Abstract
Background
Risk factors for birth defects are frequently investigated using data limited to liveborn infants. By conditioning on survival, results of such studies may be distorted by selection bias, also described as “live birth bias”. However, the implications of live birth bias on risk estimation remain poorly understood.
Objectives
We sought to quantify live birth bias and to investigate the conditions under which it arose.
Methods
We used data on 3,994 birth defects cases and 11,829 controls enrolled in the National Birth Defects Prevention Study to compare odds ratio (OR) estimates of the relationship between three established risk factors (antiepileptic drug use, smoking, and multifetal pregnancy) and four birth defects (anencephaly, spina bifida, omphalocele, and cleft palate) when restricted to live births as compared to among live births, stillbirths, and elective terminations. Exposures and birth defects represented varying strengths of association with live birth; all controls were liveborn. We performed a quantitative bias analysis to evaluate the sensitivity of our results to excluding terminated and stillborn controls.
Results
Cases ranged from 33% live born (anencephaly) to 99% (cleft palate). Smoking and multifetal pregnancy were associated with live birth among anencephaly (crude OR [cOR] 0.61 and cOR 3.15, respectively) and omphalocele cases (cOR 2.22 and cOR 5.22, respectively). For analyses of the association between exposures and birth defects, restricting to live births produced negligible differences in estimates except for anencephaly and multifetal pregnancy, which was two-fold higher among live births (adjusted OR [aOR] 4.93) as among all pregnancy outcomes (aOR 2.44). Within tested scenarios, bias analyses suggested that results were not sensitive to the restriction to liveborn controls.
Conclusions
Selection bias was generally limited except for high mortality defects in the context of exposures strongly associated with livebirth. Findings indicate that substantial live birth bias is unlikely to affect studies of risk factors for most birth defects.
Keywords: birth defects, stillbirth, live birth, termination of pregnancy, selection bias, live birth bias
BACKGROUND
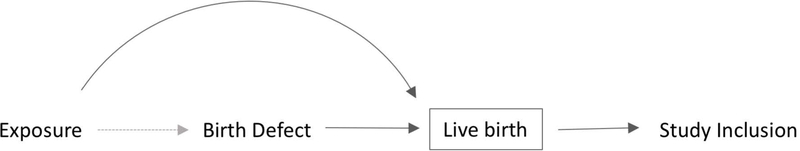
Major birth defects are common and a leading cause of perinatal mortality and lifelong morbidity and disability. 1–4 Live births are easily identified by surveillance systems via vital records and through validated algorithms in administrative data. 5 However, many birth defect cases are stillborn or undergo elective termination, particularly those with more severe conditions. 6–9 Studies among live births may be distorted by selection bias when the exposure is also independently associated with survival to live birth (Figure 1). 10 This situation has recently been referred to as “live birth bias”. 11,12
Figure 1.
Generic directed acyclic graph (DAG) of hypothetical selection bias in studies of birth defects. Solid arrows represent associations. Dashed arrow represents the association of interest. The box represents conditioning on live birth through selection into a study.
Live birth bias may occur when an exposure is associated with a) the diagnosis of a birth defect (e.g., obesity may reduce the sensitivity of ultrasound); b) survival among those with a birth defect to a time of potential detection (e.g., high doses of toxins can cause miscarriage); or c) decisions to terminate a pregnancy with a prenatally diagnosed birth defect or access to termination procedures (e.g., Medicaid does not cover abortion in some states, limiting access). 13–15
Previous investigations of live birth bias in birth defects studies have focused on chromosomal disorders and neural tube defects – both of which have relatively high frequencies of termination and stillbirth – and exposures consisting primarily of maternal demographic characteristics rather than potential teratogens. 16–20 Therefore, the conditions under which live birth bias may arise are poorly understood. Currently researchers do not know whether live birth bias is a plausible explanation for an association between, say, maternal medication use and omphalocele (over 80% of cases are live born).
Although there has been a movement towards quantifying potential bias in epidemiology studies, this is difficult to accomplish for selection bias because, by definition, data on those not included are unavailable. 21 Further, there are few analyses of associations between survival to live birth and potential exposures to inform quantitative bias analyses. 21–24
Quantification of live birth bias would inform the validity of data sources for etiologic research of birth defects restricted to live births. 5,25,26 Few studies have data for a variety of exposures on a large number of cases with specific birth defects and include systematic identification of cases also ending in termination or stillbirth, in which to conduct this quantification. Using data from the largest population-based case-control study of birth defects, we sought to quantify the degree of bias in epidemiologic studies of birth defects conducted among live births across a range of risk factors and birth defects.
METHODS
Case-Control Selection
The National Birth Defects Prevention Study (NBDPS) is a multi-state, population-based case-control study of major structural birth defects in the United States between 1997 and 2011. Study methods have been described in detail. 27 Briefly, birth defect cases were identified using active surveillance systems in 10 states (Arkansas, California, Georgia, Iowa, Massachusetts, North Carolina, New Jersey, New York, Texas, and Utah). Cases with a known cause (i.e., single gene or chromosomal disorders) were ineligible. Medical records of cases were reviewed for defect confirmation and all cases were classified by clinical geneticists using standard criteria. 28 Eligible cases could be live births, elective terminations (any gestational age), or stillbirths (gestational age at delivery ≥20 weeks’ gestation or birth weight ≥500g if gestational age unknown). Controls were live born infants without major structural or syndromic birth defects randomly selected from the same geographical location and time period as the cases through either birth certificates or birth hospital records.
Mothers of all eligible cases and controls were invited to participate in a telephone interview within 24 months of their estimated date of delivery (“due date”) about demographic, reproductive factors, pregnancy history, health behaviors, and lifestyle characteristics. Mothers of both cases and controls were ineligible to participate in the interview if the infant was not in the mother’s legal custody, the mother was deceased or incarcerated, did not speak English or Spanish, or had already participated in the NBDPS for a previous pregnancy.
Ethics Approval
Approval for this study was granted by the Institutional Review Board of the Centers for Disease Control and Prevention and Institutional Review Boards at all study sites. Informed consent was obtained from each participant at the time of the interview.
Statistical Analyses
We investigated two primary questions: (i) Do the results of analyses meaningfully differ when conducted among live births only as compared to when stillbirths and terminations are also included? and (ii) Under what conditions do analyses differ meaningfully?
Analyzing all of the large number of specific birth defects and exposures included in the NBDPS for each of these questions was not practical. Therefore, we selected representative birth defects and exposures to evaluate our questions. The strength of selection bias is dependent on how strongly the exposure and birth defect of interest each affect live birth. 29 Therefore, we selected exposures and birth defects which represent the range of strengths of association with survival to live birth. Our process for selecting these is detailed below.
Selected birth defects
To quantify how the strength of association between birth defects and survival to live birth affects selection bias, we selected four birth defects which represent the full spectrum of survival to live birth: anencephaly, low survival (high stillbirth and termination incidence); spina bifida moderate survival (moderate stillbirth and high termination incidence); omphalocele moderate survival (high stillbirth and moderate termination incidence); cleft palate without cleft lip high survival (low stillbirth and termination incidence).30
Selected exposures
To quantify how the strength of the association between exposure and survival to live birth affects selection bias, we selected three established risk factors for birth defects to represent exposures with a range of effects on survival to live birth in cases and controls: Smoking has a moderate association with oral clefts and no known association with termination for birth defects but a moderately increased risk of stillbirth. 31–36 Antiepileptic drugs (AEDs) are strongly associated with anencephaly and spina bifida and may be moderately associated with termination for birth defects due to increased surveillance for birth defects among AED users; there is no known effect on stillbirth.37–40 Multifetal pregnancies have a moderate association with anencephaly and omphalocele, and a strong inverse association with termination for birth defects as termination could negatively affect the survival of normally formed co-siblings. 41,42 However, compared to singleton pregnancies, multifetal pregnancies have a higher risk of stillbirth. 43
From the maternal interview, we obtained information about first trimester maternal smoking (any smoking vs no smoking), first trimester use of any AED (any time in the first trimester vs no use during the three months before and throughout pregnancy), and multifetal pregnancy (multiple vs singleton). AEDs were defined as any medication containing clonazepam, divalproex sodium, gabapentin, oxcarbazepine, phenytoin, phenobarbital, primidone, diazepam, topiramate, levetiracetam, lamotrigine, valproic acid, or carbamazepine. Multifetal pregnancies were based on maternal interview or if a response was missing from the interview, information abstracted from medical or vital records. If more than one infant from a multiple set had eligible birth defects, the oldest eligible infant was included in the study (n=1 cleft palate, both twins had the same defect and were live born).
Statistical models
We used Firth’s penalized logistic regression models to estimate odds ratios (OR) and 95% profile-likelihood confidence intervals (95% CIs).44,45
We quantified the strength of the relationship between 1. birth defect and live birth by estimating crude ORs for anencephaly, omphalocele, and spina bifida compared to cleft palate (since all controls were live born, we selected the defect with highest survival for comparison) and 2. exposures and live birth by estimating crude ORs for each birth defect-exposure pair.
Selection on survival was introduced by restricting analyses to the following case groups: “live births only”, “live births and stillbirths”, and “live births, stillbirths, and terminations”. We adjusted logistic models for the following a priori selected covariates: maternal age category (<25, 25–34, ≥35 years), maternal race/ethnicity (white non-Hispanic, Black non-Hispanic, Hispanic, Asian/Pacific Islander, Other), and use of a vitamin containing folic acid any time in the first trimester of pregnancy. Covariates were selected based on known associations with the exposures and birth defects as well as a sufficiently high prevalence in the population to provide confounding adjustment given small numbers of exposed birth defects cases. Given that small numbers can lead to substantial random variation, model results were considered to differ if the 95% CI of models among live births, stillbirths, and terminations excluded the OR when restricted to live births only, or to live births and stillbirths.
Missing data
Mothers missing data on smoking (n=300 control mothers, 92 case mothers) or AED use (n=238 control mothers, 81 case mothers) were excluded from those respective analyses. Cases with an unknown pregnancy outcome (n=10) were excluded from all analyses.
Sensitivity analyses
We evaluated the potential effect of clustering by study center using logistic mixed effect models with a random intercept for study center. To simplify analyses, we assumed no misclassification of exposure or outcome and no unmeasured confounding, although these potential sources of bias may be present. 35
During the study, the inclusion of cases by survival outcome differed by center and over time; some centers included only livebirths and others expanded to non-livebirths later in the study period. 27 The main analyses used data from all centers, but we conducted a sensitivity analysis excluding centers and time periods where stillborn infants and terminations were not collected (“Complete Collection Sample”).
Differences in the proportion of birth defect cases terminated among certain sub-populations may affect the degree of selection bias observed. Therefore, we repeated analyses while restricting to the following populations: 1. singleton pregnancies (increased risk of termination for birth defects) and 2. cases with isolated birth defects (decreased risk of termination for birth defects); analyses of multifetal pregnancy were excluded from this sensitivity analysis.46 Finally, since interview participation may differ by survival outcome, we compared survival by interview status for each birth defect.
Quantitative bias analysis
In a case-control study, the controls represent the distribution of the exposures in the population which gave rise to the cases (“study base”), and thus must be sampled without regard to exposure status.47 Due to the technical and legal limitations, the control population of the NBDPS was limited to live born infants. Some exposures may be associated with stillbirth or termination among controls. Therefore, NBDPS controls could in theory fail to accurately represent the study base. Further, exclusion of these survival outcomes limits the ability of our analyses to fully quantify potential selection bias. 48 Thus, we conducted a quantitative bias analysis based on published literature to evaluate the sensitivity of our results to the inclusion of only live born controls. Details of the methods and data sources used can be found in the Supplemental eMaterials.
All analyses were conducted using SAS version 9.4 (SAS Institute, Cary, NC).
RESULTS
Among 15,823 mother-infant pairs (3,994 case mothers and 11,829 control mothers) interviewed and included in our evaluation, the number of infants identified with birth defects of interest ranged from 444 for omphalocele to 1631 for cleft palate.
Strength of association between birth defects and survival to live birth
Survival to live birth (Table 1), was lowest for anencephaly (live birth 33%) and highest for cleft palate (live birth 99%). Compared to the odds of live birth for cases with cleft palate, the odds of live birth were substantially lower for anencephaly (OR 0.007, 95% CI 0.004, 0.01), omphalocele (OR 0.09, 95% CI 0.06, 0.15), and spina bifida (OR 0.10, 95% CI 0.06, 0.15).
Table 1.
Exposure distributions by survival outcome and combined survival outcomes for selected exposures and birth defects; National Birth Defects Prevention Study, 1997 – 2011.
| Survival Outcome |
Combined Survival Outcomes |
|||||
|---|---|---|---|---|---|---|
| Live births | Stillbirths | Terminations | Live births | Live births & Stillbirths | Live births, Stillbirths & Terminations | |
| N (%a) | N (%) | N (%) | N (%a) | N (%) | N (%) | |
| Anencephaly | ||||||
| Total | 219 (33.4) | 153 (23.3) | 284 (43.2) | |||
| Main Exposures | ||||||
| Smokingb | 18 (8.2) | 22 (14.4) | 36 (12.7) | 18 (8.2) | 40 (10.8) | 76 (11.6) |
| Antiepileptic Drugsb | 2 (0.9) | 1 (0.7) | 5 (1.8) | 2 (0.9) | 3 (0.8) | 8 (1.2) |
| Multifetal Pregnancyc | 26 (11.9) | 9 (5.9) | 7 (2.5) | 26 (11.9) | 35 (9.4) | 42 (6.4) |
| Omphalocele | ||||||
| Total | 381 (85.8) | 36 (8.1) | 27 (6.1) | |||
| Main Exposures | ||||||
| Smoking | 86 (22.6) | 5 (13.9) | 2 (7.4) | 86 (22.6) | 91 (21.8) | 93 (20.9) |
| Antiepileptic Drugs | 2 (0.5) | 0 (0.0) | 0 (0.0) | 2 (0.5) | 2 (0.5) | 2 (0.5) |
| Multifetal Pregnancy | 42 (11) | 1 (2.8) | 0 (0.0) | 42 (11.0) | 43 (10.3) | 43 (9.7) |
| Spina Bifida | ||||||
| Total | 1141 (88.2) | 30 (2.3) | 122 (9.4) | |||
| Main Exposures | ||||||
| Smoking | 193 (16.9) | 5 (13.9) | 2 (7.4) | 193 (16.9) | 200 (17.1) | 218 (16.9) |
| Antiepileptic Drugs | 28 (2.5) | 0 (0) | 3 (2.5) | 28 (2.5) | 28 (2.4) | 31 (2.4) |
| Multifetal Pregnancy | 42 (3.7) | 1 (3.3) | 2 (1.6) | 42 (3.7) | 43 (3.7) | 45 (3.5) |
| Cleft Palate | ||||||
| Total | 1607 (98.5) | 17 (1) | 7 (0.4) | |||
| Main Exposures | ||||||
| Smoking | 343 (21.3) | 1 (5.9) | 2 (28.6) | 343 (21.3) | 344 (21.2) | 346 (21.2) |
| Antiepileptic Drugs | 27 (1.7) | 0 (0) | 0 (0) | 27 (1.7) | 27 (1.7) | 24 (1.7) |
| Multifetal Pregnancy | 65 (4) | 1 (5.9) | 0 (0) | 65 (4) | 66 (4.1) | 66 (4) |
Row percentages
First trimester exposure
If multiple fetuses in a multifetal pregnancy had eligible defects the oldest fetus was selected for inclusion
Strength of association between exposures and survival to live birth
AEDs were not associated with odds of live birth for any defect (Table 2), but smoking was moderately associated with decreased odds of live birth among anencephaly cases (OR 0.61, 95% CI: 0.34, 1.04) and increased odds among omphalocele cases (OR 2.22, 95% CI 1.10, 5.34). Multifetal pregnancy was strongly associated with increased odds of live birth among both anencephaly (OR 3.15, 95% CI 1.71,5.92) and omphalocele cases (OR 5.22, 95% CI 1.40, 46.78).
Table 2.
Crude relative odds of exposure by survival outcome among birth defect cases
| Odds ratio (95% confidence interval) |
|||
|---|---|---|---|
| Antiepileptic Drugs | Smoking | Multifetal Pregnancya |
|
| Anencephaly | |||
| Live births | 0.77 (0.14, 3.03) | 0.61 (0.34, 1.04) | 3.15 (1.71,5.92) |
| Stillbirth or termination | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) |
| Omphalocele | |||
| Live births | 0.84 (0.07, 116.23) | 2.22 (1.10, 5.34) | 5.22 (1.40, 46.78) |
| Stillbirth or termination | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) |
| Spina Bifida | |||
| Live births | 1.13 (0.41, 4.23) | 0.97 (0.64, 1.54) | 1.70 (0.63, 6.25) |
| Stillbirth or termination | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference)) |
| Cleft Palate | |||
| Live births | 0.87 (0.12, 111.46) | 1.64 (0.59, 6.20) | 0.67 (0.17, 6.03) |
| Stillbirth or termination | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) |
If multiple fetuses in a multifetal pregnancy had eligible defects the oldest fetus was selected for inclusion
Exposure prevalence by combined survival outcomes
Although exposure prevalence varied across individual survival outcomes, there was little change between the proportion of exposed cases among live births compared to among live births, stillbirths, and terminations for most analyses (Table 1). The exception was for cases of anencphaly where we found notable differences for the exposures of multifetal pregnancy (11.9% among live births vs 6.4% among all survival outcomes)and smoking (8.2% among live births vs 11.6% among all survival outcomes.
Analyses of associations between exposures and specific birth defects among live births only compared to all survival outcomes
Except for analyses of anencephaly, estimated aORs among live births did not differ from those among live births and stillbirths, or among all survival outcomes (Figure 2). The estimated association of smoking with anencephaly among live births was further from the null than the estimate among all survival outcomes, but this analysis did not meet our criterion for difference. The aOR of anencephaly associated with multifetal pregnancy was twice as high when restricted to live births (aOR 4.93, 95% CI 3.16, 7.20) compared to among all survival outcomes (aOR 2.44, 95% CI 1.73, 3.35). The point estimate for live births was outside of the 95% CI among all survival outcomes meeting our criteria for difference.
Figure 2.
Comparison of analyses among live births, live births and stillbirths, and all survival outcomes for selected exposures and birth defects; National Birth Defects Prevention Study, 1997 – 2011.
Estimates were obtained from Firth’s logistic regression models with profile likelihood 95% confidence intervals adjusted for maternal age category, race/ethnicity, and first trimester exposure to a vitamin containing folic acid. The reference line indicates the null value. Birth defects are arranged in order of prevalence of stillbirth and termination from highest (top) to lowest (bottom). Exposures are arranged from (left) lowest association with prevalence of stillbirth and termination to (right) highest. If multiple fetuses in a multifetal pregnancy had eligible birth defects the oldest fetus was selected for inclusion.
a The 95% CI among live births only (3.16, 7.20) excludes the point estimate among all survival outcomes (live births, stillbirths, and terminations; 2.44) and slightly overlaps the upper bound of the 95% confidence interval for the latter model (1.73, 3.35).
Sensitivity Analyses
There was no difference in participation by survival for anencephaly. For the other birth defects, participation among live births was about 5% higher than for stillbirth and termination (eTable 1). Cases with multiple birth defects were more common among pregnancies ending in stillbirth or termination than live birth (eTable 2).
We did not identify a difference in the occurrence or magnitude of the bias across included survival outcomes when analyses were restricted to singletons or cases with isolated birth defects (eTables 3 – 8). Exclusion of study sites and time periods when stillbirths and terminations were not collected (“complete collection sample”) had no meaningful impact on our findings. Results of mixed effect models with a random intercept for study center did not differ meaningfully from fixed effect models, but model convergence could not be achieved for all analyses (eTables 6 – 8).
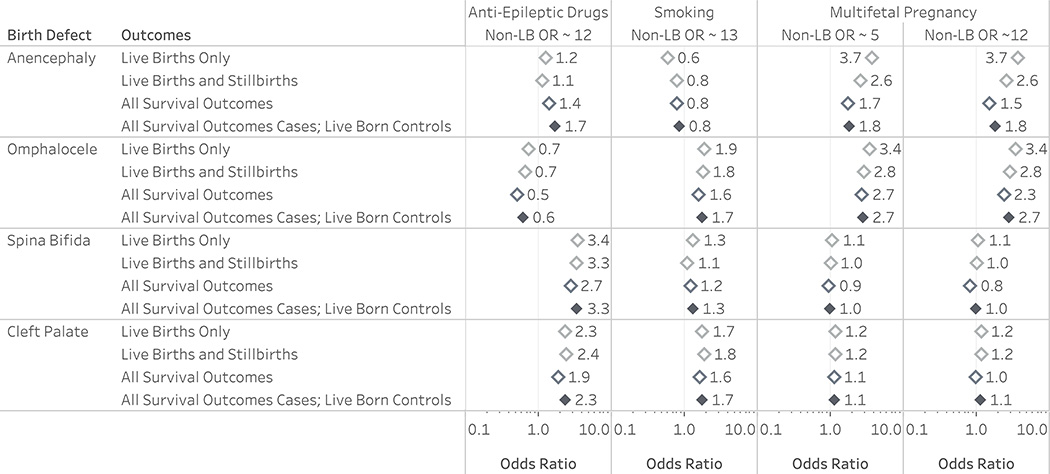
Details of the quantitative bias analysis are presented in the supplement. Most results were robust to the exclusion of stillborn and terminated controls, even when the exposure odds were 5 to 12 times higher than for liveborn controls (Figure 3; eTables 10 - 12). There was little difference in the results of analyses conducted among all survival outcomes for both cases and controls compared to our main analyses (range of difference in ORs 0.00 to 0.60).
Figure 3.
Comparison of analyses including simulated stillborn and terminated controls (open diamonds) on results of the analysis of selection bias for risk factor studies of birth defects. The reference line indicates the null value. OR is the crude exposure odds ratio for stillbirth and terminations among simulated controls. All survival outcomes for cases and live born controls only (filled diamond) shows the result that would have been obtained if only live born infants were included as controls, as in the main analysis. Full methods and results of can be found in the Supplemental eMaterials.
COMMENT
Principal findings
We found limited evidence of meaningful live birth bias in analyses of etiological risk factors for birth defects. As expected from the generic DAG, live birth bias was identified when survival to live birth was strongly associated with both the birth defect of interest and exposure among cases with that birth defect. However, when the exposure distribution among live births was similar to that among all survival outcomes – even when the prior conditions were met – any resulting bias led only to small changes in estimates (e.g., analysis of multifetal pregnancy and omphalocele). When the exposure distributions differed substantially between these populations a large bias was noted, as in the analysis of multifetal pregnancy and anencephaly. In this case, only the strength of this association was attenuated (a two-fold reduction), with no change in the direction of the relationship. However, an inversion of effects is possible.
Strengths of the Study
Examination of selection bias within this large, population-based case-control study and inclusion of a quantitative bias analysis allowed us to examine several different scenarios and quantify the bias introduced. Further, we selected exposures with well established relationships to the defects under study, and our results are consistent with previous reports conducted in various settings. 34–36,39,41,53 Consistent methods across centers helped to ensure the quality of case ascertainment and classification. Identified controls later found to have major birth defects were included as cases if they met the study criteria or excluded; thus, misclassification of the outcome is expected to be rare. Survival outcome had little effect on interview participation for cases.
Limitations of the Data
Our study has several limitations. Spontaneous abortions before 20 weeks’ gestation were not captured due to both technological and practical limitations. Therefore, we cannot rule out the possibility that exclusion of miscarried cases and controls could lead to meaningful bias; this may explain the protective association found for maternal smoking and anencephaly in our study and others.16,34,35 Identification of terminated cases by surveillance systems is likely incomplete, but the proportion of missing cases is unknown. 17 However, our findings and results of our sensitivity analysis suggest that large numbers of terminations need to be missing differentially by exposure status in order to substantially alter our results. Our criterion for defining the presence of bias are stringent and partially dependent on power; thus, we may miss true selection bias. It is possible that the wide confidence interval for the analysis of smoking and anencephaly among live births may have led us to erroneously conclude that there was no selection bias for this exposure and birth defect pairing. However, objective criteria prevent this determination from being simply based on opinion.
Although self-reported medication use is subject to errant recall, antiepileptic drugs have been found to be reported with near-perfect accuracy compared to prenatally reported medication use and dispensing records. 54,55 Additionally, self-report of smoking during pregnancy correlates well with biomarker-based estimates of smoking, but some exposure misclassification is possible. 56,57 While recall bias is a concern in retrospective studies of birth defects, bias has been found to manifest only in extreme circumstances. 58
Interpretation
Our results suggest that so long as live births are representative of the exposure distribution among all cases which are live born, stillborn, and terminated, live birth bias is unlikely to substantially alter the results of a risk factor analysis for birth defects. Although there were small differences in the exposure distributions for omphalocele cases between live births and all survival outcomes, little difference was seen for spina bifida and cleft palate cases. Consistent with this observation, there was little difference in the ORs estimated for these defects in live births vs all survival outcomes.
As with previous studies, strong selection bias was restricted to analyses of anencephaly. 16–20,49 Because only 33% of anencephaly cases were live born, this small subset is less likely to be representative of all cases than most birth defects where well over 80% are live born. Therefore, severe live birth bias is most likely to occur among birth defects with a similarly low probability of live birth (e.g., bilateral renal agenesis, trisomy 13). 30,50 However, the exposure distributions for anencephaly did not always differ between live births and all survival outcomes, suggesting that not all analyses of high mortality birth defects will be affected by live birth bias.
Importantly, however, the above observations can only be said to apply to analyses of fetuses who survive to the point where birth defect diagnosis is possible. Exposure distributions among conceptions miscarried or electively terminated prior to the point of possible birth defect diagnosis (approximately 11 weeks’ gestation) may differ substantially from survivors for whom birth defects are diagnosed or ruled out. 16,49,51,52 As approximately 20% of pregnancies end in an early loss – a proportion likely higher for more severe birth defects – exclusion of these cases may alter the exposure distribution among all cases. 11,49 Thus, miscarriages may induce some degree of selection bias which is not accounted for in our study.
We also found that the restriction of NBDPS controls to live births is unlikely to contribute to selection bias in our study or to alter the results of our analyses. Even under extreme assumptions about the strength of the relationship between exposure and stillbirth or second trimester elective termination results were similar. Thus, we believe our main results are robust to the exclusion of electively terminated and stillborn controls.
These results are expected to generalize to studies of administrative data since the NBDPS utilized population-based cumulative incidence sampling to efficiently sample from the underlying cohort. 47 NBDPS controls have previously been found to be generally representative of the underlying study populations, except for the prevalence of multifetal pregnancies.48 Correcting to the population level in the study base shifted associations with multifetal pregnancy towards the null but did not alter the occurrence of live birth bias. Studies investigating risk factors for chromosomal disorders with high incidence of stillbirth or termination or which investigate associated structural defects in populations including chromosomal disorders risk live birth bias. A low proportion of liveborn cases will result in highly imprecise results and low power. Finally, we cannot be sure that our results apply to all possible exposures. Publication of the associations of various potential risk factors by survival outcome (individually and combined) and birth defect would aid researchers in assessing the potential for live birth bias.
CONCLUSIONS
We found limited evidence of meaningful live birth bias in studies of etiologic risk factors for some representative birth defects. Nonetheless, inclusion of birth defect cases resulting in termination or stillbirth in studies of risk factors for birth defects decreases the likelihood of selection bias and improves precision of estimates
Supplementary Material
SYNOPSIS.
Study question
To what degree does selection bias impact studies investigating risk factors for birth defects, when analysis is restricted to live births only?
What is already known
Previous reports suggest that findings from studies of neural tube defects may be distorted by selection (“live birth”) bias.
What this study adds
We observed that selection bias substantial enough to change the conclusions of a research question rarely occurred when evaluation of birth defects, including spina bifida and birth defects of other organ systems, was restricted to live births. Studies of risk factors for birth defects in which most cases are live born are unlikely to be seriously affected by live birth bias.
SOCIAL MEDIA QUOTE.
Live birth bias arose when survival was strongly associated with both the birth defect of interest and exposure among cases. Yet when exposure among live born cases was similar to that among all survival outcomes, resulting bias led only to small changes in estimates.
Acknowledgments
Coding of drug information in the National Birth Defects Prevention Study used the Slone Drug Dictionary under license from the Slone Epidemiology Center of Boston University, Boston, MA. We thank the California Department of Public Health, Maternal Child and Adolescent Health Division for providing surveillance data from California for this study. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention or the California Department of Public Health.
Funding
This project was supported through Centers for Disease Control and Prevention (CDC) cooperative agreements (U01DD001037, U01DD000493, 5U01DD000491) under PA #96043, PA #02081, FOA #DD09-001, FOA #DD13-003, and NOFO #DD18-001 to the Centers for Birth Defects Research and Prevention participating in the National Birth Defects Prevention Study (NBDPS) and/or the Birth Defects Study To Evaluate Pregnancy exposureS (BD-STEPS)], the National Institutes of Health (T32HD060454 to DH), and the Health Resources and Services Administration of the U.S. Department of Health and Human Services (T03MC07648 to DH).
REFERENCES
- 1.Boyle B, Addor M-C, Arriola L, Barisic I, Bianchi F, Csáky-Szunyogh M, et al. Estimating Global Burden of Disease due to congenital anomaly: an analysis of European data. Archives of Disease in Childhood - Fetal and Neonatal Edition. 2017;103(1):F22–F28. doi: 10.1136/archdischild-2016-311845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hoyert DL, Gregory ECW. Cause of Fetal Death: Data From the Fetal Death Report, 2014. National Vital Statistic Report. 2016;65(7):1–25. [PubMed] [Google Scholar]
- 3.Gregory ECW, MacDorman MF. Fetal and Perinatal Mortality: United States, 2013. National Vital Statistic Report. 2015;64(8):1–24. [PubMed] [Google Scholar]
- 4.Botto LD, Moore CA, Khoury MJ, Erickson JD. Neural-tube defects. New England Journal of Medicine. 1999;341(20):1509–1519. doi: 10.1056/NEJM199911113412006. [DOI] [PubMed] [Google Scholar]
- 5.Palmsten K, Huybrechts KF, Kowal MK, Mogun H, Hernández-Díaz S. Validity of maternal and infant outcomes within nationwide Medicaid data. Pharmacoepidemiology and Drug Safety. 2014;23(6):646–655. doi: 10.1002/pds.3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Groen H et al. Stillbirth and neonatal mortality in pregnancies complicated by major congenital anomalies: findings from a large European cohort. Prenatal Diagnosis. 1–33 (2017). doi: 10.1002/pd.5148 [DOI] [PubMed] [Google Scholar]
- 7.Svensson E, Ehrenstein V, Nørgaard M, et al. Estimating the Proportion of All Observed Birth Defects Occurring in Pregnancies Terminated by a Second-trimester Abortion. Epidemiology. 2014;25(6):866–871. doi: 10.1097/EDE.0000000000000163. [DOI] [PubMed] [Google Scholar]
- 8.Johnson CY, Honein MA, Dana Flanders W, Howards PP, Oakley GP, Rasmussen SA. Pregnancy termination following prenatal diagnosis of anencephaly or spina bifida: a systematic review of the literature. Birth Defects Research Part A Clinical and Molecular Teratology. 2012;94(11):857–863. doi: 10.1002/bdra.23086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schechtman KB, Gray DL, Baty JD, Rothman SM. Decision-making for termination of pregnancies with fetal anomalies: analysis of 53,000 pregnancies. Obstetrics & Gynecology. 2002;99(2):216–222. [DOI] [PubMed] [Google Scholar]
- 10.Hernan MA, Hernández-Díaz S, Robins JM. A Structural Approach to Selection Bias. Epidemiology. 2004;15(5):615. doi: 10.1097/01.ede.0000135174.63482.43. [DOI] [PubMed] [Google Scholar]
- 11.Suarez EA, Landi SN, Conover MM, Jonsson Funk M. Bias from restricting to live births when estimating effects of prescription drug use on pregnancy complications: A simulation. Pharmacoepidemiology and Drug Safety. 2018;27(3):307–314. doi: 10.1002/pds.4387. [DOI] [PubMed] [Google Scholar]
- 12.Liew Z, Olsen J, Cui X, Ritz B, Arah OA. Bias from conditioning on live birth in pregnancy cohorts: an illustration based on neurodevelopment in children after prenatal exposure to organic pollutants. International Journal of Epidemiology. 2015;44(1):345–354. doi: 10.1093/ije/dyu249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Best KE, Tennant P, Bell WR, Rankin J. Impact of maternal body mass index on the antenatal detection of congenital anomalies. Brittish Journal of Obstetrics & Gynaecology. 2012;119(12):1503–1511. doi: 10.1111/j.1471-0528.2012.03462.x. [DOI] [PubMed] [Google Scholar]
- 14.Missmer SA, Suarez L, Felkner M, Wang E, Merrill AH Jr, Rothman KJ, et al. Exposure to Fumonisins and the Occurrence of Neural Tube Defects along the Texas–Mexico Border. Environmental Health Perspectives. 2006;114(2):237–241. doi: 10.1289/ehp.8221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hutcheon JA, Bodnar LM, Simhan HN. Medicaid Pregnancy Termination Funding and Racial Disparities in Congenital Anomaly–Related Infant Deaths. Obstetrics & Gynecology. 2015;125(1):163–169. doi: 10.1097/AOG.0000000000000583. [DOI] [PubMed] [Google Scholar]
- 16.Regal RR, Hook EB. Interrelationships of Relative Risks of Birth Defects in Embryonic and Fetal Deaths, in Livebirths, and in All Conceptuses. Epidemiology. 1992;3(3):247–252. doi: 10.2307/3703159. [DOI] [PubMed] [Google Scholar]
- 17.Cragan JD, Khoury MJ. Effect of Prenatal Diagnosis on Epidemiologic Studies of Birth Defects. Epidemiology. 2000; [DOI] [PubMed] [Google Scholar]
- 18.Velie EM, Shaw GM. Impact of Prenatal Diagnosis and Elective Termination on Prevalence and Risk Estimates of Neural Tube Defects in California, 1989–1991. American Journal of Epidemiology. 1996;144(5):473–479. [DOI] [PubMed] [Google Scholar]
- 19.Parks SE, Campbell K, Ramadhani TA. Importance of including all pregnancy outcomes to reduce bias in epidemiologic studies of neural tube defects-Texas, 1999 to 2005. Birth Defects Res Part A Clin Mol Teratol. 2011;91(3):185–191. doi: 10.1002/bdra.20772. [DOI] [PubMed] [Google Scholar]
- 20.Howards PP, Johnson CY, Honein MA, FLANDERS WD, National Birth Defects Prevention Study. Adjusting for bias due to incomplete case ascertainment in case-control studies of birth defects. American Journal of Epidemiology. 2015;181(8):595–607. doi: 10.1093/aje/kwu323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lash TL, Fox MP, MacLehose RF, Maldonado G, McCandless LC, Greenland S. Good practices for quantitative bias analysis. International Journal of Epidemiology. 2014;43(6):1969–1985. doi: 10.1093/ije/dyu149. [DOI] [PubMed] [Google Scholar]
- 22.Westreich D Berksonʼs Bias, Selection Bias, and Missing Data. Epidemiology. 2012;23(1):159–164. doi: 10.1097/EDE.0b013e31823b6296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Infante-Rivard C, Cusson A. Reflection on modern methods: selection bias—a review of recent developments. International Journal of Epidemiology. 2018;47(5):1714–1722. doi: 10.1093/ije/dyy138. [DOI] [PubMed] [Google Scholar]
- 24.Arnold BF, Ercumen A, Benjamin-Chung J, Colford JM Jr. Negative Controls to Detect Selection Bias and Measurement Bias in Epidemiologic Studies. Epidemiology. 2016;27(5):637–641. doi: 10.1097/EDE.0000000000000504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huybrechts KF, Palmsten K, Avorn J, et al. Antidepressant Use in Pregnancy and the Risk of Cardiac Defects. New England Journal of Medicine. 2014;370(25):2397–2407. doi: 10.1056/NEJMoa1312828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thorpe PG, Gilboa SM, Hernández-Díaz S, Lind J, Cragan JD, Briggs G, et al. Medications in the first trimester of pregnancy: most common exposures and critical gaps in understanding fetal risk. Pharmacoepidemiology and Drug Safety. 2013;22(9):1013–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reefhuis J, Gilboa SM, Anderka M, Browne ML, Feldkamp ML, Hobbs CA, et al. The national birth defects prevention study: A review of the methods. Birth Defects Research Part A Clinical and Molecular Teratology. 2015;103:656–669. doi: 10.1002/bdra.23384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rasmussen SA, Olney RS, Holmes LB, Lin AE, Keppler Noreuil KM, Moore CA. Guidelines for case classification for the national birth defects prevention study. Birth Defects Research Part A Clinical and Molecular Teratology. 2003;67(3):193–201. doi: 10.1002/bdra.10012. [DOI] [PubMed] [Google Scholar]
- 29.Rothman KJ, Greenland S, Lash TL. Modern Epidemiology. 2008.
- 30.Gunnerbeck A, Bonamy A-KE, Wikström A-K, Granath F, Wickström R, Cnattingius S. Maternal Snuff Use and Smoking and the Risk of Oral Cleft Malformations - A Population-Based Cohort Study. PLoS ONE. 2014;9(1):e84715. doi: 10.1371/journal.pone.0084715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Feldkamp ML, Srisukhumbowornchai S, Romitti PA, Olney RS, Richardson SD, Botto LD. Self-reported maternal cigarette smoke exposure during the periconceptional period and the risk for omphalocoele. Paediatric and Perinatal Epidemioogyl. 2014;28(1):67–73. doi: 10.1111/ppe.12093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xuan Z, Zhongpeng Y, Yanjun G, et al. Maternal active smoking and risk of oral clefts: a meta-analysis. Oral Surgery Oral Medicine Oral Pathology Oral Radiology. 2016;122(6):680–690. doi: 10.1016/j.oooo.2016.08.007. [DOI] [PubMed] [Google Scholar]
- 33.Meng X, Sun Y, Duan W, Jia C. Meta-analysis of the association of maternal smoking and passive smoking during pregnancy with neural tube defects. International Journal of Gynecology & Obstetrics. 2018;140(1):18–25. doi: 10.1002/ijgo.12334. [DOI] [PubMed] [Google Scholar]
- 34.Shaw GM, Carmichael SL, Vollset SE, Yang W, Finnell RH, Blom H, et al. Mid-pregnancy cotinine and risks of orofacial clefts and neural tube defects. Journal of Pediatrics. 2009;154(1):17–19. doi: 10.1016/j.jpeds.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 35.Hackshaw A, Rodeck C, Boniface S. Maternal smoking in pregnancy and birth defects: a systematic review based on 173 687 malformed cases and 11.7 million controls. Human Reproduction Update. 2011;17(5):589–604. doi: 10.1093/humupd/dmr022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Diav-Citrin O, Shechtman S, Bar-Oz B, Cantrell D, Arnon J, Ornoy A. Pregnancy outcome after in utero exposure to valproate: evidence of dose relationship in teratogenic effect. CNS Drugs. 2008;22(4):325–334. [DOI] [PubMed] [Google Scholar]
- 37.Hernández-Díaz S, Werler MM, Walker AM, Mitchell AA. Folic Acid Antagonists during Pregnancy and the Risk of Birth Defects. New England Journal of Medicine. 2000;343(22):1608–1614. doi: 10.1056/NEJM200011303432204. [DOI] [PubMed] [Google Scholar]
- 38.Werler MM, Ahrens KA, Bosco JLF, et al. Use of Antiepileptic Medications in Pregnancy in Relation to Risks of Birth Defects. Annals of Epidemiology. 2011;21(11):842–850. doi: 10.1016/j.annepidem.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Levy A, Matok I, Gorodischer R, Sherf M, Wiznitzer A, Uziel E, et al. Bias toward the null hypothesis in pregnancy drug studies that do not include data on medical terminations of pregnancy: the folic acid antagonists. Journal of Clinical Pharmacology. 2012;52(1):78–83. doi: 10.1177/0091270010390806. [DOI] [PubMed] [Google Scholar]
- 40.Boyle B, McConkey R, Garne E, Loane M, Addor MC, Bakker MK, et al. Trends in the prevalence, risk and pregnancy outcome of multiple births with congenital anomaly: a registry-based study in 14 European countries 1984–2007. Brittish Journal Obstetrics & Gynaecology. 2013;120(6):707–716. doi: 10.1111/1471-0528.12146. [DOI] [PubMed] [Google Scholar]
- 41.Dawson AL, Tinker SC, Jamieson DJ, Hobbs CA, Berry RJ, Rasmussen SA, et al. Twinning and major birth defects, National Birth Defects Prevention Study, 1997–2007. Journal of Epidemiology & Community Health. 2016;70(11):1114–1121. doi: 10.1136/jech-2015-206302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stillbirth Collaborative Research Network Writing Group. Association between stillbirth and risk factors known at pregnancy confirmation. The Journal of the American Medical Association. 2011;306(22):2469–2479. doi: 10.1001/jama.2011.1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Greenland S, Daniel R, Pearce N. Outcome modelling strategies in epidemiology: traditional methods and basic alternatives. International Journal of Epidemiology. 2016;45(2):565–575. doi: 10.1093/ije/dyw040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Greenland S, Mansournia MA, Altman DG. Sparse data bias: a problem hiding in plain sight. BMJ. April 2016:i1981–i1986. doi: 10.1136/bmj.i1981. [DOI] [PubMed] [Google Scholar]
- 45.Gregory ECW & MacDorman MF Fetal and Perinatal Mortality: United States, 2013. Natl Vital Stat Rep 64, 1–24 (2015). [PubMed] [Google Scholar]
- 46.Pryde PG, Isada NB, Hallak M, Johnson MP, Odgers AE, Evans MI. Determinants of parental decision to abort or continue after non-aneuploid ultrasound-detected fetal abnormalities. Obstetrics & Gynecology. 1992;80(1):52–56. [PubMed] [Google Scholar]
- 47.Wacholder S, McLaughlin JK, Silverman DT, Mandel JS. Selection of controls in case-control studies. I. Principles. American Journal of Epidemiology. 1992;135(9):1019–1028. [DOI] [PubMed] [Google Scholar]
- 48.Cogswell ME, Bitsko RH, Anderka M, et al. Control selection and participation in an ongoing, population-based, case-control study of birth defects: the National Birth Defects Prevention Study. American Journal of Epidemiology. 2009;170(8):975–985. doi: 10.1093/aje/kwp226. [DOI] [PubMed] [Google Scholar]
- 49.Hook EB and Regal RR. Representative and misrepresentative associations of birth defects in livebirths. Conditions under which relative risks greater than unity in livebirths necessarily imply relative risks greater than unity in all conceptuses. American Journal of Epidemiology. 1993;137(6):660–675. [DOI] [PubMed] [Google Scholar]
- 50.Parker SE, Mai CT, Campbell K, Canfield MA, Rickard R, Wang Y, Meyer RE, et al. Updated national birth prevalence estimates for selected birth defects in the United States, 2004–2006. Birth Defects Research Part A Clinical and Molecular Teratology. 2010;88(12):1008–1016. doi: 10.1002/bdra.20735. [DOI] [PubMed] [Google Scholar]
- 51.Jatlaoui TC. Jatlaoui C, Shah J, Mandel MG, Krashin JW, Suchdev DB, Jamieson DJ, Pazol K. Abortion Surveillance — United States, 2014. Morbidity and Mortality Weekly Report Surveillence Summary. 2017;66(24):1–48. doi: 10.15585/mmwr.ss6624a1. [DOI] [PubMed] [Google Scholar]
- 52.Wilcox AJ, Weinberg CR, O’Connor JF, Baird DD, Schlatterer JP, Canfield RE, et al. Incidence of early loss of pregnancy. New England Journal of Medicine. 1988;319(4):189–194. doi: 10.1056/NEJM198807283190401. [DOI] [PubMed] [Google Scholar]
- 53.Mines D, Tennis P, Curkendall SM, Li DK, Peterson C, Andrews EB, et al. Topiramate use in pregnancy and the birth prevalence of oral clefts. Pharmacoepidemiol Drug Saf. 2014;23(10):1017–1025. doi: 10.1002/pds.3612. [DOI] [PubMed] [Google Scholar]
- 54.Cheung K, Marroun El H, Elfrink ME, Jaddoe VWV, Visser LE, Stricker BHC. The concordance between self-reported medication use and pharmacy records in pregnant women. Pharmacoepidemiology and Drug Safety. 2017;26(9):1119–1125. doi: 10.1002/pds.4264. [DOI] [PubMed] [Google Scholar]
- 55.Pisa FE, Casetta A, Clagnan E, Michelesio E, Vecchi Brumatti L, Barbone F. Medication use during pregnancy, gestational age and date of delivery: agreement between maternal self-reports and health database information in a cohort. BMC Pregnancy Childbirth. 2015;15(1):310. doi: 10.1186/s12884-015-0745-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pickett KE, Rathouz PJ, Kasza K, Wakschlag LS, Wright R. Self-reported smoking, cotinine levels, and patterns of smoking in pregnancy. Paediatr Perinat Epidemiol. 2005;19(5):368–376. doi: 10.1111/j.1365-3016.2005.00660.x. [DOI] [PubMed] [Google Scholar]
- 57.McDonald SD, Perkins SL, Walker MC. Correlation between self-reported smoking status and serum cotinine during pregnancy. Addictive Behaviors. 2005;30(4):853–857. doi: 10.1016/j.addbeh.2004.08.016. [DOI] [PubMed] [Google Scholar]
- 58.Tinker SC, Gilboa S, Reefhuis J, Jenkins MM, Schaeffer M, Moore CA. Challenges in Studying Modifiable Risk Factors for Birth Defects. Curr Epidemiol Rep. 2014;2(1):23–30. doi: 10.1007/s40471-014-0028-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.