A total of 3612 ha of seagrass habitat was restored to the Virginia coast along with services like blue carbon and fisheries.
Abstract
There have been increasing attempts to reverse habitat degradation through active restoration, but few large-scale successes are reported to guide these efforts. Here, we report outcomes from a unique and very successful seagrass restoration project: Since 1999, over 70 million seeds of a marine angiosperm, eelgrass (Zostera marina), have been broadcast into mid-western Atlantic coastal lagoons, leading to recovery of 3612 ha of seagrass. Well-developed meadows now foster productive and diverse animal communities, sequester substantial stocks of carbon and nitrogen, and have prompted a parallel restoration for bay scallops (Argopecten irradians). Restored ecosystem services are approaching historic levels, but we also note that managers value services differently today than they did nine decades ago, emphasizing regulating in addition to provisioning services. Thus, this study serves as a blueprint for restoring and maintaining healthy ecosystems to safeguard multiple benefits, including co-benefits that may emerge as management priorities over time.
INTRODUCTION
The degradation of coastal habitats worldwide through anthropogenic influences has resulted in the loss of critical services that underpin the welfare of all Earth’s inhabitants (1, 2). The growing desire to protect and restore these services have led to the development of habitat restoration strategies to reverse the downward trend (3), including notable successes such as seagrasses in Tampa Bay, salt marshes in San Francisco Bay, and oysters in the Chesapeake Bay (4). Many of these successes are achieved through passive measures (such as nutrient reductions in Tampa Bay) (5), although there are increasing efforts to actively transplant foundational species to restore habitats, such as oysters and seagrasses (4). Unfortunately, many other efforts are not successful and therefore go unreported. Despite setbacks, recent syntheses suggest that some optimism is warranted, as restorations with sustained long-term and cooperative efforts on the scale of one to two decades can yield successful recovery (3, 6, 7).
A major challenge to restoration in general is determining what constitutes “success.” Metrics of reporting for most restoration projects have been defined primarily using habitat attributes, e.g., plant species diversity, biomass, areal coverage, or shoot densities (8, 9). Yet, the ultimate motivation for ecological restoration is not often the habitat itself (with the exception of harvestable resources, such as timber), but instead the emergent services that habitat provides (e.g., improved water quality, food and fisheries production, and carbon storage) (3, 10). Moreover, successful habitat restoration needs to be conducted within a robust theoretical framework that identifies the stressors that have led to the degradation and mitigates or compensates for those stressors before attempting a restoration [sensu (11)]. Often, success of restoration efforts is based on the initial establishment of the foundational species and its short-term (a few years) persistence, when environmental stressors acting over a longer time period, such as climate change, may compromise the habitat and/or its capacity to provide services and lead to long-term failure (3). Overcoming these challenges, therefore, requires a blend of historical knowledge, sustained commitment, and synthesis of expertise to promote successful outcomes for not only a single habitat but also an entire ecosystem.
Here, we report on an unparalleled large-scale seagrass restoration effort along the mid-western Atlantic that has been maintained annually for over 20 years. The inshore lagoons of Virginia, USA, once supported vast meadows of eelgrass (Zostera marina) beds that provided numerous ecosystem functions and services. In turn, these underpinned several lucrative commercial and recreational industries that were well recognized among the regional population. By 1933, a pandemic slime mold disease along the entire east coast of the United States and the west coast of Europe (12), in combination with a devastating hurricane, completely eradicated all eelgrass in the Virginia coastal lagoons. Along with the total loss of habitat was the disappearance of the brant goose (Branta bernicla), a popular game fowl, the elimination of the commercially valuable fishery for the bay scallop (Argopecten irradians), and the loss of eelgrass wrack used for fertilizer, insulation, and packing material.
For over 70 years, eelgrass was not documented in the Virginia coastal lagoons even while populations recovered in many other affected locations. However, water quality monitoring and process-based modeling of light availability in these bays (13), alongside the discovery of several very small (<2 m2) natural patches of eelgrass in one bay in the late 1990s, highlighted that seed recruitment limitation, not degraded environmental conditions, was the primary deterrent to recovery of eelgrass in this region (14). This realization led to the establishment of a seed-based restoration in 2001, resulting in the rapid recovery of eelgrass habitat in the Virginia coastal bays at a scale rarely observed in marine restoration ecology
RESULTS
Eelgrass habitat
The large-scale seed restoration effort, where 74.5 million seeds were broadcast into 536 individual restoration plots totaling 213 ha, has so far resulted in a total 3612 ha of vegetated bottom from virtually no coverage before the restoration (Fig. 1A). The majority (56% or 2028 ha) occurs in just one bay, South Bay, while the remaining 44% (1584 ha) is spread among three nearby bays: Cobb, Spider Crab, and Hog Island Bays (Figs. 1 to 3). The rapid development of plants in the restoration plots from seeds that germinated, established, and grew to adult plants in each plot (Fig. 1C) eventually produced flowers with seeds in subsequent years that dispersed naturally outside the individual plots. The rapid spread and growth from natural expansion from the restored plots highlighted the fact that the environmental conditions remained favorable for unassisted growth in these bays even into the 21st century (Figs. 1B and 2).
Fig. 1. Eelgrass cover and seedling data for the coastal bays.
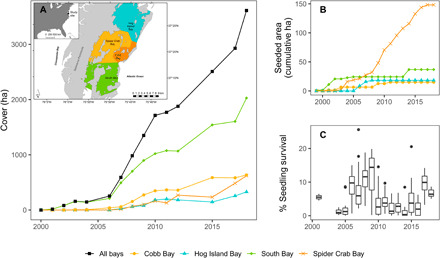
(A) Cover (in hectares) of eelgrass in each of the four coastal lagoons (South Bay, Hog Island Bay, Spider Crab Bay, and Cobb Bay: see inset) and all four bays combined (inset shows the geographic layout of these locations). (B) Cumulative seeded area for each bay. (C) Seedling establishment rates for seeds broadcast into the coastal bays in the preceding years; rates were not measured in each bay in each year, so data reflect the aggregate across all sites measured within a year.
Fig. 3. Aerial image of South, Cobb, and Spider Crab bays showing seagrass cover (dark areas) in 2018 and location of seed restoration plots (yellow boxes).
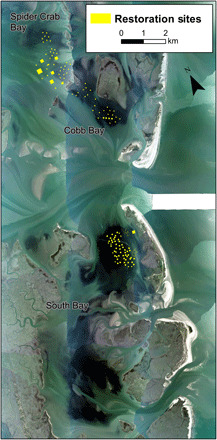
Fig. 2. Seagrass cover in the four bays for four time periods: 2001, 2006, 2010, and 2018.
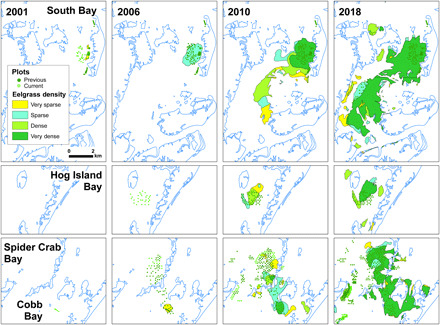
Cover estimates (very sparse, 1 to 10%; sparse, 11 to 40%; moderate, 41 to 70%; dense, 70 to 100%) indicated by color in each polygon. Small squares in each box represent restoration plots (light green are plots done that year; dark green are plots done in previous years).
Water quality
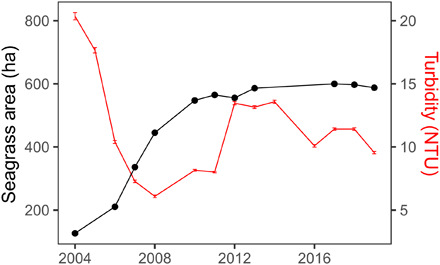
We witnessed a substantial decrease in mean turbidity levels during the summer months since the restoration was initiated within the meadow (Fig. 4). Comparisons of turbidity levels inside and outside of the vegetated areas at these sites have demonstrated a pattern of significantly lower turbidities associated with seagrass presence and increasing bed development not found in immediately adjacent, unvegetated areas of similar depths (14). Increasing bed area and seagrass density within the restored meadow during the first 5 years of seagrass restoration were associated with a marked decrease in summertime turbidity levels as measured continuously every 15 min at a fixed station located within the restored meadow (Fig. 4). Multiple regression revealed a significant negative relationship between total area of the bed surrounding the station and mean turbidity after accounting for year (after log-log transformation; β = −0.69, R2 = 0.42). There was some resurgence in turbidity levels for several years once bed development reached an asymptote; however, these increased turbidity levels were still substantially lower than those observed initially when the meadow development was low. The enduring capacity of the restored South Bay eelgrass meadow is evidenced by another, more recent, multiyear period of declining turbidity beginning in 2014.
Fig. 4. Mean turbidity [Nephelometric Turbidity unit (NTU)] ± 1 SE from continuous environmental monitoring from a YSI in the main bed of South Bay during summer months (May to August, reflecting peak annual seagrass biomass) against total area of seagrass obtained from the aerial survey immediate to the environmental sonde (northern part of South Bay).
Biogeochemical cycling
Sediment carbon and nitrogen stocks have increased exponentially with the expansion of the meadow (Fig. 5, A and B). Through time, carbon and nitrogen content in the mature seagrass sediments (>9 years) were 1.3× and 2.2× greater, respectively, than carbon and nitrogen content in the newly colonized sediments (1 to 5 years), suggesting that this storage capacity is increasing with meadow age.
Fig. 5. Ecosystem services associated with the restoration of eelgrass over time. Mean (solid lines) and 95% confidence intervals (dotted lines) over time (mT = metric tons).
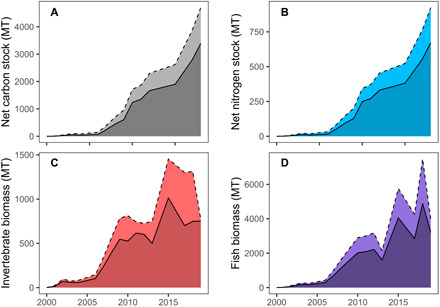
(A) Net sediment carbon stocks. (B) Net sediment nitrogen stocks (net stock = seagrass sediment stock − unvegetated sediment stock). For sediment nutrient stocks, measurements were taken in beds of varying ages and these values were matched with the corresponding year since the beginning of the restoration. (C) Total invertebrates. (D) Total fish biomass. For faunal communities, data were collected in various years, and averages/standard deviations were used to interpolate values for years in which no data were available. Both measurements were expressed per unit area and extrapolated to the total bed area for each year.
Faunal communities
Total epifaunal invertebrate biomass and total finfish biomass increased rapidly with the restoration (Fig. 5, C and D). Epifaunal invertebrate communities contained a diverse mix of crustacean, decapod, and gastropod species that were indistinguishable from natural beds in nearby Chincoteague Bay as early as 3 years after restoration and, by later years, contained a number of unique species not found in this reference area (15). Fish communities were likewise characterized by a rich and abundant assemblage dominated largely by silver perch (Bairdiella chrysoura). The next most abundant fish was pinfish (Lagodon rhomboides), a subtropical species that has been historically rare north of Cape Hatteras, North Carolina, but which our data show is now increasing in abundance in the Virginia coastal bays, perhaps as a function of warming waters. The stabilization of both fish predators and their epifaunal prey through time suggests that the bed has reached a mature and stable state with respect to a diverse and abundant food web.
Scallop restoration
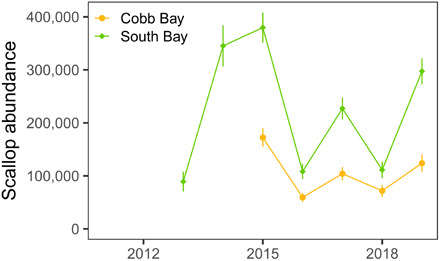
In 2008, a restoration program for bay scallops, which rely on the seagrass habitat to settle on as juvenile recruits, was initiated using broodstock from North Carolina. Annual seeding efforts have now resulted in a wild population inhabiting the seagrass beds as revealed by yearly quantitative surveys (Fig. 6). In addition, bay scallops have been observed under nets in clam aquaculture beds up to 20 km away from where they are set out in spawning cages, suggesting natural dispersal of larvae from the system via water currents.
Fig. 6. Estimates of total bay scallop abundances in South and Cobb bays based on annual surveys begun in 2013.
DISCUSSION
Eelgrass restoration on the Eastern Shore of Virginia is potentially a prime model for restoration in the 21st century, as the project focused not only on reviving this essential habitat but also on charting the cascading effects for ecosystem functioning and spurring additional restoration efforts of a commercially important species in the bay scallop. These combined efforts by academic, nonprofit, and citizen groups stand as one of the more successful marine restorations for seagrasses and rivals other large-scale marine restorations in terms of scope, rapidity, dedication, and organization (4, 16). It is also part of a growing movement toward “ocean optimism,” highlighting that active reversal of degraded ecosystems is possible over reasonable time scales (years to decades) (3).
A strong a priori understanding of the causes of decline, assessment of best practices toward restoration, and a sustained commitment to long-term monitoring and research are all essential components that allowed seagrass to thrive in these coastal bays once again. Our study demonstrates that the approach adopted here, as is currently underway in Australia (17), coupled to vast knowledge gained from numerous past seagrass projects and seagrass restoration protocols (16, 18, 19) can lead to recovery of key foundational species in alignment with emerging sustainability goals.
The success of this restoration stems from several sources: directly from the large-scale seeding efforts over time and indirectly through positive feedbacks that promoted resilience and recovery. A recent global synthesis of seagrass restoration projects concluded that larger and more densely planted restoration plots were more likely to succeed by stabilizing sediments and overcoming stochastic environmental stress at the plot level (20). This project is a leading demonstration of those principles, with the seeds in large-scale (0.2 to 0.4 ha) seed plots, surviving as a result of the biotic and abiotic nature of sedimentary environment (21, 22, 23), rapidly growing to form a dense continuous meadow, and improving water quality (turbidity). In the Virginia bays, the seeding effort occurred over only 6% of the total restored area; however, the relatively large size and high seed density of the initial seeded plots were likely key to initiating those initial positive feedbacks. The value of a long-term commitment (20+ years) to annual seeding efforts should also not be discounted. The development of inherent, self-stabilizing feedbacks can require years of meadow expansion and growth (20), and many environmental or other factors can temporarily slow or reverse this progression (24). These characteristics contrast the current study with many other seagrass restoration efforts, in which an average of 460 seeds (or adult plants) was broadcast in <1 m2 plots and monitored for only 12 months (16).
These impressive rates of change were further facilitated by the unique seed dispersal characteristics of this species and the morphological characteristics of the bays these plants now occupy. Eelgrass in this region can produce 10 million seeds per hectare or more. Once released, seeds settle rapidly, do not move far from where they fall (21), and are quickly buried via biotic and abiotic processes (21, 23), thereby expanding the edges of existing patches. In addition, the enclosed nature of these bays coupled to the shallow water depth allows seeds to entrain there.
Habitat suitability models coupled with historical records have shown that the eelgrass currently occupies only a fraction of its estimated historical distribution in these coastal lagoons: approximately 33 km2 versus 116 km2 (25). Thus, restoration is far from complete, and seeding is now focusing on bays where seagrass is currently not present. The restored meadows are now self-sustaining, but recent work suggests that maximizing eelgrass coverage within the entire region will require continued restoration effort, particularly in bays that are predicted to host meadows but where current hydrodynamics may be preventing eelgrass propagule recruitment and initial establishment (25).
The marked recovery of eelgrass here in the Virginia coastal bays stands in contrast to the continuing decline of eelgrass in the adjoining Chesapeake Bay and in many areas around the world (26). In Chesapeake Bay, eelgrass distribution changed markedly first in 1972 following the passage of a tropical storm, retreating from almost half of its distributional range, and declined an additional 29% through 2015, largely due to nutrient and sediment inputs in concert with warming temperatures (27). Similarly, eelgrass in the coastal bays of Maryland just north of the Virginia coastal bays has also declined by almost 50% since the early 2000s (27) due to deteriorating water quality conditions (28). These coastal bays are generally cooler than areas in the Chesapeake Bay and more oligotrophic than the Maryland bays, all of which have contributed to the higher cover there than in these other places (29). The reduced pollution is, in large part, due to the limited human population on the Eastern Shore, which has led to little management in the region (as opposed to many other parts of the Chesapeake watershed). Ultimately, the Virginia coastal bays may stand as the single most expansive eelgrass habitat between North Carolina and Long Island Sound and serve as a key steppingstone for the movement of fauna, including juveniles of many key fisheries species, along the east coast of the United States.
A key distinguishing feature of this restoration is that the coastal lagoons remained unvegetated for the better part of a century, allowing perceptions about seagrass, and the services it provides (30), to evolve markedly between when it was lost and when it returned. Before 1933, managers prioritized what the Millennium Ecosystem Assessment (31) terms “provisioning services,” specifically physical goods. In the early 1900s, eelgrass beds were an important economic engine, as a source of food for the highly prized and sought-after brant, a source of fertilizer and home insulation, and support of the bay scallop fishery (30). Upon recognizing the consequences of its loss for the local economy, one author wrote: “[With] it went the wildfowl, the cream of salt-water fishing, most of the clams and crabs, and all of the scallops. Speed its return, for nature deserves it if we don’t!” (32).
In contrast, modern managers now additionally emphasize “regulating services” as justification for restoration. Water quality, nursery function, forage/secondary production, and carbon and nitrogen sequestration were not widely considered in the early 1900s but today are some of the cited motivations for the conservation and restoration of seagrass habitats (33). In addition to these changes in benefit values over time, we also note that widespread adoption of the “weed-less propeller” has removed a key factor behind the antipathy toward eelgrass that was common in the region before 1933, as evidence in another contemporaneous quote: “it clogged propellers, choked clam rakes, hid seafood, tore up fish nets by sheer weight, messed up fishing lines and anchor cables and littered bathing beaches. How often we called down the wrath of heaven on this long, slimy green ribbon of hades!” (32).
Aesthetic values are also important metrics, and engagement of volunteers and other public support for our project is motivated not only by the quantifiable services listed above but also by the priceless value of natural beauty such habitats provide and the sense of community that their protection can impart (34). This project provided opportunities to hundreds of individuals to understand the challenges and successes in the restoration process and see firsthand the value of this important habitat. In this restoration effort alone, volunteers contributed over 3500 hours in collecting more than 10 million seeds and constituted an integral part of its overall success.
Seagrasses are effective at modifying and enhancing their own environment by baffling waves and slowing currents, causing particles to fall out of the water column and preventing sediment resuspension (35). This positive feedback ultimately leads to improved water clarity and also enhances carbon and nitrogen burial in sediments (36). This restoration study demonstrates the rapid change in turbidities associated with meadow development and expansion not found in adjacent unvegetated areas, and that after less than two decades carbon and nitrogen burial rates in these restored meadows are now comparable to rates in undisturbed ecosystems (36). As long as these ecosystems do not revert to their pre-vegetated state, these sediment stocks can remain buried for decades to centuries (37). This successful project in Virginia has been the first to show the potential of restoration to reinstate the ecosystem service of carbon and nitrogen sequestration in seagrass meadows (38) and contribute to emerging “blue carbon” initiatives to promote natural carbon capture under the Paris Agreement (37). In March 2020, the General Assembly of Virginia passed legislature allowing the restoration of underwater grasses to count toward carbon offset credits (SB783).
Faunal response to the restoration effort was initially marked, with values rapidly matching those from other areas and saturating in less than a decade (15). It seems that, unlike the seagrass itself, these mobile fishes and invertebrates are not limited in their capacity to colonize this restored habitat and reach a stable equilibrium in less than a decade. The considerable secondary production in these beds also fuels the growth and development of many juvenile fishes and crabs, which mature in the coastal lagoons before migrating offshore to join adult populations, where they are then commercially fished.
One exception is the bay scallop, an iconic species highly dependent on seagrass habitat. Bay scallops have a unique life cycle where juveniles recruit to and settle in the canopy of eelgrass and only migrate to the benthos as they mature (30). That bay scallop populations have only returned to a fraction of their estimated historical values [0.04 to 0.4 m−2 from restorations in other locations versus 1 to 2 m−2 from historical catch data (30)], which suggests that further intervention may be necessary to fully restore this species, including greater seagrass area and improved genetic diversity of the broodstock (39). The goal of a harvestable population of bay scallops on the Virginia coast may be one of the ultimate measures of success in this endeavor, as has been the case for other faunal restorations, such as otters in the Aleutians and wolves in Yellowstone (40, 41). However, any eventual harvest of scallops will require a delicate balance between sustaining the restored population while simultaneously depleting it, as is the case for other shellfishes, such as oysters (42).
As the world settles into the era of the Anthropocene, and regulatory agencies worldwide seek to conserve and recover valuable ecosystem services (43), our study provides a positive example that successful marine restorations are possible on the scales that contribute directly to human well-being. It addresses key deliverables for the United Nation’s Decade on Ecosystem Restoration (2021–2030) (44) and the Decade of Ocean Science for Sustainable Development, including recovery of a threatened marine habitat (seagrasses), conservation of biodiversity, provisioning of habitat, and sequestration of carbon, as well as engagement of local, academic, and citizen groups toward action and education. Such examples are sorely needed to mobilize and incentivize other restoration efforts toward these international goals. Furthermore, the Virginia coastal bays may act as a bastion against climate change and declining water quality impacts on eelgrass, even as these same factors influence the nearby Chesapeake Bay. With continued efforts, as have been sustained for the past 20 years, we expect eelgrass to continue to expand and provide critical services for the mid-western Atlantic well into the 21st century.
METHODS
Seagrass restoration
In each year from 1999 to 2018, Z. marina seeds were collected from established beds in the Chesapeake Bay and subsequently from established restored beds in the coastal bays in the spring of each year during the peak period of seed release from the flowering plants [more detailed methods are in (45)]. Seeds were maintained in temperature-controlled water baths during the summer to minimize losses from predation and natural mortality and then hand-broadcasted into predetermined unvegetated plots each fall (generally late September) in each of the four bays just before the normal period of seed germination in this region (mid-November) at seed densities determined to insure establishment of a cohort of seedlings that would grow into a dense bed. Seeds were evenly spread across each restoration plot by two individuals broadcasting seeds from a moving boat across eight evenly spaced lines in the plot. Seeds settle rapidly and do not move far from where they settle (21). Seeding sites for each year were randomly selected in each bay on the basis of an initial assessment of test plots to insure that plants would survive. The number of sites seeded each year was based on the availability of seeds collected each year. Seed densities chosen for plots were based on potential growth rates of surviving seeds that would yield a 75 to 100% cover of a seeded plot in 3 to 4 years and seeded at densities of 25 to 50 seeds per square meter. Field assessment of seedling establishment in selected restored plots was made in April of the following year after the previous fall broadcast to ensure that plants were present in the predetermined plots. Divers counted the number of seedlings along two 0.5-m-wide diagonals across each selected plot, and the total number of seedlings was adjusted to the area of the plot. The percentage of established seedlings was calculated from the total number of seeds broadcast in the surveyed plot. Summary data are made available with this publication.
Seagrass cover
Seagrass cover was mapped from aerial imagery acquired in late spring at a scale of 1:24,000 initially using a standard mapping camera with panchromatic black and white photography and then a digital mapping camera to obtain multispectral imagery. Acquisition of imagery followed specific guideline to obtain optimal imagery of the eelgrass beds [see detailed methods in (11)]. Z. marina beds were mapped and categorized as very sparse (1 to 10% cover), sparse (11 to 40% cover), moderate (41 to 70% cover), or dense (70 to 100% cover) on the basis of a visual estimate of the percent cover. Summary data are made available with this publication.
Water quality
We used water quality data from sensors deployed at two fixed stations using identical YSI 6600 EDS (YSI Inc., Yellow Springs, Ohio) multisensor sondes that measured turbidity, chlorophyll fluorescence, temperature, salinity, pH, and dissolved oxygen within the seagrass canopy at a depth of 25-cm above the bottom at 15-min intervals. The initial sensor was placed in South Bay in 2003, and a second station was added in July 2011 in Spider Crab Bay. All sondes were pre- and post-calibrated either before cruise sampling or during biweekly sonde switchouts at each of the fixed stations. All data were subject to quality assurance reviews to ensure data compliance with YSI and other standards. Details are provided in (14), and data are publicly available at http://vecos.vims.edu/.
Carbon/nitrogen stocks
To account for the density dependence of sediment carbon and nitrogen stocks over time (38, 46), we collected sediments from different areas of the restored meadow that encompassed a temporal gradient from newly established to mature seagrass sites. Samples were collected in 2013 from 67 sites distributed across 7 km2 of the South Bay meadow, encompassing seed plots from restoration in 2001 and areas of natural expansion following restoration (38). Four replicate 5-cm-depth sediment cores were collected from each site; this depth is conservative but captures the majority of the restoration effect (46). Sites were binned according to the time since seagrass establishment using the following age brackets: 0 years (unrestored), 1 to 5 years, 5 to 9 years, and >9 years. These age brackets were determined from changes in shoot density and sediment accretion rates over time (36). Sediment C and N concentrations (mg C cm−3) in the restored seagrass meadow were integrated over the 5-cm depth to produce areal C and N stocks and were averaged across sites within each age bracket. All data are publicly available at https://dx.doi.org/10.6073/pasta/de298295eec8e19fa6f337d88748889f.
Annual, landscape-scale C and N stocks were calculated by scaling the C and N concentrations by the annual area of restored seagrass, determined from the time series of aerial imagery. We used the C and N concentrations in recently established meadow (age 1 to 5 years) for the lower bound and in mature meadow (age >9 years) for the upper bound of the landscape-scale C and N stocks; average meadow age of the restoration fell between these two bounds due to continuous expansion of the meadow area over time. Bare sediment stock was subtracted from the upper and lower estimates of total C and N stocks in the restored meadow to determine the enhancement of C and N stock due to seagrass restoration.
Epifaunal invertebrates
Epifauna were sampled monthly beginning in 2001 in the seagrass bed, first using a suction sampler (periods from 2001 to 2003 and from 2010 to 2013) and later by a mesh bag (2015–2019). During the earlier period, a 0.33-m2 ring was placed over the sediment surface and the contents were suctioned into a 0.8-mm mesh bag for 2 min. The number of suction samples taken was dictated by the area of seagrass present, ranging from n = 2 to 18. After 2013, we alternately placed a small mesh bag (300-μm mesh size, 75 cm by 20 cm with a 20-cm opening) over several seagrass leaves, clipping the leaves at the base, and ensuring that leaves and fauna were in the bag before closing it. Sixteen bag samples were taken per month, in eight pairs randomly located throughout the bed.
In both cases, samples were placed on ice in the field, returned to the laboratory, and frozen. Processing entailed defrosting the sample and then identifying and enumerating all fauna. For the later samples taken using the mesh bags only, we subsequently passed the fauna through a nested series of sieves and used the size-fractionated abundances for different functional groups to estimate biomass [in mg Ash Free Dry Weight (AFDW)] based on the equations in (47). We then took the average biomass for each species from the size-fractionated samples and multiplied by the abundances of the corresponding suction samples to estimate biomass from these earlier samples. For both sets of samples, we converted total epifaunal biomass to grams and scaled by the total area of bottom sampled to yield units of grams per square meter. All data are made available with this publication.
We next multiplied the mean community biomass across all samples (taken during a single day) by 30 days for an estimate of monthly biomass and then again multiplied these values by 5 to calculate biomass for the entire summertime period during which sampling was routinely conducted (May to September). We then multiplied these values (in grams per square meter per summer) by total area of seagrass in square meters in each year to obtain total epifaunal community biomass across the entire bed. For years lacking samples, we interpolated values by averaging production across all years and multiplied this average value by the area of the bed in each year, assuming that epifaunal productivity scales linearly with bed area.
Fishes
Fishes in the seagrass beds in the coastal lagoons in Virginia were collected using a 4.9-m otter trawl (1.9-cm mesh wings and 0.6-cm cod end liner, 0.3 m–by–0.7 m doors) towed from a shallow draft vessel through seagrass habitats during daytime high tides. Trawls were conducted from May to September beginning in 2012. Six replicate trawls were taken on each sampling day. Each tow was 2 min in duration, and tows were nonoverlapping. Tow length was recorded with a GPS unit (Garmin Series). Fishes were brought onboard and identified to species level, enumerated, and measured (total length in centimeters of first individuals up to 10 of each species randomly selected from each trawl); individuals that could not be identified in the field were taken back to the laboratory for further identification. Handling was conducted following approved Institutional Animal Care and Use Committee protocols.
For standing biomass, we first converted abundance of fish to 100 m of tow length. We then obtained length-weight regression (W = a*Lb) for all species from FishBase (48). We multiplied average size of first 10 individuals by the coefficients a and b to obtain average weight per individual. Next, we multiplied average weight per individual by the total number of individuals (abundance) to obtain total biomass. All data are made available with this publication.
Then, we converted this value to square meters by multiplying by the width of the trawl (4.9 m) and dividing by 490 (average length of the tow). We multiplied the average biomass per day by 30 days and again by 5 months to yield the total community biomass per square meter per summer. Last, we multiplied this value by total area of seagrass in square meters in each year to obtain total biomass across the entirety of the bed. For years in which fish were not trawled (any year before 2012), we interpolated values by averaging biomass across all years of the trawl survey and multiplied this average value by the bed area in each unsurveyed year.
Bay scallop populations
We collected several hundred adult A. irradians from Bogue Sound, North Carolina. Juveniles spawned from these adults were held in flow-through seawater tables until they reached ~5 mm in shell height and then placed in mesh bags and cages to exclude predators and held for up to 24 months within the eelgrass meadows so that spawn from these scallops might recruit naturally. We used diver surveys to assess restored scallop populations, targeting adult scallops (>25 mm). These surveys were conducted by randomly selecting 320 locations across all three of the four coastal bay regions. At each of these sample locations, three to five divers swam along transects arranged in a stellate pattern around an anchored research skiff. At 1- to 2-m intervals along each transect (for a total of 10 to 15 replicates), the divers randomly placed a 1-m2 quadrat and thoroughly searched the area for adult scallops by touch. The total area and the total number of scallops were used to estimate overall density of scallops per square meter. Summary data are made available with this publication.
Supplementary Material
Acknowledgments
We greatly acknowledge the contributions of numerous staff, students, and volunteers from the University of Virginia’s Anheuser-Busch Coastal Research Center, TNC staff, and VIMS staff and students who have contributed to this project over the past two decades. Their dedication, patience, and perseverance are greatly appreciated. We want to especially acknowledge B. Truitt for his enthusiasm and initial support for all the work here. This is contribution number 3924 from the Virginia Institute of Marine Science and contribution number 60 from MarineGEO. Funding: Funding was provided by grants from numerous agencies, notably National Science Foundation grants DEB-0621014, DEB-1237733, and DEB- 1832221 to the Virginia Coast Reserve LTER project; the Coastal Programs of the Virginia Department of Environmental Quality funded by Coastal Zone Management Act of 1972, as amended, administered by NOAA’s Office of Ocean and Coastal Resource Management; the Virginia Recreational Fishing License Fund; the American Recovery and Reinvestment Act with funding to NOAA, grant NA09NMF4630308; Virginia Sea Grant; The Nature Conservancy, U.S. Army Corps of Engineers; and Virginia Department of Transportation, as well as private grants from the Allied-Signal Foundation, Norfolk-Southern, and the Keith Campbell Foundation for the Environment. J.S.L. was supported by the Michael E. Tennenbaum Secretarial Scholar gift to the Smithsonian Institution. Author contributions: All authors collected data. J.S.L. analyzed all data. R.J.O. and J.S.L. wrote the first draft of the manuscript with input from all authors. Competing interests: K.S.M. serves on the Research Advisory and Education Committee of Virginia Sea Grant. The authors declare that they have no other competing interests. Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. Additional data related to this paper may be requested from the authors.
SUPPLEMENTARY MATERIALS
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/6/41/eabc6434/DC1
REFERENCES AND NOTES
- 1.Jackson J. B., Kirby M. X., Berger W. H., Bjorndal K. A., Botsford L. W., Bourque B. J., Bradbury R. H., Cooke R., Erlandson J., Estes J. A., Hughes T. P., Kidwell S., Lange C. B., Lenihan H. S., Pandolphi J. M., Peterson C. H., Steneck R. S., Tegner M. J., Warner R. R., Historical overfishing and the recent collapse of coastal ecosystems. Science 293, 629–637 (2001). [DOI] [PubMed] [Google Scholar]
- 2.Halpern B. S., Walbridge S., Selkoe K. A., Kappel C. V., Micheli F., D’agrosa C., Bruno J. F., Casey K. S., Ebert C., Fox H. E., Fujita R., Heinemann D., Lenihan H. S., Madin E. M. P., Perry M. T., Selig E. R., Spalding M., Steneck R., Watson R., A global map of human impact on marine ecosystems. Science 319, 948–952 (2008). [DOI] [PubMed] [Google Scholar]
- 3.Duarte C. M., Agusti S., Barbier E., Britten G. L., Castilla J. C., Gattuso J.-P., Fulweiler R. W., Hughes T. P., Knowlton N., Lovelock C. E., Lotze H. K., Predragovic M., Poloczanska E., Roberts C., Worm B., Rebuilding marine life. Nature 580, 39–51 (2020). [DOI] [PubMed] [Google Scholar]
- 4.DeAngelis B. M., Sutton-Grier A. E., Colden A., Arkema K. K., Baillie C. J., Bennett R. O., Benoit J., Blitch S., Chatwin A., Dausman A., Gittman R. K., Greening H. S., Henkel J. R., Houge R., Howard R., Hughes A. R., Lowe J., Scyphers S. B., Sherwood E. T., Westby S., Grabowski J. H., Social factors key to landscape-scale coastal restoration: Lessons learned from three U.S. case studies. Sustainability 12, 869 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Greening H., Janicki A., Sherwood E. T., Pribble R., Johansson J. O. R., Ecosystem responses to long-term nutrient management in an urban estuary: Tampa Bay, Florida, USA. Estuar. Coast. Shelf Sci. 151, A1–A16 (2014). [Google Scholar]
- 6.MacNeil M. A., Graham N. A., Cinner J. E., Wilson S. K., Williams I. D., Maina J., Newman S., Friedlander A. M., Jupiter S., Polunin N. V., McClanahan T. R., Recovery potential of the world’s coral reef fishes. Nature 520, 341–344 (2015). [DOI] [PubMed] [Google Scholar]
- 7.Rezek R. J., Furman B. T., Jung R. P., Hall M. O., Bell S. S., Long-term performance of seagrass restoration projects in Florida, USA. Sci. Rep. 9, 15514 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zedler J. B., Kercher Z., Wetland resources: Status, trends, ecosystem services, and restorability. Annu. Rev. Environ. Resour. 30, 39–74 (2005). [Google Scholar]
- 9.Suding K. N., Toward an era of restoration in ecology: Success, failures, and opportunities ahead. Annu. Rev. Ecol. Evol. Syst. 42, 465–487 (2011). [Google Scholar]
- 10.Zedler J. B., Progress in wetland restoration ecology. Trends Ecol. Evol. 15, 402–407 (2000). [DOI] [PubMed] [Google Scholar]
- 11.Allan J. D., McIntyre P. B., Smith S. D. P., Halpern B. S., Boyer G. L., Buchsbaum A., Burton G. A., Campbell L. M., Chadderton W. L., Ciborowski J. J. H., Doran P. J., Eder T., Infante D. M., Johnson L. B., Joseph C. A., Marino A. L., Prusevich A., Read J. G., Rose J. B., Rutherford E. S., Sowa S. P., Steinman A. D., Joint analysis of stressors and ecosystem services to enhance restoration effectiveness. Proc. Natl. Acad. Sci. U.S.A. 110, 372–377 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Muehlstein L. K., Porter D., Short F. T., Labyrinthula zosterae sp. nov., the causative agent of wasting disease of eelgrass, Zostera marina. Mycologia 83, 180–191 (1991). [Google Scholar]
- 13.Lawson S. E., Wiberg P. L., McGlathery K. J., Fugate D. C., Wind-driven sediment suspension controls light availability in a shallow coastal lagoon. Estuar. Coasts 30, 102–112 (2007). [Google Scholar]
- 14.Orth R. J., Moore K. A., Marion S. R., Wilcox D. J., Parrish D. B., Seed addition facilitates eelgrass recovery in a coastal bay system. Mar. Ecol. Prog. Ser. 448, 177–195 (2012). [Google Scholar]
- 15.Lefcheck J. S., Marion S. R., Orth R. J., Actively restored ecosystems as a refuge for biological diversity: A case study from eelgrass (Zostera marina L.). Estuar. Coasts 40, 200–212 (2017a). [Google Scholar]
- 16.van Katwijk M. M., Thorhaug A., Marbà N., Orth R. J., Duarte C. M., Kendrick G. A., Althuizen I. H. J., Balestri E., Bernard G., Cambridge M. L., Cunha A., Durance C., Giesen W., Han Q., Hosokawa S., Kiswara W., Komatsu T., Lardicci C., Lee K. S., Meinesz A., Nakaoka M., O’Brien K., Paling E. I., Pickerell C., Ransijn A. M. A., Verduin J. J., Global review of seagrass restoration and the importance of large-scale planting. J. Appl. Ecol. 53, 567–578 (2016). [Google Scholar]
- 17.Statton J., Ruiz-Montoya L., Orth R. J., Dixon K. W., Kendrick G. A., Identifying critical recruitment bottlenecks limiting seedling establishment in a degraded seagrass ecosystem. Sci. Rep. 7, 14786 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.M. S. Fonseca, W. J. Kenworthy, G. W. Thayer, “Guidelines for the conservation and restoration of seagrasses in the United States and adjacent waters” (NOAA Coastal Ocean Program Decision Analysis Series No. 12, NOAA Coastal Ocean Office, 1998).
- 19.M. Fonseca, D. Dale, V. Fay, L. Hibbert, J. Karazsia, D. Rydene, M. Sramek, R. Swafford and P. Wilber, “Frequently asked questions regarding the conservation and restoration of seagrasses in the NOAA, NMFS Southeast Region” (Internal Working Document, Habitat Conservation Division, National Marine Fisheries Service Southeast Region, 2016).
- 20.Carr J., D’Odorico P., McGlathery K. J., Wiberg P., Stability and bistability of seagrass ecosystems in shallow coastal lagoons: Role of feedbacks with sediment suspension and light availability. J. Geophys. Res. 115, G03011 (2010). [Google Scholar]
- 21.Orth R. J., Luckenbach M. L., Moore K. A., Seed dispersal in a marine macrophyte: Implications for colonization and restoration. Ecology 75, 1927–1939 (1994). [Google Scholar]
- 22.Harwell M. C., Orth R. J., Influence of a tube-dwelling polychaete on the dispersal of fragmented reproductive shoots of eelgrass. Aquat. Bot. 70, 1–7 (2001). [Google Scholar]
- 23.Blackburn N. J., Orth R. J., Seed burial in Zostera marina (eelgrass): The role of infauna. Mar. Ecol. Prog. Ser. 474, 135–145 (2013). [Google Scholar]
- 24.Aoki L. R., McGlathery K. J., Wiberg P. L., Al-Haj A., Depth affects seagrass restoration success and resilience to marine heat wave disturbance. Estuar. Coasts 43, 316–328 (2020). [Google Scholar]
- 25.Oreska M. P. J., McGlathery K. J., Wiberg P., Orth R. J., Wilcox D. J., Niche plasticity observed for Zostera marina (eelgrass) based on the differential success of restored and naturally-occurring meadow patches: Implications for seagrass restoration. Estuar. Coasts (in review). [Google Scholar]
- 26.Waycott M., Duarte C. M., Carruthers T. J. B., Orth R. J., Dennison W. C., Olyarnik S., Calladine A., Fourqurean J. W., Heck K. L. Jr., Hughes A. R., Kendrick G. A., Kenworthy W. J., Short F. T., Williams S. L., Accelerating loss of seagrasses across the globe threatens coastal ecosystems. Proc. Natl. Acad. Sci. U.S.A. 106, 12377–12381 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lefcheck J. S., Wilcox D. J., Murphy R. R., Marion S. R., Orth R. J., Multiple stressors threaten a critical foundation species in Chesapeake Bay. Glob. Chang. Biol. 23, 3474–3483 (2017). [DOI] [PubMed] [Google Scholar]
- 28.Wazniak C. E., Hall M. R., Carruthers T. J. B., Sturgis B., Dennison W. C., Orth R. J., Linking water quality to living resources in a mid-Atlantic lagoon system, USA. Ecol. Appl. 17, S64–S78 (2007). [Google Scholar]
- 29.Moore K. A., Shields E. C., Parrish D. B., Orth R. J., Eelgrass survival in two contrasting systems: Role of turbidity and summer water temperatures. Mar. Ecol. Prog. Ser. 448, 247–258 (2012). [Google Scholar]
- 30.Oreska M. P. J., Truitt B., Orth R. J., Luckenbach M. W., The bay scallop, Argopecten irradians, industry collapse in Virginia and its implications for the successful seagrass-scallop management. Mar. Policy 75, 116–124 (2017). [Google Scholar]
- 31.Millennium Ecosystem Assessment, Ecosystems and Human Well-Being: Biodiversity Synthesis Report (Island Press, 2005). [Google Scholar]
- 32.E.V. Connett, Duck Shooting Along the Atlantic Tidewater (William Morrow & Company, 1947). [Google Scholar]
- 33.Unsworth R. K. F., Nordlund L. M., Cullen-Unsworth L. C., Seagrass meadows support global fisheries production. Conserv. Lett. , e12566 (2018). [Google Scholar]
- 34.Westman W. W., How much are nature’s services worth? Science 197, 960–964 (1977). [DOI] [PubMed] [Google Scholar]
- 35.Hansen J. C. R., Reidenbach M. A., Wave and tidally driven flows in eelgrass beds and their effect on sediment suspension. Mar. Ecol. Prog. Ser. 448, 271–287 (2012). [Google Scholar]
- 36.Oreska M. P. J., McGlathery K. J., Porter J. H., Seagrass blue carbon accumulation at the meadow-scale. PLOS ONE 12, e0176630 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fourqurean J. W., Duarte C. M., Kennedy H., Marbà N., Holmer M., Mateo M. A., Apostolaki E. T., Kendrick G. A., Krause-Jensen D., McGlathery K. J., Serrano O., Seagrass ecosystems as a globally significant carbon stock. Nat. Geosci. 5, 505–509 (2017). [Google Scholar]
- 38.McGlathery K. J., Reynolds L. K., Cole L. W., Orth R. J., Marion S. R., Schwarzschild A., Recovery trajectories during state changes from bare sediment to eelgrass dominance. Mar. Ecol. Prog. Ser. 448, 209–221 (2012). [Google Scholar]
- 39.Peterson C. H., Summerson H. C., Luettich R. A. Jr., Response of bay scallops to spawner transplants: A test of recruitment limitation. Mar. Ecol. Prog. Ser. 132, 93–107 (1996). [Google Scholar]
- 40.Estes J. E., Smith N. S., Palmisano J. F., Sea otter predation and community organization in the Western Aleutian Islands, Alaska. Ecology 59, 822–833 (1978). [Google Scholar]
- 41.Ripple W. J., Beschta R. L., Trophic cascades in Yellowstone: The first 15 years after wolf reintroduction. Biol. Conserv. 145, 201–213 (2012). [Google Scholar]
- 42.Beck M. W., Brumbaugh R. D., Airoldi L., Carranza A., Coen L. D., Crawford C., Defeo O., Edgar G. J., Hancock B., Kay M. C., Lenihan H. S., Luckenbach M. W., Toropova C. L., Zhang G., Guo X., Oyster reefs at risk and recommendations for conservation, restoration, and management. Bioscience 61, 107–116 (2011). [Google Scholar]
- 43.Costanza R., d’Arge R., de Groot R., Farber S., Grasso M., Hannon B., Limburg K., Naeem S., O’Neill R. V., Paruelo J., Raskin R. G., Sutton P., van den Belt M., The value of the world’s ecosystem services and natural capital. Nature 387, 253–260 (1997). [Google Scholar]
- 44.Waltham N. J., Elliott M., Lee S. Y., Lovelock C., Duarte C. M., Buelow C., Simenstad C., Negelkerken I., Claassens L., Wen C. K.-C., Barletta M., Connolly R. M., Gillies C., Mitsch W. J., Ogburn M. B., Purandare J., Possingham H., Sheaves M., UN Decade on Ecosystem Restoration 2021–2030—What chance for success in restoring coastal ecosystems? Front. Mar. Sci. 7, 71 (2020). [Google Scholar]
- 45.Marion S. R., Orth R. J., Innovative techniques for large scale collection, processing, storage, and dispersal of eelgrass (Zostera marina) seeds. Restor. Ecol. 18, 514–526 (2010). [Google Scholar]
- 46.Greiner J. T., McGlathery K. J., Gunnell J., McKee B. A., Seagrass restoration enhances “blue carbon” sequestration in coastal waters. PLOS ONE 8, e72469 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Edgar G. J., The use of the size structure of benthic macrofaunal communities to estimate faunal biomass and secondary production. J. Exp. Mar. Biol. Ecol. 137, 195–214 (1990). [Google Scholar]
- 48.R. Froese, D. Pauly, FishBase (2011); http://www.fishbase.se/search.php.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/6/41/eabc6434/DC1


