Abstract
Background:
Castleman Disease (CD) is a rare polyclonal lymphoproliferative disorder of unclear etiology. Standard therapy for unicentric CD is surgical resection. Radiotherapy can be used, however its efficacy is poorly characterized.
Patients and Methods:
We reviewed patients with histologically confirmed CD undergoing definitive local therapy at our institution between 1990 and 2017. Overall survival (OS) was determined from the date of diagnosis. Local progression free survival (LPFS) and distant failure free survival (DFFS) were determined from the date of first definitive therapy. The Kaplan-Meier method was used to analyze survival.
Results:
44 patients (29 female and 15 male) were identified with a median age at diagnosis of 40 years (range 14–70 years). Thirty-five (80%) patients received surgery alone, 3 (7%) had surgery followed by radiotherapy, and 6 (14%) had radiotherapy alone. Thirty-nine patients (89%) had a single area of involvement, and 3 patients (7%) had limited regional involvement. Two patients (5%) had multicentric CD and received consolidative radiotherapy. The 3-year OS, LPFS, and DFFS were 92%, 100%, and 100%, respectively. No distant failures were observed. The median radiation dose was 3960 cGy (range 3600–5940 cGy) in 22 fractions (range 18–33).
Conclusions:
Unicentric CD is readily amenable to cure with local therapy. Surgical excision is preferred, but radiation appears to be an effective alternative for patients when surgery is high risk or not feasible. Patients with oligo- or multi- centric CD may experience prolonged disease free survival with consolidative radiotherapy after partial response to systemic therapy.
Keywords: Lymphoproliferative disorder, giant lymph node hyperplasia, angiofollicular lymph node hyperplasia, rare diseases
Microabstract
Optimal management of Castleman Disease is poorly understood. We reviewed the outcomes of local therapy in managing Castleman Disease at our institution. Surgery and radiotherapy were both effective for local control and are curative for unicentric disease. Consolidative local therapy after systemic therapy may offer a new management option for patients with multicentric CD.
Introduction
Castleman Disease (CD) is a poorly understood clinical entity of lymph node hyperplasia with characteristic histologic patterns. It is generally regarded as a benign condition, although some descriptions regard it as partially neoplastic1. The spectrum of disease extends from unicentric CD, involving a single lymph node group, to multicentric CD, with widespread disease. Multicentric CD is either idiopathic, associated with polyneuropathy organomegaly endocrinopathy M-protein and skin changes (POEMS) syndrome2, or mediated by human herpesvirus-8 (HHV-8) infection (often co-occurring with Human Immunodeficiency Virus (HIV)). Multicentric CD is typically a severely symptomatic process often with life threatening complications3,4. Oligocentric CD is a more recently categorized clinical entity of limited regional or contiguous lymph node group involvement, which may be asymptomatic or present with mild symptoms compared to multicentric CD5. Unicentric CD tends to be asymptomatic although mild symptoms are reported in some patients. Neither unicentric CD nor oligocentric CD is associated with any known virus6,7.
HHV-8 associated multicentric CD is initially treated with rituximab on account of CD20 expression by the HHV-8 infected plasmablasts which are responsible for the secretion of viral IL-6 leading to the symptom cascade8. Somewhat analogous in HHV-8-negative multicentric CD, host cellular IL-6 rather than viral can be blocked for therapeutic advantage leading to the approval of an anti-IL6 antibody, siltuximab9.
The optimal management strategies in unicentric CD are primarily derived from reports of single cases and small case series due to the rarity of this condition. Surgical excision has been considered the gold standard of management for unicentric CD6, typically with radiotherapy reserved for unresectable cases10–12. Additionally, the role of local therapy such as surgery or radiotherapy as consolidation after systemic therapy for oligocentric or multicentric CD has not been described. In this report, we explore our experience with local therapy in the management of 44 histologically confirmed CD patients.
Materials and Methods
We queried our institutional records for patients with a diagnosis of CD between 1990 and 2017. Patients were excluded if they did not have confirmation of diagnosis by in house pathology review. Patients were considered to have unicentric CD if only one lymph node group was involved. Patients were considered oligocentric if two lymph node groups on the same side of the diaphragm were involved. Definitive local therapy was defined as either definitive excisional surgery or radiotherapy to all known sites of active disease, including those with post chemotherapy adjuvant radiation. Patients who did not undergo local therapy by this definition were excluded. Overall survival (OS) was determined from the date of diagnosis. Local progression free survival (LPFS) and distant failure free survival (DFFS) were determined from the date of first definitive therapy. The Kaplan-Meier method was used to analyze survival.
Results
Patient and treatment characteristics
Patient clinical and treatment characteristics are presented in Table 1. The majority of patients (76%) were female, and the mediastinum was the most common overall location of disease. Of our 44 patients, 38 underwent surgery, 3 of whom also had adjuvant radiotherapy for disease in the neck, and after recurrence in the axilla and pelvis. Six patients had radiotherapy alone: 5 for disease in the mediastinum and one for disease in the neck. The median radiation dose was 3960 cGy (range 3600–5940) in a median of 22 fractions (range 18–33).
Table 1:
Patient Clinical Characteristics
| Surgery (n=38) | Radiotherapy alone (n=6) | ||
|---|---|---|---|
| Median age (range) | 40 years(15–70) | 42 years(24–61) | |
| Number Female (%) | 29 (76.3%) | 6 (100%) | |
| Histologic Subtype | Hyaline Vascular | 34 (89.5%) | 2 (33.3%) |
| Plasmacytic | 3 (7.9%) | 1(16.7%) | |
| Not otherwise specified | 1 (2.6%) | 3 (50.0%) | |
| Location of Disease | Mediastinum | 10 (26.3%) | 5 (83.3%) |
| Retroperitoneum | 8 (21.1%) | - | |
| Axilla | 6 (15.8%) | - | |
| Neck | 5 (13.2%) | 1 (16.7%) | |
| Other | 9 (23.7%) | - |
Survival analyses
Three-year OS was 92.3% with OS curves shown in Figure 1. No patients in this series died of CD. Although our cohort is small, OS appeared similar between patients treated with surgery alone, radiation alone, or surgery followed by adjuvant RT (p=0.641). Local progression was observed in one patient from the surgery alone group, and none in the few treated with radiation alone initially (p=0.779). LPFS curves are shown in Figure 2. No distant failures were observed (Figure 3).
Figure 1:
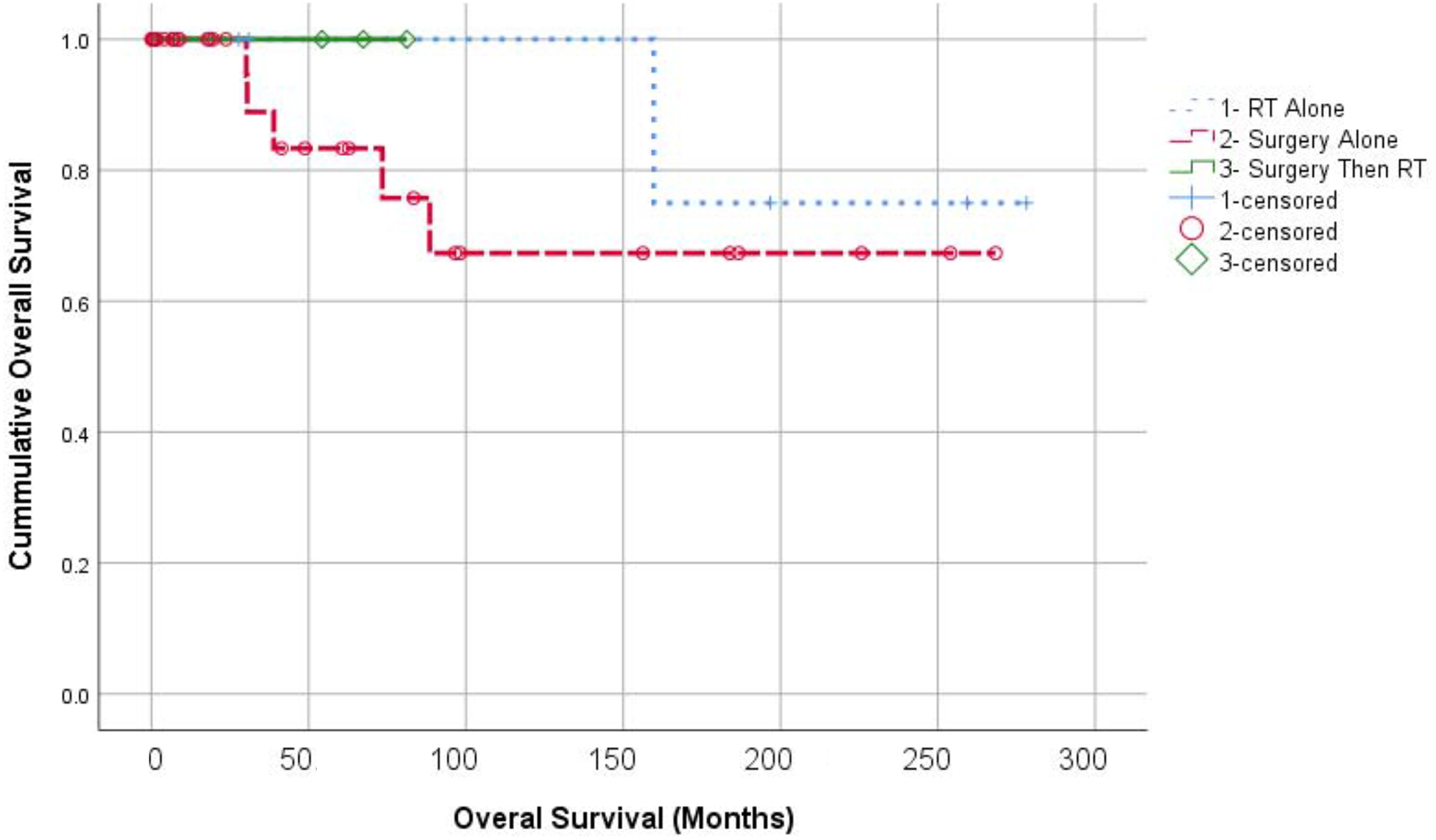
Kaplan Meier analysis of overall survival according to treatment groups. RT alone (+); Surgery alone (O); and Surgery with adjuvant RT (diamonds).
Figure 2:
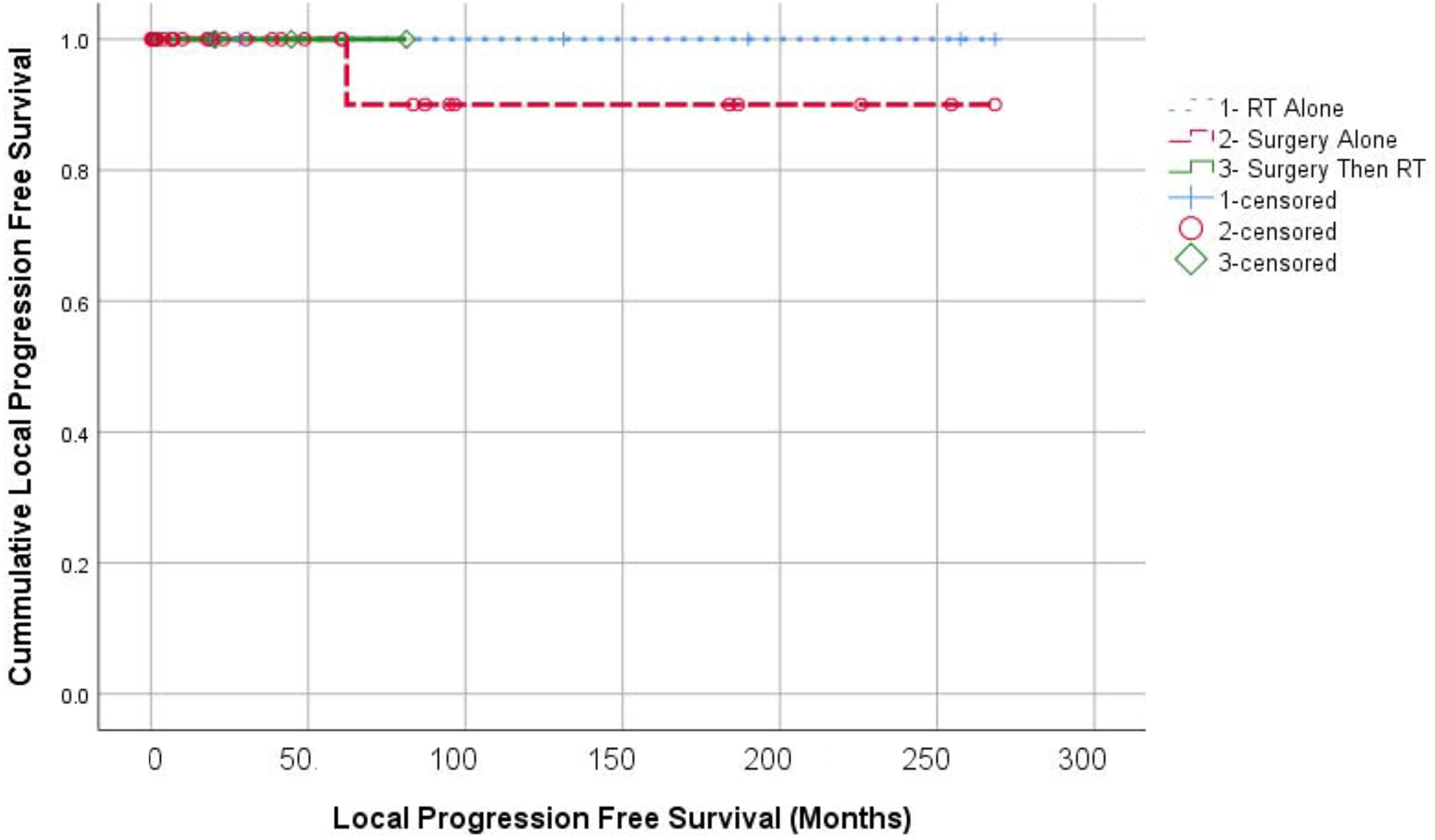
Kaplan Meier analysis of local progression free survival according to treatment groups. RT alone (+); Surgery alone (O); and Surgery with adjuvant RT (diamonds).
Figure 3:
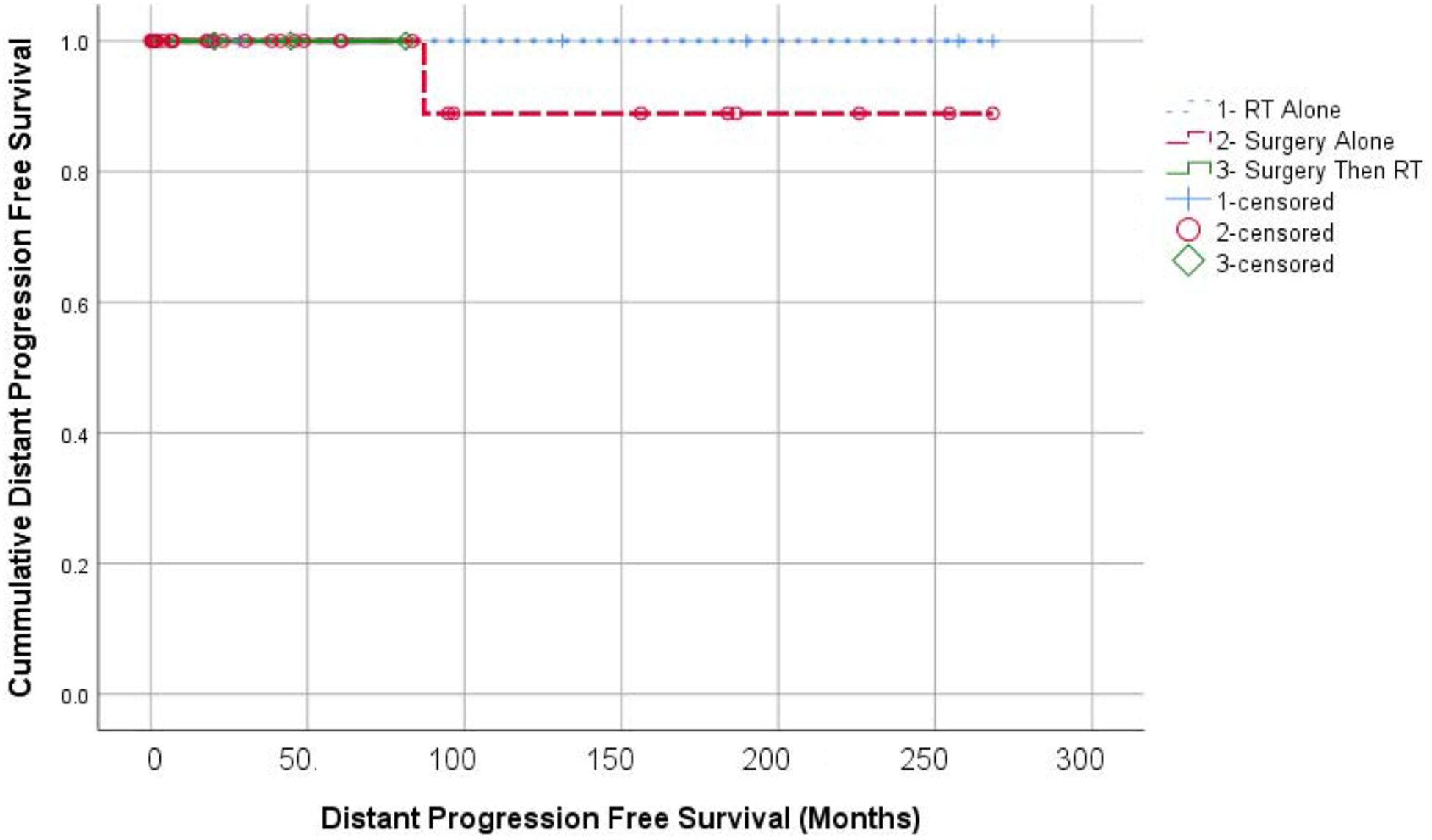
Kaplan Meier analysis of distant progression free survival according to treatment groups. RT alone (+); Surgery alone (O); and Surgery with adjuvant RT (diamonds).
Consolidative radiotherapy after systemic therapy
One patient with multicentric CD received induction chemotherapy prior to radiation. She was diagnosed at age 27 with bulky mediastinal, axillary, and diaphragmatic adenopathy and separate extranodal pleural-based lesions described as “parenchymal based disease”. She had pleural effusions and rapid progression of pulmonary symptoms. She also had night sweats and weight loss prior to therapy. She was HIV-negative, and presumed to have idiopathic multicentric CD however HHV-8 was not tested. After she underwent 6 cycles of cyclophosphamide, 750mg/m2, her symptoms resolved. On 18F-Fluorodeoxyglucose (FDG) positron emission tomography (PET) scan following chemotherapy, she had significant residual FDG avid pericardial adenopathy with standardized uptake value (SUV) of 13.0. She underwent consolidative radiotherapy to the residual FDG avid adenopathy, 3960 cGy in 22 fractions. She is now 16 years post therapy without evidence of CD.
Another patient with oligocentric CD was treated first with systemic therapy and then consolidative radiotherapy. She had an 18 month history of vague symptoms including a widespread intermittent rash, migratory joint swelling, unexplained debilitating fatigue, and anemia. Mediastinal and cervical adenopathy was identified, leading to a diagnosis of oligocentric CD with supporting evidence of elevated IL-6, erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP). She also had mild hepatosplenomegaly. Her systemic symptoms resolved promptly after treatment with IL-6 monoclonal antibody siltuximab, and she went on to maintenance treatment over 6 months.. ESR and CRP normalized, as did the hepatosplenomegaly. PET scan showed resolution of most of her disease, but there was a persistent FDG avid subcarinal lymph node with an SUV of 5.6 (SUV 9.9 at initial staging). Attempts to discontinue siltuximab were met with recurrence of her profound fatigue. She received 40 Gy in 20 fractions to the remaining subcarinal focus of FDG-avidity using intensity modulated radiation therapy with concurrent siltuximab. Siltuximab was discontinued after radiotherapy, and she is without symptom recurrence for 16 months. Her imaging is presented in Figure 4.
Figure 4:
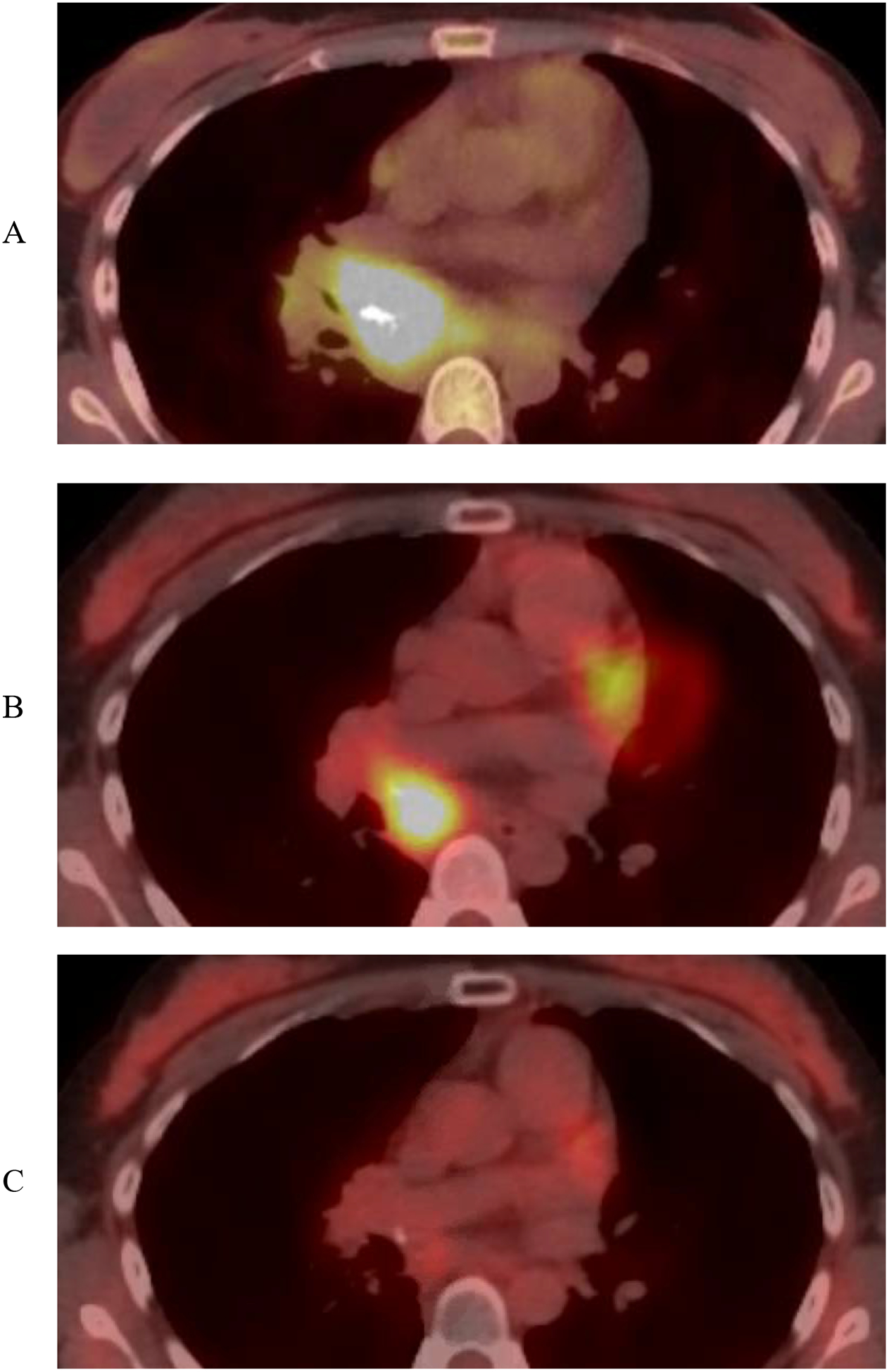
PET/CT imaging of a patient with oligocentric CD at initial staging (A) who had radiographic resolution of most sites of disease but persistent FDG avidity in the mediastinum after 6 cycles of siltuximab and debilitating symptomatology with attempted siltuximab discontinuation (B). She went on to receive 40 Gy consolidative radiation in 20 fractions with complete metabolic response and symptom free discontinuation of siltuximab (C).
Surgical Outcomes
Only one case of local recurrence was observed in this set of patients. This occurred in a male with unicentric CD in the axilla who underwent excisional biopsy of the two involved nodes. He had radiographic evidence of recurrence 5 years later, and repeat excisional biopsy, this time only one of several involved nodes re-demonstrated CD. He continues to be managed expectantly without additional local therapy and has been asymptomatic.
Adjuvant Radiation Therapy
Three patients had surgery followed by radiation. A 44 year old woman with an extensive axillary and supraclavicular mass underwent excisional biopsy. After the diagnosis of hyaline vascular CD was made she underwent a more extensive subtotal resection and was recommended adjuvant radiotherapy. She underwent mini-mantle field RT, 3960cGy in 22 fractions. She was symptomatic prior to local therapy with night sweats and proximal muscle weakness, and these both resolved after treatment. At last follow up she was symptom free and without evidence of disease 44 months after treatment. A 27 year old man with left cervical adenopathy underwent first an excisional biopsy than a cervical lymph node dissection for hyaline vascular CD. He underwent adjuvant RT to the left neck, 4500cGy in 25 fractions. At last follow up 20 months after treatment he was without evidence of disease. A 31 year old woman with hyaline vascular CD of the pelvis underwent an exploratory laparotomy which removed approximately 80% of her disease. A second surgery was attempted but due to high vascularity of the tumors biopsies were taken, and meaningful additional resection was not performed. She underwent 3960 cGy in 22 fractions to the hemipelvis and paraaortic lymph nodes. At 81 months follow up she was without evidence of recurrence.
Patient Deaths
Five patients in our cohort died with details of their CD diagnosis and treatment detailed in Table 2.
Table 2:
Details of deceased patients
| Age at diagnosis (years) | Local Therapy | Subtype | OS (months) | Comorbidiites | |
|---|---|---|---|---|---|
| Patient 1 | 38.9 | Surgery | Unicentric | 88.5 | HIV, Kaposi Sarcoma, High grade Lymphoma |
| Patient 2 | 59.2 | Surgery | Unicentric | 35.2 | monoclonal gammopathy of unknown significance |
| Patient 3 | 61.6 | Radiation | Multicentric | 159.7 | Esophageal cancer |
| Patient 4 | 70.4 | Surgery | Unicentric | 73.4 | Ovarian cancer |
| Patient 5 | 54.9 | Surgery | Unicentric | 30.5 | Neuroendocrine tumor |
Discussion
In this report we have demonstrated high rates of cure of unicentric CD treated with either complete surgical excision or radiotherapy. This is concordant with prior reports in the literature demonstrating unicentric CD is a local condition which is readily curable with local therapy. Additionally, we report patients with oligo- and multi-centric CD cytoreduced to limited stage with systemic therapy who appear to have been successfully treated with consolidative radiotherapy.
Reports of surgical excision as curative management of unicentric CD are common and recurrence is unlikely after full excision6,11. While surgery is curative and should be regarded as the preferred modality when it represents minimal risk, a recent report of 273 patients with CD including 57 with unicentric CD indicated curative resection was not possible in 21% of those with unicentric CD13. Given surgical resection is not always feasible without undue risk, understanding the efficacy of alternative local modalities is important.
Reports of treating CD patients with radiotherapy are limited and mixed in their conclusions. The strategy reported by Boutboul, et al, describes a watch and wait approach for patients in whom resection was not feasible or upfront medical management in an attempt to reduce disease6. Ultimately, 8 patients were treated with unspecified radiotherapy doses and plans with 4 complete responses (CR) and 4 partial responses (PR), though it is unclear if CT or PET was used for response assessment. A report of the MD Anderson experience described 4 patients with unicentric CD undergoing radiotherapy, with 3 CRs and one PR by CXR and clinical recurrence11. In 2016, Chan et al, summarized case reports and small series of 17 patients treated with radiotherapy in the management of CD, and overall 35% achieved a CR and 41% a PR8. These reports spanned several decades and included varied methodologies of imaging; however, it is notable that no patient was judged to have relapsed or progressive disease. Given this, a PR as judged by size criteria may represent successful treatment of unicentric CD, and retrospective series evaluating response to radiotherapy should be interpreted with this in mind.
We report on two patients, one with oligocentric CD and one multicentric CD, who had PR to systemic therapy siltuximab and cyclophosphamide, respectively. To our knowledge, ours is the first report of patients receiving consolidative radiotherapy to partially responding sites of disease after systemic therapy. Importantly, treatment recommendations for oligocentric disease were not included in the recent expert panel guidelines7. In our patient with with oligocentric CD, radiotherapy to only a small residual focus of FDG avid unresectable disease with concurrent siltuximab produced a complete radiographic response and ability to discontinue siltuximab without symptom recurrence for 16 months at the time of this analysis. While continued follow up is needed to determine whether this approach is curative, it does suggest a potential new paradigm of managing oligo- and multi-centric patients with isolated foci of residual disease. The reporting of additional experiences with multimodality management of CD are needed in order to better understand the long term outcomes of this and similar strategies for treating this rare disease.
No patient deaths in our cohort were definitely related to CD, although it must be acknowledged that retrospective analysis of cause of death is usually not reliable. All 5 deaths occurred in patients with cancer, as detailed in Table 2. Our center is a cancer hospital which undoubtedly influences this observation.
It is important to recognize that many patients with unicentric CD may not require therapy. Recognition of a pathologically enlarged lymph node appropriately prompts excisional biopsy which as demonstrated in this and other series usually results in both diagnosis and cure of the condition. Boutboul, et al, reported on observation of 13 patients with unresectable, asymptomatic disease6. One patient progressed and underwent radiotherapy however the others remained stable under the reported period, in one case for 17 years. In our series, it is not clear whether patients had any symptoms that prompted surgery, however in line with other large series, we suspect a majority were asymptomatic with surgery deployed for diagnostic purposes. In cases of unresectable disease, aggressive local therapy with radiation should be considered for patients with symptoms or as consolidation after systemic therapy. Asymptomatic patients may be suitable for observation.
Conclusions
In conclusion, unicentric CD is readily amenable to cure with local therapy. Surgical excision is preferred, but radiation appears to be an effective alternative for patients in whom surgery is not feasible and should be considered. Consolidative radiotherapy after PR to systemic therapy including anti-IL-6 targeted agents may expand the indications for local therapy in managing CD. Continued creative strategies to improve outcomes in patients with oligo- and multi-centric CD are needed.
Funding:
This work was supported by the National Institutes of Health NCI core grant P30 CA 008748.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Surgical excision is curative and the preferred treatment for unicentric Castleman Disease, however many patients have surgically unresectable disease. Radiotherapy has been used in these patients, but the outcomes reported in the literature are mixed. Moreover, consolidative local therapy after partial response to systemic therapy in cases of multi- and oligo-centric Castleman Disease have not been reported. We find excellent outcomes with both surgery and definitive radiation for unicentric Castleman Disease. Excitingly, in a symptomatic patient with oligocentric disease and partial response to systemic therapy, consolidative radiation to a partially responding site resulted in long term disease free survival. These findings will help guide local therapy choices in unicentric Castleman Disease and generate investigation into new strategies for patients with multi- and oligo-centric disease.
References
- 1.Wang W, Medeiros LJ. Castleman Disease. Surg Pathol Clin. 2019;12(3):849–863. doi: 10.1016/j.path.2019.03.003 [DOI] [PubMed] [Google Scholar]
- 2.Keddie S, Lunn MP. POEMS syndrome. Curr Opin Neurol. 2018;31(5):551–558. doi: 10.1097/WCO.0000000000000610 [DOI] [PubMed] [Google Scholar]
- 3.Pria AD, Pinato D, Roe J, Naresh K, Nelson M, Bower M. Relapse of HHV8-positive multicentric Castleman disease following rituximab-based therapy in HIV-positive patients. Blood. 2017;129(15):2143–2147. doi: 10.1182/blood-2016-10-747477 [DOI] [PubMed] [Google Scholar]
- 4.Yu L, Tu M, Cortes J, et al. Clinical and pathological characteristics of HIV- and HHV-8-negative Castleman disease. Blood. 2017;129(12):1658–1668. doi: 10.1182/blood-2016-11-748855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Rhee F, Greenway A, Stone K. Treatment of idiopathic castleman disease. Hematol Oncol Clin North Am. 2018;32(1):89–106. doi: 10.1016/j.hoc.2017.09.008 [DOI] [PubMed] [Google Scholar]
- 6.Boutboul D, Fadlallah J, Chawki S, et al. Treatment and outcome of Unicentric Castleman Disease: a retrospective analysis of 71 cases. Br J Haematol. 2019;186(2):269–273. doi: 10.1111/bjh.15921 [DOI] [PubMed] [Google Scholar]
- 7.van Rhee F, Voorhees P, Dispenzieri A, et al. International, evidence-based consensus treatment guidelines for idiopathic multicentric Castleman disease. Blood. 2018;132(20):2115–2124. doi: 10.1182/blood-2018-07-862334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chan K-L, Lade S, Prince HM, Harrison SJ. Update and new approaches in the treatment of Castleman disease. J Blood Med. 2016;7:145–158. doi: 10.2147/JBM.S60514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Rhee F, Casper C, Voorhees PM, et al. A phase 2, open-label, multicenter study of the long-term safety of siltuximab (an anti-interleukin-6 monoclonal antibody) in patients with multicentric Castleman disease. Oncotarget. 2015;6(30):30408–30419. doi: 10.18632/oncotarget.4655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Neuhof D, Debus J. Outcome and late complications of radiotherapy in patients with unicentric Castleman disease. Acta Oncol. 2006;45(8):1126–1131. doi: 10.1080/02841860600812701 [DOI] [PubMed] [Google Scholar]
- 11.Chronowski GM, Ha CS, Wilder RB, Cabanillas F, Manning J, Cox JD. Treatment of unicentric and multicentric Castleman disease and the role of radiotherapy. Cancer. 2001;92(3):670–676. doi: [DOI] [PubMed] [Google Scholar]
- 12.Karaca F, Usul Afşar C, Erkurt E, et al. The role of radiotherapy among the therapeutic options for castleman’s disease. Turk J Haematol. 2014;31(2):197–198. doi: 10.4274/tjh.2013.0164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oksenhendler E, Boutboul D, Fajgenbaum D, et al. The full spectrum of Castleman disease: 273 patients studied over 20 years. Br J Haematol. 2018;180(2):206–216. doi: 10.1111/bjh.15019 [DOI] [PubMed] [Google Scholar]


