Abstract
Background:
Observational studies have documented lower risks of coronary heart disease and diabetes among moderate alcohol consumers relative to abstainers, but only a randomized clinical trial can provide conclusive evidence for or against these associations.
Aim:
The purpose of this study was to describe the rationale and design of the Moderate Alcohol and Cardiovascular Health Trial, aimed to assess the cardiometabolic effects of one alcoholic drink daily over an average of six years among adults 50 years or older.
Methods:
This multicenter, parallel-arm randomized trial was designed to compare the effects of one standard serving (~11–15 g) daily of a preferred alcoholic beverage to abstention. The trial aimed to enroll 7800 people at high risk of cardiovascular disease. The primary composite endpoint comprised time to the first occurrence of non-fatal myocardial infarction, non-fatal ischemic stroke, hospitalized angina, coronary/carotid revascularization, or total mortality. The trial was designed to provide >80% power to detect a 15% reduction in the risk of the primary outcome. Secondary outcomes included diabetes. Adverse effects of special interest included injuries, congestive heart failure, alcohol use disorders, and cancer.
Results:
We describe the design, governance, masking issues, and data handling. In three months of field center activity until termination by the funder, the trial randomized 32 participants, successfully screened another 70, and identified ~400 additional interested individuals.
Conclusions:
We describe a feasible design for a long-term randomized trial of moderate alcohol consumption. Such a study will provide the highest level of evidence for the effects of moderate alcohol consumption on cardiovascular disease and diabetes, and will directly inform clinical and public health guidelines.
Keywords: Randomized controlled trial, ethanol, cardiovascular diseases, diabetes mellitus type 2, geriatric, research design
Introduction
Despite the well-understood adverse effects of excessive alcohol use,1 no consensus exists about the risks and benefits of moderate alcohol use on cardiovascular disease (CVD), cancer, or overall health. A lower risk of coronary heart disease (CHD) and myocardial infarction (MI) among moderate drinkers compared to abstainers has been reported in over 100 observational studies.2,3 In addition, numerous short-term randomized controlled trials have shown beneficial effects on a range of biomarkers that could explain these associations.4 However, studies of genetic polymorphisms that influence alcohol consumption and metabolism have yielded inconsistent results.5–8 Moderate alcohol consumption has also been associated with a lower risk of total mortality and type II diabetes,9–11 but uncertainty about the size and dose–response curves of these associations remains.3
At the same time, several non-cardiovascular health outcomes have plausible strong adverse relations with moderate drinking that bear directly on the safety of any clinical trial of alcohol consumption. Alcohol consumption appears linearly associated with an increase in the risk of breast cancer.12 There is evidence for a similar relationship with other cancers,13 especially oropharyngeal and esophageal cancers for which substantial synergy appears to exist between alcohol intake, cigarette smoking, and genetic susceptibility.14 Although heavy drinking can cause a direct cardiomyopathy,15 the association of lighter alcohol consumption with heart failure is positive in some analyses3 but inverse in others.16 Even moderate alcohol consumption may increase risk of injury17 and, in some cases, perhaps lead to subsequent alcohol abuse.1,18 In addition, an increased risk of overweight and obesity due to daily drinking must be considered.19
Given the potential limitations of causal inference based upon observational studies and the quite limited scope and duration of previous randomized trials of moderate alcohol consumption,20 equipoise exists about the health effects of moderate drinking. For example, differences in the associations of alcohol consumption with risk of CVD have been noted in different geographic regions.21,22 Given the widespread prevalence of alcohol consumption, this uncertainty is untenable and provides the rationale for a long-term, international, multicenter randomized trial of moderate drinking compared with no or very low alcohol intake on CVD, diabetes, and mortality among adults of above-average risk for these outcomes. The alcohol intervention to be studied was based upon observational evidence for dose and beverage type, previous clinical trial evidence for feasibility, and US and international guidelines for safety. Of note, calls for a randomized trial of alcohol consumption are now over a decade old.23,24 Here, we summarize the protocol for this study, which is also publicly available at www.clinicaltrials.gov as NCT03169530 and in the supplementary material.
Sponsorship
The trial organization for the Moderate Alcohol and Cardiovascular Health Trial (MACH15) resembled that of other large National Institutes of Health (NIH)-sponsored multicenter clinical trials and was designed to protect against possible conflicts of interest in accordance with previously published recommendations.25 The National Institute for Alcohol Abuse and Alcoholism (NIAAA) initiated and sponsored this trial following an extensive and rigorous peer review of both the application for funding and, separately, the accompanying protocol.
The Foundation for the National Institutes of Health (FNIH) supported the trial financially and managed contact between public and private organizations on behalf of NIH. The funds provided by FNIH for this project were contributed to FNIH by the brewing and distilling industries following contract negotiations that established an intellectual and financial firewall between MACH15 investigators and private contributors. The corporations providing support agreed to have, and had, no contact with trial investigators about any aspect of the study after their commitment of funding, and they agreed to receive no data or updates until they became publicly available. Ultimately, however, the most important safeguard for impartiality lies in the execution of a rigorous, transparent protocol following independent, expert peer review, and in the conduct of the statistical analyses as described in the protocol.
Trial organization
The organizational structure of MACH15 is presented in Figure 1. It consisted of several integrated coordinating centers: the Administrative Core at Beth Israel Deaconess Medical Center (BIDMC); the Biospecimen Repository Center and the Statistical Coordinating Center (SCC) at the Harvard TH Chan School of Public Health; the Data Management Center and US Clinical Coordinating Center at Wake Forest University Health Sciences (WFUHS); and the International Clinical Coordinating Center at Julius Clinical (JC) in the Netherlands. Scientific leadership was provided by the Steering Committee, with external oversight provided by Institutional Review Boards/Ethics Committees (IRB/EC) and a Data and Safety Monitoring Board (DSMB) originally appointed by NIAAA. Seven vanguard clinical sites across four continents initiated trial approval locally. The vanguard sites were: WFUHS, Wake Forest, NC, USA; Johns Hopkins ProHealth Clinical Research Center, Baltimore, MD, USA; JC, Zeist, the Netherlands; Center for Bioethics and Research, Nigeria; Institute for Clinical Effectiveness and Health Policy, Argentina; University of Copenhagen, Denmark; and Hospital Clinic – University of Barcelona, Spain.
Figure 1.
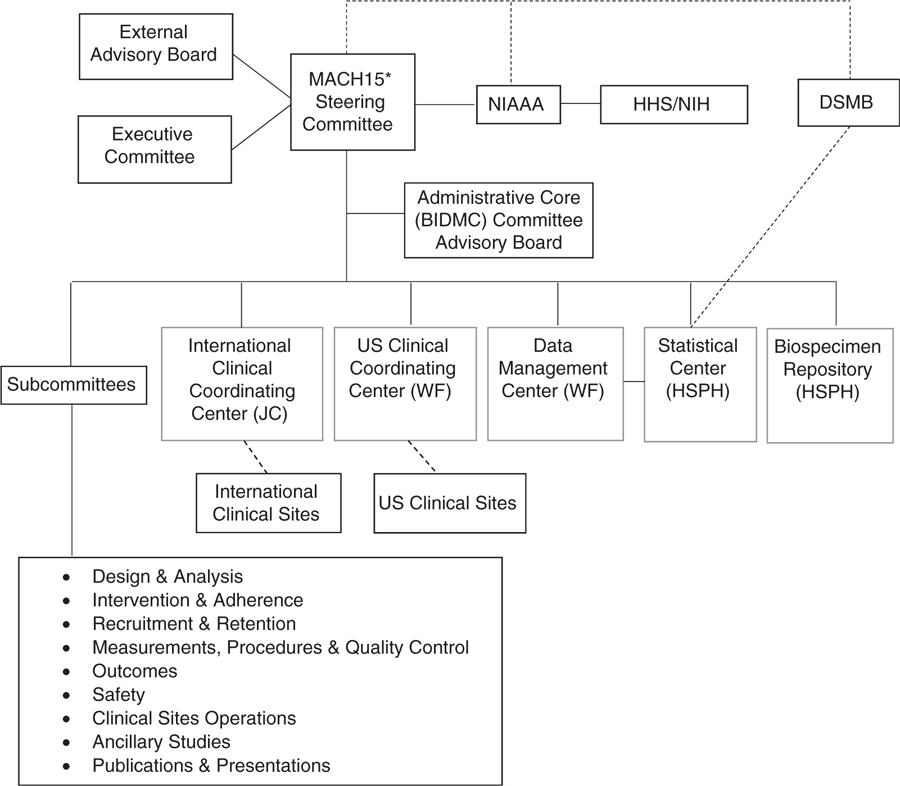
MACH15 organizational structure during the vanguard phase.
MACH15: Moderate Alcohol and Cardiovascular Health Trial; NIAAA: National Institute on Alcohol Abuse and Alcoholism; DSMB: Data and Safety Monitoring Board; JC: Julius Clinical, Zeist, Netherlands; WF: Wake Forest University School of Medicine, Winston-Salem, NC, USA; HSPH: Harvard TH Chan School of Public Health, Boston, MA, USA.
Study design
MACH15 was a two-arm, balanced-design, randomized trial. Recruitment was planned for an initial nine-month vanguard phase at the seven clinical sites listed above, followed by the addition of approximately nine clinical sites. Participants were expected to be recruited over a three-year period and followed for 4.5–7.5 years with a common closeout date, with an average follow-up of six years. Participants were stratified by clinical site and, after completing a two-week washout period, randomly assigned to either one standard serving of alcohol daily or abstention. Although randomization was generally individual, the protocol called for all consenting eligible members in a single household to be randomized to the same arm to maximize adherence.
The overall goal for recruitment was 7800 clinic- and population-based participants, approximately 500 at each of the 16–20 clinical sites around the world. Recruitment goals included ~50% women and demographic characteristics representative of the community surrounding each of the clinical sites. Due to the international nature of the trial, clinic-specific enrollment targets were determined based upon the demographics of each clinical site.
Eligibility, recruitment, and retention
A multifaceted approach to screening and enrollment is essential to achieve the recruitment goal for any clinical trial. For this multicenter international trial, recruitment strategies targeted both existing populations within the clinical practice sphere of the individual field centers and free-living individuals from outside these practice settings. Recruitment used specific community resources to promote recruitment of women and minority or under-served populations to ensure adequate representation. Recruitment strategies differed by site due to cultural differences and ranged from outreach by community research workers (in Nigeria) to broad regional mailings (in Baltimore) and recruitment through primary care networks (in the Netherlands). Further details on recruitment and screening strategies can be found in the study protocol (supplementary material).
Upon contact with interested participants and pre-screening, field centers scheduled screening visits to ensure eligibility. Subsequently, individuals meeting all eligibility criteria completed a two-week washout period where they abstained from drinking alcohol. Adherence was measured by self-reporting during and after the washout period. This ensured that prospective participants understood and were willing to comply with lifestyle changes regarding alcohol consumption, and provided for uniformity in current abstention at the time of baseline biospecimen collection. Clinical sites were allowed to institute a run-in period prior to the washout period, in which prospective participants consumed one drink (~11–15 g) of alcohol daily for seven to 14 days. Participants who successfully completed the two-week abstinence period were then scheduled for their baseline randomization visit. A central recruitment database on the study website (now closed) enabled sites to track all relevant screening inclusion and exclusion data to enable the comparative evaluation of site-specific recruitment strategies, to monitor recruitment progress trial-wide, and to adapt strategies for recruitment when indicated.
MACH15 inclusion and exclusion criteria, presented in Tables 1 and 2, were made as direct as possible to ensure standard implementation across all clinical sites. The objectives of inclusion and exclusion criteria were to enroll a trial population that would yield adequate event rates for statistical power, maximize safety, and, of least importance, promote generalizability. Importantly, the Office for Human Research Protections states that ethical review “should not consider possible long-range effects of applying knowledge gained in the research (e.g., the possible effects of the research on public policy).”26 Therefore, reduction of individual risk supersedes concerns like generalizability or public health relevance, and MACH15 investigators prioritized this mandate in minimizing risk to participants. Eligibility criteria were developed to facilitate the identification and inclusion of participants who would adhere to the trial protocol, were at above-average risk for CVD, were not at above-average risk for breast cancer, and were at minimal risk for alcoholism. These criteria were essentially identical to those used in a pilot study that preceded this trial.27
Table 1.
MACH15 inclusion criteria.
| To be eligible for the MACH15 trial, a women must meet all of the following criteria and a man must meet criteria 1, 3, and 4 |
|---|
| 1. ≥50 years old at screening |
| 2. Postmenopausal, defined as 12 consecutive months without menstruation |
| 3. Not alcohol naïve, defined by having consumed at least one drink of alcohol in the past five years |
| 4. High risk for the occurrence of a new cardiovascular disease event (one of the following): |
| a) American Heart Association (AHA)/American College of Cardiology Risk Score ≥15% within the past 24 months (among those without clinical or subclinical cardiovascular disease (CVD)) |
| b) Clinical CVD (more than six months prior to randomization), defined by: |
| 1) Previous myocardial infarction, percutaneous coronary intervention, coronary artery bypass grafting, carotid endarterectomy, carotid stenting; |
| 2) Peripheral artery disease with revascularization; |
| 3) Acute coronary syndrome with or without resting electrocardiogram (ECG) change, ECG changes on a graded exercise test, or positive cardiac imaging study; |
| 4) Prior stroke documented on brain imaging or with a persistent deficit. |
| c) Subclinical CVD, confirmed in available medical records: |
| 1) At least a 50% diameter stenosis of a coronary, carotid, or lower extremity artery; |
| 2) Coronary artery calcium score ≥400 Agatston units; |
| 3) Ankle brachial index ≤0.90; |
| 4) Left ventricular hypertrophy by ECG (based on computer reading), echocardiogram report, or other cardiac imaging procedure report; |
| 5) Abdominal aortic aneurysm ≥5 cm with or without repair. |
| d) Age ≥75 years |
Table 2.
MACH15 exclusion criteria.
| An individual who has any of the following criteria will be excluded from participation in MACH15 |
|---|
| 1. High alcohol consumption, defined by any one of the following: |
| a) Alcohol Use Disorders Identification Test score >5 at screening; |
| b) drinking, on average, >7 alcoholic beverages/week during the past six months; |
| c) drinking six or more alcoholic beverages on one occasion during the past six months. |
| 2. Yale–Brown Obsessive Compulsive Scale-heavy drinking total score of ≥6 on questions 7, 8, and 10 |
| 3. Within the six months prior to randomization, cardiovascular disease event (myocardial infarction, revascularization procedure, or stroke) |
| 4. AHA Class III-IV heart failure |
| 5. History of alcohol or substance abuse (medical record confirmed or self-reported history) |
| 6. Other intolerance or allergy to alcohol |
| 7. Dual antiplatelet therapy |
| 8. History of gastric bypass surgery |
| 9. Any serious chronic liver disease (e.g. active hepatitis B and C infections) or liver tests (aspartate aminotransferase, alanine aminotransferase, and gamma-glutamyl transpeptidase) >2 times the upper limit of the normal range using local standards |
| 10. Personal history of any colon or liver cancer |
| 11. Any other cancer with a life expectancy of less than three years |
| 12. Diagnosed with breast cancer that required either surgery or removal of breast tissue or chemotherapy |
| 13. Mother or sister ever diagnosed with breast cancer that required either surgery or removal of breast tissue or chemotherapy |
| 14. Estimated glomerular filtration rate <30 ml/min/1.73m2 or end-stage renal disease |
| 15. Ongoing use of any medication for which alcohol consumption is contraindicated |
| 16. A Patient Health Questionnaire ≥15 at screening or a positive response on question 9 dealing with suicidal ideation |
| 17. History of any organ transplant |
| 18. Unintentional weight loss >10% in last six months |
| 19. Currently participating in another cardiovascular outcome intervention trial |
| 20. Not willing or able to provide a name and contact information for at least one additional contact person other than self |
| 21. Diagnosis of dementia |
| 22. Investigator discretion regarding appropriateness of participation or concern about intervention adherence. Examples include: moderate–severe psychiatric illness, behavioral concerns regarding likelihood of low adherence to trial protocol, a medical condition likely to limit survival to less than three years, or an advanced chronic disease, such as cognitive impairment without a dementia diagnosis or any condition that requires 24-hour care |
| 23. Not willing or able to provide a signed and dated informed consent form |
| 24. Unable to successfully complete the washout period |
| 25. Not willing or able to comply with all trial procedures |
An important consideration with which trial investigators grappled was the limits of previous alcohol consumption allowed. For safety, never- and problem drinkers were both excluded. To minimize the degree of behavior change required for adherence, the limits of previous drinking were set exactly at the two doses represented in the study intervention – near abstention and one standard drink daily – as described below.
Intervention
Dose
Although maximum caps on drinking in national guidelines differ substantially, the NIAAA currently recommends limits of 14 drinks per week for men under 65 years of age and seven drinks per week for women and for men aged 65 years and older. In the absence of contraindications, a dose of ~11–15 g daily (i.e. one standard drink of regular beer, white wine, red wine, or spirits28) would be viewed as within recommended limits by authorities in all participating countries, including the US NIAAA and the US Department of Agriculture Dietary Guidelines.29
Beverage choice
The specific type of alcoholic beverage type consumed appears to be less important than the frequency with which alcohol is consumed, at least for CHD.30,31 The effect of alcohol on high-density lipoprotein cholesterol (HDL-C), a marker used in MACH15 for population-level compliance, also does not differ by beverage type in short-term controlled experiments.32,33 Moreover, differential impact of beverage type has not been observed on insulin sensitivity, platelet function, tissue plasminogen activator, and other components of clotting and fibrinolysis.30,34 In contrast, insufficient evidence exists on the potential health effects of other components of alcoholic beverages33,35 (i.e. polyphenols) to restrict the trial to a single beverage. By allowing maximal flexibility in beverage type, thus mimicking natural history, the trial aimed to maximize adherence over time and increase generalizability.
The MACH15 trial included two intervention arms: one assigned participants to consume a standard daily drink (by US standards) containing 11–15 g, and the other assigned participants to near-complete abstention from alcohol. These doses were chosen explicitly to maximize contrast between arms; given the uncertain nature of the dose–response curves for CHD and mortality, even once-weekly consumption could have physiological effects. On average, participants in the alcohol arm could choose between consuming approximately 150 ml (~5 ounces) of wine, 350 ml (~12 ounces) of beer, or 45 ml (~1.5 ounces) of spirits. Where minor differences in the alcohol content of beverages existed (e.g. the size of beer cans in the US and Denmark), participants were allowed to consume one serving of the local standard.
Participants in the intervention arm were asked to consume alcohol only after activities that required dexterity and alertness were completed for the day. They were advised to consume alcohol with meals as part of a healthy diet, but no specific dietary manipulation occurred. Participants in the abstention arm were asked to abstain from drinking alcohol nearly completely to ensure a sharp contrast between the two trial arms; however, a modest degree of tolerance towards alcohol consumption in the abstention arm was specifically allowed to promote adherence. Participants in each group received eight “day-off” passes per year that formally permitted a single-day crossover and were distributed annually beginning at randomization. In the alcohol arm, these passes could be used to abstain from drinking alcoholic beverages on days when illness or travel precluded consumption. In the abstention arm, these passes could be used to drink one alcoholic beverage on holidays and special occasions. Pass usage was assessed regularly using all forms of communication noted below. Passes could only be used twice monthly, never on the same day, and without rollover. This innovation greatly increased the feasibility and patient willingness to participate in either arm of the trial.
Participants in the alcohol arm were expected to incur increased costs over their usual intake if asked to drink daily, and the trial allowed for compensation of costs or beverages. Due to the regulatory differences in alcohol policies between states, countries, and regions, clinical sites had flexibility in terms of providing reimbursement for or distribution of alcohol. For compensation, a limit was set to 75% of the monthly cost of the most commonly consumed alcoholic beverage in the region (e.g. Bud LightⓇ in the US) to avoid influencing informed consent or encouraging excessive use. Table 3 displays the models for alcohol provision and the sites that used them. In all cases, the distribution of or reimbursement for alcohol required participants’ adherence to the protocol and frequent engagement with trial staff, providing additional protection from escalation in use. In addition, sites had discretion as to whether to provide other retention incentives to participants in both the alcohol and abstention arm, such as small non-alcoholic gifts or remuneration of transport or parking expenses.
Table 3.
Models for the distribution of the alcohol intervention.
| Intervention | Description | Site |
|---|---|---|
| Post-pay reimbursement | Participants reimbursed for alcohol purchases up to the pre-determined monthly amount. Payments occurred at regular intervals, contingent upon the submission of itemized, dated receipts that account for equal to or greater than the reimbursement amount. | Julius Clinical, The Netherlands WFUHS, Wake Forest, NC, USA |
| Gift/debit card | Participants provided with a reusable gift card for a local store that provides alcohol. The gift card received periodic installments of study funds, allowing the participant to make purchases of amounts up to the cost defined for that time period. | Institute for Clinical Effectiveness and Health Policy, Argentina |
| Store account | The study will establish participant accounts with local alcohol providers, whereby participant purchases were billed directly to the study, given set monthly spending restrictions. | Johns Hopkins ProHealth Clinical Research Center, Baltimore, MD, USA |
| Order via study staff | Participant placed an order every 1–3 months with a member of the clinical site staff, who arranges the store’s provision of these items via store pick-up or delivery and payment. | Hospital Clinic de Barcelona, Spain University of Copenhagen, Denmark Julius Clinical, The Netherlands Center for Bioethics and Research, Nigeria |
Adherence
The intensity of the intervention was similar in both arms of the trial. Beginning at the initiation of the pre-randomization run-in period and continuing throughout follow-up, in-person clinic visits were supplemented by random, individualized, automated participant contact to assess the number and the types of drinks consumed. These automated contacts occurred twice weekly (one weekend and one weekday) in months one–three; once weekly in months four–six; and once every two weeks starting at month seven until the end of the trial, provided the participant demonstrated adherence and complete engagement. The automated contacts prompted participants to provide information on the amount and type of alcohol consumed in the prior 24 hours. Given varied levels of familiarity and comfort with electronic communication, participants could choose their preferred method of communication – smartphone application, email, or text message – and could change that format at any time. If automated contact attempts were left unanswered or if adherence decayed, field center staff initiated follow-up with an off-schedule telephone call, as shown in Figure 2. Adherence to the intervention was also examined by study staff on quarterly telephone contacts using the Timeline Follow-back (seven day) recall form36 and the Yale–Brown Obsessive Compulsive Scale-heavy drinking (Y-BOCS-hd).37 The Y-BOCS-hd tracks changes in alcohol craving over time to identify participants at early risk for developing problem drinking.
Figure 2.
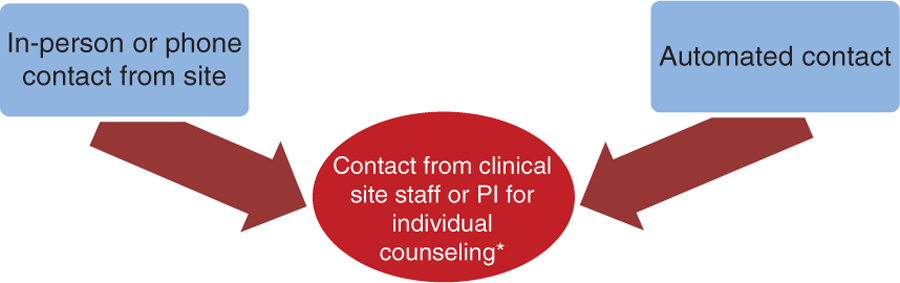
Participant contact. PI: local site primary investigator.
*Occurs only if participant is non-adherent or unresponsive.
The baseline visit included formal counseling and motivational interviewing methods that have been used successfully in other clinical trials.38 At each subsequent in-person clinic visit and telephone call, key concepts in adherence were reiterated.39,40 Participants received adherence counselling by clinical staff using individualized motivational interviewing techniques, if they: (a) were less than 85% adherent in the alcohol arm; (b) less than 100% adherent in the abstention arm, excluding passes; or (c) consumed alcohol excessively (Table 4). Specific steps to improve adherence were then triggered and involved intensive health coaching, increased automated contacts by the adherence system, and telephone or in-person discussions with the participant.
Table 4.
Triggers for adherence counseling.
| Intervention arm | Women | Men | Action |
|---|---|---|---|
| Daily alcohol | |||
| Low adherence (<85%) | No alcohol >1 day in 7 days of contact OR consumption of >1 alcoholic beverage >1 day in 7 days of contact | No alcohol >1 day in 7 days of contact OR consumption of >1 alcoholic beverage >1 day in 7 days of contact | Adherence counseling |
| Excessive alcohol consumption | >10 drinks in 7 days of contact | >14 drinks in 7 in days of contact | Intensive alcohol Counseling |
| Abstention | |||
| Low adherence (<100%) | Any alcohol consumption beyond or not in accordance with vouchers | Any alcohol consumption beyond or not in accordance with vouchers | Adherence counseling |
| Excessive alcohol consumption | >10 drinks in 7 days of contact | >14 drinks in 7 days of contact | Intensive alcohol counseling |
In addition to individual monitoring, overall group adherence to the intervention was assessed using HDL-C as an indirect biological marker (see below). Newer, more direct biomarkers of adherence,41,42 such as urine biomarkers and hair samples, were to be collected on a site-specific basis in a sample of participants to document compliance.
Hypotheses and outcomes
The primary hypothesis of the trial was that one standard serving of alcohol consumption daily would reduce the risk of CVD incidence and mortality, compared to abstention from alcohol in the targeted age group. This hypothesis was to be tested using a composite primary outcome that included time to first occurrence of non-fatal MI, non-fatal ischemic stroke, hospitalized angina, coronary/carotid revascularization, or total mortality.
In addition, two secondary hypotheses were pre-specified: (a) one standard serving of alcohol daily reduces the rate of incident diabetes compared to abstention from alcohol among participants free of diabetes at enrollment; and (b) one standard serving of alcohol consumption daily reduces the rate of CVD or CVD mortality compared to abstention from alcohol. Compared with the primary outcome, the latter hypothesis tested a composite outcome representing total CVD.
Finally, three tertiary hypotheses were pre-specified: (a) among participants free of pre-diabetes or diabetes at enrollment, one standard serving of alcohol daily reduces the rate of pre-diabetes compared to abstention from alcohol; (b) one standard serving of alcohol consumption daily reduces the rate of non-fatal MI, non-fatal ischemic stroke, or cardiovascular death, compared to abstention from alcohol; and (c) one standard serving of alcohol consumption daily reduces the rate of each of the individual components of the primary and secondary outcomes compared to abstention from alcohol (non-fatal MI, non-fatal ischemic stroke, hospitalized angina, coronary/carotid revascularization, and cardiovascular and total mortality). The second of the tertiary hypotheses tested a composite outcome representing “hard” CVD.
The choice of total mortality in the primary endpoint was intentional but bears examination. It was intended to minimize the effect of competing risk, in which non-fatal events might appear to be lower among consumers of alcohol because they died from other causes more rapidly.43
Measurements, ascertainment, and follow-up
Clinical outcomes were ascertained in both arms two and four weeks post-randomization, and every three months thereafter, during telephone calls or in-person clinic visits by assessors using a structured interview to minimize ascertainment bias. Clinical outcomes were to be identified through self-reports and laboratory measures, followed by the collection of medical records. The medical records included information about hospitalization admission and history, laboratory results, electrocardiogram, results of imaging techniques, and/or reports of revascularization or surgery procedures, collected by unmasked clinical site staff. Masked Medical Safety Officers were then to use their clinical judgment to review and adjudicate each case using pre-specified definitions and operational rules. In addition, a subset of outcome events was to be centrally validated by independent adjudicators. MACH15 used criteria, definitions for outcomes, and operational rules based on other recent cardiovascular trials, such as SPRINT44 and LIFE,45 and updated them as necessary. Clinical site staff used available registries and contact information to assess vital status annually among participants who did not respond to staff contact, but had not with-drawn consent.
Quality control
Data integrity and quality are among the highest priorities in clinical trials, including this one. The quality assurance activities to be conducted included: (a) the development and dissemination of manuals and uniform procedures to ensure data integrity; and (b) risk-based monitoring and analytic activities that assessed performance during data collection and processing. The detailed quality control procedures are described in the study protocol (supplementary material).
For quality control of outcome ascertainment, a sample of outcomes were reviewed and adjudicated centrally. At the beginning of the trial, if at least 10 composite specific outcomes (four MIs, two hospitalizations for angina, two strokes, one revascularization, one death) at each clinical site were validated with the full agreement of the Outcomes Subcommittee, then local classification and adjudication would no longer require full central review. Otherwise, central adjudication was to occur until clinical sites consistently achieved full agreement with central classification and adjudication. In either case, an annual 10% random quality control sample of outcomes was to be reviewed and validated centrally by the Outcomes Subcommittee.
MACH15 used several different proven training models for study staff: central training for clinical site staff, web-based training, on-site training by Clinical Coordinating Center personnel, and a train-the-trainer approach. Telephone calls and web-based platforms were used for periodic refresher trainings. The first central training session was conducted in October 2017, prior to the start of recruitment, for clinic investigators and staff. Each clinical site staff member was also required to complete a data handling training program before being given an ID and password to use the MACH15 website.
Statistical analysis, sample size, and power
The primary analysis in MACH15 assessed the significance of any difference observed in the distribution of time from randomization until occurrence of the primary endpoint, using censored survival data analysis methods for clustered data (i.e. in households). All randomized participants were grouped according to their intervention assigned at randomization in accordance with the intention-to-treat principle. Kaplan–Meier estimates of proportion of participants who remained event-free at pre-specified time points, and the associated confidence intervals, would be calculated in each arm, and the log-rank test for clustered data,46–48 stratified by clinical site, would assess the statistical significance of any differences observed. Hazard ratios and 95% confidence intervals would be calculated using Cox regression for clustered data,49–51 adjusted for clinical site. Log–log plots of survival and effect modification by time since randomization were to be assessed to evaluate the proportional hazards assumption. If the assumption was not justified, in secondary analysis, the study would report time-specific effect estimates and consider the use of accelerated failure time models, which may provide a more parsimonious fit to the data. The analysis of the secondary and tertiary endpoints would be conducted as described above.
Although randomization ensures that, on average, treatment groups are balanced with respect to potential risk factors for CVD or death, this may not be true in any given trial due to chance. Thus, the success of randomization was to be evaluated by comparing the baseline characteristics of participants in the treatment groups, and then to re-assess treatment effects in secondary analysis after adjusting for the risk factors associated with the strongest imbalances. To adjust as finely as possible for confounding by measured risk factors, an additional pre-specified secondary analysis would adjust for all measured risk factors.
Non-adherence to the assigned treatment is another potential challenge for obtaining an unbiased treatment effect. Secondary sensitivity analysis would include per protocol and as treated analyses52 by using instrumental variables methods,53,54 by potential outcomes approaches,55 by comparing the compliance estimates, and by computing log-rank tests weighted by time-varying probability of adherence.56 The “contamination-adjusted intention-to-treat” approach uses instrumental variable analysis to adjust for non-adherence in a two-stage approach. The potential outcomes approach is an estimation procedure for the intention-to-treat effect that maintains randomization-based properties under more plausible, non-ignorable noncompliance, and non-ignorable missing-outcome conditions. The log-rank tests weighted by time-varying probability of adherence involves modeling the probability of non-adherence given available covariate data, and then uses the inverse of these estimated probabilities, suitably stabilized, to reweight the log-rank test statistic to mimic the study population that would have been observed, had there been no non-adherence.
Effect modification by pre-specified risk factors for CVD was to be evaluated by calculating stratum-specific point and interval hazard ratios and by assessing the statistical significance of any differences observed through a partial likelihood-ratio test for clustered survival data. The pre-specified subgroups included age (<70 versus 70+ years), sex, HDL-C, and baseline CVD risk (tertiles of each), geographic region, and prevalent diabetes at enrollment. No a priori effect modifiers were hypothesized, and the study was not specifically powered to detect any.
Sample size calculations involved: extensive explorations over a range of primary endpoint definitions and baseline rates; baseline adherence rates and improvements to these due to household network effects; pro-portions of households with one, two, and three eligible and enrolled members; intra-household correlation coefficients; and minimum expected effect sizes. We assumed that the primary combined CVD incidence endpoint rate in the abstention arm would be 0.026/person-year, modestly higher than the 2.2% annual rate observed in SPRINT,44 increased slightly because, unlike SPRINT, MACH15 included diabetic participants and total mortality as an outcome. We assumed 1% annual complete loss to follow-up in both groups, and 10% non-adherence at any point in time over follow-up for participants who are the sole person in their household to be randomized. For these participants, this leads to an effective hazard ratio of 0.838, under an assumed true hazard ratio of 0.82.2 Under these assumptions, to provide a minimum of 80% power at a two-sided 0.05 significance level for detecting a 18% relative decrease in the intervention group endpoint rate, for an individually randomized trial design with one participant per household, the trial required 3900 participants per arm,57 for a total of 7800. Adherence was expected to improve among households with multiple enrolled members. Assuming that non-adherence would be 10% in single households and 9% in multiple-participant households, then the following further assumptions would guarantee 80% power: an intraclass correlation coefficient = 0.005, a proportion of households with ≥2 members equal to 70%, a proportion of additional household members eligible to be 30%, and a sample size of 7800 participants. The gain in power due to the expected modest improvement in adherence was offset by the reduction in power due to the modest within-household clustering assumed. Although we conservatively powered the trial for an expected relative risk (RR) of 0.82, evidence suggests that a RR as low as 0.75 might be plausible.2 In that situation, the trial would have adequate power for baseline rates as low as approximately 0.0125/person-year, significantly lower than the observed rate in SPRINT.44
To determine that minimal annual adherence rates were achieved to ensure adequate power, an early comparison of HDL-C, known to be a useful marker at the group level,58 was instituted. Specifically, the SCC would compare the change in HDL-C from baseline to the six-month visit between the two trial arms after 1000 participants have been enrolled. Based on a two-sample t-test for comparing the difference between the changes in the intervention and abstention groups, the minimum detectable change in HDL-C with 80% power was 0.89 to 3.1 mg/dl, assuming no change in the abstention group and that the standard deviation of the change in the HDL-C would fall between 5 mg/dl and 8.5 mg/dl, corresponding to a 50% range around the median value, 6.7 mg/dl, reported previously.58
For comparison purposes, we also calculated the sample size needed in a six-year trial with an additional three-year period of enrollment (i.e. like MACH15) to detect the observed risk of any alcohol-related cancer and of breast cancer, using published definitions and estimates of RR59 and interpolating RRs for ~15 gm/d (i.e. the dose used in MACH15 if the strongest alcoholic beverages were consistently consumed). The sample size needed to detect the observed RR for any alcohol-related cancer (RR of 1.13) is ~60,000 individuals. For breast cancer, the sample size needed to detect a RR of 1.1, assuming 2% five-year risk, is ~350,000 women.
Safety monitoring
MACH15 has been through a thorough reviewing process: the trial was approved by NIAAA, a DSMB and a Safety Subcommittee was appointed to monitor and review safety during the study, and an IRB/EC approval was a prerequisite at all sites. Safety was designed to be thoroughly monitored throughout a participant’s interaction with the MACH15 trial from the screening process through the end of trial follow-up. Both serious and non-serious adverse events (AEs) were to be documented through case report forms on the secure MACH15 website and reported to the DSMB, to the sponsor, and to each clinical site’s IRB/EC.
Specific AEs potentially related to alcohol consumption were of special interest.60 Participants were specifically queried about these events at their regular contacts and visits – exactly as often as the primary and secondary endpoints. These included total and site-specific cancer, excluding non-melanoma types of skin cancer; road or water accidents that require medical attention; major falls requiring medical attention in the hospital or emergency ward; fall-related fractures; atrial fibrillation requiring hospitalization; congestive heart failure requiring hospitalization; and hemorrhagic stroke requiring hospitalization. In addition, investigators reported increases in liver tests to >2 times the upper limit of normal, alcohol use disorder, and unexpected events for which the clinician believed that participation caused or contributed to the event.
The Safety Subcommittee was established to review trial data related to the overall safety of trial participation, address IRB/EC issues related to participant safety that may arise, review clinical practice-related issues, and oversee the clinical safety of all trial participants. The Safety Subcommittee was chaired by a Central Monitor, a masked physician, responsible for the review of all serious AEs that might require DSMB and sponsor reporting. With the assistance of the SCC, this committee also discussed AE reports, ensured consistency in AE coding and reporting, and reviewed potential trends.
Web-based trial management
All MACH15 data were collected and data entered directly into web-based forms and saved to a Structured Query Language database at the BIDMC. The web-based system incorporated real-time quality control checks, including verification of eligibility prior to randomization. Documentation of the data entry system was maintained at the BIDMC, along with training materials for clinical site staff that were available for download. Site-specific reports of aggregate participant demographics, recruitment goals, screening yield, and others were available on the website in real time. All data were password-protected, and access to each portion of the website was limited on an individual basis to correspond with an individual’s role in the trial. Person-identifiable information were kept separate from all other information and linked only by the pseudo-anonymous study-ID for each participant. As a result, the trial met or exceeded security recommendations specified by the National Institute of Standards and Technology61 and met European data protection regulations.62
Ancillary studies
Ancillary studies enhance the value and productivity of any trial and spur continued interest among the diverse group of investigators who are critical to the success of the trial. The MACH15 trial encouraged investigators to develop ancillary studies in conjunction with other investigators, within and outside of MACH15. An Ancillary Studies Committee reviewed each proposal to ensure that its objectives did not duplicate nor interfere with the main MACH15 trial objectives. As of April 2018, seven ancillary studies had been proposed and were under review, including proposals related to mutation load and carcinogenesis, metabolomics, and sleep.
Ethical approval, recruitment rates, and study termination
MACH15 received IRB approval in the following countries: USA (Maryland, North Carolina), The Netherlands, Nigeria, Argentina (two of four clinical centers), and, conditionally, in Denmark. The parent approval was received from the Committee on Clinical Investigations at the Beth Israel Deaconess Medical Center in Boston (protocol 2017P000333). The remaining approval numbers are available from the supplementary material. All participants provided written informed consent. Recruitment of participants for MACH15 was initiated in February 2018 at four vanguard sites: the Johns Hopkins ProHealth Clinical Research Center, Baltimore, MD, USA; the Wake Forest School of Medicine, NC, USA; the Center for Bioethics and Research, Nigeria; and JC, the Netherlands, as shown in Figure 3. At these four sites, a total of 751 individuals were pre-screened during telephone interviews. Screening was completed and written informed consent was signed by a total of 390 individuals, but 279 were ineligible after the screening visit. Among the remaining eligible volunteers, 32 participants completed the 14-day alcohol-free run-in and were randomized to alcohol or abstention from February to May 2018. The remaining 75 subjects were still in the screening or the run-in period of the trial, of whom four volunteers were already deemed fully eligible after run-in but had not been randomized yet. These numbers indicate that the recruitment goal of 7800 participants at approximately these and another nine sites over three years was readily achievable. In May 2018, the study was terminated by the NIH and funding withdrawn.
Figure 3.
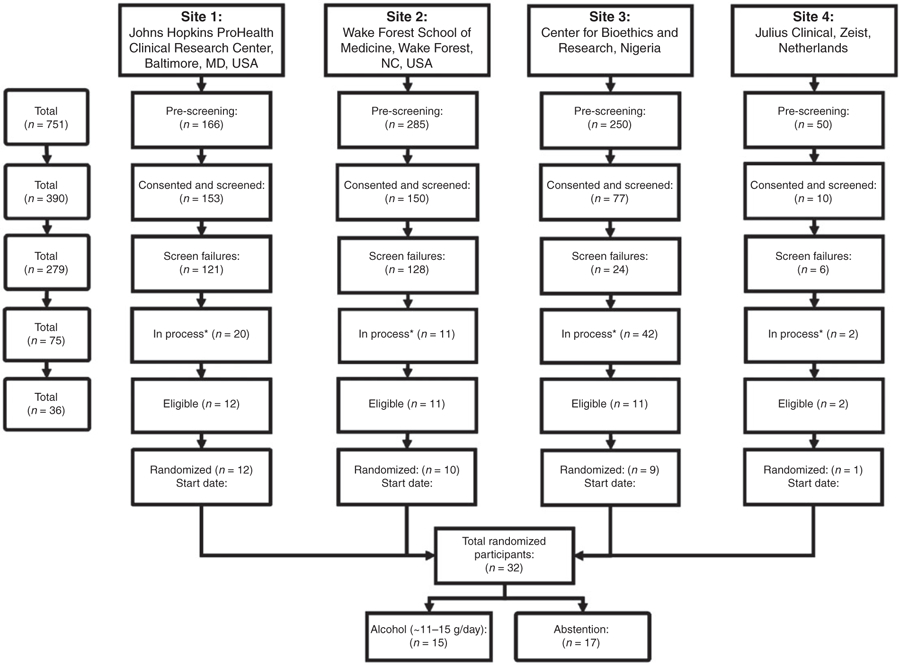
Flowchart illustrating recruitment rates.
*In process refers to participants who were in the process of eligibility assessment, e.g. awaiting blood sample analysis or participating in the washout or run-in period.
Discussion
In this balanced-design randomized trial, the doses of alcohol assigned in the two arms – ~0 and ~11–15 g daily – were designed to test the prospective relationship, if any, of alcohol with CVD and mortality. Because of the steep descending limb of the alcohol– CVD relationship, even modest levels of alcohol intake above abstention in the control group could influence trial outcomes and reduce statistical power. At the same time, given the apparently flat portion of the alcohol–CVD relationship between 15 and 30 g daily, little evidence suggested that doses above ~15 g would provide greater cardiovascular benefit. Therefore, participants were asked and counseled to consume nearly no alcohol in the abstention arm and one standard serving in the intervention arm.
Although observational studies are not perfectly consistent in the relative effects of different alcoholic beverages, their similarities greatly outweigh their differences, both in direct effects on biomarkers and on risk of chronic disease. Moreover, clinical practice and national guidelines do not differentiate among beverages in establishing safe limits to drinking. Because drinking preferences differ between individuals and even within individuals over time, maximal flexibility in beverage type increases the likelihood of adherence among individuals assigned to alcohol consumption. Further, all successful longer-term studies have used commercially available beverages30,63; and the only parallel-arm trial that used an artificial beverage containing grain ethanol demonstrated poor adherence over six months.27 Therefore, participants were not restricted in the type of commercially available alcoholic beverage they chose to consume in MACH15 but only in the amount of alcohol consumed. This flexibility in beverage choice should also increase generalizability of results.
One limitation of the MACH15 design was an inability to test various doses or to individualize them. For example, the tested dose could differ for men and women64 or be tailored to a person’s body size. On the other hand, the safety and feasibility of testing doses of alcohol above 15 g daily have not been demonstrated, for no study of three months or longer has employed doses beyond ~15 g of alcohol daily, and the ~15 g limit has previously been studied successfully for as long as 24 months.65
Although alcohol consumption has not been tested in any long-term randomized trial, and short-term trials of its use within recommended limits have rarely demonstrated any clear signals of harm, there is, nonetheless, potential for harm.66 For example, alcohol is an addictive substance, although the risk of acquiring addiction differs substantially based upon a host of environmental and personal characteristics.13,67 Alcohol is sedating, even with recommended limits, and may interfere with the ability to drive or operate dangerous or delicate machinery. In addition, alcohol can increase the sedative effects of any medication that causes drowsiness, including drugs for anxiety and depression. In addition, liver cirrhosis, classically associated with long-term heavy drinking, has also been associated with even moderate alcohol intake, although this could reflect susceptibility among individuals with predisposing forms of underlying liver disease such as non-alcoholic fatty liver disease68 or infection with hepatitis viruses.69 On the other hand, a recent meta-analysis found a linear relationship between alcohol and cirrhosis with no safe limit for women and increased risk for men with two drinks per day.70 In addition, alcohol may also impair sleep quality.71
An important question also surrounds the ability for any trial to identify cancer risks from alcohol. Unless a trial intentionally recruited subjects at high risk for cancer – an unequivocally unethical proposition – no randomized trial of any realistic size would be powered to detect an increase in risk of breast cancer of the magnitude observed in observational studies. Specifically, the sample size needed in a trial like ours to detect the expected risk of any alcohol-related cancer is ~60,000 individuals – a full 10,000 individuals larger even than the Women’s Health Initiative Dietary Modification Trial. For breast cancer, the sample size needed to detect a RR of 1.1 is ~350,000 women – that is, once men were included, it would need to be literally the largest individually randomized trial ever conducted. Given that reality, suggestions that breast cancer should be a primary outcome appear ill-advised. Instead, to maximize safety, MACH15 specifically excluded volunteers with a family history of cancers that could be affected by alcohol, resulting in an effective margin of safety in the range of 10–100 cancers. Nonetheless, cancers recorded as AEs in MACH15 would provide the best available evidence for estimation of cancer risk at moderate levels of alcohol intake. Moreover, were MACH15 to validate the observed effects of alcohol on outcomes like CHD or diabetes, it would provide strong indirect support for the observed effects on cancer that derive from literally the same cohort studies. It must also be underlined that conducting human trials with an aim of inducing adverse effects is illegal in most countries and violates international conventions; the safety margin defined here is, therefore, central for the ethical conduct of a trial of alcohol consumption.
Although observational evidence generally suggests that alcohol consumption tends to be associated with lower risk of CHD across a wide variety of populations, ethical, practical, and clinical considerations suggest that this hypothesis is most efficiently studied in high-risk individuals. From a practical standpoint, a high-risk population with a high background rate of events requires a smaller potential sample size, which minimizes the number of participants needed to recruit and follow. This population also tends to have more extensive contact with health care systems, leading to improved recruitment, and may have a particularly vested interest in cardiovascular prevention strategies, which may enhance adherence. Ethically, a high-risk population minimizes the number of participants placed at risk in a randomized trial, and with declines in problem drinking behavior with older age, tends to reduce potential harms from alcohol while maximizing potential benefits. From a clinical and public health perspective, these individuals are also the most likely to benefit if the primary or secondary hypotheses of MACH15 are confirmed, for they stand to benefit the most from any given decrease in RR and would, therefore, have the most favorable risk–benefit ratio for alcohol consumption.
At the same time, results from a global, diverse, high-risk population are apt to be generalizable to a wide variety of lower-risk populations in terms of RR reduction, even if any absolute risk reduction is necessarily smaller. To accomplish this enhanced generalizability, we formed a team of highly experienced clinical trials researchers to establish clinical, data, and biospecimen coordinating centers. Our field centers included some of the most experienced clinical trial researchers of the world.
The question of whether to include congestive heart failure in the primary outcome is important, although it was designated an AE of special interest – and, hence, monitored and ascertained actively. Alcohol intake at 80 g or more over periods of at least 20 years can result in alcoholic cardiomyopathy,72 but risk at lower doses is far less clear. Indeed, the only randomized trial to test the longitudinal effect of alcohol on left ventricular function demonstrated improved ejection fraction and myocardial performance in diabetic adults with a recent MI assigned to drink red wine.73 The substantial variability in diagnostic testing for heart failure across sites, particularly relative to ischemic events, further led the trial investigators to treat congestive heart failure as its own event.
There are no definitively recognized benefits of alcohol consumption on any specific health condition. At the same time, alcohol is widely used in social situations and specific rites and rituals, and is consumed by approximately half of the world’s adults. That it has never been tested in a long-term clinical trial reflects profound scientific inertia or non-scientific factors that so commonly surround the topic of alcohol. To address this profound deficit in the world’s understanding of this ubiquitous macronutrient, this trial would be the first to test the potential risks and benefits of daily alcohol consumption relative to abstention on risk of CVD, diabetes, mortality, and several other outcomes. The initial recruitment rate demonstrates that such a study is achievable and the overall organization of MACH15 and its approval by ethical review boards and safety monitoring boards in countries across four continents testifies to the feasibility and timeliness of MACH15.
Supplementary Material
Acknowledgements
The authors would like to thank the volunteers for their willingness to devote considerable time and effort to the trial. We would also like to thank the many trial investigators and staff members at the clinical sites and coordinating centers who contributed to the design and conduct of this study and provided the motivation for this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: this work was supported by U.S. Department of Health and Human Services/National Institutes of Health grant (grant number U10AA025286).
Footnotes
Declaration of conflicting interests
The author(s) declared the following potential conflicts of interest with respect to research, authorship, and/or publication of this article: LOD, JNE, and TLW received a Semper Ardens grant from the Carlsberg Foundation running 2015– 2021. The funder had no role in any phases of the study conduction or in the decision to prepare or publish the manuscript. LOD also reports funding from the Danish Innovation Foundation (governmental). RE reports grants from the Spanish Institute of Health “Carlos III”; Ministry of Agriculture, Fisheries and Food, Government of Spain; Autonomic Government of Catalonia, Spain; University of Barcelona, Spain; Cerveza y Salud, Spain; Fundacion Dieta Mediterranea, Spain; European Union, Brussels; NIAAA, USA. Additionally, RE received personal fees for given lectures from Brewers of Europe, Belgium; Fundacion Cerveza y Salud, Spain; Pernaud-Ricard, Mexico; Instituto Cervantes from Albuquerque/USA, Milan/Italy, and Tokyo/Japan; Lilly Laboratories and Uriach Laboratories, Spain; and the Wine and Culinary International Forum, Spain. JHK and KJM attended meetings funded by NIAAA and private-sector members of the alcohol industry in 2013–2014 prior to any application for or receipt of grant funding for the trial. MMM has worked for NIAAA and has also received reimbursement for travel from the International Alliance for Responsible Drinking. ISC reports funding between 2011 and 2015 from the Dutch Ministry of Economic Affairs, Agriculture and Innovation; the Dutch Foundation for Alcohol Research (SAR); and the Netherlands Organization for Applied Scientific Research. Their joint aim was to independently study the health effects of moderate alcohol consumption. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscripts during that period. The remaining authors DS, LCL, PK, CAA, SNA, LJA, JWJB, JWC, HJE, DDG, PEG, WI, ML, EBR, and JDW declare no conflicts of interest.
References
- 1.Gunzerath L, Faden V, Zakhari S, et al. National Institute on Alcohol Abuse and Alcoholism report on moderate drinking. Alcohol: Clin Exp Res 2004; 28: 829–847. [DOI] [PubMed] [Google Scholar]
- 2.Ronksley PE, Brien SE, Turner BJ, et al. Association of alcohol consumption with selected cardiovascular disease outcomes: a systematic review and meta-analysis. BMJ 2011; 342: d671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wood AM, Kaptoge S, Butterworth AS, et al. Risk thresholds for alcohol consumption: combined analysis of individual-participant data for 599 912 current drinkers in 83 prospective studies. Lancet 2018; 391: 1513–1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brien SE, Ronksley PE, Turner BJ, et al. Effect of alcohol consumption on biological markers associated with risk of coronary heart disease: systematic review and meta-analysis of interventional studies. BMJ (Clinical research ed) 2011; 342: d636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holmes MV, Dale CE, Zuccolo L, et al. Association between alcohol and cardiovascular disease: Mendelian randomisation analysis based on individual participant data. BMJ (Clinical research ed) 2014; 349: g4164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Takeuchi F, Yokota M, Yamamoto K, et al. Genome-wide association study of coronary artery disease in the Japanese. Eur J Hum Genet 2012; 20: 333–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Millwood IY, Walters RG, Mei XW, et al. Conventional and genetic evidence on alcohol and vascular disease aetiology: a prospective study of 500 000 men and women in China. Lancet 2019; 393: 1831–1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Han H, Wang H, Yin Z, et al. Association of genetic polymorphisms in ADH and ALDH2 with risk of coronary artery disease and myocardial infarction: a meta-analysis. Gene 2013; 526: 134–141. [DOI] [PubMed] [Google Scholar]
- 9.Carlsson S, Hammar N and Grill V. Alcohol consumption and type 2 diabetes. Diabetologia 2005; 48: 1051–1054. [DOI] [PubMed] [Google Scholar]
- 10.Joosten MM, Chiuve SE, Mukamal KJ, et al. Changes in alcohol consumption and subsequent risk of type 2 diabetes in men. Diabetes 2011; 60: 74–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Di Castelnuovo A, Costanzo S, Bagnardi V, et al. Alcohol dosing and total mortality in men and women: an updated meta-analysis of 34 prospective studies. Arch Intern Med 2006; 166: 2437–2445. [DOI] [PubMed] [Google Scholar]
- 12.Smith-Warner SA, Spiegelman D, Yaun S-S, et al. Alcohol and breast cancer in women: a pooled analysis of cohort studies. JAMA 1998; 279: 535–540. [DOI] [PubMed] [Google Scholar]
- 13.Corrao G, Bagnardi V, Zambon A, et al. Exploring the dose-response relationship between alcohol consumption and the risk of several alcohol-related conditions: a meta-analysis. Addiction 1999; 94: 1551–1573. [DOI] [PubMed] [Google Scholar]
- 14.Prabhu A, Obi KO and Rubenstein JH. The synergistic effects of alcohol and tobacco consumption on the risk of esophageal squamous cell carcinoma: a meta-analysis. Am J Gastroenterol 2014; 109: 822. [DOI] [PubMed] [Google Scholar]
- 15.Piano MR. Alcoholic cardiomyopathy: incidence, clinical characteristics, and pathophysiology. Chest 2002; 121: 1638–1650. [DOI] [PubMed] [Google Scholar]
- 16.Larsson SC, Wallin A and Wolk A. Alcohol consumption and risk of heart failure: meta-analysis of 13 prospective studies. Clin Nutr 2018; 37: 1247–1251. [DOI] [PubMed] [Google Scholar]
- 17.Cherpitel CJ, Tam T, Midanik L, et al. Alcohol and non-fatal injury in the U.S. general population: a risk function analysis. Accid Anal Prev 1995; 27: 651–661. [DOI] [PubMed] [Google Scholar]
- 18.Dawson DA and Grant BF. The “gray area” of consumption between moderate and risk drinking. J Stud Alcohol Drugs 2011; 72: 453–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beulens JW, Sierksma A, Schaafsma G, et al. Kinetics of homocysteine metabolism after moderate alcohol consumption. Alcohol: Clin Exp Res 2005; 29: 739–745. [DOI] [PubMed] [Google Scholar]
- 20.Mukamal KJ, Clowry CM, Murray MM, et al. Moderate alcohol consumption and chronic disease: the case for a long-term trial. Alcohol Clin Exp Res 2016; 40: 2283–2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leong DP, Smyth A, Teo KK, et al. Patterns of alcohol consumption and myocardial infarction risk: observations from 52 countries in the INTERHEART case-control study. Circulation 2014; 130: 390–398. [DOI] [PubMed] [Google Scholar]
- 22.Smyth A, Teo KK, Rangarajan S, et al. Alcohol consumption and cardiovascular disease, cancer, injury, admission to hospital, and mortality: a prospective cohort study. Lancet 2015; 386: 1945–1954. [DOI] [PubMed] [Google Scholar]
- 23.Freiberg MS and Samet JH. Alcohol and coronary heart disease: the answer awaits a randomized controlled trial. Circulation 2005; 112: 1379–1381. [DOI] [PubMed] [Google Scholar]
- 24.Kloner RA and Rezkalla SH. To drink or not to drink? That is the question. Circulation 2007; 116: 1306–1317. [DOI] [PubMed] [Google Scholar]
- 25.Cohen JE, Zeller M, Eissenberg T, et al. Criteria for evaluating tobacco control research funding programs and their application to models that include financial support from the tobacco industry. Tob Control 2009; 18: 228–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.US Department of Health and Human Services. Subpart A – Basic HHS Policy for Protection of Human Subjects. Maryland: US Department of Health and Human Services, 2018. [Google Scholar]
- 27.Mukamal KJ, Na B, Mu L, et al. Lessons and challenges from a 6-month randomized pilot study of daily ethanol consumption: research methodology and study design. Curr Dev Nutr 2017; 1: e000505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.US Department of Health and Human Services and US Department of Agriculture (USDA). 2015–2020 dietary guidelines for Americans (Appendix 9: Alcohol). 8th ed. Washington D.C.: USDA, 2015. [Google Scholar]
- 29.US Department of Health and Human Services and US Department of Agriculture (USDA). 2015–2020 dietary guidelines for Americans. 8th ed. Washington D.C.: USDA, 2015. [Google Scholar]
- 30.Rimm EB, Klatsky A, Grobbee D, et al. Review of moderate alcohol consumption and reduced risk of coronary heart disease: is the effect due to beer, wine, or spirits? BMJ (Clinical research ed) 1996; 312: 731–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wine Cleophas T., beer and spirits and the risk of myocardial infarction: a systematic review. Biomed Pharmacother 1999; 53: 417–423. [DOI] [PubMed] [Google Scholar]
- 32.van der Gaag MS, van Tol A, Scheek LM, et al. Daily moderate alcohol consumption increases serum paraoxonase activity; a diet-controlled, randomised intervention study in middle-aged men. Atherosclerosis 1999; 147: 405–410. [DOI] [PubMed] [Google Scholar]
- 33.Shai I, Wainstein J, Harman-Boehm I, et al. Glycemic effects of moderate alcohol intake among patients with type 2 diabetes. Diabetes Care 2007; 30: 3011–3016. [DOI] [PubMed] [Google Scholar]
- 34.Kim SH, Abbasi F, Lamendola C, et al. Effect of moderate alcoholic beverage consumption on insulin sensitivity in insulin-resistant, nondiabetic individuals. Metab Clin Exp 2009; 58: 387–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Estruch R, Sacanella E, Mota F, et al. Moderate consumption of red wine, but not gin, decreases erythrocyte superoxide dismutase activity: a randomised cross-over trial. Nutr Metab Cardiovasc Dis 2011; 21: 46–53. [DOI] [PubMed] [Google Scholar]
- 36.Sobell LC and Sobell MB. Timeline follow-back In: Litten RZ and Allen JP (eds) Measuring Alcohol Consumption. Totowa, NJ: Humana Press, 1992, pp. 41–72. [Google Scholar]
- 37.Goodman WK, Price LH, Rasmussen SA, et al. The Yale-Brown obsessive compulsive scale: I. Development, use, and reliability. Arch Gen Psychiatry 1989; 46: 1006–1011. [DOI] [PubMed] [Google Scholar]
- 38.Carroll KM. Enhancing retention in clinical trials of psychosocial treatments: practical strategies. NIDA Res Monogr 1997; 165: 4–24. [PubMed] [Google Scholar]
- 39.Estruch R, Ros E, Salas-Salvadó J, et al. Primary prevention of cardiovascular disease with a Mediterranean diet. N Engl J Med 2013; 368: 1279–1290. [DOI] [PubMed] [Google Scholar]
- 40.Svetkey LP, Stevens VJ, Brantley PJ, et al. Comparison of strategies for sustaining weight loss: the weight loss maintenance randomized controlled trial. JAMA 2008; 299: 1139–1148. [DOI] [PubMed] [Google Scholar]
- 41.Paul R, Tsanaclis L, Murray C, et al. Ethyl glucuronide as a long-term alcohol biomarker in fingernail and hair. Matrix comparison and evaluation of gender bias. Alcohol Alcohol 2019; 54: 402–407. [DOI] [PubMed] [Google Scholar]
- 42.Kintz P 2014 consensus for the use of alcohol markers in hair for assessment of both abstinence and chronic excessive alcohol consumption. Forensic Sci Int 2015; 249: A1–A2. [DOI] [PubMed] [Google Scholar]
- 43.Kent DM, Alsheikh-Ali A and Hayward RA. Competing risk and heterogeneity of treatment effect in clinical trials. Trials 2008; 9: 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ambrosius WT, Sink KM, Foy CG, et al. The design and rationale of a multicenter clinical trial comparing two strategies for control of systolic blood pressure: the Systolic Blood Pressure Intervention Trial (SPRINT). Clinical Trials 2014; 11: 532–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dahlöf B, Devereux RB, Kjeldsen SE, et al. Cardiovascular morbidity and mortality in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet 2002; 359: 995–1003. [DOI] [PubMed] [Google Scholar]
- 46.Mantel N Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep 1 1966; 50: 163–170. [PubMed] [Google Scholar]
- 47.Peto R and Peto J. Asymptotically efficient rank invariant test procedures. J R Stat Soc Ser A 1972: 185–207.
- 48.Jung S-H and Jeong J-H. Rank tests for clustered survival data. Lifetime data Anal 2003; 9: 21–33. [DOI] [PubMed] [Google Scholar]
- 49.Cox DR. Regression models and life-tables. J R Stat Soc Series B Stat Methodol 1972; 34: 187–220. [Google Scholar]
- 50.Glidden DV and Vittinghoff E. Modelling clustered survival data from multicentre clinical trials. Stat Med 2004; 23: 369–388. [DOI] [PubMed] [Google Scholar]
- 51.Gangnon RE and Kosorok MR. Sample-size formula for clustered survival data using weighted log-rank statistics. Biometrika 2004; 91: 263–275. [Google Scholar]
- 52.Sheiner LB and Rubin DB. Intention-to-treat analysis and the goals of clinical trials. Clin Pharm Ther 1995; 57: 6–15. [DOI] [PubMed] [Google Scholar]
- 53.Dunn G, Maracy M and Tomenson B. Estimating treatment effects from randomized clinical trials with noncompliance and loss to follow-up: the role of instrumental variable methods. Stat Methods Med Res 2005; 14: 369–395. [DOI] [PubMed] [Google Scholar]
- 54.Sussman JB and Hayward RA. An IV for the RCT: using instrumental variables to adjust for treatment contamination in randomised controlled trials. BMJ (Clinical research ed) 2010; 340: c2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Frangakis CE and Rubin DB. Addressing complications of intention-to-treat analysis in the combined presence of all-or-none treatment-noncompliance and subsequent missing outcomes. Biometrika 1999; 86: 365–379. [Google Scholar]
- 56.Mark SD and Robins JM. A method for the analysis of randomized trials with compliance information: an application to the Multiple Risk Factor Intervention Trial. Control Clin Trials 1993; 14: 79–97. [DOI] [PubMed] [Google Scholar]
- 57.Schoenfeld D The asymptotic properties of nonparametric tests for comparing survival distributions. Biometrika 1981; 68: 316–319. [Google Scholar]
- 58.Rimm EB, Williams P, Fosher K, et al. Moderate alcohol intake and lower risk of coronary heart disease: meta-analysis of effects on lipids and haemostatic factors. BMJ (Clinical research ed) 1999; 319: 1523–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Praud D, Rota M, Rehm J, et al. Cancer incidence and mortality attributable to alcohol consumption. Int J Cancer 2016; 138: 1380–1387. [DOI] [PubMed] [Google Scholar]
- 60.Miller SI, Kreek KM, Lieber CS, et al. Recommended council guidelines on ethyl alcohol administration in human experimentation. Bethesda: National Institute on Alcohol Abuse and Alcoholism, 1989. [Google Scholar]
- 61.Regenscheid A and Scarfone K. Recommendations of the National Institute of Standards and Technology. NIST Special Publication 2011; 800: 155. [Google Scholar]
- 62.Regulation 2016/679 of the European Parliament and of the Council of 27 April. Brussels, 2016, pp.1–88. [Google Scholar]
- 63.Marques-Vidal P, Duclmetière P, Evans A, et al. Alcohol consumption and myocardial infarction: a case-control study in France and Northern Ireland. Am J Epidemiol 1996; 143: 1089–1093. [DOI] [PubMed] [Google Scholar]
- 64.Briasoulis A, Agarwal V and Messerli FH. Alcohol consumption and the risk of hypertension in men and women: a systematic review and met-analysis. J Clin Hypertens 2012; 14: 792–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gepner Y, Golan R, Harman-Boehm I, et al. Effects of initiating moderate alcohol intake on cardiometabolic risk in adults with type 2 diabetes: a 2-year randomized, controlled trial. Ann Intern Med 2015; 163: 569–579. [DOI] [PubMed] [Google Scholar]
- 66.Mukamal KJ, Phillips RS and Mittleman MA. Beliefs, motivations, and opinions about moderate drinking: a cross-sectional survey. Fam Med 2008; 40: 188. [PubMed] [Google Scholar]
- 67.Kaner E, Heather N, Brodie J, et al. Patient and practitioner characteristics predict brief alcohol intervention in primary care. Br J Gen Pract 2001; 51: 822–827. [PMC free article] [PubMed] [Google Scholar]
- 68.Aberg F, Puukka P, Salomaa V, et al. Risks of light and moderate alcohol use in fatty liver disease: follow-up of population cohorts. Hepatology (Baltimore, Md). Epub ahead of print 20 July 2019 DOI: 10.1002/hep.30864. [DOI] [PubMed] [Google Scholar]
- 69.Poynard T, Bedossa P and Opolon P. Natural history of liver fibrosis progression in patients with chronic hepatitis C. Lancet 1997; 349: 825–832. [DOI] [PubMed] [Google Scholar]
- 70.Roerecke M, Vafaei A, Hasan OSM, et al. Alcohol consumption and risk of liver cirrhosis: a systematic review and meta-analysis. Am J Gastroenterol 2019; 114: 1574–1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kong F, Li H, Xu G, et al. Association of dietary behaviors and sleep quality: results from the adults chronic diseases and risk factors survey of 2015 in Ningbo, China. Int J Environ Res Public Health 2018; 15: 1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Urbano-Marquez A, Estruch R, Navarro-Lopez F, et al. The effects of alcoholism on skeletal and cardiac muscle. N Engl J Med 1989; 320: 409–415. [DOI] [PubMed] [Google Scholar]
- 73.Marfella R, Cacciapuoti F, Siniscalchi M, et al. Effect of moderate red wine intake on cardiac prognosis after recent acute myocardial infarction of subjects with type 2 diabetes mellitus. Biabet Med 2006; 23: 974–981. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


