Abstract
Background:
Preconception lifestyle and health play a pivotal role in positively impacting the health of a pregnancy, and this includes the reduction of exposure to endocrine-disrupting chemicals such as phthalates. We have previously demonstrated that women planning a pregnancy with assisted reproductive technology (ART) have lower phthalate metabolite concentrations than their non-ART-using counterparts.
Objective:
To determine if women who intended to become pregnant had lower phthalate metabolite concentrations than those who had an unintended pregnancy, or if change in phthalate exposure across pregnancy differed between these two groups.
Methods:
721 women enrolled in The Infant Development and Environment Study (TIDES), a multicenter U.S. prospective pregnancy cohort; 513 (71%) indicated their pregnancy was planned. Urine samples from first and third trimester visits were analyzed for 10 specific-gravity-adjusted, natural-log-transformed phthalate metabolites. Simple and multivariable linear regression, adjusting for center, race, age, income, marital status, and parity, were employed to determine whether phthalate metabolite concentrations differed by pregnancy intention.
Results:
In bivariate analyses, the geometric mean concentrations of all first trimester and most third trimester phthalates were higher in women with unplanned pregnancies. However, after covariate adjustment, only first trimester monoisobutyl phthalate (MiBP) remained associated with pregnancy intention, and the association changed direction such that unplanned pregnancies had lower MiBP concentrations (ß −0.18, 95% CI −0.35, −0.02).
Conclusions:
We did not find evidence of a difference in phthalate exposure between pregnancy planners and non-planners.
Keywords: Pregnancy, Phthalic Acids, Pregnancy, Unplanned, Endocrine Disruptors, Preconception Care
Social media quote
While unadjusted results indicated that unintended pregnancies have higher levels of exposure to certain prenatal phthalates, accounting for covariates nullified associations. Therefore, this study ultimately found no evidence that pregnancy intention was independently associated with prenatal phthalate exposure.
(with Figure 2)
Figure 2 –
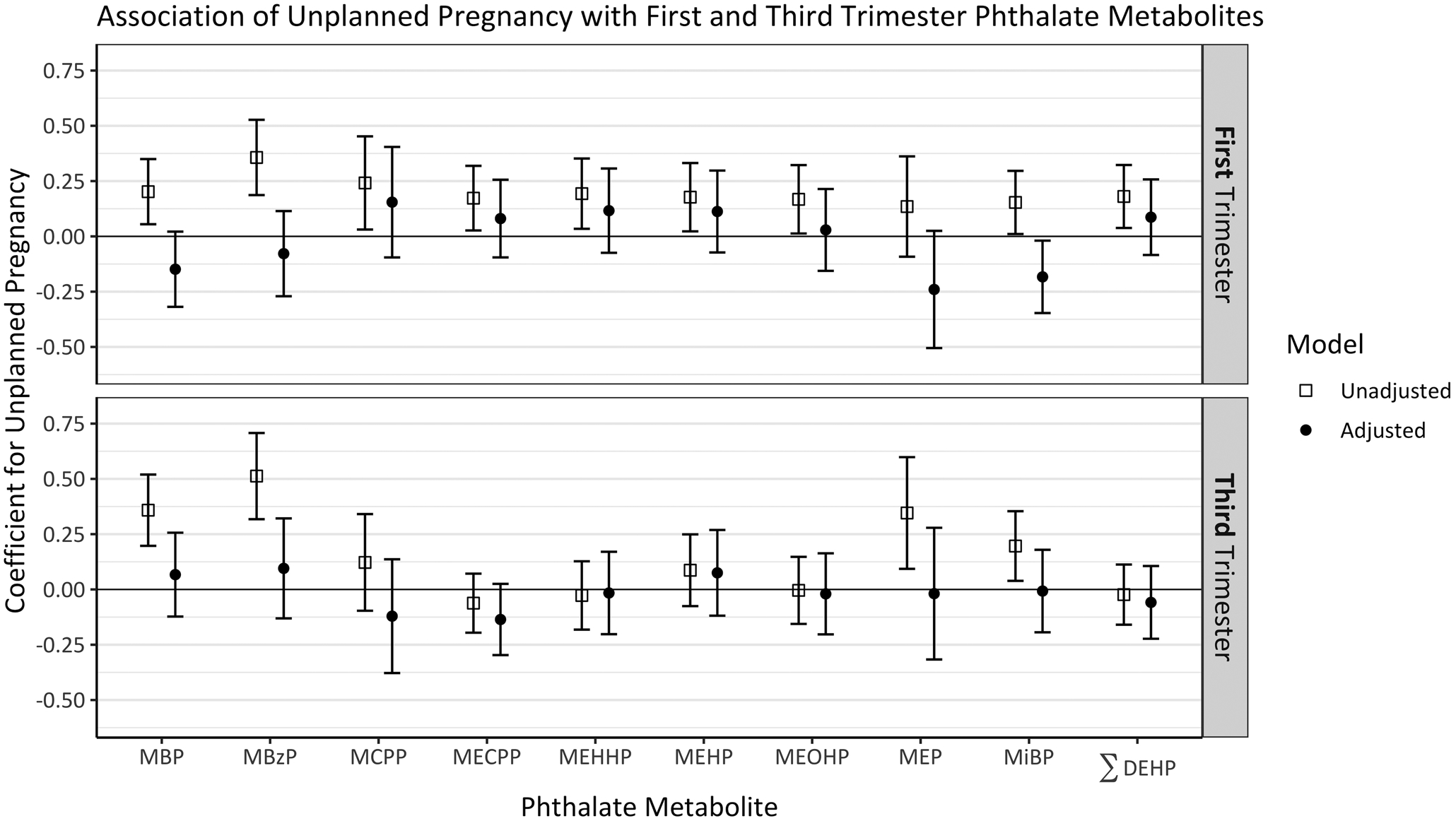
Coefficients and 95% confidence intervals for an indicator of unplanned pregnancy in simple (open square) and multivariable (solid circle) linear regressions, with separate models for each ln-transformed metabolite. Adjusted models included center, black race, age, income, marital status, and parity.
BACKGROUND
Pregnancy planning is a known predictor of the degree to which women alter their lifestyles before conception and during pregnancy. In the United States, where about half of all pregnancies are unplanned1, women with planned pregnancies are less likely to use illicit drugs, smoke cigarettes, and drink alcohol in early pregnancy, and more likely to engage in healthful behaviors such as taking folic acid compared to women with unplanned pregnancies2–5. The reasons for this are unknown; women with unintended pregnancies learn they are pregnant later than those with intended pregnancies, but also women with unintended pregnancies are less likely to change behaviors once they know they are pregnant2,6. Few studies have explored whether environmental chemical exposures differ by pregnancy intention, and none have considered the relationship between pregnancy intention and prenatal exposure to phthalates7.
Phthalates are ubiquitous plastic-softening chemicals that are commonly found in food and beverage containers, hygiene and beauty products, cosmetics, and plastic packaging. Exposure to phthalates can occur through ingestion, inhalation, or skin absorption, after which phthalates are metabolized and leave the body primarily through urine8,9. Phthalates are known to be endocrine disruptors in animals, acting as anti-androgens10; exposure to phthalate esters in utero causes male reproductive abnormalities in rat offspring11. Phthalates are also capable of inducing oxidative stress in the testes12,13, another potential mechanism for the causal relationship in rats. Phthalates can act as anti-androgens in humans, as well. Prenatal phthalate exposure has been linked to preterm birth and pregnancy complications, as well as adverse reproductive, neurodevelopmental, metabolic, and respiratory outcomes14–22.
While prenatal environmental exposures have been studied, the topic of how these exposures differ by pregnancy intention has been largely neglected. One retrospective survey found that women with unintended pregnancies had greater odds of being exposed to secondhand cigarette smoke at home, both before and after pregnancy confirmation2. This study also found that among women with no previous live births, those with unintended pregnancies were more likely to continue or initiate hot tub or sauna use during pregnancy2. In The Infant Development and Environment Study (TIDES), a large multicenter U.S. pregnancy cohort, we previously observed that women with a history of infertility who used assisted reproductive technology (ART) to conceive had lower first trimester concentrations of all four metabolites of diethylhexyl phthalate (DEHP) and the molar sum DEHP compared to their non-ART-using counterparts (adjusted geometric mean ratio for ΣDEHP: 0.83, 95% CI 0.71, 0.98). Perhaps women undergoing ART, who are by definition pregnancy planners, might reduce use of plastics in an effort to increase their chances of pregnancy16.
The current study’s primary aim was to determine whether women with planned pregnancies have lower measured concentrations of phthalate metabolites in the first or third trimester, which might suggest greater awareness of the risks associated with plastic exposure and an intentional avoidance of phthalate-containing products and foods. We hypothesized that this difference would be more marked during the first trimester, when the gap in knowledge about perinatal environmental health is likely to be greatest. Therefore, we also examined how phthalate metabolite concentrations changed over the course of a pregnancy. The extent to which pregnancy planners demonstrate greater awareness of environmental risks, adjusting for sociodemographic factors, was also examined.
METHODS
TIDES Overview
The Infant Development and Environment Study (TIDES) is a prospective pregnancy cohort study with a focus on prenatal phthalate exposure and child health outcomes. Participants were recruited between 2010 and 2012 at four university medical centers: University of California, San Francisco, University of Rochester Medical Center, University of Minnesota, and University of Washington/Seattle Children’s Hospital. Eligibility criteria included: age 18 years or older, able to read and write in English (or Spanish, in California), less than 13 weeks pregnant, pregnancy not medically threatened, and planning to deliver in a study hospital. The institutional review board (IRB) of each participating institution (including the coordinating center at Icahn School of Medicine at Mount Sinai) approved study protocols before implementation. All participants provided written informed consent.
In the first and third trimester, at routine clinical visits, participants gave urine samples for measurement of phthalate metabolite concentrations and completed questionnaires on medical and reproductive history, behaviors, and potential exposures to plastics. In the first trimester, these questions included the statements, “Chemicals in the environment can pose health risks” and “Chemicals in the environment are in so many things that it’s impossible to avoid them,” to which participants could indicate a degree of agreement on a five-point Likert scale. Other questions addressed frequency of behaviors, including purchasing eco-friendly or chemical-free products in the realms of personal care, home, and food and how often participants check recycling codes, with a response scale from “never” to “always.” These questions were developed de novo, because we knew of no validated questionnaire on environmental health attitudes and behaviors at the time23.
Pregnancy intention status of the index pregnancy was determined by a yes/no question on the first trimester survey: “Was this pregnancy planned?” A “no” response defined an unplanned, or unintended, pregnancy. In a separate question, participants were asked whether they were “definitely trying to get pregnant,” “willing to have a child whenever you got pregnant,” “did not wish to get pregnant,” or “don’t know.” Three respondents said they were definitely trying but also that the pregnancy was unplanned; they were excluded from analyses. We then used this question to partition unplanned pregnancies into two categories: indifferent (“willing to have a child whenever”) and unwanted (“did not wish to get pregnant” or “don’t know”). Further details about the TIDES cohort and survey design are published elsewhere15,23. TIDES participants who had a first or third trimester urine sample and had a live birth in the study were eligible for inclusion in the current analysis.
Urine sample analysis
Urine samples were collected in the first and third trimester in phthalate-free polypropylene cups. Specific gravity was measured within 30 minutes of collection, using a hand-held refractometer that had been calibrated with deionized water. Samples were then stored at −80°C in phthalate-free cryovials, before being shipped to laboratories at the Centers for Disease Control and Prevention (CDC) and the University of Washington (UW) for analysis. The CDC lab measured first trimester phthalate metabolites in mothers carrying male fetuses, some of the first trimester phthalate metabolites in mothers carrying female fetuses, and third trimester phthalates in all TIDES participants, whereas the UW lab measured only first trimester phthalate metabolites in mothers carrying female fetuses. The CDC lab method entailed enzymatic deconjugation of the metabolites from their glucuronidated form, automated online solid-phase extraction, separation with high performance liquid chromatography, and detection by isotope-dilution tandem mass spectrometry24. At the UW lab, glucuronidated phthalate monoesters also underwent enzymatic deconjugation, followed by online solid-phase extraction (SPE) and reversed high-performance liquid chromatography-electrospray ionization-tandem mass spectrometry (HPLC-ESI-MS/MS)25. Additional details on lab analyses for TIDES are published elsewhere15.
This analysis considered concentrations of 10 phthalate metabolites: monobutyl phthalate (MBP), monobenzyl phthalate (MBzP), mono-(3-carboxypropyl) phthalate (MCPP), mono-(2-ethyl-5-carboxypentyl) phthalate (MECPP), mono-(2-ethyl-5-hydroxyhexyl) phthalate (MEHHP), mono-(2-ethylhexyl) phthalate (MEHP), mono-(2-ethyl-5-oxohexyl) phthalate (MEOHP), monoethyl phthalate (MEP), monoisobutyl phthalate (MiBP), and the molar sum ΣDEHP.
Statistical methods
Phthalate metabolite concentrations from both timepoints were adjusted for specific gravity using the formula , where pc is the corrected urinary phthalate metabolite concentration (ng/mL), p is the observed concentration of the individual urine sample (ng/mL), SpGmed is the median specific gravity of all TIDES samples at that timepoint, and SpG is the specific gravity for the individual26. When available, machine-read values were used for concentrations below the limit of detection; when unavailable, values were imputed with the formula , where LOD is the lower limit of detection27. Concentrations were natural-log-transformed to normalize right-skewed distributions. The molar sum ΣDEHP was computed by summing its component phthalate metabolites divided by their molecular weights, i.e. .
For each phthalate at each timepoint, we compared transformed phthalate metabolite concentrations for planned and unplanned pregnancies through simple and multivariable linear regressions, adjusting for study center, black race, continuous age of the mother at first trimester survey, annual household income, marital status, and parity, with categorizations shown in Table 1. These covariates were chosen because they were all strongly associated with both pregnancy intention and at least three natural-log-transformed phthalate metabolites at each trimester. We considered other categorizations of race, but ultimately dichotomized the variable to black, non-black, because there was evidence of a difference between black and white participants in phthalate metabolite concentrations, adjusting for the other covariates, but not between white participants and other racial groups, indicating that these groups could be combined. An indicator of a college education or higher was also associated with pregnancy intention and multiple metabolites, but was highly correlated with the other covariates (generalized variance inflation factor=2.15) and therefore was not included in final models, but was included in sensitivity analyses. We tested for collinearity with an accepted measure for predictors with different dimensions: , where GVIF is the generalized variance inflation factor and df is the degrees of freedom28. All analyses were complete-case analyses. We explored alternate definitions of pregnancy intention with these models in sensitivity analyses.
Table 1 –
Characteristics of TIDES participants, by pregnancy intent
| Unplanned (n=208) | ||||
|---|---|---|---|---|
| Planned (n=513) | Indifferent (n=144) | Unwanted (n=64) | Total (n=721) | |
| Center | ||||
| San Francisco, CA | 158 (30.8%) | 24 (16.7%) | 9 (14.1%) | 191 (26.5%) |
| Minneapolis, MN | 160 (31.2%) | 30 (20.8%) | 10 (15.6%) | 200 (27.7%) |
| Rochester, NY | 77 (15.0%) | 74 (51.4%) | 39 (60.9%) | 190 (26.4%) |
| Seattle, WA | 118 (23.0%) | 16 (11.1%) | 6 (9.4%) | 140 (19.4%) |
| Race | ||||
| White | 402 (78.4%) | 78 (54.2%) | 30 (46.9%) | 510 (70.7%) |
| Black | 27 (5.3%) | 36 (25.0%) | 21 (32.8%) | 84 (11.7%) |
| Other | 84 (16.4%) | 30 (20.8%) | 13 (20.3%) | 127 (17.6%) |
| Age (years) | ||||
| Mean (SD) | 31.9 (4.72) | 28.2 (5.90) | 27.9 (6.64) | 30.8 (5.44) |
| Median [Min, Max] | 32.0 [19.0, 45.0] | 28.0 [18.0, 44.0] | 28.0 [18.0, 40.0] | 31.0 [18.0, 45.0] |
| Annual Household Income | ||||
| <$15,000 | 30 (5.8%) | 43 (29.9%) | 24 (37.5%) | 97 (13.5%) |
| $15,000–$45,000 | 66 (12.9%) | 44 (30.6%) | 21 (32.8%) | 131 (18.2%) |
| $45,001–$75,000 | 96 (18.7%) | 30 (20.8%) | 8 (12.5%) | 134 (18.6%) |
| >$75,000 | 321 (62.6%) | 27 (18.8%) | 11 (17.2%) | 359 (49.8%) |
| Education | ||||
| Less than college | 61 (11.9%) | 72 (50.0%) | 37 (57.8%) | 170 (23.6%) |
| Graduated college | 452 (88.1%) | 72 (50.0%) | 27 (42.2%) | 551 (76.4%) |
| Marital Status | ||||
| Separated/Divorced/Single | 22 (4.3%) | 54 (37.5%) | 34 (53.1%) | 110 (15.3%) |
| Married/Living as married | 491 (95.7%) | 90 (62.5%) | 30 (46.9%) | 611 (84.7%) |
| Parity | ||||
| 0 | 280 (54.6%) | 81 (56.2%) | 23 (35.9%) | 384 (53.3%) |
| 1 | 177 (34.5%) | 37 (25.7%) | 18 (28.1%) | 232 (32.2%) |
| 2+ | 56 (10.9%) | 26 (18.1%) | 23 (35.9%) | 105 (14.6%) |
To gauge potential environmental health knowledge and attitude differences between planned and unplanned pregnancies, we compared responses to the relevant questions from the first trimester survey administered to all TIDES participants. We dichotomized responses to “strongly agree” versus all other choices and “always/usually” versus all other choices23. Multivariable logistic regression, adjusting for center, age, black race, income, marital status, and education, again with categorization as shown in Table 1, was used. Predicted probabilities were estimated through marginal standardization29 and used to compute adjusted risk ratios.
All analyses were conducted in R version 3.5.2 (Vienna, Austria).
RESULTS
Women who delivered a live-born infant and provided a urine sample in the first or third trimester were eligible for this analysis; there were a total of 782 pregnancies eligible. Analyses were then restricted to those with complete, non-contradictory data on pregnancy planning status and pre-specified covariates, yielding a final sample size of 721 (Figure 1). Of these, 208 (29%) reported that their pregnancy was not planned. Women with unplanned pregnancies were younger, lower income, less educated, more likely to be non-white, and less likely to be married or living as married than women with planned pregnancies (Table 1). First trimester phthalate metabolite concentrations were available for 690 participants, and third trimester concentrations were available for 689 participants; 658 participants had concentrations from both the first and third trimester.
Figure 1 –
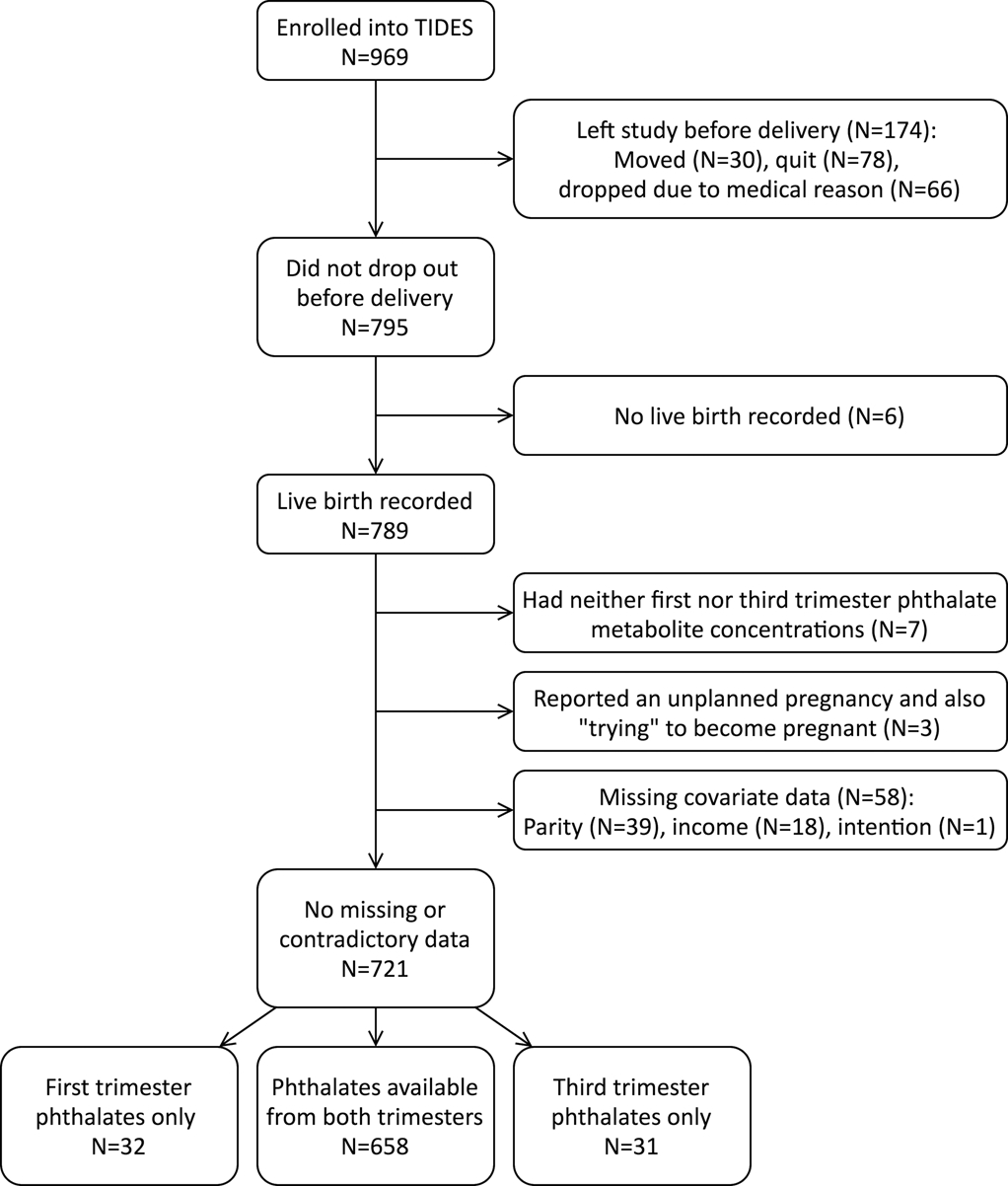
Participant flow diagram, from enrollment to analysis.
Phthalate metabolite geometric means were consistently higher in unintended pregnancies, across all metabolites in the first trimester and most in the third. In the first trimester, pregnancy intention was most strongly associated with MBzP (geometric mean ratio 1.43, 95% CI 1.21, 1.69). In the third trimester, the associations were strongest for MBzP (GMR 1.67, 95% CI 1.37, 2.03) and MBP (GMR 1.43, 95% CI 1.22, 1.68) (Figure 2, eTable 1).
After adjusting for study center, age, black race, income, marital status, and parity, unintended pregnancy was associated only with first trimester MiBP, and the direction of the association was the reverse of the bivariate model (ß −0.18, 95% CI −0.35, −0.02). There was no evidence of an association between unintended pregnancy and third trimester phthalate metabolites, after adjusting for covariates (Figure 2, eTable 2).
Five phthalates – MBP, MBzP, MECPP, MEP, and MiBP – had higher metabolite concentrations in the third trimester urine samples compared to the first, with the strongest associations in MBP, MBzP, MECPP, and MiBP. The other five phthalates had lower concentrations in the third trimester, with the strongest associations for MEHHP and MEHP. Unplanned pregnancy was somewhat associated with an increase in MBP and MiBP metabolites from the first to third trimester, adjusting for covariates (eTable 2).
When examining knowledge of environmental health risks, we did not find evidence of a difference between women with unintended pregnancies and women with intended pregnancies, adjusting for study center, age, black race, income, marital status, and education (Table 2).
Table 2 –
Environmental health attitudes and behaviors from first trimester questionnaire, by pregnancy intent, with adjusted risk ratios for unplanned pregnancy compared to planned pregnancy as a predictor of the attitude or behavior
| Attitude/Behavior | Unplanned (%) | Planned (%) | Total (%) | aRR (95% CI)a |
|---|---|---|---|---|
| Strong agreement that chemicals in the environment pose health risks | 121 (58%) | 324 (63%) | 445 (62%) | 1.11 (0.96, 1.26) |
| Strong agreement that chemicals in the environment are impossible to avoid | 37 (18%) | 145 (28%) | 182 (25%) | 0.88 (0.56, 1.20) |
| Always/usually buy eco-friendly or chemical-free personal care products | 87 (42%) | 236 (46%) | 323 (45%) | 1.01 (0.79, 1.22 |
| Always/usually buy eco-friendly or chemical-free household products | 81 (39%) | 214 (42%) | 295 (41%) | 1.09 (0.84, 1.33) |
| Always/usually eat organic, eco-friendly, or chemical-free food | 78 (38%) | 271 (53%) | 349 (48%) | 0.99 (0.81, 1.18) |
| Always/usually make sure food and drink comes in safe plastic | 53 (25%) | 284 (55%) | 337 (47%) | 0.97 (0.62, 1.31) |
| Always/usually check recycling code on the bottle | 49 (24%) | 114 (22%) | 163 (23%) | 1.17 (0.70, 1.64) |
Adjusting for study center, age, black race, income, marital status, and education of a college degree or higher
We evaluated modeling choices and comparability between labs through several sensitivity analyses. First, we included education in the multivariable linear regressions of each phthalate metabolite; coefficients for unplanned pregnancy were unchanged. Second, we excluded parity and marital status; coefficients for unplanned pregnancy were again unchanged. Third, we evaluated robustness by lab of analysis, by running the paired t-tests of phthalate concentrations across pregnancy including only the CDC samples for mothers of male fetuses (n=321); there were no major changes. We also ran our first trimester phthalate models separately for each lab and observed a positive association between unplanned pregnancy and MEHP in the samples analyzed at the CDC (ß 0.24, 95% CI 0.00, 0.47); all other results were unchanged. Finally, when we considered an alternate categorization of pregnancy intention, we found evidence that unwanted pregnancies differed from other pregnancies in MBzP change from first to third trimester, adjusting for center, age, black race, income, marital status, and parity. This held true comparing unwanted pregnancies to planned pregnancies (ß 0.42, 95% CI 0.07, 0.78) or unwanted to planned and indifferent combined (ß 0.40, 95% CI 0.06, 0.74) (not shown in tables). Previously, the association between unplanned pregnancy and change in MBzP was null (eTable 2).
COMMENT
Principal findings
Initially, we observed consistently positive associations between unplanned pregnancy and phthalate metabolites in bivariate models, across all phthalates in the first trimester and most in the third. However, after adjusting for socioeconomic and demographic covariates, almost all associations dissipated. In the multivariable models, first trimester MiBP had the strongest association with unplanned pregnancy, and this association was inverse, with higher metabolite concentrations in pregnancy planners. Based on the number of phthalates considered, this non-null relationship could be spurious and is not likely to be representative of a larger trend in the relationship between phthalates and pregnancy intention. We did not find evidence to support the hypothesis that the association between pregnancy intention and phthalate metabolite concentrations would be stronger in the first trimester than the third (Figure 2).
Strengths of the study
Strengths of this study include the large sample size and multi-site design. Virtually nothing is known about how pregnancy intention could be associated with phthalate exposure.
Limitations of the data
Potential weaknesses include the external validity of the study. Our cohort percentage of unplanned pregnancies (29%) was lower than in the United States overall (about 50%) and represents only a subset of unplanned pregnancies at large. TIDES participants were recruited in their first trimester and excluded if they did not plan to keep the child, meaning our results cannot be extended to non-planners who did not seek prenatal care or who terminated the pregnancy. Most participants were also surveyed nine years ago, in 2011, before a host of studies published their findings on the effects of phthalate exposure during pregnancy, and awareness of those effects might be higher in women who are pregnant now, though public awareness is slow to follow empirical evidence. One analysis of National Health and Nutrition Examination Survey (NHANES) data showed a 2.2-fold decrease in combined exposure to six phthalate parent compounds from 2005 to 201434.
Interpretation
In our study, the demographic traits associated with unplanned pregnancy were also associated with higher phthalate concentrations in the TIDES dataset, making confounding by socioeconomic characteristics a serious concern. It is also possible that most women, regardless of pregnancy planning status, remain relatively unaware of what phthalates are and what risks they pose. Regarding our secondary question, of whether pregnancy planning is associated with greater awareness of environmental health, we did not find any evidence of differences between women with planned and unplanned pregnancies in attitudes toward plastic safety, though perhaps participants were not operating under shared ideas of what a “safe” or “eco-friendly” product is, given that this survey was developed de novo.
Perhaps phthalates are fundamentally different from the exposures that have been shown to be associated with pregnancy planning, such as alcohol consumption. The research on phthalates is more recent, and educational campaigns to reduce exposure to plastics have not been widely adopted. One study of women seeking fertility care in Rochester found that only 29% had ever heard of phthalates30. For those who are aware of the risks, phthalate exposure is more challenging than other chemical exposures (e.g., cigarettes) to control or even personally monitor. Interestingly, the Rochester study found that knowledge about phthalates was not related to metabolite concentrations30. This motivates policy interventions, in addition to awareness campaigns.
When we considered alternate definitions of pregnancy intention, we found that unwanted pregnancies experienced a more positive change in MBzP concentrations over the course of the pregnancy, compared to planned pregnancies alone or planned and indifferent pregnancies combined. This is concerning due to the risks associated with MBzP exposure during pregnancy, including increased risk of hypertensive diseases of pregnancy for the mother31 and altered reproductive development in male offspring32,33, though the multiple testing inherent in this sensitivity analysis requires that we interpret this result with caution.
Conclusions
In conclusion, we did not find evidence that pregnancy intention is associated with phthalate metabolite concentrations. This might suggest that pregnancy planners either did not attempt to modify their plastic usage or were not successful. The results of our study reinforce that racial and socioeconomic disparities are associated with differing levels of phthalate exposure in pregnancy and that pregnancy intention status might be reflective of these disparities. Our study also contributes novel findings regarding the need to increase preconception and prenatal knowledge about the harms of plastic exposure to the developing fetus.
Supplementary Material
Synopsis.
Study question:
Is pregnancy intention associated with maternal prenatal phthalate metabolite levels?
What’s already known:
Modifying pre-pregnancy behavior might reduce harmful chemical exposure. Prenatal phthalate exposure has been linked to preterm birth and pregnancy complications, as well as adverse reproductive, neurodevelopmental, metabolic, and respiratory outcomes in offspring.
What this study adds:
In a large, multicenter, prospective pregnancy cohort study, women with unplanned pregnancies have higher concentrations of phthalate metabolites than women with planned pregnancies, especially in the first trimester. The associations attenuate, however, after adjusting for socioeconomic factors and parity.
Acknowledgments
Funding for TIDES was provided by the following grants from the National Institute of Environmental Health Sciences: R01 ES016863-04 and R01 ES016863-02S4. This research was supported in part by the Intramural Research Program of the National Institute of Environmental Health Sciences, National Institutes of Health (ZIA ES103313).
Contributor Information
Grace R. Lyden, University of Minnesota School of Public Health, Division of Biostatistics, Minneapolis, MN, USA.
Emily S. Barrett, Rutgers School of Public Health, Department of Epidemiology, Environmental and Occupational Health Sciences Institute, Piscataway, NJ, USA
Sheela Sathyanarayana, University of Washington School of Public Health, Department of Environmental and Occupational Health Sciences, Seattle, WA, USA.
Nicole R. Bush, University of California, San Francisco, Department of Psychiatry, Department of Pediatrics, San Francisco, CA, USA
Shanna H. Swan, Mount Sinai School of Medicine, Division of Preventive Medicine and Community Health, New York, NY, USA
Ruby H.N. Nguyen, University of Minnesota School of Public Health, Division of Epidemiology and Community Health, Minneapolis, MN, USA
References
- 1.Finer, Lawrence B; Zolna MR Declines in Unintended Pregnancy in the United States, 2008–2011. New England Journal of Medicine 2016;374:843–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dott M, Rasmussen SA, Hogue CJ, Reefhuis J. Association between pregnancy intention and reproductive health related behaviors before and after pregnancy recognition, National Birth Defects Prevention Study, 1997–2002. Maternal and Child Health Journal 2010;14:373–381. [DOI] [PubMed] [Google Scholar]
- 3.Hellerstedt WL, Pirie PL, Lando HA, Curry SJ, Mcbride CM, Grothaus LC, et al. Differences in Preconceptional and Prenatal Behaviors in Women with Intended and Unintended Pregnancies. American Journal of Public Health 1998;88:663–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pryor J, Patrick SW, Sundermann AC, Wu P, Hartmann KE. Pregnancy Intention and Maternal Alcohol Consumption. Obstetrics and Gynecology 2017;129:727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Than LC, Honein MA, Watkins ML, Yoon PW, Daniel KL, Correa A. Intent to become pregnant as a predictor of exposures during pregnancy: Is there a relation? The Journal of Reproductive Medicine 2005;50:389–96. [PubMed] [Google Scholar]
- 6.Naimi TS, Lipscomb LE, Brewer RD, Gilbert BC. Binge drinking in the preconception period and the risk of unintended pregnancy: implications for women and their children. Pediatrics 2003;111:1136–41. [PubMed] [Google Scholar]
- 7.Toivonen KI, Oinonen KA, Duchene KM. Preconception health behaviours: A scoping review. Preventive Medicine 2017;96:1–15. [DOI] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention. Phthalates Factsheet. https://www.cdc.gov/biomonitoring/Phthalates_FactSheet.html (last accessed March 2019).
- 9.U.S. Department of Health and Human Services. Guidance for Industry: Limiting the Use of Certain Phthalates as Excipients in CDER-Regulated Products.; 2012.
- 10.Gray LE, Ostby J, Furr J, Price M, Veeramachaneni DNR, Parks L. Perinatal exposure to the phthalates DEHP, BBP, and DINP, but not DEP, DMP, or DOTP, alters sexual differentiation of the male rat. Toxicological Sciences 2000;58:350–365. [DOI] [PubMed] [Google Scholar]
- 11.Foster PMD, Gray E, Leffers H, Skakkebæk NE. Disruption of reproductive development in male rat offspring following in utero exposure to phthalate esters. International Journal of Andrology 2006;29:140–147. [DOI] [PubMed] [Google Scholar]
- 12.Lee E, Ahn MY, Kim HJ, Kim IY, Han SY, Kang TS, et al. Effect of Di(n-butyl) Phthalate on Testicular Oxidative Damage and Antioxidant Enzymes in Hyperthyroid Rats. Environmental Toxicology 2007;22:245–255. [DOI] [PubMed] [Google Scholar]
- 13.Kasahara E, Sato EF, Miyoshi M, Konaka R, Hiramoto K, Sasaki J, et al. Role of oxidative stress in germ cell apoptosis induced by di(2-ethylhexyl)phthalate. Biochemical Journal 2002;365:849–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ferguson KK, McElrath TF, Meeker JD. Environmental Phthalate Exposure and Preterm Birth. JAMA Pediatrics 2014;168:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Swan SH, Sathyanarayana S, Barrett ES, Janssen S, Liu F, Nguyen RHN, et al. First trimester phthalate exposure and anogenital distance in newborns. Human Reproduction 2015;30:963–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alur S, Wang H, Hoeger K, Swan SH, Sathyanarayana S, Redmon BJ, et al. Urinary phthalate metabolite concentrations in relation to history of infertility and use of assisted reproductive technology. Fertility and Sterility 2015;104:1227–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sathyanarayana S, Butts S, Wang C, Barrett E, Nguyen R, Schwartz SM, et al. Early prenatal phthalate exposure, sex steroid hormones, and birth outcomes. Journal of Clinical Endocrinology and Metabolism 2017;102:1870–1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim Y, Ha EH, Kim EJ, Park H, Ha M, Kim JH, et al. Prenatal exposure to phthalates and infant development at 6 months: Prospective mothers and children’s environmental health (MOCEH) study. Environmental Health Perspectives 2011;119:1495–1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Engel SM, Miodovnik A, Canfield RL, Zhu C, Silva MJ, Calafat AM, et al. Prenatal phthalate exposure is associated with childhood behavior and executive functioning. Environmental Health Perspectives 2010;118:565–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ashley-Martin J, Dodds L, Arbuckle TE, Ettinger AS, Shapiro GD, Fisher M, et al. A birth cohort study to investigate the association between prenatal phthalate and bisphenol A exposures and fetal markers of metabolic dysfunction. Environmental Health: A Global Access Science Source 2014;13:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Whyatt RM, Perzanowski MS, Just AC, Rundle AG, Donohue KM, Calafat AM, et al. Asthma in inner-city children at 5–11 years of age and prenatal exposure to phthalates: The columbia center for children’s environmental health cohort. Environmental Health Perspectives 2014;122:1141–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Berger K, Eskenazi B, Balmes J, Holland N, Calafat AM, Harley KG. Associations between prenatal maternal urinary concentrations of personal care product chemical biomarkers and childhood respiratory and allergic outcomes in the CHAMACOS study. Environment International 2018;121:538–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barrett ES, Sathyanarayana S, Janssen S, Redmon JB, Nguyen RHN, Kobrosly R, et al. Environmental health attitudes and behaviors: findings from a large pregnancy cohort study. European Journal of Obstetrics & Gynecology and Reproductive Biology 2014;176:119–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Silva MJ, Samandar E, Preau JL, Reidy JA, Needham LL, Calafat AM. Quantification of 22 phthalate metabolites in human urine. Journal of Chromatography B 2007;860:106–112. [DOI] [PubMed] [Google Scholar]
- 25.Calafat AM. Phthalate Metabolites Method 6306.03. Atlanta, GA; 2010. [Google Scholar]
- 26.Boeniger MF, Lowry LK, Rosenberg J. Interpretation of Urine Results Used to Assess Chemical Exposure With Emphasis on Creatinine Adjustments: A Review. American Industrial Hygiene Association Journal 1993;54:615–627. [DOI] [PubMed] [Google Scholar]
- 27.Hornung RW, Reed LD. Estimation of Average Concentration in the Presence of Nondetectable Values. Applied Occupational and Environmental Hygiene 1990;5:46–51. [Google Scholar]
- 28.Fox J, Monette G, Fox J, Monette G. Generalized Collinearity Diagnostics Generalized Collinearity Diagnostics. 2016;1459:178–183. [Google Scholar]
- 29.Muller CJ, Maclehose RF. Estimating predicted probabilities from logistic regression: Different methods correspond to different target populations. International Journal of Epidemiology 2014;43:962–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pilato A, Chen C, Thurston S, Vitek W, Hoeger K, Barrett ES. Phthalate exposure, reproductive hormones, and lifestyle behaviors in women seeking fertility care. Fertility and Sterility 2017;108:e321. [Google Scholar]
- 31.Werner EF, Braun JM, Yolton K, Khoury JC, Lanphear BP. The association between maternal urinary phthalate concentrations and blood pressure in pregnancy: The HOME Study. Environmental Health: A Global Access Science Source 2015;14:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Swan SH, Main KM, Liu F, Stewart SL, Kruse RL, Calafat AM, et al. Decrease in anogenital distance among male infants with prenatal phthalate exposure. Environmental Health Perspectives 2005;113:1056–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Watkins DJ, Sánchez BN, Téllez-Rojo MM, Lee JM, Mercado-García A, Blank-Goldenberg C, et al. Impact of phthalate and BPA exposure during in utero windows of susceptibility on reproductive hormones and sexual maturation in peripubertal males. Environmental Health: A Global Access Science Source 2017;16:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reyes JM, Price PS. Temporal Trends in Exposures to Six Phthalates from Biomonitoring Data: Implications for Cumulative Risk. Environmental Science and Technology 2018;52:12475–12483. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


