clin. 2015;65:401‐421. [DOI] [PubMed] [Google Scholar]
- 3. Hong A, Lee CS, Jones D, et al. Rising prevalence of human papillomavirus‐related oropharyngeal cancer in Australia over the last 2 decades. Head Neck. 2016;38:743‐750. [DOI] [PubMed] [Google Scholar]
- 4. Chaturvedi AK, Engels EA, Pfeiffer RM, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol. 2011;29:4294‐4301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dahlstrom KR, Li G, Tortolero‐Luna G, Wei Q, Sturgis EM. Differences in history of sexual behavior between patients with oropharyngeal squamous cell carcinoma and patients with squamous cell carcinoma at other head and neck sites. Head Neck. 2011;33:847‐855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. D'Souza G, Kreimer AR, Viscidi R, et al. Case‐control study of human papillomavirus and oropharyngeal cancer. N Engl J Med. 2007;356:1944‐1956. [DOI] [PubMed] [Google Scholar]
- 7. Bahl A, Kumar P, Dar L, et al. Prevalence and trends of human papillomavirus in oropharyngeal cancer in a predominantly north Indian population. Head Neck. 2014;36:505‐510. [DOI] [PubMed] [Google Scholar]
- 8. D'Souza G, McNeel TS, Fakhry C. Understanding personal risk of oropharyngeal cancer: risk‐groups for oncogenic oral HPV infection and oropharyngeal cancer. Ann Oncol. 2017;28:3065‐3069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sun CX, Bennett N, Tran P, et al. A Pilot Study into the Association between Oral Health Status and Human Papillomavirus‐16 Infection. Diagnostics (Basel). 2017;7(1):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tezal M, Scannapieco FA, Wactawski‐Wende J, et al. Local inflammation and human papillomavirus status of head and neck cancers. Arch Otolaryngol Head Neck Surg. 2012;138:669‐675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Frisch M, Biggar RJ, Goedert JJ. Human papillomavirus‐associated cancers in patients with human immunodeficiency virus infection and acquired immunodeficiency syndrome. J Natl Cancer Inst. 2000;92:1500‐1510. [DOI] [PubMed] [Google Scholar]
- 12. Koshiol J, Lindsay L, Pimenta JM, Poole C, Jenkins D, Smith JS. Persistent human papillomavirus infection and cervical neoplasia: A systematic review and meta‐analysis. Am J Epidemiol. 2008;168:123‐137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Munoz N, Hernandez‐Suarez G, Mendez F, et al. Persistence of HPV infection and risk of high‐grade cervical intraepithelial neoplasia in a cohort of Colombian women. Br J Cancer. 2009;100:1184‐1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Agalliu I, Gapstur S, Chen Z, et al. Associations of Oral alpha‐, beta‐, and gamma‐Human papillomavirus types with risk of incident head and neck cancer. JAMA Oncol. 2016;2:509‐606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kreimer AR, Johansson M, Waterboer T, et al. Evaluation of human papillomavirus antibodies and risk of subsequent head and neck cancer. J Clin Oncol. 2013;31:2708‐2715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ahn SM, Chan JY, Zhang Z, et al. Saliva and plasma quantitative polymerase chain reaction‐based detection and surveillance of human papillomavirus‐related head and neck cancer. JAMA Otolaryngol Head Neck Surg. 2014;140:846‐854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tang KD, Kenny L, Frazer IH, Punyadeera C. High‐risk human papillomavirus detection in oropharyngeal cancers: Comparison of saliva sampling methods. Head Neck. 2019;41:1484‐1489. [DOI] [PubMed] [Google Scholar]
- 18. Tang KD, Vasani S, Taheri T, et al. An occult HPV‐driven oropharyngeal squamous cell carcinoma discovered through a saliva test. Front Oncol. 2020;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chuang AY, Chuang TC, Chang S, et al. Presence of HPV DNA in convalescent salivary rinses is an adverse prognostic marker in head and neck squamous cell carcinoma. Oral Oncol. 2008;44:915‐919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tang KD, Baeten K, Kenny L, Frazer IH, Scheper G, Punyadeera C. Unlocking the potential of saliva‐based test to detect HPV‐16‐driven oropharyngeal cancer. Cancers (Basel). 2019;11(4):473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tang KD, Menezes L, Baeten K, et al. Oral HPV16 prevalence in oral potentially malignant disorders and oral cavity cancers. Biomolecules. 2020;10(2):223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Silness J, Loe H. Periodontal disease in pregnancy. Ii. Correlation between oral hygiene and periodontal condtion. Acta Odontol Scand. 1964;22:121‐135. [DOI] [PubMed] [Google Scholar]
- 23. Greene JC, Vermillion JR. The simplified oral hygiene index. J Am Dent Assoc. 1964;68:7‐13. [DOI] [PubMed] [Google Scholar]
- 24. Biggs JW. Book reviews : Oral health surveys (Basic Methods) — 3rd Edition. Published by WHO, Geneva, 1987. Pp 53. ISBN 92 4 154216 0. J R Soc Health. 1988;108(3):113. [Google Scholar]
- 25. Lo Frisco C, Cutler R, Bramson JB. Periodontal screening and recording: perceptions and effects on practice. J Am Dent Assoc. 1993;124(7):226‐232. [DOI] [PubMed] [Google Scholar]
- 26. Preshaw PM. Detection and diagnosis of periodontal conditions amenable to prevention. BMC Oral Health. 2015;15(Suppl 1):S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lechner M, Jones OS, Breeze CE, Gilson R. Gender‐neutral HPV vaccination in the UK, rising male oropharyngeal cancer rates, and lack of HPV awareness. Lancet Infect Dis. 2019;19:131‐132. [DOI] [PubMed] [Google Scholar]
- 28. Chaturvedi AK, Graubard BI, Broutian T, et al. Effect of Prophylactic Human Papillomavirus (HPV) Vaccination on Oral HPV Infections Among Young Adults in the United States. J Clin Oncol. 2018;36:262‐267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Oh J, Zwetchkenbaum S. Oral HPV infection is common worldwide, but risk of infection differs by sex, continent, population at infection, and year. J Evid Based Dent Pract. 2019;19:101348. [DOI] [PubMed] [Google Scholar]
- 30. Rautava J, Syrjanen S. Human papillomavirus infections in the oral mucosa. J Am Dent Assoc. 2011;142:905‐914. [DOI] [PubMed] [Google Scholar]
- 31. Tezal M. Interaction between chronic inflammation and oral HPV infection in the etiology of head and neck cancers. Int J Otolaryngol. 2012;2012:575242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Dalla Torre D, Burtscher D, Solder E, Rasse M, Puelacher W. The correlation between the quality of oral hygiene and oral HPV infection in adults: A prospective cross‐sectional study. Clin Oral Investig. 2019;23:179‐185. [DOI] [PubMed] [Google Scholar]
- 33. Zhao M, Rosenbaum E, Carvalho AL, et al. Feasibility of quantitative PCR‐based saliva rinse screening of HPV for head and neck cancer. Int J Cancer. 2005;117:605‐610. [DOI] [PubMed] [Google Scholar]
- 34. Gipson BJ, Robbins HA, Fakhry C, D'Souza G. Sensitivity and specificity of oral HPV detection for HPV‐positive head and neck cancer. Oral Oncol. 2018;77:52‐56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mooij SH, Boot HJ, Speksnijder AG, et al. Six‐month incidence and persistence of oral HPV infection in HIV‐negative and HIV‐infected men who have sex with men. PLoS One. 2014;9:e98955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. D'Souza G, Fakhry C, Sugar EA, et al. Six‐month natural history of oral versus cervical human papillomavirus infection. Int J Cancer. 2007;121:143‐150. [DOI] [PubMed] [Google Scholar]
- 37. Rintala M, Grenman S, Puranen M, Syrjanen S. Natural history of oral papillomavirus infections in spouses: A prospective Finnish HPV Family Study. J Clin Virol. 2006;35:89‐94. [DOI] [PubMed] [Google Scholar]
- 38. Pierce Campbell CM, Kreimer AR, Lin HY, et al. Long‐term persistence of oral human papillomavirus type 16: the HPV infection in Men (HIM) study. Cancer Prev Res (Phila). 2015;8:190‐196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Beachler DC, Guo Y, Xiao W, et al. High oral human papillomavirus type 16 load predicts long‐term persistence in individuals with or at risk for HIV infection. J Infect Dis. 2015;212:1588‐1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Nordfors C, Vlastos A, Du J, et al. Human papillomavirus prevalence is high in oral samples of patients with tonsillar and base of tongue cancer. Oral Oncol. 2014;50:491‐497. [DOI] [PubMed] [Google Scholar]
- 41. Fakhry C, Blackford AL, Neuner G, et al. Association of Oral Human Papillomavirus DNA Persistence With Cancer Progression After Primary Treatment for Oral Cavity and Oropharyngeal Squamous Cell Carcinoma. JAMA Oncol. 2019;5:985‐992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Rettig EM, Wentz A, Posner MR, et al. Prognostic implication of persistent human papillomavirus type 16 DNA detection in oral rinses for human papillomavirus‐related oropharyngeal carcinoma. JAMA Oncol. 2015;1:907‐915. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Figure S1
Table S1
Table S2


