Abstract
Non-Hodgkin lymphoma comprises a heterogeneous group of hematologic malignancies, with about 60 subtypes that arise via various pathogenetic mechanisms. Although establishing etiology for specific NHL subtypes has been historically difficult given their relative rarity, environmental exposures have been repeatedly implicated as risk factors across many subtypes. Large-scale epidemiologic investigations have pinpointed chemical exposures in particular, but causality has not been established, and the exact biologic mechanisms underpinning these associations are unclear. Here we review chemical exposures that have been associated with development of NHL subtypes and discuss their biologic plausibility, based on current research.
Keywords: Hematologic cancers, environmental risk factors, carcinogenesis
Introduction
Non-Hodgkin lymphoma (NHL) is the most common hematologic malignancy in the world1 and is comprised of about 60 distinct subtypes that are heterogeneous in their clinical and biologic features. While NHL subtype classification is vitally important for diagnosis and treatment, researchers face challenges in acquiring adequate sample sizes to conduct subtype-specific analyses of possible risk factors, due to the relative rarity of each subtype. In the past decade, several large epidemiologic studies have succeeded in identifying risk factors based on subtype, most notable of which is the InterLymph Consortium – an international collaborative group that uses pooled analyses to conduct sufficiently powered analyses of environmental and non-environmental factors associated with lymphoma development.2 By definition, environmental exposures affect large groups with underlying commonalities and encompass a broad range of physical and social factors, including built environment, communicable disease, and psychosocial trauma. InterLymph and others have demonstrated both heterogeneity and commonality of risk factors among NHL subtypes. Non-modifiable risk factors that have most commonly been associated with NHL, regardless of subtype, include family history of hematopoietic malignancy and congenital immunodeficiency.3,4 Importantly, associations with environmental factors such as infections, radiation, and chemical exposure have been identified in several NHL subtypes. In particular, mounting evidence suggests that biologic disruption from environmental exposure to solvents, flame retardants, pesticides and herbicides, and hair dye may be key factors in NHL pathogenesis, but identifying molecular features consequent to environmental exposure and implicated in the etiology of NHL has proved challenging.5,6 Filling this knowledge gap will require integration of findings from large-scale population research, molecular characterization of potential carcinogenic agents, and studies of lymphomagenesis mechanisms. This review seeks to link epidemiologic studies to available evidence from basic science research to provide a more clear understanding of the role chemical exposures play in the molecular pathogenesis of NHL subtypes.
Environmental Exposures and Overall NHL Risk
Previous research has presented compelling evidence that link environmental factors and development of NHL. Several infectious agents – such as Epstein-Barr virus (EBV), human herpes virus 8 and human T-lymphotropic virus type I – are well-established in the etiology of lymphoma, either by transforming normal lymphocytes, causing immunodeficiency syndromes, or promoting chronic immune stimulation.7 Human immunodeficiency virus (HIV) infection has a clear link to certain aggressive NHLs, with the proportion of NHL that can be attributed to the HIV epidemic varying by subtype (e.g., Burkitt lymphoma: 20-30%, primary central nervous system lymphoma: ~20%, diffuse large B cell lymphoma [DLBCL]: ~5%).8 However, the HIV epidemic only accounts for a small proportion of the increased incidence of NHL overall in the last few decades.9–12 The increase in overall NHL incidence since the 1980’s is widely hypothesized to be the result of changes in environmental exposures over time, but culprit exposures and their etiologic links to NHL risk overall and by subtype remain poorly defined.10–12
Many studies have demonstrated positive associations between NHL risk and occupations with historically high chemical exposures or with specific groups of chemicals, particularly pesticides, herbicides, and volatile organic compounds. However, effect estimates vary based on method of exposure ascertainment, disease classification, and study design. In a meta-analysis of 29,605 cancer cases and 3,478,748 participants, NHL was the only cancer type to consistently demonstrate a statistically significant association between external exposure to and blood level of dioxins, a group of chemically related persistent environmental pollutants, and cancer mortality (standardized mortality ratio [SMR] = 1.18, 95% CI: 1.01-1.37); however, estimates based on blood levels of the exposure were imprecise due to the small number of cases.13 Additionally, the Consortium of Agricultural Cohort Studies (AGRICOH), an international pooling effort to examine agricultural exposures and health outcomes by following farmers and pesticide applicators prospectively, has conducted large-scale studies assessing pesticide exposure and NHL risk. Investigators conducted a meta-analysis of 316,270 farmers from three cohort studies and found that pig farming, an occupation where individuals may be exposed to high concentrations of bioaerosols like organic dust in addition to pesticides,14 was protective against NHL when compared to farmers who did not report farming livestock (hazard ratio [HR]=0.86, 95% CI: 0.77-0.96); there was no such association with farming involving other types of livestock.15 Interestingly, when exposures were grouped by pesticide chemical types among the entire cohort of farmers, researchers found an elevated risk in NHL among those who reported ever being exposed to terbufos (meta-HR = 1.18, 95% CI: 1.00-1.39), but a decreased risk with organochlorine insecticides (meta-HR = 0.84, 95% CI: 0.74-0.99) and phenoxy herbicides (meta-HR = 0.81, 95% CI: 0.67-0.98).16
In March 2015, a group of 17 experts from the International Agency for Research on Cancer produced a monograph detailing the carcinogenicity of pesticides, which included glyphosate – the main ingredient in the herbicide Roundup (Monsanto), and the most commonly used pesticide active ingredient in USA agriculture.17,18 NHL was highlighted in this monograph, based on the wealth of research associating NHL risk with pesticides. Although the group concluded glyphosate was “probably carcinogenic to humans,” and that animal models exhibited sufficient evidence of carcinogenicity, there was hesitancy to invoke causality due to the lack of studies on humans. In the following year, four outside expert panel reviews subsequently disagreed with the panel, concluding no association between glyphosate and NHL based on current research.19 Despite this refutation, as of summer 2019, Monsanto had been charged with three class action lawsuits (the highest reaching $2 billion) citing Roundup exposure as cause of claimants’ NHL.
While there are several theories for the underlying biologic mechanism(s) by which glyphosate might impact lymphomagenesis – such as genotoxicity, immunosuppression, or oxidative stress20 – findings from epidemiologic studies are mixed. A recent meta-analysis that included the 2018 Agricultural Health Study (AHS), a cohort of pesticide applicators that have been followed prospectively, and five case-control studies found the relative risk of NHL to increase by 41% among those highly exposed to glyphosate-based herbicides (meta-risk ratio [RR] = 1.41, 95% CI: 1.13-1.75).20 However, studies on the AHS population alone, which includes 515 incident NHL cases, have consistently observed no association between reported glyphosate exposure and NHL risk, regardless of the latency period (i.e., 5-, 10-, 15-, and 20-year lag times).21,22
A more convincing example linking NHL to chemical exposure can be found in the history of hair dye reformulation in the early 1980s. After mutagenic effects of several commonly used hair dye ingredients were observed in murine models, there was widespread reformulation of hair dye to exclude these chemicals. Several groups have since examined personal use of hair dye pre- and post-1980.23 In a European study of 2,302 incident lymphoma cases and 2,417 hospital- or population-based controls, a 37% increase in risk of developing lymphoma was observed among those who reported starting using hair dye before 1980 (odds ratio [OR] =1.37, 95% CI: 1.09-1.72), with an increased risk of 62% among those who used hair dye only before 1980 (OR=1.62, 95% CI: 1.10-2.40).24 Interestingly, when hair dye use was classified as ever versus never used, women were at higher risk for NHL (OR=1.24, 95% CI: 1.01-1.53) than men (OR=1.06, 95% CI: 0.77-1.46). Furthermore, a US population-based multicenter study of 1,321 cases and 1,051 controls demonstrated that increased NHL risk due to hair dye exposure was modified by variations in genes responsible for metabolizing aromatic amines – chemicals found in pre-1980 hair dyes.25 While these studies are informative, effects are still inconsistent. This could, in part, be a result of failing to delineate chemical risk factors by NHL subtype, which has the potential to attenuate risks or mask them completely. More recent analyses have incorporated a larger number of cases to assess associations between environmental exposures and subtype-specific risk.
Environmental Exposures by NHL Subtype
Diffuse large B-cell lymphoma
Diffuse large B-cell lymphoma (DLBCL) is an aggressive lymphoma that represents the most common subtype of NHL, responsible for about 25% of mature neoplasms. Occurring more frequently in adults, the rate of DLBCL increases dramatically after the age of 50.26 Due to its relatively high prevalence, DLBCL currently has the strongest data supporting chemical risk factors.
Epidemiology of Environmental Exposures and DLBCL
Established risk factors for DLBCL include HIV infection, EBV reactivation, family history of hematologic malignancy, history of autoimmune disease, hepatitis C virus seropositivity, Coxiella burnetii infection, and high body mass index as a young adult.27–30 Epidemiologic studies have demonstrated an increased risk of DLBCL with certain occupations or exposure to specific chemicals, although these associations have been inconsistent depending on the study population and how the exposure is classified. Table 1 summarizes studies of occupational exposure and risk of common NHL subtypes.28,31–33 The InterLymph Consortium, which analyzed 4,667 DLBCL cases and 22,639 controls, found an increased risk in field crop and vegetable farm workers (OR=1.78, 95% CI: 1.22-2.60), seamstress/embroiderers (OR=1.49, 95% CI: 1.13-1.97), and hairdressers (OR=1.65, 95% CI: 1.12-2.41) among women, while working as a material handling equipment operator increased risk in men (OR=1.58, 95% CI: 1.02-2.44).28 However, the InterLymph Consortium found no association between personal use of hair dye and DLBCL among women in an independent study of 1,543 DLBCL cases and 5,799 controls, irrespective of timing or duration of use, and in spite of associations with other NHL subtypes.32
Table 1.
Studies linking occupation with risk of NHL subtypes
| NHL Subtype | Author | Casesa | Controlsb | Exposure Assessment | Exposure | OR (95% CI) |
|---|---|---|---|---|---|---|
| DLBCL | Cerhan et al.28 | 1,347 | 5,138 | Self-report | Farm workerc | 1.78 (1.22-2.60) |
| 1,462 | 5,786 | Self-report | Seamstressc | 1.49 (1.13-1.97) | ||
| 1,420 | 5,534 | Self-report | Women’s hairdresserc | 1.65 (1.12-2.41) | ||
| 1,457 | 5,768 | Self-report | Material handling equipment operatord | 1.59 (1.02-2.44) | ||
| BL | Mbulaiteye et al.73 | 120 | 10,221 | Self-report | Cleaner | 3.49 (1.13-10.7) |
| FL | Linet et al.61 | 1,790 | 10,625 | Self-report | Spray painter | 2.66 (1.36-5.24) |
| CLL/SLL | Slager et al.78 | 1,042 | 9,695 | Self-report | Farmer | 1.23 (1.04, 1.45) |
| 1,042 | 8,795 | Self-report | Hairdresser | 1.77 (1.05-2.98) | ||
| MZL | Bracci et al.83 | 599 | 7,422 | Self-report | Carpenter | 2.34 (1.23-4.45) |
| 639 | 8,150 | Self-report | Metalworker | 3.56 (1.67-7.58) | ||
| 639 | 8,150 | Self-report | Teacher | 0.58 (0.37-0.88) | ||
| Mester et al.86 | 12 | 108 | Self-report | Wholesale retail trade industry | 3.0 (1.5-5.9) | |
| MCL | Smedby et al.88 | 201 | 5,847 | Self-report | Ever lived on a farm | 1.40 (1.03-1.90) |
| 286 | 8,139 | Self-report | Material-handling equipment operator | 3.05 (1.47-6.31) | ||
| 286 | 7,931 | Self-report | Electrician or electronics worker | 1.63 (1.09-2.44) | ||
| TCL | Xu et al.93 | 88 | 305 | Self-report | Farmer | 4.15 (1.74-9.87) |
| 88 | 305 | Self-report | Crop producer | 2.81 (1.49-5.29) | ||
| Wang et al.91 | 363 | 11,490 | Self-report | Ever lived or worked on a farm | 0.72 (0.55-0.95) | |
| 328 | 9,921 | Self-report | Textile worker | 1.58 (1.05-2.38) | ||
Total exposed/unexposed cases
Totalexposed/unexposed controls
Among females
Among males
Abbreviations: NHL = non-Hodgkin lymphoma; DLBCL = diffuse large B-cell lymphoma; BL = Burkitt lymphoma; FL = follicular lymphoma; CLL/SLL = chronic lymphocytic leukemia/small lymphocytic lymphoma; MZL = marginal zone lymphoma; MCL = mantle cell lymphoma; TCL = T-cell lymphoma OR = odds ratio; CI = confidence interval.
Several groups have identified important associations between environmental pollutants and DLBCL risk. Such studies motivated a meta-analysis that examined 21 pesticide chemical groups and 80 active ingredients extracted from 44 papers. A summary of pesticide exposure and risk of common NHL subtypes is found in Table 2. Investigators discovered a positive association between DLBCL and exposure to phenoxy herbicides (meta RR=2.0, 95%CI: 1.1-3.7)34, but studies that subdivided NHL by subtype were limited. Farmers in the AGRICOH consortium who were diagnosed with DLBCL demonstrated a positive association with exposure to the pesticide and organic pollutant glyphosate (HR: 1.36, 95% CI: 1.00-1.85).16 Finally, another study measured polychlorinated biphenyl, a persistent organic pollutant and flame retardant to which humans are typically exposed through diet and certain occupations such as manufacturing, in the plasma of 35 DLBCL cases and 409 controls. Investigators observed an increasing risk in DLBCL across exposure tertiles (3rd tertile: OR=3.1, 95% CI:1.2-8.3), making it one of the few studies to demonstrate risk of DLBCL in a dose-responsive manner.35
Table 2.
Studies linking pesticide and herbicide exposure with risk of common NHL subtypes
| NHL Subtype | Author | Casesa | Controlsb | Exposure | Exposure Assessment | Exposure Metric | Effect Estimate (95% CI) |
|---|---|---|---|---|---|---|---|
| DLBCL | Schinasi et al.34,‡ | NR | NR | Phenoxy herbicides | Various | Various | meta RR: 2.0 (1.1-3.7) |
| Eriksson et al.31,‡ | 239 | 1,016 | Phenoxyacetic acids | Self-report | Occupational exposure > 45 days | OR: 2.16 (1.08-4.33) | |
| Leon et al.16,† | 434 | 313,840 | Glyphosate | Self-report; crop-exposure matrices | Ever exposed | HR: 1.36 (1.00-1.85) | |
| BL | Buckley et al.74 | 61 | 268 | Use of household exterminator | Self-report | Use more than once per week | OR: 8.0 (p < 0.05) |
| 61 | 268 | Contact with insecticides or herbicides | Self-report | Ever exposed | OR: 4.7 (p < 0.05) | ||
| 61 | 268 | Occupational exposure to pesticides in the mother | Self-report | Ever exposed | OR: 9.6 (p < 0.05) | ||
| FL | Schroeder et al.68 | 622c | 1245 | Lindane | Self-report | Ever exposed | OR: 2.3 (1.3-3.9) |
| Dieldrin | Self-report | Ever exposed | OR: 3.7 (1.9-7.0) | ||||
| Toxaphene | Self-report | Ever exposed | OR: 3.0 (1.5-6.1) | ||||
| Phthalimide | Self-report | Ever exposed | OR: 2.9 (1.1-7.5) | ||||
| Chiu et al.69 | 172c | 1432 | Crop insecticides | Self-report | Exposure > 12 years | OR: 3.0 (1.1-8.2) | |
| Herbicides | Self-report | Exposure > 17 years | OR: 2.9 (1.1-7.9) | ||||
| Fumigants | Self-report | Ever use | OR: 5.0 (1.7-14.5) | ||||
| Eriksson et al.22,‡ | 165 | 1,016 | DDT | Self-report (occupations) | Occupational exposure > 37 days | OR: 2.14 (1.05-4.40) | |
| 165 | 1,016 | Mercurial seed dressing | Self-report (occupations) | Occupational exposure > 12 days | OR: 3.61 (1.20-10.9) | ||
| Fritschi et al.62 | 227 | 694 | Occupational exposure to organophosphates | Self-report; industrial hygienists; pesticide-crop matrix | Substantial | OR: 4.28 (1.41-13.0) | |
| CLL/SLL | Eriksson et al.22,‡ | 195 | 1,016 | Glyphosate | Self-report | Occupational exposure > 10 days | OR: 3.35 (1.42-7.89) |
| 195 | 1,016 | Herbicides | Self-report | Occupational exposure > 20 days | OR: 2.27 (1.28-4.01) | ||
| Cocco et al.33,‡ | 9 | 21 | Organophosphates | Self-report; industrial hygienists | Ever occupationally exposed | OR: 2.7 (1.2-6.0) | |
| Alavanja et al.80, † | 34 | 106 | Metaxyl | Self-report; imputation | Ever occupationally exposed | RR: 1.6 (1.0-2.5) | |
| 31 | 72 | Terbufos | Self-report; imputation | High occupational exposure group | RR: 1.6 (1.0-2.5) | ||
| 15 | 79 | DDT | Self-report; imputation | High occupational exposure group | RR: 2.6 (1.3-4.8) | ||
| Leon et al.16,† | 497 | 313,840 | Deltamethrin | Self-report; crop-exposure matrix | Ever exposed; crop-exposure matrices | HR: 1.48 (1.06-2.07) | |
Total exposed/unexposed cases
Total exposed/unexposed controls
Outcomes are t(14;18)-positive NHL versus (t14;18)-negative NHL
Studies included in meta-analysis
Overlapping cohorts
Abbreviations: NHL = non-Hodgkin lymphoma; DLBCL = diffuse large B-cell lymphoma; BL = Burkitt lymphoma; FL = follicular lymphoma; CLL/SLL = chronic lymphocytic leukemia/small lymphocytic lymphoma; DDT = dichlorodiphenyltrichloroethane; NR = not reported; OR = odds ratio; RR = risk ratio; CI = confidence interval.
Occupational exposure to trichloroethylene, an industrial solvent, has not been associated with DLBCL risk in the general population.36 However, a considerable association with chlorinated solvents was observed among female DLBCL cases who harbored a single nucleotide polymorphism (SNP) in either of two genes: IL10 (OR=3.31, 95% CI: 1.80-6.08), a gene that codes for the immunoregulatory cytokine interleukin-10, and MGMT (OR=4.24, 95% CI: 2.03-8.86), a key DNA repair gene.37,38 This suggests a gene-environment interaction whereby chemical exposure-related lymphomagenesis may be mediated by abnormalities in inflammatory milieu or DNA repair.
Studies on occupational risk factors have begun to provide some insight into the role of chemical exposures in lymphomagenesis in DLBCL, but the relationship between non-occupational or passive exposure and disease risk is unclear. Using spatial analysis, two groups have observed a positive association in DLBCL and passive exposure to volatile organic compounds (VOC) using the Environmental Protections Agency’s Toxic Release Inventory and residential proximity to release sites.39,40 First, an increased risk of DLBCL was observed in relation to residential distance and years lived near a chemical facility (e.g., for 10 years lived within 2 miles of a chemical facility, OR=1.9, 95% CI:1.2-2.9). In the latter study, investigators found that DLBCL risk decreased by 0.58% for each mile of increased residential distance from formaldehyde release sites. In a similar study that focused on spatial relationships exclusively with benzene, a VOC and known carcinogen, authors observed an elevated risk of DLBCL in certain exposure zones (e.g., exposure level 5 vs. level 1; RR=1.66, 95% CI: 1.46-1.88).41 However, total DLBCL cases were not reported. Additionally, all groups relied on distance to toxic release sites as a proxy for personal exposure, consequently lacking quantitative measures of exposure. Table 3 summarizes studies of VOC exposure and risk of common NHL subtypes. While this evidence is helpful in disentangling occupational status and chemical exposures in the role of lymphomagenesis, research that incorporates quantitative individual level exposures and temporal analysis could more definitively evaluate the relationship between passive exposure of VOCs and DLBCL risk.
Table 3.
Studies linking VOC exposure with risk of common NHL subtypes
| NHL Subtype | Author | Casesa | Controlsb | Genetic Predisposition | Exposure | Exposure Assessment | Exposure Metric | Effect Estimate (95% CI) |
|---|---|---|---|---|---|---|---|---|
| DLBCL | Deng et al.37, † | 160 | 717 | IL10 polymorphism | Occupational exposure to organic solvents | Self-report; job-exposure matrix | Ever exposed | OR=3.31 (1.80-6.08) |
| Jiao et al.38, † | 518 | 597 | MGMT polymorphism | Occupational exposure to chlorinated solvents | Self-report; job-exposure matrix | Ever exposed | OR=4.24 (2.03-8.86) | |
| De Roos et al.39 | 1321 | 1057 | -- | Residence near chemicals and allied products facility | Residence | 10 years lived within 2 miles of a facility | OR=1.9 (1.2-2.9) | |
| Bulka et al.40 | 3851 | N/A | -- | Residence near Toxic Release Inventory site (formaldehyde) | Residence | Distance near site 10 years prior to diagnosis | β = −0.0058 (p<0.001) | |
| Switchenko et al.41 | NR | N/A | -- | Residence near Toxic Release Inventory site (benzene) | Residence | Distance near site 10 years prior to diagnosis | RR=1.66 (1.46-1.88) | |
| FL | Jiao et al.38, † | 518 | 597 | MGMT polymorphism | Occupational exposure chlorinated solvents | Self-report; job-exposure matrix | Ever exposed | OR=4.44 (1.86-10.61) |
| Cocco et al.36 | 4279 | 3788 | -- | Occupational exposure to trichloroethylene | Self-report; industrial hygienists; job exposure matrix | Frequency of work time > 31% | OR=1.8 (1.1-2.9) | |
| Cocco et al.63 | 2348 | 2462 | -- | Occupational exposure to benzene, toluene, xylene (combined) | Self-report; industrial hygienists | Ever exposed | OR=1.7 (1.2-2.5) | |
| Styrene | Self-report; industrial hygienists | Ever exposed | OR=2.6 (1.3-5.2) | |||||
| CLL/SLL | Cocco et al.63 | 367 | 2179 | -- | Occupational exposure to organic solvents | Self-report; industrial hygienists | Ever exposed | OR=1.5 (1.1.−1.9) |
Total exposed/unexposed cases
Total exposed/unexposed controls
Overlapping cohorts
Abbreviations: NHL = non-Hodgkin lymphoma; DLBCL = diffuse large B-cell lymphoma; FL = follicular lymphoma; VOC = volatile organic compounds; OR = odds ratio; CI = confidence interval.
Epigenetic Consequences of Environmental Exposures in DLBCL Pathogenesis
DLBCL is clinically and genetically heterogeneous, further complicating investigation into its etiology. Advances in functional genomics subclassify DLBCL based on two distinct cells-of-origin: activated B cell-like (ABC) and germinal center B cell-like (GCB), with ABC-DLBCL exhibiting a worse overall survival.42 In addition to clinical differences, GCB and ABC DLBCL harbor unique genetic abnormalities that have been summarized elsewhere.43,44 Next-generation sequencing techniques and studies using cell line and animal models have begun to uncover drivers in DLBCL development.42 Mutations in genes responsible for chromatin remodeling, B-cell receptor signaling, the NF-κB pathway, as well as aberrant somatic hypermutation and DNA methylation have been repeatedly implicated.27,45–49 It is important to consider these subtype-specific genetic lesions when analyzing the link between environmental exposures and DLBCL pathogenesis, since GCB and ABC lymphomas likely develop via separate oncogenic pathways.50 Exposure to benzene has been linked to hematologic malignancies and aberrant DNA methylation, but its mechanism in NHL remains elusive.51 As reduction in methylation of lysine 4 on histone H3 (H3K4) has been observed in human bone marrow in response to chronic benzene exposure,52 epigenetic modifications could represent a mechanism by which benzene exposure contributes to DLBCL pathogenesis. Intriguingly, the chromatin modifying gene KMT2D (also referred to as MLL2), which codes for a set of methyltransferases responsible for catalyzing H3K4 methylation, is recurrently mutated in DLBCL – an event thought to occur early in carcinogenesis.53 In a murine model, conditional deletions of KMT2D during B cell development reduced global H3K4 methylation in DLBCL cells and increased B cell proliferation. These findings indicate that KMT2D may act as a tumor suppressor gene and highlight the importance in regulation of histone methylation in DLBCL development.54
Hypermethylation of promoter regions may lead to gene-silencing of cancer-related genes and is tightly correlated with tumor development in human and animal models.55,56 Compromising the activity of DNA damage repair proteins leads to genomic instability,57,58 thus setting the stage for oncogenesis. In a study that measured benzene concentrations and methylation patterns of genes implicated in carcinogenesis in peripheral blood, investigators found a higher degree of methylation in the promotor region of MGMT in healthy, benzene-exposed workers compared to healthy, unexposed workers.59 As previously mentioned, certain SNPs in MGMT have been shown to interact with chlorinated solvent exposure to increase DLBCL risk, highlighting this gene as a key suspect in chemical exposure-mediated lymphomagenesis whose dysregulation may occur via several distinct mechanisms.
Since certain individuals, such as those who work in manufacturing, are likely to be exposed to multiple VOCs simultaneously, chemical exposures may contribute to DLBCL pathogenesis through multiple inter-related pathways. A summary of environmental exposures and the evidenced biologic pathways is summarized in Table 4. Experiments in animal models or cell lines using demethylating agents to rescue the epigenetic effects of VOCs in healthy cells could better define the causal pathways by which these exposures lead to DLBCL, either individually or in combination.
Table 4.
Possible biologic mechanisms underlying associations between chemical exposures and risk of NHL subtypes
| NHL Subtype | Risk Factor | Potential Mechanism | OR† (95% CI) | Molecular Evidence |
|---|---|---|---|---|
| DLBCL | Organic solvents | Gene-environment interaction with SNP in genes affecting inflammatory environment | 3.31 (1.80-6.08)37 | • Interaction between organic solvent exposure and SNP in IL10 promoter region • Chemical exposure ascertained by self-report and job-exposure matrix |
| Gene-environment interaction with SNP in genes affecting DNA repair | 4.24 (2.03-8.86)38 | • Interaction in chlorinated solvent exposure and exonic SNP in MGMT • Chemical exposure ascertained by self-report and verified by industrial hygienists |
||
| Benzene | Reduced H3K4 methylation | -- | • Benzene exposure reduces H3K4 methylation in human bone marrow • Decreased methylation of H3K4 via conditional deletions of KMT2Dleads to increased B cell proliferation in DLBCL cells |
|
| Impaired DNA damage repair | -- | • SNP in MGMT interacts with organic solvents to increase DLBCL risk • High degree of promoter methylation of MGMT in peripheral blood of benzene-exposed workers |
||
| FL | Pesticides | Gene-environment interaction with t(14; 18) | 3.7 (1.9-7.0)68 5.0 (1.7-14.5)69 |
•Pesticide use more prevalent in cases harboring t(14; 18) • No association with pesticides in t(14; 18)-negative NHL cases • Chemical exposure ascertained by self-report |
| Hair dye | Gene-environment interactions with SNPs in several genes affecting DNA repair | -- | Interaction between hair dye use before 1980 and SNPs in the following DNA repair genes: | |
| 3.28 (1.27-8.50)64 |
BRCA2: non-synonymous SNP in exon 10 • Chemical exposure ascertained by self-report |
|||
| 2.70 (1.30-5.65)64 |
WRN: DNA helicase proteins; SNP in exon • Chemical exposure ascertained by self-report |
|||
| 2.76 (1.32-5.77)64 |
XRCC3: homologous recombination protein; non-synonymous SNP • Chemical exposure ascertained by self-report |
|||
| 2.07 (1.10-3.90)64 |
XRCC4: non-homologous end-joining protein; intronic splice-site SNP • Chemical exposure ascertained by self-report |
|||
| 1.93 (1.00-3.72)64 |
ERCC1: nucleotide excision repair protein • Chemical exposure ascertained by self-report |
|||
| 2.28 (1.12-4.64)64 |
RAD23B: non-synonymous SNP • Chemical exposure ascertained by self-report |
|||
| 1.96 (1.06-3.63)64 |
MGMT: exonic SNP alters enzyme substrate affinity • Chemical exposure ascertained by self-report |
|||
ORs refer to the effect observed in individuals harboring the genetic variant who were exposed to the listed chemical compared to individuals who were never exposed and do not harbor genetic variant.
Abbreviations: NHL = non-Hodgkin lymphoma; DLBCL = diffuse large B-cell lymphoma; FL = follicular lymphoma; SNP = small nucleotide polymorphism; OR = odds ratio; CI = confidence interval.
Follicular Lymphoma
Follicular lymphoma (FL), the most common indolent NHL, is a disease that originates from germinal center B cells and typically follows a slow clinical progression 26. FL most commonly presents in the sixth decade of life and, unlike most NHL subtypes, has similar incidence in men and women.60
Epidemiology of Environmental Exposures and FL
Cigarette smoking, Sjögren’s syndrome, history of blood transfusions, family history of hematologic malignancies and use of hair dye among women are factors that have been most commonly associated with FL incidence.61 InterLymph corroborated most of these findings and additionally observed an inverse association with history of blood transfusions (OR=0.78, 95% CI: 0.68-0.89) and a positive association with occupation as a spray painter (OR=2.66, 95% CI: 1.36-5.24).29,61 Other groups have observed positive associations in additional occupations, especially those associated with exposure to certain pesticides (ORs ranging from 2.14-4.28), 21, 55 organic solvents (OR=1.7, 95% CI: 1.2-2.5),31,36,62,63 and trichlorethylene (OR=1.8, 95% CI: 1.1-2.9).27 Personal hair dye use has also repeatedly been linked to risk of FL, but only if use occurred before 1980.32,64
Gene-Environment Interactions in FL Pathogenesis
Present in about 85% of cases, the chromosomal translocation t(14; 18) is the genetic hallmark of FL that results in constitutive overexpression of the anti-apoptotic protein BCL2.65 Overexpression of BCL2 alone is insufficient for malignant transformation. 65 but there is convincing evidence of gene-environment interactions between t(14; 18) and agricultural pesticides. Researchers have observed higher prevalence of t(14; 18) in 43 healthy participants who were highly exposed to benzene 66 and in a group of 56 individuals occupationally exposed to pesticides 67 compared to those who were unexposed, indicating that exposure to these chemicals may promote this translocation. In two studies that ascertained t(14; 18) status in tumor specimens, marked difference was observed in pesticide use among NHL cases with the translocation as compared to cases without. The first, a case-control study that included 182 biopsies and was restricted to white men, described positive associations with dieldrin, toxaphene, lindane, and phthalimide (a fungicide) in NHL cases that harbored t(14; 18) (dieldrin; OR=3.7, 95% CI: 1.9-7.0).68 The second study was a population-based case control study of 172 tumors from farmers in Nebraska that found a positive association with crop insecticides, herbicides, and fumigants in NHL cases with t(14; 18) (fumigants; OR=5.0, 95% CI:1.7-14.5).69 Remarkably, there was no association with any agricultural pesticides in t(14; 18)-negative cases in either study.
Benzene exposure has also been shown to potentially induce t(14; 18), but findings have been inconsistent. When structural changes in chromosomes were examined using fluorescence in situ hybridization (FISH) in cells from 43 workers highly exposed to benzene and 44 controls, translocations between chromosome 14 and 18 were detected only in the highly exposed participants.66 On the other hand, a later study conducted by the same group in the same population assessed translocations by quantitative polymerase chain reaction and found that benzene was associated with decreased incidence of t(14;18).70 The authors posited that these discrepancies are likely due to different target cell populations as well as differences in the sensitivities of the two assays. In addition to identifying an important methodological distinction in outcome classification where t(14;18) is concerned, the exact role of benzene in promoting pathogenetic translocations requires further investigation.
An additional gene-environment interaction impacting FL risk may also exist between chemical exposures and genetic variation in DNA repair genes. A population-based case-control study of Connecticut women that included 119 FL cases and 597 controls found that those who used hair dye before 1980 and harbored SNPs in well-characterized DNA repair genes had an increased risk of FL compared to those who did not harbor the same SNPs.64 These findings suggest that FL susceptibility to chemical exposures may be modified by particular genotypes. Reminiscent of DLBCL, KMT2D mutations are very common in FL, but research on the stage of carcinogenesis at which this mutation occurs is conflicting.54,71 To our knowledge, no research has investigated the biologic mechanism behind lymphomagenesis in FL in relation to chemical exposures and KMT2D mutations, and thus more work is needed to characterize possible gene-environment interactions in FL risk.
Burkitt lymphoma
Epidemiology of Environmental Exposures and BL
Burkitt lymphoma (BL) is an aggressive B-cell lymphoma with a 5-year survival between 44% and 48% 26. It is divided into three subtypes: endemic, which is associated with EBV infection; immunodeficiency-associated, seen in immunosuppressed patients (such as those with HIV infection); and sporadic.26 Genetically, BL is characterized by t(8; 14), a translocation resulting in the deregulation of the oncogene MYC, a master regulator responsible for a multitude of processes, including cellular growth regulation.72 Unlike most other NHL subtypes, BL has a bimodal age distribution, with age-specific incidence peaks during childhood and in the sixth decade of life. Additionally, BL also manifests sex differences, with about a 3-fold increased risk in males.26
Until recently, little was known regarding risk factors for sporadic BL. InterLymph investigators evaluated 295 cases of sporadic BL and 21,818 controls, stratified by age group to account for the bimodal distribution of age at diagnosis. They found that history of eczema among individuals without other atopic conditions, taller height, and employment as a cleaner were associated with an increased risk among younger participants. Sporadic BL was also associated with history of hepatitis C virus seropositivity among participants 50 years old and older, but this observation was based on only three exposed cases.73 A case-control study of children age 20 or younger found a considerable association between use of household exterminators (OR=8.0, p < 0.05), frequency of contact with insecticides or herbicides (OR=4.7, p < 0.05), and occupational pesticide exposure in the mother (OR=9.6, p < 0.05); however, this analysis was based on only 61 BL cases, questions regarding pesticide exposure were brief and relatively nonspecific, and we are unable to evaluate the precision around these estimates since confidence intervals were not reported.74 Although research has been conducted to help understand the role of chromosomal structure differences associated with BL development and one could hypothesize that early exposure to pesticides might increase risk for acquiring oncogenic changes leading to this disease, there is a dearth of published research work relating such molecular events to chemical exposures in BL. Unfortunately, this is also the case for the remainder of NHL subtypes discussed in this review.
Chronic lymphocytic leukemia/small lymphocytic leukemia
Epidemiology of Environmental Exposures and CLL
Chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) comprise an NHL subtype defined by the presence of a clonal population of CD5+ CD23+ B cells, with CLL appearing primarily in the peripheral blood and bone marrow and SLL in the lymph nodes.27 CLL/SLL is relatively common in Western countries and, until recently, was very rare in Asian populations. A study using data from the Taiwan National Cancer Registry and Surveillance, Epidemiology, and End Results Program observed a strong birth-cohort effect in Taiwanese individuals, but not Caucasians.75 Interestingly, an increase in CLL/SLL incidence in the Taiwanese temporally corresponded to progressive westernization of lifestyle in Taiwan. In a cohort study of 125 U.S.-born and 435 foreign-born Asians compared with 18,973 non-Hispanic whites in California, researchers found that CLL/SLL incidence was lower among women living in ethnic enclaves or with higher socioeconomic status, while no associations were observed in men.76 Although these findings provide persuasive evidence of an environmental component in CLL/SLL etiology, others speculate the observed differences stem from ascertainment bias.77
Several environmental exposures have been implicated in increasing CLL risk. InterLymph confirmed previously described risk factors for CLL, including usual adult height, hepatitis C virus seropositivity, working on a farm, and family history of any hematological malignancy.78 Increased risk among hairdressers was a novel association identified by InterLymph, and previous research has implicated hair dye use before 1980.32,78 A population-based case-control study found that exposure to heterocyclic amines, a byproduct of cooking meat at high temperatures, resulted in a three-fold increase risk in CLL/SLL compared to other NHL subtypes.79 Moreover, Epilymph, a case-control study comprised of 2,348 incident lymphomas and 2,462 controls from six European countries, found an increased risk in CLL/SLL among those who reported ever being exposed to organic solvents occupationally (OR=1.5, 95% CI: 1.1-1.9).
A large proportion of chemical exposures linked with CLL/SLL risk can be attributed to pesticides. Epilymph found that risk of CLL/SLL was elevated by occupational exposure to inorganic or organic pesticides, with nearly three times the increased risk if the pesticide was an organophosphate (OR=2.7, 95% CI: 1.2-6.0).33 However, estimates were based on only nine CLL/SLL cases. Another study linked glyphosate (OR=3.35, 95% CI: 1.42-7.89) – which is structurally similar to organophosphate pesticides but does not inhibit cholinesterase activity – and herbicides in general (OR=2.27, 95% CI: 1.28-4.01) to CLL/SLL risk.31 In a US-based prospective cohort of farmers and commercial pesticide applicators, researchers observed an elevated risk and dose-response trend associated with terbufos, an organophosphorus insecticide (high exposure group; RR=1.6, 95%: 1.0-2.5) and dichlorodiphenyltrichloroethane (DDT) (high exposure group; RR=2.6, 95% CI: 1.3-4.8).80 Researchers also observed elevated CLL/SLL risk in those exposed to the acylalanine fungicide metalaxyl (RR=1.6, 95% CI: 1.0-2.5), although this association did not display an exposure-response trend.80 Lastly, an international consortium of farmers demonstrated an elevated risk of CLL/SLL with deltamethrin exposure, an insecticide (HR = 1.48, 95% CI: 1.06-2.07).16 Effects of organophosphate pesticides in CLL/SLL may be explained by their potential to induce malignant characteristics as has been shown in some solid tumors. For instance, epithelial breast cancer cell lines exposed to the organic pesticides parathion or malathion in vitro exhibited increased growth capabilities and invasive characteristics compared to untreated cells.81 Although several lines of evidence suggest a role for chemical exposures in the pathogenesis of CLL/SLL, the molecular mechanisms underlying these links remain undefined.
Marginal zone lymphoma
Epidemiology of Environmental Exposures and MZL
Marginal zone lymphoma (MZL) is an indolent NHL subtype that accounts for between 5% and 10% of NHLs.60,82 Certain autoimmune, inflammatory, and infectious diseases have been the most strongly implicated risk factors for MZL.29,83,84 For instance, in an InterLymph investigation on autoimmune conditions as NHL risk factors, Sjögren’s syndrome was associated with a nearly 1000-fold increased risk in 307 parotid gland MZLs.85 Interestingly, in a population-based case-control study in Germany, occupations in wholesale retail trade of public administration were found to have a higher risk of MZL. This may reflect those workers’ higher exposure to the general public, which could increase their likelihood of contracting an infectious disease; however, researchers were only able to examine 38 MZL cases.86 On the other hand, occupation as a teacher has been inversely associated with MZL risk, although the reasons for this protective effect remain unclear.29 Genetic risk factors associated with MZL include family history of hematologic cancer or NHL. In terms of environmental exposures, InterLymph discovered a positive association with permanent hair dye use in splenic MZL and occupation as a metalworker in nodal MZL. Given MZL’s strong association with states of immune dysregulation, it is possible that certain chemical exposures induce states of chronic inflammation that contribute to lymphomagenesis.
Mantle cell lymphoma
Epidemiology Environmental Exposures and MCL
Mantle cell lymphoma (MCL) is a rare, aggressive, and largely incurable NHL subtype. Genetically, MCL is characterized by the presence of t(11; 14), resulting in the overexpression of cyclin D1.87 Accounting for between 2% and 10% NHL, few risk factors have been consistently identified across epidemiologic studies, besides a two-to-three times higher risk in men compared to women.26 In its multivariate analyses of 557 MCL cases and 13,766 controls, InterLymph identified an increased risk among those with residence on a farm or a family history of hematologic malignancy.88 This limited evidence suggests a multifactorial etiology comprised of immune-related environmental exposures and genetic susceptibility for this NHL subtype.
T-cell lymphomas
T-cell lymphomas are a group of rare and highly heterogeneous malignancies that make up about 5%-10% of all NHLs.89 Exhibiting significant variability in clinical, genetic and immunologic factors, T-cell lymphomas can be diagnosed as early as childhood until about the seventh decade of life.26 Several studies have found a remarkable increased risk of T-cell NHL among those with celiac disease and a marginally increased risk with use of phenytoin, an anticonvulsant drug.90 In InterLymph’s pooled analysis of 584 histologically confirmed peripheral T-cell lymphomas (PTCL, the most common T-cell lymphoma) and 15,912 controls, researchers confirmed celiac disease as a strong risk factor and observed modest positive associations with family history of hematologic malignances, eczema, psoriasis, smoking 40 or more years, employment as a textile worker, and employment as an electrical fitter. Interestingly, in contrast to other NHL subtypes, having ever lived or worked on a farm was found to decrease risk in PTCL.91 Exposure to farms is typically associated with pesticide and/or herbicide exposure, which has been repeatedly shown to increase risk of other NHL subtypes. It is possible that there may be an unmeasured exposure associated with farming that explains a protective effect in PTCL. Conversely, a case-control study evaluating lifestyle and environmental factors of nasal natural killer/T-cell lymphoma (NKTCL) – a T-cell lymphoma that strongly associated with EBV infection92 - in East Asia observed several positive associations, some of which were related to farming. After adjustment for age, sex, and country of residence, researchers found that the risk of NKTCL was markedly increased with employment as a farmer or crop producer, pesticide use, residence near a garbage-burning plant, and history of smoking. Crop producers who reported minimizing exposure by using gloves and glasses or sprinkling downwind at the time of pesticide use were found to have a lower NKTCL risk compared to those who did not take precautions.93 However, that study was limited to 88 cases. Since NCKTL occurs far more frequently in Asian and Latin American countries than Western countries,94 it is possible that effects of environmental exposures are modified by ancestral genotypes.
Conclusions and Future Directions
Although there is a growing body of research dedicated to understanding the effects of chemical exposures on lymphomagenesis, further investigation is needed. Inconsistencies in environmental risk factor estimates may reflect the lack of subtype-specific analyses, given the apparent variability in oncogenic pathways among subtypes. Thus, it is essential to account for the unique genetic abnormalities observed in different NHL subtypes when attempting to establish the causality of chemical agents such as pesticides, herbicides, and organic solvents.
Inconsistencies may also be due to exposure classification and ascertainment. Due to the rarity of NHL subtypes, case-control studies are the most viable study design for subtype-specific analyses. However, in a large majority of these studies, exposure status is collected after the occurrence of the disease, typically by self-report, and is likely to introduce recall bias. This results in exposure misclassification, which may be differential by disease status, thus biasing the effect in an unpredictable direction.95 Such biases may explain the lack of dose-response data and conflicting estimates across studies and populations in studies of chemical exposures and NHL risk. Investigators can begin to provide parameters regarding uncertainty by quantifying the impact of the misclassification by conducting quantitative bias analyses.
As with any review, it is important to note that this synopsis is based wholly on published research and is thus prone to publication bias.96 Wherever possible, we have included notable negative findings from high-quality studies and highlighted exposures for which associations with NHL risk are controversial. Additionally, while we do not believe statistical significance should be used as a benchmark for worthy studies, in an attempt to clearly catalogue associations with NHL subtypes in a large number of chemical exposures, we considered inclusion of results from high-quality studies that were statistically significant and had meaningful effect sizes. As such, these results should be interpreted with caution.
Molecular epidemiology and benchwork science have awarded us high-dimensional genetic information that has proved valuable in identifying populations with increased susceptibility to cancers.97 Some of the strongest evidence presented in this review – such as risk estimates that are heavily modified by chromosome translocation status – stems from gene-environment interactions, but these findings have yet to inform changes in public health policy. Models for such changes to policy can be motivated by the world of solid tumor research. Numerous studies have identified considerable interaction between alcohol dehydrogenase polymorphisms and alcohol consumption in gastrointestinal cancer risk among East Asian populations.98–101 While these findings have yet to rise to the level of public health policy change, researchers and clinicians have outlined several modalities of cancer prevention.102 If future research establishes high-penetrance SNPs in association with NHL subtypes, chemically-exposed populations could benefit greatly from added surveillance.103,104 In helping to establish causality, and thus influence environmental policy, consideration should also be placed on proximal drivers, such as the transcriptome and metabolome. Likewise, research efforts to better characterize these gene-environment interactions could focus on how implicated genetic variants function in xenobiotic metabolism, immune regulation, and/or DNA repair in vulnerable populations.6 A framework for the types of interdisciplinary studies needed to define pathways of chemical exposure-related lymphomagenesis is illustrated in Figure 1.
Figure 1.
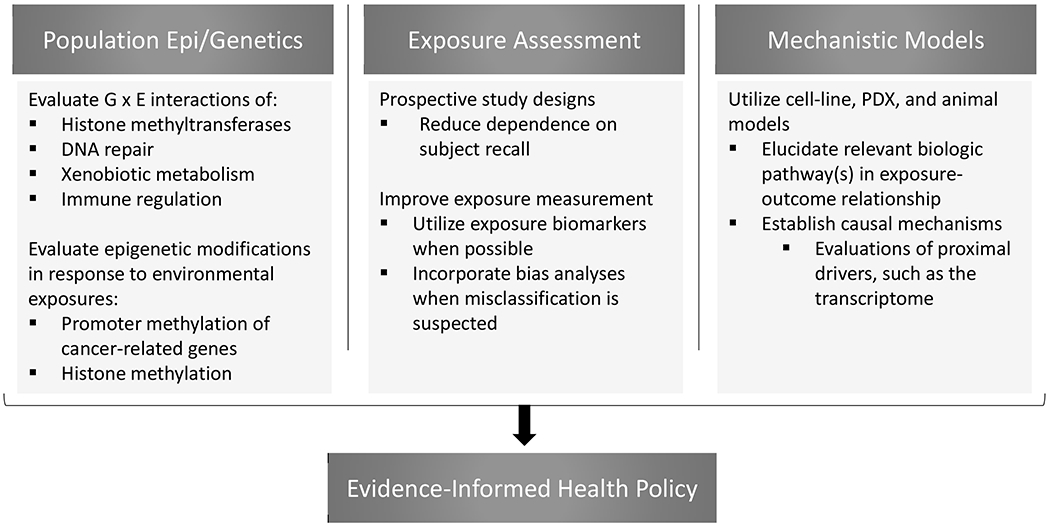
Recommended future approaches to addressing important gaps of knowledge in the association between chemical exposures and NHL subtypes.
As evidenced by the hair dye formulation change in 1980, establishing molecular mechanisms of pathogenesis is an important first step in promoting public health measures that prevent non-communicable disease. An example of public health policies that prevent cancer can be found in Egypt. Efforts aimed at eradication of schistosomiasis, a parasitic disease caused by flatworms that increases bladder cancer risk, has decreased the proportion of cancer patients treated for bladder cancer from 27.6% to 11.7% through targeted education efforts and infection control.105,106 If causal links between chemical exposures and NHL can be established, changes in the stringency of health policies surrounding occupational exposures could have profound effects on NHL incidence. Additionally, educating workers on potential risks associated with chemical exposures may aid in primary and secondary prevention efforts.
Large-scale epidemiologic studies and molecular basic science research have begun to elucidate the role of chemical exposures in the pathogenesis of NHL overall and by subtype. Further work that incorporates environmental epidemiology, population genetics, and lymphoma biology is needed to establish causality of chemical agents and implement appropriate preventative measures.
Acknowledgments
C.R.F. has served as a consultant for: Abbvie, AstraZeneca, Bayer, BeiGene, Celgene (unpaid), Denovo Biopharma, Genentech/Roche (unpaid), Gilead, OptumRx, Karyopharm, MEI Pharmaceuticals, Pharmacyclics/Janssen, Spectrum. C.R.F. has received research funding from: Abbvie, Acerta, BeiGene, Celgene, Gilead, Genentech/Roche, Janssen Pharmaceutical, Millennium/Takeda, Pharmacyclics, TG Therapeutics, Burroughs Wellcome Fund, Eastern Cooperative Oncology Group, National Cancer Institute, and the V Foundation. J. L. Koff received the 2018 AACR Lymphoma Research Fellowship, supported by Pharmacyclics, an AbbVie Company, and Janssen Biotech, Inc., Grant Number 18-40-48-KOFF
Abbreviations:
- NHL
Non-Hodgkin lymphoma
- DLBCL
Diffuse large B-cell lymphoma
- FL
Follicular lymphoma
- CLL/SLL
Chronic lymphocytic leukemia and small lymphocytic lymphoma
- MZL
Marginal zone lymphoma
- BL
Burkitt lymphoma
- MCL
Mantle cell lymphoma
- PTCL
Peripheral T-cell lymphoma
- NKTCL
Nasal NK/T-cell lymphoma
- DDT
Dichlorodiphenyltrichloroethane
- HIV
Human immunodeficiency virus
- OR
Odds ratio
- RR
Risk ratio
- HR
Hazard ratio
- CI
Confidence interval
- SNP
small nucleotide polymorphism
- EBV
Epstein-Barr virus
References
- 1.Global Cancer Observatory: Cancer Today. Lyon, France: International Agency for Research on Cancer; 2018. (Accessed 27 August 2019, at https://gco.iarc.fr/today.) [Google Scholar]
- 2.Morton LM, Sampson JN, Cerhan JR, et al. Rationale and Design of the International Lymphoma Epidemiology Consortium (InterLymph) Non-Hodgkin Lymphoma Subtypes Project. JNCI Monographs 2014;2014:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grulich AE, Vajdic CM, Cozen W. Altered Immunity as a Risk Factor for Non-Hodgkin Lymphoma. Cancer Epidemiology Biomarkers & Prevention 2007;16:405–8. [DOI] [PubMed] [Google Scholar]
- 4.Wang SS, Slager SL, Brennan P, et al. Family history of hematopoietic malignancies and risk of non-Hodgkin lymphoma (NHL): a pooled analysis of 10 211 cases and 11 905 controls from the International Lymphoma Epidemiology Consortium (InterLymph). Blood 2007;109:3479–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bassig BA, Lan Q, Rothman N, Zhang Y, Zheng T. Current Understanding of Lifestyle and Environmental Factors and Risk of Non-Hodgkin Lymphoma: An Epidemiological Update. Journal of Cancer Epidemiology 2012;2012:1–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kelly RS, Vineis P. Biomarkers of susceptibility to chemical carcinogens: the example of non-Hodgkin lymphomas. British Medical Bulletin 2014;111:89–100. [DOI] [PubMed] [Google Scholar]
- 7.Hartge P, Smith MT. Environmental and behavioral factors and the risk of non-Hodgkin lymphoma. Cancer Epidemiol Biomarkers Prev 2007;16:367–8. [DOI] [PubMed] [Google Scholar]
- 8.Shiels MS. Proportions of Kaposi Sarcoma, Selected Non-Hodgkin Lymphomas, and Cervical Cancer in the United States Occurring in Persons With AIDS, 1980-2007. JAMA 2011;305:1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Groves FD, Linet MS, Travis LB, Devesa SS. Cancer Surveillance Series: Non-Hodgkin’s Lymphoma Incidence by Histologic Subtype in the United States From 1978 Through 1995. JNCI Journal of the National Cancer Institute 2000;92:1240–51. [DOI] [PubMed] [Google Scholar]
- 10.Clarke CA, Glaser SL. Changing incidence of non-Hodgkin lymphomas in the United States. Cancer 2002;94:2015–23. [DOI] [PubMed] [Google Scholar]
- 11.Miranda-Filho A, Piñeros M, Znaor A, Marcos-Gragera R, Steliarova-Foucher E, Bray F. Global patterns and trends in the incidence of non-Hodgkin lymphoma. Cancer Causes & Control 2019;30:489–99. [DOI] [PubMed] [Google Scholar]
- 12.Cartwright R, Brincker H, Carli PM, et al. The rise in incidence of lymphomas in Europe 1985–1992. European Journal of Cancer 1999;35:627–33. [DOI] [PubMed] [Google Scholar]
- 13.Xu J, Ye Y, Huang F, et al. Association between dioxin and cancer incidence and mortality: a meta-analysis. Scientific Reports 2016;6:38012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Basinas I, Schlunssen V, Takai H, et al. Exposure to Inhalable Dust and Endotoxin Among Danish Pig Farmers Affected by Work Tasks and Stable Characteristics. 2013;57:1005–19. [DOI] [PubMed] [Google Scholar]
- 15.El-Zaemey S, Schinasi L, Ferro G, et al. Animal farming and the risk of lymphohaematopoietic cancers: a meta-analysis of three cohort studies within the AGRICOH consortium. Occupational and Environmental Medicine 2019:oemed-2018-1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leon ME, Schinasi LH, Lebailly P, et al. Pesticide use and risk of non-Hodgkin lymphoid malignancies in agricultural cohorts from France, Norway and the USA: a pooled analysis from the AGRICOH consortium. International Journal of Epidemiology 2019;48:1519–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guyton KZ, Loomis D, Grosse Y, et al. Carcinogenicity of tetrachlorvinphos, parathion, malathion, diazinon, and glyphosate. The Lancet Oncology 2015;16:490–1. [DOI] [PubMed] [Google Scholar]
- 18.Atwood D, Paisley-Jones Claire. Pesticides Industry Sales and Usage 2008-2012 Market Estimates: U.S. Environmental Protection Agency; 2017. [Google Scholar]
- 19.Williams GM, Aardema M, Acquavella J, et al. A review of the carcinogenic potential of glyphosate by four independent expert panels and comparison to the IARC assessment. Critical Reviews in Toxicology 2016;46:3–20. [DOI] [PubMed] [Google Scholar]
- 20.Zhang L, Rana I, Shaffer RM, Taioli E, Sheppard L. Exposure to glyphosate-based herbicides and risk for non-Hodgkin lymphoma: A meta-analysis and supporting evidence. Mutation Research/Reviews in Mutation Research 2019;781:186–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.De Roos AJ, Blair A, Rusiecki JA, et al. Cancer Incidence among Glyphosate-Exposed Pesticide Applicators in the Agricultural Health Study. Environmental Health Perspectives 2004;113:49–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Andreotti G, Koutros S, Hofmann JN, et al. Glyphosate Use and Cancer Incidence in the Agricultural Health Study. JNCI: Journal of the National Cancer Institute 2018;110:509–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ames BN, Kammen HO, Yamasaki E. Hair dyes are mutagenic: identification of a variety of mutagenic ingredients. Proceedings of the National Academy of Sciences 1975;72:2423–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.De Sanjosé S, Benavente Y, Nieters A, et al. Association between Personal Use of Hair Dyes and Lymphoid Neoplasms in Europe. American Journal of Epidemiology 2006;164:47–55. [DOI] [PubMed] [Google Scholar]
- 25.Morton LM, Bernstein L, Wang SS, et al. Hair dye use, genetic variation in N-acetyltransferase 1 (NAT1) and 2 (NAT2), and risk of non-Hodgkin lymphoma. Carcinogenesis 2007;28:1759–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Teras LR, Desantis CE, Cerhan JR, Morton LM, Jemal A, Flowers CR. 2016 US lymphoid malignancy statistics by World Health Organization subtypes. CA: A Cancer Journal for Clinicians 2016;66:443–59. [DOI] [PubMed] [Google Scholar]
- 27.Koff JL, Chihara D, Phan A, Nastoupil LJ, Williams JN, Flowers CR. To Each Its Own: Linking the Biology and Epidemiology of NHL Subtypes. Curr Hematol Malig Rep 2015;10:244–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cerhan JR, Kricker A, Paltiel O, et al. Medical History, Lifestyle, Family History, and Occupational Risk Factors for Diffuse Large B-Cell Lymphoma: The InterLymph Non-Hodgkin Lymphoma Subtypes Project. JNCI Monographs 2014;2014:15–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morton LM, Slager SL, Cerhan JR, et al. Etiologic Heterogeneity Among Non-Hodgkin Lymphoma Subtypes: The InterLymph Non-Hodgkin Lymphoma Subtypes Project. JNCI Monographs 2014;2014:130–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Melenotte C, Million M, Audoly G, et al. B-cell non-Hodgkin lymphoma linked to Coxiella burnetii. Blood 2016;127:113–21. [DOI] [PubMed] [Google Scholar]
- 31.Eriksson M, Hardell L, Carlberg M, Åkerman M. Pesticide exposure as risk factor for non-Hodgkin lymphoma including histopathological subgroup analysis. International Journal of Cancer 2008;123:1657–63. [DOI] [PubMed] [Google Scholar]
- 32.Zhang Y, Sanjose SD, Bracci PM, et al. Personal Use of Hair Dye and the Risk of Certain Subtypes of Non-Hodgkin Lymphoma. American Journal of Epidemiology 2008;167:1321–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pierluigi Cocco GS, Stefania Dubois, Claudia Pili, Michela Pilleri, Mariagrazia Zucca AMtM, Nikolaus Becker, Yolanda Benavente, Silvia de Sanjosé LF, Anthony Staines, Marc Maynadié, Alexandra Nieters, Paul Brennan LM, Maria Grazia Ennas, Paolo Boffetta. Lymphoma risk and occupational exposure to pesticides- results of the Epilymph study. Occupational and Environmental Medicine 2013;70:91–8. [DOI] [PubMed] [Google Scholar]
- 34.Schinasi L, Leon ME. Non-Hodgkin lymphoma and occupational exposure to agricultural pesticide chemical groups and active ingredients: a systematic review and meta-analysis. Int J Environ Res Public Health 2014;11:4449–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bertrand KA, Spiegelman D, Aster JC, et al. Plasma Organochlorine Levels and Risk of Non-Hodgkin Lymphoma in a Cohort of Men. Epidemiology 2010;21:172–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cocco P, Vermeulen R, Flore V, et al. Occupational exposure to trichloroethylene and risk of non-Hodgkin lymphoma and its major subtypes: a pooled IinterLlymph analysis. Occup Environ Med 2013;70:795–802. [DOI] [PubMed] [Google Scholar]
- 37.Deng Q, Zheng T, Lan Q, et al. Occupational solvent exposure, genetic variation in immune genes, and the risk for non-Hodgkin lymphoma. European Journal of Cancer Prevention 2013;22:77–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jiao J, Zheng T, Lan Q, et al. Occupational solvent exposure, genetic variation of DNA repair genes, and the risk of non-Hodgkin’s lymphoma. Eur J Cancer Prev 2012;21:580–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.De Roos AJ, Davis S, Colt JS, et al. Residential proximity to industrial facilities and risk of non-Hodgkin lymphoma. Environmental Research 2010;110:70–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bulka C, Nastoupil LJ, Koff JL, et al. Relations Between Residential Proximity to EPA-Designated Toxic Release Sites and Diffuse Large B-Cell Lymphoma Incidence. South Med J 2016;109:606–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Switchenko JM, Bulka C, Ward K, et al. Resolving uncertainty in the spatial relationships between passive benzene exposure and risk of non-Hodgkin lymphoma. Cancer Epidemiol 2016;41:139–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reddy A, Zhang J, Davis NS, et al. Genetic and Functional Drivers of Diffuse Large B Cell Lymphoma. Cell 2017;171:481–94.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lunning MA, Green MR. Mutation of chromatin modifiers; an emerging hallmark of germinal center B-cell lymphomas. Blood Cancer Journal 2015;5:e361–e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pasqualucci L The genetic basis of diffuse large B-cell lymphoma. Current Opinion in Hematology 2013;20:336–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dobashi A Molecular Pathogenesis of Diffuse Large B-Cell Lymphoma. J Clin Exp Hematop 2016;56:71–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dawson MA, Kouzarides T. Cancer epigenetics: from mechanism to therapy. Cell 2012;150:12–27. [DOI] [PubMed] [Google Scholar]
- 47.Jiang Y, Dominguez PM, Melnick AM. The many layers of epigenetic dysfunction in B-cell lymphomas. Current Opinion in Hematology 2016;23:377–84. [DOI] [PubMed] [Google Scholar]
- 48.Jiang Y, Hatzi K, Shaknovich R. Mechanisms of epigenetic deregulation in lymphoid neoplasms. Blood 2013;121:4271–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cerchietti L, Leonard JP. Targeting the epigenome and other new strategies in diffuse large B-cell lymphoma: beyond R-CHOP. Hematology 2013;2013:591–5. [DOI] [PubMed] [Google Scholar]
- 50.Pasqualucci L, Trifonov V, Fabbri G, et al. Analysis of the coding genome of diffuse large B-cell lymphoma. Nature Genetics 2011;43:830–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sha Y, Zhou W, Yang Z, et al. Changes in Poly(ADP-Ribosyl)ation Patterns in Workers Exposed to BTX. PLoS ONE 2014;9:e106146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yu K, Shi Y-F, Yang K-Y, et al. Decreased topoisomerase IIα expression and altered histone and regulatory factors of topoisomerase IIα promoter in patients with chronic benzene poisoning. Toxicology Letters 2011;203:111–7. [DOI] [PubMed] [Google Scholar]
- 53.Morin RD, Mendez-Lago M, Mungall AJ, et al. Frequent mutation of histone-modifying genes in non-Hodgkin lymphoma. Nature 2011;476:298–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang J, Dominguez-Sola D, Hussein S, et al. Disruption of KMT2D perturbs germinal center B cell development and promotes lymphomagenesis. Nature Medicine 2015;21:1190–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jones PA, Baylin SB. The fundamental role of epigenetic events in cancer. Nature Reviews Genetics 2002;3:415–28. [DOI] [PubMed] [Google Scholar]
- 56.Herman JG, Baylin SB. Gene Silencing in Cancer in Association with Promoter Hypermethylation. N Engl J Med 2003;349:2042–54. [DOI] [PubMed] [Google Scholar]
- 57.Ratnaparkhe M, Wong JKL, Wei P-C, et al. Defective DNA damage repair leads to frequent catastrophic genomic events in murine and human tumors. Nature Communications 2018;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Michmerhuizen AR, Pesch AM, Moubadder L, et al. PARP1 Inhibition Radiosensitizes Models of Inflammatory Breast Cancer to Ionizing Radiation. Molecular Cancer Therapeutics 2019:molcanther.0520.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ren J, Cui J-P, Luo M, et al. The prevalence and persistence of aberrant promoter DNA methylation in benzene-exposed Chinese workers. PLOS ONE 2019;14:e0220500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Roman E, Smith AG. Epidemiology of lymphomas. Histopathology 2011;58:4–14. [DOI] [PubMed] [Google Scholar]
- 61.Linet MS, Vajdic CM, Morton LM, et al. Medical History, Lifestyle, Family History, and Occupational Risk Factors for Follicular Lymphoma: The InterLymph Non-Hodgkin Lymphoma Subtypes Project. JNCI Monographs 2014;2014:26–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fritschi L, Benke G, Hughes AM, et al. Occupational Exposure to Pesticides and Risk of Non-Hodgkin’s Lymphoma. American Journal of Epidemiology 2005;162:849–57. [DOI] [PubMed] [Google Scholar]
- 63.Cocco P, T’Mannetje A, Fadda D, et al. Occupational exposure to solvents and risk of lymphoma subtypes: results from the Epilymph case-control study. Occupational and Environmental Medicine 2010;67:341–7. [DOI] [PubMed] [Google Scholar]
- 64.Guo H, Bassig BA, Lan Q, et al. Polymorphisms in DNA repair genes, hair dye use, and the risk of non-Hodgkin lymphoma. Cancer Causes & Control 2014;25:1261–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kridel R, Sehn LH, Gascoyne RD. Pathogenesis of follicular lymphoma. Journal of Clinical Investigation 2012;122:3424–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang L, Rothman N, Li G, et al. Aberrations in chromosomes associated with lymphoma and therapy-related leukemia in benzene-exposed workers. Environmental and Molecular Mutagenesis 2007;48:467–74. [DOI] [PubMed] [Google Scholar]
- 67.Roulland S, Lebailly P, Lecluse Y, Briand M, Pottier D, Gauduchon P. Characterization of the t(14;18)BCL2-IGHTranslocation in Farmers Occupationally Exposed to Pesticides. Cancer Research 2004;64:2264–9. [DOI] [PubMed] [Google Scholar]
- 68.Schroeder Jane C., Baric Ralph, Dent Georgette A., Weinberg Clarice R., Yount Boyd, Cerhan James R., Lynch Charles F., Schuman Leonarcl M., Tolbert Paige E., Rothman Nathaniel, Cantor Kenneth P,, Blair A Agricultural risk factors for t(14;18) subtypes of non-Hodgkin’s lymphoma. Epidemiology 2001;12:701–9. [DOI] [PubMed] [Google Scholar]
- 69.Chiu BCH. Agricultural pesticide use and risk of t(14;18)-defined subtypes of non-Hodgkin lymphoma. Blood 2006;108:1363–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.McHale CM, Lan Q, Corso C, et al. Chromosome Translocations in Workers Exposed to Benzene. JNCI Monographs 2008;2008:74–7. [DOI] [PubMed] [Google Scholar]
- 71.Green MR, Gentles AJ, Nair RV, et al. Hierarchy in somatic mutations arising during genomic evolution and progression of follicular lymphoma. Blood 2013;121:1604–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Love C, Sun Z, Jima D, et al. The genetic landscape of mutations in Burkitt lymphoma. Nature Genetics 2012;44:1321–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mbulaiteye SM, Morton LM, Sampson JN, et al. Medical History, Lifestyle, Family History, and Occupational Risk Factors for Sporadic Burkitt Lymphoma/Leukemia: The Interlymph Non-Hodgkin Lymphoma Subtypes Project. JNCI Monographs 2014;2014:106–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Buckley JD, Meadows AT, Kadin ME, Le Beau MM, Siegel S, Robison LL. Pesticide exposures in children with non-Hodgkin lymphoma. Cancer 2000;89:2315–21. [DOI] [PubMed] [Google Scholar]
- 75.Wu SJ, Huang SY, Lin CT, Lin YJ, Chang CJ, Tien HF. The incidence of chronic lymphocytic leukemia in Taiwan, 1986-2005: a distinct increasing trend with birth-cohort effect. Blood 2010;116:4430–5. [DOI] [PubMed] [Google Scholar]
- 76.Clarke CA, Glaser SL, Gomez SL, et al. Lymphoid Malignancies in U.S. Asians: Incidence Rate Differences by Birthplace and Acculturation. Cancer Epidemiology Biomarkers & Prevention 2011;20:1064–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yang S, Gale RP, Shi H, et al. Is there an epidemic of chronic lymphocytic leukaemia (CLL) in China? Leuk Res 2018;73:16–20. [DOI] [PubMed] [Google Scholar]
- 78.Slager SL, Benavente Y, Blair A, et al. Medical History, Lifestyle, Family History, and Occupational Risk Factors for Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma: The InterLymph Non-Hodgkin Lymphoma Subtypes Project. JNCI Monographs 2014;2014:41–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Morton LM, Wang SS, Cozen W, et al. Etiologic heterogeneity among non-Hodgkin lymphoma subtypes. Blood 2008;112:5150–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Alavanja MCR, Hofmann JN, Lynch CF, et al. Non-Hodgkin Lymphoma Risk and Insecticide, Fungicide and Fumigant Use in the Agricultural Health Study. PLoS ONE 2014;9:e109332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Calaf G, Echiburu-Chau C, Roy D. Organophosphorous pesticides and estrogen induce transformation of breast cells affecting p53 and c-Ha-ras genes. Int J Oncol 2009;35. [DOI] [PubMed] [Google Scholar]
- 82.Smith A, Howell D, Patmore R, Jack A, Roman E. Incidence of haematological malignancy by sub-type: a report from the Haematological Malignancy Research Network. British Journal of Cancer 2011;105:1684–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bracci PM, Benavente Y, Turner JJ, et al. Medical History, Lifestyle, Family History, and Occupational Risk Factors for Marginal Zone Lymphoma: The InterLymph Non-Hodgkin Lymphoma Subtypes Project. JNCI Monographs 2014;2014:52–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Suarez F Infection-associated lymphomas derived from marginal zone B cells: a model of antigen-driven lymphoproliferation. Blood 2006;107:3034–44. [DOI] [PubMed] [Google Scholar]
- 85.Ekstrom Smedby K, Vajdic CM, Falster M, et al. Autoimmune disorders and risk of non-Hodgkin lymphoma subtypes: a pooled analysis within the InterLymph Consortium. Blood 2008;111:4029–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mester B Occupation and malignant lymphoma: a population based case control study in Germany. Occupational and Environmental Medicine 2006;63:17–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jares P, Colomer D, Campo E. Molecular pathogenesis of mantle cell lymphoma. Journal of Clinical Investigation 2012;122:3416–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Smedby KE, Sampson JN, Turner JJ, et al. Medical History, Lifestyle, Family History, and Occupational Risk Factors for Mantle Cell Lymphoma: The InterLymph Non-Hodgkin Lymphoma Subtypes Project. JNCI Monographs 2014;2014:76–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Van Arnam JS, Lim MS, Elenitoba-Johnson KSJ. Novel insights into the pathogenesis of T-cell lymphomas. Blood 2018;131:2320–30. [DOI] [PubMed] [Google Scholar]
- 90.Alexander DD, Mink PJ, Adami H-O, et al. The non-Hodgkin lymphomas: A review of the epidemiologic literature. International Journal of Cancer 2007;120:1–39. [DOI] [PubMed] [Google Scholar]
- 91.Wang SS, Flowers CR, Kadin ME, et al. Medical History, Lifestyle, Family History, and Occupational Risk Factors for Peripheral T-Cell Lymphomas: The InterLymph Non-Hodgkin Lymphoma Subtypes Project. JNCI Monographs 2014;2014:66–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yamaguchi M, Suzuki R, Oguchi M. Advances in the treatment of extranodal NK/T-cell lymphoma, nasal type. Blood 2018;131:2528–40. [DOI] [PubMed] [Google Scholar]
- 93.Xu J-X, Hoshida Y, Yang W-I, et al. Life-style and environmental factors in the development of nasal NK/T-cell lymphoma: A case–control study in East Asia. International Journal of Cancer 2007;120:406–10. [DOI] [PubMed] [Google Scholar]
- 94.Aozasa K, Takakuwa T, Hongyo T, Yang W-I. Nasal NK/T-cell lymphoma: epidemiology and pathogenesis. International Journal of Hematology 2008;87:110–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lash Timothy L., Fink AK Bias Analysis of Exposure Misclassification Applying Quantitative Bias Analysis to Epidemiologic Data: Springer; 2009:85–94. [Google Scholar]
- 96.Mlinarić A, Horvat M, Šupak Smolčić V. Dealing with the positive publication bias: Why you should really publish your negative results. Biochemia Medica 2017;27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wang SS, Nieters A. Unraveling the interactions between environmental factors and genetic polymorphisms in non-Hodgkin lymphoma risk. Expert Review of Anticancer Therapy 2010;10:403–13. [DOI] [PubMed] [Google Scholar]
- 98.Yokoyama A. Alcohol and aldehyde dehydrogenase gene polymorphisms and oropharyngolaryngeal, esophageal and stomach cancers in Japanese alcoholics. Carcinogenesis 2001;22:433–9. [DOI] [PubMed] [Google Scholar]
- 99.Matsuo K, Hamajima N, Shinoda M, et al. Gene–environment interaction between an aldehyde dehydrogenase-2 (ALDH2) polymorphism and alcohol consumption for the risk of esophageal cancer. Carcinogenesis 2001;22:913–6. [DOI] [PubMed] [Google Scholar]
- 100.Yokoyama A Genetic polymorphisms of alcohol and aldehyde dehydrogenases and glutathione S-transferase M1 and drinking, smoking, and diet in Japanese men with esophageal squamous cell carcinoma. Carcinogenesis 2002;23:1851–9. [DOI] [PubMed] [Google Scholar]
- 101.Wu C, Kraft P, Zhai K, et al. Genome-wide association analyses of esophageal squamous cell carcinoma in Chinese identify multiple susceptibility loci and gene-environment interactions. Nature Genetics 2012;44:1090. [DOI] [PubMed] [Google Scholar]
- 102.Chang JS, Hsiao J-R, Chen C-H. ALDH2 polymorphism and alcohol-related cancers in Asians: a public health perspective. Journal of Biomedical Science 2017;24:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Do CB, Hinds DA, Francke U, Eriksson N. Comparison of Family History and SNPs for Predicting Risk of Complex Disease. PLoS Genetics 2012;8:e1002973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Jostins L, Barrett JC. Genetic risk prediction in complex disease. 2011;20:R182–R8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gouda IMN, Bilal D, El-Bolkainy T, El-Bolkainy MN. Bilharziasis and Bladder Cancer- A Time Trend Analysis of 9843 Patients. Journal of the Egyptian Nat Cancer 2007;19:158–62. [PubMed] [Google Scholar]
- 106.Salem S, Mitchell RE, El-Alim El-Dorey A, Smith JA, Barocas DA. Successful control of schistosomiasis and the changing epidemiology of bladder cancer in Egypt. BJU International 2011;107:206–11. [DOI] [PubMed] [Google Scholar]


