SUMMARY
The ability to recognize emotions in others and adapt one’s behavior accordingly is critical for functioning in any social context. This ability is impaired in several psychiatric disorders, such as autism and psychopathy. Recent work has identified the anterior cingulate cortex (ACC) among other brain regions involved in this process. Neural recording studies have shown that neurons in ACC are modulated by reward or shock when delivered to a conspecific and when experienced first-hand. Since previous studies do not vary reward and shock within the same experiment, it has been unclear whether the observed activity reflects how much attention is being paid to outcomes delivered to a conspecific or the valence associated with those stimuli. To address this issue, we recorded from ACC as rats performed a Pavlovian task that predicted whether reward, shock, or nothing would be delivered to the rat being recorded from or a conspecific located in the opposite chamber. Consistent with previous reports, we found that the firing of ACC neurons was modulated by aversive stimuli delivered to the recording rat and their conspecific. Activity of some of these neurons genuinely reflected outcome identity (i.e., reward or shock), however the population of neurons as a whole responded similarly for both reward and shock, as well as for cues that predicted their occurrence (i.e., reward >neutral and shock > neutral; attention). These results suggest that ACC can process information about outcomes (i.e., identity, recipient) in the service of promoting attention in some social contexts.
Keywords: anterior cingulate cortex, social behavior, empathy, reward, distress, Pavlovian, rat, prosocial, electrophysiology
eTOC Blurb
ACC is thought to contribute to empathy and prosocial behavior by signaling the emotions of others, but it might also contribute to social behavior via mechanisms related to attention. Schneider et al. show that ACC processes information about rewards and punishments for oneself and others in the service of attention.
INTRODUCTION
The significance of this study arises from a current lack of understanding regarding the neural underpinnings of social cognition. These mechanisms underlie our ability to perceive social cues and use that information to update our predictions about the environment. The importance of these functions is made clear by numerous psychiatric disorders that impair them [1–12]. Pharmacological treatments for these disorders are broad in scope and often ineffective, highlighting the need for a better understanding of the fundamental neurobiology.
Work has begun to parse out how individual brain regions contribute to the ability to recognize emotions in others. Among these areas is the anterior cingulate cortex (ACC). The involvement of ACC is not surprising, as this region is involved in non-social processes such as decision-making, attention and cognitive control, processes necessary for social cognition, which are also impaired in the aforementioned disorders [5,7–11,13,14]. Elegant work in monkeys and rats has shown, in different studies, that firing in ACC is modulated by the delivery of positive (e.g., reward) or negative (e.g., shock) outcomes to conspecifics located nearby that often mirror changes in firing that occur with first-hand experience of those same outcomes. It is thought that this shared code might allow individuals to recognize emotions in others so that appropriate action can be taken (e.g., learn from others; make pro-social choices) [15–17].
It is clear from this body of literature that ACC contributes to social cognition, but its functional role in social contexts as it relates to ACC’s known non-social functions has not been fully explored, leaving gaps in our fundamental understanding of what ACC is actually signaling in social paradigms. On one hand, ACC’s role in reward evaluation and foraging would suggest that it signals the valence of outcomes delivered to oneself and another [18–26]. On the other hand, ACC’s role in cognitive control suggests that it might play a role in driving attention towards arousing social and non-social cues in the environment [18,27–34].
One way to dissociate between valence encoding (i.e., signaling if something is good or bad) and attentional signaling (i.e., unsigned signal in response to arousing, salient or motivating stimuli) is to manipulate both appetitive and aversive stimuli within the same paradigm [35]. This takes advantage of the fact that both positive (e.g., reward) and negative (e.g., shock) outcomes – though oppositely signed – are arousing and attention-grabbing compared to neutral stimuli. Thus, if a brain area fires similarly to appetitive and aversive stimuli, then one would argue that its activity represents attention or arousal, whereas if it fires differently, its activity may instead reflect valence or emotion associated with those stimuli. The same holds true in social contexts. For example, if neurons are co-encoding first-hand pain and the observed pain of a conspecific, then it might be encoding valence or emotion (i.e., emotional mirror neuron), but only if those neurons do not respond or respond in the opposite direction during appetitive events. If, however, activity changes are in the same direction for appetitive and aversive events, then firing might better reflect changes in arousal or attention.
To dissociate between these two signals, we recorded from rat ACC in a task where presentation of auditory stimuli predicted the valence of an outcome to be delivered at the end of each trial; reward, shock or nothing. Five seconds later, a spatial visual cue indicated the recipient of that outcome: either the rat that was being recorded from (recording rat) or a conspecific located in the opposite chamber. By manipulating both reward and shock we can determine whether activity reflects attention (both reward and shock are attention-grabbing thus firing should be similar for both trial-types) or outcome identity (reward or shock). We found heterogeneous firing in ACC suggesting that ACC contributes to both of these functions; however, at the level of the entire population there was a significant positive correlation between reward-and shock-related firing when outcomes were delivered to the recording rat and the conspecific, suggesting that ACC main output function in our paradigm is to increase attention in social contexts.
RESULTS
Pavlovian Social Outcome Paradigm
Neural activity was recorded from six rats during performance of our Pavlovian Social Outcome Paradigm, in which pairs of rats were placed in opposite sides of a modified shuttle box, separated by a mesh divider that allowed rats to see, smell, and hear each other (Figure 1A). Rats arrived together, lived individually but next to each other in transparent cages, and partners remained consistent throughout the experiment. The walls opposite to the divider were equipped with a directional cue light, a food cup and a shock grid (Figure 1A). Each trial began with illumination of a house light (Figure 1B–D). Five seconds after onset of the house light, 3 different auditory stimuli (5s) predicted delivery of 3 corresponding outcomes (sucrose pellet, foot-shock, or nothing), that were delivered to either the recording rat (self) or the conspecific (other). At the time of cue presentation, either rat had a 50% chance of receiving the following outcome. Thus, during presentation of the auditory stimulus, animals could not yet accurately predict which rat would receive the outcome. This information was only made known by subsequent illumination of one of the two directional light cues located in either the recording rat’s or the conspecific’s chamber (Figure 1A). After presentation of the directional light for 5s, the outcome (reward, shock or nothing) was delivered to the same side as the illuminated light cue. Initial presentation of auditory outcomes cues followed by directional light cues, which predicted outcomes that would arrive 5 s later, were features of the task that were intended to maintain a level of uncertainty to promote attention to non-social and social cues that predicted which rat would receive what outcome. Uncertainty was also induced within each recording session by having rats perform 4 different trial blocks (60 trials per block), during which both rats received outcomes (R/R; ‘R’ designates ‘reinforced’; numerator = recording rat; denominator = conspecific), neither rat received outcomes (N/N where ‘N’ designates which rat was not reinforced), only the recording rat was reinforced (R/N) or only the conspecific was reinforced (N/R). During non-reinforced trials all stimuli were presented but shocks and reward were not delivered.
Figure 1. Pavlovian Social Outcome Task.
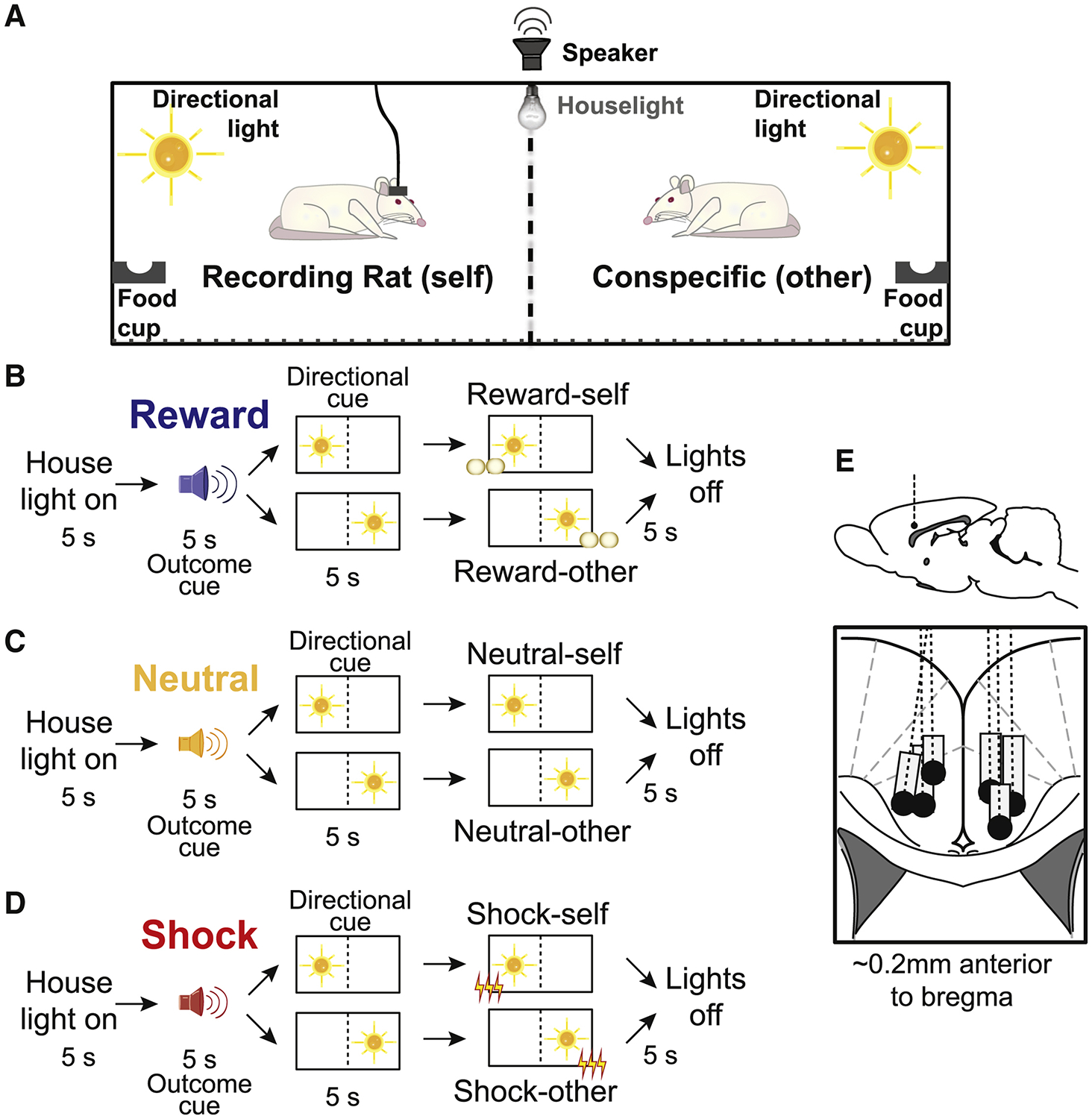
A) Schematic of behavioral chamber used in the task. Pairs of rats are placed in opposite sides of the ‘divider’ within the same shuttle-box chamber, on a grid of conductive rods. The directional light sits on the panel opposite to the ‘divider’ on each side, above the grid but low enough to be visible through the ‘divider’ from the other side. The food cup is to the left of the directional light. Both sides are diagonally mirrored in their placements of the light and food cup. B-D) Flow diagram of a trial within the task, which begins with the onset of the house lights. After 5s, an outcome cue (5s) indicates the type of outcome delivered, followed by activation of one of the directional lights (10s), indicating which side receives the outcome. Five (5) seconds after the onset of the directional light cue, the outcome is delivered to the designated side. In the following 10s the directional lights turn off, then the houselights turn off, 5s apart each. B-D) represent all 6 basic trial-types reward-self, reward-other, neutral-self, neutral-other, shock-self, and shock-other. There were 4 different trial blocks (60 trials per block; 10 trials per trial-type), during which both rats received outcomes (R/R; where ‘R’ designates ‘reinforced’; numerator = recording rat; denominator = conspecific), neither rat received outcomes (N/N; ‘N’ designates which rat was not reinforced), only the recording rat was reinforced (R/N) or only the conspecific was reinforced (N/R). During non-reinforced trials all stimuli were presented but shocks and reward were not delivered. E) Location of recording sites (Paxinos and Watson). Dashed line represents electrode placement and gray boxes mark the extent of the recording locations.
When reporting the results below we will adhere to the following terminology: ‘Self’ trials refer to trials during which the outcome was delivered to the recording rat, whereas ‘other’ trials refer to trials in which the outcome was delivered to the conspecific. ‘Reinforced’ refers to trials where outcomes were delivered, whereas ‘nonreinforced’ refers to trials where outcomes were not delivered. There were 6 trial-types (reward-self, reward-other, neutral-self, neutral-other, shock-self and shock-other), which occurred in equal proportions.
Rats correctly internalize auditory, directional light cues, and block context
Because the task was Pavlovian we used food cup bream breaks and video scoring to determine if rats understood the task. Figure 2A–H shows the average beam breaks into the food cup over trial time for the three trial-types averaged over all recordings. Bar graphs in Figure 2IL show average beam breaks during two trials epochs: the ‘directional light epoch’ (5s after onset of the directional cue) and the ‘outcome epoch’ (5s after outcome delivery).
Figure 2. Rats learn the predictive value of both outcome and directional cues, modulated by different outcome contexts.
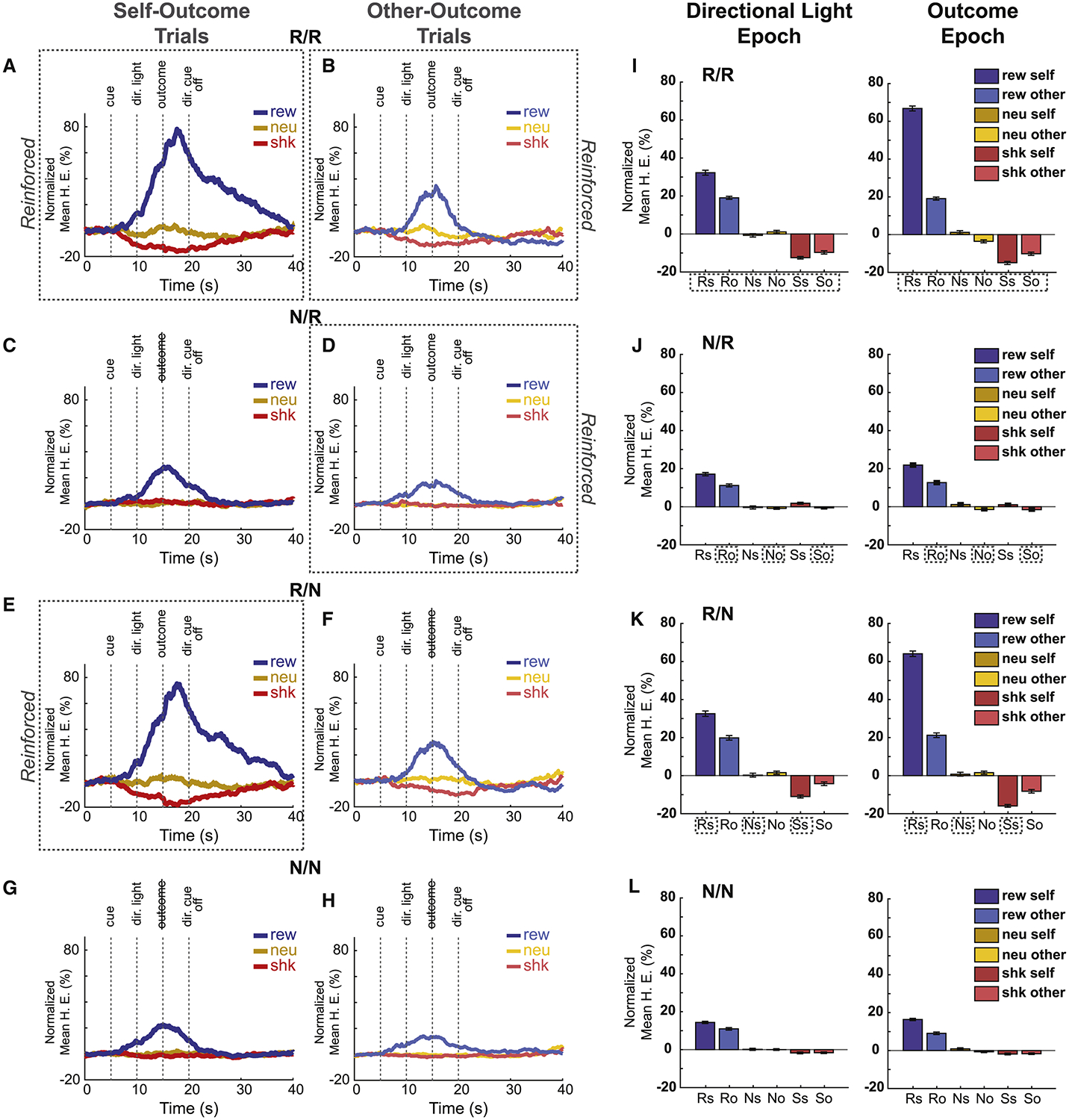
Average beam breaks from food cup entry as a percentage of trial time for each outcome type (reward – blue, neutral – orange, shock – red), across each block type (Dotted line boxes indicate whether trials were “Reinforced”) for self-(A,C,E,G) and other (B,D,F,H) outcome trial. N = 139 sessions (6 rats). Vertical dotted lines indicate task-related events (outcome cue, directional light (dir. light), outcome delivery, directional light off). I-L) Averaged food cup entry during the directional light (left) and outcome (right) epochs (5 s). Reinforcement context for each row corresponds to the block type of the same row in A-H. Trial types: reward-self (Rs, solid blue), reward-other (Ro, light-blue), neutral-self (Ns, solid orange), neutral-other (No, light-orange), shock-self (Ss, solid red) and shock-other (So, light-red). Statistics are reported in the text. There were 4 different trial blocks (60 trials per block; 10 trials per trial-type), during which both rats received outcomes (R/R; where ‘R’ designates which rat was reinforced; numerator = recording rat; denominator = conspecific), neither rat received outcomes (N/N; ‘N’ designates ‘not reinforced’), only the recording rat was reinforced (R/N) or only the conspecific was reinforced (N/R). During non-reinforced trials all stimuli were presented but shocks and reward were not delivered. 1st row of figures (A,B,I) = R/R trials; 2nd row of figures (C,D,J) = N/R trials; 3rd row of figures (E,F,K) = R/N trials; 4th row of figures (G,H,L) = N/N trials.
First, let’s consider blocks of trials where both the recording rat and the conspecific received outcomes (i.e., ‘R/R trial blocks; Figure 2, top row). As shown previously, prior to outcome delivery, beam breaks increased and decreased on reward-self (blue) and shock-self (red) trials relative to neutral (orange) trials (Figure 2A), respectively [36]. After the presentation of the directional light cue (i.e., the cue that informed the rat which animal would receive the outcome), there was a significant increase in food cup entries for reward-self compared to reward-other trials, demonstrating that rats anticipated the receipt of reward before its delivery (Figure 2I, dark vs pale blue; Wilcoxon; z = 5.326, p < 0.001). During shock trials, there was a significant decrease in beam breaks during both the directional light and outcome epochs compared to neutral, for both shock-self and shock-other trials, and the effect was stronger for shock-self (Figure 2I; red; Wilcoxon; shock-self: DL: z = −8.193, p < 0.001; Out: z = −9.738, p < 0.001; shock-other: DL: z = −7.189, p < 0.001; Out: z = −4.472, p < 0.001). Thus, in trial blocks where both rats were reinforced (R/R) food cup entries were higher and lower for reward and shock trials compared to neutral, and were stronger when the recording rats were personally going to receive the outcome.
These results demonstrate that recording rats understood the meaning of auditory and directional cues. Importantly, these effects were highly dependent on whether the recording rat was being reinforced in a given block of trials. That is, during N/R and N/N blocks increases and decreases in food cup entries relative to neutral trials were reduced relative to R/R and R/N trial blocks (Figure 2; first and third rows vs. second and fourth rows). Most interestingly, this was even true during shock-other trials in blocks where the conspecific was still receiving shock (i.e., N/R; Figure 2D,J; Wilcoxon; DL: z = −8.723, p < 0.001; Outcome: z = −8.124, p < 0.001). This suggests that suppression of food cup responding reflects behavioral reactions due to potential harm to oneself, not to the conspecific. This argument is also supported by the observation that food cup entries were significantly suppressed during shock-other trials even during trial blocks when the conspecific was not being shocked, but the recording rat was (i.e., R/N; Figure 2F,K; Wilcoxon; DL: z = −6.466, p < 0.001; Outcome: z = −7.759, p < 0.001). Overall, these results suggest that changes in behavior of the recording rats that occurred when conspecifics received shock reflected concern for oneself, as opposed to empathetic concern for the other.
Freezing and approach during shock trials
Above we show that rats understand the meaning of cues and exhibit increases and suppression of food cup entries, during reward and shock trials relative to neutral trials, respectively. To better understand the nature of these data, especially as they relate to shock trials, we scored video for freezing and approach. Figure 3A–D represents average freezing of recorded animals for each block, during the 5s-long outcome cue (‘cue’), directional cue (‘dir. cue ON’), outcome delivery (‘outcome’) and directional cue off (‘dir. cue OFF’) epochs of the task. Freezing was defined as the sudden absence of movement except for respiration. Consistent with the analysis of food cup entries, it was clear from the recording rats’ freezing behavior that they understood the meaning of the cues during trial blocks where both animals were reinforced (Figure 3A, R/R); rats froze more in shock-self and shock-other trials compared to their neutral counterparts (‘dir. cue ON’: shock-self, χ2 = 32.000, p < 0.001; shock-other, χ2 = 17.850, p < 0.001; Outcome: shock-self, χ2 = 19.154, p < 0.001; shock-other, χ2 = 3.467, p = 0.0593), and froze more often on shock-self trials compared to shock-other trial types during the directional light and outcome epochs (‘dir. cue ON’: χ2 = 8.181, p = 0.004; Outcome: χ2 = 19.154, p < 0.001). Freezing was most apparent during the directional cue light epoch indicating that rats anticipated shock delivery (Figure 3A–D).
Figure 3. Rats show increased freezing and conspecific approach during shock-self and shock-other trials.
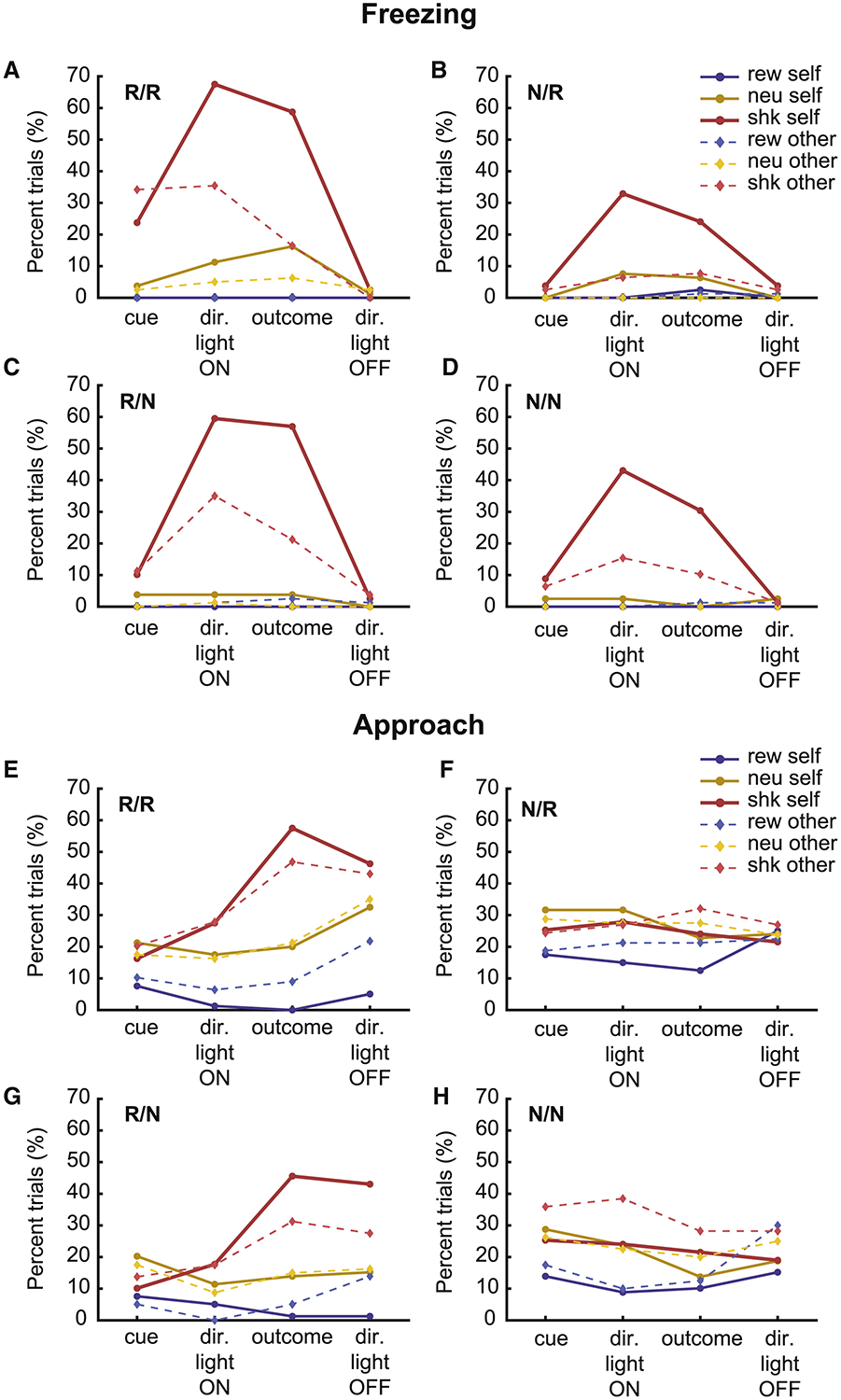
A-D) Percentage of trials recorded rats froze during each epoch, for each trial and block type. E-H) Percentage of trials recorded rats approached the gate, defined as moving towards or actively interacting at the ‘divider’. One session for each rat that contributed neural data was scored. Sessions were concatenated and counts (i.e., froze or did not freeze; approached or did not approach) were taken during each of the 5 s epochs. Counts were compared via chi-squared tests but graphs reflect percent over trials. Trial types: reward-self (solid blue), reward-other (dotted light-blue), neutral-self (solid orange), neutral-other (dotted light-orange), shock-self (solid red) and shock-other (dotted light-red). There were 4 different trial blocks (60 trials per block; 10 trials per trial-type), during which both rats received outcomes (R/R; where ‘R’ designates ‘reinforced’; numerator = recording rat; denominator = conspecific), neither rat received outcomes (N/N; ‘N’ designates which rat was not reinforced), only the recording rat was reinforced (R/N) or only the conspecific was reinforced (N/R). During non-reinforced trials all stimuli were presented but shocks and reward were not delivered. Statistics are reported in the text.
As previously reported, these results suggest that rats exhibit ‘empathetic’ behavior. However, our results were highly dependent on whether the recording rat was receiving shocks during that trial block. During trial blocks where the recording rat was not shocked, but the conspecific was (Figure 3B, N/R), freezing was significantly reduced (Shock-other in R/R vs N/R: ‘dir. cue ON’: χ2 = 15.89, p < 0.0001; ‘Outcome’: χ2 = 12.80, p = 0.0003), suggesting that when rats did not anticipate first-hand harm, they did not express behavioral reactions associated with conspecific distress. This interpretation is further supported by the observation that freezing on shock-other trials was high during trial blocks where the recording rat, but not the conspecific, received shock (Figure 3C, R/N shock-other vs neutral-other, ‘dir. cue ON’: χ2 = 24.952, p < 0.001; Outcome: χ2 = 16.801, p < 0.001).
Along with freezing, we scored conspecific approach (Figure 3E–H). Approach was defined as the movement and investigation of the recording rat in the direction of the conspecific, which has been suggested to be a measure of attention, concern, and consolation[37–42]. During trial blocks where both rats received shock (Figure 3E, R/R), the recording rat approached the conspecific more on shock-self and shock-other trials compared to neutral trials during the directional light epoch and after the outcome, with the strongest approach being observed after the shock was delivered (‘dir. cue ON’: self, χ2 = 1.734, p = 0.182; other, χ2 = 2.263, p = 0.128; Outcome: self, χ2 = 14.420, p < 0.001; other, χ2 = 7.334, p = 0.006). Notably, increases in approach were not observed on shock trials during trial blocks where reinforcement for the recording rat was omitted, even though the conspecific was still receiving shock (Figure 3F, N/R; ‘dir. cue ON’: self, χ2 = 0.179, p = 0.662; other, χ2 = 0.019, p = 0.879; Outcome: self, χ2 = 0.022, p = 0.869; other, χ2 = 0.179, p = 0.662). That is, recording rats did not approach the conspecific while it was being shocked in trial blocks where there was no first-hand threat. However, increases in conspecific approach were present in blocks where the recording rats were receiving shock but the conspecifics was not (Figure 3G, R/N; ‘dir. cue ON’: self, χ2 = 1.044, p = 0.297; other, χ2 = 2.267, p = 0.127; Outcome: self, χ2 = 13.192, p < 0.001; other, χ2 = 4.4989, p = 0.033), suggesting that it is the threat of personal shock that promoted approach on shock-other trials.
In summary, our behavioral results demonstrate that the recording rats understand the task structure. Specifically, recording rats reacted more on ‘self’ versus ‘other’ trials, thus they understood the significance of the directional light. Rats entered the food-cup the most on reward trials, and the least on shock trials, thus they learned to discriminate between predictive auditory outcome cues. In addition, recording rats froze to cues and approached the conspecific on shock trials. These results demonstrate that both reward and shock trials have opposite valence, but are both arousing and drive behavior (shock elicits freezing and conspecific approach; reward elicits food cup entries). Lastly, our results suggest that the recording rats’ reactions during shock-other trials were highly dependent on the potential for receiving shock first-hand. That is, food cup response suppression, freezing, and approach were stronger for both reinforced and non-reinforced shock-other trials during blocks of trials when the recording rat was reinforced (R/R and R/N) and they were not different from neutral trials when the recording rat was not receiving shock (N/R).
ACC firing is stronger during threat of first-hand shock on self and other trials
The average over all recorded neurons (n = 139) across trial time for each trial-type and trial block is illustrated in Figure 4A–H. As previously reported[16], we see increases in firing during shock-self (Figure 4A; red) and shock-other (Figure 4B; red) trials compared to neutral (orange) in trial blocks where both rats were shocked (R/R; top row). However, increased firing on shock-other trials was not present during trials blocks where the conspecific received shock, but the recording rat did not (N/R; Figure 4D; red versus orange). Instead, there were increases in firing on shock-other trials relative to neutral-other trials during the trial blocks where the conspecific did not receive shock but the recording rat did (R/N; Figure 4F). Thus, much like behavior, firing was stronger for both reinforced and non-reinforced shock-other trials during trial blocks when the recording rat was reinforced, and not different from neutral trials when the recording rat was not receiving shock. Therefore, firing on shock-other trials cannot reflect that the conspecific is being shocked.
Figure 4. ACC population activity during Pavlovian Social Outcome Task.
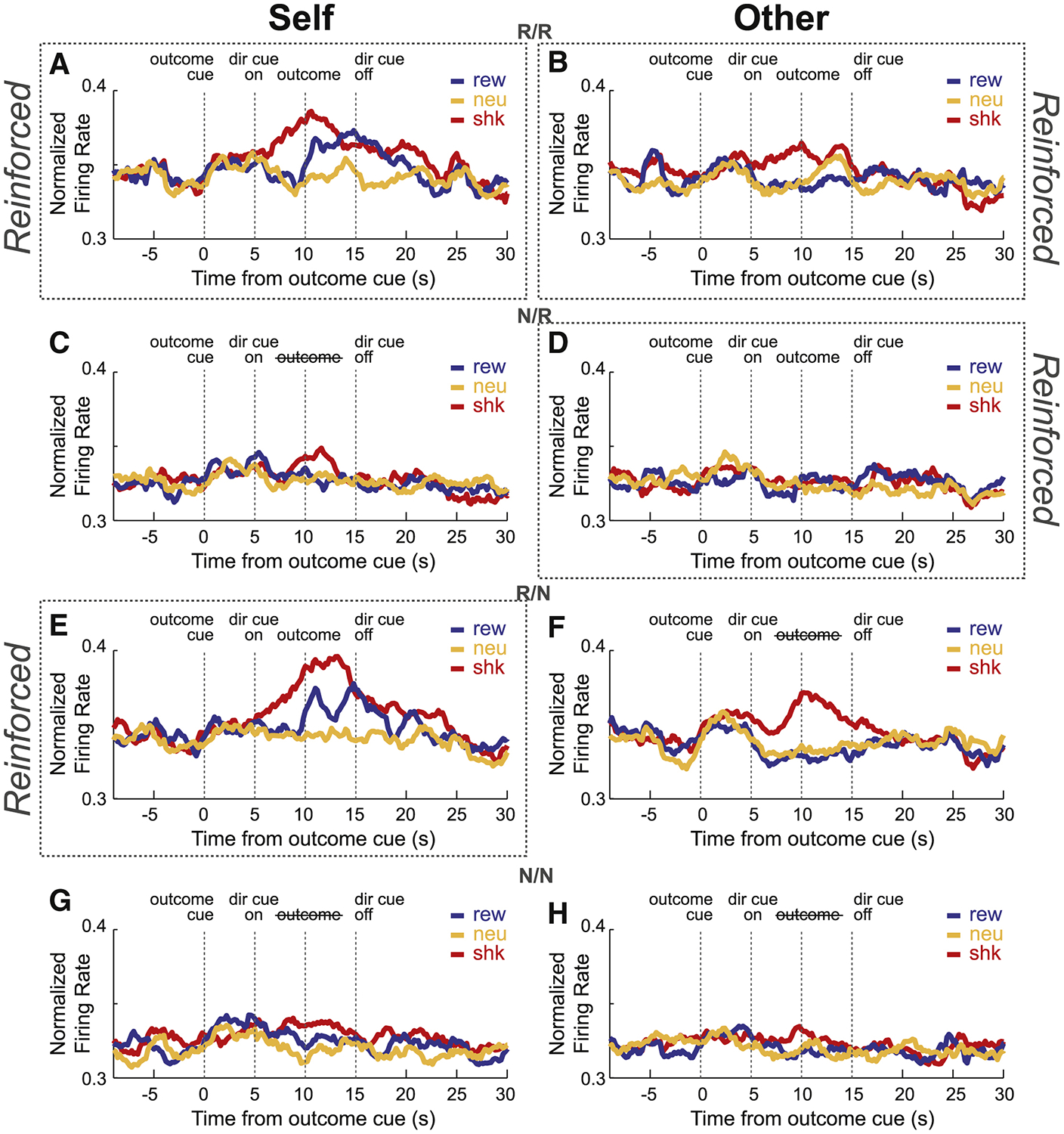
A-H) Normalized mean firing rate of all recorded neurons (n = 139), across each reinforcement block type for reward (blue), shock (red) and neutral (orange) trials (see Figures S4 and S5 for single-cell examples of ACC neurons selective to shock or reward). Each row shows neural firing for self-and other-outcome trials (as indicated above each column) for each block (Dotted line boxes indicate whether self or other trials were reinforced for that block). There were 4 different trial blocks (60 trials per block; 10 trials per trial-type), during which both rats received outcomes (R/R; where ‘R’ designates ‘reinforced’; numerator = recording rat; denominator = conspecific), neither rat received outcomes (N/N;’N’ designates which rat was not reinforced), only the recording rat was reinforced (R/N) or only the conspecific was reinforced (N/R). During non-reinforced trials all stimuli were presented but shocks and reward were not delivered. 1st row of figures (A,B) = R/R trials; 2nd row of figures (C,D) = N/R trials; 3rd row of figures (E,F) = R/N trials; 4th row of figures (G,H) =N/N trials. See Figure S1 for presentation of trial-types split into first and last half of trials per block, and Figure S3 for neural activity of ACC neurons selective during the auditory cue phase.
To quantify these effects, for each neuron we computed the normalized difference between firing on shock and neutral trials (shock index = shock – neutral/ shock + neutral) independently for self and other trials during directional light and outcome epochs for each trial block. Distributions of these shock indices for all neurons are plotted in Figure 5. During both epochs shock index distributions were shifted above zero for ‘self’ and ‘other’ trials, indicating that the majority of ACC neurons fired higher during shock compared to neutral (Wilcoxon; R/R self, DL: μ = 0.031, p < 0.001; Outcome: μ = 0.024, p = 0.024; other, DL: μ = 0.030, p < 0.001; Outcome: μ = 0.014, p = 0.0544). Further, shock indices for self and other were positively correlated indicating that neurons that tended to fire more or less strongly for shock-self trials, tended to fire more or less strongly for shock-other trials, respectively (r2 = 0.028; p = 0.046).
Figure 5. ACC neurons tend to fire more during Shock-self and Shock-other relative to neutral.
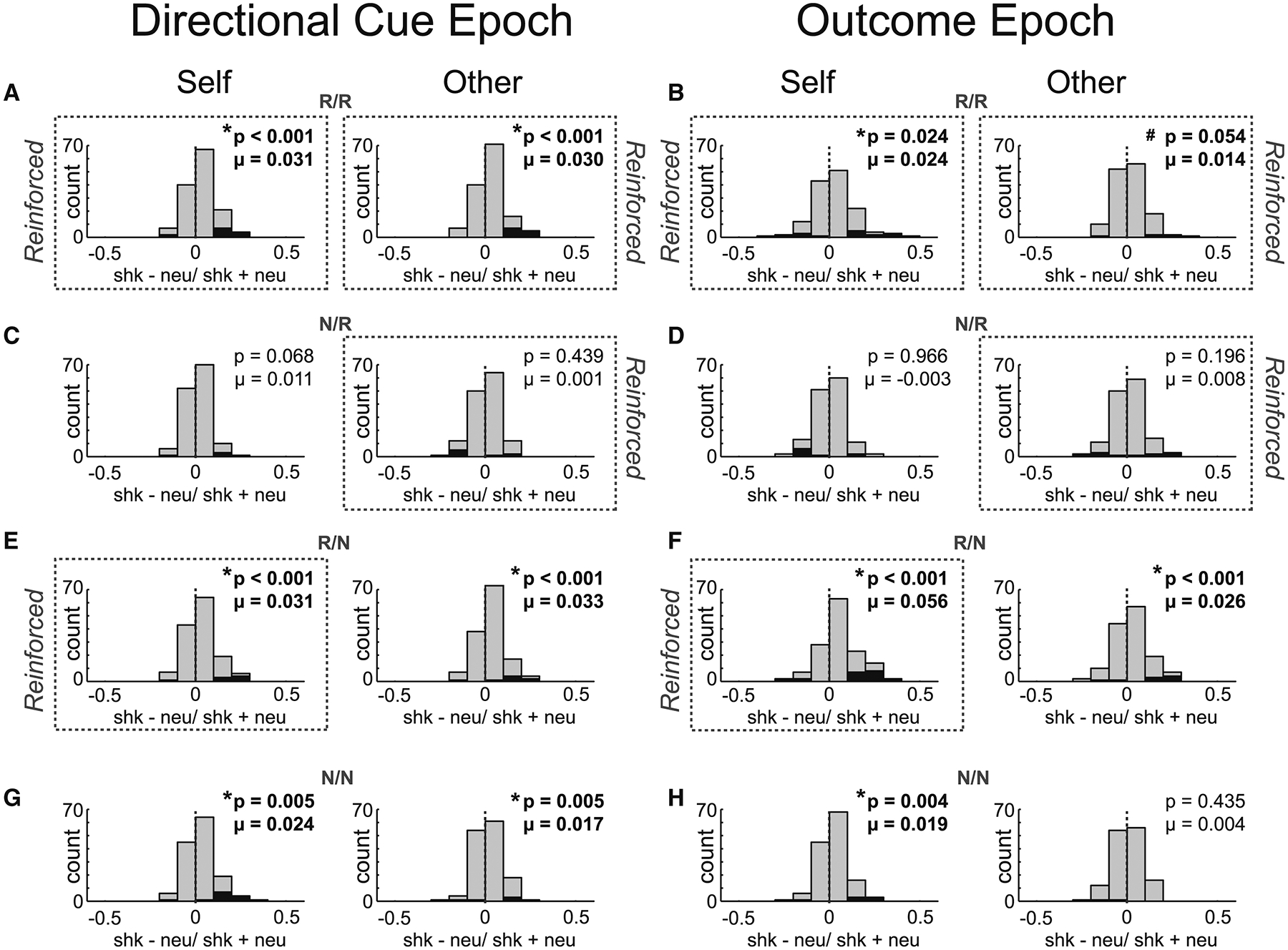
For each neuron we computed the normalized difference between firing on shock and neutral trials (shock index = shock – neutral/ shock + neutral) independently for self (left columns under ‘Self’) and other (right columns under ‘Other’) trials during directional light (A,C,E,G) and outcome epochs (B,D,F,H) for each trial block (5 s epochs). Black bars represent neurons that showed significant within-session differences between shock and neutral trials (Wilcoxon; p’s < 0.05). Distributions in trial blocks where threat of shock is likely (A-B, E-F, G-H) were significantly shifted in the positive direction, showing that many ACC neurons increased in firing during the directional light and outcome phases of shock trials, for self and other outcomes. Significant responding to other-outcome shock trials was not found in nonsocial contexts (Figure S2). There were 4 different trial blocks (60 trials per block; 10 trials per trial-type), during which both rats received outcomes (R/R; where ‘R’ designates ‘reinforced’; numerator = recording rat; denominator = conspecific), neither rat received outcomes (N/N; ‘N’ designates which rat was not reinforced), only the recording rat was reinforced (R/N) or only the conspecific was reinforced (N/R). During non-reinforced trials all stimuli were presented but shocks and reward were not delivered. 1st row of figures (A,B) = R/R trials; 2nd row of figures (C,D) = N/R trials; 3rd row of figures (E,F) = R/N trials; 4th row of figures (G,H) =N/N trials.
Consistent with the population firing (Figure 4), significant shifts in distributions on shock-other trials were not present when the conspecific was to receive shock, but there was no first-hand threat to the recording rat (i.e., N/R; Figure 5C,D; Wilcoxon; DL: μ = 0.001, p = 0.4391; Outcome: μ = 0.008, p = 0.196). Instead, distributions were significantly shifted on shock-other trials during trial blocks where recording rats received shock, even when the conspecific did not (Figure 5A,E,B,F; Wilcoxon; R/R self, DL: μ = 0.031, p < 0.001; Outcome: μ = 0.024, p = 0.024; other, DL: μ = 0.030, p < 0.001; Outcome: μ = 0.014, p = 0.0544; R/N self, DL: μ = 0.031, p < 0.001; Outcome: μ = 0.024, p < 0.001; other, DL: μ = 0.033, p < 0.001; Outcome: μ = 0.026, p < 0.001), suggesting that activity reflected behavioral reactions to the possibility of first-hand treat (i.e., suppression of food cup responding and increased freezing on shock-other during R/N trials). Consistent with this hypothesis we found the firing rate shock indices for both self and other were correlated with suppression of food cup entries (self: r2 = 0.043; p = 0.013; other: r2 = 0.030; p = 0.0403).
In conclusion, ACC neurons tended to fire higher during ‘self’ and ‘other’ shock trials when there was a threat of first-hand shock, even during shock-other trials where the conspecific did not receive shock. Notably, these increases in firing on shock-other trials were not observed during sessions where the conspecific was not present (i.e., alone sessions; R/R and R/N; Figure S2E and F; Wilcoxon; DL: μ = 0.015, p = 0.175; Outcome: μ = 0.008, p = 0.401), suggesting that conspecific presence was necessary for the observed increases in shock-other trials.
ACC firing was also elevated for reward delivered to the recording rat
The above behavioral and neural analysis suggests that ACC neurons are not signaling when shocks are to be delivered to a conspecific, but instead reflect attention paid to the conspecific (i.e., approach) on shock-other trials when there is a threat of personal shock. If this interpretation of the data is accurate and firing of ACC neurons on shock trials reflects attention, not valence, then reward trials should induce similar changes in firing.
Re-examination of Figure 4 reveals that average firing across the population is not only higher for shock compared to neutral trials, but is also higher during reward-self trials (blue). To quantify this effect and to elucidate the relationship between firing on reward-and shock-self trials, for each neuron we computed the normalized difference between firing rate on shock and neutral trials (shock index = shock – neutral/ shock + neutral) and between reward and neutral trials (reward index = reward – neutral/ reward + neutral) for self-outcome trials during the outcome epoch. For this analysis we combined data for ‘self’ trials from R/R and R/N blocks to double our sample within each session and because effects were present in both trial blocks (Figure 4). Distributions of shock and reward indices are plotted in Figure 6 and counts of significant neurons are represented by black bars (Wilcoxons; p’s < 0.05). Both reward and shock index distributions were significantly shifted above zero (shock: μ = 0.042, p < 0.001; reward: μ = 0.028, p = 0.0043) and counts of neurons that exhibited significantly higher firing for reward over neutral and shock over neutral outnumbered those showing the opposite effect (Figure 6; reward: 31 vs 14, χ2 = 6.346, p = 0.011; shock: 24 vs 4, χ2 = 14.143, p < 0.001).
Figure 6. ACC neurons tend to fire similarly for reward and shock.
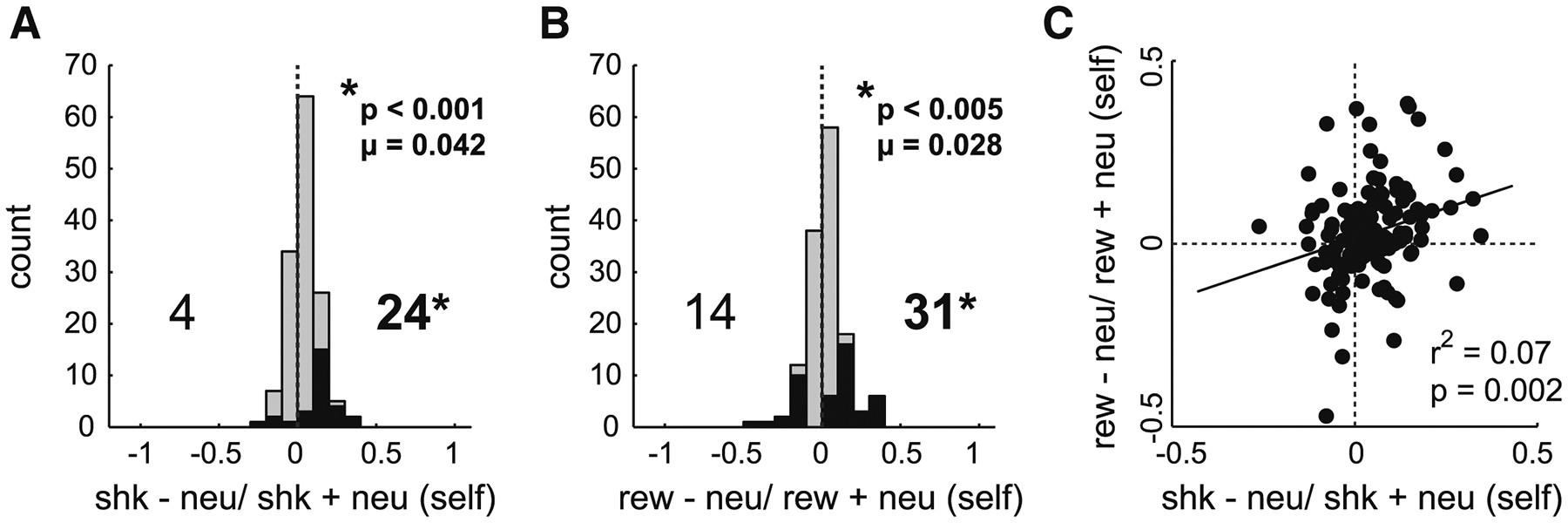
A-B) Distributions of counts for neurons selective for shock- or reward-self and other during the outcome epoch, based on calculated index scores. Index scores were obtained as the normalized difference between reward (B) or shock (A) and neutral firing rates (i.e., shock index = shock − neutral/ shock + neutral; reward index: reward − neutral/ reward + neutral) during self-outcome trials. Counts of cells firing significantly greater than or less than neutral trials are represented by black bars (Wilcoxon; p < 0.05). Data was combined across R/R and R/N trial blocks. Wilcoxon tests report significant shifts in distributions and chi-squared tests report significant differences between greater and lesser counts of neurons. C) Correlation between reward and shock trials during self-trials.
Finally, we asked whether neurons that were responsive during reward trials were also responsive during shock trials (and vice versa) during both self and other trials. That is, did neurons that tended to fire more or less strongly for reward, tend to fire more or less strongly to shock, respectively? As aforementioned, a positive correlation would suggest population-level firing represented changes in attention associated with reward and shock, whereas a negative correlation would suggest that activity reflected valence or emotion associated with those stimuli. Lastly, no correlation would suggest that ACC neurons encode reward and shock independently. We found a significant positive correlation between reward and shock indices during both self-and other-trials for both directional light and outcome epochs (DL: self: r2 = 0.102, p < 0.001; other: r2 = 0.111, p < 0.001; Outcome (Figure 6C): self: r2 = 0.07, p < 0.001; other: r2 = 0.122, p < 0.001).
Although activity at the population level in ACC was elevated for both reward and shock – suggesting that overall function of ACC is more closely aligned with attention – this does not exclude the possibility that signals in ACC were heterogeneous (see Figures S3–5 for single cell examples and additional population histograms) or that some neurons in ACC did signal reward and shock independently. For example, we found that 22 (16%) and 12 (9%) neurons increased firing to reward and shock, without significant modulation during shock and reward, respectively (Figure 7).
Figure 7. ACC neurons responsive to either shock or reward during the outcome epoch.
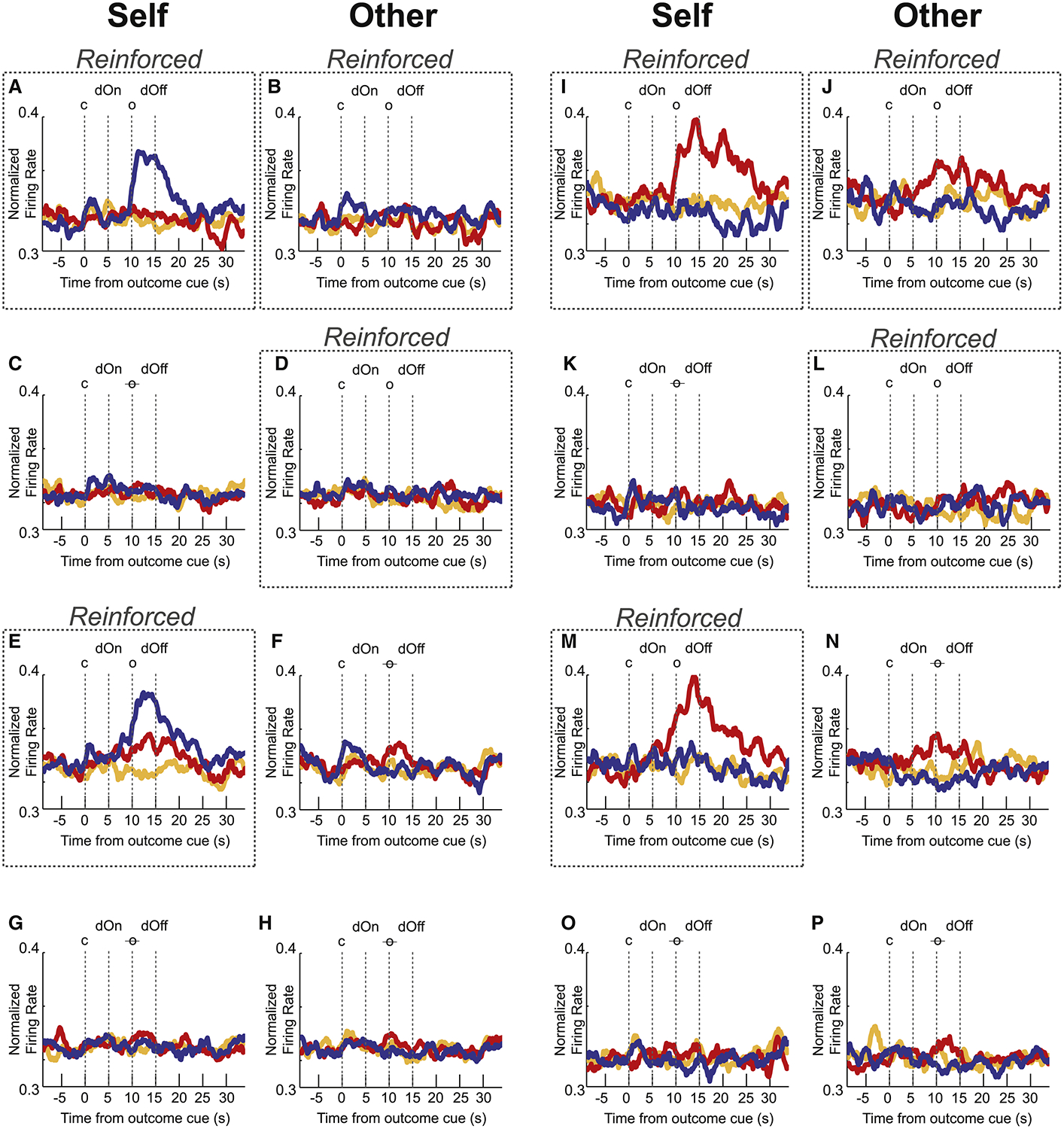
Normalized mean firing rate of a population of neurons selective to shock (I-P; n = 12(9%) or reward (A-H; n = 22(16%)) trial-types during the outcome epoch (5s), across each reinforcement block type for reward (blue), shock (red) and neutral (orange) trials. Averaged over blocks where the recording rat was reinforced (i.e., R/R and R/N), these neurons showed significantly stronger firing (Wilcoxon; p < 0.05) for either reward-self or shock-self over neutral-self but not for both. Each row shows neural firing for self-and other-outcome trials (as indicated by column) for each block (Dotted line boxes indicate whether self or other trials were reinforced for that block). c = cue; dOn = directional light on; o = outcome; o = omitted outcome (i.e., non-reinforced); dOff = directional light off.
DISCUSSION
The current state of the social neuroscience field suggests that ACC acts as an “emotional mirror neuron” system that allows an individual to perceive the emotions of another via neurons that signal both first-hand pain and the observed pain of others. This shared code might underlie observational fear learning, consolation, empathy, harm aversion and pro-social behavior, which indeed appear to be ACC-dependent. While it is true that increased firing to both first-hand and observed pain might genuinely reflect a shared emotional state, it is equally possible that increases in activity reflect heightened arousal or attention associated with distress, whether it be to oneself or another. While both mechanisms might contribute to subsequent social behaviors -such as observational learning, harm aversion and pro-social behavior -the underlying mechanisms are completely different.
Here, we show that increases in activity reported during first-hand and observed distress can reflect increased attention. Specifically, we show that increased firing to first-hand pain and a conspecific’s pain are correlated with increased firing to reward delivery. Further, we show that rat behavior and ACC firing is only modulated when the recording rat, was threatened with first-hand pain. That is, even in rats that have experienced shock, when they are safe, their behavior and firing in ACC were not modulated by conspecific shock. Even more striking is the observation that firing increases during shock-other trials when the conspecific was not being shocked but the threat of first-hand shock was present. All this suggests that ACC is signaling attention in social contexts when there was threat of personal harm.
Our data demonstrating that ACC is modulated during both shock-self and shock-other trials fits well with previous rodent work. In voles, ACC activity is high when animals console other stressed, previously shocked voles[40]. In mice, ACC perturbation impairs observational fear learning [43,44] and inhibition of ACC projecting neurons to amygdala alters amygdala’s representation of the aversive cue during observational conditioning [45]. Further, it has been shown that firing in ACC is synchronized with amygdala during observational learning [44] and that amygdala-projecting ACC neurons preferentially encode socially derived aversive cue information [45]. Lastly, in rats, neurons in ACC have been characterized as ‘emotional mirror neurons,’ as they were found to increase firing to pain inflicted to the recording rat, as well as to a conspecific, according to a potential shared code that maps the distress of another onto that of the observer [15,16].
Although our data are consistent with previous ‘shock’ work in rodents, the fact that we found very few neurons that increased during reward-other trials is inconsistent with ‘reward’ work previously reported in monkeys. In monkey ACC, neurons fire when reward is allocated to a conspecific, to oneself, or in both contexts[24]. Although recent work has shown the influence of social cues on reward learning [46], to the best of our knowledge this has not been explored in rodents, thus it is possible that rodent ACC is not responsive to rewards delivered to others. However, we speculate that the presence of shock stimuli may have diluted neural effects due to lower social engagement during reward-other trials. Future work is necessary to better understand the role of rodent ACC in observation of appetitive events.
From previous research is clear that ACC is important for recognition of social distress [18,24,44,47,48]. Our work is significant because we add to this growing literature, by uncovering the potential nature of what is being encoded by ACC in response to conspecific reward and distress, simultaneously as opposed to separately. By manipulating both reward and shock, our work suggests – at least in the context of our task and the region of ACC that we are recording from – that ACC contributes more towards directing attention, and less to the evaluation of outcomes delivered to the conspecific or the emotional tags that they carry. Examining recording sites from previous studies suggests that more rostral and ventral regions of ACC might be involved in affective processing [16], while more caudal and dorsal regions may contribute in greater part to executive function, such as attention [49,50], leaving open the possibility that other regions in ACC might carry such information.
It might be argued that the main reason why, here, ACC seems to encode social attention but not vicarious emotion, is that rats performing the current task did not exhibit empathy. This is certainly possible as we will discuss in the next paragraph, but it is important to point out that our rats did freeze, suppress food cup behavior, and approach when the other rat froze during shock-other trials, which other studies have used as evidence for empathy in rodents [16,36,37,40,42,44,46–48,51]. Moreover, also consistent with previous work, we show that when a rat is not experiencing shock they exhibit less empathetic behavior [37,42,45,47]. Importantly, previous papers have concluded that shock naive rats don’t freeze when another rat freezes because the observer rat is unable to fully empathize with what the other rat is feeling until it has experienced the pain itself. By examining behavior in well trained animals and by manipulating shock and no-shock within the same session, we are able to show that in rats that are fully aware of what the shock is, that their behavioral reactions (i.e., freezing, food cup suppression, approach) to the other rat being shocked are not because they are unfamiliar with the shock and can’t empathize, but instead, it is because they don’t feel threatened. Thus, we argue that our rats do show similar behavioral measures of empathy as found in previous work, and that under these circumstances neural activity in ACC correlates better with attention.
With that said, it is entirely possible that what ACC encodes during this social task is task-dependent. For example, in primate studies, monkeys have to choose between delivering reward to the conspecific and oneself or between the conspecific and an empty bottle [24,52]. This type of evaluation might require ACC to better encode the value that the animal places on these circumstances, by directing attention to socially-derived cues from the conspecific. Further, the nature of encoding in ACC might also be highly dependent on how the animal subsequently uses social information to alter its own behavior, which will consequently depend on the value that the animal places on outcomes delivered to the conspecific. Although many studies have shown rats to exhibit empathetic and pro-social behaviors [15,37,38,39,42,53,54,55], we have found that rats can be rather ‘self-interested’. This has been evident in our studies examining dopamine (DA) release in nucleus accumbens in a version of the Pavlovian task described here [36,51]. For example, we have shown that rats emit appetitive vocalizations and DA is released during rewards delivered to conspecific, but only early during learning. After rats experienced several trials where the conspecific received reward and they did not, vocalizations became aversive and DA was inhibited during conspecific reward delivery [51,56,57]. Further, we have shown that DA is released when the recording rat observes the conspecific receive shocks, suggesting that observation of the conspecific receiving shock, instead receiving shock itself, is an event that is better in value than expected [36]. Therefore, in tasks where rats are self-interested – such as in an appetitively/aversively competitive context – and circumstances are well-learned, ACC may contribute more to social attention. In contrast, when different task parameters promote seemingly more empathetic behaviors, then ACC activity might better reflect encoding of the affective information received from other rats. Given the evident influence of ACC-amygdala interactions on vicarious learning and decision-making tasks [44,45,58,59], differential ACC activity profiles in competitive versus non-competitive tasks may modulate downstream social decision making preferences for self-interested versus prosocial behavior.
In conclusion, here we replicate work showing that neurons in ACC respond to rewards and shocks delivered to oneself and others, but by varying valence within the same task and by omitting outcomes in different trial blocks, we demonstrate that while activity in ACC can represent specific attributes related to conspecific distress, its overall population activity reflects attention in social contexts when there is threat of personal harm.
STAR METHODS
Resource availability
Lead Contact:
Further information and requests for resources should be directed to and will be fulfilled by the Lead Contact, Dr. Matthew Roesch (mroesch@umd.edu)
Materials Availability:
This study did not generate new materials.
Data and Code Availability:
Data will be made available from the Lead Contact, Dr. Matthew Roesch upon request so that data can be provided in a format most suitable to the requester.
Experimental Model and Subject Details
Animals
Six male and six female Sprague-Dawley rats were obtained with weights 175–225g from Charles River Labs. Rats were individually housed on a 12-hr light–dark cycle and tested during the light phase. Water was available ad libitum and body weight was maintained at no less than 85% of pre-experimental levels by food restriction (14–15g of laboratory chow daily in addition to approximately 2.5g of sucrose pellets (Test Diet) consumed during daily experimental sessions). Each implanted animal was paired with the same conspecific throughout the experiment. Conspecifics were of the same age and sex, were ordered at the same time and housed next to each other in transparent cages in the animal colony. Rats were not housed in the same cage due to implants. All experiments were approved by the University of Maryland College Park Institutional Animal Care and Use Committee under university and NIH guidelines.
Surgical procedures and histology
Surgical procedures followed guidelines for aseptic technique. Electrodes were manufactured and implanted as in prior recording experiments [31,60,61]. Rats had a drivable bundle of ten 25μm diameter FeNiCr wires (Stablohm 675, California Fine Wire, Grover Beach, CA) chronically implanted in the left or right hemisphere dorsal to anterior cingulate cortex (n = 6 rats; 0.2mm anterior to bregma, 0.5mm left [n = 3] or right [n = 3] of the midline, and 1mm ventral to the brain surface, according to Paxinos and Watson; see also Figure 1E). Immediately prior to implantation, wires were freshly cut with surgical scissors to extend ~1mm beyond the cannula and electroplated with platinum (H2PtCl6, Aldrich, Milwaukee, WI) to an impedance of ~300kOhms. Cephalexin (15mg/kg p.o.) was administered twice daily for two weeks postoperatively to prevent infection.
Method Details
Pavlovian Social Outcome Task
In the reported experiments we utilize a modified version of a task previously published [36], described in detail below. Recordings were collected in a modified shuttle box chamber (Figure 1A; 16 in × 6.25in × 8.375 in; WDH; Med Associates; n = 6 rats). A modified guillotine door with wire mesh covering the opening divided the chamber in two equal compartments. Rats could see, smell and hear each other. Each trial began with illumination of a houselight (Figure 1B–D). Five seconds later, one of three auditory cues (the ‘outcome cue’) was emitted for 5 s (i.e., tone, white noise or clicker, counterbalanced across rats) gated by an Arduino [62,63]. One auditory cue indicated that reward would be delivered (i.e., reward trial), the second cue signaled that shock would be administered (i.e., shock trials) and the third cue (i.e. neutral) indicated that neither reward nor punishment would occur. After 5 s, the auditory cue was terminated simultaneously with the illumination of one of the two directional lights. This ‘directional’ cue informed the rats which side of the cage (random 50/50) would lead to a positive (reward), negative (foot-shock) or neutral outcome (nothing). After 5 s, reward or punishment or nothing was administered to the side of the box that was illuminated by the directional cue. The shock consisted of two 250 ms shocks (0.56 mA) spaced 2 s apart. Reinforcement occurred on 80% of trials. This paradigm was completely Pavlovian, thus rats had no control over what outcomes would occur or which rat would receive them. The directional light turned off 5 s after the delivery of outcomes, followed 5 s later by the houselights turning off and a final 5 s ITI before the start of the next trial.
Experimental sessions lasted 2 h, where rats underwent 4 different blocks of 60 trials (six trial types, 30s/trial, 10 trials/type; Figure 1B–D). Trials were presented in a pseudo-randomized order. The block types represent four possible combinations (context pairs) for outcome delivery for each pair of rats: ‘Both reinforced’ (R/R), where both rats are reinforced (i.e., receive outcomes); ‘both not reinforced’ (N/N), where neither rat receives outcomes; ‘only self (recording rat) reinforced ‘ (R/N), where only the recorded rat received outcomes; ‘only conspecific reinforced’ (N/R), where the conspecific received outcomes while the conspecific did not. The four blocks in every session follow one of two sequences, which alternated daily. Two sequences were established in order to counterbalance the order in which self or other are extinguished during the task. Finally, every 6 sessions, recording rats trained in a session alone, as a control for social context. In these sessions, pellet outcomes to the other were delivered to an empty beaker, and shock deliveries to the other were delivered as normal, but to an empty side.
An infrared beam was placed at the entrance to the food cup on the recording rat’s side of the cage. This beam was disrupted upon entry of the rat’s nose into the food cup, and beam breaks served as a quantitative measure of reward seeking. In our Med Associates boxes, we sampled every 10 ms to determine if the beam in the food cup was broken throughout the entire trial.
Behavioral electrophysiology
Procedures were the same as described previously [60]. Electrodes were advanced at the end of recording sessions (40 or 80μm). Neural activity was recorded using two identical Plexon Omniplex systems (Dallas, TX), connected to the animals’ implants through a commutator which allowed them to freely move about the chamber. Waveforms (>2.5:1 signal-to-noise) were extracted from active channels and recorded to disk by an associated workstation with event timestamps from the behavior computer.
Quantification and Statistical Analysis
Behavioral data analysis
For analysis of behavioral responding, infrared beam break data (10 ms sampling rate) were aggregated as proportions across 1-second bins (i.e. divided by the number of possible breaks per second to yield a percentage), collected from the MED-PC software (Med Associates). For video scoring of freezing and approach, cameras were positioned facing the recording rat. Video analysis, like IR and neural analyses, focused on four trial epochs lasting five seconds in length: auditory cue; directional light; outcome and post-outcome to houselights off. Freezing (sudden cessation of movement) and approach toward the mesh divider were assessed during these periods by two independent observers. Statistical procedures on the data were executed using MATLAB (MathWorks; Wilcoxon and Student’s t-test) and Excel (Microsoft; Chi-squared).
Electrophysiological data analysis
Units were sorted via Offline Sorter software from Plexon Inc (Dallas, TX), using a template matching algorithm and analyzed in Neuroexplorer (Plexon) and MATLAB (MathWorks). Activity was examined during two different 5 s epochs: Directional Light epoch = directional light to outcome deliver (5s); Outcome epoch: 5 s after start of outcome delivery (i.e., 5 s starting 5s after onset of directional lights). Activity in population histograms was normalized by dividing by the maximal firing rate of each neuron. All statistical procedures were executed using raw firing rates or counts, in either MATLAB (Wilcoxon) or Excel (Chi-squared). Neurons were classified as being reward-or shock-responsive by comparing reward to neutral and shock to neutral, respectively (Wilcoxon; p < 0.05).
Supplementary Material
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Bacterial and Virus Strains | ||
| Biological Samples | ||
| Chemicals, Peptides, and Recombinant Proteins | ||
| Chloroplatinic acid solution (H2PtCl6) | Sigma Aldrich | https://www.sigmaaldrich.com/catalog/product/aldrich/262587 |
| Critical Commercial Assays | ||
| Deposited Data | ||
| Data will be made available from the Lead Contact, Dr. Matthew Roesch upon request so that data can be provided in a format most suitable to the requester | mroesch@umd.edu | |
| Experimental Models: Cell Lines | ||
| Experimental Models: Organisms/Strains | ||
| Rat; Sprague-Dawley | Charles River Laboratory | N/A |
| Oligonucleotides | ||
| Recombinant DNA | ||
| Software and Algorithms | ||
| MATLAB | MathWorks | https://www.mathworks.com/products/matlab.html |
| Offline Sorter v3 | Plexon Inc. | https://plexon.com/products/offline-sorter/ |
| NeuroExplorer | Plexon Inc. | https://plexon.com/products/neuroexplorer/ |
| OmniPlex | Plexon Inc. | https://plexon.com/products/omniplex-software/ |
| Med-PC IV | Med Associates Inc. | https://www.med-associates.com/ |
| Other | ||
Anterior cingulate activity is modulated by reward and shock to self and others
Anterior cingulate fires similarly to both reward and shock, reflecting attention
Firing and behavior in response to shock to others is modulated by personal threat
Anterior cingulate cortex (ACC) signals contribute to social attention
Acknowledgements:
Special thanks are given to the McDannald lab (Boston College, MA) for their instruction and guidance with shock components of the behavioral task. This work was supported by NIMH (R01MH112504).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interests: The authors declare no competing interests.
REFERENCES
- 1.Scott-Van Zeeland AA, Dapretto M, Ghahremani DG, Poldrack RA, and Bookheimer SY (2010). Reward processing in autism. Autism Res 3, 53–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Neuhaus E, Beauchaine TP, and Bernier R (2010). Neurobiological correlates of social functioning in autism. Clinical Psychology Review 30, 733–748. [DOI] [PubMed] [Google Scholar]
- 3.Taylor BA, and DeQuinzio JA (2012). Observational Learning and Children With Autism. Behav Modif 36, 341–360. [DOI] [PubMed] [Google Scholar]
- 4.Cook JL, and Black J (2012). The Influence of Social Interaction on Cognitive Training for Schizophrenia. Front. Neurosci 6 (140). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blair RJR (2003). Neurobiological basis of psychopathy. Br J Psychiatry 182, 5–7. [DOI] [PubMed] [Google Scholar]
- 6.Gaigg SB (2012). The Interplay between Emotion and Cognition in Autism Spectrum Disorder: Implications for Developmental Theory. Front. Integr. Neurosci 6 (113). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gonzalez-Liencres C, Shamay-Tsoory SG, and Brüne M (2013). Towards a neuroscience of empathy: Ontogeny, phylogeny, brain mechanisms, context and psychopathology. Neuroscience & Biobehavioral Reviews 37, 1537–1548. [DOI] [PubMed] [Google Scholar]
- 8.Marsh AA, Finger EC, Fowler KA, Jurkowitz ITN, Schechter JC, Yu HH, Pine DS, and Blair RJR (2011). Reduced amygdala–orbitofrontal connectivity during moral judgments in youths with disruptive behavior disorders and psychopathic traits. Psychiatry Research: Neuroimaging 194, 279–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Finger EC, Marsh AA, Blair KS, Reid ME, Sims C, Ng P, Pine DS, and Blair RJR (2011). Disrupted Reinforcement Signaling in the Orbitofrontal Cortex and Caudate in Youths With Conduct Disorder or Oppositional Defiant Disorder and a High Level of Psychopathic Traits. AJP 168, 152–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bachevalier J, and Loveland KA (2006). The orbitofrontal–amygdala circuit and self-regulation of social–emotional behavior in autism. Neuroscience & Biobehavioral Reviews 30, 97–117. [DOI] [PubMed] [Google Scholar]
- 11.King JA, Blair RJR, Mitchell DGV, Dolan RJ, and Burgess N (2006). Doing the right thing: A common neural circuit for appropriate violent or compassionate behavior. NeuroImage 30, 1069–1076. [DOI] [PubMed] [Google Scholar]
- 12.Lockwood PL (2016). The anatomy of empathy: Vicarious experience and disorders of social cognition. Behavioural Brain Research 311, 255–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Machado CJ, and Bachevalier J (2006). The impact of selective amygdala, orbital frontal cortex, or hippocampal formation lesions on established social relationships in rhesus monkeys (Macaca mulatta). Behavioral neuroscience 120, 761. [DOI] [PubMed] [Google Scholar]
- 14.Yerys BE, Wallace GL, Harrison B, Celano MJ, Giedd JN, and Kenworthy LE (2009). Set-shifting in children with autism spectrum disorders: Reversal shifting deficits on the Intradimensional/Extradimensional Shift Test correlate with repetitive behaviors. Autism 13, 523–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hernandez-Lallement J, Attah AT, Soyman E, Pinhal CM, Gazzola V, and Keysers C (2020). Harm to Others Acts as a Negative Reinforcer in Rats. Current Biology 30, 949–961.e7. [DOI] [PubMed] [Google Scholar]
- 16.Carrillo M, Han Y, Migliorati F, Liu M, Gazzola V, and Keysers C (2019). Emotional Mirror Neurons in the Rat’s Anterior Cingulate Cortex. Current Biology 29, 1301–1312.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Waal FBM, and Preston SD (2017). Mammalian empathy: behavioural manifestations and neural basis. Nat Rev Neurosci 18, 498–509. [DOI] [PubMed] [Google Scholar]
- 18.Apps MAJ, Rushworth MFS, and Chang SWC (2016). The Anterior Cingulate Gyrus and Social Cognition: Tracking the Motivation of Others. Neuron 90, 692–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hayden BY, Pearson JM, and Platt ML (2009). Fictive Reward Signals in the Anterior Cingulate Cortex. Science 324, 948–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hayden BY, and Platt ML (2010). Neurons in Anterior Cingulate Cortex Multiplex Information about Reward and Action. Journal of Neuroscience 30, 3339–3346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hosokawa T, Kennerley SW, Sloan J, and Wallis JD (2013). Single-Neuron Mechanisms Underlying Cost-Benefit Analysis in Frontal Cortex. Journal of Neuroscience 33, 17385–17397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kennerley SW, Dahmubed AF, Lara AH, and Wallis JD (2009). Neurons in the Frontal Lobe Encode the Value of Multiple Decision Variables. Journal of Cognitive Neuroscience 21, 1162–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luk C-H, and Wallis JD (2013). Choice Coding in Frontal Cortex during Stimulus-Guided or Action-Guided Decision-Making. Journal of Neuroscience 33, 1864–1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chang SWC, Gariépy J-F, and Platt ML (2013). Neuronal reference frames for social decisions in primate frontal cortex. Nat Neurosci 16, 243–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barack DL, Chang SWC, and Platt ML (2017). Posterior Cingulate Neurons Dynamically Signal Decisions to Disengage during Foraging. Neuron 96, 339–347.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yoo SBM, Tu JC, Piantadosi ST, and Hayden BY (2020). The neural basis of predictive pursuit. Nat Neurosci 23, 252–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bissonette GB, Powell EM, and Roesch MR (2013). Neural structures underlying set-shifting: Roles of medial prefrontal cortex and anterior cingulate cortex. Behavioural Brain Research 250, 91–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bryden DW, Brockett AT, Blume E, Heatley K, Zhao A, and Roesch MR (2019). Single Neurons in Anterior Cingulate Cortex Signal the Need to Change Action During Performance of a Stop-change Task that Induces Response Competition. Cerebral Cortex 29, 1020–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vázquez D, Pribut HJ, Burton AC, Tennyson SS, and Roesch MR (2020). Prior cocaine self-administration impairs attention signals in anterior cingulate cortex. Neuropsychopharmacol. 45, 833–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brockett AT, Tennyson SS, deBettencourt CA, Gaye F, and Roesch MR (2020). Anterior cingulate cortex is necessary for adaptation of action plans. Proc Natl Acad Sci USA 117, 6196–6204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bryden DW, Johnson EE, Tobia SC, Kashtelyan V, and Roesch MR (2011). Attention for learning signals in anterior cingulate cortex. The Journal of neuroscience : the official journal of the Society for Neuroscience 31, 18266–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roesch MR, Esber GR, Li J, Daw ND, and Schoenbaum G (2012). Surprise! Neural correlates of Pearce-Hall and Rescorla-Wagner coexist within the brain: Neural correlates of RW and PH. European Journal of Neuroscience 35, 1190–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hayden BY, Heilbronner SR, Pearson JM, and Platt ML (2011). Surprise Signals in Anterior Cingulate Cortex: Neuronal Encoding of Unsigned Reward Prediction Errors Driving Adjustment in Behavior. Journal of Neuroscience 31, 4178–4187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ebitz RB, and Platt ML (2015). Neuronal Activity in Primate Dorsal Anterior Cingulate Cortex Signals Task Conflict and Predicts Adjustments in Pupil-Linked Arousal. Neuron 85, 628–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bissonette GB, Bryden DW, and Roesch MR (2014). You won’t regret reading this. Nat Neurosci 17, 892–893. [DOI] [PubMed] [Google Scholar]
- 36.Lichtenberg NT, Lee B, Kashtelyan V, Chappa BS, Girma HT, Green EA, Kantor S, Lagowala DA, Myers MA, Potemri D, et al. (2018). Rat behavior and dopamine release are modulated by conspecific distress. eLife 7, e38090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Atsak P, Orre M, Bakker P, Cerliani L, Roozendaal B, Gazzola V, Moita M, and Keysers C (2011). Experience Modulates Vicarious Freezing in Rats: A Model for Empathy. PLoS ONE 6, e21855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bartal IB-A, Decety J, and Mason P (2011). Empathy and Pro-Social Behavior in Rats. Science 334, 1427–1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ben-Ami Bartal I, Rodgers DA, Bernardez Sarria MS, Decety J, and Mason P (2014). Pro-social behavior in rats is modulated by social experience. eLife 3, e01385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Burkett JP, Andari E, Johnson ZV, Curry DC, de Waal FBM, and Young LJ (2016). Oxytocin-dependent consolation behavior in rodents. Science 351, 375–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lungwitz EA, Stuber GD, Johnson PL, Dietrich AD, Schartz N, Hanrahan B, Shekhar A, and Truitt WA (2014). The Role of the Medial Prefrontal Cortex in Regulating Social Familiarity-Induced Anxiolysis. Neuropsychopharmacol 39, 1009–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Meyza KZ, Bartal IB-A, Monfils MH, Panksepp JB, and Knapska E (2017). The roots of empathy: Through the lens of rodent models. Neuroscience & Biobehavioral Reviews 76, 216–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Keum S, Shin H-S Neural Basis of Observational Fear Learning: A Potential Model of Affective Empathy. Neuron, 104 (2019), pp. 78–86. [DOI] [PubMed] [Google Scholar]
- 44.Jeon D, Kim S, Chetana M, Jo D, Ruley HE, Lin S-Y, Rabah D, Kinet J-P, and Shin H-S (2010). Observational fear learning involves affective pain system and Cav1.2 Ca2+ channels in ACC. Nat Neurosci 13, 482–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Allsop SA, Wichmann R, Mills F, Burgos-Robles A, Chang C-J, Felix-Ortiz AC, Vienne A, Beyeler A, Izadmehr EM, Glober G, et al. (2018). Corticoamygdala Transfer of Socially Derived Information Gates Observational Learning. Cell 173, 1329–1342.e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.van Gurp S, Hoog J, Kalenscher T, and van Wingerden M (2019). Social Value Unblocks Pavlovian Reinforcement Learning in Male Rats. SSRN Journal. [Google Scholar]
- 47.Kim EJ, Kim ES, Covey E, and Kim JJ (2010). Social Transmission of Fear in Rats: The Role of 22-kHz Ultrasonic Distress Vocalization. PLoS ONE 5, e15077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim S, Matyas F, Lee S, Acsady L, and Shin H-S (2012). Lateralization of observational fear learning at the cortical but not thalamic level in mice. Proceedings of the National Academy of Sciences 109, 15497–15501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bush G, Luu P, and Posner MI (2000). Cognitive and emotional influences in anterior cingulate cortex. Trends in Cognitive Sciences 4, 215–222. [DOI] [PubMed] [Google Scholar]
- 50.Devinsky O, Morrell MJ, and Vogt BA (1995). Contributions of anterior cingulate cortex to behaviour. Brain 118, 279–306. [DOI] [PubMed] [Google Scholar]
- 51.Kashtelyan V, Lichtenberg NT, Chen ML, Cheer JF, and Roesch MR (2014). Observation of Reward Delivery to a Conspecific Modulates Dopamine Release in Ventral Striatum. Current Biology 24, 2564–2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Noritake A, Ninomiya T, and Isoda M (2018). Social reward monitoring and valuation in the macaque brain. Nat Neurosci 21, 1452–1462. [DOI] [PubMed] [Google Scholar]
- 53.Hernandez-Lallement J, van Wingerden M, Marx C, Srejic M, and Kalenscher T (2015). Rats prefer mutual rewards in a prosocial choice task. Front. Neurosci 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sivaselvachandran S, Acland EL, Abdallah S, and Martin LJ (2018). Behavioral and mechanistic insight into rodent empathy. Neuroscience & Biobehavioral Reviews 91, 130–137. [DOI] [PubMed] [Google Scholar]
- 55.Sato N, Tan L, Tate K, and Okada M (2015). Rats demonstrate helping behavior toward a soaked conspecific. Anim Cogn 18, 1039–1047. [DOI] [PubMed] [Google Scholar]
- 56.Oberliessen L, Hernandez-Lallement J, Schäble S, van Wingerden M, Seinstra M, and Kalenscher T (2016). Inequity aversion in rats, Rattus norvegicus. Animal Behaviour 115, 157–166. [Google Scholar]
- 57.Oberliessen L, and Kalenscher T (2019). Social and Non-social Mechanisms of Inequity Aversion in Non-human Animals. Front. Behav. Neurosci 13, 133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hernandez-Lallement J, van Wingerden M, Schäble S, and Kalenscher T (2016). Basolateral amygdala lesions abolish mutual reward preferences in rats. Neurobiology of Learning and Memory 127, 1–9. [DOI] [PubMed] [Google Scholar]
- 59.Hernandez-Lallement J, van Wingerden M, Schäble S, and Kalenscher T (2016). A Social Reinforcement Learning Hypothesis of Mutual Reward Preferences in Rats In Social Behavior from Rodents to Humans, Wöhr M and Krach S, eds. (Cham: Springer International Publishing; ), pp. 159–176. [DOI] [PubMed] [Google Scholar]
- 60.Bryden DW, and Roesch MR (2015). Executive Control Signals in Orbitofrontal Cortex during Response Inhibition. 35, 3903–3914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bryden DW, Burton AC, Kashtelyan V, Barnett BR, and Roesch MR (2012). Response inhibition signals and miscoding of direction in dorsomedial striatum. Frontiers in integrative neuroscience 6, 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wright KM, DiLeo A, and McDannald MA (2015). Early adversity disrupts the adult use of aversive prediction errors to reduce fear in uncertainty. Front. Behav. Neurosci 9 (227). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.DiLeo A, Wright KM, and McDannald MA (2016). Subsecond fear discrimination in rats: adult impairment in adolescent heavy alcohol drinkers. Learn. Mem 23, 618–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available from the Lead Contact, Dr. Matthew Roesch upon request so that data can be provided in a format most suitable to the requester.


