Abstract
Circadian rhythms integrate many physiological pathways, helping organisms to align the timing of various internal processes to daily cycles in the external environment. Disrupted circadian rhythmicity is a prominent feature of modern society, and has been designated as a probable carcinogen. Here we review multiple studies, in humans and animal models, that suggest a causal effect between circadian disruption and increased risk of cancer. We also discuss the complexity of this connection, which may depend on the cellular context.
Keywords: circadian rhythm, circadian clocks, shift work schedule, rotating shift work, jet lag syndrome, chronotherapy
INTRODUCTION
In every kingdom of life, organisms from bacteria to humans exhibit endogenous circadian rhythms in behaviour, physiology, and/or metabolism (1). Processes such as sleep/wake cycle, feeding, core body temperature, and other metabolic or immune pathways display circadian (from the Latin circa diem, meaning “about a day”) oscillations with a period of about 24 hours. Circadian rhythms are thought to have evolved in response to the daily light/dark cycle as a mechanism that attuned organisms to the natural period of the Earth’s rotation. A central clock in the suprachiasmatic nucleus (SCN) of the hypothalamus drives behavioral rhythms and synchronizes peripheral tissue clocks via incompletely defined nutritional, neural, and humoral mediators. Virtually all cells in the body possess the molecular circadian clock machinery and can sustain endogenous circadian oscillations.
At the molecular level, a cell-autonomous circadian clock in mammals consists of an autoregulatory positive and negative-feedback transcriptional network (2) with a positive arm — heterodimers of circadian locomotor output cycles kaput (CLOCK) and brain and muscle arnt-like protein 1 (BMAL1) — driving transcription of two inhibitory arms — periods (PERs) and cryptochromes (CRYs) on one hand and nuclear receptor subfamily 1 group D members 1 and 2 (NR1D1/NR1D2 also called REV-ERBα/REV-ERBβ) on the other, which feed back to inhibit the BMAL1/CLOCK heterodimer transactivation function (Figure 1a). These transcriptional-translational feedback loops drive 24-hour periodic expression of gene products leading to rhythmic physiological functions. BMAL1 plays a crucial role in this network as in its absence, circadian behaviors are abolished (3).
Figure 1: Molecular basis of circadian clock disruption and its effect on cancer.
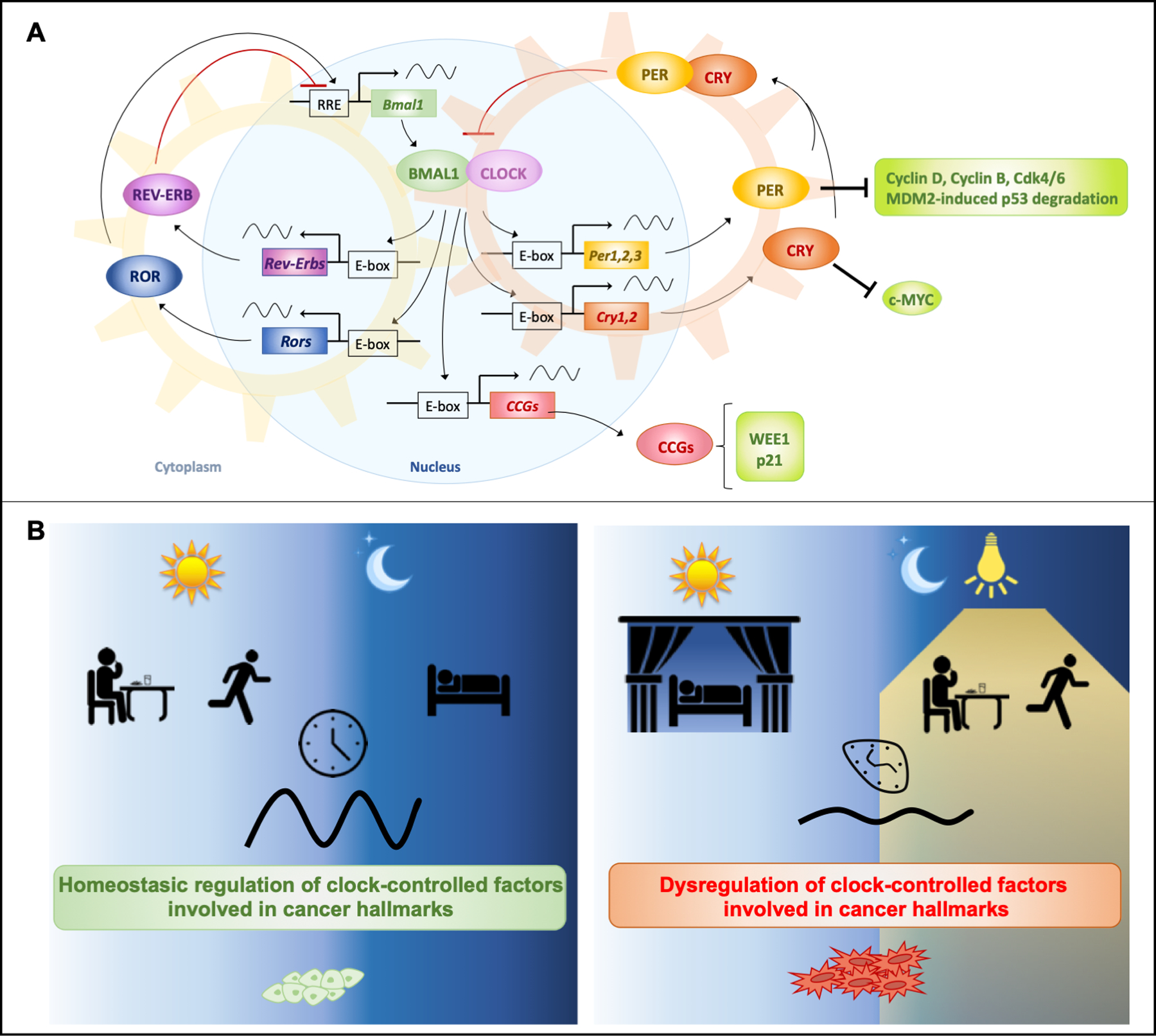
(A) Circadian clock molecular machinery and its direct regulation of cancer related factors. The mammalian clock machinery consists of the positive transcriptional elements CLOCK, or its paralog NPAS2, and BMAL1. This heterodimer activates Periods (Per1–3), Cryptochromes (Cry1, Cry2) and other clock-controlled genes (CCGs) via E-boxes. Notably, key cell cycle regulators such as Wee1 and p21 are CCGs. PER and CRY proteins accumulate in the cytoplasm, and dimerize to form a complex that migrates into the nucleus to repress CLOCK:BMAL1 transcriptional activity. This leads to repression of Pers, Crys, and CCGs, thus forming a negative feedback loop. This cycle takes about 24 hours and is re-initiated by degradation of PERs and CRYs. An additional feedback loop is interlaced with this core loop. Rev-Erbα and Rev-Erbβ (a.k.a. Nuclear receptor subfamily 1, group D members 1 and 2, or Nr1d1 and Nr1d2) and Rors (RAR-related orphan receptors), are CCGs and repress or activate transcription of Bmal1 respectively. PERs and CRYs modulate post-translational regulation of key factors of cell cycle control. (B) Misalignment with the external environment causing circadian disruption of the molecular clock and its cancer-related targets. When lifestyles are aligned with the natural solar day, circadian rhythms allow homeostatic regulation of pathways involved in cell protection (left). When chronically shifting phase from the natural solar day, circadian rhythms of the key clock factors are dampened, affecting regulation of these pathways (right).
Since the invention of the light bulb, the daily activities of humans have extended into the night, resulting in a dramatic change in lifestyle, including the increased prevalence of night shift workers. In 2015, the Occupational Health Supplement from the National Health Interview Survey (NHIS) revealed that about 12–32% of the workforce in the United States was assigned to irregular schedules, including night and rotating shifts (https://wwwn.cdc.gov/NIOSH-WHC/chart/ohs-workorg?OU=WORKSCHD_RCD&T=I&V=R2). Although these types of schedules are necessary in our modern society, especially in the healthcare industry, they can disrupt the internal circadian clock machinery and the sleep/wake cycle, and cause excessive exposure to light at night (LAN). Given that the circadian clock is a critical regulator of many physiological processes, it is not surprising that lifestyles misaligned with the natural solar day have been associated with increased risk of disease, including sleep and metabolic disorders, cardiovascular and Alzheimer’s diseases, and several cancer types. Based on evidence from epidemiological studies and experiments in animal models, the International Agency for Research on Cancer (IARC) has classified “shift-work that involves circadian disruption” as potentially “carcinogenic to humans (Group 2A)” in 2007 and again in 2019 (4). However, our understanding of the causal molecular mechanisms underlying this phenomenon remains in its infancy. Several hypotheses have been proposed to explain the shift work-cancer connection: internal desynchronization, light at night (resulting in melatonin suppression), sleep disruption or lifestyle disturbances (Figure 1b). Results from a continuously increasing number of studies suggest that multiple factors may contribute to linking shift work and adverse health consequences. Here, we recapitulate human field studies and animal studies highlighting the growing evidence suggesting a causal effect of circadian disruption on cancer risk. As we learn more about the impact of different types of circadian disruption on a variety of cancers, we will improve our understanding of the underlying causes, which will enable evidence-based lifestyle and clinical recommendations.
EPIDEMIOLOGICAL STUDIES REVEAL ADVERSE EFFECTS OF DISRUPTED CLOCKS ON CANCER
Environmental Factors Modifying Circadian Rhythms
Shift work
The first studies of shift work and human cancer were purposefully designed to test the theory that light at night (LAN) promotes breast cancer. This theory proposes that exposure to LAN lowers melatonin production by the pineal gland, which, in turn, increases estrogen production leading to higher breast cancer risk. A Fred Hutchinson Cancer Research Center case control study designed in the early 1990s and published in 2001 showed that breast cancer risk was increased among subjects who frequently did not sleep during the period of the night (OR = 1.14 for each night per week; 95% CI = 1.01 to 1.28). Furthermore, graveyard shiftwork was associated with increased breast cancer risk (OR = 1.6; 95% CI = 1.0 to 2.5), with a trend of increased risk with greater cumulative years and with more hours per week of graveyard shiftwork (5). Since then, many epidemiological studies support the association between shift work and enhanced cancer risk. However, shift work impacts many factors such as feeding behavior, sleeping patterns, and circadian clock dysregulation, that likely contribute to altered cancer risk. Thus, it is difficult to adjust for all of the potential cofounding factors of epidemiological studies.
The analyses that predominantly motivated the 2007 IARC decision classifying shift-work as a probable carcinogen came from the Nurses’ Health Studies. The Nurses’ Health Study (NHS) and Nurses’ Health Study II (NHS II) were established in 1976 and 1989, respectively, and are among the largest prospective investigations into the risk factors for major chronic diseases in women, including collected data on shift-work exposure (6, 7). A 2017 study that analyzed long-term follow-up of these two cohorts confirmed the association between long-term rotating night-shift work and higher risk of breast cancer, and found that this connection was particularly significant among women who performed shift work during young adulthood, suggesting that age at exposure could be a factor in this association (8). In this study, the interaction of shift work with menopausal status was not significant but another meta-analysis determined that pre-menopausal (but not post-menopausal) women had a higher breast cancer risk if they were current or recent night shift workers (9), reinforcing the hypothesis that circadian disruption has a more significant impact on breast cancer incidence in women who are not yet subject to the contributing factor of menopause. Since the IARC decision, results from several cohort studies and meta-analyses have been published with variable conclusions (10). Although most of the recent meta-analyses suggest night shift work may be associated with a modestly elevated risk of breast cancer in women (11–14), some cite insufficient or weak evidence for a definite link between night-shift work and breast cancer (15–17).
Although most research has focused on the increased risk of breast cancer among female night shift workers, a few studies examine different types of cancer. Studies of the impact of shift work on colorectal cancer are inconclusive (18–20). Independent cohort studies of night workers and shift workers have observed increased incidence of prostate (21), endometrial epithelial malignancies (22) and non-Hodgkin’s lymphoma (23). Results from the NHS first cohort suggest that there are modest increased risks of lung cancer associated with extended periods of night-shift work among smokers but not among nonsmokers, highlighting the issue of confounding factors (24).
Chronic jet lag, social jet lag and time zone
Jet lag is another environmental factor associated with altered circadian rhythms and higher incidence of cancers. It arises from the discrepancy between internal body clocks and the environmental light/dark cycle. Most people experience acute jet lag when travelling through multiple time zones over a short period of time, but this jet lag becomes chronic for airline pilots and flight attendants and has deleterious effects on health. Indeed, previous cancer incidence studies on airline pilots and flight attendants have indeed reported increased rates of specific cancers, predominantly skin cancer (25–28) and to a lesser extent prostate (25, 27) and breast (28, 29) cancers. Because of extended periods at high altitude, flight crews are exposed to cosmic ionizing radiation, which may contribute to their increased incidence of some cancers, especially skin cancers. But circadian rhythm disruption due to night shift work, irregular schedules and frequently crossing time zones is another factor to consider. For example, standardised incidence ratio (SIR) for malignant melanoma is higher among those who had been flying over five time zones (SIR: 25, 95% CI 6.73 to 64.00 compared to SIR: 9.09, 95% CI 0.12 to 50.58 for pilots never flying over five time zone) (26). In another study, the relative risk of prostate cancer, calculated by a Poisson regression analysis, increased with accumulating number of flight hours in long distance aircraft but not with estimated radiation dose (25), indicating a primary effect of circadian rhythm disruption.
Long-term rotating shift work and chronic jet lag are major circadian disruptors, but many if not most people in modern society are exposed to subtle chronic disruption of their biological clocks. Decades of research have established typical variation and developmental patterns in circadian behavior in human populations and individuals can be assigned “chronotypes” to classify each of us as a “morning lark”, a “night owl”, or somewhere in between (30). Regardless of classification, many people wake up early during the week for work or school and sleep later on weekends, thus regularly changing their sleep/wake timing. This form of subtle jet lag is termed “social jet lag” and is particularly common in teenagers, who have a later chronotype due to their developmental stage (31). Researchers are now starting to investigate the potential deleterious effects of social jet lag on health (32). Another common source of subtle circadian misalignment is the definition of time zones. Dawn and dusk times vary depending on longitudinal position within a time zone: moving five degrees of longitude westward across a time zone results in a 20-minute delay in sunrise. For example, when a person in Montgomery, Alabama, wakes up at 7 a.m., the sun is up, and thus that person’s body clock is in phase with the environment. But when a person living in Odessa, Texas, gets up at the same time, in the same time zone, it could still be dark outside, causing a circadian misalignment over time. A 2011 study revealed that in Russia and China, those residing in the western border of a timezone had higher cancer incidence and mortality rates, especially for female breast cancer, and lower life expectancy (33). Subsequently, Caporaso and colleagues measured a similar effect within the United States (34). Using data from the Surveillance, Epidemiology, and End Results Program (2000–2012), they examined associations between the position in a timezone and age-standardized county-level incidence rates for total cancers combined and 23 specific cancers, including four million cancer diagnoses in white residents of 607 counties in 11 US states. After adjusting for latitude, poverty, cigarette smoking, and state, they found increased incidence for cancers overall and some specific types of cancers from the east to the west in a timezone. Consistent with the previous ecological study in Russia (33), they showed an association with breast and prostate cancer and also revealed increased risk of chronic lymphocytic leukemia in both genders, stomach cancer, liver cancer and non-Hodgkin lymphoma in males, and esophageal, colorectal, and lung cancer in females associated with western timezone position. The strongest and most consistent effect was observed for chronic lymphocytic leukemia, a tumor that lacks strong extrinsic environmental risk factors and which has been shown to exhibit altered expression of specific clock genes, including BMAL1 and PER2 (35). These findings support the idea that circadian disruption increases cancer risk, and elegantly avoid the lifestyle factors that may confound analyses of shift workers. Furthermore, these studies indicate that circadian disruption is not a rare phenomenon affecting only shift workers or people exposed to chronic jet lag, but is widespread throughout the general population.
Associations between polymorphisms in clock genes and cancer in humans
Several studies have suggested a causal association between polymorphisms in clock genes and human cancer susceptibility. In 2005, a structural variant in the circadian gene PER3 (an exonic length variation associated with delayed sleep phase syndrome and diurnal preference) was found to be significantly associated with increased risk of breast cancer (36). Soon after, a 2007 study showed a robust association of the Ala394Thr polymorphism in the clock gene NPAS2 (rs2305160) with reduced risk of non-Hodgkin’s lymphoma (37). Ground-breaking work has identified a large number of polymorphisms affecting multiple clock genes (BMAL1, CLOCK, CK1ε, CRY1–2, NPAS2, and PER1–3) that are linked to increased risks in prostate, breast, ovarian and pancreatic cancers (see (38) for review). Recently, a meta-analysis of 27 eligible studies comprising 96,756 subjects and investigating 687 polymorphisms involving 14 clock genes identified 10 single nucleotide polymorphisms (SNPs) significantly associated with cancer risk, especially for breast cancer (39). Furthermore, results from a study that used multiple locus analysis to examine the effect of genetic variation in the circadian pathway, which increased the statistical power compared to single gene analysis, revealed a highly significant association between alterations in the circadian pathway overall and the risk of each malignancy investigated (e.g. breast, prostate and lung cancer) (40). The greatest impact was found for variants in ARNTL, CLOCK, RORA and RORB and variants in CRY1, CRY2, NPAS2, NR1D1, and PER3 contributed to the significant impact for at least two of the three malignancies examined. However, the majority of the studied polymorphisms are located in intronic regions (39) and their impact on clock gene expression and function is still unknown.
Together, findings from clinical epidemiological studies and genetic evidence support the hypothesis that circadian clocks can influence cancer incidence in humans.
EXPERIMENTS IN ANIMALS REINFORCE THE CONNECTION BETWEEN DISRUPTED CLOCKS AND CANCER
Physiological circadian disruption in animal models
As studies in humans are always complex due to the heterogeneity of the subjects, many researchers have turned to rodent models to investigate the impact of circadian disruption on cancer risk. Much of this work was pioneered by Dr. Francis Levi, whose team first tackled the issue using the drastic approach of physically destroying the SCN in mice using bilateral electrolytic lesions, before inoculating tumors (41). This procedure suppressed the 24-hour rhythm in rest/activity and body temperature and severely damped the corticosterone secretion rhythm in the animals. Transplantation of a Glasgow osteosarcoma or a pancreatic adenocarcinoma into mice with ablated SCNs resulted in accelerated malignant growth as compared with sham-operated animals. However, SCN lesions severely impair peripheral clock and physiological rhythms, which is an extreme case of circadian disruption that is not actually observed in humans. In order to better mimic chronic disruption of a functional circadian timing system, such as those experienced by shift workers, researchers turned to chronic jet lag (CJL) protocols in mice by altering their light schedules. Many CJL protocols consist of repeated light-phase advances which significantly disrupt circadian rhythms in physiologic outputs such as locomotor activity, body temperature and corticosterone secretion. They also severely alter rhythmic expression of core clock genes in the SCN and in peripheral tissues. Many studies using different models of cancer revealed that animals exposed to these regular light-phase advances developed more aggressive tumors and had overall poorer survival (42–47) (Table 1).
Table 1:
Effects of environmental circadian disruption on mouse models of cancer
| CJL light Schedule | Cancer Model | Impact | Reference |
|---|---|---|---|
| Advance of light onset by 8 hours (4 hours in dark) every 2 days for 10 days | Inoculation of Glasgow osteosarcoma | Faster tumor progression and poorer overall survival | (42) |
| 8-hour light-advance followed by an 8-hour light-delay in the onset of the light period every 3 days | Radiation-induced tumor development | Higher incidence of ovarian, kidney, intestinal, liver, and pancreatic tumors, osteosarcoma, and lymphoma, and overall poorer survival | (44) |
| Weekly alternating light-dark (LD) cycle throughout animal life | Breast cancer-prone p53R270H©/+ WAPCre conditional mutant mice | Increase in breast tumor development | (45) |
| Weekly switch between two rooms different only at the time of light onset for 8 hours from 4–90 week of age | C57BL/6J inbred WT mice | Enhanced spontaneous development of hepatocellular carcinoma (HCC) | (46) |
| 8-hour light advance schedule repeated every 2–3 days | K-rasLSL-G12D;p53flox/flox (KP) mouse model of lung adenocarcinoma | Enhanced lung tumor burden and decreased overall survival | (47) |
Genetically engineered mouse models of cancer (GEMMs) recapitulate cancer development driven by common oncogenic drivers. Several of these have now been found to exhibit more aggressive disease when exposed to CJL. van der Horst and colleagues exposed Tp53R270H/+;WAPCre conditional mutant mice, a model for spontaneous breast cancer development, to a weekly inversion of the light-dark (LD) cycle throughout their lives. The latency to mammary gland tumor development was reduced in the mice subjected to chronic LD inversions compared to control mice housed in standard lighting conditions (12 hours of light and 12 hours of dark, 12:12LD) (45). Furthermore, in a large scale survival study Fu and colleagues showed that CJL enhanced the spontaneous development of hepatocellular carcinoma (HCC) in C57BL/6J inbred wildtype mice (46). The underlying mechanism was very similar to that observed in obese humans with initiation of non-alcoholic fatty liver disease (NAFLD) that progresses to steatohepatitis and fibrosis before HCC detection. Mechanistically, CJL induced a profound rewiring of hepatic circadian gene expression as well as rhythmic metabolism, resulting in induction of hepatic cholestasis that activates the oncogenic program of the nuclear receptor constitutive androstane receptor (CAR), a well-known liver tumor promoter that mediates toxic bile acid signaling. These findings illustrate how disruption of metabolic homeostatis upon environmental circadian disruption can drive tumorigenesis. Given the critical importance of metabolic alterations in tumor development in general, metabolic impacts of circadian disruption likely extend beyond HCC. Physiological disruption of circadian rhythms by exposure to CJL also accelerated lung tumorigenesis in a mouse model of non-small cell lung cancer (NSCLC). Using mice with activation of oncogenic K-RAS and loss of function of tumor protein p53 (Tp53 also known as p53), the K-rasLSL-G12D/+;p53flox/flox (KP) mice, Papagiannakopoulos et al. showed enhanced tumor burden and poorer survival in mice exposed to CJL compared to mice kept in 12:12LD (47). Together, these studies reveal that environmental disruption of circadian rhythms alters the development of diverse tumor types. The underlying mechanisms are likely multifaceted and further investigations are needed to provide insight into how circadian disruption influences tumor initiation and progression.
Some general themes have emerged regarding the effects of CJL on tumor progression. Disruption occurs at several levels, including behavioral and hormonal rhythms as well as clock gene expression in the SCN, in tissues at the periphery of the tumor, and in the tumor itself. For example, alterations of immune system components by CJL may contribute to deleterious effects on tumorigenesis. In rats, a CJL paradigm consisting of ten repeated 6-hour photic advances occurring every 2 days, followed by 5–7 days of constant darkness, suppressed circadian rhythms of natural killer cell function, also attenuated their cytolytic activity, and promoted lung tumor growth following intravenous injection of mammary adenocarcinoma cells (MADB106) (48). A recent Pan-Cancer analysis demonstrated that abnormal circadian clock contributes to T cell exhaustion and global upregulation of immune inhibitory molecules such as PD-L1 and CTLA-4 (49), reinforcing the hypothesis that environmental circadian disruption can drive tumorigenesis through dysregulation of immune homeostasis both at the cell-autonomous and systemic levels.
Physiological disruption produced by altered light exposure in animal models mimics many features of circadian disruption experienced by shift workers. However, most inbred mouse strains lack the enzymes required for biosynthesis of melatonin (50). Therefore, the potential role of disrupted melatonin production caused by shift work cannot be evaluated in these studies. The robust impact of CJL on mouse models of cancer even in the absence of endogenous melatonin biosynthesis indicates that disruption of molecular circadian clocks can influence tumorigenesis, which is also supported by studies using genetic models.
Genetic circadian disruption in animal models
In order to understand the impact of disrupting the circadian clock machinery on cancer incidence, apart from other aspects of physiology that are affected by altered light exposure, researchers turned to mouse models harboring germline mutation of core clock genes (Table 2).
Table 2:
Effects of molecular circadian clock disruption on mouse models of cancer
| Mutation in clock gene | Impact on the clock machinery | Impact on cancer | Reference |
|---|---|---|---|
| Per2m/m (Ubiquitous) | Shorter circadian period followed by a loss of circadian rhythmicity in constant darkness Non-robust clock | Enhanced lymphomas in multiple organs caused by gamma irradiation | (44, 52) |
| Per1−/−;Per2−/− (Ubiquitous) | Immediate loss of circadian rhythms in constant darkness Nonfunctional clock | Enhanced lymphomas in multiple organs caused by gamma irradiation | (44) |
| Cry1−/−;Cry2−/− (Ubiquitous) | Immediately loss of circadian rhythms in constant darkness Nonfunctional clock | Enhanced lymphomas in multiple organs caused by gamma irradiation | (44) |
| Bmal1−/− (Ubiquitous) | Diurnal variation in activity abolished Nonfunctional clock | Increased lung tumor burden in Kras mutant mice | (47) |
| Bmal1−/− (Tumor-autonomous) | Cell-autonomous nonfunctional clock | Increased lung tumor burden in Kras mutant mice | (47) |
Several studies used mice homozygous for a noncoding mutation that results in greatly reduced expression of endogenous Per2 (Per2m/m), which have altered circadian clock function (51), and found an increase in tumor formation in different experimental settings (44, 47, 52, 53). For example, within 16 months after exposure to gamma radiation, Per2m/m mice developed lymphomas with 71% penetrance, teratomas and salivary gland hyperplasia with 100% penetrance, while those phenomena were observed in 5% or less of wildtype mice (52). When crossed with K-rasLSL-G12D/+ (K) or K-rasLSL-G12D/+;p53flox/flox (KP) mice, two mouse models of lung adenocarcima, mice with the hypomorphic mutant allele of Per2 presented enhanced pulmonary tumor burden and poorer survival (47). Interestingly, KP Per2 mutant cells exhibited altered glucose and glutamine metabolism compared to KP control cells indicating a metabolic dysregulation at the cell-autonomous level upon altered Per2 function, which could promote tumor growth. Similar results have been observed in mice with deletion of Per2 alone or concomitant with deletion of Per1 (44, 46). Although Per1 single deletion has not been studied in animal cancer models, in vitro and xenograft studies suggest that PER1 has a similar tumor inhibitory effect as that observed for PER2 (54). PER1 and PER2 have been shown to play important roles in cell proliferation, apoptosis and cell cycle progression by regulating the CYCLIN/CDK/CKI cell cycle network (55, 56) which could also contribute to their observed tumor-suppressive effects.
While ubiquitous deletion or mutation of the repressors Per1 and/or Per2 seems to consistently increase cancer incidence, deletion of Cry1 and/or Cry2 has divergent effects on tumor formation in different contexts. While the dual deletion of Cry1/2 improved survival and slowed tumor progression in p53-null mice (57), this same double deletion enhanced spontaneous (46) and irradiation-induced (44) formation of hepatocellular carcinomas as well as formation of cholangiocarcinomas after exposure to diethylnitrosamine (58). Cry2 deletion resulted in poorer survival in a mouse model of MYC-driven Burkitt’s lymphoma (59); Cry1 deletion has not been investigated in that model. These findings point out the complexity of the system where different molecular pathways can be targeted in different tumor models. Further investigation will be required to understand the overlapping and distinct roles of PERs and CRYs in regulating various molecular and physiological pathways that contribute to tumorigenesis.
Delineating the mechanisms by which circadian disruption influences tumorigenesis is further complicated because clock components have multiple functions beyond modulating circadian rhythms. For example, PER2 interacts with p53 and prevents its ubiquitination by the E3 ligase Mouse Double Minute 2 homolog (MDM2) and downstream proteasomal degradation (60). Consistent with the diverse impacts of their deletion on in vivo tumor models, studies have also revealed non-redundant roles for CRY1 and CRY2 in regulating DNA damage. While deletion of Cry2 alone increased cellular transformation in cooperation with multiple different oncogenic factors, deletion of Cry1 attenuated cellular transformation only in cells lacking p53 (59). Moreover, upon genotoxic stress, CRY1 is stabilized via deubiquitination by Herpes virus associated ubiquitin-specific protease (HAUSP), whereas CRY2 degradation via recruitment to FBXL3 is enhanced (61). CRY2 also has a unique role in participating in post-translational regulation and degradation of the oncogenic transcription factor c-MYC (59). These results suggest distinct functions for CRY1 and CRY2 following DNA damage and could contribute to the divergent results observed in studies using mice with dual deletion of Cry1 and Cry2. Because all of the studies of PER and CRY deletion in tumor models involved germline deletion of these circadian repressors, it is also possible that systemic impacts of the genetic manipulation play an unappreciated role in driving their impact on tumor phenotypes. To understand the tumor-autonomous roles of PERs and CRYs, studies employing conditional deletion strategies will be required.
To disrupt the clock locally, a few studies have employed tissue-specific deletion of Bmal1. Mice harboring germline deletion of Bmal1 are not an appropriate model for studying cancer because they age prematurely, due to accumulation of reactive oxygen species in tissues (62). Inducible Bmal1 deletion allows the expression of the gene during development, eliminating the early aging phenotype (63). Papagiannakopoulos et al. reported an increased lung tumor number and decreased survival upon loss of Bmal1 in an autochthonous KRAS-driven GEMM (KrasLA2/+) that gives rise spontaneously to sporadic tumors in various tissues (47). In the KrasLSL-G12D/+ (K) mouse model of lung adenocarcima, Cre-mediated deletion of Bmal1 only in the cancer cells accelerated lung tumor progression suggesting that cell-autonomous circadian disruption can impact tumor growth. Interestingly, this effect was not observed in the KP mouse model, in which p53 is deleted in combination with the activation of KRASG12D, indicating a potential p53-dependent role for BMAL1 in preventing cancer progression. Indeed, a functional interaction between BMAL1 and p53 has been proposed in prior studies (64). In a similar organ-specific targeting approach, hepatocyte-specific loss of Bmal1 enhanced the formation of spontaneous hepatocellular carcinoma (46). Multiple studies have shown that the CLOCK/BMAL1 complex influences cell cycle progression by regulating expression of the transcript encoding the G2/M inhibitor Wee1 (65), which may contribute to these observations. Conversely, genetic deletion of Bmal1 impaired proliferation, enhanced myeloid differentiation, and depleted leukemia stem cells (66), and keratinocyte-specific deletion of Bmal1 reduced the development of RAS-driven squamous tumors (67). Finally, a recent study demonstrated that BMAL1 can promote breast cancer cell invasion and metastasis by up-regulating matrix metalloproteinase 9 (Mmp9) expression (68). Therefore, Bmal1 may act on different pathways depending on the cellular context to regulate tumor initiation and/or progression. Like the divergent effects of Cry1 and Cry2 deletion in diverse tumor models, these findings demonstrate that the context in which clock function is disrupted critically impacts its influence. They also suggest that clock components could function independently from the clock machinery to regulate pathways involved in tumorigenesis in a tissue-specific manner.
CONCLUSIONS AND PERSPECTIVES
In modern society, almost all people are subjected to circadian disruption to some extent: it can be subtle, due to social jetlag or waking up before sunrise due to our location within a time zone, or it can be chronic for people who work night shifts or frequently cross time zones. Rotating shift work appears to be one of the most disruptive lifestyles in terms of discordance between circadian gene expression, physiology, and human activities. These lifestyles have been linked to higher incidence of multiple cancers as well as metabolic syndrome (69) and cardiovascular disease (70). However, lifestyles that alter circadian synchrony with the external environment also impair sleep/wake cycles and metabolism, and affect eating and smoking habits, making it difficult to decipher the contribution of circadian disruption per se to the observed impact on disease risk. Two main factors that could contribute to higher cancer incidence are disruption in metabolic and immune homeostasis at the cell-autonomous and non-automous levels. Indeed, metabolic processes and immune pathways are tightly regulated by the circadian clock and are critical regulatory mechanisms to influence tumorigenesis (71, 72). Metabolic syndrome comprises an inflammatory signature, is increased in rotating shift workers, and has been associated with increased total cancer mortality (73).
Moreover, the connection between circadian disruption and cancer appears to be bidirectional as tumors can dampen circadian rhythms locally (74, 75) and remotely in distal organs, rewiring hepatic metabolism as well (76). Further, circadian disruption, assessed by rest-activity, body temperature, sleep, and/or hormonal secretion rhythms, was found in up to 50% of patients with metastatic cancer and was associated with poor outcomes, including fatigue, anorexia, sleep disorders, and reduced progression-free and overall survival (77). Although further studies are warranted, it is very likely that circadian disruption within tumors leads to compromised protective mechanisms at the cell-autonomous level and a rewiring of processes in peripheral tissues, which can further drive tumorigenesis by supplying metabolic fuel, emphasizing the significance of tumor–host interaction.
Studies in genetically engineered mouse models have allowed researchers to specifically investigate the contribution of the clock machinery disruption by targeting key clock genes. Although the majority of the findings indicate that a more robust circadian rhythmicity leads to better outcomes, deletion of a specific clock gene has variable effects depending on the tumor model, highlighting a complex connection between molecular circadian clocks and cancer, which depends on the tissue context. Cancer is a complex disease, involving multiple pathways to overcome protective barriers. Accumulating evidence reveals that circadian clock core components play critical roles in several hallmarks of cancer, including cell proliferation, DNA damage and repair and cell death (see (78) for review).
We are only beginning to dissect the multi-faceted and complex connection between circadian disruption and cancer risk. In addition to enhanced understanding of tumor development, a better understanding of how clocks influence cancer biology and repsonse to treatment could lead to improved therapeutic outcomes. Not only do circadian clocks alter tumor biology, they also can profoundly influence drug pharmacokinetics (79) and/or targeted responses (80). Several studies have shown promise for the implementation of chronotherapy (optimizing the timing of administration for active components). A seminal study demonstrated that the toxicity of the chemotherapeutic agent cyclophosphamide dramatically depends on the time of day at which it is administered in healthy mice, and this was attributed to time of day dependent effects on B cell survival (81). A recent study found that the efficacy of the FDA-approved CDK4/6 inhibitor PD-0332991 is greater in the morning than at night, and there was reduced efficacy in both cells and mice when their circadian rhythms were disrupted (82). This highlights the importance of including endogenous circadian rhythms as a variable for drug pharmacokinetics and pharmacodynamics and although this involves complex mathematical modeling, some groups have begun to use multiscale systems chronopharmacology approaches, based on cellular and whole-body physiology in order to design patient-tailored chronotherapies (83). Many tumors have altered circadian rhythms, and assessing the molecular clock states of tumors compared to healthy tissues could also be exploited to time treatments when healthy cells will be less affected, while targeting cancer cells. Today, cancer chronotherapy research holds promise for improving medical treatment regimens with regard to both drug tolerance and efficacy, as well as with new treatment targeting the activity of the circadian system itself. Promising work on small molecules that target clock components and their efficacies in cancer-related functions is not discussed here but is well described in a recent review (84). Other avenues to explore are lifestyle recommendations (e.g. time-restricted feeding and daily exercise for example) and hospital policies and lighting conditions, aimed at reinforcing endogenous circadian rhythms in patients.
Greater understanding of the molecular effects of jet lag, shift work, and other sources of chronic circadian disruption, will help to develop strategies to minimize the increased cancer risk associated with these behaviors, including timing delivery of cancer therapies for maximum benefit and lifestyle recommendations for the prevention and/or the correction of circadian disruption.
Significance.
Accumulating evidence points to an adverse effect of circadian disruption on cancer incidence and progression, indicating that time of day could influence the effectiveness of interventions targeting cancer prevention and management.
Acknowledgments
This work was supported by R01 grant CA211187 to K.A.L.
Footnotes
The authors declare no potential conflicts of interest.
References
- 1.Bass J, and Lazar MA. Circadian time signatures of fitness and disease. Science. 2016;354(6315):994. [DOI] [PubMed] [Google Scholar]
- 2.Buhr ED, and Takahashi JS. Molecular components of the Mammalian circadian clock. Handb Exp Pharmacol. 2013(217):3–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bunger MK, Wilsbacher LD, Moran SM, Clendenin C, Radcliffe LA, Hogenesch JB, et al. Mop3 is an essential component of the master circadian pacemaker in mammals. Cell. 2000;103(7):1009–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ward EM, Germolec D, Kogevinas M, McCormick D, Vermeulen R, Anisimov VN, et al. Carcinogenicity of night shift work. The Lancet Oncology. 2019;20(8):1058–9. [DOI] [PubMed] [Google Scholar]
- 5.Davis S, Mirick DK, and Stevens RG. Night Shift Work, Light at Night, and Risk of Breast Cancer. JNCI: Journal of the National Cancer Institute. 2001;93(20):1557–62. [DOI] [PubMed] [Google Scholar]
- 6.Schernhammer ES, Laden F, Speizer FE, Willett WC, Hunter DJ, Kawachi I, et al. Rotating Night Shifts and Risk of Breast Cancer in Women Participating in the Nurses’ Health Study. JNCI: Journal of the National Cancer Institute. 2001;93(20):1563–8. [DOI] [PubMed] [Google Scholar]
- 7.Schernhammer ES, Kroenke CH, Laden F, and Hankinson SE. Night Work and Risk of Breast Cancer. Epidemiology. 2006;17(1):108–11. [DOI] [PubMed] [Google Scholar]
- 8.Wegrzyn LR, Tamimi RM, Rosner BA, Brown SB, Stevens RG, Eliassen AH, et al. Rotating Night-Shift Work and the Risk of Breast Cancer in the Nurses’ Health Studies. Am J Epidemiol. 2017;186(5):532–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cordina-Duverger E, Menegaux F, Popa A, Rabstein S, Harth V, Pesch B, et al. Night shift work and breast cancer: a pooled analysis of population-based case–control studies with complete work history. European Journal of Epidemiology. 2018;33(4):369–79. [DOI] [PubMed] [Google Scholar]
- 10.Lee H-E, Lee J, Jang T-W, Kim I-A, Park J, and Song J. The relationship between night work and breast cancer. Ann Occup Environ Med. 2018;30:11-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.He C, Anand ST, Ebell MH, Vena JE, and Robb SW. Circadian disrupting exposures and breast cancer risk: a meta-analysis. International Archives of Occupational and Environmental Health. 2015;88(5):533–47. [DOI] [PubMed] [Google Scholar]
- 12.Jia Y, Lu Y, Wu K, Lin Q, Shen W, Zhu M, et al. Does night work increase the risk of breast cancer? A systematic review and meta-analysis of epidemiological studies. Cancer Epidemiology. 2013;37(3):197–206. [DOI] [PubMed] [Google Scholar]
- 13.Wang F, Yeung KL, Chan WC, Kwok CCH, Leung SL, Wu C, et al. A meta-analysis on dose–response relationship between night shift work and the risk of breast cancer. Annals of Oncology. 2013;24(11):2724–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin X, Chen W, Wei F, Ying M, Wei W, and Xie X. Night-shift work increases morbidity of breast cancer and all-cause mortality: a meta-analysis of 16 prospective cohort studies. Sleep Medicine. 2015;16(11):1381–7. [DOI] [PubMed] [Google Scholar]
- 15.Kamdar BB, Tergas AI, Mateen FJ, Bhayani NH, and Oh J. Night-shift work and risk of breast cancer: a systematic review and meta-analysis. Breast Cancer Research and Treatment. 2013;138(1):291–301. [DOI] [PubMed] [Google Scholar]
- 16.Ijaz S, Verbeek J, Seidler A, Lindbohm M-L, Ojajärvi A, Orsini N, et al. Night-shift work and breast cancer – a systematic review and meta-analysis. Scandinavian Journal of Work, Environment & Health. 2013(5):431–47. [DOI] [PubMed] [Google Scholar]
- 17.Travis RC, Balkwill A, Fensom GK, Appleby PN, Reeves GK, Wang X-S, et al. Night Shift Work and Breast Cancer Incidence: Three Prospective Studies and Meta-analysis of Published Studies. JNCI: Journal of the National Cancer Institute. 2016;108(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Walasa WM, Carey RN, Si S, Fritschi L, Heyworth JS, Fernandez RC, et al. Association between shiftwork and the risk of colorectal cancer in females: a population-based case–control study. Occupational and Environmental Medicine. 2018;75(5):344. [DOI] [PubMed] [Google Scholar]
- 19.Wang X, Ji A, Zhu Y, Liang Z, Wu J, Li S, et al. A meta-analysis including dose-response relationship between night shift work and the risk of colorectal cancer. Oncotarget. 2015;6(28):25046–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Papantoniou K, Devore EE, Massa J, Strohmaier S, Vetter C, Yang L, et al. Rotating night shift work and colorectal cancer risk in the nurses’ health studies. International Journal of Cancer. 2018;143(11):2709–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Flynn-Evans EE, Mucci L, Stevens RG, and Lockley SW. Shiftwork and Prostate-Specific Antigen in the National Health and Nutrition Examination Survey. JNCI: Journal of the National Cancer Institute. 2013;105(17):1292–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Viswanathan AN, Hankinson SE, and Schernhammer ES. Night Shift Work and the Risk of Endometrial Cancer. Cancer Research. 2007;67(21):10618. [DOI] [PubMed] [Google Scholar]
- 23.Lahti TA, Partonen T, Kyyrönen P, Kauppinen T, and Pukkala E. Night-time work predisposes to non-Hodgkin lymphoma. International Journal of Cancer. 2008;123(9):2148–51. [DOI] [PubMed] [Google Scholar]
- 24.Schernhammer ES, Feskanich D, Liang G, and Han J. Rotating night-shift work and lung cancer risk among female nurses in the United States. Am J Epidemiol. 2013;178(9):1434–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pukkala E, Aspholm R, Auvinen A, Eliasch H, Gundestrup M, Haldorsen T, et al. Incidence of cancer among Nordic airline pilots over five decades: occupational cohort study. BMJ. 2002;325(7364):567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rafnsson V, Hrafnkelsson J, and Tulinius H. Incidence of cancer among commercial airline pilots. Occupational and Environmental Medicine. 2000;57(3):175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gudmundsdottir EM, Hrafnkelsson J, and Rafnsson V. Incidence of cancer among licenced commercial pilots flying North Atlantic routes. Environ Health. 2017;16(1):86-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McNeely E, Mordukhovich I, Staffa S, Tideman S, Gale S, and Coull B. Cancer prevalence among flight attendants compared to the general population. Environ Health. 2018;17(1):49-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Buja A, Mastrangelo G, Perissinotto E, Grigoletto F, Frigo AC, Rausa G, et al. Cancer Incidence among Female Flight Attendants: A Meta-Analysis of Published Data. Journal of Women’s Health. 2006;15(1):98–105. [DOI] [PubMed] [Google Scholar]
- 30.Roenneberg T, Pilz LK, Zerbini G, and Winnebeck EC. Chronotype and Social Jetlag: A (Self-) Critical Review. Biology (Basel). 2019;8(3):54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fischer D, Lombardi DA, Marucci-Wellman H, and Roenneberg T. Chronotypes in the US – Influence of age and sex. PLOS ONE. 2017;12(6):e0178782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wittmann M, Dinich J, Merrow M, and Roenneberg T. Social Jetlag: Misalignment of Biological and Social Time. Chronobiology International. 2006;23(1–2):497–509. [DOI] [PubMed] [Google Scholar]
- 33.Borisenkov MF. Latitude of Residence and Position in Time Zone are Predictors of Cancer Incidence, Cancer Mortality, and Life Expectancy at Birth. Chronobiology International. 2011;28(2):155–62. [DOI] [PubMed] [Google Scholar]
- 34.Gu F, Xu S, Devesa SS, Zhang F, Klerman EB, Graubard BI, et al. Longitude Position in a Time Zone and Cancer Risk in the United States. Cancer Epidemiol Biomarkers Prev. 2017;26(8):1306–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rana S, Munawar M, Shahid A, Malik M, Ullah H, Fatima W, et al. Deregulated expression of circadian clock and clock-controlled cell cycle genes in chronic lymphocytic leukemia. Molecular Biology Reports. 2014;41(1):95–103. [DOI] [PubMed] [Google Scholar]
- 36.Zhu Y, Brown HN, Zhang Y, Stevens RG, and Zheng T. Period3 Structural Variation: A Circadian Biomarker Associated with Breast Cancer in Young Women. Cancer Epidemiology Biomarkers & Prevention. 2005;14(1):268. [PubMed] [Google Scholar]
- 37.Zhu Y, Leaderer D, Guss C, Brown HN, Zhang Y, Boyle P, et al. Ala394Thr polymorphism in the clock gene NPAS2: a circadian modifier for the risk of non-Hodgkin’s lymphoma. International journal of cancer. 2007;120(2):432–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morales-Santana S, Morell S, Leon J, Carazo-Gallego A, Jimenez-Lopez JC, and Morell M. An Overview of the Polymorphisms of Circadian Genes Associated With Endocrine Cancer. Frontiers in Endocrinology. 2019;10(104). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Benna C, Helfrich-Förster C, Rajendran S, Monticelli H, Pilati P, Nitti D, et al. Genetic variation of clock genes and cancer risk: a field synopsis and meta-analysis. Oncotarget. 2017;8(14):23978–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mocellin S, Tropea S, Benna C, and Rossi CR. Circadian pathway genetic variation and cancer risk: evidence from genome-wide association studies. BMC Medicine. 2018;16(1):20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Filipski E, King VM, Li X, Granda TG, Mormont M-C, Liu X, et al. Host Circadian Clock as a Control Point in Tumor Progression. JNCI: Journal of the National Cancer Institute. 2002;94(9):690–7. [DOI] [PubMed] [Google Scholar]
- 42.Filipski E, Delaunay F, King VM, Wu M-W, Claustrat B, Gréchez-Cassiau A, et al. Effects of Chronic Jet Lag on Tumor Progression in Mice. Cancer Research. 2004;64(21):7879. [DOI] [PubMed] [Google Scholar]
- 43.Filipski E, Subramanian P, Carrière J, Guettier C, Barbason H, and Lévi F. Circadian disruption accelerates liver carcinogenesis in mice. Mutation Research/Genetic Toxicology and Environmental Mutagenesis. 2009;680(1):95–105. [DOI] [PubMed] [Google Scholar]
- 44.Lee S, Donehower LA, Herron AJ, Moore DD, and Fu L. Disrupting circadian homeostasis of sympathetic signaling promotes tumor development in mice. PloS one. 2010;5(6):e10995–e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Van Dycke Kirsten CG, Rodenburg W, van Oostrom Conny TM, van Kerkhof Linda WM, Pennings Jeroen LA, Roenneberg T, et al. Chronically Alternating Light Cycles Increase Breast Cancer Risk in Mice. Current Biology. 2015;25(14):1932–7. [DOI] [PubMed] [Google Scholar]
- 46.Kettner NM, Voicu H, Finegold MJ, Coarfa C, Sreekumar A, Putluri N, et al. Circadian Homeostasis of Liver Metabolism Suppresses Hepatocarcinogenesis. Cancer Cell. 2016;30(6):909–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Papagiannakopoulos T, Bauer Matthew R, Davidson Shawn M, Heimann M, Subbaraj L, Bhutkar A, et al. Circadian Rhythm Disruption Promotes Lung Tumorigenesis. Cell Metabolism. 2016;24(2):324–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Logan RW, Zhang C, Murugan S, O’Connell S, Levitt D, Rosenwasser AM, et al. Chronic shift-lag alters the circadian clock of NK cells and promotes lung cancer growth in rats. J Immunol. 2012;188(6):2583–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wu Y, Tao B, Zhang T, Fan Y, and Mao R. Pan-Cancer Analysis Reveals Disrupted Circadian Clock Associates With T Cell Exhaustion. Frontiers in Immunology. 2019;10(2451). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kasahara T, Abe K, Mekada K, Yoshiki A, and Kato T. Genetic variation of melatonin productivity in laboratory mice under domestication. Proc Natl Acad Sci U S A. 2010;107(14):6412–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zheng B, Larkin DW, Albrecht U, Sun ZS, Sage M, Eichele G, et al. The mPer2 gene encodes a functional component of the mammalian circadian clock. Nature. 1999;400(6740):169–73. [DOI] [PubMed] [Google Scholar]
- 52.Fu L, Pelicano H, Liu J, Huang P, and Lee CC. The Circadian Gene Period2 Plays an Important Role in Tumor Suppression and DNA Damage Response In Vivo. Cell. 2002;111(1):41–50. [DOI] [PubMed] [Google Scholar]
- 53.Mteyrek A, Filipski E, Guettier C, Okyar A, and Lévi F. Clock gene Per2 as a controller of liver carcinogenesis. Oncotarget. 2016;7(52):85832–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li H-X, Fu X-J, Yang K, Chen D, Tang H, and Zhao Q. The clock gene PER1 suppresses expression of tumor-related genes in human oral squamous cell carcinoma. Oncotarget. 2016;7(15):20574–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wood PA, Yang X, Taber A, Oh E-Y, Ansell C, Ayers SE, et al. Period 2 mutation accelerates ApcMin/+ tumorigenesis. Mol Cancer Res. 2008;6(11):1786–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gery S, Komatsu N, Baldjyan L, Yu A, Koo D, and Koeffler HP. The Circadian Gene Per1 Plays an Important Role in Cell Growth and DNA Damage Control in Human Cancer Cells. Molecular Cell. 2006;22(3):375–82. [DOI] [PubMed] [Google Scholar]
- 57.Ozturk N, Lee JH, Gaddameedhi S, and Sancar A. Loss of cryptochrome reduces cancer risk in p53 mutant mice. Proceedings of the National Academy of Sciences. 2009;106(8):2841–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mteyrek A, Filipski E, Guettier C, Oklejewicz M, van der Horst GTJ, Okyar A, et al. Critical cholangiocarcinogenesis control by cryptochrome clock genes. International Journal of Cancer. 2017;140(11):2473–83. [DOI] [PubMed] [Google Scholar]
- 59.Huber A-L, Papp SJ, Chan AB, Henriksson E, Jordan SD, Kriebs A, et al. CRY2 and FBXL3 Cooperatively Degrade c-MYC. Molecular Cell. 2016;64(4):774–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gotoh T, Vila-Caballer M, Santos CS, Liu J, Yang J, and Finkielstein CV. The circadian factor Period 2 modulates p53 stability and transcriptional activity in unstressed cells. Mol Biol Cell. 2014;25(19):3081–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Papp SJ, Huber A-L, Jordan SD, Kriebs A, Nguyen M, Moresco JJ, et al. DNA damage shifts circadian clock time via Hausp-dependent Cry1 stabilization. eLife. 2015;4:e04883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kondratov RV, Kondratova AA, Gorbacheva VY, Vykhovanets OV, and Antoch MP. Early aging and age-related pathologies in mice deficient in BMAL1, the core componentof the circadian clock. Genes Dev. 2006;20(14):1868–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yang G, Chen L, Grant GR, Paschos G, Song W-L, Musiek ES, et al. Timing of expression of the core clock gene Bmal1 influences its effects on aging and survival. Science Translational Medicine. 2016;8(324):324ra16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jiang W, Zhao S, Jiang X, Zhang E, Hu G, Hu B, et al. The circadian clock gene Bmal1 acts as a potential anti-oncogene in pancreatic cancer by activating the p53 tumor suppressor pathway. Cancer Letters. 2016;371(2):314–25. [DOI] [PubMed] [Google Scholar]
- 65.Matsuo T, Yamaguchi S, Mitsui S, Emi A, Shimoda F, and Okamura H. Control Mechanism of the Circadian Clock for Timing of Cell Division in Vivo. Science. 2003;302(5643):255. [DOI] [PubMed] [Google Scholar]
- 66.Puram Rishi V, Kowalczyk Monika S, de Boer Carl G, Schneider Rebekka K, Miller Peter G, McConkey M, et al. Core Circadian Clock Genes Regulate Leukemia Stem Cells in AML. Cell. 2016;165(2):303–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Janich P, Pascual G, Merlos-Suárez A, Batlle E, Ripperger J, Albrecht U, et al. The circadian molecular clock creates epidermal stem cell heterogeneity. Nature. 2011;480:209. [DOI] [PubMed] [Google Scholar]
- 68.Wang J, Li S, Li X, Li B, Li Y, Xia K, et al. Circadian protein BMAL1 promotes breast cancer cell invasion and metastasis by up-regulating matrix metalloproteinase9 expression. Cancer Cell International. 2019;19(1):182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Maury E. Off the Clock: From Circadian Disruption to Metabolic Disease. Int J Mol Sci. 2019;20(7):1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Khaper N, Bailey CDC, Ghugre NR, Reitz C, Awosanmi Z, Waines R, et al. Implications of disturbances in circadian rhythms for cardiovascular health: A new frontier in free radical biology. Free Radical Biology and Medicine. 2018;119:85–92. [DOI] [PubMed] [Google Scholar]
- 71.Verlande A, and Masri S. Circadian Clocks and Cancer: Timekeeping Governs Cellular Metabolism. Trends Endocrinol Metab. 2019;30(7):445–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Man K, Loudon A, and Chawla A. Immunity around the clock. Science (New York, NY). 2016;354(6315):999–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gathirua-Mwangi WG, Monahan PO, Murage MJ, and Zhang J. Metabolic syndrome and total cancer mortality in the Third National Health and Nutrition Examination Survey. Cancer Causes Control. 2017;28(2):127–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Altman Brian J, Hsieh Annie L, Sengupta A, Krishnanaiah Saikumari Y, Stine Zachary E, Walton Zandra E, et al. MYC Disrupts the Circadian Clock and Metabolism in Cancer Cells. Cell Metabolism. 2015;22(6):1009–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Relógio A, Thomas P, Medina-Pérez P, Reischl S, Bervoets S, Gloc E, et al. Ras-mediated deregulation of the circadian clock in cancer. PLoS Genet. 2014;10(5):e1004338–e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Masri S, Papagiannakopoulos T, Kinouchi K, Liu Y, Cervantes M, Baldi P, et al. Lung Adenocarcinoma Distally Rewires Hepatic Circadian Homeostasis. Cell. 2016;165(4):896–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Innominato PF, Roche VP, Palesh OG, Ulusakarya A, Spiegel D, and Lévi FA. The circadian timing system in clinical oncology. Annals of Medicine. 2014;46(4):191–207. [DOI] [PubMed] [Google Scholar]
- 78.Shafi AA, and Knudsen KE. Cancer and the Circadian Clock. Cancer Research. 2019;79(15):3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kriebs A, Jordan SD, Soto E, Henriksson E, Sandate CR, Vaughan ME, et al. Circadian repressors CRY1 and CRY2 broadly interact with nuclear receptors and modulate transcriptional activity. Proceedings of the National Academy of Sciences. 2017;114(33):8776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tang Q, Xie M, Yu S, Zhou X, Xie Y, Chen G, et al. Periodic Oxaliplatin Administration in Synergy with PER2-Mediated PCNA Transcription Repression Promotes Chronochemotherapeutic Efficacy of OSCC. Adv Sci (Weinh). 2019;6(21):1900667-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gorbacheva VY, Kondratov RV, Zhang R, Cherukuri S, Gudkov AV, Takahashi JS, et al. Circadian sensitivity to the chemotherapeutic agent cyclophosphamide depends on the functional status of the CLOCK/BMAL1 transactivation complex. Proc Natl Acad Sci U S A. 2005;102(9):3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lee Y, Lahens NF, Zhang S, Bedont J, Field JM, and Sehgal A. G1/S cell cycle regulators mediate effects of circadian dysregulation on tumor growth and provide targets for timed anticancer treatment. PLOS Biology. 2019;17(4):e3000228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ballesta A, Innominato PF, Dallmann R, Rand DA, and Lévi FA. Systems Chronotherapeutics. Pharmacol Rev. 2017;69(2):161–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Miller S, and Hirota T. Pharmacological Interventions to Circadian Clocks and Their Molecular Bases. Journal of Molecular Biology. 2020. [DOI] [PubMed] [Google Scholar]


