Abstract
The family of hereditary cerebellar ataxias is a large group of disorders with heterogenous clinical manifestations and genetic etiologies. Among these, over 30 autosomal dominantly inherited subtypes have been identified, collectively referred to as the spinocerebellar ataxias (SCAs). Generally, the SCAs are characterized by a progressive gait impairment with classical cerebellar features, and in a subset of SCAs, accompanied by extra-cerebellar features. Beyond the common gait impairment and cerebellar atrophy, the wide range of additional clinical features observed across the SCAs is likely explained by the diverse set of mutated genes that encode proteins with seemingly disparate functional roles in nervous system biology. By synthesizing knowledge obtained from studies of the various SCAs over the past several decades, convergence onto a few key cellular changes, namely ion channel dysfunction and transcriptional dysregulation, has become apparent and may represent central mechanisms of cerebellar disease pathogenesis. This review will detail our current understanding of the molecular pathogenesis of the SCAs, focusing primarily on the first described autosomal dominant spinocerebellar ataxia, SCA1, as well as the emerging common core mechanisms across the various SCAs.
Keywords: Spinocerebellar ataxia, CAG/polyglutamine disorder, Repeat expansion, SCA1, ATXN1, Ataxin-1, Neurodegeneration
Introduction
Ataxia is a broadly defined clinical feature of many neurological disorders and is characterized by an impairment in the coordination of voluntary movements, including those associated with speech, gait, and fine motor movements, among others. Ataxia can manifest due to a variety of environmental or genetic reasons. There have been several groups of monogenic inherited ataxias that have been described, which have been collectively termed as the hereditary cerebellar ataxias. Broadly, the hereditary cerebellar ataxias are a heterogenous family of disorders that result primarily in a progressive degeneration of the tissues involved in the coordination of movement, some of which progress to affect bulbar regions involved in respiratory functions. Although each individual hereditary ataxia is considered a rare disease, the family of hereditary ataxias has been reported to have a collective prevalence of 1–9 in 100,000 based on ethnic group [1–6]; however, these numbers are likely underestimates due to diagnostic criteria variations, molecular screening limitations, as well as a large number of familial cerebellar ataxias with unknown genetic origins. The genetic etiology underlying a large majority of spinocerebellar ataxias has been described, with a variety of inheritance patterns. The hereditary ataxias have been primarily classified into two main subfamilies of disorders based on the mode of inheritance: the spinocerebellar ataxias (SCAs), inherited in an autosomal dominant manner, and the recessive ataxias, which include the spinocerebellar ataxia, autosomal recessive disorders (SCARs). Within these groups, there is a tremendous degree of genetic and clinical heterogeneity, with over 30 genetically distinct SCAs and an even greater number of recessive ataxia-causing genes that continues to increase with the identification of novel disease-causing rare mutations. In addition, forms of ataxia with X-linked and mitochondrial inheritance patterns have been described. The complex genetics underlying the hereditary ataxias have been previously reviewed in depth [2, 3, 7–10].
Among the autosomal dominantly inherited SCAs, mutations in many functionally disparate genes have been identified, ranging from genes encoding transcriptional co-regulators, ion channels, and kinases, to many others. The mutations identified in these genes are of many different types, including missense mutations, nonsense mutations, duplications/deletions, as well as repeat expansion mutations. Following the identification of a CAG repeat expansion mutation in Androgen Receptor (AR) as being causal for spinal and bulbar muscular atrophy (SBMA) [11], it was determined that a subset of highly penetrant SCAs was due to similar polyglutamine (polyQ) repeat expansion mutations, which were later named SCA1, SCA2, SCA3/Machado-Joseph Disease (MJD), SCA6, SCA7, SCA17, and dentatorubral-pallidoluysian atrophy (DRPLA). As is the case with other polyQ diseases, including Huntington’s disease (HD) [12], a subset of polyQ SCAs are characterized by trans-generational anticipation in which the unstable repeat tract can expand further in subsequent generations due to germline CAG genomic instability [13–15], and this longer repeat length is typically associated with an earlier onset of disease and more severe disease phenotypes [16]. Although the specific gene containing the repeat expansion is unique in the different polyQ SCAs, extensive past and ongoing studies on each of these disorders are beginning to reveal converging pathological molecular mechanisms that may explain common behavioral and pathological abnormalities observed across the SCAs. This review will focus on the autosomal dominant SCAs, describing the convergent and divergent molecular mechanisms underlying these various disorders, with a focus on the molecular and cellular pathogenesis of SCA1.
Spinocerebellar ataxia type 1 (SCA1)
The first step in identifying the genetic locus for a hereditary cerebellar ataxia occurred when the HLA complex of chromosome 6 was linked to a form of the disease that was clinically distinct from the previously described Friedrich’s ataxia [17], and this was subsequently validated in a larger cohort [18, 19]. Following the discovery of CAG repeat expansion mutations in patients with SBMA [11] and HD [20], the underlying genetic cause of SCA1 was first described in 1993 when the presence of an unstable CAG repeat was identified in ATXN1 using positional cloning and linkage analysis of inherited spinocerebellar ataxia patients [21–23]. The identification of this disease-causing mutation led to the beginning of molecularly defining the various hereditary cerebellar ataxias.
With a molecular definition of SCA1, genetic testing to detect ATXN1 expansions has enabled rapid and accurate diagnosis of this monogenic disorder. It has been estimated that 1–2 in 100,000 develop SCA1 during their lifetime, with SCA1 patients making up approximately 6% of all autosomal dominant cerebellar ataxias. However, as mentioned above, these numbers vary greatly across ethnic groups [3, 24]. Clinically, SCA1 is characterized by progressive muscle weakening and ataxia, with difficulties in speech and swallowing. At later stages, symptoms progress to include muscle atrophy, cognitive deficiencies, and bulbar dysfunction leading to respiratory complications, which is the primary cause of death [25]. Upon diagnosis, typically in the third or fourth decades of life, the duration of the disease ranges from 10 to 30 years; however, both age of onset and rate of disease progression are affected by the length of the CAG repeat expansion [16]. Pathologically, SCA1 patients display cerebellar and brain stem degeneration, with atrophy of the ventral pons and middle cerebellar peduncles [26], as well as profound loss of Purkinje cells of the cerebellum and neurons of the inferior olivary nucleus [27]. In addition to overt loss of these populations of cells, loss of neurons in cortical, subcortical, and spinal structures has also been reported [26, 28, 29]. As is the case with most other neurodegenerative disorders, SCA1 patients develop aberrant protein aggregates, which is replicated in animal models. However, in the case of the nuclear ataxin-1-positive inclusions observed in SCA1, it is unclear how or if these aggregates contribute to disease pathogenesis, as transgenic mice expressing non-aggregating polyQ-expanded ataxin-1 still displayed behavioral deficits and Purkinje cell degeneration [30].
Ataxin-1 in the central nervous system and in disease
The ATXN1 gene is located at 6p22.3 and encodes the 816-amino acid, 87 kDa protein, ataxin-1, which is widely expressed across neuronal and glial populations throughout the central nervous system [31, 32]. In humans, ATXN1 contains nine exons, with the first seven residing in the 5′ untranslated region (UTR) and the eighth and ninth encoding the protein-coding sequences [33]. The first protein-coding exon carries the CAG repeat tract, which is aberrantly expanded in SCA1 patients. Structurally, ataxin-1 contains several key domains in addition to its polyQ tract that mediate its normal functions and pathogenic behavior in SCA1 (Fig. 1). The C-terminus of ataxin-1 carries a highly conserved region with three functionally important domains—the 14–3–3 binding domain, the U2AF-homology ligand motif, and nuclear localization signal (NLS). Additionally, the ataxin-1/HBP-1 (AXH) domain mediates many of its protein–protein interactions, as well as its RNA-binding activity. Through these domains, ataxin-1 can bind RNA and a host of other proteins, most notably several transcription factors and splicing factors, and shuttle between the nucleus and cytoplasm [34, 35].
Fig. 1.
Protein organization of ataxin-1. Schematic displaying the key domains of human ataxin-1 protein. Post-translational modification sites are displayed above the protein with key enzymes involved in the modification displayed on the right. Interactors of ataxin-1 are displayed below the domain with which they interact
SCA1 patients carry expansions of the CAG repeat tract of ATXN1. Normally, unexpanded ATXN1 alleles contain 4–36 CAG repeats in this polyQ tract, with those containing greater than 21 repeats typically containing 1–3 interrupting CAT codons [36]. The expansion of the repeat tract of one allele of ATXN1 to 39 or more uninterrupted CAG repeats, or 43 or more CAG interrupted repeats in SCA1 patients, results in the highly penetrant, late-onset, and progressive cerebellar ataxia [23, 37, 38], while expansions between 36 and 38 trinucleotide repeats can result in ataxia without the other clinical features of SCA1 [38]. The significance of interruptions in the ATXN1 CAG tract in SCA1 pathogenesis has been a topic of interest, as interrupting codons profoundly influence the properties of the ATXN1 gene and its protein product, ataxin-1 [37, 39–41]. Sequence analysis of CAT interruptions in unexpanded and expanded alleles in SCA1 patients demonstrated that nearly all unexpanded alleles larger than 21 repeats carry 1 or more interrupting CAT codons, while mutant alleles frequently do not [37]. This finding led to the hypothesis that loss of interrupting codon(s) confers a greater propensity for aberrant expansion of the repeat tract, which can help to explain anticipation, the phenomenon of intergenerational expansion of mutant alleles [18, 42]. In addition to affecting genomic repeat instability, histidine-encoding CAT codons also modulate the pathogenicity of expanded ataxin-1 protein, as evidenced by the effect of repeat interruption configuration on disease age of onset and severity [41, 43, 44], as well as the effects on penetrance of intermediate length alleles [38, 45]. Several studies of repeat configuration have demonstrated that the presence of interrupting histidine residues reduces aggregation kinetics of expanded ataxin-1 through decreasing the rate of formation and stability of insoluble aggregates [41, 46–48], which may explain the effect of histidine interruptions on pathogenicity and penetrance.
While the exact molecular basis for the pathogenicity of polyQ-expanded ataxin-1 is not completely understood, extensive studies have suggested that the pathogenic changes result from toxic gain-of-function mechanisms rather than loss-of-function mechanisms, as complete genetic ablation of Atxn1 does not induce ataxia-like behaviors or neurodegeneration in mice [49]. Although these gain-of-function changes are thought to be the primary driver of disease-related changes, it is also believed that partial loss-of-function mechanisms may exacerbate disease phenotypes in the presence of polyQ-expanded ataxin-1 [50], as removal of the non-expanded Atxn1 allele in Atxn1154Q/2Q SCA1 knock-in mice worsens disease phenotypes [51]. Furthermore, overexpression of a protein related to ataxin-1 named brother of ataxin-1 (BOAT), also known as ataxin-1-like (ATXN1L), can suppress neurodegenerative phenotypes associated with polyQ expansion of ataxin-1 [52, 53].
Many previous studies have provided useful insights into key interactions that may underlie SCA1 pathogenesis. Due to the repeat nature of the SCA1-causing mutation, an early question in the field was whether the mechanism of pathogenicity of the polyQ expansion was through aberrantly increasing the interactions between ataxin-1 and its native binding partners or if the expansion resulted in novel disease-specific interactions. The initial efforts to understand these novel gain-of-function mechanisms involved the assessment of protein interactors of ataxin-1, in particular, those whose behaviors were altered in response to polyQ expansion. These efforts led to a wealth of knowledge regarding ataxin-1 functions within its native protein complexes. For example, it was determined that ataxin-1 interacts with 14–3–3 proteins through its 14–3–3 binding domain in the conserved C-terminus region and that this interaction was enhanced with polyQ expansion of ataxin-1 [54]. This interaction stabilizes ataxin-1, enhancing its levels and promoting nuclear inclusion formation, which may be involved in the subsequent neurodegeneration observed in SCA1. In addition to the C-terminus of ataxin-1 mediating its binding to 14–3–3 proteins, it was also determined that nearby residues are involved in the interactions between ataxin-1 and various splicing factors, including RBM17 [51] and U2AF65 [55]. Importantly, the ability of ataxin-1 to localize to the nucleus via its C-terminus NLS has been shown to be necessary for disease pathogenesis, as mutation of the NLS completely abrogates toxicity in a transgenic model of SCA1 [30].
In addition to these interactions through the functional motifs of its C-terminus, ataxin-1 interacts with several proteins through its AXH domain, and many of these interactions are involved in disease mechanisms. Ataxin-1 was found to be abundant in large protein complexes containing the transcriptional repressor Capicua (CIC), whose transcriptional repression activity is altered in the presence of expanded ataxin-1. The ataxin-1–CIC interaction occurs via the AXH domain of ataxin-1 [56]. Follow-up studies have examined how SCA1 mutations affect the interaction between CIC and ataxin-1 [51, 53, 56–58]. Similarly, the AXH domain of ataxin-1 mediates its interactions with Tip60/retinoid acid orphan-related receptor alpha (RORα) [59, 60], with Gfi-1 [61], and ATXN1L [53]. The incorporation of expanded ataxin-1 into these complexes alters the stability and activity of the native complexes, as well as the levels of ataxin-1 protein itself. By modulating the levels of these interacting partners or their binding to ataxin-1, including through deletion of the AXH domain itself or alteration of polyQ tract length, many aspects of the disease process can be modulated [53, 57, 59, 61]. Interestingly, both the AXH domain and the polyQ tract of ataxin-1 interact with leucine-rich acidic nuclear protein (LANP) in a repeat length-dependent manner, and the expression pattern of LANP has been suggested to potentially explain selective neuronal vulnerability in SCA1 [62]. Functionally, mutant ataxin-1 is thought to compete for LANP binding with the transcriptional repressor P120E4F (E4F) [63], and this gain-of-function may explain the neuritic pathology observed in Purkinje cells, as LANP reduction in SCA1 knock-in mice reversed molecular layer thinning [64, 65]. Collectively, these data, together with the critical role of post-translational modification of ataxin-1 that is discussed in the following section, reveal that although the expanded polyQ tract of ataxin-1 is necessary for SCA1 pathogenesis, it is not sufficient, as these other key domains are crucial.
Although ataxin-1 associates within these various signaling complexes, it was recently concluded that the primary driver for SCA1 pathogenesis in the cerebellar Purkinje cells is ataxin-1’s interaction with CIC, as mutagenesis of key amino acid residues involved in the ataxin-1–CIC interaction, V591A and S602D, prevented toxicity in a Purkinje cell-specific SCA1 transgenic mouse model [58, 66]. This interaction was proposed to mediate a gain-of-function mechanism central to pathology, as cerebellar Cic knock-out was not sufficient to drive neurodegenerative phenotypes, in line with the previous data showing that haploinsufficiency of Cic reduced disease severity in SCA1 knock-in mice [57]. Although structural studies have shown that these mutations are sufficient to abolish the binding of ataxin-1 with CIC [58], it is unclear whether these mutations similarly disrupt its interactions with other binding partners that also associate via the AXH domain or additional functions of ataxin-1.
Beyond its causal role in SCA1, ataxin-1 has been implicated in the other neurodegenerative diseases. Interestingly, intermediate length polyQ expansions of ataxin-1 (≥ 32 repeats) that do not expand to the pathogenic SCA1-causing length are associated with a greater risk for developing sporadic amyotrophic lateral sclerosis (sALS) [67, 68]. However, the mechanism for this remains completely elusive. In addition, a genome-wide association study (GWAS) of a cohort of late-onset Alzheimer’s disease (LOAD) patients revealed a SNP in an intronic region of ATXN1 that is associated with greater risk and earlier onset of LOAD [69]. Follow-up studies in mice have revealed that loss of Atxn1 may contribute to AD-related phenotypes through increasing the proteolytic cleavage of amyloid precursor protein (APP) via an increase in the transcription of the BACE1, encoding the β-secretase enzyme [70, 71]. Whether the BACE1-related mechanisms attributed to ataxin-1 loss-of-function are involved in SCA1 pathogenesis is not known. These studies warrant a closer investigation into extra-cerebellar regions of the brain that may be affected by these changes, which may explain the cognitive decline observed in SCA1 patients [8].
Post-translational modifications of ataxin-1
Across numerous other neurodegenerative diseases, including several other polyQ repeat expansion disorders, pathogenicity of disease-causing or disease-associated proteins has been observed to be modulated by post-translational modifications [72–75]. In addition to the CAG repeat tract, the AXH domain, and the conserved motifs near the C-terminus of ataxin-1, phosphorylation at several specific amino acid residues of ataxin-1 has been shown to modulate ataxin-1’s functions and to be crucial for the SCA1 pathogenesis [76–78]. The best characterized of these sites is serine 776 (S776) in the C-terminal region of ataxin-1. The functional significance of phosphorylation at S776–SCA1 pathogenesis has been demonstrated through models in which mutations at this residue prevent its phosphorylation (S776A) or the constitutive phospho-mimetic (S776D) form. In cell culture and Purkinje cell-specific transgenic animal models harboring polyQ expansion mutation of ataxin-1, S776A mutation alone substantially reduced nuclear ataxin-1 inclusion formation and significantly ameliorated disease induction [79]. Interestingly, transgenic mice expressing an ATXN1 transgene with a wild-type length polyQ tract (30Q), but carrying a phosphomimetic S776D mutation, displayed many similar features of the ataxin-1 [82Q] transgenic mouse model with the absence of cell death [80]. These data suggest that S776 phosphorylation contributes to neuronal dysfunction, while the expanded polyQ tract renders the Purkinje cells susceptible to eventual degeneration [80].
Mechanistically, phosphorylation of ataxin-1 at S776 by Akt kinase and other kinases increases interactions of phospho-ataxin-1 with the molecular chaperone protein 14–3–3 [54], increasing the stability of ataxin-1 and preventing dephosphorylation by phosphatases [81]. The stabilization of mutant ataxin-1 subsequently increases the propensity for aggregate formation, which may or may not be involved in the toxic gain-of-function mechanisms observed in SCA1 [36]. The increased binding of ataxin-1 with 14–3–3 also decreases ataxin-1’s binding to the splicing factor U2AF65 in the large spliceosome complex, which may favor ataxin-1’s self-association, as well as affect U2AF65-mediated splicing [55, 82]. In addition to increasing the stability of cytoplasmic ataxin-1 through 14–3–3 interactions, the phosphorylation at S776 alters the distribution of ataxin-1 from its native protein complexes, increasing the interaction of ataxin-1 with RBM17, while decreasing its incorporation into separate CIC-containing complexes [51]. This polyQ length-dependent shift from large CIC-containing complexes to preferential incorporation into RBM17-containing protein complexes explains, in part, the gain- and loss-of-function mechanisms at play in SCA1 [51]. Similarly, the interaction between ataxin-1 and Tip60 increases upon S776 phosphorylation, enhancing pathogenesis by disrupting RORα-mediated gene expression [59, 60].
Because the rescue observed in transgenic animals carrying S776A mutation was incomplete and the mutation did not completely abolish phospho-ataxin-1 [79], it was predicted that there may be other residues within ataxin-1 that are the sites of phosphorylation and may also contribute to pathogenicity of polyQ-expanded ataxin-1. Follow-up studies identified a phospho-site near the polyQ tract at S239 with consensus recognition motifs for various kinases, including ERK, a component of the MAPK signaling pathway [83]. This site was later proposed to be a site of phosphorylation by Nemo-like kinase (Nlk), a MAPK-like kinase, and reduction of Nlk reduced polyQ-expanded ataxin-1-associated toxicity in a Drosophila and mouse model of SCA1 [84]. The precise mechanism through which S239 phosphorylation contributes to SCA1 pathogenesis is not understood, but may provide an alternative avenue for future therapeutic approaches.
To capitalize on the knowledge that ataxin-1 phosphorylation is crucial for SCA1 pathogenesis, further efforts have been placed into identifying the specific kinases responsible for phosphorylation of ataxin-1 [85]. These approaches have revealed a host of protein kinases capable of phosphorylating ataxin-1 at various residues. Although the initial studies identified Akt as the kinase that phosphorylates ataxin-1 at S776 in cell culture and Drosophila [54, 85, 86], it was later determined that its reduction did not decrease ataxin-1-S776 phosphorylation in SCA1 transgenic mice [87], suggesting an alternate or redundant kinase for ataxin-1 in mammals. Rather, protein kinase A (PKA) was found to be an in vivo kinase of mammalian ataxin-1 at S776 and its reduction delayed disease onset in SCA1 mice [87, 88]. In a parallel unbiased Drosophila and cell culture genetic screen for modifiers of mutant ataxin-1 levels, multiple components of the RAS-MAPK-MSK1 pathway altered mutant ataxin-1 levels through MSK1 phosphorylation of S776 [89]. A forward genetic screen in Drosophila identified Pak3 as a modulator of ataxin-1 levels, and reduction of Pak3 or related kinase Pak1 reduced ataxin-1 levels and suppressed polyQ-expanded ataxin-1-associated neurotoxicity independent of S776 [90].
In addition to being phosphorylated, ataxin-1 interacts with several key molecules involved in protein ubiquitination, quality control, and folding. First, ataxin-1 interacts with UbcH6, an E2 ubiquitin-conjugating enzyme [91]. Ubiquitination of ataxin-1 by UbcH6 suppresses its transcriptional repression, while short hairpin RNA (shRNA)-mediated reduction of UbcH6 increases its repression by altering the degradation of ataxin-1 [92]. Furthermore, ataxin-1 co-localizes with co-chaperone/E3 ligase C-terminus of Hsc-70 interacting protein (CHIP) and molecular chaperones in cell culture and human SCA1 post-mortem tissue [93, 94], which has been shown to assist in the degradation of Hsp70- and Hsp90-bound substrates [95]. This ubiquitination of ataxin-1 by CHIP catalyzes the ubiquitination and eventual degradation of unexpanded and polyQ-expanded ataxin-1 [93] and the sequestration of ataxin-1 into detergent-insoluble aggregates [94]. These result in a neuroprotective effect in a Drosophila model of SCA1. Interestingly, the effect of CHIP on ataxin-1 is dependent on phosphorylation at S776 [94]. Finally, ataxin-1 also interacts with ataxin-1 interacting ubiquitin-like protein (A1Up), which carries an N-terminal ubiquitin-like region [96]. This interaction affects the ability of A1Up to interact with the 19S proteasome [97]. Collectively, these interactions suggest a key role of the ubiquitin–proteasome pathway in mediating the processing of ataxin-1.
Several studies have examined the effect of altering ataxin-1 processing on SCA1-related phenotypes. Genetic reduction of Ube3a, which encodes the E3 ubiquitin ligase E6-AP, in Purkinje cell-specific ataxin-1 [82Q] transgenic mice resulted in a reduction of ataxin-1 aggregation, but exacerbated Purkinje cell pathology [98]. This suggested that the formation of nuclear inclusions is not essential for pathology, but rather, sequestering of ataxin-1 into insoluble aggregates may prevent it from inducing deleterious effects. In the other studies, overexpression of chaperone HDJ-2 resulted in reduced ataxin-1 aggregation in vitro [99], and overexpression of stress-induced Hsp70 in vivo ameliorated motor and pathological phenotypes in SCA1 transgenic mice [100]. Seemingly paradoxically, a follow-up study determined that Hsc70/Hsp70 mediates the stabilizing effect of Akt-induced phosphorylation of S776 on ataxin-1 [86]. Overall, these studies suggest that Hsc70 may be involved in conferring a less pathogenic conformation for ataxin-1. These studies underscore the complex roles of various ataxin-1 conformational species in SCA1 pathogenesis.
Beyond changes in phosphorylation and ubiquitination, ataxin-1 is known to be regulated through a number of other post-transcriptional and post-translational modifications. Some of these additional modifications of ataxin-1 include SUMOylation [101–103] and transglutamination [104, 105]. The importance of ataxin-1 post-translational modifications has been comprehensively reviewed previously [76–78]. Finally, in addition to the post-translational modifications of ataxin-1 protein described above, the post-transcriptional regulation of ATXN1 mRNA levels by the RNA-binding protein PUMILIO1 has recently been described, where Pumilio1 haploinsufficiency results in increased ataxin-1 levels [106].
Transcriptomic approaches to understanding core pathogenic mechanisms of SCA1
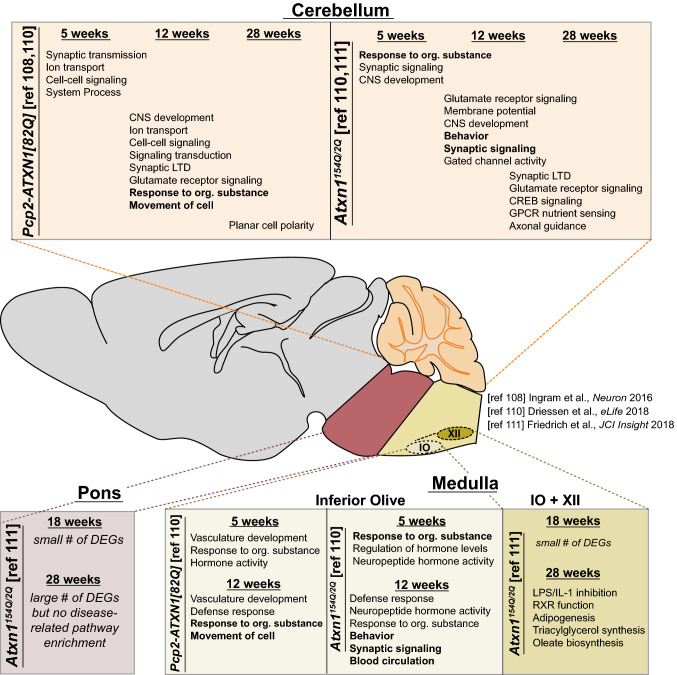
Due to the well-documented interactions of ataxin-1 with several key regulators of transcription, including CIC [51, 56, 58, 66], Tip60/RORα [59, 60], Gfi-1 [61], and SMRT [107], it has been posited that the pathogenic effects of polyQ-expanded ataxin-1 could manifest through altered activity of transcriptional regulation complexes in which ataxin-1 is found. To more broadly characterize the time-dependent, tissue-specific alterations that may explain the pathogenesis of SCA1, multiple groups have employed unbiased transcriptomic approaches across multiple SCA1 animal models. These studies have identified transcriptional signatures of the affected tissues that offer insights into key mechanisms of SCA1 (Fig. 2). Weighted gene co-expression network analysis (WGCNA) of mRNA sequencing data from the cerebellum of SCA1 Purkinje cell-specific transgenic mice at early, moderate, and late stages of disease revealed two gene modules associated with cerebellar disease pathology [108]. Of these modules, one contained genes pertinent to glutamatergic receptor and synaptic long-term depression, many of which are enriched or exclusively expressed in Purkinje cells and contain CIC-binding sites in their promoter regions. Interestingly, some of these genes are similarly altered in SCA1 knock-in mice and other SCA animal models, providing the further evidence for the relevance of these changes to SCA pathogenesis. The second module identified in this study contained genes associated with planar cell polarity, which has been suggested to be crucial for appropriate nervous system development and function [109]. The differential expression of these genes likely represents non-cell-autonomous changes in additional cerebellar populations of cells, potentially due to compensatory pathways in response to primary Purkinje cell insults. This study also examined the transcriptional changes in Purkinje cell-specific ataxin-1[30Q] S776D animals, which develop ataxia, but do not display progressive Purkinje cell pathology [108]. Notably, Cck was significantly upregulated in these animals and targeted deletion of Cck or its receptor, Cck1r, was sufficient to induce progressive Purkinje cell degeneration, suggesting a neuroprotective role of Cck in the cerebellum of these mice.
Fig. 2.
Summary of SCA1 transgenic and knock-in mouse RNA-sequencing. Summary of findings from transcriptional profiling of the cerebellum, pons, and medulla of SCA1 Purkinje cell-specific transgenic mouse (Pcp2-ATXN1[82Q]) and Atxn1154Q/2Q (knock-in) mice throughout disease [108, 110, 111]. Pathways in bold represent commonly perturbed pathways across multiple tissues within a given model at that time point. RNA-sequencing of the medulla was performed on either the inferior olivary nucleus (IO) alone [110], or the IO and hypoglossal nucleus (IO + XII) [111]
This unbiased assessment of SCA1-associated changes in Purkinje cell-specific mouse models has been extended to the cerebellum of the SCA1 knock-in mouse model and beyond to other affected tissues mainly within the brainstem [110, 111]. By performing comparisons of transcriptional changes across disease models, multiple tissues, and different time points, a clearer perspective of the core pathogenic mechanisms unique to each affected tissue at each stage of disease is beginning to emerge. Although the circuitry connecting the cerebellum and brainstem regions is tightly interconnected and proper function is essential for appropriate motor coordination, the mechanisms at play in each tissue at different stages of disease are somewhat overlapping. However, the majority of changes between the two affected tissues are largely distinct. While the cerebellar changes at the early stages of disease affect genes related to neurotransmission and processes that affect neuronal conduction, the molecular changes observed within the inferior olive involve more immune-related alterations, as enriched pathways include genes associated with the defense response [110]. Importantly, these immune-related alterations in microglia of the inferior olive appear to be non-cell-autonomous, as the changes occur both in the SCA1 knock-in model and the Purkinje cell-specific transgenic mouse model [110]. These unbiased approaches provide molecular insights into the region-specific mechanism of SCA1 pathogenesis in different affected tissues [112].
Collectively, these studies have revealed critical insights of clinical significance regarding the complex molecular underpinnings of SCA1. As described above, it has become apparent that distinct transcriptional changes occur in various SCA1-affected tissues and manifest at different times during disease progression. Multiple transcriptomics analyses have suggested that in the cerebellum, gene alterations are more strongly driven by CIC-dependent mechanisms, while less of an enrichment was observed in brainstem tissues [108, 110, 111]. Furthermore, within an affected tissue, various transcriptional changes can result from the different structural features of ataxin-1 in SCA1. Data from transcriptomics studies may be helpful in reconciling the earlier finding that the Purkinje cell-specific ataxin-1[30Q] S776D animals display ataxia-like motor impairments without progressive Purkinje cell loss. This animal model suggests that the transcriptional changes underlying ataxia are linked to ataxin-1 phosphorylation and that the Purkinje cell degeneration results from expansion of the polyQ tract. Profiling of differentially enriched pathways between these animals revealed long-term depression and glutamate receptor signaling as key divergent pathways, which may be central to Purkinje cell degeneration, warranting further experimental validation. Clinically, these data suggest that multiple molecular mechanisms that differentially contribute to various aspects of disease are at play and must be considered when designing novel therapeutic strategies. Therefore, successful approaches will target an upstream effector of affected pathways, such as an antisense oligonucleotide (ASO) targeting ATXN1 transcript for degradation, or employ combinatorial therapies that simultaneously target all major changes.
Regional and cell type vulnerability
Historically, studies of affected tissues in SCAs have focused primarily on cellular and molecular changes within the cerebellum, and more recently, the brainstem, as these regions contain the populations of cells most obviously lost in SCA1 human post-mortem tissue [113–115]. However, in patients, it is well established that clinical phenotypes beyond gait impairments are present during a patient’s lifetime, including dysarthria, dysphagia, nystagmus, hypotonia, chorea, bulbar dysfunction, and cognitive impairments, among other clinical manifestations [8, 116]. Although it is widely accepted that neurons within the cerebellum and brainstem degenerate through the course of SCA1, this presence of diverse clinical symptoms suggests that additional neuron subtypes and central nervous system tissues may also be affected, beyond the cerebellum and brainstem. Recent studies have identified that other neuronal populations, including CA2 neurons of the hippocampus [70] and spinal cord motor neurons [117] are also adversely affected in SCA1 knock-in mice, and their dysfunction correlates to the emergence of additional behavioral deficits [118]. Further examination of these other tissues will provide useful insights into the degree of impairment of tissues not classically associated with the disease and the common or unique mechanisms underlying these changes, which will help to explain the diverse constellation of symptoms observed in SCA1 patients. These studies may also provide insights into protective mechanisms present in unaffected or less affected tissues.
While neurodegenerative diseases are defined by a loss of particular populations of neurons, the contribution of non-neuronal cells to these diseases is becoming increasingly apparent in most neurodegenerative disorders [119], including ALS [120–123], Alzheimer’s disease [124–126], and Huntington’s disease [127–129]. In the case of SCA1, transgenic mice in which polyQ-expanded ataxin-1 expression is limited to Purkinje cells, as well as SCA1 knock-in mice, display an early activation of astrocytes and microglia, suggesting a non-cell-autonomous induction of neuroinflammation [130, 131]. Importantly, these glial changes occur at time points prior to overt loss of Purkinje cells and correlate well with the course of disease. Although neuroinflammation is commonly observed as a result of neurodegeneration, the emergence of these early, secondary changes in astrocytes and microglia suggests that they may contribute, at least in part, to critical pathogenic mechanisms causal for the human disease [132]. To this end, a preclinical approach geared towards reducing the microglial number or activation via the colony-stimulating factor 1 (CSF1R) inhibitor PX3397 has been shown to ameliorate SCA1 phenotypes in vivo [133]. Interestingly, inhibition of NF-κB inflammatory signaling in microglia induced motor deficits in mice without altering pathogenesis in SCA1 transgenic mice [134]. On the other hand, reduction of NF-κB in astrocytes modulated the course of disease in SCA1 transgenic mice in a bi-phasic manner in which the early reduction of pro-inflammatory signaling exacerbated disease phenotypes, while the late-stage inhibition ameliorated Purkinje cell deficits and improved motor functions [130].
Beyond inflammatory changes in glial populations, changes in the other populations have been reported. Although SCA1 is a progressive neurodegenerative disease, developmental changes in specific stem cell populations of the cerebellum have been observed in mice that express mutant ataxin-1, including Prom1-positive multipotent stem cells that give rise to postnatal astrocytes, stellate cells, and basket cells of the cerebellum [135, 136]. Furthermore, changes in oligodendrocytes, the myelin-producing cells of the central nervous system, have been suggested to occur in SCAs. Diffusion tensor imaging of SCA patients has revealed prominent white matter loss in regions of the cerebellum in SCA1 patients, and the degree of white matter changes correlated with the clinical score on the Scale for the Assessment and Rating of Ataxia (SARA) score [137]. This finding suggests that an impairment of myelination or oligodendrocyte function may contribute to cerebellar circuitry dysfunction and ataxic phenotypes. It is important to note that, as is the case with most neurodegenerative disease-associated proteins, ataxin-1 is ubiquitously expressed; therefore, its polyQ expansion mutation may have unique consequences in different cell types. Whether these glial-related changes represent a primary or secondary mechanism of SCA1 disease pathogenesis remains an outstanding question. Because of intricate cerebellar circuitry, changes in the fate of cells within the cerebellum that interact with the Purkinje cells are likely to alter the weight of specific inputs onto Purkinje cells or its activity onto deep cerebellar nuclei, thereby modulating its output, which likely contributes to the behavioral phenotypes observed in SCA1.
Other autosomal dominant cerebellar ataxias and core pathogenic mechanisms
Following the identification of CAG repeat expansion mutations in ATXN1 as the genetic cause for SCA1, polyQ mutations in six independent genes (ATXN2, ATXN3, CACNA1A, ATXN7, TBP, and ATN1) were subsequently identified as causal for different subtypes of SCA, later named SCA2 [138–140], SCA3/MJD [141], SCA6 [142], SCA7 [143], SCA17 [144, 145], and DRPLA [146], respectively, based on the mutated gene. In addition to these most common SCAs attributed to polyQ expansion mutations, there were many other monogenic SCAs inherited in an autosomal dominant fashion identified to be caused by the other types of mutations. Among these, there are SCAs attributed to noncoding 5′ UTR CAG repeat expansions (SCA12), other trinucleotide repeat expansions (SCA8), pentanucleotide repeat expansions (SCA10, SCA31), hexanucleotide repeat expansions (SCA36), chromosomal duplications (SCA20, SCA39), as well as missense mutations (SCA5, SCA13, SCA14, SCA19, SCA21, SCA22, SCA23, SCA26, SCA27, SCA28, SCA29, SCA34, SCA35, SCA38, SCA40, and SCA41). Interestingly, although the SCAs described the above result in a progressive ataxia involving cerebellar degeneration [114, 115], the genes in which the mutations occur encode proteins with diverse functions. The clinical, molecular, and pathological heterogeneities across all the SCAs are likely due to the variable nuanced functions of the mutated genes. However, the emergence of some common clinical and pathological features suggests possible core mechanisms that may be at play across the SCAs. Understanding these convergent and divergent mechanisms will provide useful insights into the specific cellular pathways of interest when studying the disease and developing therapeutic approaches. By examining the genes in which SCA-causing mutations are found combined with employing molecular genetics approaches, several key cellular mechanisms upon which these changes may converge have been identified, including Purkinje cell excitability changes and transcriptional dysregulation.
Purkinje cell excitability and membrane conductance
Purkinje cells spontaneously fire at a pacemaking frequency of about 40 Hz. This activity is directly modified through excitatory synaptic inputs from climbing fibers projecting from the inferior olivary nucleus and the parallel fibers of cerebellar granule neurons, as well as local inhibitory neurons that include basket cells and stellate cells [147]. Because the Purkinje cells are the sole source of output from the cerebellar cortex, their electrical properties and precise modulation are crucial for proper cerebellar function and coordination of motor function. Human genetics studies have revealed that mutations in several genes, whose protein products encode ion channels, including CACNA1A (SCA6), KCNC3 (SCA13), ITPR1 (SCA15, SCA16, SCA29), KCND3 (SCA19, SCA22), TRPC3 (SCA41), and CACNA1G (SCA42), are causal for a subset of SCAs. As expected, these genes are highly expressed in Purkinje cells of the cerebellum. The evidence for Purkinje cell dysfunction being central to these diseases is underscored by the fact that in addition to disease-causing mutations in ion channels, ion-channel dysfunction has been identified to occur in other SCAs, including in several polyQ SCAs due to mutations in genes whose products are not ion channels themselves [108, 148–155]. These changes in neuronal physiology in SCAs have been recently reviewed in detail elsewhere [156]. Collectively, these studies reveal that perturbations of Purkinje cell excitability, as well as synaptic transmission onto Purkinje cells, disrupt the computational circuitry within the cerebellum and are often sufficient to induce ataxia-like behaviors that may commonly underlie a large proportion of the hereditary cerebellar ataxias.
Transcriptional dysregulation
As described in detail above, a primary molecular mechanism through which SCA1-causing polyQ expansion mutations of ataxin-1 produce deleterious effects is through modifying the interaction of ataxin-1 into its native transcriptional regulator protein complexes. Beyond SCA1, several other polyQ SCAs are attributed to mutations in proteins that function in transcriptional regulator complexes, including ataxin-7, TBP, and ATN1, the proteins whose polyQ expansion cause SCA7, SCA17, and DRPLA, respectively. In the case of SCA7, ataxin-7 incorporates into the SAGA-like, the TFTC, and STAGA complexes; however, its polyQ expansion produces a dominant-negative effect of inhibiting the histone acetyltransferase activity of these complexes, thereby disturbing downstream gene expression [157, 158]. TBP, the protein mutated in SCA17, is a general transcription factor that normally binds to the TATA-box upstream of transcription start sites, subsequently recruiting the other proteins involved in transcription, including TBP-associated factors (TAFs) and RNA Polymerase I, II, and III. PolyQ-expanded TBP has been shown to bind fewer TATA boxes within the DNA [159], and although TBP is a general transcription factor, its changes in interactions with other transcription factors and their altered occupancy at specific loci are thought to underlie SCA17 pathology [160].
CACNA1A, the gene that is polyQ-expanded in SCA6, encodes the α1A subunit of the voltage-gated P/Q type channel. Interestingly, it also encodes a second cistron that can be translated in a cap-independent manner via an internal ribosome entry site independently of the full-length protein, giving rise to a stable C-terminal fragment of α1A subunit (α1ACT) [161, 162]. This C-terminal fragment can translocate to the nucleus, where it functions as a transcription factor involved in Purkinje cell development [161, 163]. While the polyQ tract is present in both the full-length calcium channel subunit, as well as the α1ACT fragment, a primary mechanism through which the polyQ-induced toxicity in SCA6 occurs is through inhibition of α1ACT activity as a transcription factor [161, 164]. Similarly, polyQ expansion of ATN1, the protein mutated in DRPLA, inhibited transcriptional activity of its interacting partner CREB-binding protein [165].
Although only a subset of SCA-causing proteins function as bona fide transcriptional regulators or transcription factors, transcriptional dysregulation can be observed much more broadly across other SCAs. For example, while the primary function of ataxin-3 is as a deubiquitinase, it is well documented that robust changes in transcriptional regulators occur in animal models of SCA3, including changes in major transcription factor signaling complexes, such as CREB [166, 167]. As the affected transcriptional complexes and factors are different across the various SCAs, a cross-examination of the converging downstream transcriptional changes across these disorders will provide further insights into commonly affected cellular mechanisms that may underlie SCA pathogenesis.
Conclusion
The family of hereditary cerebellar ataxias is comprised of a large number of genetically distinct disorders that share some common clinical and pathological features, as described above. In addition to ion-channel dysfunction and transcriptional dysregulation, several other pathways have been implicated in SCA pathogenesis, including protein quality control, RNA metabolism, and mitochondrial dysfunction, among others. In the case of perturbed protein quality control, key cellular protein clearance pathways have been shown to be affected in specific SCAs [168, 169]. Furthermore, the formation of ubiquitin-positive inclusions containing the expanded disease-causing protein has been observed across some of the polyQ SCAs [144, 170–175]; however, the pathological contribution of these aggregates to the disease mechanism is a topic of debate. In some instances, inclusions correlate with disease severity [170], while in others, are suggested to potentially play a protective role in the disease [176]. A more detailed interrogation of the existence and functional consequences of specific oligomeric aggregated intermediates of each of the polyQ-expanded proteins is required before a unifying hypothesis regarding a toxic oligomeric species can be made.
There are currently no available disease course-modifying therapies for any SCA. Due to the large degree of etiological and clinical heterogeneity across the different SCAs, tailored therapies within a particular SCA based on the existing knowledge of disease mechanisms from preclinical models may be a viable approach to therapeutic development. In recent years, ASOs have gained traction as a primary therapeutic approach in preclinical models of neurodegenerative disorders. Because toxic gain-of-function mechanisms conferred by the mutant proteins are thought to underlie disease pathogenesis, harnessing the ability of ASOs to specifically target an mRNA of interest for RNase H-mediated degradation allows for the reduction of toxic mutant polyQ-expanded proteins, ameliorating disease-related phenotypes [111, 177–182]. Since the design of allele-selective ASOs is challenging, potential deleterious effects of reduction of the wild-type allele also must be considered when implementing non-allele-selective ASOs in the SCAs. Further testing of ASO-based approaches along with continued efforts toward a better understanding of the cellular and molecular changes in each SCA will provide critical information in the development of therapeutic agents for SCAs and related disorders.
Acknowledgements
We thank all members of the Lim laboratory for useful feedback, critiques, and comments. This work was supported by National Institutes of Health Grants NS083706 (J.L.), NS088321 (J.L.), MH119803 (J.L.), AG066447 (J.L.), T32 NS007224 (L.T.), Lo Graduate Fellowship for Excellence in Stem Cell Research (L.T.), and the Gruber Science Fellowship (L.T.).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ruano L, Melo C, Silva MC, Coutinho P. The global epidemiology of hereditary ataxia and spastic paraplegia: a systematic review of prevalence studies. Neuroepidemiology. 2014;42:174–183. doi: 10.1159/000358801. [DOI] [PubMed] [Google Scholar]
- 2.Manto MU. The wide spectrum of spinocerebellar ataxias (SCAs) Cerebellum. 2005;4:2–6. doi: 10.1080/14734220510007914. [DOI] [PubMed] [Google Scholar]
- 3.Schöls L, Bauer P, Schmidt T, Schulte T, Riess O. Autosomal dominant cerebellar ataxias: clinical features, genetics, and pathogenesis. Lancet Neurol. 2004;3:291–304. doi: 10.1016/S1474-4422(04)00737-9. [DOI] [PubMed] [Google Scholar]
- 4.Brusco A, et al. Molecular genetics of hereditary spinocerebellar ataxia: mutation analysis of spinocerebellar ataxia genes and CAG/CTG repeat expansion detection in 225 Italian families. Arch Neurol. 2004;61:727–733. doi: 10.1001/archneur.61.5.727. [DOI] [PubMed] [Google Scholar]
- 5.Zortea M, et al. Prevalence of inherited ataxias in the province of Padua, Italy. Neuroepidemiology. 2004;23:275–280. doi: 10.1159/000080092. [DOI] [PubMed] [Google Scholar]
- 6.Joo BE, Lee CN, Park KW. Prevalence rate and functional status of cerebellar ataxia in Korea. Cerebellum. 2012;11:733–738. doi: 10.1007/s12311-011-0332-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anheim M, Tranchant C, Koenig M. The autosomal recessive cerebellar ataxias. N Engl J Med. 2012;366:636–646. doi: 10.1056/NEJMra1006610. [DOI] [PubMed] [Google Scholar]
- 8.Klockgether T, Mariotti C, Paulson HL. Spinocerebellar ataxia. Nat Rev Dis Primers. 2019;5:24. doi: 10.1038/s41572-019-0074-3. [DOI] [PubMed] [Google Scholar]
- 9.Paulson HL, Shakkottai VG, Clark HB, Orr HT. Polyglutamine spinocerebellar ataxias—from genes to potential treatments. Nat Rev Neurosci. 2017;18:613–626. doi: 10.1038/nrn.2017.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jayadev S, Bird TD. Hereditary ataxias: overview. Genet Med. 2013;15:673–683. doi: 10.1038/gim.2013.28. [DOI] [PubMed] [Google Scholar]
- 11.La Spada AR, Wilson EM, Lubahn DB, Harding AE, Fischbeck KH. Androgen receptor gene mutations in X-linked spinal and bulbar muscular atrophy. Nature. 1991;352:77–79. doi: 10.1038/352077a0. [DOI] [PubMed] [Google Scholar]
- 12.Ridley RM, Frith CD, Crow TJ, Conneally PM. Anticipation in Huntington’s disease is inherited through the male line but may originate in the female. J Med Genet. 1988;25:589–595. doi: 10.1136/jmg.25.9.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paulson HL. The spinocerebellar ataxias. J Neuroophthalmol. 2009;29:227–237. doi: 10.1097/WNO0b013e3181b416de. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nance MA. Clinical aspects of CAG repeat diseases. Brain Pathol. 1997;7:881–900. doi: 10.1111/j.1750-3639.1997.tb00892.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.La Spada RA. Trinucleotide repeat instability: genetic features and molecular mechanisms. Brain Pathol. 1997;7:943–963. doi: 10.1111/j.1750-3639.1997.tb00895.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ranum LPW, et al. Molecular and clinical correlations in spinocerebellar ataxia type I: evidence for familial effects on the age at onset. Am J Hum Genet. 1994;55:244–252. [PMC free article] [PubMed] [Google Scholar]
- 17.Yakura H, Wakisaka A, Fujimoto S, Itakura K. Letter: hereditary ataxia and HL-A genotypes. N Engl J Med. 1974;291:154–155. doi: 10.1056/NEJM197407182910314. [DOI] [PubMed] [Google Scholar]
- 18.Zoghbi HY, Pollack MS, Lyons LA, Ferrell RE, Daiger SP, Beaudet AL. Spinocerebellar ataxia: variable age of onset and linkage to human leukocyte antigen in a large kindred. Ann Neurol. 1988;23:580–584. doi: 10.1002/ana.410230609. [DOI] [PubMed] [Google Scholar]
- 19.Zoghbi HY, Sandkuijl LA, Ott J, Daiger SP, Pollack M, O’Brien WE, Beaudet AL. Assignment of autosomal dominant spinocerebellar ataxia (SCA1) centromeric to the HLA region on the short arm of chromosome 6, using multilocus linkage analysis. Am J Hum Genet. 1989;44:255–263. [PMC free article] [PubMed] [Google Scholar]
- 20.Macdonald M. A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington’s disease chromosomes. Cell. 1993;72:971–983. doi: 10.1016/0092-8674(93)90585-e. [DOI] [PubMed] [Google Scholar]
- 21.Ranum LPW, Duvick LA, Rich SS, Schut LJ, Litt M, Orr HT. Localization of the autosomal dominant HLA-linked spinocerebellar ataxia (SCA1) locus, in two kindreds, within an 8-cM subregion of chromosome 6p. Am J Hum Genet. 1991;49:31–41. [PMC free article] [PubMed] [Google Scholar]
- 22.Zoghbi HY, et al. The gene for autosomal dominant spinocerebellar ataxia (SCA1) maps telomeric. Am J Hum Genet. 1991;49:23–30. [PMC free article] [PubMed] [Google Scholar]
- 23.Orr HT, et al. Expansion of an unstable trinucleotide CAG repeat in spinocerebellar ataxia type 1. Nat Genet. 1993;4:221–226. doi: 10.1038/ng0793-221. [DOI] [PubMed] [Google Scholar]
- 24.Moseley ML, et al. Incidence of dominant spinocerebellar and Friedreich triplet repeats among 361 ataxia families. Neurology. 1998;51:1666–1671. doi: 10.1212/wnl.51.6.1666. [DOI] [PubMed] [Google Scholar]
- 25.Orr HT, Zoghbi HY. Trinucleotide repeat disorders. Annu Rev Neurosci. 2007;30:575–621. doi: 10.1146/annurev.neuro.29.051605.113042. [DOI] [PubMed] [Google Scholar]
- 26.Genis D, Matilla T, Volpini V, Rosell J, Davalos A, Ferrer I, Molins A, Estivill X. Clinical, neuropathologic, and genetic studies of a large spinocerebellar ataxia type 1 (SCA1) kindred: (CAG)n expansion and early premonitory signs and symptoms. Neurology. 1995;45:24–30. doi: 10.1212/wnl.45.1.24. [DOI] [PubMed] [Google Scholar]
- 27.Robitaille Y, Lopes-Cendes I, Becher M, Rouleau G, Clark AW. The neuropathology of CAG repeat diseases: review and update of genetic and molecular features. Brain Pathol. 1997;7:901–926. doi: 10.1111/j.1750-3639.1997.tb00893.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rub U, et al. Spinocerebellar ataxia type 1 (SCA1): new pathoanatomical and clinico-pathological insights. Neuropathol Appl Neurobiol. 2012;38:665–680. doi: 10.1111/j.1365-2990.2012.01259.x. [DOI] [PubMed] [Google Scholar]
- 29.Martins CR, Jr, Martinez ARM, de Rezende TJR, Branco LMT, Pedroso JL, Barsottini OGP, Lopes-Cendes I, Franca MC., Jr Spinal cord damage in spinocerebellar ataxia type 1. Cerebellum. 2017;16:792–796. doi: 10.1007/s12311-017-0854-9. [DOI] [PubMed] [Google Scholar]
- 30.Klement IA, Skinner PJ, Kaytor MD, Yi H, Hersch SM, Clark HB, Zoghbi HY, Orr HT. Ataxin-1 nuclear localization and aggregation. Cell. 1998;95:41–53. doi: 10.1016/s0092-8674(00)81781-x. [DOI] [PubMed] [Google Scholar]
- 31.Servadio A, Koshy B, Armstrong D, Antalffy B, Orr HT, Zoghbi HY. Expression analysis of the ataxin-1 protein in tissues from normal and spinocerebellar ataxia type 1 individuals. Nat Genet. 1995;10:94–98. doi: 10.1038/ng0595-94. [DOI] [PubMed] [Google Scholar]
- 32.Zhang Y, et al. An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J Neurosci. 2014;34:11929–11947. doi: 10.1523/JNEUROSCI.1860-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Banfi S, et al. Identification and characterization of the gene causing type 1 spinocerebellar ataxia. Nat Genet. 1994;7:513–520. doi: 10.1038/ng0894-513. [DOI] [PubMed] [Google Scholar]
- 34.Irwin S, Vandelft M, Pinchev D, Howell JL, Graczyk J, Orr HT, Truant R. RNA association and nucleocytoplasmic shuttling by ataxin-1. J Cell Sci. 2005;118:233–242. doi: 10.1242/jcs.01611. [DOI] [PubMed] [Google Scholar]
- 35.Yue S, Serra HG, Zoghbi HY, Orr HT. The spinocerebellar ataxia type 1 protein, ataxin-1, has RNA-binding activity that is inversely affected by the length of its polyglutamine tract. Hum Mol Genet. 2001;10:25–30. doi: 10.1093/hmg/10.1.25. [DOI] [PubMed] [Google Scholar]
- 36.Zoghbi HY, Orr HT. Pathogenic mechanisms of a polyglutamine-mediated neurodegenerative disease, spinocerebellar ataxia type 1. J Biol Chem. 2009;284:7425–7429. doi: 10.1074/jbc.R800041200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chung MY, Ranum LP, Duvick LA, Servadio A, Zoghbi HY, Orr HT. Evidence for a mechanism predisposing to intergenerational CAG repeat instability in spinocerebellar ataxia type I. Nat Genet. 1993;5:254–258. doi: 10.1038/ng1193-254. [DOI] [PubMed] [Google Scholar]
- 38.Zuhlke C, Dalski A, Hellenbroich Y, Bubel S, Schwinger E, Burk K. Spinocerebellar ataxia type 1 (SCA1): phenotype-genotype correlation studies in intermediate alleles. Eur J Hum Genet. 2002;10:204–209. doi: 10.1038/sj.ejhg.5200788. [DOI] [PubMed] [Google Scholar]
- 39.Pearson CE, Eichler EE, Lorenzetti D, Kramer SF, Zoghbi HY, Nelson DL, Sinden RR. Interruptions in the triplet repeats of SCA1 and FRAXA reduce the propensity and complexity of slipped strand DNA (S-DNA) formation. Biochemistry. 1998;37:2701–2708. doi: 10.1021/bi972546c. [DOI] [PubMed] [Google Scholar]
- 40.Kraus-Perrotta C, Lagalwar S. Expansion, mosaicism and interruption: mechanisms of the CAG repeat mutation in spinocerebellar ataxia type 1. Cerebellum Ataxias. 2016;3:20. doi: 10.1186/s40673-016-0058-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Menon RP, et al. The role of interruptions in polyQ in the pathology of SCA1. PLoS Genet. 2013;9:e1003648. doi: 10.1371/journal.pgen.1003648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schut JW. Hereditary ataxia. Arch Neurol Psychiatry. 1950;63:535–568. [Google Scholar]
- 43.Quan F, Janas J, Popovich BW. A novel CAG repeat configuration in the SCA1 gene: implications for the molecular diagnostics of spinocerebellar ataxia type 1. Hum Mol Genet. 1995;4:2411–2413. doi: 10.1093/hmg/4.12.2411. [DOI] [PubMed] [Google Scholar]
- 44.Matsuyama Z, Izumi Y, Kameyama M, Kawakami H, Nakamura S. The effect of CAT trinucleotide interruptions on the age at onset of spinocerebellar ataxia type 1 (SCA1) J Med Genet. 1999;36:546–548. [PMC free article] [PubMed] [Google Scholar]
- 45.Goldfarb LG, et al. Unstable triplet repeat and phenotypic variability of spinocerebellar ataxia type 1. Ann Neurol. 1996;39:500–506. doi: 10.1002/ana.410390412. [DOI] [PubMed] [Google Scholar]
- 46.Sen S, Dash D, Pasha S, Brahmachari SK. Role of histidine interruption in mitigating the pathological effects of long polyglutamine stretches in SCA1: a molecular approach. Protein Sci. 2003;12:953–962. doi: 10.1110/ps.0224403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jayaraman M, Kodali R, Wetzel R. The impact of ataxin-1-like histidine insertions on polyglutamine aggregation. Protein Eng Des Sel. 2009;22:469–478. doi: 10.1093/protein/gzp023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Calabresi V, Guida S, Servadio A, Jodice C. Phenotypic effects of expanded ataxin-1 polyglutamines with interruptions in vitro. Brain Res Bull. 2001;56:337–342. doi: 10.1016/s0361-9230(01)00600-1. [DOI] [PubMed] [Google Scholar]
- 49.Matilla A, et al. Mice lacking ataxin-1 display learning deficits and decreased hippocampal paired-pulse facilitation. J Neurosci. 1998;18:5508–5516. doi: 10.1523/JNEUROSCI.18-14-05508.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Crespo-Barreto J, Fryer JD, Shaw CA, Orr HT, Zoghbi HY. Partial loss of ataxin-1 function contributes to transcriptional dysregulation in spinocerebellar ataxia type 1 pathogenesis. PLoS Genet. 2010;6:e1001021. doi: 10.1371/journal.pgen.1001021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lim J, Crespo-Barreto J, Jafar-Nejad P, Bowman AB, Richman R, Hill DE, Orr HT, Zoghbi HY. Opposing effects of polyglutamine expansion on native protein complexes contribute to SCA1. Nature. 2008;452:713–718. doi: 10.1038/nature06731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mizutani A, Wang L, Rajan H, Vig PJ, Alaynick WA, Thaler JP, Tsai CC. Boat, an AXH domain protein, suppresses the cytotoxicity of mutant ataxin-1. EMBO J. 2005;24:3339–3351. doi: 10.1038/sj.emboj.7600785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bowman AB, et al. Duplication of Atxn1l suppresses SCA1 neuropathology by decreasing incorporation of polyglutamine-expanded ataxin-1 into native complexes. Nat Genet. 2007;39:373–379. doi: 10.1038/ng1977. [DOI] [PubMed] [Google Scholar]
- 54.Chen H-K, et al. Interaction of Akt-phosphorylated ataxin-1 with 14-3-3 mediates neurodegeneration in spinocerebellar ataxia type 1. Cell. 2003;113:457–468. doi: 10.1016/s0092-8674(03)00349-0. [DOI] [PubMed] [Google Scholar]
- 55.de Chiara C, Menon RP, Strom M, Gibson TJ, Pastore A. Phosphorylation of S776 and 14-3-3 binding modulate ataxin-1 interaction with splicing factors. PLoS ONE. 2009;4:e8372. doi: 10.1371/journal.pone.0008372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lam YC, et al. ATAXIN-1 interacts with the repressor Capicua in its native complex to cause SCA1 neuropathology. Cell. 2006;127:1335–1347. doi: 10.1016/j.cell.2006.11.038. [DOI] [PubMed] [Google Scholar]
- 57.Fryer JD, et al. Exercise and genetic rescue of SCA1 via the transcriptional repressor Capicua. Science. 2011;334:690–693. doi: 10.1126/science.1212673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kim E, Lu HC, Zoghbi HY, Song JJ. Structural basis of protein complex formation and reconfiguration by polyglutamine disease protein ataxin-1 and Capicua. Genes Dev. 2013;27:590–595. doi: 10.1101/gad.212068.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gehrking KM, Andresen JM, Duvick L, Lough J, Zoghbi HY, Orr HT. Partial loss of Tip60 slows mid-stage neurodegeneration in a spinocerebellar ataxia type 1 (SCA1) mouse model. Hum Mol Genet. 2011;20:2204–2212. doi: 10.1093/hmg/ddr108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Serra HG, et al. RORalpha-mediated Purkinje cell development determines disease severity in adult SCA1 mice. Cell. 2006;127:697–708. doi: 10.1016/j.cell.2006.09.036. [DOI] [PubMed] [Google Scholar]
- 61.Tsuda H, et al. The AXH domain of Ataxin-1 mediates neurodegeneration through its interaction with Gfi-1/Senseless proteins. Cell. 2005;122:633–644. doi: 10.1016/j.cell.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 62.Matilla A, Koshy BT, Cummings CJ, Isobe T, Orr HT, Zoghbi HY. The cerebellar leucine-rich acidic nuclear protein interacts with ataxin-1. Nature. 1997;389:974–978. doi: 10.1038/40159. [DOI] [PubMed] [Google Scholar]
- 63.Cvetanovic M, Rooney RJ, Garcia JJ, Toporovskaya N, Zoghbi HY, Opal P. The role of LANP and ataxin 1 in E4F-mediated transcriptional repression. EMBO Rep. 2007;8:671–677. doi: 10.1038/sj.embor.7400983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Opal P, Garcia JJ, Propst F, Matilla A, Orr HT, Zoghbi HY. Mapmodulin/leucine-rich acidic nuclear protein binds the light chain of microtubule-associated protein 1B and modulates neuritogenesis. J Biol Chem. 2003;278:34691–34699. doi: 10.1074/jbc.M302785200. [DOI] [PubMed] [Google Scholar]
- 65.Cvetanovic M, Kular RK, Opal P. LANP mediates neuritic pathology in spinocerebellar ataxia type 1. Neurobiol Dis. 2012;48:526–532. doi: 10.1016/j.nbd.2012.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rousseaux MWC, et al. ATXN1-CIC complex is the primary driver of cerebellar pathology in spinocerebellar ataxia type 1 through a gain-of-function mechanism. Neuron. 2018;97(1235–1243):e5. doi: 10.1016/j.neuron.2018.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Conforti FL, et al. Ataxin-1 and ataxin-2 intermediate-length PolyQ expansions in amyotrophic lateral sclerosis. Neurology. 2012;79:2315–2320. doi: 10.1212/WNL.0b013e318278b618. [DOI] [PubMed] [Google Scholar]
- 68.Lattante S, et al. ATXN1 intermediate-length polyglutamine expansions are associated with amyotrophic lateral sclerosis. Neurobiol Aging. 2018;64:157e1–157e5. doi: 10.1016/j.neurobiolaging.2017.11.011. [DOI] [PubMed] [Google Scholar]
- 69.Bertram L, et al. Genome-wide association analysis reveals putative Alzheimer’s disease susceptibility loci in addition to APOE. Am J Hum Genet. 2008;83:623–632. doi: 10.1016/j.ajhg.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Suh J, et al. Loss of ataxin-1 potentiates alzheimer’s pathogenesis by elevating cerebral BACE1 transcription. Cell. 2019;178(1159–1175):e17. doi: 10.1016/j.cell.2019.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang C, Browne A, Child D, Divito JR, Stevenson JA, Tanzi RE. Loss of function of ATXN1 increases amyloid beta-protein levels by potentiating β-secretase processing of β-amyloid precursor protein. J Biol Chem. 2010;285:8515–8526. doi: 10.1074/jbc.M109.079079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Todd TW, Kokubu H, Miranda HC, Cortes CJ, La Spada AR, Lim J. Nemo-like kinase is a novel regulator of spinal and bulbar muscular atrophy. Elife. 2015;4:e08493. doi: 10.7554/eLife.08493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Humbert S, Bryson EA, Cordelières FP, Connors NC, Datta SR, Finkbeiner S, Greenberg ME, Saudou F. The IGF-1/Akt pathway is neuroprotective in Huntington’s disease and involves huntingtin phosphorylation by Akt. Dev Cell. 2002;2:831–837. doi: 10.1016/s1534-5807(02)00188-0. [DOI] [PubMed] [Google Scholar]
- 74.Gioeli D, Black BE, Gordon V, Spencer A, Kesler CT, Eblen ST, Paschal BM, Weber MJ. Stress kinase signaling regulates androgen receptor phosphorylation, transcription, and localization. Mol Endocrinol. 2006;20:503–515. doi: 10.1210/me.2005-0351. [DOI] [PubMed] [Google Scholar]
- 75.Chen S, Kesler CT, Paschal BM, Balk SP. Androgen receptor phosphorylation and activity are regulated by an association with protein phosphatase 1. J Biol Chem. 2009;284:25576–25584. doi: 10.1074/jbc.M109.043133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lagalwar S, Orr HT. Regulation of ataxin-1 phosphorylation and its impact on biology. Methods Mol Biol. 2013;1010:201–209. doi: 10.1007/978-1-62703-411-1_13. [DOI] [PubMed] [Google Scholar]
- 77.Orr HT. SCA1-phosphorylation, a regulator of ataxin-1 function and pathogenesis. Prog Neurobiol. 2012;99:179–185. doi: 10.1016/j.pneurobio.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ju H, Kokubu H, Lim J. Beyond the glutamine expansion: influence of posttranslational modifications of ataxin-1 in the pathogenesis of spinocerebellar ataxia type 1. Mol Neurobiol. 2014;50:866–874. doi: 10.1007/s12035-014-8703-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Emamian ES, Kaytor MD, Duvick LA, Zu T, Tousey SK, Zoghbi HY, Clark HB, Orr HT. Serine 776 of ataxin-1 is critical for polyglutamine-induced disease in SCA1 transgenic mice. Neuron. 2003;38:375–387. doi: 10.1016/s0896-6273(03)00258-7. [DOI] [PubMed] [Google Scholar]
- 80.Duvick L, et al. SCA1-like disease in mice expressing wild-type ataxin-1 with a serine to aspartic acid replacement at residue 776. Neuron. 2010;67:929–935. doi: 10.1016/j.neuron.2010.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lai S, O'Callaghan B, Zoghbi HY, Orr HT. 14-3-3 Binding to ataxin-1(ATXN1) regulates its dephosphorylation at Ser-776 and transport to the nucleus. J Biol Chem. 2011;286:34606–34616. doi: 10.1074/jbc.M111.238527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Menon RP, Soong D, de Chiara C, Holt MR, Anilkumar N, Pastore A. The importance of serine 776 in ataxin-1 partner selection: a FRET analysis. Sci Rep. 2012;2:919. doi: 10.1038/srep00919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Vierra-Green CA, Orr HT, Zoghbi HY, Ferrington DA. Identification of a novel phosphorylation site in ataxin-1. Biochim Biophys Acta. 2005;1744:11–18. doi: 10.1016/j.bbamcr.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 84.Ju H, et al. Polyglutamine disease toxicity is regulated by nemo-like kinase in spinocerebellar ataxia type 1. J Neurosci. 2013;33:9328–9336. doi: 10.1523/JNEUROSCI.3465-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kaytor MD, Byam CE, Tousey SK, Stevens SD, Zoghbi HY, Orr HT. A cell-based screen for modulators of ataxin-1 phosphorylation. Hum Mol Genet. 2005;14:1095–1105. doi: 10.1093/hmg/ddi122. [DOI] [PubMed] [Google Scholar]
- 86.Jorgensen ND, Andresen JM, Pitt JE, Swenson MA, Zoghbi HY, Orr HT. Hsp70/Hsc70 regulates the effect phosphorylation has on stabilizing ataxin-1. J Neurochem. 2007;102:2040–2048. doi: 10.1111/j.1471-4159.2007.04678.x. [DOI] [PubMed] [Google Scholar]
- 87.Jorgensen ND, et al. Phosphorylation of ATXN1 at Ser776 in the cerebellum. J Neurochem. 2009;110:675–686. doi: 10.1111/j.1471-4159.2009.06164.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Perez Ortiz JM, et al. Reduction of protein kinase A-mediated phosphorylation of ATXN1-S776 in Purkinje cells delays onset of Ataxia in a SCA1 mouse model. Neurobiol Dis. 2018;116:93–105. doi: 10.1016/j.nbd.2018.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Park J, et al. RAS-MAPK-MSK1 pathway modulates ataxin 1 protein levels and toxicity in SCA1. Nature. 2013;498:325–331. doi: 10.1038/nature12204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bondar VV, et al. PAK1 regulates ATXN1 levels providing an opportunity to modify its toxicity in spinocerebellar ataxia type 1. Hum Mol Genet. 2018;27:2863–2873. doi: 10.1093/hmg/ddy200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hong S, Lee S, Cho SG, Kang S. UbcH6 interacts with and ubiquitinates the SCA1 gene product ataxin-1. Biochem Biophys Res Commun. 2008;371:256–260. doi: 10.1016/j.bbrc.2008.04.066. [DOI] [PubMed] [Google Scholar]
- 92.Lee S, Hong S, Kang S. The ubiquitin-conjugating enzyme UbcH6 regulates the transcriptional repression activity of the SCA1 gene product ataxin-1. Biochem Biophys Res Commun. 2008;372:735–740. doi: 10.1016/j.bbrc.2008.05.125. [DOI] [PubMed] [Google Scholar]
- 93.Al-Ramahi I, et al. CHIP protects from the neurotoxicity of expanded and wild-type ataxin-1 and promotes their ubiquitination and degradation. J Biol Chem. 2006;281:26714–26724. doi: 10.1074/jbc.M601603200. [DOI] [PubMed] [Google Scholar]
- 94.Choi JY, et al. Co-chaperone CHIP promotes aggregation of ataxin-1. Mol Cell Neurosci. 2007;34:69–79. doi: 10.1016/j.mcn.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 95.Quintana-Gallardo L, Martin-Benito J, Marcilla M, Espadas G, Sabido E, Valpuesta JM. The cochaperone CHIP marks Hsp70- and Hsp90-bound substrates for degradation through a very flexible mechanism. Sci Rep. 2019;9:5102. doi: 10.1038/s41598-019-41060-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Davidson JD, Riley B, Burright EN, Duvick LA, Zoghbi HY, Orr HT. Identification and characterization of an ataxin-1-interacting protein: A1Up, a ubiquitin-like nuclear protein. Hum Mol Genet. 2000;9:2305–2312. doi: 10.1093/oxfordjournals.hmg.a018922. [DOI] [PubMed] [Google Scholar]
- 97.Riley BE, Xu Y, Zoghbi HY, Orr HT. The effects of the polyglutamine repeat protein ataxin-1 on the UbL-UBA protein A1Up. J Biol Chem. 2004;279:42290–42301. doi: 10.1074/jbc.M406284200. [DOI] [PubMed] [Google Scholar]
- 98.Cummings CJ, et al. Mutation of the E6-AP ubiquitin ligase reduces nuclear inclusion frequency while accelerating polyglutamine-induced pathology in SCA1 mice. Neuron. 1999;24:879–892. doi: 10.1016/s0896-6273(00)81035-1. [DOI] [PubMed] [Google Scholar]
- 99.Cummings CJ, Mancini MA, Antalffy B, DeFranco DB, Orr HT, Zoghbi HY. Chaperone suppression of aggregation and altered subcellular proteasome localization imply protein misfolding in SCA1. Nat Genet. 1998;19:148–154. doi: 10.1038/502. [DOI] [PubMed] [Google Scholar]
- 100.Cummings CJ, Sun Y, Opal P, Antalffy B, Mestril R, Orr HT, Dillmann WH, Zoghbi HY. Over-expression of inducible HSP70 chaperone suppresses neuropathology and improves motor function in SCA1 mice. Hum Mol Genet. 2001;10:1511–1518. doi: 10.1093/hmg/10.14.1511. [DOI] [PubMed] [Google Scholar]
- 101.Ryu J, Lee DH. Dual-specificity phosphatase 18 modulates the SUMOylation and aggregation of Ataxin-1. Biochem Biophys Res Commun. 2018;502:389–396. doi: 10.1016/j.bbrc.2018.05.178. [DOI] [PubMed] [Google Scholar]
- 102.Ryu J, Cho S, Park BC, Lee DH. Oxidative stress-enhanced SUMOylation and aggregation of ataxin-1: Implication of JNK pathway. Biochem Biophys Res Commun. 2010;393:280–285. doi: 10.1016/j.bbrc.2010.01.122. [DOI] [PubMed] [Google Scholar]
- 103.Riley BE, Zoghbi HY, Orr HT. SUMOylation of the polyglutamine repeat protein, ataxin-1, is dependent on a functional nuclear localization signal. J Biol Chem. 2005;280:21942–21948. doi: 10.1074/jbc.M501677200. [DOI] [PubMed] [Google Scholar]
- 104.Vig PJ, Wei J, Shao Q, Hebert MD, Subramony SH, Sutton LT. Role of tissue transglutaminase type 2 in calbindin-D28k interaction with ataxin-1. Neurosci Lett. 2007;420:53–57. doi: 10.1016/j.neulet.2007.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.D'Souza DR, Wei J, Shao Q, Hebert MD, Subramony SH, Vig PJ. Tissue transglutaminase crosslinks ataxin-1: possible role in SCA1 pathogenesis. Neurosci Lett. 2006;409:5–9. doi: 10.1016/j.neulet.2006.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gennarino VA, et al. Pumilio1 haploinsufficiency leads to SCA1-like neurodegeneration by increasing wild-type ataxin1 levels. Cell. 2015;160:1087–1098. doi: 10.1016/j.cell.2015.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tsai CC, Kao HY, Mitzutani A, Banayo E, Rajan H, McKeown M, Evans RM. Ataxin 1, a SCA1 neurodegenerative disorder protein, is functionally linked to the silencing mediator of retinoid and thyroid hormone receptors. Proc Natl Acad Sci USA. 2004;101:4047–4052. doi: 10.1073/pnas.0400615101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ingram M, et al. Cerebellar transcriptome profiles of ATXN1 transgenic mice reveal SCA1 disease progression and protection pathways. Neuron. 2016;89:1194–1207. doi: 10.1016/j.neuron.2016.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tissir F, Goffinet AM. Shaping the nervous system: role of the core planar cell polarity genes. Nat Rev Neurosci. 2013;14:525–535. doi: 10.1038/nrn3525. [DOI] [PubMed] [Google Scholar]
- 110.Driessen TM, Lee PJ, Lim J. Molecular pathway analysis towards understanding tissue vulnerability in spinocerebellar ataxia type 1. Elife. 2018;7:e39981. doi: 10.7554/eLife.39981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Friedrich J, et al. Antisense oligonucleotide-mediated ataxin-1 reduction prolongs survival in SCA1 mice and reveals disease-associated transcriptome profiles. JCI Insight. 2018;3(21):e123193. doi: 10.1172/jci.insight.123193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Jafar-Nejad P, Ward CS, Richman R, Orr HT, Zoghbi HY. Regional rescue of spinocerebellar ataxia type 1 phenotypes by 14-3-3epsilon haploinsufficiency in mice underscores complex pathogenicity in neurodegeneration. Proc Natl Acad Sci USA. 2011;108:2142–2147. doi: 10.1073/pnas.1018748108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Seidel K, Siswanto S, Brunt ER, den Dunnen W, Korf HW, Rub U. Brain pathology of spinocerebellar ataxias. Acta Neuropathol. 2012;124:1–21. doi: 10.1007/s00401-012-1000-x. [DOI] [PubMed] [Google Scholar]
- 114.Koeppen AH. The pathogenesis of spinocerebellar ataxia. Cerebellum. 2005;4:62–73. doi: 10.1080/14734220510007950. [DOI] [PubMed] [Google Scholar]
- 115.Koeppen AH, Ramirez RL, Bjork ST, Bauer P, Feustel PJ. The reciprocal cerebellar circuitry in human hereditary ataxia. Cerebellum. 2013;12:493–503. doi: 10.1007/s12311-013-0456-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Klockgether T. Update on degenerative ataxias. Curr Opin Neurol. 2011;24:339–345. doi: 10.1097/WCO.0b013e32834875ba. [DOI] [PubMed] [Google Scholar]
- 117.Takechi Y, et al. Impairment of spinal motor neurons in spinocerebellar ataxia type 1-knock-in mice. Neurosci Lett. 2013;535:67–72. doi: 10.1016/j.neulet.2012.12.057. [DOI] [PubMed] [Google Scholar]
- 118.Orengo JP, van der Heijden ME, Hao S, Tang J, Orr HT, Zoghbi HY. Motor neuron degeneration correlates with respiratory dysfunction in SCA1. Dis Model Mech. 2018;11:dmm032623. doi: 10.1242/dmm.032623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Verkhratsky A, Parpura V, Pekna M, Pekny M, Sofroniew M. Glia in the pathogenesis of neurodegenerative diseases. Biochem Soc Trans. 2014;42:1291–1301. doi: 10.1042/BST20140107. [DOI] [PubMed] [Google Scholar]
- 120.Philips T, Rothstein JD. Glial cells in amyotrophic lateral sclerosis. Exp Neurol. 2014;262(Pt B):111–120. doi: 10.1016/j.expneurol.2014.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kang SH, Li Y, Fukaya M, Lorenzini I, Cleveland DW, Ostrow LW, Rothstein JD, Bergles DE. Degeneration and impaired regeneration of gray matter oligodendrocytes in amyotrophic lateral sclerosis. Nat Neurosci. 2013;16:571–579. doi: 10.1038/nn.3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Yamanaka K, et al. Astrocytes as determinants of disease progression in inherited amyotrophic lateral sclerosis. Nat Neurosci. 2008;11:251–253. doi: 10.1038/nn2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Boillee S, Yamanaka K, Lobsiger CS, Copeland NG, Jenkins NA, Kassiotis G, Kollias G, Cleveland DW. Onset and progression in inherited ALS determined by motor neurons and microglia. Science. 2006;312:1389–1392. doi: 10.1126/science.1123511. [DOI] [PubMed] [Google Scholar]
- 124.Hansen DV, Hanson JE, Sheng M. Microglia in Alzheimer’s disease. J Cell Biol. 2018;217:459–472. doi: 10.1083/jcb.201709069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Roth AD, Ramirez G, Alarcon R, Von Bernhardi R. Oligodendrocytes damage in Alzheimer’s disease: beta amyloid toxicity and inflammation. Biol Res. 2005;38:381–387. doi: 10.4067/s0716-97602005000400011. [DOI] [PubMed] [Google Scholar]
- 126.Henstridge CM, Hyman BT, Spires-Jones TL. Beyond the neuron-cellular interactions early in Alzheimer disease pathogenesis. Nat Rev Neurosci. 2019;20:94–108. doi: 10.1038/s41583-018-0113-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Shin JY, Fang ZH, Yu ZX, Wang CE, Li SH, Li XJ. Expression of mutant huntingtin in glial cells contributes to neuronal excitotoxicity. J Cell Biol. 2005;171:1001–1012. doi: 10.1083/jcb.200508072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hsiao HY, Chern Y. Targeting glial cells to elucidate the pathogenesis of Huntington’s disease. Mol Neurobiol. 2010;41:248–255. doi: 10.1007/s12035-009-8097-5. [DOI] [PubMed] [Google Scholar]
- 129.Huang B, Wei W, Wang G, Gaertig MA, Feng Y, Wang W, Li XJ, Li S. Mutant huntingtin downregulates myelin regulatory factor-mediated myelin gene expression and affects mature oligodendrocytes. Neuron. 2015;85:1212–1226. doi: 10.1016/j.neuron.2015.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kim JH, Lukowicz A, Qu W, Johnson A, Cvetanovic M. Astroglia contribute to the pathogenesis of spinocerebellar ataxia Type 1 (SCA1) in a biphasic, stage-of-disease specific manner. Glia. 2018;66:1972–1987. doi: 10.1002/glia.23451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Cvetanovic M, Ingram M, Orr H, Opal P. Early activation of microglia and astrocytes in mouse models of spinocerebellar ataxia type 1. Neuroscience. 2015;289:289–299. doi: 10.1016/j.neuroscience.2015.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ferro A, Sheeler C, Rosa JG, Cvetanovic M. Role of microglia in ataxias. J Mol Biol. 2019;431:1792–1804. doi: 10.1016/j.jmb.2019.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Qu W, Johnson A, Kim JH, Lukowicz A, Svedberg D, Cvetanovic M. Inhibition of colony-stimulating factor 1 receptor early in disease ameliorates motor deficits in SCA1 mice. J Neuroinflammation. 2017;14:107. doi: 10.1186/s12974-017-0880-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ferro A, Qu W, Lukowicz A, Svedberg D, Johnson A, Cvetanovic M. Inhibition of NF-κB signaling in IKKβF/F;LysM Cre mice causes motor deficits but does not alter pathogenesis of spinocerebellar ataxia type 1. PLoS ONE. 2018;13:e0200013. doi: 10.1371/journal.pone.0200013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Cvetanovic M, Hu YS, Opal P. Mutant ataxin-1 inhibits neural progenitor cell proliferation in SCA1. Cerebellum. 2017;16:340–347. doi: 10.1007/s12311-016-0794-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Edamakanti CR, Do J, Didonna A, Martina M, Opal P. Mutant ataxin1 disrupts cerebellar development in spinocerebellar ataxia type 1. J Clin Invest. 2018;128:2252–2265. doi: 10.1172/JCI96765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Mandelli ML, De Simone T, Minati L, Bruzzone MG, Mariotti C, Fancellu R, Savoiardo M, Grisoli M. Diffusion tensor imaging of spinocerebellar ataxias types 1 and 2. AJNR Am J Neuroradiol. 2007;28:1996–2000. doi: 10.3174/ajnr.A0716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Pulst SM, et al. Moderate expansion of a normally biallelic trinucleotide repeat in spinocerebellar ataxia type 2. Nat Genet. 1996;14:269–276. doi: 10.1038/ng1196-269. [DOI] [PubMed] [Google Scholar]
- 139.Imbert G, et al. Cloning of the gene for spinocerebellar ataxia 2 reveals a locus with high sensitivity to expanded CAG/glutamine repeats. Nat Genet. 1996;14:285–291. doi: 10.1038/ng1196-285. [DOI] [PubMed] [Google Scholar]
- 140.Sanpei K, et al. Identification of the spinocerebellar ataxia type 2 gene using a direct identification of repeat expansion and cloning technique, DIRECT. Nat Genet. 1996;14:277–284. doi: 10.1038/ng1196-277. [DOI] [PubMed] [Google Scholar]
- 141.Kawaguchi Y, et al. CAG expansions in a novel gene for Machado-Joseph disease at chromosome 14q32.1. Nat Genet. 1994;8:221–228. doi: 10.1038/ng1194-221. [DOI] [PubMed] [Google Scholar]
- 142.Zhuchenko O, et al. Autosomal dominant cerebellar ataxia (SCA6) associated with small polyglutamine expansions in the alpha 1A-voltage-dependent calcium channel. Nat Genet. 1997;15:62–69. doi: 10.1038/ng0197-62. [DOI] [PubMed] [Google Scholar]
- 143.David G, et al. Cloning of the SCA7 gene reveals a highly unstable CAG repeat expansion. Nat Genet. 1997;17:65–70. doi: 10.1038/ng0997-65. [DOI] [PubMed] [Google Scholar]
- 144.Nakamura K, et al. SCA17, a novel autosomal dominant cerebellar ataxia caused by an expanded polyglutamine in TATA-binding protein. Hum Mol Genet. 2001;10:1441–1448. doi: 10.1093/hmg/10.14.1441. [DOI] [PubMed] [Google Scholar]
- 145.Zuhlke C, et al. Different types of repeat expansion in the TATA-binding protein gene are associated with a new form of inherited ataxia. Eur J Hum Genet. 2001;9:160–164. doi: 10.1038/sj.ejhg.5200617. [DOI] [PubMed] [Google Scholar]
- 146.Koide R, et al. Unstable expansion of CAG repeat in hereditary dentatorubral-pallidoluysian atrophy (DRPLA) Nat Genet. 1994;6:9–13. doi: 10.1038/ng0194-9. [DOI] [PubMed] [Google Scholar]
- 147.Ito M. Cerebellar circuitry as a neuronal machine. Prog Neurobiol. 2006;78:272–303. doi: 10.1016/j.pneurobio.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 148.Hansen ST, Meera P, Otis TS, Pulst SM. Changes in Purkinje cell firing and gene expression precede behavioral pathology in a mouse model of SCA2. Hum Mol Genet. 2013;22:271–283. doi: 10.1093/hmg/dds427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Chopra R, Bushart DD, Shakkottai VG. Dendritic potassium channel dysfunction may contribute to dendrite degeneration in spinocerebellar ataxia type 1. PLoS ONE. 2018;13:e0198040. doi: 10.1371/journal.pone.0198040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Shakkottai VG, do Carmo Costa M, Dell'Orco JM, Sankaranarayanan A, Wulff H, Paulson HL. Early changes in cerebellar physiology accompany motor dysfunction in the polyglutamine disease spinocerebellar ataxia type 3. J Neurosci. 2011;31:13002–13014. doi: 10.1523/JNEUROSCI.2789-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Jeub M, Herbst M, Spauschus A, Fleischer H, Klockgether T, Wuellner U, Evert BO. Potassium channel dysfunction and depolarized resting membrane potential in a cell model of SCA3. Exp Neurol. 2006;201:182–192. doi: 10.1016/j.expneurol.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 152.Chen X, Tang TS, Tu H, Nelson O, Pook M, Hammer R, Nukina N, Bezprozvanny I. Deranged calcium signaling and neurodegeneration in spinocerebellar ataxia type 3. J Neurosci. 2008;28:12713–12724. doi: 10.1523/JNEUROSCI.3909-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Liu J, Tang TS, Tu H, Nelson O, Herndon E, Huynh DP, Pulst SM, Bezprozvanny I. Deranged calcium signaling and neurodegeneration in spinocerebellar ataxia type 2. J Neurosci. 2009;29:9148–9162. doi: 10.1523/JNEUROSCI.0660-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Bushart DD, Chopra R, Singh V, Murphy GG, Wulff H, Shakkottai VG. Targeting potassium channels to treat cerebellar ataxia. Ann Clin Transl Neurol. 2018;5:297–314. doi: 10.1002/acn3.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Shakkottai VG, et al. Enhanced neuronal excitability in the absence of neurodegeneration induces cerebellar ataxia. J Clin Invest. 2004;113:582–590. doi: 10.1172/JCI20216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Bushart DD, Shakkottai VG. Ion channel dysfunction in cerebellar ataxia. Neurosci Lett. 2019;688:41–48. doi: 10.1016/j.neulet.2018.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.McMahon SJ, Pray-Grant MG, Schieltz D, Yates JR, 3rd, Grant PA. Polyglutamine-expanded spinocerebellar ataxia-7 protein disrupts normal SAGA and SLIK histone acetyltransferase activity. Proc Natl Acad Sci USA. 2005;102:8478–8482. doi: 10.1073/pnas.0503493102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Palhan VB, et al. Polyglutamine-expanded ataxin-7 inhibits STAGA histone acetyltransferase activity to produce retinal degeneration. Proc Natl Acad Sci. 2005;102:8472–8477. doi: 10.1073/pnas.0503505102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Friedman MJ, Wang CE, Li XJ, Li S. Polyglutamine expansion reduces the association of TATA-binding protein with DNA and induces DNA binding-independent neurotoxicity. J Biol Chem. 2008;283:8283–8290. doi: 10.1074/jbc.M709674200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Friedman MJ, Shah AG, Fang ZH, Ward EG, Warren ST, Li S, Li XJ. Polyglutamine domain modulates the TBP-TFIIB interaction: implications for its normal function and neurodegeneration. Nat Neurosci. 2007;10:1519–1528. doi: 10.1038/nn2011. [DOI] [PubMed] [Google Scholar]
- 161.Du X, Wang J, Zhu H, Rinaldo L, Lamar KM, Palmenberg AC, Hansel C, Gomez CM. Second cistron in CACNA1A gene encodes a transcription factor mediating cerebellar development and SCA6. Cell. 2013;154:118–133. doi: 10.1016/j.cell.2013.05.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Du X, et al. α1ACT is essential for survival and early cerebellar programming in a critical neonatal window. Neuron. 2019;102:770–785. doi: 10.1016/j.neuron.2019.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Kordasiewicz HB, Thompson RM, Clark HB, Gomez CM. C-termini of P/Q-type Ca2+ channel α1A subunits translocate to nuclei and promote polyglutamine-mediated toxicity. Hum Mol Genet. 2006;15:1587–1599. doi: 10.1093/hmg/ddl080. [DOI] [PubMed] [Google Scholar]
- 164.Tsou WL, Qiblawi SH, Hosking RR, Gomez CM, Todi SV. Polyglutamine length-dependent toxicity from α1ACT in Drosophila models of spinocerebellar ataxia type 6. Biol Open. 2016;5:1770–1775. doi: 10.1242/bio.021667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Nucifora FC, Jr, et al. Interference by huntingtin and atrophin-1 with cbp-mediated transcription leading to cellular toxicity. Science. 2001;291:2423–2428. doi: 10.1126/science.1056784. [DOI] [PubMed] [Google Scholar]
- 166.Chou AH, Yeh TH, Ouyang P, Chen YL, Chen SY, Wang HL. Polyglutamine-expanded ataxin-3 causes cerebellar dysfunction of SCA3 transgenic mice by inducing transcriptional dysregulation. Neurobiol Dis. 2008;31:89–101. doi: 10.1016/j.nbd.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 167.Toonen LJA, et al. Transcriptional profiling and biomarker identification reveal tissue specific effects of expanded ataxin-3 in a spinocerebellar ataxia type 3 mouse model. Mol Neurodegener. 2018;13:31. doi: 10.1186/s13024-018-0261-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Alves S, et al. The autophagy/lysosome pathway is impaired in SCA7 patients and SCA7 knock-in mice. Acta Neuropathol. 2014;128:705–722. doi: 10.1007/s00401-014-1289-8. [DOI] [PubMed] [Google Scholar]
- 169.Chai Y, Koppenhafer SL, Shoesmith SJ, Perez MK, Paulson HL. Evidence for proteasome involvement in polyglutamine disease: localization to nuclear inclusions in SCA3/MJD and suppression of polyglutamine aggregation in vitro. Hum Mol Genet. 1999;8:673–682. doi: 10.1093/hmg/8.4.673. [DOI] [PubMed] [Google Scholar]
- 170.Seidel K, et al. On the distribution of intranuclear and cytoplasmic aggregates in the brainstem of patients with spinocerebellar ataxia type 2 and 3. Brain Pathol. 2017;27:345–355. doi: 10.1111/bpa.12412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Skinner PJ, Koshy BT, Cummings CJ, Klement IA, Helin K, Servadio A, Zoghbi HY, Orr HT. Ataxin-1 with an expanded glutamine tract alters nuclear matrix-associated structures. Nature. 1997;389:971–974. doi: 10.1038/40153. [DOI] [PubMed] [Google Scholar]
- 172.Paulson HL, et al. Intranuclear inclusions of expanded polyglutamine protein in spinocerebellar ataxia type 3. Neuron. 1997;19:333–344. doi: 10.1016/s0896-6273(00)80943-5. [DOI] [PubMed] [Google Scholar]
- 173.Becher MW, et al. Dentatorubral and pallidoluysian atrophy (DRPLA). Clinical and neuropathological findings in genetically confirmed North American and European pedigrees. Mov Disord. 1997;12:519–530. doi: 10.1002/mds.870120408. [DOI] [PubMed] [Google Scholar]
- 174.Holmberg M, et al. Spinocerebellar ataxia type 7 (SCA7): a neurodegenerative disorder with neuronal intranuclear inclusions. Hum Mol Genet. 1998;7:913–918. doi: 10.1093/hmg/7.5.913. [DOI] [PubMed] [Google Scholar]
- 175.Paul S, Dansithong W, Figueroa KP, Scoles DR, Pulst SM. Staufen1 links RNA stress granules and autophagy in a model of neurodegeneration. Nat Commun. 2018;9:1–4. doi: 10.1038/s41467-018-06041-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Todd TW, Lim J. Aggregation formation in the polyglutamine diseases: protection at a cost? Mol Cells. 2013;36:185–194. doi: 10.1007/s10059-013-0167-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Scoles DR, et al. Antisense oligonucleotide therapy for spinocerebellar ataxia type 2. Nature. 2017;544:362–366. doi: 10.1038/nature22044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Niu C, et al. Antisense oligonucleotides targeting mutant Ataxin-7 restore visual function in a mouse model of spinocerebellar ataxia type 7. Sci Transl Med. 2018;10:eaap8677. doi: 10.1126/scitranslmed.aap8677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Hu J, et al. Allele-selective inhibition of ataxin-3 (ATX3) expression by antisense oligomers and duplex RNAs. Biol Chem. 2011;392:315–325. doi: 10.1515/BC.2011.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Toonen LJA, Rigo F, van Attikum H, van Roon-Mom WMC. Antisense oligonucleotide-mediated removal of the polyglutamine repeat in spinocerebellar ataxia type 3 mice. Mol Ther Nucleic Acids. 2017;8:232–242. doi: 10.1016/j.omtn.2017.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.McLoughlin HS, et al. Oligonucleotide therapy mitigates disease in spinocerebellar ataxia type 3 mice. Ann Neurol. 2018;84:64–77. doi: 10.1002/ana.25264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Moore LR, et al. evaluation of antisense oligonucleotides targeting ATXN3 in SCA3 mouse models. Mol Ther Nucleic Acids. 2017;7:200–210. doi: 10.1016/j.omtn.2017.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]