Abstract
Cholesterol is a risk factor for breast cancer. However, it is still unclear whether the cholesterol biosynthesis pathway plays any significant role in breast carcinogenesis. 24‐Dehydrocholesterol reductase (DHCR24) is a key enzyme in the cholesterol synthesis pathway. Although DHCR24 is reported to have different functions in different cancers, it is not clear whether DHCR24 is involved in breast cancer. In this study, we found that DHCR24 expression was higher in breast cancer especially in luminal and HER2 positive breast cancer tissues compared with normal breast. Changes in DHCR24 expression altered cellular cholesterol content without affecting the adherent growth of breast cancer cells. However, DHCR24 knockdown reduced whereas DHCR24 overexpression enhanced breast cancer stem‐like cell populations such as mammosphere and aldehyde dehydrogenase positive cell numbers. In addition, DHCR24 overexpression increased the expression of the Hedgehog pathway‐regulated genes. Treating DHCR24 overexpressing breast cancer cell lines with the Hedgehog pathway inhibitor GANT61 blocked DHCR24‐induced mammosphere growth and increased mRNA levels of the Hedgehog regulated genes. Furthermore, expression of a constitutively activated mutant of Smoothened, a key hedgehog signal transducer, rescued the decreases in mammosphere growth and Hedgehog regulated gene expression induced by knockdown of DHCR24. These results indicate that DHCR24 promotes the growth of breast cancer stem‐like cells in part through enhancing the Hedgehog signaling pathway. Our data suggest that cholesterol contribute to breast carcinogenesis by enhancing Hedgehog signaling and cancer stem‐like cell populations. Enzymes including DHCR24 involved in cholesterol biosynthesis should be considered as potential treatment targets for breast cancer.
Keywords: breast cancer, cholesterol, DHCR24, Hedgehog pathway, stem‐like cells
Cholesterol is a risk factor for breast cancer. Our study uncovers that 24‐dehydrocholesterol reductase (DHCR24), a key enzyme in the cholesterol synthesis pathway, promotes the growth of cancer stem‐like cell populations by enhancing the Hedgehog signaling pathway in ER+ and HER2+ breast cancer. Our data suggest that cholesterol contribute to breast carcinogenesis by enhancing Hedgehog signaling and cancer stem‐like cell population.
1. INTRODUCTION
Breast cancer is the most common cancer in women worldwide. There are more than 2.08 million new breast cancer cases worldwide, including more than 620 000 deaths in 2018 alone. 1 Breast cancer can be classified into 3 main subtypes clinically that includes luminal, HER2 positive (+), and triple negative breast cancer (TNBC). 2 , 3 Although significant progress has been made in treating different types of breast cancer effectively through development of new targeted therapies, drug resistance, recurrence, and metastasis remain the major challenges in the clinic.
Cancer stem cells play critical roles in drug resistance and cancer metastasis. 4 , 5 Hedgehog pathway is an important signaling pathway regulating the growth and self‐renewal of normal and cancer stem cells (CSC). 6 , 7 , 8 Upon hedgehog binding to its receptor PTCH1 on the cell surface, the key signal transducer Smoothened (SMO) is activated and promotes nuclear translocation of the transcription factor Gli, consequentially turning on the transcription of hedgehog regulated target genes including Gli and PTCH1. 9 Hyperactivity of Hedgehog signaling promotes the occurrence and progression of a variety of cancers including breast cancer. 10 Recent studies using Hedgehog pathway inhibitor GANT61 showed that the Hedgehog signaling pathway plays a role in the expansion of breast cancer stem‐like cells. 11 , 12 Cholesterol and its derivatives are essential for Hedgehog signaling. Cholesterol can covalently modify Hedgehog and SMO to evoke proper activation of the Hedgehog pathway. 13 , 14 Cholesterol can also non‐covalently bind and activate SMO. Cholesterol‐binding structural regions in SMO include transmembrane (TM)2 and TM3 in the seven‐transmembrane domains of SMO, 15 and the extracellular cysteine‐rich domain (CRD). 16
Cholesterol is a risk factor for breast cancer. High cholesterol levels in plasma are associated with a high risk of breast cancer and it is an independent risk factor for breast cancer incidence and recurrence. 17 Patients with pre‐diagnostic use of lipid‐lowering drugs, such as statins, are associated with a reduced risk of breast cancer. 18 Studies using a breast tumor animal model showed that mice on a high‐fat diet have significantly higher blood cholesterol and enhanced tumor growth compared with mice on a normal diet. Importantly, statins can block breast tumor growth in mice on a high‐fat diet, 19 , 20 suggesting the importance of cholesterol or the cholesterol biosynthesis pathway in breast tumorigenesis. However, it is still less clear how intracellularly biosynthesized cholesterol contributes to breast carcinogenesis.
Cholesterol synthesis is a multi‐step metabolic pathway.DHCR24/seladin‐1, with the full name 3β‐dehydrocholesterol‐Δ24‐reductase, plays a key role in the Bloch and Kandutsch‐Russell pathways of cholesterol synthesis, respectively. DHCR24 catalyzes desmosterol to cholesterol in the Bloch pathway, and lanosterol to 24,25‐dihydrolanosterol in the Kandutsch‐Russell pathway. 21 , 22 DHCR24 is expressed in all cholesterol‐synthesizing cells. 21 The role of DHCR24 in cancer has not been well studied. Limited research has shown that DHCR24 plays the opposite roles in different cancers. In endometrial cancer, DHCR24 can promote the invasion of endometrial cancer cells. 23 In contrast, in prostate cancer, the expression of DHCR24 is lower in advanced tumors compared with in early tumors and normal tissues. DHCR24 inhibits the growth of prostate cancer cells. 24 , 25 However, the role of DHCR24 in breast cancer is still unknown.
In this report, we found that DHCR24 expression is higher in breast tumors compared with normal tissues. DHCR24 promotes the growth of a CSC‐like population in breast cancer cells without affecting the growth of the bulk of breast cancer cells. In addition, we uncovered DHCR24 through activation of the Hedgehog pathway to enhance breast cancer stem cell properties. The results of our study suggest that cholesterol contributes to breast carcinogenesis by enhancing the cancer stem‐like cell population.
2. MATERIALS AND METHODS
2.1. Data mining of The Cancer Genome Atlas database (TCGA)
TCGA is a publicly funded project and contains transcriptome data for various cancers including breast cancer. The mRNA expression profiling data of breast cancer and matched adjacent non‐tumor tissue in TCGA were downloaded (http://cancergenome.nih.gov/). The differences in DHCR24 mRNA expression between adjacent non‐tumor tissues and breast cancer were calculated and presented as a scatter plot and histogram.
2.2. Cell culture and reagents
Breast cancer cell lines MCF7, ZR‐75‐1, T47D, SKBR3, BT474, AU565, HCC1954, MDA‐MB‐231, Hs578t were obtained from the American Tissue Culture Collection (ATCC). The SUM149PT cell line was from BioIVT. MCF10A cell line was a gift from Dr. Joan Brugge (Harvard Medical School). 293T17 cell line was as described. 26 BT474 cells were cultured in Dulbecco's modified Eagle's medium (DMEM) (Gibco) supplemented with 10% fetal bovine serum (FBS) (Lonsera), 100 units/mL penicillin and 100 μg/mL streptomycin (Beyotime Institute of Biotechnology). AU565 cells were cultured in DMEM: Nutrient Mixture F‐12 (DMEM/F‐12) (Gibco) supplemented with 10% FBS, 100 units/mL penicillin and 100 μg/mL streptomycin. SUM149PT cells were cultured in DMEM/F‐12 supplemented with 5% FBS, 5 μg/mL insulin, 1 μg/mL hydrocortisone, 100 units/mL penicillin and 100 μg/mL streptomycin. 293T17, MCF7 cells were cultured in DMEM supplemented with 5% FBS, 100 units/ml penicillin and 100 μg/mL streptomycin. SUM149PT and AU565 cells were grown in a tissue culture incubator with an atmosphere of 5% CO2 in air, whereas BT474, MCF7, and 293T17 cells were grown in tissue culture incubator with 7.5% CO2 in air. GANT61 was purchased from Selleck Chemicals. Methyl‐β‐cyclodextrin was purchased from Sigma.
2.3. Construction of expression vectors
For knockdown of DHCR24, two different DHCR24 short hairpin RNAs (shRNAs) (sh11 and sh12) were inserted into the PLKO.1‐puro vector using standard DNA cloning techniques. The 2 DHCR24 shRNA sequences are: sh11, 5'‐GCTCTCGCTTATCTTCGATAT‐3'; sh12, 5′‐GCAGAGCTCTACATCGACATT‐3'. The PLKO.1‐control shRNA plasmid was used as described. 27 For overexpression of DHCR24, DHCR24 cDNA was inserted into the pBabe‐hygro plasmid using PCR and standard DNA cloning techniques. To construct the constitutively activated mutant of SMO, pcDNA3.1‐SMO‐3xFlag plasmid was purchased from YouBio and used as a template to generate pcDNA3.1‐SMOW535L‐3xFlag plasmid using the Mut Express® II Fast Mutagenesis Kit V2 System (Vazyme). The Flag‐SMOW535L insert was amplified by standard PCR and inserting into the pBabe‐puro plasmid. 27 Primer sequences for point mutagenesis are: forward 5′‐GAGCACCTtGGTCTGGACCAAGGCCACGCTG‐3′; reverse 5′‐CAGACCaAGGTGCTCATGGCGATGCCAGTTCC‐3′. Primer sequences for subcloning into pBabe‐puro plasmid are: forward 5′‐CCGGAATTCGCTAGTT‐AAGCTTGGTAC‐3′; reverse 5′‐CAGGTCGACTCACTTGTCATCGTCATC‐3′. All of the generated plasmids were verified by DNA sequencing.
2.4. Virus production and infection
For the production of lentivirus, 293T17 cells were cotransfected with PLKO.1 plasmid together with 2 packaging plasmids, pCMV‐VSV‐G and delta 8.9 as described. 27 For the production of retrovirus, 293T17 cells were cotransfected with retroviral plasmid together with 2 packaging plasmids, pCMV‐VSV‐G and gag/pol as described. 26 Harvested virus supernatants were filtered through a 0.45 μm low protein‐binding sterile filter. Breast cancer cells were incubated with lentiviral or retroviral supernatants in the presence of 8 μg/mL of polybrene (Sigma) and selected with 1.3 μg/mL puromycin (Invitrogen) for 3 days or with 40 μg/mL hygromycin (Invitrogen) for 5 days before being used for experiments.
2.5. Cholesterol assay
Cellular cholesterol levels were analyzed using the Amplex™ Red Cholesterol Assay kit (Invitrogen) according to the manufacturer’s instructions. Total cellular protein content was determined by bicinchonic acid (BCA) assay kit (Beyotime). Cells were starved without serum overnight, and lysed in 0.5% Triton X‐100 buffer (including 20 mmol/L Tris, 100 mmol/L NaCl, 1 mmol/L EDTA). Then, 50 μL of cell lysate and 50 μL Amplex Red reagent were added to the 96‐well plate. After 30 min incubation at 37°C in the dark, the sample fluorescence was measured using a Varioskan flash microplate reader (excitation at 560 nm and emission at 590 nm). Cholesterol levels were calculated according to a standard curve, normalized by total cellular protein, and expressed as μg of cholesterol per mg of protein.
2.6. Colony formation assay
Colony formation assay was performed to assess the ability of a single cell to grow into a colony in tissue culture plates. Breast cancer cells were cultured in 24‐well culture plates for 6 d to form colonies. Colonies were fixed with 10% neutral formalin, and stained with 0.5% crystal violet solution. The dye was extracted by adding 10% acetic acid. Absorbance at 540 nm was measured using a Varioskan flash microplate reader. All experiments were repeated 3 times.
2.7. Quantitative (q)RT‐PCR for mRNA
Total RNA was extracted from cells using TRIzol Reagent (Invitrogen) following the manufacturer's instructions. First‐strand cDNA synthesis and amplification were performed using PrimeScript™ RT reagent kit with gDNA Eraser (TaKaRa). Q‐PCR was used to quantify the expression of Gli3, PTCH1, Bmi‐1, mRNAs using SYBR® Premix Ex TaqII (Bio‐Rad). The primers for q‐PCR are listed below: Gli3, forward 5′‐ACTTCCGCCTTATCTAGTAGCC‐3′, reverse 5′‐CCACGGGTTGCTGAGATCAT‐3′; PTCH1, forward 5′‐GAAGAAG‐ GTGCTAATGTCCTGAC‐3′, reverse 5′‐GTCCCAGACTGTAATTTCGCC‐3′; Bmi‐1, forward 5′‐GGATCCTCATCCTTCTGCTGATGCTG‐3′, reverse 5′‐GAATTCGCATCACAGTCATTGCTGCT‐3′.
2.8. Western blot
Breast cancer cell lines were lysed in 1× SDS sample buffer (62.5 mmol/L Tris‐HCl pH 6.8, 2% SDS, 0.002% bromophenol blue, 10% glycerol, 5% β‐mercaptoethanol). Equal amounts of cell lysates were resolved by 10% SDS‐PAGE and transferred to Immobilon‐P membranes (Millipore), immunoblotted using the appropriate primary and the HRP‐conjugated secondary antibodies (Beyotime Institute of Biotechnology), and developed using enhanced chemiluminescence (ECL) reagents. ECL signals were captured by the ChemiDoc MP imaging system (Bio‐Rad) and were analyzed using Image Lab 5.0 software (Bio‐Rad). Antibodies recognizing DHCR24 and β‐Actin were purchased from Cell Signaling Technology. Flag‐antibody (M2 clone) was from Beyotime.
2.9. Aldehyde dehydrogenase (ALDH)‐positive cell analysis
The aldehyde dehydrogenase (ALDH)‐positive (+) cell population was analyzed by flow cytometry using the ALDEFLUOR™ assay kit (StemCell Technologies) according to the manufacturer’s instruction. Cells (1 × 106) were starved in serum‐free culture overnight, resuspended in ALDEFLUOR™ assay buffer, mixed with the activated ALDEFLUOR™ reagent, and incubated for 35 min at 37°C. For the ALDH negative control, ALDEFLUOR™ DEAB reagent (an ALDH inhibitor) was mixed with cell samples containing activated ALDEFLUOR™ reagent. ALDH+ cells were analyzed by FACS Aria (BD Biosciences) using FlowJ10 software.
2.10. Mammosphere culture assay
Twenty‐four well plates were coated with 100 μL of 12 mg/mL polyhema at 37°C overnight. Detached breast cancer cells (5000) were resuspended in mammosphere culture medium: DMEM/F12, 20 ng/mL bFGF (MultiSciences), 20 ng/mL EGF (MultiSciences), 5 μg/mL insulin (Solarbio), B27 supplement (Gibco), 0.5% BSA (Sangon Biotech), seeded in triplicates into each polyhema coated well and cultured at 37°C. Cells were replenished with fresh media every 3 days. Mammospheres with a diameter greater than 50 μm were quantified using Nikon NIS‐Element software. The total numbers of mammospheres in 6 random fields under 4x objective lens were determined for each well. The experiments were repeated at least 3 times.
2.11. Statistical analysis
Statistical analyses were performed using GraphPad Prism (version 6.0). Student T test was used to compare data between 2 groups. One‐way ANOVA with Bonferroni multiple comparison test correction was used to analyze data among multiple groups. Two‐way ANOVA was used to analyze differences with 2 independent factors. All statistical tests were two‐sided, and P < 0.05 was considered statistically significant.
3. RESULTS
3.1. DHCR24 expression is higher in ER‐positive and HER2 positive breast cancer
Although there are reports in the literature that DHCR24 can participate in the promotion of tumorigenesis and development, it is still unclear whether DHCR24 plays any role in breast cancer. We first analyzed DHCR24 mRNA expression in the breast cancer cohort from the TCGA database, which included mRNA expression data in breast cancer tissues from 1178 patients. Among them, 112 cases contained breast tumor tissue with adjacent normal tissue. The analysis revealed that DHCR24 mRNA expression was significantly higher in breast tumors compared with normal tissues (Figure 1A) or adjacent normal tissues (Figure 1C). In addition, DHCR24 mRNA expression was significantly higher in luminal A, luminal B or HER2 positive (+) breast tumor tissues compared with normal breast tissues. Interestingly, DHCR24 mRNA levels were similar in TNBC tissues and normal breast tissues (Figure 1B). Furthermore, DHCR24 protein levels were examined in different subtypes of breast cancer cell lines available in our laboratory (Figure 1D). Consistent with results from the analysis of TCGA database, DHCR24 protein levels were significantly higher in HER2+ (BT474, SKBR3, and AU565) and ER‐positive (ZR‐75‐1 and T47D) breast cancer cell lines compared with the normal breast epithelial cell line MCF10A and TNBC cell lines (SUM149PT, MDA‐MB‐231, and Hs578t) (Figure 1D).
FIGURE 1.
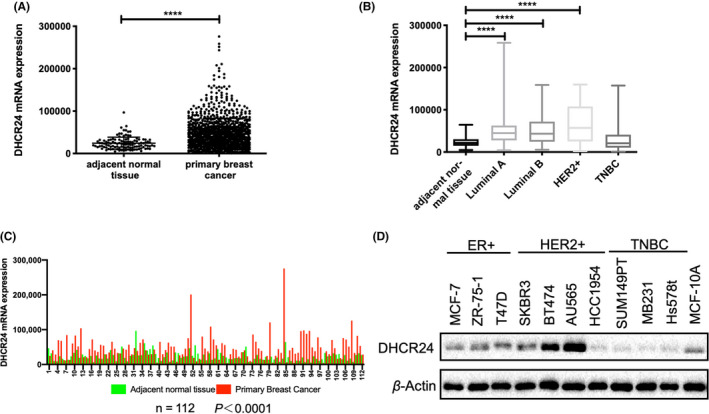
DHCR24 expression is higher in ER‐positive and HER2‐positive breast cancer. mRNA expression data from the breast cancer cohort in the TCGA database were downloaded and analyzed (A‐C). A, mRNA expression levels of DHCR24 in 1100 breast cancer tissues and 112 non‐tumor tissues. ****P < .0001. B, DHCR24 mRNA expression levels are significantly higher in luminal A, luminal B or HER2 + breast cancer compared with normal breast tissues or triple negative breast tumor tissues after analyzing data in TCGA database. **** P < .0001. C, DHCR24 mRNA expression is higher in breast tumor tissues compared with the adjacent normal breast tissues (n = 112). ****P < .0001. D, Immunoblot analysis of DHCR24 protein levels in the indicated ER+, HER2+, or triple negative breast cancer (TNBC) cell lines. The experiment was repeated 3 times
3.2. Changes in the expression level of DHCR24 alter cellular cholesterol levels without affecting the adherent growth of the breast cancer cell lines
To explore the role of DHCR24 in breast cancer, we first examined whether DHCR24 expression affected the growth of breast cancer cells. DHCR24 was knocked down by 2 different DHCR24 shRNAs in BT474 and AU565 cell lines. In addition, DHCR24 was overexpressed using the pBabe‐hygro retroviral vector containing DHCR24 cDNA in BT474, SUM149PT, and MCF7 cell lines. Western blot analysis confirmed that DHCR24 expression was decreased in BT474 and AU565 cell lines by DHCR24 shRNAs (Figure 2A), and increased in BT474, SUM149PT, and MCF7 cell lines (Figure 2B).
FIGURE 2.
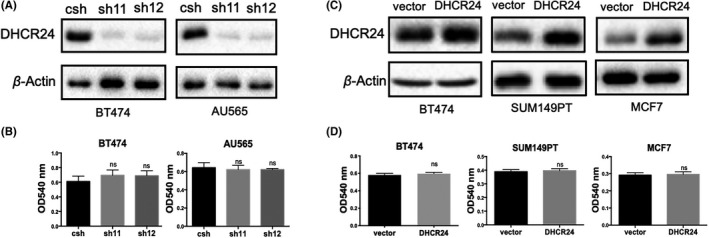
Changes in the expression of DHCR24 does not affect the adherent growth of the breast cancer cell lines. A, Knockdown of DHCR24 expression in breast cancer cell lines BT474 and AU565 using PLKO.1 lentivirus expressing control shRNA (csh) and 2 different DHCR24 shRNAs (sh11 and sh12). B, Overexpression of DHCR24 in breast cancer cell lines BT474, SUM149PT, and MCF7 using vector only pBabe‐puro retrovirus or pBabe‐puro retrovirus expressing DHCR24. DHCR24 levels in the indicated breast cancer cell lines were verified by western blot analysis using DHCR24 antibody and β‐Actin antibody as a loading control. DHCR24 knockdown (C) or overexpression (D) does not affect the adherent growth of the indicated breast cancer cell lines. Breast cancer cell lines were subjected to colony formation
Since DHCR24 is a key enzyme in the intracellular cholesterol biosynthesis pathway, 21 , 22 cholesterol levels were measured in breast cancer cells using the Amplex Red Cholesterol Assay kit. Cholesterol levels were decreased by more than 60% in 2 cell lines (BT474 and AU565) with DHCR24 knockdown compared with control knockdown (Figure S1A). Conversely, cholesterol levels were increased by c. 17%, 35%, and 50% in 3 cell lines (BT474, MCF7, and SUM149PT) respectively with DHCR24 overexpression compared with vector control (Figure S1B). The effect of changes in DHCR24 expression on adherent growth of cells was explored using a colony formation assay. The results showed that neither DHCR24 knockdown (Figure 2C) nor overexpression (Figure 2D) affected the adherent growth of these breast cancer cells.
3.3. DHCR24 promotes the growth of CSC‐like population in breast cancer cells
A published report showed that knockdown of enzymes such as SCD, LSS, HMGCS1 that are involved in the cholesterol biosynthesis pathways affects the tumorsphere growth of lung and colon cancer cells. 28 Tumorsphere or mammosphere is often enriched with CSCs. We hypothesized that DHCR24 may affect the growth of breast CSCs.
DHCR24 knockdown cell lines (BT474 and AU565) and DHCR24 overexpression cell lines (BT474, SUM149PT and MCF7) were cultured under mammosphere culture conditions for 5 d. The numbers of mammospheres were significantly reduced in cells expressing 2 different DHCR24 shRNAs compared with cells expressing control shRNA (Figure 3A). In contrast, the numbers of mammospheres were significantly increased in cells with DHCR24 overexpression compared with cells containing the vector control (Figure 3B). These data indicated that DHCR24 is important for the growth of mammosphere in breast cancer cells. In addition, we also examined the content of ALDH+ cells that are enriched for breast CSCs. 29 ALDH can maintain cell homeostasis by catalyzing the conversion of toxic aldehydes to non‐toxic carboxylic acids, which is particularly important for the self‐renewal of stem cells and CSCs. 30 The ALDH+ cell population was analyzed by flow cytometry using an ALDEFLUOR kit. The percentages of ALDH+ cell populations were greatly decreased in BT474 cells expressing 2 different DHCR24 shRNAs compared with cells expressing control shRNA (Figure 3C). Conversely, the percentages of ALDH+ cell populations were significantly enhanced in MCF7 cells with DHCR24 overexpression compared with cells containing the vector control (Figure 3D). These data showed that DHCR24 is critical for maintaining the ALDH+ cell population. Together, our results demonstrated that DHCR24 promotes the growth of stem cell‐like populations in breast cancer cells.
FIGURE 3.

DHCR24 promotes the growth of breast CSC‐like population. A, B, DHCR24 promotes mammosphere growth of breast cancer cells. BT474 and AU565 expressing csh or DHCR24 shRNAs (sh11 and sh12) (A), BT474, SUM149PT and MCF7 expressing vector only or exogenous DHCR24 (B) plated in triplicate wells were subjected to mammosphere formation assay as described in the method section. Mammospheres with diameter>50 μm were counted from 6 random fields under 4x magnification objective lens, and the average number of mammospheres per well was shown. Scale bars, 50 μm. C, D, DHCR24 increases the ALDH+ cell population in breast cancer cells. BT474 cells expressing csh or DHCR24 shRNAs (sh11 and sh12) (C) and MCF7 cells expressing vector alone or DHCR24 were pre‐treated without (−) or with (+) ALDH inhibitor DEAB. ALDH+ cell population was analyzed by flow cytometry analysis using the ALDEFLUOR assay kit. *P < .05, **P < .01, ***P < .001, two‐tailed Student T test. Data shown are representative from 3 independent experiments
3.4. DHCR24 promotes gene expression of the Hedgehog pathway in breast CSC‐like population
The Hedgehog signaling pathway plays an important role in regulating the growth of normal stem cells and tumor stem cells. 6 Recent studies using Hedgehog pathway inhibitor GANT61 suggested that the Hedgehog signaling pathway plays a role in the expansion of breast cancer stem‐like population cells. 11 , 12 Considering the key role of cholesterol in activation of the Hedgehog signaling pathway, we speculated that DHCR24 may promote the growth of stem cell‐like populations in breast cancer cells through the Hedgehog signaling pathway. To examine the effect of changes in DHCR24 expression on Hedgehog pathway‐regulated gene expression in CSC cells, DHCR24 knockdown cell lines (BT474 and AU565) and DHCR24 overexpression cell lines (SUM149PT and MCF7) were cultured in mammosphere culture conditions for 10 d before being subjected to quantitation of Gli3 and PTCH1 mRNA levels. The data showed that knockdown of DHCR24 by 2 different shRNAs caused significant decreases in Gli3 and PTCH1 mRNA levels compared with control shRNA in BT474 and AU565 cells (Figure 4A). Conversely, DHCR24 overexpression notably increased Gli3 and PTCH1 mRNA levels compared with vector alone control in SUM149PT and MCF7 cells (Figure 4B). These results showed that DHCR24 can enhance Hedgehog signaling in breast cancer stem‐like cells.
FIGURE 4.
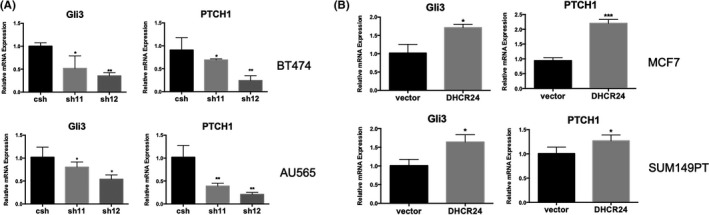
DHCR24 promotes gene expression of the hedgehog pathway in breast CSC‐like population. A, DHCR24 knockdown reduces gene expression of the hedgehog signaling pathway in BT474 and AU565 cells. B, DHCR24 overexpression increases gene expression of the hedgehog signaling pathway in MCF7 and SUM149PT cells. Cells were plated in triplicate wells under mammosphere growth conditions for 10 d, and analyzed for Gli3 and PTCH1 mRNA levels by q‐PCR. *P < .05,**P < .01,***P < .001, one‐way ANOVA. Data shown are representative from 3 independent experiments
3.5. Treatment with Gli inhibitor reduces DHCR24‐enhanced CSC‐like cell populations and Hedgehog regulated gene expression
GANT61 is a hexahydropyrimidine derivative that inhibits the effector molecule Gli1/2 in the Hedgehog pathway. 31 To explore whether DHCR24‐induced breast cancer CSC‐like properties are mediated by the Hedgehog pathway, two cell lines (SUM149PT and MCF7) overexpressing DHCR24 were subjected to mammosphere growth assay in the absence or presence of 5 μmol/L GANT61 for 5 d. While DHCR24 overexpression increased mammosphere numbers in both cell lines compared with cells containing vector control, addition of GANT61 to cells with DHCR24 overexpression caused significant reduction in mammosphere numbers, to a level that similar to that in cells containing vector only and treated with vehicle control (Figure 5A,B). Similarly, results from flow cytometry analysis using an ALDEFLUOR kit revealed that DHCR24‐enhanced ALDH+ cell populations were significantly inhibited in MCF7 (Figure S2A) and SUM149PT (Figure S2B) cells treated with GANT61 compared with vehicle control.
FIGURE 5.
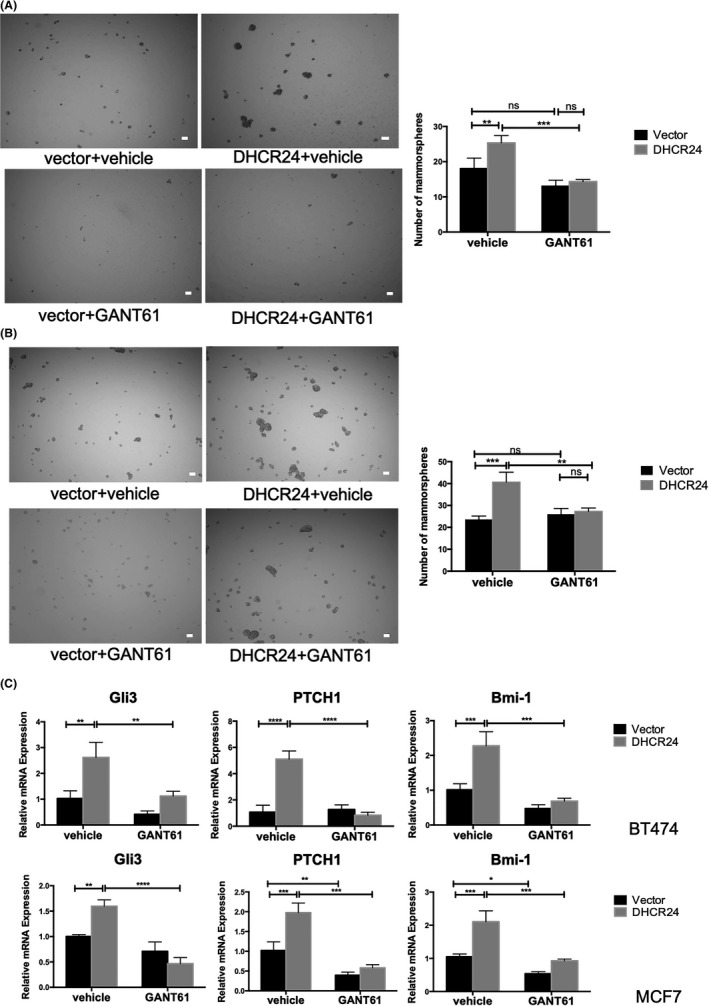
Treatment with Gli inhibitor reduces DHCR24‐enhanced mammosphere growth and hedgehog regulated gene expression in breast cancer cells. A, Treatment with Gli inhibitor GANT61 reduces DHCR24‐enhanced mammosphere growth. BT474 and MCF7 infected with pBabe vector and pBabe expressing DHCR24 were plated in triplicate wells under mammosphere growth condition in the absence or presence of GANT61 (5 μM) for 5 d. Mammosphere numbers were quantified as described in 3B, Treatment with GANT61 decreased DHCR24‐enhanced gene expression of the hedgehog pathway. C, Indicated cell lines were plated in triplicate wells under mammosphere growth condition for 10 d, and analyzed for Gli3, PTCH1, and Bmi‐1 mRNA levels by q‐PCR. *P < .05, **P < .01, ***P < .001, two‐way ANOVA. Data shown are representative from 3 independent experiments
In addition, we examined the effect of GANT61 treatment on the mRNA expression of Gli3, PTCH1, and Bmi‐1 in mammospheres by qRT‐PCR (Figure 5C). The results showed that the GANT61 treatment notably inhibited mRNA levels of Gli3, PTCH1, and Bmi‐1 compared with vehicle control in DHCR24 overexpressing cells. Compared with vehicle control, GANT61 treatment caused a much smaller decrease in mRNA levels of Gli3, PTCH1, and Bmi‐1 in vector alone cells. This result showed that Gli inhibitor reduced DHCR24‐enhanced gene expression of the Hedgehog signaling pathway. Together, these results support our hypothesis that the Hedgehog signaling pathway mediates DHCR24‐enhanced growth of the breast cancer stem‐like cells.
3.6. SMO‐activated mutant can rescue the decrease in CSC‐like cell populations and inhibition of the Hedgehog pathway caused by DHCR24 knockdown
To further verify that DHCR24 promotes the expansion of stem cell‐like cells in breast cancer cells through the Hedgehog signaling pathway, we overexpressed a constitutively activated SMO mutant SMOW535L 32 , 33 in BT474 and AU565 cell lines expressing control shRNA or DHCR24 shRNAs (sh11, sh12) ( Figure 6A,B). These cell lines were first subjected to mammosphere assay. Mammosphere numbers were significantly decreased in DHCR24 knockdown BT474‐sh11 cells compared with BT474‐csh control cells, whereas the numbers of mammospheres were significantly increased in BT474‐sh11 cells after being expressed with the activated mutant SMOW535L compared with vector control (Figure 6C). Similarly, compared with vector alone control, the expression of SMOW535L also significantly enhanced the numbers of mammospheres in DHCR24 knockdown AU565‐sh11 and AU565‐sh12 cell lines (Figure 6D). In addition, results from flow cytometry analysis using the ALDEFLUOR kit showed that expression of SMOW535L significantly increased the ALDH+ cell population in MCF7 (Figure S3A, B) and AU565 (Figure S3C, D) cells expressing DHCR24 shRNA compared with vector control. These results indicated that expression of the SMO‐activated mutants can rescue the reduced CSC‐like cell populations induced by DHCR24 knockdown.
FIGURE 6.
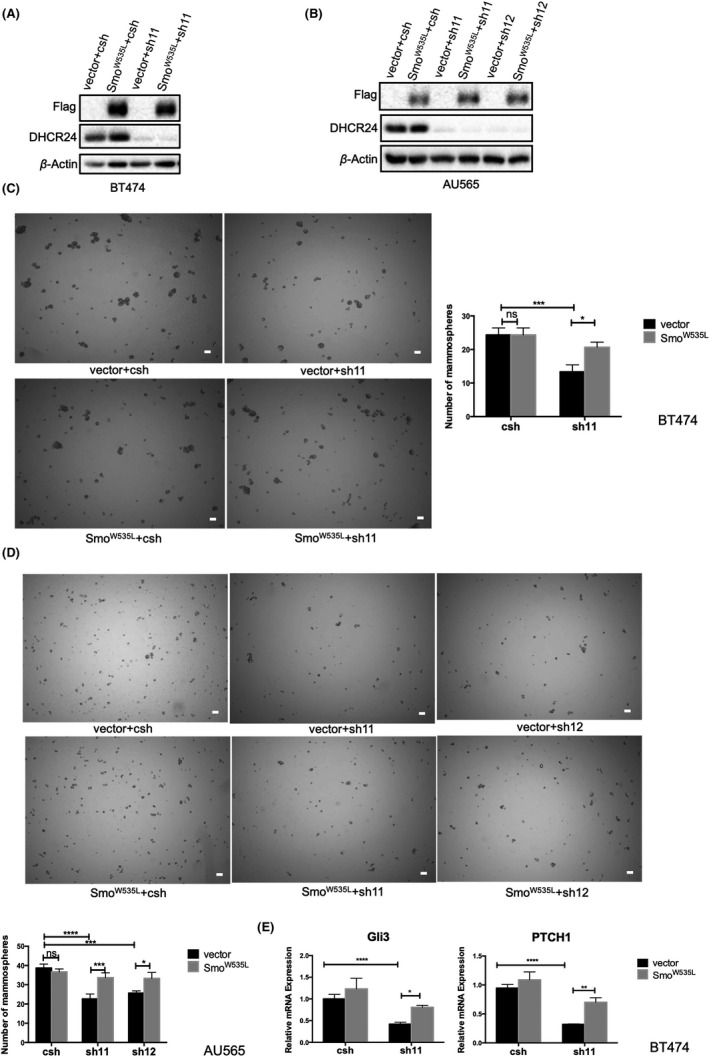
Expression of the constitutively activated SMO mutant rescues decreased mammosphere growth and Hedgehog regulated gene expression induced by DHCR24 knockdown in breast cancer cells. A, B, Expression of the activated SMO mutant W535L (SMOW535L) in breast cancer cells. BT474 (A) and AU565 (B) cells were infected with pBabe‐Hygro vector alone and pBabe‐Hygro Flag‐SMOW535L retroviruses and selected with hygromycin before infected with PLKO.1 lentiviruses expressing control shRNA (csh) and DHCR24 shRNA (sh11) respectively. The expression of SMOW535L and DHCR24 in indicated cell lines were analyzed by western blot using Flag and DHCR24 antibodies with β‐Actin antibody as a loading control. C, D, Expression of the activated SMO mutant SMOW535L rescues decreased mammosphere growth induced by DHCR24 knockdown. Indicated BT474 (C) and AU565 (D) cell lines were subjected to mammosphere growth assay. Scale bars, 50 μm. (E) Expression of the activated SMO mutant SMOW535L rescues hedgehog regulated gene expression inhibited by DHCR24 knockdown. Indicated BT474 cell lines were grown in mammosphere growth condition in triplicates for 10 d and analyzed for Gli3 and PTCH1 mRNA expression by q‐PCR. *P < .05, **P < .01, ***P < .001, two‐way ANOVA. Data shown are representative from 3 independent experiments
In addition, we also examined the effects of SMOW535L expression on the mRNA levels of Gli3 and PTCH1 in mammosphere. Gli3 and PTCH1 mRNA levels were significantly reduced in DHCR24 knockdown BT474‐sh11 cells compared with BT474‐csh control cells (Figure 6E). Similar to the effects on mammosphere growth (Figure 6D), expression of the activated mutant SMOW535L significantly increased Gli3 and PTCH1 mRNA levels in BT474‐sh11 cells compared with vector alone control. These results demonstrated that expression of the activated SMO‐mutants also rescued decreased mRNA levels of Gli3 and PTCH1 induced by DHCR24 knockdown (Figure 6E).
4. DISCUSSION
Breast cancer is a highly heterogeneous cancer. Like most cancers, the exact cause of its occurrence has not been fully understood. Multiple studies have revealed that obesity and cholesterol are risk factors for breast cancer. 34 Our study revealed a novel function of DHCR24, a key enzyme in the intracellular cholesterol synthesis pathway, in breast cancer. We found that DHCR24 mRNA levels are significantly higher in breast cancer tissues especially in the luminal and HER2+ breast cancer compared with the adjacent normal tissues (Figure 1). Our data showed that DHCR24 promotes the growth of breast cancer stem‐like cells (Figure 3) without affecting the adherent growth of the bulk of breast cancer cells (Figure 2). Our study revealed that DHCR24 enhances breast CSC‐like properties through the Hedgehog pathway. First, DHCR24 knockdown or overexpression decreased or increased the number of breast CSC‐like populations (Figure 3) and the expression of hedgehog pathway‐regulated genes (Gli3, PTCH1) (Figure 4), respectively. Secondly, the Hedgehog pathway inhibitor GANT61 31 can prevent the increase in mammosphere numbers and the increased expression of Gli3 and PTCH1 caused by the DHCR24 overexpression (Figure 5). Finally, the expression of the constitutively activated SMO mutant (SMOW535L) can rescue the reduction of mammosphere numbers and Gli3 and PTCH1 gene expression caused by DHCR24 knockdown (Figure 6).
Recent studies indicated that Hedgehog pathway is involved in maintaining the characteristics of breast CSCs. 27 , 28 Two reports showed that inhibition of the Hedgehog pathway with a pharmacological drug GANT61 caused a decrease in the number of breast cancer stem‐like cells. GANT61 is known to have significant anti‐cell growth activity in various tumor types, and it can also reduce the cancer stem‐like cell populations in ER‐positive and TNBC cells. 11 , 12 However, it is not clear how other cellular proteins regulate breast CSC‐like population through crosstalk with the Hedgehog pathway. Our results indicated that DHCR24 maintains breast tumor stem cell‐like properties through enhancing the Hedgehog pathway. A recent report showed that TSPAN8, a member of the tetraspanins family glycoproteins, can promote breast cancer cell stemness by interacting with PTCH1 and sustaining Hedgehog signaling. 4
Our results suggested that DHCR24 promotes breast CSC‐like cell populations by controlling intracellular synthesis of cholesterol, which contributes to the activation of SMO and subsequent Hedgehog signaling. Consistent with DHCR24 being a key enzyme in the cholesterol synthesis pathway, we found that knockdown and overexpression of DHCR24 significantly reduced and increased cellular cholesterol contents respectively in breast cancer cells (Figure S1). Furthermore, depleting cellular cholesterol with methyl‐β‐cyclodextrin prevented the ability of DHCR24 overexpression to enhance mammosphere growth (Figure S4A, B) and gene expression of the Hedgehog pathway (Figure S4C) in BT474 and MCF7 cells. Recent studies have demonstrated that cholesterol plays key roles in Hedgehog pathway activation. Cholesterol binds SMO non‐covalently through the TM2 and TM3 regions in the seven‐helix transmembrane domain and its extracellular CRD, causing conformational change and activation of SMO to transmit Hedgehog signal. 35 , 36 SMOW535L is the constitutively activated SMO mutant, whose activation is independent of Hedgehog. The mutation site of W535L is not located in the cholesterol‐binding regions of SMO. 32 , 33 The fact that the expression of the activated mutant SMOW535L in DHCR24 knockdown breast cancer cells can rescue the defects, such as the reductions of Gli3 and PTCH1 mRNAs, mammosphere growth ( Figure 6), and ALDH+ cell population (Figure S3) further supports the idea that DHCR24 acts upstream of SMO and regulates SMO activation. Consistent with our finding, a recent study using CRISPR/Cas9 gene knockout screening in NIH3T3 fibroblasts has revealed that multiple enzymes including LSS, DHCR7, SC5D, and DHCR24 in the cholesterol synthesis pathway are critical for intracellular cholesterol content and Gli transcriptional activation in NIH3T3 cells. 36 Interestingly, another recent study showed that knockdown of enzymes involved in cholesterol synthesis can reduce mammosphere growth of breast cancer cells although the effects of knockdown of these enzymes on Hedgehog signaling were not examined in this study. 37
Because our analysis revealed that DHCR24 expression is higher in luminal (ER‐positive) and HER2+ breast cancer than TNBC, this suggests that different enzymes involved in cholesterol biosynthesis promote breast cancer stem cell characteristic in different subtypes of breast cancer. Our data certainly support a role of DHCR24 in maintaining cancer stem‐like cell properties in ER‐positive or HER2+ breast cancer. A recent study showed that knockdown of FDFT1 and CYB5R3, two enzymes involved in cholesterol synthesis, inhibited the growth of mammospheres in TNBC cells, suggesting the critical roles of these 2 enzymes in promoting CSCs in TNBC. 37 In addition, the protein levels of FDFT1 and CYB5R3 were elevated in mammospheres compared with the bulk of ER negative breast cancer cells. 37 Interestingly, we also found that DHCR24 protein levels were notably elevated in mammospheres compared to the bulk of ER+ (MCF7 and BT474) or HER2+ (HCC1954 and BT474) breast cancer cells (data not shown). This may also explain why knockdown of DHCR24 did not affect the bulk population growth of breast cancer cells (Figure 2B,D). It will be interesting to determine how the expression of enzymes involved in cholesterol biosynthesis is upregulated in breast cancer stem‐like cells in future.
In summary, our study uncovered that DHCR24 promotes the growth of cancer stem‐like cell populations by enhancing the Hedgehog signaling pathway in ER+ and HER2+ breast cancer. Considering the involvement of CSCs in drug resistance and metastasis, drugs targeting enzymes involved in cholesterol synthesis pathway including DHCR24 should be explored to treat breast cancer patients with recurrence.
DISCLOSURE STATEMENT
The authors have no conflict of interest.
Supporting information
Figs S1‐S4
ACKNOWLEDGMENTS
This study was supported by grants from the National Natural Science Foundation of China (81972463, 81971291), Natural Science Foundation of Zhejiang Province, China (LY18H080003, LY20C080001), Wenzhou Medical University Startup funds, and in part supported by the Key Discipline of Zhejiang Province in Medical Technology (First Class, Category A).
Qiu T, Cao J, Chen W, et al. 24-Dehydrocholesterol reductase promotes the growth of breast cancer stem-like cells through the Hedgehog pathway. Cancer Sci. 2020;111:3653–3664. 10.1111/cas.14587
Contributor Information
Hongzhi Li, Email: lhz@wmu.edu.cn.
Haihua Gu, Email: haihuagu@wmu.edu.cn.
REFERENCES
- 1. Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394‐424. [DOI] [PubMed] [Google Scholar]
- 2. Gruver AM, Portier BP, Tubbs RR. Molecular pathology of breast cancer: The journey from traditional practice toward embracing the complexity of a molecular classification. Arch Pathol Lab Med. 2011;135(5):544‐557. [DOI] [PubMed] [Google Scholar]
- 3. Nagaraj G, Ellis MJ, Ma CX. The natural history of hormone receptor‐positive breast cancer: Attempting to decipher an intriguing concept. Oncology. 2012;26(8): 696‐697, 700. [PubMed] [Google Scholar]
- 4. Zhu R, Gires O, Zhu L, et al. TSPAN8 promotes cancer cell stemness via activation of sonic Hedgehog signaling. Nat Commun. 2019;10(1):2863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shiozawa Y, Nie B, Pienta KJ, et al. Cancer stem cells and their role in metastasis. Pharmacol Ther. 2013;138(2):285‐293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Briscoe J, Therond PP. The mechanisms of Hedgehog signalling and its roles in development and disease. Nat Rev Mol Cell Biol. 2013;14(7):416‐429. [DOI] [PubMed] [Google Scholar]
- 7. Jiang J, Hui CC. Hedgehog signaling in development and cancer. Dev Cell. 2008;15(6):801‐812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cochrane CR, Szczepny A, Watkins DN, et al. Hedgehog signaling in the maintenance of cancer stem cells. Cancers (Basel). 2015;7(3):1554‐1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chen Y, Jiang J. Decoding the phosphorylation code in Hedgehog signal transduction. Cell Res. 2013;23(2):186‐200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wu F, Zhang Y, Sun B, et al. Hedgehog signaling: From basic biology to cancer therapy. Cell Chem Biol. 2017;24(3):252‐280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Koike Y, Ohta Y, Saitoh W, et al. Anti‐cell growth and anti‐cancer stem cell activities of the non‐canonical hedgehog inhibitor GANT61 in triple‐negative breast cancer cells. Breast Cancer. 2017;24(5):683‐693. [DOI] [PubMed] [Google Scholar]
- 12. Kurebayashi J, Koike Y, Ohta Y, et al. Anti‐cancer stem cell activity of a hedgehog inhibitor GANT61 in estrogen receptor‐positive breast cancer cells. Cancer Sci. 2017;108(5):918‐930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Porter JA, Young KE, Beachy PA. Cholesterol modification of hedgehog signaling proteins in animal development. Science (New York, N.Y.). 1996;274(5285):255‐259. [DOI] [PubMed] [Google Scholar]
- 14. Xiao X, Tang JJ, Peng C, et al. Cholesterol modification of smoothened is required for hedgehog signaling. Mol Cell. 2017;66(1):154‐162 e110. [DOI] [PubMed] [Google Scholar]
- 15. Hedger G, Koldso H, Chavent M, et al. Cholesterol interaction sites on the transmembrane domain of the hedgehog signal transducer and class F G protein‐coupled receptor smoothened. Structure. 2019;27(3):549‐559 e542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Luchetti G, Sircar R, Kong JH, et al. Cholesterol activates the G‐protein coupled receptor smoothened to promote hedgehog signaling. Elife. 2016;5:e20304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kitahara CM, Berrington de Gonzalez A, Freedman ND, et al. Total cholesterol and cancer risk in a large prospective study in Korea. J Clin Oncol. 2011;29(12):1592‐1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Baek AE, Nelson ER. The contribution of cholesterol and its metabolites to the pathophysiology of breast cancer. Horm Cancer. 2016;7(4):219‐228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kaiser J. Cholesterol forges link between obesity and breast cancer. Science. 2013;342(6162):1028. [DOI] [PubMed] [Google Scholar]
- 20. Nelson ER, Wardell SE, Jasper JS, et al. 27‐Hydroxycholesterol links hypercholesterolemia and breast cancer pathophysiology. Science. 342(6162)1094‐1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zerenturk EJ, Sharpe LJ, Ikonen E, et al. Desmosterol and DHCR24: unexpected new directions for a terminal step in cholesterol synthesis. Prog Lipid Res. 2013;52(4):666‐680. [DOI] [PubMed] [Google Scholar]
- 22. Spann NJ, Glass CK. Sterols and oxysterols in immune cell function. Nat Immunol. 2013;14(9):893‐900. [DOI] [PubMed] [Google Scholar]
- 23. Dai M, Zhu XL, Liu F, et al. Cholesterol synthetase DHCR24 induced by insulin aggravates cancer invasion and progesterone resistance in endometrial carcinoma. Sci Rep. 2017;7:41404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Battista MC, Guimond MO, Roberge C, et al. Inhibition of DHCR24/ seladin‐1 impairs cellular homeostasis in prostate cancer. Prostate. 2010;70(9):921‐933. [DOI] [PubMed] [Google Scholar]
- 25. Bonaccorsi L, Luciani P, Nesi G, et al. Androgen receptor regulation of the seladin‐1/DHCR24 gene: Altered expression in prostate cancer. Lab Invest. 2008;88(10):1049‐1056. [DOI] [PubMed] [Google Scholar]
- 26. Fang Z, Li T, Chen W, et al. Gab2 promotes cancer stem cell like properties and metastatic growth of ovarian cancer via downregulation of miR‐200c. Exp Cell Res. 2019;382(1):111462. [DOI] [PubMed] [Google Scholar]
- 27. He L, Du Z, Xiong X, et al. Targeting androgen receptor in treating HER2 positive breast cancer. Sci Rep. 2017;7:14584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Song M, Lee H, Nam MH, et al. Loss‐of‐function screens of druggable targetome against cancer stem‐like cells. FASEB J. 2017;31(2):625‐635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ginestier C, Hur MH, Charafe‐Jauffret E, et al. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell. 2007;1(5):555‐567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhou L, Sheng D, Wang D, et al. Identification of cancer‐type specific expression patterns for active aldehyde dehydrogenase (ALDH) isoforms in ALDEFLUOR assay. Cell Biol Toxicol. 2019;35(2):161‐177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Borah A, Pillai SC, Rochani A, et al. GANT61 and curcumin loaded PLGA nanoparticles for GLI‐1 and PI3K/Akt mediated inhibition in breast adenocarcinoma. Nanotechnology. 2020;31(18):185102. [DOI] [PubMed] [Google Scholar]
- 32. Atwood SX, Sarin KY, Whitson RJ, et al. Smoothened variants explain the majority of drug resistance in basal cell carcinoma. Cancer Cell. 2015;27(3):342‐353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Xie J, Murone M, Luoh S, et al. Activating Smoothened mutations in sporadic basal‐cell carcinoma. Nature. 1998;391(6662):90‐92. [DOI] [PubMed] [Google Scholar]
- 34. Garcia‐Estevez L, Moreno‐Bueno G. Updating the role of obesity and cholesterol in breast cancer. Breast Cancer Res. 2019;21(1):35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Huang P, Nedelcu D, Watanabe M, et al. Cellular cholesterol directly activates smoothened in hedgehog signaling. Cell. 2016;166(5):1176‐1187 e1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kinnebrew M, Iverson EJ, Patel BB, et al. Cholesterol accessibility at the ciliary membrane controls hedgehog signaling. Elife. 2019;8:e50051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ehmsen S, Pedersen MH, Wang G, et al. Increased cholesterol biosynthesis is a key characteristic of breast cancer stem cells influencing patient outcome. Cell Rep. 2019;27(13):3927‐3938 e3926. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figs S1‐S4


