Summary
When two or more bacterial species inhabit a shared niche, often, they must compete for limited nutrients. Iron is an essential nutrient that is especially scarce in the marine environment. Bacteria can use the production, release, and re-uptake of siderophores, small molecule iron chelators, to scavenge iron. Siderophores provide fitness advantages to species that employ them by enhancing iron acquisition, and moreover, by denying iron to competitors incapable of using the siderophore-iron complex. Here, we show that cell-free culture fluids from the marine bacterium Vibrio fischeri ES114 prevent growth of other vibrio species. Mutagenesis reveals the aerobactin siderophore as the inhibitor. Our analysis reveals a gene, that we name aerE, encodes the aerobactin exporter, and LuxT is a transcriptional activator of aerobactin production. In co-culture, under iron-limiting conditions, aerobactin production allows V. fischeri ES114 to competitively exclude Vibrio harveyi, which does not possess aerobactin production and uptake genes. By contrast, V. fischeri ES114 mutants incapable of aerobactin production lose in competition with V. harveyi. Introduction of iutA, encoding the aerobactin receptor, together with fhuCDB, encoding the aerobactin importer are sufficient to convert V. harveyi into an “aerobactin cheater”.
Keywords: siderophore, Vibrio fischeri, aerobactin, iron, competition, genes, regulator
Introduction
Microbial competition is a driving force in ecosystems in which limited resources exist (reviewed by Hibbing et al., 2010; Foster and Bell, 2012; Stubbendieck and Straight, 2016; Ghoul and Mitri, 2016). Marine bacteria, in particular, must survive in seawater that is frequently depleted for essential nutrients (Bristow et al., 2017). Success in inhabiting heterogeneous and/or nutrient-poor marine environments often relies on exploitation of transient microscale nutrient “hot spots” that are associated with detritus, marine snow particles, and marine phytoplankton and zooplankton (reviewed by Stocker and Seymour, 2012). In such environments, key limiting nutritional resources specify the particular competitive interactions that take place. Competitive strategies can be indirect, for example, rapid capture of a limiting resource (Pfeiffer et al., 2001; Khare and Tavazoie, 2015), while direct competition strategies include the production of antibiotics or other toxic compounds (Abrudan et al., 2015), the formation of surface-associated biofilms that physically exclude competitors from nutritious territory (Nadell and Bassler, 2011), and the contact-dependent delivery of toxic effector molecules into competitor cells via type VI secretion (Logan et al., 2018).
In marine environments, vibrios are a ubiquitous and abundant bacterial genera (reviewed by Thompson et al., 2004). To explore mechanisms underlying interspecies marine bacterial competition, here, we developed a dual-species co-culture model containing two Vibrionaceae species. We focused on two well-studied vibrios, Vibrio harveyi and Vibrio fischeri, that are known to occur together in nature (Ramesh et al., 1989; Ruby and Lee, 1998). Each species can exist free-living in the ocean and associated with marine animals, including in the case of V. fischeri, as a symbiont (McFall-Ngai and Ruby, 1991; Alcaide, 2003). In this initial study, we restricted our analyses to their free-living lifestyles using shaking liquid cultures for co-incubations. Attractive features of the V. fischeri-V. harveyi co-culture model are that both species grow under identical laboratory conditions, and many tools exist for genetic modifications, allowing us to precisely manipulate each species and quantitatively measure the consequences. Moreover, a variety of endogenous and heterologous reporter genes are available for monitoring each strain in isolation and in combination.
Surprisingly, in our initial attempt to characterize the co-culture system, we found that V. fischeri produces and releases an inhibitor that prevents the growth of V. harveyi. We discovered that the inhibitor is the siderophore aerobactin. Siderophores are small molecule high-affinity iron chelators that many bacteria produce and secrete to scavenge iron (reviewed by Neilands, 1995). In this case, aerobactin produced by V. fischeri is released into the culture medium, it chelates the available iron, and as a consequence, V. harveyi cannot grow.
Iron is an essential nutrient for virtually all organisms and, germane to the current work, is required by bacteria as a cofactor for enzymes involved in DNA synthesis, electron transport, and the TCA cycle (Crosa et al., 2004). Iron acquisition presents a challenge to most bacteria because, despite its abundance, it has poor solubility in the presence of oxygen at physiological pH (Hem, 1972). More extreme is the situation in the marine environment: dissolved iron concentrations in the ocean are typically in pM to nM ranges, below the level required for growth of most bacteria (Rijkenberg et al., 2014). The remarkable finding that atmospherically-transported Saharan dust enables proliferation of vibrio bacteria by delivering dissolved iron to surface marine environments further demonstrates the exquisite scarcity of iron in the ocean (Westrich et al., 2016).
Vibrio species commonly produce one or more siderophores to acquire iron (Thode et al., 2018). V. harveyi encodes genes for the biosynthesis of two siderophores, anguibactin and amphi-enterobactin (Naka et al., 2013; Zane et al., 2014). V. fischeri siderophore production has been demonstrated (Graf and Ruby, 2000; Cordero et al., 2012), and indeed, our scanning of the V. fischeri genome sequence using AntiSMASH, a tool that identifies biosynthetic gene clusters (Medema et al., 2011), revealed biosynthetic genes for one hydroxamate siderophore, aerobactin. Aerobactin biosynthetic genes were first characterized in enteric species (de Lorenzo et al., 1986; de Lorenzo and Neilands, 1986; Bailey et al., 2018), and we use these earlier findings to underpin our analyses of the genes involved in V. fischeri siderophore production recognizing that the structure of V. fischeri aerobactin could differ from that of other species.
Iron exists in two states, the reduced ferrous (Fe2+) form and the oxidized ferric (Fe3+) form. While ferrous (Fe2+) iron is soluble, ferric (Fe3+) iron primarily exists in insoluble ferric hydroxides. Siderophores have high affinity for ferric (Fe3+) iron, the abundant environmental form of iron at neutral pH in the presence of oxygen (Crosa et al., 2004). In Gram-negative bacteria, once synthesized, siderophore export typically occurs via a membrane-spanning transporter protein (reviewed by Saha et al., 2013). Released siderophores bind to the otherwise unavailable ferric (Fe3+) iron. Subsequently, siderophore-iron (Fe3+) complexes are recognized by specific outer membrane (OM) receptors of the TonB-dependent receptor family, with TonB providing the energy for import across the OM. A periplasmic binding protein shuttles the siderophore-iron (Fe3+) complex to an inner-membrane ABC transporter that delivers the complex to the cytoplasm. Finally, iron must be released from the siderophore either by reducing iron from the ferric (Fe3+) form to the ferrous (Fe2+) form, promoting its release, or via enzymatic cleavage of the siderophore (reviewed by Payne et al., 2016).
In environments harboring multi-species bacterial communities, siderophores are known to play roles in competition for iron (Weaver and Kolter, 2004; Schiessl et al., 2017; Bruce et al., 2017). Siderophore production enables iron capture and use for the producer while simultaneously denying competitors access to an essential nutrient. When two or more species each produce siderophores, the species that produces the siderophore with the highest affinity for iron can enjoy a competitive advantage (Joshi et al., 2006). However, because siderophores are released from cells, they are considered public goods and are susceptible to exploitation by non-producing cells. Indeed, many bacterial species possess genes for siderophore binding and uptake, but they lack genes for siderophore production. Such siderophore-non-producing bacteria are considered “cheaters”, capable of using heterologous siderophores without expending the energy required for their synthesis (Traxler et al., 2012; Cordero et al., 2012; Leinweber et al., 2017; Butaitė et al., 2017).
Here, we identify the siderophore aerobactin as a small molecule inhibitor secreted by V. fischeri that prevents growth of V. harveyi. While V. harveyi cannot produce or import aerobactin, we identify other vibrio species that are natural aerobactin cheaters: they do not produce aerobactin, but they use it. We identify the V. fischeri genes required for production, release, and uptake of aerobactin, revealing AerE as a transporter that is used for aerobactin export. We pinpoint the minimal subset of genes required to convert V. harveyi into an aerobactin cheater. We use mutagenesis to identify regulators of aerobactin production, revealing LuxT as a transcriptional activator of V. fischeri siderophore biosynthesis genes. We investigate the mechanism underlying natural variation in siderophore production between two V. fischeri strains. Finally, we demonstrate that in dual-species co-culture, siderophore production allows V. fischeri to establish a niche and exclude growth of its competitor V. harveyi, conferring a fitness advantage to V. fischeri when grown under iron-limiting conditions.
Results
V. fischeri ES114 produces a siderophore that prevents growth of other vibrio species
Marine environments are estimated to have 104 to 106 prokaryotic cells per milliliter (Whitman et al., 1998), and metagenomic studies have revealed that there exists marked microbial phylogenetic diversity (Sogin et al., 2006; Sunagawa et al., 2015). Vibrios are a ubiquitous and abundant bacterial genera in the marine environment (reviewed by Thompson et al., 2004). How vibrios thrive in multi-species marine environments raises questions concerning cooperation and competition. To explore the requirements for dual-species growth and whether interspecies interactions play roles in promoting or preventing cohabitation, we focused on two well-studied vibrio species, V. harveyi and V. fischeri. We grew cultures of our wild-type (WT) V. harveyi strain, BB120, (called V. harveyi from here forward), V. fischeri ES114, and V. fischeri MJ11 alone and in the presence of 10% (v/v) cell-free culture fluids from the other strains/species. V. fischeri ES114 culture fluids prevented V. harveyi growth in minimal marine medium, whereas identical preparations from V. fischeri MJ11 did not (Fig. 1A). The inability to grow was not specific to V. harveyi: V. fischeri ES114 culture fluids also prevented growth of Photobacterium angustum S14 and Vibrio cholerae C6706, while no diminishment of growth yield of Vibrio parahaemolyticus BB22OP and Vibrio vulnificus ATCC 29306 occurred (Fig. 1B). The growth inhibition activity was specific to V. fischeri ES114, as culture fluids prepared from all the other tested strains did not inhibit V. harveyi growth (Fig. S1).
Fig. 1.
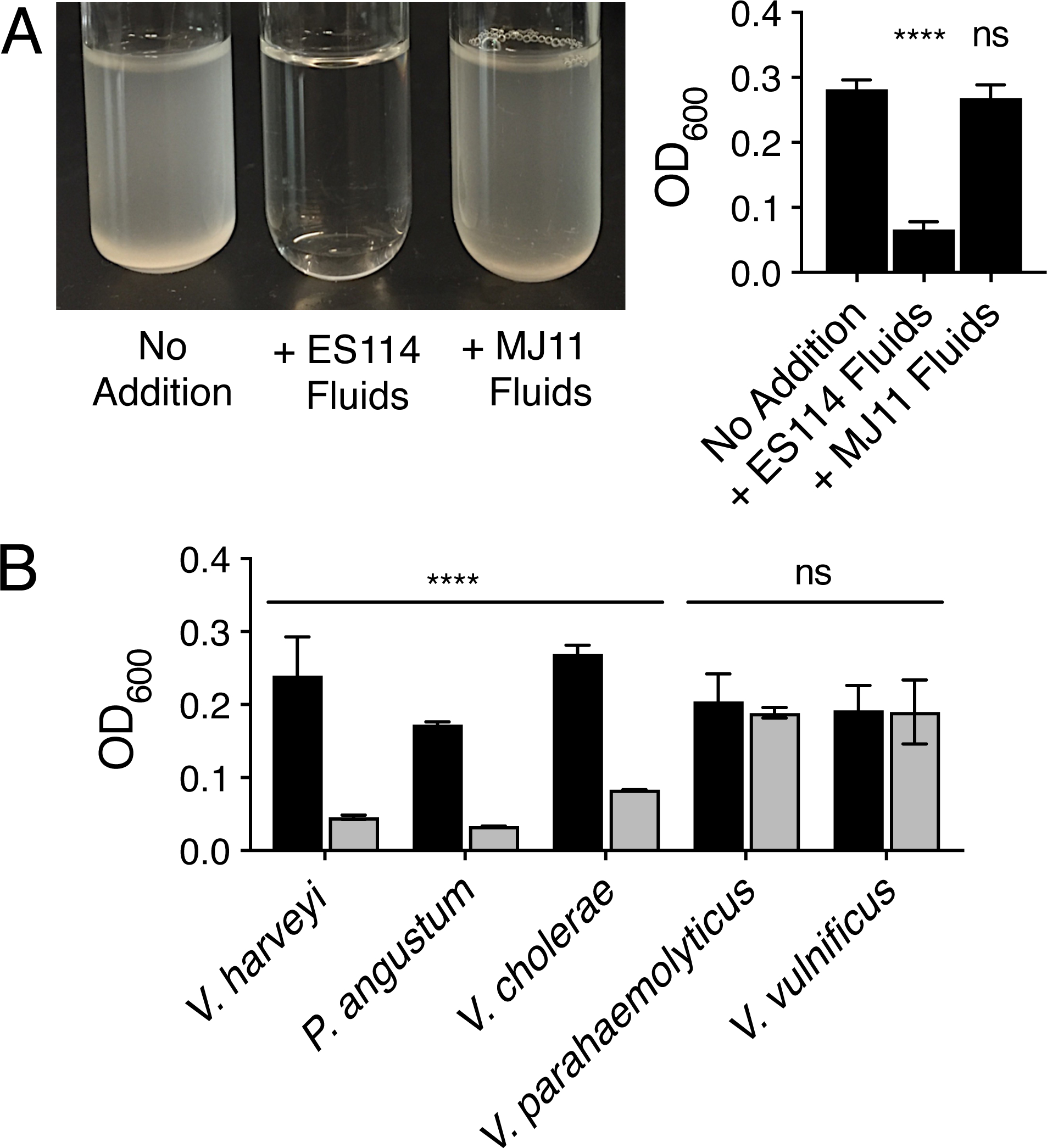
V. fischeri ES114 culture fluids prevent growth of vibrio species in minimal marine medium. A. Representative V. harveyi BB120 cultures (left) and the corresponding optical density (OD600) readings (right) following growth for 16 h in minimal marine medium supplemented with 10% (v/v) of the indicated culture fluid. ES114 and MJ11 denote V. fischeri ES114 and V. fischeri MJ11, respectively. For the no addition control, 10% (v/v) sterile minimal marine medium was supplied instead of culture fluids. B. Growth of the indicated bacterial species after 16 h in minimal marine medium supplied with 10% (v/v) sterile minimal marine medium (black) or 10% (v/v) culture fluids from V. fischeri ES114 grown in minimal marine medium (gray). In A and B, error bars represent standard deviations for three biological replicates. Unpaired two-tailed t tests were performed comparing the treated conditions to the no addition controls. P-values: ns ≥ 0.05, **** < 0.0001.
V. fischeri ES114 culture fluids retained the ability to prevent V. harveyi growth following boiling or filtration through a 10,000 MWCO membrane, suggesting that the inhibitor is a small molecule (Fig. S2A). V. fischeri ES114 culture fluids only inhibited V. harveyi growth when V. fischeri ES114 was cultured in minimal marine medium but not when it was grown in rich medium (Fig. S2B). The culture fluids prevented V. harveyi growth when isolated from V. fischeri ES114 grown to an OD600 of approximately 0.7 or higher (Fig. S2C). Finally, the presence of V. fischeri ES114 culture fluids prevented the growth but did not kill V. harveyi. While below the level of detection by OD600 measurements, a small increase in V. harveyi cell density could be detected by counting colony forming units (CFUs) (Fig. S2D). These results indicate that the inhibitory substance produced by V. fischeri ES114 accumulates during growth, and moreover, is either regulated by nutrients, that a component present in rich medium masks or destroys the inhibitor, or that V. harveyi can overcome inhibition if the exogenously-supplied culture fluids contain additional nutrients.
To identify the inhibitor, we conducted a random transposon mutagenesis screen with the goal of isolating V. fischeri ES114 mutants incapable of inhibitor production. Our rationale was that culture fluids from such mutants would not prevent the growth of V. harveyi. We isolated 6,720 V. fischeri ES114 insertion mutants. V. harveyi was grown in the presence of 50% culture fluids from the V. fischeri ES114 insertion mutant strains, and bioluminescence was measured as an indicator of V. harveyi growth. We also measured the final OD600 of each V. fischeri ES114 mutant to identify mutants harboring transposon insertions that caused severe growth defects. These mutants were eliminated from the screen because we assumed that culture fluids from such mutants would likely possess low amounts of inhibitor, and thus allow growth of V. harveyi. Sixty-five V. fischeri ES114 mutants were identified without obvious growth defects and whose culture fluids allowed growth of V. harveyi as indicated by light production of 10-fold or more above background (Fig. S3A). False positive hits were eliminated in our follow-up analysis: cell-free cultures fluids from the 65 V. fischeri ES114 candidate mutant strains were prepared and tested, this time at 5%, for the ability to prevent V. harveyi growth. Seventeen V. fischeri ES114 transposon insertion mutants appeared defective in production of the growth-inhibitory substance (Fig. S3B).
The genes harboring transposon insertions in the 17 V. fischeri ES114 mutants were identified by arbitrarily-primed PCR and sequencing of the transposon-chromosome junctions (Fig. S3B). One operon, iucABCD, encoding the biosynthetic enzymes for the siderophore aerobactin, harbored 5 of the transposon insertions (Fig. 2A). Aerobactin is a hydroxamate siderophore originally identified in the Gram-negative bacterium Aerobacter aerogenes (now known as Enterobacter aerogenes) (Gibson and Magrath, 1969). Aerobactin production is common to enteric bacteria such as Escherichia coli, Shigella, and Salmonella spp. (reviewed by Payne et al., 2016). Aerobactin is known to be made by Vibrio mimicus (Moon et al., 2004), Vibrio hollisae (Okujo and Yamamoto, 1994; Suzuki et al., 2006), and other marine vibrio isolates, including environmental isolates of V. fischeri (Cordero et al., 2012).
Fig. 2.
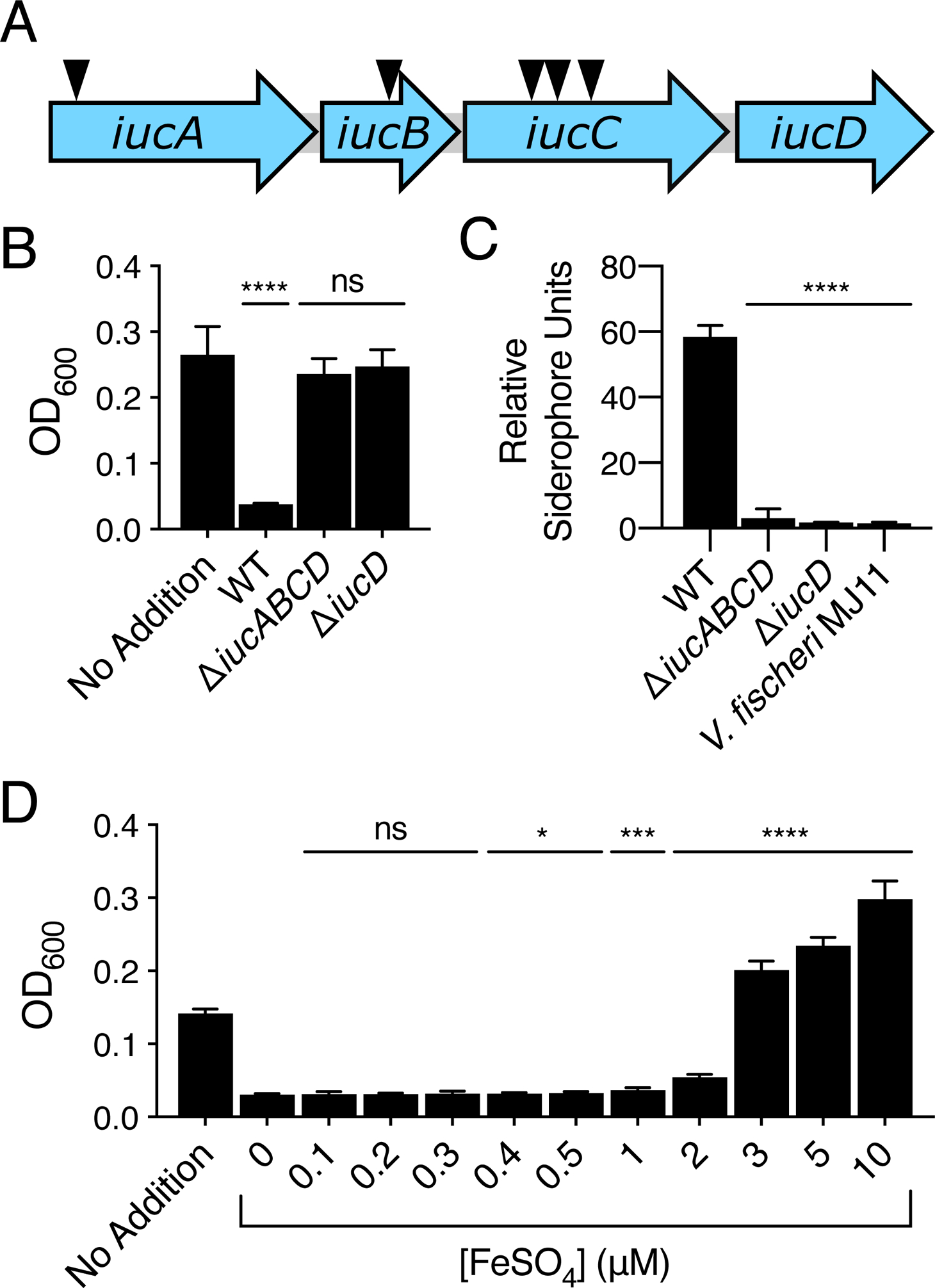
V. fischeri ES114 produces a siderophore that inhibits V. harveyi growth. A. Schematic of the V. fischeri ES114 aerobactin biosynthetic operon. The arrows depict the locations of five independent transposon insertions identified in the screen. B. V. harveyi growth in minimal marine medium supplemented with 10% (v/v) culture fluids from WT V. fischeri ES114 or the indicated V. fischeri ES114 mutant strain. In the case of the no addition control, 10% (v/v) minimal marine medium was added. C. CAS assay quantitation of siderophore present in culture fluids collected from WT V. fischeri ES114, the indicated mutant V. fischeri ES114 strains, or V. fischeri MJ11. D. V. harveyi growth in minimal marine medium supplemented with 10% (v/v) culture fluids prepared from WT V. fischeri ES114. The indicated concentrations of ferrous (Fe2+, no siderophore required) sulfate were added simultaneously to the cell-free culture fluids. In the case of the no addition control, 10% (v/v) minimal marine medium was added. In B, C, and D, error bars represent standard deviations for three biological replicates. Unpaired two-tailed t tests were performed comparing the treated conditions to the no addition control (B), mutants to WT (C), and iron-treated samples to the untreated (0 μM) control sample (D). P values: ns ≥ 0.05, * < 0.05, *** < 0.001, **** < 0.0001.
We hypothesized that, in minimal marine medium, V. fischeri ES114 releases aerobactin. Thus, when its culture fluids are added to V. harveyi, iron sequestration prevents V. harveyi growth. To test this notion, we deleted the entire iucABCD operon from V. fischeri ES114. We also deleted only iucD, encoding a lysine monooxygenase that functions in the first step of aerobactin biosynthesis (de Lorenzo et al., 1986). Culture fluids from both mutant V. fischeri ES114 strains did not prevent V. harveyi growth (Fig. 2B). We used the liquid chrome azurol S (CAS) siderophore detection dye assay to measure siderophore in the culture fluids (Schwyn and Neilands, 1987; Payne, 1994). WT V. fischeri ES114 culture fluids possessed siderophore whereas fluids from the ΔiucABCD and ΔiucD mutants did not (Fig. 2C). Also, consistent with our above finding that culture fluids isolated from V. fischeri MJ11 do not inhibit V. harveyi growth, V. fischeri MJ11 culture fluids possessed no detectable siderophore (Fig. 2C). To test if inhibition of V. harveyi growth was indeed due to chelation of iron by aerobactin, we added increasing concentrations of iron together with the 10% WT V. fischeri ES114 culture fluids to V. harveyi. In this experiment, soluble ferrous (Fe2+) iron was provided, and from here forward, we distinguish the form of iron supplied in our experiments as either “ferrous (Fe2+, no siderophore required)” or “ferric (Fe3+, siderophore required)”. Addition of 3 μM or higher ferrous (Fe2+, no siderophore required) iron rescued V. harveyi growth (Fig. 2D). We conclude that iron chelation is responsible for the inhibition of V. harveyi growth when V. fischeri ES114 culture fluids are present. As shown in Fig. S3 and described below, 8 additional V. fischeri ES114 genes were identified in the mutagenesis screen that potentially encode components that decrease aerobactin production via regulation of iucABCD, reduction in secretion or recycling of aerobactin, or alteration of metabolism to indirectly influence siderophore biosynthesis or activity.
Fur represses aerobactin production in V. fischeri ES114
It was curious that growth inhibition of V. harveyi occurred only when culture fluids were obtained from V. fischeri ES114 grown in minimal marine medium but not in rich medium (Fig. S2B). We suspected that either V. fischeri ES114 does not produce aerobactin when grown in rich medium, and/or sufficient iron is present in rich medium to overcome aerobactin chelation. To explore these possibilities, we quantified transcription of the V. fischeri ES114 siderophore biosynthetic genes under the two conditions. There was only low level iucA expression in V. fischeri ES114 grown in rich medium. In minimal marine medium, iucA transcription increased with increasing cell density until late exponential phase. Specifically, at OD600 = 0.7, iucA transcription was 30-fold higher in V. fischeri ES114 grown in minimal marine medium than when it was grown to the same OD600 in rich medium (Fig. S4). Activation of iucABCD expression could occur in minimal marine medium or repression could occur in rich medium. A likely candidate for controlling transcription by a repressive mechanism is Fur (ferric uptake regulator), the major transcriptional regulator of iron transport in Gram-negative bacteria. Under iron-replete conditions, typically, Fur binds ferrous iron (Fe2+, no siderophore required), the complex binds so-called Fur box DNA elements, and transcription is repressed (Bagg and Neilands, 1987). Thus, Fur-regulated genes are derepressed under iron-limiting conditions. Aerobactin production is Fur-regulated in E. coli (de Lorenzo et al., 1987), and Fur boxes have been identified neighboring the aerobactin biosynthetic genes in V. hollisae (Suzuki et al., 2006). The CAS assay shows that ferrous iron (Fe2+, no siderophore required) concentrations of 2 μM or higher repressed siderophore production in V. fischeri ES114, and deletion of fur relieved repression (Fig. 3A). When introduced on a plasmid, mVenus fused to the iucABCD promoter was likewise repressed in response to increasing ferrous iron (Fe2+, no siderophore required) concentration. No repression occurred in the absence of Fur (Fig. 3B). Using a colorimetric ferene dye assay for iron, we found that the concentration of iron in our minimal marine medium is ~0.3 μM (Fig. S5) Thus, in minimal marine medium, limited iron availability coupled with high siderophore production by V. fischeri ES114 impair V. harveyi growth in the presence of V. fischeri ES114 culture fluids. Increased iron availability and/or Fur-mediated repression of siderophore production in V. fischeri ES114 allows V. harveyi growth.
Fig. 3.
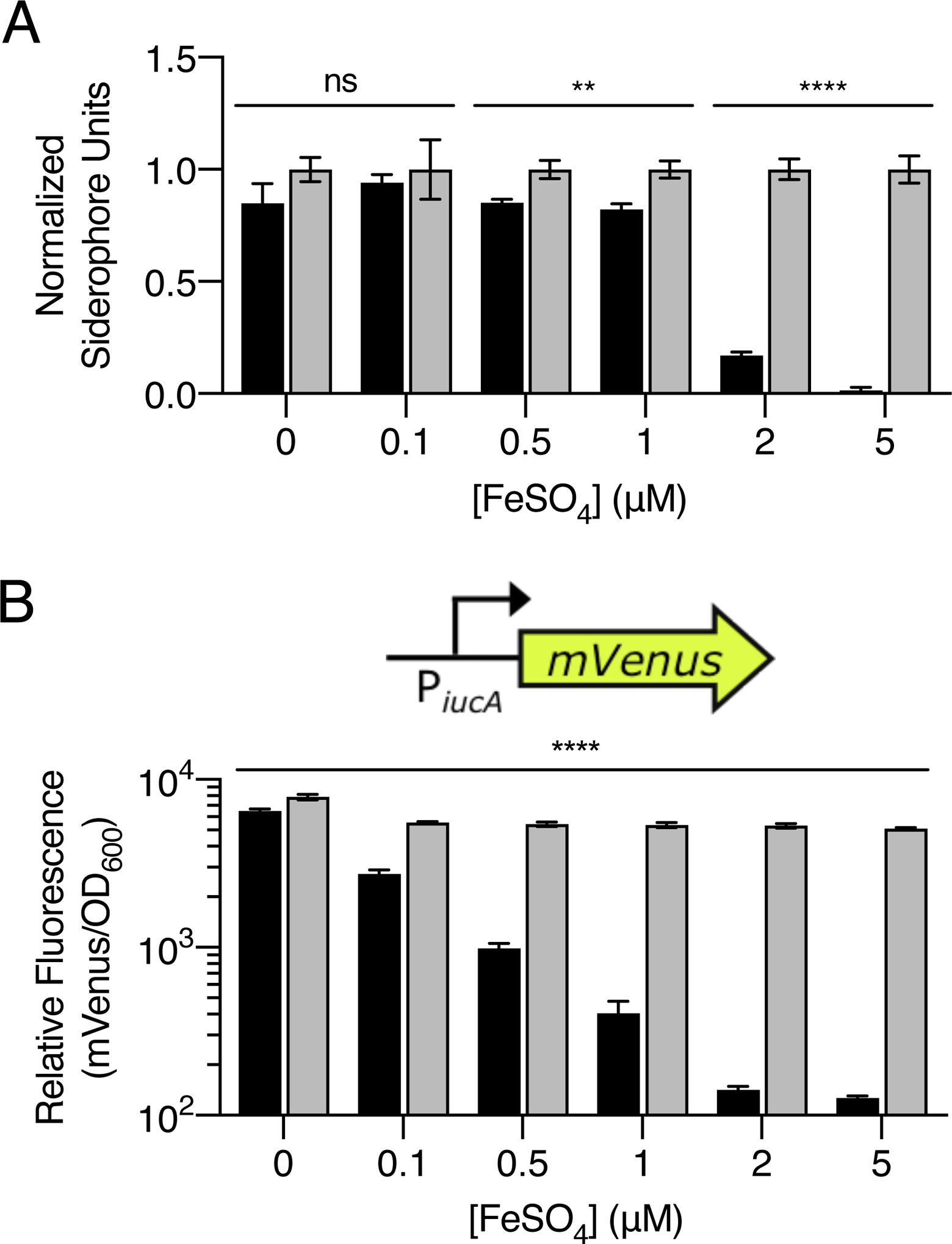
Fur represses V. fischeri ES114 siderophore production under iron-replete conditions. A. CAS assay quantitation of siderophore present in culture fluids prepared from WT (black) or Δfur (gray) V. fischeri ES114 grown in minimal marine medium supplemented with the indicated concentrations of ferrous (Fe2+, no siderophore required) sulfate. To account for increased growth in response to increasing iron concentrations, values were normalized to a siderophore unit value of 1 for the Δfur mutant at each iron concentration. B. Relative fluorescence values (mVenus/OD600) for WT (black) or Δfur (gray) V. fischeri ES114 strains carrying an iucA’-mVenus transcriptional reporter on a plasmid. The strains were grown in minimal marine medium supplemented with the indicated concentrations of ferrous (Fe2+, no siderophore required) sulfate. Values represent the relative fluorescence at OD600 = 0.4 for each condition. For A and B, error bars represent standard deviations for three biological replicates. Unpaired two-tailed t tests were performed comparing WT to Δfur for each treatment. P values: ns ≥ 0.05, ** < 0.01, **** < 0.0001.
Identification of activators of aerobactin production in V. fischeri ES114
As mentioned, our mutagenesis screen identified transposon insertions in 8 genes in addition to those in the aerobactin biosynthetic operon (Fig. S3B). Three genes, cca, mnmG, and trmE, are involved in tRNA modification. While we do not understand the connection these genes have to aerobactin production, we speculate they may alter the availability of the required L-lysine substrate. We did not study these three genes further. We constructed in-frame deletions in the 5 remaining genes and examined the effects on aerobactin production. Deletion of 4 of the 5 genes, luxT, yebK, fre, and glpK, significantly reduced aerobactin production in V. fischeri ES114 (Fig. 4A). Consistent with this finding, increased V. harveyi growth occurred in the presence of culture fluids prepared from the 4 V. fischeri ES114 mutants (Fig. 4B). LuxT, YebK, Fre, and GlpK are therefore activators of aerobactin production in V. fischeri ES114. luxT and yebK encode DNA-binding transcriptional regulators. fre and glpK encode flavin reductase and glycerol kinase, respectively. Deletion of any of these 4 genes did not alter the growth of V. fischeri ES114 (Fig. S6). The fifth gene, glpF, encoding the glycerol uptake facilitator protein did not fit the above pattern, as in-frame deletion of glpF did not reduce aerobactin production (Fig. 4A,B). glpF is located in an operon upstream of glpK. We presume that transposon insertion in glpF is polar on glpK, which explains this result. We were surprised that our mutagenesis screen did not identify glnD, as a previous report showed that transposon insertion in glnD reduces siderophore production by V. fischeri ES114 (Graf and Ruby, 2000). To investigate this discrepancy, we deleted glnD from V. fischeri ES114. The mutant exhibits only a modest decrease in siderophore production and in iucA transcription (Fig. S7A,B, respectively). Culture fluids from the ΔglnD mutant strain retain the ability to inhibit V. harveyi growth (Fig. S7C). Together, these results explain why glnD was not revealed in our screen.
Fig. 4.
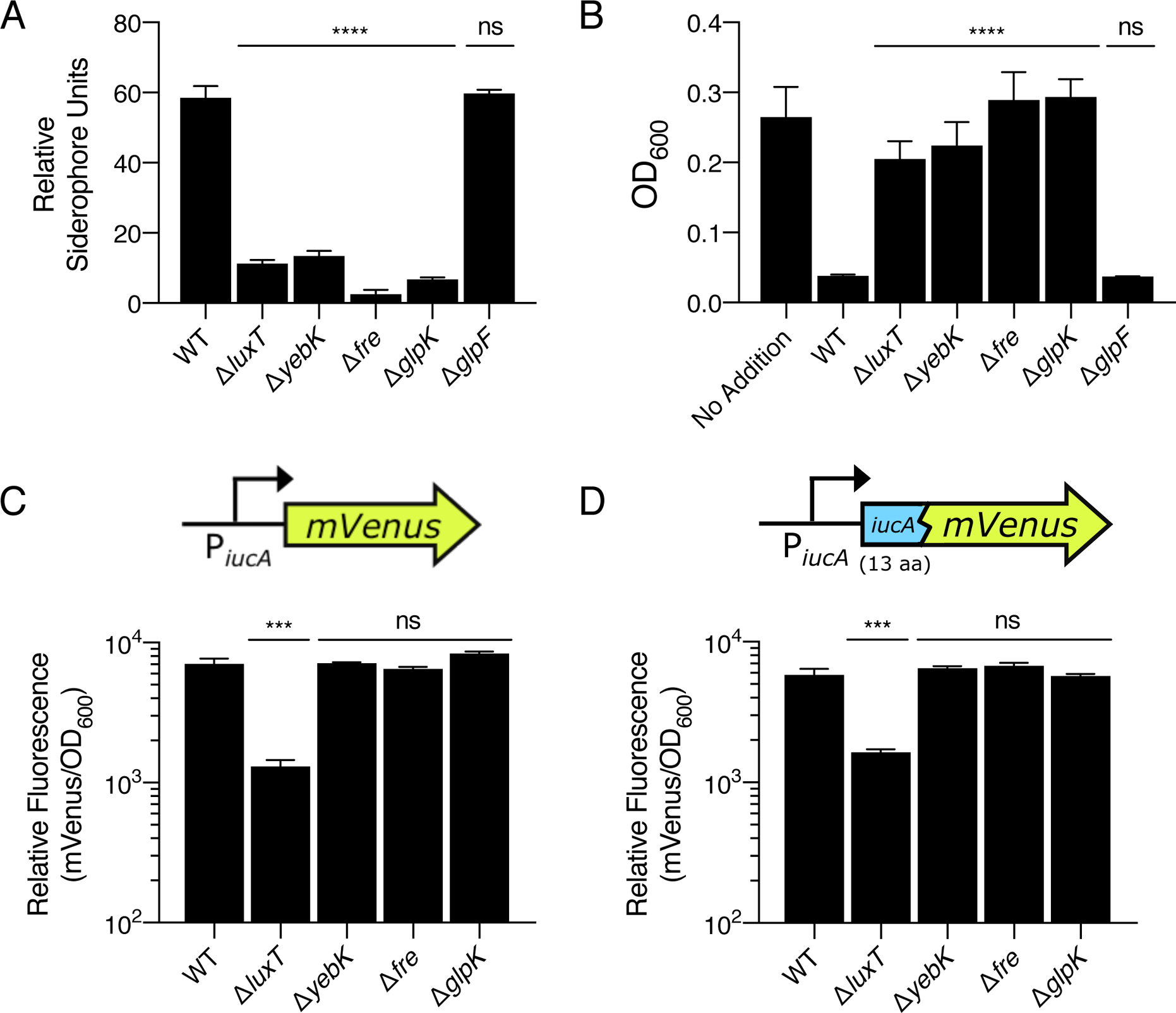
LuxT, YebK, Fre, and GlpK are activators of siderophore production in V. fischeri ES114. A. CAS assay quantitation of siderophore levels in culture fluids collected from the indicated V. fischeri ES114 strains. B. V. harveyi growth after 16 h in minimal marine medium supplemented with 10% (v/v) culture fluids prepared from the indicated V. fischeri ES114 strains. In the case of the no addition control, 10% (v/v) sterile minimal marine medium was added. C. Relative fluorescence values (mVenus/OD600) of the indicated V. fischeri ES114 strains carrying an iucA’-mVenus transcriptional reporter on a plasmid. Values represent relative fluorescence when OD600 = 0.4 for each sample. D. As in C, except that the strains harbor a translational iucA’-’mVenus reporter. In all panels, error bars represent standard deviations of three biological replicates. Unpaired two-tailed t tests were performed comparing mutants to WT (A, B, C, and D). P values: ns ≥ 0.05, *** < 0.001, **** < 0.0001.
To examine the mechanisms underlying control of aerobactin production by LuxT, YebK, Fre, and GlpK, we introduced a plasmid harboring an iucA’-mVenus transcriptional fusion or an iucA’-’mVenus translational fusion into WT V. fischeri ES114 and the four deletion mutant strains. Only deletion of luxT significantly decreased iucABCD transcription (Fig. 4C), and as a consequence, the corresponding activity of the translational reporter was also reduced (Fig. 4D). The strains carrying deletions in yebK, fre, and glpK did not exhibit altered expression of either reporter (Fig. 4C,D). In the Discussion, we provide hypotheses for the role of each of these genes.
LuxT, a member of the TetR transcription factor family (reviewed by Ramos et al., 2005), was originally identified as a repressor of luxO encoding the central response regulator in the V. harveyi quorum-sensing (QS) system (Freeman and Bassler, 1999; Lin, Miyamoto, and Meighen, 2000a; Lin, Miyamoto, and Meighen, 2000b). To test if LuxT regulates siderophore production in a QS-dependent manner, we measured siderophore production in V. fischeri ES114 ΔainS, ΔainR, and ΔlitR ΔluxR strains encoding a QS autoinducer synthase, QS autoinducer receptor, and the two QS master transcriptional activators, respectively (Engebrecht and Silverman, 1984; Gilson et al., 1995; Fidopiastis et al., 2002; Kimbrough and Stabb, 2013). There was no difference in siderophore production in the mutants compared to WT, indicating that LuxT regulates siderophore production independently of QS (Fig. S8).
We tested whether LuxT control of iucABCD transcription is direct. We used a strategy in which we overexpressed luxT in WT and Δfur E. coli MG1655 harboring the iucA’-mVenus transcriptional reporter plasmid. There was no change in reporter output when luxT expression was induced, indicating that either LuxT functions indirectly to control iucABCD transcription or LuxT functions together with some other V. fischeri ES114 cofactor that is not present in E. coli (Fig. S9A). qRT-PCR confirmed that luxT was transcribed from the overexpression vector in E. coli as there was a 40-fold increase in luxT transcript levels in the strain supplied with the arabinose inducer compared to the isogenic uninduced strain (Fig. S9B). E. coli Fur repressed iucA transcription, confirming its role in aerobactin repression and the validity of our heterologous system (Fig. S9A). Below, we discuss possible mechanisms by which LuxT could regulate iucABCD.
Aerobactin cheating: requirements for aerobactin recognition and uptake
In communities, public goods can be exploited by cheaters who acquire advantages through use of the good but who do not pay the energetic cost of goods production. In the context of siderophores, a cheater need only possess genes required for recognition and uptake of the siderophore-iron (Fe3+) complex. Often, siderophore biosynthetic genes exist within larger gene clusters harboring genes encoding ancillary functions for reception and transport (reviewed by Crosa and Walsh, 2002). Such is the case for V. fischeri ES114: iucABCD are present in a 9-gene cluster (Fig. 5A). These 9 genes appear to be conserved and co-inherited in aerobactin producing vibrio strains (Cordero et al., 2012). In the cluster, iutA, encoding the OM receptor for the aerobactin-iron (Fe3+) complex, is immediately downstream of the iucABCD biosynthetic genes (de Lorenzo et al., 1986). The fhuCDB genes are upstream of iucABCD and encode an inner membrane ABC transporter in which FhuD is a periplasmic binding protein, FhuB is a membrane permease, and FhuC is an ATP-binding protein (Wooldridge et al., 1992). A gene (VF_A0157) encoding a major facilitator superfamily (MFS) transporter resides upstream of fhuCDB and is oriented in the opposite direction. This gene is called shiF in Shigella, but no function has been ascribed to it (Forman et al., 2007). Here, we refer to this gene as aerE for aerobactin exporter, a function we demonstrate below.
Fig. 5.
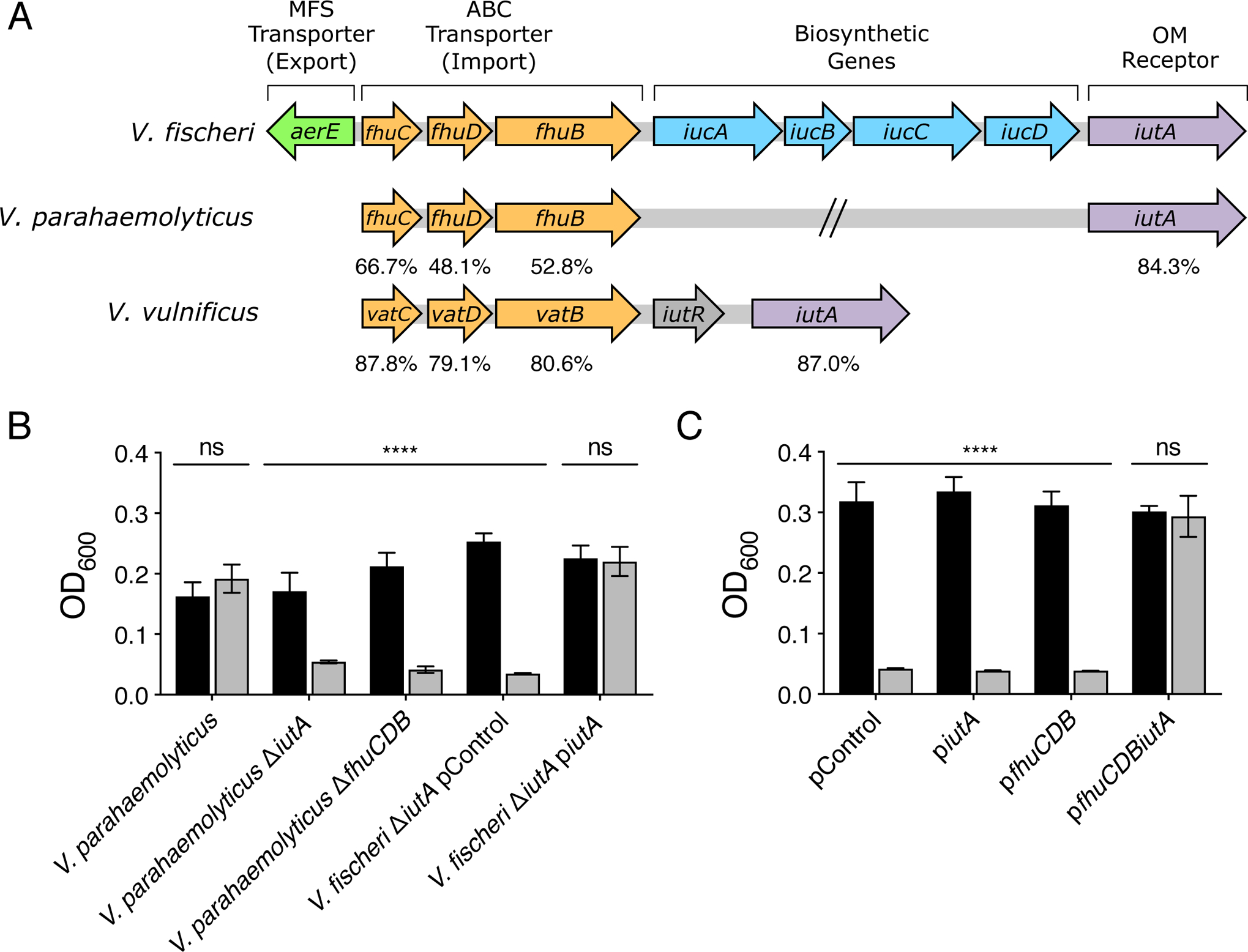
IutA and FhuCDB are sufficient for aerobactin cheating. A. The V. fischeri ES114 aerobactin biosynthetic gene cluster, depicted to scale. Homologs identified in V. parahaemolyticus BB22OP and V. vulnificus YJ016 are shown along with the percent positive similarity in amino acid sequence to the corresponding V. fischeri ES114 homolog. B. Growth after 16 h of the indicated V. parahaemolyticus BB22OP and V. fischeri ES114 strains in minimal marine medium supplied with 10% (v/v) minimal marine medium (black) or culture fluids from WT V. fischeri ES114 (gray). The pControl plasmid is the empty parent vector. piutA carries the V. fischeri ES114 iutA gene expressed from its native promoter. C. Growth after 16 h of V. harveyi harboring the indicated plasmids in minimal marine medium supplemented with 10% (v/v) minimal marine medium (black) or V. fischeri ES114 culture fluids (gray). The pControl plasmid is the empty parent vector. piutA, pfhuCDB, and pfhuCDBiutA carry the indicated genes expressed from their native promoters. In B and C, error bars represent standard deviations for three biological replicates. Unpaired two-tailed t tests were performed comparing the no addition control samples to the treated samples for each condition. P values: ns ≥ 0.05, **** < 0.0001.
V. parahaemolyticus and V. vulnificus grew in the presence of V. fischeri ES114 culture fluids (Fig. 1B), but culture fluids prepared from these species did not inhibit V. harveyi growth (Fig. S1). Based on these findings, we hypothesize that V. parahaemolyticus and V. vulnificus are aerobactin cheaters. Support for this idea comes from inspection of genome sequences. The genome of V. vulnificus ATCC 29306, the strain used here, has not been sequenced, however the genomes of the related strain V. vulnificus YJ016 and of V. parahaemolyticus BB22OP (used here) show that neither possesses the iucABCD biosynthetic genes. Both species encode fhuCDB and iutA homologs (Fig. 5A). We predict that the IutA homolog would recognize and import aerobactin across the OM, and the FhuCDB homologs would transport aerobactin across the inner membrane. Previous studies have shown the requirement for these genes in aerobactin uptake in V. parahaemolyticus and V. vulnificus strains (Funahashi et al., 2003; Tanabe et al., 2005; Funahashi et al., 2009). To verify this activity in our V. parahaemolyticus strain, we deleted either iutA or fhuCDB. Indeed, when V. fischeri ES114 culture fluids were administered to a V. parahaemolyticus ΔiutA or ΔfhuCDB strain, no growth of the recipient occurred (Fig. 5B). Moreover, deletion of iutA in V. fischeri ES114 made the strain incapable of growth in the presence of its own WT culture fluids. This defect was complemented by in trans expression of iutA (Fig. 5B). Together, these data demonstrate the necessity IutA and FhuCDB for uptake of extracellular aerobactin in both aerobactin producing and aerobactin cheating strains. To further explore the requirements to be an aerobactin cheater, we turned to V. harveyi. We reasoned that, because V. harveyi does not encode homologs of any of the nine genes in the aerobactin cluster, we could use V. harveyi as a tool to identify the minimal components required for cheating. We introduced plasmids into V. harveyi encoding subsets of genes from the V. fischeri ES114 aerobactin cluster. Expression of either iutA or fhuCDB was insufficient to rescue V. harveyi growth in the presence of V. fischeri ES114 culture fluids. However, V. harveyi harboring both iutA and fhuCDB could grow in their presence (Fig. 5C). We conclude that IutA and FhuCDB, encoding, respectively, the siderophore OM receptor and siderophore importer are sufficient to convert a non-aerobactin-producing species into an aerobactin cheater.
AerE is an aerobactin exporter
The protein encoded by the first gene in the V. fischeri ES114 aerobactin cluster (Fig. 5A), that we name aerE, encodes a MFS transporter. aerE homologs are typically present in aerobactin gene clusters but their roles in iron acquisition and utilization, if any, have not been defined. It has been speculated that homologs of aerE encode aerobactin exporters, however, the authors noted uncertainty based on the existing data. Specifically, Forman et al. (2007) reported that E. coli deleted for the aerE homolog remained capable of aerobactin secretion, and Genuini et al. (2019) showed that fluids from an E. coli mutant deleted for the gene possessed less aerobactin than those from WT.
We sought to clearly define the role of AerE. Deletion of aerE from V. fischeri ES114 did not reduce the relative level of siderophore present in culture fluids as assessed by the CAS assay (Fig. 6A). However, deletion of aerE did cause a growth defect in V. fischeri ES114 when the strain was grown in minimal marine medium but not when grown in rich medium (Fig. S10A,B, respectively). We interpret these results to mean that, in minimal marine medium, aerobactin is produced and accumulates to a toxic level in the ΔaerE strain. By contrast, in rich medium, because Fur represses siderophore production (Fig. S2B), no toxicity occurs (Fig. S10B). To test for increased intracellular aerobactin in the V. fischeri ES114 ΔaerE strain, we measured aerobactin levels in cells (i.e., not in culture fluids). Approximately 40-fold more siderophore was in the cytoplasm of the V. fischeri ES114 ΔaerE mutant than in the WT (Fig. 6B). Deletion of fhuCDB or iutA encoding the aerobactin importer and OM receptor, respectively, did not alter intracellular aerobactin levels (Fig. 6B). We conclude that aerE and its homologs (i.e., shiF) encode aerobactin exporters. We can think of two possibilities to explain the finding that culture fluids from the V. fischeri ES114 ΔaerE mutant possess normal levels of siderophore (Fig. 6A): either aerobactin-toxicity-mediated cell death and, consequently, lysis, causes siderophore release into the mutant culture fluids, or V. fischeri ES114 harbors an additional aerobactin export mechanism(s). We assessed cell death using Sytox Green, a stain that only permeates cells with compromised membranes. We did not detect increased lysis of the ΔaerE V. fischeri ES114 strain relative to WT V. fischeri ES114 (Fig. S11).
Fig. 6.
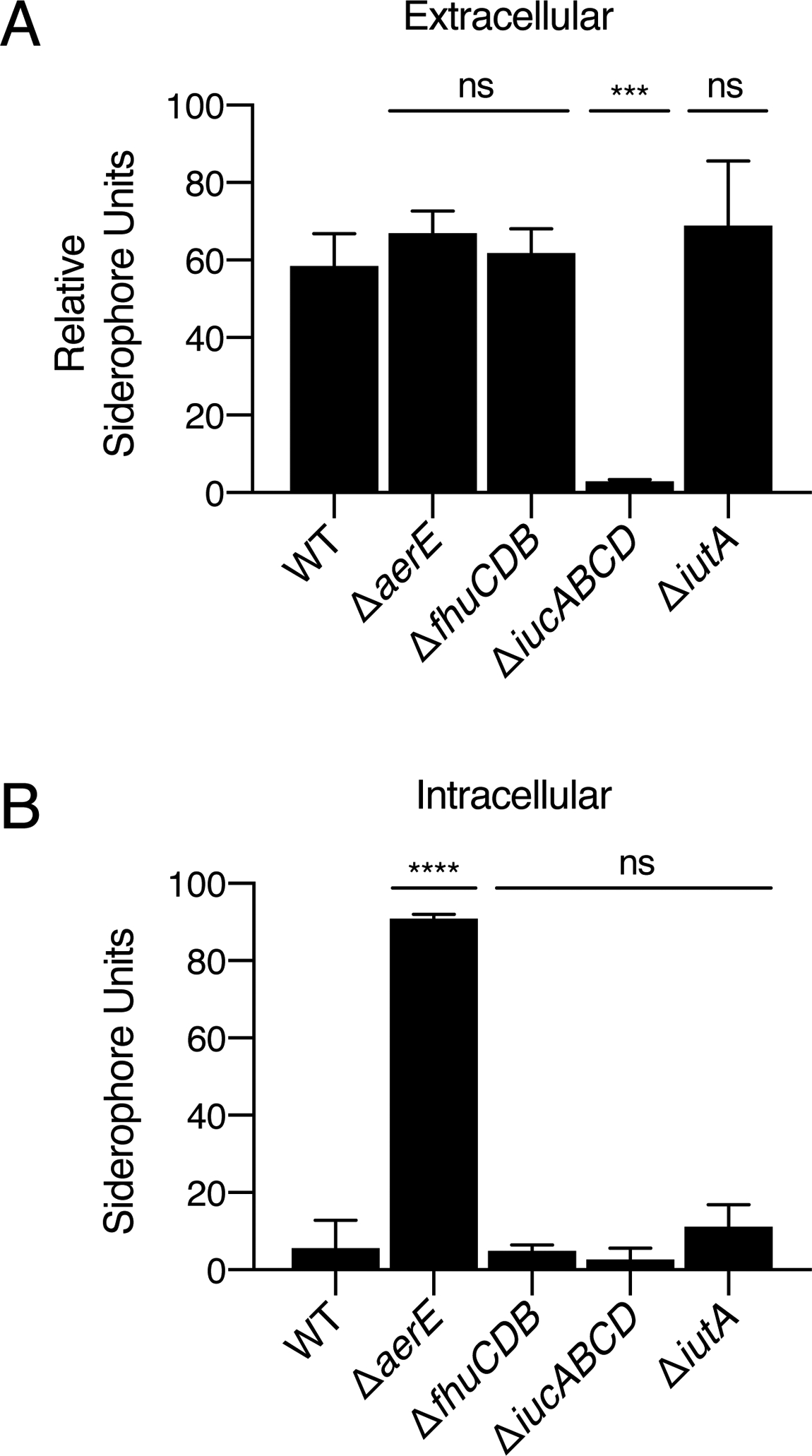
aerE encodes an MFS transporter that exports aerobactin. A. CAS assay quantitation of siderophore in culture fluids collected from the indicated V. fischeri ES114 strains grown in minimal marine medium. B. CAS assay quantitation of intracellular siderophore levels in the strains from A. In A and B, error bars represent standard deviations for three biological replicates. Unpaired two-tailed t tests were performed comparing mutants to WT. P values: ns ≥ 0.05, *** < 0.001, **** < 0.0001.
Variation between siderophore-producing and non-producing V. fischeri strains
V. fischeri ES114 and V. fischeri MJ11 share a high level of conservation between their genomes. Specifically, V. fischeri MJ11 encodes over 90% of the open-reading frames present in V. fischeri ES114, and shared sequences encode proteins with a median amino acid identity of 98.8% (Mandel et al., 2009). The divergence between the two strains raises interesting evolutionary questions and is, furthermore, responsible for establishing host-specificity: V. fischeri ES114 is a symbiont of the squid, Euprymna scolopes, whereas V. fischeri MJ11 is a symbiont of the fish, Monocentris japonica (Boettcher and Ruby, 1990; Mandel et al., 2009). The striking variability in siderophore production by V. fischeri ES114 and V. fischeri MJ11 strains that do and do not produce detectable aerobactin, respectively (Fig. 2C and Pankey et al., 2017), is not due to a lack of aerobactin biosynthetic genes in V. fischeri MJ11. Its genome encodes the entire aerobactin cluster, and there are no obvious frameshift or nonsense mutations present that would abrogate function (Fig. S12A).
To assess the possibility that the difference in V. fischeri ES114 and V. fischeri MJ11 aerobactin phenotypes is due to differences in gene expression, we used qRT-PCR to quantify mRNA levels of aerE, fhuC, iucA, and iutA. In order to compare transcript levels, qRT-PCR primers were designed to bind DNA sequences that are 100% identical between the two strains. Surprisingly, transcription of all four genes from the non-producing V. fischeri MJ11 strain was equal to or higher than the corresponding gene in the producing V. fischeri ES114 strain (Fig. S12B). Thus, differences in transcription cannot underpin the two phenotypes. With respect to the encoded proteins, the IucABCD biosynthetic proteins are less similar (mean 96.4% identity) between the two strains than the proteins encoded by the flanking genes (mean 98.6% identity) (Fig. S12A). We hypothesized that the biosynthetic enzymes encoded by iucABCD may be more efficient in V. fischeri ES114 than in V. fischeri MJ11. To test this possibility, we overexpressed the iucABCD operon from each strain in a V. fischeri ES114 ΔiucABCD mutant and measured siderophore production using the CAS assay. Overexpression of V. fischeri ES114 iucABCD fully restored the ΔiucABCD defect. However, overexpression of the V. fischeri MJ11 iucABCD genes drove only partial restoration of siderophore production (Fig. 7A). In the reciprocal experiment, overexpression of iucABCD from V. fischeri ES114 drove a marked enhancement in siderophore production by V. fischeri MJ11 over that which occurred when its own iucABCD genes were overexpressed (Fig. 7B). Thus, the difference in siderophore production between V. fischeri ES114 and V. fischeri MJ11 does not stem from differences in transcriptional regulation, rather, the difference apparently arises at the protein level, perhaps due to differences in post-transcriptional regulation, biosynthetic enzymatic activity, or protein stability.
Fig. 7.
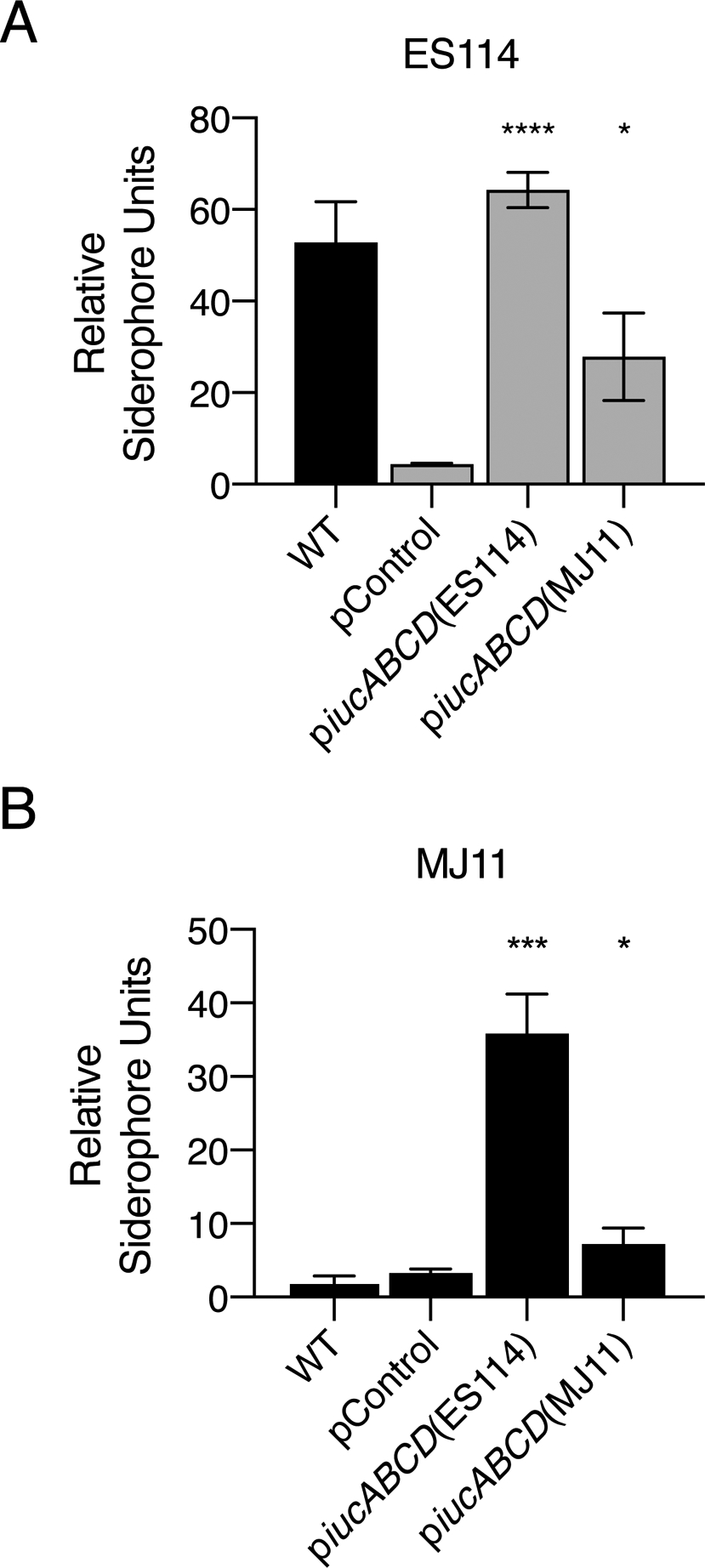
Differences in aerobactin production between V. fischeri ES114 and V. fischeri MJ11 stem from differences at the protein level. A. CAS assay quantitation of siderophore levels in culture fluids from WT (black bar) or ΔiucABCD (gray bars) V. fischeri ES114 harboring the indicated plasmids. The pControl plasmid is the empty parent vector. The piucABCD(ES114) and piucABCD(MJ11) plasmids carry iucABCD from the indicated strain under the IPTG inducible tac promoter. Cultures were grown in minimal marine medium with 0.5 mM IPTG to induce expression. B. CAS assay quantitation of siderophore levels in V. fischeri MJ11 culture fluids. Plasmids and growth conditions as in A. In A and B, error bars represent standard deviations for three biological replicates. Unpaired two-tailed t tests were performed comparing the strains harboring piucABCD(ES114) and piucABCD(MJ11) to the pControl strain. P values: * < 0.05, *** < 0.001, **** < 0.0001.
Aerobactin production confers a fitness advantage to V. fischeri ES114 in co-culture with V. harveyi when iron is limiting
The competitive exclusion principle states that if two species occupy the same ecological niche, the species that multiplies most rapidly, even if only by a small margin, will displace the other species, driving it to extinction given sufficient time (Hardin, 1960). In bacteria, exclusion of the less fit strain can be followed using long-term co-culture growth experiments (Veldkamp and Jannasch, 1972; Bruger and Waters, 2016; Sexton and Schuster, 2017). With our new understanding that aerobactin is an inhibitor of V. harveyi growth under iron-limiting conditions, we sought to test its role in dual-species co-culture and whether its presence or absence alters the outcome in competitions between V. fischeri ES114 and V. harveyi.
Growth rates of each species were first analyzed individually. In our minimal marine medium, V. fischeri ES114 and V. harveyi have similar growth rates, but V. harveyi grows to an overall higher final cell density (Fig. S13A). In an attempt to mimic natural marine environments in which siderophores could play roles in competition, iron was depleted from the medium by treatment with Chelex 100 resin. This process made it so that iron was undetectable in the medium as measured by a colorimetric ferene dye assay (Fig. S5). The limit of detection was approximately 0.02 μM. Next, ferric (Fe3+, siderophore required) iron was added back to the treated medium at 10 μM to provide an iron source that can be accessed by aerobactin and enable significant growth of WT V. fischeri ES114. We call this iron-depleted medium. WT V. fischeri ES114 grew to 6-fold higher final cell density than V. harveyi in the iron-depleted medium. The V. fischeri ES114 ΔiucABCD mutant did not exhibit higher growth capacity than V. harveyi in the iron-depleted medium (Fig. S13B). Therefore, aerobactin is required for growth of V. fischeri ES114 to high cell density in iron-depleted medium. V. harveyi harbors genes enabling production of two siderophores. Nonetheless, growth of V. harveyi was negligible in the iron-depleted medium as 6-fold lower OD600 was achieved than in minimal marine medium (compare red lines in Fig. S13A,B). The inability of siderophores to promote V. harveyi growth under iron-depleted conditions is likely due to QS repression of siderophore gene expression which restricts siderophore production to low cell density (Lilley and Bassler, 2000). Indeed, culture fluids from WT V. harveyi possess 18-fold less siderophore activity than culture fluids from WT V. fischeri ES114, as detected by the CAS assay (Fig. S14).
We competed V. harveyi against either WT or ΔiucABCD V. fischeri ES114 under four different growth conditions. In each case, the two species were combined in a 1:1 ratio, inoculated into the growth medium, and allowed to grow for 24 h. Fitness was measured by plating and counting CFUs of each species at the start and the end of the co-culture. The V. fischeri ES114 and V. harveyi colony morphologies are distinct, so they are easily distinguished on agar plates. Additionally, on agar plates, V. harveyi produces light and V. fischeri ES114 does not, aiding in species discrimination. The selection rate constants were calculated, the preferred analysis method for competitions under starvation conditions (Travisano and Lenski, 1996; Lenski, 2019). A value of 1 indicates that V. fischeri ES114 increased in cell density about 1 natural log more than V. harveyi. A value of 0 indicates that the two species increased equally in cell density. A value of −1 means that V. harveyi increased about 1 natural log more than V. fischeri ES114.
In minimal marine medium, V. harveyi outcompeted both WT and ΔiucABCD V. fischeri ES114 (Fig. 8). This result can be explained by the fact that V. harveyi grows to a higher cell density than V. fischeri ES114 in minimal marine medium (Fig. S13A). Apparently, aerobactin production plays no role under this condition because iron is sufficiently abundant for both species to grow without the need to produce a siderophore (Fig. S13A). Additionally, when V. fischeri ES114 was grown in monoculture in this condition, it did not activate siderophore production until it achieved an OD600 of 0.7, about 6 h into growth (Figs. S2C and S4) indicating that by the time V. fischeri does produce aerobactin, the growth window in which aerobactin can suppress V. harveyi doublings has passed. CAS assays confirmed that V. fischeri ES114 does indeed produce siderophore in our competitive setup (Fig. S15). Next, when competed in minimal marine medium supplemented with 10% (v/v) WT V. fischeri ES114 culture fluids, both WT and ΔiucABCD V. fischeri ES114 out-competed V. harveyi. Both V. fischeri strains increased by at least 4 natural logs more than V. harveyi (Fig. 8). No growth of V. harveyi was detected in either competition presumably because the aerobactin in the culture fluids had sequestered the iron. Both V. fischeri ES114 strains grew because irrespective of whether the strain produced (WT) or did not produce (ΔiucABCD) aerobactin, the siderophore supplied in the exogenous culture fluids could be used for iron acquisition. However, when competed in minimal marine medium supplemented with culture fluids from the ΔiucABCD V. fischeri ES114 strain, neither V. fischeri ES114 strain showed a growth advantage over V. harveyi (Fig. 8). This result shows that extracellular aerobactin is key for conferring the growth advantage to V. fischeri ES114 under our conditions. Finally, when competed in the iron-depleted medium, WT V. fischeri ES114 exhibited a significant fitness advantage over V. harveyi, increasing by about 2.3 natural logs more than its V. harveyi competitor. However, the ΔiucABCD mutant V. fischeri ES114 strain lost the competition: V. harveyi increased about 2.7 natural logs more than the V. fischeri ΔiucABCD strain (Fig. 8). Therefore, when iron is limiting, as it is in natural marine environments, aerobactin production confers a competitive advantage to V. fischeri ES114 over V. harveyi. Presumably, in the ocean, the ability of V. fischeri ES114 to successfully outcompete other non-cheater, non-aerobactin producing bacteria depends on the release of aerobactin for iron acquisition.
Fig. 8.
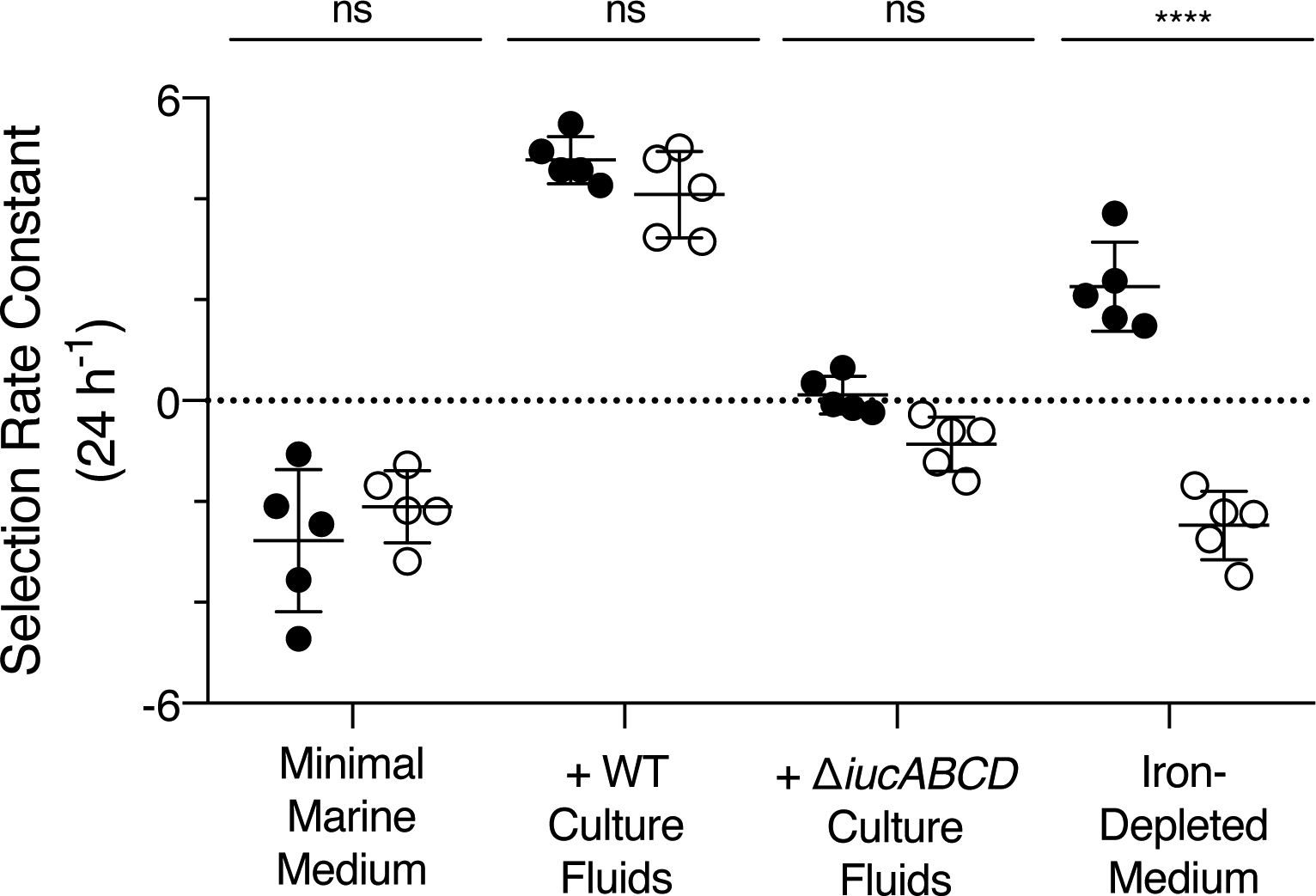
V. fischeri ES114 aerobactin production confers a competitive advantage against V. harveyi when iron is limiting. Competition experiments between V. harveyi and either WT (black circles) or ΔiucABCD (white circles) V. fischeri ES114 under the indicated growth conditions. For each competition, the selection rate constant (24 h−1) is plotted for the V. fischeri ES114 strain compared to V. harveyi. Error bars represent standard deviations of five biological replicates. Unpaired two-tailed t tests were performed comparing the V. harveyi/V. fischeri ES114 WT competition to the V. harveyi/V. fischeri ES114 ΔiucABCD competition for each growth condition. P values: ns ≥ 0.05, **** < 0.0001.
Discussion
When two bacterial species coexist and both species require the same limiting nutrient(s) to grow, competition is favored. The bacterial species that grows most rapidly can drive competitive exclusion of the other species. Different competitive strategies have evolved. In this study, we identified one strategy that can be used by the marine bacterium V. fischeri ES114. By producing and secreting the siderophore aerobactin, V. fischeri ES114 improves its ability to acquire iron and, therefore, reproduce, while simultaneously denying iron to a competing species, inhibiting its growth. Indeed, aerobactin production alone is sufficient to dictate the fate of V. fischeri ES114 when in competition with another vibrio species under iron-depleted conditions.
Siderophores, because they are secreted, are considered public goods, products that are costly for an individual cell to produce, but that can provide benefits to members of a community. Public goods producing bacteria are vulnerable to cheaters, bacteria that benefit from using public goods without paying the metabolic cost of producing them. As shown here and elsewhere, bacterial cheaters that possess genes for siderophore recognition and uptake exist. With respect to aerobactin, the IutA OM receptor and the FhuCDB importer represent the minimal components required to cheat. To become a cheater, an aerobactin producing strain could lose the iucABCD biosynthetic genes. Alternatively, the genes encoding IutA and FhuCDB could be acquired by horizontal gene transfer. Horizontal transfer is known to have distributed aerobactin genes across multiple vibrio phylogenetic lineages (Thode et al., 2018). Siderophore cheating is common in vibrios, and the number of siderophore receptors encoded in a vibrio genome typically exceeds the number of siderophores an individual strain can produce. For example, one strain of Vibrio tasmaniensis encodes 7 different siderophore receptor genes, but it only harbors the biosynthetic genes to produce 1 siderophore. A strain of Vibrio nereis encodes 6 receptors, but it cannot produce any siderophores (Thode et al., 2018). These examples emphasize the pressure on vibrios to scavenge iron, and are presumably relevant to interspecies interactions that occur in the ocean.
Prior to the present work, V. fischeri ES114 was known to produce a siderophore (Graf and Ruby, 2000), however the genes involved in siderophore biosynthesis and uptake had not been characterized. V. fischeri ES114 possesses a 9-gene cluster harboring aerobactin biosynthesis, recognition, and import genes. Here, we assign a function to the first gene in the cluster, aerE, by showing it encodes a MFS transporter that exports aerobactin. Not surprisingly, aerE is conserved in aerobactin producing vibrio strains (Fig. S16A,B and Cordero et al., 2012), because, to profit from siderophore production, a cell must secrete the siderophore so it can perform its extracellular iron-scavenging function. Interestingly, aerE is also conserved in aerobactin cheating vibrio strains. While there are exceptions, most aerobactin-cheating vibrios possess a cluster that contains aerE, fhuCDB, and iutA, all residing in their usual relative positions, however, the biosynthetic iucABCD genes are replaced by iutR, encoding a GntR family transcriptional regulator (Fig. S16C and Cordero et al., 2012). In V. vulnificus M2799, IutR is a repressor of iutA (Tanabe et al., 2005).
Given that aerE sits in the most distal position in the aerobactin gene cluster, it is reasonable to assume that horizontal transfer of a cassette lacking aerE but including the fhu and iutA genes could have occurred. However, that is not what most vibrio aerobactin cheater genomes show. We hypothesize that acquisition of aerE by aerobactin cheater strains provides two possible advantages over acquisition of the minimal fhuCDB and iutA cheater gene set. First, possession of AerE makes an aerobactin cheater immune to cytoplasmic aerobactin toxicity. We say this based on our assessment of the growth defect displayed by the V. fischeri ES114 ΔaerE mutant (Fig. S10A). Second, possession of AerE may enable aerobactin recycling by cheater vibrios, fostering higher overall iron acquisition and, in turn, a superior growth advantage during competitive situations.
We do not yet understand how V. fischeri ES114, as an aerobactin producer, thrives in low-iron environments containing aerobactin cheaters. We note that V. fischeri ES114 possesses homologs of peuA (VF_A0191) and desA (VF_A0784) encoding putative receptors for the siderophores enterobactin and deferoxamine B, respectively (Tanabe et al., 2011; Cordero et al., 2012; Tanabe et al., 2014). It is possible that V. fischeri ES114 switches between siderophore-producing and siderophore-cheating based on iron availability and whether or not other vibrios are present that can supply siderophores (aerobactin, enterobactin, or deferoxamine B). Indeed, mechanisms to down-regulate production of siderophores that act as public goods are predicted to evolve in the presence of competitors, whereas siderophores that are privatized and cannot be exploited by competitors are upregulated (Niehus et al., 2017).
Regarding possession of aerobactin genes, our growth curve analyses show a shorter lag phase for V. fischeri ES114 mutants deficient in siderophore production, secretion, and import, than for WT V. fischeri ES114 likely due to the energetic cost to WT V. fischeri ES114 of producing and using these components (Fig. S10A,B). The fact that V. fischeri maintains these functions, despite the cost, further supports the notion that they play a crucial role in survival. Aerobactin production by V. fischeri ES114 could be especially relevant during colonization of its symbiotic host. Microarray analyses revealed that genes for V. fischeri siderophore production are upregulated ~5-fold in the E. scolopes host where V. fischeri ES114 exists in monoculture (McFall-Ngai and Ruby, 1991; Wier et al., 2010). Presumably V. fischeri ES114 must produce aerobactin in the host as cheating using one of its other siderophore receptors is not a feasible strategy.
Here we also identified four activators of aerobactin production in V. fischeri ES114: Fre, YebK, LuxT, and GlpK (Fig. 4A,B) and we speculate on their potential roles. First, flavin reductase, encoded by fre, reduces flavin using NAD(P)H (Spyrou et al., 1991). Siderophore recycling and iron release often occur via reduction of the bound ferric (Fe3+) iron to the soluble ferrous (Fe2+) form for which siderophores have low affinity. Indeed, reduced flavin can transfer electrons to promote the reduction of ferric (Fe3+) iron when it is bound to a siderophore (Coves and Fontecave, 1993; reviewed by Fontecave et al., 1994; Schröder et al., 2003). Perhaps, in V. fischeri ES114, Fre participates in such a manner to recycle aerobactin, driving an increase in extracellular siderophore production as detected by the CAS assay. Next, YebK, is a transcriptional regulator with no known function. Unexpectedly, while YebK promotes aerobactin production, it is not via transcriptional activation of iucABCD (Fig. 4A,C). YebK is a member of the MurR/RpiR family, and it has homology to HexR in Pseudomonas putida. In P. putida, HexR is a repressor of the hex regulon encoding enzymes required for carbohydrate metabolism by the Entner-Doudoroff pathway. HexR also represses the genes encoding glucokinase and glyceraldehyde-3-phosphate dehydrogenase (Hager et al., 2000; del Castillo et al., 2008). Evidence exists that HexR detects oxidative stress (Kim et al., 2008; Kim and Park, 2014). If YebK functions analogously to HexR, YebK could participate in metabolic pathways to increase the substrates required for aerobactin biosynthesis or to promote its recycling. Additionally, if YebK does indeed detect oxidative stress, it could provide a regulatory mechanism to control iron acquisition during oxidative stress conditions. It could be beneficial to repress iron uptake under oxidative stress because ferrous (Fe2+) iron reacts with hydrogen peroxide in the Fenton reaction to generate harmful hydroxyl radicals that damage DNA (Imlay et al., 1988).
Next is LuxT, a TetR family transcriptional regulator that activates iucABCD transcription. In V. parahaemolyticus, the LuxT homolog SwrT activates lateral flagella (laf) genes required for swarming motility by an indirect mechanism: SwrT represses swrZ, encoding a GntR-type transcriptional regulator that, in turn, represses laf (Jaques and McCarter, 2006). V. fischeri has a swrZ homolog that is a candidate to encode the component that acts between LuxT and iucABCD. Finally, GlpK: glycerol kinase catalyzes the first step in glycerol metabolism (reviewed by Lin, 1976). In our mutant collection, the connection between GlpK and aerobactin production is the most difficult to rationalize. The minimal marine medium used in our experiments has glycerol as the carbon source and amino acids are also present. While highly speculative, we suspect that glpK mutation demands that V. fischeri ES114 use amino acids, rather than glycerol, for growth, altering metabolic pathways that decrease the availability of a substrate required for aerobactin production.
Finally, by identifying aerobactin as a growth inhibitory molecule for V. harveyi and eliminating its production in V. fischeri ES114, we have established a convenient new co-culture model system for studying two well-characterized vibrios. Moreover, both vibrio species produce a variety of public goods including extracellular proteases, chitinases, and QS autoinducers, all of which can be monitored in real time. This co-culture system, in which either or both species can be genetically manipulated, provides a route to the quantitative investigation of both competitive and cooperative interspecies interactions that occur in nature.
Experimental Procedures
Bacterial strains and culture conditions
V. fischeri strains were derived from the E. scolopes light organ isolate V. fischeri ES114 (Boettcher and Ruby, 1990) and the M. japonica isolate MJ11 (Mandel et al., 2009). All V. harveyi strains were derivatives of V. harveyi BB120 (BAA-1116) (Bassler et al., 1997). V. parahaemolyticus strains were derived from V. parahaemolyticus BB22OP (also known as LM5312) (McCarter, 1998). Strains are listed in Table S1. E. coli S17–1 λpir was used for cloning, and E. coli MG1655 was used for heterologous gene expression. Vibrio strains were grown aerobically with shaking at 30°C in either “rich” Luria-Marine (LM) medium or “minimal marine” Autoinducer Bioassay (AB) medium containing 0.4% vitamin-free casamino acids (Difco) (Greenberg et al., 1979; Bassler et al., 1994). E. coli strains were grown aerobically with shaking in LB medium at either 37°C or 30°C. Unless otherwise indicated, erythromycin, chloramphenicol, kanamycin, ampicillin, and polymyxin B were added to final concentrations of 5 μg mL−1, 10 μg mL−1, 100 μg mL−1, 100 μg mL−1, and 50 μg mL−1, respectively. The plasmid pEVS170 was maintained in E. coli by growth in BHI medium (Difco) supplemented with erythromycin at a concentration of 150 μg mL−1. Induction from the Ptac promoter was performed with 0.5 mM Isopropyl β-D-1-thiogalactopyranoside (IPTG), and induction of the PBAD promoter was with 0.2% (v/v) arabinose. Plasmids were transformed into E. coli by electroporation using a Bio-Rad Micro Pulser. Plasmids were introduced into vibrios by conjugation with E. coli S17–1 λpir on LB plates. V. fischeri exconjugants were selected on agar containing ampicillin, and V. harveyi and V. parahaemolyticus exconjugants were selected on agar with polymxyin B. CFU assessment was performed using serial dilutions and plating.
DNA manipulation and mutant construction
PCR reactions relied on either KOD Hot Start DNA Polymerase (Sigma) or iProof DNA Polymerase (Bio-Rad). Oligonucleotides were obtained from Integrated DNA Technologies and are listed in Table S2. All cloning was performed using isothermal DNA assembly (IDA) (Gibson et al., 2009) using Gibson Assembly Master Mix (New England Biolabs). Plasmids used in this study are listed in Table S3. Sequencing of plasmid DNA and PCR products was conducted at Genewiz. Vibrio chromosomal mutations were generated using the suicide vector pRE112 (Edwards et al., 1998) containing the counter-selectable sucrose marker sacB as previously described (Chaparian et al., 2016). Briefly, 1 kb regions of DNA flanking the gene(s) of interest were cloned in tandem into pRE112 and transformed into E. coli S17–1 λpir. Following conjugation with the recipient vibrio strain, colonies that had undergone the single crossover recombination event were selected by growth on agar plates containing chloramphenicol. The second crossover event was selected by plating these strains on LM agar plates containing 15% sucrose. Colonies were screened for chloramphenicol sensitivity, and the chromosomal alterations were confirmed by PCR. The E. coli Δfur allele came from the Keio Collection (Baba et al., 2006) and was transferred into E. coli MG1655 by phage P1vir transduction (Silhavy et al., 1984). Expression of the piutA and pfhuCDBiutA constructs from their native promoters was accomplished by including approximately 300 bp of upstream DNA in the cloned product. In the case of pfhuCDBiutA, the insert was amplified from the V. fischeri ES114 ΔiucABCD strain.
Cell-free culture fluid preparations and assays
Unless otherwise specified, cell-free culture fluids were isolated as follows. Overnight cultures of the donor V. fischeri or other vibrio strains grown in rich medium were pelleted via centrifugation at 21,100 × g (Eppendorf 5424) and resuspended in minimal marine medium. Fresh minimal marine medium (5 mL) was inoculated with the washed cells, normalizing each culture to a starting OD600 = 0.01 as measured by a DU800 spectrophotometer (Beckman Coulter). Following growth for 16 h, the OD600 was measured and the cells were pelleted by centrifugation at 2,808 × g for 15 min at 4°C (Eppendorf 5810 R). The clarified supernatants were collected and filtered through 0.22 μm filter units (MilliporeSigma™ SLGP033RB). To assay for growth inhibition of V. harveyi or other vibrio species, the cell-free culture fluids were aliquotted into clear-bottom 96-well plates (Corning) in quadruplicate technical replicates at 10% (v/v) of the total 200 μL volume. The remainder of the volume was supplied from a 1:1000 dilution of the recipient vibrio species that had been grown overnight in rich medium and washed and resuspended in minimal marine medium. Provision of 10% (v/v) sterile minimal marine medium was used as the no addition control. The plates were covered with gas-permeable sealing membranes (Breathe-Easy) and incubated with shaking at 30°C for 16 h. Growth of the recipient strains was monitored by measurement of OD600 using an Envision 2103 Multilabel Reader (Perkin Elmer). When growth inhibition was assayed over time, 25 mL cultures were used. Samples of culture fluids (1 mL) were collected every 2 h and OD600 measurements were made. To characterize the inhibitor substance, size exclusion examination of the cell-free culture fluids was performed using 10,000 MWCO Amicon Ultra-15 Centrifugal Filter Units (UFC901024).
V. fischeri ES114 transposon mutagenesis screen
A library of V. fischeri ES114 transposon mutants was generated as previously described (Lyell et al., 2008). Briefly, the mini-Tn5 delivery vector pEVS170 was conjugated into V. fischeri ES114 using triparental mating with E. coli CC118λpir containing the helper plasmid pEVS104 (Stabb and Ruby, 2002). Following incubation overnight at 30°C, V. fischeri ES114 colonies harboring transposon insertions were selected by growth at room temperature on LM agar plates containing erythromycin. Isolated mutant colonies were arrayed into 96-well plates containing rich medium with erythromycin and covered with gas permeable sealing membranes. Following overnight growth at 30°C with shaking, 1 μL of each mutant culture was inoculated into a new 96-well plate containing 200 μL of minimal marine medium. The original plates were stored at −80°C for later access to mutants of interest. The newly-inoculated plates were covered with gas permeable sealing membranes and incubated for 16 h at 30°C with shaking. The plates were subjected to centrifugation at 2,808 × g for 15 min (Eppendorf 5810 R). The resulting culture fluids were supplied at 50% (v/v) to the recipient V. harveyi strain by transferring 100 μL to wells of new 96-well plates containing 100 μL of a 1:1000 dilution of a V. harveyi culture that had been grown overnight and washed in minimal marine medium. For simplification purposes, we did not filter the remaining mutant V. fischeri ES114 cells out of the culture fluids, however, polymyxin B was added to the cultures to prevent further growth of V. fischeri ES114 while allowing V. harveyi growth. After incubation for 16 h with shaking at 30°C, V. harveyi bioluminescence was assessed for each well as a proxy for growth using an Envision 2103 Multilabel Reader. Values were normalized by dividing each well by the average bioluminescence of the entire plate. V. fischeri ES114 makes almost no bioluminescence under laboratory conditions so the presence of any residual cells did not contribute to the bioluminescence reading. The insertion sites of the transposons of interest were identified using arbitrarily-primed PCR and sequencing as previously described (O’Toole et al., 1999; Brennan et al., 2013).
Chrome azurol S siderophore detection assay
The CAS liquid assay (Schwyn and Neilands, 1987) was used to measure siderophore activity in cell-free culture fluids from different vibrio strains. The CAS assay solution was prepared as previously described (Payne, 1994). 0.5 mL of CAS assay solution was added to 0.5 mL of cell-free culture fluids prepared from vibrio strains grown in minimal marine medium. After mixing, 10 μL of shuttle solution (0.2 M 5-sulfosalicylic acid) was added and the samples were mixed. Samples were incubated at room temperature for 30 min, after which A630 measurements were taken using a DU800 spectrophotometer. Minimal marine medium was used as the blank. Siderophore units were calculated as [A630(medium control) – A630(culture fluid)]/A630(medium control) × 100 (Payne, 1994). Values were divided by the OD600 of the cultures from which the culture fluids were isolated to calculate siderophore units relative to cell density. To measure intracellular siderophore levels, 50 mL cultures were grown in minimal marine medium as described above. After 8 h, the cells were pelleted by centrifugation at 2,808 × g for 15 min at 4°C, concentrating and normalizing each to OD600 = 40 in 600 μL. After 3 washes with 600 μL minimal marine medium, the cells were lysed by boiling for 15 min. Cell debris was removed from the lysates by centrifugation for 2 min at 21,100 × g. 500 μL of lysate was used in the CAS assay. Because the V. fischeri aerobactin structure is unknown, we do not possess the pure compound to generate a standard curve. Thus, concentrations of aerobactin cannot be quantified in our CAS assays, so we restrict our analyses to comparisons of relative levels within each experiment.
Quantitative real-time PCR analysis
Cultures of V. fischeri grown overnight in rich medium were washed in minimal marine medium as above and diluted to an OD600 = 0.005 in 25 mL of fresh minimal marine or rich medium. RNA was harvested every 2 h over growth from three independent cultures using the RNeasy mini kit (Qiagen #4104) and OD600 was measured at each timepoint. The RNA samples were treated in two sequential DNase reactions with the TURBO DNA-free Kit (ThermoFisher, AM1907). cDNA was generated as described (Tu and Bassler, 2007) using SuperScript III Reverse Transcriptase (ThermoFisher, 18080085) with 1 μg of harvested RNA per sample. Real-time PCR was performed in 384-well reaction plates using a QuantStudio 6 Flex Real-Time PCR detection system (ThermoFisher) and PerfeCTa SYBR Green FastMix (Quanta, 95074) as previously described (Tu and Bassler, 2007). Reactions (10 μL) were analyzed in quadruplicate technical replicates. Data were analyzed by a comparative ΔΔCT method in which the relative amount of the indicated transcript was normalized to the internal hfq control gene. qRT-PCR primers are listed in Table S2.
Fluorescent reporter and growth curve assays
To measure iucABCD expression, transcriptional and translational reporter fusions to the fluorescent protein mVenus were constructed. For the transcriptional reporter, a region of approximately 300 bp upstream of iucA encompassing the promoter was cloned upstream of mVenus translated from a consensus ribosome-binding site. The translational reporter included the same 300 bp promoter fragment, but mVenus was cloned in frame following the DNA encoding the first thirteen amino acids of IucA. Primers used for reporter construction are listed in Table S2. Reporter activity was measured using a BioTek Synergy Neo2 Multi-Mode reader (BioTek, Winooski, VT, USA). Specifically, overnight cultures of V. fischeri ES114 grown in rich medium were washed as above and inoculated into minimal marine medium containing chloramphenicol, normalizing each culture to a starting OD600 of 0.005. 150 μL were transferred to a clear-bottom 96-well plate in quadruplicate technical replicates. 50 μL of mineral oil (Sigma) was added to prevent evaporation. In the plate reader, the cultures were grown with constant shaking at 30°C. Both OD600 and fluorescence (excitation 515 nm/emission 528 nm) were measured every 15 min for 24 h. For normalization purposes, relative fluorescence values (mVenus/OD600) are reported for each strain at OD600 = 0.4. Growth curve assays were similarly conducted: vibrio cultures were grown in clear-bottom 96-well plates (Corning) as above in the indicated growth medium. The plates were incubated with constant shaking at 30°C, and OD600 was measured every 15 min for 24 h. To measure piucA’-mVenus transcriptional reporter activity in E. coli, recombinant strains were sub-cultured 1:1000 in 1 mL of LB, and grown 6 h with shaking at 30° C. The cells were pelleted by centrifugation and resuspended in sterile PBS prior to fluorescence and OD600 measurements using a BioTek Synergy Neo2 Multi-Mode reader. RNA was collected at the 6 h timepoint for qRT-PCR analysis as described above. Lysis was assayed using Sytox Green (ThermoFisher) in which 1 μM dye was added to cultures grown for 16 h in minimal marine medium. Fluorescence (excitation 504 nm/emission 523 nm) and OD600 were measured using a BioTek Synergy Neo2 Multi-Mode reader.
Competition experiments
An iron-depleted growth condition was established for competition experiments by treating 10X minimal marine medium (lacking MgSO4) three times with Chelex 100 resin in batch (Bio-Rad). MgSO4 was left out of the 10X minimal marine medium because Chelex 100 can bind Mg2+ ions. 5 g of resin were added to 100 mL of the 10X solution and incubated with stirring for 1 h. The solution was decanted from the resin, and the procedure was repeated two more times. Iron depletion was monitored using a ferene colorimetric dye assay as previously described (Folsom et al., 2014). Briefly, 100 μL of iron-detection reagent was added to 1 mL of medium, and the mixture was incubated for 30 min at room temperature. Purified Milli-Q water (Sigma) was treated identically and used as the blank. Iron concentrations were determined by measuring absorbance at 593 nm (A593) using a DU800 spectrophotometer followed by comparison to a standard curve generated using an iron standard (Sigma). After three Chelex 100 treatments, iron levels were below the level of detection of the ferene assay, approximately 0.02 μM. To make 100 mL of iron-depleted medium, 10 mL of Chelex 100-treated 10X minimal marine medium was combined with 1.23 g MgSO4 and purified Milli-Q water was added to a volume of 100 mL. A trace metals solution lacking iron (Cold Spring Harbor Protocols) was added at 1X and the medium was filtered through a 0.22 μm filter. 10 μM ferric (Fe3+, siderophore required) citrate was added to provide a source of iron that is accessible to siderophore. Following overnight growth in rich medium, 500 μL of five V. fischeri and five V. harveyi cultures were pelleted by centrifugation (21,100 × g for 2 min), washed twice with iron-depleted medium, and resuspended in iron-depleted medium. The V. fischeri and V. harveyi suspensions were combined at a 1:1 ratio in 1 mL of the indicated growth medium to a total OD600 of 0.005 (0.0025 OD600 V. fischeri and 0.0025 OD600 V. harveyi). A portion of this starting culture was collected for serial dilution and plating on agar plates to assess CFUs. Next, 150 μL of each co-culture were transferred to a 96-well plate. 50 μL of mineral oil was added to prevent evaporation. The cultures were grown with shaking for 24 h at 30°C. Serial dilutions and plating on agar plates were performed to measure the final cell numbers of both species. V. fischeri and V. harveyi colonies were distinguished by differences in colony morphology and bioluminescence emission. Selection rate constants, r, for V. fischeri were calculated as a measure of relative performance by subtracting the Malthusian parameter of V. harveyi (mVh) from the Malthusian parameter of V. fischeri (mVf). The Malthusian parameter is calculated as mi = ln(x1/x0) for competitor i, where x0 and x1 are the cell densities (CFU mL−1) at the start (x0) and the end (x1) of the 24 h growth period. The selection rate constant of V. fischeri is calculated as r = mVf −mVh.
Bioinformatic analyses
The V. fischeri ES114 genome was scanned for siderophore biosynthetic genes using AntiSMASH with relaxed strictness (Medema et al., 2011). Bioinformatic searches for homologs of genes in the V. fischeri ES114 aerobactin gene cluster were performed using exhaustive BLASTx searches with an expect threshold of 10. The amino acid sequences of the identified homologs were compared in Geneious by pairwise ClustalW alignment. Similarities between homologs are reported as either % pairwise identity in amino acid sequence or % pairwise positive (BLSM62).
Statistical methods
All statistical analyses were performed using GraphPad Prism software in which unpaired two-tailed t tests were performed comparing the means of two groups, as indicated in the figure legends. Error bars correspond to standard deviations of the means.
Supplementary Material
Acknowledgements
We thank members of the Bassler laboratory, Dr. Ned Wingreen, and Dr. Mohamed Donia for insightful discussions and suggestions. This work was supported by the Howard Hughes Medical Institute, National Institutes of Health (NIH) Grant 5R37GM065859, and National Science Foundation Grant MCB-1713731 (to B.L.B). M.E. was supported by NIH graduate training grant NIGMS T32GM007388.
Footnotes
Conflict of interest
The authors declare that they have no conflict of interest.
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- Abrudan MI, Smakman F, Grimbergen AJ, Westhoff S, Miller EL, van Wezel GP, and Rozen DE (2015) Socially mediated induction and suppression of antibiosis during bacterial coexistence. Proc Natl Acad Sci USA 112: 11054–11059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcaide E (2003) Numerical taxonomy of Vibrionaceae isolated from cultured amberjack (Seriola dumerili) and surrounding water. Curr Microbiol 46: 184–189. [DOI] [PubMed] [Google Scholar]
- Baba T, Ara T, Hasegawa M, Takai Y, Okumura Y, Baba M, et al. (2006) Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Molecular Systems Biology 2: 2006.0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagg A, and Neilands JB (1987) Ferric uptake regulation protein acts as a repressor, employing iron (II) as a cofactor to bind the operator of an iron transport operon in Escherichia coli. Biochemistry 26: 5471–5477. [DOI] [PubMed] [Google Scholar]
- Bailey DC, Alexander E, Rice MR, Drake EJ, Mydy LS, Aldrich CC, and Gulick AM (2018) Structural and functional delineation of aerobactin biosynthesis in hypervirulent Klebsiella pneumoniae. J Biol Chem 293: 7841–7852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassler BL, Greenberg EP, and Stevens AM (1997) Cross-species induction of luminescence in the quorum-sensing bacterium Vibrio harveyi. J Bacteriol 179: 4043–4045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassler BL, Wright M, and Silverman MR (1994) Multiple signalling systems controlling expression of luminescence in Vibrio harveyi: sequence and function of genes encoding a second sensory pathway. Molecular Microbiology 13: 273–286. [DOI] [PubMed] [Google Scholar]
- Boettcher KJ, and Ruby EG (1990) Depressed light emission by symbiotic Vibrio fischeri of the sepiolid squid Euprymna scolopes. J Bacteriol 172: 3701–3706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan CA, Mandel MJ, Gyllborg MC, Thomasgard KA, and Ruby EG (2013) Genetic determinants of swimming motility in the squid light-organ symbiont Vibrio fischeri. MicrobiologyOpen 2: 576–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bristow LA, Mohr W, Ahmerkamp S, and Kuypers MMM (2017) Nutrients that limit growth in the ocean. Curr Biol 27: R474–R478. [DOI] [PubMed] [Google Scholar]
- Bruce JB, Cooper GA, Chabas H, West SA, and Griffin AS (2017) Cheating and resistance to cheating in natural populations of the bacterium Pseudomonas fluorescens. Evolution 71: 2484–2495. [DOI] [PubMed] [Google Scholar]
- Bruger EL, and Waters CM (2016) Bacterial Quorum Sensing Stabilizes Cooperation by Optimizing Growth Strategies. Appl Environ Microbiol 82: 6498–6506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butaitė E, Baumgartner M, Wyder S, and Kümmerli R (2017) Siderophore cheating and cheating resistance shape competition for iron in soil and freshwater Pseudomonas communities. Nat Commun 8: 414–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaparian RR, Olney SG, Hustmyer CM, Rowe-Magnus DA, and van Kessel JC (2016) Integration host factor and LuxR synergistically bind DNA to coactivate quorum-sensing genes in Vibrio harveyi. Molecular Microbiology 101: 823–840. [DOI] [PubMed] [Google Scholar]
- Cordero OX, Ventouras LA, DeLong EF, and Polz MF (2012) Public good dynamics drive evolution of iron acquisition strategies in natural bacterioplankton populations. Proc Natl Acad Sci USA 109: 20059–20064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coves J, and Fontecave M (1993) Reduction and mobilization of iron by a NAD(P)H:flavin oxidoreductase from Escherichia coli. Eur J Biochem 211: 635–641. [DOI] [PubMed] [Google Scholar]
- Crosa JH, and Walsh CT (2002) Genetics and Assembly Line Enzymology of Siderophore Biosynthesis in Bacteria. Microbiol Mol Biol Rev 66: 223–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosa JH, Mey AR, and Payne SM (2004) Iron Transport in Bacteria. ASM Press. [Google Scholar]
- de Lorenzo V, and Neilands JB (1986) Characterization of iucA and iucC genes of the aerobactin system of plasmid ColV-K30 in Escherichia coli. J Bacteriol 167: 350–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lorenzo V, Bindereif A, Paw BH, and Neilands JB (1986) Aerobactin biosynthesis and transport genes of plasmid ColV-K30 in Escherichia coli K-12. J Bacteriol 165: 570–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lorenzo V, Wee S, Herrero M, and Neilands JB (1987) Operator sequences of the aerobactin operon of plasmid ColV-K30 binding the ferric uptake regulation (fur) repressor. J Bacteriol 169: 2624–2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Castillo T, Duque E, and Ramos JL (2008) A set of activators and repressors control peripheral glucose pathways in Pseudomonas putida to yield a common central intermediate. J Bacteriol 190: 2331–2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards RA, Keller LH, and Schifferli DM (1998) Improved allelic exchange vectors and their use to analyze 987P fimbria gene expression. Gene 207: 149–157.9511756 [Google Scholar]
- Engebrecht J, and Silverman M (1984) Identification of genes and gene products necessary for bacterial bioluminescence. Proc Natl Acad Sci USA 81: 4154–4158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fidopiastis PM, Miyamoto CM, Jobling MG, Meighen EA, and Ruby EG (2002) LitR, a new transcriptional activator in Vibrio fischeri, regulates luminescence and symbiotic light organ colonization. Molecular Microbiology 45: 131–143. [DOI] [PubMed] [Google Scholar]
- Folsom JP, Parker AE, and Carlson RP (2014) Physiological and proteomic analysis of Escherichia coli iron-limited chemostat growth. J Bacteriol 196: 2748–2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontecave M, Coves J, and Pierre JL (1994) Ferric reductases or flavin reductases? Biometals 7: 3–8. [DOI] [PubMed] [Google Scholar]
- Forman S, Nagiec MJ, Abney J, Perry RD, and Fetherston JD (2007) Analysis of the aerobactin and ferric hydroxamate uptake systems of Yersinia pestis. Microbiology (Reading, Engl) 153: 2332–2341. [DOI] [PubMed] [Google Scholar]
- Foster KR, and Bell T (2012) Competition, not cooperation, dominates interactions among culturable microbial species. Curr Biol 22: 1845–1850. [DOI] [PubMed] [Google Scholar]
- Freeman JA, and Bassler BL (1999) A genetic analysis of the function of LuxO, a two-component response regulator involved in quorum sensing in Vibrio harveyi. Molecular Microbiology 31: 665–677. [DOI] [PubMed] [Google Scholar]
- Funahashi T, Tanabe T, Aso H, Nakao H, Fujii Y, Okamoto K, et al. (2003) An iron-regulated gene required for utilization of aerobactin as an exogenous siderophore in Vibrio parahaemolyticus. Microbiology (Reading, Engl) 149: 1217–1225. [DOI] [PubMed] [Google Scholar]
- Funahashi T, Tanabe T, Shiuchi K, Nakao H, and Yamamoto S (2009) Identification and characterization of genes required for utilization of desferri-ferrichrome and aerobactin in Vibrio parahaemolyticus. Biol Pharm Bull 32: 359–365. [DOI] [PubMed] [Google Scholar]
- Genuini M, Bidet P, Benoist J-F, Schlemmer D, Lemaitre C, Birgy A, and Bonacorsi S (2019) ShiF acts as an auxiliary factor of aerobactin secretion in meningitis Escherichia coli strain S88. BMC Microbiol 19: 298–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghoul M, and Mitri S (2016) The Ecology and Evolution of Microbial Competition. Trends in Microbiology 24: 833–845. [DOI] [PubMed] [Google Scholar]
- Gibson DG, Young L, Chuang RY, Venter JC, Hutchison CA, and Smith HO (2009) Enzymatic assembly of DNA molecules up to several hundred kilobases. Nat Methods 6: 343–345. [DOI] [PubMed] [Google Scholar]
- Gibson F, and Magrath DI (1969) The isolation and characterization of a hydroxamic acid (aerobactin) formed by Aerobacter aerogenes 62-I. Biochim Biophys Acta 192: 175–184. [DOI] [PubMed] [Google Scholar]
- Gilson L, Kuo A, and Dunlap PV (1995) AinS and a new family of autoinducer synthesis proteins. J Bacteriol 177: 6946–6951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf J, and Ruby EG (2000) Novel effects of a transposon insertion in the Vibrio fischeri glnD gene: defects in iron uptake and symbiotic persistence in addition to nitrogen utilization. Molecular Microbiology 37: 168–179. [DOI] [PubMed] [Google Scholar]
- Greenberg EP, Hastings JW, and Ulitzur S (1979) Induction of luciferase synthesis in Beneckea harveyi by other marine bacteria. Arch Microbiol 120: 87–91. [Google Scholar]
- Hager PW, Calfee MW, and Phibbs PV (2000) The Pseudomonas aeruginosa devB/SOL homolog, pgl, is a member of the hex regulon and encodes 6-phosphogluconolactonase. J Bacteriol 182: 3934–3941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardin G (1960) The competitive exclusion principle. Science 131: 1292–1297. [DOI] [PubMed] [Google Scholar]
- Hem JD (1972) Chemical Factors that Influence the Availability of Iron and Manganese in Aqueous Systems. Geol Soc America Bull 83: 443–450. [Google Scholar]
- Hibbing ME, Fuqua C, Parsek MR, and Peterson SB (2010) Bacterial competition: surviving and thriving in the microbial jungle. Nat Rev Microbiol 8: 15–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imlay JA, Chin SM, and Linn S (1988) Toxic DNA damage by hydrogen peroxide through the Fenton reaction in vivo and in vitro. Science 240: 640–642. [DOI] [PubMed] [Google Scholar]
- Jaques S, and McCarter LL (2006) Three new regulators of swarming in Vibrio parahaemolyticus. J Bacteriol 188: 2625–2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi F, Archana G, and Desai A (2006) Siderophore cross-utilization amongst rhizospheric bacteria and the role of their differential affinities for Fe3+ on growth stimulation under iron-limited conditions. Curr Microbiol 53: 141–147. [DOI] [PubMed] [Google Scholar]
- Khare A, and Tavazoie S (2015) Multifactorial Competition and Resistance in a Two-Species Bacterial System. PLoS Genet 11: e1005715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Jisun, and Park W. (2014) Oxidative stress response in Pseudomonas putida. Appl Microbiol Biotechnol 98: 6933–6946. [DOI] [PubMed] [Google Scholar]
- Kim Juhyun, Jeon CO, and Park W. (2008) Dual regulation of zwf-1 by both 2-keto-3-deoxy-6-phosphogluconate and oxidative stress in Pseudomonas putida. Microbiology (Reading, Engl) 154: 3905–3916. [DOI] [PubMed] [Google Scholar]
- Kimbrough JH, and Stabb EV (2013) Substrate specificity and function of the pheromone receptor AinR in Vibrio fischeri ES114. J Bacteriol 195: 5223–5232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leinweber A, Fredrik Inglis R, and Kümmerli R (2017) Cheating fosters species coexistence in well-mixed bacterial communities. ISME J 11: 1179–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenski RE (2019) The E. coli long-term experimental evolution project site. http://myxo.css.msu.edu/ecoli.
- Lilley BN, and Bassler BL (2000) Regulation of quorum sensing in Vibrio harveyi by LuxO and Sigma-54. Molecular Microbiology 36: 940–954. [DOI] [PubMed] [Google Scholar]
- Lin EC (1976) Glycerol dissimilation and its regulation in bacteria. Annu Rev Microbiol 30: 535–578. [DOI] [PubMed] [Google Scholar]
- Lin YH, Miyamoto C, and Meighen EA (2000a) Purification and characterization of a luxO promoter binding protein LuxT from Vibrio harveyi. Protein Expr Purif 20: 87–94. [DOI] [PubMed] [Google Scholar]
- Lin YH, Miyamoto C, and Meighen EA (2000b) Cloning and functional studies of a luxO regulator LuxT from Vibrio harveyi. Biochim Biophys Acta 1494: 226–235. [DOI] [PubMed] [Google Scholar]
- Logan SL, Thomas J, Yan J, Baker RP, Shields DS, Xavier JB, et al. (2018) The Vibrio cholerae type VI secretion system can modulate host intestinal mechanics to displace gut bacterial symbionts. Proc Natl Acad Sci USA 115: E3779–E3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyell NL, Dunn AK, Bose JL, Vescovi SL, and Stabb EV (2008) Effective mutagenesis of Vibrio fischeri by using hyperactive mini-Tn5 derivatives. Appl Environ Microbiol 74: 7059–7063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandel MJ, Wollenberg MS, Stabb EV, Visick KL, and Ruby EG (2009) A single regulatory gene is sufficient to alter bacterial host range. Nature 458: 215–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarter LL (1998) OpaR, a homolog of Vibrio harveyi LuxR, controls opacity of Vibrio parahaemolyticus. J Bacteriol 180: 3166–3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFall-Ngai MJ, and Ruby EG (1991) Symbiont recognition and subsequent morphogenesis as early events in an animal-bacterial mutualism. Science 254: 1491–1494. [DOI] [PubMed] [Google Scholar]
- Medema MH, Blin K, Cimermancic P, de Jager V, Zakrzewski P, Fischbach MA, et al. (2011) antiSMASH: rapid identification, annotation and analysis of secondary metabolite biosynthesis gene clusters in bacterial and fungal genome sequences. Nucleic Acids Res 39: W339–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon YH, Tanabe T, Funahashi T, Shiuchi KI, Nakao H, and Yamamoto S (2004) Identification and Characterization of Two Contiguous Operons Required for Aerobactin Transport and Biosynthesis in Vibrio mimicus. Microbiol Immunol 48: 389–398. [DOI] [PubMed] [Google Scholar]
- Nadell CD, and Bassler BL (2011) A fitness trade-off between local competition and dispersal in Vibrio cholerae biofilms. Proc Natl Acad Sci USA 108: 14181–14185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naka H, Actis LA, and Crosa JH (2013) The anguibactin biosynthesis and transport genes are encoded in the chromosome of Vibrio harveyi: a possible evolutionary origin for the pJM1 plasmid–encoded system of Vibrio anguillarum? MicrobiologyOpen 2: 182–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neilands JB (1995) Siderophores: structure and function of microbial iron transport compounds. J Biol Chem 270: 26723–26726. [DOI] [PubMed] [Google Scholar]
- Niehus R, Picot A, Oliveira NM, Mitri S, and Foster KR (2017) The evolution of siderophore production as a competitive trait. Evolution 71: 1443–1455. [DOI] [PubMed] [Google Scholar]
- O’Toole GA, Pratt LA, Watnick PI, Newman DK, Weaver VB, and Kolter R (1999) Genetic approaches to study of biofilms. Methods Enzymol 310: 91–109. [DOI] [PubMed] [Google Scholar]
- Okujo N, and Yamamoto S (1994) Identification of the siderophores from Vibrio hollisae and Vibrio mimicus as aerobactin. FEMS Microbiol Lett 118: 187–192. [DOI] [PubMed] [Google Scholar]
- Pankey MS, Foxall RL, Ster IM, Perry LA, Schuster BM, Donner RA, et al. (2017) Host-selected mutations converging on a global regulator drive an adaptive leap towards symbiosis in bacteria. Elife 6: 2937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne SM (1994) Detection, isolation, and characterization of siderophores. Methods Enzymol 235: 329–344. [DOI] [PubMed] [Google Scholar]
- Payne SM, Mey AR, and Wyckoff EE (2016) Vibrio Iron Transport: Evolutionary Adaptation to Life in Multiple Environments. Microbiol Mol Biol Rev 80: 69–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer T, Schuster S, and Bonhoeffer S (2001) Cooperation and competition in the evolution of ATP-producing pathways. Science 292: 504–507. [DOI] [PubMed] [Google Scholar]
- Ramesh A, Loganathan BG, and Venugopalan VK (1989) Seasonal distribution of luminous bacteria in the sediments of a tropical estuary. J Gen Appl Microbiol 35: 363–368. [Google Scholar]
- Ramos JL, Martínez-Bueno M, Molina-Henares AJ, Terán W, Watanabe K, Zhang X, et al. (2005) The TetR Family of Transcriptional Repressors. Microbiol Mol Biol Rev 69: 326–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rijkenberg MJA, Middag R, Laan P, Gerringa LJA, van Aken HM, Schoemann V, et al. (2014) The distribution of dissolved iron in the West Atlantic Ocean. PLoS ONE 9: e101323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruby EG, and Lee KH (1998) The Vibrio fischeri-Euprymna scolopes Light Organ Association: Current Ecological Paradigms. Appl Environ Microbiol 64: 805–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha R, Saha N, Donofrio RS, and Bestervelt LL (2013) Microbial siderophores: a mini review. J Basic Microbiol 53: 303–317. [DOI] [PubMed] [Google Scholar]
- Schiessl KT, Janssen EM-L, Kraemer SM, McNeill K, and Ackermann M (2017) Magnitude and Mechanism of Siderophore-Mediated Competition at Low Iron Solubility in the Pseudomonas aeruginosa Pyochelin System. Front Microbiol 8: 1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schröder I, Johnson E, and de Vries S (2003) Microbial ferric iron reductases. FEMS Microbiol Rev 27: 427–447. [DOI] [PubMed] [Google Scholar]
- Schwyn B, and Neilands JB (1987) Universal chemical assay for the detection and determination of siderophores. Anal Biochem 160: 47–56. [DOI] [PubMed] [Google Scholar]
- Sexton DJ, and Schuster M (2017) Nutrient limitation determines the fitness of cheaters in bacterial siderophore cooperation. Nat Commun 8: 230–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silhavy TJ, Berman ML, and Enquist LW (1984) Experiments with Gene Fusions. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- Sogin ML, Morrison HG, Huber JA, Mark Welch D, Huse SM, Neal PR, et al. (2006) Microbial diversity in the deep sea and the underexplored “rare biosphere”. Proc Natl Acad Sci USA 103: 12115–12120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spyrou G, Haggård-Ljungquist E, Krook M, Jörnvall H, Nilsson E, and Reichard P (1991) Characterization of the flavin reductase gene (fre) of Escherichia coli and construction of a plasmid for overproduction of the enzyme. J Bacteriol 173: 3673–3679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stabb EV, and Ruby EG (2002) RP4-based plasmids for conjugation between Escherichia coli and members of the Vibrionaceae. Methods Enzymol 358: 413–426. [DOI] [PubMed] [Google Scholar]
- Stocker R, and Seymour JR (2012) Ecology and physics of bacterial chemotaxis in the ocean. Microbiol Mol Biol Rev 76: 792–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stubbendieck RM, and Straight PD (2016) Multifaceted Interfaces of Bacterial Competition. J Bacteriol 198: 2145–2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunagawa S, Coelho LP, Chaffron S, Kultima JR, Labadie K, Salazar G, et al. (2015) Ocean plankton. Structure and function of the global ocean microbiome. Science 348: 1261359–1261359. [DOI] [PubMed] [Google Scholar]
- Suzuki K, Tanabe T, Moon YH, Funahashi T, Nakao H, Narimatsu S, and Yamamoto S (2006) Identification and transcriptional organization of aerobactin transport and biosynthesis cluster genes of Vibrio hollisae. Res Microbiol 157: 730–740. [DOI] [PubMed] [Google Scholar]
- Tanabe T, Funahashi T, Miyamoto K, Tsujibo H, and Yamamoto S (2011) Identification of genes, desR and desA, required for utilization of desferrioxamine B as a xenosiderophore in Vibrio furnissii. Biol Pharm Bull 34: 570–574. [DOI] [PubMed] [Google Scholar]
- Tanabe T, Kato A, Shiuchi K, Miyamoto K, Tsujibo H, Maki J, et al. (2014) Regulation of the expression of the Vibrio parahaemolyticus peuA gene encoding an alternative ferric enterobactin receptor. PLoS ONE 9: e105749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanabe T, Naka A, Aso H, Nakao H, Narimatsu S, Inoue Y, et al. (2005) A novel aerobactin utilization cluster in Vibrio vulnificus with a gene involved in the transcription regulation of the iutA homologue. Microbiol Immunol 49: 823–834. [DOI] [PubMed] [Google Scholar]
- Thode SK, Rojek E, Kozlowski M, Ahmad R, and Haugen P (2018) Distribution of siderophore gene systems on a Vibrionaceae phylogeny: Database searches, phylogenetic analyses and evolutionary perspectives. PLoS ONE 13: e0191860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson FL, Iida T, and Swings J (2004) Biodiversity of Vibrios. Microbiol Mol Biol Rev 68: 403–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travisano M, and Lenski RE (1996) Long-term experimental evolution in Escherichia coli. IV. Targets of selection and the specificity of adaptation. Genetics 143: 15–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traxler MF, Seyedsayamdost MR, Clardy J, and Kolter R (2012) Interspecies modulation of bacterial development through iron competition and siderophore piracy. Molecular Microbiology 86: 628–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu KC, and Bassler BL (2007) Multiple small RNAs act additively to integrate sensory information and control quorum sensing in Vibrio harveyi. Genes Dev 21: 221–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldkamp H, and Jannasch HW (1972) Mixed culture studies with the chemostat. J Appl Chem 22: 105–123. [Google Scholar]
- Weaver VB, and Kolter R (2004) Burkholderia spp. alter Pseudomonas aeruginosa physiology through iron sequestration. J Bacteriol 186: 2376–2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westrich JR, Ebling AM, Landing WM, Joyner JL, Kemp KM, Griffin DW, and Lipp EK (2016) Saharan dust nutrients promote Vibrio bloom formation in marine surface waters. Proc Natl Acad Sci USA 113: 5964–5969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitman WB, Coleman DC, and Wiebe WJ (1998) Prokaryotes: the unseen majority. Proc Natl Acad Sci USA 95: 6578–6583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wier AM, Nyholm SV, Mandel MJ, Massengo-Tiassé RP, Schaefer AL, Koroleva I, et al. (2010) Transcriptional patterns in both host and bacterium underlie a daily rhythm of anatomical and metabolic change in a beneficial symbiosis. Proc Natl Acad Sci USA 107: 2259–2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wooldridge KG, Morrissey JA, and Williams PH (1992) Transport of ferric-aerobactin into the periplasm and cytoplasm of Escherichia coli K12: role of envelope-associated proteins and effect of endogenous siderophores. J Gen Microbiol 138: 597–603. [DOI] [PubMed] [Google Scholar]
- Zane HK, Naka H, Rosconi F, Sandy M, Haygood MG, and Butler A (2014) Biosynthesis of amphi-enterobactin siderophores by Vibrio harveyi BAA-1116: identification of a bifunctional nonribosomal peptide synthetase condensation domain. J Am Chem Soc 136: 5615–5618. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


