Abstract
Objective:
The pathologic nature of pediatric renal artery occlusive lesions causing renovascular hypertension has been the subject of numerous anecdotal reports. This study was undertaken to define the character of childhood renal artery stenoses. A better understanding of this disease is particularly germane given its unknown etiology and the limited success of certain contemporary treatment options.
Methods:
Renal artery specimens obtained during open operations in children being treated for renovascular hypertension from 2004-2016 were studied. Excluded from study were arteries subjected to earlier open or endovascular operations. Histologic preparations employing hematoxylin-eosin, Movat, Masson’s Trichrome or Verhoeff Van Gieson stains allowed characterization of the intima, media, and adventitial tissues. External and luminal diameters were measured. Microscopic data were correlated with preoperative arteriographic images. The histologic and morphologic findings were assessed in regard to coexistent nonrenal arterial and aortic lesions as well as known syndromic diseases.
Results:
Thirty-three stenotic renal arteries from 28 children were subjected to examination. Stenoses involved the proximal-ostial renal arteries (24), central renal arteries (7) and distal segmental renal arteries (2). Ostial stenoses commonly exhibited pre-occlusive concentric hyperplasia of intimal tissues, frequent internal elastic lamina disruptions, and a diminutive and discontinuous media. Central and distal renal stenoses most often exhibited lesser intimal cellular hyperplasia and more noticeable fibrodysplasia of the media and adventitia. The mean external and luminal diameters of the renal arteries having ostial stenoses were all smaller than the expected renal artery size for a given age. Abdominal aortic coarctation or hypoplastic aortas occurred in 14 children. NF-1 affected 4 children with ostial renal artery disease and 1 child with mid-renal artery disease, but there were no distinguishing features unique to their stenoses.
Conclusions:
Pediatric renal artery stenotic disease affects exceedingly small arteries. Ostial lesions frequently exhibit extensive luminal encroachments characterized by cellular hyperplasia of intimal tissues and scant medial smooth muscle. Central and distal renal arterial stenoses were characterized most often by extensive fibrodysplasia of the media and adventitia. The early success and durability of catheter-based angioplasty may be compromised by the cellular abnormalities of pediatric renal artery occlusive disease observed in this investigation.
Table of Contents Summary
Single-center, retrospective (histopathologic) review
Small arterial diameter and predictable cellular abnormalities were identified in samples of 33 stenotic pediatric renal arteries, surgically treated for renovascular hypertension. These findings may compromise the early success and durability of catheter-based angioplasty in the treatment of developmental proximal-ostial pediatric renal artery occlusive disease.
INTRODUCTION
Pediatric renal artery stenotic disease is an uncommon disease responsible for refractory renin-mediated hypertension. The clinical manifestations of this disease is well known, yet the etiology of the underlying arterial stenoses remains unknown and their histologic and morphologic character have not been clearly established. The pathologic nature of pediatric renal artery occlusive lesions causing renovascular hypertension has been the subject of numerous anecdotal reports.
The impetus for this study was to define the anatomic histopathology of stenotic renal artery disease in children, as a means of providing insight as to why certain contemporary procedures, including catheter-based interventions, have resulted in less salutatory outcomes than anticipated, despite early technical successes. It is anticipated that the study’s findings will serve in creating rational guidelines for treating children with renovascular hypertension.
METHODS
Forty-nine stenotic renal arteries were subjected to histologic examination after being obtained from 42 children during surgical procedures for renovascular hypertension at the University of Michigan from 2004 to 2016. In total, 33 specimens obtained from 28 children represented well prepared intact stenotic segments and are the basis of this report, as the underlying pathology could not be accurately characterized amongst the 14 other children having 16 renal artery specimens that were fragmented, represented incomplete arterial rings, or had been inadequately prepared for the current study. Excluded from study were those arteries previously subjected to catheter-based angioplasty with or without stenting, and those representing failures of prior open surgical procedures. This retrospective review was approved by the Institutional Review Board at the University of Michigan (HUM0006223), and a waiver of informed consent was granted.
The renal artery’s histologic character was correlated with the preoperative arteriographic images of the affected artery. Stenotic lesions were categorized as to their location within the renal vasculature: 1) ostial-proximal main renal artery, 2) central main renal artery including 1st order branching, and 3) distal segmental renal artery involving 2nd order branches and beyond. Concomitant aortic narrowings, stenotic disease of the splanchnic arteries, as well as the presence of any syndromic disease were noted.
Standard histologic preparations included 10% neutral formalin fixation, paraffin embedding and sectioning, followed by tissue staining: hematoxylin-eosin (H&E), Movat, Verhoeff Van Gieson, and Masson’s trichrome staining. The artery’s cellular and interstitial characteristic were recorded, including the status of luminal endothelial cell lining, the appearance of intact or disrupted elastic fibers, unusual accumulations of collagen fibers and other ground substances, round or spindle-shaped intimal and medial cells, and the presence of inflammatory cells. The external diameter and inner (luminal) diameter of each fixed artery were measured, and compared to the estimated normal renal artery dimensions by age, determined by correlating known renal artery diameters and kidney lengths, to known kidney sizes at given ages.
Given that the excised lengths of the stenotic segments were never more than 2 or 3 mm in length, any perfusion fixation would have been technically impossible. Nevertheless, luminal diameters of the ostial and central renal arterial stenoses, estimated by conventional catheter-based arteriographic means did not appears to differ from the histologic specimen measurements. Any potential reductions would have been exceedingly small, given that the luminal diameters by either measurement technique were less than 0.8 mm in 26 of 31 stenoses, and never exceeded 1.2 mm.
The external diameters could not be accurately measured on preoperative MRAs or duplex ultrasound studies. Nevertheless, accurate measurements of the external diameter were possible on three of five preoperative CT studies. Reductions of the external diameter in these cases were estimated to be 10%, 10%, and 4%. The latter observation was consistent with the calculated 9.9% diameter reduction of fresh arterial rings following formalin-paraffin preparation in the experimental setting.(1)
The three layers of the arterial wall were characterized and the dominant pathologic features were assigned to each artery. Abnormal intimal features included differing degrees of eccentric or concentric cellular hyperplasia, denoted as intimal fibrodysplasia (IF), manifest by encroachments into the lumen, as well as disruption and duplication of the internal elastic lamina (IEL). Abnormal media included medial fibrodysplasia (MF) manifest by disorganized smooth muscle and increases in collagen ground substances, at times with the appearance of a thickened media. In other renal arteries, scant or discontinuous media created the appearance of a thinned media. Abnormal findings within the adventitia included fibrodysplasia (AF), evident by unusual concentrations of elastin fibers and in some instances increases in the ground substances.
RESULTS
Twenty-eight children, with a mean age of 6.8±4.3 years, had 33 intact renal arteries sufficient for accurate anatomic characterization. There was no sex predilection (15 boys and 13 girls). Bilateral renal artery stenotic disease affected 17 of these children, although adequately prepared bilateral stenotic arterial segments were available for study in only 5 children. Stenoses affecting 8 arteries exhibited marked abnormalities involving all three anatomic layers of the arterial wall, the intima, media, and adventitia, 21 stenoses affected at least two of the artery’s three anatomic layers, and 4 stenoses exhibited involvement of only one layer. Such changes reflected a complex array of pathologic findings (Figure 1). The site of the stenosis within the renal artery deserves comment in that its location appeared to favor the presence of specific changes in each of the arterial wall layers.
Figure 1:

A. Focal ostial stenosis at the origin of the renal artery (arrow) in a child with a normal aorta (reformatted CTA). B. Focal ostial stenosis in a renal artery originating in the region of an abdominal aortic coarctation (CTA).
Proximal-Ostial Renal Artery Lesions
The most frequent stenotic lesion location was in the proximal renal artery, near or at the ostium of its aortic origin, being observed in 21 children (Table I). These stenoses were usually concentric and focal, affecting the proximal 5 to 10 mm of the renal artery (Figure 1). The histologic appearance of these stenoses were often very complex with involvement of more than just one layer of the arterial wall (Figure 2).
Table 1. Proximal-Ostial Renal Artery Specimens: 24 in 21 Children.
[M-male, F-female; c-concentric, e-eccentric; min-minimal, mod-moderate, extn-extensive; IF-intimal fibrodysplasia (cellular accumulations), IEL-internal elastic lamina, dupl-duplication, disrp-disrupted; sctM-scant media, discnM-discontinuous media, MF-media fibrodysplasia (disorganized smooth muscle, increased ground substances); AF-adventitial fibrodysplasia (increased cellular and ground substances); Ao-aorta, AbdAoCoarc-abnormal aortic coarctation; SMA=-superior mesenteric artery, CA-celiac artery; NF-1-neurofibromatosis-1, NoDA-no distinct abnormality]
| Age (yr) Sex | Intima | Media | Adventitia | Dominant Abnormality | Comment | |
|---|---|---|---|---|---|---|
| 1 | 1.5M | eIF(mod) | MF(mod), sctM | NoDA | MF, sctM | --- |
| 2 | 1.5M | cIF(extn), IEL disrp | sctM | NoDA | IF | SMA branch and hepatic artery branch stenoses |
| 3 | 2M | 1-cIF(mod), IEL disrp, luminal thrombus 2-cIF(extn), IEL disrp/dupl |
discnM NoDA |
NoDA NoDA |
IF, sctM IF |
AbdAoCoarc (suprarenal) |
| 4 | 3F | cIF(extn), IEL dupl, luminal thrombus | MF(min) | NoDA | IF | AbdAoCoarc (occluded infrarenal Ao), SMA stenosis |
| 5 | 3F | cIF(extn), IEL disrp/dupl | MF(mod), sctM | NoDA | IF | AbdAoCoarc (intraranal), NF-1 |
| 6 | 4F | cIF(extn), IEL disrp | discnM | AF(min) | IF | AbdAoCoarc (infrarenal) CA and SMA stenoses |
| 7 | 4F | 1-cIF(extn), IEL disrp 2-eIF(mod), IEL disrp |
discnM discnM |
AF(estn) AF(extn) |
IF, AF IF, AF |
Diminutive Ao (infrarenal), SMA stenosis |
| 8 | 5M | cIF(min), IEL disrp/dupl | MF(mod), discnM | NoDA | MF, discnM | NF-1 |
| 9 | 6F | cIF(extn) | NoDA | NoDA | IF | SMA stenosis |
| 10 | 6M | cIF(extn) | discnM | NoDA | IF | AbdAoCoarc (suprarenal) |
| 11 | 7F | eIF(extn), IEL disrp/dupl | MF(extn), sctM | AF(extn) | MF, AF | William’s Syndrome, contralateral polycystic kidney |
| 12 | 7M | cIF(extn), IEL disrp | MF(extn) | NoDA | MF | --- |
| 13 | 7M | cIF(mod), IEL disrp/dupl | MF(extn), discnM | NoDA | IF, discnM | AbdAoCoarc (intrarenal), SMA stenosis |
| 14 | 8F | eIF(extn), IEL disrp/dupl | MF(extn), discnM | NoDA | IF, discnM | AbdAoCoarc (infrarenal), Bicuspid Aortic Valve |
| 15 | 8M | 1-cIF(extn), IEL disrp 2-eIF(mod), IEL disrp |
MF(mod), sct/discnM MF(mod), discnM |
NoDA NoDA |
IF IF |
--- |
| 16 | 9M | cIF(extn), IEL disrp | MF(extn), sctM | NoDA | IF | AbdAoCoarc (intrarenal), CA and SMA stenoses |
| 17 | 11M | eIF(min), IEL disrp | sctM | NoDA | sctM | AbdAoCoarc (infrarenal), SMA stenosis, NF-1 |
| 18 | 12F | eIF min), IEL disrp/dupl | MF(extn), sctM | NoDA | MF | Diminutive Ao (infrarenal) |
| 19 | 13M | cIF(mod), IEL disrp | MF(mod), sctM | NoDA | IF | NF-1 |
| 20 | 15M | cIF(extn), IEL disrp, luminal thrombus | discnM | NoDA | IF | AbdAoCoarc (intrarenal), SMA stenosis |
| 21 | 16F | cIF(mod) | MF(extn), discnM | NoDA | MF | AbdAoCoarc (infrarenal) |
Figure 2:

A complex renal artery stenosis exhibiting abnormalities in all three vessel layers: intimal fibrodysplasia (star), duplication of the internal elastic lamina (arrow), disorganized fibrodysplastic media, and disproportionate accumulations of elastin in the adventitia. (Movat, original magnification x 4)
The dominate pathologic abnormality affecting 21 of the 24 ostial stenoses resided in the intima. The cellular hyperplasia of IF was extensive or moderate in 14 and 7 of the 24 ostial lesions, respectively (Figure 3). In addition, marked disruptions and duplications of the IEL accompanied the IF in 20 renal arteries (Figure 4). Spindle-shaped cells suggesting smooth muscle cells or myofibroblasts were occasionally noted within the hyperplastic tissues comprising the IF.
Figure 3:

Extensive intimal fibrodysplasia encroaching in the lumen was the most dominant abnormality in 14 of the 24 ostial stenoses. Prominent collagen noted in intima and media tissues. (Movat, original magnification x 10)
Figure 4:

Disruption and duplication of the internal elastic lamina associated with intimal fibrodysplasia was observed in 20 of the 24 ostial stenoses. (Movat, original magnification x 10)
The media of ostial stenoses was distinctly abnormal in 10 arteries, including 6 that exhibited extensive MF (Figure 5). The remaining 4 arteries exhibited scant or discontinuous media (Figure 6). Renal artery ostial stenoses in close proximity to their aortic origin (the transition zone from an elastic to a muscular vessel) were observed to have an intermingling of both discontinuous smooth muscle cells and elastic tissue within the media (Figure 7). Adventitial structures in arteries with ostial stenoses were relatively normal in appearance with the exception of 3 arteries exhibiting AF (Figure 8).
Figure 5:

Medial fibrodysplasia manifest by collagenous matter separating smooth muscle occurred in 6 of the 24 ostial stenoses. (Masson trichrome, original magnification x 4)
Figure 6:

Scant, discontinuous media evident in 4 of the 24 ostial stenosis with minimal intimal fibrodysplasia. (Movat, original magnification x 4)
Figure 7:

Intermingling of both discontinuous smooth muscle cells and elastic tissue within the fibrodysplastic media evident in the transition zone of ostial stenoses occurring in close proximity to the renal artery’s aortic origin. Masson trichrome, original magnification x 20.
Figure 8:

Adventitial fibrodysplasia associated with extensive intimal fibrodysplasia in an ostial stenosis. (H&E, original magnification x 4)
Ostial renal artery stenoses were observed in very small arteries, having mean external and luminal diameters of 2.2mm±0.6 and 0.4mm±0.3, respectively. These stenotic arterial segments were smaller than that the estimated size of renal arteries expected in normal children of the same age (Figure 9).
Figure 9:

Graphic demonstration of the external and luminal diameters of pediatric renal artery stenoses, compared to expected luminal diameters at a given age (representing an angiographic luminal diameter). Assessments excluded measurement when multiple renal arteries existed, with the exception of ostial lesions in 3 children with diminutive accessory renal arteries.
Although 13 of the 21 children with ostial lesions had bilateral renal artery stenoses, only 3 had both renal arteries available for this study. In cases of bilaterality, the dominant abnormality was symmetric. Concomitant aortic narrowings accompanied the ostial renal stenoses in the form of distinct abdominal aortic coarctations in 11 of children and diminutive aortas in 2 children. Normal aortas accompanied the renal artery ostial stenoses in the remaining 8 children. Stenotic lesions affected the ostia of the celiac artery (CA) and superior mesenteric artery (SMA) in 7 children with ostial renal artery stenoses, including 5 having aortic narrowings. One child had William’s syndrome, but her stenotic tissue was indistinguishable from the stenoses affecting the other children’s arteries. Similarly, 4 children with ostial stenoses had neurofibromatosis type 1 (NF1). In comparing children with isolated renal artery occlusive disease to those with a more advanced phenotype or known syndrome, there were no distinguishing histopathologic differences, with the exception of the presence of occasional nonconstricting neural elements (Wagner-Meissner bodies) in the outer adventitia in NF1 children’s arteries.
Central Renal Artery Lesions
Stenotic renal arteries in the mid-portion of the main renal artery in 4 children and the early-portion of a first order branch in a 5th child (Figure 10) exhibited a spectrum of pathologic abnormalities (Table II). The media of 3 arteries exhibiting the distal renal artery stenoses exhibited marked MF as their dominant histologic feature (Figure 11). Marked AF associated with extensive IF occurred in three other stenoses (Figure 12).
Figure 10:

Central mid-renal artery stenosis in a first order branch of the main renal artery (selective renal arteriogram).
Table 2. Distal Renal Artery Specimens: 7 in 5 Children.
[M-male, F-female; c-concentric, e-eccentric; min-minimal, mod-moderate, extn-extensive; IF-intimal fibrodysplasia (cellular accumulations), IEL-internal elastic lamina, dupl-duplication, disrp-disrupted; sctM-scant media, discnM-discontinuous media, MF-media fibrodysplasia (disorganized smooth muscle, increased ground substances); AF-adventitial fibrodysplasia (increased cellular and ground substances); Ao-aorta, AbdAoCoarc-abnormal aortic coarctation; SMA=-superior mesenteric artery, CA-celiac artery; NF-1-neurofibromatosis-1, NoDA-no distinct abnormality]
| Age (yr) Sex | Intima | Media | Adventitia | Dominant Abnormality | Comment | |
|---|---|---|---|---|---|---|
| 1 | 2F | 1 cIF(extn), IEL disrp, luminal thrombus 2 cIF(extn) |
sct/discM sctM |
AF(extn) AF(mod) |
IF, AF IF |
CA and SMA stenosis, NF-1 |
| 2 | 3M | 1 cIF(min), IEL disrp 2 NoDA |
MF(mod) MF(mod) |
NoDA NoDA |
MF MF |
SMA, Inferior mesenteric artery, Hepatic artery irregular stenoses |
| 3 | 4F | cIF(extn), IEL disrp | MF(mod), discnM | AF(extn) | IF, AF | --- |
| 4 | 5M | NoDA | sct/discnM | NoDA | sct/discnM | --- |
| 5 | 15F | eIF(mod), IEL disrp/dupl | MF(mod), discnM | AF(extn)) | AF | --- |
Figure 11:

Medial fibrodysplasia with collagenous matter separating smooth muscle was the dominant finding in 3 central renal artery stenoses. (Masson trichrome, original magnification x 10)
Figure 12:

Adventitial fibrodysplasia (arrow) manifest as homogenous tissue surrounding discontinuous media was the dominant abnormality in 3 central renal artery stenoses, all of which were associated with extensive intimal fibrodysplasia (star). (Movat, original magnification x 4)
Central renal artery stenoses were also observed in very small arteries. The mean external and luminal diameters of the stenotic segments affecting the central renal arteries was 2.8mm±0.4 and 0.9mm±0.2, respectively. These stenotic segments, like the ostial lesions, were smaller than the size of renal arteries expected in normal children of the same age (Figure 9).
Three of the 5 patients with central renal artery lesions had bilateral renal artery stenoses, but only 2 had a segment of the contralateral renal artery available for study. Unlike the children with proximal renal artery stenoses, none of this group had aortic narrowings, although 2 had ostial stenoses of their splanchnic artery origins. The only child with NF1 in this group had no distinguishing histopathologic abnormalities of her stenotic renal arteries compared to the stenoses in the other four children.
Distal-Segmental Renal Artery Lesions
Two patients had stenoses affecting the more distal branches of the renal arteries (Figure 13). Segmental renal artery stenoses exhibited extensive adventitial abnormalities (Table III). The dominant finding in one artery was extensive AF with minimal changes in the intima or media (Figure 14). The second artery manifest similar AF, but it was associated with marked IF and the presence of luminal thrombus (Figure 15).
Figure 13:

Segmental renal artery stenosis, nearing occlusion (selective renal arteriogram).
Table 3. Segmental Renal Artery Specimens: 2 in 2 Children.
[M-male, F-female; c-concentric, e-eccentric; min-minimal, mod-moderate, extn-extensive; IF-intimal fibrodysplasia (cellular accumulations), IEL-internal elastic lamina, dupl-duplication, disrp-disrupted; sctM-scant media, discnM-discontinuous media, MF-media fibrodysplasia (disorganized smooth muscle, increased ground substances); AF-adventitial fibrodysplasia (increased cellular and ground substances); Ao-aorta, AbdAoCoarc-abnormal aortic coarctation; SMA=-superior mesenteric artery, CA-celiac artery; NF-1-neurofibromatosis-1, NoDA-no distinct abnormality]
| Age (yr) Sex | Intima | Media | Adventitia | Dominant Abnormality | Comment | |
|---|---|---|---|---|---|---|
| 1 | 4M | cIF(extn), luminal thrombus | NoDA | AF(extn) | IF, AF | --- |
| 2 | 7F | NoDA | NoDA | AF(extn) | AF | AbdAoCoarc (suprarenal), NF-1 |
Figure 14:

Extensive adventitial fibrodysplasia of a segmental renal artery stenosis with no abnormalities of the intima or media (luminal tissue represents a preparation artifact). (Masson trichrome, original magnification x 4)
Figure 15:
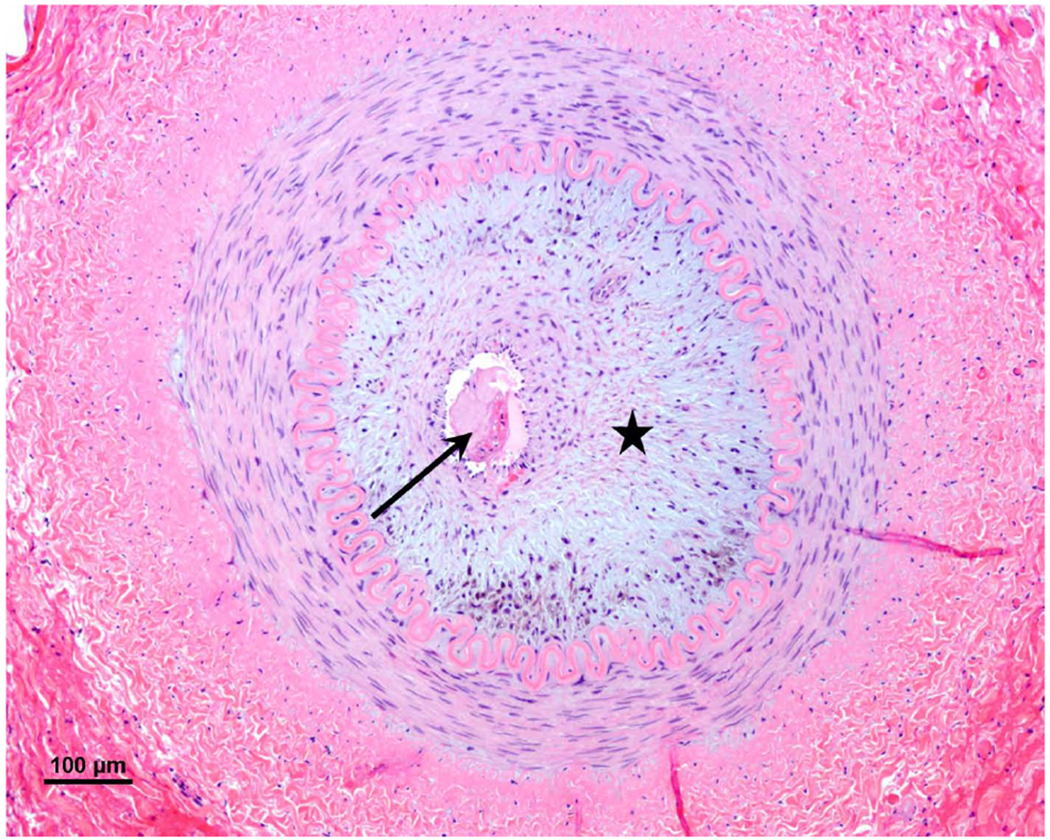
Adventitial fibrodysplasia of a segmental renal artery stenosis with marked intimal fibrodysplasia (star), normal media, intact internal elastic lamina, and the presence of luminal thrombus (arrow). (H&E, original magnification x 10)
DISCUSSION
Pediatric renal artery stenoses are a recognized cause of excessive renin release from the kidney resulting in a refractory form of hypertension. The clinical presentation of this disease is well known, but the underlying etiology of the renal artery occlusive lesions is not understood. In part, this relates to the paucity of reports on the specific anatomic character of the renal artery stenoses, themselves. The histologic description of a diseased renal artery responsible for hypertension in a child was first reported in 1938(2). That artery had some features similar to those described in the present report, but the authors attributed the altered renal blood flow to an obstructive plug of luminal tissue. That was a misinterpretation. The plug represented a preparation artifact (telescoping of the specimen), as was observed in the present study (Figure 14).
Since that first report, there has been only one systematic study regarding the histologic findings in pediatric renal artery occlusive disease. That study, published in 1991, reported the microscopic appearance of 12 stenotic renal arteries in children, 9 surgical and 3 autopsy specimens(3). There were marked similarities to the present study; bilateral renal artery disease affected 75% of the cases, 50% had concomitant aortic anomalies, and 25% had NF1. The histologic features were also similar, but were categorized differently, as medial fibrous dysplasia, perimedial elastic dysplasia, intimal fibrous dysplasia and medial muscular dysplasia. This former report included nine histologic studies in four patients, that exhibited many of the same features presently reported, albeit with different terminology. Three other publications have presented scattered photographs of pediatric renal artery occlusive disease; one focusing on the lesion’s histopathology(4), and two on the accompanying clinical disease(5, 6). The histologic character of these three cases were near identical to those of the present study.
It is likely that there will be fewer opportunities to examine surgical specimens of stenotic renal arteries in children in the future, given the increasing use of catheter-based angioplasty in the treatment of their renovascular hypertension. Nevertheless, an abundance of material involving children’s renal arteries currently exists in the form of radiographic images, clinical records, and renal artery specimens, at the University of Michigan. Given the availability of this resource, the authors chose to reexamine all renal artery specimens obtained during surgical procedures performed in children over a 12-year time period. Paraffin blocks of unprepared stenotic arteries for those having only H&E staining were re-cut and subjected to elastin staining as a means to better define the anatomic nature of these lesions.
The findings of the current study suggest that certain histopathologic differences exist depending on its location within the renal artery. All of the ostial lesions exhibited some degree of abnormal cellular hyperplasia within the intima, contributing to the arterial stenoses. This form of IF was concentric in 19 of the 24 ostial stenoses and it is speculated that it may have been present elsewhere in a nearby unexamined segment of the 5 arteries exhibiting eccentric narrowings. Although 5 renal arteries exhibited luminal thrombus, in only one child’s artery did recanalization of the thrombus appear to contribute to the IF. Cellular accumulations within the intima of children are not rare. It is generally recognized that luminal injury from intra-arterial catheter trauma, or arteries manipulated during operative procedures, may result in IF. But none of the former interventions had occurred in the current cohort of children of this study.
It may be speculated that cellular hyperplasia within the intima is the result of a growth-factor stimulus that causes proliferation of the occasional cell seen residing between the luminal endothelium and IEL Such growth-factors may be a consequence of high blood flow disturbances through a narrowed renal artery at its origin. This may occur if the embryonic renal artery at its origin never grew, but the kidney mass did, resulting in increased volume blood flow with a corresponding increase in shear-stresses through the vessel’s ostium. Such rheologic factors accompanying high blood flow through the narrowed vessel would predictably initiate a progressive and accelerated proliferation of intimal cells. No existing interventions, pharmacologic or radiation-related (brachytherapy), are recognized in preventing such secondary intimal fibroplasia in children. Unfortunately, removal of the cellular intimal tissue (as with cutting-balloon angioplasty) does not alter the underlying medial and adventitial structures of the stenosis, and the re-accumulation of intimal fibrodysplastic tissue is inevitable.
Medial abnormalities were also common in ostial stenoses, although MF was the dominant feature in only 5 specimens. Less obvious was a disorganization of the media’s smooth muscle, often accompanied with increases in interstitial collagen and other ground substances. The occasional appearance of scant and discontinuous layers of medial smooth muscle cells certainly appeared abnormal. Nevertheless, when interspersed with elastin tissue this may simply have represented the normal transition zone from predominately elastin lamellae of the aorta to the smooth muscle lamellae in the media of a normal renal artery(7).
The unusual incidence currently reported of abdominal aortic coarctation in children with the ostial stenoses may support a growth-failure (developmental) nature of ostial stenoses. Abdominal aortic coarctation is well known to be associated with ostial stenoses of the renal artery as well as splanchnic arteries(8). Whatever causes the aorta to be narrowed, it appears to occur at the same time of embryonic fusion of the two dorsal aortas around the 25th day of gestation. This event transpires at the same time the multiple mesonephric arteries to each kidney decrease in number and attach to the newly formed single dorsal aortic channel. It remains unknown as to what genetic or other factors may disturb this process and result in both a narrowing of the aorta and its branch origins.
The stenotic renal arteries in this experience were small, especially those accompanying ostial lesions, and that observation deserves comment. The external size of renal arteries in normal growing children has not been previously directly published, but their arteriographic diameters in relation to kidney lengths are known(9), and kidney lengths related to age are known(10, 11). These two findings provide a means of estimating the average renal artery diameter in growing children, as presented in this report (Figure 9). Given that an arteriographic-defined luminal diameter is going to be less than the external dimeter, the normal renal artery estimates of the current study may be understated. The regular morphologic undulations of the IEL in the study’s ex vivo specimens, resulting from a circumferential constriction of the stenotic segments, also means that the true in vivo external renal artery diameters would be greater under systemic arterial pressure. Nevertheless, even a moderate increase in measured diameter would not discount the fact that these were unusually small stenotic arteries. The frequent ‘hour-glass’ narrowing of ostial renal artery stenosis observed during an operative procedure supports the tenet that they are indeed small arteries.
All of the lesions encountered in this study were focal lesions, be they involving the ostia, central, or distal renal artery, with one exception. One patient exhibited multiple narrowings with no intervening mural dilations over a few cm. of the central renal artery. Similar stenoses were observed in his hepatic as well as superior and inferior mesenteric arteries. Although an arteritis was suspected, the dominant finding was medial fibroplasia with no inflammatory cells. The central renal and distal renal artery stenoses in the current report were not easily categorized and their etiology even more speculative. Stenoses affecting the mid-portion of the renal artery often exhibited IF as a dominant feature, but the media and adventitial manifest very different processes. In the later tissues either MF or AF were most evident, but both never occurred together in the same artery, as co-dominant findings. The authors’ overall operative experience suggests that central renal artery stenoses in children are not as common as ostial stenoses, having been encountered in 15 of 97 children undergoing open surgical procedures for renovascular hypertension(6). Underlying events leading to central renal artery stenoses in this series are unknown.
Segmental pediatric renal artery stenoses have not been characterized histologically in the published literature. Although marked AF was observed in the current report, there is little available evidence to suggest the cause of these lesions. The authors have witnessed segmental renal artery occlusions secondary to embolism from thrombus dislodged from an umbilical catheter placed for neonatal exchange transfusions, but none of the current study’s children had undergone such a procedure.
Neurofibromatosis affected children in each of the renal artery’s anatomic sites exhibiting a stenosis. The association on NF1 with renal artery stenoses and abdominal aortic narrowing has been well documented by the authors prior publications(6, 8). However, the specific underlying defect leading to the vascular abnormalities in NF1 patients remain undefined(12). Similarly, it has been recognized that renal artery stenoses similar to those described in this report have been observed in children whose mothers experienced a rubella infection during pregnancy(13, 14). Yet none of the children in this study whose stenotic renal arteries were examined was the product of a complicated pregnancy, including rubella or other notable maternal viral infections.
Pediatric renal artery disease appears to be distinct from the fibrodysplastic diseases noted in adult renal arteries(15–17). It is of interest, that in the past it has been generally recognized that renal artery IF in adults often involves younger patients as a focal lesion and occurs without a female predilection, as occurs in the other forms of adult arterial dysplasia. It may be that some adults exhibiting renal artery IF had their disease at a young age, with its recognition occurring only when the stenoses progressed to cause difficult to control hypertension. While some may propose that pediatric renal artery disease is a related phenotype of adult fibromuscular dysplasia, it should be recognized that the classic adult features of female predilection, young adult age, and multifocal typical string of beads type of medial fibrodysplasia have no counterpart in the current study’s children. However, the perimedial form of adult renal artery dysplasia with excesses in elastic tissue may be similar to the renal artery AF observed in 8 of this study’s children. None of the renal artery stenoses examined in this study exhibited features that might reflect segmental arterial mediolysis(18), although the later may be misinterpreted in assessing the anatomic character of pediatric renal artery lesions.
Failure of endoluminal catheter-based interventions with balloon angioplasty, with or without stenting, in the treatment of ostial stenoses in children has been reported by the authors(19). These failures, both occurring immediately and later, may be explained, in part, by the current reports’ findings. Extensive cellular hyperplasia of IF occurring in a small artery with concomitant MF would seem a difficult target for endovascular treatment. The authors’ examinations of explanted renal arteries following unsuccessful endovascular interventions (unpublished data) confirms the presence of severe dysplastic changes, most evident in a proliferative IF, and supports the tenet that pediatric ostial stenoses are not easily treated by endovascular means.
The current study had four inherent limitations. First, given the very small arteries involved and the need to use considerable care during the renal artery reconstruction procedures, the samples removed for histologic study may not have been the most representative of the entire stenotic lesion. However, the degree of in vivo luminal narrowing could not have been less that noted in the acquired samples. Second, a potential ascertainment bias may exist in type of samples studied based on the selection of children undergoing open surgical procedures at the University of Michigan. However, the patients’ stenoses reported in this paper are likely similar to those at other referral institutions undertaking both open surgical procedures as well as endovascular interventions. The choice of an open or endovascular intervention often depends on the wishes of the referring doctor and technical expertise of the treating doctors. The stenoses in the current report were clearly defined by location (ostial, central, distal renal artery), morphology (concentric, eccentric) and dimensions (luminal and external diameters). The latter facts should allow others to compare their clinical material with those from the authors’ institution. Third, the small numbers of renal arteries examined, especially of the distal renal artery, make definitive statements as to the histopathology of all pediatric renal artery disease inappropriate. Fourth, the microscopic appearance of these stenoses did not define their etiology. Further analysis is necessary to define molecular mechanisms of arterial fibrodysplasia in these lesions. Nevertheless, this study lays the foundation for bettering our understanding of pediatric renal artery occlusive disease.
CONCLUSION
Occlusive lesions of the renal arteries causing renovascular hypertension in children are uncommonly examined, but exhibit a broad spectrum of histologic abnormalities. Pediatric renal artery stenotic disease affects exceedingly small arteries. The most common pathology affecting nearly all stenoses is IF. It is the dominant abnormality in ostial renal artery stenoses. Disorders of the media in the form of MF or disruptions of the smooth muscle layers are common in many pediatric renal artery stenoses, but are uncommon as a dominant feature of the stenosis. Adventitial abnormalities are less well characterized, and although they may affect any anatomic segment of the renal artery, it is more likely to be a dominate feature of central and segmental renal artery stenoses. Future study will be required to document the cellular proliferation rates in intimal tissues, the character of the medial cell as secretory myofibroblasts, vascular smooth muscle, or some other cell type, the presence or absence of apoptosis affecting medial cells, as well as the type and quantity of abnormal proteins within different segments of the arterial wall. The underlying cause of pediatric renal artery stenoses remain poorly understood, but many appear related to developmental misadventures during embryonic growth. The early success and durability of catheter-based angioplasty may be compromised by the complex cellular abnormalities of pediatric renal artery occlusive disease observed in this investigation.
ARTICLE HIGHLIGHTS.
Type of Research:
Single-center, retrospective (histopathologic) review
Key Findings:
Samples from 33 stenotic renal arteries from 28 children were subjected to histopathologic examination. Pediatric renal artery stenosis affected exceedingly small arteries. Proximal-ostial lesions were most common and exhibited extensive luminal encroachments characterized by cellular hyperplasia of intimal tissues and scant medial smooth muscle.
Take home Message:
The exceedingly small diameter of pediatric renal arteries affected by developmental occlusive disease alongside predictable cellular abnormalities may compromise the early success and durability of catheter-based angioplasty.
Acknowledgments
Funding: Supported in part from the Zangara Research Fund and Robson Research Fund, both at the University of Michigan; and NIH grant, R01HL 139672.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
COI: The authors have no conflict of interest or conflict of competing interests relevant to this study.
REFERENCES
- 1.Dobrin PB. Effect of histologic preparation on the cross-sectional area of arterial rings. J Surg Res. 1996;61(2):413–5. [DOI] [PubMed] [Google Scholar]
- 2.Leadbetter WF BC. Hypertension in unilateral renal artery disease. J Urol. 1938;39:611–26. [Google Scholar]
- 3.Devaney K, Kapur SP, Patterson K, Chandra RS. Pediatric renal artery dysplasia: a morphologic study. Pediatr Pathol. 1991;11(4):609–21. [DOI] [PubMed] [Google Scholar]
- 4.Westenend PJ, Smedts F, de Jong MC, Lommers EJ, Assmann KJ. A 4-year-old boy with neurofibromatosis and severe renovascular hypertension due to renal arterial dysplasia. Am J Surg Pathol. 1994;18(5):512–6. [DOI] [PubMed] [Google Scholar]
- 5.Schurch W, Messerli FH, Genest J, Lefebvre R, Roy P, Carter P, et al. Arterial hypertension and neurofibromatosis: renal artery stenosis and coarctation of abdominal aorta. Can Med Assoc J. 1975;113(9):879–85. [PMC free article] [PubMed] [Google Scholar]
- 6.Stanley JC, Criado E, Upchurch GR Jr., Brophy PD, Cho KJ, Rectenwald JE, et al. Pediatric renovascular hypertension: 132 primary and 30 secondary operations in 97 children. J Vasc Surg. 2006;44(6):1219–28; discussion 28-9. [DOI] [PubMed] [Google Scholar]
- 7.Janzen J, Lanzer P, Rothenberger-Janzen K, Vuong PN. The transitional zone in the tunica media of renal arteries has a maximal length of 10 millimetres. Vasa. 2000;29(3):168–72. [DOI] [PubMed] [Google Scholar]
- 8.Stanley JC, Criado E, Eliason JL, Upchurch GR Jr., Berguer R, Rectenwald JE. Abdominal aortic coarctation: surgical treatment of 53 patients with a thoracoabdominal bypass, patch aortoplasty, or interposition aortoaortic graft. J Vasc Surg. 2008;48(5):1073–82. [DOI] [PubMed] [Google Scholar]
- 9.Taber P, Korobkin MT, Gooding CA, Palubinskas AJ, Neuhauser EB. Growth of the abdominal aorta and renal arteries in childhood. Radiology. 1972;102(1):129–34. [DOI] [PubMed] [Google Scholar]
- 10.Han BK, Babcock DS. Sonographic measurements and appearance of normal kidneys in children. AJR Am J Roentgenol. 1985;145(3):611–6. [DOI] [PubMed] [Google Scholar]
- 11.Rosenbaum DM, Korngold E, Teele RL. Sonographic assessment of renal length in normal children. AJR Am J Roentgenol. 1984;142(3):467–9. [DOI] [PubMed] [Google Scholar]
- 12.Lie JT. Vasculopathies of Neurofibromatosis Type 1 (von Recklinghausen Disease). Cardiovasc Pathol. 1998;7(2):97–108. [DOI] [PubMed] [Google Scholar]
- 13.Esterly JR, Oppenheimer EH. Vascular lesions in infants with congenital rubella. Circulation. 1967;36(4):544–54. [DOI] [PubMed] [Google Scholar]
- 14.Fortuin NJ, Morrow AG, Roberts WC. Late vascular manifestations of the rubella syndrome. A roentgenographic-pathologic study. Am J Med. 1971. ;51(1):134–40. [DOI] [PubMed] [Google Scholar]
- 15.Harrison EG Jr., McCormack LJ. Pathologic classification of renal arterial disease in renovascular hypertension. Mayo Clin Proc. 1971;46(3):161–7. [PubMed] [Google Scholar]
- 16.Plouin PF, Perdu J, La Batide-Alanore A, Boutouyrie P, Gimenez-Roqueplo AP, Jeunemaitre X. Fibromuscular dysplasia. Orphanet J Rare Dis. 2007;2:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stanley JC, Gewertz BL, Bove EL, Sottiurai V, Fry WJ. Arterial fibrodysplasia. Histopathologic character and current etiologic concepts. Arch Surg. 1975; 110(5):561–6. [DOI] [PubMed] [Google Scholar]
- 18.Slavin RE, Inada K. Segmental arterial mediolysis with accompanying venous angiopathy: a clinical pathologic review, report of 3 new cases, and comments on the role of endothelin-1 in its pathogenesis. Int J Surg Pathol. 2007;15(2):121–34. [DOI] [PubMed] [Google Scholar]
- 19.Eliason JL, Coleman DM, Criado E, Kershaw DB, Blatt NB, Williams DM, et al. Remedial operations for failed endovascular therapy of 32 renal artery stenoses in 24 children. Pediatr Nephrol. 2016;31(5):809–17. [DOI] [PubMed] [Google Scholar]


