Abstract
Myopia is induced when a growing eye wears a diffuser that deprives it of detailed spatial vision (form deprivation, FD). In chickens with optic nerve section (ONS), FD myopia still occurs, suggesting that the signals underlying myopia reside within the eye. As avian eyes differ from mammals, we asked whether local mechanisms also underlie FD myopia in a mammalian model. Young guinea pigs underwent either sham surgery followed by FD (SHAM+FD, n=7); or ONS followed by FD (ONS+FD, n=7); or ONS without FD (ONS, n=9). FD was initiated 3 days after surgery with a diffuser that was worn on the surgically treated eye for 14 days. Animals with ONS+FD developed −8.9 D of relative myopia and elongated by 135 μm more than in their untreated eyes after 2 weeks of FD. These changes were significantly greater than those in SHAM+FD animals (−5.5 D and 40 μm of elongation after 14 days of FD), and reflected exaggerated elongation of the posterior chamber. The myopia reversed when FD was discontinued, despite ONS, but eyes did not recover back to normal (30 days after surgery, ONS+FD eyes still retained −3 D of relative myopia when SHAM+FD animals had returned to normal). No long-term residual myopia was present after ONS alone, ruling out a surgical artefact. Although the gross mechanism signaling myopic ocular growth and its recovery in the young mammalian eye does not require an intact optic nerve, its fine-tuning is disrupted by ONS.
Keywords: Myopia, Optic Nerve, Form Deprivation
Graphical Abstract
Myopic eyes grow too long so images are short-focused. What controls this excessive growth? We know that across species, deprivation of detailed vision (form deprivation, FD) increases the rate of eye elongation and induces myopia. When the form deprivation ceases, these myopic eyes then slow their growth and recover from their myopia. We show here that in young mammalian eyes, this bidirectional response still occurs after the optic nerve is cut, implicating local retinal control of eye growth. However, the eye grows excessively after optic nerve section (ONS) showing that the fine tuning of eye growth requires an intact optic nerve.
1 |. INTRODUCTION
High myopia remains one of the leading causes of blindness in the world today, the result of steep increases in its prevalence over the last few decades to reach epidemic levels in East Asia (Holden et al., 2016). These rapid changes point to visual environmental influences on refractive error development. The notion that eye growth regulation is guided by visual input is also supported by strong evidence from a variety of animal studies, including early studies involving young chicks (Wallman, Gottlieb, Rajaram, & Fugate-Wentzek, 1987). Specifically, visual form deprivation (FD), achieved either by closing the lids (lid suture) or covering the eye with opaque diffusers leads to increased eye elongation and axial myopia (Troilo et al., 2019; Wallman & Winawer, 2004). The analogous conditions in young babies are ptosis and cataract, which are also known to similarly derail ocular growth (Gusek-Schneider & Martus, 2001; Huo et al., 2012; Pan, Cheng, Saw, Wang, & Wong, 2013). These growth patterns contrast with those under normal visual conditions in which young eyes typically lose their neonatal refractive errors through a coordinated active growth process known as emmetropization.
Elucidation of the mechanisms underlying these visual effects on eye growth is critical to the development of much needed anti-myopia treatments designed to prevent or slow the excessive eye growth underlying most myopias. One important question that has been the focus of a number of studies, mostly involving the chick, is the role of the retina versus the brain in eye growth regulation. To address this question, both surgical and chemical lesion techniques have been employed to disconnect the retina from the brain, and/or to disrupt other neural pathways linking the eye and to brain (Wildsoet, 2003).
Surgical lesioning of the optic nerve (optic nerve section, ONS) was used in the two earliest studies seeking to isolate the role of the retina in eye growth regulation (Troilo, Gottlieb, & Wallman, 1987; Wildsoet & Pettigrew, 1988). Both studies involved young chicks, one involving partial (hemisector) deprivation and the other lid suture; in both cases, ONS did not prevent FD myopia. These results are also consistent with those of later follow-up FD studies, which mostly used full field diffusers (Choh, Lew, Nadel, & Wildsoet, 2006; Troilo & Wallman, 1991; Wildsoet & Wallman, 1995).
The only primate lesioning study addressing the role of the brain in eye growth regulation is a seminal study involving chiasmal sections (Raviola & Wiesel, 1985). This choice of lesion site was necessary to avoid disruption of the retinal vasculature, which enters the globe via the optic nerve in primates. In contrast, the blood supply to the pecten, which represents the source of retinal nutrients in the bird eye, enters independently of the optic nerve (De Schaepdrijver, Simoens, Lauwers, & De Geest, 1989). Interestingly, this early primate study reported significant differences between species. Lid suture myopia still developed in two rhesus macques in which the occipital lobe had been removed, and in three other rhesus macaques after lesioning of the optic chiasm (Raviola & Wiesel, 1985). However, both eyes of the one stumptail macque subjected to intracranial section of the optic chiasm, became more hyperopic, instead of myopic after unilateral lid fusion (Raviola & Wiesel, 1985). The reason for this species difference is unclear, but may reflect divergence in retinal or accommodation mechanisms, since these two species also differ in their response to atropine (Raviola & Wiesel, 1985).
While the chick has been a widely used animal model in myopia research, especially in studies of eye growth regulation and the effects of ONS on eye growth in particular, important structural differences between avian versus mammalian and primate eyes, the high cost and ethical concerns over the use of primates in such research, and the limited access to tree shrews have motivated the adoption of rodents as accessible mammalian models, with the most popular being guinea pigs and mice. In relation to the question of local retinal regulation of eye growth, there is only one relevant study involving optic nerve crush in C57BL/6 mice. Surprisingly, the sham surgery alone affected corneal power, this presumed surgical artifact possibly reflecting the difficulty in operating on such small eyes (axial length < 2.8 mm) (Gong et al., 2020).
The study reported here exploits the guinea pig, as a representative mammalian model with pertinent ocular characteristics similar to human eyes, and of a relatively large eye size, with an axial length of approximately 8.5 mm. As a model for further exploring the question of local retinal control, the guinea pig has the additional advantage of having limited retinal vasculature (De Schaepdrijver et al., 1989), allowing the optic nerve to be sectioned without compromising blood flow to the retina. We specifically explored the effects of ONS on FD myopia and recovery from these FD-induced changes in young guinea pigs. In the guinea pig, as in chicks, ONS did not prevent FD myopia, although we found significant differences between the responses to FD of eyes undergoing ONS versus sham (no ONS) surgeries, which could not be easily ascribed to the ONS surgery per se. Preliminary versions of some of this data have been previously reported in abstract form (Wildsoet & McFadden, 2010).
2 |. MATERIALS AND METHODS
2.1 |. Animals and housing
Pigmented guinea pigs (Cavia porcellus, sourced from the University of Newcastle) were housed with their mothers and littermates as previously described (McFadden, Howlett, & Mertz, 2004) in opaque plastic boxes (65 × 45 cm and 20 cm high) with open wire lids. Diurnal lighting (12h on/ 12h off cycle) was provided by white light emitting diodes affixed above each box, with an intervening opaque perspex barrier serving to diffuse the light; this combination achieved a luminance of ~400 lux at center of each box. The study was approved by the Animal Care and Ethics Committee at the University of Newcastle and conformed to NSW legislative requirements and the National Health and Medical Research Council, Australian Code for the Care and Use of Animals for Scientific Purposes.
2.2 |. Experimental design
Twenty-four guinea pigs were randomly assigned to one of three treatments, all of which were monocular: Optic nerve section alone (ONS), ONS followed by FD (ONS+FD), or sham surgery followed by FD (SHAM+FD). In all cases, the fellow eyes were left untreated as controls. Within litters, animals were approximately equally distributed across the two FD groups. Animals underwent ONS or sham surgery at 5 days of age, and in the case of the two FD groups, diffusers were fitted to the same eye 3 days later and the FD treatment maintained for 14 days, after which diffusers were removed and animals monitored for a further 33 days. Refractive error and additional ocular biometric data were collected from both eyes of each animal, both before and repeatedly after the surgery for the ONS only group and immediately before, during and after termination of the FD treatment in the case of the other two groups. Group treatment details, as well as the timing of measurements are summarized in Table 1.
TABLE 1.
Experimental design and duration of treatments. All animals underwent monocular surgery, either optic nerve section (ONS) or sham surgery (SHAM), at 5 days of age, and two groups underwent form deprivation (FD) of the same eye.
| Group | Optical Treatment | Period of Treatment (days) |
Age of Measurement (days) | Measurement Time relative to Surgery (days) |
|---|---|---|---|---|
| ONS+FD (N=8) |
FD | 0 | 8 | 3 |
| 7 | 15 | 10 | ||
| 14 | 22 | 17 | ||
| Recovery from FD | 18 | 40 | 35 | |
| 33 | 55 | 50 | ||
| SHAM+FD (N=7) |
FD | 0 | 8 | 3 |
| 7 | 15 | 10 | ||
| 14 | 22 | 17 | ||
| Recovery from FD | 18 | 40 | 35 | |
| 33 | 55 | 50 | ||
| ONS only (N=9) | NIL | 3 | −2 | |
| 8 | 3 | |||
| 15 | 10 | |||
| 22 | 17 | |||
| 38 | 33 | |||
| 64 | 59 |
2.3 |. Optic nerve section (ONS) and sham surgeries
Surgeries were undertaken under aseptic conditions with the aid of a Zeiss operating microscope. Guinea pigs were anaesthetized with a mixture of ketamine and xylazine (50 mg/kg; 5 mg/kg respectively), given as an IP injection, and subsequently received an intraorbital injection of 0.1 ml lidocaine hydrochloride (Xylocaine® 2%) as a retrobulbar block. The optic nerve was accessed by blunt dissection through a small window in the temporal conjunctiva, after gently rotating the globe with the aid of a bulbar conjunctival anchoring suture. In the case of optic nerve section, a small incision was then made in the dural sheath of the optic nerve to access the nerve fibers, which were teased free and cut. The same procedure was followed for animals undergoing sham surgery, except that the optic nerve was left intact after visualization. Non-preserved artificial tears (TheraTears, Seton US) were applied to the cornea during the surgery to prevent drying. At the end of the surgery, broad-spectrum antibiotic drops (Fusidic acid, 1%, 10mg/g, Conoptal®, Bayer) were applied to the surgical site, the anchoring suture removed, and the bulbar conjunctiva repositioned over the orbital entry site. Ophthalmic antibiotic gel (Conoptal®, Bayer) was generously applied to the cornea during recovery from anesthesia.
ONS is known to disrupt the direct pupil response to light. Therefore, in all animals both the direct and consensual pupil responses were assessed daily for 3 days after surgery and at less regular intervals thereafter up to 17 days of age. Pupil responses were qualitatively assessed in awake, handheld animals using a bright white LED as the stimulus and an infrared (IR) video system to record responses. The pupils of ONS eyes prior to light stimulation were relatively larger than those of their fellows, and in all cases direct pupil responses were no longer detected confirming the success of the surgery (Fig. 1); as expected consensual responses appeared normal. The sham surgery had no effect on pupil responses. To rule out adverse retinal effects of the surgery, all eyes were also examined using direct ophthalmoscopy, daily for 3 days post-surgery and after FD for Groups 1 and 2 and more regularly in animals not form deprived. In all cases, eyes retained their normal bright pink/salmon fundus color and no other abnormalities were observed.
FIGURE 1.
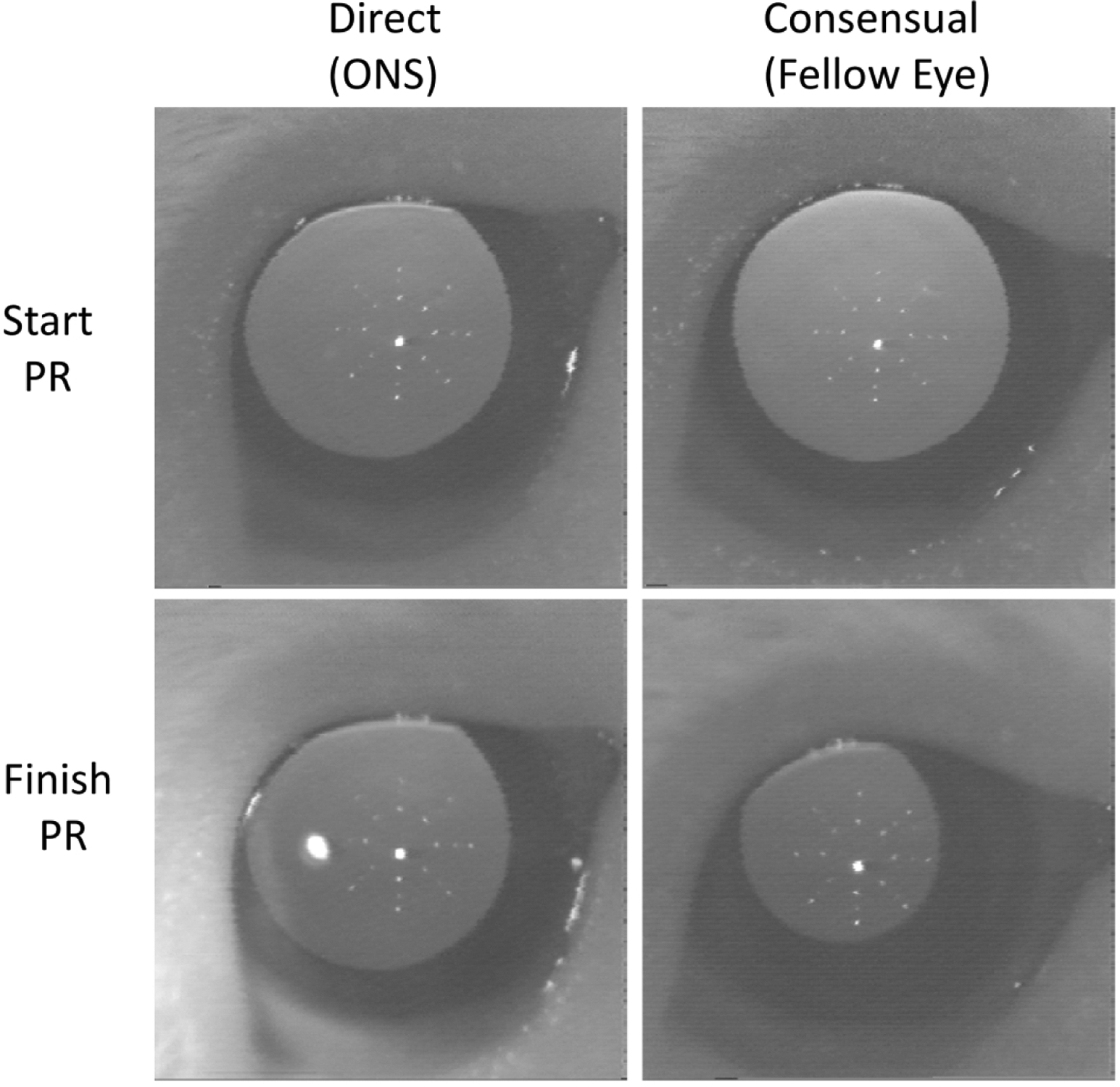
Example of pupil responses (PR) recorded after monocular ONS surgery using an IR camera. The pupil was illuminated with IR light, and the PR was stimulated with a white LED. The start PR was recorded just prior to the white light onset; the finish PR represents the maximum PR reached during 5 sec of continuous white light stimulation. The white LED was used to stimulate only the ONS eye (direct response), with the contralateral (fellow) eye (consensual response) simultaneously recorded. The direct response was abolished (bottom left), but not the consensual PR (bottom right).
2.4 |. Ocular measurements
To evaluate the changes induced by the surgeries and FD treatments, refractive errors, corneal powers, and axial ocular dimensions were recorded at regular intervals, starting 3 days after the surgery, to allow time for the conjunctival surgical wound to heal. The measurement schedule is summarized in Table 1.
Corneal curvature:
The corneal radius of curvature was measured as previously described (Howlett & McFadden, 2006). In brief, measurements were made in both eyes of handheld awake, non-cyclopleged animals using a custom-designed videokeratometer. The radius of curvature of the anterior cornea (r) was derived from the average of 12 points distributed within the pupil area and corneal power (F) then derived using an assumed corneal refractive index of 1.376.
Refractive error:
Following corneal curvature measurements, one drop of 1% cyclopentolate was instilled in each eye of each animal. Refractive errors were measured by streak retinoscopy 1 to 1.5 h later, after cycloplegia had been achieved. Results for both horizontal and vertical meridians were averaged to estimate the spherical equivalent refractive error (Howlett & McFadden, 2006).
Axial ocular dimensions:
Animals were then anesthetized (1.5% isoflurane in oxygen) for measurement of on-axis ocular dimensions (Howlett & McFadden, 2006; McFadden et al., 2004). A high frequency A-scan (20 MHz) ultrasonography system, as previously described, was used to capture axial dimensional data from both eyes. The dimensions of the main ocular compartments - anterior and posterior vitreous chamber depths, and crystalline lens thickness, as well as the thicknesses of the three layers making up the back wall of the eye - retina choroid, and sclera, were derived from captured traces. “Anterior chamber depth” was defined as the axial distance from the anterior surface of the cornea to the anterior surface of the crystalline lens. “Vitreous chamber depth” was defined as the axial distance between the posterior surface of the lens and the anterior surface of the retina. “Ocular length” was defined as the total distance from the anterior corneal surface to the posterior scleral surface.
2.5 |. Data analysis
The data are presented as mean ± the standard error of the mean (SE). Interocular difference data represent values for treated eyes minus those of fellow (untreated) eyes. Such differences in refractive error are also referred to as “relative” myopia; likewise references to “relative” ocular distances refer to the associated interocular difference. The statistical significance of differences between treated eyes and their fellows were evaluated using the matched pairs t-tests. Differences between groups were evaluated using 2-way ANOVAs followed by the Holm-Sidak multiple comparison tests applied to interocular differences at each time point. R values are based on Pearson product moment correlation. Statistical analyses made use of IBM SPSS V 25 and SigmaPlot V 14.
3 |. RESULTS
3.1 |. Summary
Optic nerve section (ONS), did not prevent either the induction of, or recovery from, form deprivation (FD) myopia. However, the responses of ONS eyes to FD were exaggerated compared to those of eyes undergoing sham surgery and they did not fully recover from the induced myopia. Eyes undergoing ONS alone exhibited transient, low myopic shifts in refractive errors, although they were minimally affected overall. The effects of the various treatments are described in more detail in the following sections.
3.2 |. FD myopia in eyes with optic nerve section (ONS) compared to sham surgery
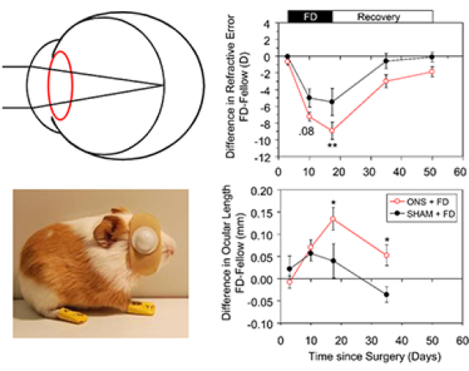
Eyes became myopic and elongated in response to FD imposed with diffusers, despite sectioning of their optic nerves. In animals in which the optic nerve was cut, significant myopia developed after only 7 days of FD. The mean interocular difference in refractive error after 7 days was −7.2 D (p < 0.001, Table 2, Fig. 2a, right panel) and ONS+FD eyes had already significantly elongated (interocular difference in ocular length of +71 μm, p = 0.005, Table 2, Fig. 2b, right panel). The interocular difference in refractive error further increased to −8.9 D after 2 weeks of FD, with ONS+FD eyes exhibiting −6.9 D of myopia (p = 0.007, Table 2, Fig. 2a, left panel). This myopia reflected increased elongation of FD eyes relative to their fellow eyes (interocular difference of 135 μm, p = 0.002, Fig. 2b, left panel).
TABLE 2.
Summary of mean data (+/− Standard Error (SE)) for treated and fellow eyes in each of the three treatment groups for the principal ocular parameters recorded at each measurement time point. Both interocular differences and the significance of these differences are shown. P values are from matched pairs t-tests.
| Group | Measure Point | Age (days after surgery) | Eye | Refractive Error (D) | Anterior Chamber Depth (mm) | Lens Thickness (mm) | Vitreous Chamber Depth (mm) | Ocular Length (mm) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SE | Mean | SE | Mean | SE | Mean | SE | Mean | SE | ||||
| ONS + FD | FD0 | 8 (+3) |
Fellow | 4.0 | 0.6 | 1.113 | 0.024 | 1.228 | 0.027 | 3.073 | 0.037 | 7.964 | 0.051 |
| ONS+FD | 3.4 | 0.7 | 1.079 | 0.024 | 1.191 | 0.026 | 3.074 | 0.042 | 7.956 | 0.043 | |||
| Difference | −0.6 | 0.4 | −0.033 | 0.021 | −0.037 | 0.023 | 0.001 | 0.020 | −0.008 | 0.029 | |||
| P | <0.01 | <0.05 | <0.05 | ns | ns | ||||||||
| FD7 | 15 (+10) |
Fellow | 3.9 | 0.2 | 1.187 | 0.028 | 3.575 | 0.016 | 3.124 | 0.034 | 8.248 | 0.046 | |
| ONS+FD | −3.3 | 1.1 | 1.165 | 0.027 | 3.588 | 0.024 | 3.235 | 0.033 | 8.318 | 0.046 | |||
| Difference | −7.2 | 1.1 | -0.022 | 0.014 | 0.012 | 0.021 | 0.111 | 0.025 | 0.071 | 0.016 | |||
| P | <0.001 | ns | ns | <0.01 | <0.01 | ||||||||
| FD14 | 22 (+17) |
Fellow | 2.0 | 0.3 | 1.212 | 0.027 | 3.676 | 0.024 | 3.195 | 0.037 | 8.443 | 0.048 | |
| ONS+FD | −6.9 | 1.5 | 1.199 | 0.020 | 3.719 | 0.021 | 3.325 | 0.041 | 8.578 | 0.067 | |||
| Difference | −8.9 | 1.6 | -0.013 | 0.016 | 0.043 | 0.019 | 0.130 | 0.019 | 0.135 | 0.025 | |||
| P | <0.01 | ns | ns | <0.001 | <0.01 | ||||||||
| Recovery | 40 (+35) |
Fellow | 2.4 | 0.2 | 1.269 | 0.028 | 3.887 | 0.013 | 3.208 | 0.035 | 8.754 | 0.058 | |
| ONS+FD | −0.6 | 0.8 | 1.239 | 0.022 | 3.909 | 0.020 | 3.295 | 0.055 | 8.806 | 0.062 | |||
|
Difference P |
−3.0 <.05 |
0.9 |
−0.030 ns |
0.019 |
0.022 ns |
0.017 |
0.087 <0.05 |
0.026 |
0.052 ns |
0.023 | |||
| SHAM + FD | FD0 | 8 (+3) |
Fellow | 3.7 | 0.6 | 1.111 | 0.024 | 1.226 | 0.027 | 3.025 | 0.037 | 7.935 | 0.051 |
| Sham+FD | 3.3 | 0.7 | 1.054 | 0.024 | 1.163 | 0.026 | 3.048 | 0.042 | 7.923 | 0.043 | |||
| Difference | 0.0 | 0.4 | −0.026 | 0.021 | −0.029 | 0.023 | 0.042 | 0.020 | 0.022 | 0.029 | |||
| P | ns | ns | ns | ns | ns | ||||||||
| FD7 | 15 (+10) |
Fellow | 3.2 | 0.2 | 1.163 | 0.014 | 3.596 | 0.021 | 3.100 | 0.035 | 8.218 | 0.042 | |
| Sham+FD | −1.8 | 0.5 | 1.159 | 0.015 | 3.609 | 0.016 | 3.168 | 0.030 | 8.286 | 0.040 | |||
| Difference | −5.0 | 0.6 | 0.005 | 0.011 | 0.013 | 0.018 | 0.038 | 0.023 | 0.057 | 0.017 | |||
| P | <0.001 | ns | ns | ns | <0.05 | ||||||||
| FD14 | 22 (+17) |
Fellow | 2.8 | 0.5 | 1.232 | 0.025 | 3.674 | 0.018 | 3.166 | 0.035 | 8.432 | 0.037 | |
| Sham+FD | −4.3 | 1.8 | 1.225 | 0.021 | 3.701 | 0.016 | 3.208 | 0.040 | 8.501 | 0.055 | |||
| Difference | −5.5 |
1.0 |
0.004 |
0.015 |
0.026 |
0.025 | −0.009 |
0.020 | 0.040 | 0.039 | |||
| P | <0.01 | ns | ns | ns | ns | ||||||||
| Recovery | 40 (+35) |
Fellow | 2.1 | 0.3 | 1.295 | 0.030 | 3.888 | 0.033 | 3.160 | 0.035 | 8.727 | 0.036 | |
| Sham+FD | 0.1 | 1.6 | 1.266 | 0.035 | 3.896 | 0.035 | 3.209 | 0.053 | 8.738 | 0.070 | |||
|
Difference |
−0.6 |
0.8 | −0.029 |
0.017 |
0.010 |
0.017 | −0.008 |
0.011 |
-0.036 |
0.018 | |||
| P | ns | ns | ns | ns | ns | ||||||||
| ONS Alone | 3 (−2) |
Fellow | 5.1 | 1.1 | 1.068 | 0.023 | 3.342 | 0.024 | 2.966 | 0.023 | 7.731 | 0.034 | |
| ONS | 5.9 | 1.0 | 1.060 | 0.025 | 3.311 | 0.019 | 2.954 | 0.026 | 7.681 | 0.048 | |||
| Difference |
0.8 ns |
0.9 | −0.008 ns |
0.012 | −0.031 ns |
0.015 |
-0.012 ns |
0.012 |
-0.037 ns |
0.021 | |||
| P | |||||||||||||
| 8 (+3) |
Fellow | 4.0 | 0.9 | 1.118 | 0.016 | 3.428 | 0.016 | 3.002 | 0.022 | 7.907 | 0.042 | ||
| ONS | 3.8 | 0.8 | 1.097 | 0.020 | 3.434 | 0.020 | 3.017 | 0.021 | 7.901 | 0.044 | |||
| Difference | −0.2 |
0.5 | −0.021 |
0.007 |
0.006 |
0.010 |
0.016 |
0.016 |
-0.006 |
0.018 | |||
| P | ns | <0.05 | ns | ns | ns | ||||||||
| 15 (+10) |
Fellow | 3.3 | 0.3 | 1.172 | 0.017 | 3.547 | 0.027 | 3.029 | 0.032 | 8.115 | 0.040 | ||
| ONS | 2.1 | 0.6 | 1.140 | 0.021 | 3.556 | 0.019 | 3.099 | 0.022 | 8.138 | 0.038 | |||
| Difference | −1.1 |
0.4 | −0.032 |
0.011 |
0.009 |
0.016 |
0.070 |
0.017 |
0.023 |
0.022 | |||
| P | <0.05 | <0.05 | ns | <0.01 | ns | ||||||||
| 22 (+17) |
Fellow | 3.9 | 0.4 | 1.237 | 0.020 | 3.648 | 0.021 | 3.061 | 0.028 | 8.301 | 0.037 | ||
| ONS | 2.2 | 0.6 | 1.214 | 0.019 | 3.635 | 0.028 | 3.119 | 0.021 | 8.312 | 0.036 | |||
| Difference | −1.6 | 0.5 | −0.023 | 0.012 | −0.012 | 0.019 | 0.058 | 0.014 | 0.011 | 0.013 | |||
| P | <0.05 | ns | ns | <0.01 | ns | ||||||||
| 38 (+33) |
Fellow | 3.8 | 0.3 | 1.317 | 0.011 | 3.813 | 0.020 | 3.097 | 0.025 | 8.605 | 0.037 | ||
| ONS | 1.4 | 0.8 | 1.296 | 0.014 | 3.823 | 0.023 | 3.094 | 0.025 | 8.589 | 0.036 | |||
| Difference | −2.4 |
0.6 | −0.022 |
0.013 |
0.010 |
0.016 | −0.003 |
0.021 | −0.017 |
0.025 | |||
| P | <0.01 | ns | ns | ns | ns | ||||||||
FIGURE 2.
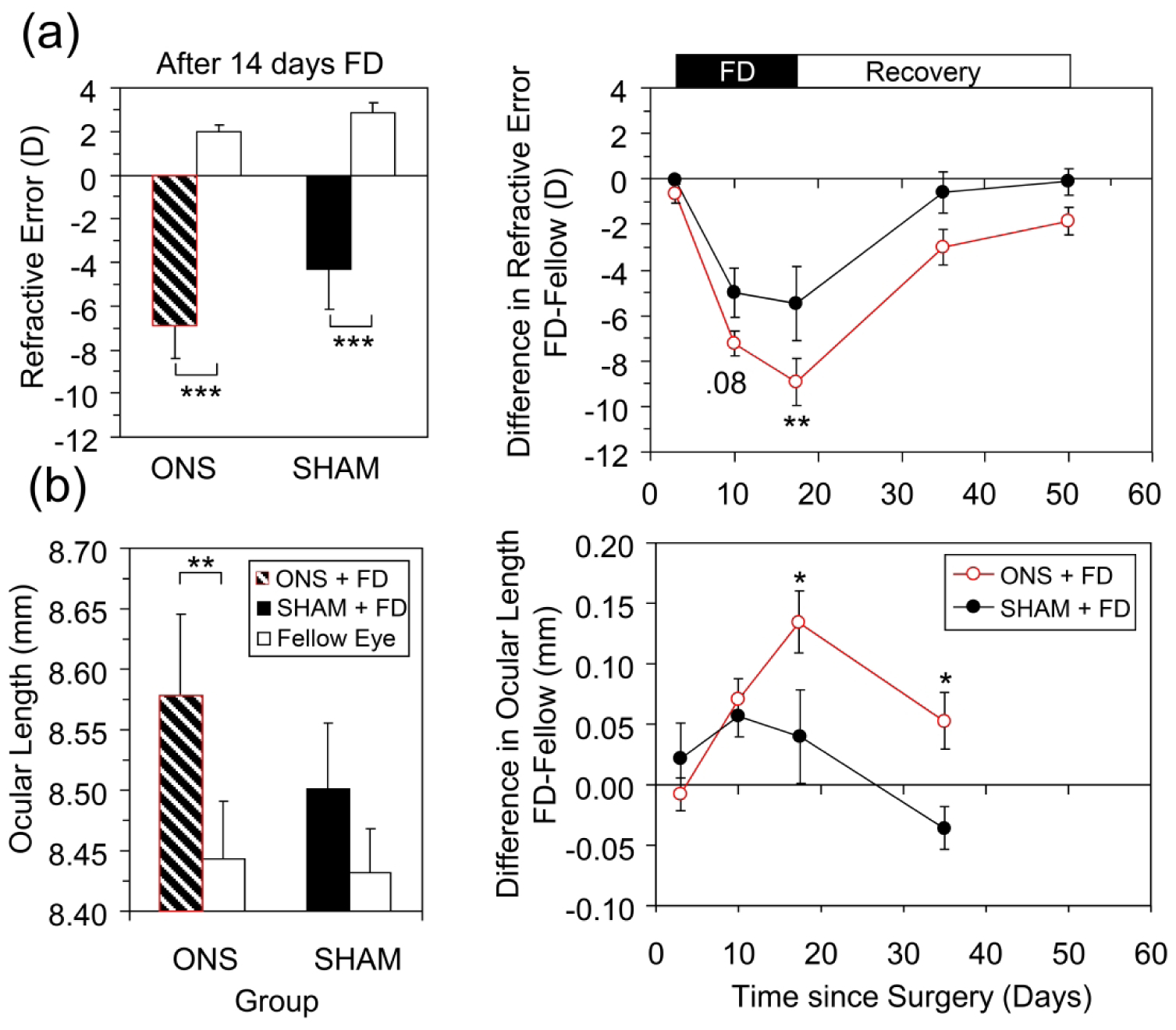
Effects of monocular ONS compared with sham surgery (SHAM) on response to FD treatment; (a) Refractive error and (b) Ocular length. The left panels show mean data for treated and fellow eyes after 14 days of FD. The right panels show mean interocular differences before, during (Black bar at top) and after termination of FD treatment (White bar at top). P values are from Matched pairs t-tests (left panels) or Holm Sidak Comparisons (right panels): *, p < 0.05; **, p < 0.01; ***, p < 0.001.
Relative to animals undergoing sham surgery and subsequently form deprived, animals in which the optic nerve was cut developed more myopia in response to FD. Specifically, while SHAM eyes exhibited significant relative myopia (−5.5 D) after 14 days of FD and also had increased ocular lengths (by 40 μm) (Fig 2a and 2b, left panels), the induced myopia was only 62% of that recorded in ONS animals. Specifically, the mean interocular difference in refractive error was greater for ONS compared to SHAM animals by −3.4 D (p < 0.01, Fig. 2a, right panel), and ONS animals also recorded a much larger mean interocular difference in ocular length (135 μm vs. 40 μm, p < 0.05, Fig. 2b, right panel).
The relative myopia induced in ONS+FD animals primarily reflected significant increases in vitreous chamber depth relative to that of untreated fellow eyes (Fig. 3a). In these animals, the mean interocular difference in vitreous chamber depth recorded after 14 days of FD closely matched the equivalent relative difference in ocular length (130 of 135 μm respectively). In contrast, increased vitreous chamber elongation did not consistently account for the relative myopia recorded in the SHAM+FD animals (Fig. 3a). In both groups, there was no significant difference in relative corneal power after FD (e.g., SHAM+FD: 1.39 ± 0.5 D; ONS+FD: 1.36 ± 1.2 D, FD7). Relative lens thickness and relative anterior chamber depth (AC) changes over the 14-day FD period did not statistically differ between the two groups (AC: +20 vs. +30 μm; Lens: +80 vs. +55 μm; ONS+FD vs. SHAM+FD respectively, FD14-FD0).
FIGURE 3.
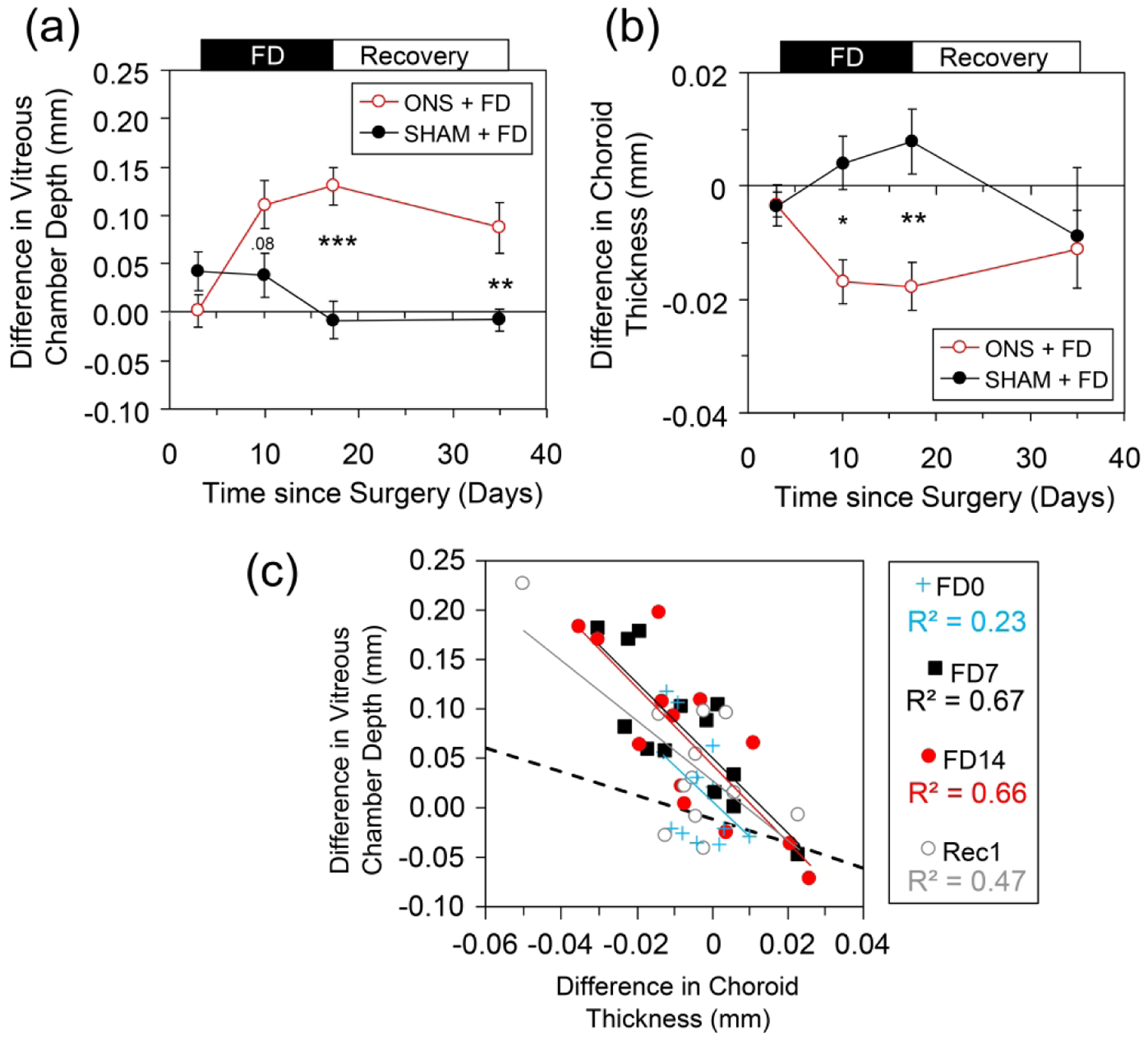
Effect of monocular FD in animals with ONS compared to Sham surgery, expressed as the mean interocular differences in: (a) vitreous chamber depth, and (b) choroidal thickness. Surgery undertaken on day zero in the eyes that were subsequently form deprived. FD was terminated 17 days after surgery. (c) Correlation between interocular differences in vitreous and choroidal thickness in all FD animals; the line of best fit to all data points is shown. The changes in vitreous chamber depth are much larger than those in choroidal thickness and the data does not correspond to a 1:1 relationship as indicated by the dashed line. P values are from Holm Sidak comparisons: *, p < 0.05; **, p < 0.01; ***, p < 0.001.
ONS+FD and SHAM+FD groups showed significant differences in choroidal thickness responses (Fig. 3b, F1,43 = 4.9, p < 0.05). ONS+FD eyes significantly thinned their choroids relative to their fellows (Fig. 3b, Table 3), thinning on average by 10 μm over the FD treatment period (p = 0.003). In contrast, the FD+SHAM eyes did not thin their choroids, and instead their choroids thickened slightly, by 8 μm over the same period (FD14 - FD0), although this change was not significant (Fig. 3b).
TABLE 3.
Summary of mean data (+/− SE) for treated and fellow eyes in each of the three treatment groups for the thicknesses of the posterior layers of the eye at each measurement time point. Interocular differences and the significance of these differences are also shown. P values are from matched pairs t-tests.
| Group | Measure Point | Age (days after surgery) | Eye | Retinal Thickness (Hm) | Choroid Thickness (Hm) | Scleral Thickness (H-m) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SE | Mean | SE | Mean | SE | |||||
| ONS + FD | FD0 | 8 (+3) |
Fellow | 156 | 3 | 92 | 11 | 116 | 4 | |
| O NS+FD | 156 | 2 | 88 | 6 | 113 | 4 | ||||
| Difference | 0 | 2 | −3 | 4 | −3 | 4 | ||||
| P | ns | ns | ns | |||||||
| FD7 | 15 (+10) |
Fellow | 153 | 1 | 93 | 7 | 115 | 2 | ||
| ONS+FD | 147 | 1 | 76 | 4 | 107 | 5 | ||||
| Difference | −6 | 1 | −17 | 4 | −8 | 4 | ||||
| P | <0.001 | <0.01 | ns | |||||||
| FD14 | 22 (+17) |
Fellow | 150 | 1 | 97 | 6 | 114 | 2 | ||
| ONS+FD | 144 | 2 | 79 | 2 | 112 | 2 | ||||
| Difference | −6 | 1 | −18 | 4 | −2 | 1 | ||||
| P | <0.01 | <0.01 | ns | |||||||
| Recovery | 40 (+35) |
Fellow | 154 | 1 | 106 | 7 | 129 | 4 | ||
| ONS+FD | 146 | 1 | 95 | 7 | 122 | 4 | ||||
| Difference | −8 | 1 | −11 | 7 | −7 | 4 | ||||
| P | <0.001 | ns | ns | |||||||
| SHAM + FD | FD0 | 8 (+3) |
Fellow | 155 | 3 | 102 | 11 | 112 | 4 | |
| Sham+FD | 157 | 2 | 92 | 6 | 112 | 4 | ||||
| Difference | 1 | 2 | −4 | 4 | 1 | 4 | ||||
| P | ns | ns | ns | |||||||
| FD7 | 15 (+10) |
Fellow | 152 | 2 | 95 | 7 | 112 | 3 | ||
| Sham+FD | 150 | 1 | 92 | 5 | 108 | 4 | ||||
| Difference | −1 | 1 | 4 | 5 | −1 | 1 | ||||
| P | ns | ns | ns | |||||||
| FD14 | 22 (+17) |
Fellow | 152 | 1 | 97 | 6 | 111 | 3 | ||
| Sham+FD | 151 | 2 | 97 | 6 | 118 | 6 | ||||
| Difference | 1 | 1 | 8 | 6 | 6 | 3 | ||||
| P | ns | ns | ns | |||||||
| Recovery | 40 (+35) |
Fellow | 154 | 2 | 110 | 9 | 119 | 6 | ||
| Sham+FD | 148 |
2 |
97 |
7 |
122 |
3 |
||||
| Difference |
−5 |
3 |
−9 |
12 |
4 |
4 | ||||
| p | ns | ns | ns | |||||||
| ONS Alone | 3 (−2) |
Fellow | 162 | 3 | 83 | 3 | 109 | 4 | ||
| ONS | 163 | 2 | 83 | 3 | 111 | 4 | ||||
| Difference | 1 | 1 | 0 | 3 | 2 | 2 | ||||
| p | ns | ns | ns | |||||||
| 8 (+3) |
Fellow | 158 | 1 | 91 | 3 | 111 | 2 | |||
| ONS | 156 | 1 | 87 | 3 | 110 | 2 | ||||
| Difference | −1 | 1 | −4 | 4 | −1 | 2 | ||||
| p | ns | ns | ns | |||||||
| 15 (+10) |
Fellow | 154 | 2 | 93 | 5 | 120 | 2 | |||
| ONS | 152 | 1 | 82 | 4 | 110 | 3 | ||||
| Difference | -3 | 1 | −11 | 4 | −10 | 2 | ||||
| p | ns | <0.05 | <0.01 | |||||||
| 22 (+17) |
Fellow | 151 | 1 | 84 | 3 | 120 | 2 | |||
| ONS | 149 | 1 | 85 | 3 | 110 | 3 | ||||
| Difference | -3 | 1 | 2 | 4 | −10 | 3 | ||||
| p | ns | ns | <0.01 | |||||||
| 38 (+33) |
Fellow | 152 | 1 | 94 | 8 | 132 | 3 | |||
| ONS | 149 | 1 | 97 | 3 | 130 | 6 | ||||
| Difference | −3 | 1 | 3 | 7 | −2 | 7 | ||||
| p | ns | ns | ns | |||||||
The interocular differences in choroidal thickness and vitreous chamber depth were significantly correlated across all animals (r = −0.73, p < 0.001), as well as within each group (ONS+FD: r = −0.73, p < 0.001; SHAM+FD: r = −0.52, p = 0.01). This relationship was strongest during the form deprivation period, and also significant afterwards during the recovery (Rec) period (correlation coefficient at FD0: r = −0.48; p = 0.08; FD7: r = −0.80, p = 0.002; FD14: r = −0.81, p = 0.001; Rec: r = −0.67, p = 0.014, Fig. 3c).
While the thinning of the choroid in response to FD contributed to the increase in vitreous chamber depth of FD+ONS eyes, the changes in choroidal thickness were much smaller than the changes in vitreous chamber depth. For example, by day 14 in ONS animals, the choroid of FD eyes was 18 μm thinner than that of fellow eyes (Table 3, Fig. 3b), while their vitreous chambers had elongated by 130 μm (Table 2, Fig. 3a).
The retina was also found to be relatively thin in the FD+ONS eyes (interocular difference between −6 and −8 μm, Table 3), although these small changes, even when combined with choroidal and scleral changes, were insufficient to account for the enlarged vitreous chambers (FD7: −31 Vs. +111 μm; FD14: −26 Vs. +130 μm). Retinal thinning was not observed in the SHAM+FD eyes (Table 3). This difference may reflect the greater enlargement of ONS+FD eyes as larger eyes tend to have thinner retina (r = −0.36, p < 0.001 for interocular differences in vitreous chamber and retinal thickness across all animals).
3.3 |. Reversal of ocular changes during recovery from FD after ONS
After diffusers were removed at 22 days of age, both ONS+FD and SHAM+FD animals reversed the refractive changes induced by FD, showing similar progressive decreases in their myopia over time (Fig. 2a, right panel). For example, after 18 days without diffusers, the direction of the changes in interocular differences in refractive error had reversed (ONS+FD, +5.9 D; SHAM+FD, +4.9 D, Recovery-FD14, p < 0.001 in both cases) and this recovery was similar in the two groups (p = 0.59). In the case of the SHAM animals, these changes were sufficient to eliminate the pre-existing induced myopia, i.e., the interocular difference in refractive error was now minimal (Table 2, 40 days of age). In contrast, in the case of the ONS+FD animals, which were more myopic at the end of the FD treatment period, significant myopia remained (Table 2). Specifically, after 18 days of recovery from FD, the interocular refractive error difference was reduced to −3.0 D (p = 0.017), and after a total of 33 days of “recovery”, it was reduced to −1.86 ± 0.6 D (p = 0.017, Fig. 2a, right panel).
The recovery from induced myopia observed when diffusers were removed, was associated with a reversal in the relative rate of ocular elongation that now significantly slowed in these still growing, young eyes. In both groups of animals, similar reductions in the rate of ocular elongation occurred. After 18 days without diffusers, interocular differences in ocular length were reduced by −76 μm in SHAM animals (p = 0.077) and by −82 μm in the ONS animals (p = 0.034), relative to values at the end of the FD treatment period. Because the ONS eyes had elongated more over the FD treatment period than SHAM eyes, the interocular difference in ocular length for the ONS group remained greater than that of the SHAM group despite 18 days of recovery (Fig. 2b, right panel), consistent with the greater residual myopia in the ONS group.
During recovery from FD, interocular differences in both anterior chamber depth and lens thickness, also changed in the opposite direction to that observed during the FD treatment period (AC: +20 vs. +30 μm (FD) and −17 vs. −32 (Rec); Lens: +80 vs. +55 μm (FD) and −21 vs. −16 μm (Rec); ONS vs. SHAM respectively for FD14-FD0 (FD) and Recovery-FD14 (Rec)). Finally, even though FD+ONS and FD+SHAM animals exhibited distinctly different choroidal response patterns the during FD, both groups exhibited similar interocular differences by the end of the recovery period, reversing the earlier changes (Fig. 3b).
3.4 |. Effects of ONS surgery alone
Animals that underwent monocular ONS surgery but no FD treatment showed no differences between their ONS and fellow eyes in their refractive errors, either before (at 3 days of age) or 3 days after surgery (at 8 days of age; Table 2, Fig. 4a open triangles). However, a small amount of relative myopia gradually developed over the following month, reaching a maximum of −2.4 D by 33 days post-surgery (Fig. 4a), before slowly dissipating to be negligible by 59 days post-surgery. This myopia reflected a relative, albeit transient, increase in vitreous chamber depth in ONS eyes over this time period (Table 2). In addition, the ONS surgery caused a consistently smaller anterior chamber, which was significantly smaller between 3–10 days after surgery (Table 2). In individual animals, smaller relative anterior chambers were associated with a relatively thinner crystalline lens (r = −0.51, p = 0.001), although interocular differences in crystalline lens thickness did not reach statistical significance at any time point. These effects of the ONS surgery are likely to have had small, if transient, effects on the optics of the eye.
FIGURE 4.
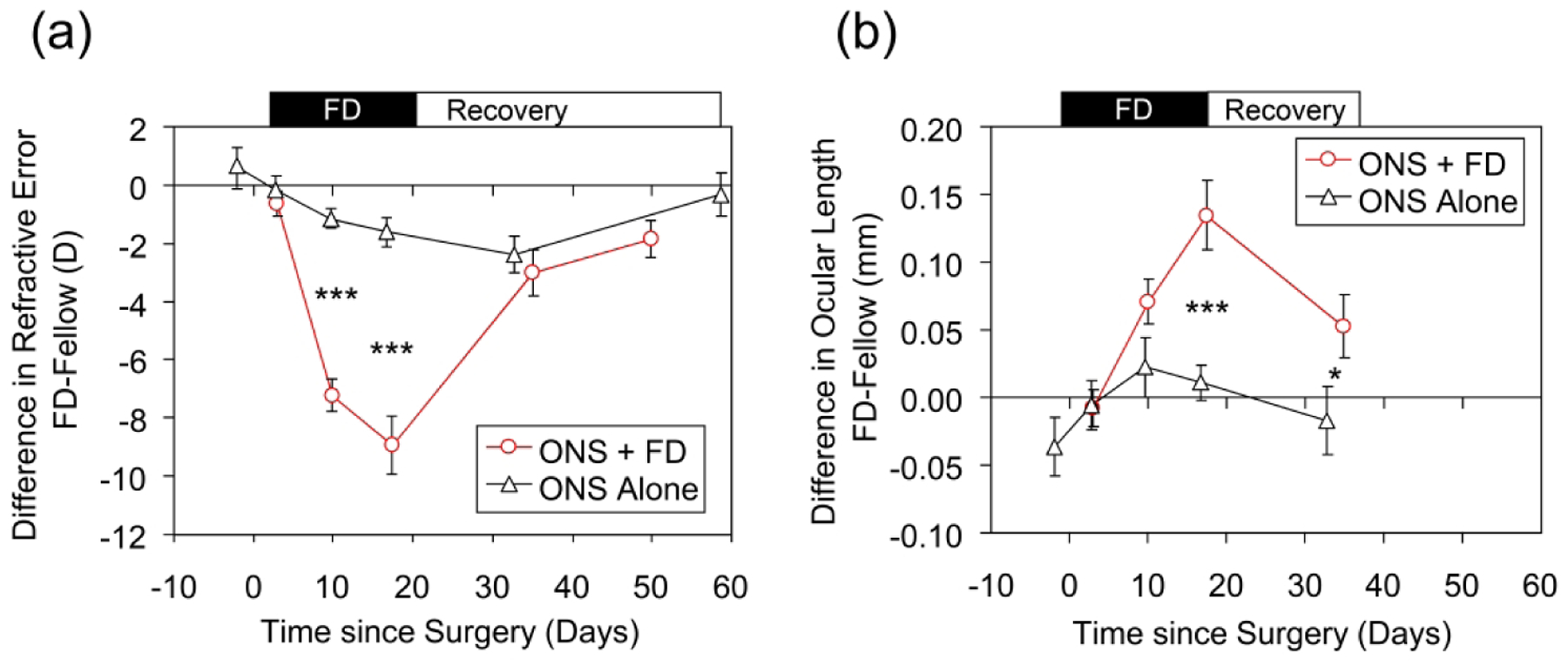
Effect of monocular ONS alone (Black symbols), or ONS combined with FD (red symbols), shown as mean interocular differences in: (a) Refractive error and (b) Ocular length. Surgery was undertaken at time zero; FD terminated on Day 17. P values are from Holm Sidak comparisons: *, p < 0.05; ***, p < 0.001.
ONS had no significant effects on retinal thickness (Table 3), eliminating the surgery itself as the source of the relative retinal thinning observed in the ONS+FD group. However, transient, significant thinning of the choroid and sclera (each interocular difference of approximately −10 μm) was observed in ONS eyes at 10 days post-surgery (Table 3). Thus some of the choroidal thinning observed in the ONS+FD group may have been due to the ONS surgery, although it was not sustained in the ONS only group. The interocular differences in the thickness of the choroid and sclera were not related to each other directly, but were each correlated with the relative differences in vitreous chamber depth (r = −0.6, p < 0.001; r = −0.5, p = 0.008 respectively) consistent with larger eyes having thinner choroids and scleras.
3.5 |. ONS surgical effects on FD-induced myopia and recovery
Compared to the amount of myopia induced by FD in ONS eyes, the relative myopia recorded in ONS only animals was small, only 18% of that recorded at the end of the FD period in animals that also underwent FD treatment (−1.6 D, ONS vs. −8.9 D, FD+ONS, p < 0.001, Fig. 4a). Thus the greater myopia that occurred in animals form-deprived after ONS is unlikely to represent an artifact of ONS per se.
The residual relative myopia observed in FD+ONS animals after 18 and 33 days of recovery (−3.0 D and −1.9 D respectively) was similar in amount to that observed in animals undergoing ONS alone, at similar times post-surgery (Fig. 4a), raising the possibility that this residual myopia was a byproduct of ONS surgery. However, the origin of the residual relative myopia was different in the two groups. In animals undergoing only ONS surgery, neither ocular length nor vitreous chamber depth was affected 33 days after surgery (Table 2); instead the myopia reflected a small relative and marginally significant increase in corneal power in ONS eyes (0.7 ± 0.3 D, p = 0.056), although no significant interocular differences in corneal power were recorded at earlier time points (0.1; 0.7; 0.2; 0.3 D at 3, 8, 15 and 22 days after surgery respectively, p > 0.3 in all cases). It is possible that optical changes contributed to the residual myopia since the relative anterior chamber and lens thickness of ONS only eyes were observed to be affected at earlier time points, and a shallower anterior chamber was still present 33 days after surgery, although no longer statistically significant. These observations in the ONS only group contrasts with the clear axial origin of the residual myopia in the ONS+FD group at 33 days. At this time point, the ONS+FD eyes were also significantly longer than the ONS only eyes (Fig. 4b, p = 0.04), reflecting greater relative vitreous chamber elongation (difference between the groups of 82 μm, p = 0.006). Therefore, the excessive myopia and enhanced axial elongation induced by FD in ONS eyes cannot be attributed to a surgical artifact, nor is the failure of FD+ONS eyes to show full recovery and residual myopia due to ONS per se.
4. |. DISCUSSION
4.1 |. ONS does not prevent FD myopia but increases the gain of the response
While ONS did not prevent the development of FD myopia, eyes undergoing ONS elongated at a faster rate compared to those undergoing sham surgery, and thus became more myopic over the FD treatment period, (ONS+FD: −8.9 D vs. SHAM+FD: −5.5 D). Eyes undergoing ONS alone showed only low myopia (ONS: −1.1 D). The amount of myopia recorded in the animals undergoing sham surgery closely approximates that previously observed in our animals with FD for 2 weeks but otherwise untreated (Howlett & McFadden, 2006), and ONS alone without FD did not cause excessive elongation. Thus the exaggerated response to FD of the ONS group is unlikely to be a surgical artifact, e.g., related to compromised ocular blood flow. Ophthalmoscopy observations also tend to rule out the latter possibility.
Parallels can be drawn between the above results from young guinea pigs and observations from equivalent studies in chickens. Specifically, ONS eyes showed increased axial elongation in response to FD, compared to eyes subjected to FD without ONS. For example, chick eyes fitted with diffusers 10 days after ONS showed exaggerated changes in their vitreous chamber depths relative to chicks of the same age undergoing FD but not ONS (FD: 125 μm vs. FD+ONS: 560 μm) (Choh et al., 2006). This effect of ONS is still evident but not as large when FD was initiated only 4 days after the ONS surgery (FD vs FD+ONS: −14 D vs. 17 D, 550 vs. 600 μm change in vitreous chamber) (Wildsoet, 2003). Additionally, in an earlier study, it was noted that partial (lateral temporal) binocular FD in young chicks induced greater myopia in the eye which received unilateral ONS+FD compared to the eye that was only form deprived (−15 D Vs. −4.4 D respectively) (Troilo & Wallman, 1991).
Thinning of the retinal and/or choroidal layers contributed to the increase in vitreous chamber depth and hence in optical axial length (distance from the anterior cornea to retina). For example, choroidal thinning accounted for between 33–55% of the optical axial length increases and between 42–69% of the vitreous chamber expansion in one chick study (Choh et al., 2006). Similarly in the current guinea pig study, changes in choroidal thickness were well correlated with the changes in vitreous chamber depth, albeit smaller in scale, accounting for 13.8% of the changes. In chicks, anterior chamber enlargement in FD eyes was a more variable feature of the above studies, and was also not a consistent feature of the guinea pig studies described here. Taken together, these studies suggest that ONS surgery increases the gain of the myopic response to FD, with changes in vitreous chamber elongation dominating and accompanied by choroidal thinning. It is possible that without an intact optic nerve, the eye may accelerate its growth in response to form deprivation to a maximum rate with limited inhibitory control.
4.2 |. The Effects of ONS surgery
The parallel between chicks and guinea pigs did not extend to eyes that were subject to ONS alone. In young guinea pigs ONS alone induced a small amount of transient myopia. In contrast, chick eyes subjected to the ONS surgery but not form deprived, show reduced axial elongation and increased hyperopia, with interocular differences being apparent shortly after ONS surgery (reduction in vitreous chamber depth by 0.05%) (Wildsoet, 2003) and becoming larger over time, with refractive error differences in excess of +10 D between ONS and untreated fellow eyes reported after 5 days post-surgery, and relative reductions in vitreous chamber depth of greater than 300 μm (Troilo et al., 1987; Wildsoet & Wallman, 1995).
Some of the hyperopia and reduction in eye length observed in the ONS eyes of chicks can be attributed to ONS-induced choroidal thickening (Choh et al., 2006; Wildsoet, 2003; Wildsoet & Wallman, 1995; Wildsoet & Pettigrew, 1988). Choroidal thickening is a well-established ocular response to myopic defocus (which induces hyperopia), observed across most species studies to-date, including chicks (Wildsoet & Wallman, 1995), guinea pigs (Howlett & McFadden, 2006) and humans (Chakraborty, Read, & Collins, 2012), although chicks are noteworthy for their much larger choroidal responses, being an order of magnitude greater compared to the other species (Wildsoet & Wallman, 1995). At least in chicks, the choroidal thickening is thought to be causally linked to ocular growth inhibition (Nickla & Wallman, 2010). The mechanism underlying the choroidal thickening response in chick eyes after ONS remains to be resolved, although curiously, the choroids of guinea pig eyes show a tendency to thin rather than thicken after ONS.
In the current study in guinea pig eyes, ONS surgery caused a transient myopia that slowly developed but resolved over time. This trajectory suggests it was not due to a sudden change in tension on the globe, but is consistent with possible transient scleral remodeling (such as might be caused by scar tissue) and associated minor transient mechanical effects on the posterior globe. Alternatively, eyes subjected to ONS alone also showed transient changes in the anterior chamber, which was correlated with crystalline lens thinning, suggesting ONS may cause transient changes in the anterior globe as previously noted in mice (Gong et al., 2020). Nonetheless, in the current study, these effects were small and transient and could not explain the changes in ONS eyes that responded to FD with an increased gain in the rate of posterior eye elongation.
4.3 |. Recovery from FD myopia does not require an intact optic nerve
Despite the exaggerated response to FD in eyes subjected to ONS surgery, these eyes were able to appropriately slow their growth when diffusers were removed. This suggests that there are mechanisms that are not disrupted by ONS that can either decode the sign of defocus or detect anisometropic differences between the two eyes and respond appropriately. However, ONS eyes did not fully recover from induced myopia after the FD treatment was terminated, with some residual myopia still present one month after diffusers were removed. We have previously reported rapid recovery in young guinea pigs after 11 days of monocular FD, with interocular ocular length differences no longer significant after just 3 days of recovery (Howlett & McFadden, 2006). In the case of ONS+FD eyes, residual eye elongation was still present 18 days after the FD treatment was terminated. Corneal changes may have contributed to this residual myopia, as the effect of ONS itself was to transiently increase the power of the cornea by 0.7 D, contrasting with the effect in mice of optic nerve crush, which leads to corneal flattening rather than steepening (Gong et al., 2020). Nonetheless, it is perhaps noteworthy that the ONS+FD eyes were also more myopic at the start of the recovery process, so it is possible that the capacity to recover from induced myopia had simply been exceeded.
The observed ability of guinea pig eyes subjected to ONS to switch from accelerated to relatively inhibited growth after diffusers were removed, is consistent with previous observations in chicks of recovery from the effects of partial or complete FD through inhibition of ocular growth, despite ONS. As in guinea pigs, such recovery responses are not entirely normal in chicks. However, different from guinea pig eyes, the eyes of chicks overshoot the normal emmetropic set-point after ONS, becoming more hyperopic than normal (Troilo & Wallman, 1991).
4.4 |. Possible underlying causes
There are multiple possible causes of the ONS effects observed in the current study. Apart from disconnecting the retina from higher visual centers, in chicks, ONS eliminates centrifugal fibers descending from higher centers that synapse with retinal amacrine cells (Miles, 1972; Wildsoet, 2003). Such centrifugal fibers are also present in mammals and primates, and while they are relatively sparse compared to numbers in the chicken (10 or fewer in man vs. 10,000 or more in the chicken) (Reperant et al., 2006) their efferent fibers branch widely within the retina (Gastinger, Tian, Horvath, & Marshak, 2006). In the guinea pig retina, 2–3 fibers with many collateral branches have been followed from the optic disc to the inner plexiform layer (Ventura, 1959).
ONS also induces degeneration of the retinal ganglion cells (RGCs) (chicks, (Chong, Wildsoet, & Choh, 2013); guinea pigs, (McFadden, Zeng, Walker, Metse, & Wildsoet, 2015)) which may be preceded by subtle changes in their afferent connections (McFadden et al., 2015). These changes in the retinal circuitry could alter the ability of the retina to sense optical defocus and/or alter signals generated. Thus the observed differences in the responses of ONS versus SHAM eyes to FD may not be related to the elimination of influences from higher visual centers, but rather a product of disruption to RGC integrity and/or associated neuronal connections.
Additionally, across species, ONS interferes with defocus-induced accommodation and causes pupil dilation in the lesioned eye, although the consensual pupil light reflex is generally preserved (Li & Howland, 1999), as was the case in our guinea pigs. The open loop nature of wearing a diffuser, which severely degrades retinal images, thereby eliminates the possibility of visual feedback influences on eye growth. None-the-less, the lack of a pupil response in ONS eyes would likely have increased the luminance experienced through diffusers by the retinas of ONS eyes relative to those of eye undergoing sham surgery. Extended exposure to high light levels during rearing has been reported to retard the development of FD myopia in chicks (Ashby, Ohlendorf, & Schaeffel, 2009) and monkeys (Smith, Hung, & Huang, 2012) and may even cause hyperopia, not the increased myopia we report here. Furthermore, these inhibitory effects of light on myopia development are typically linked with very high luminance levels (>15,000 lux), which cannot be emulated by the pupil effects of ONS reported in our guinea pigs, which were raised in 400 lux white light. Therefore, it seems unlikely that neither the transient myopia in eyes subjected to ONS alone, nor the increased myopia reported in ONS+FD eyes here, is the result of increased luminance per se.
As an alternative to ONS surgery, a number of studies, mostly in chicks, have used either chemical lesioning or pharmacological intervention to silence the retina-brain signal pathways. Do they offer any additional insight into the findings in relation to ONS reported here in guinea pig? As a chemical lesioning agent, to eliminate retinal ganglion cells, colchicine has been given as intravitreal injections to day-old chicks (Morgan, 1981). However, colchicine appears to also eliminate dopaminergic amacrine cells (Fischer, Morgan, & Stell, 1999). In addition, it disrupts microtubules in myelinated axons of the optic nerve (Davidson, Green, & Wong, 1983), as well as in other ocular tissues, so it is not possible to rule out direct effects on the cornea, choroid and sclera, as mediators or contributors to some of the effects observed. For example, injected eyes exhibit larger than normal vitreous chambers, as well as increased equatorial and axial dimensions (Choh, Padmanabhan, Li, Sullivan, & Wildsoet, 2008; Fischer et al., 1999), yet these eyes show reduced responses to form deprivation myopia (Fischer et al., 1999). The crystalline lenses and retinas of these eyes are also thinner than normal (Choh et al., 2008).
Serial intravitreal injections of tetrodotoxin (TTX) have also been used to silence retinal ganglion cells. It may be relevant that TTX, which does not induce RGC degeneration per se, tends to reduce the response to FD, at least in tree shrews and chicks, rather than cause enhanced growth as reported here after ONS. Specifically, In tree shrews, TTX reduced the response to lid-suture myopia, although TTX alone also resulted in smaller than normal eyes, with reductions in both the anterior and posterior vitreous chambers (Norton, Essinger, & McBrien, 1994). As an explanation for the latter changes, the authors speculate that the injections may have led to changes in the mechanical forces mediating eye enlargement. It is noteworthy that they used the same injection site for repeated injections, which may have affected intraocular pressure. In chicks, TTX reduced the growth of the anterior segment and crystalline lens, but did not prevent FD-induced vitreous chamber elongation (McBrien, Moghaddam, Cottriall, Leech, & Cornell, 1995). The net effect of these changes was a reduction in, but not elimination of, FD-induced myopia. TTX treated chicks also do not fully recover from FD myopia, with the vitreous chamber remaining excessively elongated (McBrien et al., 1995), serving to offset the hyperopia otherwise expected from the anterior segment reductions; instead refractive errors appeared relatively normal (McBrien et al., 1995).
The fact that in multiple species, the eye can develop FD myopia and recover its refractive state despite ONS or TTX treatment argues that a link between the retina and the brain is not required for the eye to sense and recover from an anisometropic myopic refractive error. However, across species, the ability to refine the refractive error is impaired by such interventions.
5. |. CONCLUSION
We found that, in guinea pigs, as in chickens, the eye is still able to become myopic in response to form deprivation and also recover from induced myopia after communication between the retina and brain is eliminated through ONS, arguing that eye growth regulation is largely mediated by local (ocular) mechanisms. However, the gain control with form deprivation was significantly altered by ONS, although which of the multiple consequences of ONS underlies the latter effect is currently unknown. The poor eye growth gain control with ONS was observed under open loop conditions in which visual feedback was unavailable. Whether ONS affects the ability of the mammalian eye to correctly respond to defocus under closed loop visual feedback conditions, such as simulated experimentally with defocusing lenses, remains to be determined.
ACKNOWLEDGMENTS
Authors are grateful to Grant support from The University of Newcastle G0900214 (SM), International Sciences Linkage, CG120160, DIISR (Australian Govt) (SM) and NIH/NEI R01 EY012392 (CW) for funding this study.
Funding Information
CF Wildsoet (PI) National Eye Institute of National Institute of Health, USA, R01 EY012392, Mechanisms underlying emmetropization and local eye growth regulation.
SA McFadden (CI) SA McFadden University of Newcastle Grant G0900214. Retinal mechanisms underlying myopia.
SA McFadden (CI) International Sciences Linkage, CG120160, DIISR (Australian Govt), Etiology of myopia.
SPECIAL ACKNOWLEDGEMENTS (Prefer to be upfront if Editors agree)
The authors would like to dedicate this paper to the memory of Professor Jack Pettigrew, whose openness to challenging established dogma lead to the first chick-based studies involving optic nerve section, which were the inspiration for the guinea pig study reported here.
Abbreviations
- FD
Form Deprivation
- ONS
Optic Nerve Section
Footnotes
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
ETHICAL APPROVAL
The study was approved by the Animal Care and Ethics Committee at the University of Newcastle and conformed to NSW legislative requirements and the National Health and Medical Research Australian Code for the Care and Use of Animals for Scientific Purposes.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Ashby R, Ohlendorf A, & Schaeffel F (2009). The effect of ambient illuminance on the development of deprivation myopia in chicks. Investigative Ophthalmology & Visual Science, 50(11), 5348–5354. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/19516016. doi: 10.1167/iovs.09-3419 [DOI] [PubMed] [Google Scholar]
- Chakraborty R, Read SA, & Collins MJ (2012). Monocular myopic defocus and daily changes in axial length and choroidal thickness of human eyes. Exp Eye Res, 103, 47–54. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/22971342. doi: 10.1016/j.exer.2012.08.002 [DOI] [PubMed] [Google Scholar]
- Choh V, Lew MY, Nadel MW, & Wildsoet CF (2006). Effects of interchanging hyperopic defocus and form deprivation stimuli in normal and optic nerve-sectioned chicks. Vision Res, 46(6–7), 1070–1079. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/16212999. doi: 10.1016/j.visres.2005.08.020 [DOI] [PubMed] [Google Scholar]
- Choh V, Padmanabhan V, Li WS, Sullivan AB, & Wildsoet CF (2008). Colchicine attenuates compensation to negative but not to positive lenses in young chicks. Exp Eye Res, 86(2), 260–270. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/18078935. doi: 10.1016/j.exer.2007.10.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong S, Wildsoet C, & Choh V (2013). Life and Death of Retinal Cells in Optic Nerve Sectioned Chick Eyes. Investigative Ophthalmology & Visual Science, 54(15), 6096–6096. Retrieved from http://dx.doi.org/. [Google Scholar]
- Davidson C, Green WR, & Wong VG (1983). Retinal atrophy induced by intravitreous colchicine. Investigative Ophthalmology & Visual Science, 24(3), 301–311. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/6187703. [PubMed] [Google Scholar]
- De Schaepdrijver L, Simoens P, Lauwers H, & De Geest JP (1989). Retinal vascular patterns in domestic animals. Res Vet Sci, 47(1), 34–42. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/2772405. [PubMed] [Google Scholar]
- Fischer AJ, Morgan IG, & Stell WK (1999). Colchicine causes excessive ocular growth and myopia in chicks. Vision Res, 39(4), 685–697. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/10341956. doi: 10.1016/s0042-6989(98)00178-3 [DOI] [PubMed] [Google Scholar]
- Gastinger MJ, Tian N, Horvath T, & Marshak DW (2006). Retinopetal axons in mammals: emphasis on histamine and serotonin. Curr Eye Res, 31(7–8), 655–667. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/16877274. doi: 10.1080/02713680600776119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong X, Wu XH, Liu AL, Qian KW, Li YY, Ma YY, … Weng SJ (2020). Optic nerve crush modulates refractive development of the C57BL/6 mouse by changing multiple ocular dimensions. Brain Res, 1726, 146537 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/31672473. doi: 10.1016/j.brainres.2019.146537 [DOI] [PubMed] [Google Scholar]
- Gusek-Schneider GC, & Martus P (2001). Stimulus deprivation myopia in human congenital ptosis: a study of 95 patients. J Pediatr Ophthalmol Strabismus, 38(6), 340–348. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/11759772. [DOI] [PubMed] [Google Scholar]
- Holden BA, Fricke TR, Wilson DA, Jong M, Naidoo KS, Sankaridurg P, … Resnikoff S (2016). Global Prevalence of Myopia and High Myopia and Temporal Trends from 2000 through 2050. Ophthalmology, 123(5), 1036–1042. Retrieved from http://www.sciencedirect.com/science/article/pii/S0161642016000257. doi: 10.1016/j.ophtha.2016.01.006 [DOI] [PubMed] [Google Scholar]
- Howlett MH, & McFadden SA (2006). Form-deprivation myopia in the guinea pig (Cavia porcellus). Vision Res, 46(1–2), 267–283. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/16139323. doi: 10.1016/j.visres.2005.06.036 [DOI] [PubMed] [Google Scholar]
- Huo L, Cui D, Yang X, Wan W, Liao R, Trier K, & Zeng J (2012). A retrospective study: form-deprivation myopia in unilateral congenital ptosis. Clin Exp Optom, 95(4), 404–409. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/22494158. doi: 10.1111/j.1444-0938.2012.00716.x [DOI] [PubMed] [Google Scholar]
- Li T, & Howland HC (1999). A true neuronal consensual pupillary reflex in chicks. Vision Res, 39(5), 897–900. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/10341943. [DOI] [PubMed] [Google Scholar]
- McBrien NA, Moghaddam HO, Cottriall CL, Leech EM, & Cornell LM (1995). The effects of blockade of retinal cell action potentials on ocular growth, emmetropization and form deprivation myopia in young chicks. Vision Research, 35(9), 1141–1152. Retrieved from http://www.sciencedirect.com/science/article/pii/004269899400237G. doi: 10.1016/0042-6989(94)00237-G [DOI] [PubMed] [Google Scholar]
- McFadden SA, Howlett MH, & Mertz JR (2004). Retinoic acid signals the direction of ocular elongation in the guinea pig eye. Vision Res, 44(7), 643–653. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/14751549. doi: 10.1016/j.visres.2003.11.002 [DOI] [PubMed] [Google Scholar]
- McFadden SA, Zeng G, Walker L, Metse AP, & Wildsoet CF (2015). Changes in Cell Density in the Retinal Ganglion Cell Layer After Optic Nerve Section in Young Guinea Pigs. Investigative Ophthalmology & Visual Science, 56(7), 3623–3623. doi:https://doi.org/ [Google Scholar]
- Miles FA (1972). Centrifugal control of the avian retina. 3. Effects of electrical stimulation of the isthmo-optic tract on the receptive field properties of retinal ganglion cells. Brain Res, 48, 115–129. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/4645201. [DOI] [PubMed] [Google Scholar]
- Morgan IG (1981). Intraocular colchicine selectively destroys immature ganglion cells in chicken retina. Neurosci Lett, 24(3), 255–260. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/6168980. doi: 10.1016/0304-3940(81)90167-1 [DOI] [PubMed] [Google Scholar]
- Nickla DL, & Wallman J (2010). The multifunctional choroid. Prog Retin Eye Res, 29(2), 144–168. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/20044062. doi: 10.1016/j.preteyeres.2009.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton TT, Essinger JA, & McBrien NA (1994). Lid-suture myopia in tree shrews with retinal ganglion cell blockade. Vis Neurosci, 11(1), 143–153. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/8011577. doi: 10.1017/s0952523800011184 [DOI] [PubMed] [Google Scholar]
- Pan CW, Cheng CY, Saw SM, Wang JJ, & Wong TY (2013). Myopia and age-related cataract: a systematic review and meta-analysis. Am J Ophthalmol, 156(5), 1021–1033 e1021. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/23938120. doi: 10.1016/j.ajo.2013.06.005 [DOI] [PubMed] [Google Scholar]
- Raviola E, & Wiesel TN (1985). An animal model of myopia. N Engl J Med, 312(25), 1609–1615. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/4000200. doi: 10.1056/NEJM198506203122505 [DOI] [PubMed] [Google Scholar]
- Reperant J, Ward R, Miceli D, Rio JP, Medina M, Kenigfest NB, & Vesselkin NP (2006). The centrifugal visual system of vertebrates: a comparative analysis of its functional anatomical organization. Brain Res Rev, 52(1), 1–57. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/16469387. doi: 10.1016/j.brainresrev.2005.11.008 [DOI] [PubMed] [Google Scholar]
- Smith EL 3rd, Hung LF, & Huang J (2012). Protective effects of high ambient lighting on the development of form-deprivation myopia in rhesus monkeys. Investigative Ophthalmology & Visual Science, 53(1), 421–428. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/22169102. doi: 10.1167/iovs.11-8652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troilo D, Gottlieb MD, & Wallman J (1987). Visual deprivation causes myopia in chicks with optic nerve section. Curr Eye Res, 6(8), 993–999. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/3665562. doi: 10.3109/02713688709034870 [DOI] [PubMed] [Google Scholar]
- Troilo D, Smith EL 3rd, Nickla DL, Ashby R, Tkatchenko AV, Ostrin LA, … Jones L (2019). IMI - Report on Experimental Models of Emmetropization and Myopia. Investigative Ophthalmology & Visual Science, 60(3), M31–M88. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/30817827. doi: 10.1167/iovs.18-25967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troilo D, & Wallman J (1991). The regulation of eye growth and refractive state: an experimental study of emmetropization. Vision Res, 31(7–8), 1237–1250. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/1891815. [DOI] [PubMed] [Google Scholar]
- Ventura J, Mathieu M (1959). Exogenous fibres of the retina Tr. Can. Ophthalmol. , 22, 184–196. [Google Scholar]
- Wallman J, Gottlieb MD, Rajaram V, & Fugate-Wentzek LA (1987). Local retinal regions control local eye growth and myopia. Science, 237(4810), 73–77. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/3603011. [DOI] [PubMed] [Google Scholar]
- Wallman J, & Winawer J (2004). Homeostasis of eye growth and the question of myopia. Neuron, 43(4), 447–468. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/15312645. doi: 10.1016/j.neuron.2004.08.008 [DOI] [PubMed] [Google Scholar]
- Wildsoet C (2003). Neural pathways subserving negative lens-induced emmetropization in chicks--insights from selective lesions of the optic nerve and ciliary nerve. Curr Eye Res, 27(6), 371–385. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/14704921. [DOI] [PubMed] [Google Scholar]
- Wildsoet C, & Wallman J (1995). Choroidal and scleral mechanisms of compensation for spectacle lenses in chicks. Vision Res, 35(9), 1175–1194. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/7610579. doi: 10.1016/0042-6989(94)00233-c [DOI] [PubMed] [Google Scholar]
- Wildsoet CF, & McFadden SA (2010). Optic Nerve Section Does Not Prevent Form Deprivation-Induced Myopia or Recovery From it in the Mammalian Eye. ARVO Meeting Abstracts, Investigative Ophthalmology & Visual Science, 51(13), 1737. doi:https://doi.org/ [Google Scholar]
- Wildsoet CF, & Pettigrew JD (1988). Experimental myopia and anamalous eye growth patterns unaffected by optic nerve section in chickens: Evidence for local control of eye growth Clinical Vision Sciences, 3(2), 99–107. Retrieved from https://espace.library.uq.edu.au/view/UQ:718523. [Google Scholar]


