Abstract
Formation of amyloid plaques is the hallmark of Alzheimer’s disease. Our early studies show that lead (Pb) exposure in PDAPP transgenic mice increases β-amyloid (Aβ) levels in the cerebrospinal fluid (CSF) and hippocampus, leading to the formation of amyloid plaques in mouse brain. Aβ in the CSF is regulated by the blood-CSF barrier (BCB) in the choroid plexus. However, the questions as to whether and how Pb exposure affected the influx and efflux of Aβ in BCB remained unknown. This study was conducted to investigate whether Pb exposure altered the Aβ efflux in the choroid plexus from the CSF to blood, and how Pb may affect the expression and subcellular translocation of two major Aβ transporters, i.e., the receptor for advanced glycation end-products (RAGE) and the low density lipoprotein receptor protein-1 (LRP1) in the choroid plexus. Sprague-Dawley rats received daily oral gavage at doses of 0, 14 (low-dose), and 27 (high-dose) mg Pb/kg as Pb acetate, 5 d/wk, for 4 or 8 wks. At the end of Pb exposure, a solution containing Aβ40 (2.5 μg/mL) was infused to rat brain via a cannulated internal carotid artery. Subchronic Pb exposure at both dose levels significantly increased Aβ levels in the CSF and choroid plexus (p<0.05) by ELISA. Confocal data showed that 4-wk Pb exposures prompted subcellular translocation of RAGE from the choroidal cytoplasm toward apical microvilli. Furthermore, it increased the RAGE expression in the choroid plexus by 34.1% and 25.1% over the controls (p<0.05) in the low- and high- dose groups, respectfully. Subchronic Pb exposure did not significantly affect the expression of LRP1; yet the high-dose group showed LRP1 concentrated along the basal lamina. The data from the ventriculo-cisternal perfusion revealed a significantly decreased efflux of Aβ40 from the CSF to blood via the blood-CSF barrier. Incubation of freshly dissected plexus tissues with Pb in artificial CSF supported a Pb effect on increased RAGE expression. Taken together, these data suggest that Pb accumulation in the choroid plexus after subchronic exposure reduces the clearance of Aβ from the CSF to blood by the choroid plexus, which, in turn, leads to an increase of Aβ in the CSF. Interaction of Pb with RAGE and LRP1 in choroidal epithelial cells may contribute to the altered Aβ transport by the blood-CSF barrier in brain ventricles.
Keywords: Lead, beta-amyloid, choroid plexus, RAGE, LRP1
1. Introduction
Alzheimer’s disease (AD) is the leading neurodegenerative disorder and one of the most common causes of dementia worldwide. Accumulation of β-amyloid (Aβ) within extracellular spaces of the brain is believed to be the critical event in AD etiology [1, 2]. Aβ can also stimulate hyperphosphorylation of tau, which facilitates the formation of neurofibrillary tangles in nerve terminals [3]. From a kinetic point of view, the processes that may contribute to the aggregation of Aβ in brain extracellular space include, but not limited to, (i) an increased supply of Aβ peptides from brain internal sources, (ii) a decreased degradation by Aβ-cleavage enzymes in the brain, (iii) disrupted influx or efflux transport of Aβ through brain barrier systems, or (iv) the combination of all of the above.
The choroid plexus in brain ventricles, where the blood-cerebrospinal fluid (CSF) barrier is located, regulates the material transport between the blood and CSF[4]. The tight junctions between choroidal epithelial cells provide the structural basis of the barrier between two fluid compartments, with the apical microvilli stretching into the CSF and the basal lamina facing the blood circulation. The choroid plexus functions to secrete the CSF, transport nutrients and peptides into the CSF and remove brain metabolites and unwanted materials from the CSF, so as to maintain the homeostasis of the central milieu. Aβ in the brain extracellular space can freely enter into the CSF, because of a direct continuity between the cerebral interstitial fluid and CSF [5]. Several reports in literature have established that Aβ in the CSF can be transported by the blood-CSF barrier [6–8]. Early studies in this group have shown that the choroidal epithelial cells are capable of transporting Aβ in either direction of the blood-CSF barrier, but more favorable in transporting Aβ from the CSF to blood[6]. Moreover, the choroid plexus possesses various enzymes that participate in Aβ degradation [9]. Noticeably, Aβ has been detected in the choroid plexus of AD patients [10] and the CSF concentration of Aβ42 to Aβ40 has been suggested as a biomarker for AD diagnosis [11].
Since more than 90% of AD cases are sporadic AD, it is reasonable to postulate that environmental exposure to toxic materials may contribute to the pathogenesis of AD. Lead (Pb), an environmental neurotoxin, is known to be associated with detrimental effects in the nervous system. Workers occupationally exposed to Pb display an increase in neurodegeneration and cortical atrophy, as well as behavioral deficits similar to the manifestation in AD patients [12–15]. Animal studies also demonstrate that Pb exposure during early life stage is associated with alterations in the expression and regulation of amyloid precursor protein (APP) in mice, rats, and non-human primates, with increased memory impairments later in life [16–18]. Evidence from this group has shown that chronic Pb exposure in PDAPP transgenic mice increases Aβ levels in the CSF and brain hippocampus and facilitates the aggregation of Aβ in mouse brain. Moreover, our synchrotron X-ray fluorescent microscopic data reveal a clear presence of Pb in the formed amyloid plaques [19, 20].
The choroid plexus is one of the main targets for Pb accumulation in human brains [21]. Our own study in rodents suggests a substantial accumulation of Pb in the choroid plexus upon acute exposure to Pb [22]. Far more than simply being sequestered in plexus tissue, Pb in the choroid plexus has been shown to alter the choroid plexus functions such as by reducing the production of an Aβ-transporter transthyretin[23], altering thyroxin transport by the blood-CSF barrier[4], and interfering with the expression of low density lipoprotein receptor protein-1 (LRP1) [24]. Thus, it is possible and even likely that Pb accumulated in the choroid plexus after exposure may interfere with the transport processes in the blood-CSF barrier that govern Aβ movement between the two fluid compartments and impair the Aβ homeostasis in the CSF.
To the best of our knowledge, the primary transporter at the blood-brain barrier that takes up Aβ (i.e., influx) from the blood to brain parenchyma is RAGE, whereas the main transporter for the efflux of Aβ from brain to the blood is LRP1 [25, 26]. While the presence of RAGE and LRP1 in the choroid plexus has been verified in literature [24, 27], their subcellular distribution in choroidal epithelia and how Pb may interact with these two transporters and therefore affect Aβ transport by the blood-CSF barrier remains unknown. Thus, the purposes of the present study were to investigate (1) whether in vivo subchronic Pb exposure in rats affected the influx transport of Aβ from the blood to the choroid plexus and CSF by in situ brain infusion of Aβ40; (2) whether the subchronic Pb exposure impaired the efflux of Aβ40 through the blood-CSF barrier by using an in situ ventriculo-cisternal (VC) perfusion technique; (3) whether subchronic Pb exposure altered the expression and subcellular location of RAGE and LRP1 in the choroid plexus; and (4) whether in vitro Pb exposure had the similar impact on the expression and cellular trafficking of RAGE in the choroid plexus. Understanding the regulatory mechanism of Aβ at the blood-CSF barrier with or without Pb exposure will help uncover the mechanisms by which Pb exposure contributes to the pathoetiology of AD.
2. Material and methods
2.1. Materials
Chemical reagents were purchased from the following sources: Mn chloride tetrahydrate (MnCl2·4H2O) from Fisher Scientific (Pittsburgh, PA); Cu chloride (CuCl2), calcium chloride (CaCl2), Dextran-70, hydroxyethyl piperazineethanesulfonic acid (HEPES), monoclonal anti-mouse β-actin antibody, 2-mercaptoethanol, phenylmethylsulfonyl fluoride (PMSF), polyacrylamide and tetramethyl-ethylenediamine (TEMED) from Sigma Chemicals (St Louis, MO); ultrapure nitric acid from VWR international (Chicago, IL); Fluor Alexa-488 conjugated secondary antibody from Life Technologies (Carlsbad, CA); protease inhibitor cocktail from Calbiochem (San Diego, CA); Tris base, glycine, sodium dodecyl sulfate (SDS), 2xLaemmli sample buffer, Triton X-100, and clarity Western ECL substrate from Bio-Rad (Hercules, CA); Aβ40 pure PTD human protein and Aβ40 human ELISA kit from Invitrogen (Waltham, MA); anti-RAGE antibody and anti-LRP1 antibody from Abcam (Cambridge, MA); Anti-Aβ40 antibody from Biolegend (San Diego, CA); rat LRP1/CD91 ELISA Kit and rat AGER/RAGE ELISA Kit from LifeSpan BioSciences (Seattle WA); Radioactive 14C-sucrose (specific activity: 495 mCi/mmol) from Moravek Biochemicals (Brea, CA); and Eco-lite-(+) scintillation cocktail from MP Biomedicals (Irvine, CA). All reagents were of analytical grade, HPLC grade, or the best available pharmaceutical grade.
2.2. Animals, Pb administration, and experimental design
Male and female Sprague Dawley rats were purchased from the Harlan Sprague Dawley Inc. (Indianapolis, IN). At the time of use, the rats were 10 weeks old weighing 220–250 g. Upon arrival, the rats were housed in a temperature-controlled room under a 12-h light/12-h dark cycle and allowed to acclimate for one week prior to experimentation. Rats had free access to deionized water and pellet rat chow (Teklad Dlobal 18% Protein Rodent Diet, 2018s; Envigo). The study was conducted in compliance with standard animal use practices and approved by the Animal Care and Use Committee of Purdue University.
Pb acetate (PbAc) was dissolved in sterile saline. Rats received oral gavages at the dose of 14 and 27 mg Pb/kg (as low and high dose groups, respectively), once daily, 5 days per week, for 4 or 8 consecutive weeks. The dose was determined based on earlier Pb neurotoxicity studies conducted in this laboratory [20]. The same volume of saline was given to the control group.
Two separate experiments were designed to investigate the uptake and clearance of Aβ by the choroid plexus following subchronic Pb exposure. Experiment 1 was designed to characterize the effect of Pb on the transport of Aβ from blood to the choroid plexus. After subchronic Pb exposure, rat brains were in situ infused with Aβ40, followed by determination of Aβ40 concentrations in the choroid plexus and CSF, and the subcellular location and expression of RAGE and LRP1 in the choroid plexus (Fig. 1A). Experiment 2 was designed to study Pb-induced alterations in Aβ clearance by the blood-CSF barrier. At the end of subchronic Pb exposure, an in situ ventriculo-cisternal (VC) perfusion technique was used to determine changes of Aβ40 clearance by the blood-CSF barrier (Fig. 1B). Detailed technical approaches for in situ brain infusion and in situ VC perfusion are provided below.
Fig. 1. Schematic illustration of the experiment design.
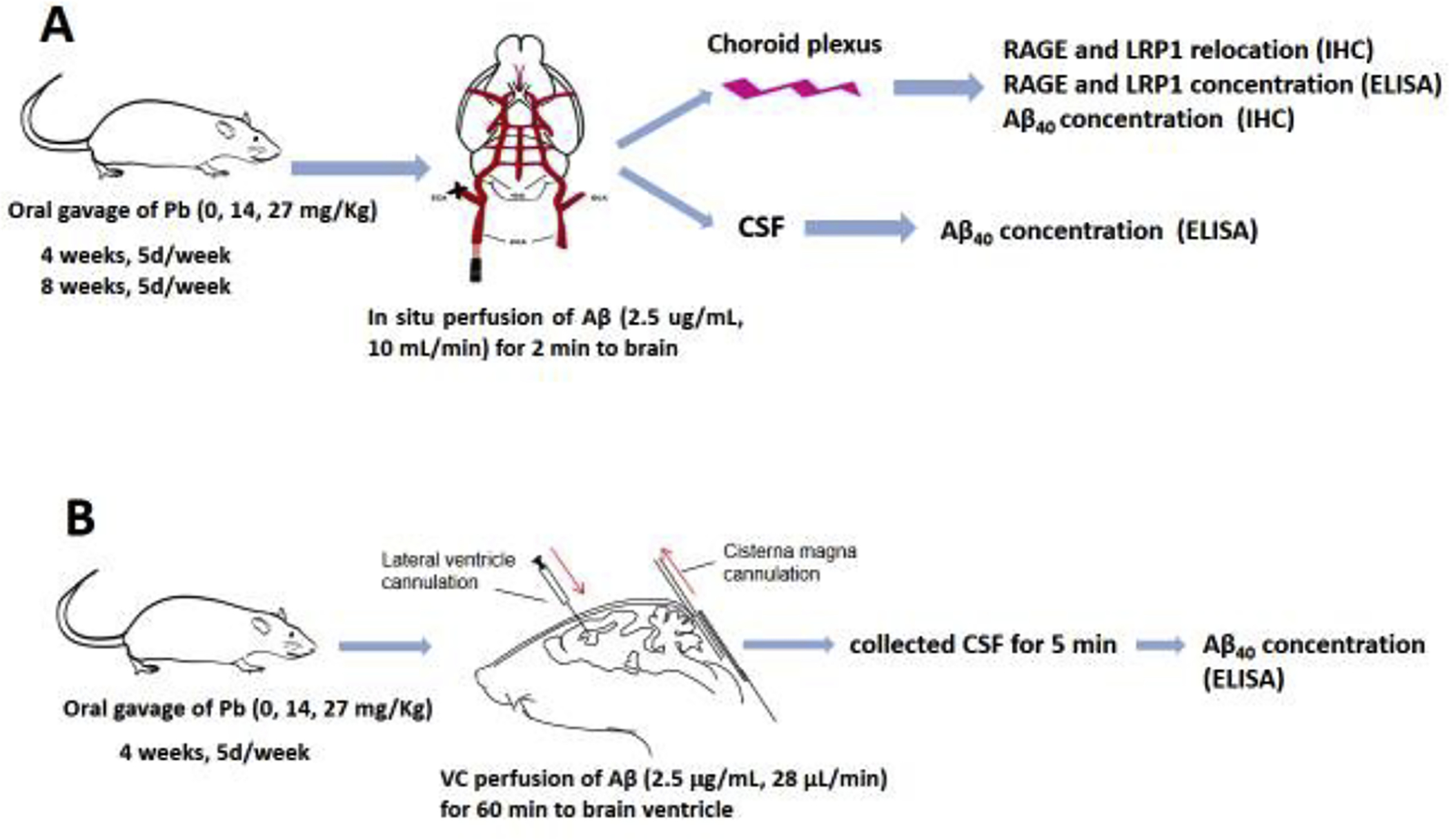
(A). In situ brain infusion. Rats received oral gavages at the doses indicated for 4 or 8 weeks. The in situ brain infusion of Aβ40 was performed 24 hours after the last dose. The CSF and choroid plexus were collected for determination of Aβ40 concentration and the expression of RAGE and LRP1. (B). Ventriculo-cisternal (VC) perfusion. Rats received oral gavages at the doses indicated for 4 weeks. The in situ VC perfusion was performed 24 hours after the last dose. Aβ40 along with a space marker [14C]sucrose was infused into the lateral ventricle; the CSF outflow was collected from the cisterna magna to determine the concentration both of [14C]sucrose and Aβ40.
2.3. Determination of Pb concentrations by atomic absorption spectrophotometry (AAS)
Brain samples (200 mg of wet weight) were digested in a MARSX press microwave-accelerated reaction system with 0.20 mL ultrapure concentrated nitric acid at 200°C for 4 hrs. Blood samples (200 μL) were digested with nitric acid in the oven at 55°C overnight. The AAS technique to quantify Pb has been used in this lab since 1996 [28], following the procedure by Fernandez and Hilligoss[29]. An Agilent Technologies 200 Series SpectrAA with a GTA 120 graphite tube atomizer was used to quantify Pb concentrations. Digested samples were diluted by 50, 500, or 1000 times with 1.0 % (vol/vol) ultrapure HNO3 in order to keep the reading within the concentration range of standard curves. The standard curves were prepared daily at concentrations of 0, 4, 8, 12, 16, and 20 μg/L with correlation coefficient of r2=0.9869. The detection limit was 1.35 ng Pb/ml of assay solution. The intra-day and inter-day precisions of the method were 1.5 % and 2.9 %, respectively.
2.4. In situ brain infusion
The in situ brain infusion technique has been well established and routinely used in our laboratory [30, 31]. Twenty-four hours after the last gavage, the rat was anesthetized with ketamine/xylazine (75:10 mg/kg, l mg/kg, ip), and placed on an electrical warming pad. The right common carotid artery was exposed and a small cut on the artery was made. After insertion of a polyethylene catheter (PE-10) tubing (toward the brain), ligations of the pterygopalatine, occipital, superior thyroid and external carotid arteries were made to ensure the perfusate entering exclusively to the internal carotid artery. The brain was perfused with a 95% O2 −5% CO2 saturated and continuously gassed 37°C Ringer’s solution (in 1000 mL: NaCl 7.31 g, KCl 0.356 g, NaHCO3 2.1 g, KH2PO4 0.166 g, MgSO4.7H2O 0.3 g, glucose 1.5 g, sodium pyruvate 0.11 g, and CaCl2 0.278 g, pH 7.4) at a flow rate of 9 mL/min (Heidolph Pump drive 5201). The “Aβ ” solution containing 25 μg/ml Aβ40 in pre-gassed Ringer’s solution in a separate second syringe was perfused in the cannulated internal carotid artery via a second syringe pump (Harvard Compact Infusion Pump, Model 11 Plus) at a flow rate of 1 mL/min. The total flow rate of the perfusion was therefore 10 mL/min. To prevent recirculation of the rat blood, the left ventricle of the heart was cut at the start of the perfusion.
After 2 min of “Aβ” perfusion, the Harvard syringe pump was turned off and the brain vascular system was washed for 1 min with the Ringer solution to remove Aβ40 adsorbed to the luminal surface. At the end of brain infusion, the CSF sample was collected from the cisterna magna; blood collected from the caudal vein; the brain removed from the skull, and the choroid plexus subsequently dissected. Collected samples were stored at −80°C for future analyses.
2.5. Determination of intracellular location of RAGE and LRP1 in the choroid plexus
At the end of the in situ infusion of Aβ40, the choroid plexus from one of the lateral ventricles was used for immunofluorescent staining to determine the cellular location of RAGE and LRP1 in the tissue and quantify Aβ40 by the signal intensity. The plexus from the other lateral ventricles was removed to extract proteins for measurement of the RAGE and LRP1 concentrations by enzyme-linked immunosorbent assay (ELISA).
Choroid plexus tissues were fixed with 4% paraformaldehyde for 15 min, followed by permeabilization with 0.3% Triton X-100+PBS for 30 min at room temperature and then blocked in normal goat serum for 1 hr at room temperature. The tissues were double-immunostained at 4°C with primary antibodies against LRP1 (1:500 for 1-day) and Aβ (1:500 for 4 days), or primary antibodies against RAGE (1:500 for 2-days) and Aβ (1:500 for 4 days), followed by incubation with goat anti-rabbit Alexa-488 conjugated secondary antibody (1:800) and goat anti-mouse Texas Red (1:800) at room temperature for 1 h. The plexus tissues were mounted to the objective slides using prolong Gold Anti-Fade to avoid fluorescent bleaching for confocal microscopy examination. The negative control was established by using only the secondary antibody to reflect nonspecific staining on the background. Image were obtained by a Nikon inverted confocal laser-scanning microscope and viewed with a 488-nm laser and a 562-nm laser source for excitation with magnification at 600×. The field containing epithelium with underlying vasculature was selected for analyses.
2.6. Quantification of RAGE and LRP1 in the plexus and Aβ40 in CSF by ELISA
The plexus tissues were homogenized in homogenization buffer containing 20mM Tris, pH 7.5, 5 mM EGTA, 1% Triton X-100, 1% SDS, Protease Inhibitor Cocktail (Calbiochem, San Diego, CA). The protein concentration was determined by using a Bradford assay according to the manufacturer’s instruction. Samples were then diluted 1:10 with sample dilution buffer (1% BSA with 0.05% Tween-20). Levels of RAGE and LRP1 were determined by rat AGER/RAGE ELISA Kit and rat LRP1/CD91 ELISA Kit purchased from LifeSpan BioSciences. The CSF samples were diluted 1:3 with the dilution buffer. Concentrations of Aβ40 were determined by Aβ40 human ELISA kit from Invitrogen.
2.7. In vitro plexus tissue incubation with Pb and RAGE intracellular trafficking
To verify the effect of Pb exposure on the subcellular relocation of RAGE in the choroidal epithelial cells, the freshly isolated choroid plexus tissues were incubated in the artificial CSF (aCSF) containing PbAc (50 μM) for 30 or 60 min, followed by incubation with Aβ40 (5 μM) for 1 h. At the end of the incubation, plexus tissues were fixed in 4% paraformaldehyde for immunofluorescent staining and confocal microscopic studies.
2.8. In situ ventriculo-cisternal (VC) perfusion
The VC perfusion technique has been well established and routinely used in our laboratory[30–33]. Prior to surgery, animals were fully anesthetized with ketamine/xylazine (75:10 mg/mL, 1 mL/kg, i.p.) and placed in a stereotaxic frame. A longitudinal incision was made in the scalp to expose the surface of the skull. A cranial burr hole (1 mm) was drilled into the skull of the right hemisphere with the coordinates of 0.8 mm posterior to bregma and 1.4 mm lateral to the midline. This was followed by the insertion of a guide cannula at 3.5 mm vertical from the skull surface (Fu et al., 2016; Wang et al., 2008). An internal cannula connected to PE50 tubing was inserted into the guide cannula for lateral ventricle perfusion controlled by the pump-driven syringe filled with pre-gassed artificial CSF (aCSF). The aCSF containing 0.5 μCi/mL of [14C] sucrose and 2.5 μg/mL of Aβ40 was delivered to the lateral ventricle at a rate of 27 μL/min by a syringe pump (Harvard Compact Infusion Pump, Model 11 Plus). A 26G butterfly needle was inserted into the cisterna magna to collect the perfusion outflow. The cisternal outflow samples were collected at 5 min intervals throughout the perfusion time (60 min). The CSF volume was determined by measuring its weight assuming the CSF density was 1 g/mL. Core body temperature was maintained at 37°C during the surgery using a rectal probe feedback-controlled heating pad. CSF outflow samples were analyzed for Aβ40 concentrations by ELISA; the radioactivity of [14C] sucrose was counted on a Packard Tri-Carb 2900 TR Liquid Scintillation Analyzer (counting efficiency for [14C]: 94%) after the samples were mixed with Eco-lite cocktail.
3.9. Statistical analyses
All data are presented as mean + SD. Statistical analyses of the differences between control and Pb-exposed groups were carried out by a Student’s t-test. Comparisons of differences between the control and Pb-exposed groups within the 4- and 8-week time points were analyzed by one-way ANOVA with post hoc comparisons by the Dunnett’s test. All the statistical analyses were conducted using IBM SPSS for Windows (version 22.0). The differences between two means were considered significant if p values were equal or less than 0.05.
3. Results
3.1. Increased Pb concentration in blood and brain tissues in Pb-exposed animals
Following subchronic oral exposure to Pb at 14 or 27 mg Pb/kg, the blood Pb levels (BLL) were between 10–25 μg/dL. There was a dose-related increase in BLL, as the BLL in the high exposure group was significantly higher than that in the low exposure group (p<0.05). However, the duration of Pb exposure, i.e., 4-week vs. 8-week, did not affect BLL (Table 1). Pb concentrations in brain tissues also showed a dose-dependent increase in both 4-week and 8-week exposure groups. Consistent with our previous finding[22], subchronic Pb exposure significantly increased Pb concentrations in the choroid plexus (Table 1). By per gram of wet tissue basis, the choroid plexus contained Pb at nearly 80 fold higher levels compared to brain tissues.
Table 1.
Pb concentrations in blood and brain tissues following subchronic in vivo Pb exposure
| Weeks | Tissue | Control | Pb (14 mg/kg) | Pb (27 mg/kg) |
|---|---|---|---|---|
| 4 | Blood (μg/dL) | 0.29±0.27 | 15.82±5.39** | 24.76±6.25**,# |
| Choroid Plexus (μg/g) | 1.58±0.16 | 45.06±14.95** | 64.14±5.75**,# | |
| Brain Tissue (μg/g) | 0.02±0.001 | 0.27±0.021** | 0.48±0.012**,# | |
| 8 | Blood (μg/dL) | 0.32±0.14 | 10.21±3.29** | 23.12±2.73**,# |
| Choroid Plexus (μg/g) | 1.32±0.26 | 38.49±15.68** | 62.59±8.03**,# | |
| Brain Tissue (μg/g) | 0.02±0.01 | 0.29±0.14* | 0.54±0.23**,# |
Note: Rats received PbAc by oral gavage once daily for 4 or 8 weeks. Data represent mean ± S.D., n= 8.
p < 0.05,
p < 0.01, as compared to controls;
p < 0.05, as compared with the low dose group (14 mg/kg).
3.2. Increased Aβ40 levels in the CSF and the choroid plexus after in situ brain infusion of Aβ40 in Pb-exposed animals
ELISA analyses revealed that Aβ40 levels in the CSF were increased in a Pb-exposure dose-related fashion after in situ brain infusion of Aβ40 in the 4-week exposure group (Fig. 2A); the increases were 16.5% and 21.1% in the low and high exposure groups, respectively. Prolonged Pb exposure for 8 weeks did not further increase Aβ concentrations in the CSF (Fig. 2A).
Fig. 2. Uptake of Aβ40 in the CSF and choroid plexus following subchronic Pb exposure.
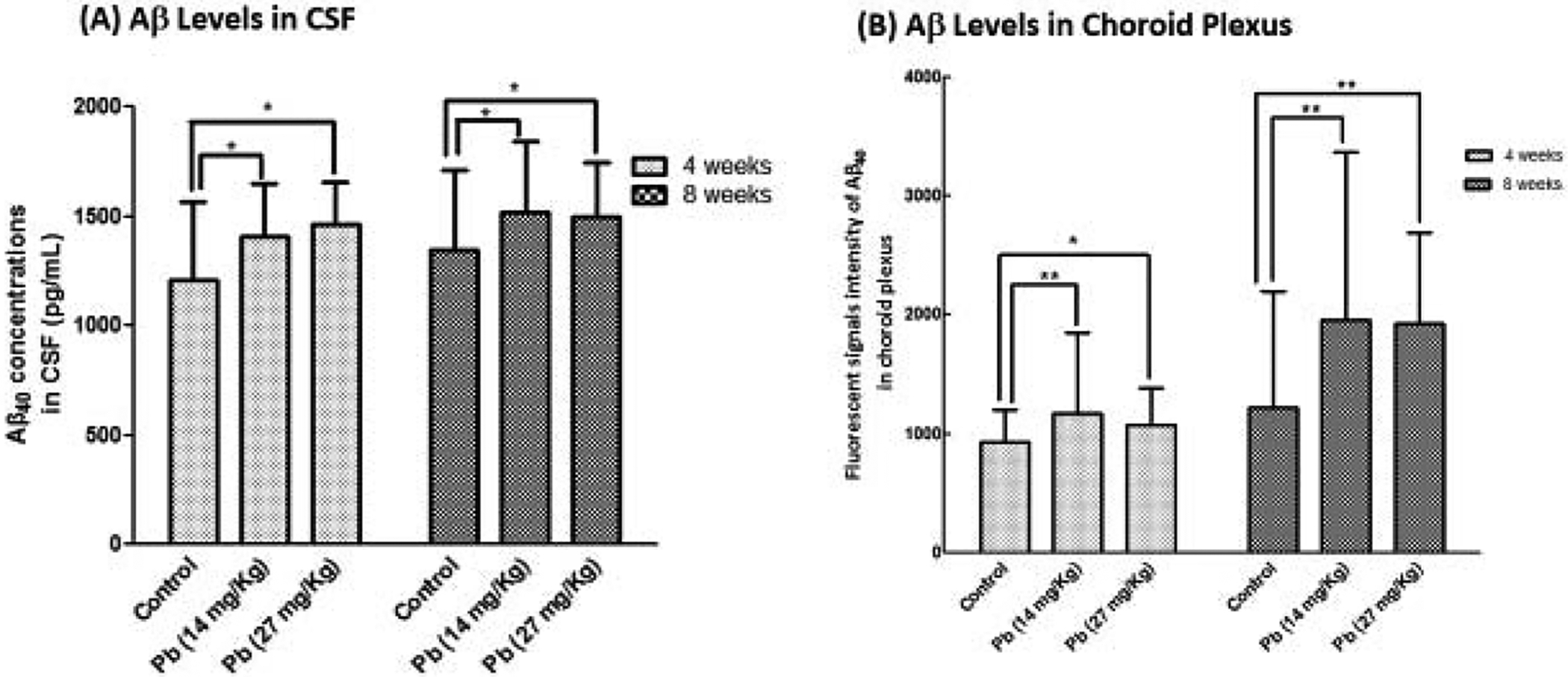
See Fig. 1A for exposure regimen. (A). Aβ40 levels in the CSF as determined by the ELISA following Pb treatment and Aβ40 infusion. (B). Aβ40 levels in the choroid plexus tissue as determined by fluorescent intensity following Pb treatment and Aβ40 perfusion. Data represent mean ± SD, n = 7; *: p<0.05, **: p<0.001.
Data from quantitation of the Aβ40 fluorescent intensity in the choroid plexus showed that Pb exposure for 4 weeks significantly increased Aβ in the choroid plexus by 26.1% (p<0.001) and 15.8% (p<0.05) in the low and high exposure groups, respectively. After 8-week exposure, Aβ signals in the choroid plexus were increased by 60.7% (p<0.001) and 58.0% (p<0.001) in the low and high exposure groups, respectively (Fig. 2B). These observations suggested that the choroid plexus was able to acquire Aβ40 from the blood and transport Aβ40 from blood to CSF, and Pb accumulated in the choroid plexus may facilitate Aβ uptake by the choroid plexus.
3.3. Increased expression of RAGE in the choroid plexus in Pb-exposed animals
RAGE is known to mediate the influx of Aβ from blood to brain at the BBB, while LRP1 does the reversal to efflux Aβ from brain to blood[32]. To determine Pb effect on RAGE and LRP1, ELISA was used to quantify the protein expression in the choroid plexus. Data in Fig. 3A demonstrate that after 4-weeks Pb exposure, the expression of RAGE was increased by 34.1% and 25.1% in the low and high Pb exposure groups (p<0.05), respectively. Prolonged exposure for 8 weeks did not further increase the RAGE expression (Fig. 3A). In contrast, Pb exposure for 4 weeks did not affect the expression of LRP1 in the choroid plexus (Fig. 3B). However, an 8-week treatment showed a 22–24% increase of LRP1 expression in the choroid plexus (p<0.05).
Fig. 3. Expression of RAGE and LRP1 in the choroid plexus following subchronic Pb exposure.
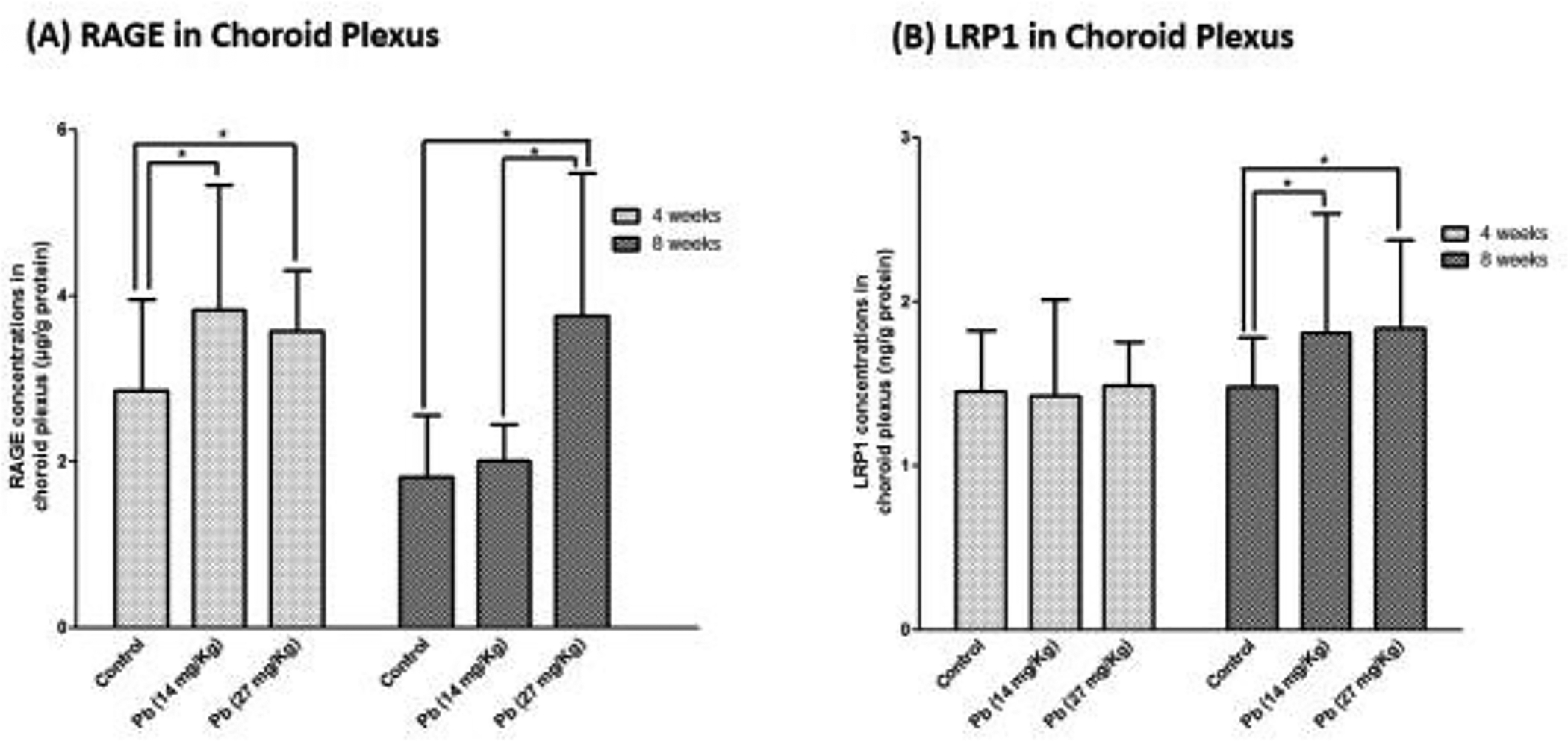
See Fig. 1A for exposure regimen. (A). RAGE expression in the choroid plexus following Pb treatment and Aβ40 infusion. (B). LRP1 expression in the choroid plexus following Pb treatment and Aβ40 infusion. Data represent mean ± SD, n = 7; *:p<0.05, **: p<0.001.
3.4. Intracellular trafficking of RAGE and LRP1 in choroid plexus in Pb-exposed animals
The choroid plexus separates two fluid compartments, with the apical microvilli in touch with the CSF and the basal lamina facing the blood. Thus, the status of intracellular trafficking of Aβ transporters, i.e., RAGE and LRP1, is essential to understanding of how the blood-CSF barrier regulates the Aβ homeostasis in the CSF. The confocal data in Fig. 4A showed that under the normal condition (i.e., control), RAGE signals were evenly distributed inside the choroidal epithelial cells around the nuclei. Subchronic in vivo Pb exposure for 4 weeks (without in situ brain infusion of Aβ) appeared to render the RAGE signals moving toward the choroidal microvilli; this became even more apparent in the high exposure group (Fig. 4A). Similarly, the LRP1 signals in the control animals were also evenly distributed around the nuclei in choroidal cells; in vivo Pb exposure without in situ Aβ infusion did not seem to affect the intracellular location of LRP1 (Fig. 4A).
Fig. 4. Intracellular trafficking of RAGE and LRP1 in choroid plexus following subchronic Pb exposure.
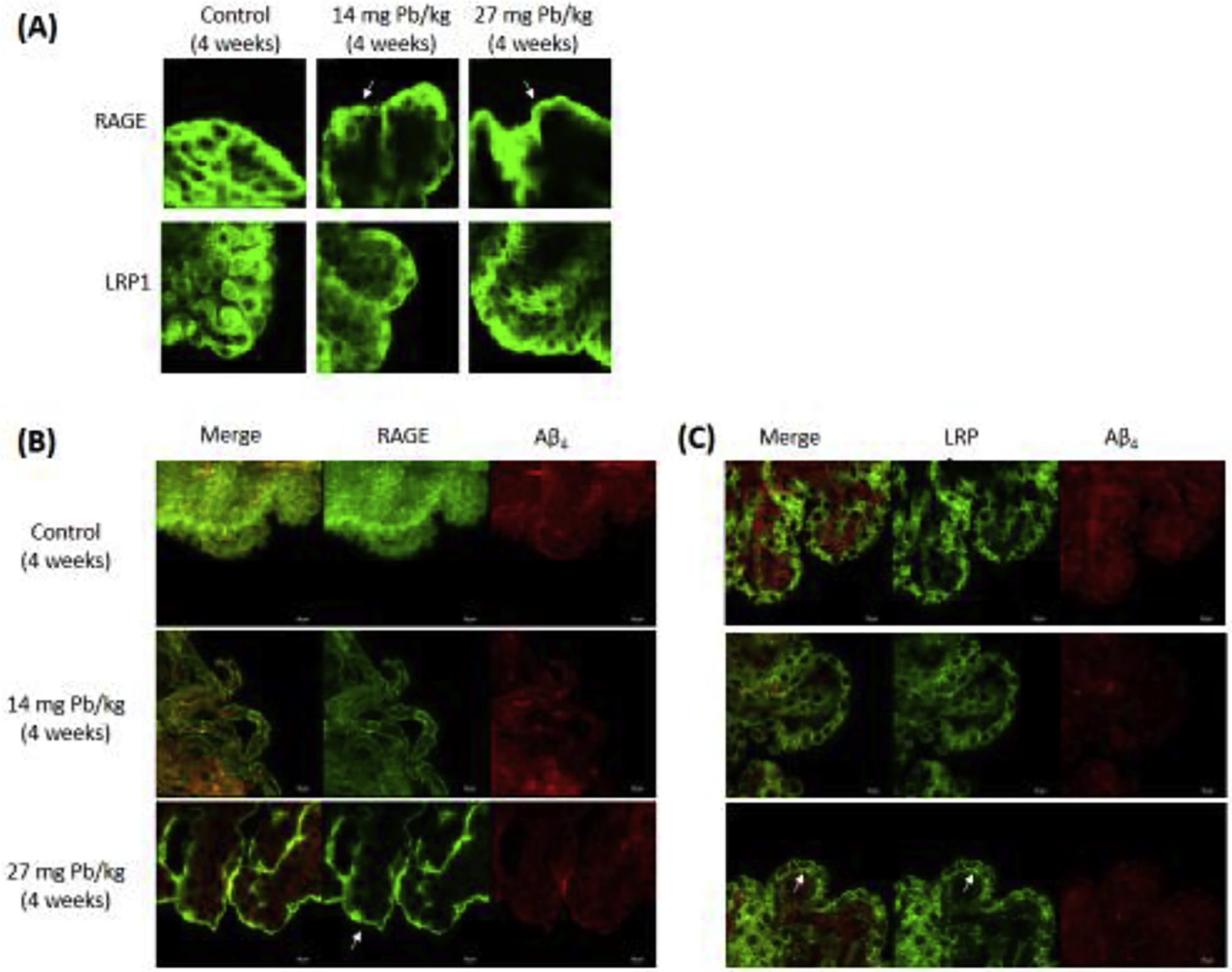
See Fig. 1A for exposure regimen. (A). Intracellular translocation of RAGE and LRP1 following Pb exposure for 4 weeks. Pb exposure moved RAGE signal from the cytoplasm toward the apical microvilli (arrow heads), while LRP1 signals were translocated to the basolateral membrane. (B). Double stain RAGE or LRP1 with Aβ40 following subchronic 4-week Pb exposure and Aβ40 infusion.
Subchronic Pb exposure followed by in situ brain infusion of Aβ prompted the subcellular translocation of RAGE from the cytoplasm toward the apical microvilli (Fig. 4B). The same treatment did not affect the LRP1 movement at the low dose; however, at the high dose with Aβ infusion, LRP1 signals apparently were concentrated in the basal lamina region facing the blood (Fig. 4C).
3.5. Increased expression of RAGE in plexus tissues following in vitro Pb incubation
To further verify the effect of Pb exposure on the expression of RAGE in the choroid plexus, the freshly isolated plexus tissues were incubated with Pb for 30 or 60 min, followed by incubation with 10 μM Aβ40. The immunofluorescent data did not show an evident subcellular relocation of RAGE following Pb exposure (Fig. 5A). By quantifying the intensity of RAGE fluorescent signals, incubation with 10 and 50 μM Pb for 30 min resulted in 21% (p<0.05) and 50% (p<0.001) increases in RAGE expression, respectively. Incubation with 10 and 50 μM Pb for 50 min led to an even larger expression of RAGE by 102% (p<0.001) and 93% (p<0.001), respectively (Fig. 5B).
Fig. 5. intracellular trafficking of RAGE in the choroid plexus following in vitro Pb incubation.
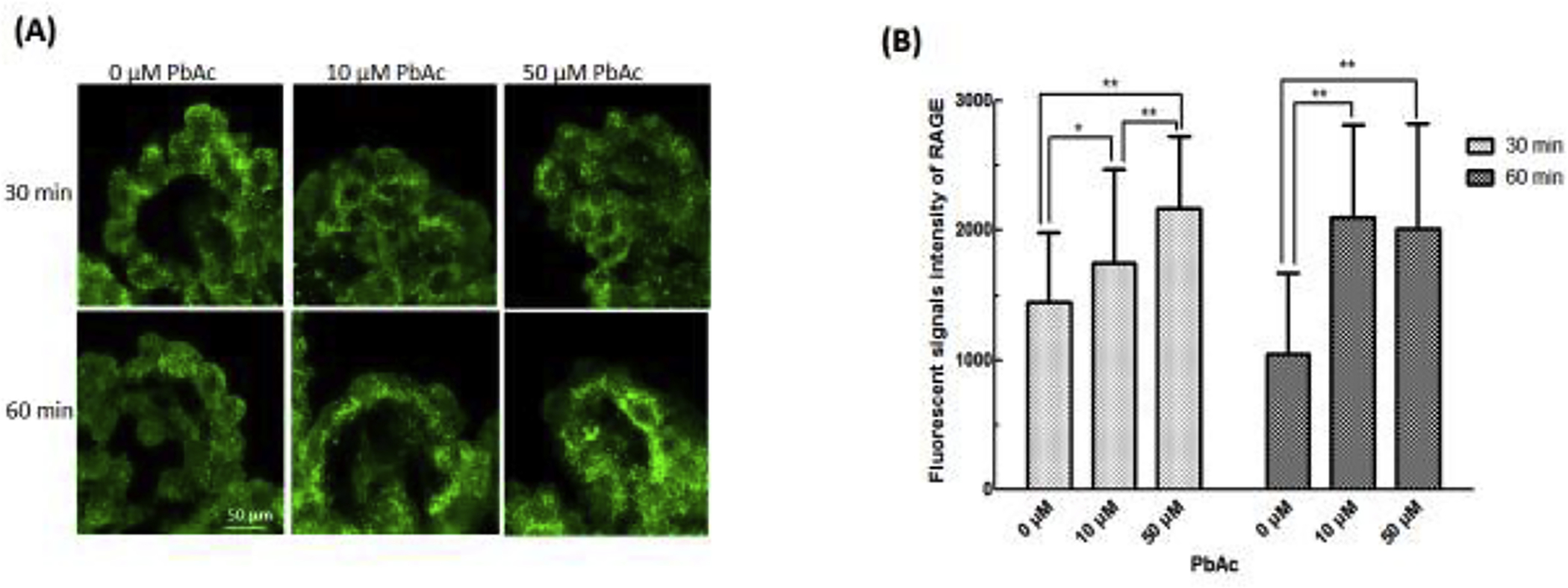
Freshly dissected rat choroid plexus tissues were incubated with 0, 10 and 50 μM PbAc in aCSF for 30 and 60 min, followed by incubation with Aβ40 (10 μM) in aCSF for 1 hr. (A). No subcellular relocation of RAGE was observed following PbAc and Aβ40 exposure. (B). Increased fluorescent signals intensity of RAGE after incubation with 10 μM or 50 μM Pb in aCSF for 30 or 60 min. Data represent mean ± SD, n = 7; *:p<0.05, **: p<0.001.
3.6. Reduced Aβ40 clearance by the BCB in Pb-exposed animals
The in situ VC perfusion technique allows determining the clearance of Aβ40 by the choroid plexus. With a slow perfusion of Aβ40 along with a space marker [14C]sucrose into the lateral ventricle and constant monitoring of the CSF outflow from the cisterna magna, the lost Aβ40 in the distance from the ventricle to cisterna magna, after correcting for the diffusion factor by [14C]sucrose, is considered to be removed by the choroid plexus’s efflux mechanism. Data from counting [14C]sucrose radioactivity showed that subchronic Pb exposure did not change the kinetic behavior of this space marker (Fig. 6A). In control animals, Aβ40 in the CSF outflow reached the steady-state concentration (Css) of 56 pg/mL. Subchronic Pb exposure, however, greatly increased Aβ40 concentrations in the CSF outflow; the Css was 821 pg/mL and 374 pg/mLin the low and high exposure groups, increased by 11.7 and 5.34 fold, respectively. A higher percentage of Aβ40 recovered from the CSF outflow implied a lower in vivo uptake of Aβ40 molecules by the choroid plexus. Thus, it seemed likely that in vivo Pb treatment significantly reduced the efflux of Aβ40 by the blood-brain barrier.
Fig. 6. Aβ40 clearance by the choroid plexus by the VC perfusion.
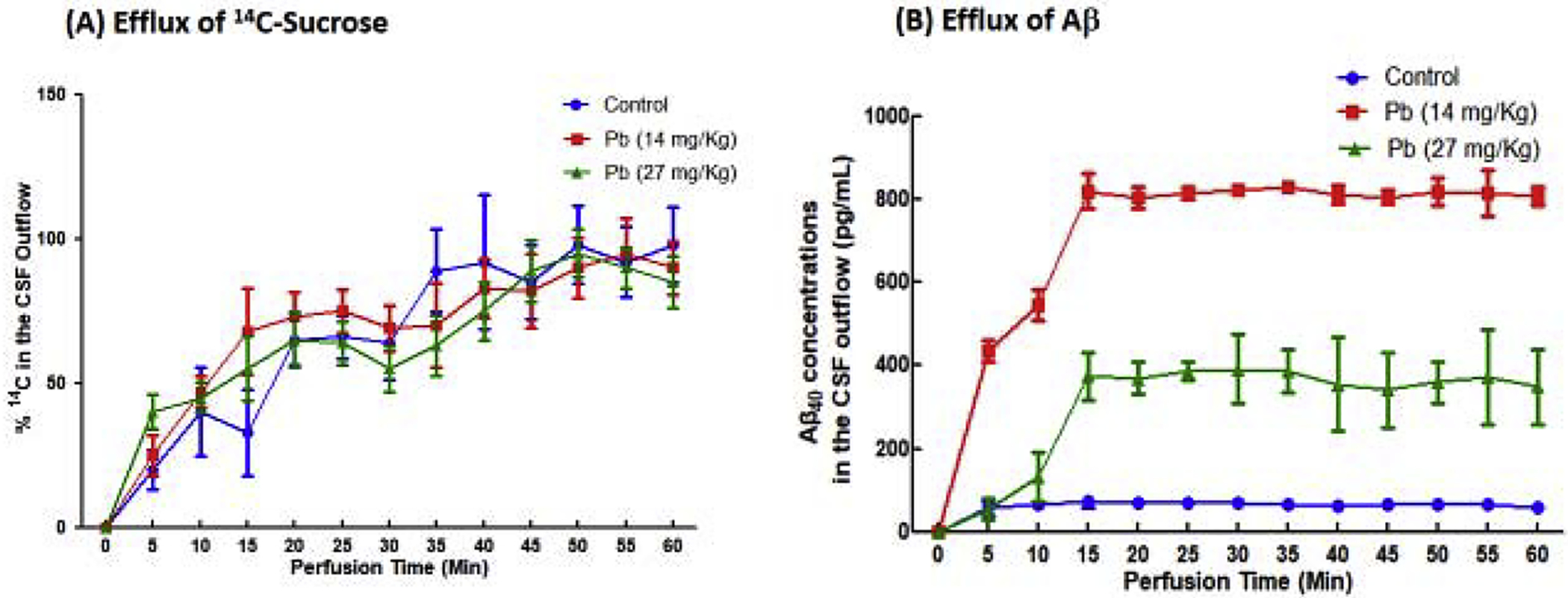
Aβ40 together with space marker [14C]sucrose was infused into the lateral ventricle; the CSF outflow was collected from the cisterna magna to determine the concentration both of [14C]sucrose and Aβ40. (A). % 14C radioactivity in the CSF outflow. (B). Aβ40 concentrations in the CSF outflow. Data represent means ± SD, n=6; *: p<0.05, as compared with the control.
4. Discussion
The data from current study indicated that: (1) by in situ brain infusion of Aβ40, the uptake of Aβ40 from blood to the choroid plexus and CSF was significantly increased in Pb-exposed animals as compared to controls; (2) the efflux of Aβ40 from the CSF to blood by the VC perfusion was significantly decreased; (3) subchronic Pb exposure significantly upregulated the expression of RAGE in the choroid plexus and promoted its intracellular trafficking from the cytoplasm toward the microvilli; and (4) subchronic Pb exposure did not significantly affect the expression LRP1; yet it translocated LRP1 to the basal lamina in choroidal epithelial cells.
Previous studies from our group have established that Pb exposure in Tg-SWDI APP transgenic mice results in elevated levels of Aβ in brain tissues and CSF; further analyses by synchrotron X-ray fluorescent microscopy reveals the presence of Pb ions in amyloid plaques [20]. Evidently, the increased Pb ions in brain parenchyma following Pb exposure participate directly in the formation of amyloid plaques. The data presented in this study support these previous findings, and further indicate that beyond the direct interaction with amyloid proteins, Pb exposure may increase the influx of Aβ from blood to the CSF (based on the in situ brain infusion data) and hamper the removal process of Aβ in the choroid plexus (based on the VC perfusion data). Thus, a combination of interactions of Pb with Aβ molecules and with their uptake and removal processes seems likely to potentiate the formation of amyloid plaques in brain in Pb-exposed animals.
The choroid plexus has long been known to accumulate Pb. Friedheim and his colleagues are among the first to report a significant age-related Pb accumulation in human choroid plexus based on the autopsy data from 51 New York residents (Friedheim et al., 1983). Another human study by Manton et al. (1984) further shows that the Pb concentration is 100 fold higher in human choroid plexus than in their brain cortex. Animal studies also support a dose-dependent and time-related accumulation of Pb in the choroid plexus (O’Tuama et al., 1976; Zheng et al., 1991, 2001). In the current study, the blood Pb concentration reached 10.2 μg/dL and 23.1 μg/dL following 8-week Pb oral gavages in low and high dose groups, respectively, which was at the similar blood Pb level (18 μg/dL) after 12-week chronic Pb exposure in drinking water (50 mg/mL) (Zheng et al., 2001). Similarly, Pb concentration in the choroid plexus in the current subchronic exposure dose regimen was greatly increased as compared to controls. A high accumulation of Pb in brain choroid plexus allows Pb to selectively interact with the cellular processes that govern Aβ transport between the blood and CSF. Our early studies also show that the choroid plexus has the capability to acquire Aβ from the CSF [6]. Acute high-dose exposure to Pb causes an increased uptake of Aβ from the CSF to the choroid plexus, and the effect is due partly to the suppressed expression of LRP1 by Pb exposure [24]. The results from our present study support the view that Pb accumulated in the choroid plexus interferes with the Aβ regulation by the blood-CSF barrier.
Brain Aβ is mainly regulated by the brain barrier systems including the blood-brain barrier between the blood and brain parenchyma and the blood-CSF barrier between the blood and CSF. By using in situ brain infusion technique, we demonstrated that Pb exposure significantly increased the CSF concentration of Aβ40. This increase could be due to a facilitated influx of Aβ from the blood to CSF and/or due to a hampered efflux (removal) of Aβ from the CSF which is mediated by the choroid plexus. Our previous studies have established that the choroid plexus is capable of transporting Aβ molecules between the blood and CSF in both directions; however, it favors the pathways from the CSF to blood more than those in the opposite direction (Crossgrove et al., 2006). The VC perfusion followed by assessing Aβ in the CSF efflux showed a great amount of Aβ remaining in the CSF efflux, suggesting an impaired Aβ removal by the choroid plexus. The observation that subchronic Pb exposure increased the Aβ accumulation in the choroid plexus from our in situ brain infusion study strengthens a critical role of the choroid plexus in transporting Aβ between the blood and CSF; it provides the direct evidence to support the mechanism that a detrimental effect on Aβ clearance by the blood-CSF barrier may underlie the Pb-induced formation of amyloid plaques[19].
How, then, did Pb in the choroid plexus interfere with Aβ transport by the blood-CSF barrier? In the blood–brain barrier, RAGE, a 35kD transmembrane receptor of the immunoglobulin superfamily [34, 35], is known to promote the influx of circulating Aβ from the blood to brain parenchyma [36]. Blocking RAGE uptake at the blood-brain barrier decreases Aβ concentrations in the brains of aged APP mice [37]. Literature data also suggest that the Aβ-RAGE interaction at the blood-brain barrier may accelerate the oxidative stress, leading to the inflammatory reaction in barrier cells; the damaged blood-brain barrier, in turn, facilitates more Aβ accumulation in the blood-brain barrier or brain parenchyma [38]. LRP1, on the other hand, has been shown to facilitate the efflux of Aβ in the blood-brain barrier from brain parenchyma to the blood[37]. Our data clearly showed that both RAGE and LRP1 were present in the blood-CSF barrier in the choroidal epithelial cells. Under normal conditions, both Aβ transporters seemed to be evenly distributed in cytoplasm. Subchronic Pb exposure, however, visibly moved RAGE toward the apical microvilli extending in the CSF, while it translocated LRP1 to the basolateral membrane facing the blood. The exact role of this intracellular trafficking of both transporters within choroidal epithelial cells in transporting Aβ molecules between the two fluid compartments remains unknown. Noticeably, subchronic Pb exposure significantly upregulated the expression of RAGE in the choroid plexus. Thus, it seems possible that as a consequence of Pb exposure, an increased RAGE may facilitate Aβ uptake with an ensuing Aβ accumulation in the choroid plexus, which, in turn, promotes the translocation of LRP1 so as to remove Aβ from the choroidal epithelial cells to the blood. This hypothetical mechanism, however, needs further experimental verification.
The current study has two major limitations. First, in addition to RAGE and LRP1, the choroid plexus possesses enzymes and proteins involving Aβ production and metabolism, such as amyloid precursor protein (APP), α- and β-secretase, insulin degrading enzyme (IDE), endothelin-converting enzyme-1 (ECE), and neprilysin[9]. Thus, Pb toxicity on these enzymes and proteins cannot be excluded. Second, through in situ brain infusion, Aβ may enter the CSF en route the blood-barrier barrier. Thus, an increased CSF Aβ may not be uniquely contributed by the blood-CSF barrier. A study to understand the action of Pb on the blood-brain barrier and the relationship to brain Aβ status has been planned in this lab.
In conclusion, our data presented in this report provide the first-hand evidence that subchronic Pb exposure in rats results in a substantial Pb accumulation in the choroid plexus, which, in turn, decreases the clearance of Aβ from the CSF to blood by the blood-CSF barrier in the choroid plexus, leading to an increased Aβ level in the CSF. Both RAGE and LRP1 in choroidal epithelial cells play a critical role in response to environmental insults such as Pb exposure, in regulating Aβ transport by the blood-CSF barrier. Pb interaction with RAGE and LRP1 in the choroid plexus may underlie Aβ accumulation in this tissue in brain ventricles.
Highlights:
Subchronic Pb exposure increases Aβ levels in the CSF and choroid plexus.
In vivo Pb exposure prompted subcellular translocation of RAGE in choroidal epithelia from the cytoplasm toward apical microvilli.
Subchronic Pb exposure reduces the clearance of Aβ from the CSF to blood.
Acknowledgement
Authors appreciate Dr. Gang Zhao for his assistance in animal handling during experiments. This study was supported in part by NIH/National Institute of Environmental Health Sciences Grants Number R56 ES008146 (WZ) and R01 ES027078 (WZ and YD) and the National Natural Science Foundation of China Grant Number 81701377 (XLS).
Abbreviations:
- LRP1
low density lipoprotein receptor protein-1
- RAGE
advanced glycation end-products
- Pb
lead
- CSF
cerebrospinal fluid
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement: none
References:
- [1].Nakabayashi J, Yoshimura M, Morishima-Kawashima M, Funato H, Miyakawa T, Yamazaki T,Ihara Y, Amyloid beta-protein (A beta) accumulation in the putamen and mammillary body during aging and in Alzheimer disease, Journal of neuropathology and experimental neurology. 57 (1998) 343–52. https://10.1097/00005072-199804000-00007. [DOI] [PubMed] [Google Scholar]
- [2].Bugiani O, Giaccone G, Frangione B, Ghetti B,Tagliavini F, Alzheimer patients: preamyloid deposits are more widely distributed than senile plaques throughout the central nervous system, Neuroscience letters. 103 (1989) 263–8. https://10.1016/0304-3940(89)90110-9. [DOI] [PubMed] [Google Scholar]
- [3].Hernandez F, Gomez de Barreda E, Fuster-Matanzo A, Lucas JJ,Avila J, GSK3: a possible link between beta amyloid peptide and tau protein, Experimental neurology. 223 (2010) 322–5. https://10.1016/j.expneurol.2009.09.011. [DOI] [PubMed] [Google Scholar]
- [4].Zheng W, Aschner M,Ghersi-Egea JF, Brain barrier systems: a new frontier in metal neurotoxicological research, Toxicology and applied pharmacology. 192 (2003) 1–11. https://10.1016/s0041-008x(03)00251-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Brody DL, Magnoni S, Schwetye KE, Spinner ML, Esparza TJ, Stocchetti N, Zipfel GJ,Holtzman DM, Amyloid-beta dynamics correlate with neurological status in the injured human brain, Science. 321 (2008) 1221–4. https://10.1126/science.1161591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Crossgrove JS, Li GJ,Zheng W, The choroid plexus removes beta-amyloid from brain cerebrospinal fluid, Experimental biology and medicine. 230 (2005) 771–6. https://10.1177/153537020523001011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Monro OR, Mackic JB, Yamada S, Segal MB, Ghiso J, Maurer C, Calero M, Frangione B,Zlokovic BV, Substitution at codon 22 reduces clearance of Alzheimer’s amyloid-beta peptide from the cerebrospinal fluid and prevents its transport from the central nervous system into blood, Neurobiology of aging. 23 (2002) 405–12. [DOI] [PubMed] [Google Scholar]
- [8].Serot JM, Bene MC,Faure GC, Choroid plexus, aging of the brain, and Alzheimer’s disease, Frontiers in bioscience : a journal and virtual library. 8 (2003) s515–21. [DOI] [PubMed] [Google Scholar]
- [9].Crossgrove JS, Smith EL,Zheng W, Macromolecules involved in production and metabolism of beta-amyloid at the brain barriers, Brain research. 1138 (2007) 187–95. https://10.1016/j.brainres.2006.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Kalaria RN, Premkumar DR, Pax AB, Cohen DL,Lieberburg I, Production and increased detection of amyloid beta protein and amyloidogenic fragments in brain microvessels, meningeal vessels and choroid plexus in Alzheimer’s disease, Brain research. Molecular brain research. 35 (1996) 58–68. [DOI] [PubMed] [Google Scholar]
- [11].Kanai M, Matsubara E, Isoe K, Urakami K, Nakashima K, Arai H, Sasaki H, Abe K, Iwatsubo T, Kosaka T, Watanabe M, Tomidokoro Y, Shizuka M, Mizushima K, Nakamura T, Igeta Y, Ikeda Y, Amari M, Kawarabayashi T, Ishiguro K, Harigaya Y, Wakabayashi K, Okamoto K, Hirai S,Shoji M, Longitudinal study of cerebrospinal fluid levels of tau, A beta1–40, and A beta1–42(43) in Alzheimer’s disease: a study in Japan, Annals of neurology. 44 (1998) 17–26. https://10.1002/ana.410440108. [DOI] [PubMed] [Google Scholar]
- [12].Stewart WF, Schwartz BS, Davatzikos C, Shen D, Liu D, Wu X, Todd AC, Shi W, Bassett S,Youssem D, Past adult lead exposure is linked to neurodegeneration measured by brain MRI, Neurology. 66 (2006) 1476–84. https://10.1212/01.wnl.0000216138.69777.15. [DOI] [PubMed] [Google Scholar]
- [13].Shih RA, Hu H, Weisskopf MG,Schwartz BS, Cumulative lead dose and cognitive function in adults: a review of studies that measured both blood lead and bone lead, Environmental health perspectives. 115 (2007) 483–92. https://10.1289/ehp.9786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Hu H, Shih R, Rothenberg S,Schwartz BS, The epidemiology of lead toxicity in adults: measuring dose and consideration of other methodologic issues, Environmental health perspectives. 115 (2007) 455–62. https://10.1289/ehp.9783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Jiang YM, Long LL, Zhu XY, Zheng H, Fu X, Ou SY, Wei DL, Zhou HL,Zheng W, Evidence for altered hippocampal volume and brain metabolites in workers occupationally exposed to lead: a study by magnetic resonance imaging and (1)H magnetic resonance spectroscopy, Toxicology letters. 181 (2008) 118–25. https://10.1016/j.toxlet.2008.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Wu J, Basha MR,Zawia NH, The environment, epigenetics and amyloidogenesis, Journal of molecular neuroscience : MN. 34 (2008) 1–7. https://10.1007/s12031-007-0009-4. [DOI] [PubMed] [Google Scholar]
- [17].Basha MR, Wei W, Bakheet SA, Benitez N, Siddiqi HK, Ge YW, Lahiri DK,Zawia NH, The fetal basis of amyloidogenesis: exposure to lead and latent overexpression of amyloid precursor protein and beta-amyloid in the aging brain, The Journal of neuroscience : the official journal of the Society for Neuroscience. 25 (2005) 823–9. https://10.1523/JNEUROSCI.4335-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Bihaqi SW, Early life exposure to lead (Pb) and changes in DNA methylation: relevance to Alzheimer’s disease, Reviews on environmental health. 34 (2019) 187–195. https://10.1515/reveh-2018-0076. [DOI] [PubMed] [Google Scholar]
- [19].Gu H, Wei X, Monnot AD, Fontanilla CV, Behl M, Farlow MR, Zheng W,Du Y, Lead exposure increases levels of beta-amyloid in the brain and CSF and inhibits LRP1 expression in APP transgenic mice, Neuroscience letters. 490 (2011) 16–20. https://10.1016/j.neulet.2010.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Gu H, Robison G, Hong L, Barrea R, Wei X, Farlow MR, Pushkar YN, Du Y,Zheng W, Increased beta-amyloid deposition in Tg-SWDI transgenic mouse brain following in vivo lead exposure, Toxicology letters. 213 (2012) 211–9. https://10.1016/j.toxlet.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Friedheim E, Corvi C, Graziano J, Donnelli T,Breslin D, Choroid plexus as a protective sink for heavy metals?, Lancet. 1 (1983) 981–2. https://10.1016/s0140-6736(83)92099-8. [DOI] [PubMed] [Google Scholar]
- [22].Zheng W, Perry DF, Nelson DL,Aposhian HV, Choroid plexus protects cerebrospinal fluid against toxic metals, FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 5 (1991) 2188–93. https://10.1096/fasebj.5.8.1850706. [DOI] [PubMed] [Google Scholar]
- [23].Zheng W, Blaner WS,Zhao Q, Inhibition by lead of production and secretion of transthyretin in the choroid plexus: its relation to thyroxine transport at blood-CSF barrier, Toxicology and applied pharmacology. 155 (1999) 24–31. https://10.1006/taap.1998.8611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Behl M, Zhang Y, Monnot AD, Jiang W,Zheng W, Increased beta-amyloid levels in the choroid plexus following lead exposure and the involvement of low-density lipoprotein receptor protein-1, Toxicology and applied pharmacology. 240 (2009) 245–54. https://10.1016/j.taap.2009.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Osgood D, Miller MC, Messier AA, Gonzalez L,Silverberg GD, Aging alters mRNA expression of amyloid transporter genes at the blood-brain barrier, Neurobiology of aging. 57 (2017) 178–185. https://10.1016/j.neurobiolaging.2017.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Donahue JE, Flaherty SL, Johanson CE, Duncan JA 3rd, Silverberg GD, Miller MC, Tavares R, Yang W, Wu Q, Sabo E, Hovanesian V,Stopa EG, RAGE LRP -1, and amyloid-beta protein in Alzheimer’s disease, Acta neuropathologica. 112 (2006) 405–15. https://10.1007/s00401-006-0115-3. [DOI] [PubMed] [Google Scholar]
- [27].Pascale CL, Miller MC, Chiu C, Boylan M, Caralopoulos IN, Gonzalez L, Johanson CE,Silverberg GD, Amyloid-beta transporter expression at the blood-CSF barrier is age-dependent, Fluids and barriers of the CNS. 8 (2011) 21. https://10.1186/2045-8118-8-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Zheng W, Shen H, Blaner WS, Zhao Q, Ren X,Graziano JH, Chronic lead exposure alters transthyretin concentration in rat cerebrospinal fluid: the role of the choroid plexus, Toxicology and applied pharmacology. 139 (1996) 445–50. https://10.1006/taap.1996.0186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Fernandez F, Hilligoss, An improved graphite furnace method for the determination of lead in blood using matrix modification and the L’ vov platform, Atomic Spectr. 3 (1982) 130–31. [Google Scholar]
- [30].Fu X, Zhang Y, Jiang W, Monnot AD, Bates CA,Zheng W, Regulation of copper transport crossing brain barrier systems by Cu-ATPases: effect of manganese exposure, Toxicological sciences : an official journal of the Society of Toxicology. 139 (2014) 432–51. https://10.1093/toxsci/kfu048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Monnot AD, Behl M, Ho S,Zheng W, Regulation of brain copper homeostasis by the brain barrier systems: effects of Fe-overload and Fe-deficiency, Toxicology and applied pharmacology. 256 (2011) 249–57. https://10.1016/j.taap.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Deane R, Zheng W,Zlokovic BV, Brain capillary endothelium and choroid plexus epithelium regulate transport of transferrin-bound and free iron into the rat brain, Journal of neurochemistry. 88 (2004) 813–20. https://10.1046/j.1471-4159.2003.02221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Wang X, Li GJ,Zheng W, Efflux of iron from the cerebrospinal fluid to the blood at the blood-CSF barrier: effect of manganese exposure, Experimental biology and medicine. 233 (2008) 1561–71. https://10.3181/0803-RM-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Wautier MP, Tessier FJ,Wautier JL, [Advanced glycation end products: A risk factor for human health], Annales pharmaceutiques francaises. 72 (2014) 400–8. https://10.1016/j.pharma.2014.05.002. [DOI] [PubMed] [Google Scholar]
- [35].Yu SL, Wong CK, Szeto CC, Li EK, Cai Z,Tam LS, Members of the receptor for advanced glycation end products axis as potential therapeutic targets in patients with lupus nephritis, Lupus. 24 (2015) 675–86. https://10.1177/0961203314559631. [DOI] [PubMed] [Google Scholar]
- [36].Deane R, Du Yan S, Submamaryan RK, LaRue B, Jovanovic S, Hogg E, Welch D, Manness L, Lin C, Yu J, Zhu H, Ghiso J, Frangione B, Stern A, Schmidt AM, Armstrong DL, Arnold B, Liliensiek B, Nawroth P, Hofman F, Kindy M, Stern D,Zlokovic B, RAGE mediates amyloid-beta peptide transport across the blood-brain barrier and accumulation in brain, Nature medicine. 9 (2003) 907–13. https://10.1038/nm890. [DOI] [PubMed] [Google Scholar]
- [37].Deane R, Singh I, Sagare AP, Bell RD, Ross NT, LaRue B, Love R, Perry S, Paquette N, Deane RJ, Thiyagarajan M, Zarcone T, Fritz G, Friedman AE, Miller BL,Zlokovic BV, A multimodal RAGE-specific inhibitor reduces amyloid beta-mediated brain disorder in a mouse model of Alzheimer disease, The Journal of clinical investigation. 122 (2012) 1377–92. https://10.1172/JCI58642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Schmidt AM, Sahagan B, Nelson RB, Selmer J, Rothlein R,Bell JM, The role of RAGE in amyloid-beta peptide-mediated pathology in Alzheimer’s disease, Current opinion in investigational drugs. 10 (2009) 672–80. [PubMed] [Google Scholar]


