Abstract
We previously reported bidirectional gene expression regulation of the Bone Morphogenetic Proteins (BMP2, 4, & 7) in chick retinal pigment epithelium (RPE) in response to imposed optical defocus and form-deprivation (FD). This study investigated whether there are local (regional) differences in these effects. 19-day old White-Leghorn chicks wore monocular +10 or −10 D lenses, or diffusers (FD) for 2 or 48 h, after which RPE samples were collected from both eyes, from a central circular zone (3 mm radius), and 3 mm wide annular mid-peripheral and peripheral zones in all cases. BMP2, 4, and 7 gene expression levels in RPE from treated and fellow control eyes were compared as well as differences across zones. With the +10 D lens, increased expression of both BMP2 and BMP4 genes was observed in central and mid-peripheral zones but not the peripheral zone after 2 and 48 h. In contrast, with the −10 D lens BMP2 gene expression was significantly decreased in all three zones after 2 and 48 h. Similar patterns of BMP2 gene expression were observed in all three zones after 48 h of FD. Smaller changes were recorded for BMP4 and BMP7 gene expression for both myopia-inducing treatments. That optical defocus- and FD-induced changes in BMP gene expression in chick RPE show treatment-dependent local (regional) differences suggest important differences in the nature and contributions of local retinal and underlying RPE regions to eye growth regulation.
Keywords: myopia, chick, RPE, BMP
Graphical Abstract
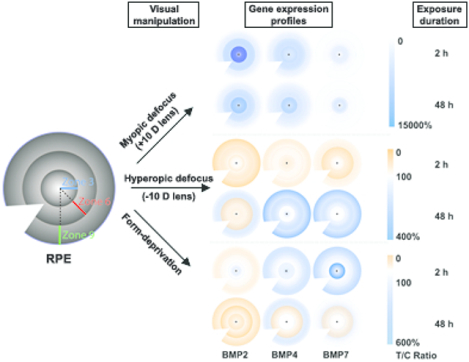
We previously reported in young chicks bidirectional gene expression regulation of the BMP2, 4, & 7 in retinal pigment epithelium (RPE) after visual manipulation, tied to the direction of induced eye growth changes. Here we report local (regional) differences in expression patterns that were also treatment specific. Both BMP2 and BMP4 genes showed the expected increased expression with imposed myopic defocus (+10 D lens) after 2 and 48 h, albeit limited to central and mid-peripheral RPE zones. In contrast, the expected decrease in BMP2 gene expression with imposed hyperopic defocus (−10 D lens) was evident across all three zones after both 2 and 48 h, with similar patterns observed after 48 h with form-deprivation. Similar but smaller changes were recorded for BMP4 and BMP7 gene expression with the latter myopia-inducing treatments.
Introduction
Myopia is a common refractive error, which in most cases is the product of excessive eye elongation, resulting in poor distance vision when not optically corrected. The change in eye length largely reflects excessive enlargement of the vitreous chamber, with potential for associated blinding complications, such as retinal detachment, macular degeneration and choroidal neovascularization.1, 2 The overall prevalence of myopia is increasing rapidly around the world as reflected in the projected global prevalence figure of 49.8% for 2050, compared to 22.9% as reported in 2000.3 Likewise, the prevalence of high myopia has increased. The comparable statistics for high myopia are 9.8% and 2.7% for 2050 and 2000 respectively. Myopia is especially common in some regions of East Asia, where prevalence figures of greater than 80% have already been reported for some populations.4
Although the etiology of the most common, juvenile form of myopia remains unresolved, it is likely that both environmental and genetic factors contribute to its onset and progression.5, 6 Studies using animal models have provided strong evidence for active emmetropization and the roles for visual environmental factors in ocular growth regulation, with dysregulated growth leading to the development of myopia under some conditions.7 Previous studies involving both young chickens and monkeys have also provided evidence of local, regional control of the posterior vitreous chamber, the main target of emmetropizing mechanisms. For example, when only one half of the retina (either nasal or temporal) was form-deprived, vitreous chamber and thus overall eye shape changes were restricted to the region of deprivation.8, 9 Likewise, half lenses (negative and positive), used to impose local focusing errors, also induce local shape changes.10–12 Together these studies point to local ocular growth regulation, with a key role of the neural retina, as the origin of growth-modulating signal cascades.7, 13, 14 That elimination of the fovea in young monkeys failed to disrupt emmetropization, as reflected in recovery from form-deprivation myopia, also suggests an important role of the peripheral retina in eye growth regulation.15–17
Although the concept of local retinal control of eye growth is now widely accepted, details of the underlying molecular and cellular mechanisms, including the assumed retina-sclera signaling cascades, are not well understood.18–23 The retinal pigment epithelium (RPE) likely serves as a relay station for growth regulatory signals, being strategically positioned between the neural retina, the assumed origin of growth modulatory signals, and the choroid and sclera, which together determine the dimensions of the vitreous chamber.24, 25 Consistent with this hypothesis, we have reported differences in the expression of a number of genes in the chick RPE that are tied to experimentally altered eye growth.26–29 The expression of genes for three members of the BMP family (BMP2, BMP4, and BMP7) has been shown to be bidirectionally regulated: increased with imposed myopia (positive lenses), which inhibits eye growth, and decreased with imposed hyperopia (negative lenses), which accelerates eye growth.26, 27 RPE BMP gene expression is also decreased with form-deprivation, which also accelerates eye growth.29 These responses are detectible after relatively short exposures, e.g., a few minutes to a couple of hours, depending on the visual stimulus, suggesting roles for BMPs in both the initiation and early stages of altered eye growth responses.25–27, 29 Their involvement is also perhaps not surprising, given that BMPs, which belong to the transforming growth factor beta (TGF-β) superfamily, are well known morphogens, with already described diverse roles in early development, both within and outside the eye, including limb patterning, retinal formation and RPE specification.28, 30–37
There are accumulating data from clinical studies showing effective control of myopia progression in children with multifocal contact lenses that aim to limit imposed myopic defocus to more peripheral retinal regions, with parallel observations in animal model studies.38–41 There is also on-going speculation that uncorrected peripheral hyperopic errors might underlie myopia, at least in some individuals.42 In the study reported here, we sought to further investigate the local (regional) processes underlying ocular growth regulation, using the chick as our model and RPE-BMP gene expression as a local signature of central, mid-peripheral and peripheral retinal signals.26, 27, 29
Materials and Methods
Animals and Lens Treatment
Eggs were obtained from the University of California, Davis (CA), and White Leghorn chickens were hatched on-site at the University of California, Berkeley (CA). Chicks were raised under a 12 h light/12 h dark cycle, with daytime room illumination averaged at 254 lux measured with an IL1700 research radiometer (International Light, Inc, USA). Chicks were given free access to food and water. At 19 days of age, each chick was fitted with a monocular +10 or −10 D lens, or a diffuser, each of which was worn for a period of 2 or 48 h. The contralateral (fellow) eyes of treated chicks served as controls. Age-matched untreated chicks were also included as additional controls. For each treatment group, reported data represent results from at least three independent experiments, with each repetition involving 3 – 4 chicks. Experiments were carried out according to the guidelines of the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and were approved by the Animal Care and Use Committee (ACUC) at the University of California, Berkeley.
RPE Sample Collection
To minimize any diurnal or circadian influences on the data, experimental chicks were always sacrificed in the afternoon, between noon to 5 pm. After they were sacrificed, both treated (T) and fellow control (C) eyes were quickly enucleated. The anterior portion of the eye was first cut away, after which the pecten was cut away from the remaining posterior eyecup and the retina then gently separated from the RPE. Next, the remaining wall of the eyecup containing RPE-choroid-sclera was divided into three concentric zones centered on the posterior pole, with the aid of 6 and 12 mm tissue punches (Acuderm Inc, FL) and fine scissors. Finally, the RPE from each zone was isolated from the choroid by gentle pipetting and collected into Eppendorf tubes. This procedure generated three RPE samples per eye, corresponding to a central circular zone of 3 mm radius (zone 3, labeled T3 or C3), and two 3-mm wide annular zones, which comprised a mid-peripheral (3–6 mm) zone (zone 6, labeled T6 or C6), and a peripheral (6–9 mm) zone (zone 9, labeled T9 or C9) (Figure 1). RPE samples were lysed with RLT buffer from RNeasy Mini kits (Qiagen, Valencia, CA), and stored at stored −80 °C for later processing.
Figure 1.
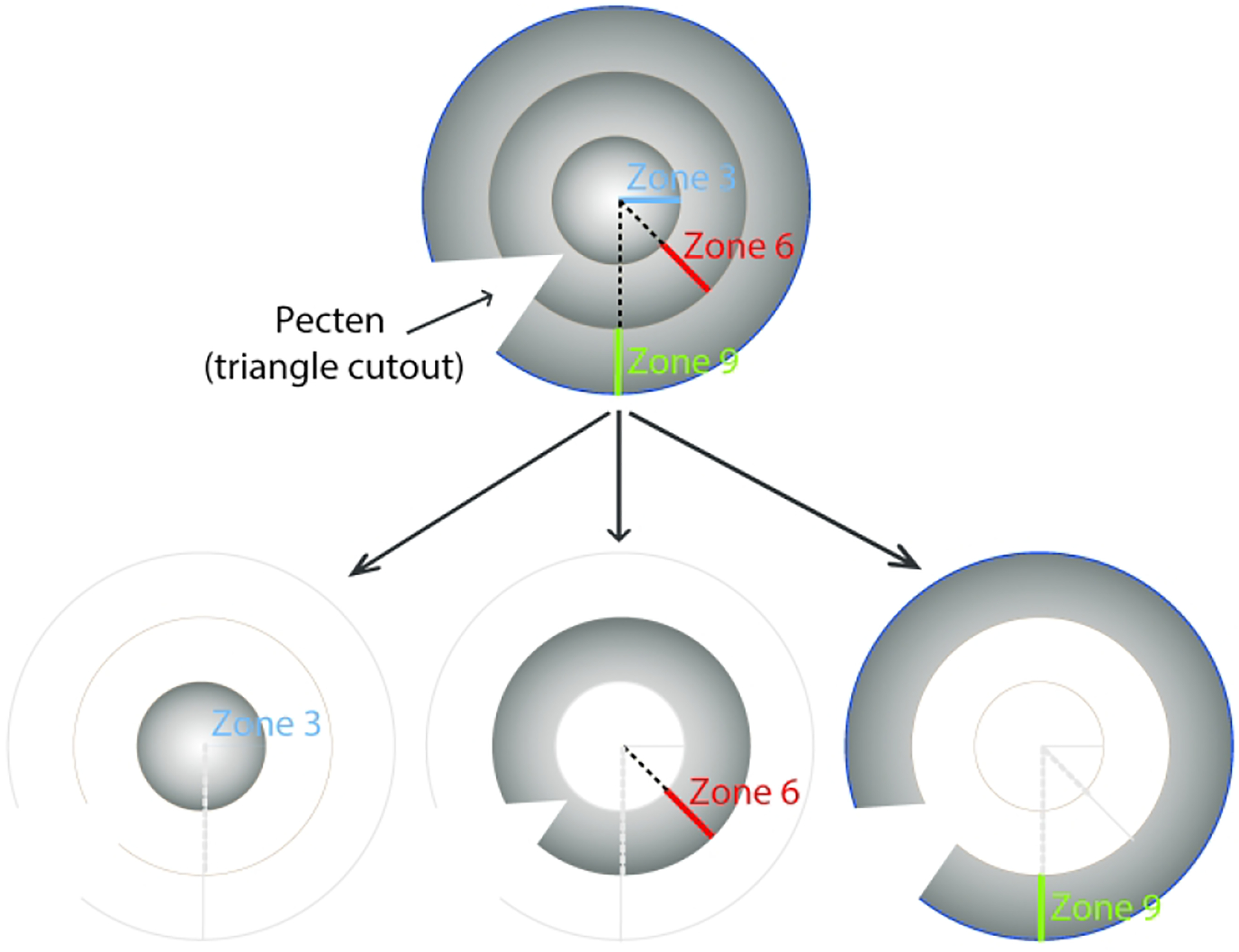
Diagram showing the origin of the three RPE samples collected from treated and fellow control eyes: a circular 6 mm diameter central zone (zone 3), and two 3-mm wide annular zones, i.e., a midperipheral zone (zone 6), and a peripheral zone (zone 9). The triangle cutout represents the location of the pecten.
RNA Purification, Reverse Transcription and Real-Time PCR
RNA from RPE samples was purified using RNeasy Mini kits (Qiagen) and on-column DNase digestion (Qiagen), according to manufacturer’s protocols. The purified RNA was then reverse transcribed to cDNA (SuperScript III First-Strand Synthesis System for RT-PCR, Invitrogen, Carlsbad, CA). Bio-Rad SYBR Green PCR Kits (Bio-Rad) and a StepOnePlus Real-Time PCR System (Applied Biosystems, RRID:SCR_015805) were used to amplify the cDNA, with all PCR reactions being performed in triplicate and the amount of cDNA utilized in each PCR reaction adjusted in accordance with expression levels. GAPDH was selected as the housekeeping gene. Details of the BMP2, BMP4, BMP7, and GAPDH primers are provided in Table 1 and previous related publications.26, 27 Mean normalized expression (MNE) levels were derived from PCR data for all three genes and samples, and regional differences in treatment effects were subsequently evaluated in terms of the mean mRNA expression levels in treated versus control eyes, as previous described.27, 43
Table 1.
Primer gene symbols, NCBI access numbers, sequences, efficiencies, and sizes of amplicons
| Gene | NCBI Access Number | Sequences (5’ - 3’) | Efficiency | Amplicon |
|---|---|---|---|---|
| BMP2 | NM_204358.1 | Forward: 5’-AGCTTCCACCACGAAGAAGTTT-3’ | 93.6 % | 96 bp |
| Reverse: 5’-CTCATTAGGGATGGAAGTTAAATTAAAGA-3’ | ||||
| BMP4 | NM_205237.3 | Forward: 5’-CCAGCAAATCAGCCGTCAT-3’ | 97.5 % | 57 bp |
| Reverse: 5’-CGGACTGGAGCCGGTAGA-3’ | ||||
| BMP7 | XM_417496.6 | Forward: 5’-GGTGGCAGGACTGGATCATC-3’ | 100 % | 64 bp |
| Reverse: 5’-GCGCATTCTCCTTCACAGTAATAC-3’ | ||||
| GAPDH | NM_204305.1 | Forward: 5’-AGATGCAGGTGCTGAGTATGTTG-3’ | 95.6 % | 71 bp |
| Reverse: 5’-GATGAGCCCCAGCCTTCTC-3’ |
Statistics
Paired Student’s t-tests were conducted to compare gene expression differences within birds, e.g., in treated compared to fellow control eyes of treated chicks, or right and left eyes of untreated chicks, for the same zones (T3 vs. C3, T6 vs. C6, and T9 vs. C9). One-way ANOVAs combined with post-hoc analysis (and Bonferroni correction), were used to compare regional differences in treatment-induced BMP gene expression level changes (i.e., zone 3 vs. zone 6 vs. zone 9).
Results
BMP Gene Expression in RPE of Untreated Chicks
For untreated chicks and all three genes (BMP2, BMP4 and BMP7), no significant differences in gene expression levels in RPE were found either between the same zone of right and left eyes (paired t-test), or between zones (one-way ANOVAs with Bonferroni correction, supplemental Table 3), although the data show large variability (p > 0.05 for all cases, Figure 2A). Specifically, the relative gene expression levels for BMP2, i.e., ratios of expression, R eye:L eye, expressed as %, were 198.6 ± 90.7%, 160.8 ± 62.9% and 227.6 ± 141.7% for zones 3, 6, and 9 respectively. Equivalent values for zones 3, 6 and 9 and BMP4 were 288.5 ± 131.6%, 140.8 ± 46.6%, and 257.3 ± 162.1%, and for BMP7, were 386.8 ± 187.3%, 124.7 ± 48.1% and 276.8 ± 185.1%.
Figure 2.
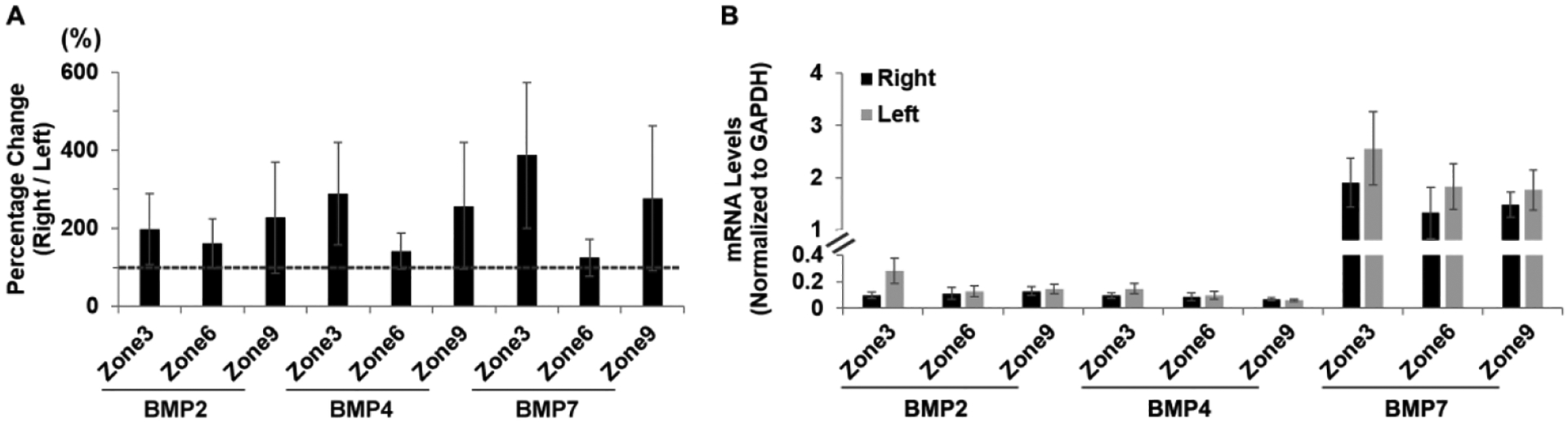
A. Gene expression levels in RPE from untreated chicks for BMP2, BMP4, and BMP7 and 3 different zones expressed as ratios of right vs. left eyes (%). B. mRNA levels normalized to GAPDH for right and left eyes, for the same three genes and zones.
RPE mRNA levels of BMP2, BMP4, and BMP7 for the right and left eyes of untreated birds normalized to levels of GADPH expression are shown in Figure 2B and summarized in Supplemental Table 1. Overall, BMP7 expression levels were higher in absolute terms compared to those of the other two genes. Thus for BMP2 and zones 3, 6 and 9, mRNA levels were 0.19 ± 0.05, 0.12 ± 0.03 and 0.14 ± 0.02 respectively (mean of results from right and left eyes). Equivalent values for BMP4 and the three zones were 0.12 ± 0.02, 0.09 ± 0.02 and 0.06 ± 0.01, and for BMP7, 2.24 ± 0.42, 1.58 ± 0.32 and 1.63 ± 0.22.
RPE-BMP Gene Expression Differences Induced by +10 D Lenses
For both the 2 and 48 h treatment durations, the +10 D lens resulted in significant increases in BMP2 and BMP4 gene expression in the central and midperipheral zones (zones 3 and 6), as well as an increase in BMP7 gene expression in the central zone (zone 3) after 2 h of treatment. Relevant data, including gene expression differences between lens-wearing and fellow control eyes, sample numbers and statistics are summarized in Table 2 and shown graphically in Figure 3A as ratios of expression (%) in treated versus control eyes. Gene expression levels measured in terms of BMP mRNA levels normalized to GAPDH are also shown for the +10 D lens-treated and control eyes in Figure 3C, D, E.
Table 2.
BMP gene expression differences induced by monocular +10 and −10 D lens treatments and form-deprivation in three RPE zones, reported as mean ratios (%) of expression in treated versus control eyes (SEMs in brackets)
| 2 h | 48 h | |||||
|---|---|---|---|---|---|---|
| Zone 3 | Zone 6 | Zone 9 | Zone 3 | Zone 6 | Zone 9 | |
| +10 D lens | n = 12 | n = 8 | n = 8 | n = 13 | n = 12 | n = 12 |
| BMP2 | 12800 [3162]*** | 3075 [786]** | 219 [65] | 7735 [3365]* | 1405 [642]* | 160 [46] |
| BMP4 | 2343 [825]*** | 1062 [256]* | 114 [26] | 2634 [1308]* | 466 [136]* | 138 [28] |
| BMP7 | 429 [124]** | 289 [151] | 87 [19] | 876 [475] | 149 [55] | 137 [29] |
| -10 D lens | n = 10 | n = 8 | n = 8 | n = 18 | n = 10 | n = 10 |
| BMP2 | 35.6 [10.0]* | 26.2 [13.9]** | 15.9 [3.6]* | 52.1 [15.3]* | 49.8 [23.9]* | 116.6 [74.0] |
| BMP4 | 70.3 [22.0]* | 88.3 [39.4] | 74.2 [31.7] | 111.9 [36.4] | 193.9 [83.1] | 332.0 [146.7] |
| BMP7 | 88.3 [22.0] | 39.7 [16.9]* | 86.7 [30.9] | 143.1 [41.8] | 291.2 [179.4] | 297.6 [140.2] |
| FD | n = 14 | n = 14 | n = 14 | n = 15 | n = 10 | n = 10 |
| BMP2 | 185.2 [76.5] | 111.9 [52.0] | 92.7 [42.5] | 27.2 [6.9]* | 18.0 [3.2]‡ | 35.7 [12.3]* |
| BMP4 | 258.2 [81.8] | 185.7 [83.4] | 243.7 [123.0] | 107.4 [44.7] | 51.3 [7.8]* | 135.4 [45.5] |
| BMP7 | 598.0 [430.5] | 166.5 [97.5] | 345.8 [164.0] | 134.6 [60.8] | 76.3 [23.6] | 158.0 [59.1] |
p < 0.05,
p < 0.01,
p < 0.001,
p = 0.055
Figure 3.
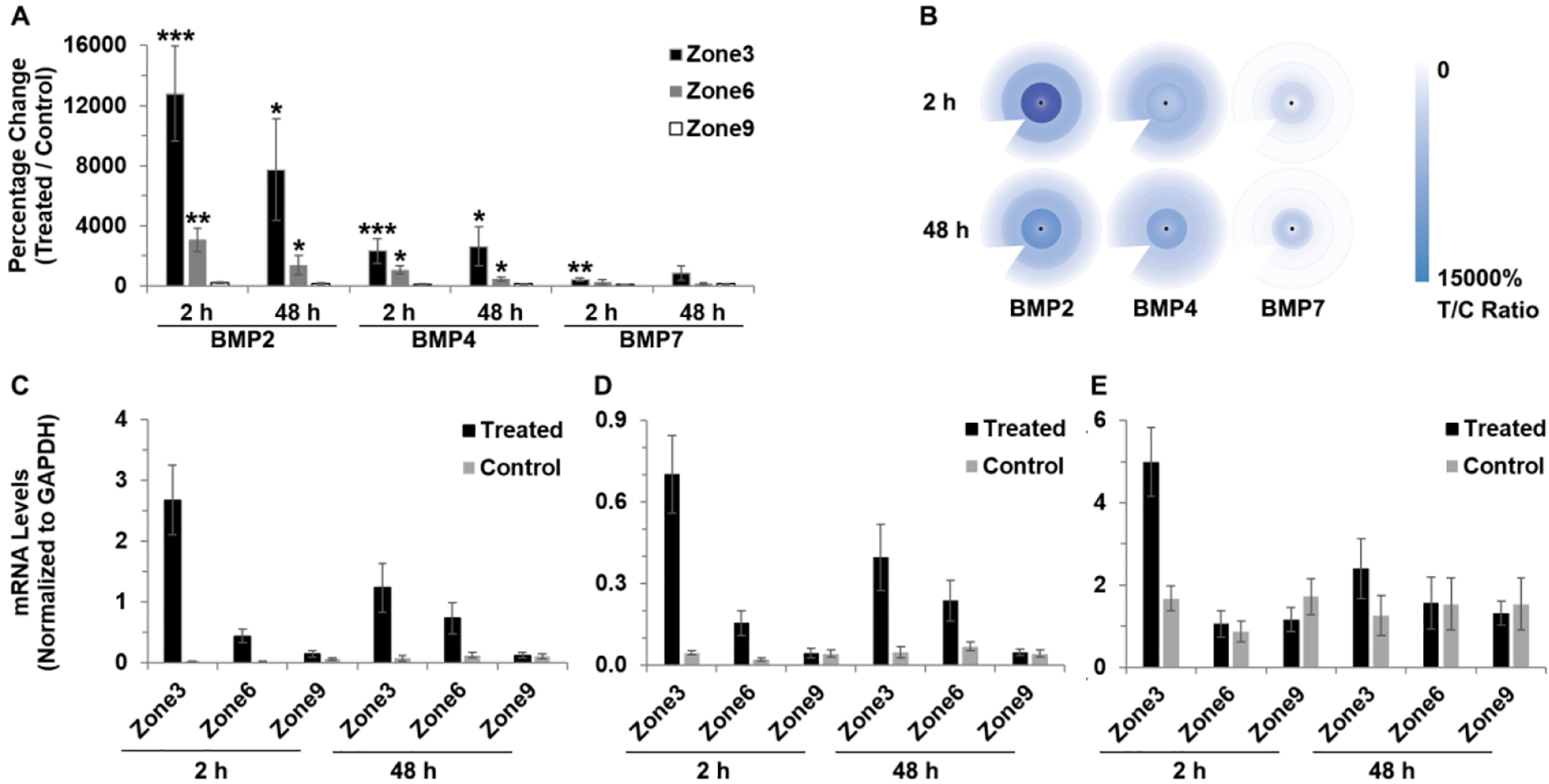
Interocular differences in BMP2, BMP4 and BMP7 gene expression in RPE after 2 and 48 h of +10 D lens wear: (A) graph showing these differences as mean ratios of expression (T/C), expressed as percentages ± SEMs, and (B) cartoon heat maps of the same; mRNA levels normalized to GADPH for treated and control eyes and 3 RPE zones: (C) BMP2, (D) BMP4, and (E) BMP7. For all 3 genes, expression significantly increased in treated compared to control eyes, but not for all treatment durations. * p < 0.05, ** p < 0.01, *** p < 0.001.
To further evaluate treatment-induced interocular differences, ratios of RPE expression levels in treated versus control eyes were derived (Table 2). Expressed in this way, BMP2 gene expression differences induced by the +10 D lens treatment proved to be exceptionally large. After 2 and 48 h of lens wear BMP2 gene expression was increased to 12800 ± 3162% (or 128-fold, p < 0.001) and 7735 ± 3365% (or 77-fold, p < 0.05) of that in the control eye in zone 3, and 3075 ± 786% (or 31-fold, p < 0.01) and 1405 ± 642% (or 14-fold, p < 0.05) in zone 6. However, interocular differences in zone 9 did not reach statistical significance for either treatment duration. BMP4 gene expression was also significantly increased in both zones 3 and 6, albeit to a lesser degree than observed for BMP2. Equivalent T:C ratios for BMP4 and 2 and 48 h lens wear were 2343 ± 825% (or 23-fold, p < 0.001) and 2634 ± 1308% (or 26-fold, p < 0.05) in zone 3, and 1062 ± 256% (or 11-fold, p < 0.05) and 466 ± 136% (or 5-fold, p < 0.05) for zone 6. For the BMP7 gene, significantly increased expression was limited to zone 3 and 2 h lens wear, i.e. by 429 ± 124% (or 4-fold, p < 0.01).
To evaluate regional differences in treatment effects on BMP2, BMP4, and BMP7 gene expression for the +10 D lens treatment, interocular ratios (T:C, expressed as %) were summarized and compared (Table 3 and supplemental Table 3). They confirmed, statistically, that the treatment effects in the central and mid-peripheral regions were larger than those in the most peripheral region, and that the differences in BMP2 and BMP4 gene expression were larger compared to BMP7 gene expression. In terms of gene expression levels normalized to GAPDH, BMP2 mRNA levels in treated eyes after 2 h of lens wear were 2.67 ± 0.57, 0.44 ± 0.22, and 0.15 ± 0.06 for zones 3, 6, and 9 respectively, with comparable values for control eyes being 0.03 ± 0.004, 0.02 ± 0.01, and 0.07 ± 0.02. mRNA levels are summarized in more detail in Supplemental Table 2.
Table 3.
Relative BMP gene expression differences induced by +10 and −10 D lens treatments and form-deprivation treatment in RPE 3 zones (interocular differences expressed as mean fold changes)
| 2 h | 48 h | |||||
|---|---|---|---|---|---|---|
| Zone 3/6 | Zone 3/9 | Zone 6/9 | Zone 3/6 | Zone 3/9 | Zone 6/9 | |
| +10 D lens | ||||||
| BMP2 | 4.2 | 58.5 | 14.1 | 5.5 | 48.4 | 8.8 |
| BMP4 | 2.2 | 20.5 | 9.3 | 5.7 | 19.0 | 3.4 |
| BMP7 | 1.5 | 6.4 | 3.3 | 5.9 | 6.4 | 1.1 |
| −10 D lens | ||||||
| BMP2 | 1.36 | 2.2 | 1.6 | 1.0 | −2.2 | −2.3 |
| BMP4 | −1.25 | −1.1 | 1.2 | −1.7 | −3.0 | −1.7 |
| BMP7 | 2.2 | 1.0 | −2.2 | −2.0 | −2.1 | −1.0 |
| Form-Deprivation | ||||||
| BMP2 | 1.65 | 2.0 | 1.2 | 1.5 | −1.3 | −2.0 |
| BMP4 | 1.39 | 1.1 | −1.3 | 2.1 | −1.3 | −2.6 |
| BMP7 | 3.59 | 1.7 | −2.1 | 1.8 | −1.2 | −2.1 |
p < 0.05,
p < 0.01.
RPE-BMP Gene Expression Differences induced by −10 D Lenses
Here, also, the lens treatment more consistently affected the expression of BMP2 than that of either BMP4 or BMP7. Specifically, BMP2 gene expression was decreased in all three zones (3, 6, and 9), after 2 h of treatment, as well as zones 3 and 6 after 48 h of treatment. In contrast, decreases in BMP4 and BMP7 gene expression were limited to zone 3 and 6 respectively, after 2 h of treatment in each case. Relevant data, including gene expression differences, sample numbers and statistics are summarized in Table 2 and shown graphically as ratios (%) of expression in treated versus control eyes in Figure 4A. mRNA levels of BMPs (normalized to those of GAPDH) in −10 D lens-treated and control eyes are also shown for each RPE zone in Figure 4C, D, E.
Figure 4.
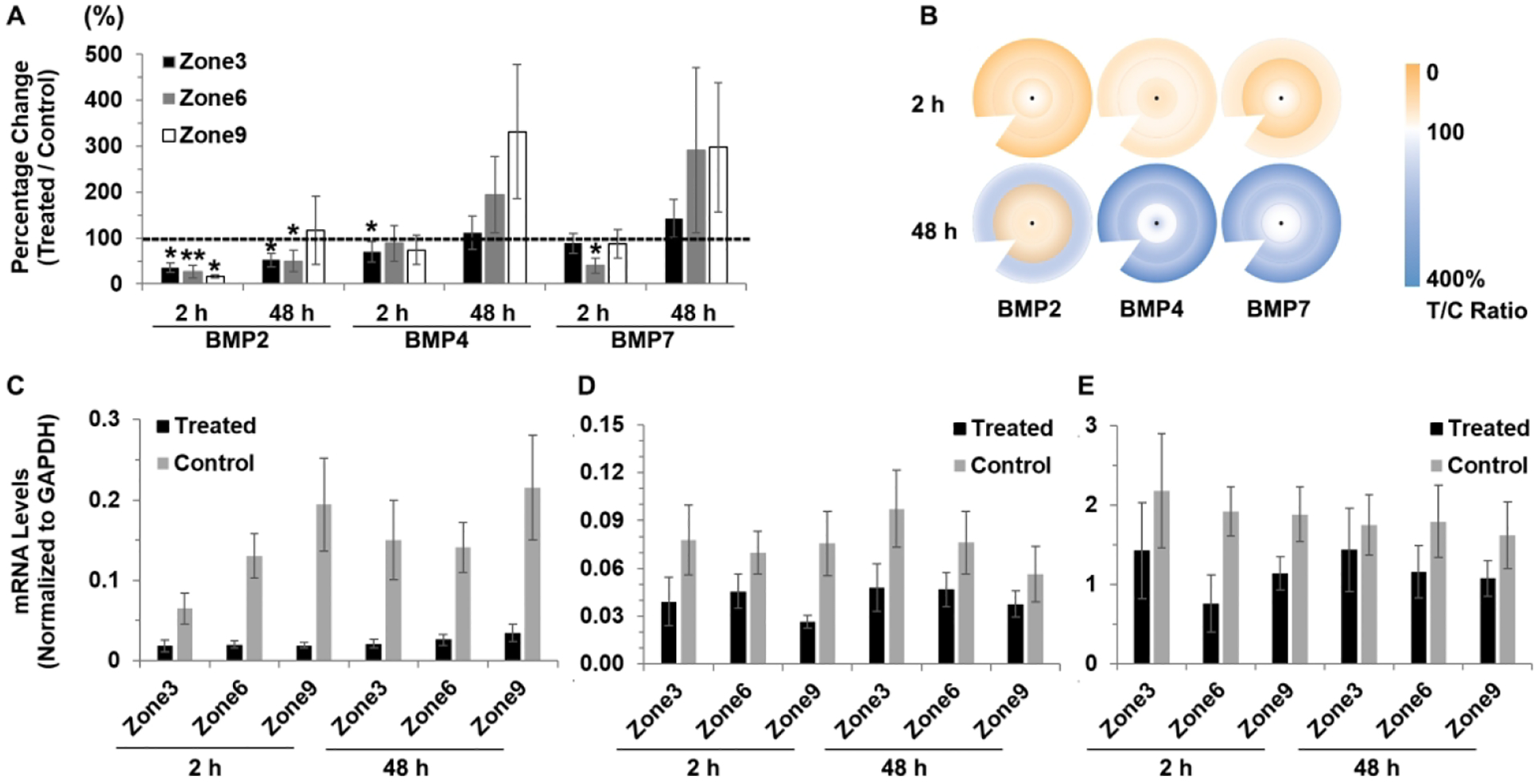
Interocular differences in BMP2, BMP4 and BMP7 gene expression in RPE after 2 and 48 h of −10 D lens wear: (A) graph showing these differences as mean ratios of expression (T/C), expressed as percentages ± SEMs, and (B) cartoon heat maps of the same; mRNA levels normalized to GADPH for treated and control eyes and 3 RPE zones: (C) BMP2, (D) BMP4, and (E) BMP7. For the BMP2 gene, expression significantly decreased in treated compared to control eyes in all three RPE zones after 2 h treatment, as well as in zones 3 and 6 after 48 h treatment; significantly decreased expression after 2 h of treatment limited to zone 3 for BMP4 and to zone 6 for BMP7. * p < 0.05, ** p < 0.01.
Gene expression ratios for BMP2 were found to be significantly decreased in the central zone (3) of treated compared to control eyes, to 35.6 ± 10.0% after 2 h of lens treatment (p < 0.05), and to 52.1 ± 15.3% after 48 h of treatment (p < 0.05). BMP2 gene expression was also significantly decreased in zone 6 after 2 and 48 h of treatment, to 26.2 ± 13.9% (p < 0.01) and 49.8 ± 23.9% (p = 0.01) respectively, while in zone 9, BMP2 gene expression was only significantly decreased after 2 h of treatment, to 15.9 ± 3.6% (p < 0.05). Significant differential BMP4 gene expression was limited to zone 3, and only after 2 h of treatment, when it was decreased to 70.3 ± 22.0% (p < 0.05), while differential gene expression of BMP7 achieved significance only for zone 6 and only after 2 h of treatment, when it was decreased to 39.7 ± 16.9% (p < 0.05).
As for the +10 D lens treatment, regional variations in the effect of the −10 D lens on gene expression for BMP2, BMP4, and BMP7 were evaluated by comparing T:C ratios for zones 3, 6, and 9 (data summarized in Tables 3 and supplemental Table 3). With the −10 D lens treatment and in contrast to the pattern reported for the +10 D lens treatment, treatment-induced differences in gene expression levels were found to be similar in all three zones. These contrasting outcomes are further reflected in mRNA data (see Supplemental Table 2). For examples, average BMP2 mRNA levels normalized to GAPDH in zones 3, 6, and 9 of treated eye after −10 D lens treatment for 2 h were 0.02 ± 0.01, 0.02 ± 0.004, and 0.02 ± 0.004, respectively. While the BMP2 mRNA levels in the 3 zones of control eyes were 0.07 ± 0.02, 0.13 ± 0.03, and 0.19 ± 0.06, respectively.
Gene Expression in Three RPE Zones of Form-Deprivation Treated Chicks
While significant differential gene expression was also observed with the form-deprivation (FD) treatment, which, like negative lenses, induces myopia, these effects generally took longer to develop than those observed with the −10 D lens, reaching significance only after 48 h of treatment. For BMP2, all three zones showed significantly decreased gene expression with 48 h of FD, while significant differences were limited to the mid-peripheral zone for BMP4 and no significant differences were recorded for BMP7. Relevant data including gene expression differences, sample numbers and statistics are summarized in Table 2 and shown graphically as ratios (%) of expression in treated versus control eyes in Figure 5A. mRNA levels of BMPs (normalized to GAPDH) in form-deprived and control eyes are also shown for each RPE zone in Figure 5C, D, E.
Figure 5.
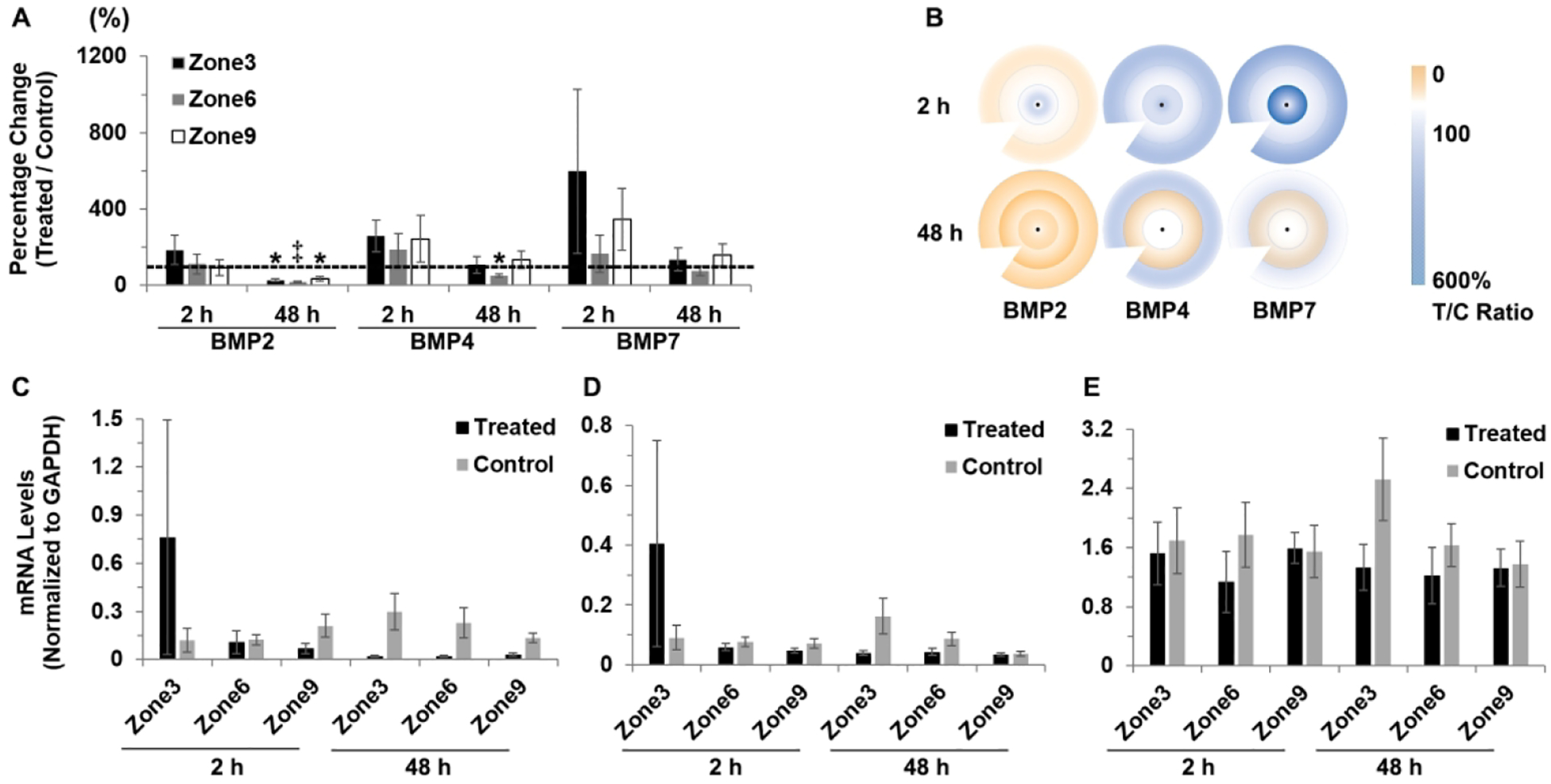
Interocular differences in BMP2, BMP4 and BMP7 gene expression in RPE after 2 and 48 h of FD lens wear: (A) graph showing these differences as mean ratios of expression (T/C), expressed as percentages ± SEMs, and (B) cartoon heat maps of the same; mRNA levels normalized to GADPH for treated and control eyes and 3 RPE zones: (C) BMP2, (D) BMP4, and (E) BMP7. Significant decrease was observed only after 48 h treatment and only for BMP2 and BMP4 gene expression in treated compared to control eyes. * p < 0.05, ‡ p = 0.055.
Expressed as ratios of gene expression in treated versus control eyes, decreases in BMP2 reached statistical significance in all three zones after 48 h of treatment, to 27.2 ± 6.9% (p < 0.05), 18.0 ± 3.2% (p = 0.055) and 35.7 ± 12.3% (p < 0.05) in zones 3, 6 and 9 respectively. For the BMP4 gene, significantly decreased expression was limited to zone 6 and 48 h of treatment (51.3 ± 7.8%; p < 0.05).
As with the two lens treatments, regional variations in the effect of form-deprivation on gene expression for BMP2, BMP4, and BMP7 were evaluated through a comparison of T:C ratios for zones 3, 6, and 9 (Table 3 and supplemental Table 3). As with the −10 D lens treatment, gene expression patterns for BMP2, BMP4, and BMP7 were similar across all three zones. Similar trends were also observed in mRNA levels normalized to GAPDH (Supplemental Table 2). After 48 h of treatment with form-deprivation, average BMP2 mRNA levels in zones 3, 6, and 9 of treated eye were 0.02 ± 0.01, 0.02 ± 0.004, and 0.03 ± 0.01, respectively, compared to levels in control eyes of 0.30 ± 0.11, 0.23 ± 0.10, and 0.14 ± 0.03, respectively.
Discussion
Early studies of optical defocus-induced gene expression differences in chick RPE from our laboratory consistently showed bidirectional gene regulation for BMP2, BMP4, and BMP7 in response to short-term exposure to positive and negative lenses.26, 27 In a later follow-up study involving the same three BMPs (BMP2, 4, and 7), form-deprivation and negative lens treatments were found to similarly affect gene expression, i.e., decrease expression.29 Taken together, the results of these BMP studies reveal a pattern of decreased gene expression when eye growth is accelerated, i.e., with negative lenses and form-deprivation, and increased expression when eye growth is slowed, as with positive lenses. The results of the current study offer an additional perspective in the form of evidence of local regional variations in BMP gene expression in chick RPE in response to both defocus of opposite signs and form-deprivation. In brief, for the +10 D lens treatment, increased BMP gene expression was most pronounced in the central and mid-peripheral regions of RPE, while there was no significant difference in expression levels between treated and fellow (control) eyes in the peripheral region. In contrast, decreased BMP gene expression, as induced by both −10 D lens and form-deprivation treatments, was more consistent across all three regions, and the treatment-induced effects were also generally not as large. It is also notable that while all three treatments induced consistent differences in gene expression after 48 h, with form-deprivation, such treatment effects only reached significance with this longer treatment period, while in contrast, significant decreases in BMP2 gene expression were observed in all three zones after just 2 h of exposure to the −10 D lens.
Investigations into the respective roles of the central and peripheral retina in eye growth regulation and myopia development represent a very active area of research in recent years.12, 15–17, 41, 44 In part such studies are motivated by interest in better understanding the mechanisms underlying currently used optical treatments for myopia control in humans, concentric multifocal contact lenses and orthokeratology lenses being two such examples.45 While there is still on-going debate about whether imposition of peripheral myopic defocus is key to such myopia control effects, improved understanding of underlying retinal mechanisms is critical to refining lens designs and improving treatment outcomes.
As noted in the introduction, in one study involving rhesus monkeys, foveal ablation was found to have minimal effects on normal emmetropization and the response to form-deprivation of axial myopia.17 In another study involving marmosets, lenses designed to impose myopic defocus on more peripheral retinal regions were found to induce axial hyperopia, and similarly, those imposing peripheral hyperopic defocus induced axial myopia.41 These studies together point to an important role for the peripheral retina in controlling eye growth and thus refractive errors, at least in primates, although confounding factors, such as the release of inflammatory mediators after foveal ablation, and alterations in on-axis defocus secondary to associated changes in spherical aberration with multifocal lenses preclude clear-cut conclusions.
In relation to understanding the ocular growth responses to multifocal lenses, related studies in chicks offer some additional insights. Specifically, one study found that when defocus was limited to either the center or periphery of the lens (central-only & peripheral-only), both lens designs induced changes in axial length and refractive errors in the eyes of young chicks, the periphery-only lens design generally inducing greater effects than both center-only and single vision lens designs.44 Nonetheless, that the central retina plays an important role in eye growth regulation, at least in chicks, is reinforced by results in another study in which form-deprivation of the central retina was found to be sufficient to induce axial myopia, even when the peripheral retina received normal vision.46 If eye growth regulation can be considered a focusing mechanism, like ocular accommodation, but with a slower time constant, then it is of potential relevance that the dependence on the higher-acuity central retina of human ocular accommodation, has been challenged more recently.47 Nonetheless, the results reported here emphasize the importance of the central retina, although they do not rule out a role for the peripheral retina in eye growth regulation. They also raise an interesting mechanistic question as to why the induced effects of positive lenses (+10 D), on gene expression show a regional bias favoring the central and mid-peripheral regions of RPE (estimated to subtend ~30 and 75 deg respectively), and thus presumably of the overlying retina, while strong regional biases were not evident in the induced effects of negative lenses and form-deprivation. Could such differences offer insights into the retinal circuits and cells involved in the processing of these opposing myopia-genic and inhibitory visual stimuli? Furthermore, in the case of imposed myopic defocus (with positive lenses), which is linked to slowed ocular growth, as little as 15 minutes of treatment appears sufficient to increase the expression of these same genes in chick RPE.29 The translatability of these observations to human myopia control warrants investigation.
This study was confined to BMPs, and the treatment periods used in the current study were kept quite short to limit induced changes in overall ocular dimensions. Thus the results are consistent with roles for BMPs in the initiation and early stages of altered eye growth. BMPs are already known to function as morphogens during visual system development.48, 49 These growth factors are also known to be involved in a wide variety of biological processes including cell proliferation, apoptosis, extracellular matrix synthesis, and to play roles in the development and patterning of various tissues, organs and limbs.50–53 Of the three genes investigated in our study, BMP2 gene expression consistently showed the largest effect of the +10 D lens treatment. Based on the known functions of BMP2, it is interesting to speculate that BMP proteins secreted from RPE might contribute to the well-characterized early choroidal thickening response to positive lens treatments.54–57 These findings for BMP2 gene expression, and specifically the link between increased RPE gene expression and treatments that slow eye growth, also make it a plausible pharmacological target for controlling myopia. However, note also that as only short treatment durations were used in this study, conclusions about the roles of BMPs in longer-term adjustments to eye growth, which are more closely tied to scleral remodeling, await further investigation.
In summary, we observed regional biases in BMP gene expression patterns in chick RPE in response to short-term treatments with form-deprivation and optical defocus stimuli of opposite signs, suggesting important, regionally-specific roles for RPE-derived BMPs in the early stages of altered eye growth responses. Further related investigations involving multifocal optical treatments, as used for myopia control, are warranted as a potential source of important insight into underlying mechanisms.
Supplementary Material
Grants:
This study is supported by National Eye Institute Grants R01EY012392 (CFW), K12EY017269 (YZ), K08EY023609 (YZ), R21EY029107 (YZ), and T35EY007139 (EE).
Footnotes
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1.Saw SM, Gazzard G, Shih-Yen EC, Chua WH (2005). Myopia and associated pathological complications. Ophthalmic Physiol Opt, 25, 381–391. [DOI] [PubMed] [Google Scholar]
- 2.Ang M, Wong CW, Hoang QV, Cheung GCM, Lee SY, Chia A, Saw SM, Ohno-Matsui K, Schmetterer L (2019) Imaging in myopia: potential biomarkers, current challenges and future developments. Br J Ophthalmol, 103, 855–862. [DOI] [PubMed] [Google Scholar]
- 3.Holden BA, Fricke TR, Wilson DA, Jong M, Naidoo KS, Sankaridurg P, Wong TJ, Naduvilath TJ, Resnikoff S (2016) Global prevalence of myopia and high myopia and temporal trends from 2000 through 2050. Ophthalmology, 123, 1036–1042. [DOI] [PubMed] [Google Scholar]
- 4.Rudnicka AR, Kapetanakis VV, Wathern AK, Logan NS, Gilmartin B, Whincup PH, Cook DG, Owen CG (2016). Global variations and time trends in the prevalence of childhood myopia, a systematic review and quantitative meta-analysis: implications for aetiology and early prevention. Br J Ophthalmol, 100, 882–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ramamurthy D, Lin, Chua SY, Saw SM (2015). A review of environmental risk factors for myopia during early life, childhood and adolescence. Clin Exp Optom, 98, 497–506. [DOI] [PubMed] [Google Scholar]
- 6.Li J, Zhang Q (2017). Insight into the molecular genetics of myopia. Mol Vis,23, 1048–1080. [PMC free article] [PubMed] [Google Scholar]
- 7.Wallman J, Winawer J (2004). Homeostasis of eye growth and the question of myopia. Neuron, 43, 447–468. [DOI] [PubMed] [Google Scholar]
- 8.Wallman J, Gottlieb MD, Rajaram V, Fugate-Wentzek LA (1987). Local retinal regions control local eye growth and myopia. Science, 237, 73–77. [DOI] [PubMed] [Google Scholar]
- 9.Smith EL 3rd., Huang J, Hung LF, Blasdel TL, Humbird TL, Bockhorst KH (2009). Hemiretinal form deprivation: evidence for local control of eye growth and refractive development in infant monkeys. Invest Ophthalmol Vis Sci, 50, 5057–5069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Diether S, Schaeffel F (1997). Local changes in eye growth induced by imposed local refractive error despite active accommodation. Vision Res, 37, 659–668. [DOI] [PubMed] [Google Scholar]
- 11.Smith EL 3rd., Hung LF, Huang J, Blasdel TL, Humbird TL, Bockhorst KH (2010). Effects of optical defocus on refractive development in monkeys: evidence for local, regionally selective mechanisms. Invest Ophthalmol Vis Sci, 51, 3864–3873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith EL 3rd., Hung LF, Huang J, Arumugam B (2013). Effects of local myopic defocus on refractive development in monkeys. Optom Vis Sci, 90, 1176–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Troilo D, Gottlieb MD, Wallman J (1987). Visual deprivation causes myopia in chicks with optic nerve section. Curr Eye Res, 6, 993–999. [DOI] [PubMed] [Google Scholar]
- 14.Wildsoet CF, Pettigrew JD (1988). Experimental myopia and anamalous eye growth patterns unaffected by optic nerve section in chickens: evidence for local control of eye growth. Clinical Vision Sciences, 3(2), 99–107. [Google Scholar]
- 15.Smith EL 3rd., Kee CS, Ramamirtham R, Qiao-Grider Y, Hung LF (2005). Peripheral vision can influence eye growth and refractive development in infant monkeys. Invest Ophthalmol Vis Sci, 46, 3965–3972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smith EL 3rd., Ramamirtham R, Qiao-Grider Y, Hung LF, Huang J, Kee CS, Coats D, Paysse E. (2007). Effects of foveal ablation on emmetropization and form-deprivation myopia. Invest Ophthalmol Vis Sci, 48, 3914–3922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang J, Hung LF, Smith EL 3rd. (2011). Effects of foveal ablation on the pattern of peripheral refractive errors in normal and form-deprived infant rhesus monkeys (Macaca mulatta). Invest Ophthalmol Vis Sci, 52, 6428–6434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tkatchenko TV, Troilo D, Benavente-Perez A, Tkatchenko AV (2018). Gene expression in response to optical defocus of opposite signs reveals bidirectional mechanism of visually guided eye growth. PLoS Biol, 16, e2006021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McGlinn AM, Baldwin DA, Tobias JW, Budak MT, Khurana TS, Stone RA (2007). Form-deprivation myopia in chick induces limited changes in retinal gene expression. Invest Ophthalmol Vis Sci, 48, 3430–3436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.He L, Frost MR, Siegwart JT Jr., Norton TT (2018). Altered gene expression in tree shrew retina and retinal pigment epithelium produced by short periods of minus-lens wear. Exp Eye Res, 168, 77–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jobling AI, Wan R, Gentle A, Bui BV, McBrien NA (2009). Retinal and choroidal TGF-beta in the tree shrew model of myopia: isoform expression, activation and effects on function. Exp Eye Res, 88, 458–466. [DOI] [PubMed] [Google Scholar]
- 22.Guo L, Frost MR, Siegwart JT Jr., Norton TT (2014). Scleral gene expression during recovery from myopia compared with expression during myopia development in tree shrew. Mol Vis, 20, 1643–1659. [PMC free article] [PubMed] [Google Scholar]
- 23.Schippert R, Brand C, Schaeffel F, Feldkaemper MP (2006). Changes in scleral MMP-2, TIMP-2 and TGFbeta-2 mRNA expression after imposed myopic and hyperopic defocus in chickens. Exp Eye Res, 82, 710–719. [DOI] [PubMed] [Google Scholar]
- 24.Rymer J, Wildsoet CF (2005). The role of the retinal pigment epithelium in eye growth regulation and myopia: a review. Vis Neurosci, 22, 251–261. [DOI] [PubMed] [Google Scholar]
- 25.Zhang Y, Wildsoet CF (2015). RPE and choroid mechanisms underlying ocular growth and myopia. Prog Mol Biol Transl Sci, 134, 221–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang Y, Liu Y, Wildsoet CF (2012). Bidirectional, optical sign-dependent regulation of BMP2 gene expression in chick retinal pigment epithelium. Invest Ophthalmol Vis Sci, 53, 6072–6080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang Y, Liu Y, Ho C, Wildsoet CF (2013). Effects of imposed defocus of opposite sign on temporal gene expression patterns of BMP4 and BMP7 in chick RPE. Exp Eye Res, 109, 98–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang Y, Raychaudhuri S, Wildsoet CF (2016). Imposed optical defocus induces isoform-specific up-regulation of TGFbeta gene expression in chick retinal pigment epithelium and choroid but not neural retina. PLoS One, 11, e0155356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang Y, Phan E, Wildsoet CF (2019). Retinal defocus and form-deprivation exposure duration affects RPE BMP gene expression. Sci Rep, 9, 7332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bier E, De Robertis EM (2015). EMBRYO DEVELOPMENT. BMP gradients: A paradigm for morphogen-mediated developmental patterning. Science, 348, aaa5838. [DOI] [PubMed] [Google Scholar]
- 31.Badugu A, Kraemer C, Germann P, Menshykau D, Iber D (2012). Digit patterning during limb development as a result of the BMP-receptor interaction. Sci Rep, 2, 991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Raspopovic J, Marcon L, Russo L, Sharpe J (2014). Modeling digits. Digit patterning is controlled by a Bmp-Sox9-Wnt Turing network modulated by morphogen gradients. Science, 345, 566–570. [DOI] [PubMed] [Google Scholar]
- 33.Fuhrmann S (2010). Eye morphogenesis and patterning of the optic vesicle. Curr Top Dev Biol, 93, 61–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Steinfeld J, Steinfeld I, Coronato N, Hampel ML, Layer PG, Araki M, Vogel-Höpker A. (2013). RPE specification in the chick is mediated by surface ectoderm-derived BMP and Wnt signalling. Development, 140, 4959–4969. [DOI] [PubMed] [Google Scholar]
- 35.Adler R, Belecky-Adams TL (2002). The role of bone morphogenetic proteins in the differentiation of the ventral optic cup. Development, 129, 3161–3171. [DOI] [PubMed] [Google Scholar]
- 36.Seko Y, Shimokawa H, Tokoro T (1995). Expression of bFGF and TGF-beta 2 in experimental myopia in chicks. Invest Ophthalmol Vis Sci, 36, 1183–1187. [PubMed] [Google Scholar]
- 37.Honda S, Fujii S, Sekiya Y, Yamamoto M (1996). Retinal control on the axial length mediated by transforming growth factor-beta in chick eye. Invest Ophthalmol Vis Sci, 37, 2519–2526. [PubMed] [Google Scholar]
- 38.Gwiazda J, Hyman L, Hussein M, Everett D, Norton TT, Kurtz D, Leske MC, Manny R, Marsh-Tootle W, Scheiman M (2003). A randomized clinical trial of progressive addition lenses versus single vision lenses on the progression of myopia in children. Invest Ophthalmol Vis Sci, 44, 1492–1500. [DOI] [PubMed] [Google Scholar]
- 39.Kang P, Fan Y, Oh K, Trac K, Zhang F, Swarbrick HA (2013). The effect of multifocal soft contact lenses on peripheral refraction. Optom Vis Sci, 90, 658–666. [DOI] [PubMed] [Google Scholar]
- 40.Bowrey HE, Zeng G, Tse DY, Leotta AJ, Wu Y, To C-H, Wildsoet CF, McFadden SA (2017). The effect of spectacle lenses containing peripheral defocus on refractive error and horizontal eye shape in the guinea pig. Invest Ophthalmol Vis Sci, ;58, 2705–2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Benavente-Perez A, Nour A, Troilo D (2014). Axial eye growth and refractive error development can be modified by exposing the peripheral retina to relative myopic or hyperopic defocus. Invest Ophthalmol Vis Sci, 55, 6765–6773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Charman WN, Radhakrishnan H (2010). Peripheral refraction and the development of refractive error: a review. Ophthalmic Physiol Opt, 30, 321–338. [DOI] [PubMed] [Google Scholar]
- 43.Simon P (2003). Q-Gene: processing quantitative real-time RT-PCR data. Bioinformatics, 19, 1439–1440. [DOI] [PubMed] [Google Scholar]
- 44.Liu Y, Wildsoet C (2011). The effect of two-zone concentric bifocal spectacle lenses on refractive error development and eye growth in young chicks. Invest Ophthalmol Vis Sci, 52, 1078–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Saw SM, Matsumura S, Hoang QV (2019). Prevention and management of myopia and myopic pathology. Invest Ophthalmol Vis Sci, 60, 488–499. [DOI] [PubMed] [Google Scholar]
- 46.Wang JC, Chun RK, Zhou YY, Zuo B, Li KK, Liu Q, To CH (2015). Both the central and peripheral retina contribute to myopia development in chicks. Ophthalmic Physiol Opt, 35, 652–662. [DOI] [PubMed] [Google Scholar]
- 47.Labhishetty V, Cholewiak SA, Banks MS (2019). Contributions of foveal and non-foveal retina to the human eye’s focusing response. J Vis, 19, 18. [DOI] [PubMed] [Google Scholar]
- 48.Wordinger RJ, Clark AF (2007). Bone morphogenetic proteins and their receptors in the eye. Exp Biol Med (Maywood), 232, 979–992. [DOI] [PubMed] [Google Scholar]
- 49.Adler R, Canto-Soler MV (2007). Molecular mechanisms of optic vesicle development: complexities, ambiguities and controversies. Dev Biol, 305, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.. Salazar VS, Gamer LW, Rosen V (2016). BMP signalling in skeletal development, disease and repair. Nat Rev Endocrinol, 12, 203–221. [DOI] [PubMed] [Google Scholar]
- 51..Matsuda S, Harmansa S, Affolter M (2016). BMP morphogen gradients in flies. Cytokine Growth Factor Rev, 27, 119–127. [DOI] [PubMed] [Google Scholar]
- 52..Zeller R, Lopez-Rios J, Zuniga A (2009). Vertebrate limb bud development: moving towards integrative analysis of organogenesis. Nat Rev Genet, 10, 845–858. [DOI] [PubMed] [Google Scholar]
- 53.Li H, Cui D, Zhao F, Huo L, Hu J, Zeng J (2015). BMP-2 is involved in scleral remodeling in myopia development. PLoS One,10, e0125219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wildsoet C, Wallman J (1995). Choroidal and scleral mechanisms of compensation for spectacle lenses in chicks. Vision Res, 35, 1175–1194. [DOI] [PubMed] [Google Scholar]
- 55.Wallman J, Wildsoet C, Xu A, Gottlieb MD, Nickla DL, Marran L, Krebs W, Christensen AM (1995). Moving the retina: choroidal modulation of refractive state. Vision Res, 35, 37–50. [DOI] [PubMed] [Google Scholar]
- 56.Marneros AG, Fan J, Yokoyama Y, Gerber HP, Ferrara N, Crouch RK, Olsen BR (2005). Vascular endothelial growth factor expression in the retinal pigment epithelium is essential for choriocapillaris development and visual function. Am J Pathol, 167, 1451–1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fitzgerald ME, Wildsoet CF, Reiner A (2002). Temporal relationship of choroidal blood flow and thickness changes during recovery from form deprivation myopia in chicks. Exp Eye Res, 74, 561–70. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


