Abstract
Tract-based spatial statistics (TBSS) of diffusion tensor imaging (DTI) studies have consistently shown diminished white matter (WM) integrity for individuals with cocaine use disorder (CUD). The present study used seed-based d mapping (SDM) to determine the extent to which a systematic difference in the WM integrity of cocaine users may exist (as compared to healthy controls). Articles from 2006 (when TBSS was first developed) to present were reviewed, with eight selected for inclusion. Meta-analysis found lower fractional anisotropy (FA) in the genu of the corpus callosum for cocaine users, with a small-to-moderate peak effect size (Hedge’s g = −0.331). Sensitivity analyses mostly supported the robustness of the obtained difference. Differences detected at exploratory thresholds for significance suggested insult to WM integrity extending beyond the corpus callosum. The present results compliment a previous ROI-based meta-analysis of DTI studies in individuals with CUD. These findings have significant implications for the potential role of neuroprotective agents in the treatment of CUD and merit additional iteration as more studies accrue in the literature.
Keywords: Cocaine use disorder, Diffusion tensor imaging, Fractional anisotropy, Meta-analysis, Seed-based d mapping, Tract-based spatial statistics
INTRODUCTION
Approximately 5.0 million adults in the United States report using illicit psychostimulants in the preceding year,1 with long-term trends indicating little progress in reducing prevalence rates. Of major concern currently is evidence indicating a re-emergence of cocaine and methamphetamine use in the US, coinciding with the existing opioid epidemic. The notable rise in drug overdoses has been referred to as the fourth wave of the epidemic,2 underscoring the lethal consequences that arise from co-use of stimulant and opioid drugs.
Cocaine and methamphetamine have long been known to have the potential to induce neurotoxicity.3 While exact mechanisms are complex and not fully understood, psychostimulants are thought to primarily exert their toxic effects by enhancing monoaminergic neurotransmission that consequently triggers alterations in mitochondrial function, oxidative stress, excitotoxicity and hyperthermia (see Gonçalves et al4 for a review). More recent studies have shown that cocaine and methamphetamine can trigger neuroinflammatory processes characterized by activation of the immune response system, which further contribute to blood brain barrier dysfunction and increased levels of peripheral toxins.5
White matter (WM) microstructure in the brain is vulnerable to the neurotoxic effects of chronic stimulant use. Diffusion tensor imaging (DTI) allows for evaluation of WM tracts in the brain. Abnormalities can be detected by quantifying the diffusion properties of tissue water. Common metrics include axial diffusivity (AD; longitudinal), radial diffusivity (RD), mean diffusivity (MD), and fractional anisotropy (FA) – a summary of eigenvalues of the diffusivity metrics that may represent density, diameter, and/or myelination of WM fibers.6 Lower FA values may therefore indicate lower integrity of WM, reflecting compromised axonal microstructure and/or alterations in myelin.
Recently, we systematically reviewed findings from DTI studies that focused on region-of-interest (ROI)-based analysis of WM integrity in stimulant (primarily cocaine) users compared to healthy controls.7 Our meta-analysis, based on 11 studies, found significantly lower FA values in subjects with stimulant use disorders: across all regions, there was a small-to-moderate overall group difference Hedge’s g = −0.37 (where Hedge’s g provides an corrected index of effect size similar to Cohen’s d). Subgroup analysis based on specific ROIs revealed a significant overall effect for the combined regions of the corpus callosum (Hedge’s g = −0.27), consistent with research regarding the high density of WM in this area of the brain.8
Tract-based spatial statistics (TBSS) provides improved sensitivity and objectivity for DTI analysis of group-level data via non-linear registration techniques and use of a single, averaged FA skeleton.9 Our previous meta-analysis7 was limited to examining specific regions of interest; TBSS enables an unbiased whole-brain voxel-wise mapping of WM and offers an estimate of whole-brain WM diffusivity metrics (i.e., AD, RD, and FA) across groups of multiple subjects. The number of studies using TBSS-based methodology to measure WM in psychostimulant users is small, relative to ROI-based studies. Differences in findings between the two methods might be expected and, in fact, have been reported in meta-analyses for heroin dependence,10,11 major depressive disorder,12 and bipolar disorder.13 To date, a TBSS meta-analysis of WM integrity in stimulant use disorders has not been published. Such an analysis could provide a more comprehensive and accurate characterization of WM tract integrity associated with chronic stimulant use.
Building on prior work,7 the goal of the present study was to investigate WM integrity in individuals with stimulant use disorders in comparison to healthy controls (HC) utilizing results from whole-brain DTI. A seed-based d-mapping (SDM) technique was used to conduct a meta-analysis of studies employing TBSS analysis of DTI imaging data.14 The focus of the present study necessarily narrowed to examine CUD, as opposed to the more broad investigation into stimulant use disorders, due to a paucity of TBSS studies of other stimulants. Similarly, a lack of reported TBSS findings on the AD, RD, MD diffusivity metrics in the studies examined here required an exclusive focus on FA. The present study examined differences in overall FA between HC and individuals with CUD, with a secondary analysis investigating the potential influence of chronicity of cocaine use.
MATERIALS AND METHODS
Eligibility criteria
Inclusion and exclusion criteria were determined by the authors with the goal of identifying matched groups of primary stimulant use in comparison to HC participants. Criteria were created in order to capture a broad range of WM imaging studies, and included the following: (1) diagnosis of stimulant use/dependence based on DSM-IV, DSM-5, ICD-9, or ICD-10 criteria, (2) simulant use disorder identified as the primary mental health disorder, (3) inclusion of one more major white matter tract (including whole brain), (4) experimental or quasi-experimental design with a healthy control comparison, (5) fractional anisotropy (FA) reported outcome, (6) full-length research article (i.e., no conference proceedings) published in a peer-reviewed journal, (7) English language text, and (8) adult human participants. The WM tract criterion (3) was branched subsequently into a group of ROI-based analysis and TBSS studies. Results from a meta-analysis on the former group were reported in a previous manuscript.7 The latter group was used for the present analysis.
Search and information sources
Articles were sourced using Pubmed, Embase, and PSYCHINFO databases. Database deduplication and article review were conducted in accordance with PRISMA recommendations for systematic review and meta-analysis.15 Abstracts were reviewed by two independent reviewers (authors HS and CB) based on inclusion/exclusion criteria, and disagreements were discussed collaboratively to derive the final pool of articles. Abstracts were reviewed using the Rayyan web application for systemic reviews.16 In cases of missing or incomplete available data,17 authors were contacted for additional information.
Selection
Studies were selected based on inclusion and exclusion criteria following abstract and article review. Though search terms were created in order to capture stimulant use for studies of primary CUD and methamphetamine use disorder (MUD) (see full search terms included in Appendix A), only one study (of those in the final group that included TBSS outcomes) evaluated MUD alone.18 This study was necessarily evaluated via exploratory sensitivity analysis in the present manuscript due to statistical complications from comparing two distinct methamphetamine-using groups to one HC group without adequate control for correlated observations (i.e., there are no so-called “random effects” available in SDM). This exploratory sensitivity analysis is provided in the Supplemental material. One other study assessed mixed stimulant use (cocaine and amphetamines); however, insufficient details were provided in the manuscript.17 All other studies (n = 8) had a stimulant use group that included CUD alone. Thus, the current manuscript focuses on those studies that specifically evaluated CUD versus HC.
Data analytic strategy
Meta-analysis was performed via Seed-based d Mapping with Permutation of Subject Images (SDM-PSI version 6.11)19 to investigate differences in white matter integrity between CUD (including abstinent, currently dependent, and poly-substance users) and HC across 8 DTI studies of FA analyzed via TBSS. Existing literature describes validation of the SDM methodology.14,20,21 Briefly, SDM incorporates data across studies to reproduce and quantitatively synthesize coordinate mapping of the brain. Reported coordinates are registered to the SDM template and t-values are converted to effect sizes. Data extracted from the 8 studies included sample sizes (for both CUD and HC groups); magnitude and valence of t-values (whereby positive valence determines higher values for the cocaine using sample and vice-versa); software package of origin (FSL, SPM, or other); X, Y, and Z coordinates (reported in Talairach or MNI space), and thresholds of significance for reported t-values. Where studies reported no significant difference in CUD and HC groups (i.e., Kelly et al22) null results were entered into the model. Meta-analytic mean differences were calculated and reported as Hedges’ g, a measure of standardized mean difference appropriate for small sample sizes.23 Peak coordinates were overlaid on the MRIcron template24 for graphical depiction. Additional analyses examined potential meta-regression effects of chronicity of cocaine use and minimum duration of abstinence prior to imaging. Further, in a hypothesis-generating fashion, follow-up exploratory analyses evaluated different thresholds of significance for differences between the CUD and HC groups. These exploratory analyses emphasized the potential value in knowledge discovery and avoiding Type II error. However, consequently, any differences found beyond the a priori significance threshold must be considered as preliminary.
Methodological quality and risk of bias
Assessment of methodological quality at the individual study level was performed using a rating form adapted from prior studies7,25 with 15 items across six domains. Domains evaluated included: study design (3 items; e.g., “Are the groups and recruitment clearly defined?”), comparability of groups (2 items; e.g., “Were the inclusion and exclusion criteria explicitly defined for all groups?”), participant characteristics (2 items; e.g., “Was comorbidity between stimulant use and use of other substances reported?”), measurement of stimulant use disorder (3 items; e.g., “Was chronicity of drug use reported?”), DTI methodology (3 items; e.g., “Did the authors provide details regarding timing of scan with respect to last drug use?”), and statistical analyses (2 items; e.g., “Were known confounding factors accounted for in the design or analysis?”). Two independent ratings of these domains compared author responses across all 15 items, with each scored dichotomously (Y/N).
Additional analyses examined study bias at the analysis-level using a jackknife sensitivity analysis for the main outcome20, in which the analysis was run multiple times, each with one study removed to establish reproducibility of the results (i.e., a leave-one-out permutation procedure). Finally, with the caveat that statistical tests of publication biases are flawed in meta-analyses with sample sizes below 10, we have provided results from the Egger test and a funnel plot for the primary meta-analysis.
RESULTS
Study selection
Eight studies, detailed in Table 1, were included in the final analysis from the initial pool of 113 studies across databases. The final analysis included 224 participants in the CUD group and 233 HC participants, for a total of N=457 participants across all included studies. Full information for the exclusion of full-text articles is detailed in the PRISMA flow diagram for study selection (Figure 1). Initially, twelve studies met criteria for inclusion in the present analysis; however, as noted above, four of these were determined to be incompatible, leaving eight.
Table 1.
Study Characteristics.
| First Author | Year | n CUD (HC) | Mean Age CUD (HC) | n M/F (HC) | Grouping Medical Comorbidity | Lifetime Drug Use History | Min. Abstinence Time (Days) | Mean Years of Use | Brand | Direction |
|---|---|---|---|---|---|---|---|---|---|---|
| Bell* | 2011 | 43 (43) | 37.4 (38.8) | 41/2 (36/7) | NONE | CUD: ANY HC: NONE | 7 | 9.3 | Siemens | 8 |
| Kelly | 2011 | 25 (24) | 35.0 (35.1) | 23/2 (20/4) | NONE | CUD: A/C HC: NONE | 14 | 11.4 | Siemens | 64 |
| Lane | 2010 | 15 (18) | 38.5 (35.2) | 10/5 (9/9) | NONE | CUD: ANY HC: NONE | 1 | 10.1 | Phillips | 21 |
| Lim‡ | 2008 | 21 (21) | 42.5 (40.9) | 11/10 11/10 | NONE | CUD: NONE HC: NONE | 4 | 18.9 | Siemens | 12 |
| van Son | 2016 | 37 (38) | 29.5 (31.1) | 37/0 38/0 | NONE | CUD: ANY HC: NONE | 15 | 4.1 | Phillips | 32 |
| Vaquero | 2017 | 30 (30) | 32.9 (32.3) | 24/6 24/6 | NONE | CUD: NO O HC: NO O | 1 | 12.9 | Siemens | 12 |
| Wakim | 2017 | 15 (21) | 50.3 (36.3) | 0/15 (0/21) | CUD: HIV+ HC: NONE | CUD: ANY HC: NONE | 60 | 11.9 | Phillips | 32 |
| Yip | 2017 | 38 (38) | 42.5 (38.1) | 25/13 28/10 | NONE | CUD: ANY HC: NONE | N/I | N/I | Siemens | 32 |
Notes. HC: Controls; CUD: Cocaine Use Disorder; M/F: Male/Female; ABS: Abstinent; CUR: Current Users; N/I: No Information; Drug Use: A: Alcohol; C: Cannabis; H: Heroin; O: Opiates
denotes studies that used a 1.5T MRI (rather than 3T);
indicates simultaneous use of any substance except nicotine;
denotes studies that were previously included in an ROI-based meta-analysis.
Figure 1. PRISMA flow diagram.
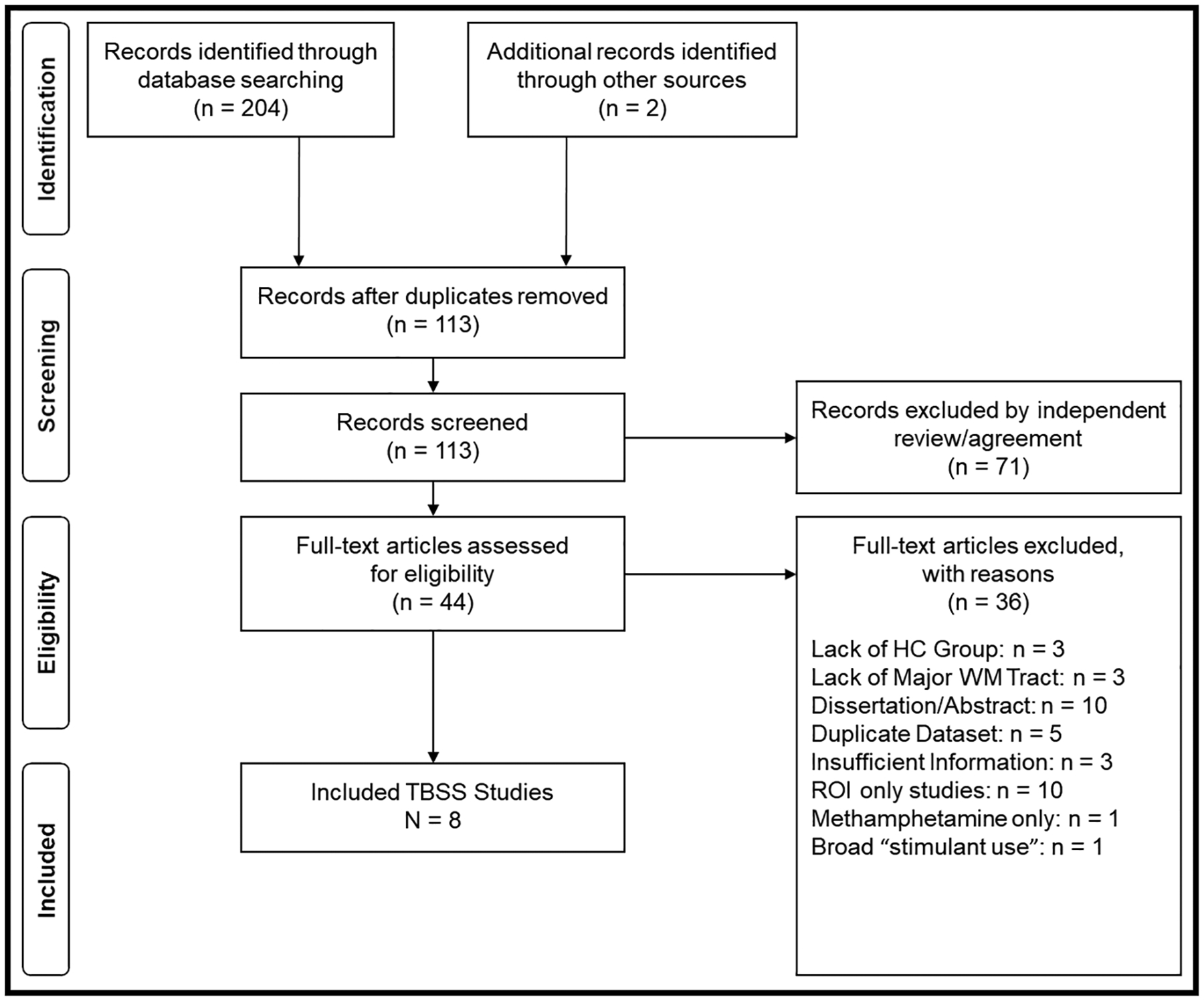
PRISMA flow chart for identifying TBSS studies.
Study characteristics and methodological quality
Results of the methodological quality review indicated acceptable study quality. The range of ratings across studies was restricted due to a ceiling effect (maximum = 15). The range of scores suggested high methodological quality (Rater 1: 12–15; Rater 2: 11–15), with seven studies receiving scores in the top quartile (>11.75) for both raters.
Differences in FA in CO and HC groups
SDM-PSI meta-analysis including all eight studies, with a primary one-tailed threshold p = 0.05, found lower FA in individuals with CUD (compared to HC) in the corpus callosum (highest peak SDM-Z = −2.588, p = 0.0048). The cluster breakdown indicated that all 97 differential voxels across two separate clusters were in the corpus callosum. Details of the local peaks are provided in Table 2. Figure 2 provides a graphical illustration of the lower FA in the corpus callosum for the CUD participants. Extracting masked values for the primary negative peak indicated a small to moderate pooled effect size Hedge’s g = −0.331 and low heterogeneity I2 = 11.47.
Table 2.
Local peaks for observed differences in the corpus callosum.
| Cluster | MNI Coordinates | SDM-Z | p | ||
|---|---|---|---|---|---|
| x | y | z | |||
| Cluster 1: Corpus Callosum (83 Voxels) | −6 | 24 | 12 | −2.588 | 0.0048 |
| −6 | 16 | 18 | −2.586 | 0.0049 | |
| −6 | 20 | 16 | −2.559 | 0.0053 | |
| −4 | 6 | 22 | −2.012 | 0.0221 | |
| 4 | 18 | 16 | −1.950 | 0.0256 | |
| −8 | 6 | 24 | −1.911 | 0.0280 | |
| 6 | 22 | 14 | −1.811 | 0.0351 | |
| 2 | 12 | 20 | −1.696 | 0.0449 | |
| Cluster 2: Corpus Callosum (14 Voxels) | 18 | 32 | 14 | −1.800 | 0.0360 |
| 18 | 34 | 10 | −1.772 | 0.0382 | |
| 18 | 36 | 6 | −1.698 | 0.0447 | |
Figure 2. Lower FA in the corpus callosum of individuals with cocaine use disorder (relative to healthy controls).

Meta-analysis found lower FA across studies in the genu of the corpus callosum for individuals with CUD relative to HC, with a maximum peak difference at MNI coordinates x = −6, y = 24, z = 12.
The leave-one-out Jackknife permutation sensitivity analysis supported the overall pattern of results, with all permutations maintaining statistical significance at the primary threshold. Moreover, all but two permutations supported significance at the exploratory conservative threshold as well (removing the Bell et al26 or the Wakim et al27 publications resulted in non-supported findings at the conservative threshold). Further, although Egger’s test of publication bias is problematic for meta-analyses with fewer than 10 studies, we note that the test did not support publication bias. A funnel plot similarly suggested a lack of publication bias.
Exploratory follow-up analyses were performed to assess group differences at different significance thresholds. To provide a more conservative estimate that is agnostic to the presumed direction of the difference in FA between individuals with CUD and HC, a one-tailed threshold p = 0.025 was tested, finding that the difference between groups was still detected (albeit, with fewer provided peak coordinates and a lower count of 36 differential voxels in only one cluster). Conversely, considering that DTI may have difficulty assessing differences in FA in less dense brain regions than the corpus callosum, thresholds of p < 0.10, 0.15, and 0.20 were also tested to provide less conservative estimates. These exploratory analyses universally supported the aforementioned differences in the corpus callosum, with some detected differences in the right hemisphere in the anterior thalamic projections, striatum, inferior network, inferior fronto-occipital fasciculus, pons, superior longitudinal fasciculus II, and superior temporal gyrus. Further details regarding these exploratory analyses are provided in the supplementary material.
Meta-regression
Chronicity of use and minimum length of abstinence pre-imaging were explored using meta-regression via the linear model function in SDM-PSI. Chronicity was reported in seven of the eight studies and ranged from 4.05 to 18.90 years (mean = 11.20 years). Abstinence was reported in the same set of studies and ranged from 1 to 60 days (mean = 7 days). Analyses using the available data did not support an effect of chronicity; however, an effect of abstinence (β = −0.021) was supported by the present study at the primary and conservative significance thresholds (α=0.05 and α=0.025, respectively). A follow-up leave-one-out Jackknife permutation analysis on the effect of abstinence suggested that the finding may be tenuous. The Wakim et al27 study provided a minimum abstinence pre-imaging value (60 days) four times greater than the next-highest study (14 in Kelly et al22), and removing the Wakim et al27 study yielded the only model with no support for the meta-regression effect. All other permutations (including Wakim et al27) provided nearly identical values for the effect of abstinence.
DISCUSSION
Summary of evidence
The present analysis identified 8 TBSS studies examining differences in FA between cocaine users and healthy controls. Meta-analysis was performed to determine a pooled effect of cocaine use on FA values across all studies. An overall significant group difference was found for peak values in the genu of the corpus callosum (g = −0.331), indicating lower FA values for CUD relative to HC. Additional differences were detected at higher, exploratory thresholds in several areas, including the right hemisphere of the anterior thalamic projections, striatum, inferior network, inferior fronto-occipital fasciculus, pons, superior longitudinal fasciculus II, and the superior temporal gyrus. Meta-regression analyses demonstrated weak support for an effect of minimum duration of abstinence pre-imaging and no effect of chronicity on FA values. All indices of publication bias (Jackknife leave-one-out permutation testing; Egger’s test; funnel plot) supported the present findings at the primary significance threshold.
Connection to linked meta-analysis
To our knowledge, this study is the first meta-analysis of TBSS studies of FA in patients with CUD. The present findings substantiate our prior ROI-based meta-analysis on the effect of stimulant use on the corpus callosum.7 First, the present peak group difference found in the corpus callosum (g = −0.331) was comparable to the difference in the same region that was determined by the ROI meta-analysis (g = −0.270), and chronicity similarly did not moderate the findings. However, the heterogeneity in the ROI-based meta-analysis was significant, and subsequent random-effects modeling did not discern significant group differences for overall FA. The present study capitalized on TBSS improvements in whole-brain voxel-wise mapping of WM to find a statistically reliable group difference between CUD and HC while also providing a straightforward agreement with the previous meta-analysis.
Implications for brain structure, cognitive function, and treatment response
The overarching idea behind the present study is that chronic cocaine use may damage white matter integrity in the brain over time via axonal injury and demyelination. The current meta-analysis provided a quantitative synthesis of recent whole-brain studies examining differences in measured FA between cocaine using individuals and healthy controls, finding that a systematic difference appears to exist in the genu of the corpus callosum. Damage to the corpus callosum may thus be implicated in some of the longer-term cognitive effects of chronic cocaine use, described in a comprehensive review28 as a general level of impairment to response inhibition, recall, cognitive flexibility, behavior adaptation, reward processing, and psychomotor performance.
Similar research in the neuroanatomy of chronic cocaine users (including some of the articles included in the present meta-analysis) has found lower gray matter17,29,30 and lower cortical thickness.31 Longitudinal studies have shown that reducing cocaine use leads to some recovery of these abnormalities in gray matter32 and cortical thickness,31 replete with improved cognitive performance. However, the neuroplasticity of WM is more nebulous, with preliminary research33 showing that some tracts may recover while others might not, particularly those involved in decision making (i.e., the prefrontal cortex). Such persistent deficits have self-evident consequences for treating cocaine use disorder, whereby impaired decision making and cocaine abuse demonstrate an intractable recursive pattern. Conversely, Xu et al34 found that individuals with better WM integrity at the onset of treatment experienced superior response (i.e., longer abstinence). Understanding changes in the white matter of chronic cocaine users with respect to abstinence and/or neuroprotective pharmacotherapy may have significant implications for improving decision making and response to treatment via breakdown of this negative feedback loop.
Limitations
The primary limitation of the present study involves the ability to detect significant differences in FA values for brain regions less dense than the corpus callosum. We found differences in regions other than the corpus callosum only when using higher exploratory thresholds for statistical significance. This limitation may be a consequence of the small number of studies (n = 8) that were included in the meta-analysis, or alternatively, that any actual differences between groups are not large enough to be detected by DTI. Additionally, as DTI techniques continue to be refined (e.g., diffusion kurtosis imaging (DKI)),35 improved signal-to-noise ratios may provide more accurate and/or sensitive estimates of WM tracts with less density and more fiber crossing.
The sample size for the present analysis was relatively low and should be repeated as more TBSS studies of individuals with CUD emerge. This is a pervasive concern across meta-analyses of TBSS studies, which either have accrued too slowly in the literature or been underreported (potentially due to null findings). Further, although differences between studies in scanners, software (FSL vs. SPM), and mapping systems (i.e., Talairach vs. MNI) are handled natively by the SDM software, the potential heterogeneity inherent to these differences may have influenced the estimation precision in the present study.
Inspecting differences in FA between cocaine users and healthy controls requires special attention to the potentially confounding role of alcohol use. The studies included in the present meta-analysis each contended with this issue in different ways. Two studies36,37 excluded cocaine-dependent participants with comorbid or historical lifetime alcohol dependence. Three studies allowed a number of comorbid alcohol-dependent participants (3/43 in Bell et al;26 11/25 in Kelly et al22; 2/16 in Wakim et al27). One study explicitly ensured the same lifetime rates of alcohol dependence across groups,38 another allowed a limited number of alcohol-dependent participants to both groups,29 and finally the remaining study39 distinctly evaluated a polysubstance-using group, but noted that the control comparison group also demonstrated some level of lifetime alcohol use as well. Although these studies demonstrated a high level of experimental and statistical control for the role of alcohol, the present meta-analysis may be limited by the influence of comorbid alcohol use on white matter.
Heterogeneity in the patient samples across articles may have influenced the results (e.g., the chronic cocaine users evaluated by Lim et al29 may be systematically different from the HIV+ cocaine users assessed by Wakim et al27); however, this concern is somewhat mitigated, as statistical heterogeneity in the meta-analysis was low. Further, the Wakim et al27 article compared HC to an all-female group of HIV-positive individuals with cocaine use disorder; no other studies featured a comparably idiosyncratic sample. It is possible that the insult to white matter that occurs within these individuals due to their underlying medical comorbidity is worse than the insult to white matter found in the other studies, potentially driving a disproportionate amount of the observed pooled effect. However, as noted in the leave-one-out jackknife sensitivity analysis, removal of this study did not result in changes to inference at the primary statistical significance threshold (α=0.05).
Due to the amended exclusionary criteria adopted during the current study, the findings here have limited external validity for individuals using different primary drugs than cocaine. While the pattern of lower FA values could rationally extend beyond cocaine to other stimulants, the present study was not able to make this evaluation. The impact of the present analysis may be limited to the extent that the effects of cocaine on FA values do not generalize to other stimulants.
Conclusions
WM abnormalities are likely responsible for some part of the cognitive impairments observed in chronic cocaine using individuals. WM integrity in CUD has been implicated in deficits to attention, working memory, impulse control, and poorer treatment outcomes34,36,40. Pharmacological interventions that impact WM structure (e.g., n-acetylcysteine41; pioglitazone42) may improve cognition and/or treatment outcomes, and as such warrant further investigation. Future research should also investigate differences in FA between healthy controls and other substance use disorders, particularly alcohol.
The present study enhances literature on WM in cocaine users by using a whole-brain approach to take advantage of new developments in DTI. The findings here synthesize previous research concerning WM integrity in CUD and merit iteration as more studies accrue in the literature. Additional research may help further explicate the relationship between substance use and WM integrity.
Supplementary Material
ACKNOWLEDGEMENTS
Funding for this study was provided by the National Institute of Drug Abuse (NIDA P50 DA009262) and the UTHealth Louis A. Faillace Endowment (SDL). The funding agency had no further role in the design of the study; in the collection, analysis, or interpretation of data; in the writing of the report; or in the decision to submit the manuscript for publication. The authors declare no conflict of interest.
REFERENCES
- 1.Center for Behavioral Health Statistics and Quality. 2017 National Survey on Drug Use and Health. Rockville, MD; 2017. [Google Scholar]
- 2.Hall FS, Miczek KA. Emerging threats in addiction: Will novel psychoactive substances contribute to exacerbating the ongoing drug overdose epidemic? Psychopharmacology (Berl). 2019;236(3):839–843. doi: 10.1007/s00213-019-05271-7 [DOI] [PubMed] [Google Scholar]
- 3.Carvalho M, Carmo H, Costa VM, et al. Toxicity of amphetamines: An update. Arch Toxicol. 2012;86(8):1167–1231. [DOI] [PubMed] [Google Scholar]
- 4.Gonçalves J, Baptista S, Silva AP. Psychostimulants and brain dysfunction: A review of the relevant neurotoxic effects. Neuropharmacology. 2014;87:135–149. doi: 10.1016/j.neuropharm.2014.01.006 [DOI] [PubMed] [Google Scholar]
- 5.Kousik SM, Napier TC, Carvey PM. The effects of psychostimulant drugs on blood brain barrier function and neuroinflammation. Front Pharmacol. 2012;3 June. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johansen-Berg H, Behrens TEJ. Diffusion MRI: From Quantitative Measurement to in-Vivo Neuroanatomy. (Johansen-Berg H, Behrens T, eds.).; 2009. doi: 10.1016/B978-0-12-374709-9.00002-X [DOI] [Google Scholar]
- 7.Beard CL, Schmitz JM, Soder HE, et al. Regional differences in white matter integrity in stimulant use disorders: A meta-analysis of diffusion tensor imaging studies. Drug Alcohol Depend. 2019;201:29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abhinav K, Yeh FC, Pathak S, et al. Advanced diffusion MRI fiber tracking in neurosurgical and neurodegenerative disorders and neuroanatomical studies: A review. Biochim Biophys Acta - Mol Basis Dis. 2014;1842(11):2286–2297. [DOI] [PubMed] [Google Scholar]
- 9.Smith SM, Jenkinson M, Johansen-Berg H, et al. Tract-based spatial statistics: Voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31(4):1487–1505. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?db=pubmed&cmd=Retrieve&dopt=AbstractPlus&list_uids=16624579%5Cnpapers3://publication/doi/10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 10.Qiu Y, Jiang G, Su H, et al. Progressive White Matter Microstructure Damage in Male Chronic Heroin Dependent Individuals: A DTI and TBSS Study. PLoS One. 2013;8(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wollman SC, Alhassoon OS, Stern MJ, et al. White matter abnormalities in long-term heroin users: A preliminary neuroimaging meta-analysis. Am J Drug Alcohol Abuse. 2015;41(2):133–138. [DOI] [PubMed] [Google Scholar]
- 12.Chen G, Hu X, Li L, et al. Disorganization of white matter architecture in major depressive disorder: A meta-analysis of diffusion tensor imaging with tract-based spatial statistics. Sci Rep. 2016;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nortje G, Stein DJ, Radua J, Mataix-Cols D, Horn N. Systematic review and voxel-based meta-analysis of diffusion tensor imaging studies in bipolar disorder. J Affect Disord. 2013;150(2):192–200. [DOI] [PubMed] [Google Scholar]
- 14.Müller VI, Cieslik EC, Laird AR, et al. Ten simple rules for neuroimaging meta-analysis. Neurosci Biobehav Rev. 2018;84:151–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264–269. [DOI] [PubMed] [Google Scholar]
- 16.Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan-a web and mobile app for systematic reviews. Syst Rev. 2016;5(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ersche KD, Jones PS, Williams GB, Turton AJ, Robbins TW, Bullmore ET. Abnormal brain structure implicated in stimulant drug addiction. Science (80- ). 2012;335(6068):601–604. [DOI] [PubMed] [Google Scholar]
- 18.Uhlmann A, Fouche JP, Lederer K, Meintjes EM, Wilson D, Stein DJ. White matter microstructure and impulsivity in methamphetamine dependence with and without a history of psychosis. Hum Brain Mapp. 2016;37(6):2055–2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Albajes-Eizagirre A, Solanes A, Vieta E, Radua J. Voxel-based meta-analysis via permutation of subject images (PSI): Theory and implementation for SDM. Neuroimage. 2019;186:174–184. doi:age.2018.10.077 [DOI] [PubMed] [Google Scholar]
- 20.Radua J, Mataix-Cols D, Phillips ML, et al. A new meta-analytic method for neuroimaging studies that combines reported peak coordinates and statistical parametric maps. Eur Psychiatry. 2012;27(8):605–611. doi: 10.1016/j.eurpsy.2011.04.001 [DOI] [PubMed] [Google Scholar]
- 21.Radua J, Mataix-Cols D. Meta-analytic methods for neuroimaging data explained. Biol Mood Anxiety Disord. 2012;2(1). doi: 10.1186/2045-5380-2-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kelly C, Zuo XN, Gotimer K, et al. Reduced interhemispheric resting state functional connectivity in cocaine addiction. Biol Psychiatry. 2011;69(7):684–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hedges LV., Olkin I. Statistical Methods for Meta-Analysis. San Diego, CA: Academic Press; 1985. [Google Scholar]
- 24.Rorden C, Brett M. Stereotaxic display of brain lesions. Behav Neurol. 2000;12(4):191–200. doi: 10.1155/2000/421719 [DOI] [PubMed] [Google Scholar]
- 25.Hirst RB, Beard CL, Colby KA, Quittner Z, Mills BM, Lavender JM. Anorexia nervosa and bulimia nervosa: A meta-analysis of executive functioning. Neurosci Biobehav Rev. 2017;83:678–690. [DOI] [PubMed] [Google Scholar]
- 26.Bell RP, Foxe JJ, Nierenberg J, Hoptman MJ, Garavan H. Assessing white matter integrity as a function of abstinence duration in former cocaine-dependent individuals. Drug Alcohol Depend. 2011;114(2–3):159–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wakim KM, Molloy CJ, Bell RP, Ross LA, Foxe JJ. White matter changes in HIV+ women with a history of cocaine dependence. Front Neurol. 2017;8(OCT). doi: 10.3389/fneur.2017.00562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Spronk DB, van Wel JHP, Ramaekers JG, Verkes RJ. Characterizing the cognitive effects of cocaine: A comprehensive review. Neurosci Biobehav Rev. 2013;37(8):1838–1859. doi: 10.1016/j.neubiorev.2013.07.003 [DOI] [PubMed] [Google Scholar]
- 29.Lim KO, Wozniak JR, Mueller BA, et al. Brain macrostructural and microstructural abnormalities in cocaine dependence. Drug Alcohol Depend. 2008;92(1–3):164–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaag AM, van Wingen GA, Caan MWA, Homberg JR, van den Brink W, Reneman L. White matter alterations in cocaine users are negatively related to the number of additionally (ab)used substances. Addict Biol. 2017;22(4):1048–1056. [DOI] [PubMed] [Google Scholar]
- 31.Hirsiger S, Hänggi J, Germann J, et al. Longitudinal changes in cocaine intake and cognition are linked to cortical thickness adaptations in cocaine users. NeuroImage Clin. 2019;21. doi: 10.1016/j.nicl.2019.101652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Parvaz MA, Moeller SJ, d’Oleire Uquillas F, et al. Prefrontal gray matter volume recovery in treatment-seeking cocaine-addicted individuals: a longitudinal study. Addict Biol. 2017;22(5):1391–1401. doi: 10.1111/adb.12403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.He Q, Li D, Turel O, Bechara A, Hser YI. White matter integrity alternations associated with cocaine dependence and long-term abstinence: Preliminary findings. Behav Brain Res. 2020;379:112388. doi: 10.1016/j.bbr.2019.112388 [DOI] [PubMed] [Google Scholar]
- 34.Xu J, Devito EE, Worhunsky PD, Carroll KM, Rounsaville BJ, Potenza MN. White matter integrity is associated with treatment outcome measures in cocaine dependence. Neuropsychopharmacology. 2010;35(7):1541–1549. doi: 10.1038/npp.2010.25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jensen JH, Helpern JA, Ramani A, Lu H, Kaczynski K. Diffusional kurtosis imaging: The quantification of non-Gaussian water diffusion by means of magnetic resonance imaging. Magn Reson Med. 2005;53(6):1432–1440. doi: 10.1002/mrm.20508 [DOI] [PubMed] [Google Scholar]
- 36.Lane SD, Steinberg JL, Ma L, et al. Diffusion tensor imaging and decision making in cocaine dependence. PLoS One. 2010;5(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vaquero L, Cámara E, Sampedro F, et al. Cocaine addiction is associated with abnormal prefrontal function, increased striatal connectivity and sensitivity to monetary incentives, and decreased connectivity outside the human reward circuit. Addict Biol. 2017;22(3):844–856. [DOI] [PubMed] [Google Scholar]
- 38.Yip SW, Morie KP, Xu J, et al. Shared Microstructural Features of Behavioral and Substance Addictions Revealed in Areas of Crossing Fibers. Biol Psychiatry Cogn Neurosci Neuroimaging. 2017;2(2):188–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van Son D, Wiers RW, Catena A, Perez-Garcia M, Verdejo-García A. White matter disruptions in male cocaine polysubstance users: Associations with severity of drug use and duration of abstinence. Drug Alcohol Depend. 2016;168:247–254. [DOI] [PubMed] [Google Scholar]
- 40.Moeller FG, Hasan KM, Steinberg JL, et al. Reduced anterior corpus callosum white matter integrity is related to increased impulsivity and reduced discriminability in cocaine-dependent subjects: Diffusion tensor imaging. Neuropsychopharmacology. 2005;30(3):610–617. doi: 10.1038/sj.npp.1300617 [DOI] [PubMed] [Google Scholar]
- 41.Klauser P, Xin L, Fournier M, et al. N-acetylcysteine add-on treatment leads to an improvement of fornix white matter integrity in early psychosis: a double-blind randomized placebo-controlled trial. Transl Psychiatry. 2018;8(1). doi: 10.1038/s41398-018-0266-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schmitz JM, Green CE, Hasan KM, et al. PPAR-gamma agonist pioglitazone modifies craving intensity and brain white matter integrity in patients with primary cocaine use disorder: A double-blind randomized controlled pilot trial. Addiction. 2017;112(10):1861–1868. doi: 10.1111/add.13868 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


