Abstract
Objective:
The value of hepatocellular carcinoma (HCC) surveillance is defined by the balance of benefits, i.e. early tumor detection, and potential harms, related to false positive and indeterminate results. Although physical harms can be observed in 15–20% of patients with cirrhosis undergoing HCC surveillance, prior cost effectiveness analyses have not incorporated costs of harms. We aimed to evaluate the cost-effectiveness of HCC surveillance including both benefits and harms.
Design:
We constructed a Markov model to compare surveillance strategies of ultrasound (US), US and alpha fetoprotein (AFP), and no surveillance in 1 million simulated patients with compensated cirrhosis. Harms included imaging and biopsy in patients undergoing surveillance for HCC. Model inputs were based on literature review and costs were derived from the Medicare fee schedule, with all costs inflated to 2018 dollars. The primary outcome was the incremental cost-effectiveness ratio (ICER) per incremental quality-adjusted life-year (QALY).
Results:
In the base case analysis, US and AFP was the dominant strategy over both US alone and no surveillance. In a probabilistic sensitivity analysis, US and AFP was the most cost-effective strategy in 80.1% of simulations at a willingness-to-pay threshold of $100,000 per QALY. In our threshold analyses, an HCC incidence >0.4% per year and surveillance adherence >19.5% bi-annually was necessary for US and AFP to be cost effective compared to no surveillance.
Conclusion:
Accounting for both surveillance-related benefits and harms, US and AFP is more cost-effective for HCC surveillance than US alone or no surveillance in patients with compensated cirrhosis.
Keywords: liver cancer, ultrasound, alpha fetoprotein, screening
INTRODUCTION
Despite improvements in treatment over time, hepatocellular carcinoma (HCC) continues to be a highly morbid tumor with an incidence-to-mortality ratio that approaches 1.(1) It is the fastest increasing cause of cancer-related death over the last decade in the United States and the third leading cause of cancer-related death worldwide.(1–3) However, prognosis for HCC is highly dependent on tumor stage at diagnosis. Whereas patients detected at late stages are only eligible for palliative therapies and have a median survival less than one year, patients detected at an early stage can undergo curative therapy and achieve 5-year survival exceeding 70%.(4, 5)
Over 90% of HCC in the United States and Europe occur in the setting of cirrhosis, and HCC is the leading cause of death in patients with compensated cirrhosis.(6) Given an identifiable at-risk population and selective availability of curative treatments for HCC detected at an early stage, several professional societies including the American Association for the Study of Liver Diseases (AASLD) and National Comprehensive Cancer Network (NCCN) recommend routine HCC surveillance using an abdominal ultrasound with or without a serum blood test, alpha fetoprotein (AFP), in patients with cirrhosis.(7, 8)
There is increasing recognition that the value of cancer screening programs must consider both benefits and potential harms of screening. Although this is best accomplished through a randomized controlled trial (RCT), a previous attempt at an RCT was prematurely closed given inadequate enrollment.(9) Therefore, we are forced to depend on level II and modeling data to evaluate the value of HCC surveillance. Several cohort studies have evaluated the benefits of HCC surveillance and demonstrated an association with early detection and improved survival, even after adjusting for lead time and length time bias.(10–12) Although screening harms have been well described for many cancer screening programs, there have only been two studies to date describing potential harms of HCC surveillance in patients with cirrhosis.(13, 14) Both studies reported diagnostic evaluation due to false positive or indeterminate screening exams in approximately 20% of patients, with some patients undergoing repeated CT/MRI exams or invasive evaluation, such as liver biopsy.(13, 14) Prior decision and cost-effectiveness analyses preceded these data on screening harms so failed to comprehensively account for the consequences of false positive or indeterminate surveillance results. Herein, we present a cost effectiveness analysis evaluating HCC surveillance using ultrasound with or without AFP, incorporating recent data on benefits and harms, in a cohort of patients with compensated cirrhosis.
METHODS
Model Overview
We developed a probabilistic decision analytical microsimulation state-transition Markov model(15) using dedicated software (TreeAge version 19.1.1, Williamstown, MA). The analysis was performed according to published guidelines of economic analyses.(16, 17) We simulated a cohort of 1,000,000 patients with diagnosed compensated cirrhosis at cohort entry, who were followed at a referral center over their lifetime horizon. We modeled only compensated cirrhosis because of prior analyses showing limited effectiveness of surveillance in patients who are decompensated (i.e. Child Pugh class B and C).(18)
Surveillance Strategies
Three surveillance strategies were modelled: no surveillance, semi-annual ultrasound, and semi-annual ultrasound with AFP. Patients in the no-surveillance arm would experience the natural history of cirrhosis. Irrespective of surveillance strategy, for patients who developed HCC, there is a chance of incidental tumor detection, dependent on tumor stage and the degree of hepatic decompensation, across all three arms. Otherwise, the sensitivity and specificity of each modality used across arms governed the likelihood of early detection and surveillance consequences. False-negative results followed the progressive natural history of HCC until detected incidentally, symptomatically, or by follow-up imaging. False positive results underwent evaluation by a variable number of CT/MRI scans or liver biopsy that were conditional on the trigger (AFP versus ultrasound) and history of prior false positive results. True positive nodules were also evaluated by variable number of CT/MRI, with or without liver biopsy, for confirmation.
Modeling Parameters
Surveillance test-characteristics and clinical outcomes were dependent on the patient’s disease severity and the duration in a given health-state. All health-states and the potential inter-state transitions are depicted in Supplementary Figure 1 and the work-up of patients with ultrasound detected liver nodules is depicted in Supplemental Figure 2. The time-dependent risk of decompensation was modeled using a cumulative risk function competing with the risk of death.(19) Once patients developed HCC staged at diagnosis, with subsequent survival, utilities and costs assigned to each particular stage. For patients with undiagnosed HCC, their utilities were commensurate with their stage of cirrhosis. Death due to HCC and/or decompensation (when present) was modeled in a similar fashion accounting for the competing risk of liver transplantation for persons selected for the liver transplant waitlist. Table 1 details the model parameters as well as their sources. Transition probabilities are modelled as beta, triangular, or tabular distributions. We assumed that time spent evaluating a false-positive surveillance result was associated with a 5% absolute reduction in health-state utility while patients were in the false positive state. Costs were modeled as triangular distributions. All costs (inflated to 2018 US dollars), life-years and utilities were discounted at a rate of 3% per annum.
Table 1.
Model Input Parameters
| Input Variable | Value | Range | References |
|---|---|---|---|
| Hepatocellular Carcinoma Incidence | |||
| Incidence in compensated cirrhosis (yearly) | 2% | 1%–3% | (22, 36–38) |
| Incidence in decompensated cirrhosis (yearly) | 4% | 2%–6% | (22, 36–38) |
| Screening effectiveness and Diagnostics | |||
| Sensitivity of ultrasound alone for > early stage HCC | 84% | 76%–92% | (21) |
| Sensitivity of ultrasound alone for early stage HCC | 45% | 30%–62% | (21) |
| Specificity of ultrasound alone | 91% | 86%–94% | (21) |
| Sensitivity of US with AFP for > early stage HCC | 97% | 91%–99% | (21) |
| Sensitivity of US with AFP for early HCC | 63% | 48%–75% | (21) |
| Specificity of US with AFP | 84% | 77%–89% | (21) |
| Ultrasound leads to CT/MRI due to false positive | 12% | 8%–16% | (13, 14) |
| AFP leads to CT/MRI because of a false positive | 8% | 5%–11% | (13, 14) |
| Number of CT/MRI completed prior to diagnosis of HCC | 1.5 | 1–3 | (13, 14) |
| Number of CT/MRI completed without diagnosis of HCC for false positive AFP | 1.2 | 1.0–1.5 | (14) |
| Number of CT/MRI completed without diagnosis of HCC for false positive US | 2.5 | 2–4 | (13, 14) |
| Proportion of patients biopsied | 0.3% | 0.1%–0.5% | (13, 14) |
| Biopsy bleeding or biliary injury | 0.6% | 0.3%–0.9% | (14) |
| Death from biopsy | 0.08% | 0.04%–.10% | (14) |
| Biopsy false negative | 30% | 22%–36% | (14) |
| Disease Progression | |||
| Decompensated cirrhosis to palliative care (annually) | 4% | 2%–10% | (39) |
| Advanced HCC to palliative care (annually) | 70% | 60%–80% | (40) |
| Early stage HCC to intermediate stage HCC (no treatment; annual) | 5% | 3%–9% | (41) |
| Intermediate stage HCC to advanced stage HCC (no treatment; annual) | 15% | 12%–18% | (42) |
| Transplant | |||
| Liver transplant for HCC (probability at 6 months) | 14.1% | 10%–18% | (43) |
| Liver transplant for HCC (probability at 12 months) | 60% | 20%–80% | (43) |
| Liver transplant for HCC (probability at 24 months) | 90.6% | 75%–100% | (43) |
| Liver transplant wait time | 4.2 months | 1.6–7.7 months | (44) |
| Costs (2018 US Dollars) | |||
| Abdominal US | $142.35 | 133.31–194.52 | (45) |
| AFP | $18.64 | $16.32–$20.71 | (46) |
| MRI Abdomen with and without contrast | $498.78 | $441.32–$648.29 | (45) |
| CT Abdomen with and without contrast | $273.54 | $236.67–$346.40 | (45) |
| Liver Biopsy | $1,034.87 | $846.05–$1223.70 | (45) |
| Liver biopsy complications | $5,449 | $1,385–$35,273 | (47) |
| Liver transplant (first year) | $354,672 | $283,543–$364,672 | (45, 48) |
| Liver transplant (after first year) | $49,906 | $44,814–$54,447 | (45, 48) |
| HCC early stage costs (annual) | $64,798 | $35,512–$131,951 | (49) |
| HCC intermediate stage costs (annual) | $93,522 | $42,091–$168,287 | (49) |
| HCC advanced stage costs (annual) | $84,405 | $36,480–$115,797 | (49) |
| Compensated cirrhosis costs (annual) | $26,024 | $5,157–$312,287 | (50) |
| Decompensated cirrhosis costs (annual) | $68,604 | $13,810–$823,248 | (50) |
| Palliative care costs (daily) | $193 | $151–$744 | (51) |
| Utilities | |||
| HCC early stage | 0.72 | 0.62–0.82 | (52) |
| HCC intermediate stage | 0.69 | 0.62–0.78 | (53), (52) |
| HCC advanced stage | 0.65 | 0.52–0.78 | (53), (52) |
| Compensated cirrhosis | 0.78 | 0.71–0.89 | (53), (52) |
| Decompensated cirrhosis | 0.60 | 0.46–0.71 | (53), (52) |
| Decompensated cirrhosis and HCC | 0.57 | 0.46–0.68 | (53), (52) |
| Post-Liver transplant (year 1) | 0.69 | 0.55–0.78 | (54) |
| Post-Liver transplant (subsequent years) | 0.79 | 0.62–0.91 | (54) |
| Palliative Care | 0.40 | 0.37–0.42 | (55) |
Model Procedures
The goal of this analysis was to model two outcomes simultaneously based on the generation of discounted costs (2018 US dollars) and discounted quality adjusted life years (QALY) that accrue over time. As there is uncertainty intrinsic to all input parameters (i.e. confidence intervals or ranges of values), our primary analysis was a micro-simulation that accounted for 1,000,000 unique combinations within the input parameter distributions. We then conducted a probabilistic sensitivity analysis that is a second-order evaluation of uncertainty where each of the 1,000,000 simulated patients could experience 10,000 random samples within each parameter’s distribution. The end result of this analysis is the probability of cost-effectiveness (cost per QALY) for a given strategy in the overall set of simulations. We calculated the incremental cost-effectiveness ratios (ICER) for the competing strategies and interpreted the results with reference to the contemporary willingness-to-pay (WTP) threshold. The WTP threshold is the amount per person that society is willing to pay to adopt a new clinical strategy for an additional QALY over the current acceptable strategy. It is generally considered to be 2–3 times the individual share of gross domestic product.(20) We discuss most results in terms of a WTP of $100,000 but also assess the probability of cost-effectiveness for each strategy across a range of WTP up to $200,000 in ‘cost-acceptability curves’. We performed two threshold analyses, varying incidence of HCC and consistent adherence to HCC surveillance, to determine the parameters at which there was a transition in the preferred strategy seen in the base case.
RESULTS
Base Case
In our base case analysis, we modeled a 2% annual HCC incidence rate for a cohort of 1,000,000 patients with compensated cirrhosis, resulting in a 23% lifetime-risk of HCC. The proportion of HCC diagnosed at an early stage, as defined by Milan criteria, was 74%, 83%, and 91% for no-surveillance, US alone, and US with AFP respectively. The average lifetime discounted costs and QALYs are described in Table 1. The number of CT/MRI required for each diagnosis of HCC, were 1.4, 8.0, and 9.7 for the no surveillance, US alone, and US with AFP arms, respectively. The survival of each arm was 10.93 years for US and AFP, 10.92 in US alone, and 10.84 in the no surveillance arm. US with AFP was the dominant strategy given that it yielded more QALYs (6.02) at the lowest cost.
Sensitivity analyses
When the strategies were evaluated over a range of potential screening-related harms (i.e. minimum or maximum number of CT/MRIs to arrive at an HCC diagnosis), the benefits of US with AFP surveillance were attenuated but without altering the overall rankings (Table 2).
Table 2.
Cost effectiveness of HCC surveillance strategies with base case inputs
| Average Overall Cost (USD) | Incremental Cost (USD) | Average Overall QALYs | Incremental QALYs | Cost per incremental QALY | |
|---|---|---|---|---|---|
| US with AFP | 1,254,173.20 | 0.00 | 6.02 | 0.00 | - |
| US Alone | 1,257,879.04 | 3,705.84 | 6.00 | −0.02 | Dominated |
| No surveillance | 1,266,358.65 | 12,185.45 | 5.98 | −0.04 | Dominated |
| Maximum cross-sectional imaging before diagnosis | |||||
| US with AFP | 1,256,329.35 | 0.00 | 6.02 | 0.00 | - |
| US Alone | 1,260,328.23 | 3,998.88 | 5.99 | −0.03 | Dominated |
| No surveillance | 1,268,173.63 | 11,844.29 | 5.99 | −0.03 | Dominated |
| Minimum cross-sectional imaging before diagnosis | |||||
| US with AFP | 1,251,775.31 | 0.00 | 6.03 | 0.00 | - |
| US Alone | 1,254,641.51 | 2,866.21 | 6.02 | −0.01 | Dominated |
| No surveillance | 1,265,725.43 | 13,950.12 | 5.98 | −0.05 | Dominated |
For all values within the modeled parameters, US with AFP remained a dominant strategy compared to US alone and no surveillance. This included all ranges of modeled harms and benefits associated with surveillance (i.e. across all sensitivity analyses). We conducted several one-way sensitivity analyses to establish the salient model parameters in the comparison between US alone and no surveillance strategies. In Figure 1, we used a tornado diagram to illustrate how the cost-effectiveness of US alone screening relative to no surveillance varies according to the incidence of HCC and the screening test characteristics within the modelled variable parameters. This analysis demonstrates that higher rates of HCC and higher sensitivity/specificity for US alone improve the cost-effectiveness of US alone surveillance. However, across the range of all modelled parameters, the incremental cost-per-QALY of US alone is within the traditional WTP-threshold of $100,000 per-QALY. In Supplemental Table 1, we show the results of the one-way sensitivity analysis for all the input parameters. The cost effectiveness of US vs no surveillance is sensitive to incidence of HCC in compensated cirrhosis, specificity of ultrasound, cost of compensated cirrhosis care, and probability of liver transplantation. In a probabilistic sensitivity analysis (Figure 2) as the willingness-to-pay per incremental QALY rises, the proportion of modelled samples where US with AFP is most cost-effective rises while no surveillance falls. No surveillance is the preferred strategy in 15.3% of samples while US with AFP is preferred in 73.2% at a WTP-threshold of $50,000, compared to 10.1% and 80.3% of cases, respectively, at a WTP-threshold of $100,000. Finally, we varied incidental detection of early HCC within the no-surveillance arm from 0–25% for every cycle. At 0%-incidental probability, the proportion of early HCC detected for the population is 32% (occurring entirely during the decompensated health-state), rising to 58% early detection at 10%, and 74% at 25% incidental detection.
Figure 1.
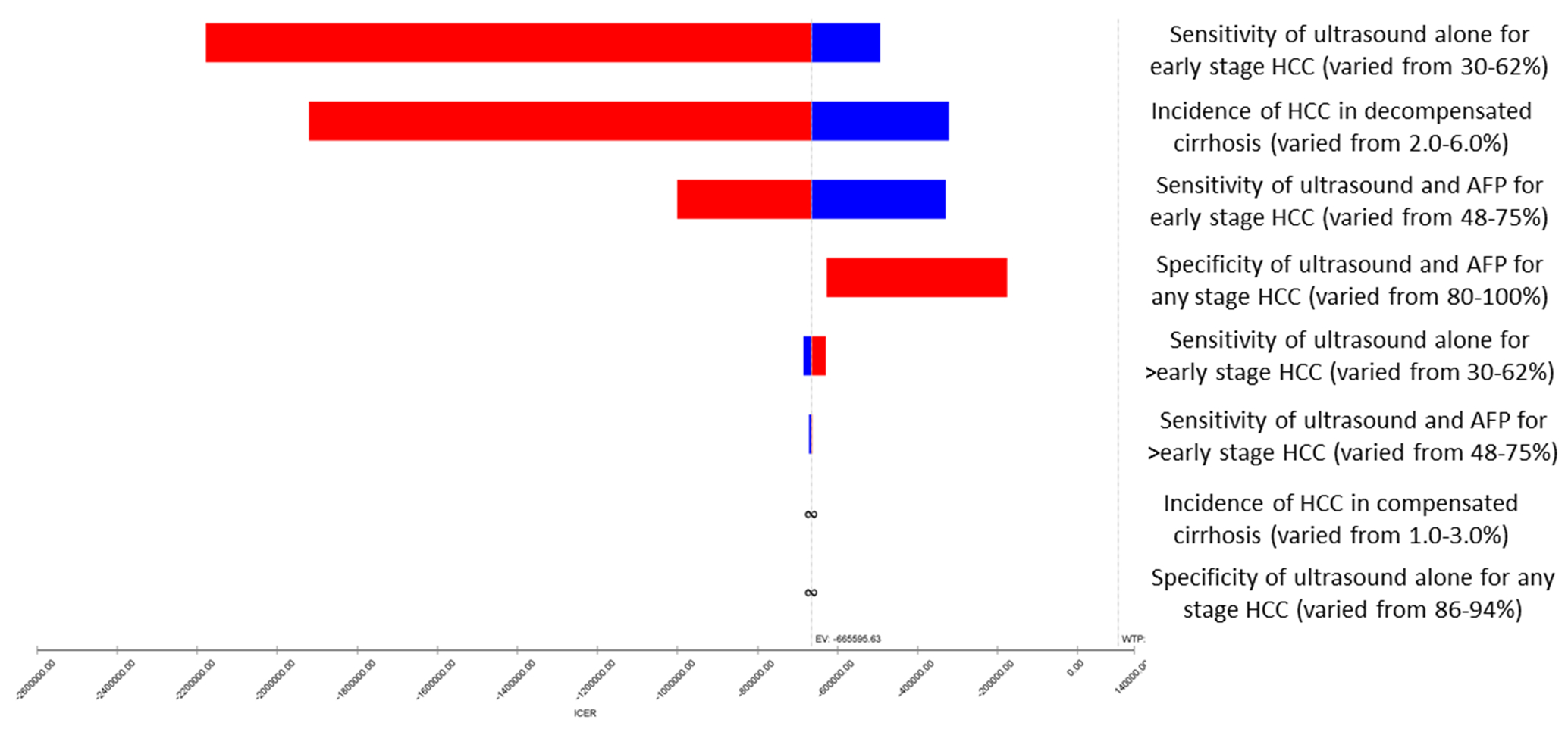
Tornado plot showing one-way sensitivity analyses for ultrasound versus no surveillance
Figure 2.
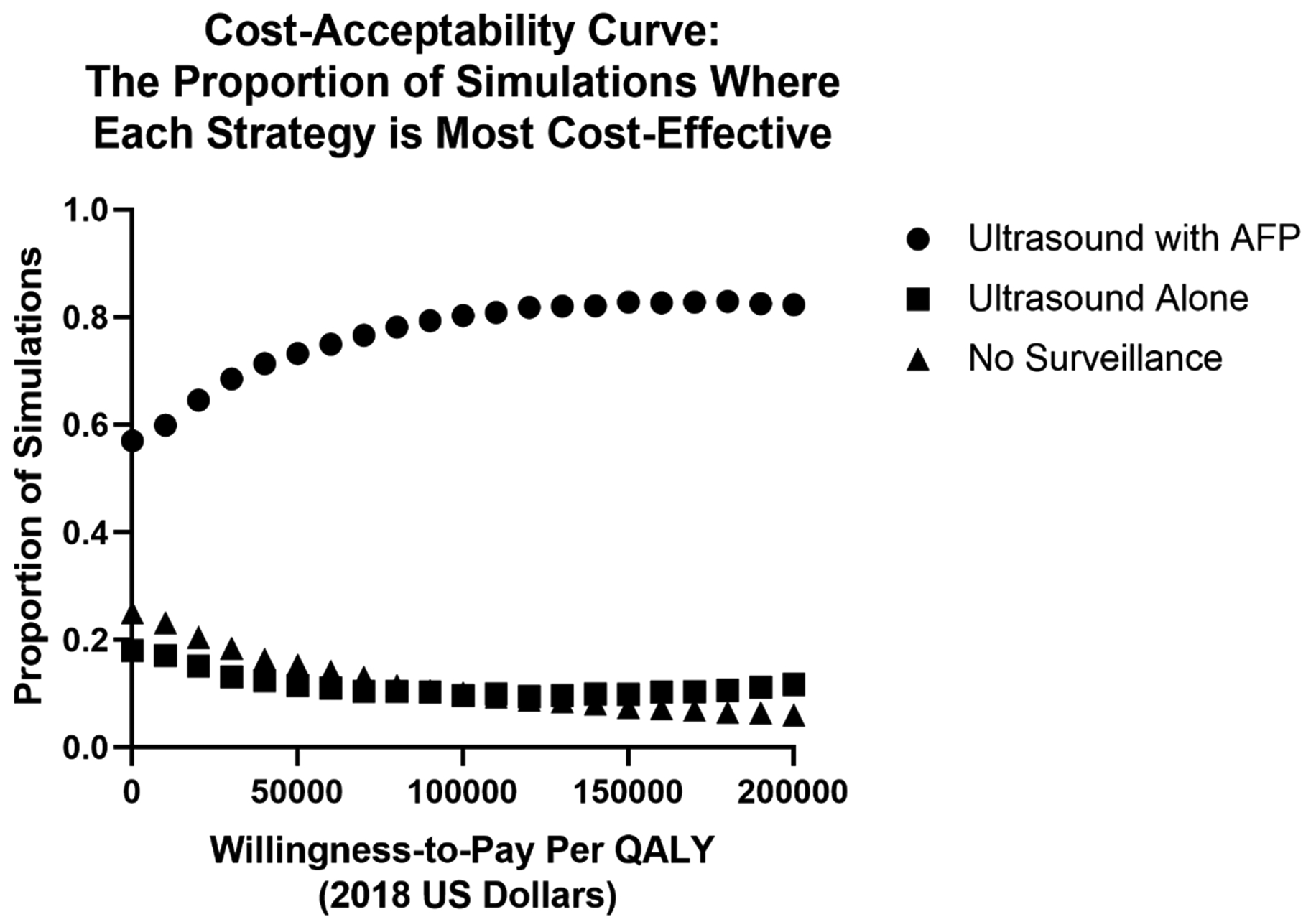
Cost-effectiveness acceptability curves for no surveillance, ultrasound surveillance, and surveillance with ultrasound and alpha fetoprotein.
Threshold Analysis
In two threshold analyses, we established the HCC incidence and surveillance adherence thresholds for our model. First, in Figure 3A, we demonstrate a threshold-analysis for HCC incidence. The cost-per-QALY is shown across a range of HCC incidence from 0% to 5% per year. As the incidence of HCC rises above zero, the cost per QALY of screening declines. The HCC incidence threshold at which US with AFP falls below $100,000/QALY compared to no surveillance is 0.4%/year, with US and AFP yielding the lowest cost-per-QALY for all HCC incidences >0.7%/year.
Figure 3a.
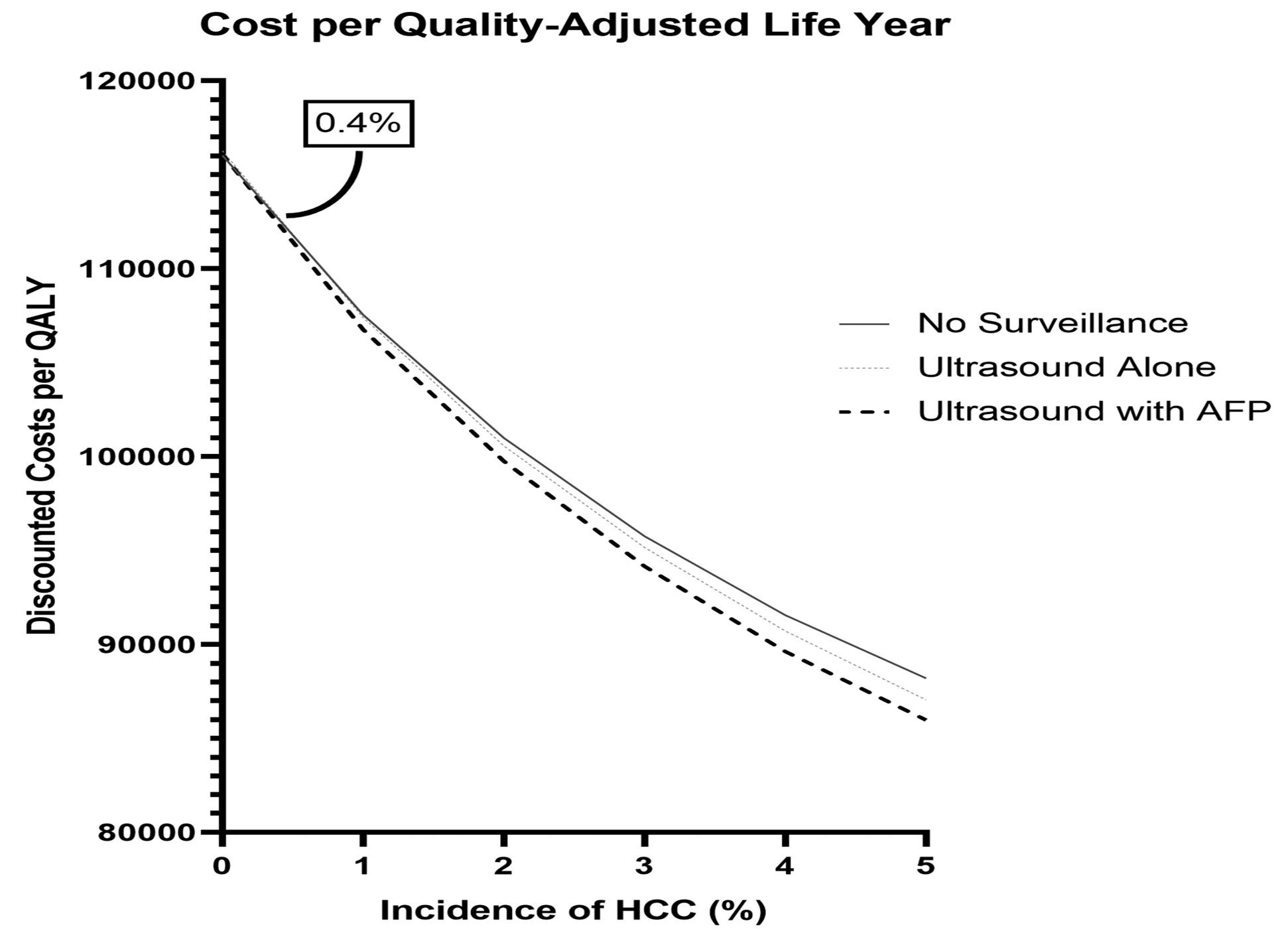
Threshold analysis of the cost per quality adjusted life year with varying hepatocellular carcinoma incidence
Second, In Figure 3B, we show the cost-per-QALY across a range of consistent adherence rates for HCC surveillance (18%-100%). As adherence to screening allocation rises, this cost-per-QALY falls. For all adherence rates >19.5%, US with AFP yields the lowest cost-per-QALY. US with AFP has an ICER below $100,000/QALY at all adherence rates and becomes dominant compared to no-surveillance (i.e. is more QALYs at lower cost) when consistent adherence exceeds 59%.
Figure 3b.
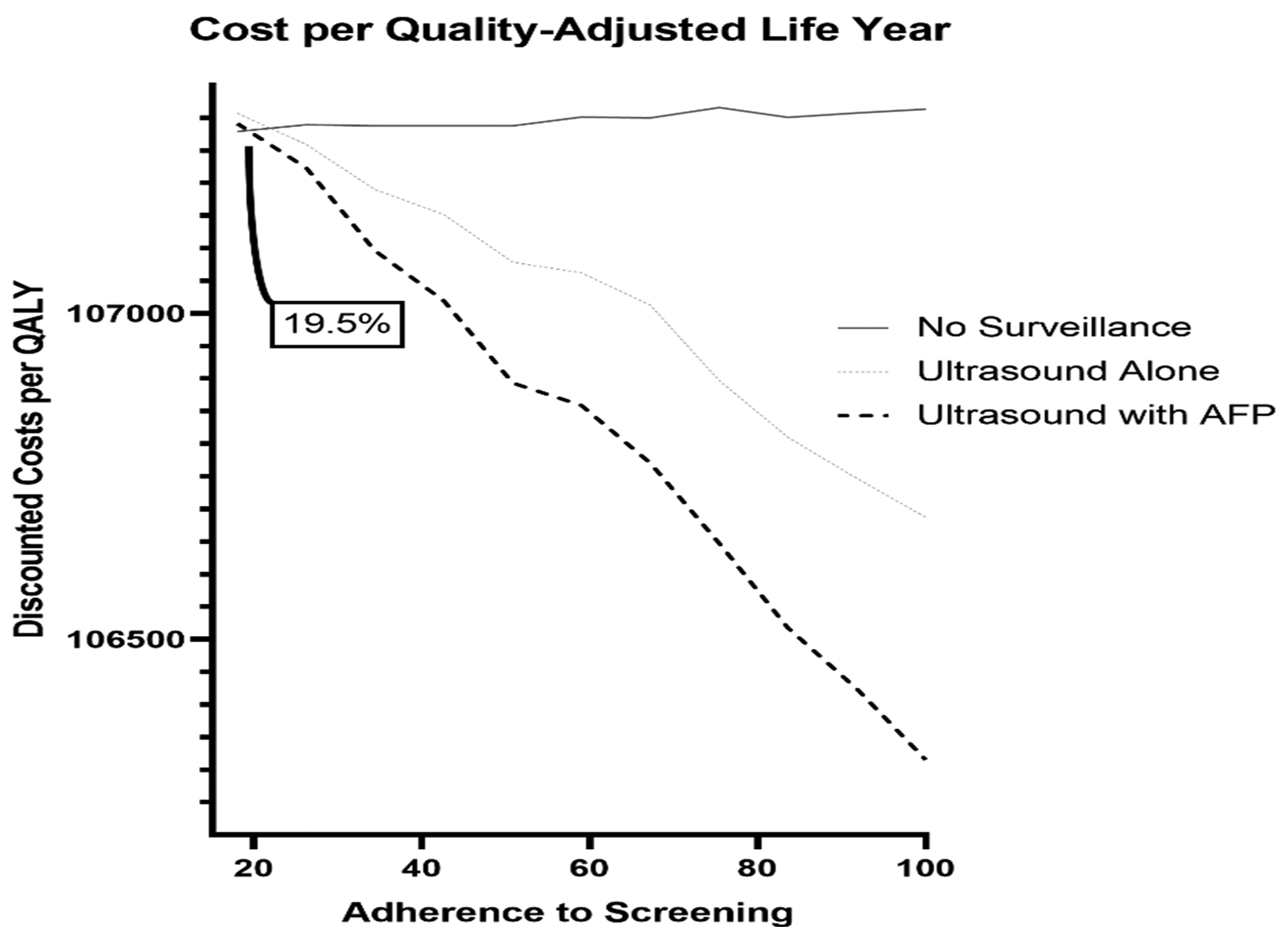
Threshold analysis of the cost per quality adjusted life year with varying adherence to hepatocellular carcinoma surveillance strategies
DISCUSSION
HCC surveillance is associated with improved early detection of HCC and overall improved survival.(11, 21) However, emerging data on potential surveillance-related harms including false positive results that can lead to unnecessary testing and psychological distress have called into question the value of HCC surveillance.(13) Our study, modeling cost-effectiveness of surveillance integrating potential screening harms, advances knowledge about HCC surveillance cost-effectiveness. First, we found that US with AFP was the cost-effective strategy compared to both no surveillance and US alone. Specifically, US with AFP maximizes early detection of HCC with the lowest costs and highest QALYs. Sensitivity analyses demonstrate that US with AFP becomes a superior strategy when the incidence of HCC is >0.4% and is robust to sensitivity analyses across the range of published screening test characteristics.
What these data mean for screening research
First, early detection of HCC for a population at risk of HCC is beneficial. Although the mechanisms underlying the benefits of surveillance are not explicitly clear from this study, our findings demonstrate that surveillance increases the rate of early detection and that the cost-effectiveness of screening is robust to the harms of screening (false-positives, additional testing). However, these benefits are less clear for persons with a low (≤0.4% risk of HCC), such as those with F3 fibrosis in the setting of HCV or NASH where the annual incidence of HCC is 0.3%-0.5%(22–24). Surveillance in this population of patients without cirrhosis remains controversial, and our study indicates that there may be a subgroup of patients with advanced fibrosis, without cirrhosis, where HCC surveillance is cost-effective. Second, while the specificity of US with AFP is lower than US alone, data have suggested that differential use of follow-up diagnostic testing may mitigate the physical harms and resultant costs related to false positive AFP results.(11, 21) Further, US with AFP has improved sensitivity for early stage HCC detection relative to US alone, driving its cost-effectiveness. US and AFP were associated with the most CT/MRI tests for each diagnosis of HCC, however this did not negatively affect its relative cost-effectiveness. Third, we demonstrated that adherence to surveillance has a significant impact on the cost-effectiveness of any surveillance strategy. Although this is important given prior studies highlighting underuse of HCC surveillance in clinical practice(25, 26), the threshold of adherence for HCC surveillance using US with AFP to remain cost-effective is relatively low at 19.5%, highlighting the dominance of this surveillance strategy. We found that US with AFP surveillance is dominant over no surveillance once consistent surveillance adherence exceeds 59%, setting an aspirational threshold to maximize surveillance value.
Several published modeling studies have evaluated the cost-effectiveness of ultrasound surveillance(27, 28); however, no prior study has included real world harms data. Many of the existing cost-effectiveness studies have shown that surveillance with US alone was the most cost-effective strategy when compared to other competing strategies, including US with AFP and multiphasic cross sectional modalities.(27, 29, 30) Our results are discordant with the prior literature, given inclusion of more recent data detailing the sensitivity of ultrasound with AFP as well as the integration of surveillance harms instead of simply relying on specificity measurements. Published cohort studies have shown that many patients with false positive AFP results are simply monitored instead of undergoing diagnostic testing, whereas those with ultrasound are more likely to follow-up testing.(14)
Ideally, in order to minimize harms of surveillance, while maximizing benefits, the highest intensity surveillance should be directed to those patients at highest risk. A precision approach to surveillance has been modeled to be the most cost-effective, however we currently lack well validated stratification tools for HCC risk.(31) Certain populations, such as those with elevated body mass index and those with decompensated cirrhosis, have lower quality imaging using US and may benefit from surveillance using multiphasic cross-sectional imaging, however this strategy deserves further study.(32) While development of risk stratification methods and evaluation of novel surveillance techniques are underway, further efforts must be undertaken to increase the consistent receipt of surveillance in patients with cirrhosis and certain populations with chronic hepatitis B. Interventions to better identify undiagnosed cirrhosis are needed, as up to 25% of patients with HCC are unaware they have cirrhosis prior to their HCC diagnosis.(33, 34)
Our study has notable strengths and weaknesses. Markov modeling has known limitations based on assumption and the quality of the input data. Several of the input sources were from single sources, however the robustness of the findings across the model ranges, suggest additional inputs would not dramatically affect our results. Surveillance harms have psychological consequences for patients and may decrease QALYs, however we currently lack data on the patient-reported impact of surveillance harms so we made estimates on decrements in QALYs with surveillance-related harms while patients were in a false positive state. Despite increased harms associated with US and AFP, it remained the most cost-effective strategy. Finally, our cost-effectiveness analysis was restricted to currently available strategies given limited phase III data evaluating benefits and harms for novel biomarker panels that are currently undergoing evaluation.(35)
In conclusion, we have shown that HCC surveillance using US with AFP is the most cost-effectiveness strategy, compared to US alone and no surveillance, across a wide range of parameters. Surveillance using US and AFP is cost-effective at an adherence threshold of 19.5% and becomes dominant over no surveillance once adherence >59%, highlighting the need for interventions to improve recognition of cirrhosis and increase surveillance utilization among at-risk patients. While awaiting newer imaging and serum-based surveillance modalities, our data highlight the need for interventions to promote surveillance using US with AFP among at-risk patients.
Supplementary Material
Study Highlights.
What is Known
HCC surveillance is associated with early detection and improved survival
The optimal surveillance strategy, when accounting for harms, is unknown
What is New Here
When accounting for real world harms, surveillance with US and AFP is the dominant surveillance strategy
HCC surveillance with US and AFP is cost-effective with incidence greater than 0.4% and biannual adherence greater than 19.5%.
Grant Support:
This project was supported by National Cancer Institute R01 CA222900, R01 CA212008, U01 CA230669 and U01 CA230694. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Veterans Administration or the National Institutes of Health.
Footnotes
Conflicts of Interest: Dr. Singal has been on advisory boards and served as a consultant for Wako Diagostics, Roche, Exact Sciences, Glycotest, Bayer, Eisai, BMS, Exelixis, and Merck. Dr Tapper served on advisory boards and was a consultant for Bausch Health, Rebiotix, Mallinckrodt, Kaleido, Novartis, and Allergan. Dr. Parikh has served on advisory boards for Bayer, Eisai, Exelixis, Wako; Research Grants: TARGET Pharmasolutions, Bayer, Exact Sciences, Glycotest and a consultant for Exelixis, Bristol-Myers Squibb, Eli Lilly, Freenome.
References
- 1.Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: a cancer journal for clinicians 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- 2.Tapper EB, Parikh ND. Mortality due to cirrhosis and liver cancer in the United States, 1999–2016: observational study. bmj 2018;362:k2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.White DL, Thrift AP, Kanwal F, et al. Incidence of hepatocellular carcinoma in all 50 United States, from 2000 through 2012. Gastroenterology 2017;152:812–820. e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Toni EN, Schlesinger-Raab A, Fuchs M, et al. Age independent survival benefit for patients with hepatocellular carcinoma (HCC) without metastases at diagnosis: a population-based study. Gut 2020;69:168–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Njei B, Rotman Y, Ditah I, et al. Emerging trends in hepatocellular carcinoma incidence and mortality. Hepatology 2015;61:191–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Trinchet JC, Bourcier V, Chaffaut C, et al. Complications and competing risks of death in compensated viral cirrhosis (ANRS CO12 CirVir prospective cohort). Hepatology 2015;62:737–750. [DOI] [PubMed] [Google Scholar]
- 7.Marrero JA, Kulik LM, Sirlin CB, et al. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Clinical Liver Disease 2019;13:1. [DOI] [PubMed] [Google Scholar]
- 8.Benson AB, D’Angelica MI, Abbott DE, et al. NCCN Guidelines Insights: Hepatobiliary Cancers, Version 2.2019: Featured Updates to the NCCN Guidelines. Journal of the National Comprehensive Cancer Network 2019;17:302–310. [DOI] [PubMed] [Google Scholar]
- 9.Poustchi H, Farrell GC, Strasser SI, et al. Feasibility of conducting a randomized control trial for liver cancer screening: is a randomized controlled trial for liver cancer screening feasible or still needed? Hepatology 2011;54:1998–2004. [DOI] [PubMed] [Google Scholar]
- 10.van Meer S, de Man RA, Coenraad MJ, et al. Surveillance for hepatocellular carcinoma is associated with increased survival: Results from a large cohort in the Netherlands. J Hepatol 2015;63:1156–63. [DOI] [PubMed] [Google Scholar]
- 11.Singal AG, Pillai A, Tiro J. Early detection, curative treatment, and survival rates for hepatocellular carcinoma surveillance in patients with cirrhosis: a meta-analysis. PLoS Med 2014;11:e1001624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Choi DT, Kum HC, Park S, et al. Hepatocellular Carcinoma Screening Is Associated With Increased Survival of Patients With Cirrhosis. Clin Gastroenterol Hepatol 2019;17:976–987 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Konerman MA, Verma A, Zhao B, et al. Frequency and Outcomes of Abnormal Imaging in Patients With Cirrhosis Enrolled in a Hepatocellular Carcinoma Surveillance Program. Liver Transpl 2019;25:369–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Atiq O, Tiro J, Yopp AC, et al. An assessment of benefits and harms of hepatocellular carcinoma surveillance in patients with cirrhosis. Hepatology 2017;65:1196–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hunink MGM WM, Wittenberg E, Drummond MF, Pliskin JS, Wong JB, Glasziou PP. Decision making in health and medicine: Integrating evidence and values. Cambridge, UK: Cambridge University Press; 2014. [Google Scholar]
- 16.Petrou S, Gray A. Economic evaluation using decision analytical modelling: design, conduct, analysis, and reporting. Bmj 2011;342. [DOI] [PubMed] [Google Scholar]
- 17.Siegel JE, Weinstein MC, Russell LB, et al. Recommendations for reporting cost-effectiveness analyses. JAMA 1996;276:1339–1341. [DOI] [PubMed] [Google Scholar]
- 18.Del Poggio P, Olmi S, Ciccarese F, et al. Factors that affect efficacy of ultrasound surveillance for early stage hepatocellular carcinoma in patients with cirrhosis. Clinical Gastroenterology and Hepatology 2014;12:1927–1933. e2. [DOI] [PubMed] [Google Scholar]
- 19.D’Amico G, Garcia-Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. Journal of hepatology 2006;44:217–231. [DOI] [PubMed] [Google Scholar]
- 20.Neumann PJ, Cohen JT, Weinstein MC. Updating cost-effectiveness—the curious resilience of the $50,000-per-QALY threshold. New England Journal of Medicine 2014;371:796–797. [DOI] [PubMed] [Google Scholar]
- 21.Tzartzeva K, Obi J, Rich NE, et al. Surveillance Imaging and Alpha Fetoprotein for Early Detection of Hepatocellular Carcinoma in Patients With Cirrhosis: A Meta-analysis. Gastroenterology 2018;154:1706–1718 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kanwal F, Kramer JR, Mapakshi S, et al. Risk of Hepatocellular Cancer in Patients With Non-Alcoholic Fatty Liver Disease. Gastroenterology 2018;155:1828–1837 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ioannou GN, Beste LA, Green PK, et al. Increased Risk for Hepatocellular Carcinoma Persists Up to 10 Years After HCV Eradication in Patients With Baseline Cirrhosis or High FIB-4 Scores. Gastroenterology 2019;157:1264–1278 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cardoso AC, Moucari R, Figueiredo-Mendes C, et al. Impact of peginterferon and ribavirin therapy on hepatocellular carcinoma: incidence and survival in hepatitis C patients with advanced fibrosis. J Hepatol 2010;52:652–7. [DOI] [PubMed] [Google Scholar]
- 25.Singal AG, Yopp A, C SS, et al. Utilization of hepatocellular carcinoma surveillance among American patients: a systematic review. J Gen Intern Med 2012;27:861–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Singal AG, Li X, Tiro J, et al. Racial, social, and clinical determinants of hepatocellular carcinoma surveillance. Am J Med 2015;128:90 e1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Andersson KL, Salomon JA, Goldie SJ, et al. Cost effectiveness of alternative surveillance strategies for hepatocellular carcinoma in patients with cirrhosis. Clin Gastroenterol Hepatol 2008;6:1418–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang JD, Mannalithara A, Piscitello AJ, et al. Impact of surveillance for hepatocellular carcinoma on survival in patients with compensated cirrhosis. Hepatology 2018;68:78–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lima PH, Fan B, Berube J, et al. Cost-Utility Analysis of Imaging for Surveillance and Diagnosis of Hepatocellular Carcinoma. AJR Am J Roentgenol 2019:1–9. [DOI] [PubMed] [Google Scholar]
- 30.Saab S, Ly D, Nieto J, et al. Hepatocellular carcinoma screening in patients waiting for liver transplantation: a decision analytic model. Liver Transpl 2003;9:672–81. [DOI] [PubMed] [Google Scholar]
- 31.Goossens N, Singal AG, King LY, et al. Cost-Effectiveness of Risk Score-Stratified Hepatocellular Carcinoma Screening in Patients with Cirrhosis. Clin Transl Gastroenterol 2017;8:e101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Simmons O, Fetzer DT, Yokoo T, et al. Predictors of adequate ultrasound quality for hepatocellular carcinoma surveillance in patients with cirrhosis. Aliment Pharmacol Ther 2017;45:169–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Singal AG, Yopp AC, Gupta S, et al. Failure rates in the hepatocellular carcinoma surveillance process. Cancer prevention research 2012;5:1124–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Walker M, El-Serag HB, Sada Y, et al. Cirrhosis is under-recognised in patients subsequently diagnosed with hepatocellular cancer. Aliment Pharmacol Ther 2016;43:621–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Berhane S, Toyoda H, Tada T, et al. Role of the GALAD and BALAD-2 Serologic Models in Diagnosis of Hepatocellular Carcinoma and Prediction of Survival in Patients. Clin Gastroenterol Hepatol 2016;14:875–886 e6. [DOI] [PubMed] [Google Scholar]
- 36.Mancebo A, Gonzalez-Dieguez ML, Cadahia V, et al. Annual incidence of hepatocellular carcinoma among patients with alcoholic cirrhosis and identification of risk groups. Clin Gastroenterol Hepatol 2013;11:95–101. [DOI] [PubMed] [Google Scholar]
- 37.Lok AS, Seeff LB, Morgan TR, et al. Incidence of hepatocellular carcinoma and associated risk factors in hepatitis C-related advanced liver disease. Gastroenterology 2009;136:138–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Papatheodoridis GV, Chan HL, Hansen BE, et al. Risk of hepatocellular carcinoma in chronic hepatitis B: assessment and modification with current antiviral therapy. J Hepatol 2015;62:956–67. [DOI] [PubMed] [Google Scholar]
- 39.Su F, Yu L, Berry K, et al. Aging of Liver Transplant Registrants and Recipients: Trends and Impact on Waitlist Outcomes, Post-Transplantation Outcomes, and Transplant-Related Survival Benefit. Gastroenterology 2016;150:441–53 e6; quiz e16. [DOI] [PubMed] [Google Scholar]
- 40.Parikh ND, Marshall VD, Singal AG, et al. Survival and cost‐effectiveness of sorafenib therapy in advanced hepatocellular carcinoma: An analysis of the SEER–Medicare database. Hepatology 2017;65:122–133. [DOI] [PubMed] [Google Scholar]
- 41.Mehta N, Sarkar M, Dodge JL, et al. Intention to treat outcome of T1 hepatocellular carcinoma with the “wait and not ablate” approach until meeting T2 criteria for liver transplant listing. Liver Transpl 2016;22:178–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Frangakis C, Geschwind JF, Kim D, et al. Chemoembolization decreases drop-off risk of hepatocellular carcinoma patients on the liver transplant list. Cardiovasc Intervent Radiol 2011;34:1254–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ishaque T, Massie AB, Bowring MG, et al. Liver transplantation and waitlist mortality for HCC and non-HCC candidates following the 2015 HCC exception policy change. Am J Transplant 2019;19:564–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mehta N, Dodge JL, Hirose R, et al. Increasing Liver Transplantation Wait-List Dropout for Hepatocellular Carcinoma With Widening Geographical Disparities: Implications for Organ Allocation. Liver Transpl 2018;24:1346–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Medicare Fee Schedule. [cited 2019 August 17]; Available from: https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/PhysicianFeeSched/index
- 46.Medicare Fee Schedule. [cited 2019 August 15]; Available from: https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/ClinicalLabFeeSched/Clinical-Laboratory-Fee-Schedule-Files
- 47.Myers RP, Fong A, Shaheen AA. Utilization rates, complications and costs of percutaneous liver biopsy: a population-based study including 4275 biopsies. Liver Int 2008;28:705–12. [DOI] [PubMed] [Google Scholar]
- 48.Best JH, Veenstra DL, Geppert J. Trends in expenditures for Medicare liver transplant recipients. Liver transplantation 2001;7:858–862. [DOI] [PubMed] [Google Scholar]
- 49.Tapper EB, Catana AM, Sethi N, et al. Direct costs of care for hepatocellular carcinoma in patients with hepatitis C cirrhosis. Cancer 2016;122:852–8. [DOI] [PubMed] [Google Scholar]
- 50.Gordon SC, Pockros PJ, Terrault NA, et al. Impact of disease severity on healthcare costs in patients with chronic hepatitis C (CHC) virus infection. Hepatology 2012;56:1651–60. [DOI] [PubMed] [Google Scholar]
- 51.Hospice Services Payment System. [cited 2019 September 9]; Available from: http://www.medpac.gov/docs/default-source/payment-basics/medpac_payment_basics_17_hospice_final4ea311adfa9c665e80adff00009edf9c.pdf?sfvrsn=0
- 52.Chong CA, Gulamhussein A, Heathcote EJ, et al. Health-state utilities and quality of life in hepatitis C patients. Am J Gastroenterol 2003;98:630–8. [DOI] [PubMed] [Google Scholar]
- 53.Lim KC, Wang VW, Siddiqui FJ, et al. Cost-effectiveness analysis of liver resection versus transplantation for early hepatocellular carcinoma within the Milan criteria. Hepatology 2015;61:227–37. [DOI] [PubMed] [Google Scholar]
- 54.Rana A, Ackah RL, Webb GJ, et al. No Gains in Long-term Survival After Liver Transplantation Over the Past Three Decades. Ann Surg 2019;269:20–27. [DOI] [PubMed] [Google Scholar]
- 55.Ock M, Lim SY, Lee HJ, et al. Estimation of utility weights for major liver diseases according to disease severity in Korea. BMC Gastroenterol 2017;17:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


