Dear Editor,
A 29-year-old Chinese male with recurrent alveolar soft part sarcoma of the tongue metastatic to lung presented with fever and rash (Figure 1a). He underwent glossectomy and adjuvant radiotherapy, complicated by recurrence and abscess with sinus tract formation on the right neck. Current therapy included ipilimumab 3mg/kg and nivolumab 1mg/kg (completed 9 cycles; last dose two days before admission), and cabozantinib 40mg daily (held for six weeks due to sinus tract in the neck).
Figure 1.
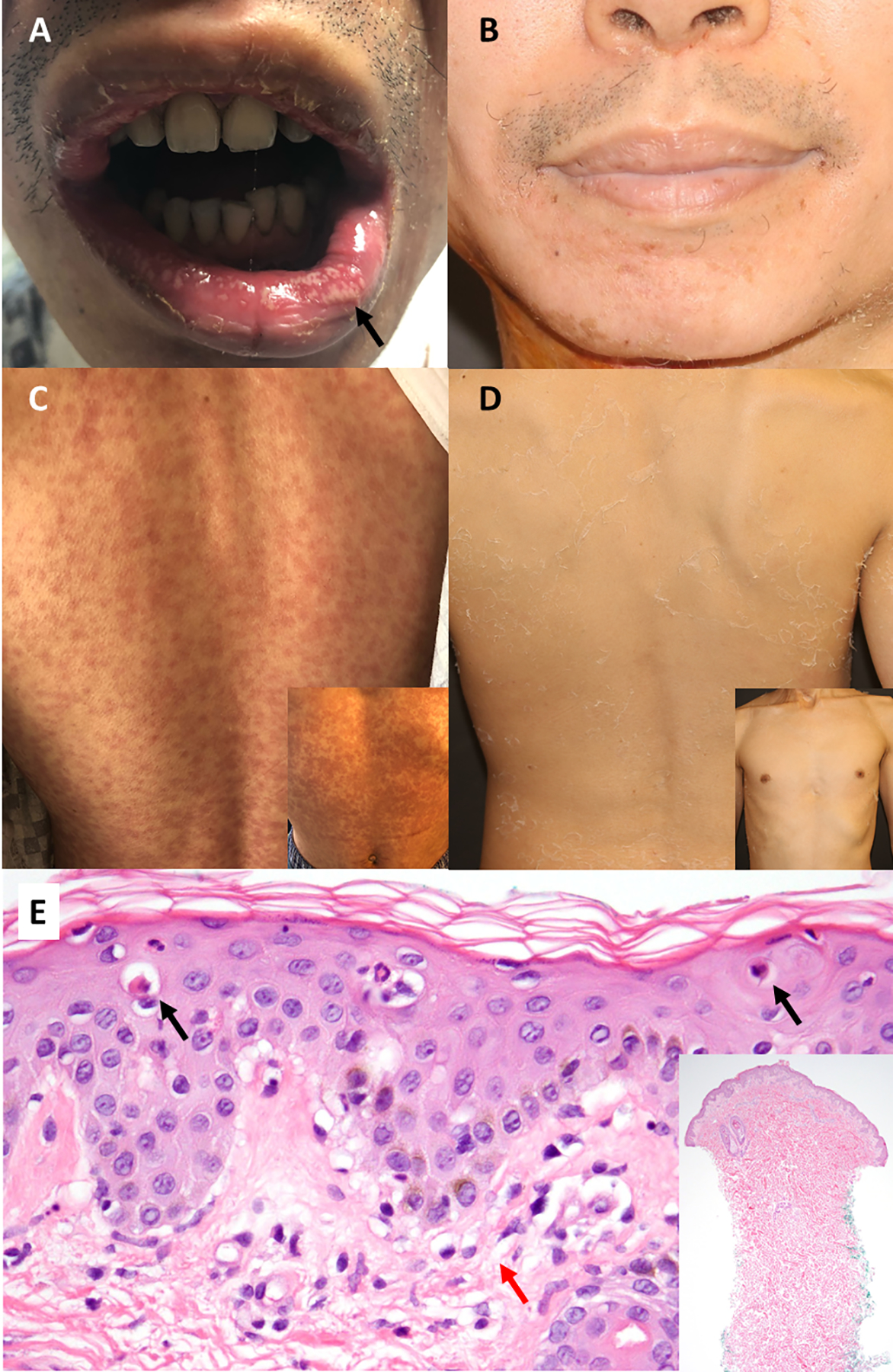
Initial presentation showing morbilliform eruption diffusely over torso with associated mucosal pustules (1a, 1c). One week following tocilizumab administration (4mg/kg intravenously, 1 dose) with complete resolution of the cutaneous eruption, leaving behind dry desquamation (1b, 1d). Punch biopsy demonstrating scattered necrotic keratinocytes (black arrows) and vacuolar interface dermatitis at the basal layer (red arrow) with occasional eosinophils consistent with drug hypersensitivity eruption (1e).
He received doxycycline and amoxicillin/clavulanic acid for a superinfected malignant wound on the neck, and fluconazole for oral thrush. Four days later, he returned tachycardic and febrile to 39.3C with erythematous macules and papules diffusely over the torso and extremities along with facial oedema and blepharitis. The right jawline had a sinus tract with purulent drainage. He had elevated lactic acid (2.1 [ref 0.3–1.3 mM/L]), normal white blood count with 91% neutrophils, and no eosinophilia or renal/hepatic dysfunction. Infectious workup including blood/urine cultures and chest X-ray were unremarkable. Of note, he had diarrhoea and was norovirus positive; other viral testing was negative.
Skin biopsy demonstrated vacuolar interface dermatitis with scattered keratinocyte necrosis and occasional eosinophils consistent with a drug hypersensitivity eruption (Figure 1e). He had no known drug allergies. The recent amoxicillin/clavulanic acid was favoured given its increased incidence of morbilliform eruptions1.
He was treated with clobetasol 0.05% spray, avoiding systemic steroids given the neck abscess and norovirus infection. On day two, he developed pustules on the lips with mucosal pain and odynophagia (Fig. 1b). His xerophthalmia progressed to intermittent blurry vision and rash and pruritus worsened. Inflammatory biomarker analysis showed significantly elevated IL-6 (376.8 pg/mL [ref < 5]), IL-10 (103 pg/mL [ref <18]), TNFα (49 pg/mL [ref <22]), and elafin (35 pg/mL [ref 4.9–23.8]). IL-1 was normal (2 pg/mL [ref < 36]). C-reactive protein (CRP) was 6.82 mg/dL ([ref <0.30]). Given the significantly elevated IL-6, he was treated with one dose tocilizumab (Actemra®, Roche AG, Basel, Switzerland) 4mg/kg intravenously.
The next day the rash was less erythematous, fevers subsided, and no additional ocular or oral symptoms developed. Two days after administration, mouth pain resolved, pruritus decreased, and he was discharged with clobetasol 0.05% spray, carboxymethylcellulose 0.5% ophthalmic drops, and dexamethasone mouth rinse. By one week, all mucocutaneous symptoms resolved without sequelae (Figures 1c, 1d). His CRP, a surrogate marker for IL-6 activity, normalized, IL-6 decreased to 19 pg/mL, and other biomarkers normalized (TNFα: 8 pg/mL, IL-10: <9 pg/mL, IL-1: < 1pg/mL, and elafin: 8 ng/mL).
We describe the novel use of tocilizumab, an IL-6R inhibitor, for rapid and complete resolution of a severe cutaneous drug hypersensitivity reaction in a patient with underlying malignancy and active infection. Steroid-induced side effects are numerous, especially in immunosuppressed patients, and may interfere with on-going oncologic therapy. Identifying targeted, steroid-sparing medications capable of rapidly ameliorating SCARs with elevated levels of inflammatory biomarkers is of critical importance.
IL-6 is an acute phase reactant and mediator of fever elevated in immune-mediated inflammatory syndromes;2 serum levels correlate with disease activity. IL-6 induces acute phase proteins produced by the liver, including CRP, which is an independent indicator of systemic inflammation and participates in tissue damage3.
Recently, IL-6 was identified as a biomarker for severe adverse drug eruptions4. Targeted anticancer therapies and immunotherapy may result in SCARs with atypical presentations and significant overlap, making their classification difficult5, and their progression challenging to predict. IL-6 elevation occurs early and may precede fulminant development of the drug eruption, providing a prognostic tool for monitoring and diagnostic avenue for treatment with targeted therapy4. As CRP is not specific to IL-6 elevation, we recommend checking IL-6 levels at onset of the drug reaction and using CRP as a surrogate marker of IL-6 activity for monitoring response.
Tocilizumab, a humanized recombinant monoclonal antibody against soluble and membrane-bound IL-6R, is FDA-approved for treatment of cytokine release syndrome (CRS), rapidly ameliorating fevers and symptoms of CRS without apparent effect on oncologic therapy6.
Similarly, targeted inhibition of IL-6 activity may reduce tissue damage in SCARs with elevated IL-6 levels, and is an enticing alternative to steroids. Our patient had an infected sinus tract, and high dose systemic steroids may worsen infection and delay wound healing. Sequelae of steroids are numerous,7 and may be avoided with more specific, targeted anti-inflammatory treatments. TNFα-inhibitors have been an effective alternative to systemic steroids for SCARs, with faster re-epithelialization and lower incidence of gastrointestinal side effects8.
Herein, we observed rapid and complete resolution of a severe drug hypersensitivity reaction after treatment with tocilizumab. Within 24 hours of administration, fevers and progression of mucocutaneous symptoms ceased. Targeted therapies directed at the causative pathogenic mediators are coveted to spare the deleterious effects of broad-acting immunosuppressive medications, such as corticosteroids. Tocilizumab may represent a steroid-sparing therapeutic option for patients with elevated IL-6 secondary to severe drug hypersensitivity reactions.
Funding sources:
This research was funded in part through the NIH/NCI Cancer Center Support Grant P30 CA008748.
Footnotes
Conflicts of interest: Dr. Alina Markova is on the advisory board for AstraZeneca and receives research funding from Incyte.
References
- 1.Blumenthal KG, Peter JG, Trubiano JA et al. Antibiotic allergy. Lancet 2019; 393: 183–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nishimoto N, Kishimoto T. Interleukin 6: from bench to bedside. Nat. Clin. Pract. Rheumatol 2006; 2: 619–26. [DOI] [PubMed] [Google Scholar]
- 3.Yao X, Huang J, Zhong H et al. Targeting interleukin-6 in inflammatory autoimmune diseases and cancers. Pharmacol. Ther 2014; 141: 125–39. [DOI] [PubMed] [Google Scholar]
- 4.Shiohara T, Mizukawa Y, Aoyama Y. Monitoring the acute response in severe hypersensitivity reactions to drugs. Curr. Opin. Allergy Clin. Immunol 2015; 15: 294–9. [DOI] [PubMed] [Google Scholar]
- 5.Chen CB, Wu MY, Ng CY et al. Severe cutaneous adverse reactions induced by targeted anticancer therapies and immunotherapies. Cancer Manag. Res 2018; 10: 1259–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davila ML, Riviere I, Wang X et al. Efficacy and toxicity management of 19–28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci. Transl. Med 2014; 6: 224ra25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Waljee AK, Rogers MA, Lin P et al. Short term use of oral corticosteroids and related harms among adults in the United States: population based cohort study. BMJ 2017; 357: j1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang CW, Yang LY, Chen CB et al. Randomized, controlled trial of TNF-alpha antagonist in CTL-mediated severe cutaneous adverse reactions. J. Clin. Invest 2018; 128: 985–96. [DOI] [PMC free article] [PubMed] [Google Scholar]


