Abstract
Small conductance calcium-activated potassium channels (SKs) are solely activated by intracellular Ca2+ and their activation leads to potassium efflux, thereby repolarizing/hyperpolarizing membrane potential. Thus, these channels play a critical role in synaptic transmission, and consequently in information transmission along the neuronal circuits expressing them. SKs are widely but not homogeneously distributed in the central nervous system (CNS). Activation of SKs requires submicromolar cytoplasmic Ca2+ concentrations, which are reached following either Ca2+ release from intracellular Ca2+ stores or influx through Ca2+ permeable membrane channels. Both Ca2+ sensitivity and synaptic levels of SKs are regulated by protein kinases and phosphatases, and degradation pathways. SKs in turn control the activity of multiple Ca2+ channels. They are therefore critically involved in coordinating diverse Ca2+ signaling pathways and controlling Ca2+ signal amplitude and duration. This review highlights recent advances in our understanding of the regulation of SK2 channels and of their roles in normal brain functions, including synaptic plasticity, learning and memory, and rhythmic activities. It will also discuss how alterations in their expression and regulation might contribute to various brain disorders such as Angelman Syndrome, Alzheimer’s disease and Parkinson’s disease.
Introduction
Small conductance Ca2+-activated K+ channels (SKs) belong to the family of Ca2+-activated K+ channels. Based on channel properties, Ca2+-activated K+ channels are classified into 3 subtypes (Figure 1A; members of Ca2+-activated K+ channels are in bold): big-conductance K+ channels (BK, aka KCa1.1, KCNMA1) with single channel conductance of 100–200 pS, small conductance K+ channels (SK1-3, aka KCa2.1-2.3, KCNN1−3) with 10–20 pS single channel conductance, and intermediate-conductance K+ channels (IK, aka KCa3.1/SK4/KCNN4) (Adelman, Maylie et al. 2012). SKs are widely expressed in the CNS and play critical roles in various brain functions due to their unique channel properties and locations. SKs are activated by increased intracellular [Ca2+] ([Ca2+]i), one of the most common downstream events in synaptic transmission, resulting from either a Ca2+ influx through plasma membrane Ca2+ permeable ionotropic N-methyl-D-aspartate glutamate receptor (NMDAR) (Ngo-Anh, Bloodgood et al. 2005), or Ca2+ release from intracellular Ca2+ stores, and calcium binding to constitutively bound calmodulin (CaM). Activation of SKs leads to rapid K+ efflux and membrane repolarization/hyperpolarization; the resulting shunting of membrane potential changes the function of other ion channels, e.g., NMDAR, thereby further regulating information transmission along neuronal circuits expressing these channels. Emerging evidence has positioned SKs in the center of a signaling cascade regulating information flow along CNS circuits and indicated that abnormal function within this cascade is involved in several neurological and neuropsychiatric disorders. Furthermore, due to their widespread distribution in multiple organs, these channels are also critically involved in various physiological functions in several peripheral systems, such as the cardiovascular, intestinal, renal, and immune systems. In this review, we will focus on the distribution, post-translational regulation, and function of SK2 channels in normal brain function and in various brain diseases. The authors apologize to the authors of many excellent papers that we failed to cite here due to space limitation.
Figure 1. Ca2+-activated potassium channels.
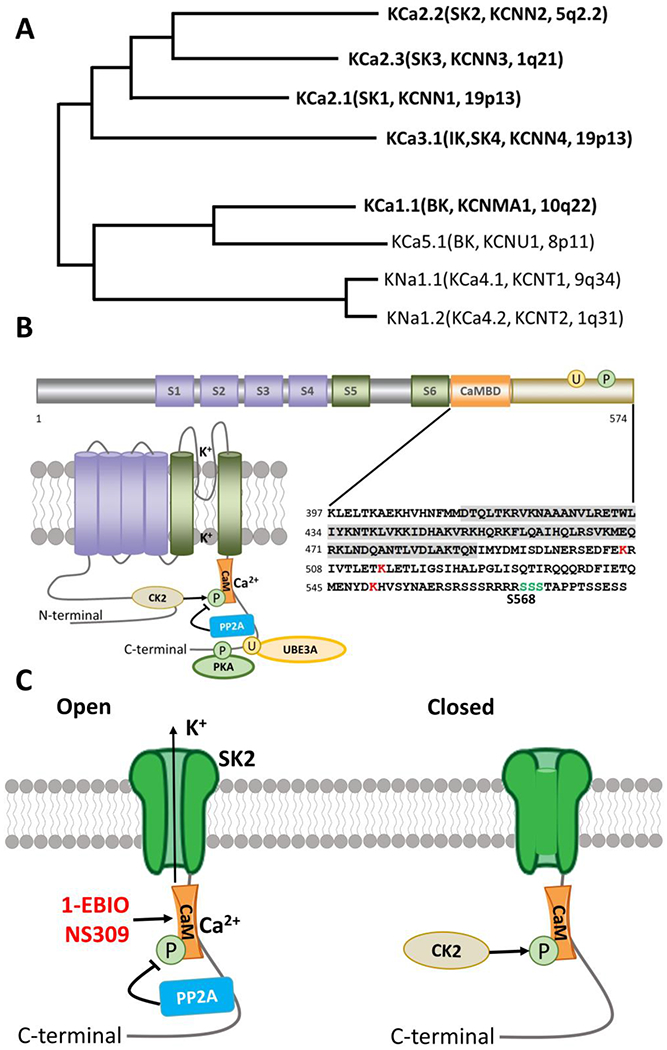
A. Phylogenetic tree for Ca2+ activated K channels. Modified from Aldrich R, Chandy KG, Grissmer S, Gutman GA, Kaczmarek LK, Wei AD, Wulff H. Calcium- and sodium-activated potassium channels, introduction. Last modified on 22/01/2015. Accessed on 16/08/2019. IUPHAR/BPS Guide to PHARMACOLOGY, http://www.guidetopharmacology.org/GRAC/FamilyIntroductionForward?familyld=69 (Aldrich, Chandy et al. 2019)
B. Functional domains, topography, and C-terminal sequence of SK2 channels. Cylinders represent α-helical transmembrane segments (S1-S6) with green (S5,S6) indicating the pore-lining segments. The C-terminal calmodulin binding domain (CaMBD) is shown in orange and shaded in detailed sequence (lower right panel). Major sites of PKA phosphorylation (P) and Ube3a ubiquitination sites (U) are indicated by green and red respectively in the detailed sequence (lower right panel). Phosphorylation and dephosphorylation of CaM by CK2 and PP2A are also indicated in the lower left panel.
C. Open and closed versions of the SK2 channel. PP2A and CK2 alter the Ca2+ sensitivity of the SK channels by dephosphorylating or phosphorylating SK-associated CaM. 1-EBIO and NS309 act by enhancing the Ca2+ sensitivity of SK channels.
1. SK channel distribution and function in the CNS
A major function of SKs is to mediate the medium afterhyperpolarization (mAHP), thereby influencing synaptic transmission, neuronal firing frequency, synaptic plasticity, and learning and memory, although the contribution of SK channels to the mAHP in hippocampal subfield CA1 (Cornu Ammonis 1) pyramidal cells has been controversial (Stocker, Krause et al. 1999, Bond, Herson et al. 2004, Gu, Vervaeke et al. 2005, Chen, Benninger et al. 2014). The three subtypes of SKs exhibit different but also overlapping regional and subcellular distributions in the CNS. The allosteric blocker apamin displays some selectivity between SK channel subtypes, with SK2 being the most sensitive, followed by SK3 and then SK1 (Kohler, Hirschberg et al. 1996). Both SK1 and SK2 channels are highly expressed in neocortex, hippocampus, and cerebellum; in many cases both channels are found in the same neurons (Sailer, Hu et al. 2002, Sailer, Kaufmann et al. 2004), which may provide the basis for the preferential assembly of heteromeric SK1/SK2 channels (Church, Weatherall et al. 2015). SK1 can also form heteromeric channels with IK channels; these heteromeric channels have unique pharmacological and biophysical properties, which are distinct from those seen with homomeric channels (Higham, Sahu et al. 2019). It is noteworthy that metabotropic glutamate receptor type 5 (mGluR5) and SK2 channels have been shown to co-assemble in heterologous cells and rat primary hippocampal neurons, wherein mGluR5 receptor activation increases SK2 currents (Garcia-Negredo, Soto et al. 2014). Furthermore, co-expression of these two proteins not only increased their plasma membrane targeting, but also increased mGlu5 receptor function. It remains to be determined whether the interaction of these two membrane proteins plays any roles at the neuronal circuit and behavioral levels.
Within the hippocampus, the highest expression levels of SK1 channels are found in stratum radiatum in hippocampal CA1 and CA3 subregions, and in the molecular layer of the dentate gyrus, while SK2 channel expression is highest in stratum radiatum and stratum oriens in CA1 and CA2. Low to medium levels of SK1 and SK2 channels are also found in the amygdala. Low levels of SK3 channels are present in cortex, hippocampus, and other brain regions (Rimini, Rimland et al. 2000, Stocker and Pedarzani 2000, Tacconi, Carletti et al. 2001, Sailer, Hu et al. 2002, Chen, Gorman et al. 2004, Sailer, Kaufmann et al. 2004). At the cellular level, SK1 and SK2 channels are primarily found in neuronal cell bodies and dendrites. In hippocampal CA1 pyramidal neurons, SK2 channels are present in dendritic spines in close proximity to NMDARs (Lin, Lujan et al. 2008, Allen, Bond et al. 2011).
Two SK2 isoforms, SK2-long (SK2-L) and SK2-short (SK2-S), have been identified, with SK2-L having a 207 amino acid N-terminal extension, as compared to SK2-S (Allen, Bond et al. 2011). Both SK2-L and SK2-S isoforms are expressed in CA1 pyramidal neurons. SK2-L is necessary for synaptic localization, as SK2-S channels are excluded from the post-synaptic density of dendritic spines in mice lacking the SK2-L isoform (Allen, Bond et al. 2011). It has recently been reported that the synaptic scaffold protein, MPP2 (membrane palmitoylated protein 2), is crucial for SK2 synaptic localization and function in synaptic plasticity (Kim, Lujan et al. 2016). Postmortem study of human brains has detected SK2 proteins in stratum oriens, radiatum and lacunosum moleculare of hippocampal CA1 and CA2 areas. SK2 channels have also been found in human amygdala, with the highest expression in the basolateral nucleus, and in neocortex, with the highest expression in layer V (Willis, Trieb et al. 2017). Interestingly, a recent report showed that in layer V pyramidal neurons, somatic and dendritic SK channels have opposite effects on neuronal excitability, with activation of somatic SK channels inhibiting while activation of the dendritic channels enhancing neuronal output (Bock, Honnuraiah et al. 2019). This difference could be due to different interacting proteins since shRNA-mediated MPP2 knockdown only affects SK2 channels in dendritic spines and not in soma (Kim, Lujan et al. 2016). Purkinje cells exhibit an axo-somato-dendritic gradient of SK2 expression with a 12-fold increase from the soma to the dendritic spines, although SK2 channels are excluded from the PSDs in dendritic spines of Purkinje neurons (Lujan, Aguado et al. 2018). In mouse locus coeruleus (LC) neurons, although SK1, SK2 and SK3 channels are all expressed, SK2 channels are the predominantly expressed variant. SK channels are essential regulators of the intrinsic pacemaker function of LC neurons; in these neurons, the AHP is mostly mediated by the outward currents generated by SK channels (Matschke, Rinne et al. 2018).
2. SK channel properties and their modulation
SKs exhibit six transmembrane segments (S1-6), a P loop region, and intracellular N and C terminal domains (Figure 1B) (Faber 2009); the K+-selective pore is formed between S5 and S6. Calmodulin (CaM) is constitutively bound to the CaM-binding domain (CaMBD) of the C terminus of SK channels and provides the Ca2+ sensing mechanism (Xia, Fakler et al. 1998, Schumacher, Rivard et al. 2001). Binding of Ca2+ to CaM induces a conformational change of the channel, which results in its opening (Xia, Fakler et al. 1998, Fanger, Ghanshani et al. 1999, Schumacher, Rivard et al. 2001). Notably, E-F hands 1 and 2 in the N terminus of CaM are necessary and sufficient for Ca2+-dependent gating in SK2 channels, whereas the C-terminal domains are necessary for Ca2+-independent, constitutive binding (Keen, Khawaled et al. 1999). Several lines of investigation have shown that SK2 channels form a complex with a protein kinase, casein kinase 2 (CK2), and a protein phosphatase, phosphatase 2A (PP2A) [reviewed in (Adelman, Maylie et al. 2012)]. Like CaM, both CK2 and PP2A are constitutively bound to SK channels. By phosphorylating SK2-associated CaM at Thr80, CK2 reduces SK channel Ca2+ sensitivity and increases channel closing rate, whereas PP2A reverses CK2-induced channel changes by dephosphorylation of Thr80 (Figure 1C). Interestingly, phosphorylation and dephosphorylation by CK2 and PP2A at Thr80 is “state-dependent”: phosphorylation occurs only when the channel is closed while dephosphorylation happens when the channel is open (Adelman 2016). Targeting the binding of CaM to the CaMBD has led to the identification of a class of positive channel modulators, including 1-Ethyl-2-benzimidazolinone (1-EBIO) (Figure 1C) (Pedarzani, Mosbacher et al. 2001). CK2-mediated reduction of SK channel Ca2+ sensitivity contributes to M1-type muscarinic receptor (M1R)-induced facilitation of synaptic transmission in hippocampal CA1 pyramidal neurons (Giessel and Sabatini 2010). However, M1R-induced facilitation of theta burst stimulation (TBS)-induced long-term potentiation (LTP) at Schaffer collateral synapses in hippocampus depends on SK channel inhibition induced by protein kinase C (PKC), instead of by CK2 (Buchanan, Petrovic et al. 2010). The reason for this discrepancy is currently unknown.
Direct phosphorylation of some of the predicted phosphorylation sites of the SK channels has also been shown to modulate their expression and functions (Kohler, Hirschberg et al. 1996). In particular, protein kinase A (PKA)-mediated phosphorylation of serine residues 568-570 (Figure 1B) in the C-terminal domain of SK2 channels reduces their surface localization in COS cells (Ren, Barnwell et al. 2006). In amygdala pyramidal neurons, PKA-mediated phosphorylation of SK channels also reduces their targeting to the plasma membrane (Faber, Delaney et al. 2008), although the exact residues involved in this process have not yet been identified. PKA-induced SK channel internalization has also been observed in dopaminergic neurons in the substantia nigra (Estep, Galtieri et al. 2016). On the other hand, Rab4-and Rab11-mediated forward trafficking is important for membrane insertion of SK channels (Honrath, Krabbendam et al. 2017). We recently showed that lysine residues (K506/K514/K550, Figure 1B) in the C-terminal domain of SK2 can be ubiquitinated by the E3 ligase, UBE3A, which results in SK2 protein endocytosis and degradation (Figure 2A) (Sun, Zhu et al. 2015). Moreover, Ube3a-mediated SK2 ubiquitination hinders Rab11-mediated SK2 recycling to synaptic membranes following internalization resulting from theta-burst stimulation (TBS)-induced long term potentiation (LTP) (Figure 2A) (Sun, Liu et al. 2020). Posttranslational downregulation of dendritic SK2 channels has also been shown to contribute to brain-derived neurotrophic factor (BDNF)-induced facilitation of LTP (Kramar, Lin et al. 2004) and deafferentation-induced reactive hyperexcitability in hippocampal CA1 pyramidal neurons (Cai, Wei et al. 2007). At the system level, circuit-specific down-regulation of SK2 channels in amygdala neurons correlates with enhanced anxiety-like behavior, which can be corrected by virus-mediated expression of SK2 (Zhang, Liu et al. 2019), further underlining the importance of maintaining optimal synaptic localization and levels of SK channels.
Figure 2. Regulation of synaptic SK2 levels and function in synaptic transmission and plasticity.
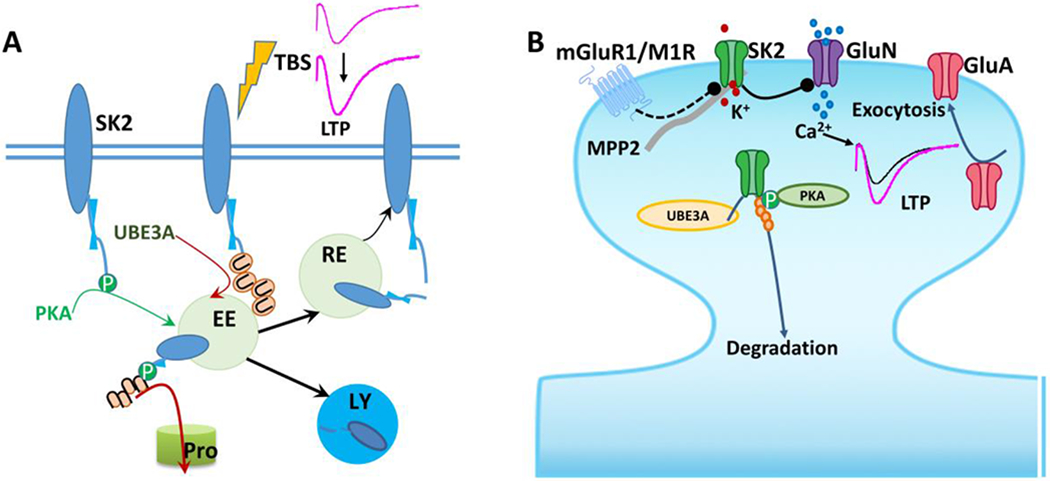
A. Synaptic SK2 channels are regulated by PKA phosphorylation and Ube3a ubiquitination; both modifications target SK2 channel for endocytosis. Some of the endocytosed SK2 channels could recycle back to synaptic membranes, while enhanced ubiquitination and phosphorylation target them for degradation in proteasomes, and possibly lysosomes as well. EE, early endosomes; RE, recycling endosomes; LY, lysosomes; Pro, proteasomes. Modified from Sun et al. 2020 (Sun, Liu et al. 2020).
B. SK2 channel-mediated hyperpolarization shunts AMPAR (GluA)-induced depolarization, thereby inhibiting NMDAR (GluN) activation. Removal of SK2 channels by phosphorylation or ubiquitination and inhibition of SK2 channel activity by metabotropic receptors (mGluR1 or M1R) facilitate NMDAR opening and LTP induction. LTP-induced endocytosis of synaptic SK2 channels occurs in parallel with and depends on exocytosis of GluA1-containing AMPARs. The synaptic scaffold protein, MPP2 (membrane palmitoylated protein 2), is crucial for SK2 synaptic localization and function in synaptic plasticity.
3. SK2 channels play important roles in synaptic plasticity and learning and memory
Glutamate receptors, including ionotropic AMPA (α-Amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid) and NMDA receptors and metabotropic receptors (mGluRs), mediate most of the excitatory synaptic transmission in the CNS and play critical roles in synaptic plasticity and cognitive functions. Besides mediating the mAHP [(Stocker, Krause et al. 1999, Bond, Herson et al. 2004); but see (Gu, Vervaeke et al. 2005, Chen, Benninger et al. 2014)] and the repolarization of Ca2+-mediated plateau potentials (Cai, Liang et al. 2004), SKs regulate the amplitude of excitatory post-synaptic potentials (EPSP) by limiting membrane depolarization, as demonstrated in hippocampal and amygdala neurons (Bloodgood and Sabatini, 2007; Faber et al., 2005; Ngo-Anh et al., 2005). In hippocampal CA1 pyramidal neurons, AMPA receptor (AMPAR)-mediated postsynaptic depolarization results in the removal of Mg2+ from NMDAR-channels, allowing channel opening and Ca2+ influx. Ca2+ then activates SK2 and the resulting outward K+ current partially repolarizes the membrane and reinstates Mg2+ blockade of NMDAR-channels (Ngo-Anh, Bloodgood et al. 2005). This local negative feedback loop between SK2 channels and NMDARs has been proposed to play an important role in regulating neuronal firing frequency and controlling the threshold for LTP induction at CA3 to CA1 synapses in hippocampus (Hammond, Bond et al. 2006, Lin, Lujan et al. 2008). Ca2+ influx through NMDARs is responsible for the induction of LTP and long-term depression (LTD) at these synapses, with large amounts of Ca2+ influx leading to LTP, whereas a smaller amount of Ca2+ influx results in LTD (Malenka and Nicoll 1993). Therefore, by controlling [Ca2+]i homeostasis, SK2 channels effectively influence bidirectional synaptic plasticity. Indeed, Stackman et al (Stackman, Hammond et al. 2002) showed that blocking SK channels with apamin changed the stimulation frequency for LTD induction from 10 Hz to 5 Hz (Stackman, Hammond et al. 2002), in an NMDAR-dependent manner. Recently, it has been reported that inhibition of SK channels, paradoxically by Ca2+ released from lysosomes, is required for mGluR1-induced LTP in hippocampal CA1 pyramidal neurons, and counterintuitively in a PP2A-dependent manner (Foster, Taylor et al. 2018). Although the precise mechanism is unknown, this result further emphasizes the importance of SK channels in synaptic plasticity, since mGluR1 activation is commonly linked to LTD instead of LTP.
Accumulating evidence indicate that events that trigger synaptic plasticity also regulate synaptic SK2 channel levels through the activation of PKA and Ube3a. In mouse hippocampus, LTP induction results in endocytosis of synaptic SK2 channels (Lin, Lujan et al. 2008, Sun, Zhu et al. 2015), which contributes to the enhancement of EPSPs at potentiated synapses (Lin, Lujan et al. 2008). This process is partially due to PKA-mediated phosphorylation of three serine residues (Ser568–570) (Lin, Lujan et al. 2008) and to Ube3a-mediated ubiquitination (Sun, Zhu et al. 2015) in the C-terminal domain of SK2 channels (Figure 2). LTP-induced endocytosis of synaptic SK2 channels occurs in parallel with and depends on PKA-dependent exocytosis of GluA1-containing AMPARs (Lin, Lujan et al. 2008), since blocking GluA1-containing AMPA receptor exocytosis also prevents SK2 channel endocytosis (Lin, Lujan et al. 2010). However, how traffickingcoupling of these two synaptic proteins in opposite directions occurs remains unknown. As mentioned earlier, Ube3a-mediated SK2 ubiquitination also contributes to LTP-induced removal of SK2 channels (Sun, Zhu et al. 2015) and hinders their recycling back to synaptic membranes and promotes their degradation (Figure 2B). We have recently reported that LTP induced by different stimulation patterns depends on different cell signaling pathways; while high-frequency stimulation (HFS)-induced LTP depends on PKA activation, TBS-induced LTP does not (Zhu, Liu et al. 2015). It is conceivable that, although TBS-induced LTP does not depend on PKA activation, TBS could still induce PKA activation and trigger SK2 internalization. This notion is supported by the observation that, although application of PKA inhibitors did not block TBS-induced LTP, it did block LTP-induced synaptic SK2 removal (Lin, Lujan et al. 2008). Together, these results suggest that higher levels of PKA activation could compensate for the lack of Ube3a-mediated SK2 regulation during LTP induction. It also appears that Ube3a contributes more to controlling basal levels of synaptic SK2, while PKA-induced SK2 internalization occurs preferentially during synaptic activation. Furthermore, we recently showed that while the two types of SK2 channel posttranslational modifications by Ube3a and PKA could occur independently, PKA-mediated phosphorylation facilitates Ube3a-mediated ubiquitination of SK2 (Sun, Liu et al. 2020). Thus, cooperation between PKA and Ube3a provides further fine-tuning of synaptic SK2 levels.
Another type of synaptic plasticity in which SK channels may play important roles is spike-timing-dependent (STD) plasticity (STDP). The canonical STDP is a process under which neuronal connection strength is adjusted according to its “activation history”: a synaptic connection is time-dependently strengthened if the presynaptic stimulation precedes (positive) the postsynaptic neuronal spike, whereas it is weakened if presynaptic stimulation follows (negative) the postsynaptic spike. In layer V pyramidal neurons of somatosensory cortex, low-frequency (0.2 Hz) single AP-EPSP (action potential-excitatory postsynaptic potential) pairing at both positive and negative intervals did not lead to STDP (Jones, To et al. 2017), which is consistent with the literature (Markram, Lubke et al. 1997, Kampa, Letzkus et al. 2006). However, in the presence of the SK2 channel blocker, apamin, positive pairing induced LTP while negative pairing induced LTD. In this case, spine localized SK2 channels fine-tuned STDP induction by regulating NMDAR (Jones, To et al. 2017). Tigaret et al. reported that, at mature hippocampal Schaffer collateral synapses, STDP-induced LTP requires potentiation of NMDARs by mGluR1-mediated inhibition of SK channels (Tigaret, Olivo et al. 2016). A subsequent study showed that activation of M1 receptors facilitates STD-induced LTP by reducing SK channel activity (Tigaret, Chamberlain et al. 2018). Furthermore, M1R-mediated inhibition of SK channels has been linked to LTP induced by patterns of activity taking place during behavioral exploration, while mGluR1-mediated inhibition of SK channels was associated with sharp wave ripple-stimulated LTP (Tigaret, Chamberlain et al. 2018). Thus, SK channels play critical roles in synaptic plasticity induced by different stimulation patterns or under different neuronal “activity history”.
In a recent review, Titley et al. (Titley, Brunei et al. 2017) argued that activity-induced alterations in neuronal intrinsic excitability is critical for learning and memory, in an SK channel dependent manner. This “neurocentric view of learning” posits that changes in intrinsic excitability, such as through downregulation of SK channels, reduce AHP amplitude, which in turn leads to a prolonged enhancement of EPSPs, followed by increased probability of spike generation, and thus in a long-lasting synaptic strength enhancement and information encoding. The validity of this hypothesis notwithstanding, the critical roles of SK channels in cognitive functions are clear. The unique properties of SK channels, including their activation by calcium, their slow deactivation kinetics, their regulation by neuromodulators, kinases and phosphatases, and ubiquitination enable them to regulate neuronal activity at multiple levels in circuits expressing them. Indeed, in vivo animal experiments have shown that apamin treatment facilitates hippocampus-dependent learning and memory (Stackman, Hammond et al. 2002, Faber, Delaney et al. 2005, Ngo-Anh, Bloodgood et al. 2005, Allen, Nakayama et al. 2011, Chakroborty, Kim et al. 2012). Genetic deletion of SK2 channels, but not of SK1 or SK3 channels, abolishes the effect of apamin (Bond, Herson et al. 2004), confirming the inhibitory effect of SK2 channels in learning and memory.
4. Roles of SK2 in rhythmic and pacemaker activity and related behaviors
Rhythmic activities in the brain are critical for coding and storing information in the brain (Lisman and Jensen 2013). The hippocampus exhibits three main classes of rhythms: theta (~4–12 Hz), sharp wave-ripples (~150–200 Hz ripples superimposed on ~0.01–3 Hz sharp waves), and gamma (~25–100 Hz) (Colgin 2016). The theta rhythm is predominant during locomotion and attention, and is necessary for memory formation (Winson 1978). Hippocampal gamma activity appears to facilitate memory encoding as well, although this is still debated (Jutras, Fries et al. 2009, Colgin and Moser 2010). Sharp wave–ripples are thought to be responsible for stabilizing and consolidating memories among other functions [reviewed in (Colgin 2016)].
Combe et al. (Combe, Canavier et al. 2018) recently showed that CA1 pyramidal neurons fire preferentially in the slow gamma range regardless of whether the input occurs at fast or slow gamma frequencies, suggesting the existence of a filter regulating CA1 outputs in response to CA3 inputs. This filtering effect was greatly attenuated when SK channels were blocked with apamin, suggesting the involvement of SK channels. Likewise, M1R stimulation also attenuates this selectivity, indicating that M1R-mediated inhibition of SK channels contributes to rhythmic selective transmission at hippocampal CA3 to CA1 synapses. Alternating neuronal synchrony between hippocampal subregions may be instrumental in information routing within the hippocampal formation or in shifting hippocampal function between memory encoding and memory retrieval (Colgin and Moser 2010). It has recently been shown that SK3 channels control the frequency and precision of pacemaker spiking of dopamine (DA) neurons in the substantia nigra, which play important roles in movement, reward, and cognition (Wolfart, Neuhoff et al. 2001). The selective coupling of SK and T-type Ca2+ channels is critical for maintaining pacemaker activity and preventing intrinsic burst-firing of these DA neurons (Wolfart and Roeper 2002). Similarly, apamin-sensitive SK channels are involved in controlling the firing pattern of cerebellar Purkinje neurons, which are essential for motor coordination and maintenance of balance (Womack and Khodakhah 2003).
5. SK2 channels in brain disorders
We recently reported that SK2 channels were involved in alterations in synaptic plasticity and impairment in learning and memory in a mouse model of Angelman syndrome (AS), a neurogenetic disorder with severe developmental delay and cognitive and motor disability. TBS-induced LTP is reduced, while low-frequency (LFS)-induced LTD is enhanced in hippocampal slices from AS mice, as compared to wild-type (WT) mice; both changes can be reversed by apamin (Sun, Zhu et al. 2015). However, HFS-induced LTP remains intact (Figure 3). The effects of apamin on both LTP impairment and LTD enhancement are NMDAR-dependent. Apamin treatment also significantly improves the performance of AS mice in the fear-conditioning paradigm (Sun, Zhu et al. 2015). Interestingly, studies of transgenic mice that overexpress SK2 channels reveal that induction of LTP with three 50 Hz tetani is reduced in slices from SK2-overexpressed mice relative to WT slices, while induction of LTP with three 100 Hz trains is equivalent in WT and SK2-overexpressed slices (Hammond, Bond et al. 2006). These results are consistent with the notion that, by regulating NMDAR activity, increased synaptic levels of SK2 channels modify the response of hippocampal circuits to different stimulation patterns, and thus affect different cognitive functions. However, in contrast to the enhanced LTD in AS slices, SK2 overexpression does not affect the induction of LTD. The discrepancies may arise because of different ages of animals used in the two studies. Hammond et al. prepared hippocampal slices from 3-to 5-week-old SK2-overexpressed or WT littermate mice, while we prepared hippocampal slices from 3-month-old control WT or AS mice. It has been reported that the magnitude of LTD declines in an age-dependent manner, with sustained LTD in slices from 3-to 5-week-old mice and only a transient synaptic depression in slices from adult mice (Dudek and Bear 1993). Whether SK2 is involved in the postnatal decline in LTD magnitude is an intriguing question. Of note, it has recently been shown that spaced training improved water maze spatial learning by AS mice (Lauterborn, Schultz et al. 2019). We have shown that although one TBS failed to induce LTP, two TBS applied within 45 min was able to induce LTP in AS mice (Sun, Zhu et al. 2015). We subsequently showed that the effect of two TBS was due to PKA activation-induced SK2 endocytosis, as it was blocked by a PKA inhibitor and occluded with apamin (Sun, Liu et al. 2020). Whether down-regulation of SK2 contributes to improved performance in spaced learning remains a possibility to be confirmed.
Figure 3. TBS-induced LTP but not HFS-induced LTP is impaired in hippocampal CA1 region of AS mice.
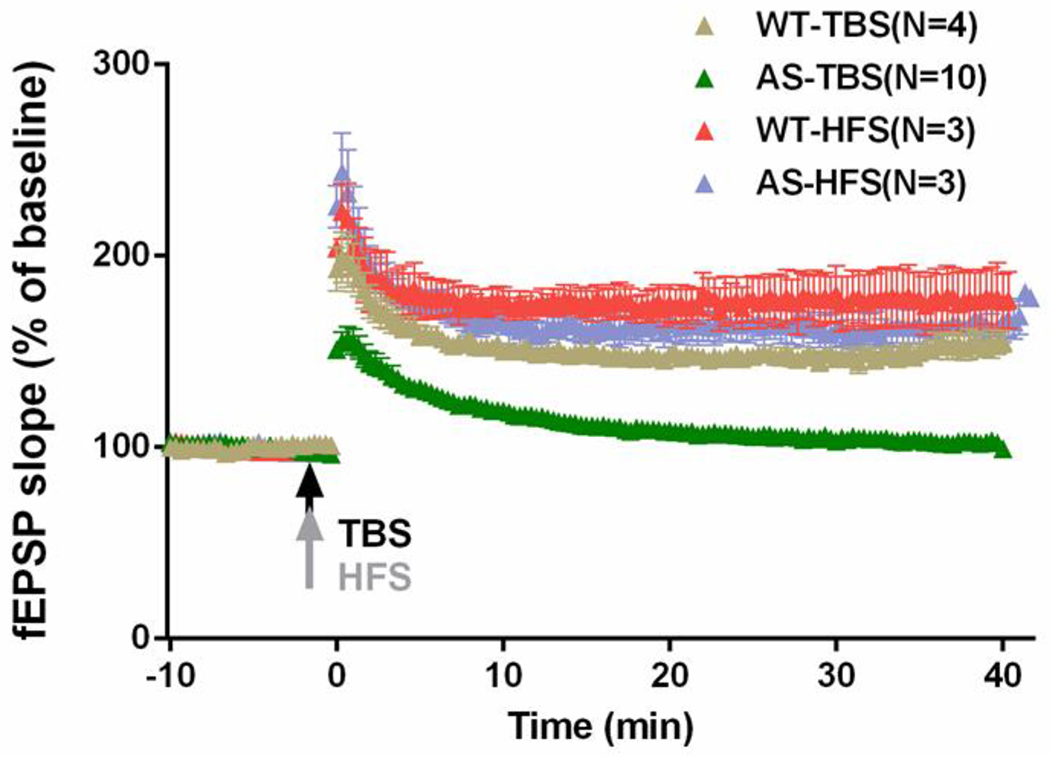
Acute hippocampal slices from wildtype (WT) and Ube3a deficienct (AS) mice were first recorded for 10 min with a stable baseline; the slices were then stimulated with either TBS (10 bursts of 4 pulses at 100 Hz delivered at 5 Hz) or HFS (100 Hz, 1 s) and fEPSP were further recorded for another 40 min.
Since SK channels play critical roles in synaptic transmission, rhythmic activity, and other functions in the CNS, it is thus not surprising that abnormal levels and/or functions of these channels are linked to various CNS disorders (Figure 4). It has also been recently reported that abnormal function of BK channels is associated with another autism spectrum disorder (ASD)-related disorder, the Fragile X syndrome (FXS) (Verkerk, Pieretti et al. 1991, Deng, Rotman et al. 2013, Brager and Johnston 2014, Contractor, Klyachko et al. 2015); in this case, treatment with a selective BK channel opener reduced behavioral deficits in Fmr1 knockout mice (Hebert, Pietropaolo et al. 2014).
Figure 4. Changes in levels and activity of SK channels lead to alterations in neuronal firing frequency/patterns and synaptic transmission, which are potentially involved in several neurological disorders.
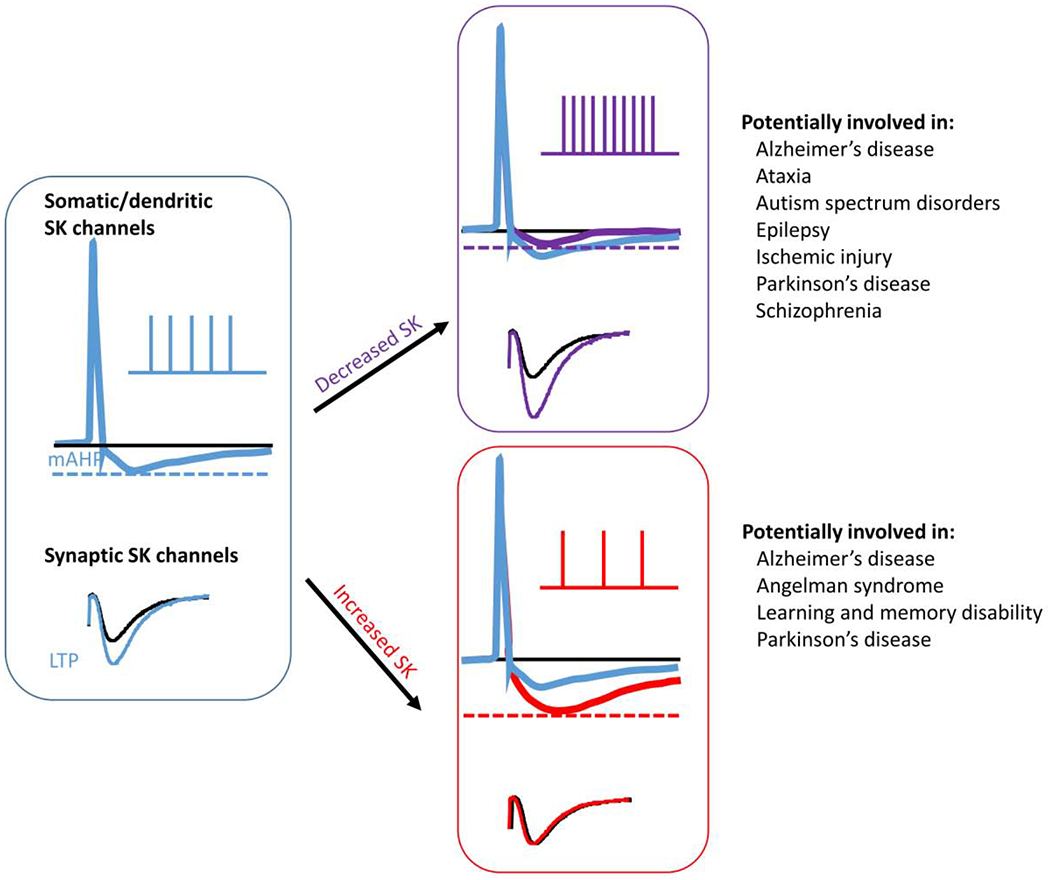
See text for details.
Alterations in SK channels have also been reported in various neurodegenerative diseases. In one mouse model of Alzheimer’s disease (AD), the TgCRND8 mice, attention deficits have been linked to increased apamin-sensitive SK activity (Proulx, Fraser et al. 2015), resulting from enhanced functional coupling of nicotinic receptor-mediated calcium signals to SK2 channels, which in turn inhibits nicotinic receptor-mediated excitation of prefrontal attentional circuitry (Proulx, Power et al. 2019). Over-activation of SK channels has also been shown to mediate the abnormal signaling initiated by amyloid precursor protein (APP) intracellular domain (AICD)-induced changes in GluN2B-enriched NMDARs at immature excitatory synapses (Pousinha, Mouska et al. 2017). In this regard, SK channel blockers should be beneficial for reversing the learning and memory deficits found in AD patients. However, it has also long been proposed that excitotoxicity induced by NMDAR over-activation contributes to the pathogenesis of AD. If this were to be true, activation of SK channels to inhibit NMDAR and change intrinsic firing frequency could be beneficial for AD patients (Zadori, Veres et al. 2014). This notion is further supported by the findings that reduced SK2 channel levels contribute to glutamate toxicity and ischemia-induced neuronal death (Dolga, Terpolilli et al. 2011).
The pathogenesis of Parkinson’s disease (PD), the second most common age-related neurodegenerative disease, is associated with DA neuronal loss (Surmeier, Guzman et al. 2010) and has also been related to SK and other K+ channels (reviewed in (Wang, Zeng et al. 2008, Chen, Xue et al. 2018)), which is consistent with the critical roles that SK channels play in neurotransmission of DA neurons in the SNc (substantia nigra pars compacta, see earlier discussion). In an animal study with the frissonnant mutant mice, a proposed model for PD, deletion of SK2 expression has been linked to altered neuronal AHP and firing patterns and impaired locomotion and constant rapid tremor (Szatanik, Vibert et al. 2008). However, the precise role of SK channels in the etiology of PD remains elusive, in part due to contradictory experimental evidence. For instance, reduced SK channel activity has been proposed to cause some of the NMDAR overactivation-induced symptoms in PD [reviewed in (Chen, Xue et al. 2018)]. Along this line, activation of SK channels by NS309 in human DA neurons inhibited spontaneous firing by enhancing mAHP (Ji and Shepard 2006), thereby reducing neurotoxicity (Dolga, de Andrade et al. 2014), whereas inhibition of SK channels with NS8953 reduced the number of dopaminergic neurons (Benitez, Belalcazar et al. 2011). These results suggest that increasing SK channel activity could potentially preserve DA neurons and be beneficial to PD patients. On the other hand, several studies have provided evidence that blockade of SK channels by apamin is neuroprotective in the in vivo MPTP-induced PD model (Doo, Kim et al. 2010, Kim, Yang et al. 2011, Alvarez-Fischer, Noelker et al. 2013) and alleviates cognitive and motor deficits induced by partial striatal DA lesions in rats (Chen, Deltheil et al. 2014). A possible interpretation for these apparently contradictory results in PD pathogenesis is that SK channel positive or negative modulators could be beneficial depending on the stage (early or late) of the disease.
Dysregulation of SK channels has also been implicated in other neurological diseases. It has been proposed that altered neuronal excitability due to increased SK channel currents, as shown in rats with reduced DISC-1 expression, may be linked to schizophrenia (El-Hassar, Simen et al. 2014). ∆Disc1 mutant mice have also been shown to exhibit an upregulated expression of SK2, which is associated with a prolonged interspike interval in the hippocampal CA1 field (Sultana, Ghandi et al. 2018). A spontaneous mutation of the SK3 channel gene (KCNN3) was identified in human schizophrenia patients (Miller, Rauer et al. 2001). Down-regulation of SK channels has been associated with seizures occurring in several brain diseases. In the pilocarpine model of epilepsy in rats, the expression and function of SK channels were significantly reduced (Oliveira, Skinner et al. 2010). Reduced SK activity has also been linked to seizure activity in Dravet syndrome (Isom 2019, Ritter-Makinson, Clemente-Perez et al. 2019). Animal studies showed that cognitive impairment caused by cerebral hypoperfusion could be reversed by down-regulation of SK1,2,3 channels resulting from melatonin treatment (Al Dera, Alassiri et al. 2019). In contrast, decreased SK3 levels have been reported to be associated with hypobaric hypoxia-induced learning and memory impairment (Kushwah, Jain et al. 2018).
Apamin sensitive SK channels have also been implicated in reward seeking and drug abuses. For instance, reduced SK3-mediated current and increased neuronal excitability in the nucleus accumbens core represent critical mechanisms that facilitate motivation to seek alcohol after abstinence (Hopf, Bowers et al. 2010), while in hippocampal pyramidal neurons, decreased synaptic SK2 channel levels appear to be critical for alcohol-associated plasticity (Mulholland, Becker et al. 2011). It has recently been reported that chronic intermittent ethanol exposure increases synaptic excitability in the ventral, but not the dorsal, domain of the hippocampus, which is associated with ventral hippocampal alterations in synaptosomal expression of SK2 and GluA2 subunits (Ewin, Morgan et al. 2019). Likewise, in ventral tegmental area (VTA) dopaminergic neurons, reduced SK channel activity appears to be responsible for increased probability of burst firing induced by withdrawal from intermittent ethanol exposure (Hopf, Martin et al. 2007). On the other hand, apamin treatment rescues striatal plasticity and behavioral alterations in cannabinoid tolerance, suggesting that an increased SK2 activity is involved in drug tolerance (Nazzaro, Greco et al. 2012). Increased SK2 channel-mediated negative feedback of NMDAR has also been proposed to participate in context-dependent sensitization to morphine (Fakira, Portugal et al. 2014). Additionally, SK2-mediated neuronal plasticity in nucleus accumbens neurons is abolished by cocaine withdrawal (Ishikawa, Mu et al. 2009). These results indicate that SK channel activity is altered by drug-induced plasticity and may play important roles in reward-seeking behaviors.
Conclusions
Ca2+-activated SK channels play important roles in synaptic transmission and plasticity through their interactions with multiple membrane Ca2+ channels, including NMDAR. Emerging evidence has indicated that diverse signals regulate SK channels activity by altering their Ca2+ sensitivity. Recent research has emphasized the regulation of the synaptic localization of SK channels by multiple protein complexes, including the scaffolding protein MPP2, the protein kinase PKA, and the ubiquitination E3 ligase UBE3A. Activity-dependent regulation of SK channel function and synaptic distribution has been shown to be critically involved in learning and memory, spike duration and frequency, and rhythmic activity. However, the roles of SKs in neurological diseases are often controversial due to insufficient research. Thus, further investigation of the protein complexes orchestrating the dynamic regulation of synaptic SK channel trafficking and distribution would undoubtedly benefit not only our understanding of CNS function but also of various brain disorders.
Highlights.
Ca2+-activated small conductance K+ (SK) channels are widely expressed in the CNS.
Protein levels and functions of SK channels are regulated by phosphorylation and ubiquitination.
SK channels play important roles in synaptic plasticity and brain rhythmic activity.
Dysfunction of SK channels has been implicated in several brain disorders.
Acknowledgements:
This work was supported by an MH101703 from NIMH to XB and NS104078 from NINDS to MB. XB is also supported by funds from the Daljit and Elaine Sarkaria Chair.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Adelman JP (2016). “SK channels and calmodulin.” Channels (Austin) 10(1): 1–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adelman JP, Maylie J and Sah P (2012). “Small-conductance Ca2+-activated K+ channels: form and function.” Annu Rev Physiol 74: 245–269. [DOI] [PubMed] [Google Scholar]
- Al Dera H, Alassiri M, Eleawa SM, AlKhateeb MA, Hussein AM, Dallak M, Sakr HF, Alqahtani S and Khalil MA (2019). “Melatonin Improves Memory Deficits in Rats with Cerebral Hypoperfusion, Possibly, Through Decreasing the Expression of Small-Conductance Ca(2+)-Activated K(+) Channels.” Neurochem Res 44(8): 1851–1868 [DOI] [PubMed] [Google Scholar]
- Aldrich R, Chandy KG, Grissmer S, Gutman GA, Kaczmarek LK, Wei AD and Wulff H (2019). “Calcium-and sodium-activated potassium channels (version 2019.4) in the IUPHAR/BPS Guide to Pharmacology Database.” IUPHAR/BPS Guide to Pharmacology CITE 2019(4). [Google Scholar]
- Allen D, Bond CT, Lujan R, Ballesteros-Merino C, Lin MT, Wang K, Klett N, Watanabe M, Shigemoto R, Stackman RW Jr., Maylie J and Adelman JP (2011). “The SK2-long isoform directs synaptic localization and function of SK2-containing channels.” Nat Neurosci 14(6): 744–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen D, Nakayama S, Kuroiwa M, Nakano T, Palmateer J, Kosaka Y, Ballesteros C, Watanabe M, Bond CT, Lujan R, Maylie J, Adelman JP and Herson PS (2011). “SK2 channels are neuroprotective for ischemia-induced neuronal cell death.” J Cereb Blood Flow Metab 31(12): 2302–2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Fischer D, Noelker C, Vulinovic F, Grunewald A, Chevarin C, Klein C, Oertel WH, Hirsch EC, Michel PP and Hartmann A (2013). “Bee venom and its component apamin as neuroprotective agents in a Parkinson disease mouse model.” PLoS One 8(4): e61700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benitez BA, Belalcazar HM, Anastasia A, Mamah DT, Zorumski CF, Masco DH, Herrera DG and de Erausquin GA (2011). “Functional reduction of SK3-mediated currents precedes AMPA-receptor-mediated excitotoxicity in dopaminergic neurons.” Neuropharmacology 60(7-8): 1176–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock T, Honnuraiah S and Stuart GJ (2019). “Paradoxical excitatory impact of SK channels on dendritic excitability.” J Neurosci. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond CT, Herson PS, Strassmaier T, Hammond R, Stackman R, Maylie J and Adelman JP (2004). “Small conductance Ca2+-activated K+ channel knock-out mice reveal the identity of calcium-dependent afterhyperpolarization currents.” J Neurosci 24(23):5301–5306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brager DH and Johnston D (2014). “Channelopathies and dendritic dysfunction in fragile X syndrome.” Brain Res Bull 103: 11–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan KA, Petrovic MM, Chamberlain SE, Marrion NV and Mellor JR (2010). “Facilitation of long-term potentiation by muscarinic M(1) receptors is mediated by inhibition of SK channels.” Neuron 68(5): 948–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai X, Liang CW, Muralidharan S, Kao JP, Tang CM and Thompson SM (2004). “Unique roles of SK and Kv4.2 potassium channels in dendritic integration.” Neuron 44(2): 351–364. [DOI] [PubMed] [Google Scholar]
- Chakroborty S, Kim J, Schneider C, Jacobson C, Molgo J and Stutzmann GE (2012). “Early presynaptic and postsynaptic calcium signaling abnormalities mask underlying synaptic depression in presymptomatic Alzheimer’s disease mice.” J Neurosci 32(24):8341–8353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Deltheil T, Turle-Lorenzo N, Liberge M, Rosier C, Watabe I, Sreng L, Amalric M and Mourre C (2014). “SK channel blockade reverses cognitive and motor deficits induced by nigrostriatal dopamine lesions in rats.” Int J Neuropsychopharmacol 17(8): 1295–1306. [DOI] [PubMed] [Google Scholar]
- Chen MX, Gorman SA, Benson B, Singh K, Hieble JP, Michel MC, Tate SN and Trezise DJ (2004). “Small and intermediate conductance Ca(2+)-activated K+ channels confer distinctive patterns of distribution in human tissues and differential cellular localisation in the colon and corpus cavernosum.” Naunyn Schmiedebergs Arch Pharmacol 369(6): 602–615. [DOI] [PubMed] [Google Scholar]
- Chen S, Benninger F and Yaari Y (2014). “Role of small conductance Ca(2)(+)-activated K(+) channels in controlling CA1 pyramidal cell excitability.” J Neurosci 34(24): 8219–8230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Xue B, Wang J, Liu H, Shi L and Xie J (2018). “Potassium Channels: A Potential Therapeutic Target for Parkinson’s Disease.” Neurosci Bull 34(2): 341–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church TW, Weatherall KL, Correa SA, Prole DL, Brown JT and Marrion NV (2015). “Preferential assembly of heteromeric small conductance calcium-activated potassium channels.” Eur J Neurosci 41(3): 305–315. [DOI] [PubMed] [Google Scholar]
- Colgin LL (2016). “Rhythms of the hippocampal network.” Nat Rev Neurosci 17(4):239–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colgin LL and Moser EI (2010). “Gamma oscillations in the hippocampus.” Physiology (Bethesda) 25(5): 319–329. [DOI] [PubMed] [Google Scholar]
- Combe CL, Canavier CC and Gasparini S (2018). “Intrinsic Mechanisms of Frequency Selectivity in the Proximal Dendrites of CA1 Pyramidal Neurons.” J Neurosci 38(38): 8110–8127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contractor A, Klyachko VA and Portera-Cailliau C (2015). “Altered Neuronal and Circuit Excitability in Fragile X Syndrome.” Neuron 87(4): 699–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng PY, Rotman Z, Blundon JA, Cho Y, Cui J, Cavalli V, Zakharenko SS and Klyachko VA (2013). “FMRP regulates neurotransmitter release and synaptic information transmission by modulating action potential duration via BK channels.” Neuron 77(4): 696–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolga AM, de Andrade A, Meissner L, Knaus HG, Hollerhage M, Christophersen P, Zischka H, Plesnila N, Hoglinger GU and Culmsee C (2014). “Subcellular expression and neuroprotective effects of SK channels in human dopaminergic neurons.” Cell Death Dis 5: e999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolga AM, Terpolilli N, Kepura F, Nijholt IM, Knaus HG, D’Orsi B, Prehn JH, Eisel UL, Plant T, Plesnila N and Culmsee C (2011). “KCa2 channels activation prevents [Ca2+]i deregulation and reduces neuronal death following glutamate toxicity and cerebral ischemia.” Cell Death Dis 2: e147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doo AR, Kim ST, Kim SN, Moon W, Yin CS, Chae Y, Park HK, Lee H and Park HJ (2010). “Neuroprotective effects of bee venom pharmaceutical acupuncture in acute 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced mouse model of Parkinson’s disease.” Neurol Res 32 Suppl 1: 88–91. [DOI] [PubMed] [Google Scholar]
- Dudek SM and Bear MF (1993). “Bidirectional long-term modification of synaptic effectiveness in the adult and immature hippocampus.” J Neurosci 13(7): 2910–2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Hassar L, Simen AA, Duque A, Patel KD, Kaczmarek LK, Arnsten AF and Yeckel MF (2014). “Disrupted in schizophrenia 1 modulates medial prefrontal cortex pyramidal neuron activity through cAMP regulation of transient receptor potential C and small-conductance K+ channels.” Biol Psychiatry 76(6): 476–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estep CM, Galtieri DJ, Zampese E, Goldberg JA, Brichta L, Greengard P and Surmeier DJ (2016). “Transient Activation of GABAB Receptors Suppresses SK Channel Currents in Substantia Nigra Pars Compacta Dopaminergic Neurons.” PLoS One 11(12): e0169044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewin SE, Morgan JW, Niere F, McMullen NP, Barth SH, Almonte AG, Raab-Graham KF and Weiner JL (2019). “Chronic Intermittent Ethanol Exposure Selectively Increases Synaptic Excitability in the Ventral Domain of the Rat Hippocampus.” Neuroscience 398: 144–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faber ES (2009). “Functions and modulation of neuronal SK channels.” Cell Biochem Biophys 55(3): 127–139. [DOI] [PubMed] [Google Scholar]
- Faber ES, Delaney AJ, Power JM, Sedlak PL, Crane JW and Sah P (2008). “Modulation of SK channel trafficking by beta adrenoceptors enhances excitatory synaptic transmission and plasticity in the amygdala.” J Neurosci 28(43): 10803–10813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faber ES, Delaney AJ and Sah P (2005). “SK channels regulate excitatory synaptic transmission and plasticity in the lateral amygdala.” Nat Neurosci 8(5): 635–641. [DOI] [PubMed] [Google Scholar]
- Fakira AK, Portugal GS, Carusillo B, Melyan Z and Moron JA (2014). “Increased small conductance calcium-activated potassium type 2 channel-mediated negative feedback on N-methyl-D-aspartate receptors impairs synaptic plasticity following context-dependent sensitization to morphine.” Biol Psychiatry 75(2): 105–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanger CM, Ghanshani S, Logsdon NJ, Rauer H, Kalman K, Zhou J, Beckingham K, Chandy KG, Cahalan MD and Aiyar J (1999). “Calmodulin mediates calcium-dependent activation of the intermediate conductance KCa channel, IKCa1.” J Biol Chem 274(9): 5746–5754. [DOI] [PubMed] [Google Scholar]
- Foster WJ, Taylor HBC, Padamsey Z, Jeans AF, Galione A and Emptage NJ (2018). “Hippocampal mGluR1-dependent long-term potentiation requires NAADP-mediated acidic store Ca(2+) signaling.” Sci Signal 11(558). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Negredo G, Soto D, Llorente J, Morato X, Galenkamp KM, Gomez-Soler M, Fernandez-Duenas V, Watanabe M, Adelman JP, Shigemoto R, Fukazawa Y, Lujan R and Ciruela F (2014). “Coassembly and Coupling of SK2 Channels and mGlu5 Receptors.” J Neurosci 34(44): 14793–14802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giessel AJ and Sabatini BL (2010). “M1 muscarinic receptors boost synaptic potentials and calcium influx in dendritic spines by inhibiting postsynaptic SK channels.” Neuron 68(5): 936–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu N, Vervaeke K, Hu H and Storm JF (2005). “Kv7/KCNQ/M and HCN/h, but not KCa2/SK channels, contribute to the somatic medium after-hyperpolarization and excitability control in CA1 hippocampal pyramidal cells.” J Physiol 566(Pt 3): 689–715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond RS, Bond CT, Strassmaier T, Ngo-Anh TJ, Adelman JP, Maylie J and Stackman RW (2006). “Small-conductance Ca2+-activated K+ channel type 2 (SK2) modulates hippocampal learning, memory, and synaptic plasticity.” J Neurosci 26(6): 1844–1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert B, Pietropaolo S, Meme S, Laudier B, Laugeray A, Doisne N, Quartier A, Lefeuvre S, Got L, Cahard D, Laumonnier F, Crusio WE, Pichon J, Menuet A, Perche O and Briault S (2014). “Rescue of fragile X syndrome phenotypes in Fmr1 KO mice by a BKCa channel opener molecule.” Orphanet J Rare Dis 9: 124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higham J, Sahu G, Wazen RM, Colarusso P, Gregorie A, Harvey BSJ, Goudswaard L, Varley G, Sheppard DN, Turner RW and Marrion NV (2019). “Preferred Formation of Heteromeric Channels between Coexpressed SK1 and IKCa Channel Subunits Provides a Unique Pharmacological Profile of Ca(2+)-Activated Potassium Channels.” Mol Pharmacol 96(1): 115–126. [DOI] [PubMed] [Google Scholar]
- Honrath B, Krabbendam IE, Culmsee C and Dolga AM (2017). “Small conductance Ca(2+)-activated K(+) channels in the plasma membrane, mitochondria and the ER: Pharmacology and implications in neuronal diseases.” Neurochem Int 109: 13–23. [DOI] [PubMed] [Google Scholar]
- Hopf FW, Bowers MS, Chang SJ, Chen BT, Martin M, Seif T, Cho SL, Tye K and Bonci A (2010). “Reduced nucleus accumbens SK channel activity enhances alcohol seeking during abstinence.” Neuron 65(5): 682–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopf FW, Martin M, Chen BT, Bowers MS, Mohamedi MM and Bonci A (2007). “Withdrawal from intermittent ethanol exposure increases probability of burst firing in VTA neurons in vitro.” J Neurophysiol 98(4): 2297–2310. [DOI] [PubMed] [Google Scholar]
- Ishikawa M, Mu P, Moyer JT, Wolf JA, Quock RM, Davies NM, Hu XT, Schluter OM and Dong Y (2009). “Homeostatic synapse-driven membrane plasticity in nucleus accumbens neurons.” J Neurosci 29(18): 5820–5831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isom LL (2019). “Is Targeting of Compensatory Ion Channel Gene Expression a Viable Therapeutic Strategy for Dravet Syndrome?” Epilepsy Curr 19(3): 193–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji H and Shepard PD (2006). “SK Ca2+-activated K+ channel ligands alter the firing pattern of dopamine-containing neurons in vivo.” Neuroscience 140(2): 623–633. [DOI] [PubMed] [Google Scholar]
- Jones SL, To MS and Stuart GJ (2017). “Dendritic small conductance calcium-activated potassium channels activated by action potentials suppress EPSPs and gate spike-timing dependent synaptic plasticity.” Elife 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jutras MJ, Fries P and Buffalo EA (2009). “Gamma-band synchronization in the macaque hippocampus and memory formation.” J Neurosci 29(40): 12521–12531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampa BM, Letzkus JJ and Stuart GJ (2006). “Requirement of dendritic calcium spikes for induction of spike-timing-dependent synaptic plasticity.” J Physiol 574(Pt 1): 283–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keen JE, Khawaled R, Farrens DL, Neelands T, Rivard A, Bond CT, Janowsky A, Fakler B, Adelman JP and Maylie J (1999). “Domains responsible for constitutive and Ca(2+)-dependent interactions between calmodulin and small conductance Ca(2+)-activated potassium channels.” J Neurosci 19(20): 8830–8838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim G, Lujan R, Schwenk J, Kelley MH, Aguado C, Watanabe M, Fakler B, Maylie J and Adelman JP (2016). “Membrane palmitoylated protein 2 is a synaptic scaffold protein required for synaptic SK2-containing channel function.” Elife 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JI, Yang EJ, Lee MS, Kim YS, Huh Y, Cho IH, Kang S and Koh HK (2011). “Bee venom reduces neuroinflammation in the MPTP-induced model of Parkinson’s disease.” Int J Neurosci 121(4): 209–217. [DOI] [PubMed] [Google Scholar]
- Kohler M, Hirschberg B, Bond CT, Kinzie JM, Marrion NV, Maylie J and Adelman JP (1996). “Small-conductance, calcium-activated potassium channels from mammalian brain.” Science 273(5282): 1709–1714. [DOI] [PubMed] [Google Scholar]
- Kushwah N, Jain V, Dheer A, Kumar R, Prasad D and Khan N (2018). “Hypobaric Hypoxia-Induced Learning and Memory Impairment: Elucidating the Role of Small Conductance Ca(2+)-Activated K(+) Channels.” Neuroscience 388: 418–429. [DOI] [PubMed] [Google Scholar]
- Lauterborn JC, Schultz MN, Le AA, Amani M, Friedman AE, Leach PT, Gall CM, Lynch GS and Crawley JN (2019). “Spaced training improves learning in Ts65Dn and Ube3a mouse models of intellectual disabilities.” Transl Psychiatry 9(1): 166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin MT, Lujan R, Watanabe M, Adelman JP and Maylie J (2008). “SK2 channel plasticity contributes to LTP at Schaffer collateral-CA1 synapses.” Nat Neurosci 11(2): 170–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin MT, Lujan R, Watanabe M, Frerking M, Maylie J and Adelman JP (2010). “Coupled activity-dependent trafficking of synaptic SK2 channels and AMPA receptors.” J Neurosci 30(35): 11726–11734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman JE and Jensen O (2013). “The theta-gamma neural code.” Neuron 77(6): 1002–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lujan R, Aguado C, Ciruela F, Arus XM, Martin-Belmonte A, Alfaro-Ruiz R, Martinez-Gomez J, de la Ossa L, Watanabe M, Adelman JP, Shigemoto R and Fukazawa Y (2018). “SK2 Channels Associate With mGlu1alpha Receptors and CaV2.1 Channels in Purkinje Cells.” Front Cell Neurosci 12: 311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malenka RC and Nicoll RA (1993). “NMDA-receptor-dependent synaptic plasticity: multiple forms and mechanisms.” Trends Neurosci 16(12): 521–527. [DOI] [PubMed] [Google Scholar]
- Markram H, Lubke J, Frotscher M and Sakmann B (1997). “Regulation of synaptic efficacy by coincidence of postsynaptic APs and EPSPs.” Science 275(5297): 213–215 [DOI] [PubMed] [Google Scholar]
- Matschke LA, Rinne S, Snutch TP, Oertel WH, Dolga AM and Decher N (2018). “Calcium-activated SK potassium channels are key modulators of the pacemaker frequency in locus coeruleus neurons.” Mol Cell Neurosci 88: 330–341. [DOI] [PubMed] [Google Scholar]
- Miller MJ, Rauer H, Tomita H, Rauer H, Gargus JJ, Gutman GA, Cahalan MD and Chandy KG (2001). “Nuclear localization and dominant-negative suppression by a mutant SKCa3 N-terminal channel fragment identified in a patient with schizophrenia.” J Biol Chem 276(30): 27753–27756. [DOI] [PubMed] [Google Scholar]
- Mulholland PJ, Becker HC, Woodward JJ and Chandler LJ (2011). “Small conductance calcium-activated potassium type 2 channels regulate alcohol-associated plasticity of glutamatergic synapses.” Biol Psychiatry 69(7): 625–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazzaro C, Greco B, Cerovic M, Baxter P, Rubino T, Trusel M, Parolaro D, Tkatch T, Benfenati F, Pedarzani P and Tonini R (2012). “SK channel modulation rescues striatal plasticity and control over habit in cannabinoid tolerance.” Nat Neurosci 15(2): 284–293. [DOI] [PubMed] [Google Scholar]
- Ngo-Anh TJ, Bloodgood BL, Lin M, Sabatini BL, Maylie J and Adelman JP (2005). “SK channels and NMDA receptors form a Ca2+-mediated feedback loop in dendritic spines.” Nat Neurosci 8(5): 642–649. [DOI] [PubMed] [Google Scholar]
- Oliveira MS, Skinner F, Arshadmansab MF, Garcia I, Mello CF, Knaus HG, Ermolinsky BS, Otalora LF and Garrido-Sanabria ER (2010). “Altered expression and function of small-conductance (SK) Ca(2+)-activated K+ channels in pilocarpine-treated epileptic rats.” Brain Res 1348: 187–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedarzani P, Mosbacher J, Rivard A, Cingolani LA, Oliver D, Stocker M, Adelman JP and Fakler B (2001). “Control of electrical activity in central neurons by modulating the gating of small conductance Ca2+-activated K+ channels.” J Biol Chem 276(13): 9762–9769. [DOI] [PubMed] [Google Scholar]
- Pousinha PA, Mouska X, Raymond EF, Gwizdek C, Dhib G, Poupon G, Zaragosi LE, Giudici C, Bethus I, Pacary E, Willem M and Marie H (2017). “Physiological and pathophysiological control of synaptic GluN2B-NMDA receptors by the C-terminal domain of amyloid precursor protein.” Elife 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proulx E, Fraser P, McLaurin J and Lambe EK (2015). “Impaired Cholinergic Excitation of Prefrontal Attention Circuitry in the TgCRND8 Model of Alzheimer’s Disease.” J Neurosci 35(37): 12779–12791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proulx E, Power SK, Oliver DK, Sargin D, McLaurin J and Lambe EK (2019). “Apamin Improves Prefrontal Nicotinic Impairment in Mouse Model of Alzheimer’s Disease.” Cereb Cortex. [DOI] [PubMed] [Google Scholar]
- Ren Y, Barnwell LF, Alexander JC, Lubin FD, Adelman JP, Pfaffinger PJ, Schrader LA and Anderson AE (2006). “Regulation of surface localization of the small conductance Ca2+-activated potassium channel, Sk2, through direct phosphorylation by cAMP-dependent protein kinase.” J Biol Chem 281(17): 11769–11779. [DOI] [PubMed] [Google Scholar]
- Rimini R, Rimland JM and Terstappen GC (2000). “Quantitative expression analysis of the small conductance calcium-activated potassium channels, SK1, SK2 and SK3, in human brain.” Brain Res Mol Brain Res 85(1-2): 218–220. [DOI] [PubMed] [Google Scholar]
- Ritter-Makinson S, Clemente-Perez A, Higashikubo B, Cho FS, Holden SS, Bennett E, Chkhaidze A, Eelkman Rooda OHJ, Cornet MC, Hoebeek FE, Yamakawa K, Cilio MR, Delord B and Paz JT (2019). “Augmented Reticular Thalamic Bursting and Seizures in Scn1a-Dravet Syndrome.” Cell Rep 26(1): 54–64 e56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sailer CA, Hu H, Kaufmann WA, Trieb M, Schwarzer C, Storm JF and Knaus HG (2002). “Regional differences in distribution and functional expression of small-conductance Ca2+-activated K+ channels in rat brain.” J Neurosci 22(22): 9698–9707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sailer CA, Kaufmann WA, Marksteiner J and Knaus HG (2004). “Comparative immunohistochemical distribution of three small-conductance Ca2+-activated potassium channel subunits, SK1, SK2, and SK3 in mouse brain.” Mol Cell Neurosci 26(3): 458–469 [DOI] [PubMed] [Google Scholar]
- Schumacher MA, Rivard AF, Bachinger JP and Adelman JP (2001). “Structure of the gating domain of a Ca2+-activated K+ channel complexed with Ca2+/calmodulin.” Nature 410(6832): 1120–1124. [DOI] [PubMed] [Google Scholar]
- Stackman RW, Hammond RS, Linardatos E, Gerlach A, Maylie J, Adelman JP and Tzounopoulos T (2002). “Small conductance Ca2+-activated K+ channels modulate synaptic plasticity and memory encoding.” J Neurosci 22(23): 10163–10171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocker M, Krause M and Pedarzani P (1999). “An apamin-sensitive Ca2+-activated K+ current in hippocampal pyramidal neurons.” Proc Natl Acad Sci U S A 96(8): 4662–4667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocker M and Pedarzani P (2000). “Differential distribution of three Ca(2+)-activated K(+) channel subunits, SK1, SK2, and SK3, in the adult rat central nervous system.” Mol Cell Neurosci 15(5): 476–493. [DOI] [PubMed] [Google Scholar]
- Sultana R, Ghandi T, Davila AM, Lee CC and Ogundele OM (2018). “Upregulated SK2 Expression and Impaired CaMKII Phosphorylation Are Shared Synaptic Defects Between 16p11.2del and 129S:∆disc1 Mutant Mice.” ASN NEURO 10: 1759091418817641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J, Liu Y, Zhu G, Cato C, Hao X, Qian L, Lin W, Adhikari R, Luo Y, Baudry M and Bi X (2020). “PKA and Ube3a regulate SK2 channel trafficking to promote synaptic plasticity in hippocampus: Implications for Angelman Syndrome.” Sci Rep 10(1): 9824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J, Zhu G, Liu Y, Standley S, Ji A, Tunuguntla R, Wang Y, Claus C, Luo Y, Baudry M and Bi X (2015). “UBE3A Regulates Synaptic Plasticity and Learning and Memory by Controlling SK2 Channel Endocytosis.” Cell Rep 12(3): 449–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J, Zhu G, Liu Y, Standley S, Ji AX, Tunuguntia R, Wang Y, Claus C, Luo Y, Baudry M and Bi X (2015). “UBE3A regulates synaptic plasticity and learning and memory by controlling SK2 channel endocytosis.” Cell Rep 12: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surmeier DJ, Guzman JN and Sanchez-Padilla J (2010). “Calcium, cellular aging, and selective neuronal vulnerability in Parkinson’s disease.” Cell Calcium 47(2): 175–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szatanik M, Vibert N, Vassias I, Guenet JL, Eugene D, de Waele C and Jaubert J (2008). “Behavioral effects of a deletion in Kcnn2, the gene encoding the SK2 subunit of small-conductance Ca2+-activated K+ channels.” Neurogenetics 9(4): 237–248. [DOI] [PubMed] [Google Scholar]
- Tacconi S, Carletti R, Bunnemann B, Plumpton C, Merlo Pich E and Terstappen GC (2001). “Distribution of the messenger RNA for the small conductance calcium-activated potassium channel SK3 in the adult rat brain and correlation with immunoreactivity.” Neuroscience 102(1): 209–215. [DOI] [PubMed] [Google Scholar]
- Tigaret CM, Chamberlain SEL, Sadowski J, Hall J, Ashby MC and Mellor JR (2018). “Convergent Metabotropic Signaling Pathways Inhibit SK Channels to Promote Synaptic Plasticity in the Hippocampus.” J Neurosci 38(43): 9252–9262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tigaret CM, Olivo V, Sadowski J, Ashby MC and Mellor JR (2016). “Coordinated activation of distinct Ca(2+) sources and metabotropic glutamate receptors encodes Hebbian synaptic plasticity.” Nat Commun 7: 10289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titley HK, Brunel N and Hansel C (2017). “Toward a Neurocentric View of Learning.” Neuron 95(1): 19–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkerk AJ, Pieretti M, Sutcliffe JS, Fu YH, Kuhl DP, Pizzuti A, Reiner O, Richards S, Victoria MF, Zhang FP and et al. (1991). “Identification of a gene (FMR-1) containing a CGG repeat coincident with a breakpoint cluster region exhibiting length variation in fragile X syndrome.” Cell 65(5): 905–914. [DOI] [PubMed] [Google Scholar]
- Wang G, Zeng J, Ren R and Chen S (2008). “Potassium channels in the basal ganglia: promising new targets for the treatment of Parkinson’s disease.” Front Biosci 13: 3825–3838. [DOI] [PubMed] [Google Scholar]
- Willis M, Trieb M, Leitner I, Wietzorrek G, Marksteiner J and Knaus HG (2017). “Small-conductance calcium-activated potassium type 2 channels (SK2, KCa2.2) in human brain.” Brain Struct Funct 222(2): 973–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winson J (1978). “Loss of hippocampal theta rhythm results in spatial memory deficit in the rat.” Science 201(4351): 160–163. [DOI] [PubMed] [Google Scholar]
- Wolfart J, Neuhoff H, Franz O and Roeper J (2001). “Differential expression of the small-conductance, calcium-activated potassium channel SK3 is critical for pacemaker control in dopaminergic midbrain neurons.” J Neurosci 21(10): 3443–3456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfart J and Roeper J (2002). “Selective coupling of T-type calcium channels to SK potassium channels prevents intrinsic bursting in dopaminergic midbrain neurons.” J Neurosci 22(9): 3404–3413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Womack MD and Khodakhah K (2003). “Somatic and dendritic small-conductance calcium-activated potassium channels regulate the output of cerebellar Purkinje neurons.” J Neurosci 23(7): 2600–2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia XM, Fakler B, Rivard A, Wayman G, Johnson-Pais T, Keen JE, Ishii T, Hirschberg B, Bond CT, Lutsenko S, Maylie J and Adelman JP (1998). “Mechanism of calcium gating in small-conductance calcium-activated potassium channels.” Nature 395(6701): 503–507. [DOI] [PubMed] [Google Scholar]
- Zadori D, Veres G, Szalardy L, Klivenyi P, Toldi J and Vecsei L (2014). “Glutamatergic dysfunctioning in Alzheimer’s disease and related therapeutic targets.” J Alzheimers Dis 42 Suppl 3: S177–187. [DOI] [PubMed] [Google Scholar]
- Zhang WH, Liu WZ, He Y, You WJ, Zhang JY, Xu H, Tian XL, Li BM, Mei L, Holmes A and Pan BX (2019). “Chronic Stress Causes Projection-Specific Adaptation of Amygdala Neurons via Small-Conductance Calcium-Activated Potassium Channel Downregulation.” Biol Psychiatry 85(10): 812–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu G, Liu Y, Wang Y, Bi X and Baudry M (2015). “Different patterns of electrical activity lead to long-term potentiation by activating different intracellular pathways.” J Neurosci 35(2): 621–633. [DOI] [PMC free article] [PubMed] [Google Scholar]


