Abstract
Background:
Combination checkpoint inhibitor immunotherapy has recently become a new frontline standard of care for metastatic renal cell carcinoma (mRCC). Previously published data has demonstrated a survival benefit of cytoreductive nephrectomy (CN) in the interferon era, but the utility of CN in conjunction with the use of tyrosine kinase inhibitors (TKIs) has been controversial.
Patients:
In the current case series, we report on five patients with IMDC intermediate- or poor-risk initially unresectable metastatic renal cell carcinoma who successfully underwent CN.
Results:
All patients received induction combination checkpoint inhibitor immunotherapy (CPI) ipilimumab-nivolumab with or without pembrolizumab-axitinib. Three patients underwent robotic CN, and one patient underwent open and laparoscopic CN, respectively. Three of the five patients were found to have significant fibrosis and desmoplastic reaction at the time of nephrectomy, requiring surgical expertise and increased operative time.
Conclusions:
Herein we highlight the feasibility of this approach and the post-CPI surgical challenge of CN. The role of CN in patients receiving systemic therapy with CPI is not yet defined and is quickly evolving.
Keywords: frontline therapy, kidney cancer, stage 4, systemic therapy, surgery
Introduction
There were an estimated 73,820 new cases of renal cell carcinoma (RCC) and 14,770 deaths in 20191. Approximately 16% of patients present with metastatic disease, and the 5-year survival of advanced RCC is still poor, around 12%1, 2. However, the therapeutic landscape of metastatic renal cell carcinoma (mRCC) has rapidly evolved over the last several years3, 4. For patients with IMDC intermediate or poor risk clear cell RCC who are candidates for checkpoint inhibitor immunotherapy (CPI), both combination anti-CTLA-4 therapy with ipilimumab and anti-PD-1 therapy with nivolumab, as well as the anti-PD-1 inhibitor pembrolizumab in combination with the oral tyrosine kinase inhibitor axitinib, are now approved for first line therapy5.
The phase III CheckMate 214 trial randomized 847 patients in a 1:1 fashion to receive either ipilimumab 3 mg/kg IV and nivolumab 1 mg/kg IV every 3 weeks for four cycles followed by nivolumab monotherapy 3 mg/kg IV every 2 weeks or sunitinib monotherapy (50 mg daily, 4 weeks on, 1 week off)6. After 42 months of follow-up, the median overall survival among intermediate and poor risk patients in the ipilimumab-nivolumab arm was 47.0 months compared with 26.6 months in the sunitinib arm (HR 0.66, 95% CI 0.55 – 0.80, p < 0.0001), with a complete response rate of 10% and a partial response rate of 32%7. 68% of intermediate/poor risk patients (121 of 179) had an ongoing response to therapy, with the median duration of response not yet reached. In the KEYNOTE-426 randomized phase III clinical trial, pembrolizumab-axitinib combination therapy also demonstrated improved efficacy over sunitinib8. While the survival data for this study are not yet mature, after 12.8 months of follow-up, 89.9% of patients in the pembrolizumab-axitinib group were alive at 12 months compared with 78.3% of patients in the sunitinib group (HR for disease progression or death, 0.69; 95% CI, 0.57 to 0.84; p < 0.001). Comparable clinical benefits were also observed with avelumab-axitinib in the Phase III JAVELIN Renal 101 trial9.
In addition to advances in systemic therapy for mRCC, the role of cytoreductive nephrectomy (CN) remains unclear due to the rapid evolution of systemic treatment for mRCC. In the cytokine era, several trials demonstrated a survival benefit with CN in conjunction with interferon-based therapy versus interferon alone10, 11. In that context, CN was associated with an improvement in overall survival of about 5.8 months12. Moreover, in the targeted therapy era, it is known that CN is likely to benefit patients who have limited sites of extra-renal metastatic disease and an absence of poor risk features.13–16 However, the utility of CN for patients receiving frontline CPI for advanced RCC is not yet defined, specifically since the approval of ipilimumab-nivolumab in 2018 and the approval of pembrolizumab-axitinib and avelumab-axitinib in 2019 as front-line treatments for mRCC. In the current case series, we present five patients with intermediate or poor risk mRCC with an initially unresectable primary tumor who underwent elective CN following induction therapy consisting of ipilimumab-nivolumab with or without pembrolizumab-axitinib.
Results
Patients included in this case series were consented at the Siteman Cancer Center at Washington University in St. Louis under an IRB-approved protocol. The clinical characteristics of the patients included in this case series are delineated below and summarized in Table 1.
Table 1.
| Patient | Disease extent at diagnosis | Neoadjuvant CPI therapy | Radiographic response after neoadjuvant CPI | Pathology results at time of cytoreductive nephrectomy (CN) | Current status |
|---|---|---|---|---|---|
| #1 (IMDC poor risk) |
Right renal primary, retroperitoneal lymph nodes, lung nodules, low-volume osseous metastases |
|
Near-CR of pulmonary metastases, near-CR of retroperitoneal lymphadenopathy, PR of primary renal lesion | Right nephrectomy: Extensive necrosis and histiocytic response, consistent with treatment effect; no viable tumor identified Marked interstitial nephritis, segmental and global glomerulosclerosis in peritumoral non-neoplastic kidney parenchyma, not involving distant renal parenchyma One lymph node with no evidence of malignancy (0/1) |
Active surveillance, NED |
| #2 (IMDC poor risk) |
Right renal primary (large, heterogeneously enhancing), lung nodules, large lytic metastasis in the right scapula |
|
Stable/persistent infiltrative, multinodular mass within the right kidney, mixed response in the lungs and stability of osseous metastases. | Right nephrectomy: Clear cell renal cell carcinoma (5.2 cm), WHO/ISUP grade 4, with extensive treatment effect, invading renal sinus fat, resection margins uninvolved
|
Interval development of solitary CNS metastasis s/p radiosurgery Initiated second line cabozantinib |
| #3 (IMDC intermediate risk) |
12.3 cm right renal mass with tumor thrombus invading the IVC and liver invasion, mediastinal lymphadenopathy, and pulmonary nodules |
|
Slight increase in the size of the right renal mass, marked increase in the adenopathy around the renal mass and in the kidney, and slight decrease in the size of pulmonary nodules | Tumor thrombus excision with clear cell renal cell carcinoma, involving vessel wall Renal cell carcinoma (12 cm), clear cell type, WHO/ISUP grade 4 with rhabdoid features and extensive necrosis, invading adrenal gland by direct extension, extensively involving perinephric and hilar soft tissue Carcinoma abuts but does not invade liver and diaphragm Renal cortical scars, patchy interstitial chronic inflammation and focal global glomerulosclerosis (25%) |
Interval development metastases involving the lumbar spine and psoas muscle Initiated second line axitinib and pembrolizumab |
| #4 (IMDC intermediate risk) |
7.3 × 9.1 × 8.7 cm mass in the left kidney as well as multifocal osseous metastatic disease involving the sternum, cervical spine, thoracic spine, left acetabulum, and left tibia. |
|
Persistent osseous metastatic disease with progressive epidural tumor involvement of the thoracic spine.
|
Left nephrectomy: Clear cell renal cell carcinoma (6.0 cm), WHO/ISUP grade 2, confined to the kidney, resection margins uninvolved |
Resumed axitinib and pembrolizumab; ongoing clinical response |
| #5 (IMDC intermediate risk) |
Left hilar mass, multiple pleural and parenchymal metastatic lesions, and 12.6 × 12.3 cm centrally necrotic left renal mass with associated tumor thrombus. |
|
Near complete resolution of pulmonary and left hilar metastatic disease, PR of the primary left renal mass with decrease in size to 9.9 cm. | Kidney, left, radical nephrectomy: Clear cell renal cell carcinoma with extensive treatment effect, WHO/ISUP grade 4 with rhabdoid features, resection margins uninvolved Marked interstitial chronic inflammation with interstitial fibrosis/tubular atrophy No evidence of malignancy in one lymph node (0/1) Adrenal gland, left, radical nephrectomy. No evidence of viable carcinoma |
Plan to continue nivolumab monotherapy |
Patient #1:
51-year-old man who presented with abdominal discomfort in 2018. A CT scan of the chest, abdomen and pelvis demonstrated a large a large right-sided renal mass measuring 8.6 × 8.1 × 9.1 cm as well as numerous large, necrotic retroperitoneal lymph nodes, bilateral pulmonary nodules, and osseous metastasis involving the right proximal femur (Figure 1). Retroperitoneal lymph node biopsy confirmed metastatic carcinoma most consistent with clear cell RCC. The patient initiated combination CPI in June 2018 with ipilimumab 1 mg/kg and nivolumab 3 mg/kg every 3 weeks for 4 cycles and subsequently transitioned to nivolumab monotherapy, 480 mg IV every 4 weeks. He received 4 cycles of nivolumab monotherapy, after which he was found to have a marked radiographic response to therapy with interval reduction of the primary renal mass to 4.2 × 3.0 cm in December 2018 and near resolution of metastatic disease in the chest. He then underwent elective robotic CN and lymphadenectomy in January 2019. He then resumed nivolumab monotherapy in February 2019 for a total of 11 cycles post-operatively and subsequently transitioned to active surveillance. Most recent interval imaging in February 2020 demonstrated no evidence of disease.
Figure 1.
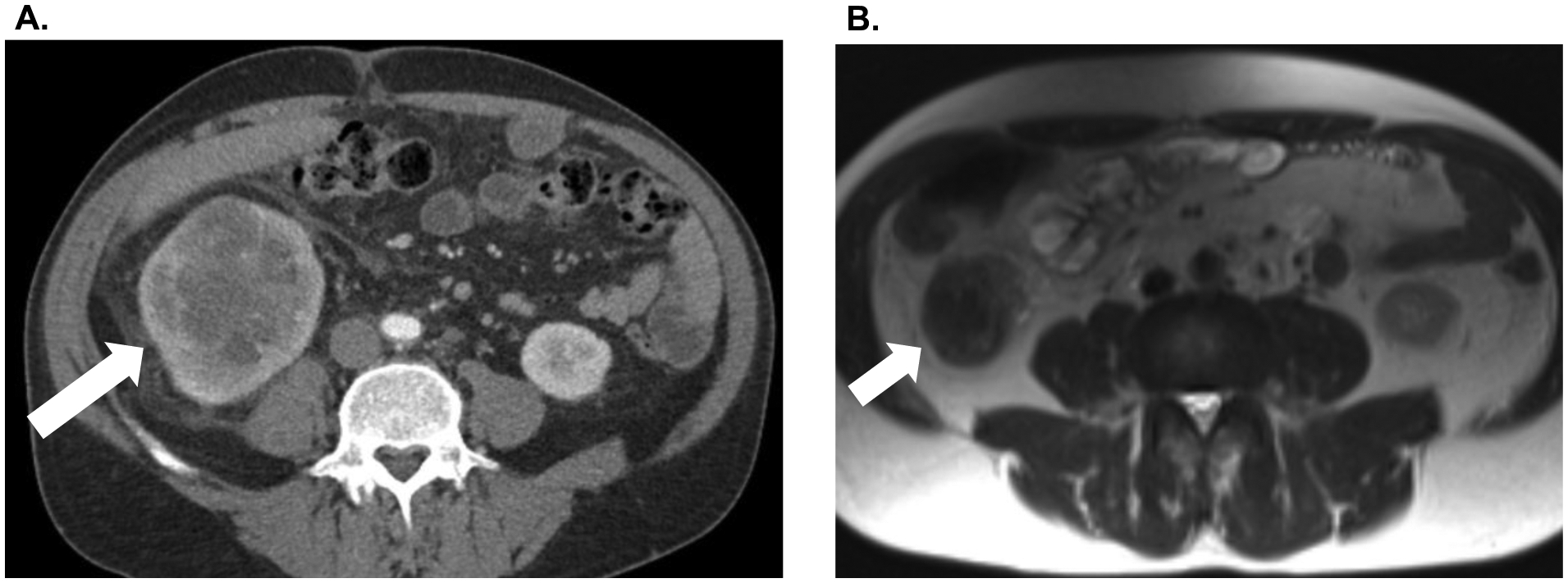
A. Patient #1 pre-treatment CT abdomen/pelvis demonstrating large right renal mass. B. Marked interval reduction in the size of the primary renal mass after four cycles of ipilimumab/nivolumab combination therapy and four cycles of nivolumab monotherapy.
Patient #2:
84-year-old man who presented with anorexia and fatigue in 2018. Initial imaging was consistent with metastatic disease with multiple pulmonary nodules, a large lytic mass involving the inferior right scapula, and a large heterogeneously enhancing right renal mass. Right renal biopsy demonstrated grade 4 clear cell RCC. He initiated combination CPI in December 2018 with ipilimumab 1 mg/kg and nivolumab 3 mg/kg every 3 weeks for 4 cycles and subsequently transitioned to nivolumab monotherapy, 480 mg IV every 4 weeks. He received 2 cycles of nivolumab monotherapy. The infiltrative, multinodular mass within the right kidney persisted on subsequent imaging. He had a mixed response in the lungs and stability of osseous metastases. He then underwent robotic CN and IVC thrombectomy in May 2019. Following CN, he received an additional 10 cycles of nivolumab monotherapy through early February 2020. Most recently he developed a right frontal CNS metastasis that was treated with radiosurgery. He has since initiated second line cabozantinib.
Patient #3:
55-year-old man who presented with gross hematuria in 2018. He was found to have a 12.3 cm right renal mass with tumor thrombus invading the IVC and liver, mediastinal lymphadenopathy, and pulmonary nodules. He underwent renal biopsy and was found to have grade 4 clear cell RCC with rhabdoid differentiation. He initiated combination CPI in June 2018 with ipilimumab 1 mg/kg and nivolumab 3 mg/kg every 3 weeks for 4 cycles and subsequently transitioned to nivolumab monotherapy, 480 mg IV every 4 weeks. He received 7 cycles of nivolumab monotherapy. Subsequent imaging demonstrated slight increase in the size of the right renal mass, marked increase in the adenopathy around the renal mass and in the kidney, and slight decrease in the size of pulmonary nodules. He then underwent open CN, IVC thrombectomy, and partial hepatectomy in April 2019. Following CN, he received an additional 7 cycles of nivolumab monotherapy through December 2019. He then developed disease recurrence in the lumbar spine requiring decompressive laminectomy. He initiated second line therapy with axitinib and pembrolizumab in mid-December 2019, with partial response to therapy as of March 2020.
Patient #4:
42-year-old man who presented with progressive back pain in late 2018. He was subsequently found to have a 7.3 × 9.1 × 8.7 cm mass in the left kidney as well as multifocal osseous metastatic disease involving the sternum, cervical spine, thoracic spine, left acetabulum, and left tibia. A T10 biopsy confirmed mRCC. He initiated combination CPI in February 2019 with ipilimumab 1 mg/kg and nivolumab 3 mg/kg every 3 weeks. After 3 cycles, he had persistent osseous metastatic disease and progressive epidural tumor involvement of the thoracic spine. In April 2019, he underwent extensive thoracic laminectomy and excision of epidural tumor, as well thoracic spine corpectomy and T5–T7 and T9–T11 spinal fusion followed by adjuvant radiation therapy. Subsequently, he was transitioned to second line therapy with axitinib and pembrolizumab in May 2019. He received axitinib 5 mg PO BID and pembrolizumab 200 mg IV every 4 weeks for 13 cycles. On interval imaging, the left renal mass had decreased to 4.7 × 4.4 × 4.6 cm, with overall stable osseous metastatic disease (Figure 2). He underwent laparoscopic CN in February 2020 and subsequently resumed axitinib and pembrolizumab with ongoing clinical benefit.
Figure 2.
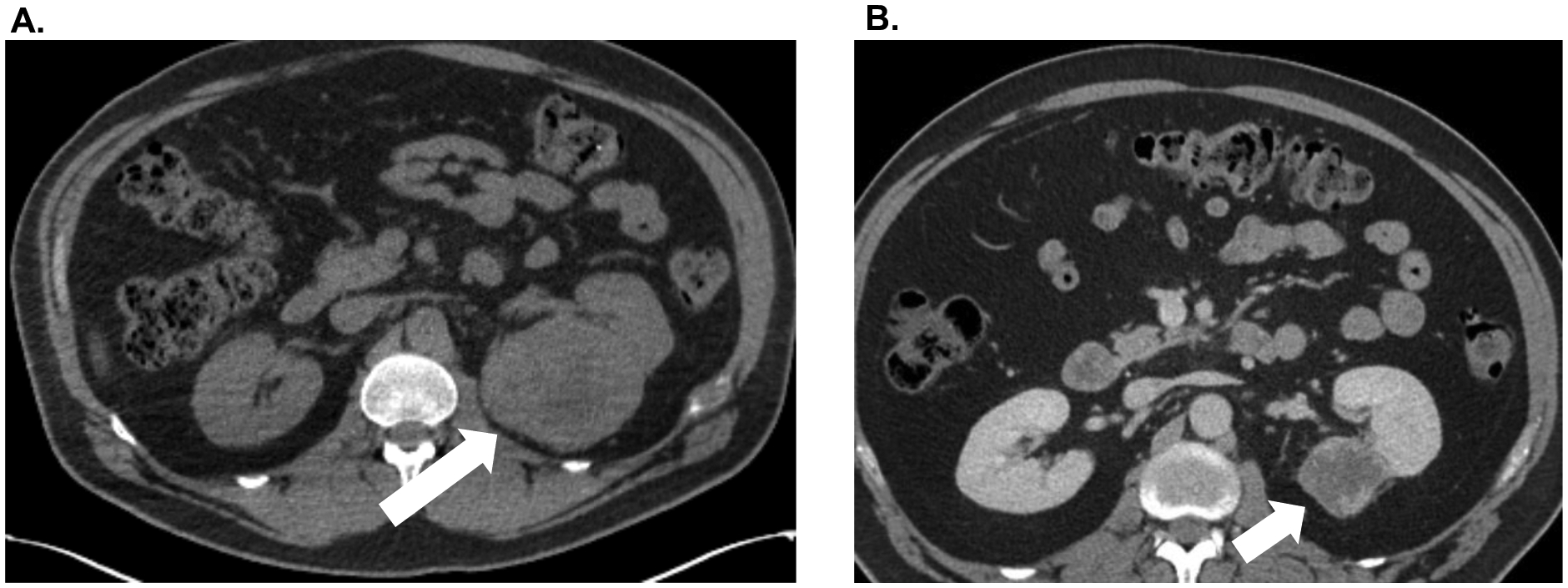
A. Patient #4 pre-treatment CT abdomen/pelvis demonstrating large right renal mass. B. Significant interval reduction in the size of the primary renal mass after three cycles of ipilimumab/nivolumab combination therapy followed by pembrolizumab/axitinib for 13 cycles.
Patient #5:
75-year-old man who presented in September 2019 with cough and hemoptysis. He was found to have a left hilar mass and multiple pleural and parenchymal metastatic lesions, as well as a 12.6 × 12.3 cm centrally necrotic left renal mass with associated tumor thrombus. He initiated combination CPI in June 2019 with ipilimumab 1 mg/kg and nivolumab 3 mg/kg every 3 weeks for 4 cycles and then transitioned to nivolumab monotherapy, 480 mg IV every 4 weeks. He received one cycle of nivolumab, and interval imaging demonstrated near complete resolution of pulmonary and left hilar metastatic disease, with the primary left renal mass decreased in size to 9.9 cm (Figure 3). He underwent robotic CN and lymphadenectomy in February 2020 and subsequently resumed nivolumab monotherapy 480 mg IV every 4 weeks in March 2020.
Figure 3.
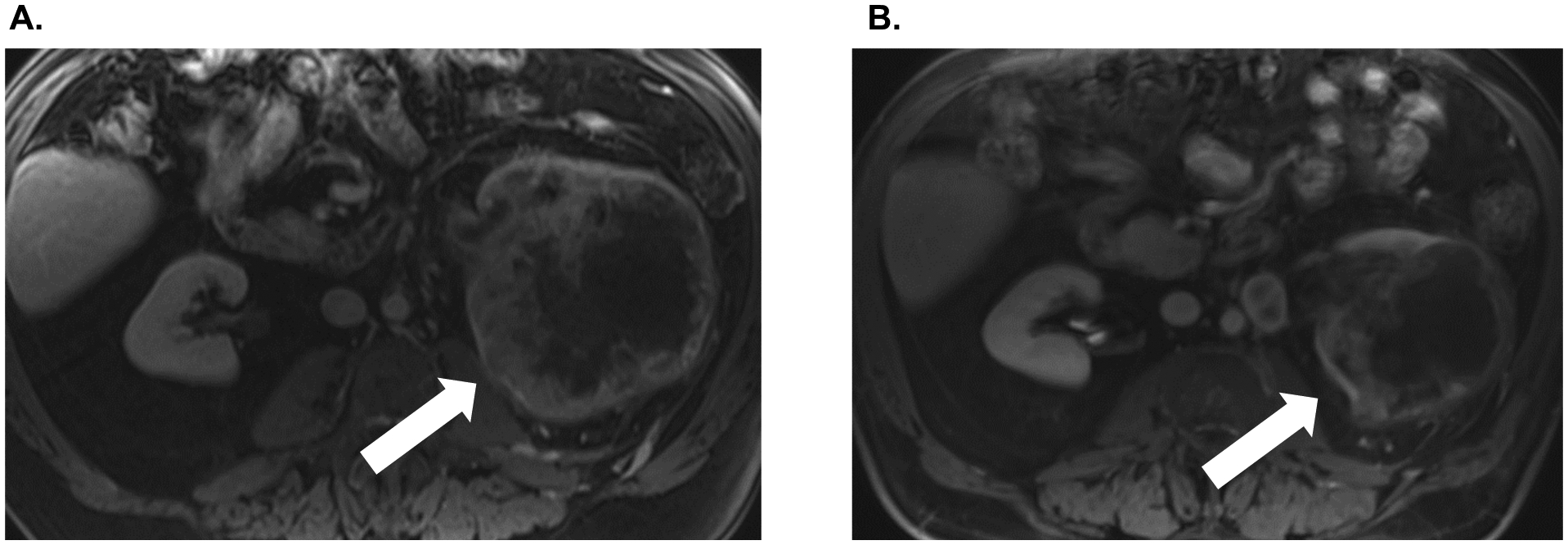
A. Patient #5 pre-treatment MRI of the abdomen demonstrating large right renal mass. B. Significant interval reduction in the size of the primary renal mass after four cycles of ipilimumab/nivolumab combination therapy and one cycle of nivolumab monotherapy.
Discussion
The landscape of mRCC has been rapidly evolving over the last several years, as has the data surrounding the applicability of CN in the modern era. The phase III CARMENA trial demonstrated that sunitinib alone was non-inferior to CN followed by sunitinib with respect to overall survival16. The randomized phase II SURTIME trial sought to evaluate the impact of three cycles of neoadjuvant sunitinib prior to CN versus CN followed by sunitinib. However, this study was underpowered and accrued poorly, and the primary endpoint of the 28-week progression free rate did not differ between the two arms17. Moreover, the data surrounding neoadjuvant systemic therapy prior to CN in advanced RCC has been primarily limited to the targeted tyrosine kinase inhibitor agents to date. There has been a modest reported benefit of downsizing of previously unresectable tumors, with an average of less than 20% size reduction18, 19. One significant concern of the use of neoadjuvant tyrosine kinase inhibitors is the potential for impaired wound healing and an increased risk of hemorrhage, which has been reported in one series20.
In the current case series, we present five cases of patients who received frontline treatment with combination CPI for mRCC with initially unresectable primary tumors who subsequently underwent successful elective CN before continuing with systemic therapy. To our knowledge, this is the first such reported series in the literature.
Three of the five patients (#1, #2, #5) in our series had at least stable disease or a partial response to initial CPI therapy on pre-operative imaging prior to undergoing CN. Of note, on pathological examination of the resection specimens for these patients, all three were noted to have extensive treatment effect. One patient (#1) in whom there was no remaining viable tumor at the time of CN is currently still NED almost two years after his initial diagnosis. While the majority of the patients in this case series have only been followed for two years or less since their diagnosis of metastatic disease, it will be of interest to determine over time whether their survival is equal or improved to patients of the same risk categories without CN in the CheckMate-214 population. It is also important to consider the potential risks and limitations of employing CPI prior to CN. In our patient series, three of the patients had substantial desmoplastic tissue reactions that contributed to additional operative time and difficulty. Two of these cases were particularly challenging due to the degree of fibrosis and desmoplastic reaction, with particularly difficult hilar dissections. In one case, the anatomy was very distorted and the superior mesenteric artery was inadvertently clipped, as it was thought to be the renal artery. Fortunately, this was recognized immediately and the clip was removed, but this could have been a lethal complication. Having surgical experience in such cases can be very helpful in the avoidance of intraoperative complications. Notably, despite the difficult dissection planes, there was no need for conversion to an open approach in any of the laparoscopic or robotic cases. The benefit to the patients is likely to be greatest if the nephrectomy can safely be performed laparoscopically or robotically. These are challenging cases that require extra time in the operating room, and ultimately, the surgical approach should be at the discretion of the surgeon based on his/her experience and comfort with the approach.
Moreover, it is noteworthy that the extent of desmoplastic reaction correlated with the extent of treatment effect noted on pathologic specimen examination and was most prevalent in patients #1, #2, and #5. This treatment effect also highlights the importance of considering timing of CN. From our limited patient series, it is not clear whether longer exposure to CPI alone increases the likelihood of desmoplastic reaction and increased tissue fibrosis, or whether this is rather related to the degree of immune infiltration and response to treatment.
Ongoing clinical trials such as the Danish NORDIC-SUN study (NCT03977571), “Deferred Cytoreductive Nephrectomy in Synchronous Metastatic Renal Cell Carcinoma” are of great interest and will help shed light on several unanswered questions in this therapeutic space. In this multicenter phase III clinical trial, 400 patients will be randomized to standard induction ipilimumab 1 mg/kg IV and nivolumab 3 mg/kg IV every 3 weeks for four cycles followed by maintenance nivolumab 480 mg IV every 4 weeks versus standard induction ipilimumab 1 mg/kg IV and nivolumab 3 mg/kg IV every 3 weeks for four cycles followed by CN and then subsequently initiation of maintenance nivolumab. The primary endpoint of this study is overall survival. The anticipated readout of this trial is in 2025, and we await these results with great interest.
The current cases series demonstrates successful, albeit challenging, elective CN in patients with intermediate-and poor-risk mRCC with initially unresectable primary tumors who received frontline CPI therapy. CPI followed by CN is feasible and represents a promising strategy to further augment the significant benefit of first line immunotherapy for patients with advanced RCC.
Key Take Home Points:
The use of cytoreductive nephrectomy (CN) in the era of checkpoint inhibitor therapy (CPI) for metastatic renal cell carcinoma (mRCC) is not yet established
We present five mRCC patients with unresectable primary tumors who received CPI followed by successful CN, with one patient currently NED
Highlights:
Case series of 5 patients with metastatic RCC and unresectable primary tumors
Patients received frontline combination checkpoint inhibitor immunotherapy (CPI)
All patients successfully underwent cytoreductive nephrectomy (CN) following CPI
3 of 5 patients had significant fibrosis and desmoplastic reaction at time of CN
Clinical Practice Points.
The management of metastatic renal cell carcinoma (mRCC) is rapidly evolving. Combination checkpoint inhibitor immunotherapy (CPI) with nivolumab and ipilimumab (CheckMate-214) has led to a substantial survival benefit for patient with intermediate or poor risk mRCC. Other combinations with pembrolizumab-axitinib (KEYNOTE-426) and avelumab-axitinib (JAVELIN Renal 101) have also shown promising results. In this rapidly evolving field with the increasing use of frontline CPI, the role of cytoreductive nephrectomy (CN) is less well defined. Previously, during the use of interferon, CN was associated with a survival benefit, but this was not recapitulated in more recent trials utilizing tyrosine kinase inhibitors (TKIs). Thus, evaluating the use of CN in patients who receive frontline CPI for mRCC with an initially unresectable primary tumor is an important area of unmet clinical need. In this case series, we present five patients who received first line CPI; all were later able to undergo successful resection of their primary tumors. However, it is important to note the particular surgical skill and expertise required to perform CN in these patients. Three of the five patients had significant fibrosis and desmoplastic reaction at the time of surgery, which required additional operative time and skill to address. Nonetheless, the potential clinical benefit of sequential therapy with CPI followed by CN is substantial – one patient currently remains NED. In carefully selected patients, this combination strategy may render some patients with advanced mRCC free to disease, and may portend a further shift in the treatment paradigm for this patient population.
Acknowledgements:
JJH is supported by NIH R01 CA223231.
Funding: This work was supported by the National Institutes of Health R01 CA223231.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: none
Disclosures: The authors have no relevant conflicts of interest to disclose.
References
- 1.Cancer Stat Facts: Kidney and Renal Pelvis Cancer. NIH SEER Program. 2019;https://seer.cancer.gov/statfacts/html/kidrp.html.
- 2.Saad AM, Gad MM, Al-Husseini MJ, Ruhban IA, Sonbol MB, Ho TH. Trends in Renal-Cell Carcinoma Incidence and Mortality in the United States in the Last 2 Decades: A SEER-Based Study. Clinical Genitourinary Cancer. 2019;17:46–57.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hsieh JJ, Purdue MP, Signoretti S, et al. Renal cell carcinoma. Nat Rev Dis Primers. 2017;3:17009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang JJ, Hsieh JJ. The Therapeutic Landscape of Renal Cell Carcinoma: From the Dark Age to the Golden Age. Semin Nephrol. 2020;40:28–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Monteiro FSM, Soares A, Debiasi M, et al. First-Line Treatment for Metastatic Renal Cell Carcinoma in the immuno-oncology era: Systematic Review and Network Meta-Analysis. Clinical Genitourinary Cancer. 2020. [DOI] [PubMed]
- 6.Motzer RJ, Tannir NM, McDermott DF, et al. Nivolumab plus Ipilimumab versus Sunitinib in Advanced Renal-Cell Carcinoma. N Engl J Med. 2018;378:1277–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tannir NMea. Overall survival and independent review of response in CheckMate 214 with 42-month follow-up: First line nivolumab + ipilimumab (N+I) versus sunitinib (S) in patients (pts) with advanced renal cell carcinoma (aRCC). Journal of Clinical Oncology. 2020;38:Abstract 609. [Google Scholar]
- 8.Rini BI, Plimack ER, Stus V, et al. Pembrolizumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N Engl J Med. 2019;380:1116–1127. [DOI] [PubMed] [Google Scholar]
- 9.Motzer RJ, Penkov K, Haanen J, et al. Avelumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N Engl J Med. 2019. [DOI] [PMC free article] [PubMed]
- 10.Flanigan RC, Salmon SE, Blumenstein BA, et al. Nephrectomy followed by interferon alfa-2b compared with interferon alfa-2b alone for metastatic renal-cell cancer. N Engl J Med. 2001;345:1655–1659. [DOI] [PubMed] [Google Scholar]
- 11.Mickisch GH, Garin A, van Poppel H, et al. Radical nephrectomy plus interferon-alfa-based immunotherapy compared with interferon alfa alone in metastatic renal-cell carcinoma: a randomised trial. Lancet. 2001;358:966–970. [DOI] [PubMed] [Google Scholar]
- 12.Flanigan RC, Mickisch G, Sylvester R, Tangen C, Van Poppel H, Crawford ED. Cytoreductive nephrectomy in patients with metastatic renal cancer: a combined analysis. J Urol. 2004;171:1071–1076. [DOI] [PubMed] [Google Scholar]
- 13.Motzer RJ, Mazumdar M, Bacik J, Berg W, Amsterdam A, Ferrara J. Survival and prognostic stratification of 670 patients with advanced renal cell carcinoma. J Clin Oncol. 1999;17:2530–2540. [DOI] [PubMed] [Google Scholar]
- 14.Heng DY, Xie W, Regan MM, et al. Prognostic factors for overall survival in patients with metastatic renal cell carcinoma treated with vascular endothelial growth factor-targeted agents: results from a large, multicenter study. J Clin Oncol. 2009;27:5794–5799. [DOI] [PubMed] [Google Scholar]
- 15.Bex A, Ljungberg B, van Poppel H, Powles T, European Association of U. The Role of Cytoreductive Nephrectomy: European Association of Urology Recommendations in 2016. Eur Urol. 2016;70:901–905. [DOI] [PubMed] [Google Scholar]
- 16.Mejean A, Ravaud A, Thezenas S, et al. Sunitinib Alone or after Nephrectomy in Metastatic Renal-Cell Carcinoma. N Engl J Med. 2018;379:417–427. [DOI] [PubMed] [Google Scholar]
- 17.Bex A, Mulders P, Jewett M, et al. Comparison of Immediate vs Deferred Cytoreductive Nephrectomy in Patients With Synchronous Metastatic Renal Cell Carcinoma Receiving Sunitinib: The SURTIME Randomized Clinical Trial. JAMA Oncol. 2019;5:164–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Amin C, Wallen E, Pruthi RS, Calvo BF, Godley PA, Rathmell WK. Preoperative tyrosine kinase inhibition as an adjunct to debulking nephrectomy. Urology. 2008;72:864–868. [DOI] [PubMed] [Google Scholar]
- 19.Bex A, van der Veldt AA, Blank C, et al. Neoadjuvant sunitinib for surgically complex advanced renal cell cancer of doubtful resectability: initial experience with downsizing to reconsider cytoreductive surgery. World J Urol. 2009;27:533–539. [DOI] [PubMed] [Google Scholar]
- 20.Thomas AA, Rini BI, Stephenson AJ, et al. Surgical resection of renal cell carcinoma after targeted therapy. J Urol. 2009;182:881–886. [DOI] [PubMed] [Google Scholar]


