Abstract
Purpose:
124I-PU-H71 is an investigational first-in-class radiologic agent specific for imaging tumor epichaperome formations. The intracellular epichaperome forms under cellular stress and is a clinically validated oncotherapeutic target. We conducted a first-in-human study of microdose 124I-PU-H71 for positron emission tomography (PET) to study in vivo biodistribution, pharmacokinetics, metabolism, and safety; and the feasibility of epichaperome-targeted tumor imaging.
Experimental Design:
Adult patients with cancer (n=30) received 124I-PU-H71 tracer (201±12 MBq, <25 μg) IV bolus followed by PET/CT scans and blood radioassays.
Results:
124I-PU-H71 PET detected tumors of different cancer types (breast, lymphoma, neuroblastoma, genitourinary, gynecologic, sarcoma, and pancreas). 124I-PU-H71 was retained by tumors for several days while it cleared rapidly from bones, healthy soft tissues, and blood. Radiation dosimetry is favorable and patients suffered no adverse effects.
Conclusions:
Our first-in-human results demonstrate the safety and feasibility of non-invasive in vivo detection of tumor epichaperomes using 124I-PU-H71 PET, supporting clinical development of PU-H71 and other epichaperome-targeted therapeutics.
Keywords: PET, PU-H71, cancer, heat shock protein 90, epichaperome
Introduction
New biomarker assays are urgently needed to optimize clinical development of new investigational cancer pharmacotherapeutics targeting tumor epichaperome formations. The epichaperome is defined by increased strength (thermodynamic stability) of molecular interactions within an integrated functional network of intracellular chaperome units, with the 90-kDa heat shock protein (HSP90) chaperone molecule as its keystone, induced by cellular stress associated with malignant transformation (1). This stress-induced epichaperome augments the fitness of several oncogenic protein networks that support cancer cell survival.
HSP90 is an important oncotherapeutic target well-credentialed by early-phase clinical trials with HSP90-targeted drugs and preclinical research (2-6), but recently researchers recognized HSP90 is an important oncotherapeutic target only when part of a tumor epichaperome formation (1,7-10). HSP90 is the most abundant chaperome member in human cells (1), but in most healthy cells and in many tumors, HSP90 exists in dynamic complexes or non-complexed forms biochemically and functionally distinct from HSP90 integrated into tumor epichaperome formations (1).
Clinical experience with first-generation HSP90 inhibitors (notably 17-AAG and 17-DMAG) revealed a need for improved drug safety and efficacy, with oncologists calling for co-development of new target- and drug-specific pharmacometric assays that can help identify patients whose tumors are most likely to respond to these drugs and inform therapeutic dosing strategy, to optimize intratumoral drug concentrations and thereby optimize dose-dependent tumor response (4-6,11-13). HSP90 expression in tumors does not predict tumor sensitivity to HSP90 inhibitors, nor do plasma pharmacokinetics provide a surrogate for tumor pharmacokinetics/drug binding, upon which tumor response depends (1,14,15).
To overcome such therapeutic and diagnostic limitations, we developed PU-H71, a small molecule inhibitor that selectively interacts with HSP90 within epichaperome complexes (epichaperome/HSP90), with negligible binding to HSP90 in healthy cells and tumors without epichaperome formations (1,10,16). After entering tumor cells by diffusion across plasma membranes, PU-H71 binds selectively to intracellular HSP90 ATPase binding sites that have a structural configuration characteristic of HSP90 incorporated into tumor epichaperomes; such HSP90 ATPase configurations are absent in healthy tissue cells (10). PU-H71 competitively inhibits ATP binding to HSP90, disabling its HSP90 ability to incorporate within multimeric chaperome complexes, thereby destabilizing epichaperome formations composed of hyperconnected chaperome networks that integrate and enable multiple vital tumor cellular processes (7). As a therapeutic, non-radioactive compound, PUH71 is administered via intravenous infusion. PU-H71 demonstrated safety and efficacy in preclinical models (17-21). One clinical phase 1 therapy dose escalation trial has been reported, to date (NCT01581541) (16), in which patients received 10 to 470 mg/m2/day on days 1 and 8, in 21-day cycles, for up to 6 cycles. Results from multiple other phase 1/2 PUH71 therapy trials are pending (www.clinicaltrials.gov) (16). Iodine-124 PU-H71 (124I-PU-H71) is a positron-emitting form of PU-H71 for non-invasive positron emission tomography (PET). 124I-PU-H71 and PU-H71 are identical in molecular structure, both with an iodine atom (positron-emitting I-124 and stable I-127, respectively), making 124I-PU-H71 a true PU-H71 tracer. 124I-PU-H71 PET offers a first-of-kind in vivo assay of tumor PU-H71 pharmacokinetics, epichaperome/HSP90 expression, and target engagement, advancing clinical development of PU-H71 therapy by PET applications toward optimizing patient selection, therapeutic dosing design, and response assessment (1).
We began a first-in-human trial to explore the feasibility of in vivo PET tumor imaging using microdose 124I-PU-H71 and to learn its in vivo pharmacokinetics, biodistribution, metabolism, and radiation dosimetry. To our knowledge, this is the first clinical trial of an epichaperome-targeted imaging agent. Following our preliminary report (1), we now provide the complete results of our trial.
Materials and Methods
Radiopharmaceutical preparation
[124I]-PU-H71 was prepared at the MSK Radiochemistry and Molecular Imaging Probes Facility in compliance with requirements specified in the Chemistry, Manufacturing, and Controls section of an FDA acknowledged IND (IND#110291), following published protocol (22). Briefly, 25 μg tin precursor (tert-butyl (3-(6-amino-8-((6-(trimethylstannyl)benzo[d][1,3]dioxol-5-yl)thio)-9H-purin-9-yl)propyl)(isopropyl)carbamate) was dissolved in 20 μL of methanol and added to a solution of radioactive sodium iodide [124I]-NaI in 50 μL 0.1 N sodium hydroxide solution. 15 μL of chloramine-T solution (1.5 mg/mL in acetic acid) was added and the solution was gently shaken and heated at 50 °C for 5 minutes to ensure complete radioiodination. Solution was allowed to cool to room temperature. 10 μL of methionine methyl ester (0.5 g/mL) in water and 10 μL of concentrated HCl were added and solution was heated at 50 °C for 30 min for removal of Boc protecting group. Reaction mixture was cooled to room temperature and purified using preparatory HPLC. Product was collected and solvent removed under reduced pressure using rotary evaporator. Final product was formulated in 5% ethanol in saline (0.9%) and passed through 0.22 μm sterilizing filter into pyrogen-free sterile final product vial. QC samples were withdrawn and used for quality control analysis. Purity was analyzed using HPLC under similar conditions and identity of the radioactive compound was confirmed by co-elution with non-radioactive standard using analytical HPLC (Supplementary Fig. 1). Sterility and endotoxin (LAL) testing were performed to ensure product safety. Average yields (isolated) of [124I]-PU-H71 were 55 (± 6) % with purity >98%. Specific activity of final formulation was ~980 mCi/μmol (range: 427 to 1403 mCi/μmol).
Patients
The study was approved by the institutional review board (NCT01269593) and performed under an investigational new drug application approved by U.S. Food and Drug Administration. Informed written consent was obtained from patients. The study was conducted in accordance with the Declaration of Helsinki.
Adult patients with solid malignancy or lymphoma were eligible (eligibility criteria detailed in Supplementary Table 1). For thyroid protection against possible free radioiodine, patients ingested seven drops of saturated solution of potassium iodide (SSKI) at least two hours prior to radiotracer injection repeating SSKI dose daily for two weeks post-injection. To detect thyroid injury, serum thyroid-stimulating hormone (TSH) assay was obtained <1-week pre-injection, as baseline; with follow-up TSH assay 6-12 months post-injection. Patients were monitored for signs and symptoms of toxicity at each imaging time-point. Patients reported any adverse symptoms experienced in the following 30 days to study investigators.
Plasma analyses
For radiotracer injection and blood sampling, separate peripheral intravenous (IV) lines were placed. Blood samples were collected 10, 20, and 30 minutes and 1-2 or 3-4 hours after tracer injection, and additionally 20-24 hours in a subset (n=7). For quantification of tracer clearance and metabolism, whole blood and/or plasma samples were radioassayed by well counter and radioHPLC using standard techniques (details in Supplementary Methods).
PET image acquisition
Patients received 201±12 MBq 124I-PU-H71 by IV injection over 1-2 minutes; no IV or oral radiographic contrast was administered. To facilitate bowel tracer clearance, patients ingested bisacodyl 15 mg orally on three consecutive nights post-injection. Scans were obtained at 3-4 (3.6±0.4) and 20-24 (22.5±1.7) hours post-injection, using a single Discovery DSTE™ PET-CT imaging system (General Electric, USA). Scout X-ray established patient anatomic landmarks. CT scans were acquired for attenuation correction and anatomic localization of PET data, spanning from skull vertex to proximal thighs. CT data was acquired with 140 kVp, current scaled according to body weight up to 80 mA, pitch of 1.75:1, reconstructed slice thickness of 3.75 mm, and 0.8 s per rotation. CT data was reconstructed in a 512 x 512 matrix using filtered back-projection algorithm.
To analyze early changes in biodistribution, 15 patients had additional PET scans at 0, 10, and 20 minutes post-injection using a single pre-injection CT scan for attenuation correction and anatomic localization of PET data. To evaluate the duration of tumor target binding, patients voluntarily participated in additional PET-CT time-points at 40-80 (n=21, 52.5±10.1) hours and/or approximately one week (n=9, 160.3±24.3 hours) post-injection. PET emission data was acquired for 45-60 minutes total, in craniocaudal direction from head to proximal thighs in approximately 6-7 PET bed positions. For 0, 10, and 20-minute post-injection PET scans, PET acquisition time was distributed equally over bed positions. For later time-points, in the initial 7 patients, PET image acquisition time was distributed equally over bed positions. To optimize visualization of tumor uptake in the subsequent 23 patients, PET acquisition spanned the body in 10 minutes with imaging of selected body region of interest (ROI), in one bed position, for an additional 35-50 minutes. To identify optimal imaging duration per bed position, PET images for 10 patients were reconstructed from subsets of the PET ROI data, in increasing 5-minute increments up to 50 minutes total (5, 10, 15 minutes, etc.). Tumor sites were confirmed from the most recent CT, MRI, and/or FDG PET/CT scans obtained as per standard of care <4 weeks prior to trial enrollment, with no interim treatment prior to the PUH71 PET scans. Tumor SUVs (standard uptake values) measured from reconstructed PET data subsets were plotted as a function of PET scan duration, to identify the minimum scan duration time (minutes/bed) at which tumor SUV values became repeatable/consistent with values measured from longer-duration scans. SUVs represent the PET-measured tissue uptake quantified as the fraction of the injected tracer dose per mL of tissue, normalized to patient mass (grams); we used the common SUVpeak parameter, which reports the average voxel SUVs within the one-cm3 spherical region of highest tumor uptake. PET images were reconstructed using an ordered subset expectation maximization iterative algorithm. Emission data corrected for randoms, detector inhomogeneity, scatter, attenuation, deadtime, and decay. Images were reconstructed to a 128 x 128 matrix over a 70-cm field of view. PET emission scans were acquired in two-dimensional (2D) mode for all patients except two patients for whom 3D PET was performed. For the 3D PET image reconstructions, an additional correction for cascade coincidences (prompt gammas) was applied using an in-house algorithm based on a scaled random distribution (see Supplementary Methods).
Biodistribution and radiation dosimetry
PET/CT data was analyzed by an experienced nuclear medicine physician (M.D.) and medical physicist (P.Z.) using PET-CT image display software (HERMES Gold4.4-B, HERMES Medical Solutions AB, Stockholm, Sweden; and AW Centricity Imaging-PACS/AW Suite, GE Healthcare Integrated IT Solutions, Barrington, IL). We quantified tracer biodistribution within tumors and organs of interest. For radiation dosimetry, organ-specific time-integrated activity coefficients, ã(rS, TD), were determined as input data for OLINDA/EXM (OLINDA/EXM v.1, Vanderbilt University, Nashville, TN), an FDA-approved radiation dosimetry software package (23), to obtain calculations of absorbed and effective dose. Model of tracer excretion is described in the next paragraph. The standard Voiding Bladder Model, as implemented in OLINDA, estimated human urinary bladder dosimetry (24) from PET-derived fractional urinary and clearance half-time values. Disintegrations occurring in the small and large intestinal compartments were calculated from the PET-derived fractional biliary excreta used as input for the ICRP 100 Human Alimentary Tract model (25) implemented using SAAM II (version 2.2.1; SAAM Institute, University of Washington), modifying compartmental transfer coefficients to account for prokinetic effects of bisacodyl supplements provided to patients (26) (see Supplementary Methods for additional details).
Tracer excretion
To quantitatively model urinary and biliary tracer excretion visualized by PU-H71 PET, total urinary activity at each PET scan/time-point was measured by 3D VOI encompassing urinary bladder, noting no visually detectable radio-excreta in the upper urinary tract in any patient. Bladder wall tissue uptake was not evident (thus, it did not hinder VOI measurements). Total excreted biliary-fecal activity within hepatobiliary tree and bowels was also measured by 3D VOI. Bowel and biliary vessel wall uptake were negligible, relative to excreta activity. Tracer lost from the body by defecation or urination was tracked, indirectly, by monitoring the amount of activity in the entire field of view (FOV) of each serial, decay-corrected PET dataset, using the total activity present in the initial PET scan immediately after tracer injection as the normalizing factor to which subsequent PET FOV activities were compared (as detailed in Supplementary Methods).
Statistics
For quantitative tumor analyses, the tumor with the highest SUV was selected from each patient. Tumors were qualitatively dichotomized as avid or not avid based on PET appearance at the 24-hour time-point; tumors with tracer retention greater than bloodpool tracer retention were considered avid. Graphs were plotted and statistical analyses performed with SAS Studio 3.5 (SAS Institute, Cary, NC). Experimental data were summarized by descriptive statistics and data distributions were assessed for normality. Tumor uptake and blood radioassay values at paired time-points were compared using Wilcoxon signed-rank test. Data frequency comparisons were performed by Fisher exact test. Continuous data from independent samples were compared by Mann-Whitney U test. Data relationships were assessed by linear regression analysis with R2 statistic where appropriate. P < 0.05 were considered statistically significant. All statistical tests were two-sided.
Results
Patients
Patient and disease characteristics are shown in Table 1. Thirty patients were recruited with various cancers: breast cancer (n=10); cervix (n=1); chronic lymphocytic leukemia (n=1); Hodgkin lymphoma (n=1); non-Hodgkin lymphoma (n=4); neuroblastoma (n=2); ovarian cancer (n=4); pelvic clear cell carcinoma of Mullerian origin (n=1); pleomorphic sarcoma (n=1); prostate cancer (n=3); and pancreas cancer (n=2). All patients were compliant with thyroid-protective SSKI dosing. No signs or symptoms of adverse effects from the PUH71 tracer occurred, and an absence of thyroid toxicity was demonstrated by clinical and serum assays in the minimum six-month follow-up period.
Table 1.
Patient and tumor characteristics, including tumor histology and tracer avidity on [124I] PU-H71 PET. SUV, standard uptake value (calculated as SUVpeak; see Methods); NA, not applicable or data not available; CLL, chronic lymphocytic leukemia; NHL, non-Hodgkin lymphoma.
| Pt | Cancer | Age | Sex | PU-H71- Avid† |
SUV 4 h |
T:B‡ 4 h |
T:M‡ 4 h |
SUV 24 h |
T:B‡ 24 h |
T:M‡ 24 h |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Breast | 52 | F | Y | 1.8 | 2.1 | 1.5 | 1.3 | 3.6 | 3.3 |
| 2 | Breast | 49 | F | Y | 2.7 | 9.6 | 7.7 | 1.0 | 5.9 | 5.6 |
| 3 | Breast | 68 | F | NA* | NA* | NA* | NA* | NA* | NA* | NA* |
| 4 | Breast | 50 | F | Y | 1.5 | 1.7 | 2.7 | 0.9 | 2.3 | 5.2 |
| 5 | Breast | 66 | F | Y | 2.2 | 3.6 | 4.9 | 2.1 | 7.8 | 17.5 |
| 6 | Breast | 58 | F | N | 0.9 | 1.7 | 1.2 | 0.3 | 1.3 | 1.3 |
| 7 | Breast | 60 | F | Y | 1.2 | 1.7 | 2.4 | 1.5 | 4.8 | 5.1 |
| 8 | Breast | 35 | F | N | 1.1 | 1.8 | 1.8 | 0.3 | 1.0 | 1.6 |
| 9 | Breast | 43 | F | Y | 1.6 | 3.1 | 6.0 | 0.6 | 5.6 | 11.2 |
| 10 | Breast | 44 | F | Y | 11.0 | 14.4 | 22.0 | 3.8 | 13.1 | 20.0 |
| 11 | Cervix | 56 | F | Y | 2.5 | 6.1 | 5.1 | 0.8 | 1.7 | 8.0 |
| 12 | CLL | 65 | M | Y | 0.64 | 1.5 | 2.1 | 0.4 | 4.0 | 6.7 |
| 13 | Hodgkin | 42 | F | NA** | NA** | NA** | NA** | NA** | NA** | NA** |
| 14 | NHL | 47 | F | N | 0.8 | 1.2 | 1.2 | 0.1 | 0.6 | 1.2 |
| 15 | NHL | 62 | F | Y | 1.1 | 1.8 | 2.8 | 0.6 | 3.0 | 6.0 |
| 16 | NHL | 65 | F | N | 0.7 | 1.5 | 1.9 | 0.2 | 1.2 | 2.3 |
| 17 | Neuroblastoma | 31 | F | N | 0.7 | 2.2 | 2.4 | 0.1 | 1.2 | 1.5 |
| 18 | Neuroblastoma | 25 | M | Y | 1.6 | 2.7 | 5.3 | 0.6 | 3.2 | 6.4 |
| 19 | NHL | 82 | M | Y | 0.9 | 1.8 | 2.0 | 0.4 | 0.9 | 1.0 |
| 20 | Ovarian | 75 | F | NA*** | NA*** | NA*** | NA*** | NA*** | NA*** | NA*** |
| 21 | Ovarian | 58 | F | N | 0.9 | 0.9 | 1.0 | 0.4 | 0.6 | 1.1 |
| 22 | Ovarian | 59 | F | Y | 3.4 | 5.0 | 5.4 | 1.0 | 5.1 | 6.7 |
| 23 | Ovarian | 27 | F | N | 1.6 | 2.5 | 2.0 | 0.7 | 2.4 | 6.0 |
| 24 | Pancreas | 76 | F | Y | 0.8 | 4.2 | 4.0 | 0.8 | 19.8 | 8.8 |
| 25 | Pancreas | 66 | M | N | 1.7 | 1.6 | 3.3 | 0.6 | 0.7 | 2.1 |
| 26 | Müllerian∥ | 57 | F | Y | 3.2 | 4.2 | 5.2 | 1.8 | 4.0 | 6.2 |
| 27 | pleomorphic sarcoma | 71 | M | N | NA**** | NA**** | NA**** | 1.1 | NA**** | 2.5 |
| 28 | Prostate | 57 | M | NA***** | NA***** | NA***** | NA***** | NA***** | NA***** | NA***** |
| 29 | Prostate | 71 | M | N | 2.1 | 1.3 | 3.0 | 1.6 | 1.8 | 4.6****** |
| 30 | Prostate | 67 | M | N | 1.3 | 2.9 | 3.8 | 0.5 | 1.3 | 1.4 |
Abbreviations: Pt: patient, F: female, M: male, Y: yes, N: No, SUV: standardized uptake value.
Tumor avidity for PU-H71 (Y, yes; N, no). Avidity defined by demonstrated tracer uptake and retention greater than blood pool and background tissues on PU-H71PET at 24-hour time-point.
Tumor:Blood pool ratio. Tumor avidity on PU-H71 PET quantified by conventional standardized uptake value (SUVpeak) and the ratio of tumor SUVpeak to blood pool SUVpeak, measured from PET images obtained approximately 24 hours post-injection (see Methods).
Any systemic anti-cancer treatment <4 weeks prior to PU-H71 PET; days between last treatment dose and the PU-H71 PET study are shown in parentheses.
Pelvic clear cell carcinoma of Müllerian origin
Patient 3 had subcentimeter cutaneous tumor.
Patient 13 had renal lymphomatous tumors; excreted radiotracer obscured PET evaluation.
Patient 20 had ill-defined subcentimeter peritoneal tumor infiltrate.
Patient 27: 4h PET scan not obtained; 24h PET scan technical artifact limiting bloodpool SUV measurement.
Patient 28: PET evaluation of tumor site obscured by adjacent physiologic bowel activity.
Patient 29. SUV values attributable to PET noise artifact.
Blood kinetics and metabolism
Plasma radiotracer clearance and exposure were similar between patients, despite significant variation in tumor tracer retention, as discussed below. This indicates plasma pharmacokinetics are not a surrogate for tumor pharmacokinetics, consistent with our preclinical data (27). Tracer (i.e., parent 124I-PU-H71 and radiometabolites) cleared rapidly from systemic blood circulation as a bi-exponential function of time. A rapid initial phase (t1/2 α 8.6 ±4.8 min; 95% CI [6.9–10.3]) was followed by a slow terminal phase that began around the 4-hour time-point (t1/2 β 163.9 ±155.1 min; 95% CI [108–219]). Plasma radiotracer exposure was 1.7±0.6 % (95% CI [1.5–1.9]) ID•g•h/mL from 10 minutes to 4 hours post-injection. Radiotracer became predominantly radiometabolites within 1 hour p.i. (Table 2). PUH71 metabolites are not pharmacologically active. Average tracer blood:plasma ratio was consistent (0.76 ±0.04) across time-points (up to 4 hours, p.i.). Blood tracer levels were scant by 24 hours post-injection (24-hour bloodpool SUV 0.2 ±0.1 average/SD; 95% CI [0.16–0.24]; see Table 3 and Fig. 1).
Table 2.
124I-PU-H71 is rapidly cleared from plasma and undergoes in vivo metabolism.
| Plasma t1/2 α (min) |
Plasma t1/2 α (min) |
Plasma t1/2 β (min) |
Plasma t1/2 β (min) |
Blood t1/2 α (min) |
Blood t1/2 α (min) |
Blood t1/2 β (min) |
Blood t1/2 β (min) |
||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| GM | GSD | GM | GSD | GM | GSD | GM | GSD | ||||
| Tracer (total) | 8.3 | 4.4 | 521.2 | 325.4 | 8.2 | 3.6 | 354.3 | 153.3 | |||
| 124I-PU-H71 | 7.0 | 2.1 | 264.1 | 2.4 | 7.9 | 1.5 | 366.2 | 1.2 | |||
| 10 min | 20 min | 30 min | 1h | 4h | |||||||
| GM | GSD | GM | GSD | GM | GSD | GM | GSD | GM | GSD | ||
| Free I-124 | 0-4 MIN | 5.9% | 2.6% | 14.8% | 1.5% | 10.0% | 6.1% | 13.2% | 6.2% | 22.3% | 4.4% |
| Radio-metabolites | 5-17 MIN | 13.0% | 1.7% | 22.0% | 1.6% | 28.1% | 1.3% | 33.5% | 1.4% | 36.5% | 1.1% |
| Intact PU-H71 | 18-20 MIN | 69.2% | 1.3% | 51.7% | 1.2% | 44.9% | 1.3% | 32.3% | 1.5% | 22.7% | 1.6% |
| Waste | 21-30 MIN | 5.3% | 1.6% | 6.0% | 2.0% | 5.5% | 2.0% | 4.1% | 1.7% | 3.9% | 1.9% |
Note: Data shown are relative amounts of intact parent compound and radiometabolites in plasma supernatant at 10, 20, and 30 minutes and 1 and 4 hours after tracer injection (population average ± SD). Parent and metabolites were separated by radioHPLC, identified by characteristic radioHPLC elution times. The amount of each radioHPLC eluted peak was quantified by scintillation well-counting, correcting for background activity and physical decay. Relative amounts were calculated as the percentage of the sum of total activity of identified radioHPLC peaks in each sample. GM, geometric mean; GSD, geometric standard deviation factor.
Table 3.
124I-PU-H71 accumulates in tumors, with negligible retention in most normal organs.
| Organ | 4 hour (n=30) | 24 hour (n=30) | ||
|---|---|---|---|---|
| Mean SUV | Mean SD | Mean SUV | Mean SD | |
| Aortic blood pool | 0.5 | 0.2 | 0.2 | 0.1 |
| Appendicular skeleton* (humerus)* | 0.5 | 0.3 | 0.3 | 0.3 |
| Axial skeleton* | 1.0 | 0.3 | 0.3 | 0.3 |
| Brain | 0.1 | 0.1 | 0.1 | 0.2 |
| Kidney | 1.5 | 0.6 | 0.6 | 0.4 |
| Liver | 6.4 | 2.3 | 2.3 | 1.7 |
| Lung | 0.4 | 0.2 | 0.2 | 0.1 |
| Muscle, skeletal | 0.4 | 0.2 | 0.2 | 0.1 |
| Myocardium | 2.8 | 1.0 | 1.7 | 1.1 |
| Spleen | 2.0 | 1.2 | 0.7 | 0.4 |
| Thyroid | 2.3 | 1.3 | 1.1 | 0.9 |
| Tumors | 1.9 | 2.0 | 0.9 | 0.8 |
Note: Biodistribution and tumor uptake at 4 and 24 hours after tracer injection. Shown are study population (n=30 patients) average and standard deviation values for tracer uptake in major organs and tracer-avid tumors in terms of standardized uptake value (SUV average; see Methods). Thyroid uptake likely represents free radioiodide uptake (product of in vivo tracer metabolism); hepatic uptake likely represents tracer metabolism and hepatobiliary excretion.
Tracer uptake in humerus considered representative of compact bone/osteogenic cell tracer avidity and the spine representative of tracer uptake in bone red marrow (see Results).
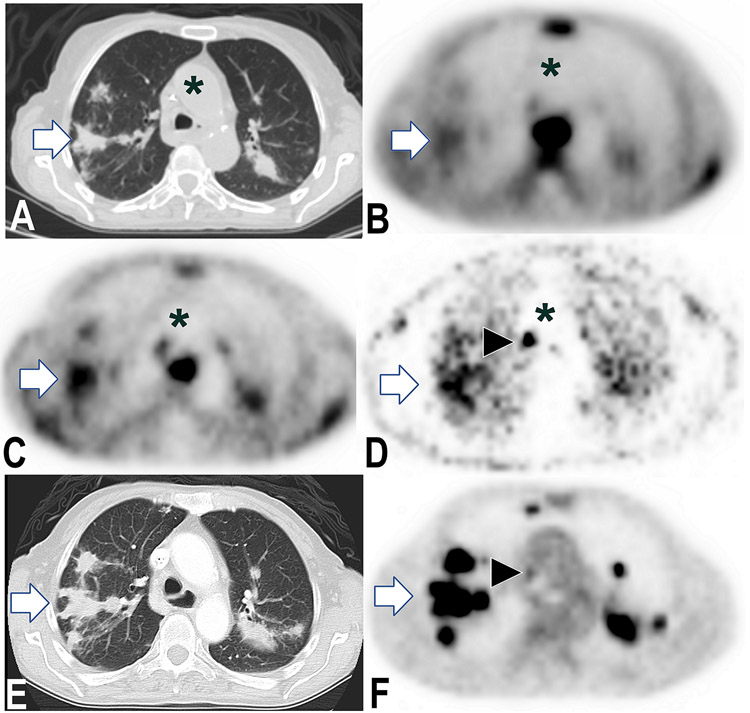
Figure 1.
Metastases demonstrate sustained PU-H71 retention one week post-injection. 76-year-old female with stage IV pancreatic cancer, on three-month chemotherapy vacation. PET and CT images show corresponding axial planes of chest. 124I-PU-H71 PET images obtained 3, 24, and 192 hours post-injection (B, C, and D, respectively) show rapid tracer clearance from blood (asterisk, aorta) with sustained PU-H71 retention by pulmonary metastases (e.g., right lung mass, arrow) and nodal metastases (e.g., arrowhead). Between 3 and 24 h (B and C), tumor:bloodpool SUV ratio value increased 371% (3 and 24 h ratios 4.2 and 19.8, respectively). Corresponding images from standard F-18 fluorodeoxyglucose (FDG) PET/CT images (E and F) obtained 2 days prior.
Tumor pharmacokinetics and tracer biodistribution
PET demonstrated the feasibility of tumor detection in humans by epichaperome-targeted imaging, visualizing tumor-selective PU-H71-targeting in vivo (Figs. 1-2, Supplementary Figs. 4-5). Our clinical PET data indicates the presence of tumor epichaperome formations in a variety of cancer types, as previously demonstrated preclinically (1). PU-H71-avid tumors were detected in 58% of patients (n=26, Table 1), significantly higher in PU-H71 concentration than non-avid tumors, in terms of SUV, tumor:bloodpool and tumor:muscle ratios at 24 hours (Supplementary Table 2). Evaluated tumors ranged in size from 0.8 to 4.5 cm (1.6 ± 0.5 cm average/SD). Contrary to rapid PU-H71 clearance from blood over a few hours, seen in all patients (as above), prolonged tracer retention in PU-H71-avid tumors was evident on PET imagery over a period of days (up to one week post-injection, Fig. 1). Across the patient population, rapid blood clearance yielded tumor:bloodpool ratios of radiotracer concentrations up to 14.4 at 4 hours (3.2± 3.0 average/SD) and up to 13.1 at 24 hours (3.9 ± 4.4 average/SD) post-injection (Figs. 1 and 2, Supplementary Fig. 2), consistent with specific PU-H71-binding to tumors, observed sustained for days beyond drug clearance from plasma.
Figure 2.
Typical 124I-PU-H71 biodistribution. 57-year-old female with clear cell carcinoma of Müllerian origin, including biopsied malignant pulmonary mass (arrow). Three-dimensional maximum intensity projection (MIP) PET images obtained 1, 4, and 24 hours post-injection 326 MBq 124I-PU-H71. PET image intensity normalized to pulmonary mass SUVmax. Malignant pulmonary tumor demonstrates sustained PU-H71 retention, while tracer clears from systemic organs, including bone marrow (arrowhead), myocardium (white asterisk), and skeletal musculature (black asterisk). Predominant hepatic metabolism with biliary-fecal excretion evident.
Plasma tracer pharmacokinetics did not predict tumor PU-H71 tracer retention at the 24-hour time-point, indicating PU-H71 PET data offers a new, non-trivial in vivo tumor pharmacokinetic biomarker. Plasma tracer clearance and exposure were similar between patients (plasma AUC coefficient of variation 36.6%) with scant circulating tracer by 24 hours. In contrast, tumor tracer retention (SUV) at 24 hours post-injection varied considerably between patients (24-hour tumor SUV range 0.1-3.8; average/SD 0.9 ± 0.8 average/SD, with coefficient of variation 88.9%) and did not correlate with plasma tracer exposure (Supplementary Fig. 3). Differences in tumor retention (24-hour SUV) were not explainable by differences in plasma drug availability. Overall tumor SUV decreased between 4 to 24 hours by 50.7 ± 27.0% (range: −25.0 to +87.5%; SUV units 1.0±1.4; p=0.00002), consistent with reversible tracer binding, with tumor drug-efflux rate varying between patients (Table 1).
Duration of tumor cell PU-H71 retention, in cancer cell lines and patient-derived tumor specimens, quantitatively correlates with epichaperome levels, and in turn with tumor PU-H71 sensitivity (1). Tumor PU-H71 retention in our population demonstrated a trend toward an average increase in tumor:bloodpool SUV ratio of 25.7 ± 99.9% from 4 to 24 hours post-injection (median 4.8%; range: −72.1 to +371.4%; see Figs. 1 and 3; p=0.9). Tumor-selective PU-H71 retention was statistically evident in terms of an average increase tumor:muscle SUV ratio of 52.0 ± 87.3% from 4 to 24 hours post-injection (p=0.02, Z = −2.2963; median 20.9%; range: −63.2 to +257.1%) indicating tumors retain PU-H71 more than avidly than healthy musculature; with significant variability in PU-H71 avidity between tumors (Figs. 3 and 4). Preclinical data indicate tumors vary in expression of epichaperome formations, with corresponding variability in tumor sensitivity to targeted therapy (1).
Figure 3.
Tumor retention of PU-H71 tracer varied considerably among patients. Tumor retention is expressed as tumor:muscle SUV ratio (y-axis) measured on PET at 4 and 24 hours post-tracer injection.
Figure 4.
Tumor retention of PU-H71 tracer varied considerably among patients. Tumor retention was expressed as tumor SUV (y-axis) or tumor:muscle SUV ratio (y-axis) measured on PET at 24 hours post-tracer injection. Patient ID number corresponds to Table 1.
Normal organs overall demonstrated low PU-H71 tracer concentrations (Table 3) with progressive tracer clearance at each time-point, indicating the absence of epichaperome formations in most healthy tissues. Among normal organs, I-124 tracer residence time was greatest in the liver, thyroid, and myocardium, showing equal or greater average uptake than tumors. Hepatic tracer uptake is attributable to drug metabolism and visualized hepatobiliary clearance. Thyroid uptake, probably uptake of free radioiodine, was nontoxic dosimetrically (Supplementary Table 3) and clinically, with no thyrotoxicity detected by serum TSH assays at follow-up. Activity was initially prominent in bone red marrow and spleen, clearing by the second and third post-injection days, respectively (Fig. 2). Mild tracer accumulation was visualized in the choroid plexi. Brain uptake was scant, consistent with blood-brain barrier drug impermeability. Other tissues demonstrated variable mild uptake, present in some but not all patients; uptake that was probably physiological, with no clinical evidence of pathology; including variable uptake in pancreas, extraocular musculature, and adipose tissues.
Hepatobiliary excretion of tracer predominated (45.4 ±15.8%ID; 95% CI [39.8–51.1]) with a lesser amount of urinary excretion (29.5 ±10.9%ID; 95% CI [25.6–33.4]). Onset of tracer excretion was prompt, with biliary-to-duodenum transit of excreted tracer and bladder accumulations detectable by the first or second PET scans (before 10-20 minutes post-injection).
Radiation dosimetry
PU-H71 tracer dosimetry was favorable, with an effective dose of 0.5 ± 0.1 (95% CI [0.46–0.54]) mSv/MBq. Lower large intestines were the critical organ (Supplementary Table 3; 2.0±0.5 mGy/MBq; 95% CI [1.8–2.2]), attributable to observed biliary-fecal tracer excretion. Despite in vivo production of free I-124 radiometabolite, identified by radioHPLC analysis, thyroid-absorbed doses were low, consistent with effective thyroid blockade by prescribed SSKI. No patients demonstrated thyroid injury, clinically, or significant change in serum TSH assay values versus baseline (p=ns) 6-12 months after tracer injection.
Discussion
Our first-in-human trial demonstrated for the first time, to our knowledge, the safety and feasibility of in vivo radiologic detection of cancerous tumors in human subjects using an epichaperome-/HSP90-targeted imaging agent (1). Epichaperome-targeted PET imaging with 124I-PU-H71 offers a new diagnostic approach to the urgent, unmet clinical need for target-specific clinical biomarkers in the development of epichaperome-targeted pharmacotherapy, with potential clinical applications to guide patient selection, optimize dosing strategy, and understand responses in ongoing early-phase cancer therapy trials.
Over 20 HSP90 inhibitors have entered clinical trials with typical cancer response rates of ~20% as monotherapy after standard therapy has failed (4-6,11-13). Because minor response rates with early HSP90 inhibitors occur in patients with the most prevalent types of solid cancers, lymphoma, and myeloma, the absolute number of cancer patients who could potentially benefit from epichaperome/HSP90-targeted therapy can reasonably be estimated as approaching one million people, annually, worldwide (28). Such potential clinical impact cannot be overlooked and makes clearly evident the need for new diagnostics to identify this important subset of responders, pretreatment, and optimize therapeutic dosing. Target- and drug-specific pharmacodynamic and pharmacokinetic assays are vital in early-phase trials to iteratively optimize cancer drug development (29-32).
Tumor response to epichaperome inhibitors depends upon whether the tumor has formed epichaperomes and upon tumor pharmacokinetics (1). Invasive tissue assays of HSP90 and related biomarkers, to date, have proven impractical and of dubious predictive or pharmacometric value (4-6,12). Blood-based HSP90-related assays—e.g., pharmacodynamic (PD) monitoring of peripheral blood cells—and monitoring of plasma drug concentrations of HSP90 inhibitors have validated no surrogate response marker nor identified an optimal dosing strategy (4-7).
PU-H71 PET offers a fundamentally new approach to trial pharmacometrics for epichaperome-targeted therapeutics (4,5,7,11-13) with potential applications in patient selection, image-guided dosing design, and tumor response analyses. The (non-radioactive) small molecule PU-H71 is one of a new generation of inhibitors now entering clinical trials, selectively targeting HSP90 inhabiting the tumor epichaperome (NCT01393509, NCT03166085, NCT03373877, NCT01581541). Chiosis and colleagues demonstrated that tumor sensitivity to PU-H71 is determined specifically by tumor HSP90 integration into epichaperome networks, not by total tumor HSP90 protein expression, and that tumor PU-H71 avidity is a biomarker of tumor epichaperome (1,33). 124I-PU-H71 has ideal characteristics as a tracer of therapeutic PU-H71. The radiotracer and therapeutic drug are the same molecule, differing only in the isotope of an endogenous iodine atom. After intravenous injection, intratumoral drug is almost entirely intact parent PU-H71, with spontaneous efflux of metabolites in HPLC-MS/MS of tumor specimens (34). Thus, tumor signal detected by PET represents intact 124I-PU-H71 and can be used to quantify tumor molar concentrations of PU-H71 therapy; we are currently obtaining biopsy-based clinical validation of PET measurements of in vivo tumor drug concentrations in human subjects receiving PU-H71 therapy (NCT01393509) (35). PU-H71 PET will thereby enable non-invasive monitoring of tumor dose, advancing pharmacometric dose-response analyses beyond correlations with injected dose or plasma exposure. Targeted PET imaging can offer clinical in vivo patient data on targeted drug biodistribution, changes in tumor target expression, and the extent of tumor target engagement by drug, across multiple time-points (36,37).
This trial successfully accomplished the standard first step for introducing a new radiolabeled drug, into human usage: testing the safety and in vivo behavior of the compound, in a pilot study (38,39). PET detected PU-H71-avid tumors in most patients (58%), consistent with the percentage of tumors found in preclinical research to express the epichaperome.(1,10) Our trial data demonstrates that tumor retention of PU-H71, which varied considerably between patients, could not be predicted by plasma PU-H71 pharmacokinetics. Plasma PK/drug clearance, determined from serial blood sampling, is a conventional pharmacometric gauge of systemic exposure to drug used as a basis for rational dosing strategy design (32). Yet plasma PK do not predict tumor sensitivity to PU-H71 or to early HSP90 inhibitors (1,7). PU-H71 plasma clearance was similar in all patients, including both patients with and without sustained tumor PU-H71 avidity; and animal models show plasma concentrations are an inaccurate surrogate for in vivo tumor drug concentrations (1,27). Because tumor response to HSP90 inhibitors is dose-dependent (1,27)—dependent upon tumor dose, not merely plasma dose—PU-H71 PET offers an attractive tumor PK biomarker for discovering how to properly dose patients in early phase trials and routine practice, to optimize tumor dose toward anti-tumor efficacy (30,32).
Tumor PU-H71 avidity is an investigational biomarker of tumor cell epichaperome formation, validated in preclinical models, including cancer cell lines and patient-derived tumor specimens (1,7). PU-H71 specifically binds the ATPase of HSP90 integrated within the epichaperome as found in tumor cells, inducing tumor cell death, whereas PU-H71 has negligible HSP90 binding or cellular pharmacodynamic effect absent the epichaperome—thereby avoiding damage to normal tissues (1). Inhibition of HSP90 ATPase does not appear sufficient to induce tumor cell death in the absence of cellular epichaperome formation that integrates HSP90 (1). Our PET trial data confirms the in vivo selectivity of PU-H71 for the tumor epichaperome with distinct tracer retention by tumors detectable even one week post-injection. Hepatobiliary-fecal tracer excretion potentially limits the detection of tracer-avid tumors in the liver and bowels, in contrast to otherwise rapid systemic PU-H71 clearance from healthy tissues. We are testing the hypothesis that tumor pharmacokinetics from PU-H71 PET correlate with tumor response in ongoing PU-H71 therapy trials (NCT01393509 and NCT03166085) to obtain clinical validation of the strong correlation we observe between tumor PU-H71 avidity and response in preclinical models (1). Preliminary preclinical and clinical data (personal communication, Dr. Chiosis) indicates PU-H71 PET has potential application as a target biomarker for other HSP90-targeted pharmacotherapeutics that have selectively targeted the ATP-binding region of HSP90, to date (40).
First-in-human trial data presented herein demonstrates the safety and feasibility of non-invasive in vivo tumor detection using a first-in-class investigational PET biomarker of the tumor epichaperome, 124I-PU-H71. Tumors across a wide histologic spectrum of cancer types demonstrated PU-H71 avidity. Our trial results support ongoing phase 1/2 clinical trials testing the ability of epichaperome-targeted PET imaging to address the urgent unmet need for new pharmacometrics that can optimize patient selection and dosing strategy with epichaperome/HSP90-targeted therapeutics, toward improved patient outcomes. PU-H71 PET validation is expected to enhance HSP90/epichaperome pharmacometrics with clinical applications as a radiologic tumor epichaperome/HSP90 biomarker; guiding patient selection for HSP90-targeted therapy (predicting tumor sensitivity based upon tumor 124I-PU-H71 avidity); enabling dose-response analyses based upon time-integrated tumor dose, not merely plasma exposure; and personalizing PU-H71 therapeutic dosing strategy based upon tumor pharmacokinetics, not merely plasma pharmacokinetics.
Supplementary Material
Statement of Translational Relevance.
The epichaperome is a well-validated new oncotherapeutic target. Epichaperome-targeted PET offers first-of-kind pharmacometrics that promise to optimize clinical development of this important new class of cancer drugs.
Statement of Significance.
This article publishes results from the first-in-human trial of 124I-PU-H71, the first clinical imaging agent for in vivo assay of the tumor epichaperome, a key oncotherapeutic target.
Acknowledgments
We thank Ms. Leah Bassity for editorial assistance. We are indebted to Dr. Rashid Ghani and his MSKCC Radiopharmacy staff, MSKCC Radiology RN Louise Harris, and MSKCC Radiology research team members Tamara Torres, Brian Krichevsky, Alicia Lashley and Crystal Gansukh for their assistance in conducting the patient studies.
Financial Support: This research was funded in part through the NIH/NCI Cancer Center Support Grant P30 CA008748, as well as by NIH grants R01 CA172546, P01 CA186866, R56 AG061869, P50 CA192937, R21 CA167803, and R35 CA232130. Samus Therapeutics Inc., the Jane H. Gordon Breast Cancer Research Fund, the Breast Cancer Research Fund, Susan G. Komen for the Cure, and the Experimental Therapeutics Center of MSK also provided support for this research.
Footnotes
Disclosure of Potential Conflicts of Interest: Memorial Sloan Kettering Cancer Center holds the intellectual rights to PU-H71 and [124I]-PU-H71 (PCT/US06/03676, PCT/US2012/045861), which have been licensed to Samus Therapeutics. NP, TT, GC, JSL and SML are listed as the inventors on the licensed intellectual property. MD, NP, TT, DZ, SO, GC, JSL and SML have received financial royalties for their IP contributions. GC has partial ownership of Samus Therapeutics and serves a member of its scientific advisory board. GC and LN are co-founders of, and have financial interests in, Samus Therapeutics, Inc. MD is listed on a patent concerning 124I-PU-H71 unrelated to the current trial. SML reports receiving commercial research grants from Genentech, Inc., WILEX AG, Telix Pharmaceuticals Limited, and Regeneron Pharmaceuticals, Inc.; holding ownership interest/equity in Elucida Oncology Inc.; and holding stock in ImaginAb, Inc. He is the inventor and owner of issued patents both currently unlicensed and licensed by MSK to Samus Therapeutics, Inc., Elucida Oncology, Inc., and Y-mAbs Therapeutics, Inc. SML serves or has served as a consultant to Cynvec LLC, Eli Lilly & Co., Prescient Therapeutics Limited, Advanced Innovative Partners LLC, Gerson Lehrman Group, Progenics Pharmaceuticals, Inc., and Janssen Pharmaceuticals, Inc. The other authors of this study declare that they have no competing interests.
References
- 1.Rodina A, Wang T, Yan P, Gomes ED, Dunphy MP, Pillarsetty N, et al. The epichaperome is an integrated chaperome network that facilitates tumour survival. Nature 2016;538(7625):397–401 doi 10.1038/nature19807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Solit DB, Basso AD, Olshen AB, Scher HI, Rosen N. Inhibition of heat shock protein 90 function down-regulates Akt kinase and sensitizes tumors to Taxol. Cancer Res 2003;63(9):2139–44. [PubMed] [Google Scholar]
- 3.Solit DB, Zheng FF, Drobnjak M, Munster PN, Higgins B, Verbel D, et al. 17-Allylamino-17-demethoxygeldanamycin induces the degradation of androgen receptor and HER-2/neu and inhibits the growth of prostate cancer xenografts. Clin Cancer Res 2002;8(5):986–93. [PubMed] [Google Scholar]
- 4.Solit DB, Ivy SP, Kopil C, Sikorski R, Morris MJ, Slovin SF, et al. Phase I trial of 17-allylamino-17-demethoxygeldanamycin in patients with advanced cancer. Clin Cancer Res 2007;13(6):1775–82 doi 13/6/1775 [pii] 10.1158/1078-0432.CCR-06-1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Banerji U, O'Donnell A, Scurr M, Pacey S, Stapleton S, Asad Y, et al. Phase I pharmacokinetic and pharmacodynamic study of 17-allylamino, 17-demethoxygeldanamycin in patients with advanced malignancies. J Clin Oncol 2005;23(18):4152–61 doi 23/18/4152 [pii] 10.1200/JCO.2005.00.612. [DOI] [PubMed] [Google Scholar]
- 6.Jhaveri K, Ochiana SO, Dunphy MP, Gerecitano JF, Corben AD, Peter RI, et al. Heat shock protein 90 inhibitors in the treatment of cancer: current status and future directions. Expert Opin Investig Drugs 2014;23(5):611–28 doi 10.1517/13543784.2014.902442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Joshi S, Wang T, Araujo TLS, Sharma S, Brodsky JL, Chiosis G. Adapting to stress - chaperome networks in cancer. Nature reviews Cancer 2018. doi 10.1038/s41568-018-0020-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dart A Tumorigenesis: Networking: a survival guide. Nature reviews Cancer 2016;16(12):752 doi 10.1038/nrc.2016.125. [DOI] [PubMed] [Google Scholar]
- 9.Kourtis N, Lazaris C, Hockemeyer K, Balandran JC, Jimenez AR, Mullenders J, et al. Oncogenic hijacking of the stress response machinery in T cell acute lymphoblastic leukemia. Nat Med 2018;24(8):1157–66 doi 10.1038/s41591-018-0105-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang T, Rodina A, Dunphy MP, Corben A, Modi S, Guzman ML, et al. Chaperome heterogeneity and its implications for cancer study and treatment. J Biol Chem 2019;294(6):2162–79 doi 10.1074/jbc.REV118.002811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Solit DB, Osman I, Polsky D, Panageas KS, Daud A, Goydos JS, et al. Phase II trial of 17-allylamino-17-demethoxygeldanamycin in patients with metastatic melanoma. Clin Cancer Res 2008;14(24):8302–7 doi 14/24/8302 [pii] 10.1158/1078-0432.CCR-08-1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ramanathan RK, Egorin MJ, Eiseman JL, Ramalingam S, Friedland D, Agarwala SS, et al. Phase I and Pharmacodynamic Study of 17-(Allylamino)-17-Demethoxygeldanamycin in Adult Patients with Refractory Advanced Cancers. Clin Cancer Res 2007;13(6):1769–74 doi 10.1158/1078-0432.ccr-06-2233. [DOI] [PubMed] [Google Scholar]
- 13.Scher HI, Sawyers CL. Biology of progressive, castration-resistant prostate cancer: directed therapies targeting the androgen-receptor signaling axis. J Clin Oncol 2005;23(32):8253–61 doi 23/32/8253 [pii] 10.1200/JCO.2005.03.4777. [DOI] [PubMed] [Google Scholar]
- 14.Yuno A, Lee MJ, Lee S, Tomita Y, Rekhtman D, Moore B, et al. Clinical Evaluation and Biomarker Profiling of Hsp90 Inhibitors. Methods Mol Biol 2018;1709:423–41 doi 10.1007/978-1-4939-7477-1_29. [DOI] [PubMed] [Google Scholar]
- 15.Scaltriti M, Dawood S, Cortes J. Molecular pathways: targeting hsp90--who benefits and who does not. Clin Cancer Res 2012;18(17):4508–13 doi 10.1158/1078-0432.CCR-11-2138. [DOI] [PubMed] [Google Scholar]
- 16.Speranza G, Anderson L, Chen AP, Do K, Eugeni M, Weil M, et al. First-in-human study of the epichaperome inhibitor PU-H71: clinical results and metabolic profile. Invest New Drugs 2017. doi 10.1007/s10637-017-0495-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guo A, Lu P, Lee J, Zhen C, Chiosis G, Wang YL. HSP90 stabilizes B-cell receptor kinases in a multi-client interactome: PU-H71 induces CLL apoptosis in a cytoprotective microenvironment. Oncogene 2017;36(24):3441–9 doi 10.1038/onc.2016.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Giulino-Roth L, van Besien HJ, Dalton T, Totonchy JE, Rodina A, Taldone T, et al. Inhibition of Hsp90 Suppresses PI3K/AKT/mTOR Signaling and Has Antitumor Activity in Burkitt Lymphoma. Mol Cancer Ther 2017;16(9):1779–90 doi 10.1158/1535-7163.MCT-16-0848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goldstein RL, Yang SN, Taldone T, Chang B, Gerecitano J, Elenitoba-Johnson K, et al. Pharmacoproteomics identifies combinatorial therapy targets for diffuse large B cell lymphoma. J Clin Invest 2015;125(12):4559–71 doi 10.1172/JCI80714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kucine N, Marubayashi S, Bhagwat N, Papalexi E, Koppikar P, Sanchez Martin M, et al. Tumor-specific HSP90 inhibition as a therapeutic approach in JAK-mutant acute lymphoblastic leukemias. Blood 2015;126(22):2479–83 doi 10.1182/blood-2015-03-635821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bhagwat N, Koppikar P, Keller M, Marubayashi S, Shank K, Rampal R, et al. Improved targeting of JAK2 leads to increased therapeutic efficacy in myeloproliferative neoplasms. Blood 2014;123(13):2075–83 doi 10.1182/blood-2014-01-547760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Taldone T, Zatorska D, Ochiana SO, Smith-Jones P, Koziorowski J, Dunphy MP, et al. Radiosynthesis of the iodine-124 labeled Hsp90 inhibitor PU-H71. Journal of labelled compounds & radiopharmaceuticals 2016;59(3):129–32 doi 10.1002/jlcr.3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stabin MG, Siegel JA. Physical models and dose factors for use in internal dose assessment. Health Phys 2003;85(3):294–310. [DOI] [PubMed] [Google Scholar]
- 24.Cloutier RJ, Smith SA, Watson EE, Snyder WS, Warner GG. Dose to the fetus from radionuclides in the bladder. Health Phys 1973;25(2):147–61. [DOI] [PubMed] [Google Scholar]
- 25.International Commission on Radiological Protection. ICRP 100: Human Alimentary Tract Model for Radiological Protection In: Valentin J, editor. Annals of the ICRP; Volume 362006 p 61–7. [DOI] [PubMed] [Google Scholar]
- 26.Ewe K, Ueberschaer B, Press AG, Kurreck C, Klump M. Effect of lactose, lactulose and bisacodyl on gastrointestinal transit studied by metal detector. Alimentary pharmacology & therapeutics 1995;9(1):69–73. [DOI] [PubMed] [Google Scholar]
- 27.Caldas-Lopes E, Cerchietti L, Ahn JH, Clement CC, Robles AI, Rodina A, et al. Hsp90 inhibitor PU-H71, a multimodal inhibitor of malignancy, induces complete responses in triple-negative breast cancer models. Proc Natl Acad Sci U S A 2009;106(20):8368–73 doi 0903392106 [pii] 10.1073/pnas.0903392106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.World Health Organisation. August 20, 2010. www.who.int. August 20, 2010.
- 29.Workman P, Aboagye EO, Chung YL, Griffiths JR, Hart R, Leach MO, et al. Minimally invasive pharmacokinetic and pharmacodynamic technologies in hypothesis-testing clinical trials of innovative therapies. J Natl Cancer Inst 2006;98(9):580–98 doi 98/9/580 [pii] 10.1093/jnci/djj162. [DOI] [PubMed] [Google Scholar]
- 30.Peck CC, Cross JT. Getting the Dose Right: Facts, a Blueprint, and Encouragements. Clin Pharmacol Ther 2007;82(1):12–4. [DOI] [PubMed] [Google Scholar]
- 31.U.S. Food and Drug Administration, Center for Drug Evaluation and Research (CDER), Center for Biologics Evaluation and Research (CBER). Guidance for Industry: Population Pharmacokinetics. In: U.S. Department of Health and Human Services, editor 1999. [Google Scholar]
- 32.Powell JR, Gobburu JV. Pharmacometrics at FDA: evolution and impact on decisions. Clin Pharmacol Ther 2007;82(1):97–102 doi 6100234 [pii] 10.1038/sj.clpt.6100234. [DOI] [PubMed] [Google Scholar]
- 33.Moulick K, Ahn JH, Zong H, Rodina A, Cerchietti L, Gomes DaGama EM, et al. Affinity-based proteomics reveal cancer-specific networks coordinated by Hsp90. Nat Chem Biol 2011;advance online publication doi http://www.nature.com/nchembio/journal/vaop/ncurrent/abs/nchembio.670.html#supplementary-information. [DOI] [PMC free article] [PubMed]
- 34.Taldone T, Pillarsetty N, Dunphy MPS, Gerecitano JF, Caldas-Lopes E, Beattie B, et al. Development of a noninvasive assay to determine drug concentration in tumor during hsp90 inhibitor therapy. . Cancer Research, 2015 106th Annual Meeting of the American Association for Cancer Research (AACR; ) 2015 April 18-22 2015;Vol 75, No 15_suppl (Aug 1, 2015 Supplement), 2015: 5444. [Google Scholar]
- 35.Gerecitano JF, Modi S, Gajria D, Taldone T, Alpaugh M, DaGama EG, et al. Using 124I-PU-H71 PET imaging to predict intratumoral concentration in patients on a phase I trial of PU-H71. Journal of Clinical Oncology 2013;31(15_suppl):11076- doi 10.1200/jco.2013.31.15_suppl.11076. [DOI] [Google Scholar]
- 36.Saleem A, Harte RJ, Matthews JC, Osman S, Brady F, Luthra SK, et al. Pharmacokinetic evaluation of N-[2-(dimethylamino)ethyl]acridine-4-carboxamide in patients by positron emission tomography. J Clin Oncol 2001;19(5):1421–9. [DOI] [PubMed] [Google Scholar]
- 37.Scheibe PO, Vera DR, Eckelman WC. What is to be gained by imaging the same animal before and after treatment? Nuclear medicine and biology 2005;32(7):727–32. [DOI] [PubMed] [Google Scholar]
- 38.U.S. Food and Drug Administration, U.S. Dept. of Health and Human Services, Center for Drug Evaluation and Research. Guidance for Industry, Investigators, and Reviewers: Exploratory IND Studies. Available online: wwwfdagov/cder/guidanceindexhtm 2006.
- 39.International Commission on Radiological Protection. ICRP 105: Radiological Protection in Medicine. Ann ICRP; 2007;37(6). [DOI] [PubMed] [Google Scholar]
- 40.Chene P ATPases as drug targets: learning from their structure. Nat Rev Drug Discov 2002;1(9):665–73 doi 10.1038/nrd894nrd894 [pii]. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.