Abstract
Introduction:
Renovascular hypertension (RVH) associated with renal artery and abdominal aortic narrowings is the third most common cause of pediatric hypertension. Untreated children may experience major cardiopulmonary complications, stroke, renal failure and death. The impetus of this study was to describe the increasingly complex surgical practice for such patients with an emphasis on anatomic phenotype and contemporary outcomes following surgical management, as a means of identifying those factors responsible for persistent or recurrent hypertension necessitating reoperation.
Methods:
A retrospective analysis was performed of consecutive pediatric patients with RVH undergoing open surgical procedures at the University of Michigan from 1991 to 2017. Anatomic phenotype and patient risk factors were analyzed to predict outcomes regarding blood pressure control and the need for secondary operations using ordered and binomial logistic multinomial regression model, respectively.
Results:
One hundred sixty-nine children (76 girls, 93 boys) underwent primary index operations at a median age of 8.3 years. Thirty-one children (18%) had neurofibromatosis type I, 76 (45%) had abdominal aortic coarctations, and 28 (17%) had a single functioning kidney. Prior to treatment at the University of Michigan, 51 children experienced failed previous open operations (15) or endovascular interventions (36) for RVH at other institutions.
Primary surgical interventions (342) included: main renal artery (136) and segmental renal artery (10) aortic reimplantation; renal artery bypass (55); segmental renal artery embolization (10); renal artery patch angioplasty (8); resection with reanastomosis (4); partial or total nephrectomy (25). Non-renal artery procedures included: patch aortoplasty (32), aorto-aortic bypass (32), and splanchnic arterial revascularization (30). Nine patients required reoperation in the early post-operative period.
During a mean follow-up of 49 months, secondary interventions were required in 35 children (21%) including both open surgical (37) and endovascular (14) interventions. Remedial interventions to preserve primary renal artery patency or a nephrectomy if such was impossible, were required in 22 children (13%). The remaining secondary procedures were performed to treat previously untreated disease that became clinically evident during follow-up. Age at operation and abdominal aortic coarctation were independent predictors for reoperation. The overall experience revealed hypertension to be cured in 74 children (44%), improved in 78 (46%) and unchanged in 17 (10%). Children undergoing remedial operations were less likely (33%) to be cured of hypertension. There was no perioperative mortality or renal insufficiency requiring dialysis following either primary or secondary interventions.
Conclusions:
Contemporary surgical treatment of pediatric RVH provides a sustainable overall benefit to 90% of children. Interventions in the very young (ie: <3 years) and concurrent abdominal aortic coarctation increase the likelihood of reoperation. Patients undergoing remedial surgery after earlier operative failures are less likely to be cured of hypertension. Judicious post-operative surveillance is imperative in children surgically treated for RVH.
Table of Contents Summary
Surgical revascularization of renal artery stenosis and abdominal aortic coarctation provided a sustainable hypertension benefit in 90% of children in this retrospective study of 169 patients with renovascular hypertension. Interventions in the very young and concurrent abdominal aortic coarctation increase the likelihood of reoperation, mandating judicious post-operative surveillance.
INTRODUCTION
Pediatric renovascular hypertension (RVH) secondary to renal artery occlusive lesions and abdominal aortic coarctations risks significant morbidity and mortality.(1, 2) The pathophysiology and etiology of both diseases remain poorly defined. Moreover, the low frequency of these entities limit existing data to institutional series and anecdotal case reports. The optimal management of these children, including indications for invasive treatment, remains ill-defined. To date there remain no randomized trials comparing medical management to surgical revascularization for pediatric RVH. (1, 3–8) Moreover, the role of endovascular interventions as first-line or ‘bridging’ option for treatment of pediatric RVH has not been defined.(9–13)
A previous report from the University of Michigan on the surgical treatment of 97 children having RVH, demonstrated a 97% benefit regarding blood pressure control.(1) The authors’ surgical experience has nearly doubled since 2005, and has become increasingly complicated in that prior open surgical or endovascular failures now account for a third of those referred for definitive care. The impetus of this study was to describe the increasingly complex surgical care of such patients with an emphasis on anatomic phenotype and contemporary outcomes following their surgical management as a means of identifying those factors responsible for persistent or recurrent hypertension necessitating reoperation.
METHODS
The medical records of consecutive children, 18 years or younger, with RVH undergoing surgical procedures at the University of Michigan from 1991 to 2017 were reviewed. Children operated on prior to 1991 were excluded due to many instances of limited follow-up. Currently, all data regarding interventions for pediatric renovascular disease treated at the University of Michigan is prospectively entered into an institutional registry. This series included 50 children (of the aforementioned 97) who have been previously reported, specifically treated after 1991 with complete records,(1), and 119 more recent and heretofore unreported cases.
Refractory hypertension unresponsive to optimal drug therapy was the basis for surgical intervention in all but one patient. For reference, normal blood pressure in a pediatric patient exists when it is < 90th percentile for sex, age, height. Hypertension is defined as an average systolic or diastolic blood pressure ≥ 95th percentile for sex, age, and height across three separate measurements, and is categorized using published standards of pediatric blood pressure.(14)
Postoperatively, hypertension outcomes were classified as cured if children were normotensive requiring no antihypertensive medications at the time of last follow-up; improved if their blood pressures were within normotensive ranges while on a less intensive antihypertensive regimen; and therapeutic failures if their diastolic pressures were higher than the normal levels and not 15% lower than preoperative levels or if ACE inhibitors were required for blood pressure control. This retrospective review was approved by the Institutional Review Board at the University of Michigan (HUM0006223) and a waiver of informed consent was granted.
The association of each independent variable (age, aortic reconstruction, neurofibromatosis Type I (NF1), abdominal aortic coarctation, prior renal artery intervention, and number of antihypertensive medications) with the occurrence of a reoperation was estimated using bivariate logistic regression. This was followed by a multiple logistic regression to estimate the effect of the independent variable on the odds of undergoing a reoperation. Pairwise comparison of marginal effects was used to see if the probability of reoperation differed by the type of aortic reconstruction. The variable change in medications pre- to post-intervention was modeled using simple ordinal logistic regression to estimate the effect of each independent variable. This was followed by a multiple ordinal logistic regression to estimate the effect of each independent variable. Kaplan-Meier survival curves were used to estimate the potential for reoperation along with a log rank test to detect if such potential differed significantly if associated with an aortic coarctation reconstruction, a prior renal artery procedure, or the presence of NF1. Cox proportional hazards modeling was used to assess the impact of age and initial number of medications on the potential for reoperation. All statistical analyses were done using STATA version 15.1 (College Station, TX). Statistically significance was defined as p<0.05.
RESULTS
A total of 169 children with RVH were treated surgically at the University of Michigan from 1991 to 2017. (Table I). This surgical cohort demonstrated a slight male predominance (n=93, 55%). The median age of children at the time their hypertension was recognized was 5 years (SD±60 months). The median age at the time of their primary operation at the University of Michigan was 8.2 years (SD±63 months, range 3 months – 18 years), and the median weight was 24.95 kg (SD±20, range 5.2 to 97.6). Prior interventions for RVH had been performed in 51 (30%) of the series’ children at other institutions before they were referred and received treatment at the University of Michigan.
Table 1:
Demographics and Preoperative Risk Factors
| Variable | Total Cohort (N=169) |
|---|---|
| Male Gender (N, %) | 93 (55%) |
| Age at Time of Michigan Surgical Intervention (Mean ± SD) | 8.96 ± 5.53 years |
| Weight at Time of Michigan Intervention (Mean ± SD) | 58.33 ± 16.8 kg |
| Number of Initial Antihypertensive Medications (Mean ± SD) | 2.70 ± 1.58 |
| Neurofibromatosis Type 1 | 31 (18.34%) |
| Abdominal Aortic Coarctation (N, %) | 76 (44.97%) |
| Prior Intervention Performed Elsewhere for RVH before Michigan Intervention (N, %) | 51 (30.18%) – Open surgical (14), Endovascular (35), Combination (2) |
kg = kilograms, RVH = renin-mediated renovascular hypertension
Historical Risk Factors:
NF1 affected 31 (18%) of the series’ children. Additional concurrent diagnoses included: Williams Syndrome (6), Scoliosis (5), Moyamoya Disease (5), Klippel-Trenaunay Syndrome (1), Beals syndrome (1), von Willebrand disease (1), Takayasus arteritis (1), Noonan syndrome (1), gonadal dysgenesis (1), Alagille syndrome (1), macrocephaly (1), Monosomy 16p, trisomy 21q, and alpha thalassemia (1).
Five patients had a neoplastic disease, including: a single yolk sac tumor (1), Wilm’s tumor (1), hemangioendothelioma (1), neuroblastoma, and a retroperitoneal neurofibroma (1). Hypertension was incidentally diagnosed in 90 asymptomatic children. The remaining 79 children were symptomatic, of which headache was most common manifestation (Table II). Eighteen patients were younger than 3 years at the time of their primary operation at the University of Michigan. In this subgroup of the very young, both malignant hypertension (N=5, 28%) and failure to thrive (N=4, 22%) were relatively common. In contrast, these manifestations in children older than 3 years were less common (N=4 to 5%). The mean number of antihypertensive medications pre-operatively was 2.70 (SD ± 1.58). Mean Creatinine preoperatively was 0.62 mg/100mL (SD± 0.31). Among children having preoperative echocardiograms 68% (69) exhibited left ventricular hypertrophy.
Table 2:
Clinical Manifestations
| Symptom or Sign | N=79 (53% of Total Cohort) |
|---|---|
| Headache | 25 |
| Failure to Thrive | 11 |
| Malignant Hypertension (including hypertensive encephalopathy) | 13 |
| Lower Extremity Symptoms (claudication, leg fatigue with walking)* | 11 |
| Neurologic Events or Symptoms | Stroke – 6, Stroke with Moyamoya – 4, Seizuref - 3 |
| Abdominal Symptoms (pain, nausea, recurrent emesis) | 9 |
| Epistaxis | 6 |
| Facial nerve palsy | 4 |
| Visual change (including hypertensive retinopathy) | 4 |
| Agitation, Irritability | 3 |
| Hematuria (including microscopic) | 3 |
| Dyspnea on Exertion | 2 |
All cases associated with abdominal aortic coarctations
Exclusive of diagnosed seizure disorder
Vascular Anatomy:
Renal artery lesions were often bilateral (N=91, 54%), being frequently ostial (N=112, 66%) in most cases. (Figure 1, Table III). Multiple renal arteries occurred in 21% of patients (36). Twenty-eight children (17%) had a single functioning kidney at the time of the primary operation; related to ischemic atrophy (12), prior nephrectomy (13), or congenital absence (3). Nearly, half the children (83) had splanchnic arterial stenoses or occlusions affecting the celiac artery, superior mesenteric artery, or both. Four individuals had evidence of diffuse arterial dysplasia with ‘beading’ of the ileocolic (1), hepatic (4) and splenic (2) arteries. Almost half the children (76) had a concurrent abdominal aortic coarctation (Figure 2–4); six had a concurrent thoracic isthmic aortic coarctation, and one had a bicuspid aortic valve. Concurrent abdominal (large vessel) arterial aneurysms affected the aorta (2), common iliac artery (1), hypogastric artery (1), renal artery (9), inferior mesenteric artery (1), or a ureteric collateral artery (1).
Figure 1:
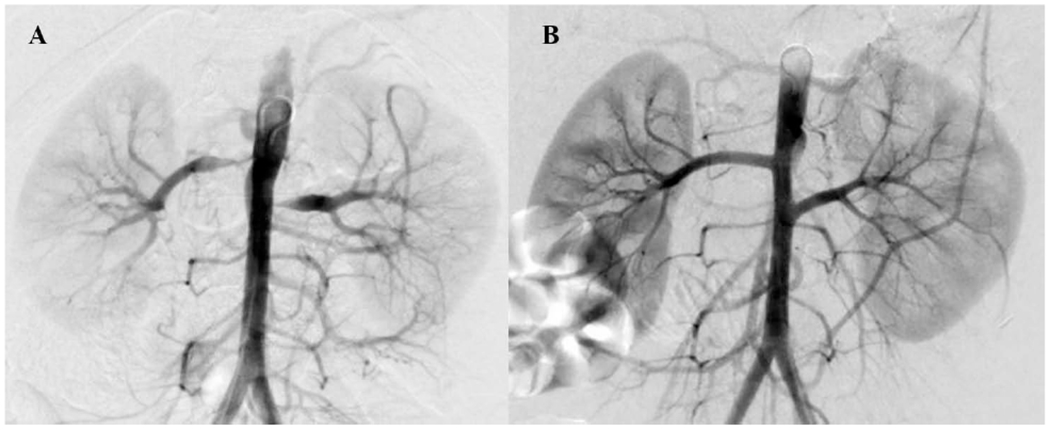
(A) Preoperative arteriogram of an 8-year-old boy with medically refractory renovascular hypertension and bilateral renal artery ostial stenosis. (B) Postoperative completion arteriogram following bilateral renal artery reimplantation.
Table 3:
Renal Vascular Anatomy and Associated Pathology
| Renal Artery Anatomy | Entire Cohort (N=169) | Absence of Abdominal Aortic Coarctation (N=93, 55% of total cohort) | Abdominal Aortic Coarctation (N=76, 45% of total cohort) | Presence of NF1 (N=31, 18% of total cohort) | Absence of NF1 (N=138, 82% of total cohort) |
|---|---|---|---|---|---|
| Bilateral Stenoses | 91 (54%) | 35 (38%) | 56 (74%) | 18 (58%) | 73 (53%) |
| Multiple Renal Arteries | 36 (21%) | 19 (20%) | 17 (22%) | 4 (13%) | 32 (23%) |
| Ostial Stenosis | 112 (66%) | 52 (56%) | 60 (79%) | 28 (90%) | 84 (61%) |
| Mid-renal Artery Stenosis | 14 (8%) | 1 (1%) | 13 (17%) | 0 | 14 (10%) |
| Distal Artery Stenosis | 6 (4%) | 6 (6%) | 0 | 1 (3%) | 5 (4%) |
| Segmental Arterial Stenosis | 17(10) | 13 (14%) | 4 (5%) | 2 (6%) | 15 (11%) |
| Diffuse Renal Arterial Dysplasia | 593%) | 3 (3%) | 2 (3%) | 1 (3%) | 4 (3%) |
| Focal Renal Artery Occlusion | 26(15%) | 17 (18%) | 9 (12%) | 3 (10%) | 23 (17%) |
NF1 = Neurofibromatosis Type 1
Figure 2:
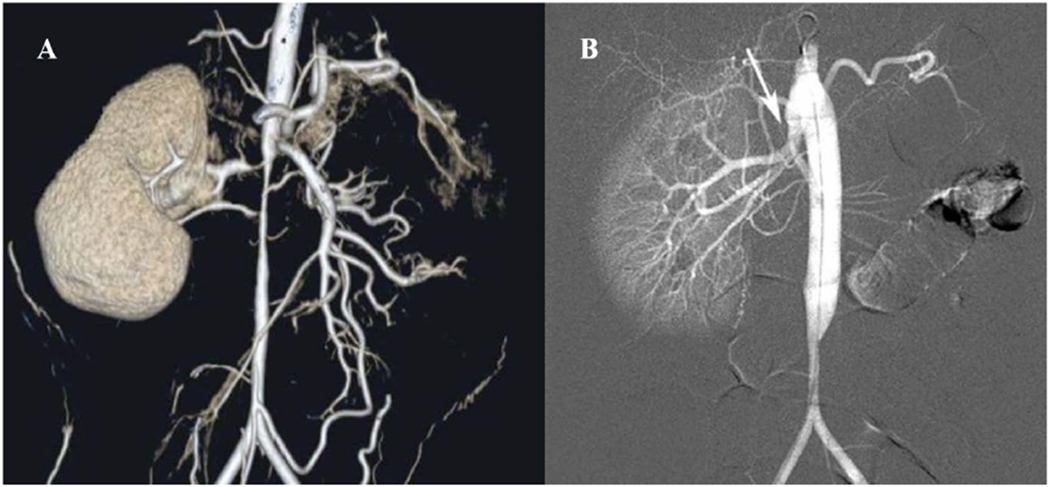
(A) Preoperative CT-arteriogram of a 7-year-old boy with a single right kidney and medically refractory renovascular hypertension demonstrating supra-renal abdominal aortic coarctation with ostial stenosis of the two right renal arteries and the superior mesenteric artery (SMA). (B) Postoperative completion arteriogram following aorto-aortic bypass with SMA intimectomy and reimplantation of the right superior and inferior renal arteries onto the transected aorta (arrow). He remains cured of hypertension now 9 years out from surgical revascularization.
Figure 4:
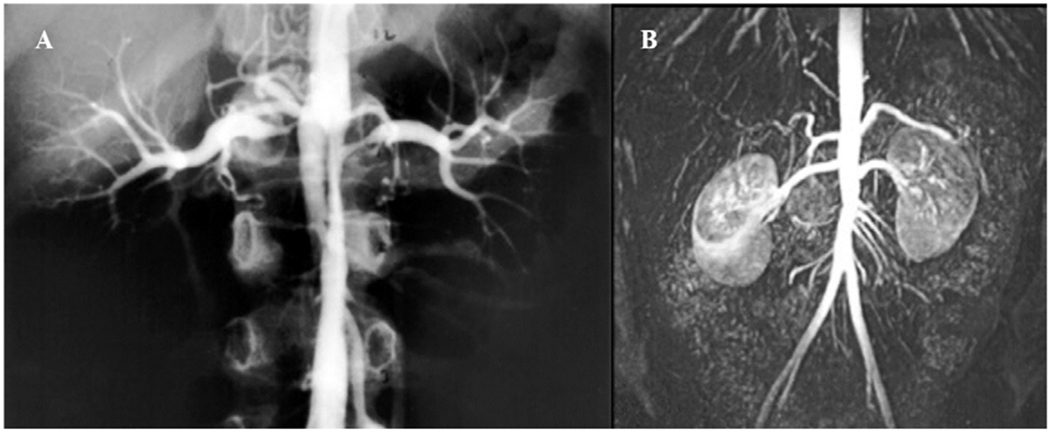
(A) Preoperative arteriogram of a 6-year-old boy with medically refractory renovascular hypertension demonstrating interrenal abdominal aortic coarctation and bilateral ostial renal artery stenoses. (B) Postoperative surveillance MR-angiogram following patch aortoplasty and bilateral renal artery reimplantation. He remains cured of hypertension more than 20 years out from surgical revascularization.
Primary Interventions.
A total of 342 primary surgical interventions were performed: 248 directed to renal artery occlusive disease, 64 to aortic narrowings, and 30 to occlusive lesions of the splanchnic arteries (Table IV). Renal artery reimplantation onto the aorta was performed most commonly (136, 55% of primary renal artery procedures) (Figure 1B), followed by aorto-renal bypass (50, 20% of primary renal artery procedures). Hypogastric artery was utilized for conduit in 89% of the renal artery bypasses and patch angioplasties. Saphenous vein, splenic artery, common iliac artery and expanded polytetrafluoroethylene (ePTFE) grafts were uncommonly used in these procedures. Partial (21) or complete (4) nephrectomy was performed in 12% and 4%, respectively, in children having irreparable renal atrophy (non-function) or renal artery disease deemed technically unreconstructable (ie: diffuse, segmental arterial dysplasia or absent distal target for revascularization during intra-operative exploration). An additional 12 nephrectomies were performed for the 12 non-functional atrophic kidneys referenced above. Among the series’ 76 patients with abdominal aortic coarctations, 64 required concurrent aortic surgery with an aorto-aortic bypass (32) (Figure 2B, 3B) or patch aortoplasty (32) (Figure 4B). The preferred conduit in these reconstructions was ePTFE. Concurrent splanchnic revascularizations were performed in 17% of the series’ patients.
Table 4:
Primary Surgical Interventions at Michigan
| Intervention Site | N (Number of procedures) | Notes |
|---|---|---|
| Renal Artery | Renal Reimplantation Aortic (134), SMA (2), Segmental Renal Artery (10) |
|
| Renal Bypass Aortorenal (50)*, Iliorenal (2), Splenorenal (1), Interposition Renorenal (2) |
Conduit Hypogastric Artery with the following exceptions: GSV (2), ePTFE (2),CIA (1). | |
| Renal Artery Patch Angioplasty (8) | Conduit Hypogastric Artery, Exception: GSV-1, splenic artery-1. | |
| Resection with Reanastomosis (4) | ||
| Absolute Alcohol Embolization (10) | ||
| Kidney | Partial Nephrectomy 4, Total Nephrectomy 21. | |
|
Aorta (N 64, 38% of cohort) |
Patch Aortoplasty (32), Aortic Bypass (32)f | Preferred Conduit ePTFE |
| Splanchnic Arteries (N 28, 17% of cohort) |
Superior Mesenteric Artery Intervention: Hepato-SMA bypass (2), Aorto-SMA bypass (3), SMA Reimplantation (15), SMA Ligation (2), SMA Patch Angioplasty by celiac reimplantation onto SMA (2), SMA Intimectomy (2). Celiac Artery Intervention: Aneurysm Resection with hepatosplenic anastomosis (1), Reimplantation (3). |
Preferred Conduit Hypogastric Artery |
SMA = superior mesenteric artery, GSV = great saphenous vein, ePTFE = expanded polytetrafluoroethylene, CIA = common iliac artery
a single case complicated by multi-lobed aneurysm required ex-vivo reconstruction
ascending aortic inflow in four cases
Figure 3:
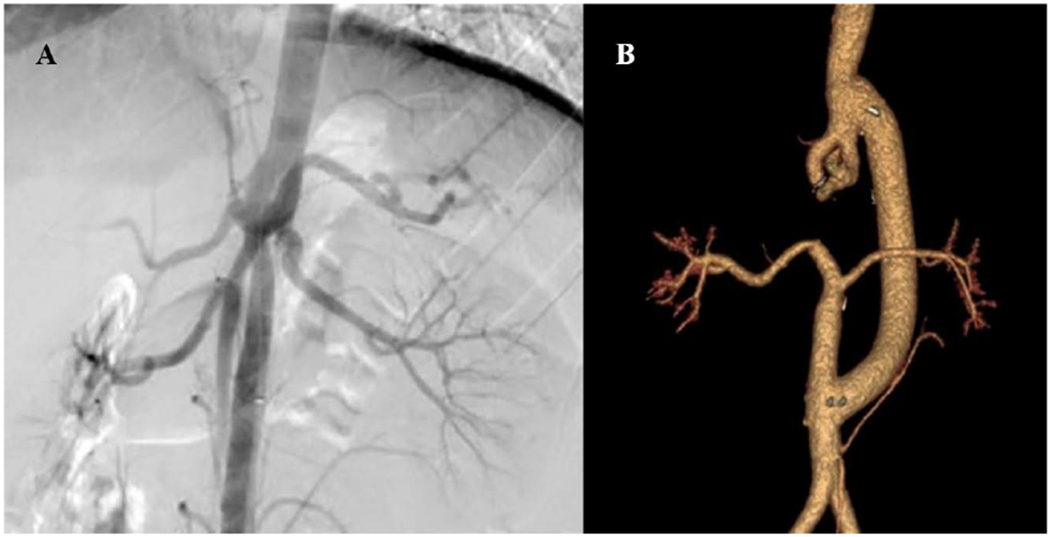
(A) Preoperative arteriogram of a 15-year-old boy with medically refractory renovascular hypertension and lower extremity exertional fatigue demonstrating partial duplication of the aorta and abdominal aortic coarctation with bilateral ostial renal artery stenoses. (B) Postoperative completion CT-arteriogram following aorto-aortic bypass using 16 mm ePTFE graft, ligation and division of dysplastic and narrowed aorta distal to the superior mesenteric artery, and bilateral renal artery reimplantation onto the transected terminal aorta.
Length of stay after these primary procedures averaged 13.6 days (SD ±9.1). Surgical morbidity was not life threatening, but relevant (Table V). Nine patients (5%) required reoperation in the early post-operative period (within 30 days) primarily related to graft thrombosis or bleeding. Chemical pancreatitis, with or without an accompanying ileus was the most common complication affecting 20 patients (12%), followed by infections (7%), and pulmonary complications (7%). Renal insufficiency requiring dialysis during the study period did not occur and there were no cases of infection involving the site of the renal, aortic or splanchnic arterial procedures. There was no mortality during this study period.
Table 5:
Perioperative Complications
| System | Complication |
|---|---|
| Reoperation | Renal graft thrombosis requiring nephrectomy (2) and revision (1), Post-operative hemorrhage (4), Fascial dehiscence (1), Delayed abdominal closure (1). |
|
Infectious Note: No cases of graft infection or wound infection |
Bacteremia (2), UTI (4), C Diff (3), Vaginal yeast infection (1), Thrush (1), Cellulitis associated with peripheral IV (1). |
| Gastrointestinal | Chemical pancreatitis and/or prolonged (>7d) ileus (20), GI bleed (2). |
| Respiratory | Pneumonia (7, of which 3 patients required prolonged or re-intubation), ARDS with prolonged intubation (1), Viral URI (1), Pneumothorax requiring tube thoracostomy (1), Pleural effusion requiring tube thoracostomy (1). |
| Hematologic | Perinephric hematoma requiring transfusion (2), Phlebitis (3), DVT (2), PE (1), Digital thromboembolism (1), HIT (1). |
| Miscellaneous | Partial thickness skin loss (1), Decubitus ulcer (1), MCA stroke (1), Chylous ascites requiring delayed thoracic duct ligation (1), Intraoperative splenic laceration requiring splenectomy (1). |
UTI = urinary tract infection, C Diff = clostridium difficile colitis, GI = gastrointestinal, ARDS = acute respiratory distress syndrome, URI = upper respiratory infection, DVT = deep venous thrombosis, PE = pulmonary embolism, HIT = heparin-induced thrombocytopenia, MCA = middle cerebral artery
Secondary Interventions.
During a mean follow-up of 49 months, 35 children (21%) required at least one reoperation, including both open surgical (37) and endovascular (14) procedures directed at the renal arteries (36) or aorta (15), and fewer directed at the splanchnic arteries (Table VI). The relative incidence of reoperation was higher in patients with concurrent abdominal aortic narrowings (N=19, 24%) and NF1 (N=9, 29%). The secondary interventions to preserve primary patency, or a nephrectomy if such was impossible, were required following index operation in 22 children (13% of total cohort), while the remaining subsequent operations were not a ‘reoperation’, but rather required to treat evolving aortic, renal or mesenteric vascular bed that became clinically evident following the index operation. Among the 64 patients undergoing concurrent aortic operations at the time of their primary surgery, 12.5% (4 of 32) of those having a patch aortoplasty required reoperation, and 18.8% (6 of 32) of those with a prior aortic bypass required reoperation. Specifically, three patients in each of the former cohorts required the secondary intervention to preserve aortic patency (9%). Follow-up into adulthood was available for 38 patients, with the oldest patient being 34 years at the date of last follow-up. There were no adult complications identified, nor reinterventions required after age 18y.
Table 6:
Secondary Surgical Interventions at Michigan
| Remedial Interventions for failure of Primary Procedure at Michigan | Open Surgical Interventions | Endovascular Interventions |
|---|---|---|
|
Remedial Renal Revacularization Renal bypass: Aorto-renal (8), Splenorenal (1), Ilio-renal (2). Renal Reimplantation – aortic (2), SMA (1), Renal artery patch angioplasty (2), Renal thromboendarterectomy (2), Renal intimectomy (1). Nephrectomy (3) Remedial Aortic Revascularization Aortic bypass: Aorto-aortic (2), Aorto-iliac (1),_Thoraco-abdominal aortic bypass (2). Patch aortoplasty (3) Remedial Mesenteric Revacularization Mesenteric bypass: SMA-hepatic artery bypass (1), Aorto-SMA bypass (1), Aorto-celiac bypass (to address inflow to splenorenal bypass) (1). SMA reimplantation (2), Splenic artery reimplantation (to address inflow to splenorenal bypass) (1). |
Renal/anastomotic angioplasty (9), Renal artery stenting (2), Segmental renal angioplasty (1), Segmental renal artery embolization (1), Thrombolysis of thrombosed aortorenal bypass (1), Aortic balloon angioplasty (2), Aortic stenting (4). | |
| Secondary Interventions For Disease Progression Unrelated to Primary Operation | ||
| Renal Reimplantation (contralateral to index renal revascularization) (4), Segmental renal artery reimplantation (1), Segmental renal artery embolization (1), SMA reimplantation (2), Aortic patch angioplasty (6), Thoracoabdominal aortic bypass (2). | Renal angioplasty (1), Segmental embolization (3). |
SMA = superior mesenteric artery
Median time to the first secondary operation in the entire series was 14 months (Range 2 to 159 months, SD ±38.43). Seventeen children (10%) required at least two reoperations including both open surgical (9) and endovascular (8) procedures, among whom seven necessitated a third open surgery. One child, initially operated on at 5 years of age, required 10 additional procedures over the next 14 years, including open (5) and endovascular (5) interventions. There were no cases of late renal failure requiring renal replacement therapy and no operative mortality following any reoperation.
Logistic regression modeling identified younger age at operation (adjusted odds ratio 0.86, 95% CI: 0.77-0.95) and abdominal aortic coarctation (adjusted odds ratio 6.01, 95% CI:1.70-21.24) to be independent predictors of reoperation. (Appendix Table 1) The pairwise comparison of the effects of aortic bypass and that of patch on the probability of reoperation was not statistically significant. Log rank tests documented that the hazards of reoperation did not differ significantly related to: abdominal aortic coarctation, aortic reconstruction, NF1, or preoperative intervention. (Figure 5) Age at operation significantly affected hazards of a reoperation. Specifically, as age increased by one year, the rate of reoperation decreased by about 10% (HR=0.90; 95% CI: 0.83-0.97).
Figure 5:
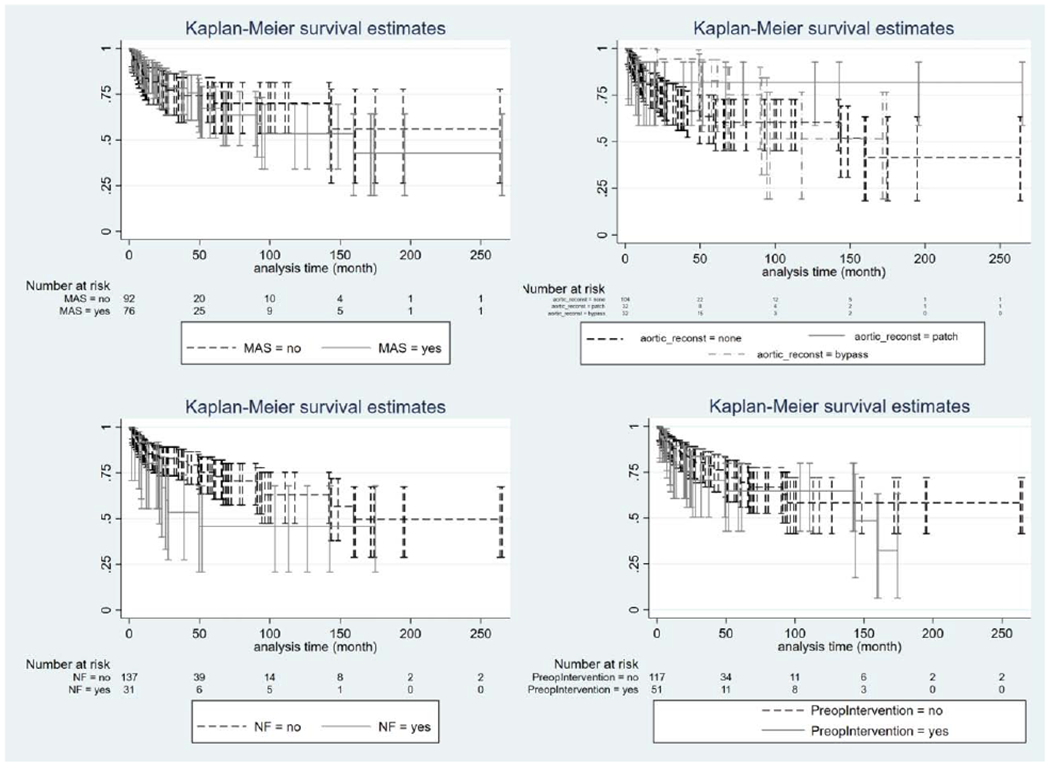
Kaplan Meier Curve (survival analysis): Log rank tests documented that the hazards of reoperation did not differ significantly related to (A) abdominal aortic coartctation, (B) concurrent aortic reconstruction, (C) Neurofibromatosis Type 1, and (D) Preoperative intervention. (MAS = abdominal aortic coarctation, reconst = reconstruction, NF = Neurofibromatosis Type 1)
Among the series’ 169 patients, 74 (44%) were cured of hypertension, 78 (46%) were improved, and 17 (10%) were unchanged with regard to their hypertension. (Table VII) Overall, the mean number of antihypertensive medications at follow-up was 0.99 ±1.16, compared to preoperative number of 2.7 ±0.58. NF1 was identified by univariate analysis to be negatively associated with hypertension benefit (odds ratio 0.42, 95% CI: 0.2-0.91). Multiple ordinal regression modeling identified prior intervention (adjusted odds ratio 0.49, CI: 0.25-0.98) as the single independent factor predicting that children undergoing remedial operations were less likely to be cured (33%) of their hypertension. (Appendix Table 2)
Table 7:
Postoperative Hypertension Benefits
| Cure (%) | Improved (%) | Failure (unchanged) (%) | |
|---|---|---|---|
| Total Cohort (N=169)* | 74 (44%) | 78 (46%) | 17 (10%) |
| Among Patients with Concurrent Abdominal Aortic Coarctation (N=76) | 28 (37%) | 37 (49%) | 11 (14%) |
| Among Patients without Abdominal Aortic Coarctation (N=93) | 46 (49%) | 41 (44%) | 6 (6%) |
| Among Patients with a Prior Intervention for RVH (51) | 17 (33%) | 29 (57%) | 5 (10%) |
| Among Patients without a Prior Intervention for RVH (N=118) | 57 (48%) | 49 (42%) | 12 (10%) |
| Among Patients with NF1 (N=31) | 9 (29%) | 16 (52%) | 6 (19%) |
| Among Patients without NF1 (N=138) | 65 (47%) | 62 (45%) | 11 (8%) |
| Among Patients with NF1 and Concurrent Aortic Treatment (N=19) | 3 (16%) | 11 (58%) | 5 (26%) |
| Among Patients <3 Years of Age at Operation (N=21) | 7 (33%) | 12 (57%) | 2 (10%) |
| Among Patients ≥3 Years of Age at Operation (N=148) | 67 (45%) | 66 (45%) | 15 (10%) |
RVH = renin-mediated renovascular hypertension, NF1 = Neurofibromatosis Type 1
Includes primary index and secondary operations
DISCUSSION
Renin-mediated hypertension secondary to renal artery stenoses and abdominal aortic coarctation is an uncommon cause of high blood pressure in childhood. The authors’ institutional history of treating occlusive lesions of the renal arteries and abdominal aorta in pediatric patients has extended for more than 4 decades. Postoperative blood pressure control has appeared best when treating patients with isolated renal artery narrowings, as evident in the first University of Michigan report in 1973 citing 95% cure and 5% failure rates among 22 children undergoing renal artery reconstructive surgery.(15) By 1981, that surgical experience increased to 34 children having isolated renal artery stenoses and six (15%) with coexistent renal artery stenoses and abdominal coarctations.(16) In those having isolated renal artery disease, the hypertension ‘cure’, ‘improved’, and ‘failure’ rates were 88%, 9% and 3%, respectively, in contrast to 67%, 33%, and 0%, respectively, among those undergoing concurrent aortic reconstructions.
An expanded University of Michigan report in 1995 validated this discrepancy in hypertension benefit, noting ‘cure’, ‘improved’ and ‘failure’ rates of 88%, 10% and 2%, among children treated for isolated renal artery disease and 60%, 40% and 0% for those undergoing coexisting aortic interventions.(17) The authors subsequently observed a decline in ‘cure rate’ over time from 81% (1963-72) to 58% (1994-2006).(1) In addition, the authors’ reported a ‘cure rate’ of only 25% for remedial operations performed in 24 children following failed endovascular therapy.(18)
The University of Michigan experience has become increasingly complicated in that failures of previous open surgical and endovascular procedures performed elsewhere, now affect approximately a third of children with RVH referred to the authors for treatment. In addition, the complexity of the disease being treated has increased with concurrent abdominal aortic coarctations affecting 45% of the current series’ children. In comparison to our earlier experience, the overall hypertension ‘cure rate’ has decreased to 44%. Although this is below ‘cures’ generally reported in the past by high volume centers, it is in line with the 46% cure rate reported by a contemporary report of 37 children from Great Ormond Street Hospital for Children in London, UK.(19) It is hypothesized that this reflects the increased rates of the performance of secondary revascularizations undertaken as salvage procedures after earlier failed interventions.(19–21) While NF1 and failure of an initial intervention were identified by univariate analysis to be associated with lesser blood pressure benefits, prior failures were the single independent predictor that children undergoing secondary operations were less likely to be ‘cured’ of their hypertension. Such may represent an effect of the prior intervention or existence of more resistant underlying disease. Further study is needed to clarify this observation.
Abdominal aortic coarctation is a rare disease and the resulting blood pressure elevations can be severe and often difficult to control medically. The authors have previously reported salutary outcomes exceeding 90% among patients with the aortic narrowings having a mean age of 11.9 years.(22) Among the former report’s patients exhibiting hypertension, cure and improved rates were 56% and 38% respectively. The present data expands on this phenotype, supporting hypertension benefit in 86% of treated patients with abdominal aortic coarctation (37% cure, 49% improvement). Nevertheless, there remain significant knowledge gaps regarding the pathogenesis of this disease, its clinical management. The question exists as to whether the aortic procedure, itself, may cause abnormal flow patterns to the kidneys that initiates persistent excessive renin release.(23) Such events may account for the poorer outcomes following aortic procedures observed in clinical practice.
The Hospital for Sick Children in Toronto, Canada recently reported a 30-year experience with renal and aortic stenosis that included 93 pediatric-aged patients of whom 51 had abdominal aortic coarctations.(3) One-third of the children underwent open surgical interventions, but 53 underwent endovascular management of their renal artery lesions as a ‘first intervention’. The probability of undergoing an endovascular ‘first intervention’ varied among the children. Those with abdominal aortic coarctations had a 60% lower risk of being treated by endovascular means compared to those with isolated renal artery stenoses. Importantly, 65% of that series’ children remained hypertensive with a median follow-up of 2 years.
Secondary interventions to preserve primary patency of a renal artery revascularization, often at anastomotic suture lines, or nephrectomy for a failed reconstruction, was undertaken in 13% of the current series. This supports the importance of regular surveillance post-operatively, especially during periods of rapid childhood growth. Moreover, as evident in the current study, secondary interventions are invariably more complex. Although most earlier reports have not emphasized progression of renal artery or aortic branch disease, this report did identify 13 children (18%) who required an operation for evolving disease that became clinically relevant following primary index operation, as exemplified by patients whose previously normal contralateral main renal artery or segmental renal artery become stenotic over time and with growth resulting in clinically significant recurrent renin-mediated hypertension.
Endovascular interventions are increasingly reported as a means of treating pediatric renovascular disease. While technically feasible, the blood pressure benefits remain inferior to open revascularizations with benefit in many series ranging from 54 to 69%.(12, 13, 23) Endovascular restenosis has been well described following pediatric renal artery angioplasty, ranging from 7 to 44% interventions, being more frequent following treatment of renal stenoses in children having defined syndromes like NF1. Additionally, the benefits from secondary open operations in these latter children have limited benefits.(3, 24, 25) Our current practice considers renal artery angioplasty for mid-distal-renal artery and segmental stenoses that appear multi-focal, web-like, or beaded by arteriogram or with IVUS, while we continue to favor primary reimplantation for ostial/proximal main renal artery stenosis and open surgical revascularization of unifocal stenoses of the mid-, distal-, or segmental renal arteries that appear hypoplastic by arteriogram or IVUS. Also, regardless of age, angioplasty is favored for recurrent stenosis at suture lines from neointimal hyperplasia. It is the authors opinion that renal and aortic stenting should be avoided in pediatric patients, and only employed for salvage cases. Treatment decisions are made collaboratively by our multi-disciplinary team, that includes representation from vascular surgery, pediatric nephrology, and interventional radiology. The surgical approach to renal revascularization at the University of Michigan has varied little in recent decades.(1) In situ reconstruction is favored to preserve important collaterals and limit intra-operative warm ischemia, even in cases of distal/segmental disease and multiple renal arteries. Ex vivo reconstruction was employed for a single patient in this series with a complex, distal left renal artery aneurysm with multiple existing segmental arteries. Reimplantation onto the aorta of the normal renal artery, beyond its stenosis, when possible, remains the preferred surgical technique for renal revascularization. Care is taken to carefully spatulate the renal artery to create generous orifice for reimplantation. The anastomoses are completed with an interrupted fine monofilament sutures to permit future growth in infants and the very young. In children requiring an aorto-renal bypass, vein grafts are avoided due to the risk of late aneurysmal changes. Hypogastric artery is favored as a conduit in these instances. Concerns have previously been raised regarding the procurement of the hypogastric artery in children, regarding the risk of pelvic ischemia. In the University of Michigan experience, there have been no cases of buttock claudication or genitourinary dysfunction, and five children (4 girls, 1 boy) having excision of their internal iliac artery have gone on to successfully have children.
Concurrent aortic revascularization was common in the present series’ children. Patch aortoplasty, when feasible, remains the authors’ preferred technique for aortic revascularization. However, aorto-aortic bypass, often originating from the supra-celiac abdominal aorta or descending thoracic aorta is favored in children having a very narrow abdominal aortic coarctations. In these cases, an arterial patch-aortic anastomotic suture lines would nearly overlap. The type of aortic revascularization (patch or bypass) did not significantly impact blood pressure benefits post-operatively. The preferred conduit for both a patch aortoplasty and an aortic bypass is ePTFE, given the recognized later aneurysmal deterioration of Dacron. Whenever possible, patches and grafts are sized sufficiently large enough so as not to become constrictive as the child grows into adulthood. The authors recommend regular post-operative surveillance, to include the following: 1) Regular monitoring of blood pressure and renal function by primary nephrologist; 2) Annual renal duplex to monitor renal size/mass and velocities; 3) Annual ABI to assess lower extremity perfusion in cases of aortic reconstruction; 4) Cross-sectional imaging every other year to assess aortic reconstructions, during years of long-growth; and 5) Ambulatory blood pressure monitoring and echocardiogram for concerns related to poorly controlled hypertension and / or symptoms. Any concern for recurrent stenosis and recurrent hypertension should prompt further investigation with catheter-based arteriogram.
Limitations to this single-center retrospective study are intrinsic to the study design and compounded by the diverse geographical location of patient residence and common international/out-of-state referral patterns. Specifically, some patients were lost to long-term follow-up, introducing the potential for type II error in that some patients may have required secondary procedures elsewhere. 105 patients (62%) have not had follow-up at our center or correspondence within the preceding 24 months. Importantly, while the authors recommend judicious post-operative surveillance, as above, this is often performed locally for those international and out-of-state patients. As such, records are not consistently or widely available for all patients, despite contemporary efforts and requests for such, limiting available follow-up data. Additionally, ‘cure’ as used in this study applies to a hypertension cure during available follow-up, as defined above. This is a limited definition and does not consider longitudinal follow-up into late adulthood. Clinically speaking, use of the word ‘cure’ should be severely tempered for cases of childhood arterial dysplasia. Cohort development and longer prospective longitudinal follow-up is urgently required to enhance our understanding of the natural history of pediatric renovascular hypertension with medical management and revascularization that considers patient phenotype and optimize indications for and timing of invasive treatment.
CONCLUSION
The contemporary surgical treatment of pediatric RVH due to renal artery occlusive disease and aortic coarctation requires an individualized approach that considers the patient’s age, the severity of the hypertension, the underlying disease, and the vascular anatomy including that of extra-renal disease. Open revascularization of both renal artery stenoses and abdominal aortic coarctations at a high-volume referral center provide sustainable benefits in nearly 90% of children. Nevertheless, interventions in the very young (ie: <3 years) and the presence of concurrent aortic disease increase the likelihood of a later reoperation. Those undergoing secondary procedures are less likely to be cured of hypertension. Post-operative surveillance in children treated for RVH is imperative, given the risk of restenoses and disease progression. Established criteria for the diagnosis and management of pediatric RVH must consider specific patient phenotypes to define the most appropriate interventions and identify best practices, especially when balancing the risks of open operative and endovascular interventions. Further prospective, multi-center investigation of this rare, but debilitating, condition is urgently required to improve the outcomes and quality of life for affected children.
Supplementary Material
Supplemental Table 1: Logistic regression model to predict the odds of reoperation (OR = operation, NF1 = Neurofibromatosis Type 1)
Supplemental Table 2: Ordinal regression model to predict hypertension benefit (CI = confidence interval, OR = operation, NF1 = Neurofibromatosis Type 1)
ARTICLE HIGHLIGHTS.
Type of Research:
Single-center, retrospective analysis of prospectively collected data.
Key Findings:
Surgical revascularization of 169 children with renovascular hypertension resulted in a sustainable overall benefit in hypertension in 90% of children. Interventions in the very young and in those with concurrent abdominal aortic coarctation increase the likelihood of reoperation. Patients undergoing remedial surgery after earlier operative failures are less likely to be cured of hypertension.
Take home Message:
Surgical revascularization of pediatric renovascular hypertension provides a sustainable benefit in most children, including those with abdominal aortic coarctation, with no mortality or renal insufficiency requiring dialysis.
Acknowledgments
Funding: Supported in part from the Zangara Research Fund and Robson Research Fund, both established at the University of Michigan, R01HL139672
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presented in part at the Vascular Annual Meeting, Society for Vascular Surgery, June 2, 2017
COI: The authors have no conflict of interest or conflict of competing interests.
REFERENCES
- 1.Stanley JC, Criado E, Upchurch GR Jr., Brophy PD, Cho KJ, Rectenwald JE, et al. Pediatric renovascular hypertension: 132 primary and 30 secondary operations in 97 children. J Vasc Surg. 2006;44(6):1219–28; discussion 28-9. [DOI] [PubMed] [Google Scholar]
- 2.Silverstein DM, Champoux E, Aviles DH, Vehaskari VM. Treatment of primary and secondary hypertension in children. Pediatr Nephrol. 2006;21(6):820–7. [DOI] [PubMed] [Google Scholar]
- 3.Rumman RK, Matsuda-Abedini M, Langlois V, Radhakrishnan S, Lorenzo AJ, Amaral J, et al. Management and Outcomes of Childhood Renal Artery Stenosis and Middle Aortic Syndrome. Am J Hypertens. 2018;31(6):687–95. [DOI] [PubMed] [Google Scholar]
- 4.Chung H, Lee JH, Park E, Hyun H, Ahn YH, Jae HJ, et al. Long-Term Outcomes of Pediatric Renovascular Hypertension. Kidney Blood Press Res. 2017;42(3):617–27. [DOI] [PubMed] [Google Scholar]
- 5.Meyers KE, Cahill AM, Sethna C. Interventions for pediatric renovascular hypertension. Curr Hypertens Rep. 2014;16(4):422. [DOI] [PubMed] [Google Scholar]
- 6.Agrawal H, Moodie D, Qureshi AM, Acosta AA, Hernandez JA, Braun MC, et al. Interventions in children with renovascular hypertension: A 27-year retrospective single-center experience. Congenit Heart Dis. 2018;13(3):349–56. [DOI] [PubMed] [Google Scholar]
- 7.Lobeck IN, Alhajjat AM, Dupree P, Racadio JM, Mitsnefes MM, Karns R, et al. The management of pediatric renovascular hypertension: a single center experience and review of the literature. J Pediatr Surg. 2018;53(9):1825–31. [DOI] [PubMed] [Google Scholar]
- 8.Kim HB, Vakili K, Ramos-Gonzalez GJ, Stein DR, Ferguson MA, Porras D, et al. Tissue expander-stimulated lengthening of arteries for the treatment of midaortic syndrome in children. J Vasc Surg. 2018;67(6):1664–72. [DOI] [PubMed] [Google Scholar]
- 9.Tullus K, Brennan E, Hamilton G, Lord R, McLaren CA, Marks SD, et al. Renovascular hypertension in children. Lancet. 2008;371(9622):1453–63. [DOI] [PubMed] [Google Scholar]
- 10.Zhu G, He F, Gu Y, Yu H, Chen B, Hu Z, et al. Angioplasty for pediatric renovascular hypertension: a 13-year experience. Diagn Interv Radiol. 2014;20(3):285–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alexander A, Richmond L, Geary D, Salle JL, Amaral J, Connolly B. Outcomes of percutaneous transluminal angioplasty for pediatric renovascular hypertension. J Pediatr Surg. 2017;52(3):395–9. [DOI] [PubMed] [Google Scholar]
- 12.Srinivasan A, Krishnamurthy G, Fontalvo-Herazo L, Nijs E, Keller MS, Meyers K, et al. Angioplasty for renal artery stenosis in pediatric patients: an 11-year retrospective experience. J Vasc Interv Radiol. 2010;21(11):1672–80. [DOI] [PubMed] [Google Scholar]
- 13.Shroff R, Roebuck DJ, Gordon I, Davies R, Stephens S, Marks S, et al. Angioplasty for renovascular hypertension in children: 20-year experience. Pediatrics. 2006;118(1):268–75. [DOI] [PubMed] [Google Scholar]
- 14.Flynn JT, Kaelber DC, Baker-Smith CM, Blowey D, Carroll AE, Daniels SR, et al. Clinical Practice Guideline for Screening and Management of High Blood Pressure in Children and Adolescents. Pediatrics. 2017;140(3). [DOI] [PubMed] [Google Scholar]
- 15.Fry WJ, Ernst CB, Stanley JC, Brink B. Renovascular hypertension in the pediatric patient. Arch Surg. 1973;107(5):692–8. [DOI] [PubMed] [Google Scholar]
- 16.Stanley JC, Fry WJ. Pediatric renal artery occlusive disease and renovascular hypertension. Etiology, diagnosis, and operative treatment. Arch Surg. 1981;116(5):669–76. [DOI] [PubMed] [Google Scholar]
- 17.Stanley JC, Zelenock GB, Messina LM, Wakefield TW. Pediatric renovascular hypertension: a thirty-year experience of operative treatment. J Vasc Surg. 1995;21(2):212–26; discussion 26-7. [DOI] [PubMed] [Google Scholar]
- 18.Eliason JL, Coleman DM, Criado E, Kershaw DB, Blatt NB, Williams DM, et al. Remedial operations for failed endovascular therapy of 32 renal artery stenoses in 24 children. Pediatr Nephrol. 2016;31(5):809–17. [DOI] [PubMed] [Google Scholar]
- 19.Stadermann MB, Montini G, Hamilton G, Roebuck DJ, McLaren CA, Dillon MJ, et al. Results of surgical treatment for renovascular hypertension in children: 30 year single centre experience. Nephrol Dial Transplant. 2010;25(3):807–13. [DOI] [PubMed] [Google Scholar]
- 20.Martinez A, Novick AC, Cunningham R, Goormastic M. Improved results of vascular reconstruction in pediatric and young adult patients with renovascular hypertension. J Urol. 1990;144(3):717–20. [DOI] [PubMed] [Google Scholar]
- 21.O’Neill JA Jr. Long-term outcome with surgical treatment of renovascular hypertension. J Pediatr Surg. 1998;33(1):106–11. [DOI] [PubMed] [Google Scholar]
- 22.Stanley JC, Criado E, Eliason JL, Upchurch GR Jr., Berguer R, Rectenwald JE. Abdominal aortic coarctation: surgical treatment of 53 patients with a thoracoabdominal bypass, patch aortoplasty, or interposition aortoaortic graft. J Vasc Surg. 2008;48(5):1073–82. [DOI] [PubMed] [Google Scholar]
- 23.Courtel JV, Soto B, Niaudet P, Gagnadoux MF, Carteret M, Quignodon JF, et al. Percutaneous transluminal angioplasty of renal artery stenosis in children. Pediatr Radiol. 1998;28(1):59–63. [DOI] [PubMed] [Google Scholar]
- 24.Sharma S, Thatai D, Saxena A, Kothari SS, Guleria S, Rajani M. Renovascular hypertension resulting from nonspecific aortoarteritis in children: midterm results of percutaneous transluminal renal angioplasty and predictors of restenosis. AJR Am J Roentgenol. 1996;166(1):157–62. [DOI] [PubMed] [Google Scholar]
- 25.Tyagi S, Kaul UA, Satsangi DK, Arora R. Percutaneous transluminal angioplasty for renovascular hypertension in children: initial and long-term results. Pediatrics. 1997;99(1):44–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1: Logistic regression model to predict the odds of reoperation (OR = operation, NF1 = Neurofibromatosis Type 1)
Supplemental Table 2: Ordinal regression model to predict hypertension benefit (CI = confidence interval, OR = operation, NF1 = Neurofibromatosis Type 1)


