Abstract
Low-dose aspirin is recommended by the U.S. Preventive Services Task Force for primary prevention of colorectal cancer (CRC) in certain individuals. However, broader implementation will require improved precision prevention approaches to identify those most likely to benefit. The major urinary metabolite of PGE2, 11α-hydroxy-9,15-dioxo-2,3,4,5-tetranor-prostane-1,20-dioic acid (PGE-M), is a biomarker for CRC risk, but it is unknown if PGE-M is modifiable by aspirin in individuals at risk for CRC. Adults (N=180) who recently underwent adenoma resection and did not regularly use aspirin or non-steroidal anti-inflammatory drugs were recruited to a double-blind, placebo-controlled, randomized trial of aspirin at 81 or 325 mg/day for 8–12 weeks. The primary outcome was post-intervention change in urinary PGE-M as measured by liquid-chromatography mass spectrometry. 169 participants provided paired urine samples for analysis. Baseline PGE-M excretion was 15.9±14.6 (mean±S.D. ng/mg creatinine). Aspirin significantly reduced PGE-M excretion (−4.7±14.8) compared to no decrease (0.8±11.8) in the placebo group (p=0.015) (mean duration of treatment = 68.9 days). Aspirin significantly reduced PGE-M levels in participants receiving either 81 (−15%; p=0.018) or 325 mg/day (−28%; p<0.0001) compared to placebo. In 40% and 50% of the individuals randomized to 81 or 325 mg/day aspirin, respectively, PGE-M reduction reached a threshold expected to prevent recurrence in 10% of individuals. These results support that aspirin significantly reduces elevated levels of PGE-M in those at increased CRC risk to levels consistent with lower risk for recurrent neoplasia and underscore the potential utility of PGE-M as a precision chemoprevention biomarker. The ASPIRED trial is registered as NCT02394769.
INTRODUCTION
The 2016 United States Preventive Services Task Force (USPSTF) guidelines recommend the use of aspirin for primary prevention of cardiovascular disease (CVD) and colorectal cancer (CRC) in individuals aged 50–59 years with a greater than 10% ten-year risk of CVD.(1,2) However, concerns remain about aspirin’s potential side effects (e.g. bleeding), a lack of data in specific age-groups, and the need to clarify aspirin’s chemopreventive mechanisms to improve personalized treatment.(1,2) While recommendations based on age and conditioned on CVD risk broadly identify patient populations with a higher probability for a net benefit, they lack precision, particularly related to CRC risk or potential for efficacy. We have previously proposed a paradigm that leverages established biomarkers for CRC risk, especially those that are related to aspirin’s anti-cancer mode of action, to refine efficacy biomarkers and improve precision chemoprevention strategies.(3)
As an inhibitor of the cyclooxygenase (COX) activity of prostaglandin (PG)H-synthase-1 and 2 (PTGS-1/−2, or COX-1/−2), aspirin blocks conversion of arachidonic acid to PGH2 and influences a number of downstream pathways to confer aspirin’s anti-inflammatory and anti-platelet effects.(3) Upregulation of COX expression and dysregulated conversion of arachidonic acid into bioactive PGs, the most abundant of which is PGE2,(4,5) is observed in many tumor types, including CRC.(3) However, despite the mechanistic links, reliable measurement of circulating PGE2 for biomarker development is not feasible.(6,7) Instead, prior studies aimed at understanding the relationship between PGs and cancer have focused on urinary 11α-hydroxy-9,15-dioxo-2,3,4,5-tetranor-prostane-1,20-dioic acid (PGE-M), a major enzymatic metabolite reflecting in vivo PGE2 biosynthesis. These prior studies have demonstrated that PGE-M is associated with an increased risk of colorectal neoplasia(8–10) and other cancer types, including pancreas(11,12), stomach,(13,14) lung,(15) and breast,(16,17) but not ovary.(18) We previously demonstrated in a prospective study that elevated pre-diagnostic PGE-M levels are associated with an increased risk of future advanced colorectal adenomas. Furthermore, protection associated with regular aspirin/non-steroidal anti-inflammatory drug (NSAID) use was only observed among those with elevated pre-diagnostic PGE-M.(8) Thus, PGE-M may be useful as a precision chemoprevention marker. Previous studies have shown that use of aspirin and other NSAIDs are cross-sectionally associated with lower PGE-M levels.(8,9,19) However, it is unknown if aspirin modifies baseline PGE-M levels in the target, at-risk population, and if such a reduction is dose-dependent. To address this knowledge gap, we conducted the “ASPirin Intervention for the REDuction of colorectal cancer risk” (ASPIRED) randomized, placebo-controlled trial(20) to assess the effects of aspirin at 81 and 325 mg/day[d] on urinary PGE-M in individuals at elevated risk for CRC.
METHODS
Clinical trial population and design
This study was a single-site, randomized, double-blind, placebo-controlled trial of 81 or 325 mg/day aspirin in adults aged 18–80 years old (www.clinicaltrials.gov; NCT02394769). The trial was activated in July 2015 and completed enrollment in February 2019. The detailed trial protocol has been previously published and is available via open-access.(20) Participants were drawn from the patient population of the Massachusetts General Hospital (MGH) who had undergone a screening or surveillance colonoscopy with a pathologically confirmed diagnosis of at least one colorectal adenomatous polyp (including sessile serrated adenoma, but excluding hyperplastic polyp) within 9 months of study enrollment. All subjects provided written consent and the protocol was approved by the Dana-Farber/Harvard Cancer Center Institutional Review Board (DF/HCC #14–496).
Potential participants were identified using the MGH Pathology Natural Language Search tool to generate monthly reports of individuals with adenoma that were subsequently confirmed by a member of the study team using the corresponding pathology report. The individual’s treating physician invited individuals with confirmed adenoma by mail. Interested participants were recruited and eligibility was confirmed during a phone interview. Eligible participants were those that had not taken aspirin at any dose in the last 6 months, presented with an ECOG performance status ≤2, and had the ability to understand and the willingness to sign a written, informed consent document. Individuals were excluded if they used any non-aspirin NSAID at any dose 3 or more times per week during the 2 months prior to randomization; were receiving any other investigational agents; had any prior diagnosis of gastrointestinal cancer (including esophageal, gastric, small intestine, colon, and pancreatic), or any diagnosis of other cancers (with the exception of non-melanoma skin) in which there had been an active treatment within the three years prior to randomization; had a history of inflammatory bowel disease, liver or kidney disease; had a history of aspirin intolerance or known allergic reaction to compounds of similar chemical or biological composition to aspirin; had a history of bleeding diathesis, peptic ulcer or gastrointestinal bleed, endoscopic complications, or contraindication to colonoscopy; were taking any anti-coagulant agent (e.g. warfarin) or anti-platelet agent (e.g. clopidigrel); received a prior diagnosis of familial adenomatous polyposis (FAP) or hereditary non-polyposis colorectal cancer (HNPCC; Lynch Syndrome); had any adenoma that was not completely removed during the previous colonoscopy; were pregnant or breastfeeding; were unable to swallow pills; had an uncontrolled intercurrent illness that would limit compliance with study requirements; were unable or unwilling to abstain from non-protocol use of aspirin or NSAIDs or to provide blood, urine, or stool samples or colon biopsies during the study. Participants were educated via a post-card sized handout of the brand and generic names of NSAIDs that would make them ineligible from continued participation in the study. Those that reported non-study NSAID use during the study period were removed from the study and an exit interview was performed at that time. The informed consent process was performed by a study physician.
Study intervention
Participants (N=180) were assigned to 3 arms that consisted of placebo (lactose), 81 mg, or 325 mg of generic, non-coated, aspirin (acetylsalicylic acid) in a 1:1:1 ratio using block random assignment generated by the study statistician (M.W.). Aspirin capsules were prepared by the MGH Research Pharmacy to be indistinguishable from placebo and also contained lactose filler. Participants were provided with 84 blinded capsules (12-week supply) at the baseline visit and instructed to take one capsule after the clinical visit with food and water, to be repeated daily until returning for the follow-up visit. All participants, providers, and study staff were blinded to assignments. Missed doses were not made up and reported to study staff during weekly phone calls. Adherence was measured by pill count, and additionally assessed biochemically(21) by measuring serum TXB2 levels in a subset of patients.
Study visits and assessments
Prior to randomization, participants attended a baseline clinical study visit at the MGH Gastrointestinal Unit where they provided biospecimens, including urine and blood samples and underwent an unsedated flexible sigmoidoscopy without bowel preparation for stool and biopsy collection. The follow-up (post-treatment) visit mirrored the baseline visit with identical sample collection and occurred at least 8 weeks (56 days), but no more than 12 weeks (84 days), after the baseline visit. All metadata were derived by clinical assessment, self-reported questionnaire and/or abstraction of the electronic medical record.
Endpoint ascertainment
The predefined primary outcome was the effect of aspirin at 81 or 325 mg/d on urinary PGE-M. The Eicosanoid Core Laboratory at Vanderbilt University measured PGE-M levels in baseline/pre-treatment and post-treatment urine samples using liquid chromatography-mass spectrometry (LC/MS) as previously described.(18) For secondary exploratory outcomes, concentrations of the urinary thromboxane (TX) metabolite, 11-dehydroTX B2 (TXM) and serum TXB2 and PGE2 were quantified on samples sent to the Institute of Pharmacology at Catholic University School of Medicine, Rome, Italy, using Enzyme Linked Immunosorbent Assay (ELISA) as previously described.(22–27) Additional details are provided in the supplement.
Sample size
The trial sample size was powered to detect an effect on urinary PGE-M between the placebo group and the two aspirin groups combined, regardless of compliance with the study treatment (intent-to-treat). Based on prior studies,(7,8,15) we assumed a standard deviation (SD) of 5.0 for a single measurement of PGE-M and an intra-class correlation (ICC) of 0.1. With 45 participants in the placebo group and 90 participants in the combined aspirin groups, we expected 90% (80%) power to detect a mean change of PGE-M level in the aspirin group of 4.0 (3.5) ng/mg, compared with no change in the placebo group, assuming a Type I error rate of 0.05. This minimum detectable difference in mean change was consistent with the difference in the median level of PGE-M among individuals at high risk for adenoma compared with low risk.(8) To account for drop out of up approximately 20% participants, we conservatively enrolled 60 participants in each group.
Statistical analysis
Baseline characteristics of subjects were compared between treatment arms by using Fisher’s exact test for categorical variables and one-way ANOVA and unpaired two sample t-tests for continuous variables. Intention-to-treat analyses comparing the effect of aspirin treatment (grouped) on post-treatment change in PGE-M (ΔPGE-M) compared to the change in the placebo group using an unpaired two-sample t-test was performed to test the prespecified primary outcome. For subgroup analyses according to dose, a one-way ANOVA was performed followed by individual unpaired two-sample t-tests between treatment arms. For subsequent analyses, assumptions for normality (Gaussian distribution) were checked using Shapiro-Wilk. Because normality assumptions were not met for urinary TXM or serum TXB2 and PGE2, non-parametric tests (Kruskal-Wallis and/or Mann-Whitney) were performed for these analyses. Spearman correlations were performed to compare continuous measures from urine and serum at baseline or the change in levels of related metabolites. In secondary analyses, we examined if sociodemographic, lifestyle, and medical history factors were independently associated with change in urinary PGE-M levels (ΔPGE-M) using general linear models where baseline PGE-M or ΔPGE-M (post-treatment – baseline) were modeled as the outcome and covariates were included in the model. To assess if these factors modified treatment effects on ΔPGE-M, we included a cross-product term for the variable and treatment assignment in the models to assess for multiplicative interaction while adjusting for baseline levels of PGE-M. All statistical tests were two-sided and considered significant using an α-threshold <0.05, except where otherwise noted, and performed using SAS (v.9.4, Cary, NC) or Prism8 (GraphPad, San Diego, CA). All authors had access to the study data and have reviewed and approved the final manuscript.
RESULTS
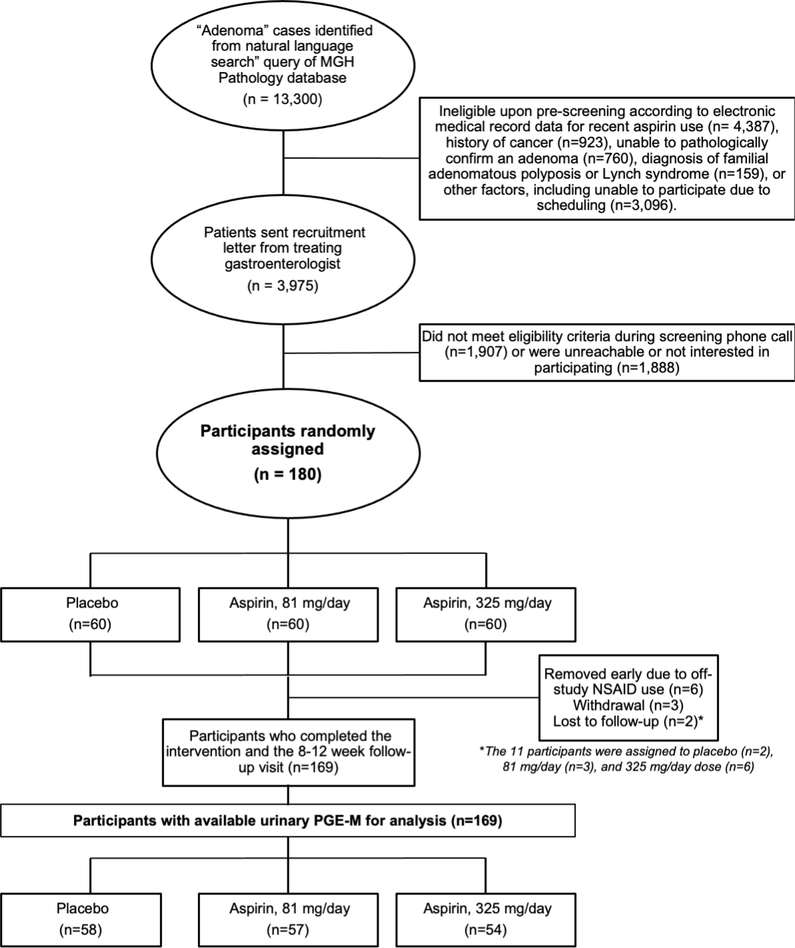
The derivation of the final trial cohort from those screened and recruited to the study is shown in Figure 1. Baseline characteristics are presented in Table 1. No significant differences were observed between treatment groups (all p > 0.05). As per protocol, six participants were removed from the study early due to reported NSAID use during the intervention period; three withdrew prior to the final visit, and two were lost-to-follow-up. No serious adverse events were reported. Minor adverse events and participant complaints documented during the study were limited and occurred at similar frequency across arms (Supplemental Table 1). This resulted in 169 participants with available pre- and post-treatment urine samples for analysis. No significant differences were observed among the remaining participants according to treatment arm.
Figure 1.
ASPIRED recruitment and participant enrollment overview (CONSORT Diagram).
Table 1.
Baseline characteristics of ASPIRED trial participants (N=180)
| Placebo (n=60) | Aspirin, 81 mg (n=60) | Aspirin, 325 mg (n=60) | |
|---|---|---|---|
| Age, yr, mean (SD) | 57.1 (9.2) | 56.1 (8.7) | 57.5 (8.3) |
| Sex, n (%)a | |||
| Female | 28 (46.7) | 29 (48.3) | 28 (46.7) |
| Race, n (%) | |||
| White | 55 (91.7) | 52 (86.7) | 53 (88.3) |
| Black/African American | 3 (5.0) | 4 (6.7) | 3 (5.0) |
| Asian | 1 (1.7) | 0 (0) | 2 (3.3) |
| More than one race | 0 (0) | 4 (6.7) | 2 (3.3) |
| Did not report | 1 (1.7) | 0 (0) | 0 (0) |
| Ethnicity, n (%) | |||
| Hispanic | 2 (3.3) | 2 (3.3) | 1 (1.7) |
| Marital Status, n (%) | |||
| Married | 40 (66.7) | 39 (65.0) | 37 (61.7) |
| Never married | 6 (10.0) | 12 (20.0) | 11 (18.3) |
| Separated | 2 (3.3) | 0 (0.0) | 1 (1.7) |
| Divorced | 8 (13.3) | 7 (11.7) | 7 (11.7) |
| Widowed | 4 (6.7) | 2 (3.3) | 4 (6.7) |
| Body Mass Index, kg/m2, mean (SD) | 26.8 (5.0) | 28.4 (4.9) | 27.5 (5.7) |
| Normal, <18.5–24.9 | 21 (35.0) | 16 (26.7) | 21 (35.0) |
| Overweight, 25.0–29.9 | 26 (43.3) | 26 (43.3) | 23 (38.3) |
| Obese, ≥30.0 | 13 (21.7) | 18 (30.0) | 16 (26.7) |
| Smoking Status, n (%) | |||
| Never | 38 (63.3) | 36 (60.0) | 32 (53.3) |
| Former | 18 (30.0) | 20 (33.3) | 19 (31.7) |
| Current | 4 (6.7) | 3 (5.0) | 8 (13.3) |
| Missing | 0 (0.0) | 1 (1.7) | 1 (1.7) |
| Alcohol Consumption, n (%) | |||
| Never | 7 (11.7) | 11 (18.3) | 11 (18.3) |
| Rarely | 14 (23.3) | 16 (26.7) | 18 (30.0) |
| 1–5 times/week | 29 (48.3) | 24 (40.0) | 23 (38.3) |
| Daily | 10 (16.7) | 8 (13.3) | 6 (10.0) |
| More than daily | 0 (0.0) | 1 (1.7) | 2 (3.3) |
| Personal Cancer History, yes, n (%) | 10 (17.0) | 6 (10.0) | 4 (6.8) |
| Family History of CRC, yes, n (%) | 13 (21.7) | 10 (16.7) | 12 (20.0) |
| Type II Diabetes, yes, n (%) | 2 (3.4) | 3 (5.0) | 2 (3.3) |
| Menopause Status (n=85)b | |||
| Pre-menopausal | 3 (10.7) | 9 (31.0) | 5 (17.9) |
| Peri-menopausal | 4 (14.3) | 1 (3.4) | 2 (7.1) |
| Post-menopausal | 20 (71.4) | 17 (58.6) | 18 (64.3) |
| Missing | 1 (3.6) | 2 (6.9) | 3 (10.7) |
| History of 81 mg aspirin use, n (%) | |||
| Never | 55 (91.7) | 50 (83.3) | 53 (88.3) |
| Intermittently (<2x/wk) | 2 (3.3) | 5 (8.3) | 5 (8.3) |
| Regularly (>2x/week) | 2 (3.3) | 2 (3.3) | 2 (3.3) |
| Missing | 1 (1.7) | 3 (5.0) | 0 (0.0) |
| History of 325 mg aspirin use, n (%) | |||
| Never | 40 (66.7) | 42 (70.0) | 42 (70.0) |
| Intermittently (<2x/wk) | 17 (28.3) | 15 (25.0) | 17 (28.3) |
| Regularly (>2x/week) | 1 (1.7) | 1 (1.7) | 1 (1.7) |
| Missing | 2 (3.3) | 2 (3.3) | 0 (0.0) |
| History of NSAID use, n (%) | |||
| Never | 18 (30.0) | 13 (21.7) | 19 (31.7) |
| Intermittently (<2x/wk) | 31 (51.7) | 36 (60.0) | 32 (53.3) |
| Regularly (>2x/week) | 10 (16.7) | 9 (15.0) | 8 (13.3) |
| Missing | 1 (1.7) | 2 (3.3) | 1 (1.7) |
| PPI Use, n (%) | |||
| Current and regular | 5 (8.3) | 6 (10.0) | 8 (13.3) |
| Missing | 7 (11.7) | 5 (8.3) | 4 (6.7) |
| H2-Blocker Use, n (%) | |||
| Current and regular | 2 (3.3) | 5 (8.3) | 2 (3.3) |
| Missing | 2 (3.3) | 1 (1.7) | 1 (1.7) |
| Antacid Use, n (%) | |||
| Current and regular | 5 (8.3) | 3 (5.0) | 3 (5.0) |
| Missing | 1 (1.7) | 0 (0.0) | 0 (0.0) |
| Statin Use, n (%) | |||
| Current and regular | 14 (23.3) | 11 (18.3) | 16 (26.7) |
| Missing | 2 (3.3) | 1 (1.7) | 1 (1.7) |
| Indication for Previous Endoscopy, n (%) | |||
| Screening | 35 (58.3) | 33 (55.0) | 30 (50.0) |
| Surveillance | 15 (25.0) | 13 (21.7) | 16 (26.7) |
| Diagnostic | 3 (5.0) | 5 (8.3) | 3 (5.0) |
| Other/Unknown | 7 (11.7) | 9 (15.0) | 11 (18.3) |
| Polyp History by location, n (%) | |||
| Right | 23 (38.3) | 25 (41.7) | 26 (43.3) |
| Left | 24 (40.0) | 14 (23.3) | 17 (28.3) |
| Both | 13 (21.7) | 21 (35.0) | 16 (26.7) |
| Unknown | 0 (0.0) | 0 (0.0) | 1 (1.7) |
| Days on treatment, mean (SD)c | 68.6 (6.5) | 68.5 (7.5) | 69.6 (6.9) |
| Pill Count Adherence, n (%)c | |||
| 95.0–100% | 52 (89.7) | 45 (79.3) | 49 (90.7) |
| 90.0–94.9% | 3 (5.2) | 9 (15.5) | 3 (5.6) |
| 80.0–89.9% | 1 (1.7) | 3 (5.2) | 2 (3.7) |
| <80.0% | 1 (1.7) | 0 (0.0) | 0 (0.0) |
| Missing | 1 (1.7) | 0 (0.0) | (0.0) |
Baseline characteristics of subjects were compared between treatment arms by using Fisher’s exact test for categorical variables and one-way ANOVA and unpaired two sample t-tests for continuous variables.
Biological sex at birth.
Question posed only to women.
Only available for individuals who completed the study and provided a pre- and post-treatment sample (n=169). Missing represents one individual who completed the study, but did not return their pill bottle to the study staff.
Urinary levels of PGE-M at baseline (pre-treatment) and post-treatment are shown in Table 2. Our primary outcome analysis considered all individuals with urine at both time points on an intention-to-treat basis per protocol without accounting for the normality of the data. In 169 subjects, the mean ± standard deviation excretion rate at baseline was 15.9 ± 14.6 ng per mg creatinine (ng/mg cr) and did not differ significantly between arms. The primary outcome that aspirin intervention at either dose resulted in a decrease in urinary PGE-M compared to placebo (p = 0.015) was successfully achieved with a mean ΔPGE-M of −4.7 ± 14.8 ng/mg cr (median = −3.5 ng/mg cr). According to dose, a mean ΔPGE-M of −4.6 ± 17.7 (p = 0.056) was observed for 81 mg/day and −4.9 ± 11.2 ng/mg cr (p = 0.01) for 325 mg/day, corresponding to a mean decrease of 15% (p = 0.018) and 28% (p < 0.0001) (median decrease = 27% and 35%, respectively) after treatment. No significant difference was observed in ΔPGE-M between the aspirin groups (p = 0.91). The mean ΔPGE-M among the placebo group was negligible (+0.8 ng/mg cr or +8.5%).
Table 2.
Urinary PGE-M concentration according to randomized intervention arm.
| Aspirin dose assignment |
||||||
|---|---|---|---|---|---|---|
| Variable | Placebo (n=58) | 81 mg/day (n=57) | P81 v. placebo | 325 mg/day (n=54) | P325 v. placebo | Paspirin (grouped) v. placebo |
| Baseline urinary PGE-M, ng/mg Cr | 15.5 (12.6) | 17.7 (17.1) | 0.44 | 14.3 (13.7) | 0.62 | 0.82 |
| Post-Intervention urinary PGE-M, ng/mg Cr | 16.4 (15.8) | 13.1 (13.4) | 0.24 | 9.4 (7.9) | 0.005 | 0.018 |
| Δ urinary PGE-M, ng/mg Cr | 0.8 (11.8) | −4.6 (17.7) | 0.056 | −4.9 (11.2) | 0.010 | 0.015 |
| % Change | 8.5 (50.6) | −15.4 (56.7) | 0.018 | −28.2 (40.3) | <0.0001 | 0.0003 |
The p-value for the primary outcome comparison is in Bold. Values are mean (SD) unless otherwise noted. P-values are generated from unpaired t-tests between groups, as noted by the subscript text, for each measure. No significant differences were observed between aspirin treatment groups (81 mg/day v. 325 mg/day), all p>0.05.
We performed several sensitivity analyses to check the robustness of the primary outcome analysis. The data was not normally distributed; however, results were similar when using non-parametric Kruskal-Wallis and Mann-Whitney tests, with the only major difference supporting a significant decrease in urinary PGE-M between placebo and 81 mg/day arms (p<0.001; Supplemental Table 2). We did observe that one individual in each of the aspirin treatment arms had abnormally high baseline urinary PGE-M. After removing these participants from the analysis, the primary outcome was not materially altered (p=0.018; Supplemental Table 3). Removing these outliers attenuated the mean decrease in absolute PGE-M levels among the aspirin intervention arms, but median values and relative percent changes remained unaltered.
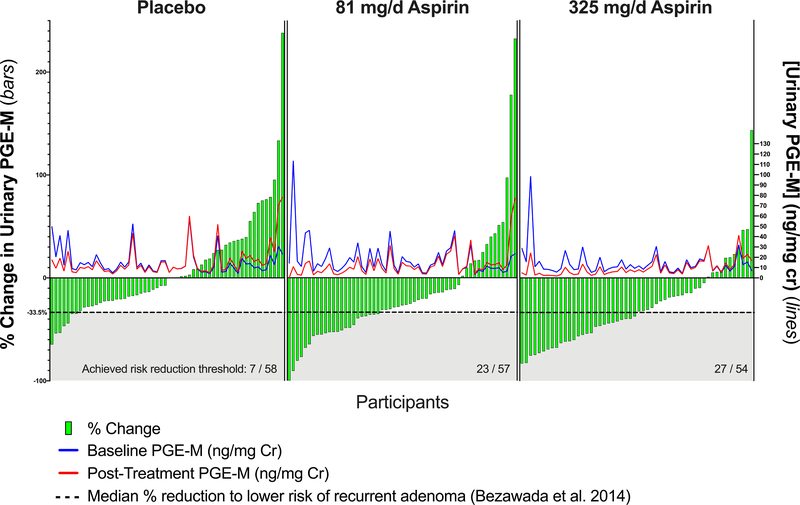
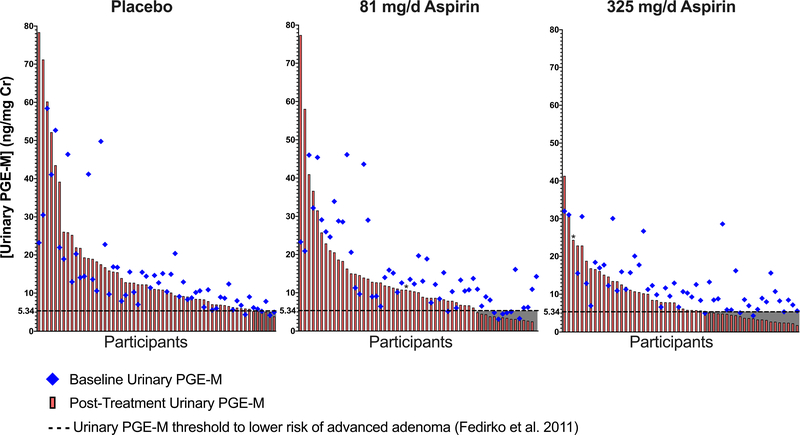
We considered the biological relevance of these changes in PGE-M. First, we previously reported that risk of advanced adenoma was restricted to individuals who were in the highest quartile of PGE-M concentration (Q4 median=9.44 ng/mg cr) at baseline compared to those in lower quartiles, including quartile 3 (median=6.28 ng/mg cr).(8) Thus, a reduction of at least 33.5% in PGE-M level may be consistent with lowered risk of advanced adenoma. Calculating the population attributable risk based on these relative risk estimates, achieving this threshold of inhibition translates to a 10% absolute risk reduction for advanced adenomas. In ASPIRED, aspirin reduced PGE-M beyond this threshold in 40% (Figure 2; p <0.001, χ2) of individuals randomized to 81 mg/day and 50.0% (p < 0.001, χ2) of individuals randomized to 325 mg/day. Second, in the Aspirin/Folate Polyp Prevention Study (AFPPS), among participants who were randomized to aspirin for 3-years, post-intervention urinary PGE-M levels below 5.34 ng/mg cr were associated with a decreased risk of any adenoma and, specifically, advanced adenoma recurrence.(19) In ASPIRED, post-treatment urinary PGE-M levels were reduced below 5.34 ng/mg cr in a significantly greater proportion of individuals randomized to 81 (Figure 3; 25%; p = 0.04, χ2) or 325 (41%; p = 0.0002, χ2) mg/day aspirin compared to placebo.
Figure 2.
Percent change in urinary PGE-M in individual ASPIRED participants according to treatment assignment in context of risk thresholds for advanced adenoma. Data from the Nurses’ Health Study (Bezawada et al.; Reference 8) suggested that individuals with the highest quartile (Q) of PGE-M at baseline (Q4, median = 9.44 ng/mg cr) were at significantly increased risk for developing advanced adenomas compared to those in lower quartiles (Q3, median = 6.28 ng/mg cr). Thus, a reduction of 33.5% based on these previously reported values may be consistent with reduced risk for advanced adenoma. The dashed line and shaded area represent the minimum threshold for change (−33.5%) at which individuals might expect a decrease in risk for recurrent neoplasia. Each green bar represents an individual’s percent change in PGE-M from baseline (left y-axis). Individual pre- and post-treatment PGE-M in ng/mg Cr appears as the red and blue trace lines, respectively (right y-axis). Aspirin intervention with 81 or 325 mg/day significantly reduced individual PGE-M levels below this threshold in a greater proportion of participants (green bars contained within gray box), 23 of 57 (40.4%) and 27 of 54 (50.0%), respectively (both p < 0.001; χ2), compared to 7 of 58 (12.0%) of those randomized to placebo.
Figure 3.
Absolute change in urinary PGE-M in individual ASPIRED participants according to treatment assignment in context of risk thresholds for recurrent advanced adenoma based on the Aspirin/Folate Polyp Prevention Study (AFPPS). The AFPPS clinical trial (Fedirko et al.; Reference 19) reported that individuals with urinary PGE-M levels below 5.34 ng/mg cr after 3 years of aspirin treatment were at significantly reduced risk of recurrent advanced adenoma compared to individuals above this threshold. Individuals are separated by treatment arm, ranked by post-treatment PGE-M level (red bar), and plotted with pre-treatment PGE-M levels (blue diamond). The dashed line and shaded area represent the minimum threshold for change (5.34 ng/mg cr) at which individuals might expect a decrease in risk for recurrent advanced neoplasia. Aspirin intervention with 81 or 325 mg/day significantly reduced PGE-M levels below this threshold in a greater proportion of individuals,14 of 57 (24.6%; p = 0.04, χ2) and 22 of 54 (40.7%; p = 0.0002, χ2), respectively, compared to 6 of 58 (10.3%) of those randomized to placebo. One individual in each of the aspirin treatment arms had abnormally high pre-treatment PGE-M levels (denoted by asterisk [*] in the figure). Pretreatment PGE-M values for these individuals equaled 113.7 and 98.5 ng/mg cr in the 81 mg/d and 325 mg/d arms, respectively.
Though the randomization resulted in no clear imbalances between arms according to baseline characteristics, we examined the impact of potential confounders on change in urinary PGE-M excretion (Supplemental Table 4). Several factors were nominally associated with increased baseline PGE-M levels: widowed or never married marital status, obesity (BMI = 30 or higher), type II diabetes, greater alcohol consumption, and ever history of non-aspirin NSAID use. However, none of these associations were significant after accounting for multiple hypothesis testing (Bonferroni-adjusted α < 0.00024; p = 0.05/21 covariates), other than type II diabetes (p = 0.0002). In addition, no significant interactions were observed between any covariate and randomized assignment that modified the effect of treatment.
Among participants who completed the study, adherence measured by pill count was high with 75% of participants exhibiting 100% adherence and only 1 participant at <80% (77%) and did not differ significantly between arms (p > 0.05). Adherence was additionally checked by measuring urinary TXM in the entire cohort (n=169) and serum TXB2 among a subset (n=30) who had serum collected according to optimized methods for TXB2 assessment (Supplemental Figure 1). At baseline, urinary TXM, an index of systemic TXA2 biosynthesis, (median: 1.7 ng/mg Cr; 1.1, 2.2), and serum TXB2, a marker of platelet COX-1 activity (median: 234.7 ng/mL; 25th, 75th percentiles: 152.3, 287.5) levels were similar across arms (p > 0.05). Compared to placebo, 81 mg/day of aspirin reduced median TXM by 71.7% (Supplemental Figure 1A; p < 0.0001) and TXB2 by 97.9% (Supplemental Figure 1B; p < 0.0001) and 325 mg/day of aspirin inhibited median and TXM by 78.0% (Supplemental Figure 1A; p < 0.0001) and TXB2 by 99.8% (Supplemental Figure 1B; p < 0.0001). The percent differences in these analytes between aspirin doses was significant (p < 0.05). Urinary TXM and serum TXB2 were correlated at baseline (Supplemental Figure 1C; Spearman, r = 0.40; p = 0.03) as was the post-aspirin change (Supplemental Figure 1D; r = 0.46; p = 0.03), though these relationships have been previously demonstrated to be relatively non-linear(28).
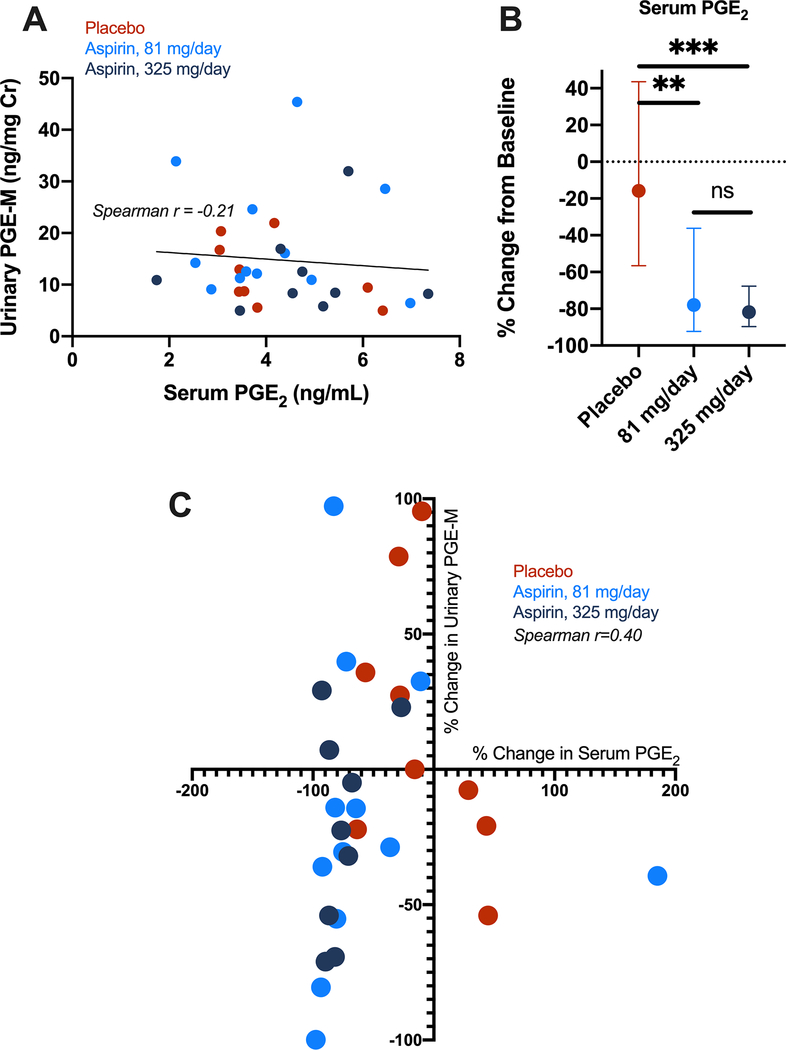
As an exploratory analysis, among the subset of 30 individuals with serum, we additionally tested pre- and post-treatment serum to determine whether aspirin inhibition of PGE2 would be similarly measured in circulation. We found that baseline serum PGE2 and urinary PGE-M concentrations were not correlated (Figure 4A), perhaps reflecting the platelet vs. non-platelet source(s) of PGE2 production. However, 81 or 325 mg/day aspirin significantly reduced serum PGE2 compared to placebo (p = 0.005 and 0.0005, respectively) and no difference between treatment arms was observed (Figure 4B) (p = 0.65). The percent change in serum PGE2 was modestly correlated to percent change in urinary PGE-M (Spearman r =0.40; p = 0.03) (Figure 4C).
Figure 4.
PGE2 measurement in serum of ASPIRED participants. A) Spearman correlation of baseline urinary PGE-M (systemic) and serum PGE2 (circulating) demonstrates measures are not well correlated. B) Aspirin intervention with 81 or 325 mg/day significantly reduces serum PGE2 from baseline compared to placebo. Mann-Whitney test, **p<0.01; ***p <0.001; ns = not significant. C) The percent decrease in urinary PGE-M, is modestly correlated with the percent change in serum PGE2 following aspirin intervention. Spearman r = 0.40; one-tailed p-value = 0.035.
DISCUSSION
Overall, standard doses of aspirin (81 or 325 mg, once daily) over 8-weeks significantly reduced pre-treatment systemic PGE2, as reflected by urinary PGE-M excretion in adults with a recent history of colorectal adenoma. Furthermore, this intervention was sufficient to reduce PGE-M excretion to levels previously associated with a reduced risk of recurrent adenoma or CRC in nearly half of the individuals randomized to aspirin. In addition, we observed profound inhibition of platelet PGE2 and platelet and systemic TXA2 biosynthesis. Combined, this work demonstrates that aspirin intervention can significantly reduce elevated PGE2 levels in patients at risk for CRC to a level consistent with reduced risk of recurrent colorectal neoplasia. These results support a causal link for aspirin’s effect on PGE2 biosynthesis as a central mechanism for its chemopreventive mode of action and that urinary PGE-M is a modifiable biomarker for CRC risk that may have utility in aspirin precision chemoprevention.
Urinary PGE-M has previously been demonstrated to be a promising biomarker to predict individual CRC risk(29), and as an efficacy marker for chemoprevention agents.(30) Here, we demonstrate that aspirin reduces PGE-M in most individuals. In the AFPPS trial which only examined urinary PGE-M only after three years of aspirin treatment, those randomized to 81 mg or 325 mg aspirin had PGE-M levels of 18% and 28% lower than those receiving placebo, respectively, which corresponds to levels 1.5–2.5 ng/mg cr lower than those receiving placebo.(19) We demonstrate strikingly similar results within individuals randomized to aspirin experiencing a mean decrease of 15% and 28% from baseline for each dose, corresponding to a mean difference of approximately −4.7 ng/mg cr. Notably, our results were also consistent with the AFPPS,(19) where no statistically significant differences were observed between 81 and 325 mg/day overall, but significant inhibition, irrespective of dose, was achieved in a much shorter timeframe. Further, our results are consistent with a randomized clinical trial in current heavy smokers (n=54) that demonstrated low-dose daily or intermittent aspirin reduced urinary PGE-M from baseline.(31) Beyond urinary PGE-M, we also demonstrate that the change in serum PGE2 measured by immunoassay appears correlated with the change in urinary PGE-M offering another potential blood-based biomarker that may be conducive to implementation in clinical settings.
By examining the change from baseline urinary PGE-M levels in at-risk individuals, our study provides an opportunity to understand personalized responses to aspirin intervention. Since prior studies employed the same method for PGE-M quantitation where the concentration was normalized to an individual’s urinary creatinine levels,(8,19) direct comparison of these normalized PGE-M levels across studies is reasonable even though they are single-timepoint, cross-sectional measures. Given that our trial cohort is comprised entirely of individuals at higher risk for CRC due to their adenoma history, it is not surprising that the observed mean baseline PGE-M of 15.9 ng/mg cr places the majority of ASPIRED participants in the highest quartile of risk according to these previous reports.(8,9,19) This finding has important implications for chemoprevention: polypectomy alone does not appear sufficient to reduce risk associated with elevated prostanoid biosynthesis in the immediate term (months after resection) for the majority of individuals diagnosed with adenoma. Biologically, this suggests that dysregulated PGE2 biosynthesis may not be restricted to neoplastic tissue and additional intervention may be required to suppress PG-mediated carcinogenesis. Future studies may be able to distinguish the major cellular source of PGE2, which could include platelets, stromal cells, or colorectal epithelium.
Although aspirin reduced PGE-M in the majority of individuals, we observed a decrease to levels consistent with reduced risk of recurrent neoplasia in approximately half of the individuals randomized to aspirin. While 81 mg/day was sufficient to achieve a 34% reduction of PGE-M in approximately 40% of individuals randomized to aspirin, more individuals reached this threshold in the 325 mg/day arm. This is even more apparent when considering the higher threshold of decreasing levels below 5.34 ng/mg cr as a benchmark for reduced risk based on the AFPPS findings,(19) where nearly twice as many individuals achieved the threshold after treatment with standard dose compared to low-dose. Therefore, while we did not observe a significant difference in urinary PGE-M levels between aspirin doses, higher doses may be more effective in achieving a response in individuals who do not respond to low-dose aspirin. Given the interindividual variability of prostanoid inhibition we observed, multiple timepoints or longer intervention may be required to better disentangle individual responses. Moreover, future studies may consider prioritizing percent change of urinary PGE-M from baseline over absolute change to more stably account for possible sources of variation. Nonetheless, this underscores the potential clinical utility for a precision prevention approach where flux in PGE-M levels could be used to identify individuals for whom aspirin is showing effects even after a short burst intervention and would likely benefit from continued use.
Conversely, this finding also highlights a subset of individuals that may be non-responsive to aspirin. Approximately 20–25% of those randomized to 81 mg/day or 325 mg/day experienced no inhibition or even an increase in PGE-M from baseline. In contrast, all but one participant experienced a strong reduction of TXM or TXB2 irrespective of dose. This may reflect the contribution of constitutive expression of COX-2 from extra-intestinal sites of PGE2 biosynthesis (e.g. kidney or brain) that may require higher doses of aspirin or more frequent dosing to sustain suppression. Therefore, there may also be clinical utility for a precision prevention approach where flux in PGE-M levels may also be used to tailor dose and duration recommendations or identify individuals who might not derive any chemoprotection, and, thus, for whom the harms associated with aspirin use might outweigh potential benefits.
Future studies should examine whether more individuals experience reduction in PGE-M below risk thresholds when provided higher doses or over longer treatment periods while closely monitoring for potential risks. Observational data supports that the chemopreventive effects for aspirin are most fully appreciated after regular use of aspirin for 5–10 years,(32,33) such that future trials with longer-term follow-up might vary the intervention period to clarify the ideal length and dose of intervention that translates into sustained inhibition of PGE-M and reduced risk of recurrent neoplasia. Similarly, use of baseline PGE-M might be used to identify participants as high, average, or low risk patients in context of existing risk markers (e.g. adenoma clinical and histopathologic features) prior to randomization so as to test whether PGE-M can be used as a sensitive risk-stratification biomarker.
We did observe that several factors, including elevated BMI, type II diabetes, and heavy alcohol consumption, were marginally associated with baseline higher urinary PGE-M levels and may contribute to the observed interindividual variation. These factors have each independently been associated with CRC risk.(34–40) BMI and obesity-related comorbidities are of particular interest considering the potential impact on aspirin bioavailability.(41,42) Given that individuals with the highest levels of PGE-M at baseline appear to derive the greatest benefit from aspirin from prospective studies,(8) these data suggest that individuals with obesity, type II diabetes, and/or consume a heavy amount of alcohol may represent populations to specifically target for aspirin following adenoma resection. While no significant interactions were observed here, larger studies should examine findings in context of these risk factors. This is especially relevant since the USPSTF confined their recommendation for aspirin use in CRC primary prevention to those individuals at 10% or greater ten-year risk for CVD,(1) which also shares these risk factors.(43) As incidence of these comorbidities continues to grow, an alternative or complementary approach to precision prevention may include incorporating additional shared risk factors for CRC and CVD, especially those that may influence eicosanoid pools, that may predict more favorable risk-benefit profiles for aspirin.
Our study has limitations. First, urinary PGE-M is a surrogate endpoint for CRC risk and is a measure of systemic PGE2 biosynthesis. Secondary endpoint analysis, including tissue gene expression, may further elucidate colon-specific roles for prostaglandin modulation by aspirin for chemoprevention. Second, our intervention period was relatively short. However, intraindividual reduction of PGE-M observed after 8–12 weeks of daily use in ASPIRED was consistent with interindividual PGE-M reduction between placebo and treatment arms in the AFPPS after 3 years of use. A longer intervention period would have presented additional challenges related to adherence or retention. Last, the trial cohort was predominantly white and additional studies will be required to support generalizability of the findings for other populations.
In conclusion, our results support that low-dose, daily aspirin over a short-term period is sufficient to downregulate PGE2 biosynthesis in many at-risk individuals to levels consistent with lower risk of CRC. However, higher doses or longer durations of treatment may be necessary to achieve significant reduction in a greater proportion of individuals. Our results support the potential utility for PGE-M for identifying individuals following adenoma resection who are more likely to derive chemopreventive benefit from aspirin. We envision that urinary PGE-M may provide a paradigm for precision prevention by which individual response can be measured and used to tailor recommendations, including whether to continue, change, or cease aspirin in a prevention setting.
Supplementary Material
Acknowledgments
The authors would like to thank the participants of the ASPIRED trial. Further, we thank the administrative, technical, nursing and clinical staff of the MGH Division of Gastroenterology. The corresponding author has full access to all the data in the study and had final responsibility for the decision to submit for publication. Funding: This clinical trial was supported by the National Cancer Institute (NCI) at the National Institutes of Health (NIH) (grant number R01CA137178) to A.T. Chan. Outcome assessments were additionally supported by Cancer Research UK (Catalyst Award – Aspirin for Cancer Prevention Collaboration) awarded to A.T. Chan and C. Patrono. D.A. Drew was supported by the NCI (T32CA009001;L30CA209764) and the National Institutes of Diabetes and Digestive and Kidney Diseases (NIDDK)(K01DK120742) and a pilot feasibility study funded by P30DK043351 at NIH. A.T. Chan is a Stuart and Suzanne Steele MGH Research Scholar.
Conflicts of Interest
No sources of support had a role in the design and conduct of the research; collecting, managing, analyzing, or interpreting data; or preparing the manuscript; nor the decision to submit the manuscript for publication. Several authors have received lecture fees or consulted for industry as described, but in no capacity directly related to these findings or the ASPIRED trial. K. Staller has consulted for Bayer AG. B. Rocca has received lecture fees from Novartis and Bayer AG. C. Patrono has received consulting and lecture fees from Acticore Biotech, Amgen, Bayer, GlaxoSmithKline and Zambon; Institutional research grants from Bayer, the European Commission and the Italian Drug Agency (AIFA); and serves as the chairperson of the Scientific Advisory Board of the International Aspirin Foundation. A.T. Chan reports consulting fees from Bayer Pharma AG, Pfizer Inc., Boehringer Ingelheim.
REFERENCES
- 1.Bibbins-Domingo K, Force USPST. Aspirin Use for the Primary Prevention of Cardiovascular Disease and Colorectal Cancer: U.S. Preventive Services Task Force Recommendation Statement. Ann Intern Med 2016;164(12):836–45 doi 10.7326/M16-0577. [DOI] [PubMed] [Google Scholar]
- 2.Dehmer SP, Maciosek MV, Flottemesch TJ, LaFrance AB, Whitlock EP. Aspirin for the Primary Prevention of Cardiovascular Disease and Colorectal Cancer: A Decision Analysis for the U.S. Preventive Services Task Force. Ann Intern Med 2016;164(12):777–86 doi 10.7326/M15-2129. [DOI] [PubMed] [Google Scholar]
- 3.Drew DA, Cao Y, Chan AT. Aspirin and colorectal cancer: the promise of precision chemoprevention. Nat Rev Cancer 2016;16(3):173–86 doi 10.1038/nrc.2016.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kortz L, Dorow J, Ceglarek U. Liquid chromatography-tandem mass spectrometry for the analysis of eicosanoids and related lipids in human biological matrices: a review. Journal of chromatography B, Analytical technologies in the biomedical and life sciences 2014;964:1–11 doi 10.1016/j.jchromb.2014.01.046. [DOI] [PubMed] [Google Scholar]
- 5.Ricciotti E, FitzGerald GA. Prostaglandins and inflammation. Arterioscler Thromb Vasc Biol 2011;31(5):986–1000 doi 10.1161/ATVBAHA.110.207449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Catella F, Nowak J, Fitzgerald GA. Measurement of renal and non-renal eicosanoid synthesis. Am J Med 1986;81(Suppl 2B):23–9. [DOI] [PubMed] [Google Scholar]
- 7.Neale JR, Dean BJ. Liquid chromatography-tandem mass spectrometric quantification of the dehydration product of tetranor PGE-M, the major urinary metabolite of prostaglandin E(2) in human urine. J Chromatogr B Analyt Technol Biomed Life Sci 2008;871(1):72–7 doi 10.1016/j.jchromb.2008.06.042. [DOI] [PubMed] [Google Scholar]
- 8.Bezawada N, Song M, Wu K, Mehta RS, Milne GL, Ogino S, et al. Urinary PGE-M levels are associated with risk of colorectal adenomas and chemopreventive response to anti-inflammatory drugs. Cancer Prev Res (Phila) 2014;7(7):758–65 doi 10.1158/1940-6207.CAPR-14-0120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cai Q, Gao YT, Chow WH, Shu XO, Yang G, Ji BT, et al. Prospective study of urinary prostaglandin E2 metabolite and colorectal cancer risk. J Clin Oncol 2006;24(31):5010–6 doi 10.1200/JCO.2006.06.4931. [DOI] [PubMed] [Google Scholar]
- 10.Johnson JC, Schmidt CR, Shrubsole MJ, Billheimer DD, Joshi PR, Morrow JD, et al. Urine PGE-M: A metabolite of prostaglandin E2 as a potential biomarker of advanced colorectal neoplasia. Clin Gastroenterol Hepatol 2006;4(11):1358–65 doi 10.1016/j.cgh.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 11.Cui Y, Shu XO, Li HL, Yang G, Wen W, Gao YT, et al. Prospective study of urinary prostaglandin E2 metabolite and pancreatic cancer risk. Int J Cancer 2017;141(12):2423–9 doi 10.1002/ijc.31007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao J, Wang J, Du J, Xu H, Zhang W, Ni QX, et al. Urinary prostaglandin E2 metabolite and pancreatic cancer risk: case-control study in urban Shanghai. PLoS One 2015;10(2):e0118004 doi 10.1371/journal.pone.0118004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dong LM, Shu XO, Gao YT, Milne G, Ji BT, Yang G, et al. Urinary prostaglandin E2 metabolite and gastric cancer risk in the Shanghai women’s health study. Cancer Epidemiol Biomarkers Prev 2009;18(11):3075–8 doi 10.1158/1055-9965.EPI-09-0680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang T, Cai H, Zheng W, Michel A, Pawlita M, Milne G, et al. A Prospective Study of Urinary Prostaglandin E2 Metabolite, Helicobacter pylori Antibodies, and Gastric Cancer Risk. Clin Infect Dis 2017;64(10):1380–6 doi 10.1093/cid/cix106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murphey LJ, Williams MK, Sanchez SC, Byrne LM, Csiki I, Oates JA, et al. Quantification of the major urinary metabolite of PGE2 by a liquid chromatographic/mass spectrometric assay: determination of cyclooxygenase-specific PGE2 synthesis in healthy humans and those with lung cancer. Anal Biochem 2004;334(2):266–75 doi 10.1016/j.ab.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 16.Cui Y, Shu XO, Gao YT, Cai Q, Ji BT, Li HL, et al. Urinary prostaglandin E2 metabolite and breast cancer risk. Cancer Epidemiol Biomarkers Prev 2014;23(12):2866–73 doi 10.1158/1055-9965.EPI-14-0685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim S, Taylor JA, Milne GL, Sandler DP. Association between urinary prostaglandin E2 metabolite and breast cancer risk: a prospective, case-cohort study of postmenopausal women. Cancer Prev Res (Phila) 2013;6(6):511–8 doi 10.1158/1940-6207.CAPR-13-0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barnard ME, Beeghly-Fadiel A, Milne GL, Akam EY, Chan AT, Eliassen AH, et al. Urinary PGE-M Levels and Risk of Ovarian Cancer. Cancer Epidemiology Biomarkers & Prevention 2019;28(11):1845 doi 10.1158/1055-9965.EPI-19-0597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fedirko V, Bradshaw PT, Figueiredo JC, Sandler RS, Barry EL, Ahnen DJ, et al. Urinary metabolites of prostanoids and risk of recurrent colorectal adenomas in the Aspirin/Folate Polyp Prevention Study (AFPPS). Cancer prevention research (Philadelphia, Pa) 2015;8(11):1061–8 doi 10.1158/1940-6207.CAPR-15-0212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Drew DA, Chin SM, Gilpin KK, Parziale M, Pond E, Schuck MM, et al. ASPirin Intervention for the REDuction of colorectal cancer risk (ASPIRED): a study protocol for a randomized controlled trial. Trials 2017;18(1):50 doi 10.1186/s13063-016-1744-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cavalca V, Rocca B, Veglia F, Petrucci G, Porro B, Myasoedova V, et al. On-pump Cardiac Surgery Enhances Platelet Renewal and Impairs Aspirin Pharmacodynamics: Effects of Improved Dosing Regimens. Clin Pharmacol Ther 2017;102(5):849–58 doi 10.1002/cpt.702. [DOI] [PubMed] [Google Scholar]
- 22.Ciabattoni G, Maclouf J, Catella F, FitzGerald GA, Patrono C. Radioimmunoassay of 11-dehydrothromboxane B2 in human plasma and urine. Biochim Biophys Acta 1987;918(3):293–7 doi 10.1016/0005-2760(87)90233-5. [DOI] [PubMed] [Google Scholar]
- 23.Pagliaccia F, Habib A, Pitocco D, Petrucci G, Zaccardi F, Di Stasio E, et al. Stability of urinary thromboxane A2 metabolites and adaptation of the extraction method to small urine volume. Clin Lab 2014;60(1):105–11 doi 10.7754/clin.lab.2013.121238. [DOI] [PubMed] [Google Scholar]
- 24.Patrignani P, Filabozzi P, Patrono C. Selective cumulative inhibition of platelet thromboxane production by low-dose aspirin in healthy subjects. The Journal of clinical investigation 1982;69(6):1366–72 doi 10.1172/jci110576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Patrignani P, Panara MR, Greco A, Fusco O, Natoli C, Iacobelli S, et al. Biochemical and pharmacological characterization of the cyclooxygenase activity of human blood prostaglandin endoperoxide synthases. Journal of Pharmacology and Experimental Therapeutics 1994;271(3):1705. [PubMed] [Google Scholar]
- 26.Patrono C, Ciabattoni G, Pinca E, Pugliese F, Castrucci G, De Salvo A, et al. Low dose aspirin and inhibition of thromboxane B2 production in healthy subjects. Thrombosis Research 1980;17(3):317–27 doi 10.1016/0049-3848(80)90066-3. [DOI] [PubMed] [Google Scholar]
- 27.Pradelles P, Grassi J, Maclouf J. Enzyme immunoassays of eicosanoids using acetylcholine esterase as label: an alternative to radioimmunoassay. Analytical Chemistry 1985;57(7):1170–3 doi 10.1021/ac00284a003. [DOI] [PubMed] [Google Scholar]
- 28.Santilli F, Rocca B, De Cristofaro R, Lattanzio S, Pietrangelo L, Habib A, et al. Platelet cyclooxygenase inhibition by low-dose aspirin is not reflected consistently by platelet function assays: implications for aspirin “resistance”. J Am Coll Cardiol 2009;53(8):667–77 doi 10.1016/j.jacc.2008.10.047. [DOI] [PubMed] [Google Scholar]
- 29.Altobelli E, Angeletti PM, Latella G. Role of Urinary Biomarkers in the Diagnosis of Adenoma and Colorectal Cancer: A Systematic Review and Meta-Analysis. J Cancer 2016;7(14):1984–2004 doi 10.7150/jca.16244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.White MN, Shrubsole MJ, Cai Q, Su T, Hardee J, Coppola J-A, et al. Effects of fish oil supplementation on eicosanoid production in patients at higher risk for colorectal cancer. Eur J Cancer Prev 2019;28(3):188–95 doi 10.1097/CEJ.0000000000000455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Garland LL, Guillen-Rodriguez J, Hsu C-H, Yozwiak M, Zhang HH, Alberts DS, et al. Effect of Intermittent Versus Continuous Low-Dose Aspirin on Nasal Epithelium Gene Expression in Current Smokers: A Randomized, Double-Blinded Trial. Cancer prevention research (Philadelphia, Pa) 2019;12(11):809–20 doi 10.1158/1940-6207.CAPR-19-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cuzick J, Thorat MA, Bosetti C, Brown PH, Burn J, Cook NR, et al. Estimates of benefits and harms of prophylactic use of aspirin in the general population. Ann Oncol 2015;26(1):47–57 doi 10.1093/annonc/mdu225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rothwell PM, Wilson M, Elwin CE, Norrving B, Algra A, Warlow CP, et al. Long-term effect of aspirin on colorectal cancer incidence and mortality: 20-year follow-up of five randomised trials. Lancet 2010;376(9754):1741–50 doi 10.1016/s0140-6736(10)61543-7. [DOI] [PubMed] [Google Scholar]
- 34.Karahalios A, English DR, Simpson JA. Weight change and risk of colorectal cancer: a systematic review and meta-analysis. American journal of epidemiology 2015;181(11):832–45 doi 10.1093/aje/kwu357. [DOI] [PubMed] [Google Scholar]
- 35.Lauby-Secretan B, Scoccianti C, Loomis D, Grosse Y, Bianchini F, Straif K, et al. Body Fatness and Cancer--Viewpoint of the IARC Working Group. N Engl J Med 2016;375(8):794–8 doi 10.1056/NEJMsr1606602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Larsson SC, Orsini N, Wolk A. Diabetes mellitus and risk of colorectal cancer: a meta-analysis. Journal of the National Cancer Institute 2005;97(22):1679–87 doi 10.1093/jnci/dji375. [DOI] [PubMed] [Google Scholar]
- 37.Yuhara H, Steinmaus C, Cohen SE, Corley DA, Tei Y, Buffler PA. Is diabetes mellitus an independent risk factor for colon cancer and rectal cancer? Am J Gastroenterol 2011;106(11):1911–22 doi 10.1038/ajg.2011.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Deng L, Gui Z, Zhao L, Wang J, Shen L. Diabetes mellitus and the incidence of colorectal cancer: an updated systematic review and meta-analysis. Dig Dis Sci 2012;57(6):1576–85 doi 10.1007/s10620-012-2055-1. [DOI] [PubMed] [Google Scholar]
- 39.De Bruijn KMJ, Arends LR, Hansen BE, Leeflang S, Ruiter R, van Eijck CHJ. Systematic review and meta-analysis of the association between diabetes mellitus and incidence and mortality in breast and colorectal cancer. Br J Surg 2013;100(11):1421–9 doi 10.1002/bjs.9229. [DOI] [PubMed] [Google Scholar]
- 40.Fedirko V, Tramacere I, Bagnardi V, Rota M, Scotti L, Islami F, et al. Alcohol drinking and colorectal cancer risk: an overall and dose-response meta-analysis of published studies. Annals of oncology : official journal of the European Society for Medical Oncology 2011;22(9):1958–72 doi 10.1093/annonc/mdq653. [DOI] [PubMed] [Google Scholar]
- 41.Petrucci G, Zaccardi F, Giaretta A, Cavalca V, Capristo E, Cardillo C, et al. Obesity is associated with impaired responsiveness to once-daily low-dose aspirin and in vivo platelet activation. J Thromb Haemost 2019;17(6):885–95 doi 10.1111/jth.14445. [DOI] [PubMed] [Google Scholar]
- 42.Rothwell PM, Cook NR, Gaziano JM, Price JF, Belch JFF, Roncaglioni MC, et al. Effects of aspirin on risks of vascular events and cancer according to bodyweight and dose: analysis of individual patient data from randomised trials. Lancet 2018;392(10145):387–99 doi 10.1016/s0140-6736(18)31133-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yusuf S, Hawken S, Ounpuu S, Dans T, Avezum A, Lanas F, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet (London, England) 2004;364(9438):937–52 doi 10.1016/S0140-6736(04)17018-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.