Abstract
Comorbidities at diagnosis among patients with chronic myeloid leukemia in chronic phase (CML‐CP) may affect their overall survival (OS) rate even in the tyrosine kinase inhibitor (TKI) era. However, the prognostic impact of comorbidities in patients with CML‐CP treated with a second‐generation TKI (2GTKI) has not been elucidated. We evaluated the effect of comorbidities on survival using the Charlson Comorbidity Index (CCI) in patients with CML‐CP treated with imatinib or a 2GTKI (nilotinib and dasatinib). From April 2010 to March 2013, 506 patients with CML‐CP were registered for the population‐based cohort study, and 452 with a median age of 56 y were assessable. Treatment groups included 139 patients receiving imatinib, 169 receiving nilotinib, and 144 receiving dasatinib. Comorbidities were diagnosed in 99 patients. CCI scores were stratified as follows: 2, 353 patients; 3, 72 patients; and ≥4, 27 patients. Treatment response did not vary relative to CCI scores. However, across the entire cohort, the OS rate was significantly lower among patients with higher CCI scores than in those with a CCI score of 2 (94.4% in score 2, 89.0% in score 3, and 72.8% in score ≥4; P < .001). Multivariate analysis identified a CCI score of ≥4 as a strong adverse prognostic factor for OS rather than the disease‐specific risk factor, older age, performance status, or selection of TKI (Wald test, P < .01). Our results demonstrated that comorbidities at diagnosis were the most important predictive factor for successful treatment, regardless of the TKI type used in CML‐CP. This trial was registered at UMIN‐CTR as 00003581.
Keywords: chronic myeloid leukemia, comorbidity, dasatinib, imatinib, nilotinib
The prognostic impact of comorbidities in chronic myeloid leukemia in patients with chronic phase CML (CML‐CP) treated with a second‐generation tyrosine kinase inhibitor (TKI) remains to be elucidated. This study demonstrated that comorbidities at diagnosis were the most important predictive factor for successful treatment, regardless of the TKI type used in CML‐CP.
Abbreviations
- 2GTKI
second‐generation tyrosine kinase inhibitor
- AA‐CCI
age‐adjusted Charlson Comorbidity Index
- ACA
additional chromosomal abnormality
- AE
adverse event
- ALT
alanine transaminase
- AP
accelerated phase
- AST
aspartate transaminase
- BP
blast crisis
- CCI
Charlson Comorbidity Index
- CCyR
complete cytogenetic response
- CI
confidence interval
- CML‐CP
chronic myeloid leukemia in chronic phase
- DASISION
Dasatinib versus Imatinib Study in Treatment‐Naive CML Patients
- DESTINY
De‐Escalation and Stopping Treatment with Imatinib, Nilotinib, or sprYcel
- DMR
deep molecular response
- ECOG
Eastern Cooperative Oncology Group
- ELTS
European Treatment and Outcome Study long‐term survival score
- ENESTnd
Evaluating Nilotinib Efficacy and Safety in Clinical Trials‐newly diagnosed patients
- EUTOS
European Treatment and Outcome Study
- IS
international scale
- JSH
Japanese Society of Hematology
- MMR
major molecular response
- NCI‐CTC
Common Toxicity Criteria of the National Cancer Institute
- NE
not estimable
- OS
overall survival
- PS
performance status
- TARGET
Timely and Appropriate Registration System for GLIVEC® Therapy
- TKI
tyrosine kinase inhibitor
- VAE
vascular adverse event
1. INTRODUCTION
BCR‐ABL1 TKIs have substantially improved the clinical outcomes of chronic myeloid leukemia in the chronic phase (CML‐CP). 1 , 2 , 3 Life expectancy after diagnosis in patients with CML‐CP of all ages was almost similar compared with that of the general population, 3 , 4 and survival in CML‐CP is more frequently determined by non‐CML‐related causes than CML itself. 5 Therefore, pretreatment status assessment at diagnosis in patients with CML‐CP has become more important for successful management in the TKIs era.
The German CML Study IV trial demonstrated that the age‐adjusted AA‐CCI score 6 , 7 is a useful tool for predicting OS in patients with CML‐CP treated with imatinib in a large clinical trial setting. 8 Nevertheless, this trial showed that comorbidities at diagnosis have no negative association with probabilities of adverse effects and treatment response, including cytogenetic and molecular response, and incidence of progression to CML in advanced phase. 8
However, 2 problems still remain. Firstly, currently available first‐line treatment options for newly diagnosed CML‐CP are second‐generation TKIs (2GTKIs) with safety profiles that are different from imatinib. Long‐term data from 2GTKI trials, including the Evaluating Nilotinib Efficacy and Safety in Clinical Trials‐newly diagnosed patients (ENESTnd) 2 and the Dasatinib versus Imatinib Study in Treatment Naive CML Patients (DASISION) trial, 3 demonstrated their benefits related to a deeper molecular response with a reduced risk of CML progression to accelerated phase/blast crisis (AP/BC) and CML‐related death. However, 2GTKIs were also associated with a higher incidence of some toxicities (ie, infection and pulmonary toxicity due to dasatinib and vascular toxicity due to nilotinib), compared with that of imatinib. 2 , 3 Therefore, the impact of comorbidities on survival in patients with CML treated with 2GTKIs may be different from that in those patients with CML receiving imatinib. A higher CCI risk score has been reportedly associated with a poor outcome in CML‐CP treated with TKIs, including 2GTKIs, but the number of enrolled patients was relatively small, and not enough patients (7.5% of all patients) received a 2GTKI treatment. 9 Secondly, a real‐world treatment study without potential selection bias is more suitable for assessing the impact of the pretreatment status on the clinical course in CML‐CP, although the German CML Study IV trial had fewer exclusion criteria than other trials. In the present study, we evaluated the effects of comorbidities on clinical outcomes in a population‐based cohort of patients with CML‐CP treated with either imatinib or a 2GTKI.
2. MATERIALS AND METHODS
2.1. Study design and treatments
The database of the Timely and Appropriate Registration System for GLIVEC® Therapy (the New TARGET) is administered by the JSH to collect the clinical data of patients with CML‐CP. 10 The New TARGET observational study 1 was designed as a prospective cohort study to evaluate the effectiveness and safety of TKIs, including 2GTKIs, in newly diagnosed patients with CML‐CP using this registry system and is supported by research funding from Novartis Pharmaceuticals and Bristol‐Myers Squibb to the JSH. The detailed treatment schedule has been described previously. 10 Briefly, patients received imatinib, nilotinib, or dasatinib as the initial therapy for CML‐CP after registration. TKI selection was based on the physicians’ decision. If a patient showed resistance to the first‐line TKI, the physician could switch to high‐dose imatinib (500‐600 mg/d) or other 2GTKIs, including bosutinib. In case of intolerance, including TKI toxicity, the physician could decide a dose reduction of the same TKI or switch to another TKI. The physician administering TKI treatment to the patient with CML‐CP would prospectively enter patient data at baseline, after 3, 6, 9, and 12 mo of therapy, and every 6 mo thereafter for the prescription status, treatment outcome, efficacy, occurrence of AEs, and safety. This study is a subgroup analysis of the New TARGET observational study 1.
2.2. Patients
Adult patients aged 18 y and older with newly diagnosed CML‐CP were registered for the New TARGET observational study 1 from 102 institutions between April 2010 and March 2013. Patients were excluded from the study if they were diagnosed with an AP/BC CML, had received interferon‐alfa, any TKIs, or hydroxyurea for more than 3 mo, or were subjected to an allogeneic hematopoietic stem‐cell transplantation before the registration, or did not start TKI treatment after registration.
2.3. Definition and evaluation of patients
The primary endpoint of the New TARGET observational study 1 was the OS rate at 5 y. OS was calculated from the first day of therapy to death or last visit. Secondary endpoints were progression‐free survival rate at 5 y, and cumulative incidences of CCyR or MMR. CCyR was defined as the absence of Ph‐positive metaphases in bone marrow samples. MMR was defined as >3‐log reduction of BCR‐ABL1 transcript [BCR‐ABL1 international scale (IS) ≤0.1%], and MR4.5 was defined as a 4.5‐log reduction of the BCR‐ABL1 transcript (BCR‐ABL1 IS ≤ 0.0032%). BCR‐ABL1 IS was regularly monitored every 3 mo in the first year and every 6 mo thereafter using the MolecularMD One‐Step qRT‐PCR BCR‐ ABL1 kit (BML Inc, Kawagoe, Japan). AEs related to the TKIs were graded according to the Common Toxicity Criteria of the National Cancer Institute (NCI‐CTC) version 4.03. This study used the CCI score 6 (without considering the age factor) to evaluate the impact of comorbidity itself at diagnosis of CML‐CP on the clinical outcome. Patients were classified into CCI risk groups 2, 3, and ≥4 for analysis according to the total scores. The lowest CCI score of 2 was based on CML. The AA‐CCI score was derived by adding one point to the summed CCI scores for each decade of age over 40. 6
2.4. Statistical analysis
Chi‐square test and Wilcoxon rank‐sum test were used to compare clinical characteristics for categorical data and continuous data, respectively. OS rates were calculated using the Kaplan‐Meier method and compared using the log‐rank test. Gray's test was used to compare cumulative incidence curves. Cox proportional hazard analyses were performed to determine prognostic indicators of OS. Wald test was conducted to assess the prognostic significance of a candidate single variable. Statistical analyses were performed using EZR, 11 a graphical user interface for R (R Foundation for Statistical Computing, Vienna, Austria). All hypothesis testing was two‐tailed with a significance level of P = .05.
3. RESULTS
3.1. Patient characteristics
Among 506 enrolled patients, 452 with a median age of 56 y (age range, 18‐92 y) were assessable. Fifty‐four patients were excluded for the following reasons: 7 inadequate information at registration, 1 double registration, 1 accelerated phase at registration, 1 withdrawal of consent, 21 no record of TKI treatment, and 23 TKI treatment before registration. The median follow‐up period was 5.4 y. Data were locked on November 10, 2018.
Patient characteristics are shown in Table 1. The CCI scores were stratified as follows: 2, 353 patients (78.1%); 3, 72 patients (15.9%); and ≥4, 27 patients (6.0%). The distribution of the disease‐specific prognostic scores (Sokal risk score and ELTS score) and Eastern Cooperative Oncology Group performance status (ECOG PS) did not vary significantly within each CCI risk group. However, higher CCI scores were significantly associated with a smaller spleen size and older age (P = .03 and P < .001, respectively; Table 1). The correlation between AA‐CCI and CCI risk score in this study was due both to the higher incidence of comorbidities and the elderly population in the group with a CCI risk score of ≥4. Among 214 patients with an AA‐CCI risk score of ≥5, 128 patients (59.8%) lacked any comorbidity (ie, patients having CML but no other diseases) and were classified as the group with CCI risk score 2. For initial treatment, 139 (30.7%) patients received imatinib, 169 (37.4%) nilotinib, and 144 (31.9%) dasatinib. Patients with higher CCI scores were significantly more frequently treated with imatinib (P = .003; Table 1). Ninety‐nine patients (21.9%) with a CCI risk score of ≥3 had various types of comorbidities, including diabetes mellitus, mild liver disease, peripheral vascular disease, myocardial infarction, renal disease, peptic ulcer disease, any tumor (excluding CML), chronic pulmonary disease, connective tissue disease, dementia, congestive heart failure, cerebrovascular accident, and diabetes with end‐organ damage (7.1%, 4.2%, 3.1%, 2.2%, 2.2%, 2.2%, 1.8%, 1.3%, 0.9%, 0.9%, 0.4%, 0.2%, and 0.2%, respectively).
TABLE 1.
Clinical characteristics relative to the CCI risk score
| Clinical features | CCI 2 | CCI 3 | CCI ≥ 4 | P‐value |
|---|---|---|---|---|
| Number of patients (%) | 353 (78.1) | 72 (15.9) | 27 (6.0) | |
| Age (y) | ||||
| Median (range) | 53 (18‐92) | 61 (21‐86) | 73 (39‐91) | <.001 |
| Gender, number of patients (%) | ||||
| Male | 224 (63.5) | 52 (72.2) | 20 (74.1) | .23 |
| Female | 129 (36.5) | 20 (27.8) | 7 (25.9) | |
| Body weight (kg) | ||||
| Median (range) | 61 (29.2‐168.8) | 62.7 (39.0‐103.4) | 65.5 (39.5‐93.0) | .46 |
| Blast in peripheral blood (%) | ||||
| Median (range) | 0.0 (0.0‐10.5) | 0.0 (0.0‐5.0) | 0.0 (0.0‐5.0) | .1 |
| Platelet counts (× 104/μL) | ||||
| Median (range) | 47.4 (3.4‐319.0) | 49.7 (4.1‐249.4) | 34.3 (9.5‐196.9) | .29 |
| Size of spleen (cm) | ||||
| Median (range) | 0.0 (0.0‐27.0) | 0.0 (0.0‐14.0) | 0.0 (0.0‐10.0) | .03 |
| AA‐CCI, number of patients (%) | ||||
| 2 | 99 (28.1) | 0 (0.0) | 0 (0.0) | <.001 |
| 3 to 4 | 126 (35.7) | 13 (18.0) | 0 (0.0) | |
| 5 to 6 | 117 (33.1) | 37 (51.4) | 5 (18.5) | |
| 7 to 10 | 11 (3.1) | 22 (30.6) | 22 (81.5) | |
| Sokal risk score, number of patients (%) | ||||
| Low | 166 (47.0) | 30 (41.7) | 10 (37) | .89 |
| Intermediate | 136 (38.5) | 29 (40.3) | 13 (48.2) | |
| High | 50 (14.2) | 13 (18.0) | 4 (14.8) | |
| Unknown | 1 (0.3) | 0 (0.0) | 0 (0.0) | |
| EUTOS long‐term survival score, number of patients (%) | ||||
| Low | 263 (74.5) | 50 (69.5) | 14 (51.9) | .15 |
| Intermediate | 69 (19.5) | 17 (23.6) | 10 (37.0) | |
| High | 21 (5.9) | 5 (6.9) | 3 (11.1) | |
| Additional chromosomal abnormality, number of patients (%) | ||||
| Absent | 334 (94.6) | 67 (93.1) | 27 (100) | .39 |
| Present | 19 (5.4) | 5 (6.9) | 0 (0.0) | |
| ECOG performance status, number of patients (%) | ||||
| 0 | 297 (84.1) | 57 (79.2) | 18 (66.7) | .09 |
| 1 | 49 (13.9) | 10 (13.9) | 8 (29.6) | |
| 2 | 6 (1.7) | 4 (5.5) | 1 (3.7) | |
| 3 | 1 (0.3) | 1 (1.4) | 0 (0.0) | |
| Selection of TKI as initial treatment, number of patients (%) | ||||
| Imatinib | 96 (27.2) | 34 (47.2) | 9 (33.3) | .003 |
| 2GTKI | 257 (72.8) | 38 (52.8) | 18 (66.7) | |
| Dasatinib | 114 (32.3) | 23 (32.0) | 7 (25.9) | |
| Nilotinib | 143 (40.5) | 15 (20.8) | 11 (40.8) | |
The P‐values were obtained from between‐group analyses. One point was added to the summed CCI scores according to each decade of age over 40 in the AA‐CCI score.
Abbreviations: 2GTKI, second‐generation tyrosine kinase inhibitor; AA‐CCI, age‐adjusted Charlson Comorbidity Index; CCI, Charlson Comorbidity Index; ECOG, Eastern Cooperative Oncology Group; EUTOS, European Treatment and Outcome Study; TKI, tyrosine kinase inhibitor.
3.2. Efficacy
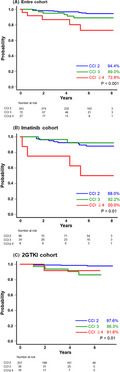
Treatment responses are presented in Table 2 (CCyR and MMR) and Figure 1A‐C (MR4.5). The CCyR and MMR rates at 12 mo did not vary substantially within each CCI risk group in the entire cohort. Similarly, differences in the cumulative incidence of CCyR and MMR were not statistically significant during the observational period in relation to the CCI scores. CCyR and MMR rates were similar among the CCI risk scores in the imatinib and 2GTKI cohorts (Table 2). Although the cumulative incidence of MR4.5 at 36 mo in patients with a CCI risk score of ≥4 was lower than in the respective patients with a CCI risk score of 2 or 3, it did not reach statistical significance (43.0% in CCI risk score 2, 44.3% in score 3, and 21.8% in score ≥4, respectively, P = .19; Figure 1A). We also compared the treatment efficacy between the imatinib and the 2GTKI cohort based on each CCI group. Among patients with a CCI risk score of 2, the treatment response rate was significantly higher in the 2GTKI cohort than in the imatinib cohort (data not shown). However, both TKI cohorts had similar treatment response rates in patients with a CCI score of 3 or ≥4, except for a cumulative incidence of CCyR in patients with a CCI score of 3 (81.8% in the imatinib cohort vs. 87.5% in the 2GTKI cohort, P = .01). In patients with a CCI risk score of ≥4, the cumulative incidence of MR4.5 at 36 mo was 0% in the imatinib cohort and 34.1% in the 2GTKI cohort (Figure 1B,C).
TABLE 2.
Treatment response relative to the CCI score
| CCI 2 | CCI 3 | CCI ≥ 4 | P‐value | |
|---|---|---|---|---|
| CCyR | % (95% CI) | % (95% CI) | % (95% CI) | |
| Entire cohort | ||||
| CCyR at 12 mo | 78.2% (73.5‐82.4) | 77.8% (66.4‐86.7) | 74.1% (53.7‐88.9) | .85 |
| Cumulative incidence of CCyR | 94.0% (90.2‐96.3) | 84.0% (73.3‐91.6) | 87.6% (60.1‐96.1) | .66 |
| Imatinib cohort | ||||
| CCyR at 12 mo | 67.7% (57.4‐76.9) | 73.5% (55.6‐87.1) | 77.8% (40.0‐97.2) | .78 |
| Cumulative incidence of CCyR | 94.3% (85.6‐97.8) | 81.8% (61.6‐91.4) | 87.5% (21.8‐98.0) | .4 |
| 2GTKI cohort | ||||
| CCyR at 12 mo | 82.0% (76.9‐86.6) | 81.6% (65.7‐92.3) | 72.2% (46.5‐90.3) | .52 |
| Cumulative incidence of CCyR | 93.8% (89.1‐96.5) | 87.5% (69.4‐94.9) | 76.5% (44.6‐90.0) | .19 |
| MMR | % (95% CI) | % (95% CI) | % (95% CI) | |
| Entire cohort | ||||
| MMR at 12 mo | 58.9% (53.6‐64.1) | 59.7% (47.5‐71.1) | 48.1% (28.7‐68.1) | .59 |
| Cumulative incidence of MMR | 95.0% (91.1‐97.2) | 90.7% (77.3‐96.2) | 89.1% (43.2‐97.9) | .64 |
| Imatinib cohort | ||||
| MMR at 12 mo | 40.6% (30.7‐51.1) | 50.0% (32.4‐67.6) | 44.4% (13.7‐78.8) | .62 |
| Cumulative incidence of MMR | 94.0% (83.5‐97.8) | 94.5% (66.2‐99.1) | 83.3% (0.26‐97.2) | .99 |
| 2GTKI cohort | ||||
| MMR at 12 mo | 65.8% (59.6‐71.5) | 68.4% (51.3‐82.5) | 50.0% (26.0‐74.0) | .37 |
| Cumulative incidence of MMR | 95.3% (90.5‐97.6) | 88.0% (70.0‐95.2) | 86.8% (30.7‐97.5) | .61 |
Abbreviations: 2GTKI, second‐generation tyrosine kinase inhibitor; CCI, Charlson Comorbidity Index; CCyR, complete cytogenetic response; CI, confidence interval; MMR, major molecular response.
FIGURE 1.
Cumulative incidence of MR4.5 over 36 mo relative to the CCI risk score. Cumulative incidence of MR4.5 over 36 mo in the entire cohort (A), the imatinib cohort (B), and the 2GTKI cohort (C). 2GTKI, second‐generation tyrosine kinase inhibitor; CCI, Charlson Comorbidity Index
There were no substantial differences in the cumulative incidence for AP and BC among the risk groups in the entire cohort (2.5% [events = 7/353] in CCI risk score 2, 4.1% [events = 2/72] in score 3, and 0% [events = 0/27] in score ≥4, P = .66). Although the cumulative incidence for AP and BC was higher with imatinib than with 2GTKI treatment (6.6% vs. 0.9%, P = .001), the incidences of disease progression in relation to the CCI risk scores were similar among both cohorts.
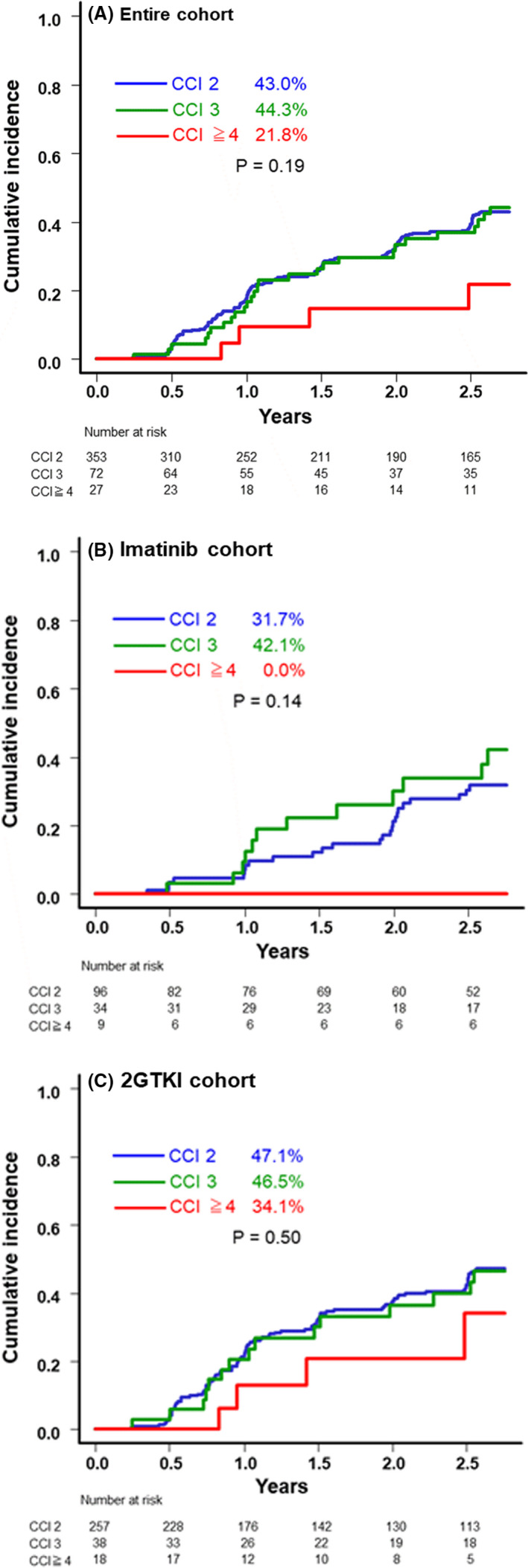
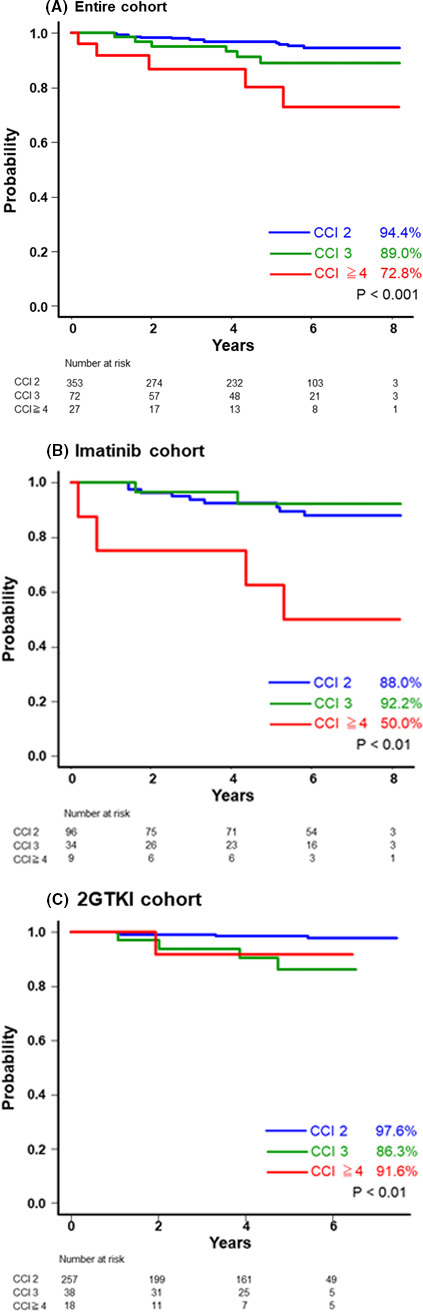
The OS rates in patients with CCI scores of 2, 3, and ≥4 within the entire cohort were 94.4%, 89.0%, and 72.8%, respectively (P < .001; Figure 2A). Although some of the respective OS rates in the 2GTKI cohort were superior compared with those in the imatinib cohort, they varied significantly between the 3 risk groups within either of these 2 cohorts (imatinib cohort: 88.0%, 92.2%, and 50.0% for patients with CCI scores of 2, 3, and ≥4, respectively, P < .01, and the 2GTKI cohort: 97.6%, 86.3%, and 91.6% for patients with CCI scores of 2, 3, and ≥4, respectively; P < .01; Figure 2B,C). Among patients with a CCI risk of ≥4, the imatinib cohort tended to have a lower OS rate (50% in the imatinib cohort vs. 91.6% in the 2GTKI cohort, P = .05). The OS rates, excluding unrelated CML deaths, did not vary substantially among the CCI groups in the entire cohort, as well as in the cohorts receiving imatinib or a 2GTKI (Figure 3A‐C).
FIGURE 2.
Kaplan‐Meier estimates of the rates of overall survival (OS) relative to the CCI score. OS in the entire cohort (A), the imatinib cohort (B), and the 2GTKI cohort (C). 2GTKI, second‐generation tyrosine kinase inhibitor; CCI, Charlson Comorbidity Index
FIGURE 3.
Kaplan‐Meier estimates of the rates of overall survival (OS), excluding CML‐unrelated death, relative to the CCI score. OS, excluding CML‐unrelated death, in the entire cohort (A), the imatinib cohort (B), and the 2GTKI cohort (C). 2GTKI, second‐generation tyrosine kinase inhibitor; CCI, Charlson Comorbidity Index
3.3. Safety data (incidence of AEs and TKI cessation)
Table 3 shows the incidence of AEs and treatment cessation relative to the CCI score. The incidence of hematological AEs (at any grade and grades 3 to 4) did not vary significantly between the CCI risk groups within the entire cohort and the 2GTKI cohort (P = .99 and P = .60 in the entire cohort, and P = .56 and P = .90 in the 2GTKI cohort, respectively), whereas a higher incidence of hematological AEs (at any grade and grade 3 to 4) was observed in patients with a CCI score of ≥4 from the imatinib cohort (P = .01 and P < .01, respectively). The details of hematological AEs are shown in [Link], [Link], [Link]. Within the entire cohort, the incidence of non‐hematological AEs (grades 3 to 4) was higher in patients with a CCI score of ≥4 than in patients from the other risk groups (P < .01; Table 3). In the imatinib cohort, pleural effusion (at any grade) and edema (at any grade) were more frequent in patients with a CCI score of ≥4 than in those from the other risk groups (P = .01 and <.01, respectively; Table S1). Within the 2GTKI cohort, the non‐hematological AEs (grades 3‐4) were more frequent in the risk group with a CCI score of ≥4 than in the other risk groups (P < .01; Table 3). Increased aspartate transaminase (AST) levels (at any grade and grades 3 to 4), pleural effusion (at any grade), and ALT levels (grades 3 to 4) were more frequent in patients with a CCI score of ≥4 than in those with a lower CCI score (P = .01, .02, .001, and <.001, respectively). We also compared the incidence of AEs between the nilotinib and the dasatinib cohort. In the nilotinib cohort, increased AST levels (at any grade and grades 3‐4) and ALT levels (at any grade and grades 3‐4) were more frequent in patients with a CCI score of ≥4 than in those with a lower CCI score (P < .01, <.001, .02, and < .001, respectively; Table S2). In the dasatinib cohort, pleural effusion (at any grade) was more frequently observed in patients with a CCI score of ≥4 than in those with a lower CCI score (P = .04; Table S3). In addition, we compared the incidence of AEs between the imatinib and the 2GTKI cohort based on each CCI group. Among patients with a CCI risk score of 2, the incidence of AEs was more frequently observed in the 2GTKI cohort than in the imatinib cohort, which mainly contributed to increased total bilirubin and lipase in the nilotinib cohort, edema in the dasatinib cohort, and rash in both cohorts (data not shown). The incidence of hematological and non‐hematological AEs at any grade was more frequently observed in the 2GTKI cohort than in the imatinib cohort among patients with a CCI risk of 3 (hematological AEs: 11.8% in the imatinib cohort vs. 36.8% in the 2GTKI cohort, P = .02; non‐hematological AEs: 8.8% in the imatinib cohort vs. 44.7% in the 2GTKI cohort, P = .001). In patients with a CCI risk of ≥4, both TKI cohorts had a similar incidence of AEs (data not shown).
TABLE 3.
The incidence of adverse events and treatment cessation relative to the CCI risk score
| CCI 2 | CCI 3 | CCI ≥ 4 | P‐value | |
|---|---|---|---|---|
| Entire cohort | ||||
| Number of patients (n = 452) | 353 (78.1) | 72 (15.9) | 27 (6.0) | |
| Hematological AEs (at any grade) | 88 (24.9) | 18 (25.0) | 7 (25.9) | .99 |
| Hematological AEs (at grade 3 to 4) | 23 (6.5) | 6 (8.3) | 3 (11.1) | .60 |
| Non‐hematological AEs (at any grade) | 113 (32.0) | 20 (27.8) | 12 (44.4) | .29 |
| Non‐hematological AEs (at grade 3 to 4) | 18 (5.1) | 1 (1.4) | 5 (18.5) | <.01 |
| VAE (at any grade) | 8 (2.3) | 3 (4.2) | 1 (3.7) | .62 |
| TKI cessation | 42 (11.9) | 16 (22.2) | 9 (33.3) | <.01 |
| Imatinib cohort | ||||
| Number of patients (n = 139) | 96 (69.1) | 34 (24.5) | 9 (6.4) | |
| Hematological AEs (at any grade) | 5 (5.2) | 4 (11.8) | 3 (33.3) | .01 |
| Hematological AEs (at grade 3 to 4) | 1 (1.0) | 3 (8.8) | 3 (33.3) | <.01 |
| Non‐hematological AEs (at any grade) | 9 (9.4) | 3 (8.8) | 3 (33.3) | .08 |
| Non‐hematological AEs (at grade 3 to 4) | 0 (0.0) | 0 (0.0) | 0 (0.0) | NE |
| TKI cessation | 16 (16.7) | 6 (17.6) | 3 (33.3) | .46 |
| 2GTKI cohort | ||||
| Number of patients (n = 313) | 257 (82.1) | 38 (12.1) | 18 (5.8) | |
| Hematological AEs (at any grade) | 83 (32.3) | 14 (36.8) | 4 (22.2) | .56 |
| Hematological AEs (at grade 3 to 4) | 22 (8.6) | 3 (7.9) | 1 (5.6) | .90 |
| Non‐hematological AEs (at any grade) | 104 (40.5) | 17 (44.7) | 9 (50.0) | .67 |
| Non‐hematological AEs (at grade 3 to 4) | 18 (7.0) | 1 (2.6) | 5 (27.8) | <.01 |
| TKI cessation | 26 (10.1) | 10 (26.3) | 6 (33.3) | .001 |
Adverse events were graded according to the Common Toxicity Criteria of the Common Toxicity Criteria of the National Cancer Institute (NCI‐CTC) version 4.03.
Abbreviations: 2GTKI, second‐generation tyrosine kinase inhibitor; AEs, adverse events; CCI, Charlson Comorbidity Index; NE, not estimable; TKI, tyrosine kinase inhibitor; VAE, vascular adverse event.
The incidence of VAEs was similar among the CCI score groups of the entire cohort (Table 3) In the imatinib cohort, the incidence of VAEs was significantly higher in the risk group with a CCI score of ≥4 than in the other risk groups, but that was only based on a single patient with a VAE in this cohort (Table S1). There is no difference in the incidence of VAEs among each CCI group in the nilotinib and the dasatinib cohort (Tables S2 and S3).
The entire cohort and the 2GTKI cohort had a higher incidence of TKI cessation in the risk group with a CCI score of ≥4 than in the other risk groups, whereas the risk groups in the imatinib cohort did not significantly differ (P < .01 in the entire cohort, P = .46 in the imatinib cohort, and P = .001 in the 2GTKI cohort; Table 3).
3.4. Cause of death relative to the CCI
Twenty‐four deaths occurred in the entire cohort (15 in the imatinib cohort and 9 in the 2GTKI cohort). In the entire cohort, all‐cause death, CML‐unrelated death, and death caused by cancer and VAE were more frequent among patients with a CCI score of ≥4 than in those with a lower CCI score (P <.01, <.001, <.001, and <.01, respectively; Table 4). In patients treated with imatinib, all‐cause death, CML‐unrelated death, and death caused by cancer, VAE, and infection were more frequent in patients with a CCI score of ≥4 than among those with CCI 2 or 3 (P < .01, <.001, <.01, <.01, and .04, respectively; Table 4). In the 2GTKI cohort, all‐cause death, CML‐related death (only 1 patient in risk group with CCI 3), and death caused by cancer and VAE were more frequent in patients with a higher CCI score than among those with a CCI of 2 (P < .01, .03, .03, and <.01, respectively; Table 4). CML‐unrelated death tended to be more frequent in patients with a CCI score of ≥3 in the 2GTKI cohort (P = .05; Table 4).
TABLE 4.
Cause of death relative to the CCI risk score
| CCI 2 | CCI 3 | CCI ≥ 4 | P‐value | |
|---|---|---|---|---|
| Number of patients (%) | Number of patients (%) | Number of patients (%) | ||
| All patients (n = 452) | 353 (78.1) | 72 (15.9) | 27 (6.0) | |
| Entire cohort | ||||
| Total number of death (all causes) | 13 (3.7) | 6 (8.3) | 5 (18.5) | <.01 |
| Cause of death | ||||
| CML‐related death | 4 (1.1) | 2 (2.8) | 0 (0.0) | .45 |
| CML‐unrelated death | 9 (2.5) | 4 (5.6) | 5 (18.5) | <.001 |
| Cancer | 4 (1.1) | 0 (0.0) | 3 (11.1) | <.001 |
| VAE | 0 (0.0) | 2 (2.8) | 1 (3.7) | <.01 |
| Infection | 3 (0.8) | 0 (0.0) | 1 (3.7) | .21 |
| Others | 2 (0.6) | 2 (2.8) | 0 (0.0) | .17 |
| Imatinib cohort | ||||
| Number of patients (n = 139) | 96 (69.1) | 34 (24.5) | 9 (6.4) | |
| Total number of death (all causes) | 9 (9.4) | 2 (5.9) | 4 (44.4) | <.01 |
| Cause of death | ||||
| CML‐related death | 4 (4.2) | 1 (2.9) | 0 (0.0) | .79 |
| CML‐unrelated death | 5 (5.2) | 1 (2.9) | 4 (44.4) | <.001 |
| Cancer | 3 (3.1) | 0 (0.0) | 2 (22.2) | <.01 |
| VAE | 0 (0.0) | 0 (0.0) | 1 (11.1) | <.01 |
| Infection | 1 (1.0) | 0 (0.0) | 1 (11.1) | .04 |
| Others | 1 (1.0) | 1 (2.9) | 0 (0.0) | .68 |
| 2GTKI cohort | ||||
| Number of patients (n = 313) | 257 (82.1) | 38 (12.1) | 18 (5.8) | |
| Total number of death (all cause) | 4 (1.6) | 4 (10.5) | 1 (5.6) | <.01 |
| Cause of death | ||||
| CML‐related death | 0 (0.0) | 1 (2.6) | 0 (0.0) | .03 |
| CML‐unrelated death | 4 (1.6) | 3 (7.9) | 1 (5.6) | .05 |
| Cancer | 1 (0.4) | 0 (0.0) | 1 (5.6) | .03 |
| VAE | 0 (0.0) | 2 (5.3) | 0 (0.0) | <.01 |
| Infection | 2 (0.8) | 0 (0.0) | 0 (0.0) | .8 |
| Others | 2 (0.6) | 1 (2.6) | 0 (0.0) | .25 |
Abbreviations: 2GTKI, second‐generation tyrosine kinase inhibitor; CCI, Charlson Comorbidity Index; CML, chronic myeloid leukemia; VAE, vascular adverse event.
3.5. Predictive factors for OS
In the multivariate analysis, including the CCI, ECOG PS, the presence of ACAs, ELTS score, age, and the choice of initial TKI treatment, the CCI score of ≥ 4 (vs. CCI score 2), PS ≥ 2 (vs. PS 0 to 1), and the selection of imatinib as first treatment (vs. 2GTKI) were significantly associated with lower OS in the entire cohort (P < .01, .01, and .04, respectively; Table 5). The hazard ratio for the CCI score of ≥4 (vs. score 2) was 5.52 (95% CI: 1.81‐16.88; P < .01). Among these variables, the CCI score of ≥4 was identified as the most powerful adverse prognostic factors for OS (Wald test, P = .01). For each TKI cohort, the CCI score of ≥4 (vs. score 2) in the imatinib cohort and the CCI score of 3 (vs. score 2) and PS ≥2 (vs. PS 0 to 1) in the 2GTKI cohort were identified as prognostic factors affecting the OS in the multivariate analysis. The CCI score of ≥4 for survival also had a strong effect on the imatinib cohort (Wald test; P < .01). A CCI score of 3 in the 2GTKI cohort tended to be related to lower survival (Wald test; P = .06).
TABLE 5.
Multivariate analysis for prognostic factor affecting overall survival
| Variable | Tested category | Reference category | Hazard ratio | Confidence interval | P‐value |
|---|---|---|---|---|---|
| Entire cohort | |||||
| CCI | 3 | 2 | 1.72 | 0.64‐4.66 | .28 |
| ≥4 | 2 | 5.52 | 1.81‐16.88 | <.01 | |
| ECOG PS | ≥2 | 0‐1 | 5.42 | 1.56‐18.85 | .01 |
| ELTS score | Intermediate | Low | 2.07 | 0.68‐6.29 | .20 |
| High | Low | 2.65 | 0.64‐10.91 | .18 | |
| ACA | Yes | No | 2.81 | 0.79‐10.02 | .11 |
| Age (y) | 61‐70 | 18‐60 | 0.86 | 0.22‐3.40 | .82 |
| ≥71 | 18‐60 | 2.22 | 0.65‐7.55 | .20 | |
| Choice of initial TKI treatment | Imatinib | 2GTKI | 2.40 | 1.03‐5.62 | .04 |
| Imatinib cohort | |||||
| CCI | 3 | 2 | 0.73 | 0.15‐3.55 | .70 |
| ≥4 | 2 | 7.66 | 1.95‐30.14 | <.01 | |
| ECOG PS | ≥2 | 0‐1 | 2.45 | 0.41‐14.6 | .32 |
| ACA | Yes | No | 4.43 | 0.93‐21.15 | .06 |
| ELTS score | Intermediate | Low | 2.10 | 0.37‐12.08 | .41 |
| High | Low | 3.65 | 0.36‐37.44 | .28 | |
| Age (y) | 61‐70 | 18‐60 | 0.49 | 0.05‐4.73 | .54 |
| ≥71 | 18‐60 | 2.19 | 0.36‐13.3 | .40 | |
| 2GTKI cohort | |||||
| CCI | 3 | 2 | 6.26 | 1.39‐28.26 | <.01 |
| ≥4 | 2 | 3.67 | 0.32‐41.71 | .29 | |
| ECOG PS | ≥2 | 0‐1 | 15.32 | 2.47‐94.89 | <.01 |
| ACA | Yes | No | NE | NE | NE |
| ELTS score | Intermediate | Low | 1.07 | 0.15‐7.45 | .95 |
| High | Low | 1.53 | 0.15‐16.04 | .72 | |
| Age (y) | 61‐70 | 18‐60 | 0.65 | 0.08‐5.42 | .69 |
| ≥71 | 18‐60 | 2.57 | 0.32‐20.39 | .37 | |
Abbreviations: 2GTKI, second‐generation tyrosine kinase inhibitor; ACA, additional chromosomal abnormality; CCI, Charlson Comorbidity Index; ECOG, Eastern Cooperative Oncology Group; ELTS, European Treatment and Outcome Study (EUTOS) long‐term survival score; NE, not estimable; PS, performance status.
4. DISCUSSION
Our study demonstrated that the comorbidities at diagnosis were the only independent negative prognostic factor for OS in a population‐based cohort of patients with CML‐CP treated with imatinib or a 2GTKI (69.3% of all patients were treated with a 2GTKI). The cumulative incidence of treatment response and progression to AP/BP were similar among the CCI risk scores in the entire cohort, as well as in the imatinib and 2GTKI cohorts. These results indicated that the life expectancy in patients with CML‐CP became almost the same as in the general population because CML was nonfatal in most of the former patients following the introduction of TKIs, and the life span of patients with CML‐CP depended on comorbidities, as in the general population.
A previous report on CML study IV, including 1519 patients with CML‐CP treated with imatinib, showed that hematological and non‐hematological AEs were slightly more frequent in patients with a higher AA‐CCI risk score than in those with a low score, but the difference was not significant. 8 In our study, hematological toxicities (at any grade and grades 3‐4) were more frequently observed in patients with CML with the higher CCI risk score in the imatinib cohort. AA‐CCI may not be an appropriate choice for predicting the effect between comorbidity itself and cumulative incidence of AEs, because approximately 60% of patients with an AA‐CCI score of ≥5 had no comorbidity (with age as the only factor) in this study. When we used an AA‐CCI risk score for reanalyzing the effect on the incidence of AEs, there was no negative association with comorbidity and adverse events in the imatinib cohort, which was in line with the previous report. 8 Conversely, the incidence of non‐hematological toxicities at grades 3‐4 was higher in patients with comorbidities than in those without comorbidities in a 2GTKI cohort. Especially, the provability of pleural effusion (at any grade) was higher in patients with a CCI score ≥4, and most patients with pleural effusion received dasatinib. A similar result has been reported in elderly patients with CML treated with second‐line dasatinib. 12
The DMR (MR4.0, BCR‐ABL1 IS ≤ 0.01%), or MR4.5 (BCR‐ABL1 IS ≤ 0.0032%) was a critical milestone for the eligibility criteria in the treatment‐free remission trial. 13 , 14 , 15 , 16 , 17 Patients with comorbidities should avoid long‐term TKI treatment because of late‐onset toxicities, such as cardiovascular events or pulmonary toxicities, and TKI cessation for treatment‐free remission might be considered whenever possible. No differences in reaching MR4.5 were reported in relation to the AA‐CCI risk score in patients with CML treated with imatinib. 8 We also demonstrated that the cumulative incidence of MR4.5 at 36 mo and the median time to MR4.5 (961 d in the imatinib cohort vs. 724 d in the 2GTKI cohort; P = .12) were similar among patients with a CCI score of ≥3 treated with imatinib or a 2GTKI, but no patient with a CCI score of ≥3 from the imatinib cohort achieved MR4.5 at 36 mo (0% in the imatinib cohort vs. 34.1% in the 2GTKI cohort; P = .13). These results from our study indicated that a DMR and the possibility of treatment‐free remission could be achieved even in patients with comorbidities by treatment with 2GTKIs in clinical practice. Some recent studies were focused on reduced TKI dosing. 18 , 19 , 20 Naqvi et al observed a favorable response rate in patients with CML‐CP treated with dasatinib at a daily dose of 50 mg, compared with the response in those patients treated with dasatinib at a standard daily dose of 100 mg, as shown in the DASISION study, although the study design was limited to a single‐arm phase 2 trial. 18 The lower rates of toxicities, including pleural effusion and interruption due to AEs were also observed in a cohort receiving dasatinib at a daily dose of 50 mg, compared with those in the classical control of the DASISION trial (dasatinib at a daily dose of 100 mg). 3 Furthermore, the De‐Escalation and Stopping Treatment with Imatinib, Nilotinib, or sprYcel (DESTINY) trial demonstrated that the de‐escalation of TKI dosing might improve the proportion of patients with stable MR4 who could successfully undergo a treatment‐free‐remission attempt. 19 , 20 These treatment approaches, which use a reduced TKI dose as an initial treatment or after a stable molecular response, may minimize treatment‐related toxicities and ensure the long‐term safety of TKI treatment, especially in patients with preexisting comorbidities.
Although we expected that the presence of comorbidities at CML diagnosis would have a significant effect on predicting the inferior survival rate in the 2GTKI cohort, compared with that in the imatinib cohort, our approach could clearly not discriminate the OS rate relative to the CCI risk score in patients initially treated with 2GTKIs. It is possible that determining a significant prognostic factor for survival in patients with CML was difficult because of the small number of death events, especially in the 2GTKI cohort in our study. Even though patients had some underlying disease at diagnosis, favorable outcomes could be obtained in patients with CML treated with 2GTKIs by administering a reduced dose with tolerable toxicities. Another factor with a potential effect on our results could be the physicians’ preference for administering imatinib to patients with a high CCI risk score in clinical practice, generating strong effects of comorbidities on survival in the imatinib cohort, compared with those in the 2GTKI cohort.
This study has some limitations. Firstly, there is a possible bias because the TKI selection, management, and monitoring frequency for AEs depended on the physician's decision based on the underlying condition or the age factor. Thus, these potential biases that may have affected our current findings could represent the disadvantages of population‐based cohorts. Secondly, the relatively small sample size of patients with a high CCI risk score may limit the accuracy of our analysis. The effect of the CCI risk score at diagnosis on clinical outcomes might have been stronger with a larger cohort, including more patients with comorbidities. Moreover, the applicability of this risk score should have been confirmed by validation in an independent test set of patients with CML receiving a 2GTKI. Thirdly, the CCI score did not include some critical factors, such as PS, hypertension, angina, and hyperlipidemia, which might be associated with CML‐unrelated death. Further studies using a risk score that included these factors might be needed to assess the long‐term safety of TKI treatment and survival in patients with CML‐CP.
However, despite these limitations, our study demonstrated that the assessment of comorbidities based on the CCI score was a meaningful tool for predicting survival in patients with CML‐CP treated with imatinib or a 2GTKI. The clinical importance for evaluating preexisting comorbidities did not change even with the introduction of a 2GTKI as front‐line therapy. Furthermore, our study provides some critical information about the efficacy and incidence of AEs according to each CCI risk score, as well as treatment cessation, which could serve as guidance for treatment decisions in clinical practice.
Clinicians should not only evaluate the leukemia‐specific prognostic risk score, but they could also consider careful management based on the comorbidity risk scores, and aim at a DMR regardless of the TKI type administered to the CML‐CP patient. Furthermore, future studies with more patients will be needed to identify a new prognostic score that includes disease‐specific factors and underlying medical conditions (ie, comorbidities, age factors, and PS).
CONFLICT OF INTEREST
The authors have no conflict of interest relative to this manuscript.
ETHICAL APPROVAL
This sub‐analysis was approved by the institutional review board of the Hamamatsu University School of Medicine and conducted in accordance with the principles of the Declaration of Helsinki. Written informed consent was obtained from each participant before enrollment for the New TARGET observational study 1, which was registered with the UMIN‐CTR (UMIN 00003581).
Supporting information
Table S1
Table S2
Table S3
ACKNOWLEDGMENTS
This sub‐analysis was performed based on previously published data from the New TARGET observational study 1 (UMIN 00003581) conducted by the JSH. The New TARGET observational study 1 was supported by research funding to JSH from Novartis Pharmaceuticals and Bristol‐Myers Squibb. We would like to express our gratitude to all the study participants and their families, and the study investigators at participating study sites. We also thank the New TARGET data center (EPS. Co.).
Ono T, Takahashi N, Kizaki M, et al. Prognostic effect of comorbidities in patients with chronic myeloid leukemia treated with a tyrosine kinase inhibitor. Cancer Sci. 2020;111:3714–3725. 10.1111/cas.14580
REFERENCES
- 1. Hochhaus A, Larson RA, Guilhot F, et al. Long‐Term Outcomes of Imatinib Treatment for Chronic Myeloid Leukemia. N Engl J Med. 2017;376:917‐927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hochhaus A, Saglio G, Hughes TP, et al. Long‐term benefits and risks of frontline nilotinib vs imatinib for chronic myeloid leukemia in chronic phase: 5‐year update of the randomized ENESTnd trial. Leukemia. 2016;30:1044‐1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cortes JE, Saglio G, Kantarjian HM, et al. Final 5‐year study results of DASISION: The Dasatinib versus imatinib study in treatment‐naive chronic myeloid leukemia patients trial. J Clin Oncol. 2016;34:2333‐2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bower H, Bjorkholm M, Dickman PW, Hoglund M, Lambert PC, Andersson TM. Life expectancy of patients with chronic myeloid leukemia approaches the life expectancy of the general population. J Clin Oncol. 2016;34:2851‐2857. [DOI] [PubMed] [Google Scholar]
- 5. Pfirrmann M, Baccarani M, Saussele S, et al. Prognosis of long‐term survival considering disease‐specific death in patients with chronic myeloid leukemia. Leukemia. 2016;30:48‐56. [DOI] [PubMed] [Google Scholar]
- 6. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373‐383. [DOI] [PubMed] [Google Scholar]
- 7. Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47:1245‐1251. [DOI] [PubMed] [Google Scholar]
- 8. Saussele S, Krauss MP, Hehlmann R, et al. Impact of comorbidities on overall survival in patients with chronic myeloid leukemia: results of the randomized CML study IV. Blood. 2015;126:42‐49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Uemura M, Imataki O, Kawachi Y, et al. Charlson comorbidity index predicts poor outcome in CML patients treated with tyrosine kinase inhibitor. Int J Hematol. 2016;104:621‐627. [DOI] [PubMed] [Google Scholar]
- 10. Kizaki M, Takahashi N, Iriyama N, et al. Efficacy and safety of tyrosine kinase inhibitors for newly diagnosed chronic‐phase chronic myeloid leukemia over a 5‐year period: results from the Japanese registry obtained by the New TARGET system. Int J Hematol. 2019;109:426‐439. [DOI] [PubMed] [Google Scholar]
- 11. Kanda Y. Investigation of the freely available easy‐to‐use software 'EZR' for medical statistics. Bone Marrow Transplant. 2013;48:452‐458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Breccia M, Latagliata R, Stagno F, et al. Charlson comorbidity index and adult comorbidity evaluation‐27 scores might predict treatment compliance and development of pleural effusions in elderly patients with chronic myeloid leukemia treated with second‐line dasatinib. Haematologica. 2011;96:1457‐1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hughes TP, Ross DM. Moving treatment‐free remission into mainstream clinical practice in CML. Blood. 2016;128:17‐23. [DOI] [PubMed] [Google Scholar]
- 14. Hochhaus A, Saussele S, Rosti G, et al. Chronic myeloid leukaemia: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow‐up. Ann Oncol. 2017;28:iv41‐iv51. [DOI] [PubMed] [Google Scholar]
- 15. Takahashi N, Nishiwaki K, Nakaseko C, et al. Treatment‐free remission after two‐year consolidation therapy with nilotinib in patients with chronic myeloid leukemia: STAT2 trial in Japan. Haematologica. 2018;103:1835‐1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Takahashi N, Tauchi T, Kitamura K, et al. Deeper molecular response is a predictive factor for treatment‐free remission after imatinib discontinuation in patients with chronic phase chronic myeloid leukemia: the JALSG‐STIM213 study. Int J Hematol. 2018;107:185‐193. [DOI] [PubMed] [Google Scholar]
- 17. Rea D, Ame S, Berger M, et al. Discontinuation of tyrosine kinase inhibitors in chronic myeloid leukemia: Recommendations for clinical practice from the French Chronic Myeloid Leukemia Study Group. Cancer. 2018;124:2956‐2963. [DOI] [PubMed] [Google Scholar]
- 18. Naqvi K, Jabbour E, Skinner J, et al. Early results of lower dose dasatinib (50 mg daily) as frontline therapy for newly diagnosed chronic‐phase chronic myeloid leukemia. Cancer. 2018;124:2740‐2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Clark RE, Polydoros F, Apperley JF, et al. De‐escalation of tyrosine kinase inhibitor dose in patients with chronic myeloid leukaemia with stable major molecular response (DESTINY): an interim analysis of a non‐randomised, phase 2 trial. Lancet Haematol. 2017;4:e310‐e316. [DOI] [PubMed] [Google Scholar]
- 20. Clark RE, Polydoros F, Apperley JF, et al. De‐escalation of tyrosine kinase inhibitor therapy before complete treatment discontinuation in patients with chronic myeloid leukaemia (DESTINY): a non‐randomised, phase 2 trial. Lancet Haematol. 2019;6:e375‐e383. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Table S2
Table S3


