Abstract
Progression on therapy in NSCLC is often evaluated radiographically; however, image-based evaluation of said therapies may not distinguish disease progression due to intrinsic tumor drug resistance or inefficient tumor penetration of the drugs. Here we report that the inhibition of mutated EGFR promotes the secretion of a potent vasoconstrictor, endothelin-1 (EDN1), which continues to increase as the cells become resistant with a mesenchymal phenotype. As EDN1 and its receptor (EDNR) is linked to cancer progression, EDNR-antagonists have been evaluated in several clinical trials with disappointing results. These trials were based on a hypothesis that the EDN1-EDNR axis activates the MAPK-ERK signaling pathway that is vital to the cancer cell survival; the trials were not designed to evaluate the impact of tumor-derived EDN1 in modifying tumor microenvironment or contributing to drug resistance. Ectopic overexpression of EDN1 in cells with mutated EGFR resulted in poor drug delivery and retarded growth in vivo but not in vitro. Intratumoral injection of rEDN significantly reduced blood flow and subsequent gefitinib accumulation in xenografted EGFR mutant tumors. Furthermore, depletion of EDN1 or the use of endothelin receptor inhibitors bosentan and ambrisentan improved drug penetration into tumors and restored blood flow in tumor-associated vasculature. Correlatively, these results describe a simplistic endogenous yet previously unrealized resistance mechanism inherent to a subset of EGFR-mutant NSCLC to attenuate TKI delivery to the tumors by limiting drug-carrying blood flow and the drug concentration in tumors.
Keywords: EGFR, Resistance, EDN1, VEGF, angiogenesis, NSCLC
INTRODUCTION
While EGFR TKIs drastically prolong survival of NSCLC patients harboring activating EGFR kinase domain mutations, acquired resistance universally develops, mediated by the emergence of the secondary T790M mutation or by focal amplification of MET, in approximately 50% and 5% of patients, respectively (1). While progression on the 1st line therapy in NSCLC can be evaluated radiographically, the image-based evaluation of the therapies may not distinguish disease progression due to drug resistance or to inefficient tumor penetration of the drugs being used.
Tumor blood vessels supply both nutrients and drugs to the tumors. Consequently, the VEGF-A-mediated tumor angiogenesis has been considered as a druggable target and anti-angiogenic agents are expected to attenuate tumor growth (2). We and others have identified an epithelial to mesenchymal transition (EMT) phenotype with mesenchymal stem cell (MSC)-like properties in EGFR TKI resistant NSCLC and the MSC-like subpopulations appear immediately following EGFR TKI exposure (3,4). While the pro-angiogenic properties of MSCs have been extensively studied, MSCs are also known to inhibit angiogenesis (5). Taken together, it remains elusive if EGFR TKI resistant tumors with a MSC-like phenotype modify blood vessels to create their survival niche.
For these reasons among others, several factors that modify tumor blood vessels have been studied for their therapeutic potentials. Since endothelin-1 (EDN1) exerts a pro-angiogenic effect on cultured endothelial cells (6,7), it was explored as a modifier of angiogenesis. However, the hemodynamic role of EDN1 in vivo is entirely different: EDN1 causes potent vasoconstriction and was originally thought to work systemically to affect blood pressure (8), promoting pulmonary hypertension and increased pulmonary vascular resistance in humans (9). Consequently, the endothelin-receptor (EDNR) inhibitors have been FDA-approved for controlling pulmonary hypertension (10).
Boldrini et al. showed that expression of EDN1 was related to poor prognosis in NSCLC patients (11) though the mechanism behind this remains unexplored. The expression of the ligand EDN1 and its receptor, EDNRA, have been linked to poor survival outcomes and increased disease progression in cancers (8,12). The endothelin signaling system consists of three different peptides, EDN1, EDN2, EDN3 and their two receptors EDNRA and EDNRB (13). EDN1 is the dominant isoform found in pulmonary endothelial cells and NSCLC cells together with EDNRA (6). The binding of EDN1 to EDNRA activates several pathways including MAPK, PI3K, and PKC pathways, promoting proliferation, cell growth, and survival (6,14). Therefore, clinical trials of anti-endothelin selective antagonists in combination with chemotherapy or TKI therapy have been constrained to unsuccessful exploration of inactivating the downstream signaling (13,15,16) in an attempt to attenuate the proliferation of cancer cells. Here, we explore if EDN1-mediated vasoconstriction results in poor drug penetration to the tumors contributing to a decreased response to EGFR TKI and eventual relapse.
MATERIALS AND METHODS
NSCLC cell lines and STR assays
NSCLC cells were obtained from the ATCC, maintained as specified and tested for mycoplasma (Genlantis). Cell lines were used for no more than 6 weeks after initial thawing. Methods to generate EGFR TKI-resistant cell lines as well as results for the STR assay analyzed by the Genetic Core of the Research Resources Center at the University of Illinois at Chicago are listed in the Supplementary Materials and Methods.
Cell viability assays and cell counting
Live cells were counted using Countess (Thermo Fisher Scientific) and an equal number of live cells were seeded in each assay to compare cell growth kinetics using the Cell Counting Kit-8 colorimetric assay (Dojindo) as previously described (4).
Western blot analysis
Lysate preparation and Western blotting were performed as described previously (4). A list of antibodies is available in the Supplementary Materials and Methods.
cDNA/shRNA constructs and lentiviral infection
cDNA/shRNA sequences and RNA depletion procedures are provided in the Supplementary Materials and Methods.
Luminex Assay
Details for Luminex-based multiplex assays are available in the Supplementary Materials and Methods.
VEGF and EDN1 Quantikine ELISA kit
Details for Quantikine ELISA assays are available in the Supplementary Materials and Methods.
Murine studies
All animal treatment studies were reviewed and approved by the Institutional Animal Care and Use Committee at Loyola University of Chicago and University of Valencia. Details for drug treatments and procedures are available in the Supplementary Materials and Methods.
Histology and immunohistochemistry
Immunohistochemistry was performed as described previously (3,4). Detailed procedures and the list of antibodies used are also available in the Supplementary Materials and Methods.
Real-time blood flow
Real-time blood flow was measured using a laser-Doppler flowmeter (PF4001 Master, Perimed), connecting a flexible microtip (0.5 mm in diameter, MT B500-OL120, Perimed, Järfälla, Sweden) of a master laser‐Doppler probe (PF418, Perimed) to the tumor.
Statistical analysis
Unless otherwise stated, comparisons of statistical significance were performed using ANOVA & Tukey’s multiple comparison test or t-test where applicable. A p<0.05 was considered statistically significant.
RESULTS
EGFR TKI resistant cells with a mesenchymal phenotype grow poorly in vivo
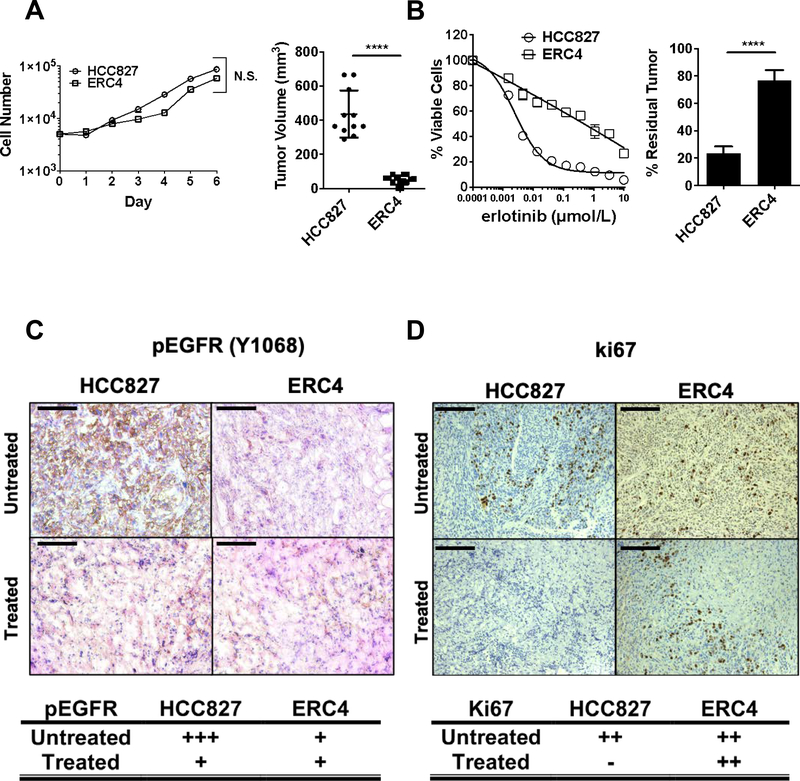
We have demonstrated that EGFR-mutant NSCLC cells grown resistant to EGFR TKIs in vitro present with an EMT phenotype (3,4). ERC4 is a mesenchymal subclone derived from epithelial HCC827 cells grown resistant to erlotinib (Fig.S1A) and is intrinsically resistant to EGFR TKI due to CXCR7 activation (3). In vitro, parental HCC827 and ERC4 proliferated at a similar rate (Fig.1A, left). In vivo, ERC4 presented a significant growth disadvantage while HCC827 produced sizable tumors on NSG mice (Fig.1A, right). Furthermore, HCC827 tumors retained their epithelial (E-cadherin) traits whereas ERC4 tumors maintained high levels of CD44 but low levels of E-cadherin (Fig.S1B).
Figure 1. Mesenchymal cells grow poorly in vivo but promote EGFR TKI resistance in vivo.
(A) (Left) In vitro proliferation assay of TKI-sensitive and resistant cell lines. 5×103 cells were seeded in 12 wells and were counted every day for 6 days. N=2. Error bars, S.D. t-test N.S. Not significant. (Right). In vivo proliferation of HCC827 and ERC4 cell lines xenografts. Tumor volumes were measured 24 days after subcutaneous xenograft. N.S. Not significant, ****p<0.0001. (B) (Left) In vitro sensitivity to EGFR TKI, gefitinib. Colorimetric in vitro proliferation assay for 72 hours with gefitinib. (Right) In vivo studies required 8 weeks of tumor growth followed by 6 days of treatment. Error bars S.D. t-test ****p<0.0001 (C) Representative IHC analyses from HCC827 and ERC4 tumors using anti-phospho-EGFR (Y1068) antibody. Scale 100 μm. Table: Scoring by board-certified pathologists, +, weak; +++, strong. (D) Representative IHC analyses from HCC827 and ERC4 tumors using anti-Ki67 antibody. Scale 100 μm. Table: Scoring by board-certified pathologists, -, negative; ++, moderate.
In vitro, HCC827 is exquisitely sensitive while ERC4 is resistant to erlotinib (Fig1B, left). Despite its growth disadvantage in vivo, ERC4 tumors were highly resistant to gefitinib, which failed to reduce the tumor volume (Fig.1B, right). In contrast, HCC827 tumors shrunk to about 80% of their size after drug treatment. Treatment with gefitinib resulted in a marked reduction in EGFR phosphorylation, a pharmacodynamic marker of drug response, in HCC827 but not ERC4 tumors (Fig.1C). While HCC827 and ERC4 tumors showed a similar positive Ki-67 staining in vivo, the gefitinib treatment dramatically reduced the Ki-67 staining in HCC827 tumors but not in ERC4 (Fig.1D).
EGFR-mutant cells differentially secrete VEGF-A and EDN1 in response to phenotypic changes
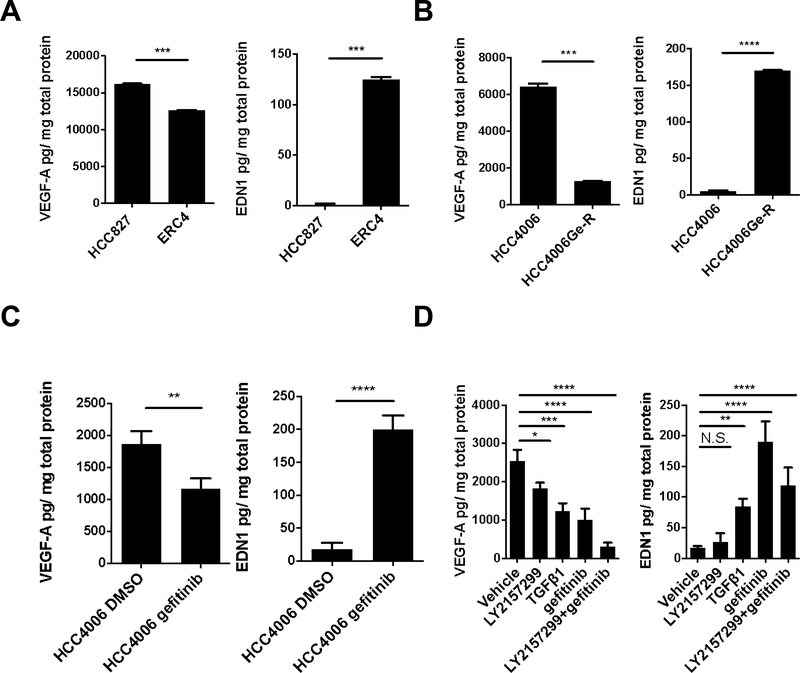
The difference in size of HCC827 and ERC4 tumors lead to the hypothesis that the mesenchymal ERC4 cells secrete factors that hamper efficient in vivo growth. To investigate if angiogenic factors are differentially expressed between the epithelial and mesenchymal tumors, we profiled the supernatants from the cells for 10 angiogenic and growth factors using a Luminex-based multiplex assay. There are two angiogenic factors that show differential expression in epithelial (TKI-sensitive) and mesenchymal (TKI-resistant) cells: VEGF-A and EDN1 (Fig.S2A). Epithelial, TKI -sensitive HCC827 cells, secrete predominantly VEGF-A while mesenchymal, TKI-resistant ERC4 cells, secrete mainly EDN1 (Fig. 2A). The same differences are seen in epithelial, TKI-sensitive HCC4006 cells compared to gefitinib resistant HCC4006Ge-R mesenchymal cells (Fig.2B&S2B) and third-generation EGFR TKI, osimertinib resistant HCC4006O-R mesenchymal cells (Fig.S2C&S2E).
Figure 2. VEGF-A and EDN1 are secreted from EGFR-mutant NSCLC cells.
(A) VEGF-A and EDN1 secretion normalized with protein concentration in HCC827 and TKI-resistant ERC4. Error bars: S.D. t-test ***p<0.001. (B) VEGF-A and EDN1 secretion normalized with protein concentration in HCC4006 and HCC4006Ge-R. Error bars: S.D. t-test ***p<0.001, ****p<0.0001. (C) VEGF-A and EDN1 secretion normalized with protein concentration after 72-hour gefitinib treatment (100 nmol/L). Error bars: S.D. t-test **p<0.01 ****p<0.0001. (D) VEGF-A and EDN1 secretion normalized with protein concentration after 72-hour vehicle (DMSO), LY2157299 (1 μmol/L), TGFβ1 (10 ng/ml), gefitinib (100 nmol/L) or LY2157299 and gefitinib treatment. Error bars: S.D. One-way ANOVA. N.S. Not significant, *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001.
We have shown that depleting E-cadherin (ΔCDH1) in HCC827 promotes EMT (Fig.S2D) and the resulting HCC827ΔCDH1 cells are resistant to EGFR TKIs (4). Consistent with the previous results, these mesenchymal cells also secreted less VEGF-A and more EDN1 than epithelial HCC827ΔNT cells (Fig.S2F). Since we discovered that EGFR mutant NSCLC cells initiate the EMT program immediately upon EGFR TKI treatment (3), we investigated if this switch from VEGF-A to EDN1 secretion was an early event or a consequence of prolonged TKI exposure. We used a short exposure to gefitinib (72 hours) to mimic an early response to treatment and it significantly attenuated VEGF-A secretion while significantly increasing EDN1 secretion in HCC4006 cells (Fig.2C). To investigate if the increase in EDN1 secretion and the decrease in the VEGF-A secretion are the consequence of the EMT, we exposed HCC4006 cells to TGFβ1 for 72 hours (Fig.2D). We have previously shown that HCC4006 cells exposed to TGFβ1 or gefitinib for 72 hours engage in the EMT program and activate the SMAD signaling (4) and the exposure decreased VEGF-A secretion and increased EDN1 secretion. The secretion of EDN1 was more prominent with gefitinib treatment and the gefitinib-induced EDN1 secretion was suppressed by the presence of TGFβ receptor inhibitor, LY2157299.
These series of experiments were performed in normoxic conditions in vitro; however, we needed to consider the hypoxic tumor-microenvironment, especially when EDN1 secretion is known to respond to hypoxic conditions (17). To test the impact of hypoxia on EDN1 secretion, we used an oxygen-controlled hood to measure VEGF-A and EDN1 secretion in both normoxic and hypoxic (1% O2) environments. Hypoxia significantly increased VEGF-A and EDN1 secretion in both epithelial HCC827 cells and mesenchymal ERC4 cells (Fig.S2G) and this increase was further enhanced by gefitinib treatment in HCC827 (Fig.S2H).
EDN1 secretion results in poor in vivo tumor growth
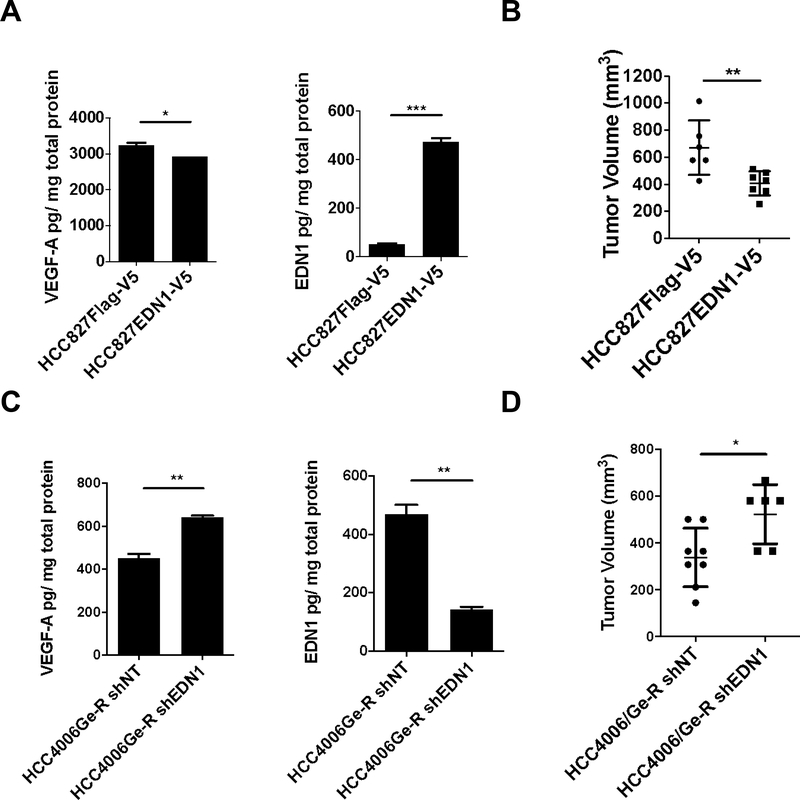
Since EDN1 is a potent vasoconstrictor, we hypothesized that EDN1 secreted from gefitinib-resistant mesenchymal cells promotes vasoconstriction thus restricting growth. To test this hypothesis, we first transduced EGFR inhibitor-sensitive HCC827 cells with lentivirus coding for Flag-V5 or EDN1-V5. EDN1 secretion was increased more than 10-fold, while that of VEGF-A remained almost unaffected (Fig.3A). In the EGFR-mutant HCC827 cells in vitro, ectopic overexpression of EDN1 did not make the cells resistant to EGFR inhibition (Fig.S3A). However, when either cell line was engrafted on the flanks of NSG mice we observed that EDN1 overexpressing tumors were significantly smaller than the control tumors (Fig.3B). We confirmed that the EDN1 overexpressing tumors secrete more EDN1 than the control tumors (Fig.S3B), and TKI treatment further induced EDN1 secretion in both conditions, although it was more prominent in EDN1 overexpressing tumors (p-value 0.08 vs 0.0001, respectively). We have also quantified the concentration of gefitinib in these tumors following drug administration (Fig.S3C) to find it is significantly lower in EDN1 overexpressing tumors than in control tumors. Accordingly, the regression of EDN1-overexpressing tumors upon gefitinib treatment was significantly less than that of control tumors (Fig.S3D). To test if exogenous addition of rEDN1 promotes EMT, HCC827 cells were exposed to rEDN1 for 72 hours. The exposure did not affect the expression of canonical EMT markers (Fig.S3E) or EGFR TKI sensitivity (Fig.S3F).
Figure 3. EDN1 expression results in poor in vivo tumor growth.
(A) VEGF-A and EDN1 secretion normalized with protein concentration in HCC827 EDN1 overexpressing cells. Error bars: S.D. t-test *<0.05, ***p<0.001. (B) 5×106 of the indicated cells were engrafted on flanks of mice and allowed to grow for 4 weeks. Error bars: S.D. t-test **p<0.01. (C) VEGF-A and EDN1 secretion normalized with protein concentration in HCC4006Ge-R EDN1 repressed cells. Error bars: S.D. t-test **p<0.01. (D) 5×106 of the indicated cells mixed in a 50:50 ratio were engrafted on flanks of NSG mice and allowed to grow for 4 weeks. Error bars: S.D. t-test *p<0.05.
Next, we wanted to see if depleting EDN1 in the mesenchymal tumors would promote better tumor growth in vivo. HCC4006Ge-R cells grow poorly in vivo (4) and overexpress EDN1 (Fig.2B). The depletion of EDN1 in the Ge-R cells with lentiviral shRNA decreased EDN1 secretion by 70% while significantly increasing VEGF-A secretion (Fig.3C) and it slightly increased sensitivity to EGFR inhibition in vitro at high concentrations (Fig.S3G). Given the reduced growth rate of the TKI-resistant mesenchymal-like HCC4006 model in vivo, we mixed both epithelial and mesenchymal subpopulations at a 50:50 ratio to develop measurable tumors in NSG mice (Fig.3D). Depleting EDN1 by shRNA in HCC4006Ge-R promoted a significant increase in tumor growth, an effect not attributable to the reversion of the mesenchymal phenotype, since EDN1 repression did not change the expression of EMT markers (Fig.S3H).
EDN1 inhibition improves the bioavailability of gefitinib in vivo
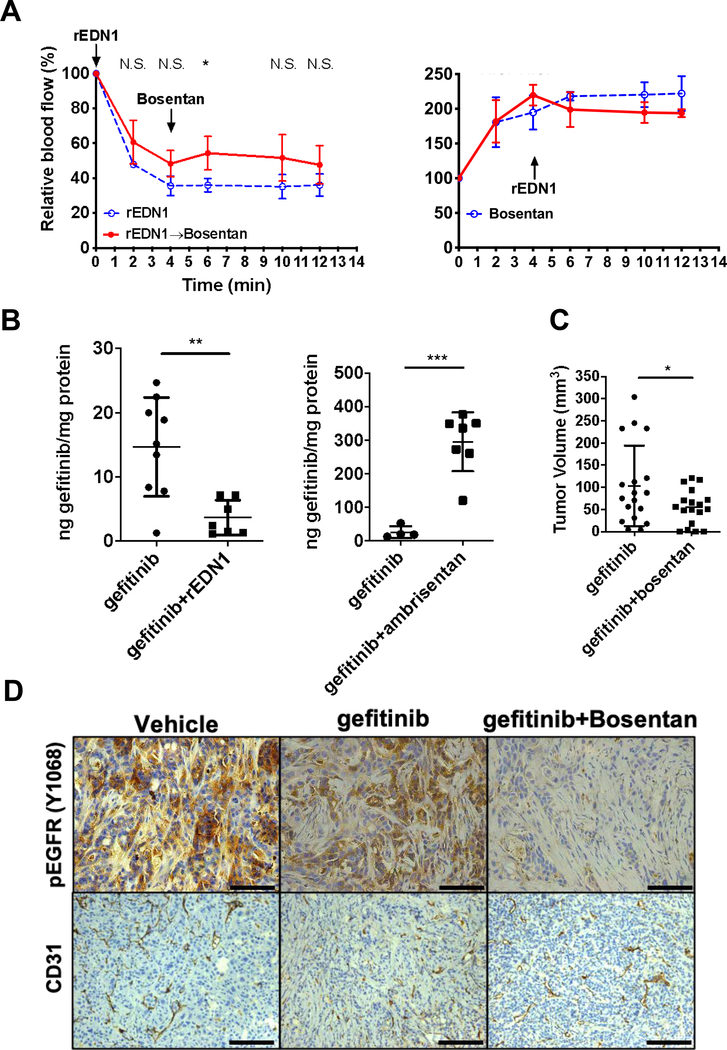
EDN1-secreting EGFR mutant cells exhibited remarkable growth retardation and EGFR inhibitor resistance in vivo but not in vitro. However, it was unclear if EDN1 modifies host vasculature to reduce not only nutrients but also the drug supply to tumors. To investigate the effect of EDN1 on the growing tumor, we used bosentan, a competitive dual EDNRA and B antagonist currently used in the management of pulmonary hypertension associated with arterial vasoconstriction. We measured the pharmacodynamic effect of bosentan and recombinant EDN1 (rEDN1) on intratumoral blood flow using a laser Doppler flow probe in contact with the tumor surface. First, HCC4006 xenografts were treated with rEDN1 (5 ng in 25 μl) intratumorally causing an immediate and sustained decrease in relative blood flow (Fig.4A left, blue). Intraperitoneal administration of bosentan (100 mg/kg) after rEDN1-injection attenuated the reduction in blood perfusion (Fig.4A left, red). Conversely, when bosentan was administered first, the blood flow increased two-fold (Fig.4A right, blue) and subsequent intratumoral injection of rEDN1 ceased, but did not reverse the bosentan-elicited vasodilation (Fig.4A right, red). In line with this observation, the blood flow in mesenchymal EDN1-secreting ERC4 tumors was significantly less than that in HCC827 (Fig.S4A, left), while there was no significant difference in the CD31-positive microvessel density between the two groups (97.7±4.0 mv/mm2 vs. 104.0±10.4 mv/mm2, respectively. p=0.3245, t-test, Fig.S4A, center) or α-SMA accumulation (Fig.S4A right).
Figure 4. EDN1 blockade improves drug delivery to the tumor.
(A) Relative blood flow in the tumors assessed using a Laser Doppler flow probe. Error bars: S.D. t-test N.S. Not significant, *p<0.05, **p<0.01. (B) (Left) Mass spectrometry-based quantification of gefitinib in HCC827 tumors after 1-hour treatment with gefitinib (20 mg/kg) or gefitinib and endothelin-1 (5 ng) normalized with total protein. Error bars: S.D. t-test **p<0.01. (Right) Mass spectrometry-based quantification of gefitinib measured in HCC827 tumors after 1-hour treatment with gefitinib (20 mg/kg) or gefitinib and ambrisentan (320 μg/kg) normalized with total protein. Error bars: S.D. t-test ***p<0.001. (C) 5×106 of HCC4006 cells were grafted on flanks of mice and allowed to grow for 2 weeks and then were treated for 17 weeks with gefitinib (20 mg/kg) or gefitinib and bosentan (200 mg/kg). Error bars: S.D. t-test *p<0.05. (D) Representative IHC analyses from HCC4006 tumors using anti-phospho-EGFR (Y1068) and CD31 antibody after gefitinib or gefitinib and bosentan treatment. Scale 100 μm.
To test if EDN1-mediated vasoconstriction and the reduced blood flow result in the decreased drug penetration, we measured gefitinib concentration in the tumors using MS following 1-hour treatment with gefitinib and vehicle, or gefitinib and rEDN1 (Fig.4B, left). Intratumoral injection of rEDN1 significantly reduced the concentration of gefitinib. Similarly, the gefitinib concentration in EGFR TKI-resistant and EDN1-secreting ERC4 tumors was significantly less than that in HCC827 tumors (Fig.S4B). To test if the structurally unrelated EDN1R inhibitors could improve gefitinib penetration in these engrafted EGFR mutant tumors, we measured gefitinib concentration using MS following 1-hour treatment with gefitinib and vehicle, or gefitinib and ambrisentan. As expected, ambrisentan significantly increased the intratumoral concentration of gefitinib (Fig.4B, right).
The exposure of EGFR mutant cells to EGFR TKI promoted the secretion of EDN1 (Fig.2C&S2H). Based on this observation, we hypothesized that concurrent EGFR and EDN1 receptor inhibition will prevent vasoconstriction and reduced EGFR TKI delivery to the tumors, improving tumor regression. Nu/Nu mice were grafted with HCC4006 cells and the tumors were allowed to grow to approximately 100 mm3, then the mice were randomized for long-term treatment with gefitinib or gefitinib and bosentan (Fig.S4C). In this long-term treatment experiment, gefitinib concentration was reduced to 20 mg/kg to prevent a steep decrease in the tumor size. After 17 weeks of treatment, the average tumor size for the gefitinib treatment was 103±90 mm3 whereas, gefitinib and bosentan combination treatment managed to reduce the average tumor size at 55±40 mm3 (Fig.4C). It should be noted that gefitinib-treated tumors relapsed and regrew on week 15 while the tumors with the combination treatment remained small toward the end of the experiment (Fig.S4C). Bosentan treatment, with or without gefitinib, was well tolerated and did not cause weight loss in the mice (Fig.S4D). In the tumors treated with gefitinib alone, EDN1 expression was 7-fold higher than vehicle-treated tumors (Fig.S4E). Chronic gefitinib-treated tumors show strong phosphorylation of EGFR while it was subtle in the tumors treated with the combination treatment (Fig.4D, top & S4G). Digital-droplet PCR showed that the proportion of the cells harboring an EGFR T790M secondary mutation did not differ between gefitinib or gefitinib and bosentan treated groups (Fig.S4F). Moreover, there were no significant differences in the microvessel density between gefitinib or gefitinib and bosentan treated groups (Fig.4D, bottom).
DISCUSSION
The molecular mechanisms underlying EGFR TKI resistance have been extensively studied to develop clinically viable strategies to overcome it. Cancer therapies usually assume that drugs penetrate the tumors efficiently as designed. For the optimal clinical response, extensive pharmacodynamic (PD) and pharmacokinetic (PK) studies have been completed for the therapeutic drugs; however, PD/PK studies using fine needle aspirations of the patients’ lung tumors following disease progression on the therapeutic response are not usually done in parallel. Consequently, the image-based evaluation of the therapies may not discern the cause for disease progression. Our results show that a subset of EGFR mutant NSCLC tumors upon EGFR TKI treatment secrete a potent vasoconstrictor, EDN1 (Fig.2C), to limit the intratumoral blood flow carrying drugs (Fig.4A,4B&4D, left), thus mimicking the acquired resistance to EGFR TKIs. Moreover, EDN1 significantly reduces intratumoral gefitinib concentration (Fig.4B, left &S4B), while the EDN receptor inhibition significantly increased it (Fig.4B, right). A representative model of this new mechanism is found in Fig.S5A.
Ectopic EDN1 expression in HCC827 significantly reduced the tumor size compared to control in vivo (Fig.3B) and helped render the anti-tumor activity of gefitinib (Fig.S3D). In contrast, depleting EDN1 from mesenchymal HCC4006Ge-R cells improved the in vivo tumor growth (Fig.3D). These occurred while modulation of EDN1 in our model had little or no effect on the proliferation and survival of EGFR-mutant cells in vitro (Fig.S3A&G). EDN1-secreting mesenchymal ERC4 (Fig.2A) showed drastic reduction of blood flow (Fig.S4A, left) without significant difference in the microvessel density between the two groups (Fig.S4A, center) and significantly lower gefitinib concentration within the tumor (Fig.S4B). In contrast, injection of rEDN1 into HCC4006 tumors significantly decreased the blood flow (Fig.4A, left) and reduced the concentration of gefitinib reaching to the tumors (Fig.4D, left). The EDN1-receptor antagonist, bosentan, prevented the rEDN1-mediated restriction of the blood flow (Fig.4A, right). Furthermore, EDNRA-specific inhibitor, ambrisentan that is not structurally related to bosentan, significantly increased the intratumoral concentration of gefitinib (Fig.4D, right). These results support the notion that EGFR mutant NSCLC cells that secrete a vasoconstrictor, EDN1, will limit the blood flow carrying drugs to the tumors.
Surprisingly, exposure of HCC4006 and HCC827 cells to gefitinib increased EDN1 secretion in vivo (Fig.S4E) and in vitro (Fig.2C&D), suggesting EGFR-mutant tumors could secrete EDN1 in response to EGFR inhibition to constrict tumor-feeding blood vessels to prevent gefitinib penetration into the tumors. This event occurs long before the tumors develop cell-intrinsic resistance mechanisms. Elevated secretion of EDN1 (Fig.2A) from ERC4 decreases blood flow (Fig.S4A, left) and gefitinib accumulation (Fig.S4B) while exposure of ERC4 to gefitinib or osimertinib activates the CXCR7-MAPK axis for the cell survival (3). Similarly, mesenchymal and EGFR multi-TKI resistant HCC4006Ge-R cells and HCC4006 cells transiently treated with gefitinib or osimertinib established EGFR TKI resistance by activating the CXCR7-MAPK axis (3). These cells secrete higher amounts of EDN1 than parental HCC4006 cells. Thus, EGFR TKI resistant cells with a mesenchymal phenotype seem to have developed two distinct mechanisms to cope with damage by gefitinib. These results suggest but do not prove that the EDN1-mediated drug resistance mechanism could occur concurrently with the evolution of cell-intrinsic resistance mechanisms. We hypothesized that bosentan would attenuate the vasoconstriction effect of the tumor-secreted EDN1, thus improving TKI distribution within the tumor. As expected, a long-term treatment of HCC4006 tumors with gefitinib and bosentan combination successfully suppressed the growth of the tumor while the tumors treated with gefitinib alone started to progress (Fig.4C&S4C). At the end of the study, the suppression of EGFR phosphorylation was better in tumors treated with both bosentan and gefitinib than in tumors treated with gefitinib alone (Fig.4D, top&S4G). We have previously described how intratumoral heterogeneity can lead to divergent resistance mechanisms (EMT and EGFR T790M) in response to gefitinib in HCC4006 cells (4). In our experiment, the frequency of the clones with T790M mutation in both gefitinib and bosentan plus gefitinib treated tumors remained very low (Fig.S4F), discarding the possible emergence of secondary resistance mutations. Consequently, the progression of the gefitinib-treated tumors at the end of 17 weeks of treatment is likely due to the reduced penetrance of gefitinib to the tumor and the EDN receptor inhibition is a viable option to induce vasodilation and ensure the delivery of gefitinib to the tumor.
We performed the experiments with the conventional gefitinib dose (50 mg/kg, oral gavage) initially with no evaluable tumors left at the end of 3–4 weeks. To evaluate the impact of EDNR inhibitor on the antitumoral effect of gefitinib, we had to reduce the dose to 20 mg/kg, oral gavage. It is unclear if the low dose (20 mg/kg) gefitinib as opposed to the conventional dose (50 mg/kg) in murine treatment prevented the emergence of T790M secondary mutations leading to resistance and metastasis. It is noteworthy that drug holiday treatment (18) or low-dose regime treatment (19) are being considered to maintain the bulk of the tumor under control delaying the appearance of drug resistance.
Clinical trials of anti-endothelin therapy have sought to inhibit the proliferation of cancer cells (13,15,16,20,21) activated by the binding of EDN1 to EDNRA (6,14). In phase III clinical trials, atrasentan and zibotentan were evaluated as single agents in patients with non-metastatic or advanced metastatic prostate cancer, and in combination with docetaxel in patients with metastatic prostate cancer. While several patients responded, the therapy was determined to be unsuccessful in patients with non-metastatic or advanced disease (22,23). Zibotentan plus carboplatin and paclitaxel was also subject to a phase II clinical trial in 120 patients with advanced ovarian cancer and no improvements in progression free survival (primary endpoint) or in secondary endpoints were reported (24). This failure is attributed to the low dose of docetaxel used in the zibotentan arm and the lack of biomarkers to stratify the patients likely to respond to the combination (6). The phase I/II trial comparing the efficacy of paclitaxel-carboplatin-atrasentan combination against paclitaxel-carboplatin show no efficacy and survival benefit in stage IIIIA and V NSCLC patients (13). The clinical trial was based on the hypothesis that atrasentan has a direct effect on tumor proliferation, inhibiting EDN receptor and downstream MAPK-ERK axis to sensitize non-specific genotypes of NSCLC for the combined chemotherapy. Consequently, it is difficult to assess if the trial results do not agree with our hypothesis that EDN1 secreted from treated tumor cells reduces the drugs to reach tumors. Overall, our results suggest that targeting the tumor’s intrinsic EDN1-EDNRA-signaling axis to suppress proliferation and survival of cancer cells themselves is not the best strategy. Rather the inhibition of the EDN1-EDNR axis ensures the tumor-feeding vessels are functionally available to deliver the EGFR TKI to the tumors, allowing the inhibition of the target. Our results suggest that further investigation is needed to understand if tumor progression on EGFR TKI may be due partially to poor drug delivery in NSCLC patients. In particular, our model (Fig.S5) may be relevant to understand why some NSCLC patients with EGFR-mutant tumors fail to respond to the EGFR TKI therapy.
Supplementary Material
STATEMENT OF SIGNIFICANCE.
EDNR antagonists can be repurposed to improve drug delivery in VEGF-A-secreting tumors which normally respond to TKI treatment by secreting EDN1, promoting vasoconstriction, and limiting blood and drug delivery.
ACKNOWLEDGMENTS
We would like to thank Dr. Enrico Benedetti, Dr. Malek Massad, Dr. Ajay Rana, and the Department of Surgery at the University of Illinois at Chicago for support. We would also like to acknowledge Drs. Nancy Zeleznik-Le and Mitchell F. Denning who served on Stephen Ollosi’s Master of Science thesis committee at Loyola University Chicago. A representative graphic of tumor EDN1 secretion and rationale for EDNR antagonist and TKI combination therapy is created with biorender.com (licensed copy, Ines Pulido).
GRANT SUPPORTS
This work is supported by NIH grant CA230778, American Cancer Society Illinois Division Basic Science Grant #254563, the American Cancer Society Research Scholar Grant 126638-RSG-14–229-01-TBG and EGFR Resisters Honorable Mention Research Award to T. Shimamura, Spanish Ministry of Science, Innovation and Universities (SAF2017–85352) and Domingo Martinez Foundation to J. Carretero, the European Regional Development Fund (FEDER) Institute of Health Carlos III of the Spanish Ministry of Science, Innovation and Universities (PI17/01282) to A. Lahoz, and NIH grant CA154778 to M. Nishimura.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked advertisement in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Research Funding: Bristol-Myers Squibb, AstraZeneca.
Footnotes
CONFLICT OF INTEREST DISCLOSURE STATEMENT
On behalf of all authors on this manuscript, we disclose the following conflicts of interest. All authors have completed separate conflict of interest forms, and we have no additional conflicts of interest to report.
Takeshi Shimamura
Consultant/Advisory Board/Ownership
Oscar Juan reports receiving honoraria from or advisory roles in Boehringer Ingelheim, Bristol-Myers Squibb, Merck Sharp & Dohme, Roche/Genetech, AstraZeneca, Pfizer, Eli Lilly, Abbvie.
REFERENCES
- 1.Chong CR, Janne PA. The quest to overcome resistance to EGFR-targeted therapies in cancer. Nat Med 2013;19:1389–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.De Palma M, Biziato D, Petrova TV. Microenvironmental regulation of tumour angiogenesis. Nat Rev Cancer 2017;17:457–74 [DOI] [PubMed] [Google Scholar]
- 3.Becker JH, Gao Y, Soucheray M, Pulido I, Kikuchi E, Rodriguez ML, et al. CXCR7 Reactivates ERK Signaling to Promote Resistance to EGFR Kinase Inhibitors in NSCLC. Cancer research 2019;79:4439–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Soucheray M, Capelletti M, Pulido I, Kuang Y, Paweletz CP, Becker JH, et al. Intratumoral Heterogeneity in EGFR-Mutant NSCLC Results in Divergent Resistance Mechanisms in Response to EGFR Tyrosine Kinase Inhibition. Cancer research 2015;75:4372–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Atiya H, Frisbie L, Pressimone C, Coffman L. Mesenchymal Stem Cells in the Tumor Microenvironment In: Birbrair A, editor. Tumor Microenvironment: Non-Hematopoietic Cells. Cham: Springer International Publishing; 2020. p 31–42. [DOI] [PubMed] [Google Scholar]
- 6.Rosano L, Spinella F, Bagnato A. Endothelin 1 in cancer: biological implications and therapeutic opportunities. Nat Rev Cancer 2013;13:637–51 [DOI] [PubMed] [Google Scholar]
- 7.Salani D, Taraboletti G, Rosano L, Di Castro V, Borsotti P, Giavazzi R, et al. Endothelin-1 induces an angiogenic phenotype in cultured endothelial cells and stimulates neovascularization in vivo. Am J Pathol 2000;157:1703–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davenport AP, Hyndman KA, Dhaun N, Southan C, Kohan DE, Pollock JS, et al. Endothelin. Pharmacol Rev 2016;68:357–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moorhouse RC, Webb DJ, Kluth DC, Dhaun N. Endothelin antagonism and its role in the treatment of hypertension. Curr Hypertens Rep 2013;15:489–96 [DOI] [PubMed] [Google Scholar]
- 10.Barton M, Yanagisawa M. Endothelin: 30 Years From Discovery to Therapy. Hypertension 2019;74:1232–65 [DOI] [PubMed] [Google Scholar]
- 11.Boldrini L, Gisfredi S, Ursino S, Faviana P, Lucchi M, Melfi F, et al. Expression of endothelin-1 is related to poor prognosis in non-small cell lung carcinoma. Eur J Cancer 2005;41:2828–35 [DOI] [PubMed] [Google Scholar]
- 12.Rosano L, Bagnato A. Endothelin therapeutics in cancer: Where are we? Am J Physiol Regul Integr Comp Physiol 2016;310:R469–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chiappori AA, Haura E, Rodriguez FA, Boulware D, Kapoor R, Neuger AM, et al. Phase I/II study of atrasentan, an endothelin A receptor antagonist, in combination with paclitaxel and carboplatin as first-line therapy in advanced non-small cell lung cancer. Clinical cancer research : an official journal of the American Association for Cancer Research 2008;14:1464–9 [DOI] [PubMed] [Google Scholar]
- 14.Iijima K, Lin L, Nasjletti A, Goligorsky MS. Intracellular signaling pathway of endothelin-1. J Cardiovasc Pharmacol 1991;17 Suppl 7:S146–9 [DOI] [PubMed] [Google Scholar]
- 15.Rosano L, Di Castro V, Spinella F, Tortora G, Nicotra MR, Natali PG, et al. Combined targeting of endothelin A receptor and epidermal growth factor receptor in ovarian cancer shows enhanced antitumor activity. Cancer research 2007;67:6351–9 [DOI] [PubMed] [Google Scholar]
- 16.Trump DL, Payne H, Miller K, de Bono JS, Stephenson J 3rd, Burris HA 3rd, et al. Preliminary study of the specific endothelin a receptor antagonist zibotentan in combination with docetaxel in patients with metastatic castration-resistant prostate cancer. Prostate 2011;71:1264–75 [DOI] [PubMed] [Google Scholar]
- 17.Stow LR, Jacobs ME, Wingo CS, Cain BD. Endothelin-1 gene regulation. FASEB J 2011;25:16–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Socinski MA, Villaruz LC, Ross J. Understanding Mechanisms of Resistance in the Epithelial Growth Factor Receptor in Non-Small Cell Lung Cancer and the Role of Biopsy at Progression. Oncologist 2017;22:3–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hirano R, Uchino J, Ueno M, Fujita M, Watanabe K. Low-dose Epidermal Growth Factor Receptor (EGFR)- Tyrosine Kinase Inhibition of EGFR Mutation-positive Lung Cancer: Therapeutic Benefits and Associations Between Dosage, Efficacy and Body Surface Area. Asian Pac J Cancer Prev 2016;17:785–9 [DOI] [PubMed] [Google Scholar]
- 20.Bagnato A, Loizidou M, Pflug BR, Curwen J, Growcott J. Role of the endothelin axis and its antagonists in the treatment of cancer. Br J Pharmacol 2011;163:220–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morris CD, Rose A, Curwen J, Hughes AM, Wilson DJ, Webb DJ. Specific inhibition of the endothelin A receptor with ZD4054: clinical and pre-clinical evidence. Br J Cancer 2005;92:2148–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fizazi K, Higano CS, Nelson JB, Gleave M, Miller K, Morris T, et al. Phase III, randomized, placebo-controlled study of docetaxel in combination with zibotentan in patients with metastatic castration-resistant prostate cancer. J Clin Oncol 2013;31:1740–7 [DOI] [PubMed] [Google Scholar]
- 23.Miller K, Moul JW, Gleave M, Fizazi K, Nelson JB, Morris T, et al. Phase III, randomized, placebo-controlled study of once-daily oral zibotentan (ZD4054) in patients with non-metastatic castration-resistant prostate cancer. Prostate Cancer Prostatic Dis 2013;16:187–92 [DOI] [PubMed] [Google Scholar]
- 24.Cognetti F, Bagnato A, Colombo N, Savarese A, Scambia G, Sehouli J, et al. A Phase II, randomized, double-blind study of zibotentan (ZD4054) in combination with carboplatin/paclitaxel versus placebo in combination with carboplatin/paclitaxel in patients with advanced ovarian cancer sensitive to platinum-based chemotherapy (AGO-OVAR 2.14). Gynecol Oncol 2013;130:31–7 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.