Abstract
OBJECTIVE
Evidence is limited about important maternal and neonatal risk factors that affect neonatal renal function. The incidence of acute kidney injury (AKI) and identification of associated risk factors in neonates exposed to antenatal indomethacin was studied.
METHODS
A retrospective cohort of neonates exposed to antenatal indomethacin within 1 week of delivery was analyzed for development of AKI up to 15 days of life. Adjusted hazard ratios (HRs) and 95% CIs for AKI risk were calculated in time-dependent Cox proportional hazards models.
RESULTS
Among 143 neonates with mean gestational age of 28.3 ± 2.4 weeks, AKI occurred in 62 (43.3%), lasting a median duration of 144 hours (IQR, 72–216 hours). Neonates with AKI had greater exposure to postnatal NSAIDs (48.4% vs 9.9%, p < 0.001) and inotropes (37.1% vs 3.7%, p < 0.001) compared with neonates without AKI. In multivariable-adjusted models, increased AKI risk was observed with antenatal indomethacin doses received within 24 to 48 hours (HR, 1.6; 95% CI, 1.28–1.94; p = 0.036) and <24 hours (HR, 2.33; 95% CI, 1.17–4.64; p = 0.016) prior to delivery. Further, postnatal NSAIDs (HR, 2.8; 95% CI, 1.03–7.61; p = 0.044), patent ductus arteriosus (HR, 4.04; 95% CI, 1.27–12.89; p = 0.018), and bloodstream infection (HR, 3.01; 95% CI, 1.37–6.60; p = 0.006) were associated significantly with increased risk of AKI following antenatal indomethacin. Neonates with AKI experienced more bloodstream infection, severe intraventricular hemorrhage, patent ductus arteriosus, respiratory distress syndrome, and longer hospitalization.
CONCLUSIONS
Extended risk of AKI with antenatal indomethacin deserves clinical attention among this population at an already increased AKI risk.
Keywords: acute kidney injury, indomethacin, nonsteroidal anti-inflammatory drugs, prematurity, tocolytics
Introduction
Acute kidney injury (AKI), previously referred to as acute renal failure, is a syndrome that results from abrupt deterioration in glomerular filtration rate (GFR).1 In neonates, the extent of AKI can range from mild, reversible kidney dysfunction to complete, potentially irreversible, anuric kidney failure.2 Preterm neonates are at an increased risk for development of AKI due to perinatal and postnatal factors, including exposure to nephrotoxic medications (e.g., NSAIDs and aminoglycosides), hypoxia, and recurrent infections resulting in multiorgan failure. Although the exact incidence of neonatal AKI is unknown, studies have estimated an incidence of 8% to 40% in preterm neonates, with an associated mortality rate of 45% to 70%.2 Acute kidney injury diagnosed soon after birth is associated with longer hospitalizations as well as an increased risk of developing chronic kidney disease.2,3
Indomethacin, a nonselective prostaglandin synthetase inhibitor, has shown efficacy in delaying preterm birth since the early 1970s. Although commonly used in obstetric practice as a tocolytic agent in women less than 32 weeks' gestation, concerns exist for serious maternal and fetal adverse events. Maternal adverse events, including esophageal reflux, nausea, and gastritis, appear to be minor when compared to maternal adverse events associated with other tocolytic agents.4 Indomethacin rapidly crosses the placenta, resulting in fetal concentrations equivalent to maternal concentrations within 6 hours of drug exposure.5 In both term and preterm infants, the mean half-life of indomethacin is prolonged (14.7 hours and ≥24 hours, respectively) compared with adults (2.5 hours).5 Fetal adverse events, such as premature closure of the ductus arteriosus, oligohydramnios, hyperbilirubinemia, necrotizing enterocolitis (NEC), and decreased renal function, have been reported.4 Additionally, known renal effects of indomethacin include decreased renal perfusion, oliguria, anuria, fluid retention, and hyperkalemia secondary to indomethacin reduction in plasma renin activity.6
Acute kidney injury secondary to indomethacin has been reported in women who received indomethacin for tocolysis. Steiger et al7 reported 3 cases of maternal AKI in pregnant women who received indomethacin for tocolysis. In these 3 cases, all pregnant women had normal serum creatinine (SCr) concentrations prior to tocolytic therapy and subsequently developed AKI within 30 hours of indomethacin initiation. Resolution of kidney function occurred on average within 5 days after indomethacin exposure. Because indomethacin readily crosses the placenta and maternal exposure has been associated with maternal AKI, there are concerns regarding the potential for and degree of risk of antenatal indomethacin-induced neonatal AKI.
In our single-institution–based cohort, indomethacin is commonly prescribed to prevent preterm labor in pregnant women <32 weeks' gestation. The objective of this study was to determine the incidence of AKI in neonates exposed to indomethacin tocolysis and to identify maternal and neonatal risk factors for the development of AKI in these patients. We further analyzed secondary morbidities, including NEC, spontaneous intestinal perforation (SIP) and intraventricular hemorrhage (IVH).
Methods
This was a retrospective, single-center study of preterm neonates at the Children's Hospital University of Illinois, University of Illinois Hospital and Health Sciences System (UI Health).
Patient Inclusion and Exclusion Criteria. Neonates exposed to antenatal indomethacin for tocolysis within 1 week of delivery at UI Health from January 1, 2006, to October 15, 2011, were included in this study. Neonates with congenital renal malformations and those who died or were transferred within 2 weeks of delivery were excluded. A computer-generated list, which included all women who received indomethacin for tocolysis during the study period, was provided by the pharmacy information technology department. Neonates were identified for study inclusion based on evaluation of maternal medical records.
Data Collection. The mothers' electronic medical records were reviewed to determine maternal age, the cumulative indomethacin dose, and the time of last indomethacin dose. The most recent maternal SCr prior to delivery (within 1 year) was collected to determine elevations in neonatal SCr that may parallel maternal SCr. Potential maternal risk factors for the development of neonatal AKI were identified, including maternal medical diagnoses and/or medications that may affect renal function (preeclampsia, HELLP syndrome, chronic maternal hypertension, oligohydramnios, absence/reversal of end-diastolic flow in the umbilical artery on prenatal ultrasound, mode of delivery, and maternal aminoglycoside therapy).
Neonatal demographic data, including gestational age, birth weight, length, 5-minute Apgar scores, sex, race, date/time of birth, and date of death (if applicable) were collected. Potential postnatal risk factors, including medications (inotropes [dopamine/dobutamine/epinephrine], indomethacin for IVH prophylaxis, indomethacin/ibuprofen for patent ductus arteriosus [PDA] treatment, and nephrotoxic agents [aminoglycosides, vancomycin, digoxin, and amphotericin]) used within 15 days of life prior to development of AKI were also collected. Other outcomes measured through day of life 15 included the presence of culture-positive sepsis or bloodstream infection (BSI) and respiratory distress syndrome (RDS).
Markers of renal function included blood urea nitrogen, SCr, and urine output (UOP). For the purpose of this study, AKI was classified using the AKIN criteria.8 A subgroup analysis using nRIFLE8 and KDIGO10 criteria for the diagnosis of AKI was also analyzed (Table 1). The days to normalization of SCr and UOP were assessed to calculate the duration of AKI. For neonates with both SCr and UOP derangements, the duration of AKI was based on the AKI qualifying criterion that lasted the longest.
Table 1.
| Stage and Class | Serum Creatinine Criteria† | Urine Output Criteria† | ||||||
|---|---|---|---|---|---|---|---|---|
| AKIN | KDIGO | nRILFE‡ | Standard | AKIN | KDIGO | nRIFLE | Standard | |
| 1. Injury | ↑ SCr ≥0.3 OR ↑ SCr ≥1.5–2 × from baseline | ↑ SCr ≥0.3 within 48 hr OR ↑SCr ≥1.5–1.9 × baseline within 7 days | ↑ SCr by 200% OR GFR ↓ >50% | SCr >1.5 | <0.5 for >6 hr | <0.5 for 6–12 hr | < 1 × 24 hr | < 1 × 24 hr |
| 2. Failure | ↑SCr > 2–3 × from baseline | — | ↑ SCr by 300% OR GFR ↓ >75% OR SCr ≥4 (acute rise ≥4) | <0.5 for >12 hr | — | <0.7 for 24 hr OR anuric for 12 hr | — | |
| 3. Loss | ↑ SCr >3 × from baseline OR SCr ≥4 with an acute ↑ >0.5 | — | Persistent failure > 4 wk | <0.3 for 24 hr OR anuric for 12 hr | — | Persistent failure > 4 wk | — | |
AKIN, Acute Kidney Injury Network; GFR, glomerular filtration rate; KDIGO, Kidney Disease Improving Global Outcomes; nRIFLE, neonatal Risk, Injury, Failure, Loss, End-Stage; SCr, serum creatinine; UOP, urine output
* AKI is defined as stage ≥ 1 or class injury or higher.
† Units for SCr are mg/dL and units for UOP are mL/kg/hr.
‡ Use adult RIFLE SCr criteria for AKI.
Patient charts were evaluated for neonatal complications associated with indomethacin exposure including: the presence of NEC ≥stage 2, SIP, and IVH (all grades as described by Papile et al11) throughout hospital stay. The diagnosis of NEC ≥stage 2 was defined as the presence of pneumatosis intestinalis. Both NEC and SIP were confirmed by a radiologist on abdominal radiograph. Detection of IVH was based on cranial ultrasonography reports. Lastly, length of hospital stay, 28-day mortality, and overall mortality were assessed.
Outcomes. The primary outcome measures were to determine the incidence of AKI in neonates exposed to indomethacin tocolysis and to identify maternal and neonatal risk factors for the development of AKI in these neonates. The secondary objective was to determine the incidence of neonatal morbidities, such as NEC, SIP, and IVH, in neonates exposed to indomethacin tocolysis.
Statistical Analyses. To compare differences in maternal and neonatal characteristics by development of AKI, we used independent samples t tests for means and Wilcoxon rank-sum tests for medians of continuous variables. We performed χ2 tests (Fisher exact test for cells <5) for comparison of categoric variables. Hypothesis tests were considered to be statistically significant at p ≤ 0.05.
Time-dependent multivariable Cox proportional hazards models were used to estimate adjusted hazard ratios (HRs) and 95% CIs for characteristics associated with development of AKI within 15 days of life. Neonates entered the analysis at the time of birth (time scale: days) and were followed until the first of AKI, death, 15 days of life, or end of the study period (censoring time). We included covariates in our models selected a priori as confounders or predictors of AKI or found to be empirically different by AKI status. Postnatal exposures, such as treatment of PDA, were treated as unidirectional time-varying covariates, allowing for daily, continuous exposure measurement. We evaluated the proportional hazards assumption graphically (by log-log plots) as well as in covariate-specific and global tests of the models. We found no evidence suggesting violation of the proportionality assumption. All analyses were performed using Stata Statistical Software: release 15 (StataCorp LLC, College Station, TX).
Results
A total of 624 maternal medical charts were reviewed for study inclusion. Of the women who received antenatal indomethacin tocolysis, 125 women delivered newborns within 1 week of their last indomethacin dose. Multiple gestations accounted for 25 pregnancies (22 sets of twins and 3 sets of triplets). A total of 153 neonates were exposed to antenatal indomethacin within 1 week prior to birth and qualified for study inclusion. Ten neonates were excluded: 2 for renal anomalies (e.g., multicystic dysplastic kidneys, hydronephrosis and megaureters), 6 died, and 2 were transferred within 2 weeks from delivery. A total of 143 preterm neonates were included in the study analysis.
Descriptive maternal and neonatal characteristics are reported by AKI status in Table 2. The average maternal age was 26.6 ± 6.3 years and mean maternal SCr prior to delivery was 0.7 ± 0.2 mg/dL. Fetuses were exposed to a median cumulative dose of 225 mg (IQR, 112–325 mg; range, 25–1350 mg) of indomethacin prior to delivery, with the last dose of indomethacin given at a median of 25 hours (IQR, 7.6–93.3 hours; range, 0.58–156 hours) prior to birth. No statistically significant differences were seen with maternal baseline demographics between groups. However, infants with AKI had a slightly greater proportion of maternal indomethacin doses occurring within 24 hours of delivery compared with infants without AKI (51.7% vs 43.2%, p = 0.540). A total of 66% of neonates were male, with a mean gestational age of 28.3 ± 2.4 weeks and birth weight of 1266 ± 418 g. Most patients (65.7%) were African American. Neonates less than 25 weeks' gestation developed AKI at a significantly higher rate than not developing AKI (11.3% vs 1.2%, p < 0.001). They also had lower mean birth weight (1032 vs 1444 g, p < 0.001) and 5-minute Apgar score (7.3 vs 8.0, p = 0.002).
Table 2.
Characteristics of Infants With and Without Acute Kidney Injury (AKI)
| Parameter | All Infants (N = 143) | Infants With AKI (n = 62) | Infants Without AKI (n = 81) | p value* |
|---|---|---|---|---|
| Maternal characteristics | ||||
| Maternal age, mean ± SD, yr | 26.6 ± 6.3 | 26.7 ± 5.3 | 26.6 ± 7 | 0.923 |
| SCr prior to delivery, mean ± SD, mg/dL | 0.7 ± 0.2 | 0.7 ± 0.2 | 0.7 ± 0.2 | 0.974 |
| Antenatal cumulative INDO dose, median (IQR), mg | 225 (112–325) | 200 (75–300) | 250(125–350) | 0.115 |
| Time from last antenatal INDO dose to delivery, median (IQR), hr | 25.4 (7.6–93.3) | 23.1 (5.0–79.1) | 34.1 (8.9–111) | 0.348 |
| >48, n (%) | 58 (40.5) | 22 (35.5) | 36 (44.4) | |
| 24–48, n (%) | 18 (12.6) | 8 (12.9) | 10 (12.3) | 0.540 |
| <24, n (%) | 67 (46.9) | 32 (51.7) | 35 (43.2) | |
| Chronic hypertension/preeclampsia, n (%) | 16 (11.2) | 4 (6.5) | 12 (14.8) | 0.180 |
| Cesarean delivery, n (%) | 71 (49.7) | 30 (48.4) | 41 (50.6) | 0.792 |
| Neonatal characteristics | ||||
| Gestational age, mean ± SD, wk | 28.3 ± 2.4 | 26.8 ± 2.2 | 29.4 ± 1.9 | <0.001† |
| <25, n (%) | 8 (5.6) | 7 (11.3) | 1 (1.2) | |
| 25–28, n (%) | 68 (47.5) | 42 (67.7) | 26 (32.1) | <0.001† |
| >28, n (%) | 67 (46.9) | 13 (21.0) | 54 (66.7) | |
| Birth weight, mean ± SD, g | 1265.6 ± 418.2 | 1032.2 ± 326.2 | 1444.3 ± 393.4 | <0.001† |
| <1000, n (%) | 48 (33.5) | 37 (59.7) | 11 (13.6) | |
| 1000–1500, n (%) | 50 (35) | 17 (27.4) | 33 (40.7) | <0.001† |
| >1500, n (%) | 45 (31.5) | 8 (12.9) | 37 (45.7) | |
| Male, n (%) | 94 (65.7) | 42 (67.7) | 52 (64.2) | 0.658 |
| Race, n (%) | ||||
| Black | 94 (65.7) | 40 (64.5) | 54 (66.7) | |
| Hispanic | 23 (16.1) | 10 (16.1) | 13 (16) | |
| White | 9 (6.3) | 4 (6.5) | 5 (6.2) | 0.995 |
| Asian | 2 (1.4) | 1 (1.6) | 1 (1.2) | |
| Other | 15 (10.5) | 7 (11.3) | 8 (9.9) | |
| 5-minute Apgar score, mean ± SD | 7.7 ± 1.2 | 7.3 ± 1.2 | 8.0 ± 1.2 | 0.002† |
| 4 to 6, n (%) | 22 (15.4) | 13 (21) | 9 (11.1) | 0.105 |
| 7 to 9, n (%) | 121 (84.6) | 49 (79) | 72 (88.9) | |
INDO, indomethacin; SCr, serum creatinine
* To test for differences between groups we used independent samples t tests for continuous variables, Wilcoxon rank-sum test for medians, and χ2 tests for categorical variables (Fisher exact test for cells <5).
† Values are statistically significant.
The cumulative incidence of AKI within 15 days of life was 43.3%, and the conditional cumulative hazards of AKI in neonates exposed to antenatal indomethacin within 1 week of delivery are reported in Figure 1. Among the 62 neonates with AKI, 57 (91.9%) met AKI criteria based on SCr alone, 3 (4.8%) based on UOP alone, and 2 (3.2%) based on both SCr and UOP derangements. The median days from birth to the onset of AKI was 2.8 days (IQR, 1.8–9.9 days; range, 1–13 days); 37.1% occurred within 1 to 2 days, and 62.9% occurred at greater than 2 days from birth. The median time to the onset of AKI from the last maternal dose of indomethacin was 6 days (IQR, 3–11.2 days; range, 1.1–18.2 days); 51.7% of the neonates developed AKI within 24 hours, 12.9% of the neonates developed AKI within 24 to 48 hours, and 35.5% developed AKI at greater than 48 hours from the last dose of maternal indomethacin.
Figure 1.
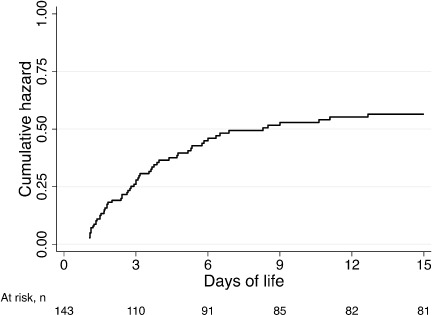
Cumulative hazards of acute kidney injury (AKI).
Acute kidney injury lasted a median duration of 144 hours (IQR, 72–216 hours; range, 8–840 hours). Of the 59 neonates with AKI based on SCr alterations, it took a median of 6 days (IQR, 3–9 days; range, 1–35 days) for the SCr to normalize. Furthermore, it took a median of 0.5 days (IQR, 0.25–1.0 days; range, 0.33–4 days) for the urine output to normalize.
The prevalence of risk factors within 15 days of life prior to development of AKI are reported in Table 3. Infants with AKI had greater exposure to NSAIDs overall (48.4% vs 9.9%, p < 0.001), but specifically for postnatal indomethacin treatment (51.6% vs 8.6%, p < 0.001) and not statistically significant for postnatal indomethacin prophylaxis (9.7% vs 2.5%, p = 0.077). Neonates with AKI also had greater exposure to inotropes (37.1% vs 3.7%, p < 0.001) but were similar with respect to use of gentamicin and vancomycin prior to AKI or in the first 15 days of life. Prior to the occurrence of AKI, neonates in the AKI group had significantly higher rates of PDA (48.4% vs 8.6%, p < 0.001). Cumulative hazard functions for AKI by time from maternal indomethacin dose to delivery, postnatal NSAIDs, and presence of PDA and BSIs are shown in Figure 2.
Table 3.
Neonatal Risk Factors Associated with Acute Kidney Injury (AKI)
| Risk Factor | All Infants (N = 143), n (%) | Infants with AKI (n = 62), n (%) | Infants without AKI (n = 81), n (%) | p value* |
|---|---|---|---|---|
| NSAIDs (any) | 38 (26.6) | 30 (48.4) | 8 (9.9) | <0.001† |
| INDO prophylactic | 8 (5.6) | 6 (9.7) | 2 (2.5) | 0.077 |
| INDO treatment | 39 (27.3) | 32 (51.6) | 7 (8.6) | <0.001† |
| Inotropes | 26 (18.2) | 23 (37.1) | 3 (3.7) | <0.001† |
| Gentamicin | 132 (92.3) | 59 (95.2) | 73 (90.1) | 0.263 |
| Vancomycin | 12 (8.4) | 3 (4.8) | 9 (11.1) | 0.232 |
| Severe IVH | 6 (4.2) | 5 (8.1) | 1 (1.2) | 0.086 |
| Patent ductus arteriosus | 37 (25.9) | 30 (48.4) | 7 (8.6) | <0.001† |
INDO, indomethacin; IVH, intraventricular hemorrhage
* To test for differences between groups we used χ2 tests for categorical variables (Fisher's exact test for cells <5).
† Values are statistically significant.
Figure 2.
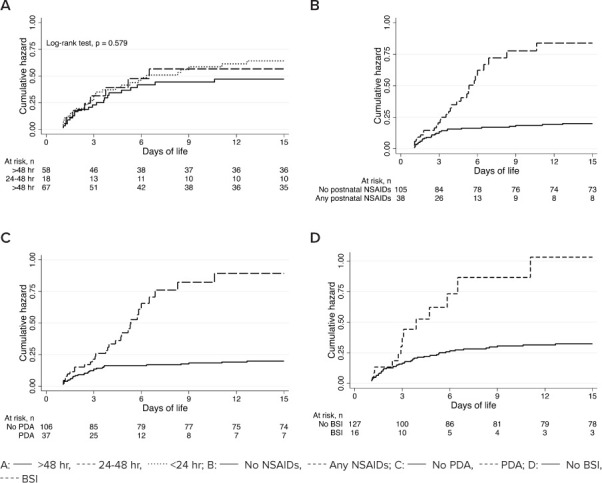
Cumulative hazards of acute kidney injury (AKI). (A) Time from maternal indomethacin dose to delivery, (B) Postnatal NSAIDs, (C) Patent ductus arteriosus (PDA), and (D) Bloodstream infection (BSI).
Results from multivariable analyses are reported in Table 4. No significant differences in risk of AKI were observed by cumulative maternal indomethacin dose, although timing of last indomethacin dose prior to delivery was associated with increased risk of AKI. Compared with neonates that were exposed to maternal indomethacin >48 hours prior to delivery, neonates exposed to indomethacin between 24 and 48 hours (HR, 1.60; 95% CI, 1.28–1.94; p = 0.036) and <24 hours (HR, 2.33; 95% CI, 1.17–4.64; p = 0.016) prior to delivery had significantly increased risk of AKI with suggestion of a time-dependent relationship with indomethacin doses and delivery (p = 0.020). These findings on timing of maternal indomethacin adjusted for other characteristics, including postnatal treatment with NSAIDs (HR, 2.80; 95% CI, 1.03–7.61; p = 0.044) and PDA (HR, 4.04; 95% CI, 1.27–12.89; p = 0.018), were significantly associated with increased risk of AKI. A trend suggesting correlation between maternal hypertension and/or preeclampsia and AKI was observed (HR, 0.18; 95% CI, 0.03–1.01; p = 0.051). In addition, findings suggested increased AKI risk with neonatal exposure to inotropes (HR, 2.25; 95% CI, 0.91–5.59; p = 0.078), although not statistically significant.
Table 4.
Cox Proportional Hazards Models Relating Maternal and Neonatal Characteristics With Development of Acute Kidney Injury (AKI)
| Parameter | Crude HR (95% CI) | p value* | Adjusted HR (95% CI) | p value* | ||
|---|---|---|---|---|---|---|
| Maternal age, yr | ||||||
| <20 | Reference | |||||
| 20–24 | 9.34 (1.25, 69.46) | 0.029† | 11.24 (1.34, 94.29) | 0.026† | ||
| 25–29 | 10.28 (1.37, 77.34) | 0.024† | 16.92 (1.80, 158.85) | 0.013† | ||
| ≥30 | 5.45 (0.74, 40.40) | 0.097 | 7.70 (0.87, 66.85) | 0.064 | ||
| Cumulative INDO dose, mg | ||||||
| <125 | Reference | |||||
| 125–199 | 1.11 (0.50, 2.48) | 0.793 | 1.15 (0.37, 3.57) | 0.809 | ||
| 200–299 | 0.95 (0.46, 1.97) | 0.887 | 1.79 (0.69, 4.65) | 0.229 | ||
| ≥300 | 0.65 (0.34, 1.25) | 0.198 | 0.76 (0.33, 1.76) | 0.521 | ||
| Time from last INDO dose to delivery, hr | ||||||
| >48 | Reference | |||||
| 24–48 | 1.23 (0.55, 2.77) | 0.611 | 1.60 (1.28, 1.94) | 0.036† | ||
| <24 | 1.33 (0.77, 2.29) | 0.301 | 2.33 (1.17, 4.64) | 0.016† | ||
| Chronic hypertension/preeclampsia | 0.32 (0.08, 1.31) | 0.112 | 0.18 (0.03, 1.01) | 0.051 | ||
| Cesarean delivery | 0.94 (0.57. 1.54) | 0.802 | 0.88 (0.49, 1.58) | 0.669 | ||
| Gestational age, wk | ||||||
| <25 | 6.34 (2.52, 15.95) | <0.001† | 0.63 (0.12, 3.38) | 0.593 | ||
| 25–28 | 4.60 (2.46, 8.59) | <0.001† | 1.54 (0.49, 4.82) | 0.460 | ||
| >28 | Reference | |||||
| Birth weight, g | ||||||
| <1000 | 7.73 (3.58, 16.70) | <0.001† | 4.21 (0.98, 18.10) | 0.053 | ||
| 1000–1500 | 2.06 (0.89, 4.78) | 0.092 | 1.03 (0.33, 3.24) | 0.963 | ||
| >1500 | Reference | |||||
| Sex (male vs female) | 1.11 (0.65, 1.89) | 0.698 | 1.32 (0.72, 2.43) | 0.375 | ||
| Race (non-black vs black) | 1.05 (0.63, 1.77) | 0.846 | 0.92 (0.49, 1.75) | 0.805 | ||
| 5-minute Apgar score (4–6 vs 7–9) | 1.79 (0.97, 3.31) | 0.062 | 0.69 (0.31, 1.56) | 0.376 | ||
| NSAIDs (any use) | 3.49 (2.11, 5.79) | <0.001† | 0.63 (0.21, 1.92) | 0.416 | ||
| INDO prophylactic | 2.39 (1.03, 5.57) | 0.043† | 0.83 (0.26, 2.68) | 0.753 | ||
| NSAID treatment | 4.42 (2.66, 7.34) | <0.001† | 2.80 (1.03, 7.61) | 0.044† | ||
| Inotropes | 4.54 (2.68, 7.68) | <0.001† | 2.25 (0.91, 5.59) | 0.078 | ||
| Gentamicin | 2.07 (0.65, 6.60) | 0.219 | 1.51 (0.41, 5.58) | 0.535 | ||
| Vancomycin | 0.42 (0.13, 1.34) | 0.144 | 0.43 (0.11, 1.64) | 0.214 | ||
| Severe IVH | 2.25 (0.90, 5.62) | 0.083 | 0.43 (0.11, 1.67) | 0.221 | ||
| Patent ductus arteriosus | 3.66 (2.21, 6.08) | <0.001† | 4.04 (1.27, 12.89) | 0.018† | ||
| Bloodstream infection | 2.81 (1.52, 5.20) | 0.001† | 3.01 (1.37, 6.60) | 0.006† | ||
HR, hazard ratio; INDO, indomethacin; IVH, intraventricular hemorrhage
* INDO prophylactic and NSAIDs treatment were fit in separate models.
† Values are statistically significant.
Neonatal comorbidities throughout hospitalization and mortality are described in Table 5. Infants with AKI had a greater occurrence of BSI during the study period (21.0% vs 3.7%, p = 0.001), as well as incidence of IVH all grades (37.1% vs 14.8%, p = 0.002) and severe grades 3/4 IVH: (11.3% vs 2.5%, p = 0.040). Neonates with AKI were also more likely to have PDA (71.0% vs 22.2%, p < 0.001) and RDS (95.2% vs 72.8%, p = 0.001) compared with neonates without AKI, but they were similar with respect to occurrence of NEC (8.1% vs 11.1%; p = 0.544). Mean length of hospitalization was also greater among neonates with AKI compared with neonates without AKI (84.6 ± 37.5 vs 52.9 ± 26.1 days, respectively, p <0 .001).
Table 5.
Neonatal Associated Comorbidities and Mortality
| Comorbidity | All Infants (N = 143) | Infants With AKI (n = 62) | Infants Without AKI (n = 81) | p value* |
|---|---|---|---|---|
| Bloodstream infection, n (%) | 16 (11.2) | 13 (21.0) | 3 (3.7) | 0.001† |
| IVH (all grades), n (%) | 35 (24.5) | 23 (37.1) | 12 (14.8) | 0.002† |
| IVH (grades 3 and 4), n (%) | 9 (6.3) | 7 (11.3) | 2 (2.5) | 0.040† |
| Patent ductus arteriosus, n (%) | 62 (43.4) | 44 (71.0) | 18 (22.2) | <0.001† |
| Necrotizing enterocolitis, n (%) | 14 (9.8) | 5 (8.1) | 9 (11.1) | 0.544 |
| Respiratory distress syndrome, n (%) | 118 (82.5) | 59 (95.2) | 59 (72.8) | 0.001† |
| Length of hospitalization, mean ± SD, days | 66.6 ± 37.5 | 84.6 ± 37.5 | 52.9 ± 26.1 | <0.001† |
| Mortality, n (%) | ||||
| 28-day | 2 (1.4) | 2 (3.2) | 0 (0.0) | 0.186 |
| Overall | 7 (4.9) | 5 (8.1) | 2 (2.5) | 0.171 |
AKI, acute kidney injury; IVH, intraventricular hemorrhage
* To test for differences between groups we used independent samples t tests for continuous variables, Wilcoxon rank-sum test for medians, χ2 tests for categorical variables (Fisher exact test for cells <5), and log rank test for equality of survivor functions.
† Values are statistically significant.
Discussion
In our retrospective cohort study of preterm neonates that were exposed to maternal indomethacin, we observed a high incidence of AKI within the first 15 days of life. Previous studies report overall neonatal rates of AKI up to 40%.2 The varying rates may be due to the different definitions of AKI used in individual studies. The incidence of AKI in our study ranges from 27% to 43% depending on the definition of AKI (27% nRIFLE; 31% Standard; 41% KDIGO; and 43% AKIN). Furthermore, the SCr of neonates at birth reflects the maternal plasma concentration, and therefore cannot be used reliably for the diagnosis of AKI within the first 24 hours of life. Hence, these values were excluded in our study. In our study, among the infants that developed AKI, 95.2% of them met AKI criteria based on SCr concentrations. Overall, AKI lasted about 6 days on average, with 1 patient lasting up to 35 days. This infant was a 24 weeks' gestation infant with a normal Apgar score and no maternal risk factors for AKI. This patient received 48 hours of gentamicin for early-onset sepsis and a 7-day course of vancomycin therapy for culture-negative clinical sepsis. Gentamicin peak and trough concentrations were therapeutic; however, vancomycin trough concentration was supratherapeutic and required dosage adjustment. The patient did not have a PDA, did not require inotropic support, and was discharged at 100 days of life. The cause of this patient's prolonged AKI is unclear.
Several case reports have described the occurrence of AKI in neonates exposed to indomethacin tocolysis.12–15 However, previous clinical trials have only indicated a probable association between antenatal indomethacin exposure and neonatal AKI.16,17 These studies, many of which were limited by a small study population, failed to examine important maternal and postnatal factors that may affect neonatal renal function. To our knowledge, this is the first study that identified risk factors for the development of neonatal AKI secondary to antenatal indomethacin exposure. In our study, AKI developed at a median age of 66.5 hours from delivery; 37% were within 24 to 48 hours, and 63% were greater than 48 hours from delivery. Infants with decreased urine output in the first 24 hours of life were not considered to have AKI unless the effect lasted beyond that time frame. This finding is similar to those of Norton and colleagues,16 who reported elevated serum creatinine and decreased urine output in preterm infants exposed to antenatal indomethacin within 48 hours of delivery. They found neonates exposed to antenatal indomethacin had significantly more renal dysfunction as evidenced by elevated SCr (>1 mg/dL) on days 1 (p = 0.03), 2 (p = 0.005), and 3 (p = 0.005) of life compared with unexposed neonates. In addition, urine output was significantly lower among the antenatal indomethacin-exposed neonates on days 1 (p = 0.003) and 2 (p = 0.003).16
We also found that administration of indomethacin closer to time of delivery was associated with development of AKI, with the greatest risk occurring with maternal indomethacin doses delivered within 24 hours of delivery. This finding is similar to a study conducted by Souter and colleagues,17 who reported that neonates born within 48 hours of indomethacin exposure were more likely to have a higher SCr at 72 hours of age compared with those not exposed (1.06 ± 0.09 vs 0.98 ± 0.07 mg/dL, p = 0.04) in a subgroup multivariate analysis of neonates born <31 weeks' gestation.
Although not statistically significant, infants born to mothers with chronic hypertension and/or preeclampsia were at higher risk for AKI. The higher risk of neonatal AKI may be due to changes in renal blood flow and placental blood flow seen in hypertensive pregnant patients. Preeclampsia and eclampsia account for 15% to 20% of maternal AKI cases.18 In addition, preeclampsia is associated with a high risk of placental abruption, which can affect oxygen delivery to the infant during fetal circulation.
In our study, neonates who are exposed to postnatal NSAIDs (e.g., indomethacin) for the treatment of PDA have nearly a 3-fold increased risk of AKI (Figure 2). NSAIDs inhibit and decrease prostaglandin synthesis, resulting in a decreased renal perfusion that results in the development of AKI. Furthermore, neonates with AKI also had greater exposure to inotropes (37.1% vs 3.7%, p < 0.001). The correlation between the use of inotropes and AKI may be a reflection of the critical condition of the neonates rather than a direct effect of the drug on renal perfusion. Critically ill infants requiring initiation of inotropic support often have decreased renal perfusion due to systemic hypotension and a reduction in cardiac output. Given that the kidneys receive ~25% of cardiac output, any reduction in cardiac output can have significant implications on renal perfusion, with a resultant decrease in GFR.19 In addition, renal vasculature vasoconstriction from high doses of vasoactive medications may result in prerenal kidney injury due to profound renal vascular bed vasoconstriction and decreased renal blood flow.
Infants with a PDA had a significantly higher risk for AKI compared with those without a PDA. In our study, we identify a 4-fold increased risk of AKI associated with a PDA (Figure 2C). An untreated PDA may have resulted in a decrease in renal function secondary to the hemodynamic effects of an open ductus arteriosus. Previous literature has described the association between persistence of a PDA and hypoperfusion secondary to alterations in blood flow and lack of compensatory mechanisms to maintain cardiac output.20 In the presence of a PDA, the left ventricle is able to maintain cardiac output secondary to the compensatory increase in stroke volume. However, the redistribution of blood flow can result in alterations in organ perfusion, potentially causing mesenteric ischemia and decreased renal and peripheral perfusion. In addition, very low birth weight infants may lack the compensatory increase in stroke volume mechanism, further compromising systemic perfusion. Therefore, treatment of a PDA may protect the neonate from developing AKI, and this could be due to prevention of hemodynamic changes that may cause damage to the kidneys.
In addition to PDA, the presence of BSI significantly increased the risk of AKI by 3-fold in our study. Several studies have identified sepsis as the most common pre-disposing factor for AKI followed by RDS and PDA.21,22 The pathogenesis of AKI in sepsis is probably related to the cytotoxic effects of inflammation and impaired microcirculation. During sepsis, the infection generally triggers a host response in which inflammatory mediators can contribute to the clearance of infection and tissue recovery but can also result in organ injury.23
Although some studies have reported an increased risk of severe IVH grades 3 and 4 in neonates exposed to antenatal indomethacin compared with those without antenatal indomethacin exposure (18%–28% vs 3%–9%, respectively),16,17,24 a meta-analysis did not detect a significant difference.25 In our study, all neonates were exposed to antenatal indomethacin, and those who developed AKI had a higher risk of severe IVH compared with those without AKI (11.3% vs 2.5%, p = 0.040). To our knowledge, we are the first study to examine the association between AKI and IVH in infants exposed to antenatal indomethacin. A total of 9 infants developed severe IVH; 6 of these cases occurred prior to the onset of AKI. None of the 9 infants received prophylactic indomethacin. A higher incidence of IVH could be expected in those not exposed to prophylactic indomethacin because of its neuroprotective effects; prostaglandin inhibition by indomethacin may protect the brain by decreasing cerebral blood flow, which occurs in response to hypoxic-ischemic insults.26 Because the kidney has an important role with blood pressure regulation, fluid balance, and electrolyte homeostasis, which may impact cerebral blood flow and perfusion, infants with AKI are at risk to develop IVH.27 Other factors that may increase the risk of severe IVH grades 3 to 4 include the lack of antenatal steroid for lung maturation, low gestational age, and birth asphyxia.28 Although antenatal steroids have been routinely prescribed to women in preterm labor at our institution, we did not evaluate the use of antenatal steroids in our study. Furthermore, we did not evaluate other potential risk factors for IVH, such as normal saline boluses, sodium bicarbonate use, and fluid intake. There was no difference in gestational age and Apgar scores between neonates who developed AKI versus those who did not.
As expected, those with AKI had a significantly longer hospitalization compared with those without AKI. Furthermore, although not statistically significant, infants with AKI had a higher 28-day mortality rate as well as overall mortality rate compared with the non-AKI group (3.2% vs 0% and 8.1% vs 2.5%, respectively). This is similar to a recent study that reported that AKI was associated with mortality at a crude HR of 0.3 (95% CI, 4.1–21) in very low birth weight neonates.29
The results of this study are limited by a number of factors. Because of the retrospective nature of this study, we were only able to gather clinical and laboratory information that was obtained as part of routine care during the study period. The selection of a relatively short observation period (15 days after birth) was used to minimize confounding variables that may cause AKI because we were specifically interested in evaluating AKI developing secondary to antenatal indomethacin use. The 15-day period was decided upon by investigators based on estimated duration of antenatal indomethacin effect. In addition, the lack of a comparator cohort of infants not exposed to antenatal indomethacin could be viewed as a limitation to this study. Although a comparator cohort would have helped identify the true incidence of AKI attributed to indomethacin, the intent of our study was to explore additional risk factors for AKI in all neonates exposed to antenatal indomethacin. A larger trial, including a matched cohort of neonates without antenatal exposure, could be beneficial in the future to further define the nephrotoxic risk specifically with in utero indomethacin exposure. However, the routine use of antenatal indomethacin in our institution made a retrospective study of this nature implausible. Furthermore, there is a lack of standardization of a clinically accepted definition of AKI in the neonatal population. Multiple AKI definitions have been proposed, including the AKIN, nRIFLE, and KDIGO. In our study, we found that the incidence of AKI differs depending on the definition used. We chose to exclude UOP derangements in the first 24 hours of life as a marker of AKI, given physiologic decreases in UOP seen immediately after birth. The decision was based on findings from Clark,30 who reported that only 21% of preterm neonates void at birth, 80% void by 8 hours of life, and 100% will void by 24 hours of life. Our study also did not evaluate for high-output renal failure; however, the occurrence of this is much less common in this population. Lastly, we failed to examine diuretic use and fluid intake during the study time frame, which may have affected the incidence of AKI because of their effect on UOP. Although diuretic use can result in elevations of UOP and therefore may have resulted in the exclusion of neonates who would have otherwise been classified as having AKI, our unit does not routinely prescribe diuretics in the first 15 days of life. Additionally, 91.9% of our patients met AKI criteria based on SCr alone (not UOP).
Identifying maternal and neonatal risk factors may allow for earlier clinical identification of infants at increased risk of AKI. Our findings have important implications for the management of neonates that are already at high risk of AKI due to maternal indomethacin exposure. Renal function should be closely monitored in neonates who were exposed to antenatal indomethacin within 24 hours of delivery, particularly when indomethacin and inotropes are prescribed in those with a PDA or a BSI. Clinicians should be cognizant of this added risk, and careful monitoring of renal function is required in these high-risk infants.
Conclusions
More than 40% of neonates exposed to indomethacin tocolysis within 1 week of delivery will develop AKI. Infants at highest risk for AKI included those who had been exposed to antenatal indomethacin within 24 hours of delivery, received indomethacin for PDA treatment, received inotropes for hypotension, and/or had a PDA. Caution is warranted when prescribing renally eliminated medications in the infants with these risk factors. The outcomes of AKI are significant, with those developing AKI having a longer length of hospitalization. Larger prospective studies should compare infants who were exposed to antenatal indomethacin to control participants to further identify risk factors for AKI. A consistent and well-established definition of AKI will allow for a better estimation of the true incidence of AKI in the neonatal population.
Acknowledgments
Preliminary data were presented at the University Health System Consortium's Pharmacy Council Meeting in New Orleans, LA, in December 2011; and at the Annual Pediatric Pharmacy Advocacy Group Meeting in Houston, TX, in April 2012.
ABBREVIATIONS
- AKI
acute kidney injury
- BSI
bloodstream infection
- GFR
glomerular filtration rate
- HR
hazard ratio
- INDO
indomethacin
- IVH
intraventricular hemorrhage
- NEC
necrotizing enterocolitis
- NSAIDs
nonsteroidal anti-inflammatory drugs
- PDA
patent ductus arteriosus
- RDS
respiratory distress syndrome
- SCr
serum creatinine
- SIP
spontaneous intestinal perforation
- UOP
urine output
Footnotes
Disclosure Dr Calip reports current employment with Flatiron Health, Inc., which is an independent subsidiary of the Roche group. The remaining authors declare no conflicts or financial interest in any product or service mentioned in the manuscript, including grants, equipment, medications, employment, gifts, and honoraria. Dr Pham has full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Ethical Approval and Informed Consent The authors assert that the study was approved by the Institutional Review Board at the University of Illinois at Chicago and informed consent was not required.
REFERENCES
- 1.Haycock GB. Management of acute and chronic renal failure in the newborn. Semin Neonatol. 2003;8(4):325–334. doi: 10.1016/S1084-2756(03)00044-7. [DOI] [PubMed] [Google Scholar]
- 2.Askenazi DJ, Ambalavanan N, Goldstein SL. Acute kidney injury in critically ill newborns: what do we know?: what do we need to learn? Pediatr Nephrol. 2009;24(2):265–274. doi: 10.1007/s00467-008-1060-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jetton JG, Boohaker LJ, Sethi SK et al. Incidence and outcomes of neonatal acute kidney injury (AWAKEN): a multicentre, multinational, observational cohort study. Lancet Child Adolesc Health. 2017;1(3):184–194. doi: 10.1016/S2352-4642(17)30069-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.ACOG Committee on Practice Bulletins--Obstetrics ACOG practice bulletin: management of preterm labor. Number 43, May 2003. Int J Gynaecol Obstet. 2003;82(1):127–135. doi: 10.1016/s0020-7292(03)00247-9. [DOI] [PubMed] [Google Scholar]
- 5.Macones GA, Marder SJ, Clothier B, Stamilio DM. The controversy surrounding indomethacin for tocolysis. Am J Obstet Gynecol. 2001;184(3):264–272. doi: 10.1067/mob.2001.111718. [DOI] [PubMed] [Google Scholar]
- 6.Blumenfeld YJ, Lyell DJ. Prematurity prevention: the role of acute tocolysis. Curr Opin Obstet Gynecol. 2009;21(2):136–141. doi: 10.1097/GCO.0b013e3283292455. [DOI] [PubMed] [Google Scholar]
- 7.Steiger RM, Boyd EL, Powers DR et al. Acute maternal renal insufficiency in premature labor treated with indomethacin. Am J Perinatol. 1993;10(5):381–383. doi: 10.1055/s-2007-994767. [DOI] [PubMed] [Google Scholar]
- 8.Mehta RL, Kellum JA, Shah SV et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11(2):R31. doi: 10.1186/cc5713. doi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ricci Z, Ronco C. Neonatal RIFLE. Nephrol Dial Transplant. 2013;28(9):2211–2214. doi: 10.1093/ndt/gft074. [DOI] [PubMed] [Google Scholar]
- 10.KDIGO Clinical Practice Guideline for Acute Kidney Injury: section 2: AKI definition. Kidney Int Suppl. 2012;2(1):19–36. doi: 10.1038/kisup.2011.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. J Pediatr. 1978;92(4):529–534. doi: 10.1016/s0022-3476(78)80282-0. [DOI] [PubMed] [Google Scholar]
- 12.Kaplan BS, Restaino I, Raval DS et al. Renal failure in the neonate associated with in utero exposure to nonsteroidal anti-inflammatory agents. Pediatr Nephrol. 1994;8(6):700–704. doi: 10.1007/BF00869093. [DOI] [PubMed] [Google Scholar]
- 13.Nishikubo T, Takahashi Y, Nakagawa Y et al. Renal impairment in very low birthweight infants following antenatal indomethacin administration. Acta Paediatr Jpn. 1994;36(2):202–206. doi: 10.1111/j.1442-200x.1994.tb03162.x. [DOI] [PubMed] [Google Scholar]
- 14.Gloor JM, Muchant DG, Norling LL. Prenatal maternal indomethacin use resulting in prolonged neonatal renal insufficiency. J Perinatol. 1993;13(6):425–427. [PubMed] [Google Scholar]
- 15.Pomeranz A, Korzets Z, Dolfin Z et al. Acute renal failure in the neonate induced by the administration of indomethacin as a tocolytic agent. Nephrol Dial Transplant. 1996;11(6):1139–1141. [PubMed] [Google Scholar]
- 16.Norton ME, Merrill J, Cooper BA et al. Neonatal complications after the administration of indomethacin for preterm labor. N Engl J Med. 1993;329(22):1602–1607. doi: 10.1056/NEJM199311253292202. [DOI] [PubMed] [Google Scholar]
- 17.Souter D, Harding J, McCowan L et al. Antenatal indomethacin--adverse fetal effects confirmed. Aust N Z J Obstet Gynaecol. 1998;38(1):11–16. doi: 10.1111/j.1479-828x.1998.tb02949.x. [DOI] [PubMed] [Google Scholar]
- 18.Prakash J, Ganiger VC. Acute kidney injury in pregnancy-specific disorders. Indian J Nephrol. 2017;7(4):258–270. doi: 10.4103/0971-4065.202406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Basile DP, Anderson MD, Sutton TA. Pathophysiology of acute kidney injury. Compr Physiol. 2012;2(2):1303–1353. doi: 10.1002/cphy.c110041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mezu-Ndubuisi OJ, Agarwal G, Raghavan A et al. Patent ductus arteriosus in premature neonates. Drugs. 2012;72(7):907–916. doi: 10.2165/11632870-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Youssef D, Abd-Elrahman H, Shehab MM, Abd-Elrheem M. Incidence of acute kidney injury in the neonatal intensive care unit. Saudi J Kidney Dis Transpl. 2015;26(1):67–72. doi: 10.4103/1319-2442.148738. [DOI] [PubMed] [Google Scholar]
- 22.Momtaz HE, Sabzehei MK, Rasuli B, Torabian S. The main etiologies of acute kidney injury in the newborns hospitalized in the neonatal intensive care unit. J Clin Neonatol. 2014;3(2):99–102. doi: 10.4103/2249-4847.134691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zarbock A, Gomez H, Kellum JA. Sepsis-induced acute kidney injury revisited: pathophysiology, prevention and future therapies. Curr Opin Crit Care. 2014;20(6):588–595. doi: 10.1097/MCC.0000000000000153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Doyle NM, Gardner MO, Wells L et al. Outcome of very low birth weight infants exposed to antenatal indomethacin for tocolysis. J Perinatol. 2005;25(5):336–340. doi: 10.1038/sj.jp.7211256. [DOI] [PubMed] [Google Scholar]
- 25.Amin SB, Sinkin RA, Glantz JC. Metaanalysis of the effect of antenatal indomethacin on neonatal outcomes. Am J Obstet Gynecol. 2007;197(5):486.e1–e10. doi: 10.1016/j.ajog.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fowlie PW, Davis PG, McGuire W. Prophylactic intravenous indomethacin for preventing mortality and morbidity in preterm infants. Cochrane Database Syst Rev. 2010;(7):CD000174. doi: 10.1002/14651858. doi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stoops C, Sims B, Griffin R, Askenazi DJ. Neonatal acute kidney injury and the risk of intraventricular hemorrhage in the very low birth weight infant. Neonatology. 2016;110(4):307–312. doi: 10.1159/000445931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ment LR, Oh W, Ehrenkranz RA et al. Antenatal steroids, delivery mode, and intraventricular hemorrhage in pre-term infants. Am J Obstet Gynecol. 1995;172(3):795–800. doi: 10.1016/0002-9378(95)90001-2. [DOI] [PubMed] [Google Scholar]
- 29.Koralkar R, Ambalavanan N, Levitan EB et al. Acute kidney injury reduces survival in very low birth weight infants. Pediatr Res. 2011;69(4):354–358. doi: 10.1203/PDR.0b013e31820b95ca. [DOI] [PubMed] [Google Scholar]
- 30.Clark DA. Times of first void and first stool in 500 newborns. Pediatrics. 1977;60(4):457–459. [PubMed] [Google Scholar]


