Abstract
SARS coronavirus-2 (SARS-CoV-2) detection in different clinical specimens has raised important insights about its pathogenesis, but some details remain to be understood. In that respect, disrupt viral control seen in solid organ transplant patients on chronic immunosuppression can help unveil pathogenic mechanisms and characterize new coronavirus disease-19 (COVID-19) immunological and clinical aspects, as well as secondary complications. We herein report a case of SARS-CoV-2 detection in ascitic fluid from a kidney transplant patient with decompensated cirrhosis and COVID-19 and then discuss about immune, cellular, and virological aspects of such clinical presentation of the disease, which also included a disseminated infection, demonstrated by viral detection in his blood sample. We subsequently discuss about the fatal outcome caused by a secondary bloodstream infection by Cryptococcus neoformans. This unprecedented case report presents ascitic fluid as a novel specimen in which SARS-CoV-2 can be detected. Immune dysregulation and cumulative risk factors may lead to secondary infections by opportunistic agents, including Cryptococcus neoformans.
Keywords: Cryptococcus, Cirrhosis, COVID, Coinfection, Viremia, Kidney transplant
Introduction
SARS coronavirus-2 (SARS-CoV-2) affects primarily upper and lower respiratory tract, but detection in different clinical specimens, including serum and stool, has raised important insights about its kinetics and pathogenesis [1].
In that respect, disruption in viral control seen in solid organ transplant patients on chronic immunosuppression [2] can help pathogenic mechanisms and characterize new coronavirus disease-19 (COVID-19) immunological and clinical aspects, as well as secondary complications.
We herein report an unprecedented case of SARS-CoV-2 detection in ascitic fluid from a kidney transplant patient with decompensated cirrhosis and COVID-19 and then discuss about immune, cellular, and virologic aspects of such clinical presentation of the disease, which also included a disseminated infection, demonstrated by viral detection in his blood sample. We then discuss about the fatal outcome caused by a secondary bloodstream infection by Cryptococcus neoformans.
Case Report
A 75-year-old white male with a history of hypertension, and a kidney transplant from a deceased donor 3 years prior, presented in the emergency department on May 18, 2020, 5 days following the onset of dry cough and progressive dyspnea, without fever or other respiratory or gastrointestinal tract symptoms. He was on immunosuppressive therapy with 4 mg tacrolimus and 5 mg prednisone and denied previous opportunistic infections. Physical examination at admission revealed an axillary temperature of 37.1 °C, blood pressure of 160/90 mmHg, heart rate of 104 per minute, respiratory rate of 25 breaths per minute, and oxygen saturation of 92% while breathing ambient air. Abdominal examination demonstrated mild ascites with collateral circulation.
He was diagnosed with liver cirrhosis 2 years ago and denied any prior decompensation, including no history of ascites. During investigation at the time, concomitant portal hypertension and thrombosis were also found but were stable since then without anticoagulation. Previous serologies were negative for HIV, hepatitis C, syphilis, and schistosomiasis. His hepatitis B serostatus was positive anti-HBs (255 UI/L), positive anti HBc, and negative HBsAg. Also, he had undetectable hepatitis B viral loads on two consecutive PCR exams.
Initial laboratory findings showed normal white cell count but severe lymphopenia (162 per mm3) as well as other cirrhosis dysfunctions including thrombocytopenia, increased international normalized ratio (INR), and hypoalbuminemia. Creatinine was 1.5 times its baseline value of 2.4 mg/dl, procalcitonin was elevated at 3.92 ng/dl, and d-dimer was above 20 μg/mL (maximum test detection). Total bilirubin was normal at 0.67 mg/dL, as were transaminases (AST: 33 U/L; ALT: 20 U/L). Evolutive laboratory exams may be seen in Table 1.
Table 1.
Evolutive blood test results on initial days of hospitalization compared with previous results
| Laboratory findings | 22/04/2020 | 18/05/2020 | 19/05/2020 | 20/05/2020 | 22/05/2020 | 23/05/2020 | |
|---|---|---|---|---|---|---|---|
| Normal range | No symptoms | Hospital day 1 Illness day 5 |
Hospital day 2 Illness day 6 |
Hospital day 3 Illness day 7 |
Hospital day 5 Illness day 9 |
Hospital day 6 Illness day 10 |
|
| Absolute white-cell count (per mm3) | 3500–10,500 | 2560 | 5700 | 4580 | 7880 | 5430 | 6730 |
| Absolute neutrophil count (per mm3) | 1700–8000 | 1802 | 5547 | 4278 | 7636 | 5197 | 6192 |
| Absolute lymphocyte count (per mm3) | 900–2900 | 586 | 162 | 229 | 150 | 114 | 269 |
| Platelet count (per mm3) | 150,000–450,000 | 45,000 | 48,000 | 44,000 | 56,000 | 64,000 | 63,000 |
| Hemoglobin (g/dL) | 13.5–17.5 | 11.7 | 11.3 | 10.3 | 10.9 | 9.9 | 9.9 |
| Hematocrit (%) | 39–50 | 34 | 32 | 30 | 32.1 | 29 | 30.2 |
| Other findings | |||||||
| Procalcitonin (ng/mL) | < 0.06 | – | – | 3.92 | – | – | – |
| Lactate dehydrogenase (U/L) | < 250 | – | 366 | – | – | – | – |
| D-dimer (ug/FEU) | < 0.5 | – | >20 | >20 | – | >20 | – |
| Troponin (pg/mL) | < 14 | – | 65 | 60 | 108 | ||
| Creatine kinase (U/L) | 39–308 | – | – | – | 135 | – | – |
| Creatinine (mg/dL) | 0.7–1.2 | 2.5 | 3.68 | 4.15 | 4.55 | 4.89 | 4.68 |
| Urea (mg/dL) | 10–50 | 80 | 126 | 142 | 184 | 218 | 234 |
| Total bilirubin (mg/dL) | 0.3–1.2 | 1.19 | – | 0.67 | – | 0.49 | 0.47 |
| Aspartate aminotransferase (U/L) | < 40 | 34 | 39 | 33 | – | 35 | 17 |
| Alanine aminotransferase (U/L) | < 41 | 36 | 22 | 20 | – | 21 | 18 |
| Gamma glutamyl transferase (U/L) | < 60 | 75 | – | 114 | – | – | – |
| Albumin (g/dL) | 3.5–5.2 | – | – | 2.6 | – | – | – |
| International normalized ratio (INR) | 0.8–1.2 | 1.35 | 1.25 | 1.34 | – | – | 1.34 |
| Alpha fetoprotein (ng/dL) | < 7 | 3.8 | 1.95 | – | – | – | |
| Sodium (mmol/L) | 137–148 | 145 | 142 | 148 | 143 | 147 | 147 |
| Potassium (mmol/L) | 3.5–5 | 4.7 | 4.6 | 5.2 | 4.4 | 4.4 | 3.2 |
| Calcium (mmol/L) | 1.15–1.32 | – | – | – | – | 1.13 | – |
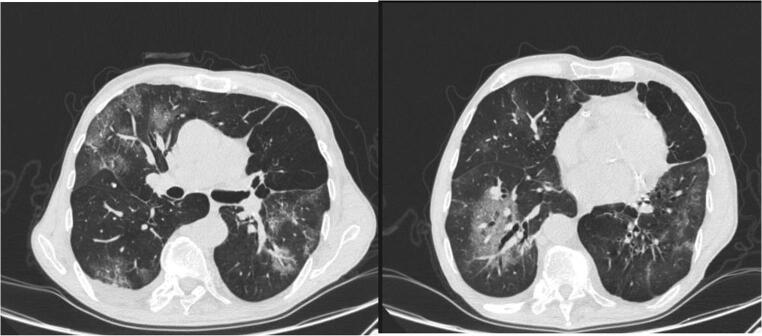
Chest computed tomography scan showed bilateral ground-glass opacities with some areas of consolidation (Fig. 1), and specimens from his nasopharyngeal swab were positive for COVID-19 on real-time reverse-transcriptase polymerase chain reaction (RT-PCR).
Fig. 1.
Chest computed tomography from day 1 of hospitalization (day 7 of illness) showing ground-glass opacities with some areas of consolidation
As supplemental oxygen (O2) with a nasal cannula was administered at 2 L/min, his pulse oximetry went up to 97%, and he was started on ceftriaxone and clarithromycin. Tacrolimus was suspended, and prednisone dose was increased to 30 mg daily.
On day 2 of hospitalization (day 8 of illness), his breathing pattern worsened, as he needed an increase in nasal oxygen flow to 6 L/min, and so did his ascites. A diagnostic and therapeutic paracentesis was performed, and 2 l of light yellow ascitic fluid were subsequently removed. Laboratory analysis showed a total cell count of 133 per mm3 (macrophages: 60%, mesotelyocytes: 24%, and lymphocytes: 16%), and no red blood cells were detected. Total protein and albumin levels were 0.8 g/dL and 0.6 g/dL, respectively. Direct smears and cultures were negative for bacteria, fungi, and mycobacteria, but RT-PCR for SARS-CoV-2 in ascitic fluid was positive. Serum and fecal samples were also positive (Table 2). A rapid serological test performed on day 10 of symptoms was positive for IgM and IgG antibodies.
Table 2.
Specimen tested for COVID-19 using RT-PCR technique according to date of exam
| Specimen | 18/05/2020 Illness day 5 |
20/05/2020 Illness day 7 |
22/05/2020 Illness day 9 |
23/05/2020 Illness day 10 |
|---|---|---|---|---|
| Nasopharyngeal swab | Positive (CT 14–15) | – | – | |
| Ascitic fluid | – | Positive (CT 34–38) | – | |
| Serum | – | – | Positive (CT 31–33) | |
| Stool | – | – | – | Positive (CT 25–26) |
Sample was considered positive if cycle threshold (CT) value is < 40
On day 4, he required intubation as his respiratory status deteriorated (PaO2/FiO2 ratio: 110). Immunosuppression was then switched to 50 mg intravenous hydrocortisone every 6 h. His ventilation parameters improved in the following days, but his graft dysfunction did not—Doppler ultrasonography of graft was normal—and he was started on hemodialysis. On day 12, despite treatment with meropenem, vancomycin, and fluconazole, he had a refractory septic shock. Blood cultures from day 12 and 16 were negative for bacteria, but both samples were positive for yeasts, which were post-mortem identified as Cryptococcus neoformans. Tracheal aspirate cultures from the same days were negative for bacteria, fungi, and mycobacteria. The patient died on day 18.
Methods
Nasal and oropharyngeal swabs were collected and stored in 2 mL of sterile Ringer’s lactate solution. Ascitic fluid was collected with a sterile syringe following abdominal punction with aseptic technique. Blood was collected in a serum separator tube and then centrifuged according to the Centers for Disease Control and Prevention (CDC) guidelines. Stool was collected with a sterile container.
Ribonucleic acid (RNA) extraction was performed in all four specimens with QIAamp Viral RNA Mini Kit (QIAGEN, Hilden, Germany), following manufacturer’s instructions. Viral detection was performed with AgPath-ID One-Step RT-PCR Reagents (ThermoFisher Scientific, Austin, USA), according to manufacturer’s instructions, in a total of 20 μL reaction volume, containing 5.0 μL of purified RNA, primers, and probes (400 nM and 200 nM, respectively), aiming at the N1 and N2 targets of the SARS-CoV-2 nucleoprotein gene in accordance with CDC guidelines. Samples with cycle threshold (Ct) values below 40 were considered positive. Rapid serological test was performed with serum sample following manufacturer’s instructions (Zhuhai Livzon Diagnostics).
Results may be seen in Table 2.
Discussion
A great variety of clinical features have been attributed to COVID-19 in both immunocompetent and immunocompromised populations, including cough and dyspnea [2, 3] similar to the ones initially reported by this patient. As disease severity progresses, pulmonary injury is found to be a common feature [3], and previous lung autopsy reports have attributed to endothelialitis with intracellular viruses [4]. Also, as it appears to incite a high rate of thrombotic events [5], it might also explain further aggravation of his previous portal vein thrombosis observed with this patient, which could possibly justify the acute worsening of his ascites on the second day of hospitalization.
Nevertheless, SARS-CoV-2 uses the angiotensin-converting enzyme 2 (ACE2) as a cell receptor to invade human cells [6], and since it is expressed in a wide variety of human tissues, including enterocytes from ileum and colon [7], it would explain detection of viral RNA in his fecal sample, and it might have contributed to local viral spread, including to peritoneum. But more importantly, macrophages also express the ACE2 receptor [8], and, despite their importance in antiviral defense mechanisms in general, more attention has been given to the hypothesis that, in the case of SARS-CoV-2, they might also enable viral anchoring, replication, and possibly spread to other organs [8]. Since macrophages were predominant in this patient’s ascitic fluid, direct viral injury might also have played a role on his initial clinical presentation. Interestingly, infectious peritonitis is a well-described condition in felines that is caused by a coronavirus through a similar immune pathway [9]. On the other hand, while reports on patients with cirrhosis and SARS-CoV-2 show a high rate of decompensation with ascites [10], viral etiology in this clinical scenario has not been very well described thus far.
At the same time, viral RNA was not detected in samples of ascitic fluid from surgical patients according to previous studies [11, 12], so it remains a matter demanding further investigation, especially because these reports present a small number of patients requiring emergency operations and no information regarding comorbidities or immune status.
In the case of solid organ transplant patients, chronic immunosuppressive therapy impairs T cell immunity [2], and disruption in viral control may lead to higher rates of viral replication [3, 13]. While this could have contributed to a disseminated, more severe infection in this patient, viral RNA has been detected in blood samples from critically ill, immunocompetent patients as well [14].
Also, transient lymphopenia is a prominent feature in SARS-CoV-2, and it can also be a marker of severe disease by inducing immune dysregulation with T cell exhaustion and depletion of the TCD4 + lymphocyte subset [15, 16]. At the same time, TCD4+ cells’ decline has a clear association with higher risk of cryptococcosis in different conditions [17, 18]. Moreover, such risk is also seen with solid organ transplant patients [19], as well as in cirrhotic patients in general, who additionally present higher incidence of disseminated infections [19]. Therefore, it can be highly debatable whether the SARS-CoV-2 infection itself and its immune dysregulation could have triggered disseminated cryptococcosis in this patient, or if he accumulated too many prior risk factors, or perhaps a combination of both. In any case, other fungal secondary infections have been reported with COVID-19 [20], and clinicians should be aware of the possibility of such scenario with Cryptococcus species as well, especially in populations with cumulative risk factors [17–19].
Conclusion
In summary, this unprecedented case report presents ascitic fluid as a novel specimen in which SARS-CoV-2 can be detected. Immune dysregulation and cumulative risk factors may lead to secondary infections by opportunistic agents, including Cryptococcus neoformans. More studies are required to better elucidate viral dynamics, immune response, and different clinical presentations in the immunocompromised, especially because of such diverse disease spectrum as seen with COVID-19.
Acknowledgments
We thank Dante Cabelho for his assistance with language.
Authors’ Contributions
Concept, design, administration, and writing: VCP and NB. Investigation and interpretation of data: VCP, AHP, LL, DDC, and NB. Supervision: OAN, JOA, and NB. All authors approve the final version of this manuscript.
Funding
This work received support from the Brazilian Coordination for the Improvement of Higher Education Personnel (CAPES) and the National Council for Scientific and Technological Development (CNPq).
Compliance with Ethical Standards
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethics Approval
This case report was approved by the Local Ethics Committee (CAAE: 33911720.3.0000.5505).
Consent for Publication
Consent for publication from this patient was not possible because he died. No close relatives were able to be reached despite several attempts. No detailed information regarding his identification was published on this manuscript.
Footnotes
This article is part of the Topical Collection on Covid-19
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Wang W, Xu Y, Gao R. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA. 2020;323(18):1843–1844. doi: 10.1001/jama.2020.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pereira M-R, Mohan S, Cohen D-J, Husain SA, Dube GK, Ratner LE, Arcasoy S, Aversa MM, Benvenuto LJ, Dadhania DM, Kapur S, Dove LM, Brown RS, Jr, Rosenblatt RE, Samstein B, Uriel N, Farr MA, Satlin M, Small CB, Walsh TJ, Kodiyanplakkal RP, Miko BA, Aaron JG, Tsapepas DS, Emond JC, Verna EC. COVID-19 in solid organ transplant recipients: initial report from the US epicenter. Am J Transplant. 2020;20:1800–1808. doi: 10.1111/ajt.15941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ackermann M, Verleden S, Kuehnel M, Haverich A, Welte T, Laenger F, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in COVID-19. N Engl J Med. 2020;383:120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Middeldorp S, Coppens M, van Haaps TF, Foppen M, Vlaar AP, Müller MCA, et al. Incidence of venous thromboembolism in hospitalized patients with COVID-19. J Thromb Haemost. 2020;18:1995–2002. doi: 10.1111/jth.14888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lu R, Zhao X, Li J, Niu P, Yang B, Wu H, Wang W, Song H, Huang B, Zhu N, Bi Y, Ma X, Zhan F, Wang L, Hu T, Zhou H, Hu Z, Zhou W, Zhao L, Chen J, Meng Y, Wang J, Lin Y, Yuan J, Xie Z, Ma J, Liu WJ, Wang D, Xu W, Holmes EC, Gao GF, Wu G, Chen W, Shi W, Tan W. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li M, Li L, Zhang Y, et al. Expression of the SARS-CoV-2 cell receptor gene ACE2 in a wide variety of human tissues. Infect Dis Poverty. 2020;9:45. doi: 10.1186/s40249-020-00662-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abassi Z, Knaney Y, Karram T, Heyman SN. The lung macrophage in SARS-CoV-2 infection: a friend or a foe? Front Immunol. 2020;11:1312. doi: 10.3389/fimmu.2020.01312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pedersen NC, Eckstrand C, Liu H, Leutenegger C, Murphy B. Levels of feline infectious peritonitis virus in blood, effusions, and various tissues and the role of lymphopenia in disease outcome following experimental infection. Vet Microbiol. 2015;175(2–4):157–166. doi: 10.1016/j.vetmic.2014.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qi X, Liu Y, Wang J, Fallowfield JA, Wang J, Li X, et al. Clinical course and risk factors for mortality of COVID-19 patients with pre-existing cirrhosis: a multicentre cohort study. Gut BMJ. 2020. 10.1016/s0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed]
- 11.Flemming S, Hankir M, Hering I, Meybohm P, Krone M, Weissbrich B, Germer CT, Wiegering A. Abdominal fluid samples (negative for SARS-CoV-2) from a critically unwell patient with respiratory COVID-19. Br J Surg. 2020;107(8):e259–e260. doi: 10.1002/bjs.11713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seeliger B, Philouze G, Benotmane I, Mutter D, Pessaux P. Is the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) present intraperitoneally in patients with coronavirus disease 2019 (COVID-19) infection undergoing emergency operations? Surgery. 2020;168(2):220–221. doi: 10.1016/j.surg.2020.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Uematsu J, Sakai SK, Kihira NS, Yamamoto H, Hirai K, Kawano M, et al. Inhibitions of human parainfluenza virus type 2 replication by ribavirin and mycophenolate mofetil are restored by guanosine and S-(4-nitrobenzyl)-6-thioinosine. Drug Discov Ther. 2019;13:314–321. doi: 10.5582/ddt.2019.01084. [DOI] [PubMed] [Google Scholar]
- 14.Chen X, Zhao B, Yueming Q, Chen Y, Xiong J, Feng Y, et al. Detectable serum severe acute respiratory syndrome coronavirus 2 viral load (RNAemia) is closely correlated with drastically elevated interleukin 6 level in critically ill patients with coronavirus disease 2019. Clin Infect Dis. 2020;ciaa449. 10.1093/cid/ciaa449. [DOI] [PMC free article] [PubMed]
- 15.Moon C. Fighting COVID-19 exhausts T cells. Nat Rev Immunol. 2020;20:277. doi: 10.1038/s41577-020-0304-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen Z, John Wherry E. T cell responses in patients with COVID-19. Nat Rev Immunol. 2020;20:529–536. doi: 10.1038/s41577-020-0402-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Silveira FP, Husain S, Kwak EJ, Linden PK, Marcos A, Shapiro R, Fontes P, Marsh JW, de Vera M, Tom K, Thai N, Tan HP, Basu A, Soltys K, Paterson DL. Cryptococcosis in liver and kidney transplant recipients receiving anti-thymocyte globulin or alemtuzumab. Transpl Infect Dis. 2007;9(1):22–27. doi: 10.1111/j.1399-3062.2006.00149.x. [DOI] [PubMed] [Google Scholar]
- 18.Warkentien T, Crum-Cianflone NF. An update on Cryptococcus among HIV-infected patients. Int J STD AIDS. 2010;21:679–684. doi: 10.1258/ijsa.2010.010182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baddley JW, Forrest GN. Cryptococcosis in solid organ transplantation. Am J Transplant. 2013;13(s4):242–249. doi: 10.1111/ajt.12116. [DOI] [PubMed] [Google Scholar]
- 20.Hughes S, Troise O, Donaldson H, Mughal N, Moore LS. Bacterial and fungal coinfection among hospitalised patients with COVID-19: a retrospective cohort study in a UK secondary care setting. Clin Microbiol Infect. 2020. 10.1016/j.cmi.2020.06.025. [DOI] [PMC free article] [PubMed]