Abstract
Chronotype has been mostly assessed with subjective scales. Objective assessment has been undertaken with actigraphy, although problems may occur in classifying chronotype. The aims of the study were to assess chronotype in school-age children using a novel integrative measurement (TAP) derived from non-invasive assessments of wrist temperature (T) physical activity (A) and body position (P) and to explore associations between chronotype, sleep disturbances, and metabolic components. Four-hundred-thirty-two children of 8–12 years were recruited from a Mediterranean area of Spain. Measurements were: (a) Chronotype objectively (7-day-rhythms of TAP) and subjectively measured (Munich-chronotype-self-reported questionnaire); (b) sleep rhythms and light exposition; (c) 7-day-diaries of food intake; (d) anthropometry and metabolic parameters; (e) academic scores. TAP acrophase was able to assess eveningness. As compared to more morning-types, more evening-types displayed lower amplitude in temperature rhythms, increased physical activity in the evening, delayed sleep and midpoint of intake and had more frequent social jet lag (P < 0.05). More evening-types had higher light intensity at 2 h before sleep and lower melatonin values (01:00 h). Eveningness associated with higher BMI and metabolic risk (higher values of insulin, glucose, triglycerides and cholesterol). Evening-types presented better grades in art. In conclusion, more evening-types, as objectively assessed, presented sleep alterations, social jet lag, obesity and higher metabolic risk.
Subject terms: Biomarkers, Risk factors
Introduction
Chronotype is a characteristic that helps to determine circadian typology1,2. Differences in the relationship between an individual’s circadian phase and external local time result in chronotypes that range from early to late3. Early chronotypes tend to perform better in the morning while late chronotypes perform better in the evening3.
In adults, evening chronotype is associated with health complications4, lower physical activity, short sleep duration and social jet lag5,6. Studies performed at earlier ages in different chronotypes are scarce and less is known about school aged children, particularly those that relate unhealthy behaviors and metabolic risk7.
Questionnaires are widely used to assess individual chronotype8. Nevertheless, the accuracy of such questionnaires depends on self-reporting, good recall, and the subject’s ability to complete them correctly and honestly9. Therefore, the use of objective tools capable to capture individual chronotype in a simple, continuous, and non-invasive form in free living conditions, is necessary, particularly in children at school ages.
Continuous monitoring of physical activity by actigraphy has been used as an objective assessment of chronotype in free living conditions, although studies have been mostly performed in pre-school children10 and adolescents11 but not in school-age children. Furthermore, actigraphy has problems in measuring midpoint of sleep because it tends to overestimate sleep and underestimate wake time12. Including wrist temperature may be of benefit because it is considered as a good sleep marker13. A recent consensus document sponsored by the National Heart Lung and Blood Institute, National Institute on Aging and the Sleep Research Society14, has stated that wrist temperature is a novel and less invasive method of measuring circadian phase timing and sleep and wake states.
TAP is an integrative variable that combines wrist temperature (T), physical activity (A) and body position (P) and has been shown to be a powerful method to assess individual chronotype, circadian system status and sleep characteristics in adults15. Compared with conventional actigraphy, TAP has been shown to be clinically superior in evaluating sleep objectively16. It improves sensitivity, specificity, and accuracy when compared with physical activity, body position or body temperature alone, and it minimizes masking effects such as those derived from environmental temperature or from device failures15. TAP has been validated in healthy and unhealthy subjects17 with dim light melatonin determinations (DLMO) and with polysomnography, in determining chronotype and circadian health18. It has also been shown that TAP, together with other non-invasive tools, is able to assess circadian health in children19. However, no studies exist evaluating the utility of TAP to determine chronotype in school-age children.
The purpose of this study is to assess TAP as a novel integrative measurement to determine chronotype and sleep patterns in school-age children and to study whether objectively assessed evening chronotypes show increased metabolic risk, social jet lag and sleep alterations as compared to morning-types.
Methods
Subjects
Four hundred thirty-two healthy children ages 8 to 12 years were recruited from three schools in a Mediterranean area of Spain between October 2014, and June 2016 (ClinicalTrials.gov ID: NCT02895282) as already described19. Approval for this study was obtained by the Ethics Committee of the University of Murcia. Written consent to participate was provided by the parents. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Recruitment procedure and methodology have been previously described19.
TAP derived chronotype and sleep variables (Table 1)
Table 1.
Sleep and circadian-related variables.
| Chronotype | Abreviations | Definition |
|---|---|---|
| Acrophase | Time period during which the daily cycle of TAP peaks | |
| Objective chronotype | Acrophase of TAP determined by Cosinor’s analysis | |
| Subjective chronoype | MCTQ | Individual chronotype assesed by Munich Chronotype Questionnaire |
| Sleep parameters | ||
| Central sleep timing | Timing of the average of the five consecutive hours of maximum values of sleep | |
| Circadian Function Index | CFI | A numerical index that determines the circadian robustness, based on three circadian parameters: Interday Stability (IS), Intraday Variability (IV) and Relative Amplitude (RA). CFI oscillates between 0 (absence of circadian rhythmicity) and 1 (robust circadian rhythm) |
| Day–night contrast | Difference between the average of measurements for the five consecutive hours with the maximum TAP and the average of measurements made for the 10 consecutive hours with the minimum TAP divided by the sum of both values | |
| Depth of sleep | Hourly average during the 5 consecutive hours of minimum values of TAP | |
| Duration of sleep | Difference between sleep bedtime and sleep awake time | |
| Interday stability | IS | Constancy of the 24 h rhythmic pattern over days. A stable rhythm is characterized by a 24 h profile that remains very similar from day to day |
| Intraday variability | IV | Fragmentation of the rhythm. Its values oscillate between 0 when the wave is perfectly sinusoidal and 2 when the wave describes a Gaussian noise |
| Regular habits | Derived from the Interday stability (IS): determines the constancy of the 24 h rhythmic pattern over the 7 days. A stable rhythm is characterized by a 24 h profile that remains very similar from day to day | |
| Relative amplitude | RA | Difference between the maximum (or minimum) value of the cosine function and mesor |
| Social jet lag | Difference in the midpoint of sleep between weekend (MSFsc) and weekdays (MSW); (Social jet lag = MSFsc—MSW). Subjects with more than 2 h of difference in the midpoint of sleep between weekend and weekdays were identified as having social jet lag21 | |
| Midpoint of food intake | Average of the seven days of the midpoint between breakfast and dinner times (first and last eating episode) | |
| TAP algorithm | TAP | The integrated TAP variable is calculated using the following procedure: we first normalized the TAP variables by calculating the 95th and 5th percentiles for each variable. Wrist temperature values were inverted since activity and position values were opposites, so that the maximum values for all 3 variables occurred at the same time of the day. Then we calculated the mean of the 3 normalized variables, where 0 corresponded to complete rest and sleep and 1 to periods of high arousal and movement |
Subjects wore a wristwatch during 7 days of study, on the non-dominant hand, that integrated a wrist temperature sensorcollecting information every 5 min, and an accelerometer sensor that measured physical activity and body position every 30 s as previously described19,20. From these measures the TAP algorithm was calculated15. Individual chronotype and sleep parameters were obtained from TAP as follows15.
Individual chronotype
Acrophase of TAP determined by Cosinor’s analysis was used as an objective biomarker of the individual chronotype (Table 1). More evening-types, neither-types and more morning-types were classified by the acrophase´s tertiles (higher values for the evening-types). An age appropriate Spanish version of the Munich Chronotype Questionnaire (MCTQ) was used to subjectively determine individual chronotype2. The MCTQ was designed to measure sleep times separately for work and free days and to estimate chronotype based on the time-based variable of the MCTQ. The midpoints of sleep were calculated for weekend (Free days) (MSF) and Weekdays (MSW). MSF was corrected as follows: MSFsc = MSF − 0.5 × (SDF − (5 × SDW + 2 × SDF)/7), where SDF was sleep duration on free days and SDW was sleep duration on work days21. Social jet lag was calculated as the difference between mid-sleep on free days and mid-sleep on work days as follows Social jet lag = MSFsc − MSW. Subjects with more than 2 h of difference in the midpoint of sleep between weekend and weekdays were identified as having social jet lag.
Sleep parameters
A 0 value of TAP indicated complete rest, whereas a 1 value corresponded to wakefulness and movement. An epoch was scored as sleep when TAP was under a default threshold, previously validated by polysomnography22. Time in movement, determined as the time in which a movement on any of three axes was detected, was used to discriminate between sleep and wake states.
From TAP the following sleep characteristics were determined by non-parametric analyses: sleep duration, circadian function index23, interdaily stability (IS), relative amplitude (RA), central sleep timing, depth of sleep, regular habits and day–night contrast (Table 1).
Light exposition
A luxmeter was programmed to collect light information continuously every 30 s. Subjects were instructed to wear the luxmeter on a lanyard over their clothing and to place it on a bedside table when asleep, as previously described24.
Daytime physical activity
Average physical activity in wakefulness was obtained from the 7-day activity record.
Food timing
A 7-day dietary record was completed that included food quantities and timing, and the midpoint of food intake.
BMI and waist circumference
BMI and waist circumference measurements were collected on the first day of the week of study as already described19.
Saliva and serum determinations
Melatonin was determined by radioimmunoassay (IBL, Germany) from two salivary samples one at night (01:00 h) and one before lunch (14:00 h). Glucose, insulin, cholesterol and triglycerides were determined from serum and saliva samples by conventional methods (Beckman Coulter Ireland Inc., Ireland).
Academic performance
Academic performance of a subpopulation was collected (n = 92). Grades for each subject were determined from overall performance on tests, as well as knowledge demonstrated during the academic year. Grades were assessed in Spanish language, mathematics, natural sciences, social sciences, English, French, artistic education, physical education and catholic religion and an average score was calculated.
All statistical analyses
All statistical analyses were performed using SPSS version 20.0 (SPSS, Chicago, Illinois, USA). Values of P < 0.05 were considered to be statistically significant. Differences between more morning-type, neither-type and more evening-type were analyzed by ANCOVA adjusted for gender, age, race, academic year, BMI and total energy intake (Table 2). In addition, Pearson correlation analyses were performed between (1) TAP acrophase and circadian characteristics (Table 2) and (2) TAP and metabolic parameters (Table 3). Linear regression was also used to test for associations between chronotype and metabolic parameters. Further adjustments for initial BMI and total energy intake were performed (Partial correlation analyses). Biomarkers in saliva and serum were log-transformed in base 10.
Table 2.
Differences between morning, neither and evening chronotypes in circadian-related variables and academic performance.
| Individual chronotype | P(1) | P(2) | P(3) | Correlation (4) | ||||
|---|---|---|---|---|---|---|---|---|
| Morning-type | Neither-type | Evening-type | r | P | ||||
| (n = 141) | (n = 141) | (n = 144) | ||||||
| Girls (%) | 48.6 | 44.7 | 58.3 | 0.059 | 0.037* | 0.021** | ||
| Characteristics | Mean ± SD | Mean ± SD | Mean ± SD | |||||
| Age (year) | 10 ± 1.18a | 10 ± 1.21a | 10 ± 1.34a | 0.623 | 0.871 | 0.979 | 0.068 | 0.164 |
| Chronotype markers | ||||||||
| Objective assessment | ||||||||
| TAP acrofase (hh:mm) | 14:26 ± 00:19a | 15:08 ± 00:10b | 15:54 ± 00:25c | < 0.001 | < 0.001 | < 0.001 | ||
| Melatonin at 01:00 h (pg/ml) | 29.88 ± 21.26a | 25.03 ± 13.97b | 24.79 ± 17.14b | 0.030 | 0.038 | 0.070 | − 0.124 | 0.013 |
| Subjective assessment | ||||||||
| MCTQ (hh:mm) | 3:50 ± 0:37a | 4:03 ± 0:36b | 4:12 ± 0:44c | < 0.001 | < 0.001 | < 0.001 | 0.225 | < 0.001 |
| Midpoint of food intake (hh:mm) | 14:56 ± 0:16a | 15:03 ± 0:20b | 15:11 ± 0:22c | < 0.001 | < 0.001 | < 0.001 | 0.319 | < 0.001 |
| Daytime activity (%) | 206.32 ± 28.87a | 206.73 ± 25.69a | 198.06 ± 28.17b | 0.015 | 0.008 | 0.074 | − 0.151 | 0.002 |
| Regular habits (%) | 91.49 ± 15.00a | 93.38 ± 15.41a | 85.85 ± 18.42b | < 0.001 | < 0.001 | 0.004 | 0.339 | < 0.001 |
| Light exposition | ||||||||
| Light acrophase (hh:mm) | 13:55 ± 0:22a | 14:20 ± 0:20b | 14:43 ± 0:24c | < 0.001 | < 0.001 | < 0.001 | 0.677 | < 0.001 |
| Light during the day (log lux) | 2.20 ± 0.45ab | 2.35 ± 0.21a | 2.12 ± 0.10b | 0.033 | 0.059 | 0.066 | − 0.163 | 0.072 |
| Light before bed time (log lux) | 0.29 ± 0.19a | 0.35 ± 0.19ab | 0.42 ± 0.19b | 0.022 | 0.030 | 0.048 | 0.285 | 0.002 |
| Sleep variables | ||||||||
| Duration | ||||||||
| Sleep duration (hh:mm) | 9:29 ± 0:38a | 9:20 ± 0:35ab | 9:11 ± 0:42b | 0.001 | 0.007 | 0.001 | − 0.169 | 0.001 |
| Short sleepers (n (%)) | 3(1) | 4(1) | 14(4) | 0.003 | 0.067 | 0.087 | ||
| Circadian Function Index (CFI) | 0.82 ± 0.08a | 0.84 ± 0.05b | 0.81 ± 0.10a | 0.007 | 0.006 | 0.011 | − 0.080 | 0.099 |
| Relative amplitude (RA) | 0.96 ± 0.11ab | 0.99 ± 0.03a | 0.94 ± 0.16b | 0.007 | 0.007 | 0.009 | − 0.144 | 0.003 |
| Interdaily stability (IS) | 0.67 ± 0.14a | 0.71 ± 0.12b | 0.66 ± 0.15a | 0.006 | 0.005 | 0.018 | − 0.061 | 0.212 |
| Sleep characteristics | ||||||||
| Central sleep timing (hh:mm) | 3:20 ± 1:14a | 3:30 ± 1:10a | 4:13 ± 1:07b | < 0.001 | < 0.001 | < 0.001 | 0.356 | < 0.001 |
| Depth of sleep (%) | 82.30 ± 12.94a | 80.33 ± 18.72ab | 76.76 ± 23.25b | 0.044 | 0.039 | 0.020 | − 0.128 | 0.008 |
| Day–night contrast (%) | 90.41 ± 13.83ab | 91.78 ± 12.35a | 88.02 ± 15.77b | 0.074 | 0.081 | 0.221 | − 0.150 | 0.002 |
| Social jet lag | ||||||||
| Social jet lag (hh:mm) | 1:12 ± 0:40a | 1:19 ± 0:38ab | 1:29 ± 0:45b | 0.003 | 0.039 | 0.010 | 0.167 | 0.001 |
| Social jet lag n (% of children) | 12 (3) | 14 (4) | 26 (7) | 0.001 | 0.002 | 0.001 | ||
| Academic performance | ||||||||
| Arts score | 5.96 ± 1.48a | 6. 69 ± 1.20b | 6.84 ± 1.08b | 0.024 | 0.050 | 0.293 | 0.264 | 0.011 |
| Average score | 7.71 ± 1.29a | 7.63 ± 1.34a | 7.70 ± 1.11a | 0.244 | 0.481 | 0.628 | 0.172 | 0.097 |
(1) Differences among chronotypes assessed by ANOVA; (2) Differences among chronotypes assessed by ANCOVA adjusted for sex, age, race, academic year and BMI. (3) Differences among chronotypes assessed using ANCOVA adjusted for sex, age, race, academic year, BMI, and total energy intake. (4) Pearson’s correlation between TAP acrophase and circadian-related variables. *Differences among chronotypes assessed using ANCOVA adjusted for age, race, academic year and BMI; ** Differences among chronotypes assessed using ANCOVA adjusted for age, race, academic year; BMI and total energy intake. Different letters indicate significant differences among chronotypes. MCTQ: Munich Chronotype Questionnaire. Social jet lag = MSF – MSW > 2 h.
Table 3.
Correlation between acrophase of TAP and metabolic parameters.
| n | r | P (1) | P(2) | Β (1) | SEM(1) | P (1) | Β (3) | SEM(3) | P(3) | |
|---|---|---|---|---|---|---|---|---|---|---|
| Serum cholesterol (mg/dl) | 73 | 0.311 | 0.007 | 0.008 | 0.043 | 0.016 | 0.007 | 0.040 | 0.016 | 0.016 |
| Serum triglycerides (mg/dl) | 73 | 0.313 | 0.008 | 0.001 | 0.074 | 0.027 | 0.008 | 0.090 | 0.025 | 0.001 |
| Saliva insulin (µUI/mL) | 125 | 0.242 | 0.007 | 0.001 | 0.195 | 0.071 | 0.007 | 0.179 | 0.077 | 0.021 |
| Saliva glucose (mg/dl) | 126 | 0.250 | 0.005 | 0.002 | 0.472 | 0.166 | 0.005 | 0.383 | 0.176 | 0.032 |
| BMI (kg/m2) | 424 | 0.099 | 0.041 | 0.578 | 0.282 | 0.041 | 0.368 | 0.178 | 0.041# | |
| Body fat of girls (%)* | 174 | 0.168 | 0.027 | 0.238 | 1.748 | 0.784 | 0.027 | − 0.434 | 0.308 | 0.161 |
(1) Pearson’s correlation test; (2) Adjusted by BMI. (3) Adjusted by BMI and total energy intake.
*Boys did not show significant differences. Biomarkers in saliva and serum were log-transformed in base 10.
#Adjusted by total energy intake.
Results
TAP as a marker of chronotype
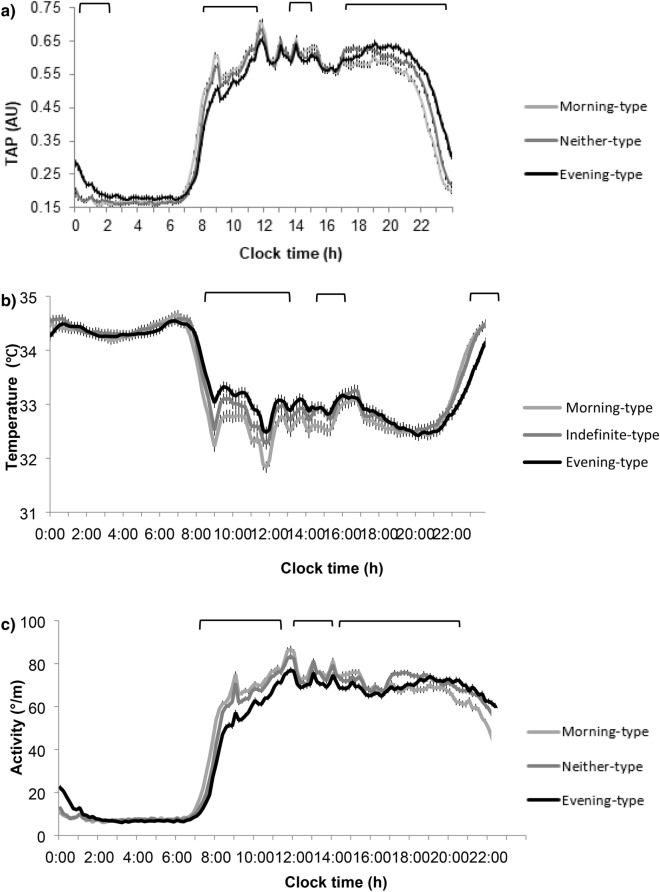
Seven-day rhythms of TAP (Fig. 1a) differed among the three objectively classified chronotypes (more morning, neither and more evening). As compared to more morning types, evening types showed a delayed pattern of TAP and lower values in the morning and higher in the evening (Fig. 1a). Similarly, subjective chronotype (derived from the Munich questionnaire), central sleep timing and midpoint of food intake, were also significantly delayed in more evening-types as compared to more morning-types (Table 2) and subjective and objective chronotypes correlated significantly with one another (r = 0.225; P < 0.001). As expected, saliva melatonin levels at 01:00 h were lower in more evening than in more morning-types (P < 0.05) (Table 2). Melatonin decreased by 3.43 (95% CI 5.963 to 0.902) pg/ml per hour of later chronotype (P = 0.008). These data suggest that TAP acrophase was correctly classifying the three independent chronotypes.
Figure 1.
Average daily patterns recorded over a seven-day period of (a) Integrative variable TAP (from peripheral temperature, activity and position) (n = 432) divided in chronotypes by tertiles, (b) temperature, (c) activity, (d) position, (e) sleep in the total population of (n = 432) and (f) light exposition in a subpopulation (n = 120) in morning, neither and evening chronotypes children. Differences among chronotypes was assessed by ANOVA. The upper brackets represents the hours at which the pattern differs significantly (P < 0.05).
More evening-types had higher values of body temperature in the morning (more sleepiness) and lower at night (more awakeness) than morning types (Fig. 1b). By contrast, evening-types had lower values of physical activity and body position during the first morning hours and higher values during the evening (P < 0.05) (Fig. 1c,d). In general, day-time physical activity was lower in evening-types as compared to neither-types and morning-types (P < 0.05) (Fig. 1c, Table 2).
Sleep characteristics
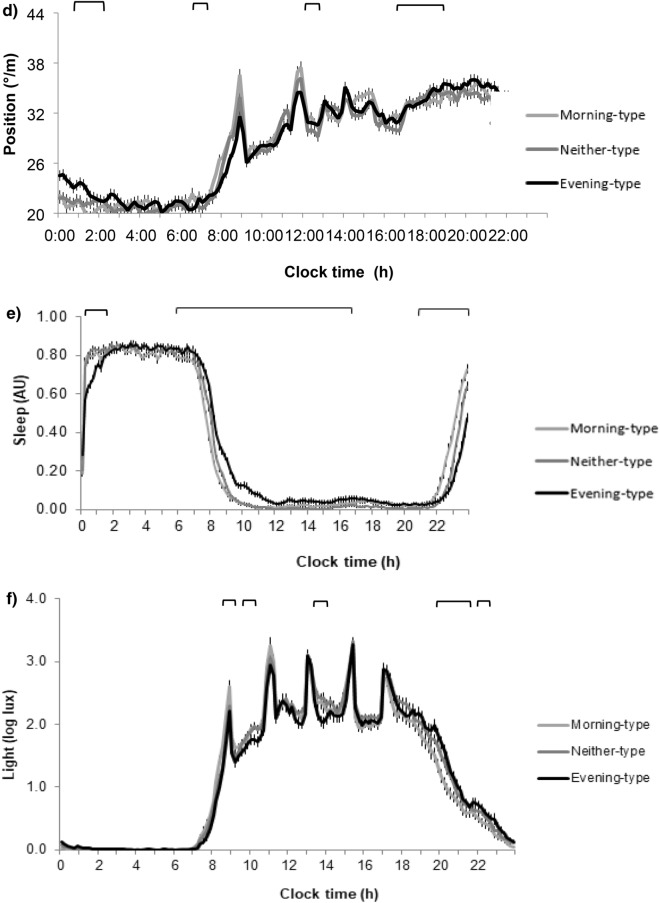
Habitual sleep duration was 09:19 ± 0:39 h. Six percent of subjects were short sleepers (duration less than 8 h) and 12% had social jet lag, (more than 2 h of difference between weekdays and weekends). Daily patterns of sleep of all subjects are presented in Fig. 1e. Delayed sleep occurred in evening-types, with higher levels of sleepiness during the day, mainly during the first hours, although sleepiness was still significantly higher until 16:00 h (P < 0.05). Evening-types had shorter sleep duration and the proportion of short sleepers was 4 times greater in evening-types than morning-types (Table 2). Evening-types had lower sleep circadian function index (P = 0.007) with decreased relative amplitude (P = 0.007) and lower interday stability (P = 0.006). Depth of sleep and day–night contrast was also decreased in evening types (Fig. 2), who showed less regular habits than the other chronotypes.
Figure 2.
Differences between morning-type, neither-type and evening-type in sleep characteristic and regular habits. Differences among chronotypes are indicated in the graphs with the Post-hoc-value of ANOVA. Different superscripts mean significant differences (P < 0.05).
Social jet lag
The differences between the weekday and weekend midpoint of sleep was 17 min higher in evening-types than in morning-types (Table 2). Furthermore, evening-types experienced social jet lag more frequently than morning-types, 7% and 3%, respectively (P = 0.001).
Light exposure
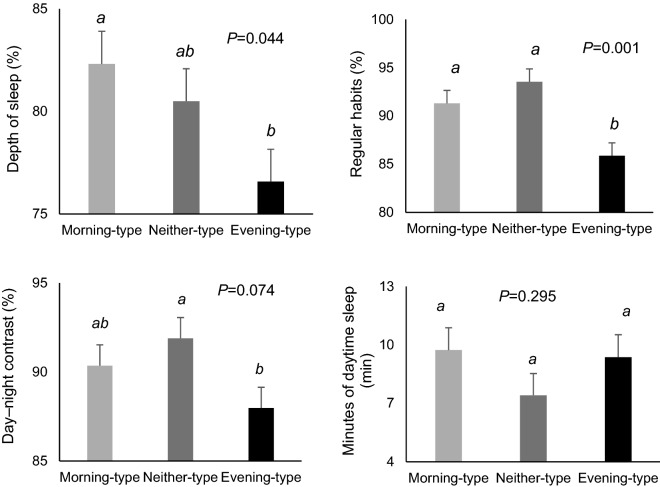
The light pattern was delayed approximately 1 h in evening-types (acrophase) (Table 2). Total light intensity was lower at daytime and higher at nighttime (Fig. 1f). Later light acrophase was associated with 0.85 (95% CI 0.41 to 1.29) hours later of TAP acrophase, and therefore with a later chronotype (P = 0.001). The light intensity, at 2 h before sleep timing, i.e. the timing in which melatonin starts to rise was 31% higher in more evening-types than in more morning-types (Fig. 3).
Figure 3.
Differences in light 2 h before bed time of morning, neither and evening chronotype children. Differences among chronotypes are indicated in the graphs with the Post-hoc-value of ANOVA. Different superscripts mean significant differences (P < 0.05).
Obesity and metabolic risk
Evening-type was associated with higher BMI and higher metabolic risk markers such as glucose, insulin, cholesterol and triglycerides levels (Table 3 and Fig. 4). A delay of 1 h in the chronotype was related with a 0.56 increase in BMI (P = 0.036). Associations with metabolic risk markers were still present after adjusting for BMI and habitual energy intake.
Figure 4.
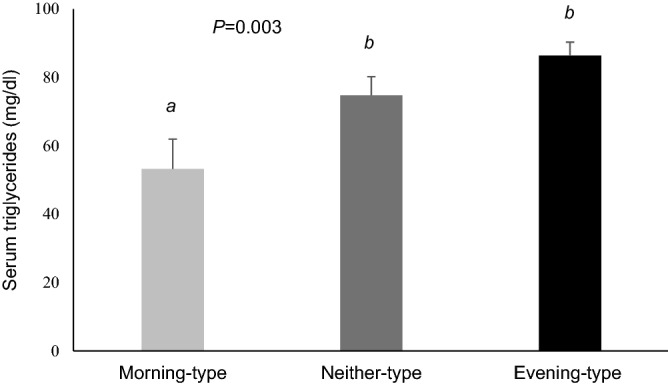
Differences in the triglycerides values of morning, neither and evening chronotype children. Differences among chronotypes are indicated in the graphs with the Post-hoc-value of ANOVA. Different superscripts mean significant differences (P < 0.05).
Academic performance
Evening types had significantly higher scores in art (P = 0.024) (Table 2), although there were no significant differences when adjusted for BMI and habitual energy intake.
Discussion
One aim of the present study was to assess TAP as a measure of chronotype in school age children. TAP simultaneously considers circadian endogenous wrist temperature and variables which are more dependent on willingness such as habitual physical activity and body position9,15. Because TAP is a non-invasive test used in free living conditions it is particularly suitable for this age group. Results from the study provide insight into evening chronotype and its association with sleep alterations, social jet lag, obesity and metabolic disturbances (higher values of basal insulin, glucose, triglycerides and cholesterol).
In the current population, subjective chronotype markers, such as central sleep timing and midpoint of intake, were delayed in more evening chronotypes as compared to more morning-types. Melatonin values at 01:00 h were significantly lower in evening-types than in morning-types, suggesting that melatonin may be still rising at those hours due to the later bedtime that characterizes evening-types.
In spite of being school-age children, with a marked schedule, we found differences in behaviors occurred mainly at night, when subjects were free to choose and endogenous trends appear. In general, more evening-types had increased physical activity in the evening while they had higher body temperature during the day which suggests an increase in sleepiness. This finding is consistent with previous studies that reported delayed physical activity patterns25 in evening-type children and presence of more episodes of daytime sleepiness26 in children with delayed behaviors. Considering that physical activity is an external synchronizer of the peripheral clocks27, these delayed behaviors may be per se affecting the circadian system function and may induce chronodisruption in these school-age children. Daytime sleepiness in youth has been associated with impairments in behavioral, mood, and performance domains28.
The duration of sleep plays an important role in school age children, given that sleep is relevant in maintaining good mental health and that short sleep has been associated with obesity29. As expected, sleep duration was decreased in evening-types. Previous studies performed in adolescents8 and preschool children10 have confirmed that late chronotypes have a decrease in sleep duration. Insufficient sleep has been associated with negative outcomes in several areas of health and functioning, including obesity, depression, school performance and risk-taking behavior30.
The “robustness” of daily sleep rhythm is determined by several parameters such as the relative amplitude, Circadian Function Index (CFI)23, interday stability and intraday variability. CFI provides information about the circadian system and facilitates objective evaluation of chronodisruption15. Lower CFI indicates less regular day-to-day rhythms, as demonstrated by a decrease in interday stability and in amplitude. In the current study, CFI of sleep was significantly lower among evening-types than morning-types, suggesting that evening-types have worse circadian function of sleep. Furthermore, evening-types had less depth of sleep, lower day–night contrast and more irregular habits.
In accordance with previous studies in adults31 and pre-school children32 findings of the present study show that evening-types experienced social jet lag more frequently than morning-types (7% and 3%, respectively). Social jet lag is a term describing misalignment between social and biological time33. Among evening chronotypes, schedules at school may interfere with individual sleep preferences and derive in chronodisruption34. During weekends, evening chronotypes are free to follow their biology and go to bed later and get up later in the morning. Social jet lag not only disrupts the amount of sleep, it also affects sleep quality, and irregular sleep is associated with poorer academic performance35. Social jet lag also affects circadian clocks and consequently the timing of hormones secretion, the activity of immune cells, and body temperature, and changes in mood at different times of day and night36 and has been shown to be a risk factor for psychological disorders37 and obesity38.
Light is the most important external synchronizer of the internal clock39. The timing of light exposure has a differential effect upon circadian phase. Early light exposure advances the cycle whereas late light delays circadian phase40. Results of the 7-day light pattern in the present study determined that evening-types presented a delayed light acrophase and lower values of light during the day. Later light acrophase was associated with approximately 1 h delay in the chronotype. Furthermore, light exposure during the last 2 h before bedtime (i.e., the timing in which melatonin starts to rise), it was 31% higher among evening-types than morning-types attaining values of 50 lx. Although there is considerable variation in individual response to light, it has been shown that light intensities of 30 lx are sufficient to suppress 50% of melatonin secretion41 and may produce a phase advance of more than three hours in the circadian pacemaker40,42. In the present study, when compared with morning types, evening types were exposed to light for shorter durations in the morning between wake time and school, which may also contribute to a more evening chronotype43. Similar findings have been reported among children in early years44.
Many studies relate eveningness to health problems4, 45,46. In adults, later chronotype is associated with greater morbidity, including higher rates of metabolic dysfunction and cardiovascular disease4 resulting in increased prevalence of metabolic syndrome, insulin resistance and sleep disturbances5. In the current study, central sleep timing was delayed approximately 1 h in evening-types. Previously, it has been reported that in children, each 1 h delay in chronotype is associated with more headaches, stomach and back aches, dizziness and worse self-rated health45. A 1 h delay in chronotype is also related to higher screen time and poor dietary habits5. Findings of the present study report that evening chronotypes have significantly higher values of basal insulin, glucose, triglycerides and cholesterol. In addition, and in agreement with previous studies performed in adolescents7 in the current population of school children, evening-types had a significantly higher BMI, which may be explained by several obesogenic behaviors, including insufficient sleep, less physical activity during the day and late eating47.
In previous studies, morningness has been positively related to intelligence, conscientiousness and learning objectives48. Early midpoint of sleep was associated with better grades48. Whereas late chronotypes are more idealistic, imaginative and intuitive49. These findings are in accordance with our results that evening-types had better grades in art, while no significant differences were found in other academic scores.
The authors present the following as limitations: (1) In the current study we detected metabolites in saliva and serum. The detection of metabolites in serum requires invasive techniques to extract a sample. Future studies might consider only detection of metabolites in saliva to avoid anxiety and stress to children because of blood extraction. (2) As an observational study conclusions of causality are limited.
Findings of the present study are a significant step in understanding chronotype and its relationship with chronodisruption and metabolic risk in children. The results show that in objectively assessed school age subjects, evening-types presented sleep alterations, social jet lag, more obesity, higher metabolic risk and better grades in art. Objective and non-invasive assessment of the individual chronotype, daily rhythms of sleep and circadian health should be included as part of a comprehensive approach to the pediatric patient. For children at risk, it is advisable to implement interventions to reduce eveningness, improve sleep and decrease social jet lag in order to decrease metabolic risk50.
Acknowledgements
We thank all children and their families and the three schools Maristas, San Antonio and San Pablo CeI for their help and support, specially to Mari Carmen Blaya, Jose Ignacio Peña and María José Martínez.
Abbreviations
- TAP
Temperature (T), activity (A) and position (P)
- IS
Interday stability
- RA
Relative amplitude
- CFI
Circadian function index
- ONTIME-Jr
Obesity, nutrigenetics, timing and mediterranean, junior
- MCTQ
Munich chronotype questionnaire
- BMI
Body mass index
Author contributions
M.G. conceptualized and designed the study, drafted the initial manuscript and critically reviewed the manuscript. J.A.M. drafted the initial manuscript and carried out analysis and interpretation of data. N.M.-L. performed the data and samples collection and drafted the initial manuscript. G.B., R.R. and M.J.R., collected data and contributed to draft the initial manuscript. A.T. contributed to the acquisition of data and drafted the initial manuscript. P.F. carried out the initial analyses and critically reviewed the manuscript for important intellectual content and made corrections. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Funding
The authors have no financial relationships relevant to this article to disclose.
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding authors on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
3/3/2021
A Correction to this paper has been published: 10.1038/s41598-021-84775-9
References
- 1.Baehr EK, Revelle W, Eastman CI. Individual differences in the phase and amplitude of the human circadian temperature rhythm: with an emphasis on morningness-eveningness. J. Sleep Res. 2000;9(2):117–127. doi: 10.1046/j.1365-2869.2000.00196.x. [DOI] [PubMed] [Google Scholar]
- 2.Roenneberg T, Kuehnle T, Juda M, et al. Epidemiology of the human circadian clock. Sleep Med. Rev. 2007;11(6):429–438. doi: 10.1016/j.smrv.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 3.Lara T, Madrid JA, Correa A. The vigilance decrement in executive function is attenuated when individual chronotypes perform at their optimal time of day. PLoS ONE. 2014;9(2):e88820. doi: 10.1371/journal.pone.0088820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Knutson KL, von Schantz M. Associations between chronotype, morbidity and mortality in the UK Biobank cohort. Chronobiol. Int. 2018;35(8):1045–1053. doi: 10.1080/07420528.2018.1454458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vera B, Dashti HS, Gomez-Abellan P, et al. Modifiable lifestyle behaviors, but not a genetic risk score, associate with metabolic syndrome in evening chronotypes. Sci. Rep. 2018;8(1):945. doi: 10.1038/s41598-017-18268-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koren D, Dumin M, Gozal D. Role of sleep quality in the metabolic syndrome. Diabetes Metab. Syndr. Obes. 2016;9:281–310. doi: 10.2147/DMSO.S95120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cespedes Feliciano, E. M. et al. Chronotype, social jet lag, and cardiometabolic risk factors in early adolescence. JAMA Pediatr. 2019. [DOI] [PMC free article] [PubMed]
- 8.Kolomeichuk SN, Teplova LI. Sleep quality and its parameters in schoolchildren. Zh Nevrol Psikhiatr Im S S Korsakova. 2017;117:92–96. doi: 10.17116/jnevro201711711292-96. [DOI] [PubMed] [Google Scholar]
- 9.Martinez-Nicolas A, Martinez-Madrid MJ, Almaida-Pagan PF, Bonmati-Carrion MA, Madrid JA, Rol MA. Assessing chronotypes by ambulatory circadian monitoring. Front. Physiol. 2019;10:1396. doi: 10.3389/fphys.2019.01396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jafar NK, Tham EK, Eng DZ, et al. The association between chronotype and sleep problems in preschool children. Sleep Med. 2017;30:240–244. doi: 10.1016/j.sleep.2016.11.015. [DOI] [PubMed] [Google Scholar]
- 11.Merikanto I, Pesonen AK, Kuula L, et al. Eveningness as a risk for behavioral problems in late adolescence. Chronobiol Int. 2017;34(2):225–234. doi: 10.1080/07420528.2016.1267739. [DOI] [PubMed] [Google Scholar]
- 12.Ancoli-Israel S, Cole R, Alessi C, Chambers M, Moorcroft W, Pollak CP. The role of actigraphy in the study of sleep and circadian rhythms. Sleep. 2003;26(3):342–392. doi: 10.1093/sleep/26.3.342. [DOI] [PubMed] [Google Scholar]
- 13.Krauchi K, Cajochen C, Werth E, Wirz-Justice A. Functional link between distal vasodilation and sleep-onset latency? Am. J. Physiol. Regul. Integr. Comp. Physiol. 2000;278(3):R741–748. doi: 10.1152/ajpregu.2000.278.3.R741. [DOI] [PubMed] [Google Scholar]
- 14.Mullington JM, Abbott SM, Carroll JE, et al. Developing biomarker arrays predicting sleep and circadian-coupled risks to health. Sleep. 2016;39(4):727–736. doi: 10.5665/sleep.5616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ortiz-Tudela E, Martinez-Nicolas A, Campos M, Rol MA, Madrid JA. A new integrated variable based on thermometry, actimetry and body position (TAP) to evaluate circadian system status in humans. PLoS Comput. Biol. 2010;6(11):e1000996. doi: 10.1371/journal.pcbi.1000996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Madrid-Navarro CJ, Escamilla-Sevilla F, Minguez-Castellanos A, et al. Multidimensional circadian monitoring by wearable biosensors in Parkinson's disease. Front. Neurol. 2018;9:157. doi: 10.3389/fneur.2018.00157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Madrid-Navarro CJ, Puertas Cuesta FJ, Escamilla-Sevilla F, et al. Validation of a device for the ambulatory monitoring of sleep patterns: A pilot study on Parkinson's disease. Front. Neurol. 2019;10:356. doi: 10.3389/fneur.2019.00356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lopez-Minguez J, Colodro-Conde L, Bandin C, Ordonana JR, Garaulet M, Madrid JA. Application of multiparametric procedures for assessing the heritability of circadian health. Chronobiol. Int. 2016;33(2):234–244. doi: 10.3109/07420528.2015.1130051. [DOI] [PubMed] [Google Scholar]
- 19.Barraco GM, Martinez-Lozano N, Vales-Villamarin C, et al. Circadian health differs between boys and girls as assessed by non-invasive tools in school-aged children. Clin. Nutr. 2019;38(2):774–781. doi: 10.1016/j.clnu.2018.03.001. [DOI] [PubMed] [Google Scholar]
- 20.Sarabia JA, Rol MA, Mendiola P, Madrid JA. Circadian rhythm of wrist temperature in normal-living subjects A candidate of new index of the circadian system. Physiol. Behav. 2008;95(4):570–580. doi: 10.1016/j.physbeh.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 21.Roenneberg T, Pilz LK, Zerbini G, Winnebeck EC. Chronotype and social jetlag: a (self-) critical review. Biology. 2019;8(3):54. doi: 10.3390/biology8030054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ortiz-Tudela E, Martinez-Nicolas A, Albares J, et al. Ambulatory circadian monitoring (ACM) based on thermometry, motor activity and body position (TAP): a comparison with polysomnography. Physiol. Behav. 2014;126:30–38. doi: 10.1016/j.physbeh.2013.12.009. [DOI] [PubMed] [Google Scholar]
- 23.Ullah S, Arsalani-Zadeh R, MacFie J. Accuracy of prediction equations for calculating resting energy expenditure in morbidly obese patients. Ann. R. Coll. Surg. Engl. 2012;94(2):129–132. doi: 10.1308/003588412X13171221501988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martinez-Nicolas A, Ortiz-Tudela E, Madrid JA, Rol MA. Crosstalk between environmental light and internal time in humans. Chronobiol. Int. 2011;28(7):617–629. doi: 10.3109/07420528.2011.593278. [DOI] [PubMed] [Google Scholar]
- 25.Gallant AR, Mathieu ME, Lundgren JD, et al. Daily physical activity patterns of children with delayed eating behaviors. J. Biol. Rhythms. 2013;28(5):332–338. doi: 10.1177/0748730413499857. [DOI] [PubMed] [Google Scholar]
- 26.Gaina A, Sekine M, Kanayama H, et al. Morning-evening preference: sleep pattern spectrum and lifestyle habits among Japanese junior high school pupils. Chronobiol. Int. 2006;23(3):607–621. doi: 10.1080/07420520600650646. [DOI] [PubMed] [Google Scholar]
- 27.Rubio-Sastre P, Gomez-Abellan P, Martinez-Nicolas A, Ordovas JM, Madrid JA, Garaulet M. Evening physical activity alters wrist temperature circadian rhythmicity. Chronobiol. Int. 2014;31(2):276–282. doi: 10.3109/07420528.2013.833215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fallone G, Owens JA, Deane J. Sleepiness in children and adolescents: clinical implications. Sleep Med. Rev. 2002;6(4):287–306. doi: 10.1053/smrv.2001.0192. [DOI] [PubMed] [Google Scholar]
- 29.Garaulet M, Martinez-Nicolas A, Ruiz JR, et al. Fragmentation of daily rhythms associates with obesity and cardiorespiratory fitness in adolescents: the HELENA study. Clin. Nutr. 2017;36(6):1558–1566. doi: 10.1016/j.clnu.2016.09.026. [DOI] [PubMed] [Google Scholar]
- 30.Shochat T, Cohen-Zion M, Tzischinsky O. Functional consequences of inadequate sleep in adolescents: a systematic review. Sleep Med. Rev. 2014;18(1):75–87. doi: 10.1016/j.smrv.2013.03.005. [DOI] [PubMed] [Google Scholar]
- 31.Zhang Z, Cajochen C, Khatami R. Social jetlag and chronotypes in the chinese population: analysis of data recorded by wearable devices. J. Med. Internet. Res. 2019;21(6):e13482. doi: 10.2196/13482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Doip Y, Ishihara K, Uchiyama M. Associations of chronotype with social jetlag and behavioral problems in preschool children. Chronobiol. Int. 2015;32(8):1101–1108. doi: 10.3109/07420528.2015.1063503. [DOI] [PubMed] [Google Scholar]
- 33.Jankowski KS. Social jet lag: Sleep-corrected formula. Chronobiol. Int. 2017;34(4):531–535. doi: 10.1080/07420528.2017.1299162. [DOI] [PubMed] [Google Scholar]
- 34.Wittmann M, Dinich J, Merrow M, Roenneberg T. Social jetlag: misalignment of biological and social time. Chronobiol. Int. 2006;23(1–2):497–509. doi: 10.1080/07420520500545979. [DOI] [PubMed] [Google Scholar]
- 35.Phillips AJK, Clerx WM, O'Brien CS, et al. Irregular sleep/wake patterns are associated with poorer academic performance and delayed circadian and sleep/wake timing. Sci. Rep. 2017;7(1):3216. doi: 10.1038/s41598-017-03171-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rudiger HW. Health problems due to night shift work and jetlag. Internist. 2004;45(9):1021–1025. doi: 10.1007/s00108-004-1257-9. [DOI] [PubMed] [Google Scholar]
- 37.Levandovski R, Dantas G, Fernandes LC, et al. Depression scores associate with chronotype and social jetlag in a rural population. Chronobiol. Int. 2011;28(9):771–778. doi: 10.3109/07420528.2011.602445. [DOI] [PubMed] [Google Scholar]
- 38.Roenneberg T, Allebrandt KV, Merrow M, Vetter C. Social jetlag and obesity. Curr. Biol. 2012;22(10):939–943. doi: 10.1016/j.cub.2012.03.038. [DOI] [PubMed] [Google Scholar]
- 39.Husse J, Eichele G, Oster H. Synchronization of the mammalian circadian timing system: light can control peripheral clocks independently of the SCN clock: alternate routes of entrainment optimize the alignment of the body's circadian clock network with external time. BioEssays. 2015;37(10):1119–1128. doi: 10.1002/bies.201500026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Boivin DB, Duffy JF, Kronauer RE, Czeisler CA. Sensitivity of the human circadian pacemaker to moderately bright light. J. Biol. Rhythms. 1994;9(3–4):315–331. doi: 10.1177/074873049400900311. [DOI] [PubMed] [Google Scholar]
- 41.Nagare R, Rea MS, Plitnick B, Figueiro MG. Effect of white light devoid of "cyan" spectrum radiation on nighttime melatonin suppression over a 1-h exposure duration. J. Biol. Rhythms. 2019;34(2):195–204. doi: 10.1177/0748730419830013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Akacem LD, Wright KP, Jr, LeBourgeois MK. Sensitivity of the circadian system to evening bright light in preschool-age children. Physiol. Rep. 2018;6(5):e13615. doi: 10.14814/phy2.13617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Harada T, Morisane H, Takeuchi H. Effect of daytime light conditions on sleep habits and morningness-eveningness preference of Japanese students aged 12–15 years. Psychiatry Clin. Neurosci. 2002;56(3):225–226. doi: 10.1046/j.1440-1819.2002.00983.x. [DOI] [PubMed] [Google Scholar]
- 44.Simpkin CT, Jenni OG, Carskadon MA, et al. Chronotype is associated with the timing of the circadian clock and sleep in toddlers. J. Sleep Res. 2014;23(4):397–405. doi: 10.1111/jsr.12142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gariepy G, Dore I, Whitehead R, Elgar FJ. More than just sleeping in: a late timing of sleep is associated with health problems and unhealthy behaviours in adolescents. Sleep Med. 2019;56:66–72. doi: 10.1016/j.sleep.2018.10.029. [DOI] [PubMed] [Google Scholar]
- 46.Maukonen M, Kanerva N, Partonen T, Mannisto S. Chronotype and energy intake timing in relation to changes in anthropometrics: a 7-year follow-up study in adults. Chronobiol. Int. 2019;36(1):27–41. doi: 10.1080/07420528.2018.1515772. [DOI] [PubMed] [Google Scholar]
- 47.Bandin C, Scheer FA, Luque AJ, et al. Meal timing affects glucose tolerance, substrate oxidation and circadian-related variables: a randomized, crossover trial. Int. J. Obes. 2015;39(5):828–833. doi: 10.1038/ijo.2014.182. [DOI] [PubMed] [Google Scholar]
- 48.Arbabi T, Vollmer C, Dorfler T, Randler C. The influence of chronotype and intelligence on academic achievement in primary school is mediated by conscientiousness, midpoint of sleep and motivation. Chronobiol. Int. 2015;32(3):349–357. doi: 10.3109/07420528.2014.980508. [DOI] [PubMed] [Google Scholar]
- 49.Simor P, Polner B. Differential influence of asynchrony in early and late chronotypes on convergent thinking. Chronobiol. Int. 2017;34(1):118–128. doi: 10.1080/07420528.2016.1246454. [DOI] [PubMed] [Google Scholar]
- 50.Harvey AG, Hein K, Dolsen MR, et al. Modifying the impact of eveningness chronotype ("night-owls") in youth: a randomized controlled trial. J. Am. Acad. Child Adolesc. Psychiatry. 2018;57(10):742–754. doi: 10.1016/j.jaac.2018.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analyzed during the current study are available from the corresponding authors on reasonable request.