Abstract
Objectives
To evaluate the efficacy and safety of sacubitril/valsartan according to dose level achieved in the PIONEER-HF trial.
Background
In patients hospitalized for acute decompensated heart failure (ADHF), in-hospital initiation and continuation of sacubitril/valsartan as compared with enalapril is well-tolerated, achieves a greater reduction in NT-proBNP, and reduces the risk of cardiovascular death or rehospitalization for HF through 8 weeks. However, not all patients achieve the target dose of sacubitril/valsartan, and its efficacy and safety in such patients are of interest.
Methods
PIONEER-HF was a randomized, double-blind, active-controlled trial of sacubitril/valsartan vs. enalapril in 881 patients stabilized during hospitalization for ADHF. Blinded study medication was administered for 8 weeks, with initial dosing selected based on the systolic blood pressure (SBP) at randomization and titrated toward a target of sacubitril/valsartan 97 mg/103 mg twice daily, or enalapril 10 mg twice daily, with an algorithm based on SBP and the investigator’s assessment of tolerability.
Results
At 4 weeks, 211 patients (60%) allocated to sacubitril/valsartan and 199 patients (55%) allocated to enalapril were dispensed the target dose. Baseline characteristics were similar in the two treatment groups within each dose level. There was no heterogeneity across dose levels in the effect of sacubitril/valsartan on the reduction in NT-proBNP (p-interaction=0.69), the reduction in cardiovascular death or rehospitalization for HF (p-interaction=0.42), or the prespecified adverse events of special interest through 8 weeks.
Conclusions
In hemodynamically stabilized patients with ADHF, the efficacy and safety of sacubitril/valsartan are generally consistent across dose levels.
Keywords: sacubitril/valsartan, heart failure, hospitalization
Introduction
Patients admitted for acute decompensated heart failure (ADHF) are at high risk for re-hospitalization for heart failure (HF) and cardiovascular death.1, 2 As such, it is increasingly recognized that initiation of evidence-based therapies during hospitalization for ADHF may decrease HF morbidity and mortality, since multiple studies have shown that initiation and adherence are enhanced when guideline-directed medical therapy (GDMT) is prescribed before hospital discharge.3, 4
In the randomized, double-blind PIONEER-HF trial (Comparison of Sacubitril/Valsartan versus Enalapril on Effect on NT-proBNP in Patients Stabilized from an Acute Heart Failure Episode) (NCT02554890), compared with enalapril, initiation of sacubitril/valsartan in patients stabilized during hospitalization for ADHF was safe, well-tolerated, and led to a significantly greater reduction in circulating N-terminal pro-brain natriuretic peptide (NT-proBNP) concentration.5 Moreover, in an exploratory analysis of adjudicated cardiovascular outcomes, sacubitril/valsartan, as compared with enalapril, significantly reduced the composite of rehospitalization for HF or cardiovascular death at 8 weeks following the initial hospitalization 4(hazard ratio [HR] 0.58, 95% confidence interval [CI] 0.39–0.87).6
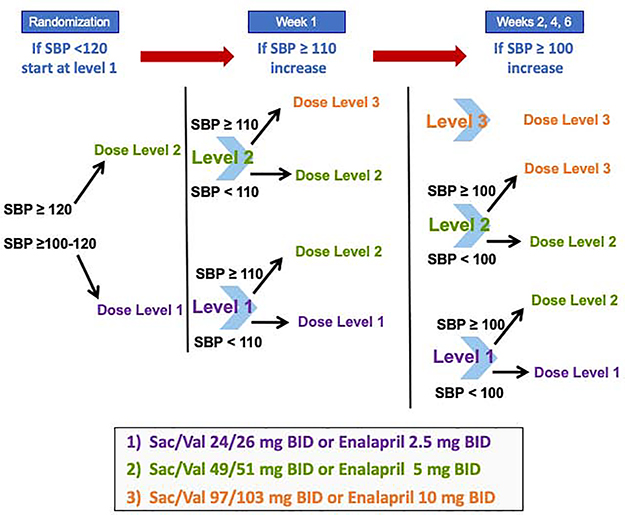
Consistent with guideline recommendations to use caution when initiating neurohormonal antagonists during hospitalization for ADHF,7 patients in the PIONEER-HF trial were started on one of two submaximal doses of sacubitril/valsartan and enalapril based on their systolic blood pressure (SBP) at the time of randomization. These doses were then to be uptitrated to target doses of 97/103 mg twice daily (sacubitril/valsartan) and 10 mg twice daily (enalapril) over the course of the study period according to a predefined dose titration algorithm (Figure 1). Nevertheless, not all patients achieved the target dose of blinded study drug.5
Figure 1. Dosing algorithm of blinded study drug in the PIONEER-HF trial.
Dosing of blinded study drug was selected based on systolic blood pressure at randomization according to a prespecified algorithm. Titration of blinded study drug dose occurred at 1, 2, 4, and 6 weeks following randomization based on SBP and tolerability. SBP, systolic blood pressure.
Whether sacubitril/valsartan confers a consistent clinical benefit at doses below the target dose in patients who have recently been hospitalized for ADHF is unknown. We therefore conducted an exploratory analysis of the efficacy and safety of sacubitril/valsartan according to dose level achieved in the PIONEER-HF trial.
Methods
Study Population
The PIONEER-HF trial was an 8-week, multicenter, randomized, double-blind, double-dummy, active-controlled trial of in-hospital initiation of sacubitril/valsartan compared with enalapril in 881 patients with heart failure with reduced ejection fraction (HFrEF) stabilized during hospital admission for ADHF.5 The primary trial results and full details of the protocol have been reported previously.5, 8 The trial included patients with left ventricular ejection fraction [LVEF] ≤40% with signs and symptoms of HF and an NT-proBNP concentration ≥1600 pg/mL or BNP concentration ≥400 pg/mL. Patients were enrolled ≥24 hours and up to 10 days after initial presentation while still hospitalized and were to be hemodynamically stable. The latter was defined by SBP ≥100 mmHg for the preceding 6 hours, with no increase in the dose of intravenous diuretics and no use of intravenous vasodilators during the preceding 6 hours, and no use of intravenous inotropes during the preceding 24 hours. All patients provided written informed consent. The study protocol was approved by the ethics committees and local institutional review boards at each participating center.
For both sacubitril/valsartan and enalapril, 3 doses of study drug were available (sacubitril/valsartan: 24/26 mg BID [dose level 1], 49/51 mg BID [dose level 2], and 97/103 mg BID [dose level 3]; enalapril: 2.5 mg BID [dose level 1], 5 mg BID [dose level 2], and 10 mg BID [dose level 3]). The starting doses during in-hospital initiation and all subsequent dose changes during the double-blind treatment period were selected using a dose titration algorithm based on SBP (Figure 1) and the investigator’s assessment of tolerability. For this analysis, patients were assessed according to the dose level achieved at 4 weeks post-randomization; therefore, the primary analytic cohort focused on those patients who were still receiving study drug at week 4 (n=715, 82%). This timepoint (i.e., midway through the trial follow-up period) was selected to provide preceding opportunity for dose optimization of study drug while still having adequate follow-up time to assess the consequences of those achieved doses. In addition, we performed two sensitivity analyses in the full trial cohort (n=875): (1) using time-varying dose level in which patients contributed person-time at a given dose level while they were taking that dose; and (2) based on the highest dose achieved at any point during the 8-week double-blind phase. Among the 160 patients not on study drug at week 4 (i.e., those included in the sensitivity analyses but not in the primary analytic cohort), 53% had last received dose level 1, 37% had last received dose level 2, and 11% had last received dose level 3.
Efficacy and Safety Outcomes
The efficacy outcomes for this analysis were: (1) the proportional change in NT-proBNP concentration from baseline through week 8; and (2) the composite of rehospitalization for HF or cardiovascular death, which was adjudicated by a blinded clinical events committee (CEC) using standard definitions.6 The key safety outcomes for this analysis were the incidences of worsening renal function (defined as an increase in the serum creatinine concentration of ≥0.5 mg/dL and a decrease in the estimated glomerular filtration rate [eGFR] of ≥25% from baseline), hyperkalemia (defined as a serum potassium concentration of ≥5.5 mmol/L), and symptomatic hypotension.
Statistical Analysis
Baseline characteristics were summarized according to study group assignment stratified by dose level achieved at 4 weeks post-randomization. Categorical variables are reported as counts and percentages, and continuous variables as medians and interquartile ranges.
A multivariable logistic regression model was used to identify independent predictors of failure to reach the target dose of blinded study drug (i.e., dose level 3) at 4 weeks post-randomization. Candidate variables were selected a priori based on clinical relevance and included age, sex, race, body-mass index (BMI), previous HF, New York Heart Association (NYHA) class, baseline SBP, baseline heart rate, LVEF at screening, NT-proBNP concentration at randomization, and baseline eGFR. Variables were selected using a backward elimination procedure, and all variables achieving a significance level of p<0.05 (older age, lower systolic blood pressure, and White race) were maintained in the final multivariable model. Adjusted odds ratios (ORs) and 95% CIs are reported.
Because patients were assessed according to dose level achieved post-randomization, all efficacy and safety analyses were, by necessity, on-treatment analyses. Consistent with the primary trial analysis, missing NT-proBNP values were treated as missing at random. The proportional change in the NT-proBNP concentration was analyzed from baseline to week 8 on a logarithmic scale using an analysis of covariance (ANCOVA) model, adjusting for the baseline value, treatment group, and variables associated with failure to reach the target dose of blinded study drug as fixed effects (age, SBP, and race). To test for heterogeneity in the biomarker response profiles of sacubitril/valsartan vs. enalapril by dose level achieved, a treatment by dose level interaction term was included as a fixed effect in the model. The cumulative event rates of re-hospitalization for HF or cardiovascular death at 8 weeks were estimated using the Kaplan-Meier (KM) method (total number of rehospitalizations for HF = 93; total number of cardiovascular deaths = 16). A landmark analysis of re-hospitalization for HF or cardiovascular death from 4 weeks post-randomization through the end of study follow-up (8 weeks post-randomization) was also performed. Hazard ratios and 95% CIs for all treatment comparisons were calculated using a Cox proportional hazards model, again adjusting for the variables associated with failure to reach the target dose of blinded study drug (age, SBP, and race). To test for heterogeneity in the treatment effect of sacubitril/valsartan vs. enalapril based on dose level achieved, a treatment by dose level interaction term was included in the Cox model.
The incidences of the key safety outcomes of worsening renal function, hyperkalemia, and symptomatic hypotension during the 8-week study period were calculated, as well as risk ratios and 95% CIs for sacubitril/valsartan vs. enalapril in each subgroup. To test for a heterogeneous treatment effect of sacubitril/valsartan vs. enalapril on each safety outcome based on dose level achieved, the Breslow-Day test for homogeneity of the risk ratios was used.
All statistical analyses were performed in R version 3.5.3. All p-values are two-sided unless otherwise specified.
Results
Dose Level Achieved
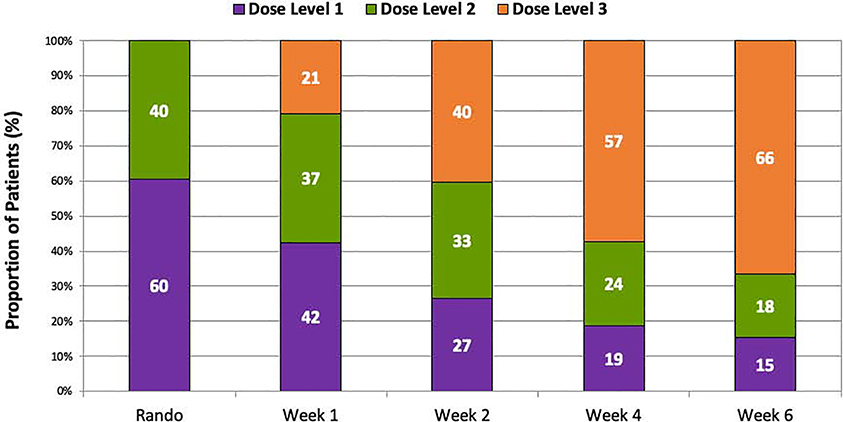
At 4 weeks post-randomization, among patients receiving study drug, 57% of patients achieved the target dose (dose level 3) of blinded study drug, 24% were on dose level 2, and 19% were on dose level 1 (Figure 2 and Supplemental Figure 1). Patients on submaximal doses of blinded study drug were older and tended to have more high-risk clinical features including lower baseline SBP, lower LVEF, and higher baseline NT-proBNP levels (all p<0.05) (Table 1). Men and women were equally likely to have achieved each of the dose levels of blinded study drug; however, White patients were significantly less likely to have achieved higher dose levels of blinded study drug as compared with Black patients. In a multivariable analysis, significant predictors of not attaining the target dose by 4 weeks post-randomization included older age, lower baseline SBP, and White race. Within each dose level, baseline characteristics were similar between patients treated with sacubitril/valsartan vs. enalapril (Table 1).
Figure 2. Distribution of study drug dose level achieved by study visit.
Shown are the proportions of each dose level dispensed among patients receiving study drug at each study visit. At week 4, 43% of patients remained on submaximal doses of study drug.
Table 1.
Baseline characteristics by dose of blinded study drug achieved at 4 weeks post-randomization.
| Variable | Dose Level 1 | Dose Level 2 | Dose Level 3 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Sac/Val (N=69) | Enalapril (N=64) | p-value | Sac/Val (N=93) | Enalapril (N=79) | p-value | Sac/Val (N=199) | Enalapril (N=211) | p-value | |
| Age, years | 66 (55–73) | 69 (63–75) | 0.070 | 62 (52–71) | 65 (52–72) | 0.930 | 59 (47–68) | 60 (50–68) | 0.256 |
| Female sex | 18 (26.1) | 19 (29.7) | 0.643 | 25 (26.9) | 20 (25.3) | 0.816 | 48 (24.1) | 64 (30.3) | 0.158 |
| Black race | 14 (20.3) | 14 (21.9) | 0.823 | 24 (25.8) | 24 (30.4) | 0.505 | 92 (46.2) | 86 (40.8) | 0.264 |
| BMI, kg/m2 | 29(24–33) | 29 (26–33) | 0.254 | 30 (26–37) | 30 (26–38) | 1.000 | 32 (27–38) | 32 (26–38) | 0.692 |
| Previous heart failure | 45 (65.2) | 41 (64.1) | 0.889 | 57 (61.3) | 49 (62.0) | 0.921 | 130 (65.3) | 122 (57.8) | 0.119 |
| Previous medication use | |||||||||
| ACEI | 26 (37.7) | 22 (34.4) | 0.692 | 31 (33.3) | 29 (36.7) | 0.643 | 73 (36.7) | 75 (35.5) | 0.810 |
| ARB | 4 (5.8) | 10 (15.6) | 0.065 | 7 (7.5) | 10 (12.7) | 0.261 | 22 (11.1) | 29 (13.7) | 0.410 |
| Beta-blocker | 41 (59.4) | 36 (56.3) | 0.711 | 48 (51.6) | 51 (64.6) | 0.087 | 119 (59.8) | 119 (56.4) | 0.486 |
| MRA | 8 (11.6) | 5 (7.8) | 0.463 | 10 (10.8) | 6 (7.6) | 0.477 | 21 (10.6) | 16 (7.6) | 0.294 |
| NYHA class | 0.205 | 0.847 | 0.323 | ||||||
| I or II | 14 (20.3) | 17 (26.6) | 24 (25.8) | 25 (31.6) | 49 (24.6) | 62 (29.4) | |||
| III | 41 (59.4) | 41 (64.1) | 60 (64.5) | 46 (58.2) | 131 (65.8) | 131 (62.1) | |||
| IV | 11 (15.9) | 6 (9.4) | 7 (7.5) | 6 (7.6) | 13 (6.5) | 16 (7.6) | |||
| Not assessed | 3 (4.3) | 0 (0.0) | 2 (2.2) | 2 (2.5) | 6 (3.0) | 2 (0.9) | |||
| SBP, mmHg | 112 (107117) | 110 (104118) | 0.185 | 116 (110125) | 112 (105125) | 0.064 | 127 (117138) | 125 (114138) | 0.301 |
| HR, bpm | 80 (72–94) | 80 (70–91) | 0.380 | 82 (71–89) | 80 (73–93) | 0.527 | 82 (74–92) | 79 (71–90) | 0.101 |
| LVEF, % | 22 (17–30) | 20 (15–28) | 0.594 | 23 (18–30) | 23 (18–30) | 0.967 | 25 (20–30) | 25 (20–30) | 0.958 |
| NT-proBNP at randomization, pg/ml | 3956 (2083 6962) |
2861(1820– 5488) | 0.208 | 3445 (15225513) | 2641 (14824805) | 0.253 | 2507 (15194487) | 2288 (11844290) | 0.334 |
| eGFR, ml/min/1.73m2 | 55 (47–70) | 56 (45–64) | 0.466 | 58 (48–76) | 58 (45–66) | 0.219 | 61 (50–74) | 62 (50–74) | 0.495 |
Categorical variables are shown as counts and percentages. Continuous variables are shown as medians with 25th-75th percentiles. ACEI, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker; BMI, body-mass index; eGFR, estimated glomerular filtration rate; HR, heart rate; IQR, interquartile range; kg, kilogram; LVEF, left ventricular ejection fraction; m2, meter-squared; min, minute; ml, milliliter; MRA, mineralocorticoid receptor antagonist; NT-proBNP, N-terminal pro-brain natriuretic peptide; NYHA, New York Heart Association; pg, picogram; sac/val, sacubitril/valsartan; SBP, systolic blood pressure.
Change in N-terminal pro-B-type natriuretic peptide by dose level achieved
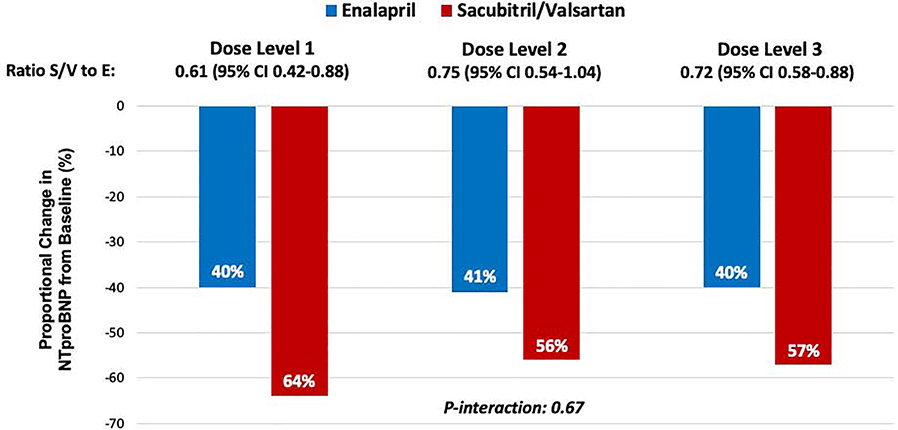
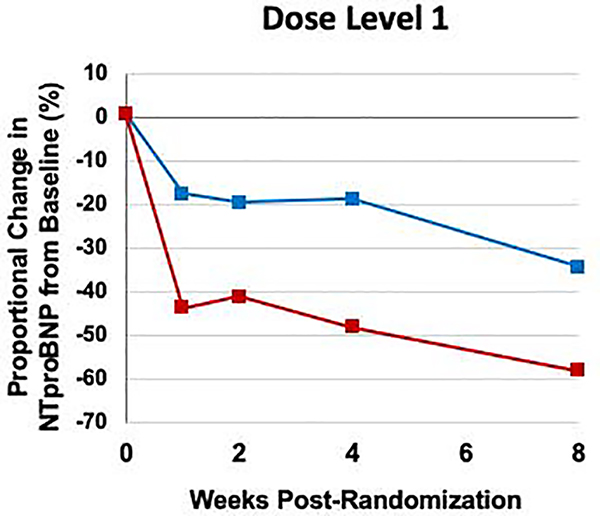
The proportional reduction in NT-proBNP concentration from baseline through week 8 was consistent regardless of dose level of blinded study drug achieved at week 4 (overall ratio of change with sacubitril/valsartan vs. enalapril 0.72, 95% CI 0.58–0.88; p-interaction = 0.67) (Figure 3). Even among patients whose dose of study drug was never escalated beyond dose level 1, there was a greater reduction in NT-proBNP concentration in patients treated with sacubitril/valsartan as compared with enalapril beginning in week 1 and continuing throughout the duration of the study (Figure 4). Furthermore, in a sensitivity analysis based on the maximum dose of blinded study drug achieved at any visit, the proportional reduction in NT-proBNP concentration with sacubitril/valsartan vs. enalapril was consistent (p-interaction = 0.86).
Figure 3. Change in NT-proBNP concentration from baseline through 8 weeks post-randomization by dose of blinded study drug achieved at week 4.
The reduction in the proportional change in NT-proBNP concentration (logarithmic scale) from baseline through week 8 with sacubitril/valsartan was consistent across all dose levels achieved. The model is adjusted for age, systolic blood pressure, and race. NT-proBNP, N-terminal pro-B-type natriuretic peptide.
Figure 4. Change in NT-proBNP concentration over time among patients who remained at dose level 1 of blinded study drug at 4 weeks post-randomization.
The reduction in NTproBNP concentration with sacubitril/valsartan vs. enalapril was consistent in patients who remained on dose level 1 of blinded study drug at 4 weeks post-randomization. The model is adjusted for age, systolic blood pressure, and race. NT-proBNP, N-terminal pro-B-type natriuretic peptide.
Hospitalization for heart failure or cardiovascular death by dose level achieved
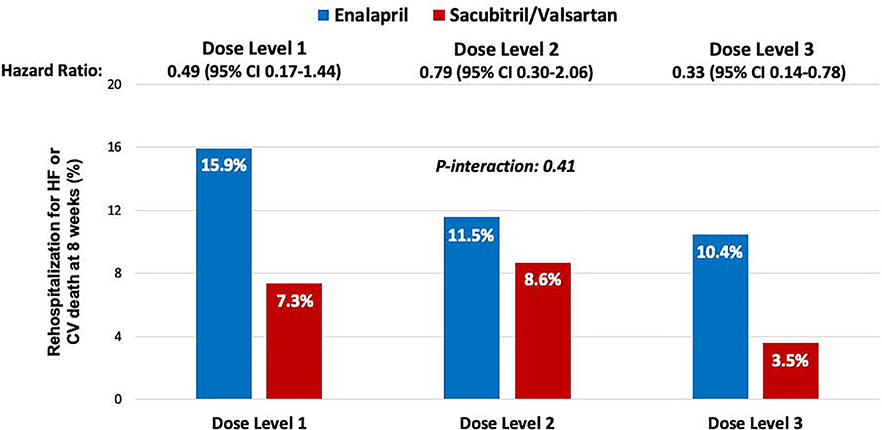
The reduction in rehospitalization for HF or cardiovascular death with sacubitril/valsartan as compared with enalapril over the course of the study was also consistent irrespective of dose level of blinded study drug achieved at week 4 (overall HR 0.58, 95% CI 0.39–0.87; p-interaction=0.41) (Central Illustration). In addition, the pattern of consistent reduction in rehospitalization for HF or cardiovascular death with sacubitril/valsartan vs. enalapril across all dose levels was observed from a landmark of 4 weeks post-randomization through the end of study follow-up (Supplemental Figure 2). In two sensitivity analyses, one based on time-varying dose level and another based on the maximum dose of blinded study drug achieved at any visit, there was similarly no heterogeneity in treatment effect (p-interaction = 0.36 and p-interaction = 0.24, respectively) (Supplemental Table 1).
Central Illustration. Effect of sacubitril/valsartan on clinical outcomes by 8 weeks post-randomization according to the dose of blinded study drug achieved at week 4.
KaplanMeier estimates of the clinical composite of cardiovascular death or rehospitalization for heart failure are shown. Treatment with sacubitril/valsartan, as compared with enalapril, significantly reduced the risk of cardiovascular death or rehospitalization for heart failure in patients who achieved the target dose of study drug and in those who did not.
Adverse events of special interest by dose level achieved
There was no heterogeneity in the risks of worsening renal function or hyperkalemia with sacubitril/valsartan vs. enalapril according to dose level of study drug achieved (p-interaction=NS for each). Owing to their lower baseline SBP, patients achieving submaximal doses of study drug had higher overall rates of symptomatic hypotension regardless of treatment group assignment; however, there was no significant difference in the risk of symptomatic hypotension with sacubitril/valsartan vs. enalapril across dose levels, and in fact, a non-significant trend towards more frequent symptomatic hypotension with sacubitril/valsartan was seen only in patients achieving dose level 3 (Table 2).
Table 2.
Key safety outcomes by dose of blinded study drug achieved at 4 weeks postrandomization.
| Safety Events, n (%) RR (95% CI) | Dose Level 1 | Dose Level 2 | Dose Level 3 | ||||
|---|---|---|---|---|---|---|---|
| Sac/V al (N=69) | Enalapril (N=64) | Sac/Val (N=93) | Enalapril (N=79) | Sac/Val (N=199) | Enalapril (N=211) | P-interaction | |
| Worsening renal function | 18 (26.1) | 16 (25.0) | 10 (10.8) | 10 (12.7) | 18 (9.0) | 27 (12.8) | 0.68 |
| 1.04 (0.58 – 1.87) | 0.85 (0.37 – 1.94) | 0.71 (0.40 – 1.24) | |||||
| Hyperkalemia | 13 (18.8) | 9 (14.1) | 14 (15.1) | 8 (10.1) | 12 (6.0) | 15 (7.1) | 0.53 |
| 1.34 (0.61 – 2.92) | 1.49 (0.66 – 3.36) | 0.85 (0.41 – 1.77) | |||||
| Symptomatic hypotension | 15 (23.4) | 15 (21.7) | 13 (14.0) | 15 (19.0) | 19 (9.5) | 11 (5.2) | 0.17 |
| 0.93 (0.49 – 1.74) | 0.74 (0.37 – 1.45) | 1.83 (0.89 – 3.75) | |||||
Worsening renal function was defined as an increase in the serum creatinine concentration of >0.5 mg/dL and a decrease in the estimated glomerular filtration rate [eGFR] of >25%. Hyperkalemia was defined as a serum potassium concentration of >5.5 mmol/L. There was no heterogeneity in the treatment effect of sacubitril/valsartan vs. enalapril on the risks of any of the key safety outcomes. CI, confidence interval; RR, risk ratio; sac/val, sacubitril/valsartan.
Discussion
HF hospitalization is an ideal time to implement GDMT for HFrEF to increase adherence and improve clinical outcomes in the vulnerable post-hospitalization period. Further, sacubitril/valsartan is known to be safe, well-tolerated, and to significantly reduce natriuretic peptide concentrations and the risk of rehospitalization for HF or CV death in HFrEF patients who are stabilized during hospitalization for ADHF.5, 6 In this analysis from the PIONEER-HF trial, we now demonstrate that the safety profile and benefits of sacubitril/valsartan appear consistent irrespective of dose level achieved during the first month post-hospitalization. Taken together, these data support the in-hospital initiation and continued post-hospitalization use of sacubitril/valsartan with the dose-titration strategy studied in PIONEER-HF even when relative hypotension precludes early up-titration to the target dose.
Achievement and maintenance of sacubitril/valsartan target dose
In the PARADIGM-HF trial (Prospective Comparison of ARNI with ACEI to Determine Impact on Global Mortality and Morbidity) of sacubitril/valsartan vs. enalapril in ambulatory patients with chronic HFrEF, all patients randomized to sacubitril/valsartan were started at the target dose of 97/103 mg twice daily;5 however, 42% of patients reduced their dose (often transiently) at some point during the study period.9 In the follow-up TITRATION trial, which tested two up-titration regimens for sacubitril/valsartan in a population similar to the PARADIGM-HF study population, the more conservative strategy of initiating low-dose sacubitril/valsartan (24/26 mg daily) with gradual up-titration over 6 weeks led to a higher chance of attaining the target dose of sacubitril/valsartan among patients transitioning from lower doses of ACEI/ARB.10 Using a similar framework, all patients in PIONEER-HF were started on submaximal doses of sacubitril/valsartan with subsequent adjustments made according to an SBP-based dose titration algorithm. However, whereas >75% of patients achieved the target dose of sacubitril/valsartan without dose interruption or down-titration in the TITRATION trial, fewer patients reached the target dose in PIONEER-HF (57% at 4 weeks and 66% at 6 weeks). This observation was similar to the findings from the TRANSITION study of pre- vs. post-discharge initiation of sacubitril/valsartan among patients stabilized during hospitalization for ADHF, in which only 48% reached target doses of sacubitril/valsartan after 10 weeks.11
The lower achieved doses of sacubitril/valsartan in PIONEER-HF and TRANSITION, as compared with TITRATION, reflects the more severe clinical profile of hospitalized HF patients. For example, as compared with stable, ambulatory HFrEF patients, those with recent admissions for ADHF tend to be older and have lower SBP,1 both of which were independently associated with failure to achieve the highest dose level of blinded study drug in PIONEER-HF. The challenges associated with achieving target doses of evidence-based neurohormonal antagonists in patients hospitalized for ADHF highlight the critical importance of understanding the efficacy and safety profiles of submaximal doses of these therapies in this population.
Clinical benefits of sacubitril/valsartan at submaximal doses
In PARADIGM-HF, blinded study drug dose reduction during the course of the trial identified patients at higher risk for the composite clinical outcome of hospitalization for HF or cardiovascular death; however, the treatment benefit of sacubitril/valsartan over enalapril following dose reduction was similar.9 Notably, all patients in PARADIGM-HF were started on the highest dose of sacubitril/valsartan (following a run-in period) and the timing of dose reduction was highly variable. By contrast, all patients in PIONEER-HF were started on a submaximal dose of blinded study drug, which was systematically increased over the course of the study; thus, patients were generally not exposed to higher dose levels of blinded study drug than the subgroup in which they were analyzed. As a result, the present analysis more effectively isolates the impact of the dose level of sacubitril/valsartan vs. enalapril achieved on the efficacy and safety outcomes, and provides reassurance that lower doses of sacubitril/valsartan in the first month following HF hospitalization offer clinical benefit.
The natriuretic peptide response profiles may provide some additional insight into the observed consistency in the clinical benefit of sacubitril/valsartan at various dose levels. Specifically, there was an early and sustained reduction in NT-proBNP concentration in patients treated with sacubitril/valsartan as compared with enalapril across all dose levels. As changes in NT-proBNP have been shown to correlate with changes in cardiac structure and function in patients treated with sacubitril/valsartan,12 it is possible that the early reductions in NT-proBNP concentration with sacubitril/valsartan observed across all dose levels in PIONEER-HF were reflective of these favorable effects.
Importantly, the results of this analysis should not be interpreted as suggesting that clinicians should target submaximal doses of sacubitril/valsartan. On the contrary, to achieve the results observed in the PIONEER-HF study, clinicians should adhere as closely as possible to the dose titration algorithms used in the trial, which targeted the maximal dose whenever tolerated. Nevertheless, these data support the notion that lower doses of sacubitril/valsartan provide clinical benefit during the vulnerable post-hospitalization period, and support the initiation GDMT with sacubitril/valsartan in hospitalized HFrEF patients and continuation of the maximum dose achieved.
Limitations
This analysis should be interpreted in the context of several limitations. First, dose level achieved at 4 weeks post-randomization is a post-randomization variable; therefore, the comparisons between patient groups achieving different dose levels in this analysis are at-risk for confounding. Nevertheless, the patient characteristics between patients treated with sacubitril/valsartan vs. enalapril at each dose level were very similar (Table 1), and the results of each of the models were unchanged after adjusting for variables associated with not attaining the target dose by 4 weeks post-randomization (age, SBP, and race). Second, dose levels of blinded study drug were dynamic over the course of the trial, so subgroup categorization based on dose levels achieved at 4 weeks post-randomization does not completely reflect the total exposure to blinded study drug over the 8-week study period. In addition, dose titration both prior to and after the 4-week timepoint may reduce heterogeneity between dose level groups. Mitigating this limitation, in two sensitivity analyses, one using a time-varying Cox model to account for dose level variation over time and the other based on the highest dose level achieved during the 8-week study period, the results were unchanged (Supplemental Table 1). Finally, the dose level subgroups were relatively small in a trial of short, fixed duration, which may limit the power to detect clinically relevant heterogeneity with respect to the safety and efficacy outcomes. We note that tests for heterogeneity are particularly underpowered statistical procedures.
Conclusions
In this analysis from the PIONEER-HF trial, we found that among patients stabilized during hospitalization for ADHF, the efficacy and safety of sacubitril/valsartan were generally consistent across various dose levels. These data support the in-hospital initiation and continued post-hospitalization use of sacubitril/valsartan broadly, including in patients who may not tolerate early up-titration to the target dose.
Supplementary Material
Clinical Perspectives.
HF hospitalization is an ideal time to implement GDMT for HFrEF to increase adherence and improve clinical outcomes in the vulnerable post-hospitalization period. The PIONEER-HF trial demonstrated that in-hospital initiation and continued post-hospitalization use of sacubitril/valsartan in patients with HFrEF stabilized during hospitalization for ADHF was safe, well-tolerated, and reduced the composite of rehospitalization for HF or CV death. Not all patients in PIONEER-HF achieved the target dose of blinded study drug during the first month post-hospitalization; however, in this analysis of the PIONEER-HF trial, we showed that the safety profile and benefits of sacubitril/valsartan appear to be generally consistent irrespective of dose level achieved using the trial-based dosing algorithm.
Translational Outlook.
These data support the in-hospital initiation and continued post-hospitalization use of sacubitril/valsartan with the dose-titration strategy studied in PIONEER-HF, which includes patients who may not tolerate early up-titration to the target dose.
Acknowledgments
Disclosures: Dr. Berg has nothing to disclose. For the work under consideration, Dr. Braunwald reports grant support to his institution from Novartis for the conduct of the PIONEER-HF Trial, for his serving on the Executive Committee of the PARADISE trial and for his participation in an Advisory Board Meeting. He also reports lectures for Novartis that were uncompensated. For outside the submitted work, Dr. Braunwald reports grants to his institution from Merck, Daiichi Sankyo, Astra Zeneca, and Glaxo Smith Kline; personal fees for consultancies with Theravance, Cardurion, and MyoKardia, and for serving on an Advisory Board for Endcadia; personal fees for lectures from Medscape, and uncompensated lectures for Medicines Company and Merck. Dr. DeVore has received research funding from AstraZeneca, Amgen, the American Heart Association, Bayer, Luitpold Pharmaceuticals, Medtronic, the National Heart, Lung, and Blood Institute, PCORI, and Novartis; and has served as a consultant for AstraZeneca, LivaNova, Mardil Medical, Novartis, and Procyrion. Dr. Lala has nothing to disclose. Dr. Pinney has received consulting fees from Abbott, CareDx, and Medtronic. Dr. Duffy is an employee of Novartis Pharmaceuticals Corp. Dr. Gurmu has received grant support from Novartis. Dr. Velazquez reports grants from Novartis, Amgen, Phillips, and NHLBI/NIH. Dr. Morrow reports grants from Abbott Laboratories, Amgen, AstraZeneca, Eisai, GlaxoSmithKline, Medicines Company, Merck, Novartis, Pfizer, Roche Diagnostics, and Takeda. He has received personal fees from Abbott Laboratories, Aralez, AstraZeneca, Bayer Pharma, GlaxoSmithKline, InCarda, Merck, Peloton, and Roche Diagnostics. The PIONEER-HF trial was funded by Novartis Pharmaceutical Corp.
Abbreviations
- ADHF
acute decompensated heart failure
- BMI
body-mass index
- eGFR
estimated glomerular filtration rate
- HF
heart failure
- HFrEF
heart failure with reduced ejection fraction
- GDMT
guideline-directed medical therapy
- NYHA
New York Heart Association
- NT-proBNP
N-terminal pro-B-type natriuretic peptide
- SBP
systolic blood pressure
Footnotes
Clinical Trial: www.clinicaltrials.gov: NCT02554890
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Solomon SD, Dobson J, Pocock S, Skali H, McMurray JJ, Granger CB, Yusuf S, Swedberg K, Young JB, Michelson EL, Pfeffer MA, Candesartan in Heart failure: Assessment of Reduction in M and morbidity I. Influence of nonfatal hospitalization for heart failure on subsequent mortality in patients with chronic heart failure. Circulation. 2007;116:1482–7. [DOI] [PubMed] [Google Scholar]
- 2.Bello NA, Claggett B, Desai AS, McMurray JJ, Granger CB, Yusuf S, Swedberg K, Pfeffer MA and Solomon SD. Influence of previous heart failure hospitalization on cardiovascular events in patients with reduced and preserved ejection fraction. Circ Heart Fail. 2014;7:590–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gattis WA, O’Connor CM, Gallup DS, Hasselblad V, Gheorghiade M, Investigators I-H and Coordinators. Predischarge initiation of carvedilol in patients hospitalized for decompensated heart failure: results of the Initiation Management Predischarge: Process for Assessment of Carvedilol Therapy in Heart Failure (IMPACT-HF) trial. J Am Coll Cardiol. 2004;43:1534–41. [DOI] [PubMed] [Google Scholar]
- 4.Curtis LH, Mi X, Qualls LG, Check DK, Hammill BG, Hammill SC, Heidenreich PA, Masoudi FA, Setoguchi S, Hernandez AF and Fonarow GC. Transitional adherence and persistence in the use of aldosterone antagonist therapy in patients with heart failure. Am Heart J. 2013;165:979–986 e1. [DOI] [PubMed] [Google Scholar]
- 5.Velazquez EJ, Morrow DA, DeVore AD, Duffy CI, Ambrosy AP, McCague K, Rocha R, Braunwald E and Investigators P-H. Angiotensin-Neprilysin Inhibition in Acute Decompensated Heart Failure. N Engl J Med. 2019;380:539–548. [DOI] [PubMed] [Google Scholar]
- 6.Morrow DA, Velazquez EJ, DeVore AD, Desai AS, Duffy CI, Ambrosy AP, Gurmu Y, McCague K, Rocha R and Braunwald E. Clinical Outcomes in Patients With Acute Decompensated Heart Failure Randomly Assigned to Sacubitril/Valsartan or Enalapril in the PIONEER-HF Trial. Circulation. 2019;139:2285–2288. [DOI] [PubMed] [Google Scholar]
- 7.Writing Committee M, Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr., Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL and American College of Cardiology Foundation/American Heart Association Task Force on Practice G. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128:e240–327. [DOI] [PubMed] [Google Scholar]
- 8.Velazquez EJ, Morrow DA, DeVore AD, Ambrosy AP, Duffy CI, McCague K, Hernandez AF, Rocha RA and Braunwald E. Rationale and design of the comParIson Of sacubitril/valsartaN versus Enalapril on Effect on nt-pRo-bnp in patients stabilized from an acute Heart Failure episode (PIONEER-HF) trial. Am Heart J. 2018;198:145–151. [DOI] [PubMed] [Google Scholar]
- 9.Vardeny O, Claggett B, Packer M, Zile MR, Rouleau J, Swedberg K, Teerlink JR, Desai AS, Lefkowitz M, Shi V, McMurray JJ, Solomon SD, Prospective Comparison of AwAtDIoGM and Morbidity in Heart Failure I. Efficacy of sacubitril/valsartan vs. enalapril at lower than target doses in heart failure with reduced ejection fraction: the PARADIGM-HF trial. Eur J Heart Fail. 2016;18:1228–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Senni M, McMurray JJ, Wachter R, McIntyre HF, Reyes A, Majercak I, Andreka P, Shehova-Yankova N, Anand I, Yilmaz MB, Gogia H, Martinez-Selles M, Fischer S, Zilahi Z, Cosmi F, Gelev V, Galve E, Gomez-Doblas JJ, Nociar J, Radomska M, Sokolova B, Volterrani M, Sarkar A, Reimund B, Chen F and Charney A. Initiating sacubitril/valsartan (LCZ696) in heart failure: results of TITRATION, a double-blind, randomized comparison of two uptitration regimens. Eur J Heart Fail. 2016;18:1193–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wachter R, Senni M, Belohlavek J, Straburzynska-Migaj E, Witte KK, Kobalava Z, Fonseca C, Goncalvesova E, Cavusoglu Y, Fernandez A, Chaaban S, Bohmer E, Pouleur AC, Mueller C, Tribouilloy C, Lonn E, J ALB, Gniot J, Mozheiko M, Lelonek M, Noe A, Schwende H, Bao W, Butylin D, Pascual-Figal D and Investigators T. Initiation of sacubitril/valsartan in haemodynamically stabilised heart failure patients in hospital or early after discharge: primary results of the randomised TRANSITION study. Eur J Heart Fail. 2019;21:998–1007. [DOI] [PubMed] [Google Scholar]
- 12.Januzzi JL Jr., Prescott MF, Butler J, Felker GM, Maisel AS, McCague K, Camacho A, Pina IL, Rocha RA, Shah AM, Williamson KM, Solomon SD and Investigators P-H. Association of Change in N-Terminal Pro-B-Type Natriuretic Peptide Following Initiation of Sacubitril-Valsartan Treatment With Cardiac Structure and Function in Patients With Heart Failure With Reduced Ejection Fraction. JAMA. 2019:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.