Abstract
Background
Human epidermal growth factor receptor 2 (HER2) has emerged as an important prognostic and therapeutic target in advanced stage and recurrent uterine serous carcinoma (USC). The significance of tumoral HER2 expression in early-stage disease has not been established.
Methods
This multi-center cohort study included women with stage I USC treated from 2000–2019. Demographic, treatment, recurrence, and survival data were collected.
Immunohistochemistry (IHC) was performed for HER2 and scored 0–3+. Equivocal IHC results (2+) were further tested with fluorescence in-situ hybridization (FISH). HER2 positivity was defined as 3+ IHC or FISH positive.
Results
One hundred sixty-nine patients with stage I USC were tested for HER2; 26% were HER2-positive. There were no significant differences in age, race, stage, adjuvant therapy, or follow-up duration between the HER2-positive and negative cohorts. Presence of lymph-vascular space invasion was correlated with HER2-positive tumors (p=0.003).
After a median follow-up of 50 months, there were 43 (25.4%) recurrences. There were significantly more recurrences in the HER2-positive cohort (50.0% vs 16.8%, p<0.001). HER2 positive tumors were associated with worse progression-free (PFS) and overall survival (OS) (p<0.001 and p=0.024). On multivariate analysis, HER2 positive tumors were associated with inferior PFS (aHR 3.50, 95%CI 1.84–6.67; p<0.001) and OS (aHR 2.00, 95%CI 1.04–3.88; p=0.039) compared to HER2-negative tumors.
Conclusions
Given its significant association with worse recurrence and survival outcomes, HER2 positivity appears to be a prognostic biomarker in women with stage I uterine serous carcinoma. These data provide support for clinical trials with anti-HER2-directed therapy in early-stage disease.
Introduction
Uterine serous carcinoma (USC) is a biologically aggressive, poor prognostic subtype of endometrial cancer associated with higher recurrence and mortality rates compared to other, more common epithelial endometrial cancer subtypes. USC represents only 10% of all endometrial cancers, but an astonishing 40% of endometrial cancer-related deaths (1). Among women with early-stage USC, the standard treatment is hysterectomy and surgical staging followed by platinum/taxane-based chemotherapy with or without vaginal brachytherapy in most cases. Even in those with uterine-limited disease who are treated with adjuvant therapy, cancer recurrences are observed in an estimated 11–22% of stage I cases (2–4). Most recurrences are systemic and not possible to salvage, highlighting the critical need for novel and improved therapies.
Advancements in understanding the genomic expression profiles of USC have driven the development of targeted therapeutic agents. One such molecular target is human epidermal growth factor receptor 2 (HER2), also known as ERBB2. HER2 is a receptor tyrosine kinase that regulates cell proliferation, survival, and differentiation. The oncogenic potential of HER2 protein overexpression and/or gene amplification is widely recognized in a variety of solid tumors, including breast, gastric, esophageal, head and neck, and gynecologic cancers (5–8). HER2 overexpression and/or amplification has been reported in 18–42% of USC (9–11). In 1998, trastuzumab, a monoclonal antibody directed at HER2, was the first drug approved by the U.S. Food and Drug Administration (FDA) for treating HER2-positive metastatic breast cancer. Since then, five additional HER2-targeting agents have been approved by the FDA (12).
HER2 has emerged as an important prognostic and therapeutic target in USC. Recent randomized data demonstrate the survival benefit of adding trastuzumab to platinum/taxane-based therapy in women with advanced or recurrent, HER2-positive USC (13). The significance of tumoral HER2 overexpression or amplification in early-stage disease has not yet been established. Therefore, our study objective was to determine whether HER2 is a prognostic biomarker in women with Stage I USC.
Methods
Patient population
This was a multi-institutional, retrospective cohort study involving three U.S. tertiary academic centers: University of Minnesota (Minneapolis, MN, USA), Johns Hopkins School of Medicine (Baltimore, MD, USA), and Yale School of Medicine (New Haven, CT, USA). Institutional Review Board approval was obtained at each of the participating institutions. Patients were identified from pathology databases and through ICD-9/10 searches and were included if they had a diagnosis of stage IA or stage IB (FIGO 2009 staging) USC between the years 2000–2019. Patients were excluded if their tumor contained less than 20% serous histology. The medical record was queried for demographic information, including age, body mass index (BMI), race/ethnicity, and Eastern Cooperative Oncology Group (ECOG) performance status. Tumor characteristics collected from pathology reports included percent serous component, depth of invasion, tumor size, involvement of a polyp, lymph-vascular space invasion (LVSI), and the extent of surgical staging. Data on adjuvant treatment, recurrence, and survival were also collected.
Pathological analysis
Paraffin-embedded tumor blocks were collected from the original hysterectomy specimen. Pathology was reviewed based on hematoxylin and eosin (H&E) staining by experienced gynecologic pathologists at each institution to confirm the diagnosis of USC. Adjacent sections were then cut, and slides were submitted for HER2 immunohistochemistry (IHC) with or without dual-probe fluorescence in-situ hybridization (FISH) testing. For patients with mixed histology, testing was performed on the tumoral serous component. All IHC and FISH slides were scored by 2 pathologists using the modified 2007 ASCO/CAP guidelines for HER2 IHC (to include tumors showing a basolateral/ lateral membrane staining pattern) and FISH interpretation in breast carcinoma (14). This classification was used as it has been validated in the Fader et al, phase II USC trial (13, 15).
At Yale School of Medicine, IHC was performed using the DAKO HercepTest kit (DAKO, Carpinteria, CA). At the University of Minnesota, this was done using the Ventana PATHWAY anti-HER2 (4B5) platform (Ventana, Oro Valley, Arizona). At Johns Hopkins, both IHC methods and antibodies were used. Tumors were classified as HER2-negative if IHC staining was 0 or 1+, and as HER2-positive if IHC staining was 3+. In cases with a score of 2+, FISH was performed to determine whether HER2 was amplified in the tumor.
Dual-probe FISH was performed using fluorescently labeled probes to centromere 17 (CEP17) and a probe to the HER2 gene. At the Johns Hopkins School of Medicine and Yale School of Medicine, FISH was performed using the Abbott Molecular PathVysion Kit (Abbott Molecular, Abbott Park, IL, USA). At the University of Minnesota, this was done using the Dako IQFISH pharmDx kit (DAKO, Carpinteria, CA). Results on enumeration of at least 20 interphase tumor cell nuclei from specified tumor areas were reported as the average HER2 copy number per cell and as the ratio of average HER2 copy number to that of CEP17. Tumors were classified as HER2-amplified in cases of a HER2/CEP17 ratio ≥ 2.0 or an average HER2 copy number/cell ≥ 6.0 (14).
Statistics
Patient demographics, clinical characteristics, and treatment variables were compared between the HER2-positive and HER2-negative cohorts. For categorical variables, comparisons were drawn using chi-square contingency tables and Fisher’s exact tests where appropriate. Continuous variables were compared using a t-test for independent samples to test equality of means, or using the Wilcoxon rank-sum test to compare medians for non-normally distributed variables. Recurrence and survival outcomes were also compared based on HER2 status. Progression-free survival (PFS) was defined as the time from surgery to documented disease recurrence or progression, last follow-up or death. Overall survival (OS) was defined as time from surgery to last follow-up or death. PFS and OS were determined in the HER2-positive and HER2-negative groups using Kaplan-Meier analysis, and log-rank tests were used to compare survival functions.
Cox proportional hazards analyses were subsequently performed to determine predictors of PFS and OS. Univariate analyses for each of PFS and OS were initially performed to assess the effects of age, race, ECOG score ≥ 2, tumor substage, lymph-vascular space invasion, HER2 status, lymphadenectomy, omentectomy, and receipt of adjuvant chemotherapy (with or without radiation therapy). Afterwards, covariates for the final multivariate models were selected using forward stepwise regression with variable exclusion if p ≥ 0.2. Statistical analyses were performed using STATA/SE 15.1 (STATA, College Station, TX, USA).
Results
Demographic and clinical features
One hundred sixty-nine patients met inclusion criteria and had tumor slides available for pathological analysis and HER2 testing. Patient demographics, clinical characteristics, and treatment variables were compared between HER2-positive and HER2-negative cohorts (Table 1). The mean age for the overall cohort was 68.0 ± 8.0 years. The distribution of patients by race and ethnic background was also similar in the HER2-positive and HER2-negative groups, with the majority of patients being white (67.4%), followed by African American/Black (25.4%). Age, race, and ECOG performance score were unassociated with HER2 status.
Table 1:
Demographic and clinicopathologic characteristics of women with stage I uterine serous carcinoma stratified by HER2 status
| HER2-negative n = 125 | HER2-positive n = 44 | p-value | |
|---|---|---|---|
| Mean Age (years) | 67.9 ± 8.1 | 68.1 ± 7.7 | 0.86 |
| Median BMI (kg/m2) | 32.0 | 28.7 | 0.13* |
| Race/Ethnicity | |||
| Caucasian/White | 81 (64.8) | 33 (75.0) | |
| Black/African American | 34 (27.2) | 9 (20.5) | |
| Asian | 1 (0.8) | 2 (4.5) | |
| Hispanic | 4 (3.2) | 0 (0.0) | |
| Other/decline to answer | 5 (4.0) | 0 (0.0) | |
| ECOG† performance status | 0.43 | ||
| 0 | 96 (76.8) | 33 (75.0) | |
| 1 | 21 (16.8) | 10 (22.7) | |
| 2+ | 8 (6.4) | 1 (2.3) | |
| History of breast cancer | 22 (17.6) | 4 (9.1) | 0.23‡ |
| Surgical approach | |||
| Minimally invasive (including robotic) | 66 (52.8) | 24 (54.6) | 0.84 |
| Laparotomy | 59 (47.2) | 20 (45.4) | |
| Omentectomy | 94 (75.2) | 34 (77.3) | 0.78 |
| Pelvic lymphadenectomy | 114 (91.2) | 43 (97.7) | 0.15 |
| Pelvic and para-aortic lymphadenectomy | 102 (81.6) | 35 (79.6) | 0.77 |
| Pelvic washings | 102 (81.6) | 38 (86.4) | 0.47 |
| Stage (FIGO 2009) | |||
| IA | 100 (80.0) | 31 (70.4) | 0.19 |
| IB | 25 (20.0) | 13 (29.6) | |
| Originating in polyp | 45 (36.0) | 17 (38.6) | 0.76 |
| Histology | |||
| Pure serous | 107 (85.6) | 33 (75.0) | 0.27 |
| Mixed serous-endometrioid | 15 (12.0) | 9 (20.4) | |
| Mixed serous-clear cell | 3 (2.4) | 2 (4.6) | |
| Myometrial invasion | 75 (60.0) | 30 (68.2) | 0.34 |
| Lymph-vascular space invasion | 23 (18.4) | 18 (40.9) | 0.003 |
| Positive peritoneal cytology (n=140) | 9 (8.8) | 3 (7.9) | 1.00 ‡ |
| Adjuvant therapy | |||
| None | 31 (24.8) | 11 (25.0) | 0.99 |
| Vaginal brachytherapy alone | 6 (4.8) | 2 (4.6) | |
| Chemotherapy alone | 34 (27.2) | 11 (25.0) | |
| Combined chemo-radiotherapy** | 54 (43.2) | 20 (45.4) | |
| Median follow-up (months) | 52 (22–95) | 43 (19–80) | 0.26* |
Data are mean ± SD, median (interquartile range), or n (%)
p-value determined using Wilcoxon rank-sum test
ECOG = Eastern Cooperative Oncology Group
p-value determined using Fisher’s exact test
61 patients received brachytherapy, 6 patients pelvic radiotherapy, 7 patients brachytherapy + pelvic radiotherapy
HER2 status was not associated with surgical staging or approach. Overall, 137 patients (81.1%) underwent comprehensive surgical staging, including both pelvic and para-aortic lymphadenectomy. Pelvic washings were collected in 140 women (82.8%), among whom 12 (8.6%) had positive peritoneal cytology. These proportions did not differ by HER2 status.
Comparing pathologic features of HER2-positive and HER2-negative groups, no significant differences were found in substage, carcinoma originating in a polyp, the proportions of pure and mixed histologies, and the presence of myometrial invasion. Overall, 130 patients (76.9%) had stage IA disease, while 39 patients (23.1%) had stage IB disease. One hundred forty patients (82.8%) had pure serous histology, 24 (14.2%) had mixed serous-endometrioid histology, and 5 (3.0%) had mixed serous-clear cell histology. HER2-positive tumors were significantly more likely to exhibit lymph-vascular space invasion compared to HER2-negative tumors (40.9% versus 18.4%; p = 0.003). Adjuvant therapy did not differ between HER2 cohorts. Most patients (70.4%) received adjuvant platinum/taxane-based chemotherapy with or without radiation therapy.
HER2 testing results
In 169 tumor samples, 44 (26.0%) were HER2-positive. Ninety-nine patients (58.6%) had tumors with IHC scores of 0 or 1+ and were classified as HER2-negative whereas 28 patients (16.6%) had tumors with an IHC score of 3+ and were classified as HER2-positive (Figure 1). The remaining 42 patients (24.9%) had tumors with 2+ IHC scores (equivocal). Dual-probe FISH was performed on these cases and 16 (38.1%) of these demonstrated HER2 amplification. Rates of HER2 positivity were similar across all 3 study sites (22.8%, 26.1%, 28.8%, p=0.75)
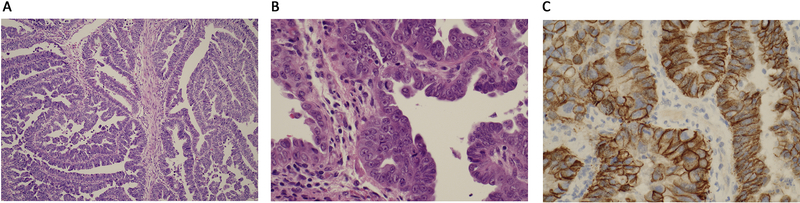
Figure 1:
Uterine serous carcinoma. a. papillary growth pattern b. malignant cells with prominent nucleoli and brisk mitotic activity c. HER2 immunohistochemical staining with 3+ staining pattern.
Recurrence and survival outcomes
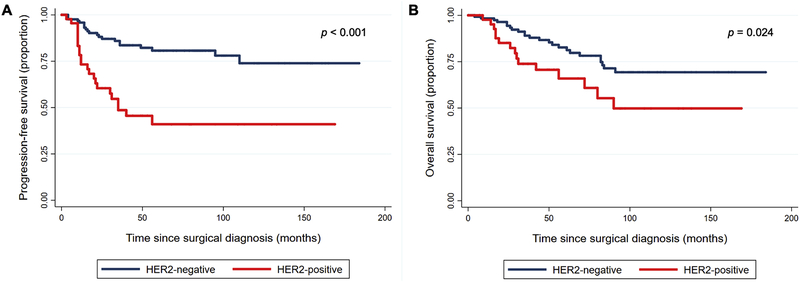
Recurrence and mortality rates were analyzed by HER2 status. With a median follow-up interval of 50 months (IQR 22 – 91 months), 43 patients (25.4%) experienced disease recurrence. Recurrence was significantly associated with HER2 status, with 50.0% of those with HER2-positive tumors experiencing disease relapse compared with 16.8% of those with HER2-negative tumors (p < 0.001) (Table 2). On Kaplan-Meier analysis, PFS was significantly worse in women with HER2-positive tumors (p < 0.001) (Figure 2A). At 36 months, the proportion of patients progression free was 0.84 (0.75–0.90) in the HER2 negative cohort and 0.49 (0.32–0.63) in the HER2 positive cohort (p<0.001) Of the 43 recurrences, 16 (37.2%) were pelvic, 17 (39.5%) were intra-abdominal, and 10 (23.3%) were extra-abdominal, with no difference by HER2 status (p = 0.24).
Table 2:
Comparison of rates of disease recurrence and death according to HER2 status
| HER2-negative n = 125 | HER2-positive n = 44 | p-value | |
|---|---|---|---|
| Recurrence | 21 (16.8 %) | 22 (50.0 %) | < 0.001 |
| Death* | 24 (19.2 %) | 15 (34.1 %) | 0.044 |
Deaths due to all causes. Nine patients died of non-uterine cancer causes including 8 in the HER2- negative cohort and 1 in the HER2-positive cohort.
Figure 2:
Kaplan-Meier curves demonstrating (A) progression-free and (B) overall survival in women with stage I uterine serous carcinoma, stratified by HER2 status
Overall, 39 patients (23.1%) died; 30 (17.8%) died of disease, and 9 (5.3%) died from other causes (Table 2). All cause mortality rates were significantly higher in women with HER2-positive tumors compared to those with HER2-negative tumors (34.1% versus 19.2%, p = 0.044). Women with HER2-positive tumors experienced significantly worse OS (log rank p = 0.024) (Figure 2B).
Univariate Cox proportional hazards analyses were performed to assess the impact of variables on PFS and OS (Supplementary Table 1).On multivariate analysis, HER2 positivity remained a strong independent risk factor for recurrence (aHR 3.50, 95% CI 1.84 – 6.67, p <0.001) and overall survival (aHR 2.00, 95% CI 1.04 – 3.88, p = 0.039) (Table 3).
Table 3:
Multivariate Cox proportional hazards analyses for predictors of progression-free and overall survival, with selection of covariates using stepwise forward selection (p<0.2)
| Progression-free survival | Overall Survival | ||||||
|---|---|---|---|---|---|---|---|
| Covariate | Adjusted Hazard Ratio (aHR) | 95% Confidence Interval | p-value | Covariate | Adjusted Hazard Ratio (aHR) | 95% Confidence Interval | p-value |
| Lymph-vascular space invasion | 1.71 | 0.80 – 3.63 | 0.17 | Age (years) | 1.038 | 0.99 – 1.08 | 0.063 |
| Stage IB | 1.71 | 0.79 – 3.67 | 0.17 | Stage IB | 2.241 | 1.11 – 4.51 | 0.029 |
| Omentectomy | 0.45 | 0.23 – 0.87 | 0.018 | Omentectomy | 0.566 | 0.28 – 1.13 | 0.11 |
| Adjuvant chemotherapy +/− radiation therapy | 0.50 | 0.25 – 0.99 | 0.046 | ECOG* score ≥ 2 | 3.099 | 1.18 – 8.14 | 0.022 |
| HER2+ | 3.50 | 1.84 – 6.67 | < 0.001 | HER2+ | 2.00 | 1.04 – 3.88 | 0.039 |
ECOG = Eastern Cooperative Oncology Group
Analyses were also performed for patients with pure serous histology only (excluding all patients with mixed histology). Results were similar. Notably, recurrence rates were higher in patients with HER2 positive tumors (45.5% vs 15.9%, p<0.001) HER2 positive tumors were associated with an increased hazard of recurrence (HR 3.44, 95% CI 1.72–6.9, p=0.001) and death (HR 1.92, 95% CI 0.89–4.16, p=0.10).
Discussion
In this study of women with stage I uterine serous carcinoma, patients with HER2 overexpression and/or gene amplification experienced significantly worse recurrence and survival outcomes than those without HER2 overexpression/amplification. HER2 positivity was associated with a three-fold greater risk of disease recurrence, and on both unadjusted and adjusted analyses, a significantly worse progression-free and overall survival. The overall cohort recurrence rate of 25% and mortality rate of 23% demonstrate the poor overall outcomes of women with early-stage USC.
Recurrences in USC are largely distant, multisite, and notoriously difficult to salvage with currently available therapies. Therefore, defining better and more targeted treatment strategies is an unmet clinical need in women with USC of all stages. This is one of the first studies to suggest that HER2 is overexpressed in 1 in 4 women with early-stage USC and may be a meaningful prognostic biomarker in this cohort. Furthermore, this study provides rationale for exploration of anti-HER2 therapy in this patient cohort.
Rates of HER2 positivity have varied dramatically in the literature, likely as a result of variable testing and reporting methods, as well as heterogeneous patient cohorts. In prior studies of HER2 expression in USC, advanced-stage tumors have represented the majority of samples, while early-stage tumors have represented a small fraction of samples. Reported rates of overexpression have ranged from 18–42%, and rates of gene amplification have varied from 16–47% (9–11, 16). Because HER2 positivity appears to be a prognostic biomarker for poor survival outcomes, higher rates of HER2 positivity might be expected in advanced-stage tumors. However, in this study, we found the rate of HER2 positivity to be comparable to that described in prior studies of predominantly advanced-stage tumors.
The oncogenic potential of the HER2 receptor is well known, although the significance of HER2 overexpression in the pathogenesis of USC remains poorly understood. In our cohort, HER2-positive tumors were significantly more likely to exhibit lymph-vascular space invasion (p = 0.003), suggesting that HER2 signaling may promote tumor invasiveness. HER2/PI3K/Akt activation has been demonstrated to drive resistance to multiple chemotherapeutic agents in breast and gastric adenocarcinoma (17, 18). Prior in vitro studies have suggested that HER2 may similarly promote chemo-resistance in USC (19). Our findings appear to be consistent with this data, as evidenced by the 50% recurrence rate reported among women with HER2-positive tumors, despite 70% receiving platinum/taxane-based adjuvant therapy.
Identifying HER2 as a biomarker in this high-risk disease has implications beyond prognosis, given that HER2 is an actionable target. Multiple anti-HER2 therapies have been described (20–25). In a recent phase II randomized trial, Fader et al evaluated 58 patients with advanced or recurrent uterine serous carcinoma overexpressing HER2 (13). Patients received carboplatin and paclitaxel with or without trastuzumab. Patients who received trastuzumab demonstrated an improved PFS of 12.6 versus 8.0 months compared to those who received placebo (p = 0.005; HR 0.44; (90% CI 0.26 – 0.76)). When examining the subgroups, the greatest reduction in recurrence risk was seen in the patients receiving primary treatment for stage III or IV disease (p = 0.013, HR 0.40, 90% CI 0.20 –0.80), suggesting that the greatest benefit of trastuzumab is in the upfront treatment of advanced stage disease. These encouraging results prompted changes in the National Comprehensive Cancer Network (NCCN) Uterine Cancer Guidelines to include trastuzumab in addition to platinum and taxane-based chemotherapy as the preferred regimen for women with advanced or recurrent HER2-positive USC (26). The findings in the current study support extending the investigation of HER2-targeted therapies to early-stage USC.
We chose to use the 2007 American Society of Clinical Oncology/College of American Pathologists (ASCO/CAP) breast cancer HER2 algorithms, noting that more recent guidelines do exist (7, 14). Based on two pre-trial pathology studies, the 2007 breast guidelines were used with specific modifications in the phase II study by Fader et al. Tumors with intense complete or lateral/basolateral membranous HER2 immunostaining in more than 30% of tumor cells were assigned a 3+ score, and 2+ score was assigned when intense complete or lateral/basolateral membrane staining was seen in ≤ 30%, or weak to moderate staining in ≥10% of tumor cells. We felt it was important to remain consistent with the limited data in this rare disease (15, 27). Ultimately, validating HER2 testing in uterine cancer will require not only consistency among testing platforms, but also correlations between testing and outcomes of targeted therapy.
Study strengths include its relatively large sample size, inclusion of only early stage patients, that most patients were comprehensively surgically staged and received adjuvant therapy, and that experienced gynecologic pathologists and HER2 specialists reviewed and tested all cases. Limitations of this study include its retrospective nature with inherent limitations and its broad time range, which was necessary in order to achieve adequate power. Additionally, the integrity of paraffin embedded tissue does diminish overtime, which could have resulted in falsely negative IHC or FISH analyses. Adjuvant treatment recommendations have changed over this time period, creating potential unrecognized bias.
Decades of research have informed the utility of HER2 testing and HER2-targeted therapy in breast cancer. We have only begun to explore role of HER2 in uterine cancer. Emerging clinical trial data are now informing our understanding of HER2 overexpression and/or amplification as a likely poor prognostic biomarker in multiple cancers, and particularly in uterine serous carcinoma. Our study provides rationale for the exploration of anti-HER2 therapies in women with early-stage, HER2-positive uterine serous carcinoma. A cooperative group trial is planned to determine the role of HER2-targeted therapy in women with primary uterine serous carcinoma of all stages.
Supplementary Material
Supplementary Table 1: Univariate Cox proportional hazards analyses for predictors of progression-free and overall survival
Highlights.
Twenty six percent of stage I uterine serous carcinoma tumors were HER2 positive
HER2 positivity was associated with a 3 fold greater risk of disease recurrence
HER2 is a potential prognostic biomarker for women with early stage disease
Acknowledgments
Financial support and sponsorship: Research reported in this publication was supported to BKE by the Eunice Kennedy Shriver National Institute of Child Health and Human Development of the National Institutes of Health under Award Number K12HD055887 and to ADS by R01 CA154460-01 and U01 CA176067-01A1 grants from NIH, the Deborah Bunn Alley Foundation, the Tina Brozman Foundation, the Discovery to Cure Foundation, the Guido Berlucchi Foundation and the Stand-up-to cancer (SU2C) convergence grant 2.0 to ADS. Additional support was received from the Stolz Family Fund and the Kosegarten Family Fund. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflicts of interest: ANF is a consultant for Merck. BKE is a consultant for Boston Scientific and receives research funding from Clovis oncology. RLS is a consultant for Astra Zeneca. AS receives research funding from Puma, Immunomedics, Gilead, Synthod, Merck, Boehinger-Ingelheim, Genentech, and Tesaro. ACM receives research funding and is a consultant for Bristol-Myers Squibb.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hamilton CA, Cheung MK, Osann K, Chen L, Teng NN, Longacre TA, et al. Uterine papillary serous and clear cell carcinomas predict for poorer survival compared to grade 3 endometrioid corpus cancers. British journal of cancer. 2006. March 13;94(5):642–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fader AN, Drake RD, O’Malley DM, Gibbons HE, Huh WK, Havrilesky LJ, et al. Platinum/taxane-based chemotherapy with or without radiation therapy favorably impacts survival outcomes in stage I uterine papillary serous carcinoma. Cancer. 2009. May 15;115(10):2119–27. [DOI] [PubMed] [Google Scholar]
- 3.Fader AN, Nagel C, Axtell AE, Zanotti KM, Kelley JL, Moore KN, et al. Stage II uterine papillary serous carcinoma: Carboplatin/paclitaxel chemotherapy improves recurrence and survival outcomes. Gynecol Oncol. 2009. March;112(3):558–62. [DOI] [PubMed] [Google Scholar]
- 4.Havrilesky LJ, Secord AA, Bae-Jump V, Ayeni T, Calingaert B, Clarke-Pearson DL, et al. Outcomes in surgical stage I uterine papillary serous carcinoma. Gynecol Oncol. 2007. June;105(3):677–82. [DOI] [PubMed] [Google Scholar]
- 5.Chua TC, Merrett ND. Clinicopathologic factors associated with HER2-positive gastric cancer and its impact on survival outcomes--a systematic review. Int J Cancer. 2012. June 15;130(12):2845–56. [DOI] [PubMed] [Google Scholar]
- 6.Thompson SK, Sullivan TR, Davies R, Ruszkiewicz AR. Her-2/neu gene amplification in esophageal adenocarcinoma and its influence on survival. Annals of surgical oncology. 2011. July;18(7):2010–7. [DOI] [PubMed] [Google Scholar]
- 7.Wolff AC, Hammond MEH, Allison KH, Harvey BE, Mangu PB, Bartlett JMS, et al. Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Focused Update. Archives of pathology & laboratory medicine. 2018. November;142(11):1364–82. [DOI] [PubMed] [Google Scholar]
- 8.Cavalot A, Martone T, Roggero N, Brondino G, Pagano M, Cortesina G. Prognostic impact of HER-2/neu expression on squamous head and neck carcinomas. Head & neck. 2007. July;29(7):655–64. [DOI] [PubMed] [Google Scholar]
- 9.Slomovitz BM, Broaddus RR, Burke TW, Sneige N, Soliman PT, Wu W, et al. Her-2/neu overexpression and amplification in uterine papillary serous carcinoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2004. August 1;22(15):3126–32. [DOI] [PubMed] [Google Scholar]
- 10.Santin AD, Bellone S, Van Stedum S, Bushen W, Palmieri M, Siegel ER, et al. Amplification of c-erbB2 oncogene: a major prognostic indicator in uterine serous papillary carcinoma. Cancer. 2005. October 1;104(7):1391–7. [DOI] [PubMed] [Google Scholar]
- 11.Morrison C, Zanagnolo V, Ramirez N, Cohn DE, Kelbick N, Copeland L, et al. HER-2 is an independent prognostic factor in endometrial cancer: association with outcome in a large cohort of surgically staged patients. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2006. May 20;24(15):2376–85. [DOI] [PubMed] [Google Scholar]
- 12.Erickson BK, Zeybek B, Santin AD, Fader AN. Targeting human epidermal growth factor receptor 2 (HER2) in gynecologic malignancies. Current opinion in obstetrics & gynecology. 2020. February;32(1):57–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fader AN, Roque DM, Siegel E, Buza N, Hui P, Abdelghany O, et al. Randomized Phase II Trial of Carboplatin-Paclitaxel Versus Carboplatin-Paclitaxel-Trastuzumab in Uterine Serous Carcinomas That Overexpress Human Epidermal Growth Factor Receptor 2/neu. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2018. July 10;36(20):2044–51. [DOI] [PubMed] [Google Scholar]
- 14.Wolff AC, Hammond ME, Schwartz JN, Hagerty KL, Allred DC, Cote RJ, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. Archives of pathology & laboratory medicine. 2007;131(1):18–43. [DOI] [PubMed] [Google Scholar]
- 15.Buza N, English DP, Santin AD, Hui P. Toward standard HER2 testing of endometrial serous carcinoma: 4-year experience at a large academic center and recommendations for clinical practice. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2013. December;26(12):1605–12. [DOI] [PubMed] [Google Scholar]
- 16.Buza N, Roque DM, Santin AD. HER2/neu in Endometrial Cancer: A Promising Therapeutic Target With Diagnostic Challenges. Archives of pathology & laboratory medicine. 2014. March;138(3):343–50. [DOI] [PubMed] [Google Scholar]
- 17.Knuefermann C, Lu Y, Liu B, Jin W, Liang K, Wu L, et al. HER2/PI-3K/Akt activation leads to a multidrug resistance in human breast adenocarcinoma cells. Oncogene. 2003. May 22;22(21):3205–12. [DOI] [PubMed] [Google Scholar]
- 18.Diaz-Serrano A, Angulo B, Dominguez C, Pazo-Cid R, Salud A, Jimenez-Fonseca P, et al. Genomic Profiling of HER2-Positive Gastric Cancer: PI3K/Akt/mTOR Pathway as Predictor of Outcomes in HER2-Positive Advanced Gastric Cancer Treated with Trastuzumab. The oncologist. 2018. September;23(9):1092–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Santin AD, Bellone S, Gokden M, Palmieri M, Dunn D, Agha J, et al. Overexpression of HER-2/neu in uterine serous papillary cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2002. May;8(5):1271–9. [PubMed] [Google Scholar]
- 20.Santin AD, Bellone S, Buza N, Schwartz PE. Regression of metastatic, radiation/chemotherapy-resistant uterine serous carcinoma overexpressing HER2/neu with trastuzumab emtansine (TDM-1). Gynecologic oncology reports. 2017. February;19:10–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Black J, Menderes G, Bellone S, Schwab CL, Bonazzoli E, Ferrari F, et al. SYD985, a Novel Duocarmycin-Based HER2-Targeting Antibody-Drug Conjugate, Shows Antitumor Activity in Uterine Serous Carcinoma with HER2/Neu Expression. Molecular cancer therapeutics. 2016. August;15(8):1900–9. [DOI] [PubMed] [Google Scholar]
- 22.English DP, Bellone S, Schwab CL, Bortolomai I, Bonazzoli E, Cocco E, et al. T-DM1, a novel antibody-drug conjugate, is highly effective against primary HER2 overexpressing uterine serous carcinoma in vitro and in vivo. Cancer medicine. 2014. October;3(5):1256–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.El-Sahwi K, Bellone S, Cocco E, Cargnelutti M, Casagrande F, Bellone M, et al. In vitro activity of pertuzumab in combination with trastuzumab in uterine serous papillary adenocarcinoma. British journal of cancer. 2010. January 5;102(1):134–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schwab CL, Bellone S, English DP, Roque DM, Lopez S, Cocco E, et al. Afatinib demonstrates remarkable activity against HER2-amplified uterine serous endometrial cancer in vitro and in vivo. British journal of cancer. 2014. October 28;111(9):1750–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schwab CL, English DP, Roque DM, Bellone S, Lopez S, Cocco E, et al. Neratinib shows efficacy in the treatment of HER2/neu amplified uterine serous carcinoma in vitro and in vivo. Gynecol Oncol. 2014. October;135(1):142–8. [DOI] [PubMed] [Google Scholar]
- 26.Koh WJ, Abu-Rustum NR, Bean S, Bradley K, Campos SM, Cho KR, et al. Uterine Neoplasms, Version 1.2018, NCCN Clinical Practice Guidelines in Oncology. Journal of the National Comprehensive Cancer Network : JNCCN. 2018. February;16(2):170–99. [DOI] [PubMed] [Google Scholar]
- 27.Buza N, Hui P. Marked heterogeneity of HER2/NEU gene amplification in endometrial serous carcinoma. Genes, chromosomes & cancer. 2013. December;52(12):1178–86. PubMed PMID: 24123408. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1: Univariate Cox proportional hazards analyses for predictors of progression-free and overall survival