Abstract
Background.
Disruptions in neural circuits underlying emotion regulation (ER) may be a mechanism linking child maltreatment with psychopathology. We examined the associations of maltreatment with neural responses during passive viewing of negative emotional stimuli and attempts to modulate emotional responses. We investigated whether the influence of maltreatment on neural activation during ER differed across development and whether alterations in brain function mediated the association between maltreatment and a latent general psychopathology (‘p’) factor.
Methods.
Youth aged 8–16 years with (n = 79) and without (n = 72) exposure to maltreatment completed an ER task assessing neural responses during passive viewing of negative and neutral images and effortful attempts to regulate emotional responses to negative stimuli. P-factor scores were defined by a bi-factor model encompassing internalizing and externalizing psychopathology.
Results.
Maltreated youth had greater activation in left amygdala and salience processing regions and reduced activation in multiple regions involved in cognitive control (bilateral superior frontal gyrus, middle frontal gyrus, and dorsal anterior cingulate cortex) when viewing negative v. neutral images than youth without maltreatment exposure. Reduced neural recruitment in cognitive control regions mediated the association of maltreatment with p-factor in whole-brain analysis. Maltreated youth exhibited increasing recruitment with age in ventrolateral prefrontal cortex during reappraisal while control participants exhibited decreasing recruitment with age. Findings were similar after adjusting for co-occurring neglect.
Conclusions.
Child maltreatment influences the development of regions associated with salience processing and cognitive control during ER in ways that contribute to psychopathology.
Keywords: Child maltreatment, cognitive control network, emotion regulation, fMRI, P-factor, salience network
Introduction
Child maltreatment – including physical and sexual abuse and witnessing domestic violence – is common and associated with numerous forms of psychopathology, including depression, anxiety, post-traumatic stress disorder (PTSD), and disruptive behavior disorders (Finkelhor, Turner, Shattuck, & Hamby, 2013; McLaughlin et al., 2012). Recent work suggests that the association of child maltreatment with psychopathology operates entirely through associations with a latent general psychopathology factor (i.e. p-factor), with no residual associations with specific disorders after accounting for p-factor (Caspi et al., 2014; Lahey, Krueger, Rathouz, Waldman, & Zald, 2017). Disruptions in emotion regulation (ER) are thought to be a transdiagnostic mechanism linking child maltreatment and psychopathology (Heleniak, Jenness, Vander Stoep, McCauley, & McLaughlin, 2016a; Weissman et al., 2019), although the underlying neurodevelopmental mechanisms are poorly understood. The present study examined how child maltreatment influences neural responses during passive viewing of negative emotional stimuli and effortful regulation of emotional responses using cognitive reappraisal. We evaluated whether these associations vary across development and determined whether alterations in neural activation mediate the association of childhood maltreatment with p-factor.
Disruptions in ER among maltreated children have been observed in self-report (Heleniak et al., 2016a; Weissman et al., 2019) and physiological measures (McLaughlin, Sheridan, Alves, & Mendes, 2014a) and maybe a core mechanism linking child maltreatment with psychopathology. Maladaptive patterns of ER may be particularly likely to occur in children who have experienced maltreatment involving a high degree of threat, such as physical and sexual abuse and witnessing chronic domestic violence. Children exposed to these forms of maltreatment often experience severe punitive reactions from caregivers in response to emotional expression (Eisenberg, Fabes, & Murphy, 1996) and have well-documented increases in emotional reactivity (Heleniak, McLaughlin, Ormel, & Riese, 2016b; Weissman et al., 2019). Moreover, children exposed to these types of maltreatment exhibit greater use of maladaptive ER strategies, like rumination (Heleniak et al., 2016a). This heightened emotional reactivity and infrequent use of adaptive regulation strategies may make it more challenging for children to regulate emotional responses effectively.
Difficulties with ER among maltreated children may reflect disruptions in neural circuitry involved in ER. Prior research has largely examined how maltreatment influences neural recruitment during tasks designed to assess emotional reactivity or implicit (i.e. automatic) forms of ER. These studies have consistently observed heightened amygdala recruitment when viewing negative faces or social images among maltreated youths relative to controls (McCrory et al, 2011; McLaughlin, Peverill, Gold, Alves, & Sheridan, 2015). Less is known about how maltreatment influences neural circuitry involved in explicit forms of ER. Only one prior study has examined neural recruitment during an explicit ER task among maltreated youths in a small sample of adolescents (McLaughlin et al., 2015). That study found heightened recruitment in the salience network (i.e. amygdala, putamen, anterior insula) among maltreated youths relative to controls during passive viewing of negative stimuli and greater recruitment of prefrontal cortex (PFC) regions in the cognitive control network – including the superior frontal gyrus (SFG), dorsal anterior cingulate cortex (dACC), and frontal pole – during active attempts to regulate emotion to negative stimuli using cognitive reappraisal. In the present study, we examined whether maltreatment is associated with neural recruitment during a similar ER task in a large sample of children and adolescents.
Considerable developmental variation exists in neural responses during emotional processing. Amygdala reactivity to negative cues decreases from childhood to adolescence (Silvers et al., 2016, 2017), whereas recruitment of PFC regions increases across age, which may support more effective ER (McRae et al., 2012; Silvers et al., 2017). The neural circuitry underlying explicit ER appears to become more efficient with age, such that attempts to regulate emotion are increasingly effective at reducing amygdala activation (Silvers et al., 2016). It is unknown how exposure to child maltreatment influences the development of neural systems underlying ER. Here, we examine whether the association between child maltreatment and neural recruitment during ER varies across childhood and adolescence.
Elevated emotional reactivity and difficulties with ER have emerged as transdiagnostic risk factors for internalizing and externalizing psychopathology in behavioral studies (Heleniak et al., 2016a; Weissman et al., 2019). In neuroimaging studies of adults, altered PFC and amygdala recruitment during ER have been consistently associated with multiple forms of psychopathology (Ball, Ramsawh, Campbell-Sills, Paulus, & Stein, 2013; Beauregard, Paquette, & Lévesque, 2006; Dillon & Pizzagalli, 2013; Lake, Finn, & James, 2017). These associations have rarely been examined in pediatric samples. One study found no group differences in the amygdala or PFC activation between depressed and control adolescents during active attempts to regulate emotion, but that greater recruitment of dorsolateral PFC and medial PFC was associated with lower self-reported distress when regulating emotion only among youths without depression (LeWinn et al., 2018). Conversely, reduced amygdala and inferior frontal gyrus (IFG) recruitment during effortful attempts to regulate negative emotion was observed in depressed adolescents relative to controls (Perlman et al., 2012) and greater dorsolateral PFC recruitment during effortful regulation was found among adolescents reporting suicidal ideation (Miller et al., 2018). The role that disruptions in neural circuitry underlying ER play as a mechanism linking child maltreatment with psychopathology remains unknown.
We examined how child maltreatment influences neural systems underlying emotional reactivity and regulation and whether these associations vary across development in a large sample of children and adolescents exposed to maltreatment. We expected to replicate previous findings showing greater activation in the amygdala and salience processing regions in response to negative emotional stimuli and greater PFC recruitment during effortful ER among children exposed to maltreatment compared to non-maltreated controls. Extending previous work, we predicted that PFC recruitment would increase from childhood to adolescence during ER, with a steeper age-related increase among maltreated youth. Finally, we hypothesized that greater recruitment of salience processing regions during emotional reactivity and regions underlying cognitive control during effortful regulation would mediate the association of childhood maltreatment with a general psychopathology factor. Finally, we evaluated whether findings were specific to threat-related maltreatment as opposed to co-occurring deprivation experiences (i.e. poverty, neglect) (McLaughlin & Sheridan, 2016).
Methods
Participants and procedure
A total of 160 children and adolescents (52% female) aged 8–16 years (M = 12.63, s.d. = 2.68) participated in a neuroimaging study examining child maltreatment, ER, and psychopathology. Families were recruited from schools, after-school and prevention programs, adoption programs, food banks, shelters, parenting programs, medical clinics, and the general community in Seattle, WA between January 2015 and June 2017. Inclusion criteria for the maltreated group included exposure to physical or sexual abuse or direct witnessing of domestic violence. Control children had an absence of exposure to maltreatment or other forms of interpersonal violence. Each maltreatment-exposed participant was matched to a control participant on age, sex, and handedness. Exclusion criteria included IQ<80, pervasive developmental disorder, active psychotic symptoms, mania, substance abuse, or safety concerns. See online Supplementary material for details on additional MRI exclusions.
The Institutional Review Board at the University of Washington approved all procedures. Legal guardians provided written informed consent; children provided written assent. See Table 1 for sample socio-demographic characteristics.
Table 1.
Distribution of socio-demographics and psychopathology by maltreatment exposure
| Maltreatment exposed (n = 79) |
Controls (n = 72) |
χ 2 | p value | |||
|---|---|---|---|---|---|---|
| % | n | % | n | |||
| Female | 54.4 | 43 | 45.8 | 33 | 1.11 | 0.29 |
|
| ||||||
| Race/ethnicity | 47.45 | <0.001 | ||||
|
| ||||||
| White | 22.8 | 18 | 69.4 | 50 | ||
|
| ||||||
| Black | 39.2 | 31 | 5.6 | 4 | ||
|
| ||||||
| Latino | 13.9 | 11 | 6.9 | 5 | ||
|
| ||||||
| Asian/Pacific islander | 7.6 | 6 | 15.3 | 11 | ||
|
| ||||||
| Biracial/Other | 16.5 | 13 | 2.8 | 2 | ||
| M | (s.d.) | M | (s.d.) | t-Value | p Value | |
|
| ||||||
| Age | 12.94 | 2.69 | 12.57 | 2.54 | −0.86 | 0.39 |
|
| ||||||
| Income-to-needs ratio | 2.19 | 2.24 | 5.52 | 2.13 | 9.05 | <0.001 |
|
| ||||||
| IQ | 103.83 | 14.49 | 115.48 | 13.96 | 6.46 | <0.001 |
|
| ||||||
| Neglect severity | 8.65 | 4.34 | 5.85 | 1.37 | −5.24 | <0.001 |
|
| ||||||
| P-factor | 0.55 | 0.66 | −0.72 | 0.60 | −12.32 | <0.001 |
Note. IQ was measured with the Wechsler Abbreviated Scale of Intelligence, 2nd Edition (WASI-2); Neglect Severity was measured with the Childhood Trauma Questionnaire-Physical Neglect subscale, p-factor refers to the extracted p-factor latent construct constructed from several internalizing and externalizing psychopathology measures outlined in Supplementary material.
Materials and measures
Maltreatment exposure
A multi-informant, multi-method approach was used to assess child maltreatment. Children were classified as experiencing physical or sexual abuse if abuse was endorsed by the child on several well-validated self-report and interview measures including the Childhood Experiences of Care and Abuse interview (Bifulco, Brown, & Harris, 1994), UCLA PTSD Reaction Index (PTSD-RI) trauma screen (Steinberg et al., 2013), or above the validated threshold on the Childhood Trauma Questionnaire (Walker et al., 1999) or reported by the parent on the Juvenile Victimization Questionnaire (Finkelhor, Hamby, Ormrod, & Turner, 2005) or PTSD-RI. Domestic violence was assessed by child-report only (see online Supplementary material for details). A total of 79 children (51.7%) experienced maltreatment (see online Supplementary Table S1 for the frequency of specific exposure types).
General psychopathology factor (p-factor)
We defined the underlying structure of psychopathology in our sample using a standard bi-factor model (Caspi et al., 2014; Lahey et al., 2017) including multiple forms of internalizing and externalizing psychopathology. Description of psychopathology measures included in the p-factor is provided in the online Supplementary material. Following prior studies, we tested two models: correlated-factors and bi-factor models (Caspi et al., 2014; Lahey et al., 2017). In the correlated-factors model, internalizing and externalizing latent constructs are defined from manifest indicators. In the bi-factor model, the latent p-factor is regressed onto the manifest indicators followed by externalizing and internalizing latent constructs regressed onto the residualized variance in these indicators (online Supplementary Fig. S1). These models were tested using confirmatory factor analyses in MPlus 8.1 with the robust maximum likelihood estimator, which is robust to non-normality of observed indicators as in the present study. Model fit was assessed using the Akaike Information Criterion (AIC), Bayesian Information Criterion (BIC), and the Sample Adjusted BIC. Both models had similar model fit with indicators loading significantly (all ps < 0.001) with a slightly better fit for the bi-factor model (online Supplementary Table S2). In the present analyses, we used the bi-factor model because (a) we were interested in creating an indicator of general psychopathology and (b) it is the most commonly used general factor model of psychopathology (Caspi et al., 2014; Lahey et al., 2017).
The latent p-factor was regressed onto manifest symptom indicators, followed by externalizing and internalizing latent constructs regressed onto the residualized variance in the manifest indicators (online Supplementary Table S2 and Fig. S1). We extracted the factor score for the p-factor latent construct for subsequent analyses.
Emotion regulation task
Participants completed an experimental task that has been widely used in adults (Ochsner et al., 2004) and adapted for pediatric samples (McRae et al., 2012; Silvers et al., 2016, 2017) (online Supplementary Fig. S2). Age-appropriate negative and neutral images from a stimulus set developed in the laboratory of the senior author were used (see online Supplementary material for details; images and normative ratings are publicly available: https://osf.io/43hfq/). Prior to viewing images, participants were given one of two instructions, ‘look’ or ‘far’, based on Silvers et al. (2012). Participants were trained on the task at a previous study visit and instructions were reviewed in the scanner. For ‘Look’ trials, participants were trained to attend to the picture and to allow their emotional reaction to unfold naturally. In ‘Far’ trials (hereafter referred to as Regulation), participants were trained to modulate their emotional response using cognitive reappraisal. Participants were taught the reappraisal strategy of psychological distancing prior to the scan (e.g. ‘imagine the situation as far away from you,’ or ‘imagine the situation is fake or not real’). Participants rated the intensity of their emotional response on each trial using a ‘feelings thermometer’ ranging from 0 to 3. Length of stimulus presentation (6–10 s) and inter-trial interval (2–6 s) were jittered. Participants completed four runs of the task, each consisting of five Look-Neutral, five Look-Negative, and five Regulation trials (20 total trials of each type). Negative images in Look and Regulation trials were matched on valence and arousal. Trials were presented in randomized order within a run.
Covariates
Income-to-needs ratio was calculated by dividing the parent-reported family income by the poverty threshold for a family of that size as indicated by the US Census Bureau. Neglect severity was measured with the CTQ-Physical Neglect subscale (Bernstein, Ahluvalia, Pogge, & Handelsman, 1997), assessing regular access to food, medical care, and general care by parents.
Image acquisition and preprocessing
See online Supplementary material for details on image acquisition. Gnu Make was used to create a preprocessing workflow incorporating multiple software packages (Askren et al., 2016). The following preprocessing steps were applied to functional images: (a) skull-stripping using AFNI’s 3dSkullStrip; (b) anatomical coregistration with Advanced Normalization Tools (ANTs); (c) motion and slice timing correction in FSL; (d) despiking using AFNI’s 3dDespike tool; and (e) smoothing with a 6 mm full-width half-max kernel using SUSAN in FSL. Nuisance regressors entered into person-level models consisted of six rigid-body motion regressors as well as time-series extracted from white matter and ventricles prior to smoothing to control for physiological noise (Behzadi, Restom, Liau, & Liu, 2007). Outlier volumes in which framewise displacement exceeded 1 mm, the derivative of variance in BOLD signal across the brain (DVARS) exceeded the upper fence, or signal intensity was >3 s.d. from the mean were excluded by regressing these volumes out of person-level models. The resulting contrast images were normalized to MNI standard space. Specifically, ANTs software was used to register the middle volume of each functional scan to each participant’s T1-weighted image and to normalize the result to a study-specific T1-weighted pediatric template and then MNI space.
Statistical analysis
Socio-economic status (SES) and race/ethnicity differed across maltreated and control participants and were included as a covariate in all analyses. To determine whether our findings were specific to maltreatment involving threat over and above the effects of deprivation, we conducted separate sensitivity analyses controlling for the presence of neglect, in addition to race/ethnicity, in all analyses of maltreatment effects. This allowed us to evaluate whether associations of maltreatment exposure with neural recruitment and psychopathology were the result of confounding by neglect. An additional sensitivity analysis controlling for cognitive ability can be found in the online Supplementary material.
Emotion intensity ratings
To examine maltreatment-related differences in behavioral ratings, we conducted a repeated-measures analysis of variance (ANOVA) with Condition (Look-Neutral, Look-Negative, Regulation) as a within-subjects factor and Group (maltreated, control) as a between-subjects factor with emotion intensity rating as the dependent variable. Similar analyses were conducted with p-factor and age as between-subjects factors (see online Supplementary material).
fMRI
Event-related regressors were created by convolving a boxcar function of phase duration and amplitude one with the standard (double-γ) hemodynamic response function for each phase of the task (instruction, stimulus, rating). Regressors for stimulus presentation were separated by trial type (Look-Neutral, Look-Negative, Regulation). A general linear model was constructed for each participant for two contrasts used in prior studies of this task (Ochsner et al., 2004; Silvers et al., 2012): Look-Negative > Neutral (i.e. emotional reactivity) and Regulation > Look-Negative (i.e. ER). Individual-level estimates of blood oxygen level-dependent (BOLD) activity were submitted to group-level random-effects models using FSL’s FLAME 1. We examined the differences in BOLD response during contrasts of interest as a function of maltreatment exposure and the p-factor in whole-brain analysis. To investigate whether the association of maltreatment with neural recruitment varied across development, we examined an interaction of age and maltreatment exposure for each contrast.
To correct for multiple comparisons in whole-brain analyses, AFNI’s 3dClustSim program was used to calculate an appropriate cluster size threshold. Recent simulations demonstrate an elevated risk of false positives using standard cluster-level correction approaches (Eklund, Nichols, & Knutsson, 2016). Recent updates to AFNI’s 3dFWHMx and 3dClustSim utilize a spatial autocorrelation function (ACF), which more accurately estimates the spatial smoothness to reduce the risk of false positives. The ACF option in 3dFWHMx was used to estimate the spatial smoothness of residuals from the individual-level analyses. The average ACF was then used with 3dClustSim to calculate the appropriate cluster size threshold with 10 000 Monte Carlo simulations. We used a conservative voxel-wise threshold of p < 0.005 and cluster-level threshold of p < 0.001. Finally, we conducted a sensitivity analysis to correct for multiple comparisons by utilizing FDR correction for the clusters we obtained in whole-brain analysis across (1) the age by maltreatment interaction; (2) maltreatment group main effects; and (3) the main effect of p-factor for Look-Negative > Look-Neutral and Regulation > Look-Negative contrasts. We report these FDR-corrected p values in the online Supplementary material.
Region of interest (ROI) analysis
We examined amygdala activation using ROI analysis, separately for the left and right amygdala. Functional ROIs were created by intersecting a structural amygdala mask from the Harvard–Oxford subcortical atlas (20% threshold) with the map of activation in the entire sample for each contrast.
Whole-brain mediation analysis
We evaluated whether the association between maltreatment and p-factor was mediated by whole-brain activation for contrasts where group differences were observed. Mediation analysis was conducted using Mediation Effect Parametric Mapping (MEPM), a widely-used whole-brain mediation toolbox (http://wagerlab.colorado.edu/tools) (Wager, Davidson, Hughes, Lindquist, & Ochsner, 2008, 2009). The threshold for significant clusters in the mediation analysis (p < 0.005) was based on prior work using this toolbox (Wager et al., 2008, 2009; Yamamoto, Woo, Wager, Regner, & Tanabe, 2015). See online Supplementary material for details.
Results
Descriptive statistics
Correlations among maltreatment, socio-demographic covariates, and p-factor are presented in online Supplementary Table S3.
Emotion regulation task performance
Emotion intensity ratings varied by condition [F(2, 274) = 96.67, p < 0.001, η2 = 0.41] and maltreatment [F(1, 137) = 5.96, p = 0.02, partial η2 = 0.04]. Intensity ratings were lowest on Look-Neutral trials, followed by Regulation (p < 0.001), and Look-Negative (p < 0.001) trials. Maltreated participants reported globally higher emotion intensity ratings than controls (p = 0.02), but both groups reported significantly lower intensity ratings during Regulation than Look-Negative (ps < 0.001; online Supplementary Table S4 and Fig. S3).
Task-related neural recruitment
Emotional reactivity
The contrast of Look-Negative > Look-Neutral revealed activation in the frontal and parietal cortices including in the left postcentral gyrus and bilateral activation in the SFG, middle frontal gyrus (MFG), IFG, precentral gyrus, lateral orbitofrontal cortex extending into the insula, and dACC (online Supplementary Table S5 and Fig. S4). This analysis also revealed bilateral activation in the lateral occipital cortex, temporal occipital fusiform, lingual gyrus, precuneus, angular gyrus, and parahippocampal gyrus. Sub-cortically, this contrast revealed activation in the bilateral amygdala, hippocampus, and thalamus.
Emotion regulation
The contrast of Regulation > Look-Negative demonstrated widespread activation in the PFC including bilateral SFG, MFG, and frontal pole extending into the left IFG and insula, and right precentral and postcentral gyrus (online Supplementary Table S5 and Fig. S4). Additionally, it revealed activation in the bilateral middle temporal gyrus, superior temporal gyrus, inferior temporal gyrus, and temporal occipital fusiform cortex.
Maltreatment exposure and neural recruitment
Emotional reactivity
In the Look-Negative > Look-Neutral contrast, control participants had greater activation than maltreated children in five clusters (online Supplementary Table S6A; Fig. 1a). Regions encompassed in these clusters included bilateral dACC and SFG, right MFG, left IFG, and left insula. Maltreatment-exposed participants exhibited greater activation than controls in one cluster that included the left precentral gyrus, supplementary motor area (SMA), and postcentral gyrus.
Fig. 1.
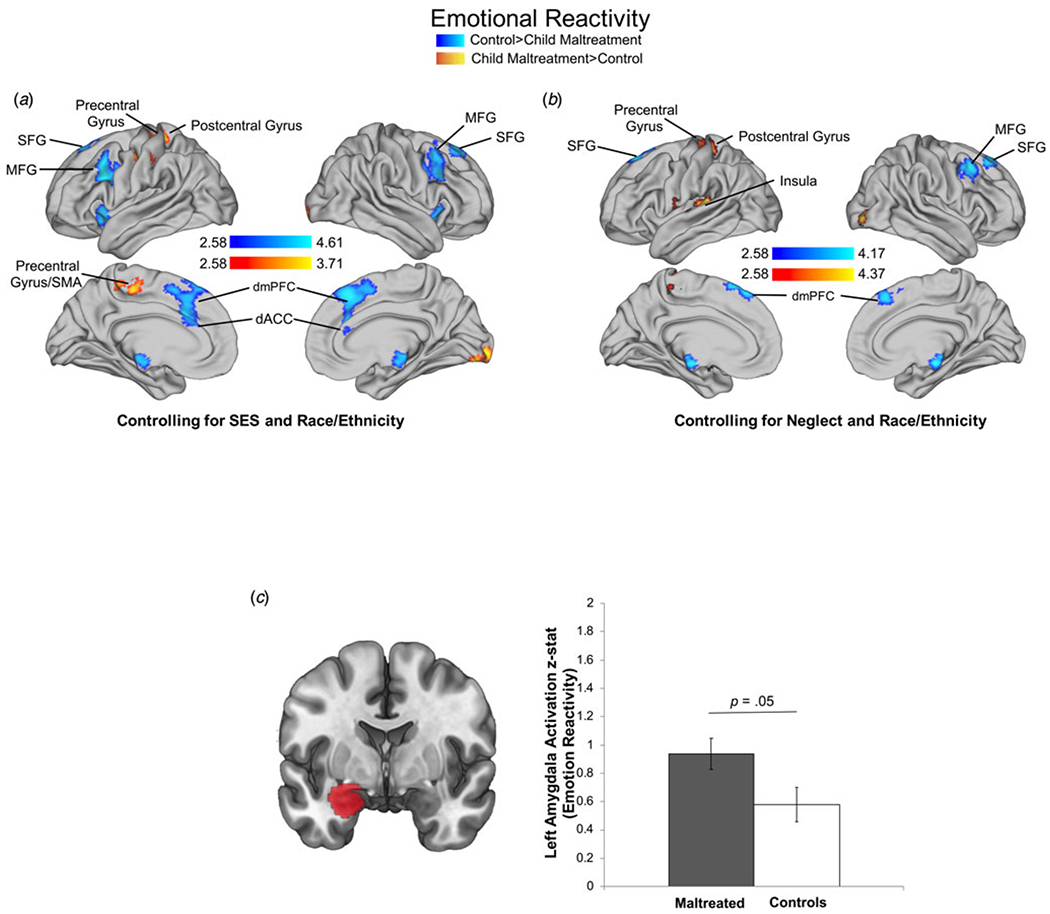
Maltreatment exposure and neural recruitment during emotional reactivity. Maltreatment main effects for whole-brain recruitment controlling for SES and race/ethnicity (a) and neglect and race/ethnicity (b), and amygdala recruitment using region of analysis (ROI) (c) during emotional reactivity (Look-Negative>Look-Neutral contrast). Cluster-level correction applied in AFNI, p < 0.005 was the primary threshold, and p < 0.001 was the cluster-level threshold. ROIs were created by masking contrasts of interest (i.e. Look-Negative > Neutral) with a mask of the amygdala from the Harvard–Oxford subcortical atlas (20% threshold; mask pictured).
In sensitivity analyses controlling for neglect, the pattern and significance of results were largely unchanged with one additional cluster emerging. Specifically, maltreatment-exposed participants exhibited significantly greater activation in the left insula, amygdala, putamen, and pallidum (online Supplementary Table S7A, Fig. 1b).
Emotion regulation
No maltreatment-related differences were found during the Regulation > Look-Negative contrast. This was unchanged after controlling for neglect.
ROI analysis
Maltreatment-exposed youth had greater left amygdala activation compared to controls in the Look-Negative > Look-Neutral contrast [F(1, 147) = 3.90, p = 0.05, partial η2 = 0.03] (Fig. 1c) but not the Regulation > Look-Negative contrast. This was unchanged after adjusting for neglect.
Variation in maltreatment-related neural recruitment across development
Emotional reactivity
Age did not interact with maltreatment to predict neural activation during the Look-Negative > Look-Neutral contrast.
Emotion regulation
Age interacted with maltreatment exposure in the Regulation > Look-Negative contrast in three clusters, including left IFG, left insula, and one cluster spanning left precentral and postcentral gyrus extending into dACC (online Supplementary Table S6B, Fig. 2a). Maltreatment-exposed youth showed increasing activation across development in these regions, whereas controls exhibited decreasing activation across age. These age-related differences were unchanged after adjusting for neglect (online Supplementary Table S7B, Fig. 2b).
Fig. 2.
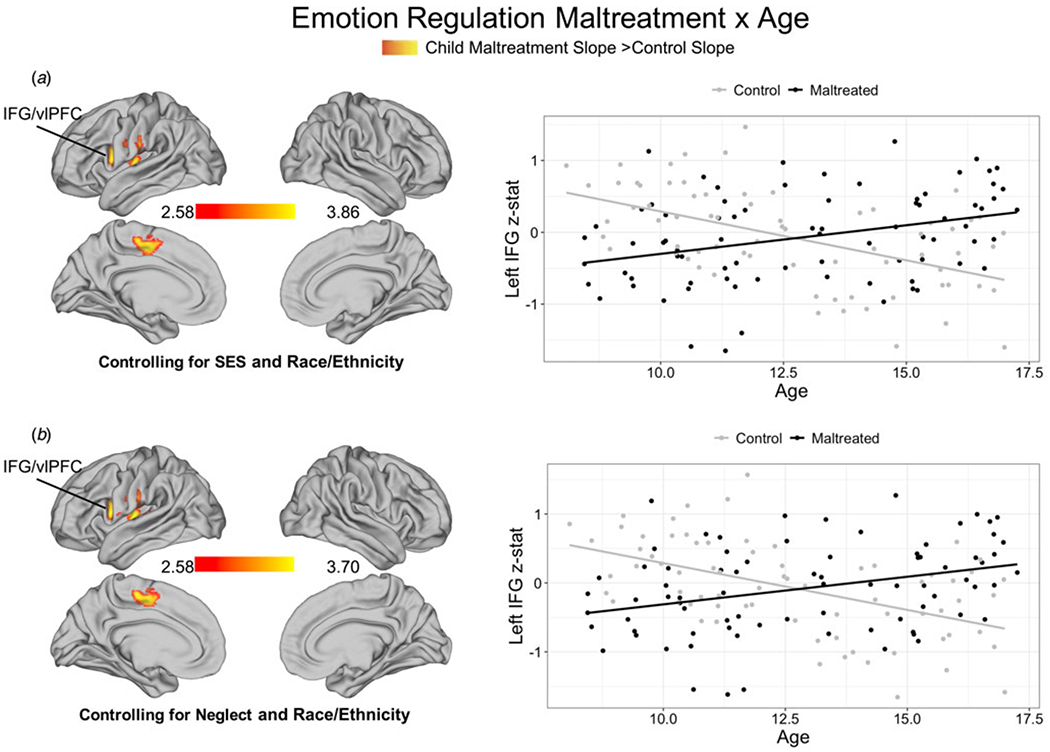
Variation in maltreatment-related neural recruitment across development during emotion regulation. Age by maltreatment moderation controlling for SES and race/ethnicity (a) and neglect and race/ethnicity (b) during emotion regulation (Decrease-Negative > Look-Negative contrast). Cluster-level correction applied in FSL, z > 23 was the primary threshold, and p < 0.01 was the cluster-level threshold.
ROI analysis
Age did not interact with maltreatment to predict amygdala activation in either the Look-Negative > Look-Neutral or Regulation > Look-Negative contrasts, with and without controls for neglect.
Neural recruitment and p-factor
Emotional reactivity
Higher p-factor scores were negatively associated with activation in the Look-Negative > Look-Neutral contrast in three PFC clusters encompassing bilateral SFG, dACC, and dorsomedial PFC; right insula, IFG, and MFG; and left IFG and frontal pole (online Supplementary Table S8, Fig. 3). P-factor scores were positively associated with activation in the Look-Negative > Look-Neutral contrast in three clusters: bilateral postcentral gyrus extending into the SMA, left precentral gyrus, and left lateral occipital cortex.
Fig. 3.
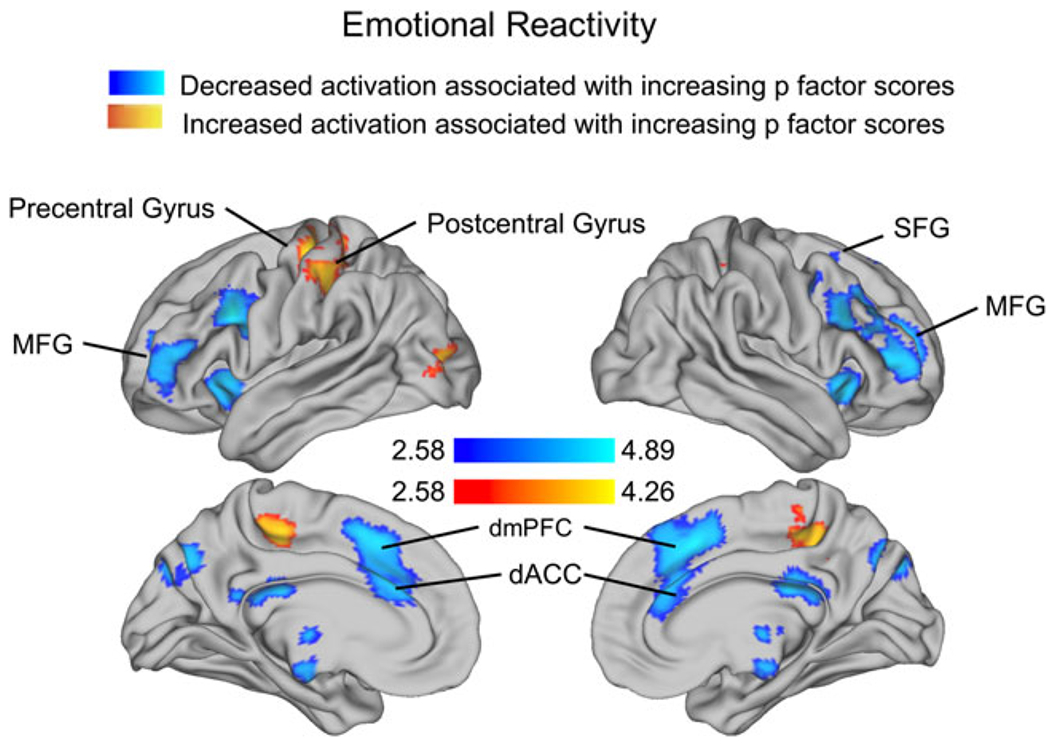
Association of p factor with neural recruitment during emotional reactivity. Cluster-level correction applied in AFNI, p < 0.005 was the primary threshold, and p < 0.001 was the cluster-level threshold.
Emotion regulation
P-factor was unrelated to activation in the Regulation > Look-Negative contrast.
ROI analysis
P-factor was unrelated to amygdala activation in either the Look-Negative > Look-Neutral or Regulation > Look-Negative contrasts.
Whole-brain mediation analyses
We conducted whole-brain mediation analyses to investigate whether the association of maltreatment exposure with p-factor scores was mediated by neural recruitment in the Look-Negative > Look-Neutral contrast. Reduced activation in left MFG, bilateral dorsomedial PFC, and left dACC and greater activation in the left postcentral gyrus and right cuneus in the Look-Negative > Look-Neutral contrast significantly mediated the association between maltreatment and p-factor scores (online Supplementary Table S9, Fig. 4).
Fig. 4.
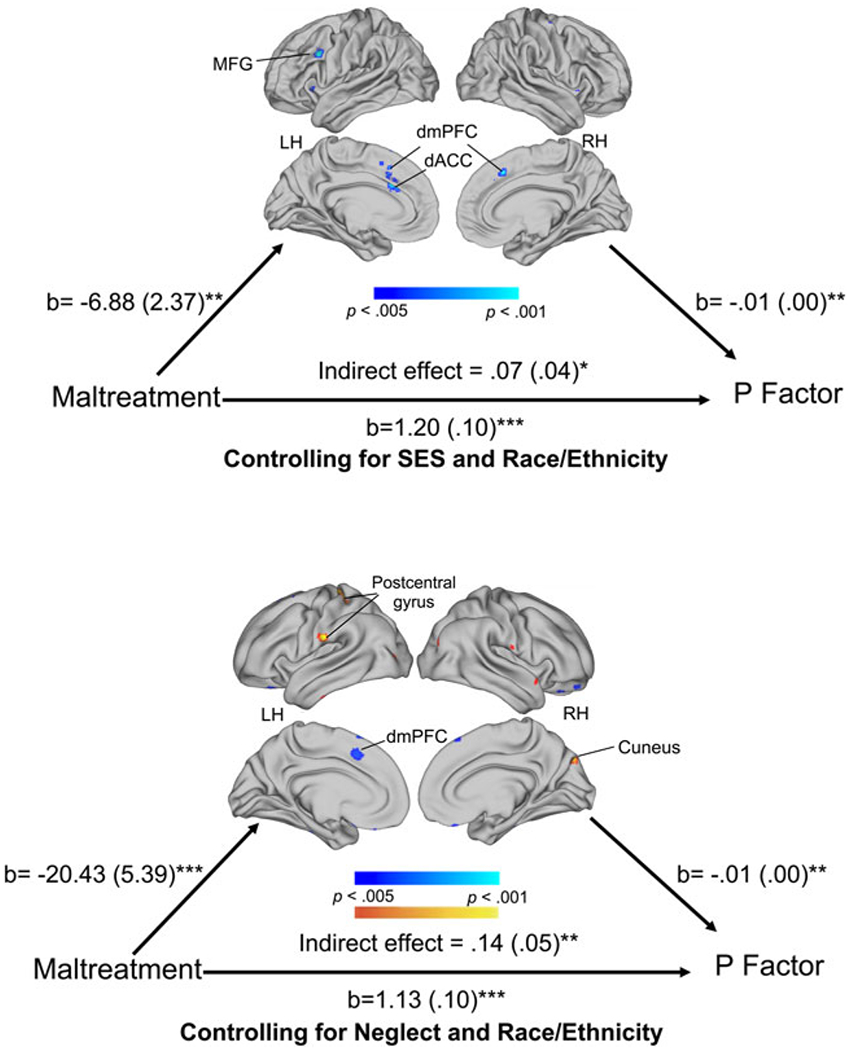
Whole-brain mediation during emotional reactivity. Depicting clusters that significantly mediated the association of child maltreatment with p factor scores during emotional reactivity (Look-Negative > Look-Neutral contrast) controlling for SES and race/ethnicity (a) and neglect and race/ethnicity (b). We used Mediation Effect Parametric Mapping (MEPM) to generate a whole-brain map of mediators with the Wager et al. (2008) Mediation Toolbox. Threshold for significant clusters was p < 0.005 with a minimum of 5 voxels per cluster.
Discussion
We examined the associations between child maltreatment and neural recruitment underlying emotional reactivity and regulation in a large sample of children and adolescents. Child maltreatment was associated with greater activation in amygdala and salience processing regions and reduced recruitment in PFC regions involved in cognitive control when passively viewing negative v. neutral images. Additionally, maltreated youth exhibited increasing age-related recruitment in the ventrolateral PFC (vlPFC) during cognitive reappraisal whereas control participants exhibited decreasing recruitment across the transition to adolescence, which may reflect a more efficient age-related pattern of neural activation among non-maltreated youths. Finally, we provided novel evidence that reduced neural recruitment in cognitive control regions during passive viewing of negative stimuli mediates the association of maltreatment with a general psychopathology factor (p-factor) in whole-brain analysis. These findings suggest that child maltreatment influences the development of regions associated with salience processing and cognitive control, which may in turn increase the risk for psychopathology.
Greater activation in left amygdala among maltreated youths relative to controls when viewing negative v. neutral images is consistent with evidence from our group (McLaughlin et al., 2015) and others (McCrory et al., 2011) documenting heightened salience of negative emotional information among youth exposed to child maltreatment. This pattern likely reflects the heightened motivational salience of threatening information in youth raised in environments characterized by danger. We also observed reduced neural recruitment in multiple PFC regions involved in cognitive control – including SFG, MFG, and dACC – in maltreated youth compared to controls when viewing negative stimuli. Activation in these regions has been consistently implicated in ER processes and may reflect the automatic use of regulation strategies in response to negative emotional information. Greater recruitment of these regions in youth without maltreatment histories could reflect the unprompted use of regulation strategies when viewing negative information in order to modulate emotional reactions. Alternatively, dACC disengagement occurs when attention is directed away from threat-related cues in attentional orienting tasks (Price et al., 2014). Reduced engagement of dACC and other regions associated with cognitive control during emotional processing could reflect greater attentional avoidance of threat-related cues among maltreated youth, a pattern that has been observed in behavioral studies (Kelly et al., 2015; Pine et al., 2005). One of these prior studies further demonstrated that the degree of attentional avoidance mediated the association of maltreatment with heightened emotional reactivity (Kelly et al., 2015). Future research is needed to evaluate whether the patterns of reduced PFC activation found here are similarly observed in tasks designed to measure attentional mechanisms; if so, it could point to an additional pathway contributing to heightened emotional reactivity in maltreated youths.
We previously reported that child maltreatment was related to greater reappraisal-related activation in dorsal ACC, SFG, and frontal pole in adolescents (McLaughlin et al., 2015). We do not replicate that pattern here, but observed age-related differences in the association of maltreatment with PFC recruitment during reappraisal. Specifically, vlPFC recruitment increased with age in maltreated youth during cognitive reappraisal, whereas control participants exhibited decreasing vlPFC recruitment with age. The vlPFC is recruited during cognitive reappraisal in both adults and children (Silvers et al., 2017). Our findings extend prior work by suggesting that greater recruitment in PFC regions involved in cognitive reappraisal among maltreated than non-maltreated youth emerges across the transition to adolescence, albeit in a different region of PFC than observed in our prior work in adolescents. These age-related increases in vlPFC recruitment during reappraisal among maltreated adolescents may reflect that greater effort is required to modulate emotional responses to negative social information, particularly during adolescence when emotional responsivity is heightened (Spear, 2009). Paired with the fact that we did not observe differences in amygdala modulation during reappraisal as a function of maltreatment, this pattern suggests that maltreated youth may not experience expected age-related increases in efficiency in effortful ER during the transition to adolescence.
Neural recruitment in cognitive control regions during passive viewing of negative stimuli mediated the association between maltreatment and p-factor. Specifically, maltreated youth exhibited reduced recruitment of multiple PFC regions involved in cognitive control – including MFG, dorsomedial PFC, and dACC – when viewing negative images, which mediated the elevations in p-factor in this group. Self-reported disruptions in emotional reactivity and regulation have been identified as a mechanism linking maltreatment exposure to youth psychopathology (Weissman et al., 2019; Heleniak et al., 2016a). We extend these behavioral findings by documenting that differences in neural recruitment during emotional reactivity similarly serve as a potential mechanism linking maltreatment and psychopathology. Specifically, reduced recruitment of PFC regions associated with cognitive control may be a mechanism linking child maltreatment with p-factor. These findings are broadly consistent with prior work suggesting that heightened negative emotionality and poor cognitive control are associated with the general psychopathology factor (Caspi et al., 2014; Lahey et al., 2017) as well as a meta-analysis documenting that alterations in the structure of dACC are related to multiple forms of psychopathology in adults (Goodkind et al., 2015).
We found no associations between reappraisal-related neural recruitment and psychopathology. However, recent work from our laboratory demonstrated that the degree of amygdala modulation during reappraisal interacted with maltreatment exposure to predict the emergence of depression symptoms over time. Specifically, maltreated youths who had greater reductions in amygdala activity during reappraisal relative to simple viewing of emotional stimuli were less likely to develop depression symptoms over time than youths with lower amygdala modulation (Rodman, Jenness, Weissman, Pine, & McLaughlin, 2019). Thus, reappraisal-related neural recruitment may act as a protective factor against the development of some forms of psychopathology in maltreated youths.
To determine whether the associations of child maltreatment with neural recruitment and psychopathology persisted after adjusting for co-occurring experiences of deprivation (McLaughlin & Sheridan, 2016), we adjusted for exposure to neglect in a sensitivity analysis. Findings were largely similar, but revealed stronger associations of maltreatment with increases in recruitment of salience processing regions. These differences are consistent with theoretical models positing that threatening early experiences are particularly likely to lead to elevations in salience network recruitment during emotional processing to facilitate threat detection (McLaughlin, Sheridan, & Lambert, 2014b)
Several limitations of the study are worth noting. Although maltreatment experiences occurred prior to study participation, we were unable to determine the directionality of the association between neural recruitment and symptoms of psychopathology. Prospective studies are needed to better understand the temporal associations between neural mechanisms of ER and trauma-related psychopathology. Further, the ER task was conducted in a controlled environment with training on reappraisal strategies. While this generally mimics clinical settings, it is possible that maltreated youth would have greater difficulty utilizing reappraisal strategies in real-world situations that have greater emotional salience.
Conclusion
Child maltreatment is associated with altered function of brain regions involved in salience processing and cognitive control during emotional reactivity and regulation. Maltreatment exposure was associated with greater recruitment of amygdala and salience processing regions and reduced PFC recruitment when viewing negative v. neutral images. Reduced PFC recruitment mediated the association between maltreatment and a general psychopathology factor. During reappraisal, maltreated youth showed increasing PFC recruitment across the transition to adolescence while non-maltreated youth showed decreasing age-related recruitment, which may reflect less efficient ER among youth with a history of maltreatment. Overall, these findings suggest that disruptions in neural systems underlying emotional processing may serve as a mechanism linking child maltreatment with psychopathology.
Supplementary Material
Acknowledgments
Financial support. This research was supported by the National Institute of Mental Health K23MH112872 (JLJ); R01-MH103291 (RAM), R01-MH106482 (RAM); R01MH116325 (ABM); F31-MH108245 (CH); the Brain & Behavior Research Foundation NARSAD Young Investigator Grant (JLJ) and Doris Duke Fellowship for the Promotion of Child Wellbeing (CH).
Footnotes
Conflict of interest. None.
Ethical standards. The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
Supplementary material. The supplementary material for this article can be found at https://doi.org/10.1017/psm2000064
References
- Askren MR, McAllister-Day TR, Roh N, Mestre Z, Dines JN, Rorman BA, … Madhyastha TM (2016). Using Make for reproducible and parallel neuroimaging workflow and quality-assurance. Frontiers in Neuroinformatics, 10, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball TM, Ramsawh HJ, Campbell-Sills L, Paulus MP, & Stein MB (2013). Prefrontal dysfunction during emotion regulation in generalized anxiety and panic disorders. Psychological Medicine, 43, 1475–1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauregard M, Paquette V, & Lévesque J (2006). Dysfunction in the neural circuitry of emotional self-regulation in major depressive disorder. Neuroreport, 17, 843–846. [DOI] [PubMed] [Google Scholar]
- Behzadi Y, Restom R, Liau J, & Liu TT (2007). A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. Neurolmage, 37, 90–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein DP, Ahluvalia T, Pogge D, & Handelsman L (1997). Validity of the Childhood Trauma Questionnaire in an adolescent psychiatric population. Journal of the American Academy of Child & Adolescent Psychiatry, 36, 340–348. [DOI] [PubMed] [Google Scholar]
- Bifulco A, Brown GW, & Harris TO (1994). Childhood Experience of Care and Abuse (CECA): A retrospective interview measure. Journal of Child Psychology and Psychiatry, 35, 1419–1435. [DOI] [PubMed] [Google Scholar]
- Caspi A, Houts RM, Belsky DW, Goldman-Mellor SJ, Harrington H, Israel S, … Moffitt TE (2014). The p factor one general psychopathology factor in the structure of psychiatric disorders? Clinical Psychological Science, 2, 119–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillon DG, & Pizzagalli DA (2013). Evidence of successful modulation of brain activation and subjective experience during reappraisal of negative emotion in unmedicated depression. Psychiatry Research: Neuroimaging, 212, 99–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg N, Fabes RA, & Murphy BC (1996). Parents’ reactions to children’s negative emotions: Relations to children’s social competence and comforting behavior. Child Development, 67, 2227–2247. [PubMed] [Google Scholar]
- Eklund A, Nichols TE, & Knutsson H (2016). Cluster failure: Why fMRI inferences for spatial extent have inflated false-positive rates. Proceedings of the National Academy of Sciences, 113, 7900–7905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelhor D, Hamby SL, Ormrod R, & Turner H (2005). The Juvenile Victimization Questionnaire: Reliability, validity, and national norms. Child Abuse & Neglect, 29, 383–412. [DOI] [PubMed] [Google Scholar]
- Finkelhor D, Turner HA, Shattuck A, & Hamby SL (2013). Violence, crime, and abuse exposure in a national sample of children and youth: An update. JAMA Pediatrics, 167, 614–621. [DOI] [PubMed] [Google Scholar]
- Goodkind M, Eickhoff SB, Oathes DJ, Jiang Y, Chang A, Jones-Hagata LB, … Etkin A (2015). Identification of a common neurobiological substrate for mental illness. JAMA Psychiatry, 72, 305–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heleniak C, Jenness JL, Vander Stoep A, McCauley E, & McLaughlin RA (2016a). Childhood maltreatment exposure and disruptions in emotion regulation: A transdiagnostic pathway to adolescent internalizing and externalizing psychopathology. Cognitive Therapy and Research, 40, 394–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heleniak C, McLaughlin KA, Ormel J, & Riese H (2016b). Cardiovascular reactivity as a mechanism linking child trauma to adolescent psychopathology. Biological Psychology, 120, 108–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly PA, Viding E, Puetz VB, Palmer AL, Mechelli A, Pingault J-B, … McCrory EJ (2015). Sex differences in socioemotional functioning, attentional bias, and gray matter volume in maltreated children: A multi-level investigation. Development and Psychopathology, 27, 1591–1609. [DOI] [PubMed] [Google Scholar]
- Lahey BB, Krueger RF, Rathouz PJ, Waldman ID, & Zald DH (2017). A hierarchical causal taxonomy of psychopathology across the life span. Psychological Bulletin, 143, 142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lake AJ, Finn PR, & James TW (2017). Neural correlates of emotion reappraisal in individuals with externalizing psychopathology. Brain Imaging and Behavior, 11, 76–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeWinn KZ, Strigo IA, Connolly CG, Ho TC, Tymofiyeva O, Sacchet MD, … Yang TT (2018). An exploratory examination of reappraisal success in depressed adolescents: Preliminary evidence of functional differences in cognitive control brain regions. Journal of Affective Disorders, 240, 155–164. [DOI] [PubMed] [Google Scholar]
- McCrory EJ, De Brito SA, Sebastian CL, Mechelli A, Bird G, Kelly PA, & Viding E (2011). Heightened neural reactivity to threat in child victims of family violence. Current Biology, 21, R947–R948. [DOI] [PubMed] [Google Scholar]
- McLaughlin KA, Green JG, Gruber MJ, Sampson NA, Zaslavsky AM, & Kessler RC (2012). Childhood adversities and first onset of psychiatric disorders in a national sample of US adolescents. Archives of General Psychiatry, 69, 1151–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA, Peverill M, Gold AL, Alves S, & Sheridan MA (2015). Child maltreatment and neural systems underlying emotion regulation. Journal of the American Academy of Child & Adolescent Psychiatry, 54, 753–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA, & Sheridan MA (2016). Beyond cumulative risk: A dimensional approach to childhood adversity. Current Directions in Psychological Science, 25, 239–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA, Sheridan MA, Alves S, & Mendes WB (2014a). Child maltreatment and autonomic nervous system reactivity: Identifying dysregulated stress reactivity patterns by using the biopsychosocial model of challenge and threat. Psychosomatic Medicine, 76, 538–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA, Sheridan MA, & Lambert HK (2014b). Childhood adversity and neural development: Deprivation and threat as distinct dimensions of early experience. Neuroscience & Biobehavioral Reviews, 47, 578–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRae K, Gross JJ, Weber J, Robertson ER, Sokol-Hessner P, Ray RD, … Ochsner KN (2012). The development of emotion regulation: An fMRI study of cognitive reappraisal in children, adolescents and young adults. Social Cognitive and affective Neuroscience, 7, 11–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AB, McLaughlin KA, Busso DS, Brueck S, Peverill M, & Sheridan MA (2018). Neural correlates of emotion regulation and adolescent suicidal ideation. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging, 3, 125–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner KN, Ray RD, Cooper JC, Robertson ER, Chopra S, Gabrieli JD, & Gross JJ (2004). For better or for worse: Neural systems supporting the cognitive down-and up-regulation of negative emotion. Neuroimage, 23, 483–499. [DOI] [PubMed] [Google Scholar]
- Perlman G, Simmons AN, Wu J, Hahn KS, Tapert SF, Max JE, … Yang TT (2012). Amygdala response and functional connectivity during emotion regulation: A study of 14 depressed adolescents. Journal of Affective Disorders, 139, 75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pine DS, Mogg K, Bradley BP, Montgomery L, Monk CS, McClure E, … Kaufman J (2005). Attention bias to threat in maltreated children: Implications for vulnerability to stress-related psychopathology. American Journal of Psychiatry, 162, 291–296. [DOI] [PubMed] [Google Scholar]
- Price RB, Siegle GJ, Silk JS, Ladouceur CD, McFarland A, Dahl RE, & Ryan ND (2014). Looking under the hood of the dot-probe task: An fMRI study in anxious youth. Depression and Anxiety, 31, 178–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodman AM, Jenness JL, Weissman DG, Pine DS, & McLaughlin KA (2019). Neurobiological markers of resilience to depression following childhood maltreatment: The role of neural circuits supporting the cognitive control of emotion. Biological Psychiatry, 86(6), 464–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvers JA, Insel C, Powers A, Franz P, Helion C, Martin R, … Ochsner KN (2017). The transition from childhood to adolescence is marked by a general decrease in amygdala reactivity and an affect-specific ventral-to-dorsal shift in medial prefrontal recruitment. Developmental Cognitive Neuroscience, 25, 128–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvers JA, Insel C, Powers A, Franz P, Helion C, Martin RE, … Ochsner KN (2016). vlPFC-vmPFC-amygdala interactions underlie age-related differences in cognitive regulation of emotion. Cerebral Cortex, 27, 3502–3514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvers JA, McRae K, Gabrieli JD, Gross JJ, Remy KA, & Ochsner KN (2012). Age-related differences in emotional reactivity, regulation, and rejection sensitivity in adolescence. Emotion (Washington, D.C.), 12, 1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP (2009). Heightened stress responsivity and emotional reactivity during pubertal maturation: Implications for psychopathology. Development and Psychopathology, 21, 87–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg AM, Brymer MJ, Kim S, Briggs EC, Ippen CG, Ostrowski SA, … Pynoos RS (2013). Psychometric properties of the UCLA PTSD reaction index: Part I. Journal of Traumatic Stress, 26, 1–9. [DOI] [PubMed] [Google Scholar]
- Wager TD, Davidson ML, Hughes BL, Lindquist MA, & Ochsner KN (2008). Prefrontal-subcortical pathways mediating successful emotion regulation. Neuron, 59, 1037–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Waugh CE, Lindquist M, Noll DC, Fredrickson BL, & Taylor SF (2009). Brain mediators of cardiovascular responses to social threat: Part I: Reciprocal dorsal and ventral sub-regions of the medial prefrontal cortex and heart-rate reactivity. Neuroimage, 47, 821–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker EA, Unutzer J, Rutter C, Gelfand A, Saunders K, VonKorff M, … Katon W (1999). Costs of health care use by women HMO members with a history of childhood abuse and neglect. Archives of General Psychiatry, 56, 609–613. [DOI] [PubMed] [Google Scholar]
- Weissman DG, Bitran D, Miller AB, Schaefer JD, Sheridan MA, & McLaughlin KA (2019). Difficulties with emotion regulation as a transdiagnostic mechanism linking child maltreatment with the emergence of psychopathology. Development and Psychopathology, 31, 899–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto DJ, Woo C-W, Wager TD, Regner MF, & Tanabe J (2015). Influence of dorsolateral prefrontal cortex and ventral striatum on risk avoidance in addiction: A mediation analysis. Drug and Alcohol Dependence, 149, 10–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


