Abstract
Extinguished responding will renew when the conditioned stimulus occurs outside the extinction context. Although studies of conditioned freezing have consistently demonstrated a role for the hippocampus in renewal, several studies have demonstrated intact renewal of conditioned suppression despite damage to the hippocampus (Frohardt et al., 2002; Todd et al., 2017; Wilson et al., 1995). Because these prior studies have examined renewal when testing occurred in the original conditioning context (“Context A”), the present conditioned suppression experiments examined the role of the hippocampus when testing occurred in a context not associated with prior conditioning (“Context C”). In Experiments 1 and 2, conditioning occurred in Context A, and extinction in Context B. Renewal of conditioned suppression was observed when the extinguished CS was tested in Context C. However, renewal was attenuated in rats with lesions of the dorsal hippocampus (DH). Summation testing failed to detect conditioned inhibition in the extinction context, suggesting instead that the context acquired negative occasion-setting properties. Attenuated renewal was not due to an inability of DH lesioned rats to discriminate contexts (Experiment 3). These experiments thus demonstrate a role for the DH in renewal of conditioned suppression when testing occurs in a neutral context.
Keywords: fear extinction, renewal, conditioned suppression, hippocampus
During extinction, responding to a previously established Pavlovian conditioned stimulus (CS) is reduced when the CS is repeatedly presented in the absence of the unconditioned stimulus (US). Although conditioned responding declines during extinction, this suppression of behavior is not permanent; responding is apt to recover under a variety of conditions (see Bouton, 2002, 2004, 2017). For instance, when an extinguished CS is presented in a context different from the one in which it was extinguished, responding will return. This recovery of responding, referred to as renewal (Bouton & Bolles, 1979), demonstrates that what is learned during extinction is intimately tied to the context.
There are several mechanisms by which the context might work to suppress responding during extinction and/or promote responding during renewal (see Nelson et al., 2011; McConnell & Miller, 2014). For example, during extinction, a direct inhibitory association between the context and the US (i.e., conditioned inhibition) would protect the CS from a complete loss of associative strength, and allow for a return of responding outside the extinction context (e.g., Rescorla, 2003; for a discussion see Delamater & Westbrook, 2014). However, tests of the associative status of the extinction context have routinely failed to detect conditioned inhibition (Bouton & King, 1983; Bouton & Swartzentruber, 1986, 1989; Nelson et al., 2011). Indeed, there is very little evidence that extinction contexts become conditioned inhibitors (for reviews see Bouton, 1993; Trask et al., 2017) except under unusual circumstances (cf. Polack, Laborda & Miller, 2012).
In the renewal context, a direct excitatory association between the context and the US might promote responding and facilitate renewal. For example, in so-called ABA renewal, responding will recover when a CS is first extinguished in Context B, and then tested back in the original conditioning context (Context A). In this case, renewal could potentially result from any residual CS excitation summating with excitation conditioned directly to Context A. Bouton and King (1983) observed ABA renewal of conditioned suppression and found no evidence of contextual excitation as measured by context-preference tests. In addition, renewal has been observed in situations where testing occurs in a neutral context, and thus contextual excitation should not be present. For instance, Bouton and Bolles (1979) observed renewal when testing occurred in a third, neutral context (ABC renewal), and Bouton and Ricker (1994) reported renewal when conditioning and extinction occurred in the same context and testing occurred in a second, neutral context (AAB renewal; see also Laborda et al., 2011).
Taken together, these findings indicate that renewal does not depend on conditioned inhibition in the extinction context, or conditioned excitation in the renewal context. Instead, it is often assumed that contexts influence responding during extinction and renewal, not through direct associations with the US, but by modulating the entire CS-US association. The extinction context might act as a “negative occasion setter” (Holland, 1992) and signal that the CS will not be followed by the US. Thus, when the extinguished CS is presented in the extinction context, responding is suppressed. But when the CS is presented outside the extinction context, either in a neutral context or the original conditioning context, the influence of the negative occasion setter is removed and responding thus renews. It is noteworthy that renewal in the original conditioning context might also benefit from Context A having “positive occasion setting” properties. In contrast to the extinction context, the conditioning context might signal that the CS will be followed by the US (Bouton & Swartzentruber, 1986). Positive occasion setting by the conditioning context may be especially likely once the CS had undergone extinction (Harris et al., 2000). Thus, through both negative and positive occasion setting, contexts can come to suppress and promote conditioned responding to extinguished cues.
There is considerable interest in identifying the neural substrates of extinction and renewal (for reviews see Delamater, 2004; Maren, 2011; Maren et al., 2013; Todd et al., 2014). In particular, an impressive body of research by Maren and colleagues has identified the hippocampus as critically important in controlling the context-dependence of extinction. For example, temporary inactivation of the hippocampus at the time of test has been shown to reduce AAB and ABC renewal of conditioned freezing (Corcoran & Maren, 2001, 2004). In addition, Knapska and Maren (2009) reported increased c-fos, a marker of neural activity, in the hippocampus when an extinguished CS was tested in either the extinction or renewal context during AAB renewal. Results such as these have led to the suggestion that the role of the hippocampus is to regulate the “gating” or occasion – setting properties of the context following extinction (Maren et al., 2013). When the hippocampus is damaged or inactivated, extinction does not remain context specific and the CS elicits low levels of responding in both the extinction and renewal context (Maren, 2011).
Nevertheless, several studies have failed to find a role for the hippocampus in extinction and renewal. For example, lesions or temporary inactivation of the dorsal hippocampus have no impact on ABA or ABC renewal of appetitive conditioning in rats (Campese & Delamater, 2013). Furthermore, although disruption of the hippocampus consistently impairs renewal of conditioned freezing, extinction and renewal in other aversive conditioning preparations does not appear to depend on the hippocampus. Specifically, pre-training lesions of the fornix or the entire hippocampus have no impact on ABA renewal of conditioned suppression (Frohardt et al., 2000; Wilson et al., 1995). Likewise, post-extinction lesions of the dorsal hippocampus also have no impact on ABA renewal of conditioned suppression (Todd et al., 2017). One source of the discrepancy within aversive conditioning paradigms, either conditioned freezing or conditioned suppression, could be that all conditioned suppression experiments examining hippocampal involvement have tested ABA renewal. In contrast, the positive findings from studies of conditioned freezing have tended to test either ABC renewal (Corcoran & Maren 2001; Experiment 2) or AAB renewal (Corcoran & Maren, 2004; Corcoran et al., 2005; Experiment 2; Ji & Maren, 2005, 2008). Indeed, even in studies of conditioned freezing a role for the hippocampus in ABA renewal is not clear, with some negative (e.g., Corcoran & Maren, 2001) and some positive results (e.g., Ji & Maren, 2005). Thus, the hippocampus may have an important role in AAB and ABC renewal of aversive conditioning, while its role in ABA renewal is either less critical or may depend on specific parameters. For example, Zelikowsky et al., (2012) reported pre-training hippocampal damage attenuates renewal, but only when the CS duration at test differed from the CS duration during extinction.
The purpose of the current experiments was to further examine the role of the hippocampus in extinction and renewal of conditioned suppression. As noted, all prior studies have found no impact of hippocampal lesions on renewal in this conditioning paradigm (Frohardt et al., 2000; Wilson et al., 1995; Todd et al., 2017). However, these studies have all tested for ABA renewal. Thus, it remains possible that the hippocampus does contribute to renewal, but only when testing occurs in a context other than the original conditioning context. To that end, we examined the impact of hippocampal lesions on ABC renewal of conditioned suppression.
In addition, to more completely understand the source of renewal in these experiments, we independently assessed the associative status of the extinction context. Although there are several ways to test the associative properties of the context (see Bouton & King, 1983; Bouton & Swartzentruber, 1986) we elected to conduct summation tests to directly assess the possible presence of conditioned inhibition. If the extinction context acquired conditioned inhibition, it would be expected to reduce responding to a separately trained transfer excitor. Conversely, a failed summation test would suggest that the extinction context suppressed responding through an occasion-setting type of mechanism, signaling that the CS will not be followed by the US. To our knowledge, no other studies examining the role of the hippocampus in extinction and renewal of aversive conditioning have also independently assessed the associative status of the extinction context, making it difficult to determine the role of the context in prior studies1.
Experiment 1
The design of Experiment 1 is depicted in Table 1. All rats first received either sham or electrolytic lesions of the dorsal hippocampus. Next, they were trained to lever press in Contexts A, B, and C. After one CS pre-exposure session, all rats then underwent aversive conditioning in Context A, in which a light-off CS was paired with footshock. Following acquisition, extinction occurred in Context B, with equal exposures to Context C. Renewal testing then occurred over the final two days, with all rats being tested in both the extinction context (B) and the renewal context (C). The order of testing was counterbalanced such that half the rats in each group were first tested in B, and half were first tested in C. Sham lesioned rats were expected to show more suppression in Context C than B. If the hippocampus mediates ABC renewal of conditioned suppression, then lesioned rats were expected to show little or no suppression in Context C.
Table 1.
Behavioral Procedure of Experiments 1 and 2.
| Pre | Cond. | Ext. | Renewal Testing |
Summation Training |
Summation Testing |
|---|---|---|---|---|---|
| AL− | AL+ | BL−, C− | BL− and CL− or CL− and BL− | A: Ck+ | B: Ck− and C: Ck− or C: Ck− and B: Ck− |
Note. “A” = Context A, “B” = Context B, and “C” = Context C. “L” = light-off CS. “Ck” = click CS. “+” = footshock (.5 mA, 1 s), “−” = nonreinforcement. Both the renewal test and summation test were within subject with test order counterbalanced. During both tests, half of each group was tested in C and then B, and half were tested in B and then C.
The associative status of the extinction context was assessed via summation testing. Following renewal testing, an auditory CS was paired with shock in Context A. This cue was then tested in both Contexts B and C. If context B acquired conditioned inhibition during extinction training, then responding to the auditory cue would be expected to be lower in Context B relative to Context C.
Method
Subjects
The subjects were 24 adult male Long Evans rats, obtained from Envigo (South Easton, MA), and were about ~60 days old upon arrival. Rats were housed individually and allowed at least 6 days to acclimate to the vivarium prior to surgery. Food was available ad libitum prior to surgery (Purina standard rat chow; Nestle Purina, St. Louis, MO). Throughout the study, rats were maintained on a 14:10 light-dark cycle and monitored and cared for in compliance with association for Assessment and Accreditation of laboratory Animal Care guidelines and the Dartmouth College Institutional Animal Care and Use Committee.
Surgery
All surgeries took place over a 4-day period that immediately followed the acclimation period. Subjects were anesthetized with isoflurane gas (1.5% - 3% in oxygen) and placed in a Kopf stereotaxis apparatus. The skin was retracted and holes were drilled through the skull above each of the intended lesion sites (see Table 2) using the rat brain atlas of Paxinos and Watson (2009). Twelve rats received bilateral electrolytic lesions (2.5 mA, 25 s at each site) of the DH prior to behavioral training. Control rats received sham lesions consisting of a craniotomy and shallow, non-puncturing burr holes to minimize damage to the underlying cortex. Rats were allowed to recover for at least 1 week and were then food restricted and maintained at 85% body weight throughout the rest of the experiment.
Table 2.
Stereotaxic coordinates for dorsal hippocampus lesions.
| Lesion | AP | ML | DV |
|---|---|---|---|
| Electrolytic DH | −2.8 | ± 1.6 and ± 2.6 | −3.7 |
| −4.2 | ± 3.6 and ± 4.2 | −3.7 (medial site) and −4.0 (lateral site) | |
| Neurotoxic DH | −2.8 | ± 1.6 | −3.7 |
| −4.2 | ± 2.6 | −3.7 |
Note. All anterior-posterior (AP), medial-lateral (ML) and dorsal-ventral (DV) measurements are derived from bregma, midline, and skull surface, respectively (measurements are in mm). DH = dorsal hippocampus.
Behavioral apparatus
One set of eight conditioning chambers served as Context A. Each chamber (ENV-007, Med Associates, Georgia, VT) was housed in its own sound attenuation chamber (Med Associates, ENV-017M; 66 cm W × 56 cm H × 56 cm D) and outfitted with an exhaust fan to provide airflow and background noise (~68 dB). The sidewalls and ceiling were made of clear acrylic plastic and the front and rear walls were made of brushed aluminum. The grid floor was composed of stainless-steel rods (5-mm diameter) spaced 1.5 cm apart (center-to-center). Each chamber was outfitted with a food cup, recessed in the center of the front wall. Retractable levers (Med Associates, ENV-112CM) were positioned to the left and right of the food cup (the left lever remained retracted throughout the course of the experiment). A speaker (Med Associates, ENV-224AM) was located 20 cm above and to the right of the food cup. A house-light (Med Associates, ENV-215M) was mounted 24 cm above the grid floor at the back of the chamber and remained off over the course of the experiment. There were three panel lights (Med Associates, ENV-221M) in the chamber that remained off over the course of the experiment: one above each lever, and one just above the food cup. The chambers were illuminated by a fourth panel light, located ~16 cm above the grid floor centered over the food cup. Prior to each session, ~1.5 mls of VapoRub ointment (Vicks, USA) was placed in the waste pan to produce a distinct olfactory cue.
An additional two sets of four conditioning chambers served as Contexts B and C (counterbalanced). These chambers were modified versions of Context A and were located in a separate room in the laboratory. Like Context A, these chambers were outfitted with a food cup, a retractable lever to the right of the food cup, a house light (which remained off), a speaker, and were illuminated by a panel light mounted ~16 cm above the grid floor and centered over the food cup. These chambers did not have the three additional panel lights or the left lever.
In one set of chambers, the sidewalls and ceiling were made of clear acrylic plastic and the front and rear walls were made of brushed aluminum. The grid floor was stainless steel rods (5-mm diameter) spaced 1.5 cm apart (center-to-center). A manila folder was taped to the outside of the door and ceiling to provide a distinct visual feature. In addition, the waste pans of these chambers were filled with sawdust bedding.
In the second set of chambers the grids of the floor were staggered such that odd- and even-numbered grids were mounted in two separate planes, one 0.5 cm above the other. The staggered grid floor provided a distinct tactile feature. The ceiling and door were covered with laminated black and white checkerboard paper (1-cm squares) to provide distinct visual cues. Three panels on the back wall were covered in black electrical tape to produce a “checkerboard” pattern. The exhaust fans in these chambers were turned off. Prior to each session, ~3 mls of 15% Anise extract (McCormick, USA) in water was placed in the waste pan (which did not include sawdust bedding) to produce a distinct olfactory cue.
The offset of the panel light mounted to the front wall served as the visual CS. During CS presentation, the panel light turned off for 60 s. The auditory stimulus was a click (74 dB, 10 Hz) generated by Med Associates stimulus generator (ANL – 926). Footshocks (1 mA, 0.5 s) were generated by a Med Associates shock generator (ENV-414) connected to each chamber. The reinforcer was a 45-mg grain based rodent food pellet (BioServ, Flemington, NJ). The apparatus was controlled by computer equipment located in an adjacent room. Surveillance cameras located inside the sound-attenuating chambers were used to monitor the rats’ behavior.
Behavioral procedures
Baseline lever-press training.
On the first day of the experiment, each rat was assigned to one chamber (Context A) and received a single 45-minute session of magazine and lever training (all experimental sessions were 45 minutes). During this session, food pellets were delivered freely on a random-time 60-s (RT 60-s) schedule. In addition, all lever presses were reinforced with a single pellet. On the next day, all rats received an identical training session in Context B, and the following day in Context C. Over the next 9 days, all rats received 3 “cycles” of further lever press training. Each cycle consisted of three daily training sessions: Day 1 in Context A, Day 2 in Context B, and Day 3 in Context C. During the first cycle, lever presses were reinforced on a random-interval 15-s (RI 15-s) schedule. The schedule increased to RI-30 s for the second cycle and then to RI-60 s for the third cycle. The RI-60 s schedule was in place for the remainder of the experiment. In total, all rats had 4 sessions of lever training (magazine and lever session, RI-15, RI-30, and RI-60) in each of Context A, B, and C.
Pre-exposure and conditioning.
Following baseline lever press training, there was a single session in Context A in which the light CS was presented 4 times with an average intertrial interval (ITI) of 9 minutes (± 25%). The first CS presentations always occurred 9 minutes after placement into the chamber. As usual, responding throughout the session was reinforced on a RI-60 s schedule.
Conditioning occurred over the course of a three-day cycle. On Day 1, all rats received 4 pairings of the CS with footshock (1 mA, 0.5 s) in Context A. The timing of CS presentations occurred in the same manner as the pre-exposure phase, and lever pressing was reinforced throughout the session on a RI-60 schedule. On Day 2, all rats received a baseline lever press training session in Context A. On Day 3, rats received an additional 4 light-shock pairings in Context A, with the same procedure as Day 1.
Extinction.
Following conditioning, each rat received a session of lever training (RI-60) in Context B, and then Context C. Extinction training then occurred over the course of 4, two-day cycles. On the first day of each cycle, all rats received 8 nonreinforced presentations of the light in Context B (no shocks were presented). The first CS was presented 9 minutes after placement into the chamber. All subsequent ITIs were 3.5 minutes. Rats were removed from the chambers 3.5 minutes following the last trial. On the second day of each cycle, all rats received a lever training session of equal duration as extinction (45 minutes) in Context C.
Renewal Test.
Renewal testing occurred on the next two subsequent days. All rats received 4 nonreinforced presentations of the CS in both Contexts B and C, with test order counterbalanced. Half of the rats in each group were first tested in B and half in C. During the test, the ITI was variable, with an average ITI of 9 minutes (± 25%). The first CS occurred 9 minutes after placement into the chamber.
Summation training and testing.
Summation training occurred over the course of two, 2-day cycles. On the first day of each cycle, all rats received a baseline lever training session in Context A. On the second day of the cycle, all rats received conditioning with the click stimulus. During this session, the CS was paired with shock, with an average intertrial interval (ITI) of 9 minutes (± 25%). The first CS always occurred 9 minutes after placement into the chamber. There was then a second cycle of baseline training and conditioning to the click. Finally, on the next two days all rats received baseline lever training in Context A.
Suppression to the click CS was then tested in Contexts B and C for all rats. Summation testing was identical to renewal testing, with the exception that the click stimulus was presented instead of the light CS. All rats were tested in both Contexts B and C over the course of two days, with test order counterbalanced.
Behavioral observations and data analysis.
Throughout the experiment, suppression to the light and click stimuli was indexed in terms of standard suppression ratios of the form x/(x+y), where x represents the number of lever presses made during the 60 s stimuli, and y represents the number of lever presses made during the 60 s period just prior to the onset of the stimuli (pre-CS). Thus, a score of .5 indicates no suppression, whereas a score of 0 indicates complete suppression. Suppression ratios and pre-CS scores were calculated and then averaged over 4-trial blocks. Assessment of suppression requires responding during the pre-CS period. Therefore, in rare instances, if a rat failed to press during the pre-CS period, the trial-block mean for both suppression ratios and pre-CS responding was calculated without that trial. For each phase of the experiment we report how frequently this occurred for each group. In Experiment 1, the mean percentage of trials excluded for Sham lesioned rats was 1.04 (Mdn = 0, SEM = .46), and for DH lesioned rats was 2.68 (Mdn = 0, SEM = 1.77). The percent trials excluded did not differ across groups, F(1, 22) = .80, p = .38. In addition, during conditioning sessions, baseline lever pressing was disrupted by shock. We therefore analyze the first trial only from those sessions. Suppression ratios and pre-CS scores were analyzed with analysis of variance (ANOVA) using a rejection region of p < .05.
Lesion verification and analysis.
After the behavioral procedures were completed rats were deeply anesthetized with an overdose of sodium pentobarbital and transcardially perfused with 0.9% saline for 2 min, followed by 10% buffered formalin for 6 min. Coronal brain sections (60 μm) were collected using a freezing microtome and were Nissl-stained using thionin. Using a compound microscope (Axioskop I, Zeiss, Inc.), we identified gross tissue damage as necrosis, missing tissue, or marked thinning of the cortex. Outlines of the lesions were drawn onto digital images adapted from Paxinos and Watson (2009) using ImageJ at 4 levels along the rostro-caudal extent of the dorsal hippocampus (−2.04, −2.76, −3.60, and −4.36 mm from bregma). From these lesion drawing, we report the average number of sections containing damage to the dorsal hippocampus, and the number of rats with damage to regions outside the hippocampus.
Results
Histology
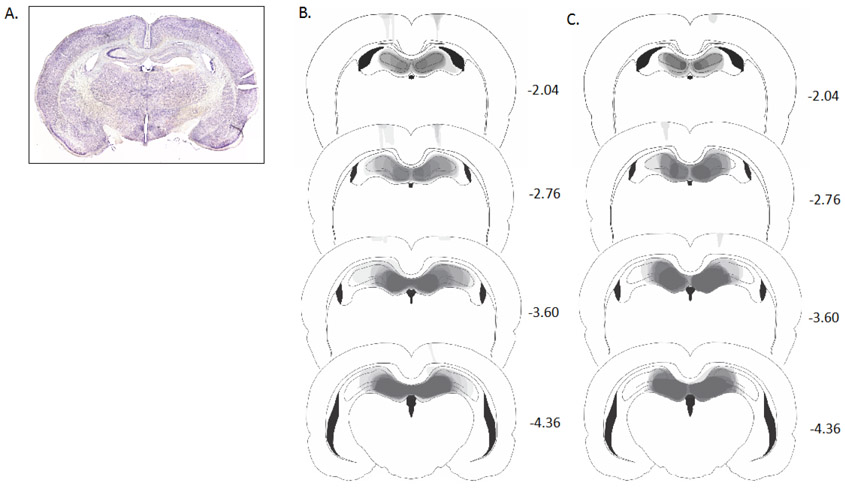
Figure 1a shows a photomicrograph of a representative DH lesion. In Figure 1b, lesions drawings are stacked onto a single atlas image for each of the 4 coronal levels. Bilateral DH damage was observed in all rats including CA1, CA2, CA3, and dentate gyrus. There was minor damage outside the hippocampus in all rats (e.g., cingulum bundle, visual cortex, motor cortex, somatosensory cortex). Damage to the hippocampus was present on 98.9 ± 3.6% of the sections collected for each subject, indicating that damage extended throughout the rostro-caudal extent of the DH.
Figure 1.
A: Photomicrograph of an electrolytic DH lesion. B: Drawings of lesions at four levels along the rostro-caudal extent of the DH. At each level, lesion drawings were stacked onto a single image. The darkness of an area indicates the number of lesions cases that include that area. Grey boxes (next to the bregma values) represent the expected darkness for overlap from all subjects. Adapted from The Rat Brain in Stereotaxic Coordinates (Compact 6th ed.), by G Paxinos and C. Watson, 2009.
Behavior
Baseline lever-press training.
Pre-training lesions of the hippocampus resulted in more rapid acquisition of lever pressing, and a higher asymptote of responding, during the initial baseline-training phase. A 2 (Group) × 12 (Session) ANOVA revealed a main effect of session, F(11, 242) = 36.68, p < .01, η2p = .63, and an interaction between group and session, F(11, 242) = 2.59, p < .01, η2p = .11. The main effect of group was not significant F(1, 22) = 3.51, p = .07. For the final session of baseline training, Sham lesioned rats averaged 12.41 (SEM = 1.15) presses per minute and DH lesioned rats averaged 21.01 (SEM = 4.65) responses per minute.
Pre-exposure.
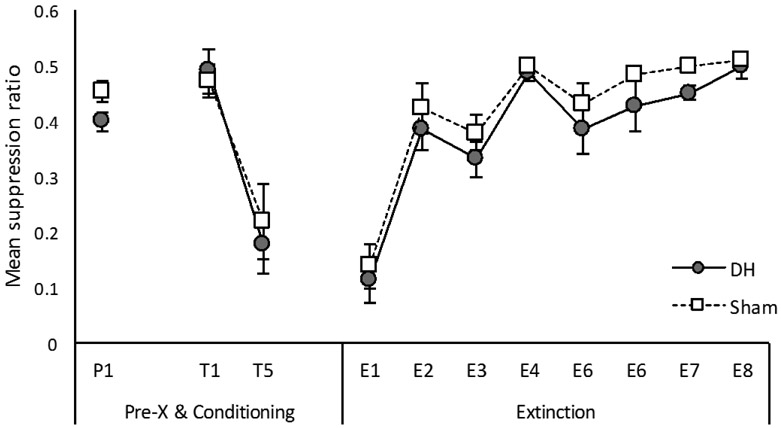
Mean suppression ratio during the pre-exposure phase of the Experiment is presented in the left portion of Figure 2. Groups did not differ during the pre-exposure phase of the experiment, F(1, 22) = .72, p = 41. The difference between groups during the pre-CS period approached significance, F(1, 22) = 4.03, p = .06. One sham lesioned rat failed to make a lever press in the pre-CS period for one trial. Average pre-CS responses for all phases of each experiment for each group are presented in Table 3.
Figure 2.
Mean suppression ratios for pre-exposure, conditioning, and extinction in Experiment 1. “P1” = suppression during the pre-exposure session in which the light stimulus was presented 4 times. “T1” and “T5” = suppression for trials 1 and 5 of conditioning, respectively. “E1” to “E8” = suppression during extinction calculated as 4-trial blocks. Sham = sham lesioned rats, DH = dorsal hippocamal lesioned rats. Error bars represent ±1 SEM.
Table 3.
Mean pre-CS responding for Experiments 1 – 3.
| Pre-Ex | Cond. Trial | Extinction (4-trial blocks) | Renewal Test |
Summation Cond. |
Summation Test |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Experiment | Group | Trial 1 | Trial 5 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | Trial 1 | Trial 5 | |||
| Exp. 1 | Sham | 12.85 (1.86) | 10.8 (0.99) | 13.81 (1.70) | 12.24 (1.95) | 13.96 (2.09) | 14.21 (2.24) | 16.22 (2.65) | 19.85 (3.41) | 18.96 (3.1) | 19.15 (2.81) | 20.52 (2.74) | 20.07 (2.92) | 16.54 (3.28) | 10.45 (3.29) | 17.29 (2.32) |
| Lesion | 22.48 (4.4) | 20.08 (4.52) | 21.0 (4.33) | 15.04 (2.66) | 15.68 (2.74) | 18.73 (4.82) | 22.08 (4.18) | 24.94 (5.83) | 22.54 (4.29) | 26.44 (6.77) | 27.19 (7.68) | 25.42 (6.26) | 22.58 (3.77) | 20.3 (3.97) | 21.71 (5.5) | |
| Exp. 2 | Sham | 17.19 (2.52) | 16.5 (2.91) | 16.25 (2.65) | 13.79 (1.96) | 15.73 (2.96) | 19.63 (4.14) | 20.13 (3.95) | 21.1 (4.65) | 20.13 (4.06) | 22.35 (4.34) | 21.90 (4.25) | 18.24 (2.80) | 15.1 (3.07) | -- | 20.18 (3.23) |
| Lesion | 16.96 (1.94) | 13.18 (1.75) | 15.72 (2.81) | 14.71 (2.17) | 15.82 (2.34) | 21.45 (3.55) | 20.23 (2.78) | 19.27 (4.27) | 20.7 (5.0) | 20.8 (3.8) | 19.91 (3.54) | 19.53 (2.45) | 24.9 (4.78) | -- | 22.64 (3.27) | |
| Exp. 3 | Sham | 13.38 (1.64) | 17.88 (2.21) | 12.66 (1.66)* | 10.41 (0.96) | 13.42 (1.22) | 17.06 (1.61) | 20.22 (3.57) | 16.64 (1.41) | 15.89 (1.67) | 25.64 (4.23) | 26.91 (4.94) | ||||
| Lesion | 16.71 (4.30) | 20.42 (4.47) | 24.14 (5.35) | 14.62 (2.74) | 17.04 (3.69) | 22.71 (3.79) | 23.89 (3.12) | 23.07 (4.01) | 24.5 (4.49) | 27.5 (3.29) | 26.92 (3.46) | |||||
Note. Mean pre-CS responding for each group in each experiment (SEM in parentheses).
indicates a significant difference between Sham and DH lesioned rats (p < .05).
Pre-Ex = pre-exposure, Cond. Trial = Conditioning, Summation Cond. = Summation Conditioning. As noted in text, pre-CS responding did not differ between contexts B and C during renewal and summation testing. Responding is therefore averaged across contexts.
Conditioning.
The acquisition of conditioned suppression is presented in the left portion of Figure 2. Only the first trial is plotted and analyzed for each conditioning session. Suppression to the CS did not differ between groups on the first trial (“T1” from conditioning day 1), F(1, 22) = .83, p = .37. By the first trial of the second day (trial 5 of conditioning, “T5”) suppression was on the response floor for both groups, which did not differ, F(1, 20) = .29, p = .60. For pre-CS scores, the group effect approached significance on trial 1, F(1, 22) = 3.98, p = .06, but was not significant for trial 5, F(1, 20) = 2.38, p = .13. Two rats failed to make a lever press in the pre-CS period for trial 5 (1 Sham, 1 DH).
Extinction.
The results of the extinction phase are presented in the right portion of Figure 2. As extinction progressed, conditioned suppression weakened for both groups at a similar rate. A 2 (Group) × 8 (Trial block) ANOVA revealed a main effect of trial block, F(7, 154) = 74.96, p < .001, η2p = .77. Neither the main effect of group nor the group by trial block interaction were significant, Fs < 1. For pre-CS scores, a 2 (Group) × 8 (Trial block) ANOVA revealed only a main effect of trial block, F(7, 154) = 8.83 p < .001, η2p = .29 (all other Fs < 1). During the first session of extinction only, 2 Sham and 3 DH lesioned rats failed to make a lever press during the pre-CS period, for a total of 3 and 6 trials, respectively.
The first and last trial from each extinction session are presented in Figure 3. For trial 1, both groups demonstrated near complete suppression and did not differ, F(1, 22) = 1.82, p = .20. By trial 32, suppression was substantially reduced, and groups did not differ, F(1, 22) = .69, p = .42. Pre-CS responding did not differ between groups for either trial 1 or trial 32, largest F(1, 22) = .84, p = .37.
Figure 3.
Spontaneous recovery in Experiment 1. Mean suppression ratios are plotted for the first and last trials of each extinction session. Spontaneous recovery (Sp. Rec.) was evident between all sessions. Sham = sham lesioned rats, DH = dorsal hippocamal lesioned rats. Error bars represent ±1 SEM.
It is also clear that spontaneous recovery occurred between some of the extinction sessions. We therefore assessed the impact of pre-training DH lesions on spontaneous recovery by comparing the last trial of each extinction session with the first trial of the next session with a 2 (Group) × 2 (Trial: Last vs. First) ANOVA. There was a significant main effect of trial for all comparisons indicating that spontaneous recovery occurred between all sessions, minimum F(1, 22) = 4.80, p = .039, η2p = .18. Neither the main effect of group nor the group by trial interaction were significant for any comparisons, largest F(1, 20) = 2.97, p = .10. Pre-CS responding was analyzed with identical ANOVAs. There was an effect of trial between extinction sessions 2 and 3, F(1, 22) = 22.01, p < .001, η2p = .50. No other main effects or interactions were significant, largest F(1, 22) = 1.57, p = .22. Two DH lesions rats failed to press during the pre-CS period of the last trial for extinction session 1 and were therefore not included in that comparison.
Renewal.
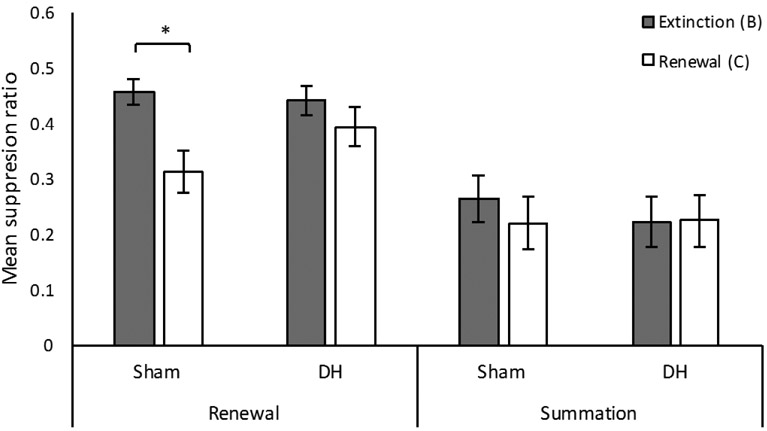
The results of the within-subject renewal test are presented in the left portion of Figure 4. For sham lesioned rats, there was more suppression in the renewal context (C) relative to the extinction context (B). However, lesions of the DH prevented renewal of suppression. The test data was analyzed with 2 (Group) × 2 (Context) ANOVA. The main effect of group was not significant, F(1, 22) = .68, p = .42. However, the main effect of context was significant, F(1, 22) = 24.43, p < .001, η2p = .53, as well as the critical interaction between context and group, F(1, 22) = 5.89, p = .024, η2p = .21. Sham lesioned rats showed more suppression in the renewal context relative to the extinction context (p < .001). However, DH lesioned rats did not show significantly more suppression in the renewal context relative to extinction context (p = .09). Analysis of pre-CS responding did not reveal any significant main effects or interactions, largest F(1, 22) = 1.34, p = .26.
Figure 4.
Results of renewal and summation testing in Experiment 1. Renewal: suppression to the light-off CS in both the extinction (B) and renewal (C) contexts. Summation: suppression to the click CS in both the extinction (B) and renewal (C) contexts. Both tests included 4 trials in each context. Error bars represent ±1 SEM. *p < .05
Because renewal is often strongest on the first test trial, we also compared the first test trial from Context B and Context C for each group separately (data not shown). Paired t-tests revealed a significant context effect for Sham lesioned rats, t(11) = 3.52, p = .005, but not for DH lesioned rats, t(11) = 1.80, p = .10. On the first test trial, mean suppression for Sham lesioned rats was .38 (SEM = .04) in Context B and .16 (SEM = .05) in Context C. For DH lesioned rats mean suppression on the first test trial was .40 (SEM = .05) in B and .29 (SEM = .06) in C. Identical analysis of pre-CS scores did not reveal a significant effect of context for either group (ps > .60).
Summation training.
Both groups acquired suppression the click stimulus (data not shown). On trial 1 of click conditioning, Sham lesioned rats showed greater suppression than DH lesioned rats, F(1, 21) = 5.89 , p = .024. Mean suppression to the click on trial 1 was .30 (SEM = .06) for Sham and .45 (SEM = .03) for DH lesioned rats. By trial 5, suppression was nearly complete for both groups, which did not differ, F(1, 19) = .35 , p = .56. Mean suppression to the click on trial 5 was .06 (SEM = .05) for Sham and .10 (SEM = .06) for DH lesioned rats. Pre-CS responding did not differ between groups on either trial 1 or 5, largest F(1, 19) = 3.70 p = .07. One sham lesioned rat failed to press during the pre-CS period for trial 1. One sham lesioned rat and 2 DH lesioned rats failed to press during the pre-CS period of trial 5.
Summation testing.
The results of the within-subject summation test are presented in the right portion of Figure 4. The click CS produced suppression, but it was equivalent in Contexts B and C and equivalent in the two groups. One DH lesioned rat failed to respond during the pre-CS period on 4 of the 8 test trials and was therefore removed from the analysis. A second DH lesioned rat failed to press during the pre-CS period for 1 trial (thus, successfully pressed for 7 of 8 test trials, and therefore remained in the analysis). Suppression during summation testing was analyzed with a 2 (Group) × 2 (Context) ANOVA. There were no significant main effects or interactions, largest F(1, 21) = .59, p = .45. Thus, suppression to the click did not differ across contexts or groups. Analysis of pre-CS responding did not reveal any significant main effects or interactions, largest F(1, 21) = 2.12, p = .15.
Discussion
In the present experiment, pre-training lesions of the dorsal hippocampus had no detectable impact on the acquisition, extinction, or spontaneous recovery of conditioned suppression (see Figures 2 and 3). These findings are consistent with prior experiments examining the effect of electrolytic fornix lesions (Wilson et al., 1995) or complete excitotoxic hippocampal lesions (Frohardt et al., 2000) on conditioned suppression. Nevertheless, electrolytic lesions of the dorsal hippocampus did weaken ABC renewal of conditioned suppression. Although conditioned suppression renewed in Context C for Sham lesioned rats, there was no evidence of renewal for rats with lesions of the dorsal hippocampus. Furthermore, as assessed via summation testing, there was no evidence of conditioned inhibition in Context B, suggesting instead that the extinction context suppressed responding by modulating the relationship between the CS and the US. To our knowledge, these results are the first to identify a role for the dorsal hippocampus in renewal of conditioned suppression.
Experiment 2
The use of electrolytic lesions in Experiment 1 leaves open the possibility that the observed deficit was due to either damage to neurons in the DH, or axonal fibers of passage. Therefore, in Experiment 2, lesions of the DH were made via infusions of N-methyl-D-asparate (NMDA), an excitotoxin that produces neuronal lesions but spares fibers of passage (Maren et al., 1997). In addition, in Experiment 2 lesions of the DH were made post-extinction training. In Experiment 1, because lesions of the DH occurred prior to training, it is not possible to differentiate between a role for the DH in the encoding or retrieval of contextual information normally acquired during extinction. Thus, in order to more closely assess the role of the DH in the retrieval of contextual information, in Experiment 2 lesions of the DH were made following extinction training.
Although there was no evidence of summation in Experiment 1, it is possible that the relatively strong associative strength of the conditioned excitor made it difficult to detect conditioned inhibition in Context B. Therefore, in Experiment 2, summation testing was conducted with a transfer excitor that first underwent less conditioning. We note that stimuli that have been both conditioned and extinguished may be especially sensitive to inhibition (Swartzentruber & Rescorla, 1994; see Rescorla, 2008). Although we considered this possibility, we decided upon the current design for two reasons. First, summation has been reported with transfer CSs that have not undergone extinction (Polack et al., 2012; Williams & Overmier, 1988; Zimmer-Hart & Rescorla, 1974). Second, the modulatory influence of negative occasion-setters can transfer to cues that have been the target of other occasion setters (see Holland, 1992). Extinction in one context (e.g., A) might thus produce a suitable target for transfer of a second context’s (e.g., B) negative occasion-setting properties. Thus, less suppression in Context B compared to context C could be interpreted as either 1) conditioned inhibition present in Context B or 2) transfer of negative occasion setting in Context B to the transfer excitor.
Method
Subjects
The subjects were 24 experimentally naïve adult male Long Evans rats, purchased from the same vendor as those in the previous experiment and maintained under the same conditions. Prior to the start of behavioral training rats were food restricted to 85% of their baseline weight.
Surgery
For the DH lesions, twelve rats received bilateral neurotoxic lesions of the DH by infusing NMDA (20 mg/ml; Sigma-Aldrich, St. Louis, MO), dissolved in 0.01 M phosphate buffered saline (PBS) at each site (see Table 2; 0.4 μL per site) using a 26 g Hamilton syringe connected to an automated pump (flow rate = 0.1 μL/min). The syringe was left in place for 1 min before and 4 min after the infusion to control the spread of the neurotoxin and minimize diffusion up the needle track. Sham lesions consisted of a craniotomy and shallow, non-puncturing burr holes to minimize damage to the underlying cortex. One lesioned rat died following surgery.
All lesions occurred during a 2-day period following the extinction phase of Experiment 2. After 9 days of recovery, all rats received a single baseline recovery session in both Context B and C, in which lever pressing was reinforced on an RI 60 s schedule and no stimuli were presented. Renewal testing began the following day.
Behavioral apparatus
The apparatus and procedures were the same as those used in Experiment 1, with the following exceptions. All of Contexts A, B, and C, were additionally illuminated with a 2.8-W bulb (with a red cover) mounted to the ceiling of the sound attenuation chamber. This additional illumination allowed for behavioral observation even when the target CS was presented (i.e., panel light off).
Behavioral procedures
The baseline lever-press training, pre-exposure, conditioning, extinction, and renewal tests phases were carried out in the same manner as Experiment 1, with the exception that all rats received an additional lever training session in B and C following recovery from surgery.
Summation training.
Summation training involved a single two-day cycle, as opposed to the two cycles in Experiment 1. Thus, all rats received one session of baseline lever training, followed by only one session of conditioning to the click. This resulted in four pairings of the click and shock, compared to the 8 pairings in Experiment 1. As in Experiment 1, all rats then received two days of lever training in Context A prior to summation testing.
Summation testing.
Summation testing was identical to Experiment 1, with the exception that only two test trials were presented in each of Context B and Context C. The first trial occurred 9 minutes after placement into the chamber, and the second trial occurred following a 6.75 minute ITI.
Behavioral observations and data analysis.
Behavioral observation and data analysis were the same as Experiment 1. In Experiment 2, the percent trials excluded because of scores of 0 in the pre-CS period did not differ across groups, F(1, 21) = 2.98, p = .10. The mean percent trials excluded for Sham lesioned rats was .81 (Mdn = 0, SEM = .51), and for DH lesioned rats was 2.85 (Mdn = 1.96, SEM = 1.10).
Lesion verification and analysis.
Dorsal hippocampal lesions were verified and analyzed using the same procedures as Experiment 1.
Results
Histology
Figure 5a shows a photomicrograph of a representative DH lesion, and lesions drawings are depicted in 5b. Bilateral DH damage was observed in all rats. There was minor damage outside the hippocampus in 7 out of 11 rats, mainly due to needle insertion in the motor or visual cortices. Damage to the hippocampus was present on 95 ± 1% of all sections collected for each subject, indicating that damage extended throughout the rostro-caudal extent of the hippocampus. This damage included CA1, CA2, CA3, and dentate gyrus.
Figure 5.
A: Photomicrograph of a neurotoxic DH lesion. B: Drawings of lesions at four levels along the rostro-caudal extent of the DH for Experiment 2. At each level, lesion drawings were stacked onto a single image. The darkness of an area indicates the number of lesions cases that include that area. Grey boxes (next to the bregma values) represent the expected darkness for overlap from all subjects. Adapted from The Rat Brain in Stereotaxic Coordinates (Compact 6th ed.), by G Paxinos and C. Watson, 2009. C: Drawings of lesions for Experiment 3.
Behavior
Baseline.
The acquisition of lever pressing proceeded similarly for all groups. A 2 (Group) × 12 (Session) ANOVA revealed a main effect of session, F(11, 231) = 63.46, p < .001, η2p = .75. Neither the main effect of group, F(1, 21) = 1.26, p = .28, nor the interaction between group and session were significant, F(11, 231) = .90, p = .544. During the last session of baseline training, rats in the (to-be) Sham group averaged 16.99 (SEM = 2.57) responses per minute and rats in the (to-be) DH lesioned group averaged 14.59 (SEM = 1.16) responses per minute.
Pre-exposure.
Mean responding during the pre-exposure phase of the experiment is presented in the left portion of Figure 6. Rats that were to receive lesions showed slightly more suppression to the CS in the pre-exposure phase, although this did not reach conventional levels of significance, F(1, 21) = 3.98, p = .06. Pre-CS responding did not differ between groups, F < 1. One rat in the DH group failed to make respond during the pre-CS period for one trial.
Figure 6.
Mean suppression ratios for pre-exposure, conditioning, and extinction in Experiment 2. “P1” = suppression during the pre-exposure session in which the light stimulus was presented 4 times. “T1” and “T5” = suppression for trials 1 and 5 of conditioning, respectively. “E1” to “E8” = suppression during extinction calculated as 4-trial blocks. Sham = sham lesioned rats, DH = dorsal hippocampal lesioned rats. Lesions occurred after extinction. Error bars represent ±1 SEM.
Conditioning.
The results of the conditioning phase are presented in the left portion of Figure 6. Groups did not differ on conditioning trial 1, F(1, 21) = .12, p = .72 or on conditioned trial 5, F(1, 21) = .22, p = .64. Likewise, there were no differences in pre-CS responding on either trial, largest F(1, 21) = 1.21, p = .35.
Extinction.
The results of the extinction phase are presented in the right portion of Figure 6. A 2 (Group) × 8 (Trial Block) ANOVA revealed a main effect of trial block, F(7, 147) = 34.82, p < .001, η2p = .63. The main effect of group was not significant, F(1, 21) = 2.96, p = .10, nor was the interaction, F < 1. On extinction day 1, 4 rats in the DH group failed to press during the pre-CS period for a total of 8 trials, and one rat in the Sham group failed to press during this period for 3 trials. Analysis of pre-CS responding revealed a main effect of trial block F(7, 147) = 6.05, p < .001, η2p = .22. Neither the main effect of group nor the interaction between group and trial block were significant, Fs < 1.
Following recovery from lesions, and prior to renewal testing, all rats had one baseline recovery session in Context B and then C, where they were allowed to lever press on a RI-60 second schedule for 45 minutes. The rate of lever pressing did not differ across groups in Context B, F(1, 21) = .01, p = .91 or in Context C, F(1, 21) = .29, p = .60. In Context B, the average rate of lever pressing was 13.32 (SEM = 1.87) for Sham and 13.63 (SEM = 1.76) for DH lesioned rats. In Context C, the average rate of lever pressing was 16.51 (SEM = 2.23) for Sham and 18.20 (SEM = 2.26) for DH lesioned rats.
Renewal.
The results of the within-subject renewal test are presented in the left portion of Figure 7. As in Experiment 1, Sham lesioned rats showed more suppression to the CS in the renewal context (C) than the extinction context (B). Further, lesions of the DH prevented renewal of suppression. The test data were analyzed with 2 (Group) × 2 (Context) ANOVA. The main effect of group was not significant, F(1, 21) = 1.27, p = .27. However, the main effect of context was significant, F(1, 21) = 9.71, p < .01, η2p = .32, as well as the critical interaction between context and group, F(1, 21) = 5.35, p = .03, η2p = .20. Sham lesioned rats showed more suppression in the renewal context relative to the extinction context (p < .01). However, DH lesioned rats did not show significantly more suppression in the renewal context relative to extinction context (p = .58). Furthermore, Sham lesioned rats had greater suppression than DH lesioned rats in the renewal context, F(1, 21) = 4.78, p = .04, but not the extinction context, F(1, 21) = .68, p = .42. One Sham lesioned rat and one DH lesioned rat failed to press during the pre-CS period during a single trial of testing. Analysis of pre-CS responding did not reveal any significant effects, largest F(1, 21) = 2.99, p = .10.
Figure 7.
Results of renewal and summation testing in Experiment 2. Renewal: suppression to the light-off CS (4 trials) in both the extinction (B) and renewal (C) contexts. Summation: suppression to the click CS (2 trials) in both the extinction (B) and renewal (C) contexts. Error bars represent ±1 SEM. *p < .05
Once again, because renewal is often strongest on the first test trial, we also compared the first test trial from Context B and C for each group separately (data not shown). Paired t-tests revealed a significant context effect for Sham lesioned rats, t(11) = 2.55, p = .03, but not for DH lesioned rats, t(10) = 1.67, p = .14. On the first test trial, mean suppression for Sham lesioned rats was .41 (SEM = .07) in Context B and .16 (SEM = .06) in Context C. For DH lesioned rats mean suppression on the first test trial was .37 (SEM = .04) in B and .26 (SEM = .05) in C. Pre-CS responding did not differ between context for Sham rats, t(11) = .62, p = .53. For DH lesioned rats, responding was numerical higher in the renewal context relative to the extinction context, however this did not reach significance, t(10) = 2.15, p = .06.
Summation training and testing.
Suppression on the first presentation of the click did not differ between groups (F < 1) nor did pre-CS responding, F(1, 20) = 3.91, p = .10. (One Sham lesioned rat failed to press during the pre-CS period for this trial),
The results of the summation test are presented in the right portion of Figure 7. The test data was analyzed with a 2 (Group) × 2 (Context) ANOVA. Neither the main effect of group, context, nor the interaction were significant, largest F(1, 21) = 3.22, p = .09. There was thus no evidence of hypothetical conditioned inhibition in the extinction context for either group. An identical analysis of pre-CS scores did not reveal any significant main effects or interactions, largest F(1, 21) = 1.12, p = .30.
Discussion
The results of Experiment 2 replicated the findings of Experiment 1. Following extinction, Sham lesioned rats demonstrated renewal of fear when testing occurred in Context C. However, lesions of the DH prevented renewal. In this case, lesions of the DH were made following extinction training, which suggests a role for the DH in the retrieval of contextual information normally needed for renewal to occur. Once again, summation testing did not detect conditioned inhibition in Context B, suggesting that renewal occurred due to a release from the negative occasion-setting properties of the extinction context. Finally, the fact that DH lesions had no impact on summation testing indicated that damage to the DH did not produce a general deficit in retrieval of conditioning, but was instead produced a deficit specific to renewal of conditioned suppression.
Experiment 3
The findings of Experiments 1 and 2 suggest that the DH is required for renewal of conditioned suppression in a neutral context. However, a more parsimonious explanation of the results is that DH lesions impaired the ability of rats to discriminate between contexts B and C during the test. A failure to differentiate between contexts B and C at the time of test would prevent renewal from occurring.
In Experiment 3, rats first received conditioning and extinction, followed by post-extinction neurotoxic lesion. This wa on contextual discrimination learning in rats that had a similar prior history as those in Experiment 2. Following recovery, instead of testing for renewal, rats underwent a contextual discrimination in which lever-pressing was reinforced with food pellets in one context, but not the other. If rats are able to discriminate between the two contexts, then lever-pressing should remain high in the reinforced context but be reduced in the non-reinforced context. If lesions impaired that ability, there should be no difference in responding in the two contexts.
Method
Subjects
The subjects were 16 experimentally naïve adult male Long Evans rats, purchased from the same vendor as those in the previous experiments and maintained under the same conditions. Prior to the start of behavioral training rats were food restricted to 85% of their baseline weight.
Surgery
The procedure for Sham (n = 9) and DH lesions (n = 7) was identical to Experiment 2.
Behavioral apparatus and procedures
The apparatus was the same as those used in Experiment 2. The baseline lever-press training, pre-exposure, conditioning, and extinction phases were carried out in the same manner as Experiments 1 and 2. Experiment 3 did not include renewal or summation testing. Following extinction, all rats proceeded to contextual discrimination training (below).
Contextual discrimination training.
Over the next four days, all rats then received two daily 45 minute sessions, one in each context. Sessions were separated by ~120 minutes. In one context, lever pressing continued to be reinforced on an RI-60 s schedule. In the other context, lever pressing was extinguished; no food pellets were delivered. Thus, it was expected that that lever pressing would be maintained in the reinforced context (“Plus”) and would decrease in the nonreinforced context (“Minus”). Notably, in both contexts, reinforcement never occurred in the first 5 minutes of the session. Thus, responding during this period allowed for a common assessment of the contingencies in place within each context, without contamination by the presence of food reinforcement. The physical identities of the chambers were counterbalanced as the “Plus” and “Minus” context, as well as whether the contexts were previously assigned as “B” or “C”.
Behavioral observations and data analysis.
Behavioral observation and data analysis were the same as Experiment 1 and Experiment 2. In Experiment 3, the percent trials excluded did not differ across groups, F(1, 14) = .77, p = .40. The mean percent trial excluded for Sham lesioned rats was 1.46 (Mdn = 0, SEM = .77), and for DH lesioned rats was 2.63 (Mdn = 2.63, SEM = 1.15).
Lesion verification and analysis.
Dorsal hippocampal lesions were verified and analyzed using the same procedures as Experiments 1 and 2.
Results
Histology
In Figure 5c, lesion drawings are stacked onto a single atlas image for each of the 4 rostro-caudal levels. Bilateral hippocampal damage was observed in all rats including CA1, CA2, CA3, and dentate gyrus. Lesion size and shape were similar to that reported in Experiment 2.
Behavior
Baseline.
The acquisition of lever pressing proceeded similarly for all groups. A 2 (Group) × 12 (Session) ANOVA revealed a main effect of session, F(11, 154) = 34.10, p < .001, η2p = .71. Neither the main effect of group nor the interaction between group and session were significant, Fs < 1. During the last session of baseline training, rats in the (to-be) Sham group averaged 15.33 (SEM = 1.56) responses per minute and rats in the (to-be) DH lesioned group averaged 18.30 (SEM = 3.47) responses per minute.
Pre-exposure.
Mean responding during the pre-exposure phase of the Experiment is presented in the left portion of Figure 8. Neither suppression nor pre-CS responding differed between groups, largest F(1, 14) = .63, p = .44.
Figure 8.
Mean suppression ratios for pre-exposure, conditioning, and extinction in Experiment 3. “P1” = suppression during the pre-exposure session in which the light stimulus was presented 4 times. “T1” and “T5” = suppression for trials 1 and 5 of conditioning, respectively. “E1” to “E8” = suppression during extinction calculated as 4-trial blocks. Sham = sham lesioned rats, DH = dorsal hippocampal lesioned rats. Lesions occurred after extinction. Error bars represent ±1 SEM.
Conditioning.
The results of the conditioning phase are presented in the left portion of Figure 8. Not surprisingly, groups did not differ on either conditioning trial 1 or conditioning trial 5, largest F(1, 14) = .113, p = .74. Although pre-CS responding did not differ on trial 1 (F < 1), there was an unexpected difference on trial 5, F(1, 14) = 5.18, p = .04.
Extinction.
The results of the extinction phase are presented in the right portion of Figure 8. Extinction was analyzed with a 2 (Group) × 8 (Trial block) ANOVA. This revealed a main effect of trial, F(7, 98) = 57.80, p < .001, η2p = .81. Neither the main effect of group nor the interaction between group and trial were significant, largest F(7, 98) = 1.95, p = .07. During the first session of extinction only, 4 DH rats failed to press during the pre-CS period for a total of 7 trials. Likewise, 3 Sham rats failed to press during this period for a total of 5 trials. For pre-CS scores, a 2 (Group) × 8 (Trial block) ANOVA revealed a main effect of trial block, F(7, 98) = 16.72, p < .001, η2p = .54. Neither the main effect of group nor the interaction between group and trial were significant, largest F(1, 14) = 1.11, p = .31.
Following recovery from lesions, and prior to the context discrimination, all rats had one baseline recovery session in Context B and then C, where they were allowed to lever press on a RI-60 second schedule for 45 minutes. The rate of lever pressing did not differ across groups in Context B, F(1, 14) = .03, p = .86 or in Context C, F(1, 14) = .04, p = .85. In Context B, the average rate of lever pressing was 19.59 (SEM = 2.51) for Sham and 18.98 (SEM = 1.86) for DH lesioned rats. In Context C, the average rate of lever pressing was 18.40 (SEM = 2.70) for Sham and 17.78 (SEM = 1.29) for DH lesioned rats.
Contextual discrimination.
Responding during the first 5 minutes in the “Plus” and “Minus” contexts is presented in Figure 9. For responding in both the “Plus” and “Minus” contexts, we calculated percent baseline of responding during the first session in each context. (Statistical conclusions drawn from the percent baseline measure did not differ from analysis of raw lever presses per minute).
Figure 9.
Contextual discrimination in Experiment 3. Mean percent baseline responding in both the reinforced “+” and non-reinforced “−” contexts. Responding is shown for the first 5 minutes of each session in which no reinforcement occurred in either context. Percent baseline is calculated relative to Session 1. Sham = sham lesioned rats, DH = dorsal hippocampal lesioned rats. Error bars represent ±1 SEM.
A 2 (Group) × 2 (Context: Plus vs. Minus) × 4 (Session) ANOVA revealed a main effect of context, F(1, 14) = 26.33, p < .001, η2p = .65, session, F(3, 42) = 10.98, p < .001, η2p = .44, and an interaction between context and session, F(3, 42) = 17.02, p = .001, η2p = .55. No other main effects or interactions were significant (all other Fs < 1). Overall, responding was significantly greater in the “Plus” context relative to the “Minus” context for sessions 3 and 4 (ps < .001).
Discussion
The main result from Experiment 3 is that both Sham and DH lesioned rats demonstrated a clear contextual discrimination, with no detectable differences between the groups. This finding is consistent with prior studies examining the role of the hippocampus in contextual discriminations (e.g., Holt & Maren, 1999; Good & Honey, 1991), and suggests the impaired renewal observed in Experiments 1 and 2 was not due to an inability of DH lesioned rats to merely discriminate Contexts B and C.
General Discussion
While prior studies have identified a role for the hippocampus in renewal of conditioned freezing, the present experiments are the first to demonstrate a role for the hippocampus in renewal of conditioned suppression. For control rats in Experiments 1 and 2, conditioned responding was reduced during extinction in Context B, but renewed when the CS was tested in a neutral, yet familiar, third context (C). However, renewal was attenuated for rats that had pre-training electrolytic lesions of the dorsal hippocampus (Experiment 1), or post-extinction neurotoxic lesions (Experiment 2). Although hippocampal lesions impaired ABC renewal, they did not impair acquisition or extinction of conditioned suppression (Experiment 1), or the ability of rats to discriminate between the extinction and renewal contexts (Experiment 3). Taken together these findings suggest a specific role for the DH in ABC renewal of conditioned suppression.
The renewal observed in Experiments 1 and 2 occurred during testing in a neutral third context (Context C) instead of the original conditioning context (Context A), which was the focus of prior studies of the renewal of conditioned suppression (Frohardt et al., 2000; Todd et al., 2017; Wilson et al., 1995). The fact that mere removal from the extinction context, without a return to the conditioning context, produced a return of responding suggests that some form of inhibition in the extinction context played a crucial role in producing renewal in sham animals here. But how should this inhibition be characterized? One possibility is that the extinction context acquired a direct inhibitory association with the US, thereby reducing responding elicited by the light-off CS. This type of mechanism would also protect the CS from a full loss of associative strength, allowing for renewal to occur in Context C (Bouton & King, 1983; Bouton & Swartzentruber, 1986; Delamater & Westbrook, 2014). However, the fact that Context B did not also reduce responding to the transfer excitor during summation testing suggests that the extinction context did not acquire conditioned inhibition. The failed summation tests also cast doubt on the notion that a configuration of context B and the CS acquired conditioned inhibition. Presumably, any inhibition conditioned to the Context B / Light CS configuration would generalize to the Context B / Click CS configuration (Pearce, 1994), and subsequently reduce responding to the transfer excitor.
A different approach would be to assume that during extinction the light-off CS and Context B generated a unique cue that acquired a direct inhibitory association with the US (Wagner & Rescorla, 1972). This inhibition would only be present in Context B, and would protect the CS from full extinction, allowing for a return of responding when the CS was tested in Context C. However, as noted by Rescorla (2008), this unique cue approach has difficulty explaining renewal when conditioning and extinction occur in the same context (e.g., AAB renewal). In this case, the CS and Context A would generate a unique cue during conditioning, but the same unique cue would also be present and undergo extinction. Without additional assumptions regarding the operation of unique cues, it is unclear why responding would subsequently increase in Context B. The fact that AAB renewal of conditioned suppression has been reported elsewhere (Bouton & Ricker, 1994), suggests that the unique cue perspective is not a full account of renewal in this paradigm.
Alternatively, it is possible that during extinction the context acquired negative occasion-setting properties (Bouton, 1993; Bouton & Swartzentruber, 1986). From this perspective, the context does not have general conditioned inhibitory properties, which would be expected to summate and reduce responding to other cues. Instead, the context specifically reduced responding to the CS that was extinguished there, by signaling that it will not be followed by the US. This signaling is specific to the extinction context, and therefore renewal occurs when the CS occurs in another context. Considering that conditioned inhibition was not detected in the current experiments, we suggest that a negative occasion-setting process is the most parsimonious account of ours results. Lesions of the hippocampus impaired this form of contextual modulation, producing extinction performance that generalized across contexts (see Holt & Maren, 1999; Maren & Holt, 2000; Maren et al., 2013; Maren, 2011).
Although we have emphasized a role for the hippocampus related to the context-dependence of extinction learning, there are alternative explanations of our findings. For example, we considered the possibility that hippocampal lesions would reduce renewal if they impaired the ability to discriminate between Contexts B and C. However, in Experiment 3, we observed intact context discrimination following neurotoxic lesions of the hippocampus, consistent with other lesion and inactivation studies (Good & Honey, 1991; Honey & Good, 1993; Holt & Maren, 1999). A second possibility is that hippocampal lesions impaired renewal due to a weakening or disruption of the original CS-US association. This seems unlikely for several reasons. For instance, in Experiment 1, by trial 5, both groups of rats had acquired essentially complete suppression (suppression ratios < .1). Likewise, in the extinction phase of Experiment 1, hippocampal lesions had no impact on responding over the entire response scale, suggesting that the original CS-US association was not impaired by lesions, even as responding was suppressed. That results also makes it unlikely that lesions impaired renewal simply by disrupting performance at a particular point on the response scale. Finally, there were no differences observed between sham and DH lesioned rats during either conditioning or summation testing with the auditory CS. Taken together, it thus seems unlikely that hippocampal lesions impaired the original CS-US association itself, consistent with other findings (Leaton & Borszcz, 1990; Frohardt et al., 2000; Talk et al., 2002; Wilson et al., 1995).
Although the present studies did not seek to compare the role of the hippocampus in ABA vs. ABC renewal directly, it is noteworthy that all prior conditioned suppression studies have found no impact of hippocampal damage on renewal when testing occurs in Context A (Frohardt et al., 2000; Wilson et al., 1995; Todd et al., 2017). One difference between ABA and ABC renewal is that in ABC renewal, the CS has never been experienced in the renewal context prior to testing. Reduced renewal under these circumstances might thus be consistent with the proposal of Maren (2014) that the hippocampus is essential for detecting unexpected events. However, Maren (2014) also noted that hippocampal lesions would be expected to attenuate ABA renewal if subjects were extensively exposed to context A, without the CS, prior to renewal testing. Contrary to this prediction, prior conditioned suppression studies have included repeated exposure to Context A alone and have nevertheless found no impact of hippocampal lesions on ABA renewal (Frohardt et al., 2000; Wilson et al., 1995; Todd et al., 2017).
A second difference between ABA and ABC renewal is that, in ABA renewal, the renewal context might further promote responding during testing presumably through positive occasion setting. It has been suggested that occasion setters might work by modulating inhibition between the CS and US (Bouton & Nelson, 1998; Swartzentruber & Rescorla, 1994); negative occasion setters activate this inhibition, whereas positive occasion setters inhibit this inhibition (see Bouton, 1997). Related to extinction and renewal, the extinction context would thus activate inhibition, leading to low levels of responding specifically in that context. This inhibition would not be fully activated in neutral contexts, resulting in renewal. Hippocampal lesions evidently cause this inhibition to remain active and thus generalize to an otherwise neutral context (C). However, when the CS is presented in the original conditioning context (A), the context might promote responding by turning off any inhibition acquired during extinction that had generalized to Context A. If the ability to “turn off” this inhibition remains intact, this might explain why hippocampal lesions do not impair ABA renewal, at least in conditioned suppression (Frohardt et al., 2000; Wilson et al., 1995; Todd et al., 2017). While this possibility requires explicit testing, it is encouraging to note that pre-training neurotoxic lesions produce enduring deficits in negative occasion setting with discrete cues, but only transiently impair positive occasion setting (Holland et al., 1999).
In summary, although prior studies have demonstrated that hippocampal damage has no impact on renewal of conditioned suppression that occurs in the original conditioning context (ABA renewal), the current experiments demonstrate that DH lesions impair renewal of conditioned suppression that occurs outside the context of conditioning (i.e., ABC renewal). Furthermore, summation testing found no evidence of conditioned inhibition in the extinction context, instead suggesting that renewal in sham lesioned rats occurred due to a release from the negative occasion-setting properties of the extinction context. Overall, the role of the DH in the current experiments is consistent with a framework in which the hippocampus is seen as critically important for the “contextualization” of extinguished fear memories, developed primarily from studies examining extinction and renewal of conditioned freezing (Holt & Maren, 1999; Maren & Holt, 2000; Maren, 2011; Maren et al., 2013).
Acknowledgments
This work was supported by National Science Foundation grant IOS1353137 (D.J.B.) and National Institute of Drug Abuse grant T32DA037202 (D.I.F.). T.P.T was supported by the National Institute of Mental Health of the National Institutes of Health under award number K01MH116158. The content is solely the responsibility of the authors and does not necessarily represent the official view of the National Institutes of Health. We thank Drs. Robert N. Leaton, Mark E. Bouton, and Eric A. Thrailkill for commenting on an earlier version of this manuscript. In addition, we thank Dr. Byron Nelson for helpful discussions regarding mechanisms of renewal. This manuscript is dedicated to the memory of David J. Bucci.
Funding:
NSF: IOS1353137 (D.J.B)
NIDA: T32DA037202 (D.I.F)
NIMH: K01MH116158 (T.P.T)
Footnotes
Some studies have attempted to isolate contextual occasion setting by matching the associative histories of the extinction and renewal contexts, presumably equating any direct context-US associations and ruling these out as a source of renewal (e.g., Lovibond et al., 1984; Maren & Hobin, 2007). For instance, if Cue X undergoes extinction in Context B, and Cue Y undergoes extinction in Context C, then both contexts should presumably be equated for any hypothetical conditioned inhibition, and potentially prevent renewal when each cue is tested in the alternative context. However, this design does not necessarily rule out conditioned inhibition as a source of renewal. If any hypothetical conditioned inhibition in one context transfers only partially to a cue extinguished in the other context, then renewal would still be observed. It is noteworthy that partial transfer has been observed in studies of conditioned inhibition with discrete cues (Rescorla, 1982; Zimmer-Hart & Rescorla, 1984; see also Pearce, 1987).
References
- Bouton ME (1993). Context, time, and memory retrieval in the interference paradigms of Pavlovian learning. Psychological Bulletin, 114, 80–99. [DOI] [PubMed] [Google Scholar]
- Bouton ME (1997). Signals for whether versus when an event will occur In Bouton ME and Fanselow MS (Eds.), Learning, motivation, and cognition: The functional behaviorism of Robert C. Bolles (pp. 385–409). Washington, D. C.: American Psychological Association. [Google Scholar]
- Bouton ME (2002). Context, ambiguity, and unlearning: Sources of relapse after behavioral extinction. Biological Psychiatry, 52, 976–986. [DOI] [PubMed] [Google Scholar]
- Bouton ME (2004). Context and behavioral processes in extinction. Learning & Memory, 11, 485–494. [DOI] [PubMed] [Google Scholar]
- Bouton ME (2017). Exticntion: Behavioral mechanisms and their implications In : Menzel R (ed.), Learning Theory and Behavior, Vol. 1 of Learning and Memory: A Comprehensive Reference, 2nd edition, Byrne JH (ed). pp. 6–83. Oxford: Academic Press. [Google Scholar]
- Bouton ME, & Bolles RC (1979). Contextual control of the extinction of conditioned fear. Learning and Motivation, 10, 445–466. [Google Scholar]
- Bouton ME, & King DA (1983). Contextual control of the extinction of conditioned fear: Tests for the associative value of the context. Journal of Experimental Psychology: Animal Behavior Processes, 9, 248–265. [PubMed] [Google Scholar]
- Bouton ME, & Nelson JB (1994). Context-specificity of target versus feature inhibition in a feature negative discrimination. Journal of Experimental Psychology: Animal Behavior Processes, 20, 51–65. [PubMed] [Google Scholar]
- Bouton ME, & Ricker ST (1994). Renewal of extinguished responding in a second context. Animal Learning & Behavior, 22, 317–324. [Google Scholar]
- Bouton ME, & Swartzentruber D (1986). Analysis of the associative and occasion-setting properties of contexts participating in a Pavlovian Discrimination. Journal of Experimental Psychology: Animal Behavior Processes, 12, 333–350. [Google Scholar]
- Bouton ME, & Todd TP (2014). A fundamental role for context in instrumental learning and extinction. Behavioural Processes, 104, 13–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campese V, & Delamater AR (2013). ABA and ABC renewal of conditioned magazine approach are not impaired by dorsal hippocampus inactivation or lesions. Behavioral Brain Research, 248, 62–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran KA, & Maren S (2001). Hippocampal inactivation disrupts contextual retrieval of fear memory after extinction. The Journal of Neuroscience, 21, 1720–1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran KA, & Maren S (2004). Factors regulating the effects of hippocampal inactivation on renewal of conditional fear after extinction. Learning & Memory, 11, 598–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran KA, Desmond TJ, Frey KA, & Maren S (2005). Hippocampal inactivation disrupts the acquisition and contextual encoding of fear extinction. The Journal of Neuroscience, 25, 8978–8987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delamater AR (2004). Experimental extinction in Pavlovian conditioning: Behavioural and neuroscience perspectives. The Quarterly Journal of Experimental Psychology, 57B, 97–132. [DOI] [PubMed] [Google Scholar]
- Delamater AR, & Westbrook RF (2014). Psychological and neural mechanisms of experimental extinction: a selective review. Neurobiology of Learning and Memory, 108, 38–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frohardt RJ, Guarraci FA, & Bouton ME (2000). The effects of neurotoxic hippocampal lesions on two effects of context after fear extinction. Behavioral Neuroscience, 114, 227–240. [DOI] [PubMed] [Google Scholar]
- Glautier S, Elgueta T, & Nelson JB (2013). Extinction context produces context inhibition and multiple-context extinction reduces response recovery in human predictive learning. Learning & Behavior, 41, 341–352. [DOI] [PubMed] [Google Scholar]
- Good M, & Honey RC (1991). Conditioning and contextual retrieval in hippocampal rats. Behavioral Neuroscience, 105, 499–509. [DOI] [PubMed] [Google Scholar]
- Harris JA, Jones ML, Bailey GK, & Westbrook RF (2000). Contextual control over conditioned responding in an extinction paradigm. Journal of Experimental Psychology: Animal Behavior Processes, 26, 174–185. [DOI] [PubMed] [Google Scholar]
- Holland PC (1992). Occasion setting in Pavlovian conditioning In: Medin DL (Ed.), The Psychology of Learning and Motivation, 28 Academic Press, San Diego, CA: pp. 69–125. [Google Scholar]
- Holland PC, & Bouton ME (1999). Hippocampus and context in classical conditioning. Current Opinion in Neurobiology, 9, 195–202. [DOI] [PubMed] [Google Scholar]
- Holt W, & Maren S (1999). Muscimol inactivation of the dorsal hippocampus impairs contextual retrieval of fear memory. The Journal of Neuroscience, 19, 9054–9062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honey RC, & Good M (1993). Selective hippocampal lesions abolish the contextual specificity of latent inhibition and conditioning. Behavioral Neuroscience, 107, 23–33. [DOI] [PubMed] [Google Scholar]
- Ji J, & Maren S (2005). Electrolytic lesions of the dorsal hippocampus disrupt renewal of conditional fear after extinction. Learning & Memory, 12, 270–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji J, & Maren S (2008). Differential roles for hippocampal areas CA1 and CA3 in the contextual encoding and retrieval of extinguished fear. Learning & Memory, 15, 244–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapska E, & Maren S (2009). Reciprocal patterns of c-Fos expression in the medial prefrontal cortex and amygdala after extinction and renewal of conditioned fear. Learning & Memory, 16, 486–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laborda MA, Witnauer JE, & Miller RR (2011). Contrasting AAC and ABC renewal: the role of context associations. Learning & Behavior, 39, 46–56. [DOI] [PubMed] [Google Scholar]
- Leaton RN, & Borszcz GS (1990). Hippocampal lesions and temporally chained conditioned stimuli in a conditioned suppression paradigm. Psychobiology, 18, 81–88. [Google Scholar]
- Lovibond PF, Preston GC, & Mackintosh NJ (1984). Context specificity of conditioning, extinction, and latent inhibition. Journal of Experimental Psychology: Animal Behavior Processes, 10, 360–375. [Google Scholar]
- Maren S (2011). Seeking a spotless mind: extinction, deconsolidation, and erasure of fear memory. Neuron, 70, 830–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S (2014). Fear of the unexpected: Hippocampus mediates novelty-induced return of extinguished fear in rats. Neurobiology of Learning and Memory, 108, 88–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S, Aharonov G, & Fanselow MS (1997). Neurotoxic lesions of the dorsal hippocampus and Pavlovian fear conditioning in rats. Behavioural Brain Research, 88, 261–274. [DOI] [PubMed] [Google Scholar]
- Maren S, Phan KL, & Liberzon I (2013). The contextual brain: implications for fear conditioning, extinction and psychopathology. Nature Reviews Neuroscience, 14, 417–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S, & Holt W (2000). The hippocampus and contextual memory retrieval in Pavlovian conditioning. Behavioural Brain Research, 110, 97–108. [DOI] [PubMed] [Google Scholar]
- Maren S, & Hobin JA (2007). Hippocampal regulation of context-dependent neuronal ativity in the lateral amygdala. Learning & Memory, 14, 318–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConnell BL, & Miller RR (2014). Associative accounts of recovery-from-extinction effects. Learning and Motivation, 46, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson JB, Sanjuan MC, Vadillo-Ruiz S, Pérez J, & León SP (2011). Experimental renewal in human participants. Journal of Experimental Psychology: Animal Behavior Processes, 37, 58–70. [DOI] [PubMed] [Google Scholar]
- Paxinos G, & Watson C (2009). The rat brain in stereotaxic coordinates (6th ed.). San Diego, CA: Academic Press. [Google Scholar]
- Pearce JM (1987). A model for stimulus generalization in Pavlovian Conditioning. Psychological Review, 94, 61–73. [PubMed] [Google Scholar]
- Pearce JM (1994). Similarity and discrimination: A selective review and a connectionist model. Psychological Review, 101, 587–607. [DOI] [PubMed] [Google Scholar]
- Polack CW, Laborda MA, & Miller RR (2012). Extinction context as a conditioned inhibitor. Learning & Behavior, 40, 24–33. [DOI] [PubMed] [Google Scholar]
- Rescorla RA (1982). Some consequences of associations between the excitor and the inhibitor in a conditioned inhibition paradigm. Journal of Experimental Psychology: Animal Behavior Processes, 8, 288–298. [Google Scholar]
- Rescorla RA (2003). Protection from extinction. Learning & Behavior, 31, 124–132. [DOI] [PubMed] [Google Scholar]
- Rescorla RA (2008). Within-subject renewal in sign tracking. The Quarterly Journal of Experimental Psychology, 61, 1793–1802. [DOI] [PubMed] [Google Scholar]
- Swartzentruber D, & Rescorla RA (1994). Modulation of trained and extinguished stimuli by facilitators and inhibitors. Animal Learning & Behavior, 22, 309–316. [Google Scholar]
- Talk AC, Gandhi CC, & Matzel LD (2002). Hippocampal function during behaviorally silent associative learning: Dissociation of memory storage and expression. Hippocampus, 12, 648–656. [DOI] [PubMed] [Google Scholar]
- Trask S, Thrailkill EA, & Bouton ME (2017). Occasion setting, inhibition, and the contextual control of extinction in Pavlovian and instrumental (operant) learning. Behavioral Processes, 137, 64–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd TP, Vurbic D, & Bouton ME (2014). Behavioral and neurobiological mechanisms of extinction in Pavlovian and instrumental learning. Neurobiology of Learning and Memory, 108, 52–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd TP, Jiang MY, DeAngeli NE, & Bucci DJ (2017). Intact renewal after extinction of conditioned suppression with lesions of either the retrosplenial cortex or dorsal hippocampus. Behavioural Brain Research, 320, 143–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner AR, & Rescorla RA Inhibition in Pavlovian conditioning: Application of a theory In Boakes RA & Halliday S (Eds.), Inhibition and learning (pp. 301–336). New York: Academic press. [Google Scholar]
- Williams DA, & Overmier JB (1988). Some types of conditioned inhibitors carry collateral excitatory associations. Learning and Motivation, 19, 348–368. [Google Scholar]
- Wilson A, Brooks DC, & Bouton ME (1995). The role of the rat hippocampal system in several effects of context in extinction. Behavioral Neuroscience, 109, 828–836. [DOI] [PubMed] [Google Scholar]
- Zelikowsky M, Pham DL, Fanselow MS (2012). Temporal factors control hippocampal contributions to fear renewal after extinction. Hippocampus, 22, 1096–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer-Hart CL, & Rescorla RA (1974). Extinction of Pavlovian conditioned inhibition. Journal of Comparative and Physiological Psychology, 86, 837–845. [DOI] [PubMed] [Google Scholar]