Abstract
Purpose of Review
Fractures are painful and disabling injuries that can occur due to trauma, especially when compounded with pathologic conditions, such as osteoporosis in older adults. It is well documented that acute pain management plays an integral role in the treatment of orthopedic patients. There is no current therapy available to completely control post-fracture pain that does not interfere with bone healing or have major adverse effects. In this review, we focus on recent advances in the understanding of pain behaviors post-fracture.
Recent Findings
We review animal models of bone fracture and the assays that have been developed to assess and quantify spontaneous and evoked pain behaviors, including the two most commonly used assays: dynamic weight bearing and von Frey testing to assess withdrawal from a cutaneous (hindpaw) stimulus. Additionally, we discuss the assessment and quantification of fracture pain in the clinical setting, including the use of numeric pain rating scales, satisfaction with pain relief, and other biopsychosocial factor measurements. We review how pain behaviors in animal models and clinical cases can change with the use of current pain management therapies. We conclude by discussing the use of pain behavioral analyses in assessing potential therapeutic treatment options for addressing acute and chronic fracture pain without compromising fracture healing.
Summary
There currently is a lack of effective treatment options for fracture pain that reliably relieve pain without potentially interfering with bone healing. Continued development and verification of reliable measurements of fracture pain in both pre-clinical and clinical settings is an essential aspect of continued research into novel analgesic treatments for fracture pain.
Keywords: Fracture pain, Nociceptive behaviors, Pain management, Animal fracture model, Opioids
Introduction
Fractures are painful and disabling injuries that can occur at any age but are particularly prevalent in 40–50% of women and 13–22% of men with osteoporosis [1,2]. The number of patients in the United States with osteoporosis or low bone density will continue to rise with the aging of the population, leading to an increased prevalence of bone fractures in future years [3]. Not only is increasing age leading to a greater prevalence of osteoporosis and associated bone fractures, it is also a risk factor for impaired fracture healing and elevated bone pain [4,5]. Fractures with impaired healing can be extremely disabling due to ongoing pain, resulting in loss of function and decreased use of the affected extremities. If pain levels reach intolerable levels, it can prevent patients from adequately loading and using the fractured bone, thus causing a loss of muscle mass and further compromising fracture healing. Indeed, it is speculated that pain is the primary determinant of noncompliance with physical therapy and rehabilitation from orthopedic injuries [6••, 7]. Inadequate pain control immediately following fracture (acute period) and up to 3 months following fracture, while the bone and soft tissues are healing (subacute period) is the greatest predictor of long term chronic pain 7 years after a fracture [8]. For this reason, adequate pain management during both the acute trauma phase and the extended recovery period of orthopedic patients is necessary to improve the quality of life of the patients and for successful bone healing.
The reasons why fracture pain persists during and following the healing process are poorly understood. While pain is nearly always anticipated during the acute period following fracture or orthopedic surgery, the extent to which patients experience pain and the duration of pain varies greatly, depending on a multitude of factors, including: age, sex, BMI, and genetics [9]. In clinicopathological terms, bone pain can be separated into two categories; subacute fracture pain and maladaptive chronic pain within the affected limb. Fracture and subacute fracture pain are important causes of restricted physical activity in older persons [10]. The development of complex regional pain syndrome (CRPS) within the affected limb is characterized by ongoing pain and signs of inflammation, including edema and vascular disturbances [11]. As illustrated in Figure 1, bone fracture pain is complex and influenced by integration of sensory, emotional, and perceptual information received by many areas of the brain [12]. Pathophysiologic stress, including depression and anxiety, poor coping strategies, and decreased self-efficacy greatly alter the perception of pain, thus these factors are important to measure and track in both pre-clinical and clinical pain studies.
Figure 1.
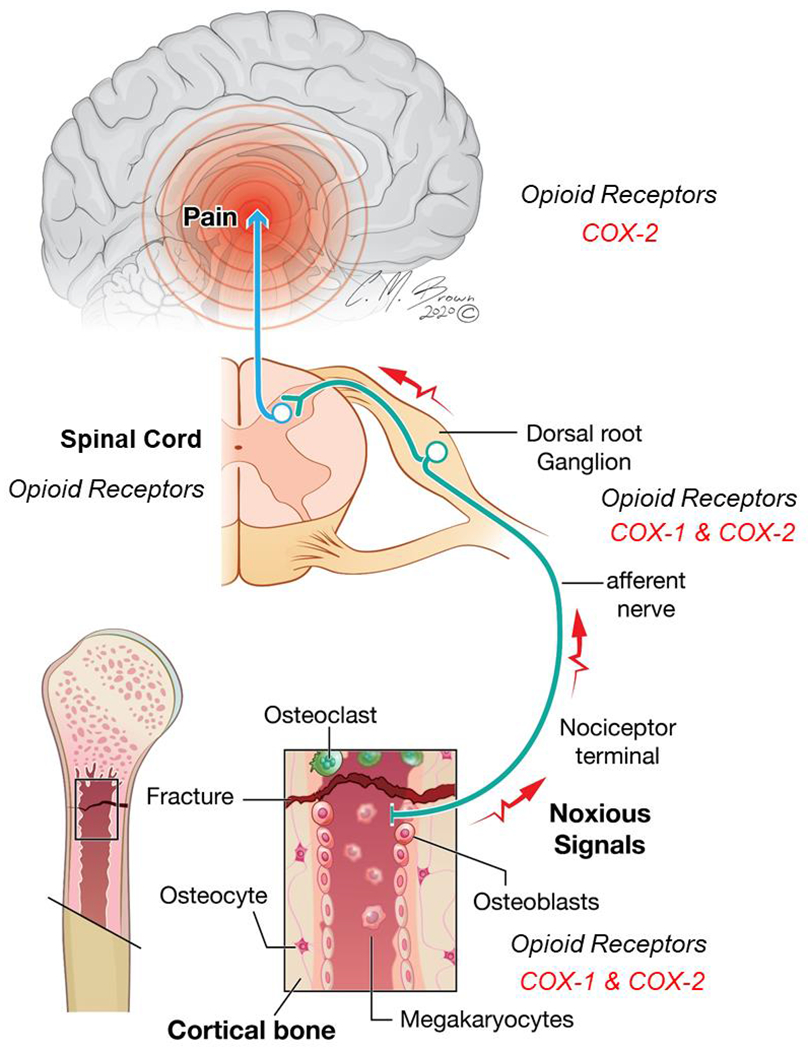
Anatomic levels of nociceptive processing following bone fracture. Upon injury, inflammatory mediators, including prostaglandins, are released locally by a variety of non-neural cells and the nervous system. Biosynthesis of prostaglandins are attributed to two different enzymes, cyclooxygenase-1 (COX-1) and cyclooxygenase-2 (COX-2), and are blocked by nonsteroidal anti-inflammatory drugs (NSAIDs). Opioid receptors are critical in the modulation of pain following fracture. Opioid receptors are expressed throughout the nociceptive neural circuitry in the peripheral nervous system, spinal cord, and critical regions of the brain involved in reward and emotion-related brain structures
Because efficacious pain management is so critical for successful bone fracture healing, it is critical to assess patient-reported pain levels within the clinical setting and fracture-induced nociceptive behaviors in animal fracture models [13]. The assays currently used to assess nociception in animal models of bone fracture have many strengths and weaknesses. Understanding the limitations of currently used behavioral assays, which endeavor to accurately and reproducibly assess post fracture pain, is crucial for interpreting studies to identity pain mechanisms and develop new effective therapies for the management of fracture pain that are superior to the current controversial first line treatments, non-steroidal anti-inflammatory drugs (NSAIDs) and opioids.
Animal Models for Fracture Pain Assessment
Animal Fracture Models Used to Create Fracture Pain
The first process in assessing nociception in an animal model is recreating a model that will elicit behavior which might be associated with pain in the rodent. There are a variety of preclinical rodent models of long bone fracture which have traditionally been used to assess fracture healing: generation of a cortical hole, osteotomy, and the 3-point, or Einhorn, model. With the cortical hole model, a surgical incision is made to expose the bone and then a drill is used to remove a small cylindrical section of bone [14]. A single hole can either be drilled through the cortical bone into the bone marrow or through both cortices to create a hole penetrating the entire thickness of the bone. Osteotomies are typically created using a saw-like device that transects the bone. A single cut can be made to mimic a fracture, or the bone can be cut in two locations to remove a small segment of bone. When a bone segment is removed, the bone must be stabilized to be the same length prior to the osteotomy using plates or a replacement material, such as an isograft or synthetic scaffold, to fill the gap of the segmental defect [15]. Osteotomies are often stabilized via an intramedullary rod or pin. Segmental bone defects, particularly critical sized bone defects that are large enough so that they will not heal without intervention, are excellent for modeling more severe traumatic bone fractures that require intensive clinical intervention. While cortical hole and osteotomies are commonly used to assess fracture healing, the first fracture model used to specifically monitor fracture pain behaviors in rodents was the 3 point, or Einhorn, model [16•, 17,18•]. The Einhorn fracture model is a 3 point break model performed by placing the bone to be fractured on a surface with two points and then the third point is centered above the bone between the two heads below. The third point is then brought down upon the bone to create the break. This model can be used to create a closed fracture in a living animal under anesthesia using the 3 point bending device or guillotine mechanism [18]. Many models first stabilize the bone with an intramedullary rod or pin before using the 3 point device to fracture the bone. All three of these fracture models could be used to study fracture pain; however, the majority of fracture pain behavior research has been conducted using the Einhorn model to create a closed femoral fracture. This is likely because the Einhorn closed fracture model simulates a simple closed fracture, which is most often seen clinically.
Animal Pain Behavior Overview
Clinical fracture pain is a perception that is influenced by psychological and experiential factors, thus an animal fracture model will not recapitulate completely all of the factors that contribute to clinical pain. Despite this limitation, considerable effort and progress has been made in objectively modeling and quantifying nociception in animals, specifically in rat and mouse models. Unlike in the clinical setting, where patients can verbalize how they perceive spontaneous and evoked pain, preclinical investigators rely upon expression of nociceptive behaviors to assess animal pain levels. Consistent animal behaviors in response to noxious stimuli are often referred to as pain behaviors, as ascending and descending pain pathways are activated by noxious stimuli in wounded animals [19]. As mentioned previously, psychological and experiential aspects of pain perception cannot be assessed in rodents but are controlled for as much as possible. Due to inherent variability in baseline nociceptive behaviors, even in inbred mice and rats, the optimal experimental design for pain behavior assessment includes a baseline measurement of each behavioral modality, prior to any noxious intervention, including bone fracture. Pain behaviors are generally described in two categories in both humans and animals, spontaneous pain behaviors and evoked pain behaviors. There are numerous behavioral tests that have been used to assess many different types of pain, including inflammatory, neuropathic, arthritic, muscle, cancer, and incisional pain. Although evoked behaviors traditionally have been used more frequently to assess nociceptive thresholds, the assessment of spontaneous pain and risk/reward behaviors has increased over the past decades to emulate the challenges faced by patients and to enhance the translatability of preclinical pain research. For this review, pain behaviors that have been used and shown to be sensitive for the assessment of fracture pain will be highlighted. Lower limb fracture pain can be assessed directly by examining the effects of body weight loading onto the fractured limb or indirectly by assessing hypersensitivity of hindpaw skin of the affected limb to mechanical or thermal stimulation [20]. Hypersensitivity of the sensory neurons innervating the bone at the fracture site constitutes primary hyperalgesia, whereas skin hypersensitivity represents secondary hyperalgesia or referred pain [21]. Secondary hyperalgesia is caused by sensitization of spinal cord neurons or neurons of higher order in the central nervous system [22]. Recent studies have shown that, while assessment of skin hypersensitivity is commonly used as a surrogate for fracture pain, the mechanisms that underlie primary and secondary hyperalgesia elicited by fracture differ and thus studies must be designed appropriately to examine whether putative therapeutics alter direct bone pain or referred pain [20].
Spontaneous Pain Behaviors for Assessing Fracture Pain
Spontaneous pain may manifest differently depending on the type of pain stimuli, whether that be inflammatory pain, visceral pain, bone pain, etc. [23]. For fracture pain, such as that from a closed femoral fracture in a mouse, pain can be assessed by observing time spent performing spontaneous pain behaviors over a set period of time, typically anywhere from 2–20 minutes depending on the study [16]. For most effective analyses, all spontaneous mouse pain behaviors are assessed through video surveillance of the animal [24]. There are some variations to these methods of observation such as recording the number of times a specific spontaneous pain behavior is performed over a period of time instead of recording the overall time spent performing the behavior [16]. The observations discussed below are used to quantify spontaneous pain because they are thought to mimic similar spontaneous or ongoing clinical pain.
Guarding and static weight bearing recapitulate behaviors commonly seen in the clinic, where patients protect an injured limb from external mechanical stimuli and the force of body weight loaded on to the affected limb [25]. Guarding of the injured limb is defined by the animal lifting and holding their limb against their body and is typically quantified by assessing the amount of time spent guarding the fractured limb [26]. In addition to guarding, a similar assessment for evaluating fracture pain is the observation of the number of spontaneous paw flinches of the fractured limb over a set period of time [16, 27]. Spontaneous paw flinches are quick, instinctive paw withdrawals or movements in reaction to spontaneous pain while guarding of the limb is typically held for a longer duration than a single paw flinch. The more hindpaw flinches and time spent guarding as well as fewer vertical stands (discussed later as rearing) indicates a higher level of fracture pain.
Possibly the most reliable indicator of fracture pain is the reduction of weight bearing with the fractured limb, so that the hindpaw of the fractured limb only rests on the floor instead of pressed flat on the ground and bearing weight. Weight bearing demonstrates the level of usage and mechanical loading of the fractured limb, which is of increased importance because load-bearing bones require mechanical loading to fully heal as previously stated. This implies weight bearing is not only important in measuring the level of pain in an animal, but also for assessing recovery and healing. Weight bearing can be measured as static weight bearing or dynamic weight bearing (also involved in gait analysis; see below). For assessing static weight bearing on the hind legs, the animal is typically placed in a small restrictive container with an inclined floor so that most weight must be placed on the hind legs. Floor sensors are placed to measure the weight placed on each hind foot individually and the distribution of weight between the two hindpaws can be determined. The greater the difference between the weight distributed on the fractured limb and the contralateral limb is an indicator of greater nociception experienced by the rodent. This model has been shown to be an effective assessment for osteoarthritis, bone cancer pain, and is applicable to fracture pain [28, 29]. A known limitation of the static weight bearing method is that it is primarily suited for unilateral hind limb injuries.
Dynamic weight bearing, gait, and locomotion assays can model how the fracture pain affects the ability of patients to ambulate; assessing altered weightbearing or limping and/or compensatory accommodations in gait to prevent or minimize pain within the affected limb. Dynamic weight bearing involves calculating the fraction of weight borne on each of the individual four limbs in freely moving animals. Typically, a dynamic weight bearing system involves a floor instrument on the bottom of the cage that records pressure data as well as foot surface area for each limb to calculate the percentage of body weight placed on each limb during movement. Software such as that from BioSeb can be used to attribute pressure readings to individual paws with high fidelity [30]. As with other behavioral assessments, the rodent is allowed to acclimate to the new cage for a set period of time before data acquisition begins. Similar to static weight bearing assessment, the dynamic weight bearing technique has been shown to be an effective nociceptive test for inflammation and cancer bone pain and is useful for fracture pain assessment [31]. Dynamic weight bearing has the added benefits of not requiring restraint of the animal and having the capacity to evaluate the weight bearing of all four limbs when compared to static weight bearing assessments.
In addition to weight bearing analyses, gait and locomotive activity have been used to assess levels of pain in rodents. Modification of many different gait parameters, such as interlimb coordination, paw pressure, paw print area, stance phase duration, swing phase duration, stride length, and swing speed can be observed in unilateral injury models. Some of these parameters, such as swing duration, have been shown to be more reliable and valid parameters than others for assessing nerve recovery or pain [32]. The first discovered method for assessing gait involved covering the animal’s paw with ink and allowing it to walk freely on a piece of paper. The paper can then be scanned and the footprints analyzed to determine limping behaviors or change in stride length [33]. Newer models use an automated gait analysis tool, such as CatWalk XT or DigiGait, to track the rodent’s paw prints as it walks along an elevated clear platform [34]. Lastly, some investigators use μCT to examine the mechanics of limb placement during movement [35]. Although gait was expected to be an ideal analysis of fracture pain in rodents, as alterations in gait are a primary symptom of lower limb fracture pain seen in humans, very limited changes in gait have been observed in rodents and gait alterations do not always correlate with nociceptive pain, such as hypersensitivity to mechanical stimulation of the hindpaw [35, 36]. Locomotive activity and distance traveled can be evaluated in a freely moving animal using video surveillance and associated tracking software or via monitoring the distance traveled on an activity wheel. Though behaviors such as diminished locomotion and wheel running activity may appear to be reflective of nociceptive behavior, interpretation of behavioral changes in exercise must be clearly defined as changes in locomotive behavior may both cause and/or be affected by changes in metabolic function, mood, or depression [37]. Moreover, reduced locomotion associated with acute inflammation does not always correlate with other measurements of pain, such as Von-Frey measurement [38]. Due to inconsistencies in the association of gait and locomotion with nociception, more emphasis has been placed on the use of dynamic weight bearing to assess fracture pain [39, 40]. Other methodologies such as the Basso Mouse Scale (BMS; detects differences in recovery after spinal cord injury) which includes joint movement, stepping ability, coordination and trunk stability [40•]. One advantage of the BMS scale is that it mainly focuses on hindlimb movement, rather than on graded changes in body support ability.
There also are changes in non-specific animal behaviors that are thought to reflect changes in quality of life and/or normal function due to spontaneous pain, which do not necessarily have a human behavioral correlate [21]. These include rearing behavior and grooming habits [41]. Rearing by the rodent, as defined as lifting both front paws off the floor at the same time, is another measure of spontaneous activity. Rearing is considered an exploratory behavior that occurs naturally in healthy mice with good affect [42]. Typically, animals in pain have been shown to have a decreased number of spontaneous rears which is consistent with a less outwardly focused animal. [24]. Rearing behaviors may have additional implications when evaluating fracture pain because most fracture models target the mouse femur or tibia, and rearing behaviors require increased weight bearing upon the hindlimbs. Rodents with increased pain will generally have excessive grooming behaviors with increased tending to the injured limb [24]. Excessive grooming has been used and verified as a measure of spontaneous nociceptive behaviors specifically for fracture pain [6]. Like other spontaneous behaviors, the number of rears and time spent grooming or attending to the fractured limb are recorded over a set period via video surveillance.
Evoked Pain Behaviors for Assessing Fracture Pain
Unlike spontaneous pain behavior observations, evoked pain behaviors are responses to an external application of some sort of noxious or non-noxious stimuli, including: heat, cold, mechanical, and electrical stimuli. These external stimuli can either be applied to the site of injury (palpation) or to another site (hindpaw withdrawal behaviors). To directly correlate with clinical practice, where orthopedic surgeons palpate the fracture site to establish whether the site is painful, preclinical investigators monitor spontaneous pain behaviors before and after palpation of the fracture site to assess fracture pain [6].
Mechanical and thermal hindpaw withdrawal behaviors are commonly used to assess fracture pain due to the common availability of testing devices. The Von Frey test is thought to be especially useful for assessing increased cutaneous mechanical sensitivity, but has also been used to measure mechanical allodynia associated with fracture pain in rodents [18, 21]. To perform the assessment, the animal is placed in a cage with a mesh bottom and is allowed to acclimate while roaming freely. The animal is then challenged with a mechanical stimulus and the force required to elicit a reflexive withdrawal response is measured. For the manual model, the plantar surface of the hindpaw is stimulated with Von Frey monofilaments with predefined bending forces ranging from 10 to 120 mN. The monofilament is applied to the bottom of the paw at a perpendicular angle until the filament for a set period (typically between 2-5 seconds). The animal is observed to gage for positive pain behaviors, withdrawing or licking the stimulated paw. There are variations in the endpoints measured with Von Frey stimulation, including assessing a response frequency to individual monofilaments to determine hyperalgesia (enhanced response to a noxious stimulus force) or allodynia (a novel response to a non-noxious stimulus force) or by determining the 50% paw withdrawal threshold (PWT) by beginning with the lowest bending force filament and gradually escalating the force applied to the paw until the force corresponding to a 50% withdrawal rate is determined [43]. Thus, a lower PWT corresponds to a higher pain level. This approach has been optimized over time to reduce the amount of test applications and control for the extent of investigator interaction with the animals [44, 45]. These methods are fairly time consuming, require repeated stimuli that can cause sensitization or learned premature withdrawal by the animal, and have large observer to observer variability [46••]. To decrease exposure of the animal to multiple stimulations, the Electronic Von Frey was developed. This device determines PWT by applying one non-bending Von Frey filament to the paw of the animal with increasing force until the animal withdraws its paw. Software accompanying the instrument records the force at which the animal removes its paw [47–50]. This method drastically reduces the number of applications needed for each animal in order to determine PWT and reduces the time required to perform the experiment; however, the test still requires an experienced researcher to help determine between false positives and true pain behaviors. Thermal hyperalgesia is also evident in animal models of fracture pain, measured by the hot plate test [18]. The hot plate test includes placing the rodent on a metal hot plate set at a constant temperature typically ranging from 50-55°C and observing how long it takes for the animal to express a nociceptive behavior such as paw withdrawal, licking, or jumping [51]. Alternatively, thermal hyperalgesia secondary to bone fracture could be measured using the Hargreaves test, where a radiant heat source is focused on a hindpaw and the temperature ramps until the animal withdraws the paw [52]. The Hargreaves test is advantageous because it allows for the fractured limb to be tested and compared to the contralateral limb. As mentioned previously, the reliability of skin hypersensitivity as a surrogate for assessing skeletal pain is still a topic of debate, since Guedon et al demonstrated that therapies such as anti-P2X3 were able to relieve skin hypersensitivity measured by evoked pain behaviors but did not relieve spontaneous skeletal pain behaviors from bone cancer pain [53•].
Clinical Fracture Pain Assessment
With the creation of pain as “the 5th vital sign” by the Joint Commission on Accreditation of Healthcare Organizations (JCAHO, now called The Joint Commission) and subsequent adoption and support from the Veterans Health Administration (VHA), there is an obligation for physicians and healthcare professionals to evaluate, treat. and alleviate a patient’s experience of pain [54]. The clinical setting has historically used a basic 11-point verbal numeric rating scale (NRS) or visual analogue scale (VAS, 0 = no pain, 10 = the worst pain ever imagined) [55, 56], which is generally a reliable and valid measure of pain intensity [57]. With pediatric populations, often based on the level of neurologic maturity and arithmetic development, the Faces Pain Scale - Revised [58, 59] is used to assess fracture pain. Orthopedic surgeons rely on these pain scales to evaluate pre-surgical, post-surgical, and change in pain during the fracture healing process. However, data have shown that these scales, while optimal for acute measurements, are not sufficient for chronic pain management and fracture evaluation [60]. With current methods there often is a discrepancy between the multidimensional aspect of pain and the unidimensional methods that are used in the evaluation of pain. The English language has evolved to portray many aspects of the quality of pain, yet often standard methods of analysis evaluate only the intensity of the pain experience [61].
Biopsychosocial Factors
While quantification of pain is assessed pre-operatively and post-operatively, there have been associations between biopsychosocial factors and chronic pain post-surgery. Several studies have shown that pre-operative factors such as depression, anxiety, self-efficacy, catastrophizing, smoking, or a history of substance abuse correlate to both acute and chronic post-surgical pain[13, 62•, 63, 64•, 65, 66]. Furthermore, data indicate that these correlations are independent of any single surgical model [64]. Therefore, patients could be pre-operatively screened for biopsychosocial factors using the Pain Health Questionnaire-Depression [67, 68]. Pain Anxiety Scale [69]. Pain Self Efficacy Questionnaire [70], and Pain Catastropliizing Scale [71], each of which is assessed with Likert Scales of varying benchmarks. Additionally, pre-operative screening for health factors such as smoking or substance abuse could predict post-surgical pain. Due to the short nature of these pre-screening questionnaires, it warrants consideration that patients pre-operatively complete these questionnaires as a new “standard of care”. Further analysis, potential screening, and intervention for these correlations could reduce the prevalence of post-surgical pain as it relates to fractures.
Pharmacological Treatment
Pain during fracture healing is common and management is complex. Currently, the two primary treatments used to manage pain for trauma-induced fracture and post-surgical pain are opioids and NSAIDs. Both drugs have been shown to have negative off-target side effects and neither has demonstrated the ability to alleviate pain completely. Narcotics are the standard of care for the majority of orthopedic patients. This choice of drugs for pain is largely due to the presence of significant perioperative pain and the necessity for adequate analgesia in patient care in the postoperative setting (Figure 1). Animal models have shown that administration of morphine produces a dose-dependent reduction in spontaneous pain behaviors such as guarding and flinch as well as increased levels of weight bearing 7 days post fracture [27]. However, the use of opioids raises many concerns including the recent identification as a major contributor to the current opioid epidemic. These concerns include preclinical and clinical studies which indicate that opioids can actually elicit dose-dependent increases in pain [40, 62, 72, 73]. Additionally, opioids are known to elicit cognitive impairment as well as tolerance and addiction. Furthermore, many of the complications and side effects associated with opioids have been shown to be more prevalent in older patient populations, which is even more concerning given the increase in prevalence of osteoporosis in older patients [74]. There are additional concerns that opioids alone or with injury can actually increase long-term pain and create opioid induced hyperalgesia (OIH) [75–78]. Opioid receptors are present on osteoblasts which has led some to hypothesize that opioids may also impair fracture healing [79].
NSAIDs (cyclooxygenase [COX] inhibitors) block the COX enzymes and reduce prostaglandins throughout the body, which is effective at relieving pain for various musculoskeletal disorders [27, 80]. However, this class of drugs also produces dose-dependent negative side effects including gastrointestinal bleeding and kidney damage. Of particular concern with fracture pain is the negative impact on skeletal health and healing of fractured bones [81]. Animal studies suggest that COX inhibition diminishes tibial bone healing due to retardation of callus formation and bone repair [81, 82]. Some clinical studies suggest that NSAIDs do not affect fracture repair, whereas others demonstrate a negative effect of the drugs [83, 84]. Current recommendations conclude that NSAID use is warranted in fracture healing, as benefits outweigh the risks [85, 86]. Due to a lack of a causal relationship to fracture nonunion, the use of NSAIDs facilitates lower dose or complete avoidance of opioid prescriptions post-fracture repair. Such regimens have been shown to lower pain scores, lower adverse effects, and improve patient satisfaction scores [87•]. Despite these results, many physicians, especially those in the United States, avoid the use of NSAIDs for patients recovering from bone fractures due to the belief that NSAIDs impair bone healing [88, 89].
The United States Compared to Other Countries
An additional aspect to address is the disparity between opioid use in the United States as compared to other countries. Two studies highlight differences in opioid prescriptions and patient satisfaction that indicate underlying psychosocial and cultural influence. A comparative study between a United States and a Dutch hospital looked at the difference in opioid prescriptions and patient satisfaction of pain management for the treatment of ankle fractures [90•]. The study analyzed two different time points: post-operative day 1 and at suture removal (approximately 10-14 days). At each time point, patients completed a 5-point Likert scale to rate their pain and satisfaction with pain management.
On post-operative day 1, patients using opioids reported significantly worse average pain scores (3.0 vs. 2.5; p < 0.05). More patients in the United States also reported consuming opioids rather than alternatives such as acetaminophen or NSAIDs (100% vs. 67%; p < 0.001). Additionally, the majority of Dutch patients (15 of 20) that did use an opioid prescription opted for tramadol, a weak opioid agonist, as compared to Americans who used strong opioid agonists, such as oxycodone or hydrocodone. On post-operative day 1, there was no difference in patient satisfaction with pain management in the opioid vs. non-opioid patient populations. Following suture removal, patients using opioids reported significantly worse average pain scores (2.6 vs. 2.1; p < 0.01). More patients in the United States continued to consume opioids rather than alternative options (70% vs. 13%; p < 0.001). At suture removal, patients who were not taking opioids in either group reported greater satisfaction with pain management than those taking opioids (4.5 vs. 4.1; p < 0.05). In both the United States and Dutch populations, results from the 5-point Likert analysis indicated that the use of opioid medications was associated with greater pain and less satisfaction with pain management.
An additional study compared United States and Vietnamese patients in the treatment of closed femoral shaft fractures [91]. At 14 days post-surgical repair, Vietnamese patients received on average less morphine equivalents than United States patients (0.9 mg/kg/day vs. 30.2 mg/kg/day). Despite substantially higher doses of opioids. United States patients reported a greater dissatisfaction with pain management. Additionally, between the United States and Vietnamese groups, there was a significant difference in anticipation and expectation of fracture pain post-repair (4% vs. 76%). Despite the increase in opioid consumption. United States patients, on average, reported greater pain levels post-fracture. Furthermore, other countries report that equal or greater satisfaction in pain management can be obtained without the use of opioid medications. These findings could be a result of one of two factors. First, increased opioid intake by Americans could increase pain scores by OIH. Alternatively, these data may indicate a role for cultural and social influences on pain perception and management [92].
Conclusions: Evolving Landscape of Pain Management in the Era of the Opioid Crisis.
Current treatment options for the management of fracture pain are insufficient. Given the importance of managing fracture pain to maximize both patient comfort and fracture healing, the development of new analgesics that do not compromise fracture healing is a prominent need currently facing the medical community. The rising incidence of fractures in the aging United States population exacerbates this issue. In order to safely develop and test potential analgesic therapies, there must be an adequate preclinical model of implementing and assessing fracture pain. Because pain is a subjective feeling, assessment of nociception in non-communicating subjects such as animals is done through observations of “pain behaviors”. While no single behavioral observation is completely sufficient to truly evaluate fracture pain, using a combination of various pain behavior observations such as spontaneous pain behaviors and evoked pain behaviors has shown to have strong correlations with nociception in non-communicating subjects (see Table 1 for a summary). The accuracy of evoked cutaneous pain behaviors with regard to assessing skeletal pain remains a topic of debate, but spontaneous pain behaviors such as dynamic weight bearing analysis and guarding or flinching behaviors continue to provide dependable insights into fracture pain in preclinical models.
Table 1.
Assessment of Fracture Pain Behaviors
| Spontaneous Pain Behaviors | ||
|---|---|---|
| Method of Pain Assessment in Animals | Key Elements Assessed | Clinical Correlate |
| Guarding/Spontaneous Flinches | Time spent lifting and holding affect limb against body/number of spontaneous paw flinches over set period of time | Patient protecting injured limb from external mechanical stimuli |
| Static Weight Bearing | Relative difference of weight place on affected limb versus healthy limb when stationary | Relative force of body weight patient loads onto affected limb while standing |
| Dynamic Weight Bearing/Gait Analysis | Fraction of weight borne on each limb while freely moving/Interlimb coordination, stance phase duration, swing phase duration, stride length, and swing speed | Presence of limp or other abnormal gait mechanics in patient |
| Locomotive Activity | Distance traveled by freely moving animal over set period time | Patient activity level |
| Rearing/Grooming Behaviors | Time spent lifting both front paws off the floor at the same time (exploratory behavior)/Time spent groom fur and tending to injured limb (note: more grooming correlates with more pain) | Patient ability/drive to perform daily activities (note: increased daily activities inversely correlates with pain) |
| Evoked Pain Behaviors | ||
| Method of Pain Assessment in Animals | Key Elements Assessed | Clinical Correlate |
| Mechanical Von Frey | Amount of mechanical force required to trigger reflexive withdrawal response | Physician physical palpation of injured limb and assessment of pain response |
| Hargreaves Test (Thermal hyperalgesia) | Temperature required to trigger reflexive withdrawal response | Not commonly performed in clinical setting |
There is variation in each individual’s response to nociceptive stimuli and likewise, varying responses to analgesic treatments, thus dependable pain assessment tools also are needed in the clinic to monitor the effectiveness of current and future therapies for each individual patient. Keeping in mind that pain is a multifactorial experience that involves both nociceptive stimuli and biopsychosocial factors, these additional components must be considered when constructing assessment tools for pain. Data suggest that pre-screening and/or post-screening for certain biopsychosocial factors could indicate consideration for alternate pain management regimens in at-risk populations for chronic post-surgical or fracture pain and become the new “standard of care”.
Due to controversy with bone healing and nonunion, current management of acute and chronic fracture pain in the United States is achieved through opioids, rather than NSAIDs. Despite data suggesting opioids produced dose-dependent increases in post-surgical pain and OIH, they remain the current standard of care. In light of international comparative studies indicating opioids produced greater pain intensity and decreased satisfaction with pain management, the future of fracture pain management could shift to alternate regimens such as low dose opioids, inclusion of NSAIDs, regional nerve blocks, and various other molecular mechanism-based targets. With continued advancement in the assessment and quantification of fracture pain in both preclinical and clinical settings, we gain the tools needed to discover new therapies and improve current management of fracture pain.
Acknowledgements
This work was supported in part by an Indiana Center for Musculoskeletal Health and Stark Neurosciences Institute Multi-Center Pilot Funding award (JCF, FAW, MAK), an Indiana University Collaborative Research Grant (MAK), NIH/NIA R01 AG060621 (MAK), NIH/NINDS R01 NS102415 (FAW), and the Indiana Clinical and Translational Sciences Institute funded, in part by Award Number UL1TR002529 from the NIH, National Center for Advancing Translational Science, Clinical and Translational Sciences Award. This material is also the result of work supported by the Richard L. Roudebush VA Medical Center, Indianapolis, IN: VA Merit #BX003751 (MAK) and #BX002209 (FAW). The contents do not represent the views of the U.S. Department of Veterans Affairs, the United States Government, or the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
- 1.Melton LJ 3rd, Chrischilles EA, Cooper C, Lane AW, Riggs BL. Perspective. How many women have osteoporosis? Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 1992:7(9): 1005–10. doi: 10.1002/jbmr.5650070902. [DOI] [PubMed] [Google Scholar]
- 2.Kanis JA, Oden A, Jolmell O, Jonsson B, de Laet C, Dawson A. The burden of osteoporotic fractures: a method for setting intervention thresholds. Osteoporosis international: a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2001; 12(5):417–27. doi: 10.1007/s001980170112. [DOI] [PubMed] [Google Scholar]
- 3.Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005–2025. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2007;22(3):465–75. doi: 10.1359/jbmr.061113. [DOI] [PubMed] [Google Scholar]
- 4.Gruber R, Koch H, Doll BA, Tegtmeier F, Einliom TA, Hollinger JO. Fracture healing in the elderly patient. Experimental gerontology. 2006;41(11):1080–93. doi: 10.1016/j.exger.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 5.Blyth FM, Cumming R, Mitchell P, Wang JJ. Pain and falls in older people. European journal of pain (London, England). 2007;11(5):564–71. doi: 10.1016/j.ejpain.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 6.••.Majuta LA, Longo G, Fealk MN, McCaffrey G, Mantyh PW. Orthopedic surgery and bone fracture pain are both significantly attenuated by sustained blockade of nerve growth factor. Pain. 2015;156(1):157–65. doi: 10.1016/j.pain.0000000000000017. [DOI] [PMC free article] [PubMed] [Google Scholar]; Recent study demonstrating utility of currently used rodent pain assessment tools to evaluate possible treatments for fracture pain.
- 7.Bukata SV, Digiovanni BF, Friedman SM, Hoyen H, Kates A, Kates SL et al. A guide to improving the care of patients with fragility fractures. Geriatric orthopaedic surgery & rehabilitation. 2011;2(1):5–37. doi: 10.1177/2151458510397504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Castillo RC, MacKenzie EJ, Wegener ST, Bosse MJ. Prevalence of chronic pain seven years following limb threatening lower extremity trauma. Pain. 2006;124(3):321–9. doi: 10.1016/j.pain.2006.04.020. [DOI] [PubMed] [Google Scholar]
- 9.Fillingim RB. Individual differences in pain responses. Current rheumatology reports. 2005;7(5):342–7. [DOI] [PubMed] [Google Scholar]
- 10.Marks R Physical activity and hip fracture disability: a review. J Aging Res. 2011;2011:741918. doi: 10.4061/2011/741918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goh EL, Chidambaram S, Ma D. Complex regional pain syndrome: a recent update. Burns Trauma. 2017;5:2. doi: 10.1186/s41038-016-0066-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rainville P Brain mechanisms of pain affect and pain modulation. Current opinion in neurobiology. 2002; 12(2): 195–204. [DOI] [PubMed] [Google Scholar]
- 13.Vranceanu A-M, Jupiter JB, Mudgal CS, Ring D. Predictors of Pain Intensity and Disability After Minor Hand Surgery. 2010;35(6):956–60. [DOI] [PubMed] [Google Scholar]
- 14.Monfoulet L, Rabier B, Chassande O, Fricain JC. Drilled hole defects in mouse femur as models of intramembranous cortical and cancellous bone regeneration. Calcified tissue international. 2010;86(1):72–81. doi: 10.1007/s00223-009-9314-y. [DOI] [PubMed] [Google Scholar]
- 15.Bigham-Sadegh A, Oryan A. Selection of animal models for pre-clinical strategies in evaluating the fracture healing, bone graft substitutes and bone tissue regeneration and engineering. Connective tissue research. 2015:56(3): 175–94. doi: 10.3109/03008207.2015.1027341. [DOI] [PubMed] [Google Scholar]
- 16.•.Koewler NJ, Freeman KT, Buus RJ, Herrera MB, Jimenez-Andrade JM, Ghilardi JR et al. Effects of a monoclonal antibody raised against nerve growth factor on skeletal pain and bone healing after fracture of the C57BL/6J mouse femur. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2007;22(11): 1732–42. doi: 10.1359/jbmr.070711. [DOI] [PubMed] [Google Scholar]; One of the first studies published utilizing rodent pain behavior measures to assess fracture pain and possible treatment.
- 17.Mitchell SAT, Majuta LA, Mantyh PW. New Insights in Understanding and Treating Bone Fracture Pain. Current osteoporosis reports. 2018;16(4):325–32. doi: 10.1007/s11914-018-0446-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.•.illville V, Laffosse JM, Fourcade O, Girolami JP, Tack I. Mouse model of fracture pain. Anesthesiology. 2008;108(3):467–72. doi: 10.1097/ALN.0b013e3181649333. [DOI] [PubMed] [Google Scholar]; One of the early studies outlining mouse model of assessing fracture pain
- 19.Chang C, Shyu BC. A fMRI study of brain activations during non-noxious and noxious electrical stimulation of the sciatic nerve of rats. Brain research. 2001;897(1-2):71–81. [DOI] [PubMed] [Google Scholar]
- 20.Guedon J-MG, Longo G, Majuta LA, Thomspon ML, Fealk MN, Mantyh PW. Dissociation between the relief of skeletal pain behaviors and skin hypersensitivity in a model of bone cancer pain. Pain. 2016; 157(6): 1239–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gregory NS, Harris AL, Robinson CR, Dougherty PM, Fuchs PN, Sluka KA. An overview of animal models of pain: disease models and outcome measures. The journal of pain : official journal of the American Pain Society. 2013; 14(11): 1255–69. doi: 10.1016/j.jpain.2013.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fuchs PN, Campbell JN, Meyer RA. Secondary hyperalgesia persists in capsaicin desensitized skin. Pain. 2000;84(2-3):141–9. [DOI] [PubMed] [Google Scholar]
- 23.Blackburn-Munro G Pain-like behaviours in animals - how human are they? Trends in pharmacological sciences. 2004;25(6):299–305. doi: 10.1016/j.tips.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 24.Brodkin J, Frank D, Grippo R, Hausfater M, Gulinello M, Achterholt N et al. Validation and implementation of a novel high-throughput behavioral phenotyping instrument for mice. Journal of neuroscience methods. 2014;224:48–57. doi: 10.1016/j.jneumeth.2013.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Santy J, Mackintosh C. A phenomenological study of pain following fractured shaft of femur. Journal of clinical nursing. 2001;10(4):521–7. [DOI] [PubMed] [Google Scholar]
- 26.Sluka KA, Westlund KN. Behavioral and immunohistochemical changes in an experimental arthritis model in rats. Pain. 1993;55(3):367–77. [DOI] [PubMed] [Google Scholar]
- 27.Freeman KT, Koewler NJ, Jimenez-Andrade JM, Buus RJ, Herrera MB, Martin CD et al. A fracture pain model in the rat: adaptation of a closed femur fracture model to study skeletal pain. Anesthesiology. 2008;108(3):473–83. doi: 10.1097/ALN.0b013e3181649351. [DOI] [PubMed] [Google Scholar]
- 28.Schott E, Berge OG, Angeby-Moller K, Hammarstrom G, Dalsgaard CJ, Brodin E. Weight bearing as an objective measure of arthritic pain in the rat. Journal of pharmacological and toxicological methods. 1994;31(2):79–83. [DOI] [PubMed] [Google Scholar]
- 29.Medhurst SJ, Walker K, Bowes M, Kidd BL, Glatt M, Muller M et al. A rat model of bone cancer pain. Pain. 2002;96(1-2): 129–40. [DOI] [PubMed] [Google Scholar]
- 30.Griffioen MA, Dernetz VH, Yang GS, Griffith KA, Dorsey SG, Renn CL. Evaluation of dynamic weight bearing for measuring nonevoked inflammatory hyperalgesia in mice. Nursing research. 2015;64(2):81–7. doi: 10.1097/nnr.0000000000000082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tetreault P, Dansereau MA, Dore-Savard L, Beaudet N, Sarret P. Weight bearing evaluation in inflammatory, neuropathic and cancer chronic pain in freely moving rats. Physiology & behavior. 2011;104(3):495–502. doi: 10.1016/j.physbeh.2011.05.015. [DOI] [PubMed] [Google Scholar]
- 32.Kappos EA, Sieber PK, Engels PE, Mariolo AV, D’Arpa S, Schaefer DJ et al. Validity and reliability of the CatWalk system as a static and dynamic gait analysis tool for the assessment of functional nerve recovery in small animal models. Brain and behavior. 2017;7(7):e00723. doi: 10.1002/brb3.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ishikawa G, Nagakura Y, Takeshita N, Shimizu Y. Efficacy of drugs with different mechanisms of action in relieving spontaneous pain at rest and during movement in a rat model of osteoarthritis. European journal of pharmacology. 2014;738:111–7. doi: 10.1016/j.ejphar.2014.05.048. [DOI] [PubMed] [Google Scholar]
- 34.Miyagi M, Ishikawa T, Kamoda H, Orita S, Kuniyoshi K, Ochiai N et al. Assessment of gait in a rat model of myofascial inflammation using the CatWalk system. Spine. 2011;36(21): 1760–4. doi: 10.1097/BRS.0b013e3182269732. [DOI] [PubMed] [Google Scholar]
- 35.Histing T, Kristen A, Roth C, Holstein JH, Garcia P, Matthys R et al. In vivo gait analysis in a mouse femur fracture model. Journal of biomechanics. 2010;43(16):3240–3. doi: 10.1016/j.jbiomech.2010.07.019. [DOI] [PubMed] [Google Scholar]
- 36.Gabriel AF, Marcus MA, Walenkamp GH, Joosten EA. The CatWalk method: assessment of mechanical allodynia in experimental chronic pain. Behavioural brain research. 2009;198(2):477–80. doi: 10.1016/j.bbr.2008.12.018. [DOI] [PubMed] [Google Scholar]
- 37.Novak CM, Burghardt PR, Levine JA. The use of a running wheel to measure activity in rodents: relationship to energy balance, general activity, and reward. Neuroscience and biobehavioral reviews. 2012;36(3): 1001–14. doi: 10.1016/j.neubiorev.2011.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cobos EJ, Ghasemlou N, Araldi D, Segal D, Duong K, Woolf CJ. Inflammation-induced decrease in voluntary wheel running in mice: a nonreflexive test for evaluating inflammatory pain and analgesia. Pain. 2012;153(4):876–84. doi: 10.1016/j.pain.2012.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Prasad J, Wiater BP, Nork SE, Bain SD, Gross TS. Characterizing gait induced normal strains in a murine tibia cortical bone defect model. Journal of biomechanics. 2010;43(14):2765–70. doi: 10.1016/j.jbiomech.2010.06.030. [DOI] [PubMed] [Google Scholar]
- 40.•.Li WW, Irvine KA, Sahbaie P, Guo TZ, Shi XY, Tawfik VL et al. Morphine Exacerbates Postfracture Nociceptive Sensitization, Functional Impairment, and Microglial Activation in Mice. Anesthesiology. 2019;130(2):292–308. doi: 10.1097/aln.0000000000002495. [DOI] [PMC free article] [PubMed] [Google Scholar]; Recent use of modern rodent behavioral models to monitor current fracture pain treatments.
- 41.Pratt D, Fuchs PN, Sluka KA. Assessment of avoidance behaviors in mouse models of muscle pain. Neuroscience. 2013;248:54–60. doi: 10.1016/j.neuroscience.2013.05.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tanaka S, Young JW, Halberstadt AL, Masten VL, Geyer MA. Four factors underlying mouse behavior in an open field. Behavioural brain research. 2012;233(1):55–61. doi: 10.1016/j.bbr.2012.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim SH, Chung JM. An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain. 1992;50(3):355–63. doi: 10.1016/0304-3959(92)90041-9. [DOI] [PubMed] [Google Scholar]
- 44.Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. Journal of neuroscience methods. 1994;53(1):55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 45.Bonin RP, Bories C, De Koninck Y. A simplified up-down method (SUDO) for measuring mechanical nociception in rodents using von Frey filaments. Mol Pain. 2014;10:26. doi: 10.1186/1744-8069-10-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.••.Deuis JR, Dvorakova LS, Vetter I. Methods Used to Evaluate Pain Behaviors in Rodents. Frontiers in molecular neuroscience. 2017; 10:284. doi: 10.3389/fnmol.2017.00284 [DOI] [PMC free article] [PubMed] [Google Scholar]; Excellent review of a multitude of pain behavioral measurements for a variety of pain states in rodents
- 47.Deuis JR, Lim YL, Rodrigues de Sousa S, Lewis RJ, Alewood PF, Cabot PJ et al. Analgesic effects of clinically used compounds in novel mouse models of polyneuropathy induced by oxaliplatin and cisplatin. Neuro-oncology. 2014; 16(10): 1324–32. doi: 10.1093/neuonc/nou048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Deuis JR, Whately E, Brust A, Inserra MC, Asvadi NH, Lewis RJ et al. Activation of kappa Opioid Receptors in Cutaneous Nerve Endings by Conorphin-1, a Novel Subtype-Selective Conopeptide, Does Not Mediate Peripheral Analgesia. ACS chemical neuroscience. 2015;6(10): 1751–8. doi: 10.1021/acschemneuro.5b00113. [DOI] [PubMed] [Google Scholar]
- 49.Deuis JR, Vetter I. The thermal probe test: A novel behavioral assay to quantify thermal paw withdrawal thresholds in mice. Temperature (Austin, Tex). 2016;3(2):199–207. doi: 10.1080/23328940.2016.1157668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lu R, Schmidtko A. Direct intrathecal drug delivery in mice for detecting in vivo effects of cGMP on pain processing. Methods in molecular biology (Clifton, NJ). 2013;1020:215–21. doi: 10.1007/978-1-62703-459-3_14. [DOI] [PubMed] [Google Scholar]
- 51.Espejo EF, Mir D. Structure of the rat’s behaviour in the hot plate test. Behavioural brain research. 1993;56(2): 171–6. [DOI] [PubMed] [Google Scholar]
- 52.Minville V, Mouledous L, Jaafar A, Couture R, Brouchet A, Frances B et al. Tibial post fracture pain is reduced in kinin receptors deficient mice and blunted by kinin receptor antagonists. J Transl Med. 2019; 17(1):346. doi: 10.1186/s12967-019-2095-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.•.Gucdon JM, Longo G, Majuta LA, Thomspon ML, Fealk MN, Mantyh PW. Dissociation between the relief of skeletal pain behaviors and skin hypersensitivity in a model of bone cancer pain. Pain. 2016; 157(6): 1239–47. doi: 10.1097/j.pain.0000000000000514. [DOI] [PMC free article] [PubMed] [Google Scholar]; Key study demonstrating differences between measured pain behaviors of skeletal pain and skin hypersensitivity associated with skeletal pain. Indicates the attenuation of skin hypersensitivity does not necessarily correlate with relief of skeletal pain.
- 54.Morone NE, Weiner DK. Pain as the fifth vital sign: exposing the vital need for pain education. Clinical therapeutics. 2013:35(11): 1728–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Litcher-Kelly L, Martino SA, Broderick JE, Stone AA. A systematic review of measures used to assess chronic musculoskeletal pain in clinical and randomized controlled clinical trials. The journal of pain : official journal of the American Pain Society. 2007;8(12):906–13. doi: 10.1016/j.jpain.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hjermstad MJ, Fayers Pm Fau - Haugen DF, Haugen Df Fau - Caraceni A, Caraceni A Fau Hanks GW, Hanks Gw Fau Loge JH, Loge Jh Fau Fainsinger R et al. Studies comparing Numerical Rating Scales, Verbal Rating Scales, and Visual Analogue Scales for assessment of pain intensity in adults: a systematic literature review. (1873-6513 (Electronic)). [DOI] [PubMed] [Google Scholar]
- 57.Jensen MP, Karoly P, Braver S. The measurement of clinical pain intensity: a comparison of six methods. Pain. 1986:27(1). [DOI] [PubMed] [Google Scholar]
- 58.Fuentes-Losada LM, Vergara-Amador E, Laverde-Cortina R. Pain management assessment in children with limb fractures in an emergency service. 2016;44(4):305–10. [Google Scholar]
- 59.Hicks CL, von Baeyer CL, Spafford PA, van Korlaar I, Goodenough B. The Faces Pain Scale – Revised: toward a common metric in pediatric pain measurement. 2001;93(2):173–83. [DOI] [PubMed] [Google Scholar]
- 60.Noback P, Cuellar D, Lombardi J, Swart E, Rosenwasser M. Evaluating Pain in Orthopedic Patients: Can the Visual Analog Scale be used as a Long-term Outcome Instrument? Journal of Pain & Relief. 2015:4(182). doi: 10.4172/2167-0846.1000182. [DOI] [Google Scholar]
- 61.Mehack R, Torgerson WS. On the Language of Pain. Anesthesiology: The Journal of the American Society of Anesthesiologists. 1971;34(1):50–9. [DOI] [PubMed] [Google Scholar]
- 62.•.Bot AGJ, Bekkers S, Amstein PM, Smith RM, Ring D. Opioid Use After Fracture Surgery Correlates With Pain Intensity and Satisfaction With Pain Relief. 2014;472(8):2542–9. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study highlights the correlations between opioids for post-surgical pain management and respective outcome and patient satisfaction.
- 63.Pinto PR, McIntyre T, Ferrero R, Almeida A, Araujo-Soares V. Predictors of acute postsurgical pain and anxiety following primary total hip and knee arthroplasty. The journal of pain : official journal of the American Pain Society. 2013; 14(5):502–15. doi: 10.1016/j.jpain.2012.12.020. [DOI] [PubMed] [Google Scholar]
- 64.•.Masselm-Dubois A, Attal N, Fletcher D, Jayr C, Albi A, Fermanian J et al. Are psychological predictors of chronic postsurgical pain dependent on the surgical model? A comparison of total knee arthroplasty and breast surgery for cancer. The journal of pain : official journal of the American Pain Society. 2013;14(8):854–64. doi: 10.1016/j.jpain.2013.02.013. [DOI] [PubMed] [Google Scholar]; Study evaluating the contribution of biopsychosocial factors to both acute and chronic post-surgical pain in orthopedic models.
- 65.Nota SPFT, Spit SA, Voskuyl T, Bot AGJ, Hageman MGJS, Ring D. Opioid Use, Satisfaction, and Pain Intensity After Orthopedic Surgery. 2015;56(5):479–85. [DOI] [PubMed] [Google Scholar]
- 66.Theunissen M, Peters Ml Fau Brace J, Brace J Fau Gramke H-F, Gramke Hf Fau Marcus MA, Marcus MA. Preoperative anxiety and catastrophizing: a systematic review and meta-analysis of the association with chronic postsurgical pain. (1536–5409 (Electronic)). [DOI] [PubMed] [Google Scholar]
- 67.Association AP. Diagnostic and statistical manual of mental health disorders . Washington, DC: Author; 2010. [Google Scholar]
- 68.Spitzer RL, Williams JBW, Kroenke K, Linzer M, deGray FV III, Halm SR et al. Utility of a New Procedure for Diagnosing Mental Disorders in Primary Care: The PRIME-MD 1000 Study. JAMA. 1994;272(22): 1749–56. [PubMed] [Google Scholar]
- 69.McCracken LM, Dhingra L. A short version of the Pain Anxiety Symptoms Scale (PASS-20): preliminary development and validity. Pain Research and Management. 2002;7(1):45–50. [DOI] [PubMed] [Google Scholar]
- 70.Nicholas MK. The pain self-efficacy questionnaire: Taking pain into account. European journal of pain (London, England). 2007;11(2):153–63. doi: 10.1016/j.ejpain.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 71.Sullivan MJ, Bishop SR, Pivik J. The pain catastrophizing scale: development and validation. Psychological assessment. 1995;7(4):524. [Google Scholar]
- 72.Wilson NM, Ripsch MS, White FA. Impact of Opioid and Nonopioid Drugs on Postsurgical Pain Management in the Rat. Pain research and treatment. 2016;2016:8364762. doi: 10.1155/2016/8364762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hayhurst CJ, Durieux ME. Differential Opioid Tolerance and Opioid-induced Hyperalgesia: A Clinical Reality. Anesthesiology. 2016;124(2):483–8. doi: 10.1097/aln.0000000000000963. [DOI] [PubMed] [Google Scholar]
- 74.Kassam AM, Gough AT, Davies J, Yarlagadda R. Can we reduce morphine use in elderly, proximal femoral fracture patients using a fascia iliac block? Geriatric nursing (New York, NY). 2018;39(1):84–7. doi: 10.1016/j.gerinurse.2017.07.003. [DOI] [PubMed] [Google Scholar]
- 75.Angst Martin SMD, Clark JDMDPD. Opioid-induced Hyperalgesia: A Qualitative Systematic Review. Anesthesiology: The Journal of the American Society of Anesthesiologists. 2006;104(3):570–87. [DOI] [PubMed] [Google Scholar]
- 76.Lee M, Silverman SM, Hansen H, Patel VB, Manchikanti L. A comprehensive review of opioid-induced hyperalgesia. Pain physician. 2011; 14(2): 145–61. [PubMed] [Google Scholar]
- 77.Wilson NM, Jung H, Ripsch MS, Miller RJ, White FA. CXCR4 signaling mediates morphine-induced tactile hyperalgesia. Brain, behavior, and immunity. 2011;25(3):565–73. doi: 10.1016/j.bbi.2010.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wilson JL, Poulin PA, Sikorski R, Nathan HJ, Taljaard M, Smyth C. Opioid Use among Same-Day Surgery Patients: Prevalence, Management and Outcomes. Pain Research and Management. 2015;20(6). doi: 10.1155/2015/897491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jain N, Himed K, Toth JM, Briley KC, Phillips FM, Khan SN. Opioids delay healing of spinal fusion: a rabbit posterolateral lumbar fusion model. The spine journal : official journal of the North American Spine Society. 2018;18(9):1659–68. doi: 10.1016/j.spinee.2018.04.012. [DOI] [PubMed] [Google Scholar]
- 80.Balano KB. Anti-inflammatory drugs and myorelaxants. Pharmacology and clinical use in musculoskeletal disease. Primary care. 1996;23(2):329–34. [DOI] [PubMed] [Google Scholar]
- 81.Kurmis AP, Kurmis TP, O’Brien JX, Dalen T. The effect of nonsteroidal anti-inflammatory drug administration on acute phase fracture-healing: a review. The Journal of bone and joint surgery American volume. 2012;94(9):815–23. doi: 10.2106/jbjs.J.01743. [DOI] [PubMed] [Google Scholar]
- 82.Lu C, Xing Z, Wang X, Mao J, Marcucio RS, Miclau T. Anti-inflammatory treatment increases angiogenesis during early fracture healing. Archives of orthopaedic and trauma surgery. 2012;132(8):1205–13. doi: 10.1007/s00402-012-1525-4. [DOI] [PubMed] [Google Scholar]
- 83.Pountos I, Georgouli T, Calori GM, Giannoudis PV. Do nonsteroidal anti-inflammatory drugs affect bone healing? A critical analysis. ScientificWorldJournal. 2012;2012:606404. doi: 10.1100/2012/606404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Geusens P, Emans PJ, de Jong JJ, van den Bergh J. NSAIDs and fracture healing. Current opinion in rheumatology. 2013;25(4):524–31. doi: 10.1097/BOR.0b013e32836200b8. [DOI] [PubMed] [Google Scholar]
- 85.Derby R,MD, Beutler A,MD. General principles of acute fracture management In: Eiff P,MD, Asplund C,MDFACSMMPH, Grayzel J,MDFAAEM, editors. UpToDate. UpToDate, Waltham, MA: (Accessed on June 16, 2019)2018. [Google Scholar]
- 86.Solomon D,MDMPH. Nonselective NSAIDs: Overview of adverse effects In: Furst D,MD, Romain P,MD, editors. UpToDate. UpToDate, Waltham, MA: (Accessed June 18, 2019)2018. [Google Scholar]
- 87.•.Post ZD, Restrepo C, K. Kahl L, van de Leur T, Purtill JJ, Hozack WJ. A Prospective Evaluation of 2 Different Pain Management Protocols for Total Hip Arthroplasty. 2010;25(3):410–5. [DOI] [PubMed] [Google Scholar]; This study highlights the efficacy of NSAIDs in avoidance or reduction of opioids for post-surgical pain management with respective outcome and patient satisfaction.
- 88.Su B, O’Connor JP. NSAID therapy effects on healing of bone, tendon, and the enthesis. Journal of applied physiology (Bethesda, Md : 1985). 2013;115(6):892–9. doi: 10.1152/japplphysiol.00053.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Roberts M, Brodribb W, Mitchell G. Reducing the pain: a systematic review of postdischarge analgesia following elective orthopedic surgery. Pain medicine (Malden, Mass). 2012;13(5):711–27. doi: 10.1111/j.1526-4637.2012.01359.x. [DOI] [PubMed] [Google Scholar]
- 90.•.Helmerhorst GTT, Lindenhovius ALC, Vrahas M, Ring D, Kloen P. Satisfaction with pain relief after operative treatment of an ankle fracture. 2012:43(11): 1958–61. [DOI] [PubMed] [Google Scholar]; One of the key international studies that contrasted analgesic regimens and overall patient satisfaction with pain management post-fracture
- 91.Carragee EJ, Vittum D, Truong TP, Burton D. Pain control and cultural norms and expectations after closed femoral shaft fractures. American journal of orthopedics (Belle Mead, NJ). 1999;28(2):97–102. [PubMed] [Google Scholar]
- 92.Koehler RM, Okoroafor UC, Cannada LK. A systematic review of opioid use after extremity trauma in orthopedic surgery. Injury. 2018;49(6): 1003–7. doi: 10.1016/j.injury.2018.04.003. [DOI] [PubMed] [Google Scholar]


