Abstract
Longitudinal data on the functioning of adults referred for possible autism as children is sparse and possibly different from datasets consisting of adult clinical referrals. 123 young adults, mean age of 26, referred for neurodevelopmental disorders in early childhood were categorized into three outcome groups: ASD diagnosis at some point and current IQ≥70 (Ever ASD-Higher IQ), ever ASD and current IQ<70 (Ever ASD-Lower IQ) and individuals who never received an ASD diagnosis (Never ASD). Independence and well-being were assessed through direct testing, questionnaires and interviews. Verbal IQ, beyond intellectual disability status, accounted for group differences in employment; autistic features (ADOS CSS) were uniquely related to adaptive skills and friendships. In many ways, the Never ASD group had similar outcomes compared to the ASD groups. However, lower well-being and fewer positive emotions were related to ASD diagnosis across IQ. The Ever ASD-Lower IQ group had the highest levels of irritability, hyperactivity, and medications. Families played a major role in supporting adults with and without ASD at all intellectual levels. Realistic ways of increasing independence should be developed through working with adults and their families, while acknowledging the contribution of individual differences in mental health, intelligence and autism symptoms across neurodevelopmental disorders.
Persons with Autism Spectrum Disorders (ASD), or autistic people, reflecting the preference of many self-advocates, (Bagatell, 2010; Kapp, Gillespie-Lynch, Sherman, & Hutman, 2013) will be adults for far longer than they are children, yet we know much less about the behavior and the trajectories of development into and during adult life than we do about those during childhood. Information about adulthood in ASD is important for many reasons, not the least of which is for developing services as well as setting goals for possible outcomes. In the current study, we present data across several aspects of functioning, including the vocational, residential, affective, and subjective outcomes of adults seen at a mean age of 26 years who were referred to autism specialist clinics for evaluation of possible autism at age 2 years or younger, as well as a supplementary sample of children referred at older ages to a similar clinic and seen on several occasions with the same protocol. We consider how autism diagnoses and cognitive abilities contribute to outcomes in adulthood, and describe the similarities and differences between adults who received an ASD diagnosis and adults who never received a diagnosis across a wide range of cognitive abilities.
Outcomes of Autistic Adults
There is a substantial literature about adult “outcomes” in ASD including several well-known longitudinal samples (e.g., Billstedt, Gillberg & Gillberg, 2011; Jones et al., 2018;, Chan et al, 2018; Howlin, Moss, Savage, & Rutter, 2013; Simonoff et al, 2019), well as many reviews and commentaries (Ayres et al, 2018; Scott et al, 2019; Taylor, 2017; Zimmerman et al, 2018). Across longitudinal and cross sectional studies, findings indicate autistic adults struggle with achieving independent living and employment, maintaining friendships, managing co-occurring mental health conditions, and have poorer quality of life (Billstedt, Gillberg & Gillberg, 2011; Roux, Shattuck, Rast, Rava, & Anderson, 2015; Sosnowy et al, 2019; Wei et al., 2017).
However, the literature is limited by a number of factors, in part because of the heterogeneity of people with ASD. First, much of the adult literature is based on record reviews or cross-sectional studies. As such, cohort differences are confounded by why a participant might be in a particular dataset at a particular age, limiting interpretations about how individuals change over time (e.g., Hewitt et al., 2012; Renty & Roeyers, 2006). Though studies on younger children with ASD have begun examining individual variation over time with more precision (Solomon et al., 2018; Visser et al., 2017), many studies on autistic adults include small samples (e.g., 5 – 30 participants; Scott et al, 2019), a wide age range of participants (Smith, Maenner, & Seltzer, 2012), limited data beyond questionnaires, medical records and caregiver interviews (e.g., Eaves & Ho, 2008) and retrospective data dependent on who could be recruited for assessment at the time (e.g., Chamak & Bonniau, 2016). In addition, a large proportion of studies focus on autistic people without intellectual disabilities (Zimmerman et al, 2018).
A second issue is that without a relevant comparison group in studies, there is a concern that the negative outcomes of autistic adults are over-emphasized and assumed to be unique. Individuals with other developmental disabilities or with early presentations of general developmental concerns, such as individuals with Down syndrome or learning disabilities, experience many similar challenges as autistic adults across development. Some studies have described similarities between outcomes of individuals with non-spectrum developmental disabilities and autistic adults, including difficulties attaining work and establishing supportive friendships (Mawhood, Howlin, & Rutter, 2000; Zwicker, Zaresani, & Emery, 2017). At the same time, however, other studies have shown that autistic adults have attenuated increases during adolescence followed by declines during adulthood in adaptive skills compared to linear increases in adults with non-spectrum developmental disabilities (Smith et al., 2012), have more difficulties getting and maintaining employment (Wei et al., 2017), and may be more liable for social difficulties (Anderson, Maye, & Lord, 2011; Shattuck, Orsmond, Wagner, & Cooper, 2011). Many of these comparisons across ASD and other disorders are complicated by variation in ability level and diagnostic accuracy across populations, as well as questions about how autism was ruled out in non-autism populations recruited from records in some studies.
Related to this issue is that cognitive ability and autism severity are often only assessed at a single point in time and assumed to be stable within individuals, which is contrary to findings on trajectories showing changes in a substantial minority (e.g., Simonoff et al., 2019; Gotham, Pickles, & Lord, 2012). These factors have not been the focus of much of the most recent research because direct assessments were seldom available across time. In addition, many studies have separated subgroups based on gross distinctions in IQ, such as intellectual disability and no intellectual disability. Previous studies (e.g., Taylor & Mailick, 2014) have typically not addressed differences that exist within the two large ranges (e.g., typically more than 50 IQ points in each group) of affected outcomes. While it is important to examine differences between groups of people who were very likely to have different objective outcomes (e.g., those with IQs below 70 and those above), it is also important to examine the role of cognitive ability within these groups. There is no reason to think that differences in cognitive function, for example, between someone with an IQ of 68 and someone with an IQ of 20, or someone with an IQ of 75 and someone with an IQ of 120, would be any less for autistic people than anyone else.
Lastly, there is has been a lack of clarity about which outcomes are considered and how outcomes are labeled for autistic adults. Characterizing outcomes by general statements, such as good, poor, fair (Howlin, Goode, Hutton, & Rutter, 2004) or as optimal and non-optimal outcomes (Fein et al., 2013; Anderson, Liang, & Lord, 2014) is a useful start, but does not provide information about individual components of adult life, which have more direct relevance to future planning. There are many possible ways to describe “outcome” in adult functioning. The World Health Organization’s (WHO) International Classification of Functioning, Disability and Health defines outcome as the goal of living a full and decent life with dignity, self-reliance and active participation in the community (WHO, 2001). Autistica, a UK-based charity that focuses on autism awareness, defines their purpose as promoting long, happy, healthy lives for autistic people (see Autistica, 2016). Two other approaches include less formal reports of self-advocates about their desires (K. A. Anderson, Sosnowy, Kuo, & Shattuck, 2018) and the recommendations of the MeASURe group (McConachie et al., 2015) in which parents identified long-term goals for their young children, with happiness and freedom from distress identified as the two highest rated goals.
Per these considerations from researchers and stakeholders, outcome was operationalized in the current paper in terms of three general domains: 1) independence and self-reliance, 2) happiness and freedom from distress and 3) autistic features. Independence was operationalized as employment and daily activities including education; type of residence and daily living adaptive scores; and happiness as self and caregiver reports of positive and negative emotions, well-being, the presence of friendship and the lack of co-occurring psychiatric disorders including depressive symptoms, anxiety, irritability and hyperactivity. Outcome measures during young adulthood are reported as they relate to current demographics, cognitive and diagnostic features.
Current Study
The goal of the present paper is to describe, for a population whom we have followed throughout childhood, how and what these young adults are doing now. Although initial recruitment was through clinic referrals, by now the participants are more like a community sample (though not a “population sample,” as seen in the SNAP cohort; Simonoff et al., 2019) because most of the families have not sought out nor received specialized autism or mental health services since their children were young. In particular, our focus here is on questions of how specific to ASD, as compared to adults with similar but different neurodevelopmental disorders, are objective outcomes in young adulthood, as well as how specific to autism are the general demographic and internal current factors that predict objective outcomes.
We first examine how participants who received an ASD diagnosis at least once through our study, distinguished by higher or lower IQ, compared to those who never had an ASD diagnosis despite numerous blinded assessments from our team. We then examine how some of these outcomes may relate to autistic features and IQ within these groups.
A strength that we capitalize on is the consideration that all individuals within our study were first assessed for autism at an early age and received repeated diagnostic assessments through adulthood—allowing us to be confident in describing the contribution autism diagnosis as well as a continuous measure of autism features (e.g., ADOS CSS scores and to some extent current ADI scores) has on development. Cognitive abilities have also been directly assessed starting in early childhood and into adulthood, recognizing the limitations of giving standardized developmental tests or IQ tests to autistic adults with severe delays (Bishop, Cuthrie, Coffing, & Lord, 2011; Bishop, Farmer, & Thurm, 2015). Because of these repeated assessments, we are able to identify similarities and differences between these groups of adults (those who had received and those who had never received a formal autism diagnosis), while also accounting for the heterogeneity that exists within the ASD and non-ASD groups, particularly in IQ and autism features. A further strength is that, for participants who can speak for themselves, we have both parent-report and self-report data about subjective aspects of adult outcome.
Methods
Participants
As shown in Figure 1S, participants were drawn from three sources. 192 children under age 3 years who were referred for possible autism to two tertiary autism programs (North Carolina and Chicago) and 21 children of the same age with non-ASD developmental delays from the same sources who made the referrals to the autism programs constituted the initial two cohorts. Three quarters of the original participants received ASD diagnoses at age 2 (Lord et al., 2006). All children and parents who could be reached were seen for face-to-face assessments at age 2, 3 (ASD referrals only), 5 (North Carolina only), 9, 19, and 26 with some young adults who had been missed at 19 or who sought out an additional evaluation seen for an assessment at 21 (See also Pickles, McCauley, Pepa, Huerta, & Lord, 2020). Beyond the face to face assessments, families and participants have been sent questionnaire packets approximately every 4 – 6 months from the time they were 11 years old until now; questionnaire data was used from the closest date to the most recent face to face assessment. In addition, this paper includes a sample of new recruits, which consists of 31 children from Michigan referred for possible autism at early ages who joined the study at approximately age 9 and were then followed concurrently with the same frequency as the rest of the sample – i.e., for an average of 16 years.
The analyses here include 123 young adults who participated in at least two face to face assessments, at least one of which was after age 18. For the current participants recruited from North Carolina and Chicago, 75% were diagnosed as having ASD (Autism and PDD-NOS) at the age 2 assessment, with a further 3% at age 5 and a further 7% receiving the ASD diagnosis at age 9 and another 6% received a new ASD diagnosis at later ages. All objective and subjective outcome data are from participants in young adulthood who had left secondary school; however, IQ tests were administered at the 19 or 21 year-old assessments when some were still in high school. Ethnic and racial minorities, almost all of whom were African American, accounted for 16% of the mostly male (85%) sample, with a mix of families from rural, suburban and urban backgrounds (61 from North Carolina, 45 from Chicago, and 17 from Michigan). Another report describing this sample (Pickles et al., 2020) empirically derives adult outcome classes and tests our ability to predict them from early childhood data.
Procedures
A battery of diagnostic and psychometric instruments was administered during visits arranged with the families and the participants at their convenience at home, school, local clinics or work places. Clinicians administering the test batteries were blind to results from previous assessments, including diagnosis, as much as possible. The research was approved by the Institutional Review Board.
At each assessment, with the exception of age 3, an overall current best estimate consensus diagnosis of 1) ASD, 2) other nonspectrum disability or psychiatric disorder (which could occur in addition to ASD) or 3) typical development was made by the team based on all available information, with diagnoses reviewed by the full research team after each visit.
Measures
Diagnostic instruments. The ADOS was administered at the most recent face to face assessment (M age 25.99, range 21.54 – 28.5). The Autism Diagnostic Interview–Revised (ADI-R; Lord, Rutter, & Le Couteur, 1994), a standard investigator-based interview, was administered to caregivers at ages 21 or 26. Using CPEA criteria, diagnostic cut-offs intended for “ever” and “most abnormal” codes were applied to ADI-R current totals from caregivers’ accounts (Lainhart et al., 2006; Risi et al., 2006).
At the same evaluation, verbally fluent adults and caregivers of all participants were also given the Social Emotional Functioning Interview (SEF-S & SEF-I; Rutter et al., 1988), a series of open-ended questions about social preferences, concerns and interests used in several previous studies (Howlin, Mawhood, & Rutter, 2000; Mawhood, Howlin, & Rutter, 2000). Questions about “work,” place of living, relationships and education were summarized using information from caregivers, and whenever possible, from the participants themselves. Medication names and dosage were reported on the Aberrant Behavior Checklist (ABC; Aman, Singh, Stewart, & Field, 1985) and confirmed in person.
Cognitive skills were assessed during the first adult visit (age 19 or older) using a hierarchy of tests described in Anderson et al. (2014). The sample was divided into three groups: more cognitively able Ever ASD-Higher IQ (VIQ≥70), less cognitively able Ever ASD-Lower IQ (VIQ<70), and never ASD, including individuals with a range of IQs – see Table 1.
Table 1.
Participant Characteristics: Simple sample means and standard deviations (), and means for sample weighted to whole recruited cohort [].
| ASD-Higher IQ | ASD-Lower IQ | Never ASD | |
|---|---|---|---|
| (n=43)a,d | (n=63)b | (n=17)c,e | |
| Gender (Females) | 2% [4%] | 22% [26%] | 35% [34%] |
| Race (Non-Caucasian) | 16% [29%] | 21% [32%] | 6% [7%] |
| Maternal Education | 2.3 (1.1) [2.5] | 2.2 (1.0) [2.4] | 2.35 (1.4) [2.59] |
| 1st VIQ | 58.1 (29.4) [53.8] | 29.2 (14.5) [29.0] | 76.8 (28.1) [27.1] |
| 1st NVIQ | 86.0 (18.2) [84.5] | 57.2 (18.9) [55.2] | 87.3 (23.6)[85.9] |
| Last VIQ | 108.5 (16.7) [104.9] | 24.0 (17.6) [23.4] | 75.7 (32.3) [74.3] |
| Last NVIQ | 102.7 (16.8) [100.4] | 33.2 (24.7) [32.2] | 80.6 (36.7) [79.7] |
| 1st ADOS CSS | 6.55 (2.56) | 7.81 (2.60) | 2.80 (1.93) |
| Last ADOS CSS | 4.78 (2.4) [5.1] | 6.6 (2.6) [6.7] | 3.75 (2.4) [3.7] |
| ADOS CSS-SA | 4.9 (2.3) [5.2] | 7.7 (1.5) [7.8] | 4.3 (2.5) [4.2] |
| ADOS CSS-RRB | 6.1 (2.5) [6.3] | 6.2 (2.4) [6.2] | 3.7 (2.2) [3.6] |
| BDI-II (I) | 6.9 (6.5) [7.8] | 3.6 (4.7) [3.5] | 5.0 (8.5) [4.9] |
| BDI-II (S) | 11.9 (9.9) [12.2] | - | 9.3 (10.5) [8.8] |
| AMAS-Anxiety (I) | 14.0 (7.7) [14.0] | 8.0 (5.9) [7.6] | 16.0 (8.8) [16.8] |
| AMAS-Anxiety (S) | 14.1 (8.7) [14.3] | - | 13.5 (5.9) [13.4] |
| PANAS-Negative (I) | 18.2 (6.7) [18.1] | 17.1 (5.9) [16.9] | 15.25 (6.5) [15.0] |
| PANAS-Negative (S) | 22.7 (8.4) [22.0] | - | 20.1 (6.6) [19.4] |
| PANAS-Positive (I) | 29.8 (6.9) [29.1] | 26.3 (8.0) [26.3] | 34.2 (8.7) [34.6] |
| PANAS-Positive (S) | 30.2 (8.6) [29.6] | - | 29.9 (8.7) [29.5] |
| WBQ (I) | 191.3 (22.1) [189.4] | 183.4 (25.8) [184.6] | 210.1 (25.8) [211.2] |
| WBQ (S) | 191.7 (25.9) [189.5] | - | 196.7 (23.3) [197.6] |
| QoL (I) | 91.7 (12.4) [89.8] | 91.4 (15.2) [92.2] | 98.1 (13.1) [97.9] |
| QoL (S) | 94.2 (11.7) [94.1] | - | 86.2 (28.3) [84.2] |
| ABC – Irritability | 4.2 (6.3) [5.5] | 8.3 (8.0) [8.4] | 4.7 (4.0) [4.7] |
| ABC – Hyperactivity | 5.0 (7.0) [6.3] | 10.0 (8.2) [10.2] | 4.0 (3.6) [4.2] |
| CBCL – Total Problems | 53.6 (8.3) [54.3] | 51.2 (8.5) [51.0] | 52.4 (11) [53.5] |
| Number of medications | 0.9 (1.2) [0.9] | 1.8 (1.3) [1.7] | 0.9 (1.6) [0.9] |
| Any Non-ASD Diagnosis | 65.8% (0.5) [69.6] | 81.6% (0.4) [82.8] | 81.2% (0.4) [81.2] |
| Work | 2.4 (1.9) [2.6] | 5.6 (1.3) [5.7] | 2.9 (2.0) [2.9] |
| Living | 1.6 (0.5) [1.6] | 2.3 (0.5) [2.3] | 1.8 (0.4) [1.8] |
| Friend | 0.8 (.95) [0.9] | 2.5 (0.75) [2.5] | 1.07 (1.2) [1.2] |
| Vineland Daily Living | 79.1 (14.2) [78.8] | 41.5 (17.1) [40.4] | 75.5 (24.1) [24.6] |
| CPEA DX from ADI-R-curr | 60% [57%] Autism | 70% [68%] Autism | 6% [5%] Autism |
| 12% [16%] ASD | 5% [6%] ASD | 12% [12%] ASD |
Notes ( ) = standard deviation, [ ] = weighted estimate of mean or proportion. (I) = informant based and (S) self-report measure. Sample size varies from measure to measure ranging between (a) 29-43, (b) 51-63 and (c) 11-17 for researcher assessed and informant based measures and (d) 26-29 and (e) 7 for self-report measures. Maternal education was coded as 1 = Graduate education, 2 = Completed college, 3 = Some college, 4= Completed high school, 5 = Did not complete high school; Work was coded as 1 = Paid full time, 2 = Paid part time, 3 = In education, 4 = Paid supervised position, 5 = Unpaid work, 6 = Day center, 7 = No work activities outside the home; Living was coded as 1 = Independent, 2 = Home with parents, 3 = Group home/Supported living; Friend was coded as 0 = One or more true friends, 1 = Peers, but limited interactions, 2 = Acquaintances only, 3 = No friends.
Other questionnaires were selected from larger batteries administered online or via paper at regular intervals in adulthood (range 19.33 to 28.42 years) providing information for our three-dimension model of outcome. Analyses included data from adulthood via parent report on the irritability and hyperactivity scales of the ABC (Aman et al., 1985; α = 0.90), and the general behavior problem totals for the Adult Behavior Checklist (CBCL; Achenbach & Rescorla, 2003 α = 0.97). Adaptive skills were assessed from parent report using the Vineland Adaptive Behavior Scales—Survey edition at the most recent face to face visit (Vineland-II; Sparrow, Cicchetti, & Balia, 2005; α = 0.94). Analyses also included data from the parent report (n=92) versions, and for participants with higher IQ the self-report versions (n = 36), of the Beck Depression Inventory-Second Edition (BDI-II; Beck, Steer, & Brown, 1996; α = 0.91), the Adult Manifest Anxiety Scale (AMAS; Reynolds, Richmond, & Lowe, 2003; α = 0.90), negative and positive attributions on the Positive and Negative Affect Schedule (PANAS; Watson, Clark, & Tellegen, 1988; α = 0.84 – 0.86), the Quality of Life Questionnaire (QLQ; Schalock, & Keith, 1993; α =0.92) and the Psychological Well Being Questionnaire (WBQ; Ryff, 1989; α = 0.71). In the case of the BDI, the PANAS and WBQ, higher numbers indicate endorsement of more items describing depressive symptoms, negative emotions (PANAS-N), pleasant emotions (PANAS-P) or positive statements about well-being. It is important to note that, in the past, the PANAS and the WBQ have typically been used only as self-report, although studies using informant report exist in other populations (e.g., Cruice, Worrall, Hickson, Murison 2005; Eadeh et al, 2019)
Work and residential living status were assessed with direct questions to participants and caregivers on the SEF-S and SEF-I about whether the adult lived with parents/caregivers at home, in group homes, other supervised living situations, or had ever lived independently and how they spent the day (e.g., employment). Ordinal variables were created to describe this information.
Statistical Analysis
Analyses were undertaken in Stata 15. Of the 244 participants defined as eligible for adult follow-up, outcome data is presented for 123 (50.4%). Attrition was associated with having lower parent education (p<.002) and being non-white (p<.001), but not associated with the participant’s gender, IQ, or ASD diagnosis when first recruited. To account for selective attrition, inverse probability weights from a logistic model with parental education (5-point scale) and race were constructed. Other than the simple unweighted descriptive statistics of Table 1, means, proportions and diagrammatic displays are based on weighted data and thus estimate the means and proportions had we been able to follow-up all 244 participants. Test statistics of group differences are based on the robust sandwich estimator of the parameter covariance matrix (Binder, 1985) and thus account for this weighting. Other than allowing for group differences in completion rates, the missingness of measures within the 123 has been assumed to be missing completely at random (MCAR). Continuous, categorical and count outcomes were analyzed using linear regression, ordinal logistic or over-dispersed Poisson regression, as appropriate. In view of the small number of participants with self-report outcomes, these measures were left unweighted with more limited analyses. Unweighted matched pairs t-tests were used to compare informant- and self-rated scales.
Results
Participants who had adult outcome data were placed into three groups: (1) 43 (35%) participants who at some point in the study received a best estimate diagnosis of Autism Spectrum Disorder (ASD) and who had a verbal IQ≥70 in adulthood. (2) 63 (52%) participants who at some point in the study received a best estimate diagnosis of ASD and who had a verbal IQ<70 during adulthood. (3) 17 (13%) participants who had never received a diagnosis of ASD despite repeated assessments. These groupings were selected in order to capture the variation of participants who moved in or out of formal ASD diagnoses from 2 to 26. In addition, all analyses were run controlling verbal IQ and nonverbal IQ (separately) with nearly identical results, consequently only those for verbal IQs are reported. As stated earlier, although IQ was used to create the two ASD groups based on intellectual disability because of differences in measures (i.e., self-report data available for the Ever ASD – Higher IQ; different ADOS modules) and in ranges of outcomes, given the variation in IQs in each of the groupings (i.e., Ever ASD – Higher IQ: 72 - 139 ; Ever IQ – Lower IQ: 2 – 69), we felt that we needed to further account for this heterogeneity within the three groupings. In general, when verbal IQ was controlled, unless otherwise indicated, differences between Lower IQ Ever ASD and Higher IQ Ever ASD were non-significant (which would be expected, and therefore are not discussed), whereas differences between Higher IQ Ever ASD and Never ASD, which are discussed, were often accentuated because of the higher IQs of the Ever ASD.
Table 1 shows the unweighted summary statistics for each measure, with weighted means presented in brackets. Multivariate logistic regression showed the Higher-IQ Ever ASD group to be less likely to be female compared to Lower IQ- Ever ASD (p=.026) and Never ASD (p=.008). There were no significant differences by race, maternal education, gender nor site, but all tests of group differences that follow adjust for these demographic differences.
Table 2 gives coefficients for contrast of the reference group (Higher-IQ-ASD) with each of the other groups for each adult outcome, adjusted for attrition and demographic characteristics at recruitment. Immediately evident is how the Higher-IQ ASD group shows larger differences that are more significant (the latter only partly due to sample size) from the Lower-IQ ASD group than from the Never ASD group.
Table 2.
Test Statistics for Outcome Contrasts with H-ASD group (reference category)
| Outcome | n | Range/Code | Coefficient Type | H-ASD v. L-ASD Comparison Coefficient (SE) | H-ASD v L-ASD Comparison p-value | H-ASD v Never ASD Comparison Coefficient (SE) | H-ASD v. Never ASD p-value |
|---|---|---|---|---|---|---|---|
| ADOS-CSS | 118 | 1 to 10 | Ordinal Regression pOR | 3.8 (0.2) | 0.012 | 0.61 (0.39) | 0.442 |
| ADI-R | 123 | 1= Aut; 2=ASD; 3=No ASD | Ordinal Regression pOR | 0.57 (0.26) | 0.218 | 11.55 (10.11) | 0.004 |
| Work | 113 | 1=Paid FT; 7=None | Ordinal Regression pOR | 27.15 (19.85) | <.001 | 1.96 (1.41) | 0.351 |
| Living | 123 | 1=Indep; 3=Grp Home | Ordinal Regression pOR | 56.02 (52.33) | <.001 | 2.72 (1.78) | 0.128 |
| Friend | 106 | 0=True Friend; 3=None | Ordinal Regression pOR | 17.23 (8.86) | <.001 | 2.08 (1.56) | 0.326 |
| Daily Living Skills | 123 | 17-112 | Regression | −39.2 (3.1) | <.001 | −7.5 (6.48) | 0.25 |
| ABC Irrit | 104 | 0-45 | Regression | 3.83 (1.69) | 0.026 | −0.43 (1.85) | 0.819 |
| ABC Hyp | 104 | 0-48 | Regression | 5.28 (1.98) | 0.009 | −1.92 (2.17) | 0.378 |
| CBCL Total | 94 | 25-77 | Regression | −2.55 (2.02) | 0.211 | −2.22 (3.74) | 0.555 |
| Num Meds | 99 | 0-7 | Poisson Regression IRR | 2.03 (0.57) | 0.012 | 1.01 (0.55) | 0.985 |
| BDI | 92 | 0-30 | Regression | −0.67 (0.27) | 0.013 | −0.49 (0.4) | 0.229 |
| AMAS | 91 | 0-28 | Regression | −0.73 (0.21) | 0.001 | 0.22 (0.37) | 0.556 |
| PANAS Pos | 92 | 0-40 | Regression | −3.85 (1.97) | 0.054 | 4.39 (3.04) | 0.152 |
| PANAS Neg | 93 | 0-40 | Regression | −0.17 (0.25) | 0.488 | −0.57 (0.35) | 0.109 |
| WBQ | 91 | 37-124 | Regression | −0.29 (0.25) | 0.238 | 0.75 (0.42) | 0.077 |
| QLQ | 102 | 46-119 | Regression | 0.03 (0.23) | 0.897 | 0.6 (0.34) | 0.08 |
Note: Test statistics aadjusted for attrition and demographic characteristics at recruitment.
pOR=Proportional Odds Ratio; IRR = Incidence Rate Ratio
As shown in Figure 1, on the ADOS-CSS, the Lower-IQ Ever ASD group scored higher than the Higher-IQ Ever ASD group which was not significantly different from the Never-ASD group (p=.203), many of whom displayed a range of autism-related behaviors as adults despite clinicians repeatedly deciding they did not meet ASD diagnostic criteria. In contrast, parent reports on the ADI-R were strikingly different, with the never ASD group having few reported symptoms (pOR=.09, p=.004 compared to HIQ-Ever) and the two ASD groups being similar (pOR=0.57, p=218).
Figure 1:
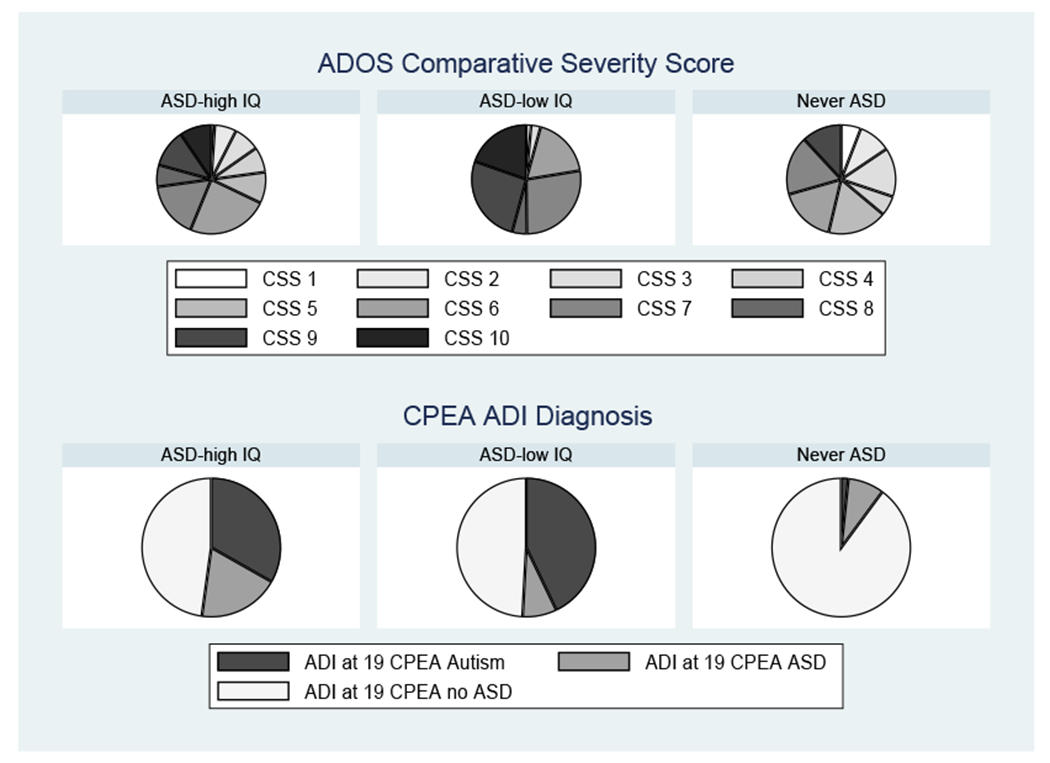
The ADOS CSS and CPEA ADI-R ASD diagnosis in adulthood.
Figure 2 displays the functioning of the three groups with respect to work situation (employment/education), domestic arrangements (place of residence) and friendships, together with standard scores on the Daily Living Skills domain of the Vineland-II. All 4 panels show marked differences between the Ever ASD-lower IQ group compared to the other groups, significant in all cases (2df p-values all <.001). In each case, the higher-IQ ASD group was not significantly different from the Never-ASD group. However, each outcome was also strongly associated with current autism features as measured with the roughly contemporaneous ADOS CSS (all p<=.007 across groups).
Figure 2:
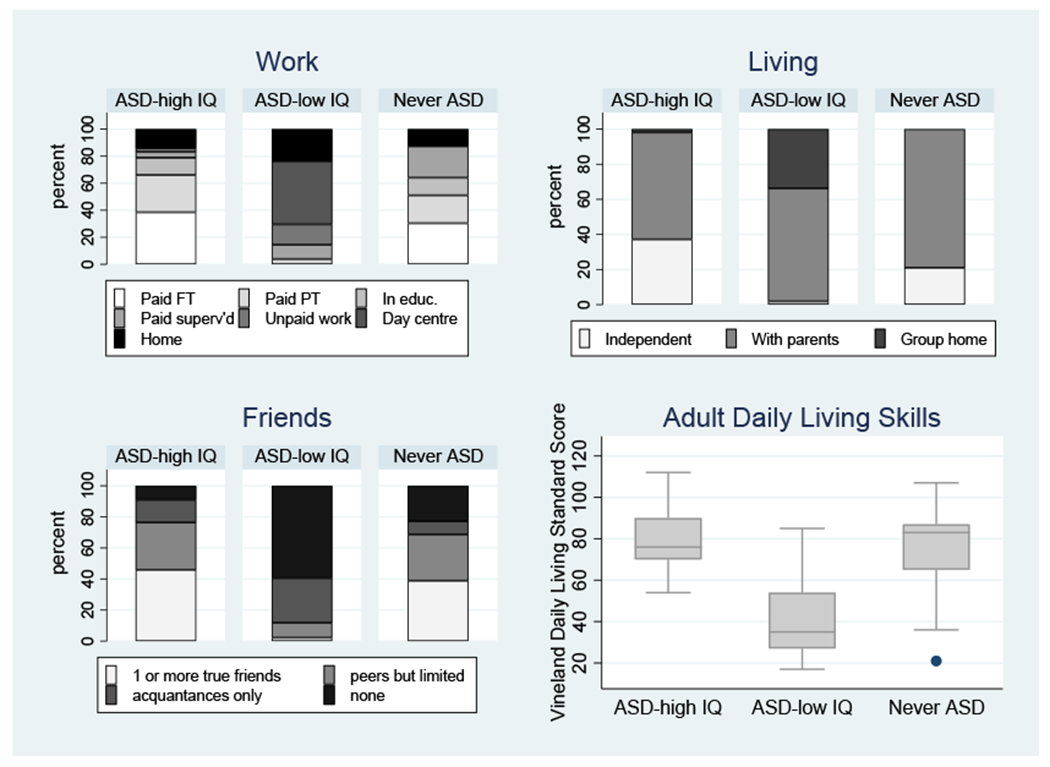
Measures of Functioning in Adult Life.
For the ABC parent report of irritability and hyperactivity scores, the principal pairwise difference among the groups was the higher scores of the Lower-IQ ASD than the Never-ASD group, with the Higher-IQ group nonsignificantly in the middle.
Shown in Table 1, there were no significant group (ASD and Never ASD) differences in the proportion of participants receiving any co-occurring mental health diagnosis in adulthood (2df p=.464) nor in the CBCL scores shown in Figure 3 (2df p=.455). However, differences in medication rates showed the Lower-IQ ASD group receiving more than twice the number of medications than the Higher-IQ ASD group (p=.012)
Figure 3:
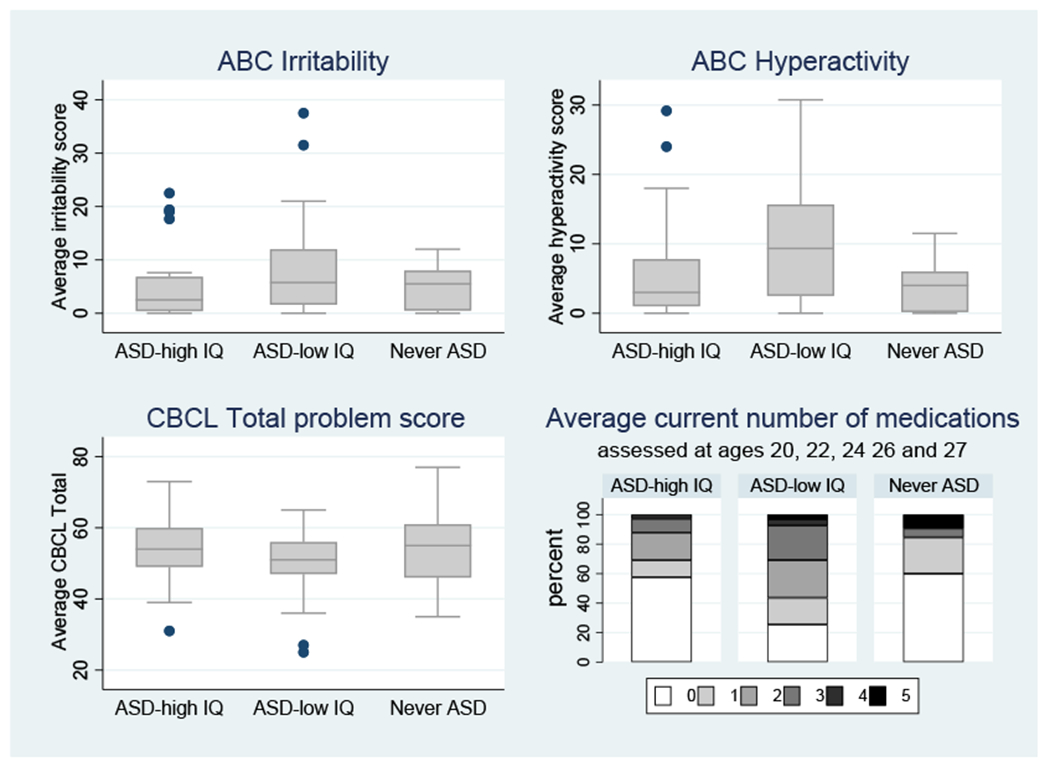
Adult Mental Health Outcomes (Irritability, Hyperactivity, Adult CBCL overall T scores).
Figure 4 compares the groups on a range of well-being related rating scales. The informant-reported attrition weighted means and 95% CI for each of the three groups are shown on the left of each panel. Groups were significantly different (overall 2df F tests p<.05) for all scales except PANAS-N and QLQ, with differences largely determined by the low ratings for the Lower-IQ ASD group compared to the Never ASD group for PANAS-P (p<.004) and WBQ (p=.010) or the Lower-IQ ASD group compared to both the Higher-IQ ASD and Never ASD for AMAS (p=.001 and p=.008) and BDI (p=0.13, p=.046). Additionally, the Higher-IQ ASD group was significantly lower than the Never ASD group on PANAS-P and WBQ.
Figure 4: Adult well-being profiles.
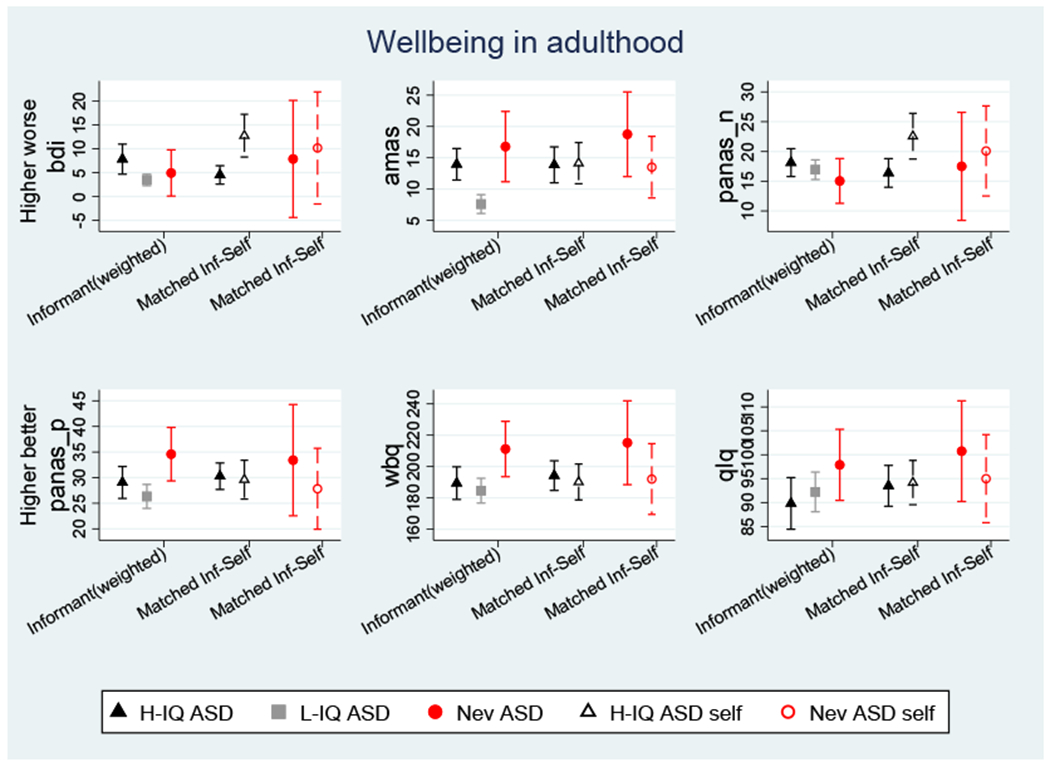
BDI = Beck Depression Inventory II, AMAS = Adult Manifest Anxiety Scale, PANAS = Positive and Negative Affect Scales, WBQ = Wellbeing Questionnaire, QLQ = Quality of life questionnaire.
Self-Report Measures
The middle of each panel compares parent- and self-report for the Higher-IQ ASD group. Simple matched t-tests indicated significantly worse self-ratings for the BDI (p=.001) and PANAS-N (p=.004). The matched ratings in the Never-ASD group, shown for completeness on the right-hand side of each panel, were considered too small (n=6 - 8) to analyze.
Controlling for IQ
While the pattern of group differences gives some indication of the substantial impact of IQ relative to ASD, additional adjustment for IQ as a covariate offers insight into the specific contribution of ASD. The similarity of the Higher and Lower-IQ ASD groups on the ADI-R current classification, became a significant difference after adjustment for verbal IQ (p=.045), with the Lower-IQ ASD group scoring an adjusted 2.25 higher. Adjustment for adult verbal IQ made the group differences between all groups non-significant for Work, Living, and Friends (2df p=.202, p=.425, p=.639 respectively) but left the Higher IQ Ever ASD group scoring relatively worse than either of the other groups for Daily Living Skills (2df p=.018, 1df versus Never ASD p=.007). The association of each outcome with ADOS CSS (all p<=.007) became non-significant after adjustment for adult verbal IQ for Work (p=.225) but not Place of Living (p=.013), Friends (p=.041) nor Daily Living Skills (p=.011). The difference in medication rates with the Lower-IQ ASD group receiving more medications than the Higher-IQ ASD group was made entirely non-significant (p=.426) by IQ adjustment, as were overall tests for BDI (p=.196), AMAS (p=.391), PANAS-P (p=.060) and WBQ (p=.056). However, with QLQ, the Higher-IQ ASD group now scored significantly lower in quality of life than both Lower-IQ ASD and Never ASD groups (p=.013 and p=.005).
Discussion
These findings just begin to scratch the surface of understanding the entry into adulthood of the 123 young people with autism and related disorders whom we have followed since childhood. Particularly striking, across all groups with ever and never ASD, is the continued major role of families in the support of daily needs of the young adults. Sixty percent of the young adults with ever diagnoses of ASD, regardless of IQ, have always lived with their parents; 80 percent of the “never ASD” group did so as well. Twenty percent of the “ever ASD lower IQ” group and 10-15% of the other two groups were home during the day with no planned activities or work. Only 30 – 40 percent of the ever ASD higher IQ and never ASD groups and none of the lower IQ ASD individuals have a friend, consistent with findings on social connectedness from the Drexel National Indicators Report (Roux, Shattuck, Rast, Rava, & Anderson, 2015) and a more recent paper on friendships (Sosnowy et al, 2019). Most of the young adults, regardless of group, relied on their families as sources of shelter, activities and relationships.
Though the sample is relatively small, it is important to note that these difficulties were also apparent for the young adults with histories of early neurodevelopmental disorders who had never had a diagnosis of ASD. Mawhood et al. (2000) reported similar findings in a follow-up study of adults with a history of early receptive language delays. Thus, gaps in adult supports and services are not unique to ASD. This is seldom discussed in the ASD literature, where comparisons are often to typically developing controls. More work with families, as well as with the young adults themselves, is needed to develop realistic ways of increasing independence and social contacts for autistic persons but also with those with other neurodevelopmental disorders.
Not surprising were differences in work outcomes between the lower and higher IQ ASD groups, the latter of which was similar to the Never ASD group, with findings consistently accounted for by IQ. In part, the strong effect of IQ on work is even more apparent because of our further analyses, going beyond general distinctions between intellectual disability and no intellectual disability. For both autistic adults and those with other neurodevelopmental disorders, people with higher verbal IQs were more likely to be employed. Although many studies have found that overall IQ predicts general measures of outcome in ASD, the finding that people with lower IQs within the average range are less likely to be employed than those with higher IQs is not surprising but an important reminder that cognitive skills, even more than autism symptoms, make a difference in options for employment.
Generally, IQ did not predict well-being nor friendship. However, measures of emotions and behaviors were related to IQ in varied directions-- higher IQs and ASD were associated with greater levels of depressive symptoms and negative emotions and lower levels of positive emotions and well-being, as well as relatively worse adaptive skills across the groups. Lower IQ was associated with higher levels of hyperactivity and irritability, particularly relevant to the Ever ASD Lower IQ group. It is important to note that the effects of IQ in these various cases may be quite different; higher intellectual ability may offer greater ability to self-regulate frustration, more opportunities to be frustrated by new challenges, or afford an individual the ability to report negative feelings.
In addition, findings replicated a recent paper reporting greater IQ-adaptive discrepancies in autistic adults with higher IQs (Alvares et al., 2019). While the frequency of depressive symptoms was more associated with IQ than CSS scores, it is still of concern that many young adults in the Ever ASD higher IQ group reported such difficulties.
Despite the limitations in independence and happiness in the three groups, some young adults previously diagnosed with autism are employed full-time, live on their own and report high well-being and positive emotions (see earlier reports in Anderson et al, 2014). There are young adults with Ever ASD and lower IQs whose parents report a relatively high level of well-being, many positive attributes, few negative ones and little irritability and hyperactivity. Overall, while correlations between self and parent-reports within individuals were not high, patterns were largely similar except that self-reports of depressive symptoms were markedly higher than those from parents. These findings thus provide only partial evidence for the validity of parent-report measures for individuals who could not complete the forms for themselves and highlight the need to develop, refine and validate non-informant based tools that can elicit reliable, well-calibrated characterizations of affective states. In addition, some of the findings have implications for treatment goals: minimizing depressive and negative feelings while increasing positive attributions particularly for the more verbally fluent adults, more systematically addressing irritability and hyperactivity, particularly in autistic people with intellectual disabilities, and more directly planning for support in developing adaptive skills across IQ ranges.
Where ASD diagnosis or symptoms contributed specifically was in the relatively lower adaptive scores for the higher IQ group and the absence of friendship and less happiness measured through positive emotions and well-being for both ASD groups compared to the Never ASD group. However, as many authors have noted, the border between ASD, ever or current, and never ASD, for young adults who had early developmental delays or who were referred for ASD assessments as children but never received a diagnosis, is not clear. In this paper, it is beyond our scope to address diagnostic change, but it is apparent in Figure 2 and Table 1 that there is considerable overlap in the difficulties experienced by adults with histories of neurodevelopmental disorders but never diagnosed with ASD and those who received ASD diagnoses, especially in social communication symptoms between the Ever ASD Higher and Never ASD groups.
In a longitudinal study, there are always multiple limitations apparent with the benefit of hindsight. Attrition has reduced the proportion of less well educated and of African-American families in our sample; we have used sample weights to make adjustments, which we feel is important, given that there were demographic differences in attrition. The small number of females and Never ASD individuals also affects the confidence of our generalizations. We are also limited by our use of informant-report on some measures that were designed for self-report (e.g., WBQ, PANAS).
In addition, participants were primarily referred for diagnostic evaluations 24 years ago and do not represent referrals today. Few children received the amount of early intervention available to some families now. The Never ASD group is diagnostically heterogeneous including mild to moderate intellectual disability, ADHD, language disorder, and major depression. A limitation in using CSS scores is that the equivalence across Module 4 (Hus & Lord, 2014) and Adapted Modules 1 and 2 is not known (Bal et al., 2020).
One commonality across our study is the under-or lack of employment and meaningful, appropriate daily activities and the continued reliance on families for daily support, even in participants without intellectual disabilities (Dudley, Klinger, Meyer, Powell, & Klinger, 2019). Within the field of autism, we need to recognize more explicitly and probably earlier in development, the role of intellectual disability and severe language delays as well as the implications of milder cognitive delays that characterized the Never ASD group and some of the participants in the Higher-IQ ASD group. More thoughtful consideration of individual differences in cognitive abilities not just early in childhood but also young adults would allow families and schools to develop realistic goals that address the implications of variation in abilities and needs (Matson & Shoemaker, 2009) and to go on to consider potential strengths and interests (e.g., Cosden, Koegel, Koegel, Greenwell, & Klein, 2006).
Recently, renewed interest in treatments for irritability and self-regulation (Charlton, Smith, Mazefsky, & White, 2019; McDougle et al., 2005), as well as those for depressive feelings, reflects awareness of the importance of emotional development in ASD. Parent-initiated movements, such as the National Council on Severe Autism (“National Council on Severe Autism,” n.d.), call attention to the need for specialized opportunities to increase the independence and happiness for individuals with autism and moderate to severe intellectual disabilities. We also need to more explicitly address the similarities, as well as the differences, in the needs of people with ASD and those with similar neurodevelopmental disorders who have both higher and lower IQs.
Supplementary Material
Acknowledgements:
This study was funded by the National Institute of Child Health and Human Development R01 HD081199 (PI: CL), and National Institute of Mental Health R01MH081873 (PI: CL). AP is partially supported by NIHR NF-SI-0617-10120 and Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King’s College London. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health and Social Care. We would like to thank Gabrielle Gunin, Kyle Frost, and Allison Megale for their assistance in data collection. CL acknowledges the receipt of royalties from the sale of the Autism Diagnostic Observation Schedule (ADOS) and the Autism Diagnostic Interview-Revised (ADIR). Royalties generated from this study were donated to a not-for-profit agency, Have Dreams. JM, LP, MH, and AP have no potential conflicts to declare. The authors would like to extend their gratitude to the participants and families for the time they have given to this project.
References
- Achenbach T, & Rescorla L (2003). Manual for the ASEBA adult forms & profiles. Burlington: University of Vermont, Research Center for Children, Youth, & Families. [Google Scholar]
- Alvares GA, Bebbington K, Cleary D, Evans K, Glasson EJ, Maybery MT, Pillar S, Uljarevic M, Varcin K, Wray J, Whitehouse AJ.(2019) The misnomer of ‘high functioning autism’: Intelligence is an imprecise predictor of functional abilities at diagnosis. Autism. 2019 June 19:1362361319852831. doi: 10.1177/1362361319852831. [DOI] [PubMed] [Google Scholar]
- Aman MG, Singh NN, Stewart AW, & Field CJ (1985). The aberrant behavior checklist: a behavior rating scale for the assessment of treatment effects. American Journal of Mental Deficiency, 89(5), 485–491. [PubMed] [Google Scholar]
- Anderson DK, Liang JW, & Lord C (2014). Predicting young adult outcome among more and less cognitively able individuals with autism spectrum disorders. Journal of Child Psychology and Psychiatry, 55(5), 485–494. 10.1111/jcpp.12178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson DK, Lord C, Risi S, Dilavore PS, Shulman C, Thurm A, … Pickles A (2007). Patterns of Growth in Verbal Abilities Among Children With Autism Spectrum Disorder. 10.1037/0022-006X.75.4.594 [DOI] [PubMed] [Google Scholar]
- Anderson DK, Maye MP, & Lord C (2011). Changes in maladaptive behaviors from midchildhood to young adulthood in autism spectrum disorder. American Journal on Intellectual and Developmental Disabilities, 116(5), 381–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson KA, Sosnowy C, Kuo AA, & Shattuck PT (2018). Transition of Individuals With Autism to Adulthood: A Review of Qualitative Studies. Pediatrics, 141(Suppl 4), S318–S327. 10.1542/peds.2016-4300I [DOI] [PubMed] [Google Scholar]
- Autistica (2016). Your questions shaping future autism research. https://www.autistica.org.uk/downloads/files/Autism-Top-10-Your-Priorities-for-Autism-Research.pdf [Google Scholar]
- Ayres M, Parr JR, Rodgers J, Mason D, Avery L, & Flynn D (2018). A systematic review of quality of life of adults on the autism spectrum. Autism, 22(7), 774–783. [DOI] [PubMed] [Google Scholar]
- Bagatell N (2010). From Cure to Community: Transforming Notions of Autism. Ethos, 38(1), 33–55. 10.1111/j.1548-1352.2009.01080.x [DOI] [Google Scholar]
- Bal VH, Maye M, Salzman E, Huerta M, Pepa L, Risi S, & Lord C (2020). The Adapted ADOS: A New Module Set for the Assessment of Minimally Verbal Adolescents and Adults. Journal of Autism and Developmental Disorders, 50(3), 719–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Steer RA, & Brown GK (1996). Manual for the Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation. [Google Scholar]
- Billstedt E, Gillberg IC, & Gillberg C (2011). Aspects of quality of life in adults diagnosed with autism in childhood: A population-based study. Autism, 15(1), 7–20. [DOI] [PubMed] [Google Scholar]
- Binder JJ (1985). On the use of the multivariate regression model in event studies. Journal of Accounting Research, 23(1), 370–383. [Google Scholar]
- Bishop SL, Farmer C, & Thurm A (2015). Measurement of nonverbal IQ in autism spectrum disorder: scores in young adulthood compared to early childhood. Journal of Autism and Developmental Disorders, 45(4), 966–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamak B, & Bonniau B (2016). Trajectories, long-term outcomes and family experiences of 76 adults with autism spectrum disorder. Journal of autism and developmental disorders, 46(3), 1084–1095. [DOI] [PubMed] [Google Scholar]
- Chan W, Smith LE, Hong J, Greenberg JS, Lounds Taylor J, & Mailick MR (2018). Factors associated with sustained community employment among adults with autism and co-occurring intellectual disability. Autism, 22(7), 794–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlton AS, Smith IC, Mazefsky CA, & White SW (2019). The Role of Emotion Regulation on Co-occurring Psychopathology in Emerging Adults with ASD. Journal of Autism and Developmental Disorders, 1–8. 10.1007/s10803-019-03983-5 [DOI] [PubMed] [Google Scholar]
- Cosden M, Koegel LK, Koegel RL, Greenwell A, & Klein E (2006). Strength-based assessment for children with autism spectrum disorders. Research and Practice for Persons with Severe Disabilities, 31(2), 134–143. [Google Scholar]
- Cruice M, Worrall L, Hickson L, & Murison R (2005). Measuring quality of life: Comparing family members’ and friends’ ratings with those of their aphasic partners. Aphasiology, 19(2), 111–129. [Google Scholar]
- Dudley KM, Klinger MR, Meyer A, Powell P, & Klinger LG (2019). Understanding Service Usage and Needs for Adults with ASD: The Importance of Living Situation. Journal of Autism and Developmental Disorders, 49(2), 556–568. 10.1007/s10803-018-3729-0 [DOI] [PubMed] [Google Scholar]
- Eadeh HM, Breaux R, Langberg JM, Nikolas MA, & Becker SP (2019). Multigroup multilevel structure of the child and parent versions of the Positive and Negative Affect Schedule (PANAS) in adolescents with and without ADHD. Psychological Assessment. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fein D, Barton M, Eigsti I-M, Kelley E, Naigles L, Schultz RT, … Tyson K (2013). Optimal outcome in individuals with a history of autism. Journal of Child Psychology and Psychiatry, 54(2), 195–205. 10.1111/jcpp.12037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotham K, Pickles A, & Lord C (2012). Trajectories of autism severity in children using standardized ADOS scores. Pediatrics, 130(5), e1278–e1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howlin P, Goode S, Hutton J, & Rutter M (2004). Adult outcome for children with autism. Journal of Child Psychology and Psychiatry, 45(2), 212–229. 10.1111/j.1469-7610.2004.00215.x [DOI] [PubMed] [Google Scholar]
- Howlin P, Mawhood L, & Rutter M (2000). Autism and developmental receptive language disorder—A follow-up comparison in early adult life. II: Social, behavioural, and psychiatric outcomes. Journal of Child Psychology and Psychiatry, 41(05), 561–578. [DOI] [PubMed] [Google Scholar]
- Howlin P, Moss P, Savage S, & Rutter M (2013). Social outcomes in mid-to later adulthood among individuals diagnosed with autism and average nonverbal IQ as children. Journal of the American Academy of Child & Adolescent Psychiatry, 52(6), 572–581. [DOI] [PubMed] [Google Scholar]
- Hus V, & Lord C (2014). The Autism Diagnostic Observation Schedule, Module 4: Revised Algorithm and Standardized Severity Scores. Journal of Autism and Developmental Disorders, 44(8), 1996–2012. 10.1007/s10803-014-2080-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones CR, Simonoff E, Baird G, Pickles A, Marsden AJ, Tregay J, … & Charman T (2018). The association between theory of mind, executive function, and the symptoms of autism spectrum disorder. Autism Research, 11(1), 95–109. [DOI] [PubMed] [Google Scholar]
- Kapp SK, Gillespie-Lynch K, Sherman LE, & Hutman T (2013). Deficit, difference, or both? Autism and neurodiversity. Developmental Psychology, 49(1), 59–71. 10.1037/a0028353 [DOI] [PubMed] [Google Scholar]
- Lainhart JE, Bigler ED, Bocian M, Coon H, Dinh E, Dawson G, et al. (2006). Head circumference and height in autism: A study by the collaborative program of excellence in autism. American Journal of Medical Genetics Part A, 140A(21), 2257–2274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C, Risi S, DiLavore PS, Shulman C, Thurm A, & Pickles A (2006). Autism from 2 to 9 years of age. Archives of general psychiatry, 63(6), 694–701. [DOI] [PubMed] [Google Scholar]
- Lord C, Rutter M, & Le Couteur A (1994). Autism Diagnostic Interview-Revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders, 24(5), 659–685. 10.1007/BF02172145 [DOI] [PubMed] [Google Scholar]
- Matson JL, & Shoemaker M (2009). Intellectual disability and its relationship to autism spectrum disorders. Research in Developmental Disabilities, 50(6), 1107–1114. 10.1016/J.RIDD.2009.06.003 [DOI] [PubMed] [Google Scholar]
- Mawhood L, Howlin P, & Rutter M (2000). Autism and Developmental Receptive Language Disorder—a Comparative Follow-up in Early Adult Life. I: Cognitive and Language Outcomes. Journal of Child Psychology and Psychiatry, 47(5), 547–559. [DOI] [PubMed] [Google Scholar]
- McConachie H, Parr JR, Glod M, Hanratty J, Livingstone N, Oono IP, … & Garland D (2015). Systematic review of tools to measure outcomes for young children with autism spectrum disorder. Health Technology Assessment, 79(41). [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDougle CJ, Scahill L, Aman MG, McCracken JT, Tierney E, Davies M, … Vitiello B (2005). Risperidone for the Core Symptom Domains of Autism: Results From the Study by the Autism Network of the Research Units on Pediatric Psychopharmacology. American Journal of Psychiatry, 162(6), 1142–1148. 10.1176/appi.ajp.162.6.1142 [DOI] [PubMed] [Google Scholar]
- National Council on Severe Autism, (n.d.). Retrieved April 1, 2019, from https://www.ncsautism.org/
- Pickles A, McCauley JB, Pepa LA, Huerta M, & Lord C (2020). The adult outcome of children referred for autism: Typology and prediction from childhood. Journal of Child Psychology and Psychiatry, doi: 10.1111/jcpp.13180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds CR , Richmond BO , & Lowe PA (2003). The Adult Manifest Anxiety Scale-Adult Version (AMAS-A). Los Angeles: Western Psychological Services. [Google Scholar]
- Risi S, Lord C, Gotham K, Corsello C, Chrysler C, Szatmari P, et al. (2006). Combining information from multiple sources in the diagnosis of autism spectrum disorders. Journal of the American Academy of Child and Adolescent Psychiatry, 45(9), 1094–1103. [DOI] [PubMed] [Google Scholar]
- Roux AM, Shattuck PT, Rast JE, Rava JA, & Anderson KA (2015). National Autism Indicators Report: Transition into Young Adulthood. Philadelphia, PA. [Google Scholar]
- Rutter M, LeCouteur A, Lord C, Macdonald H, Rios P, & Folstein S (1988). Diagnosis and Subclassification of Autism. In Diagnosis and Assessment in Autism (pp. 239–259). 10.1007/978-1-4899-0792-9_16 [DOI] [Google Scholar]
- Ryff CD (1989). Happiness is everything, or is it? Explorations on the meaning of psychological well-being. Journal of Personality and Social Psychology, 57(6), 1069–1081. [Google Scholar]
- Schalock RL & Keith KD (1993) Quality of Life Questionnaire Manual. IDS Publishing Corporation, Worthington, OH. [Google Scholar]
- Scott M, Milbourn B, Falkmer M, Black M, Bolte S, Halladay A, … & Girdler S (2019). Factors impacting employment for people with autism spectrum disorder: A scoping review. Autism, 23(4), 869–901. [DOI] [PubMed] [Google Scholar]
- Shattuck PT, Orsmond GI, Wagner M, & Cooper BP (2011). Participation in social activities among adolescents with an autism spectrum disorder. PloS one, 6(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonoff E, Kent R, Stringer D, Lord C, Briskman J, Lukito S, … & Baird G (2019). Trajectories in Symptoms of Autism and Cognitive Ability in Autism From Childhood to Adult Life: Findings From a Longitudinal Epidemiological Cohort. Journal of the American Academy of Child & Adolescent Psychiatry. [DOI] [PubMed] [Google Scholar]
- Smith LE, Maenner MJ, & Seltzer MM (2012). Developmental trajectories in adolescents and adults with autism: The case of daily living skills. Journal of the American Academy of Child & Adolescent Psychiatry, 51(6), 622–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon M, Iosif A-M, Reinhardt VP, Libero LE, Nordahl CW, Ozonoff S, … Amaral DG (2018). What will my child’s future hold? phenotypes of intellectual development in 2–8-year-olds with autism spectrum disorder. Autism Research, 11(1), 121–132. 10.1002/aur.1884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sosnowy C, Silverman C, Shattuck P, & Garfield T (2019). Setbacks and successes: How young adults on the autism spectrum seek friendship. Autism in Adulthood, 1(1), 44–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparrow SS, Cicchetti DV, & Balla DA (2005). Vineland Adaptive Behavior Scales (2nd ed). Circle Pines, MN: American Guidance Service Inc. [Google Scholar]
- Taylor JL, & Mailick MR (2014). A longitudinal examination of 10-year change in vocational and educational activities for adults with autism spectrum disorders. Developmental psychology, 50(3), 699–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser JC, Rommelse NNJ, Lappenschaar M, Servatius-Oosterling IJ, Greven CU, & Buitelaar JK (2017). Variation in the early trajectories of autism symptoms is related to the development of language, cognition, and behavior problems. Journal of the American Academy of Child & Adolescent Psychiatry, 56(8), 659–668. 10.1016/JJAAC.2017.05.022 [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA, & Tellegen A (1988). Development and validation of brief measures of positive and negative affect: the PANAS scales. Journal of Personality and Social Psychology, 54(6), 1063–1070. [DOI] [PubMed] [Google Scholar]
- Wei X, Yu JW, Wagner M, Hudson L, Roux AM, Shattuck P, & Blackorby J (2018). Job searching, job duration, and job loss among young adults with autism spectrum disorder. Journal of Vocational Rehabilitation, 48(1), 1–10. [Google Scholar]
- WHO. (2001). International classification of functioning, disability, and health. Geneva: World Health Organization. [Google Scholar]
- Zimmerman D, Ownsworth T, O’Donovan A, Roberts J, & Gullo MJ (2018). High-functioning autism spectrum disorder in adulthood: A systematic review of factors related to psychosocial outcomes. Journal of Intellectual & Developmental Disability, 43(1), 2–19. [Google Scholar]
- Zwicker J, Zaresani A, & Emery JH (2017). Describing heterogeneity of unmet needs among adults with a developmental disability: An examination of the 2012 Canadian Survey on Disability. Research in Developmental Disabilities, 65, 1–11. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


