Abstract
Aims
Wound infections involving Candida albicans can be challenging to treat because of the fungus’ ability to penetrate wound tissue and form biofilms. The goal of this study was to assess the activity of a hypochlorous acid (HOCl)-generating electrochemical scaffold (e-scaffold) against C. albicans biofilms in vitro and on porcine dermal explants (ex vivo).
Methods and Results
C. albicans biofilms were grown either on acrylic-bottom six-well plates (in vitro) or on skin tissue excised from porcine ears (ex vivo), and the polarized e-scaffold was used to generate a continuous supply of low concentration HOCl near biofilm surfaces. C. albicans biofilms grown in vitro were reduced to undetectable amounts within 24 h of e-scaffold exposure, unlike control biofilms (5.28 ± 0.034 log10 CFU cm−2; P < 0.0001). C. albicans biofilms grown on porcine dermal explants were also reduced to undetectable amounts in 24 h, unlike control explant biofilms (4.29 ± 0.057 log10 CFU cm−2; P < 0.0001). There was a decrease in the number of viable mammalian cells (35.6 ± 6.4%) in uninfected porcine dermal explants exposed to continuous HOCl-generating e-scaffolds for 24 h compared to explants exposed to nonpolarized e-scaffolds (not generating HOCl) (P < 0.05).
Conclusions
Our HOCl-generating e-scaffold is a potential antifungal-free strategy to treat C. albicans biofilms in chronic wounds.
Significance and Impact of the Study
Wound infections caused by C. albicans are difficult to treat due to presence of biofilms in wound beds. Our HOCl producing e-scaffold provides a promising novel approach to treat wound infections caused by C. albicans.
Keywords: biofilm, hypochlorous acid, Candida albicans, electrochemical scaffold, wound healing, e-scaffold
INTRODUCTION
Chronic wounds, wounds that are unable to complete the normal stages of wound healing (e.g., diabetic foot ulcers) affect 6.5 million people annually, causing $25 billion in annual healthcare costs in the United States (Sen et al. 2009; Velnar et al. 2009). Chronic wounds are often infected with both bacterial and fungal biofilm communities that contribute to delayed wound healing and require prolonged treatment regimens (Douglas 2003; Bjarnsholt et al. 2008; James et al. 2008; Dowd et al. 2011; Percival et al. 2012; Bjarnsholt 2013). Biofilm communities consist of microbial cells encased within a secreted extracellular polymeric substance (EPS) that functions as a protective layer (Van Acker et al. 2014). These communities can consume oxygen in the wound bed (James et al. 2016), contributing to hypoxia of the wound bed with resultant necrosis and excessive inflammation (Velnar et al. 2009).
Topical antimicrobial agents may be used to combat biofilms in chronic wounds, but even with these therapies bacterial and fungal, including polymicrobial biofilm communities, can be difficult to treat (O’Connell et al., 2006). The dense structure of biofilms slows the diffusion of antimicrobial agents as well as nutrients into biofilms (Brown and Gilbert 1993; Wolcott et al. 2010). This results in a deprivation of nutrients to the microbes residing in deep parts of the biofilm (i.e., those away from the biofilm-air interface), which in turn causes a decrease in metabolic activity and slows the overall growth rate of the microbes (Brown and Gilbert 1993). Reductions in the overall growth rate and metabolic activity increase the resilience of biofilms in wound beds and limit the activity of conventional antibiotics. The EPS acts as a protective shroud against external factors, guarding pathogenic microbes in wound beds (Brown and Gilbert 1993; Wolcott et al. 2010). The protective effect of EPS can be shared among microbial species in polymicrobial biofilms. For example, the resistance of Staphylococcus aureus to vancomycin increased when grown with Candida albicans biofilms or with isolated Candida matrix (Harriott and Noverr 2009). Within clinical settings, there are a variety of wound dressings available for chronic wounds, although many have not been shown to reduce biofilms in vivo (Sood et al. 2014).
Using biocides as an anti-biofilm strategy is attractive because of their microbicidal effects and the dearth of associated resistance mechanisms. Because of the rise of resistance to conventional antimicrobial agents, biocides may provide a clinical advantage as a treatment for chronic wounds (Percival et al. 2012; Van Acker et al. 2014). While research on the effect of biocides against bacteria in both planktonic and biofilm forms is extensive, parallel assessments of antifungal activity are less common (McDonnell and Russell 1999; Russell 2003).
Delivering electric current directly to wound beds has been proposed as a treatment alternative to antibiotics, topical treatments, and biocides (Kloth 2005). In addition to treating infections, exposure to electric current increases protein and collagen synthesis in fibroblast cell culture (Bourguignon and Bourguignon 1987; Kloth 2005). In prior work, Schmidt-Malan et al. (2015) grew bacterial and fungal biofilms on Teflon or titanium discs and delivered low continuous or intermittent direct electrical current as a treatment strategy. This technique with 200 μA current reduced Gram-negative bacterial biofilms in one day and Gram-positive bacterial biofilms in four days (Schmidt-Malan et al. 2015). C. albicans, however, required 2,000 μA current for four days to yield a significant reduction of fungal biofilm density (Schmidt-Malan et al. 2015). Although approaches utilizing electrical current demonstrate promising results in reducing biofilms, they are not standardized and the mechanism of action is not clear and may change over the course of treatment (Sultana et al. 2015b). Our group has developed electrochemical scaffolds (e-scaffolds) based on potentiostatic control that continuously produce small amounts of hydrogen peroxide (H2O2) or hypochlorous acid (HOCl) (Sultana et al. 2015a; Kiamco et al. 2019).
Continuous generation of small concentration of H2O2 or HOCl is advantageous because the e-scaffolds can deliver these biocides over long treatment periods. This approach circumvents the need to use high initial biocide concentrations, reducing the risk of cytotoxicity to underlying wound tissues. The e-scaffolds are three-electrode systems composed of a working and a counter electrode made of carbon fabric and a custom Ag/AgCl reference electrode. When the working electrode is polarized to −0.6 VAg/AgCl or +1.5 VAg/AgCl, there is continuous production of H2O2 or HOCl, respectively (Sultana et al. 2015a; Kiamco et al. 2019).
Common biocides used in clinical settings for wound care include H2O2, sodium hypochlorite, and HOCl (Selkon et al. 2006; Wang et al. 2007; Degnim et al. 2013). Both H2O2 and HOCl are produced by neutrophils to break down pathogenic and foreign substances and have been used to treat wound infections (Wang et al. 2007). Dilute sodium hypochlorite (Dakin’s solution) has also been used for wound debridement (Degnim et al. 2013; Mangum et al. 2018). We previously investigated infections post breast and axillary operations and found that including Dakin’s solution in postoperative care reduced bacterial colonization of drainage tubes (Degnim et al. 2013). Dakin’s solution is unstable and can therefore degrade rapidly in wound beds following single applications (Mangum et al. 2018). Compared to H2O2 and sodium hypochlorite, little is known about HOCl.
At sufficient concentrations, HOCl exposure can reduce ATP production, reduce DNA synthesis and cell wall integrity, disrupt oxidative phosphorylation, and inhibit cell division (McKenna and Davies 1988; Barrette Jr et al. 1989; McDonnell and Russell 1999; Russell 2003), with activity against bacteria including, Escherichia coli, Pseudomonas aeruginosa, and Staphylococcus aureus (Dukan and Touati 1996; Estrela et al. 2002; Degnim et al. 2013). HOCl is the predominant species in solutions at pH 4–7, but when pH exceeds 7.5, HOCl dissociates to hypochlorite ions, which have antimicrobial activity against many infectious agents, including C. albicans (Wagner et al. 1986; Estrela et al. 2002; Wang et al. 2007). The instability of pure HOCl at physiological pH has deterred its use as a primary treatment for wound biofilm infection and limited its use in periodic cleansing applications; continuous delivery methods for HOCl are not currently available.
In this work, we use an e-scaffold that produces HOCl near electrode surfaces at low concentrations for treatment of C. albicans biofilms in both in vitro and ex vivo settings using a model similar to previous work with bacterial biofilms (Kiamco et al. 2019). The concentration of HOCl was measured using a free chlorine microelectrode and shown to be dependent on the potential applied to the e-scaffold working electrode (Kiamco et al. 2019). By applying a potential >1.138VAg/AgCl to the working electrode (Bard et al. 1980), HOCl forms from oxidation of Cl− in an aqueous solution (Sandvik et al. 2013). Under these conditions, the physiological concentration of NaCl in wound beds is more than sufficient to generate HOCl through the following reactions:
Counter electrode:
| (Equation 1) |
Working electrode:
| (Equation 2) |
Cl2 gas from this reaction rapdily produces free chlorine compounds, including HOCl and OCl− (Eryilmaz and Palabiyik 2013; Sandvik et al. 2013):
| (Equation 3) |
At pH 7.0, HOCl dissolves in water to form a solution of ~76% HOCl and ~24% OCl−, the former being more reactive than the latter (Eryılmaz and Palabıyık 2013; Sandvik et al. 2013). When reaction rates are controlled, HOCl can be generated without gas formation. We used a carbon-based conductive fabric composed of nano-sized carbon fibers (10–30 nm in diameter), which is a biologically compatible material that allows HOCl generation in wound beds (Sultana et al. 2015a; Kiamco et al. 2019). The woven fabric can conform to wound surfaces, providing an adequate surface area for efficient generation of HOCl (Kiamco et al. 2019).
The goal of the present study was to evaluate the efficacy of our HOCl-producing e-scaffolds against C. albicans biofilms grown in vitro and on skin tissue excised from porcine ears (ex vivo). We report the efficacy of HOCl-producing e-scaffold against in vitro biofilms treated for 3, 6, 12 and 24 h, and against ex vivo biofilms treated for 6 and 24 h compared to untreated controls. The effect of HOCl e-scaffold treatment on mammalian tissue was assessed using cell viability and histopathology.
MATERIALS AND METHODS
Hypochlorous acid-generating electrochemical scaffold
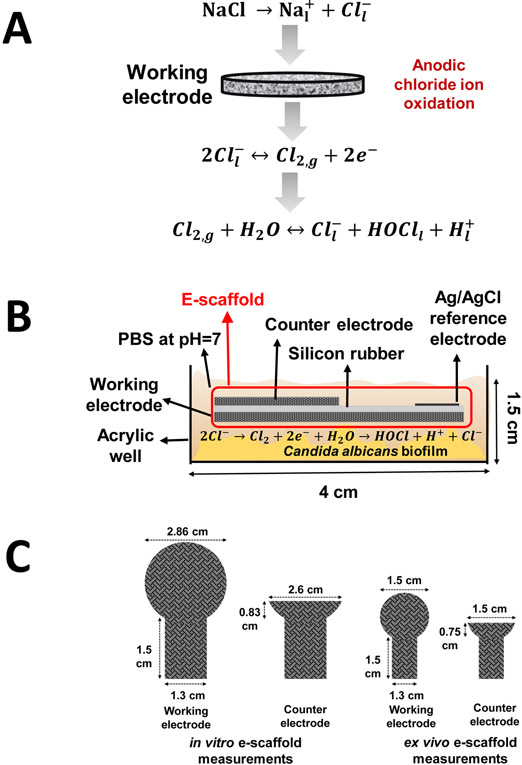
Hypochlorous acid was produced via oxidation of chloride ions to chlorine on the working electrode of the e-scaffold (Fig. 1a). The e-scaffold is a three-electrode system consisting of a working, a counter, and a reference electrode (Fig. 1b). The working and counter electrodes were constructed from conductive carbon fabric (Zoltek Companies Inc., St. Louis, MO, Panex 30 PW-06) and cut using a laser. The working electrodes for the in vitro and porcine explant biofilm models had areas of 6.42 and 1.77 cm2, respectively (Fig. 1c). The counter electrodes for the in vitro and porcine explant biofilm models had areas of 2.14 and 0.89 cm2, respectively (Fig. 1c). Before assembly, the carbon fabric was treated in 1 mol l−1 HCl overnight and washed with deionized water to improve the wettability of the e-scaffolds. The working and counter electrodes were separated by applying a thin layer of silicone (~1-2 mm thick) between them. A customized Ag/AgCl electrode was used as the reference electrode (Lewandowski and Beyenal 2013). Nylon sew snaps (Dritz, Spartanburg, SC, item #85) were used to press titanium wires (TEMCo, Amazon.com, catalog #RW0524) onto the conductive fabric, establishing electrical connection. The complete system was submerged in one well of a 6-well plate with a phosphate buffer solution (PBS) composed of 32 mmol l−1 dipotassium phosphate (K2HPO4), 18 mmol l−1 monopotassium phosphate (KH2PO4), and 0.9 % w/v sodium chloride (NaCl), pH 7.0 (Fig. 1b).
Figure 1.
a) Hypochlorous acid (HOCl) reaction mechanism occurring at the working electrode surface. b) Schematic of the hypochlorous acid-generating e-scaffold in the in vitro biofilm model. c) Dimensions of the e-scaffolds for the in vitro (left) and ex vivo (right) biofilm models.
The working electrode was positively polarized against the Ag/AgCl reference electrode. We used a custom potentiostat to polarize the working electrode to +1.5 VAg/AgCl (Renslow et al. 2011). Titanium wires connected the working and counter electrodes to external wires of the potentiostat; the reference electrode was directly connected to the external wires. The connection resistance between the electrodes and wires was less than 3 Ω. The e-scaffold was laid over C. albicans biofilms grown on an acrylic bottom 6-well plate (in vitro biofilm model) or grown on porcine tissue punches (ex vivo biofilm model). Cyclic voltammetry was used as an electrochemical conditioning step (scan window 0 VAg/AgCl to +2.0 VAg/AgCl; scan rate mV s−1 and 10 mV s−1).
C. albicans in vitro biofilm model
C. albicans IDRL-7001 (clinical wound isolate) was subcultured from −80°C stock onto tryptic soy agar (TSA, Fisher Scientific, DF0370–17-3) plates, following which plates were incubated at 37 °C for 24 h. For biofilm growth, a colony was freshly picked from a streaked plate and placed in 10 ml of tryptic soy broth (TSB) supplemented with 1 % glucose (filter-sterilized). This was incubated on a shaker at 37 °C for 24 h, following which the culture was diluted to OD600 = 1.0 and 1 ml of it was used to inoculate a 6-well plate (VWR Intl Inc, 29442-042). The inoculum was left in the well plate for a one-hour attachment period at 25°C and then washed with TSB with 1% glucose and replenished with 2 ml of TSB with 1 % glucose. The plate was then incubated at 25 °C for 48 h, with medium replacement after 24 h.
After 48 h of biofilm growth, the medium was replaced with 4 ml of PBS (~ pH 7.0) and e-scaffolds placed on top of C. albicans biofilms. An additional 3 ml of PBS was added to each well to reach a volume of 7 ml per well. Biofilms with no e-scaffold served as matched controls. Before polarization, cyclic voltammetry from 0 VAg/AgCl to +2.0 VAg/AgCl was applied to condition the e-scaffolds (scan rates 100 mV s−1 and 10 mV s−1). E-scaffolds were polarized at +1.5 VAg/AgCl for 3, 6, 9, 12 or 24 h. After polarization, e-scaffolds were removed and rinsed separately with 4 ml of fresh PBS to remove biofilm attached to the fabric. The bottoms of the wells were scraped to detach viable cells and washed twice with 2 ml of PBS, which was then combined with the 4 ml e-scaffold wash. The suspension was centrifuged, the supernatant discarded, and 1 ml of fresh PBS added. This was repeated three times to remove HOCl. Once washed, the sample was serially diluted and plated onto TSA plates. Colony-forming units (CFUs) were counted using a drop-plate count method (Sultana et al. 2015a; Kiamco et al. 2019).
C. albicans ex vivo biofilm model
The e-scaffolds were tested against biofilms grown on porcine explants. The ex vivo culture growth procedure was performed according to Sultana et al. (2015a). Ear tissue was freshly harvested from germ-free domestic white pigs (Sus scrofa domesticus), immediately cooled to 4 °C and transported to the laboratory for processing within 1 h of harvest. Pig ears were gently washed with cold low-running water and 1 ml of 10 % diluted detergent (7 ml of generic dish soap in 63 ml of deionized water) to remove dirt. Hair was removed using an electric razor, and ears rinsed with cold water and sprayed with 70 % ethanol before skin was excised with a scalpel. Each ear was cut into three equal portions to make it easier to retrieve the dermis layer. The three sections were treated the same way. Blades were changed between sections to prevent cross-contamination. A Padgett’s dermatome at a thickness of 500 μm was used to excise the dermal layer of porcine tissues. The first layer of tissue (epidermis) was discarded, and the second layer (dermis) placed into a Petri dish filled with Dulbecco’s Modified Eagle’s Medium (DMEM) (Thermo Scientific, 21063029) with the bottom of the tissue exposed to the DMEM. Areas of the ear with structural flaws (e.g., pimples, bruises, scratches) were avoided. The excised dermis sections were then punched into 12-mm-diameter discs and placed into polycarbonate transwell inserts with a 0.4-μm pore size membrane (Greiner Bio-One North America, Inc., 657641); this separated the porcine explant from the growth medium in the outer well. The growth medium consisted of DMEM supplemented with ampicillin (50 μg ml−1). Fungal biofilms were grown by inoculating the center of the explant surface with 5 μl of an overnight culture of C. albicans (OD600 = 1.0). Biofilm was allowed to establish for 48 h at 37 °C in a 5 % CO2 incubator, with the medium replaced after 48 h.
Electrochemical scaffolds for the ex vivo biofilm model were prepared as described above and laid on top of the explants; 4 ml of PBS was added to the inner wells. Similar to the in vitro biofilm model, cyclic voltammetry from 0 VAg/AgCl to +2.0 VAg/AgCl was applied to condition the e-scaffolds before polarization (scan rates included 100 mV s−1 and 20 mV s−1) and then the e-scaffolds were polarized at +1.5 VAg/AgCl for 6 or 24 h. Because of volume loss, the system was replenished with 2 ml PBS halfway through the treatment time. After 6 or 24 h of treatment, porcine punches were processed by sonicating porcine skin in 2 ml fresh PBS for 30 s, rinsing the e-scaffolds with 2 ml fresh PBS, and then combining the two solutions to perform quantitative cultures. Results of quantitative cultures from the treated porcine explants were compared to those from the infected porcine explants exposed to nonpolarized e-scaffolds and infected porcine explants not exposed to e-scaffolds.
Cell viability of porcine explants
Cell viability of noninfected porcine explants was tested to assess whether 6 or 24 h of e-scaffold exposure affected porcine tissues. Noninfected porcine explants were exposed to polarized or nonpolarized e-scaffolds or no e-scaffold for either 6 or 24 h, following which porcine cells were quantified using PrestoBlue cell viability reagent (Thermofisher, A-13262) following the manufacturer's protocol (Life Technologies). Three hundred microliters of 10 % PrestoBlue reagent with DMEM was added to treated and control porcine explants and maintained at 37 °C in 5 % CO2 for three hours. Two absorbance data points were taken for cell viability calculations, one at the oxidized peak (570 nm) and the other at the reduced peak (600 nm). The percent reduction of PrestoBlue was calculated using the two absorbance measurements and the molar extinction coefficient of oxidized and reduced PrestoBlue. The percent reductions of PrestoBlue of the porcine explants exposed to polarized and nonpolarized e-scaffolds were compared, with values normalized to those of freshly processed explants.
Histopathology
To assess damage to porcine tissues resulting from e-scaffold exposure and/or infection, infected and noninfected porcine explant punches, as well as both e-scaffold treated and non-treated porcine explant punches were examined. Explants were fixed in 10% formalin and embedded in paraffin for histopathological analysis; they were stained with hematoxylin and eosin, and resulting light micrographs subjected to treatment-blind evaluation by a board-certified anatomic pathologist (ANS).
Statistical Analysis
Data were averaged and displayed as means ± standard deviations for at least three biological replicates (each independently cultured from frozen stock and assayed on multiple days). A one-way ANOVA was used with a Tukey test to identify differences in the in vitro and ex vivo CFU data, and the explant viability data relative to the controls. P ≤ 0.05 was used as a threshold for differences to be considered statistically significant. Calculations and statistical analyses were performed using Sigma Plot© (versions 12.0 and 12.5).
RESULTS
Activity of HOCl-generating electrochemical scaffold against in vitro biofilms
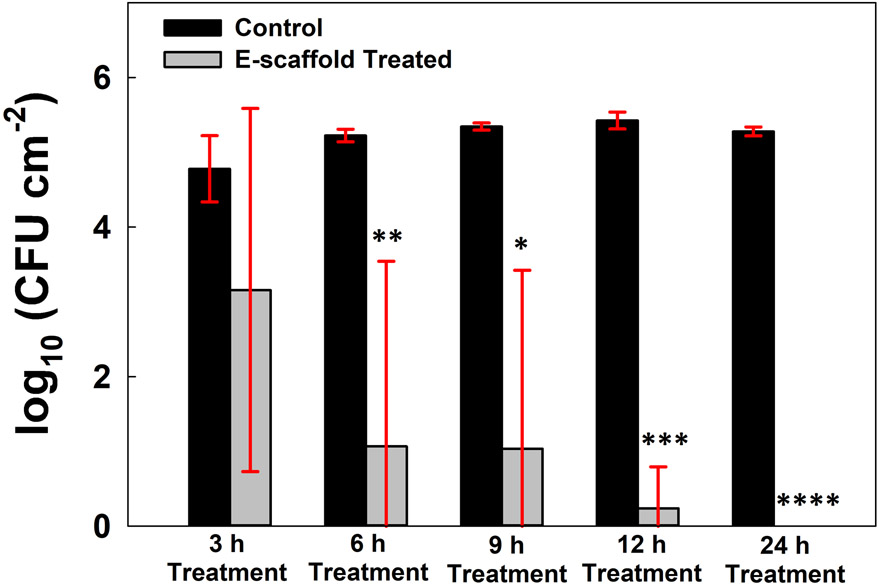
C. albicans biofilms treated with e-scaffolds showed time-dependent reductions in biofilm communities, with no viable organisms detected in quantitative cultures after 24 h (Fig. 2). Significant reductions relative to controls were evident as early as 6 h after treatment was started. After 24 h of continuous HOCl production by e-scaffolds, the number of viable fungal cells was below the detection limit of the drop-plate count quantitative culture method used.
Figure 2.
Colony forming unit counts of in vitro Candida albicans biofilms after e-scaffold treatment for 3, 6, 12 and 24 h compared to untreated controls. Data are represented as means and standard deviations of three independent biological replicates. Statistical analysis was performed using one-way ANOVA with the corresponding control group (* p ≤ 0.05, ** p ≤ 0.01, *** p ≤ 0.001 and **** p ≤ 0.0001).
Activity of HOCl-generating electrochemical scaffold against ex vivo biofilms
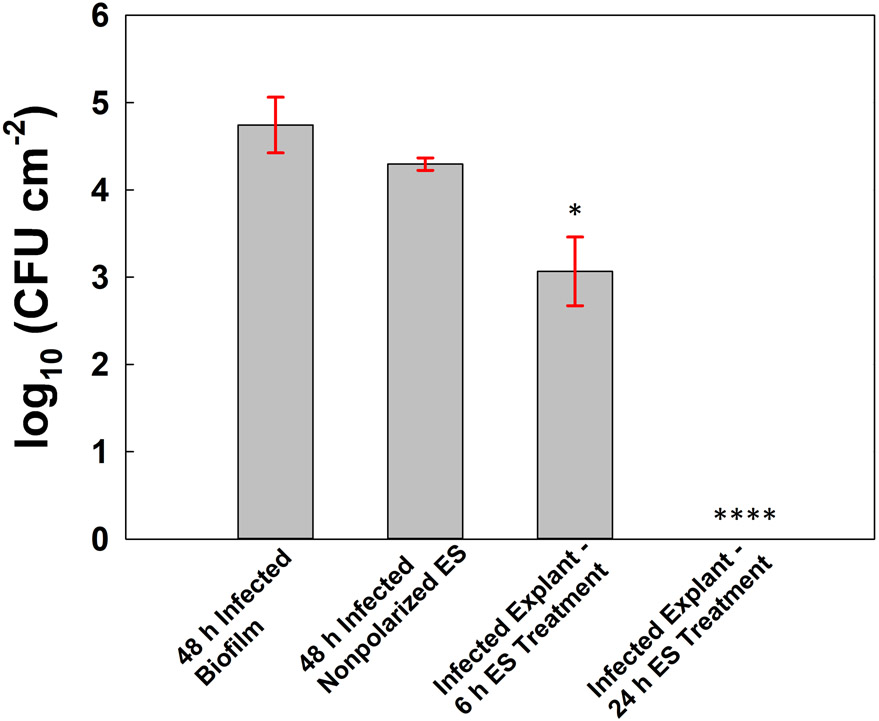
Infected porcine explants exposed to nonpolarized e-scaffolds (i.e., no potential applied) did not experience significant reductions in microbial counts compared to infected explants not exposed to e-scaffolds (reduction of 0.45 log10 cm−2, P > 0.05) (Fig. 3). Conversely, treatment with HOCl-generating e-scaffolds for 6 or 24 h reduced biofilm communities in a time-dependent manner (P ≤ 0.05) (Fig. 3). The number of viable fungal cells was below the detection limit of the quantitative culture method after 24 h of continuous HOCl e-scaffold treatment.
Figure 3.
Colony forming unit counts of ex vivo Candida albicans biofilms. From left to right: porcine explant with C. albicans biofilm (no-treatment control), porcine explant with C. albicans biofilm and nonpolarized ES (no-treatment control), infected porcine explant treated for 6 h, and infected porcine explant treated for 24 h. Data are represented as means and standard deviations of three independent replicates. Statistical analysis was performed using one-way ANOVA compared with nonpolarized e-scaffolds (* p ≤ 0.05, ** p ≤ 0.01, *** p ≤ 0.001 and **** p ≤ 0.0001).
Effect of e-scaffold treatment on cell viability of porcine explants
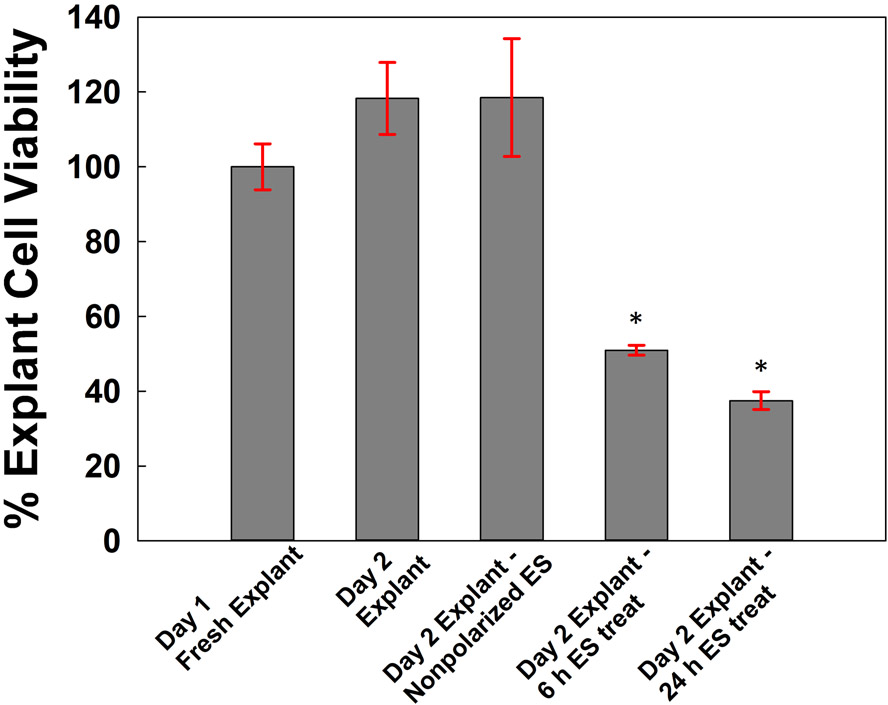
Explants exposed to nonpolarized e-scaffolds were unharmed according to the cell viability assay (Fig. 4). Porcine explants that had received 24 h of exposure to PBS (two-day-old explants) or 24 h of exposure to nonpolarized e-scaffolds were normalized to the porcine explants that had only been processed (i.e., one-day-old explants); they showed similarly high cell viabilities (109 ± 0.002 % and 105 ± 0.001 %, mean ± SD, P > 0.05). After 6 and 24 h of e-scaffold treatment, noninfected explants had percent cell viabilities of 47.2 ± 8.16 % and 35.6 ± 6.42 %, respectively, compared to the noninfected explants exposed to the nonpolarizing e-scaffolds (P < 0.02).
Figure 4.
Cell viability of porcine explant tissue assessed after processing (day 1 fresh explant), after 24 h exposure to phosphate buffered solution (day 2 explant), after 24 h of exposure to nonpolarized e-scaffolds (day 2 explant, nonpolarized ES), and after 6 h (day 2 explant, 6 h ES treatment) and 24 h (day 2 explant, 24 h treatment) of HOCl e-scaffold treatment. Data are represented as means and standard deviations of three independent biological replicates. Statistical analysis was performed using one-way ANOVA compared with the nonpolarized e-scaffold (* p ≤ 0.05).
Histopathology
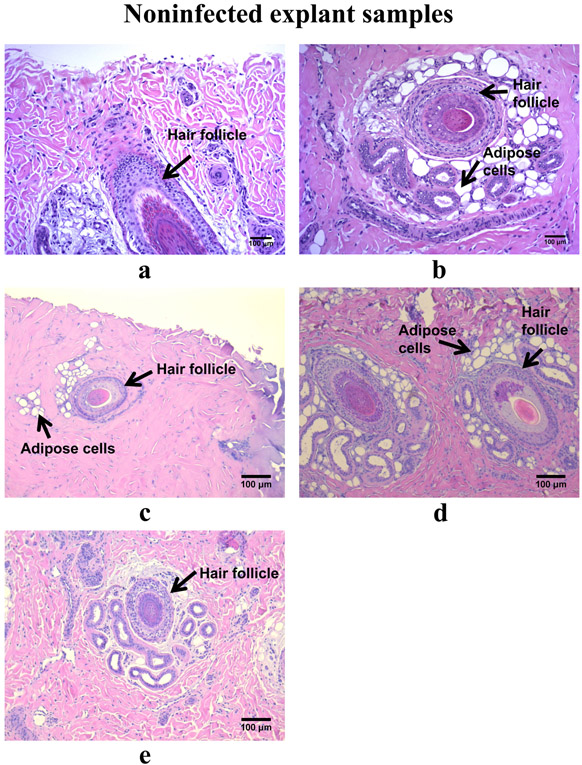
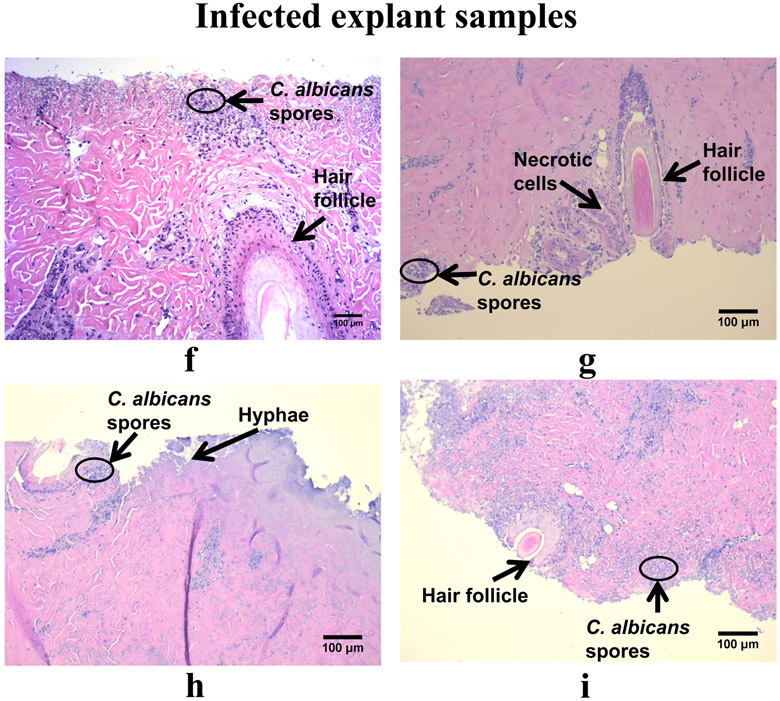
For noninfected explants, 6 or 24 h of e-scaffold treatment caused minimal tissue damage as assessed histopathologically; some adipose cells (in form of vacuoles) were visualized around hair follicles (Fig. 5a, b, c, d, e). Although these cells are common in plant, fungal, and bacterial cells, in mammalian cells they can be a marker of necrosis and cell death (Proskuryakov et al. 2003; Elmore 2007; Shubin et al. 2016). Reactive oxygen species created by HOCl exposure may have triggered cellular necrosis (Proskuryakov et al. 2003). Hematoxylin and eosin staining revealed C. albicans biofilms growing on tissues in the infected explants, with fungal hyphal invasion and fungal conidia evident in the superficial dermis (Fig. 5f, g, h, i). Minimal necrosis of cells was visible in infected explant tissues regardless of e-scaffold exposure. However, 24 h of e-scaffold exposure (either nonpolarized or polarized) was associated with the presence of a moderate number of necrotic cells (Fig. 5h and i), especially in the regions close to C. albicans hyphae.
Figure 5.
Histopathology of tissue excised from the porcine explant punches, both noninfected (a-e) and infected with Canida albicans (f-i). a) Day 1 non-infected explant without an e-scaffold. Glandular epithelium and a hair follicle are present. b) Day 1 non-infected explant after 6 h treatment. Glandular epithelium and a hair follicle are present, with minimal degeneration of tissue and adipose cells. c) Day 1 non-infected explant after 24 h treatment. Glandular epithelium and a hair follicle are present, with minimal degeneration of tissue and adipose cells. d) Day 1 non-infected explant with 24 h of nonpolarized e-scaffold exposure. Glandular epithelium and hair follicles are present, with minimal degeneration of tissue and adipose cells. e) Day 2 non-infected explant without an e-scaffold. Glandular epithelium and a hair follicle are present with minimal degeneration of tissue. f) Day 2 infected explant without an e-scaffold. C. albicans hyphae are present (mainly in the outer layer of skin) with conidia penetrating the tissue, limited necrosis, and fragmented nuclei in the glandular epithelium and hair follicle. g) Day 2 infected explant after 6 h treatment. C. albicans hyphae are present (mainly in the outer layer of skin) with conidia penetrating the tissue, limited necrosis, and fragmented nuclei in the glandular epithelium and hair follicle. h) Day 2 infected explant after 24 h treatment. C. albicans hyphae are penetrating deep (mainly in the outer layer of skin) with conidia also penetrating the tissue, limited necrosis, and fragmented nuclei in the glandular epithelium and hair follicle. i) Day 2 infected explant after 24 h of nonpolarized e-scaffold exposure. C. albicans hyphae are present (mainly in the outer layer of skin) with conidia also penetrating the tissue, limited necrosis, and fragmented nuclei in the glandular epithelium and hair follicle.
DISCUSSION
For this work, HOCl was continually produced proximal to fungal biofilms to assess the activity of a HOCl generating e-scaffold against C. albicans biofilms. In our previous studies, continuous H2O2 or HOCl e-scaffold treatment eliminated monomicrobial biofilms formed by Staphylococcus aureus [including clinical isolates of methicillin-resistant Staphylococcus aureus (MRSA)], Acinetobacter baumannii, and Pseudomonas aeruginosa (Sultana et al. 2015b; Kiamco et al. 2019; Raval et al. 2019). Herein, we show that the HOCl-producing e-scaffolds have activity against C. albicans biofilms in both in vitro and ex vivo biofilm models. As shown in Fig. 2, in vitro fungal biofilms were reduced after as little as 6 h of HOCl e-scaffold treatment. Similar results were observed in the ex vivo biofilm model, with significant reductions at both 6 and 24 h of e-scaffold treatment (Fig. 3).
One study examining the minimum bactericidal concentration (MBC) of HOCl found large variation in MBC for the same strain of C. albicans (2.7 and 0.27 μg ml−1) (Wang et al. 2007). This may be due to variations in fungal biofilm morphology. Differences in fungal morphologies (hyphal and yeast forms) and growth rates can affect the efficacy of treatments (O’Toole et al. 2000). Variation in biocidal efficacy for fungal, as well as bacterial biofilms, can also be due to variations in the chemical composition of cell walls (Russell 2003). Studies examining the effects of HOCl on bacterial biofilms have found that it requires a shorter treatment time than H2O2 or sodium hypochlorite. This includes both HOCl externally added to wound beds and electrochemically produced HOCl (Wang et al. 2007; Kiamco et al. 2019). Treatment times for externally added HOCl are similar for bacterial and fungal biofilms (Anagnostopoulos et al. 2018). As C. albicans can have differences between strains, further studies involving multiple such strains are needed to test our HOCl producing e-scaffold.
Similar to bacterial biofilms showing resistance to antibacterial treatments, fungal biofilms may also display resistance against antifungal treatments. One study compared amphotericin B, fluconazole, flucytosine, itraconazole, and ketoconazole against mature C. albicans in the biofilm and planktonic states (Hawser and Douglas 1995). Mature biofilms had lower inhibition of [3H]leucine incorporation (a measurement of antibiofilm activity) by the antifungal agents than planktonic C. albicans (Hawser and Douglas 1995). Fungal biofilms may resist antimicrobial treatments as a result of persister cells (Hawser and Douglas 1995; Bachmann et al. 2002; LaFleur et al. 2006), which neither grow nor die in the presence of antimicrobial treatment, and are phenotypically variant from metabolically active cells residing in biofilms (LaFleur et al. 2006). Delivery of HOCl from e-scaffolds may overcome these issues.
HOCl is a well-known bactericide; the amount of HOCl required to surpass cytotoxic levels is lower than that of H2O2 (Pullar et al. 2000). Reactive oxygen species produced natively by neutrophils as a part of the innate immune response, which include HOCl and H2O2, can damage host tissue, resulting in delayed tissue repair (Wilgus et al. 2013). For the current study, PrestoBlue-measured cell viability and histopathology of porcine explant tissue punches were used to evaluate the effect of HOCl-generating e-scaffolds on mammalian tissue; the former showed that for continuous HOCl e-scaffold treatment (Fig. 4) there was a 52.8% and 64.4% reduction in cell viability for 6 and 24 hours of treatment, respectively. Histology showed that prolonged exposure to HOCl-producing e-scaffolds resulted in some necrosis and cell death.
We previously showed that our HOCl-producing e-scaffold eliminated S. aureus, A. baumannii, and P. aeruginosa biofilms on porcine explants after 3, 2, and 1 h of HOCl e-scaffold treatment, respectively (Kiamco et al. 2019). In our prior study, cell viability and histopathology studies showed that 3 h of continuous HOCl e-scaffold treatment did not cause apparent host tissue damage (Kiamco et al. 2019). Another study using the e-scaffolds showed that 24 h of continuous H2O2 e-scaffold treatment did not reduce the number of viable cells in a porcine tissue punch (Sultana et al. 2015a). There is a dose-dependent relationship between HOCl concentration and toxicity to mammalian tissue (Pullar et al. 2000; Degnim et al. 2013). Histopathology showed that with prolonged treatment times, including those at which fungal biofilms were reduced to below the limit of quantification, there may be damage to the mammalian tissue. It is possibly that the higher toxicity observed here may be attributable to the use of germ-free pigs. In our prior study, we studied pigs from commercial butchers for which HOCl-produced from e-scaffolds had no apparent effect (Kiamco et al. 2019). Gnotobiotic pigs have downregulated genes required for T-cell receptor signaling compared to non-germ-free pigs (Sun et al. 2018); expression of these and other genes involved in immune function appears to be altered as a result of lack of gut microbiota (Sun et al. 2018). This might explain the difference between the cell viability experiment in previous work (Kiamco et al. 2019) and in the current study.
Histopathology images of the noninfected tissue exposed to continuous HOCl e-scaffold treatment (Fig. 5b and c) showed early stage necrosis, specifically the presence of adipose cells, while tissue explants that were both infected and exposed to continuous HOCl e-scaffold treatment (Fig. 5g and 5h) displayed limited necrosis. The damage seen in the study in the absence of fungal biofilms could be related to direct exposure of HOCl; when C. albicans biofilm was present, the biofilm was exposed to the HOCl, while the tissue was not directly exposed. C. albicans biofilms also caused damage to tissue and penetrated tissues by changing to the hyphal form. Previous studies have found that C. albicans phenotypically changes its morphology when coming in contact with biotic surfaces, inducing tissue degradation as a result of hyphal penetration (Martin et al. 2011). Moving forward, using HOCl as a biocide may be effective against pathogenic bacteria and fungi. However, the potential drawbacks of cytotoxicity should be noted; that cytotoxicity varies with biological replicates, dose and duration of continuous HOCl treatment speaks to the need to control these parameters in future studies.
Because of the unique morphological structure and growth behavior of fungal biofilms, shorter treatment durations may be beneficial. During fungal biofilm growth, cells go through a maturation phase in which their morphology shifts from yeast conidia and pseudohyphae to hyphal forms able to penetrate epithelial cells. Hyphae are smaller and thinner, and are able to penetrate and destroy surrounding tissues (Chandra et al. 2001). This means there may be more damage to infected areas than in the vicinity of bacterial biofilms. By debriding and treating wound beds early, this damage may be avoided, allowing wounds to go through the wound healing process sooner. It was previously shown that after hydro-debridement, P. aeruginosa biofilms had a larger reduction in biofilm density compared to P. aeruginosa biofilms not exposed to hydro-debridement (Wolcott et al. 2010). Adding an aggressive debridement step might allow C. albicans to be removed from the wound bed before the maturation/attachment step that makes fungal biofilms especially difficult to treat. This may allow for shorter treatment times with e-scaffolds, preventing tissue damage and necrosis due to hyphal penetration.
In summary, we report that an HOCl-generating e-scaffold is an effective approach to reduce C. albicans biofilms, with a significant reduction in CFUs after 6 h of treatment in both in vitro and ex vivo biofilm models. Collectively, the results show that an HOCl-generating e-scaffold is a promising antifungal-free strategy to treat C. albicans biofilms in chronic wounds. Future studies are needed to test the activity of HOCl-generating e-scaffolds on other strains of C. albicans and non-Candida albicans fungal biofilms, and in vivo.
ACKNOWLEDGEMENTS
This research was supported in part by NSF-CAREER award #0954186, a gift from Anthony J. and Kimberly Conroy Sawyer, and the National Institutes of Health under award number R01 AI91594. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
CONFLICT OF INTEREST
No conflict of interest declared. Beyenal and Call are part of a patent (#US20180207301A1), “Electrochemical reduction or prevention of infections” which refers to the electrochemical scaffolds described in this manuscript.
REFERENCES
- Anagnostopoulos AG, Rong A, Miller D, Tran AQ, Head T, Lee MC and Lee WW (2018) 0.01% Hypochlorous Acid as an Alternative Skin Antiseptic: An In Vitro Comparison. Dermatol Surg 44, 1489–1493. [DOI] [PubMed] [Google Scholar]
- Bachmann SP, VandeWalle K, Ramage G, Patterson TF, Wickes BL, Graybill JR and Lopez-Ribot JL (2002) In vitro activity of caspofungin against Candida albicans biofilms. Antimicrob Agents Chemother 46, 3591–3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bard AJ, Faulkner LR, Leddy J and Zoski CG (1980) Electrochemical methods: fundamentals and applications: Wiley. [Google Scholar]
- Barrette WC Jr, Hannum DM, Wheeler WD and Hurst JK (1989) General mechanism for the bacterial toxicity of hypochlorous acid: Abolition of ATP production. Biochemistry 28, 9172–9178. [DOI] [PubMed] [Google Scholar]
- Bjarnsholt T (2013) The role of bacterial biofilms in chronic infections. APMIS Suppl 121, 1–51. [DOI] [PubMed] [Google Scholar]
- Bjarnsholt T, Kirketerp-Møller K, Jensen PØ, Madsen KG, Phipps R, Krogfelt K, Høiby N and Givskov M (2008) Why chronic wounds will not heal: A novel hypothesis. Wound Repair Regen 16, 2–10. [DOI] [PubMed] [Google Scholar]
- Bourguignon GJ and Bourguignon LY (1987) Electric stimulation of protein and DNA synthesis in human fibroblasts. FASEB J 1, 398–402. [DOI] [PubMed] [Google Scholar]
- Brown MR and Gilbert P (1993) Sensitivity of biofilms to antimicrobial agents. J Appl Bacteriol 74 Suppl, 87S–97S. [DOI] [PubMed] [Google Scholar]
- Chandra J, Kuhn DM, Mukherjee PK, Hoyer LL, McCormick T and Ghannoum MA (2001) Biofilm formation by the fungal pathogen Candida albicans: Development, architecture, and drug resistance. J Bacteriol 183, 5385–5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degnim AC, Scow JS, Hoskin TL, Miller JP, Loprinzi M, Boughey JC, Jakub JW, Throckmorton A, Patel R and Baddour LM (2013) Randomized controlled trial to reduce bacterial colonization of surgical drains after breast and axillary operations. Ann Surg 258, 240–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas LJ (2003) Candida biofilms and their role in infection. Trends Microbiol 11, 30–36. [DOI] [PubMed] [Google Scholar]
- Dowd SE, Delton Hanson J, Rees E, Wolcott RD, Zischau AM, Sun Y, White J, Smith DM, Kennedy J and Jones CE (2011) Survey of fungi and yeast in polymicrobial infections in chronic wounds. J Wound Care 20, 40–47. [DOI] [PubMed] [Google Scholar]
- Dukan S and Touati D (1996) Hypochlorous acid stress in Escherichia coli: Resistance, DNA damage, and comparison with hydrogen peroxide stress. J Bacteriol 178, 6145–6150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmore S (2007) Apoptosis: A review of programmed cell death. Toxicol Pathol 35, 495–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eryılmaz M and Palabıyık I (2013) Hypochlorous acid - Analytical methods and antimicrobial activity. Tropical journal of pharmaceutical research 12, 123–126 [Google Scholar]
- Eryilmaz M and Palabiyik IM (2013) Hypochlorous Acid - Analytical Methods and Antimicrobial Activity. Tropical Journal of Pharmaceutical Research 12, 123–126. [Google Scholar]
- Estrela C, Estrela CR, Barbin EL, Spano JC, Marchesan MA and Pecora JD (2002) Mechanism of action of sodium hypochlorite. Braz Dent J 13, 113–117. [DOI] [PubMed] [Google Scholar]
- Harriott MM and Noverr MC (2009) Candida albicans and Staphylococcus aureus form polymicrobial biofilms: Effects on antimicrobial resistance. Antimicrob Agents Chemother 53, 3914–3922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawser SP and Douglas LJ (1995) Resistance of Candida albicans biofilms to antifungal agents in vitro. Antimicrob Agents Chemother 39, 2128–2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James GA, Ge Zhao A, Usui M, Underwood RA, Nguyen H, Beyenal H, deLancey Pulcini E, Agostinho Hunt A, Bernstein HC, Fleckman P, Olerud J, Williamson KS, Franklin MJ and Stewart PS (2016) Microsensor and transcriptomic signatures of oxygen depletion in biofilms associated with chronic wounds. Wound Repair Regen 24, 373–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James GA, Swogger E, Wolcott R, Pulcini E, Secor P, Sestrich J, Costerton JW and Stewart PS (2008) Biofilms in chronic wounds. Wound Repair Regen 16, 37–44. [DOI] [PubMed] [Google Scholar]
- Kiamco MM, Zmuda HM, Mohamed A, Call DR, Raval YS, Patel R and Beyenal H (2019) Hypochlorous-Acid-Generating Electrochemical Scaffold for Treatment of Wound Biofilms. Sci Rep 9, 2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloth LC (2005) Electrical stimulation for wound healing: A review of evidence from in vitro studies, animal experiments, and clinical trials. Int J Low Extrem Wounds 4, 23–44. [DOI] [PubMed] [Google Scholar]
- LaFleur MD, Kumamoto CA and Lewis K (2006) Candida albicans biofilms produce antifungal-tolerant persister cells. Antimicrob Agents Chemother 50, 3839–3846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewandowski Z and Beyenal H (2013) Fundamentals of Biofilm Research. Boca Raton, FL: CRC Press. [Google Scholar]
- Mangum LC, Franklin NA, Garcia GR, Akers KS and Wenke JC (2018) Rapid degradation and non-selectivity of Dakin’s solution prevents effectiveness in contaminated musculoskeletal wound models. Injury 49, 1763–1773. [DOI] [PubMed] [Google Scholar]
- Martin R, Wachtler B, Schaller M, Wilson D and Hube B (2011) Host-pathogen interactions and virulence-associated genes during Candida albicans oral infections. Int J Med Microbiol 301, 417–422. [DOI] [PubMed] [Google Scholar]
- McDonnell G and Russell AD (1999) Antiseptics and disinfectants: Activity, action, and resistance. Clin Microbiol Rev 12, 147–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna SM and Davies KJ (1988) The inhibition of bacterial growth by hypochlorous acid. Possible role in the bactericidal activity of phagocytes. Biochem J 254, 685–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Toole G, Kaplan HB and Kolter R (2000) Biofilm formation as microbial development. Annu Rev Microbiol 54, 49–79. [DOI] [PubMed] [Google Scholar]
- Percival SL, Hill KE, Williams DW, Hooper SJ, Thomas DW and Costerton JW (2012) A review of the scientific evidence for biofilms in wounds. Wound Repair Regen 20, 647–657. [DOI] [PubMed] [Google Scholar]
- Proskuryakov SY, Konoplyannikov AG and Gabai VL (2003) Necrosis: A specific form of programmed cell death? Exp Cell Res 283, 1–16. [DOI] [PubMed] [Google Scholar]
- Pullar JM, Vissers MC and Winterbourn CC (2000) Living with a killer: The effects of hypochlorous acid on mammalian cells. IUBMB Life 50, 259–266. [DOI] [PubMed] [Google Scholar]
- Raval YS, Mohamed A, Zmuda HM, Patel R and Beyenal H (2019) Hydrogen-Peroxide-Generating Electrochemical Scaffold Eradicates Methicillin-Resistant Staphylococcus aureus Biofilms. Glob Chall 3, 1800101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renslow R, Donovan C, Shim M, Babauta J, Nannapaneni S, Schenk J and Beyenal H (2011) Oxygen reduction kinetics on graphite cathodes in sediment microbial fuel cells. Phys Chem Chem Phys 13, 21573–21584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell AD (2003) Similarities and differences in the responses of microorganisms to biocides. J Antimicrob Chemother 52, 750–763. [DOI] [PubMed] [Google Scholar]
- Sandvik EL, McLeod BR, Parker AE and Stewart PS (2013) Direct electric current treatment under physiologic saline conditions kills Staphylococcus epidermidis biofilms via electrolytic generation of hypochlorous acid. PLoS One 8, e55118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt-Malan SM, Karau MJ, Cede J, Greenwood-Quaintance KE, Brinkman CL, Mandrekar JN and Patel R (2015) Antibiofilm Activity of Low-Amperage Continuous and Intermittent Direct Electrical Current. Antimicrob Agents Chemother 59, 4610–4615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selkon JB, Cherry GW, Wilson JM and Hughes MA (2006) Evaluation of hypochlorous acid washes in the treatment of chronic venous leg ulcers. J Wound Care 15, 33–37. [DOI] [PubMed] [Google Scholar]
- Sen CK, Gordillo GM, Roy S, Kirsner R, Lambert L, Hunt TK, Gottrup F, Gurtner GC and Longaker MT (2009) Human skin wounds: A major and snowballing threat to public health and the economy. Wound Repair Regen 17, 763–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shubin AV, Demidyuk IV, Komissarov AA, Rafieva LM and Kostrov SV (2016) Cytoplasmic vacuolization in cell death and survival. Oncotarget 7, 55863–55889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sood A, Granick MS and Tomaselli NL (2014) Wound Dressings and Comparative Effectiveness Data. Adv Wound Care (New Rochelle) 3, 511–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sultana ST, Atci E, Babauta JT, Falghoush AM, Snekvik KR, Call DR and Beyenal H (2015a) Electrochemical scaffold generates localized, low concentration of hydrogen peroxide that inhibits bacterial pathogens and biofilms. Sci Rep 5, 14908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sultana ST, Babauta JT and Beyenal H (2015b) Electrochemical biofilm control: A review. Biofouling 31, 745–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J, Zhong H, Du L, Li X, Ding Y, Cao H, Liu Z and Ge L (2018) Gene expression profiles of germ-free and conventional piglets from the same litter. Sci Rep 8, 10745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Acker H, Van Dijck P and Coenye T (2014) Molecular mechanisms of antimicrobial tolerance and resistance in bacterial and fungal biofilms. Trends Microbiol 22, 326–333. [DOI] [PubMed] [Google Scholar]
- Velnar T, Bailey T and Smrkolj V (2009) The wound healing process: An overview of the cellular and molecular mechanisms. J Int Med Res 37, 1528–1542. [DOI] [PubMed] [Google Scholar]
- Wagner DK, Collinslech C and Sohnle PG (1986) Inhibition of Neutrophil Killing of Candida-Albicans Pseudohyphae by Substances Which Quench Hypochlorous Acid and Chloramines. Infect Immun 51, 731–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Bassiri M, Najafi R, Najafi K, Yang J, Khosrovi B, Hwong W, Barati E, Belisle B, Celeri C and Robson MC (2007) Hypochlorous Acid as a Potential Wound Care Agent: Part I. Stabilized Hypochlorous Acid: A Component of the Inorganic Armamentarium of Innate Immunity. J Burns Wounds 6, e5. [PMC free article] [PubMed] [Google Scholar]
- Wilgus TA, Roy S and McDaniel JC (2013) Neutrophils and Wound Repair: Positive Actions and Negative Reactions. Adv Wound Care (New Rochelle) 2, 379–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolcott RD, Rumbaugh KP, James G, Schultz G, Phillips P, Yang Q, Watters C, Stewart PS and Dowd SE (2010) Biofilm maturity studies indicate sharp debridement opens a time- dependent therapeutic window. J Wound Care 19, 320–328. [DOI] [PubMed] [Google Scholar]