Abstract
By the time they are typically detected, neurodevelopmental disorders like autism spectrum disorder (ASD) are already challenging to treat. Preventive and early intervention strategies in infancy are critical for improving outcomes over the lifespan with significant cost savings. However, the impact of prevention and early intervention efforts is dependent upon our ability to identify infants most appropriate for such interventions. Because there may be significant overlap between prodromal symptoms across neurodevelopmental disorders and child psychopathology more broadly which may wax and wane across development, we contend that the impact of prevention and early intervention efforts will be heightened by identifying early indicators that may overlap across ASD and other commonly co-occurring disorders. This paper summarizes the existing literature on infant symptoms and identification of ASD to demonstrate the ways in which a transdiagnostic perspective could expand the impact of early identification and intervention research and clinical efforts, and to outline suggestions for future empirical research programs addressing current gaps in the identification-to-treatment pipeline. We propose four recommendations for future research that are both grounded in developmental and clinical science and that are scalable for early intervention systems: (1) development of fine-grained, norm-referenced measures of ASD-relevant transdiagnostic behavioral domains; (2) identification of shared and distinct mechanisms influencing the transition from risk to disorder; (3) determination of key cross-cutting treatment strategies (both novel and extracted from existing approaches) effective in targeting specific domains across disorders; and (4) integration of identified measures and treatments into existing service systems.
Keywords: Autism spectrum disorder, infancy, transdiagnostic, early identification, early intervention, RDoC, development
By the time they are typically detected, neurodevelopmental disorders like autism spectrum disorder (ASD) are already challenging to treat. ASD is increasingly prevalent, emerges early in development, and is associated with significant long-term impairment (Bal, Kim, Cheong, & Lord, 2015; Howlin & Magiati, 2017; Howlin, Moss, Savage, & Rutter, 2013). The economic burden resulting from elevated healthcare costs, costs to families, and costs associated with lost work represents an issue of considerable public health concern (Lavelle et al., 2014). Preventive and early intervention strategies are likely to be the most effective approaches to improving outcomes over the lifespan, with significant cost savings (Chasson, Harris, & Neely, 2007; Cidav et al., 2017; Kim, Bal, & Lord, 2018; Knudsen, Heckman, Cameron, & Shonkoff, 2006). However, the impact of these efforts is dependent upon our ability to identify infants and very young children most appropriate for such interventions.
Over the past two decades, there have been substantial efforts to uncover the earliest emerging signs of ASD. One of the key clinical implications of these early identification studies is earlier referral to evidence-based early intervention. While reliable diagnoses of ASD can be made as early as 18 months of age in some cases (Ozonoff et al., 2015; Zwaigenbaum et al., 2016), and although there are evidence-based treatments for toddlers with ASD (e.g., Carter et al., 2011; Dawson et al., 2010; Kaiser & Roberts, 2013; Kasari, Freeman, & Paparella, 2006; R. L. Koegel, Koegel, & Carter, 1999; Lovaas, 1987; McEachin, Smith, & Lovaas, 1993; see Sandbank et al., 2020 for a recent meta-analysis), the development of ASD-relevant and specific screening and intervention programs for infants has been hampered by a number of methodological and conceptual disagreements and a relative lack of fine-grained cross-disorder longitudinal comparisons. These issues have limited our understanding of early behavioral indicators and treatment targets that may be shared across, or distinct between, ASD and other emerging neurodevelopmental disorders (and, perhaps, child psychopathology more broadly).
While it is critical for diagnostic purposes to identify disorder-specific early behavioral indicators, we contend that the impact of prevention and early intervention efforts will be heightened by also identifying early indicators that may overlap across ASD and other commonly co-occurring disorders. Indeed, a range of other conditions are frequently comorbid with ASD, including attention-deficit/hyperactivity disorder (ADHD), anxiety disorders, externalizing disorders, and mood disorders (Abdallah et al., 2011; Houghton, Ong, & Bolognani, 2017). Transdiagnostic approaches focus on identifying processes that are shared across disorders and that underlie and maintain symptoms (Harvey, Watkins, Mansell, & Shafran, 2004; Nolen-Hoeksema & Watkins, 2011). This framework is frequently being utilized in the study of adult psychopathology, but has less often been applied to neurodevelopmental disorders, particularly in infancy and prior to diagnosis. These approaches could have wide-reaching effects, leading to treatments targeting impaired processes that can be applied across individuals with, or at risk for, various disorders, thereby more efficiently leveraging the limited funding allocated to early intervention services. Indeed, if key shared factors can be identified early in life across children at high risk for a range of atypical development including ASD and common comorbidities, prevention and intervention programs targeting such factors may have wider-reaching applications than those targeting disorder-specific early indicators. Transdiagnostic prevention and intervention efforts would be especially impactful during the period in infancy when a child’s outcome is still unclear and symptoms are in the process of emerging. Intervening before symptoms have become clearly instantiated is closer to a true ‘prevention’ model, which seeks to reduce the likelihood of symptoms emerging, in contrast to a diagnostic-based approach which attempts to reduce or ameliorate clinically-significant symptoms already present (Dawson, 2008; Dryden & Dryden, 2018).
The goals of this Future Directions paper are to summarize the existing literature on infant symptoms and identification of ASD, to demonstrate the ways in which a transdiagnostic perspective could address current challenges in early identification and intervention research and clinical efforts, and to outline suggestions for future empirical research programs addressing current gaps in the identification-to-treatment pipeline. Of note, we primarily focus on the first year of life, a period during which there is massive developmental plasticity and potential benefit from efficacious interventions, but a period that is also characterized by significant phenotypic heterogeneity and overlap in prodromal symptoms across neurodevelopmental disorders. We advocate for the development of a unified approach to early identification of ASD, neurodevelopmental disorders, and child psychopathology more broadly that is both grounded in developmental and clinical science and scalable for early intervention systems.
Current Approaches to Infant Identification and Intervention: What do we Know?
Infant identification
Fulfilling the promise of early intervention requires efficacious early screening and identification of infants who will benefit. Initial studies focused on early identification of ASD relied on retrospective analysis of infant/toddler home videos of diagnosed children (Osterling & Dawson, 1994; Osterling, Dawson, & Munson, 2002; Ozonoff, Iosif, et al., 2011; Werner, Dawson, Munson, & Osterling, 2005; Werner, Dawson, Osterling, & Dinno, 2000), providing crucial insights into early markers and patterns of symptom emergence and paving the way for prospective studies. However, although these studies offered some of the first opportunities to examine the early development of autism symptoms, there were significant methodological limitations (Palomo, Belinchón, & Ozonoff, 2006), resulting in a shift toward prospective high-risk infant studies given the high rates of recurrence within families, which near 20% (Ozonoff, Young, et al., 2011). These prospective studies involve the recruitment of infants at familial risk for ASD—younger siblings of diagnosed children—from early in life in order to identify early indicators. Notably, they do not come without limitations themselves, the most prominent being questions around generalizability to non-familial cases of ASD.
Despite herculean efforts, more than a decade of research employing these prospective ‘infant sibling’ designs have failed to find robust behavioral markers specific to ASD risk in infants between the ages of 0 to 12 months (Zwaigenbaum et al., 2015). Rather, differences at a group level have been documented most consistently between 12 to 24 months of age (Landa, Gross, Stuart, & Faherty, 2013; Ozonoff et al., 2010, 2008; Szatmari et al., 2016), with rare exceptions of group differences prior to 12 months (Miller et al., 2017; Nyström, Thorup, Bölte, & Falck-Ytter, 2019). Thus, although a small number of infants demonstrate overt behavioral symptoms of ASD as early as the first year of life (Bryson et al., 2007; Chawarska, Macari, & Shic, 2013; L. K. Koegel, Singh, Koegel, Hollingsworth, & Bradshaw, 2013; Rogers et al., 2014), the emergence of ASD symptoms is most often insidious, consisting of gradual declines in core social communication behaviors (Ozonoff et al., 2018; Ozonoff & Iosif, 2019). Beyond early behavioral indicators, a number of studies suggest that it may be possible to identify brain-based differences that capture increased risk before behavioral symptoms are present, including the presence of increased extra-axial cerebrospinal fluid (Shen et al., 2018, 2013), altered brain morphology (Wolff et al., 2015), differences in functional connectivity patterns (Pruett et al., 2015), and differences in microstructural properties of white matter fiber tracts (Wolff et al., 2012). The degree to which such measurements will be scalable and translatable to routine clinical practice is unclear, however.
Because many psychiatric disorders share some overlapping risk factors (Gandal et al., 2018), a growing body of research has begun to focus on the intersection between ASD and other psychiatric conditions. For example, ADHD commonly co-occurs with ASD (Leitner, 2014) and is more prevalent among family members of individuals with ASD than in the general population (Ghirardi et al., 2018; Jokiranta-Olkoniemi et al., 2016; Miller et al., 2019). Research has suggested shared genetic influences (Stergiakouli et al., 2017), and studies comparing neural, cognitive, and behavioral profiles of ASD and ADHD have revealed some similarities (Di Martino et al., 2013; Geurts et al., 2004; Semrud-Clikeman, Walkowiak, Wilkinson, & Butcher, 2010). Additionally, some children who meet criteria for ASD in preschool “evolve” to exhibit behavioral phenotypes more consistent with ADHD by middle childhood (Fein, Dixon, Paul, & Levin, 2005). In a seminal review, Johnson and colleagues (2015) highlighted key behavioral domains that may be disrupted in the early development of both ASD and ADHD, including attention regulation, temperament and self-regulation, social interaction and communication, motor skills, and sensory processing/perception (M. H. Johnson, Gliga, Jones, & Charman, 2015). Recent work from one of our own research groups has found that reduced orienting to name—a behavior typically thought to serve as a specific early indicator of ASD (and one of the earliest-documented behavioral differences among infants developing ASD; Miller et al., 2017)—may in fact be a general marker for ASD and risk for ADHD earlier in infancy but become a more specific indicator of ASD by 24 months of age (Hatch et al., 2020). Likewise, we recently described a mixture of overlapping and distinct early markers of preschool ASD- and ADHD-like latent profiles which can be difficult to disentangle early in life (Miller et al., 2020). These challenges may have significant clinical implications with respect to early identification and referral to early intervention; one study showed that diagnoses of ASD were delayed by an average of 3 years among children who received initial diagnoses of ADHD (Miodovnik, Harstad, Sideridis, & Huntington, 2015).
Similarly, anxiety disorders also frequently co-occur with ASD (Kirsch et al., 2020; see Kerns & Kendall, 2012 for a review), and anxiety symptom levels tend to be higher, on average, among unaffected family members of individuals with ASD (Howlin, Moss, Savage, Bolton, & Rutter, 2015; Shephard et al., 2017). Recent work seeking to understand the overlap in early predictors of ASD and anxiety symptoms in middle childhood has highlighted some shared predictors based on parent ratings of infant temperament, including high levels of fearfulness/shyness (Shephard et al., 2019).
These examples are intended to be illustrative, not exhaustive, but they highlight the point that it can be challenging to distinguish neurodevelopmental disorders and child psychopathology as they are emerging due to phenotypic and, possibly, etiological overlap, at least during certain periods of development. Some early behavioral indicators may overlap across these populations serving as general indices of atypical development that could be leveraged for transdiagnostic treatment development efforts. Indeed, it is likely that transdiagnostic or cross-disorder approaches to identification of early markers and disrupted processes could include ASD and a number of other conditions (e.g., schizophrenia). In our view, the overarching goals of this research are (1) to develop a deeper understanding of the pathogenesis of these conditions, and (2) to identify factors that could be tested as relevant targets of prevention and early intervention programs. Ultimately, for now we are still left asking, when and for whom should we intervene in infancy?
Infant intervention
As noted at the outset, preventive and early intervention strategies are critical to improving outcomes over the lifespan for child psychopathology and neurodevelopmental disorders (Jaffee, 2018; Sonuga-Barke & Halperin, 2010). With respect to ASD, our knowledge of early intervention in infancy is based on single-subject trials, small groups, and randomized controlled trials (RCTs) of both general developmental and ASD-specific interventions. The current early intervention system in the United States consists of programs for infants aged 0–3 years, funded via Part C of the Individuals with Disabilities Education Act of 2004 (IDEA). States have some latitude in determining specific eligibility criteria, but basic criteria include documented developmental delays in one of five specified developmental domains (cognition, motor, social-emotional, communication, or adaptive behavior) or diagnosis that typically results in developmental delays (e.g. Down syndrome, deafness, autism). States can provide services to infants deemed at-risk for delays, but only 4 states currently do so (Rosenberg, Robinson, Shaw, & Ellison, 2013). Part C programs are mandated to include parents and to be delivered in natural settings; as such, most families receive low-intensity (1–2 hours per week) services delivered via parent coaching in the home. The parent-mediated approach is also developmentally appropriate for infants when compared to other, more intensive ASD-specific approaches for toddlers.
To date, there have been only a handful of trials of ASD-specific treatment in infancy, all delivered via parent coaching. They have each focused on different groups of infants, utilized different intervention targets in terms of both child behavior and parent strategies, and used different outcome measures. Green and colleagues (Green et al., 2015, 2017, 2013) conducted a series of single-subject and randomized controlled trials testing the effects of a general developmental parent-mediated intervention, the Video Interaction for Promoting Positive Parenting (VIPP; Juffer, Bakermans-Kranenburg, & van IJzendoorn, 2008) for infants at familial risk, irrespective of behavioral symptoms at enrollment. These trials found some effects on target parent behaviors and proximal child measures of attentiveness and communication initiations, but no effects on standardized language measures or diagnostic classification at 3 years. In a subsequent RCT, Whitehouse and colleagues (2019) used the same intervention approach but targeted infants identified as at-risk for ASD based on a screening checklist. Again, they found no effects for the primary outcome on standardized measures of ASD symptoms, or secondary outcomes using standardized measures of development, behavior coding of parent-child interactions, or parent questionnaire measures of infant gesture, adaptive social functioning, or parenting sense of competence. There were some positive effects on parent-reported measures of expressive and functional language.
Other studies have focused on increasing specific “pivotal” infant behaviors, with or without also targeting parental responsivity, for symptomatic infants (Baranek et al., 2015; L. K. Koegel et al., 2013; Steiner, Gengoux, Klin, & Chawarska, 2013; Watson et al., 2017). Two single-subject studies in this area demonstrated positive effects on specific target infant behaviors: functional communication, response to name, avoidance of eye contact, and positive affect (L. K. Koegel et al., 2013; Steiner et al., 2013). Watson and colleagues (2017) conducted an RCT with 87 one-year-olds identified as ‘at risk’ via community screening to test the effects of a responsive parent coaching model targeting specific pivotal child skills across two domains: social communication and sensory regulatory. They found no significant main effect on primary child outcomes using standardized measures of ASD symptoms, adaptive functioning, or language. However, there were significant increases in parental responsiveness, one of the hypothesized mediators of developmental change; changes in parent responsiveness mediated change on the majority of child outcome measures.
Finally, in a small pilot study, Rogers et al. (2014), coached parents of infants with significant early symptoms in strategies to address 6 targeted infant ASD symptoms. Treated infants were compared to groups of infants constructed from existing datasets and matched to initial symptom level: infants with a known ASD outcome from a prior cohort, infants with ASD outcomes initially referred to treatment but who declined to participate, and high and low familial risk infant siblings with known non-ASD outcomes. Findings were mixed with respect to trajectories on standardized measures of language and cognitive development, but at 36 months, infants in the treatment group had lower scores on standardized measures of ASD symptoms, and a smaller proportion of infants with developmental quotients less than 70 or who received clinical best estimate diagnoses of ASD. In general, infants in the treatment group had more positive outcomes than infants who declined treatment, but still differed significantly from the non-ASD outcome comparison groups.
Together, these studies suggest that although there are positive impacts on various domains of functioning, general developmental interventions are not likely to be effective in reducing core ASD symptoms (Kasari, 2019). They also suggest the need for better alignment between behavioral treatment targets and active ingredients of interventions, consistent with transdiagnostic, process-focused research into early markers of neurodevelopmental disorders and child psychopathology.
Future Directions for Transdiagnostic Early Identification and Intervention: Challenges, Next Steps, and Implications
The fields of infant identification and intervention, reviewed above, face a number of challenges under the current diagnostically-oriented framework. Here, we highlight what we perceive to be the most significant barriers and describe the ways in which a rigorous and systematic transdiagnostic approach may better address these challenges. We then propose future directions for transdiagnostic early identification and intervention that will address these challenges.
Current Challenges in Infant Identification and Intervention
Measurement challenges.
Although there exist a number of high-quality ASD screening measures with appropriate sensitivity and specificity beginning in the second year of life (e.g., Modified Checklist for Autism in Toddlers, Revised with Follow-Up, Robins et al., 2020; Infant-Toddler Checklist, Wetherby, Brosnan-Maddox, Peace, & Newton, 2008; First Year Inventory, Reznick, Baranek, Reavis, Watson, & Crais, 2007; see Petrocchi, Levante, & Lecciso, 2020 for a recent systematic review), a key issue is the relative paucity of robust universal screening and evaluation tools for use within the first year (to be sure, several such measures do exist, but sensitivity and specificity values tend not to be adequate until the second year of life; Parikh, Iosif, & Ozonoff, 2020; Wetherby et al., 2008). Extending universal screening measures downward into infancy is challenging for a number of reasons. First, longitudinal data from high-risk infant siblings of children with ASD have revealed that although symptoms begin to emerge toward the end of the first year of life, there are not robust group differences between infants ultimately diagnosed with ASD and those with typical or other outcomes in the first year (for relevant reviews, see Elsabbagh & Johnson, 2016; Zwaigenbaum et al., 2015). Second, although biomarkers (e.g., EEG, MRI) may potentially be more sensitive to group differences before behavioral differences are evident (Bosl, Tager-Flusberg, & Nelson, 2018; Emerson et al., 2017; Hazlett et al., 2017; Shen et al., 2017, 2013), they are unlikely to be scalable or used universally in community-based settings. Third, there may be significant overlap between prodromal symptoms across neurodevelopmental disorders and child psychopathology more broadly (e.g., ASD, ADHD, anxiety) which may wax and wane across development (Begum Ali, Charman, Johnson, & Jones, 2020; Hatch et al., 2020; Miller et al., 2020; Shephard et al., 2019), calling into question the specificity and long-term predictive validity of these tools and ultimately necessitating the shift to a transdiagnostic perspective.
Definitional challenges.
Currently, there is a lack of consensus regarding the definition of elevated risk for ASD or optimal thresholds of prodromal features significant enough to warrant intervention. Some have argued that all infants belonging to ‘selective’ risk groups (e.g., infant siblings of children with ASD, infants with specific genetic syndromes, infants born prematurely or very low birthweight) should be referred for pre-emptive intervention, regardless of individual behavioral symptoms (Green et al., 2017). Others suggest that infants with some degree of social communication delays with emerging restricted and repetitive behaviors be considered for early intervention (Watson et al., 2017). The most stringent definitions suggest that early intervention be reserved only for infants with clear, specific symptoms of ASD (Rogers et al., 2014)—an increasingly difficult threshold to meet the younger the infant’s age. Currently under IDEA Part C, infants with significant social communication delays typically receive a mixture of speech therapy, developmental, and other allied health services (Hallam, Rous, Grove, & LoBianco, 2009; Hebbeler et al., 2007). These developmental services are not ASD-specific and thus are unlikely to exert substantial effects on core symptoms (Ingersoll, Meyer, Bonter, & Jelinek, 2014; Kasari, 2019).
Accessibility challenges.
Accessibility challenges can be separated into two categories: Those related to the measurement and definitional challenges described above, and those related to equity and geographical context. Given the lack of clear screening and assessment tools for evaluating specific ASD risk in infancy, families with concerns about ASD (or other neurodevelopmental disorders) often face extended delays between initial concerns and formal diagnosis or initiation of ASD-specific services (Zuckerman, Lindly, & Sinche, 2015). In terms of contextual factors, there are clear disparities in racial and ethnic minorities’ access to specialists and to early, evidence-based evaluations for developmental delays, ASD, and other childhood disorders (Rosenberg, Zhang, & Robinson, 2008; Smith, Gehricke, Iadarola, Wolfe, & Kuhlthau, 2020). These issues are compounded in rural areas with limited access to specialists and high-quality university-based diagnostic and intervention services (Kalkbrenner et al., 2011; Nahmias, Pellecchia, Stahmer, & Mandell, 2019). These accessibility issues are even more pronounced when considering infants at risk.
Recently, the vulnerability of our current system for evidence-based assessments—which typically involve the direct administration of standardized tools—has been highlighted under the extreme conditions of the COVID-19 global pandemic. The sudden cessation of in-person services has made it abundantly clear that new methods utilizing emerging technologies such as telehealth are desperately needed to maintain and expand access to services. There are currently very few norm-referenced assessment tools meeting IDEA requirements that can be administered remotely (Early Childhood Technical Assistance Center, 2020). These events have underscored the vulnerability of current early intervention services for very young children, who are in the developmental period most sensitive to benefits of early intervention delivery (L. K. Koegel, Koegel, Ashbaugh, & Bradshaw, 2014).
Conceptual challenges
As alluded to previously, there is variability in conceptual frameworks driving research into early markers of ASD and other conditions. Most have taken a disorder-specific approach, but it is becoming increasingly apparent that cross-disorder approaches may add value both scientifically and clinically. The NIMH RDoC aims to reduce our reliance on diagnostic categories and emphasize psychopathology-relevant behaviors in an effort to enhance knowledge of underlying processes and mechanisms, and to support personalized medicine. This framework is becoming increasingly utilized in the study of adult psychopathology, but has less-often been applied to child psychopathology and neurodevelopmental disorders. As a result, there have been growing calls to incorporate development into the RDoC framework (Garber & Bradshaw, 2020; Mittal & Wakschlag, 2017). There are a number of benefits to this framework, and also some challenges in utilizing this approach in early life including the need for the development of clinically relevant and translational measures which are appropriate to the infant and toddler periods across key transdiagnostic processes and which represent the full range of relevant behavior, from atypical to supranormal.
For example, disrupted attentional processes have been implicated in a number of neurodevelopmental and psychiatric disorders including autism, ADHD, and anxiety (Racer & Dishion, 2012). However, few measures exist to capture individual differences in this domain early in life in a psychometrically sound way that would allow for identification of clinically significant differences on the behavioral level (which, we note, is the level at which clinical decisions are currently made, including within the early intervention system). The measures that do exist are largely parent rated temperament questionnaires or more invasive methods such as eye-tracking, EEG/ERP, and MRI, which are limited with respect to clinical utility. If we are to make progress toward the goal of infant identification of a range of atypical development consistent with the RDoC framework, new measures are needed.
Expanding Behavioral Dimensions and Measurement Approaches within a Developmental Framework
Developing fine-grained measurement tools across a broader range of ASD-relevant behaviors (and beyond) has the potential to uncover distinct behavioral profiles across neurodevelopmental disorders and child psychopathology in general. This has implications for the development of process-focused interventions rather than treatments tied to specific diagnostic categories and may facilitate future randomized trials testing the effects of both existing and future treatments on specific mechanisms, underling processes, and/or domains of behavior. Novel measures of prodromal symptoms and relevant underlying processes should be designed within an integrated clinical-developmental framework. That is, they should not merely represent downward extensions of DSM symptoms, which are inherently not developmentally-oriented. Instead, good measures of core processes implicated across these childhood conditions (e.g., attention, self-regulation, social behavior) are needed.
As an example, rather than an “autism screener” for infants, the field may do well to move toward screeners or direct assessments of attention or self-regulation that could reveal early risk for a range of atypical developmental outcomes. Novel measures should be devised in a way that allows for comparison against other, same-aged infants or toddlers. Indeed, the development of norm-referenced direct assessments, including those that can be administered via distance technology, has the potential to move the field forward in clinically relevant ways. Such measures would allow not only for the measurement of deviance from same-aged peers in symptoms, behavior, and functioning, but also a better understanding of the development of fundamental processes. Similar approaches are widely used in a post-hoc fashion among older children and adolescents (e.g., neuropsychological testing); they are not diagnostic in and of themselves but provide one window into a child’s functioning. The ability to obtain comparable data early in life—prior to the onset of the full symptom set—may provide an opportunity to identify which infants are at highest risk for a range of atypical developmental outcomes. Relevant efforts are underway to develop an “NIH Infant and Toddler Toolbox” in the domains of cognition, social functioning, language, numeracy, self-regulation, and executive function (75N94019D00005, PI: Gershon). We suggest there may be subdomains of ASD-relevant behavior, such as social attention, that could be explored within the RDoC framework with an eye towards clinical relevance and integration with screening and interventions around those targets. We recognize that the range of normative behavior early in development is wide, and the development of new clinical tools as described above runs the risk of over-pathologizing. Many ASD-relevant behaviors have high base rates within typically developing infant samples. For example, motor overflow movements, wherein motor behavior from intentional actions ‘spills over’ into other incidental actions, share surface-level features with motor stereotypies. These overflow movements are observed nearly universally at some stages in infancy (Soska, Galeon, & Adolph, 2012). Other restricted and repetitive behaviors, such as intense preoccupations with specific topics, are also highly prevalent in normative samples throughout toddlerhood (Leekam et al., 2007). Thus, the development of ASD-relevant transdiagnostic measures will necessarily rely on large longitudinal investigations from infancy through adolescence in order to establish predictive validity to clinically meaningful outcomes.
Expanding the Scope and Delivery of Targeted Interventions for Infants
Development of fine-grained measurement tools across ASD-relevant behavioral domains has implications for treatment, in that such information may form the basis for treatment targets and goals. This would also have significant and far-reaching impacts on the development, validation, and implementation of novel intervention approaches. First, these measures could be utilized to develop formal process theories to guide targeted intervention approaches. Second, expanded measurement capabilities would support the identification of specific elements of existing interventions (i.e., ‘active ingredients’) likely to be shared across infants, as well as the evaluation of the effects of those components on infants’ development in terms of both direct and collateral effects. Such an approach would be consistent with transdiagnostic, process-focused research into early markers of neurodevelopmental disorders and child psychopathology. As an example, some have begun to test whether attention training among infants at risk for disrupted attentional processing (i.e., infants at familial risk for ADHD, infants at familial risk for ASD, infants who are born preterm) is feasible and effective (Forssman & Wass, 2018; Goodwin et al., 2016; Perra et al., 2020). These types of mechanisms- or process-focused approaches have shown initial evidence of generalizability to non-trained dimensions such as social communication among infants who are not at known risk, at least in the short term (Forssman & Wass, 2018). Whether long-term effects related to clinical outcomes exist is an important area for future investigations.
Many existing behavioral treatment approaches for toddlers and several of the candidate treatments for infants described in earlier sections address domains like social attention and affect regulation that are likely to be widely relevant. There is emerging work examining the efficacy of existing ASD-specific interventions in toddlers with other clinical diagnoses/genetic syndromes (e.g., Fragile X Syndrome, Tuberous Sclerosis; McDonald et al., 2020; Vismara, McCormick, Shields, & Hessl, 2019). Many evidence-based interventions for toddlers with ASD (ESDM, Rogers & Dawson, 2010; JASPER, Kasari, Gulsrud, Paparella, Hellemann, & Berry, 2015; Project IMPACT, Ingersoll & Wainer, 2013) belong to class of interventions termed “naturalistic, developmental, behavioral interventions” (NDBIs, Schreibman et al., 2015). Core shared components of various NDBIs include: 1) a focus on a broad array of developmental domains including cognition, play, language, social, and motor development; 2) learning embedded within daily living or play routines; 3) use of specific behavior analytic techniques such as a three-part learning contingencies, reinforcement, modeling, and prompting techniques; and 4) increasing balanced turn-taking and social attention of the child. NDBIs are manualized approaches with clear fidelity guidelines and measurement of child progress on specific treatment objectives. The primary difference between them is the extent to which they target specific domains, versus more comprehensive approaches targeting most developmental domains. These interventions provide fertile ground for identification of specific treatment components likely to impact infant behavior across a range of ASD-relevant domains. One of our research groups has begun to take this approach in infants with early ASD symptoms, dismantling an existing treatment (Infant Start, Rogers et al., 2014), and evaluating the direct and collateral effects of three target parent interaction techniques (“Step into the Spotlight”, “Imitation”, and “Talking to Baby”) on a range of target child behaviors (eye contact, directed vocalizations, gestures, play, irritability; Dufek, Talbott, & Rogers, 2020). These were tested in a multiple-baseline single-subjects study of 6 infants with significant ASD symptoms and their caregivers. Results were promising, with significant increases in parent fidelity scores and decreases in child ASD symptoms.
A third critical direction is the integration of implementation science approaches such as community-based participatory research into the transdiagnostic framework we are proposing. As noted by Stahmer et al. (2017), these approaches have been used most often in the domain of intervention research, helping to highlight shared goals between researchers and communities of increasing capacity and effectiveness of available community services (Drahota et al., 2016). There is a need to develop both the measures and treatments we are proposing within the context of the system that will ultimately deliver such services. Developing and testing approaches from the outset that fit within the existing service system will help to support the implementation of evidence-based practices in community settings. The transdiagnostic framework may better reflect the heterogenous populations seen in community settings.
Finally, there is a need to expand the accessibility of both identification and intervention services to families. Telehealth has an enormous potential to increase the reach of early intervention services into rural and low-resource areas, where barriers to service include long travel times for families or providers, inclement weather, and a shortage of professionals with appropriate expertise. Approximately one-quarter of the costs of early intervention services are related to transportation (J. L. Johnson, Brown, Chang, Nelson, & Mrazek, 2011). Reducing some of these costs via telehealth has the potential to increase the capacity to serve more children and deliver more frequent services. Use of telehealth has rapidly expanded within existing state Part C early intervention programs, even prior to the COVID-19 pandemic (Cason, Behl, & Ringwalt, 2012; Cole, Pickard, & Stredler-Brown, 2019). Telehealth has been used most frequently and successfully for parent coaching models versus direct instruction to children (Bearss et al., 2018; Lindgren et al., 2016; Vismara et al., 2019). Additionally, several promising tools for diagnostic screening and referral of toddlers with suspected ASD currently exist (Tele-ASD-PEDS, Corona et al., 2020; NODA, Nazneen et al., 2015). Within our own research group, we are developing a telehealth-based assessment of ASD symptoms and social communication rates for infants 6–12 months (Talbott et al., 2019). We are currently testing an expanded protocol that includes a developmental curriculum assessment including verbal and nonverbal domains and scoring of a standardized and norm-referenced measure of gross motor skills. Encouragingly, telehealth approaches have generally resulted in high family acceptability, cost-savings for systems, and increased use of evidence-based, family-centered parent coaching practices by therapists (Behl et al., 2017; Sutherland, Trembath, & Roberts, 2018) suggesting this is a feasible route to increasing access to intervention services.
Policy and Practice Implications
We would be remiss if we did not highlight relevant policy and practice implications, which are related in some way to each of the key challenges we previously identified. A dimensionally-oriented approach over the first year of life, grounded within a developmental framework, is likely scalable within existing community-based early intervention programs which, for infants, are generally oriented around domains of delay or deficit. We have provided an illustrative model (Figure 1) overlaying our proposed changes onto the existing Part C framework as well as the interaction between basic research and implementation.
Figure 1.
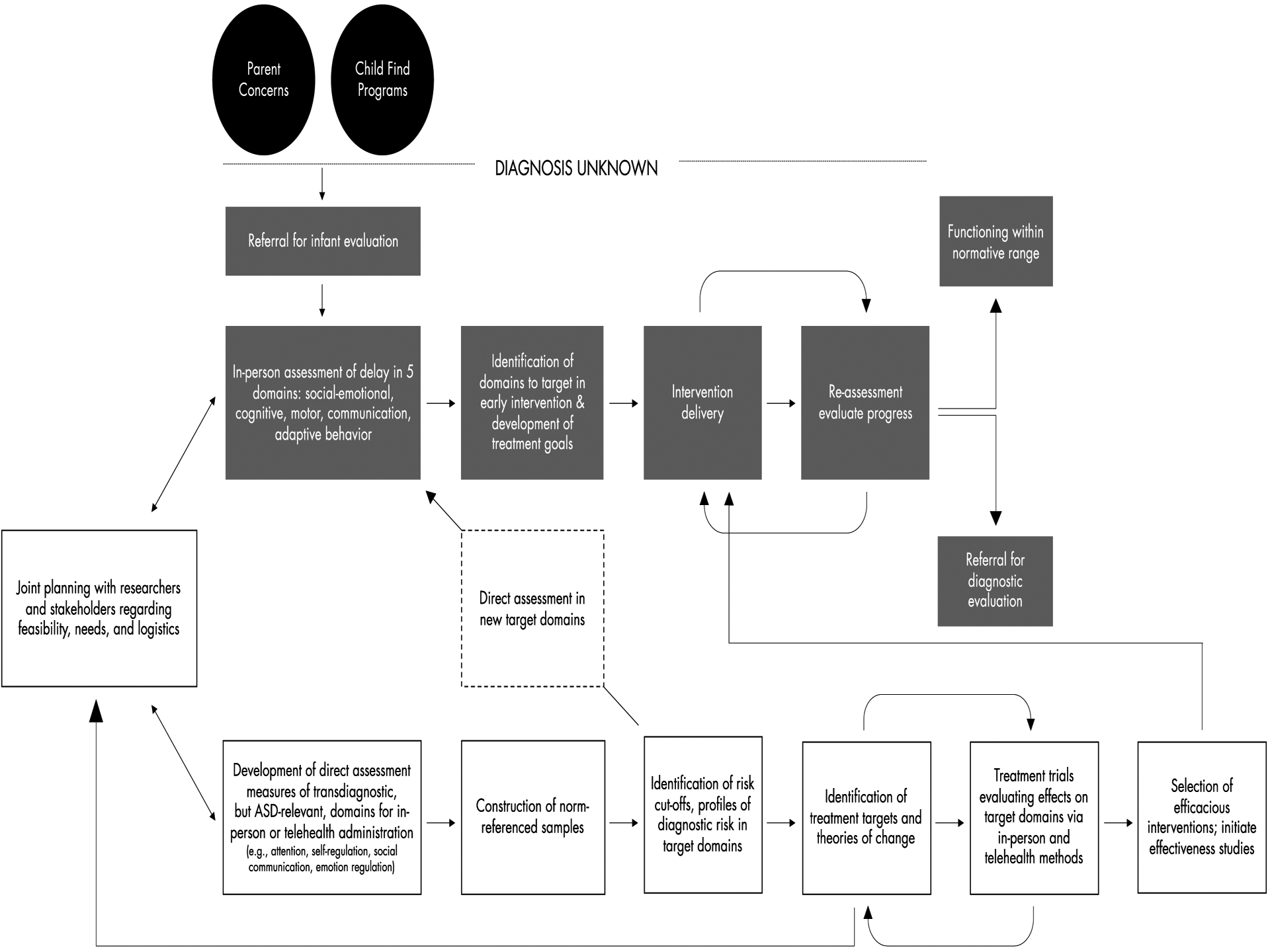
Model illustrating integration of transdiagnostic assessment and intervention for prodromal infants within the current early intervention system. Gray boxes depict the current system; white boxes depict the proposed additions.
While we contend that our proposed approach will ultimately more efficiently serve infants with prodromal risk signs, we cannot ignore the significant impact these changes may require to states’ evaluation and eligibility criteria. Identification of additional domains would require clear evidence of clinically-relevant need (e.g., adverse developmental outcomes) in order for states to expand programs. Integrating norm-referenced assessment tools in additional domains into the existing system, as we suggest, would require alignment with the five content areas already required to be evaluated by Part C regulations: (i) Physical development, (ii) Cognitive development, (iii) Communication development, (iv) Social or emotional development, or (v) Adaptive development, or policy changes at the federal level to expand eligibility criteria.
Another key consideration is balancing the benefits and burden of early identification and intervention on families when we cannot predict the diagnostic future. On the one hand, some infants and toddlers who are not ultimately diagnosed with a neurodevelopmental disorder will receive treatment. If the delivered intervention is unwanted or burdensome, the cost for families could be high. On the other hand, many infants and toddlers who do need treatment are not currently being served, despite parents’ early concerns and seeking of services (Zuckerman et al., 2015). There is some evidence that supportive parent coaching delivered through parent-mediated treatment for toddlers with ASD may in fact reduce parental anxiety, depression, and stress (Estes et al., 2014); these effects on parent mental health and overall family functioning should not be underestimated. Still, many children meeting eligibility criteria for Part C services face significant adversity and have low rates of participation in intervention services (Rosenberg et al., 2008). For example, in a sample of 1,997 toddlers being investigated for possible maltreatment, 47% had delays significant enough to quality for Part C services (Rosenberg & Smith, 2008). Finally, states already face significant challenges delivering services to eligible infants and toddlers eligible under the existing criteria (Rosenberg et al., 2013). Only 9% of 9-month-olds with delays that would make them eligible actually receive services (Feinburg, Silverstein, Donahue, & Bliss, 2011). Increased funding and capacity of the existing system is urgently needed. As a result, the costs to develop, validate, and disseminate additional norm-referenced tools that Part C is mandated to use for evaluation will likely be borne by research. It is our hope that identifying specific process-oriented mechanisms and efficacious interventions to support those mechanisms will ultimately help to improve the efficiency and capacity of the early intervention system.
Summary
A transdiagnostic approach to infant identification of ASD, neurodevelopmental disorders, and child psychopathology more broadly that is both grounded in developmental and clinical science and scalable for early intervention systems has the potential for wide-reaching impacts on research and clinical practice. Key barriers to the full implementation of such approaches at both the research and clinical levels span measurement challenges, definitional challenges, accessibility challenges, and conceptual challenges. Addressing these barriers will require an integrated effort from both clinical and developmental perspectives. We propose four recommendations for future research efforts: (1) development of fine-grained, norm-referenced measures of ASD-relevant transdiagnostic behavioral domains; (2) identification of shared and distinct mechanisms influencing the transition from risk to disorder; (3) determination of key, cross-cutting treatment strategies (both novel and extracted from existing approaches) effective in targeting specific domains across disorders; and (4) integration of identified measures and treatments into existing service systems.
Acknowledgments
This work was supported by NIH grants R21 HD100372 (Talbott) and R00 MH106642 (Miller).
References
- Abdallah MW, Greaves-Lord K, Grove J, Nørgaard-Pedersen B, Hougaard DM, & Mortensen EL (2011). Psychiatric comorbidities in autism spectrum disorders: Findings from a Danish Historic Birth Cohort. European Child and Adolescent Psychiatry, 20, 599–601. [DOI] [PubMed] [Google Scholar]
- Bal VH, Kim SH, Cheong D, & Lord C (2015). Daily living skills in individuals with autism spectrum disorder from 2 to 21 years of age. Autism, 19(7), 774–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baranek GT, Watson LR, Turner-brown L, Field SH, Crais ER, Wakeford L, … Reznick JS (2015). Preliminary efficacy of adapted responsive teaching for infants at risk of autism spectrum disorder in a community sample. Autism Research and Treatment, 2015, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bearss K, Burrell TL, Challa SA, Postorino V, Gillespie SE, Crooks C, & Scahill L (2018). Feasibility of parent training via telehealth for children with autism spectrum disorder and disruptive behavior: A demonstration pilot. Journal of Autism and Developmental Disorders, 48(4), 1020–1030. [DOI] [PubMed] [Google Scholar]
- Begum Ali J, Charman T, Johnson MH, & Jones EJH (2020). Early motor differences in infants at elevated likelihood of autism spectrum disorder and/or attention deficit hyperactivity disorder. Journal of Autism and Developmental Disorders. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behl DD, Blaiser K, Cook G, Barrett T, Callow-Heusser C, Brooks BM, … White KR (2017). A multisite study evaluating the benefits of early intervention via telepractice. Infants and Young Children, 30(2), 147–161. [Google Scholar]
- Bosl WJ, Tager-Flusberg H, & Nelson CA (2018). EEG analytics for early detection of autism spectrum disorder: A data-driven approach. Scientific Reports, 8(1), 1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryson SE, Zwaigenbaum L, Brian J, Roberts W, Szatmari P, Rombough V, & McDermott C (2007). A prospective case series of high-risk infants who developed autism. Journal of Autism and Developmental Disorders, 37(1), 12–24. [DOI] [PubMed] [Google Scholar]
- Carter AS, Messinger DS, Stone WL, Celimli S, Nahmias AS, & Yoder P (2011). A randomized controlled trial of Hanen’s “More Than Words” in toddlers with early autism symptoms. Journal of Psychology and Psychiatry, 52(7), 741–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cason J, Behl D, & Ringwalt S (2012). Overview of states’ use of telehealth for the delivery of early intervention (IDEA Part C) services. International Journal of Telerehabilitation, 4(2), 39–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chasson GS, Harris GE, & Neely WJ (2007). Cost comparison of early intensive behavioral intervention and special education for children with autism. Journal of Child and Family Studies, 16(3), 401–413. [Google Scholar]
- Chawarska K, Macari S, & Shic F (2013). Decreased spontaneous attention to social scenes in 6-month-old infants later diagnosed with autism spectrum disorders. Biological Psychiatry, 74(3), 195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cidav Z, Munson J, Estes A, Dawson G, Rogers S, & Mandell D (2017). Cost offset associated with Early Start Denver Model for children With autism. Journal of the American Academy of Child and Adolescent Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole B, Pickard K, & Stredler-Brown A (2019). Report on the use of telehealth in early intervention in Colorado: Strengths and challenges with telehealth as a service delivery method. International Journal of Telerehabilitation, 11(1), 33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corona L, Hine J, Nicholson A, Stone C, Swanson A, Wade J, … Warren Z (2020). TELE-ASD-PEDS: A telemedicine-based ASD evaluation tool for toddlers and young children. Retrieved from https://vkc.vumc.org/vkc/triad/tele-asd-peds
- Dawson G (2008). Early behavioral intervention, brain plasticity, and the prevention of autism spectrum disorder. Development and Psychopathology, 20, 775–803. [DOI] [PubMed] [Google Scholar]
- Dawson G, Rogers SJ, Munson J, Smith M, Winter J, Greenson J, … Varley J (2010). Randomized, controlled trial of an intervention for toddlers with autism: the Early Start Denver Model. Pediatrics, 125(1), e17–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Martino A, Zuo XN, Kelly C, Grzadzinski R, Mennes M, Schvarcz A, … Milham MP (2013). Shared and distinct intrinsic functional network centrality in autism and attention-deficit/hyperactivity disorder. Biological Psychiatry, 74(8), 623–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drahota A, Meza RD, Brikho B, Naaf M, Estabillo JA, Gomez ED, … Aarons GA (2016). Community-academic partnerships: A systematic review of the state of the literature and recommendations for future research. Milbank Quarterly, 94(1), 163–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dryden W, & Dryden W (2018). Two pathways to help. Single-Session Therapy (SST), 55(4), 257–258. [Google Scholar]
- Dufek S, Talbott MR, & Rogers SJ (2020). Telebaby: A telehealth invervention for caregivers of infants with early signs of autism spectrum disorder. In Meeting of the International Society for Autism Research. [Google Scholar]
- Early Childhood Technical Assistance Center. (2020). Norm-Referenced Assessment Tools for Children Birth to Age Five Years with Potential for Remote Administration for Eligibility Determination. [Google Scholar]
- Elsabbagh M, & Johnson MH (2016). Autism and the social brain: The first-year puzzle. Biological Psychiatry, 80(2), 94–99. [DOI] [PubMed] [Google Scholar]
- Emerson RW, Adams C, Nishino T, Hazlett HC, Wolff JJ, Zwaigenbaum L, … Piven J (2017). Functional neuroimaging of high-risk 6-month-old infants predicts a diagnosis of [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estes A, Vismara LA, Mercado C, Fitzpatrick A, Elder L, Greenson J, … Rogers SJ (2014). The impact of parent-delivered intervention on parents of very young children with autism. Journal of Autism and Developmental Disorders, 44(2), 353–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fein D, Dixon P, Paul J, & Levin H (2005). Brief report: Pervasive Developmental Disorder can evolve into ADHD: Case illustrations. Journal of Autism and Developmental Disorders, 35(4), 525–534. [DOI] [PubMed] [Google Scholar]
- Feinburg E, Silverstein M, Donahue S, & Bliss R (2011). The impact of race on participation in Part C early intervention services. Journal of Developmental Behavioral Pediatrics, 32(4), 284–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forssman L, & Wass SV (2018). Training basic visual attention leads to changes in responsiveness to social-communicative cues in 9-month-olds. Child Development, 89(3), e199–e213. [DOI] [PubMed] [Google Scholar]
- Gandal MJ, Haney JR, Parikshak NN, Leppa V, Ramaswami G, Hartl C, … Geschwind DH (2018). Shared molecular neuropathology across major psychiatric disorders parallels polygenic overlap. Science, 359(6376), 693–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garber J, & Bradshaw CP (2020). Developmental psychopathology and the Research Domain Criteria: Friend or foe? Journal of Clinical Child & Adolescent Psychology, 49(3), 341–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geurts HM, Verte S, Oosterlaan J, Roeyers H, Sergeant JA, Verté S, … Sergeant JA (2004). How specific are executive functioning deficits in attention deficit hyperactivity disorder and autism? Journal of Child Psychology and Psychiatry, 45(4), 836–854. [DOI] [PubMed] [Google Scholar]
- Ghirardi L, Brikell I, Kuja-Halkola R, Freitag CM, Franke B, Asherson P, … Larsson H (2018). The familial co-aggregation of ASD and ADHD: a register-based cohort study. Molecular Psychiatry, 23(2), 257–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin A, Salomone S, Bolton P, Charman T, Jones EJH, Pickles A, … Johnson MH (2016). Attention training for infants at familial risk of ADHD (INTERSTAARS): Study protocol for a randomised controlled trial. Trials, (17), 608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green J, Charman T, Pickles A, Wan MW, Elsabbagh M, Slonims V, … Johnson MH (2015). Parent-mediated intervention versus no intervention for infants at high risk of autism: A parallel, single-blind, randomised trial. The Lancet Psychiatry, 2(2), 133–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green J, Pickles A, Pasco G, Bedford R, Wan MW, Elsabbagh M, … McNally J (2017). Randomised trial of a parent-mediated intervention for infants at high risk for autism: Longitudinal outcomes to age 3 years. Journal of Child Psychology and Psychiatry, 58(12), 1330–1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green J, Wan MW, Guiraud J, Holsgrove S, McNally J, Slonims V, … Johnson M (2013). Intervention for infants at risk of developing autism: A case series. Journal of Autism and Developmental Disorders, 43(11), 2502–2514. [DOI] [PubMed] [Google Scholar]
- Hallam RA, Rous B, Grove J, & LoBianco T (2009). Level and intensity of early intervention services for infants and toddlers with disabilities: The impact of child, family, system, and community-level factors on service provision. Journal of Early Intervention, 31(2), 179–197. [Google Scholar]
- Harvey AG, Watkins E, Mansell W, & Shafran R (2004). Cognitive behavioural processes across psychological disorders: A transdiagnostic approach to research and treatment. Oxford Oxford University Press. [Google Scholar]
- Hatch B, Iosif AM, Chuang A, de la Paz L, Ozonoff S, & Miller M (2020). Longitudinal differences in response to name among infants developing ASD and risk for ADHD. Journal of Autism and Developmental Disorders. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazlett HC, Ph D, Gu H, Ph D, Munsell BC, Ph D, … Ph D (2017). Early brain development in infants at high risk for autism spectrum disorder. Nature, 542(7641), 348–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebbeler K, Spiker D, Bailey D, Scarborough A, Mallik S, Simeonsson R, & Singer M (2007). Early intervention for infants & toddlers with disabilities and their families: participants, services, and outcomes. Final report of the National Early Intervention Longitudinal Study (NEILS). [Google Scholar]
- Houghton R, Ong RC, & Bolognani F (2017). Psychiatric comorbidities and use of psychotropic medications in people with autism spectrum disorder in the United States. Autism Research, 10(12), 2037–2047. [DOI] [PubMed] [Google Scholar]
- Howlin P, & Magiati I (2017). Autism spectrum disorder: Outcomes in adulthood. Current Opinion in Psychiatry, 30(2), 69–76. [DOI] [PubMed] [Google Scholar]
- Howlin P, Moss P, Savage S, Bolton P, & Rutter M (2015). Outcomes in adult life among siblings of individuals with autism. Journal of Autism and Developmental Disorders, 45(3), 707–718. [DOI] [PubMed] [Google Scholar]
- Howlin P, Moss P, Savage S, & Rutter M (2013). Social outcomes in mid- to later adulthood among individuals diagnosed with autism and average nonverbal IQ as children. Journal of the American Academy of Child and Adolescent Psychiatry, 52(6), 572–581. [DOI] [PubMed] [Google Scholar]
- Ingersoll B, Meyer K, Bonter N, & Jelinek S (2014). A comparison of developmental social-pragmatic and naturalistic behavioral interventions on language use and social engagement in autism. Journal of Speech Language and Hearing Research, 55(5), 1301–1313. [DOI] [PubMed] [Google Scholar]
- Ingersoll B, & Wainer A (2013). Initial efficacy of project ImPACT: A parent-mediated social communication intervention for young children with ASD. Journal of Autism and Developmental Disorders, 43(12), 2943–2952. [DOI] [PubMed] [Google Scholar]
- Jaffee S (2018). Promises and pitfalls in the development of biomarkers that can promote early intervention in children at risk. Journal of Child Psychology and Psychiatry, 59(2), 97–98. [DOI] [PubMed] [Google Scholar]
- Johnson JL, Brown S, Chang C, Nelson D, & Mrazek S (2011). The cost of serving infants and toddlers under part C. Infants and Young Children, 24(1), 101–113. [Google Scholar]
- Johnson MH, Gliga T, Jones E, & Charman T (2015). Annual research review: Infant development, autism, and ADHD - Early pathways to emerging disorders. Journal of Child Psychology and Psychiatry, 56(3), 228–247. [DOI] [PubMed] [Google Scholar]
- Jokiranta-Olkoniemi E, Cheslack-Postava K, Sucksdorff D, Suominen A, Gyllenberg D, Chudal R, … Sourander A (2016). Risk of psychiatric and neurodevelopmental disorders among siblings of probands with autism spectrum disorders. JAMA Psychiatry, 73(6), 622–629. [DOI] [PubMed] [Google Scholar]
- Juffer F, Bakermans-Kranenburg MJ, & van IJzendoorn MH (2008). Promoting positive parenting: An attachment-based intervention (Juffer F, Bakermans-Kranenburg MJ, & van IJzendoorn MH, Eds.), Monographs in Parenting Series. New York, NY: Taylor & Francis Group/Lawrence Erlbaum Associates. [Google Scholar]
- Kaiser AP, & Roberts MY (2013). Parent-implemented enhanced milieu teaching with preschool children who have intellectual disabilities. Journal of Speech, Language, and Hearing Research, 56(1), 295–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalkbrenner AE, Daniels JL, Emch M, Morrissey J, Poole C, & Chen JC (2011). Geographic access to health services and diagnosis with an autism spectrum disorder. Annals of Epidemiology, 21(4), 304–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasari C (2019). Time to rethink pre-emptive interventions for infants with early signs of autism spectrum disorder. The Lancet Child and Adolescent Health, 3(9), 586–587. [DOI] [PubMed] [Google Scholar]
- Kasari C, Freeman S, & Paparella T (2006). Joint attention and symbolic play in young children with autism: a randomized controlled intervention study. Journal of Child Psychology and Psychiatry, 47(6), 611–620. [DOI] [PubMed] [Google Scholar]
- Kasari C, Gulsrud A, Paparella T, Hellemann G, & Berry K (2015). Randomized comparative efficacy study of parent-mediated interventions for toddlers with autism. Journal of Consulting and Clinical Psychology, 83(3), 554–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerns CM, & Kendall PC (2012). The presentation and classification of anxiety in autism spectrum disorder. Clinical Psychology: Science and Practice, 19(4), 323–347. [Google Scholar]
- Kim SH, Bal VH, & Lord C (2018). Longitudinal follow-up of academic achievement in children with autism from age 2 to 18. Journal of Child Psychology and Psychiatry, 59(3), 258–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsch A, Huebner A, Mehta S, Howie F, Weaver A, Myers S, … Katusic S (2020). Association of comorbid mood and anxiety disorders with autism spectrum disorder. JAMA Pediatrics, 174(1), 63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen EI, Heckman JJ, Cameron JL, & Shonkoff JP (2006). Economic, neurobiological, and behavioral perspectives on building America’s future workforce. Proceedings of the National Academy of Sciences, 103(27), 10155–10162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koegel LK, Singh AK, Koegel RL, Hollingsworth JR, & Bradshaw J (2013). Assessing and improving early social engagement in infants. Journal of Positive Behavior Interventions, 16(2), 69–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koegel LK, Koegel RL, Ashbaugh K, & Bradshaw J (2014). The importance of early identification and intervention for children with or at risk for autism spectrum disorders. International Journal of Speech-Language Pathology. [DOI] [PubMed] [Google Scholar]
- Koegel RL, Koegel LK, & Carter CM (1999). Pivotal teaching interactions for children with autism. School Psychology Review, 28(4), 576–594. [Google Scholar]
- Landa RJ, Gross AL, Stuart EA, & Faherty A (2013). Developmental trajectories in children with and without autism spectrum disorders: The first 3 years. Child Development, 84(2), 429–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavelle TA, Weinstein MC, Newhouse JP, Munir K, Kuhlthau KA, Prosser LA, … Newschaffer C (2014). Economic burden of childhood autism spectrum disorders. Pediatrics, 133(3), e520–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leekam S, Tandos J, McConachie H, Meins E, Parkinson K, Wright C, … Couteur A Le. (2007). Repetitive behaviours in typically developing 2-year-olds. Journal of Child Psychology and Psychiatry, 48(11), 1131–1138. [DOI] [PubMed] [Google Scholar]
- Leitner Y (2014). The co-occurrence of autism and attention deficit hyperactivity disorder in children - What do we know? Frontiers in Human Neuroscience, 8(268). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindgren S, Wacker D, Suess A, Schieltz K, Pelzel K, Kopelman T, … Waldron D (2016). Telehealth and autism: Treating challenging behavior at lower cost. Pediatrics, 137(February), S167–S175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovaas O (1987). Behavioral treatment and normal educational and intellectual functioning in young autistic children. Journal of Consulting and Clinical Psychology, 55(1), 3–9. [DOI] [PubMed] [Google Scholar]
- Matza LS, Paramore C, & Prasad M (2005). A review of the economic burden of ADHD. Cost Effectiveness and Resource Allocation, 3, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald N, Hyde C, Choi B, Gulsrud AC, Kasari C, Nelson CA, & Jeste S (2020). Improving developmental abilities in infants with Tuberous Sclerosis Complex: A pilot behavioral intervention study. Infants and Young Children, 33(2), 108–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEachin JJ, Smith T, & Lovaas OI (1993). Long-term outcome for children with autism who received early intensive behavioral treatment. American Journal of Mental Etardation : AJMR, 97(4), 359–372. [PubMed] [Google Scholar]
- Miller M, Austin S, Iosif A, de la Paz L, Chaung A, Hatch B, & Ozonoff S (2020). Shared and distinct developmental pathways to ASD and ADHD phenotypes among infants at familial risk. Development and Psychopathology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller M, Iosif A, Hill M, Young G, Schwichtenberg A, & Ozonoff S (2017). Response to name in infants developing autism spectrum disorder: A prospective study. Journal of Pediatrics, 183(141–146.e1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller M, Musser E, Young G, Olson B, Steiner R, & Nigg J (2019). Sibling recurrence risk and cross-aggregation of attention-deficit/hyperactivity disorder and autism spectrum disorder. JAMA Pediatrics, 173(2), 147–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miodovnik A, Harstad E, Sideridis G, & Huntington N (2015). Timing of the diagnosis of attention-deficit/hyperactivity disorder and autism spectrum disorder. Pediatrics, 136(4), e830–7. [DOI] [PubMed] [Google Scholar]
- Mittal VA, & Wakschlag LS (2017). Research domain criteria (RDoC) grows up: Strengthening neurodevelopment investigation within the RDoC framework. Journal of Affective Disorders, 216, 30–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahmias AS, Pellecchia M, Stahmer AC, & Mandell DS (2019). Effectiveness of community-based early intervention for children with autism spectrum disorder: A meta-analysis. Journal of Child Psychology and Psychiatry, 11, 1200–1209. [DOI] [PubMed] [Google Scholar]
- Nazneen N, Rozga A, Smith CJ, Oberleitner R, Abowd GD, & Arriaga RI (2015). A novel system for supporting autism diagnosis using home videos: Iterative development and evaluation of system design. JMIR MHealth and UHealth, 3(2), e68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolen-Hoeksema S, & Watkins ER (2011). A heuristic for developing transdiagnostic models of psychopathology. Perspectives on Psychological Science, 6(6), 589–609. [DOI] [PubMed] [Google Scholar]
- Nyström P, Thorup E, Bölte S, & Falck-Ytter T (2019). Joint attention in infancy and the emergence of autism. Biological Psychiatry, 86(8), 631–638. [DOI] [PubMed] [Google Scholar]
- Osterling J, & Dawson G (1994). Early recognition of children with autism: A study of first birthday home videotapes. Journal of Autism and Developmental Disorders, 32(6), 292–300. [DOI] [PubMed] [Google Scholar]
- Osterling J, Dawson G, & Munson JA (2002). Early recognition of 1-year-old infants with autism spectrum disorder versus mental retardation. Development and Psychopathology, 14, 239–251. [DOI] [PubMed] [Google Scholar]
- Ozonoff S, Gangi D, Hanzel E, Hill A, Hill M, Miller M, … Iosif A (2018). Onset patterns in autism: Variation across informants, methods, and timing. Autism Research, 11(5), 788–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozonoff S, Iosif A-M, Baguio F, Cook IC, Hill MM, Hutman T, … Young GS (2010). A prospective study of the emergence of early behavioral signs of autism. Journal of the American Academy of Child & Adolescent Psychiatry, 49(3), 256–266e2. [PMC free article] [PubMed] [Google Scholar]
- Ozonoff S, & Iosif A (2019). Changing conceptualizations of regression: What prospective studies reveal about the onset of autism spectrum disorder. Neuroscience & Biobehavioral Reviews, 100, 296–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozonoff S, Iosif AM, Young GS, Hepburn S, Thompson M, Colombi C, … Rogers SJ (2011). Onset patterns in autism: Correspondence between home video and parent report. Journal of the American Academy of Child and Adolescent Psychiatry, 50(8), 796–806.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozonoff S, Macari S, Young GS, Goldring S, Thompson M, & Rogers SJ (2008). Atypical object exploration at 12 months of age is associated with autism in a prospective sample. Autism, 12, 457–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozonoff S, Young GS, Carter A, Messinger D, Yirmiya N, Zwaigenbaum L, … Stone WL (2011). Recurrence risk for autism spectrum disorders: a Baby Siblings Research Consortium study. Pediatrics, 128(3), e488–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozonoff S, Young GS, Landa RJ, Brian J, Bryson S, Charman T, … Iosif A-M (2015). Diagnostic stability in young children at risk for autism spectrum disorder: A Baby Siblings Research Consortium study. Journal of Child Psychology and Psychiatry, 56(9), 988–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palomo R, Belinchón M, & Ozonoff S (2006). Autism and family home movies: A comprehensive review. Journal of Developmental and Behavioral Pediatrics, 27, S59–68. [DOI] [PubMed] [Google Scholar]
- Parikh C, Iosif AM, & Ozonoff S (2020). Brief Report: Use of the Infant–Toddler Checklist in infant siblings of children with autism spectrum disorder. Journal of Autism and Developmental Disorders. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perra O, Wass S, McNulty A, Sweet D, Papageorgiou K, Johnston M, … Alderdice F (2020). Training attention control of very preterm infants: Protocol for a feasibility study of the Attention Control Training (ACT). Pilot & Feasibility Studies, 6(17). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrocchi S, Levante A, & Lecciso F (2020). Systematic review of level 1 and level 2 screening tools for autism spectrum disorders in toddlers. Brain Sciences, 10(3), 1–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruett JR, Kandala S, Hoertel S, Snyder AZ, Elison JT, Nishino T, … Piven J (2015). Accurate age classification of 6 and 12 month-old infants based on resting-state functional connectivity magnetic resonance imaging data. Developmental Cognitive Neuroscience, 12, 123–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racer KH, & Dishion TJ (2012). Disordered attention: Implications for understanding and treating internalizing and externalizing disorders in childhood. Cognitive and Behavioral Practice, 19(1), 31–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reznick JS, Baranek GT, Reavis S, Watson LR, & Crais ER (2007). A parent-report instrument for identifying one-year-olds at risk for an eventual diagnosis of autism: The First Year Inventory. Journal of Autism and Developmental Disorders, 37(9), 1691–1710. [DOI] [PubMed] [Google Scholar]
- Robins ADL, Casagrande K, Barton M, Chen C-MA, Dumont-Mathieu T, & Fein D (2020). Validation of the Modified Checklist for Autism in Toddlers, Revised With Follow-up (M-CHAT-R/F). Pediatrics, 133(1), 37–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers SJ, & Dawson G (2010). Early start Denver model for young children with autism: Promoting language, learning, and engagement. New York, NY: The Guilford Press. [Google Scholar]
- Rogers SJ, Vismara LA, Wagner a. L., C McCormick, G Young, & S Ozonoff (2014). Autism treatment in the first year of life: A pilot study of infant start, a parent-implemented intervention for symptomatic infants. Journal of Autism and Developmental Disorders, 44(12), 2981–2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg SA, Robinson CC, Shaw EF, & Ellison MC (2013). Part C early intervention for infants and toddlers: Percentage eligible versus served. Pediatrics, 131(1), 38–46. [DOI] [PubMed] [Google Scholar]
- Rosenberg SA, & Smith EG (2008). Rates of Part C eligibility for young children investigated by child welfare. Topics in Early Childhood Special Education, 28(2), 68–74. [Google Scholar]
- Rosenberg SA, Zhang D, & Robinson CC (2008). Prevalence of developmental delays and participation in early intervention services for young children. Pediatrics, 121(6). [DOI] [PubMed] [Google Scholar]
- Sandbank M, Bottema-Beutel K, Crowley S, Cassidy M, Dunham K, Feldman JI, … Woynaroski TG (2020). Project Aim: Autism meta-analysis for studies of young children. Psychological Bulletin, 146(1), 1–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreibman L, Dawson G, Stahmer AC, Landa RJ, Rogers SJ, McGee GG, … Halladay A (2015). Naturalistic developmental behavioral interventions: Empirically validated treatments for autism spectrum disorder. Journal of Autism and Developmental Disorders, 45(8), 2411–2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semrud-Clikeman M, Walkowiak J, Wilkinson A, & Butcher B (2010). Executive functioning in children with asperger syndrome, ADHD-combined type, ADHD-predominately inattentive type, and controls. Journal of Autism and Developmental Disorders, 40(8), 1017–1027. [DOI] [PubMed] [Google Scholar]
- Shen MD, Kim SH, McKinstry RC, Gu H, Hazlett HC, Nordahl CW, … Styner M (2017). Increased extra-axial cerebrospinal fluid in high-risk infants who later develop autism. Biological Psychiatry, 82(3), 186–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen MD, Nordahl CW, Li DD, Lee A, Angkustsiri K, Emerson RW, … Amaral DG (2018). Extra-axial cerebrospinal fluid in high-risk and normal-risk children with autism aged 2–4 years: a case-control study. The Lancet Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen MD, Nordahl CW, Young GS, Wootton-Gorges SL, Lee A, Liston SE, … Amaral DG (2013). Early brain enlargement and elevated extra-axial fluid in infants who develop autism spectrum disorder. Brain, 136(9), 2825–2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shephard E, Bedford R, Milosavljevic B, Gliga T, Jones EJH, Pickles A, … Charman T (2019). Early developmental pathways to childhood symptoms of attention-deficit hyperactivity disorder, anxiety and autism spectrum disorder. Journal of Child Psychology and Psychiatry, 60(9), 963–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shephard E, Milosavljevic B, Pasco G, Jones EJH, Gliga T, Happé F, … Charman T (2017). Mid-childhood outcomes of infant siblings at familial high-risk of autism spectrum disorder. Autism Research, 10, 546–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KA, Gehricke JG, Iadarola S, Wolfe A, & Kuhlthau KA (2020). Disparities in service use among children with autism: A systematic review. Pediatrics, 145, S35–S46. [DOI] [PubMed] [Google Scholar]
- Sonuga-Barke EJS, & Halperin JM (2010). Developmental phenotypes and causal pathways in attention deficit/hyperactivity disorder: potential targets for early intervention? Journal of Child Psychology and Psychiatry, 51(4), 368–389. [DOI] [PubMed] [Google Scholar]
- Soska KC, Galeon MA, & Adolph KE (2012). On the other hand: Overflow movements of infants’ hands and legs during unimanual object exploration. Developmental Psychobiology, 54(4), 372–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahmer AC, Aranbarri A, Drahota A, & Rieth S (2017). Toward a more collaborative research culture: Extending translational science from research to community and back again. Autism, 21(3), 259–261. [DOI] [PubMed] [Google Scholar]
- Steiner AM, Gengoux GW, Klin A, & Chawarska K (2013). Pivotal response treatment for infants at-risk for autism spectrum disorders: A pilot study. Journal of Autism and Developmental Disorders, 43(1), 91–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stergiakouli E, Davey Smith G, Martin J, Skuse DH, Viechtbauer W, Ring SM, … St Pourcain B (2017). Shared genetic influences between dimensional ASD and ADHD symptoms during child and adolescent development. Molecular Autism, 8, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland R, Trembath D, & Roberts J (2018). Telehealth and autism: A systematic search and review of the literature. International Journal of Speech-Language Pathology, 20(3), 324–336. [DOI] [PubMed] [Google Scholar]
- Szatmari P, Chawarska K, Dawson G, Georgiades S, Landa R, Lord C, … Halladay A (2016). Prospective longitudinal studies of infant siblings of children with autism: Lessons learned and future directions. Journal of the American Academy of Child and Adolescent Psychiatry, 55, 179–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbott MR, Dufek S, Zwaigenbaum L, Bryson S, Brian J, Smith IM, & Rogers SJ (2019). Brief Report: Preliminary feasibility of the TEDI: A novel parent-administered telehealth assessment for autism spectrum disorder symptoms in the first year of life. Journal of Autism and Developmental Disorders. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vismara LA, McCormick CEB, Shields R, & Hessl D (2019). Extending the parent-delivered Early Start Denver Model to young children with Fragile X syndrome. Journal of Autism and Developmental Disorders, 49(3), 1250–1266. [DOI] [PubMed] [Google Scholar]
- Watson LR, Crais ER, Baranek GT, Turner-Brown L, Sideris J, Wakeford L, … Nowell SW (2017). Parent-mediated intervention for one-year-olds screened as at-risk for autism spectrum disorder: A randomized controlled trial. Journal of Autism and Developmental Disorders, 47(11), 3520–3540. [DOI] [PubMed] [Google Scholar]
- Werner E, Dawson G, Munson J, & Osterling J (2005). Variation in early developmental course in autism and its relation with behavioral outcome at 3–4 years of age. Journal of Autism and Developmental Disorders, 35, 337–350. [DOI] [PubMed] [Google Scholar]
- Werner E, Dawson G, Osterling J, & Dinno N (2000). Brief report: Recognition of autism spectrum disorder before one year of age: A retrospective study based on home videotapes. Journal of Autism and Developmental Disorders, 30(2), 157–162. [DOI] [PubMed] [Google Scholar]
- Wetherby AM, Brosnan-Maddox S, Peace V, & Newton L (2008). Validation of the Infant-Toddler Checklist as a broadband screener for autism spectrum disorders from 9 to 24 months of age. Autism, 12(5), 487–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehouse AJO, Varcin KJ, Alvares GA, Barbaro J, Bent C, Boutrus M, … Hudry K (2019). Pre-emptive intervention versus treatment as usual for infants showing early behavioural risk signs of autism spectrum disorder: a single-blind, randomised controlled trial. The Lancet Child & Adolescent Health, 4642(19), 1–11. [DOI] [PubMed] [Google Scholar]
- Wolff JJ, Gerig G, Lewis JD, Soda T, Styner MA, Vachet C, … Piven J (2015). Altered corpus callosum morphology associated with autism over the first 2 years of life. Brain, 138, 2046–2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff JJ, Gu H, Gerig G, Elison JT, Styner M, Gouttard S, … Piven J (2012). Differences in white matter fiber tract development present from 6 to 24 months in infants with autism. American Journal of Psychiatry, 169(6), 589–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuckerman KE, Lindly OJ, & Sinche BK (2015). Parental concerns, provider response, and timeliness of autism spectrum disorder diagnosis. Journal of Pediatrics, 166(6), 1431–1439.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwaigenbaum L, Bauman ML, Choueiri R, Fein D, Kasari C, Pierce K, … Smith Roley S (2015). Early identification and interventions for autism spectrum disorder: Executive summary. Pediatrics, 136, S1–S9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwaigenbaum L, Bryson SE, Brian J, Smith IM, Roberts W, Szatmari P, … Vaillancourt T (2016). Stability of diagnostic assessment for autism spectrum disorder between 18 and 36 months in a high-risk cohort. Autism Research, 9(7), 790–800. [DOI] [PubMed] [Google Scholar]


