Abstract
Despite prophylactic anticoagulant treatments, thrombotic complications may develop in patients with coronavirus disease 2019 (COVID-19). This study aimed to evaluate the factors influencing anti-factor Xa activity in COVID-19 patients receiving low molecular weight heparin (LMWH). We prospectively evaluated 80 COVID-19 patients, diagnosed using polymerase chain reaction test, who were admitted to our clinic and administered LMWH; LMWH (enoxaparin) was applied according to the weight, D-dimer levels, and clinical condition of patients. Anti-factor Xa activity in blood, drawn 4 h after the 3rd dose of LMWH, was measured and an activity of < 0.2 IU/mL was considered subprophylactic. Patients were followed up clinically, and anti-factor Xa activity was re-examined before discharge. Groups 1 and 2 included 13 and 67 patients with subprophylactic (mean ± SD: 0.18 ± 0.06) and prophylactic (mean ± SD: 0.43 ± 0.23) anti-factor Xa activity, respectively. The proportion of eosinophils in patients was significantly higher in group 1 than in group 2 (mean ± SD; 2.96 ± 2.55 vs 0.90 ± 1.28; p = 0.001). At the time of discharge, the eosinophilic proportion of patients was significantly higher (eosinophil %, mean ± SD; 3.06 ± 1.49 vs 2.07 ± 1.92; p = 0.001), but the activated partial thromboplastin time was significantly lower (22.34 ± 1.38 vs 24.38 ± 3.58; p = 0.01) in group 1 than in group 2. Of 14 patients with eosinophil content > 4%, 6 were in group 1 ((6/13) 46.2%), while 8 were in group 2 ((8/63) 11.9%); (p = 0.009), and all had a D-dimer level < 1 μg/mL (p = 0.03). ROC analysis for the presence of anticoagulation at subprophylactic level revealed an area under curve of 0.79 (95% CI: 0.64–0.93); p = 0.001). In conclusion; Elevated eosinophil count is related to lower anti-factor Xa activity in patients with COVID-19 receiving LMWH. The clinical significance of the subprophylactic anti-factor Xa activity should be studied in COVID-19 patients (NCT04507282).
Keywords: COVID-19, Eosinophils, Heparin, Anticoagulants, Thrombosis
Introduction
Severe acute respiratory syndrome-associated coronavirus-2 (SARS-CoV-2) causes coronavirus disease 2019 (COVID-19), which has been considered a pandemic by the World Health Organization (WHO) [1, 2]. Several reports have shown that, similar to other viral pneumonia, the incidence rate of venous thromboembolism (VTE) in COVID-19 patients, particularly those in intensive care, is high [3–6] . The cause of the hypercoagulation in COVID-19 patients has not been fully understood. Several studies have indicated an increase in some hematologic parameters that may lead to endothelial damage, immobilization-related stasis, and hypercoagulability [7–10].
Eosinophils normally make up only a small fraction of circulating leukocytes (1–3%), but their levels can vary in different disease states [11]. Eosinophil levels are clinically important because they are potent pro-inflammatory cells containing cytotoxic proteins and various enzymes (peroxidases, cationic proteins, and neurotoxins) that can influence the effectiveness of heparin [12, 13].
The International Society on Thrombosis and Hemostasis (ISTH), American Society of Hematology (ASH), and American College of Cardiology (ACC) recommend heparin prophylaxis in patients with COVID-19. However, the dose that can be used has not been clarified. Previous studies have shown that heparin prophylaxis reduces thromboembolic events in COVID-19 patients [14]. However, the efficacy of heparin prophylaxis in COVID-19 patients, as determined by laboratory data, and the factors affecting this efficacy are not known. This study aimed to evaluate the factors influencing anti-factor Xa activity in COVID-19 patients receiving low molecular weight heparin (LMWH) using laboratory data.
Methods
Study patients
After receiving approval from the Ministry of Health and the local ethics committee, we included 80 patients who were found to be COVID-19-positive by polymerase chain reaction (PCR) test in our clinic between May 15, 2020, and June 15, 2020; their written consents were obtained. The patients were followed up clinically by transferring them to the service reserved for COVID-19-positive patients.
Inclusion criteria were as follows: Patients older than 18 years, who were diagnosed with COVID-19 and were administered LMWH, and agreed to participate in the study were included.
Exclusion criteria as follows: Patients with previous coagulopathy, continuous indication of anticoagulant therapy (atrial fibrillation (AF), valve disease), glomerular filtration rate (GFR) < 30 mL/min or undergoing dialysis, or with known liver dysfunction were excluded from the study.
Diagnosis
COVID-19 was diagnosed according to the WHO interim guidelines and confirmed in our laboratory by SARS-CoV-2 RNA detection with reverse transcriptase polymerase chain reaction (RT-PCR) using nasal and pharyngeal swab samples [15].
Patients with a systolic blood pressure (SBP) of ≥ 140 mmHg and/or a diastolic blood pressure (DBP) of ≥ 90 mmHg and those using antihypertensive drugs were considered hypertensive. Patients using oral antidiabetics or insulin or exhibiting fasting blood glucose ≥ 126 mg/dl in two measurements were considered diabetic. Body mass indices (BMI) were calculated according to the following formula: BMI = body weight (kg)/square of the height (m2). GFR was calculated using the Cockcroft-Gault formula: GFR = [(140−age) × patient weight (kg)]/[72 × serum creatinine value] (× 0.85 for women) [16].
Study procedures
Demographic characteristics of the hospitalized patients with COVID-19 were recorded, and computerized tomography (CT) of thorax was evaluated. Blood samples were collected to evaluate the hematological, inflammatory, and biochemical parameters of the patients (Fig. 1). Electrocardiograms (ECG) were recorded and O2 saturation was determined.
Fig. 1.
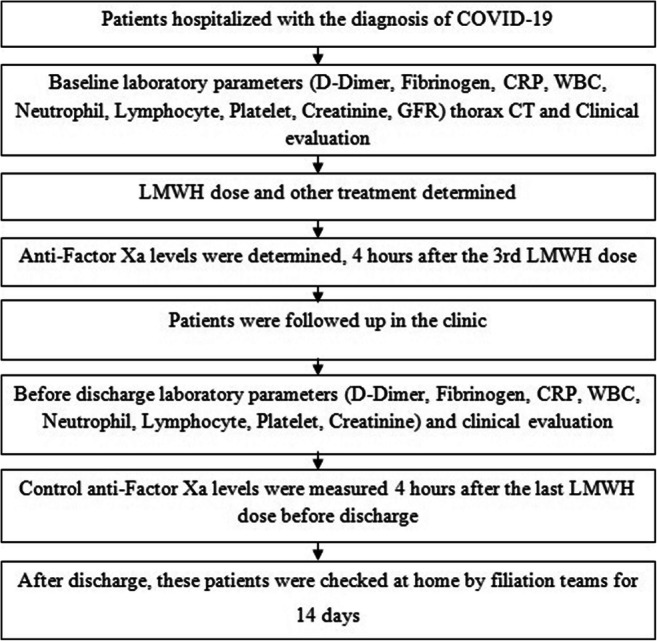
Diagram of study design
Treatment of patients with LMWH (enoxaparin) was arranged based on the results of laboratory tests, thorax CT, and clinical evaluation. LMWH dosage of 0.5 mg/kg (twice daily) was administered to patients with increased inflammation parameters (CRP (C-reactive protein) > 5 mg/L) and D-dimer levels (> 0.5 μg/mL), as well as pneumonic infiltration in thorax. LMWH dosage of 40 mg (once daily) was administered to the other patients. Other treatments were determined based on the recommendations of infectious disease specialists.
We determined the activity of anti-factor Xa in the blood collected from COVID-19 patients 4 h after the 3rd LMWH dose. An anti-factor Xa activity of < 0.2 IU/mL was defined as subprophylactic [17, 18]. According to previous studies, the threshold of anti-factor Xa activity for thromboembolic prophylaxis was 0.2 IU/mL [17, 18].
Patients with decreased O2 saturation and progressing disease state were taken to the intensive care unit. Control anti-factor Xa activity in the blood collected 4 h after administering the last LMWH dose before discharge was measured (Fig. 1).
Laboratory evaluation
Hematological parameters were examined with Mindray BC 6800 whole blood device (Mindray, China). The BC-6800 hematology analyzer used sheath flow impedance, laser scatter, and SF Cube analysis technology. The SF Cube analysis technology is three-dimensional using information from laser light scatter at two angles and fluorescent signals for cell differentiation and counting. In addition, the accuracy of cell numbers was confirmed by peripheral smear from blood samples taken from patients. Biochemical parameters were examined with Cobas C702 (Roche Diagnostics, Mannheim, Germany) device. CRP was examined with BN II nephelometer system (Siemens Healthcare Diagnostics Inc., USA). D-dimer (We used D-dimer unit. The normal range: 0–0.5 μgr/mL) and fibrinogen levels were examined by the Sysmex CS-5100 device.
The activity of anti-factor Xa was measured from the obtained plasma samples using the Berichrom Heparin kit in a Sysmex cs 5100 device in the biochemistry laboratory. The Berichrom Heparin kit is a chromogenic test (Berichrom heparin, Siemens Healthineers, Marburg, Germany). The kit contains AT III reagent. We used LMWH calibrator for calibrating the kit (Berichrom LMWH calibrator). INR (international normalized ratio), PT (prothrombin time), and aPTT (activated partial thromboplastin time) were measured as coagulation parameters. Venous blood samples in coagulation tubes were centrifuged at 5000 rpm for 10 min, and the INR, PT, and aPTT levels were measured in the biochemistry laboratory using a Sysmex cs 5100 device, Dade Actin FS, activated PTT reagent, and thromborel S reagent.
Follow-up
The patients whose general condition was stable, had reduced complaints, and had a decrease in inflammatory parameters were discharged. Patients with D-dimer values above 0.5 μg/mL during discharge were administered a single dose of LMWH (40 mg, once daily) for 30 days. Patients with lung involvement during hospitalization were given moxifloxacin (400 mg, once daily) or amoxicillin (1000 mg, twice daily) for 1 week at discharge. After discharge, these patients were examined at home by filiation teams (the team monitoring the COVID-19 patients at home) for 14 days.
Statistical analysis
The data were analyzed using the SPSS 23.0 statistics package (SPSS Inc., Chicago, IL, USA). Continuous variables have been reported as mean ± standard deviation, and categorical variables have been reported as percentages. In comparing the averages between groups, Student’s t test was used for variables with a normal distribution, and the Mann-Whitney U test was used for variables without a normal distribution. Categorical variables were compared with the chi-squared test or Fisher’s exact test. The sensitivity and specificity of eosinophil to predict subprophylactic levels of anti-factor Xa activity were analyzed by receiver operating characteristic (ROC) analysis. P values < 0.05 were considered significant.
Results
A total of 13 patients with anti-factor Xa activity < 0.2 IU/mL (subprophylactic anticoagulation) were defined as group 1, and 67 patients with anti-factor Xa activity > 0.2 IU/mL (prophylactic anticoagulation) were defined as group 2. When the baseline demographic and laboratory characteristics of the patients in groups 1 and 2 were evaluated, no significant difference was found except for the eosinophil counts and activity of anti-factor Xa (Table 1).
Table 1.
Baseline demographic and laboratory parameters of the groups
| Variable | Group 1 (13 patients) anti-factor Xa < 0.2 IU/mL | Group 2 (67 patients) Anti-factor Xa > 0.2 IU/mL | p value |
|---|---|---|---|
| Age (year) | 43.77 ± 16.77 | 45.15 ± 15.93 | 0.77 |
| Gender, n (%) | |||
| Male | 7 (53.8) | 33(49.3) | 0.76 |
| Female | 6 (46.2) | 34 (50.7) | |
| BMI (kg/m2) | 27.96 ± 4.25 | 26.26 ± 4.40 | 0.20 |
| GFR (mL/min) | 104.55 ± 26.94 | 103.13 ± 20.36 | 0.84 |
| Hypertension, n (%) | 4 (30.8) | 8 (11.9) | 0.09 |
| Diabetes mellitus, n (%) | 2 (15.4) | 7 (10.4) | 0.60 |
| Coronary artery disease, n (%) | 1 (7.7) | – | 0.16 |
| Medication, n (%) | |||
| Chloroquine | 13 (100) | 67 (100) | 1 |
| Azitromisin | 10 (76.9) | 53 (79.1) | 0.86 |
| Osetlemivir | 4 (30.8) | 6 (9.0) | 0.06 |
| Favipiravir | 3 (23.1) | 15 (22.4) | 0.95 |
| LMWH, n (%) | |||
| Single dose | 11 (84.6) | 49 (73.1) | 0.38 |
| Double dose | 2 (15.4) | 18 (26.9) | |
| CT finding, n (%) | |||
| Positive | 8 (61.5) | 43 (64.2) | 0.85 |
| Negative | 5 (38.5) | 24 (35.8) | |
| SpO2 | 97.38 ± 1.80 | 96.96 ± 2.10 | 0.49 |
| QT interval (ms) | 385.92 ± 13.00 | 390.17 ± 31.41 | 0.64 |
| WBC × 103/mL | 5.91 ± 1.31 | 5.54 ± 1.89 | 0.51 |
| Neutrophil | 3.57 ± 1.27 | 3.51 ± 1.71 | 0.91 |
| Lymphocyte | 1.76 ± 0.60 | 1.54 ± 0.66 | 0.25 |
| Eosinophil (%) | 2.96 ± 2.55 | 0.90 ± 1.28 | 0.001 |
| Eosinophil (%) | |||
| > 4 (%) | 6 (46.2) | 8 (11.9) | 0.009 |
| < 4 (%) | 7 (53.8) | 59 (88.1) | |
| Eosinophil count | 168.42 ± 147.25 | 50.32 ± 73.42 | 0.001 |
| RBC × 106/mL | 4.91 ± 0.37 | 5.15 ± 4.27 | 0.84 |
| Hemoglobin (gr/dl) | 13.86 ± 1.93 | 13.30 ± 2.30 | 0.42 |
| Platelet × 103/mL | 232.00 ± 62.21 | 197.57 ± 57.87 | 0.06 |
| Sedimentation (%) | 30.00 ± 22.86 | 32.46 ± 23.28 | 0.72 |
| CRP (mg/L) | 12.18 ± 16.66 | 25.12 ± 31.04 | 0.08 |
| Procalcitonin (μgr/L) | 0.11 ± 0.08 | 0.13 ± 0.19 | 0.60 |
| Fibrinogen (mg/dl) | 367.08 ± 134.97 | 410.00 ± 117.34 | 0.24 |
| Iron (μg/dl) | 55.92 ± 39.59 | 44.82 ± 23.48 | 0.76 |
| TIBC (μg/dl) | 264.54 ± 97.22 | 281.62 ± 75.30 | 0.47 |
| Ferritin (ng/mL) | 166.85 ± 130.83 | 220.06 ± 212.54 | 0.38 |
| Transferrin saturation (%) | 19.10 ± 14.49 | 15.06 ± 9.45 | 0.75 |
| D-dimer (μgr/mL) | 0.57 ± 0.38 | 1.21 ± 3.35 | 0.50 |
| Glucose (mg/dl) | 112.77 ± 42.29 | 106.82 ± 37.29 | 0.60 |
| BUN (mg/dl) | 12.62 ± 6.50 | 12.83 ± 4.95 | 0.89 |
| Creatinine (mg/dl) | 0.83 ± 0.39 | 0.78 ± 0.20 | 0.49 |
| AST (U/L) | 26.00 ± 9.53 | 29.69 ± 17.61 | 0.46 |
| ALT (U/L) | 25.00 ± 15.43 | 26.18 ± 18.59 | 0.83 |
| LDH (U/L) | 258.31 ± 110.76 | 252.37 ± 89.03 | 0.83 |
| Sodium (mmol/L) | 139.77 ± 3.03 | 137.15 ± 15.74 | 0.55 |
| Potassium (mEq/L) | 4.14 ± 0.49 | 4.07 ± 0.40 | 0.58 |
| Calcium (mg/dl) | 8.76 ± 0.49 | 8.46 ± 0.52 | 0.11 |
| Magnesium (mg/dl) | 1.91 ± 0.19 | 2.03 ± 0.21 | 0.07 |
| CK (U/L) | 140.77 ± 95.69 | 145.67 ± 184.75 | 0.92 |
| CK-MB (ng/mL) | 1.17 ± 0.79 | 1.11 ± 0.61 | 0.78 |
| Tn I (pg/mL) | 23.12 ± 60.26 | 5.33 ± 5.48 | 0.06 |
| BNP (pg/mL) | 60.67 ± 63.28 | 91.43 ± 129.54 | 0.40 |
| LDL (mg/dl) | 74.38 ± 20.56 | 81.55 ± 24.98 | 0.33 |
| HDL (mg/dl) | 37.46 ± 13.01 | 37.71 ± 11.39 | 0.94 |
| Triglyceride (mg/dl) | 119.23 ± 70.37 | 131.80 ± 66.85 | 0.54 |
| Total protein (g/L) | 6.72 ± 0.45 | 6.92 ± 0.47 | 0.15 |
| Albumin (g/L) | 3.96 ± 0.44 | 3.97 ± 0.33 | 0.87 |
| PT | 11.55 ± 0.91 | 11.82 ± 1.92 | 0.62 |
| aPTT (s) | 23.25 ± 3.24 | 25.62 ± 8.45 | 0.32 |
| INR | 0.95 ± 0.06 | 0.96 ± 0.19 | 0.89 |
| Baseline anti-factor Xa level (IU/mL) | 0.18 ± 0.06 | 0.43 ± 0.23 | < 0.001 |
ALT alanin aminotransferase, aPTT activated partial thromboplastin time, AST aspartate aminotransferase, BMI body mass index, BNP brain natriuretic peptide, BUN blood urea nitrogen, CAD coronary artery disease, CK creatine kinase, CK-MB creatine kinase-myocardial band, CRP C-reactive protein, CT computerized tomography, DM diabetes mellitus, GFR glomerular filtration rate, HDL high density lipoprotein, HT hypertension, INR international normalized ratio, LDH lactate dehydrogenase, LDL low density lipoprotein, LMWH low molecular weight heparin, PT prothrombin time, RBC red blood cell, SpO2, oxygen saturation, TG triglyceride, TIBC total iron binding capacity, Tn I troponin I, WBC white blood cell
Parameters that p < 0.05 are written in italics
Laboratory analysis of the blood collected before the discharge of patients revealed that eosinophil counts in group 1 were higher than in group 2, whereas aPTT and anti-factor Xa activity were lower in group 1 than in group 2 (Table 2).
Table 2.
Laboratory parameters of the patients before discharge
| Variable | Group 1 (13 patients) anti-factor Xa < 0.2 IU/mL | Group 2 (67 patients) anti-factor Xa > 0.2 IU/mL | p value |
|---|---|---|---|
| WBC × 103/mL | 6.25 ± 0.82 | 5.55 ± 1.95 | 0.08 |
| Neutrophil | 3.81 ± 1.14 | 3.26 ± 1.58 | 0.08 |
| Lymphocyte | 1.81 ± 0.69 | 1.79 ± 0.78 | 0.52 |
| Eosinophil (%) | 3.06 ± 1.49 | 2.07 ± 1.92 | 0.001 |
| Eosinophil count | 182.49 ± 95.81 | 112.18 ± 102.54 | 0.009 |
| RBC × 106/mL | 4.71 ± 0.42 | 4.41 ± 0.54 | 0.07 |
| Hemoglobin (gr/dl) | 13.26 ± 2.26 | 12.58 ± 1.95 | 0.24 |
| Platelet × 103/mL | 264.42 ± 117.14 | 226.94 ± 89.08 | 0.25 |
| Sedimentation (%) | 21.83 ± 18.86 | 35.18 ± 26.05 | 0.07 |
| CRP (mg/L) | 8.54 ± 11.47 | 19.45 ± 35.44 | 0.19 |
| Procalcitonin (μgr/L) | 0.10 ± 0.07 | 0.10 ± 0.13 | 0.96 |
| Fibrinogen (mg/dl) | 377.33 ± 145.03 | 416.98 ± 148.71 | 0.31 |
| Iron (μg/dl) | 56.58 ± 25.26 | 53.08 ± 24.71 | 0.56 |
| TIBC (μg/dl) | 266.75 ± 86.51 | 260.76 ± 80.39 | 0.96 |
| Ferritin (ng/mL) | 141.41 ± 92.12 | 294.28 ± 341.87 | 0.08 |
| Transferrin saturation (%) | 19.41 ± 10.43 | 19.92 ± 9.93 | 0.92 |
| D-dimer (μgr/mL) | 0.72 ± 0.77 | 0.78 ± 1.08 | 0.91 |
| Glucose (mg/dl) | 109.83 ± 30.53 | 108.80 ± 39.84 | 0.59 |
| BUN (mg/dl) | 13.67 ± 7.17 | 11.55 ± 4.55 | 0.43 |
| Creatinine (mg/dl) | 0.76 ± 0.25 | 0.73 ± 0.35 | 0.46 |
| AST (U/L) | 25.17 ± 10.56 | 32.13 ± 24.11 | 0.45 |
| ALT (U/L) | 27.75 ± 14.43 | 36.53 ± 27.69 | 0.54 |
| LDH (U/L) | 246.58 ± 136.74 | 264.14 ± 207.81 | 0.52 |
| Sodium (mmol/L) | 138.83 ± 4.58 | 140.58 ± 5.15 | 0.52 |
| Potassium (mEq/L) | 4.32 ± 0.49 | 4.25 ± 0.53 | 0.83 |
| Calcium (mg/dl) | 8.88 ± 0.41 | 8.54 ± 0.51 | 0.29 |
| Magnesium (mg/dl) | 1.96 ± 0.16 | 2.01 ± 0.24 | 0.55 |
| CK (U/L) | 81.08 ± 54.18 | 85.36 ± 130.24 | 0.57 |
| CK-MB (ng/mL) | 1.05 ± 0.87 | 1.07 ± 1.24 | 0.71 |
| Tn I (pg/mL) | 6.18 ± 4.01 | 9.03 ± 30.53 | 0.06 |
| BNP (pg/mL) | 65.80 ± 94.88 | 174.88 ± 196.54 | 0.44 |
| LDL (mg/dl) | 74.58 ± 21.89 | 78.30 ± 24.70 | 0.71 |
| HDL (mg/dl) | 38.83 ± 13.05 | 34.52 ± 8.87 | 0.16 |
| Triglyceride (mg/dl) | 129.75 ± 79.52 | 164.34 ± 102.70 | 0.15 |
| Total protein (g/L) | 6.72 ± 0.51 | 6.84 ± 0.51 | 0.51 |
| Albumin (g/L) | 3.97 ± 0.57 | 3.80 ± 0.52 | 0.31 |
| PT | 11.72 ± 0.59 | 11.93 ± 1.28 | 0.65 |
| aPTT (s) | 22.34 ± 1.38 | 24.38 ± 3.58 | 0.01 |
| INR | 0.96 ± 0.05 | 0.98 ± 0.11 | 0.46 |
| Control anti-factor Xa level (IU/mL) | 0.16 ± 0.04 | 0.53 ± 0.26 | < 0.001 |
ALT alanin aminotransferase, aPTT activated partial thromboplastin time, AST aspartate aminotransferase, BNP brain natriuretic peptide, BUN blood urea nitrogen, CK creatine kinase, CK-MB creatine kinase-myocardial band, CRP C-reactive protein, INR international normalized ratio, LDH lactate dehydrogenase, LDL low density lipoprotein, PT prothrombin time, RBC red blood cell, TG triglyceride, TIBC total iron binding capacity, Tn I troponin I, WBC white blood cell
Parameters that p < 0.05 are written in italics
The D-dimer values of 64 patients were < 1 μg/mL, whereas those of 16 patients were > 1 μg/mL. Patients with D-dimer values < 1 μg/mL and those with > 1 μg/mL were found to have similar activity of anti-actor Xa (baseline D-dimer: < 1 ugr/mL, 0.39 ± 0.23 vs > 1 ugr/mL, 0.40 ± 0.22, p = 0.87; before discharge: < 1 μgr/mL, 0.45 ± 0.25 vs > 1 μgr/mL, 0.62 ± 0.30, p = 0.07). However, all 14 patients with eosinophil counts > 4% were in the group with D-dimer levels < 1 μg/mL (p = 0.03). Eosinophil content and numerical values were also significantly higher in the group with D-dimer level < 1 μg/mL (82.97 ± 105.88 vs 15.65 ± 15.35; 1.47 ± 1.84 vs 0.30 ± 0.31, respectively; p = 0.01).
The AUC value in the ROC analysis for baseline eosinophil counts to show subprophylactic anti-factor Xa activity was 0.79 (range: 0.64–0.93; p = 0.001) (Fig. 2).
Fig. 2.
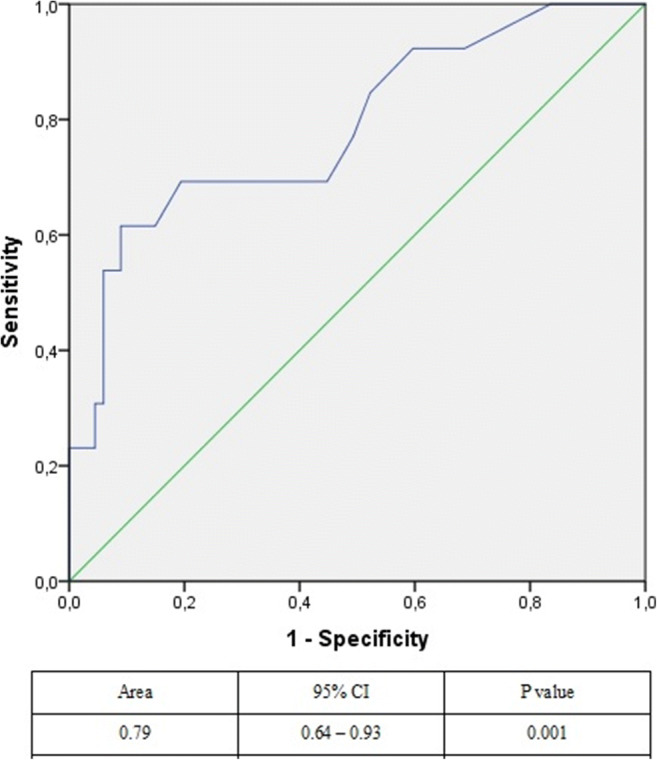
ROC analysis for baseline eosinophil counts to show subprophylactic anti-factor Xa level
Thoracic CTs of the patients were evaluated, identifying 51 patients with infection sings and 29 with no signs of infection in their thorax CT. When patients with and without thoracic CT findings were compared, age, gender, medication, eosinophil percentage > 4%, sediments, crp, fibrinogen, ferritin, AST, ALT, LDH, albumin, HDL, and calcium values were found to be significantly different between the groups (Table 3). Patients with positive CT findings mainly consisted of older male patients. Acute phase reactants of CT positive patients were found to be higher; however, D-dimer level and anti-Factor Xa activity were similar in both groups (Table 3). Cavernous sinus thrombosis was observed in one of our patients. The baseline anti-factor Xa activity of this patient was 0.19 IU/mL, the eosinophil count was 367, and the eosinophil percentage was 6.8%. The cavernous sinus thrombosis was treated with warfarin. There were no embolic complications in our other patients.
Table 3.
Demographic and laboratory parameters of CT positive and negative patients
| Variable | CT positive patients (51 patients) | CT negative patients (29 patients) | p value |
|---|---|---|---|
| Age (year) | 50.67 ± 15.80 | 34.83 ± 10.41 | < 0.001 |
| Gender, n (%) | |||
| Male | 30 (58.8) | 10 (34.5) | 0.03 |
| Female | 21 (37.5) | 19 (65.5) | |
| BMI (kg/m2) | 26.85 ± 4.35 | 25.99 ± 4.48 | 0.45 |
| Hypertension, n (%) | 10 (19.6) | 2 (6.9) | 0.12 |
| Diabetes mellitus, n (%) | 7 (13.7) | 2 (6.9) | 0.35 |
| Coronary artery disease, n (%) | 1 (2) | – | 0.44 |
| Medication, n (%) | |||
| Chloroquine | 51 (100) | 29 (100) | 1 |
| Azitromisin | 45 (88.2) | 18 (62.1) | 0.006 |
| Osetlemivir | 9 (17.6) | 1 (3.4) | 0.06 |
| Favipiravir | 17 (33.3) | 1 (3.4) | 0.002 |
| LMWH, n (%) | |||
| Single dose | 37 (72.5) | 23 (79.3) | 0.50 |
| Double dose | 14 (27.5) | 6 (20.7) | |
| SpO2 | 96.57 ± 2.37 | 97.83 ± 0.88 | 0.01 |
| QT interval (ms) | 390.02 ± 30.61 | 388.66 ± 27.44 | 0.76 |
| WBC × 103/mL | 5.46 ± 1.77 | 5.86 ± 1.87 | 0.39 |
| Neutrophil | 3.46 ± 1.63 | 3.63 ± 1.68 | 0.80 |
| Lymphocyte | 1.53 ± 0.70 | 1.66 ± 0.54 | 0.16 |
| Eosinophil (%) | 1.32 ± 2.01 | 1.08 ± 1.03 | 0.30 |
| Eosinophil (%) | |||
| > 4 (%) | 13 (25.5) | 1 (3.4) | 0.01 |
| < 4 (%) | 38 (74.5) | 28 (96.6) | |
| Eosinophil count | 71.80 ± 114.11 | 65.48 ± 64.37 | 0.24 |
| RBC × 106/mL | 4.69 ± 0.48 | 5.85 ± 6.42 | 0.66 |
| Hemoglobin (gr/dl) | 13.42 ± 2.21 | 13.35 ± 2.34 | 0.76 |
| Platelet × 103/mL | 198.24 ± 64.67 | 211.83 ± 49.25 | 0.20 |
| Sedimentation (%) | 37.67 ± 23.78 | 22.39 ± 18.50 | 0.003 |
| CRP (mg/L) | 31.14 ± 32.26 | 8.73 ± 16.31 | < 0.001 |
| Procalcitonin (μgr/L) | 0.15 ± 0.21 | 0.09 ± 0.08 | 0.28 |
| Fibrinogen (mg/dl) | 433.98 ± 126.64 | 348.59 ± 86.75 | 0.002 |
| Iron (μg/dl) | 44.16 ± 25.34 | 51.18 ± 29.18 | 0.18 |
| TIBC (μg/dl) | 269.33 ± 75.03 | 296.07 ± 84.04 | 0.06 |
| Ferritin (ng/mL) | 282.25 ± 212.23 | 86.85 ± 96.30 | < 0.001 |
| Transferrin saturation (%) | 15.34 ± 9.65 | 16.42 ± 11.90 | 0.96 |
| D-dimer (μgr/mL) | 0.98 ± 1.97 | 1.33 ± 4.44 | 0.62 |
| Glucose (mg/dl) | 114.47 ± 38.29 | 96.03 ± 34.86 | 0.001 |
| BUN (mg/dl) | 14.26 ± 5.48 | 10.23 ± 3.37 | 0.001 |
| Creatinine (mg/dl) | 0.83 ± 0.27 | 0.71 ± 0.16 | 0.03 |
| AST (U/L) | 33.67 ± 18.55 | 21.03 ± 7.43 | < 0.001 |
| ALT (U/L) | 30.82 ± 20.45 | 17.48 ± 7.32 | < 0.001 |
| LDH (U/L) | 271.94 ± 100.17 | 220.62 ± 65.56 | 0.01 |
| Sodium (mmol/L) | 138.51 ± 4.46 | 135.93 ± 23.46 | 0.28 |
| Potassium (mEq/L) | 4.10 ± 0.40 | 4.05 ± 0.45 | 0.73 |
| Calcium (mg/dl) | 8.40 ± 0.49 | 8.71 ± 0.52 | 0.02 |
| Magnesium (mg/dl) | 2.04 ± 0.23 | 1.96 ± 0.15 | 0.08 |
| CK (U/L) | 171.39 ± 208.25 | 98.24 ± 58.41 | 0.13 |
| CK-MB (ng/mL) | 1.12 ± 0.63 | 1.14 ± 0.66 | 0.92 |
| Tn I (pg/mL) | 10.18 ± 30.97 | 4.77 ± 4.27 | 0.06 |
| BNP (pg/mL) | 102.86 ± 136.50 | 56.74 ± 81.90 | 0.06 |
| LDL (mg/dl) | 80.62 ± 26.71 | 79.81 ± 19.57 | 0.85 |
| HDL (mg/dl) | 34.33 ± 9.07 | 43.96 ± 13.28 | 0.004 |
| Triglyceride (mg/dl) | 137.25 ± 68.08 | 115.44 ± 64.17 | 0.06 |
| Total protein (g/L) | 6.84 ± 0.50 | 6.97 ± 0.40 | 0.38 |
| Albumin (g/L) | 3.89 ± 0.34 | 4.11 ± 0.33 | 0.01 |
| PT | 11.89 ± 2.16 | 11.57 ± 0.81 | 0.54 |
| aPTT (s) | 25.27 ± 9.46 | 25.17 ± 3.91 | 0.26 |
| INR | 0.97 ± 0.22 | 0.94 ± 0.07 | 0.20 |
| Baseline anti-factor Xa level (IU/mL) | 0.39 ± 0.21 | 0.40 ± 0.27 | 0.94 |
| Control anti-factor Xa level (IU/mL) | 0.47 ± 0.25 | 0.50 ± 0.30 | 0.82 |
ALT, alanin aminotransferase, aPTT activated partial thromboplastin time, AST aspartate aminotransferase, BMI body mass index, BNP brain natriuretic peptide, BUN blood urea nitrogen, CAD coronary artery disease, CK creatine kinase, CK-MB creatine kinase-myocardial band, CRP C-reactive protein, DM diabetes mellitus, HDL high density lipoprotein, HT hypertension, INR international normalized ratio, LDH lactate dehydrogenase, LDL low density lipoprotein, LMWH low molecular weight heparin, PT prothrombin time, RBC red blood cell, SpO2 oxygen saturation, TG triglyceride, TIBC total iron binding capacity, Tn I troponin I, WBC white blood cell
Parameters that p < 0.05 are written in italics
During follow-up, 1 patient died and 2 patients needed intensive care unit follow-up. The average hospitalization period of the patients was 7.55 ± 3.95 days. There was no complication in the patients followed by the filiation teams for 14 days at home, and the general condition of the patients did not deteriorate.
Anti-factor Xa activity was higher in COVID-19 negative patients than in group 1 patients (COVID-19 positive patients) however eosinophil level was similar in these two groups; Anti-factor Xa activity (mean ± SD); COVID-19 negative patients: 0.78 ± 0.53 vs group 1 patients: 0.18 ± 0.06, p = 0.001, eosinophil percent (%) (mean ± SD); COVID-19 negative patients: 2.40 ± 1.28 vs group 1 patients: 2.96 ± 2.55, p = 0.54, eosinophil count (mean ± SD); COVID-19 negative patients: 217.77 ± 151.14 vs group 1 patients: 168.42 ± 147.25, p = 0.45.
Discussion
In this study, we found that increased eosinophil count is associated with the level of subprophylactic anticoagulation. Eosinophil counts evaluated for adjusting anticoagulation dose were also found to be increased in patients with low D-dimer levels. Patients with lung conditions were found to have increased inflammatory parameters and percentage of eosinophils.
COVID-19 infection has been shown to be associated with increased coagulopathy [14, 19, 20]. In these patients, the D-dimer and fibrinogen levels were increased, but aPTT level was decreased [21]. Local thrombotic events and thromboembolic complications may develop due to endothelial damage and increased coagulable condition due to COVID-19. Anticoagulant therapy reduces mortality and morbidity in COVID-19 patients [19, 20]. Various suggestions have been made about the application of anticoagulant treatment strategy. Various laboratory parameters (D-dimer) and clinical conditions of patients are effective in determining these recommendations [22].
Previous studies examined the anti-factor Xa activity after LMWH administration for VTE prophylaxis, and values below 0.2 IU/mL have been shown to be subprophylactic doses [17, 18]. However, the efficacy and dose of LMWH administered in COVID-19 patients is not clear. It is apparent that levels below the anti-factor Xa values determined in previous studies may increase the risk of VTE in COVID-19 patients, which can cause hypercoagulability. Considering this, subprophylactic anticoagulation in patients was determined in our study by taking the limit value of 0.2 IU/mL. Subprophylactic anticoagulation value was determined in 16.25% patients of the studied patients.
In the patient group with subprophylactic anticoagulation, eosinophil levels were found to be increased. Other demographic and laboratory parameters of patients with prophylactic and subprophylactic levels of anticoagulation were similar.
Eosinophils have pro-inflammatory, pleotropic, and immune regulatory properties. Eosinophils are mainly found in blood, although they are also found in the gastrointestinal tract and lungs. Lung pathology caused by eosinophils has been observed in RSV and SARS-CoV-1 viral infections [23]. Eosinophils may also contribute to the lung pathology in COVID-19 patients. In hypereosinophilic cases, the degranulation of major basic protein from eosinophils and eosinophil peroxidase causes platelet aggregation and thrombus formation [24]. Eosinophils can cause in situ thrombus formation in the lungs and veins. Patients with thoracic CT lesions had high eosinophilic inflammatory parameters. Eosinophils secrete their own chemoattractant molecules (eotaxin and platelet-activating factor) that allow more eosinophils to enter the inflammatory area, increasing inflammation and lung damage.
Enzymes released from eosinophils (peroxidases, cationic proteins, and neurotoxins) may decrease the anticoagulant activity of heparin [25, 26]. In our study, it was found that patients with high eosinophil levels had lower anticoagulant activity. Although D-dimer and fibrinogen levels were similar, patients with low anticoagulant activity only had high eosinophil levels, indicating that subprophylactic anticoagulation levels are related to eosinophils. Eosinophil counts had a good AUC (0.79) in predicting the presence of subprophylactic anticoagulation.
Our patient population was small and many of the patients were followed up for a short period of time (average: 7.5 days). Only 3 patients needed intensive care and one patient died. Therefore, the clinical outcomes of subprophylactic anticoagulation could not be evaluated. Studies involving large-scale, intensive care patients may provide information on the clinical outcomes that eosinophil counts can produce due to their subprophylactic anticoagulation property.
We compared the patients with subprophylactic anticoagulation with ten patients who had not COVID-19 diagnosis. According to the comparison, the eosinophil percent and count were similar; however, the anti-factor Xa activity was significantly lower in patients with COVID-19. These results suggest that eosinophils had more effect on anti-factor Xa activity of LMWH in COVID-19 patients. However, these two patient groups had no similar characteristics. The dose of LMWH used is not standard, so that the result should confirm with large clinical trial.
The patients who had thoracic CT lesions, more advanced disease, and were older males exhibited higher inflammatory parameters. This has been shown in previous studies [27].
Limitations
The small number of patients is the main limitation of our study, although the results of this study can be a guide for the optimization of anticoagulant therapy to decrease mortality and morbidity in COVID-19 patients. This study serves as a guide for future large-scale studies with larger patient groups. Our study groups were not included patients with morbid obese and renal failure; therefore, we need further studies with these patient groups. Besides most of our patients were followed up inpatient clinic, future studies analyzing patients in intensive care units are required.
Conclusion
Increased eosinophil counts in COVID-19 patients were found to be associated with reduced anticoagulant effect of LMWH. Hence, this study can guide to large clinical trials, and the eosinophil levels should be taken into consideration or not while determining the prophylactic anticoagulation strategy in patients with COVID-19.
Acknowledgements
We thank Harun AĞCA, Associate Professor (Uludağ University Faculty of Medicine, Department of Microbiology), for his valuable assistance for the evaluation of peripheral smear from blood samples taken from the patients.
Authors’ contribution
Study design: Selma Ari, Hasan Ari, Fahriye Vatansever Ağca, and Mehmet Melek
Data collection: Veysi Can, Ömer Furkan Demir, Özlem Şengören Dikiş, and Kağan Huysal
Biochemical analysis: Kağan Huysal
Statistical analysis: Hasan Ari, Sencer Çamci, Tamer Türk
Writing: Selma Ari, Sencer Çamci, Fahriye Vatansever Ağca, Hasan Ari, Mehmet Melek
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu Y-C, Chen C-S, Chan Y-J. The outbreak of COVID-19: an overview. J Chin Med Assoc. 2020;83:217–220. doi: 10.1097/JCMA.0000000000000270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Giannis D, Ziogas IA, Gianni P. Coagulation disorders in coronavirus infected patients: COVID-19, SARS-CoV-1, MERS-CoV and lessons from the past. J Clin Virol. 2020;127:104362. doi: 10.1016/j.jcv.2020.104362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Obi AT, Tignanelli CJ, Jacobs BN, Arya S, Park PK, Wakefield TW, Henke PK, Napolitano LM. Empirical systemic anticoagulation is associated with decreased venous thromboembolism in critically ill influenza A H1N1 acute respiratory distress syndrome patients. J Vasc Surg: Venous Lymph Disord. 2019;7:317–324. doi: 10.1016/j.jvsv.2018.08.010. [DOI] [PubMed] [Google Scholar]
- 5.Cui S, Chen S, Li X, Liu S, Wang F. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J Thromb Haemost. 2020;18:1421–1424. doi: 10.1111/jth.14830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Helms J, Tacquard C, Severac F, Leonard-Lorant I, Ohana M, Delabranche X, Merdji H, Clere-Jehl R, Schenck M, Fagot Gandet F, Fafi-Kremer S, Castelain V, Schneider F, Grunebaum L, Anglés-Cano E, Sattler L, Mertes PM, Meziani F; CRICS TRIGGERSEP Group (Clinical Research in Intensive Care and Sepsis Trial Group for Global Evaluation and Research in Sepsis) (2020) High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med 46(6):1089–1098 [DOI] [PMC free article] [PubMed]
- 7.Teuwen L-A, Geldhof V, Pasut A, Carmeliet P. COVID-19: the vasculature unleashed. Nat Rev Immunol. 2020;20:389–391. doi: 10.1038/s41577-020-0343-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Panigada M, Bottino N, Tagliabue P, Grasselli G, Novembrino C, Chantarangkul V, Pesenti A, Peyvandi F, Tripodi A. Hypercoagulability of COVID-19 patients in intensive care unit. A report of thromboelastography findings and other parameters of hemostasis. J Thromb Haemost. 2020;18:1738–1742. doi: 10.1111/jth.14850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ranucci M, Ballotta A, Di Dedda U, et al. The procoagulant pattern of patients with COVID-19 acute respiratory distress syndrome. J Thromb Haemost. 2020;18:1747–1751. doi: 10.1111/jth.14854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maier CL, Truong AD, Auld SC, Polly DM, Tanksley CL, Duncan A (2020) COVID-19-associated hyperviscosity: a link between inflammation and thrombophilia? Lancet 395(10239):1758–1759 [DOI] [PMC free article] [PubMed]
- 11.Rothenberg ME. Eosinophilia. N Engl J Med. 1998;338:1592–1600. doi: 10.1056/NEJM199805283382206. [DOI] [PubMed] [Google Scholar]
- 12.Santoro A, Zucchelli P, Trombetti E, Degli Esposti E, Sturani A, Zuccalà A, Chiarini C, Casadei-Maldini M, Mazzuca A. Effect of eosinophilia on the heterogeneity of the anticoagulant response to heparin in haemodialysis patients. Proc Eur Dial Transplant Assoc Eur Ren Assoc. 1985;21:143–149. [PubMed] [Google Scholar]
- 13.Samoszuk M, Corwin M, Hazen SL. Effects of human mast cell tryptase and eosinophil granule proteins on the kinetics of blood clotting. Am J Hematol. 2003;73(1):18–25. doi: 10.1002/ajh.10323. [DOI] [PubMed] [Google Scholar]
- 14.Barnes GD, Burnett A, Allen A, Blumenstein M, Clark NP, Cuker A, Dager WE, Deitelzweig SB, Ellsworth S, Garcia D, Kaatz S, Minichiello T. Thromboembolism and anticoagulant therapy during the COVID-19 pandemic: interim clinical guidance from the anticoagulation forum. J Thromb Thrombolysis. 2020;50:72–81. doi: 10.1007/s11239-020-02138-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aziz S, Arabi YM, Alhazzani W, Evans L, Citerio G, Fischkoff K, Salluh J, Meyfroidt G, Alshamsi F, Oczkowski S, Azoulay E, Price A, Burry L, Dzierba A, Benintende A, Morgan J, Grasselli G, Rhodes A, Møller MH, Chu L, Schwedhelm S, Lowe JJ, Bin D, Christian MD. Managing ICU surge during the COVID-19 crisis: rapid guidelines. Intensive Care Med. 2020;46:1303–1325. doi: 10.1007/s00134-020-06092-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cockcroft DW, Gault H. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 17.Wei MY, Ward SM. The anti-factor Xa range for low molecular weight heparin thromboprophylaxis. Hematol Rep. 2015;7:80–83. doi: 10.4081/hr.2015.5844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karcutskie CA, Dharmaraja A, Patel J, Eidelson SA, Padiadpu AB, Martin AG, Lama G, Lineen EB, Namias N, Schulman CI, Proctor KG. Association of anti–factor Xa–guided dosing of enoxaparin with venous thromboembolism after trauma. JAMA Surg. 2018;153:144–149. doi: 10.1001/jamasurg.2017.3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18:844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tang N, Bai H, Chen X, Gong J, Li D, Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;18:1094–1099. doi: 10.1111/jth.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Driggin E, Madhavan MV, Bikdeli B, Chuich T, Laracy J, Biondi-Zoccai G, Brown TS, der Nigoghossian C, Zidar DA, Haythe J, Brodie D, Beckman JA, Kirtane AJ, Stone GW, Krumholz HM, Parikh SA. Cardiovascular considerations for patients, health care workers, and health systems during the COVID-19 pandemic. J Am Coll Cardiol. 2020;75:2352–2371. doi: 10.1016/j.jacc.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spyropoulos AC, Levy JH, Ageno W, Connors JM, Hunt BJ, Iba T, Levi M, Samama CM, Thachil J, Giannis D, Douketis JD, the Subcommittee on Perioperative, Critical Care Thrombosis, Haemostasis of the Scientific, Standardization Committee of the International Society on Thrombosis and Haemostasis Scientific and standardization committee communication: clinical guidance on the diagnosis, prevention and treatment of venous thromboembolism in hospitalized patients with COVID-19. J Thromb Haemost. 2020;18:1859–1865. doi: 10.1111/jth.14929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lindsley AW, Schwartz JT, Rothenberg ME. Eosinophil responses during COVID-19 infections and coronavirus vaccination. J Allergy Clin Immunol. 2020;146:1–7. doi: 10.1016/j.jaci.2020.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marx C, Novotny J, Salbeck D, et al. Eosinophil-platelet interactions promote atherosclerosis and stabilize thrombosis with eosinophil extracellular traps. Blood. 2019;134:1859–1872. doi: 10.1182/blood.2019000518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fredens K, Dahl R, Venge P. In vitro studies of the interaction between heparin and eosinophil cationic protein. Allergy. 1991;46:27–29. doi: 10.1111/j.1398-9995.1991.tb00538.x. [DOI] [PubMed] [Google Scholar]
- 26.Jane Liesveld(2020) Eosinophil production and function. Merc manual professional version, Jun 2020. Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ
- 27.Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DSC, Du B, Li LJ, Zeng G, Yuen KY, Chen RC, Tang CL, Wang T, Chen PY, Xiang J, Li SY, Wang JL, Liang ZJ, Peng YX, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J, Ye CJ, Zhu SY, Zhong NS; China Medical Treatment Expert Group for Covid-19 (2020) Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med 382(18):1708–1720 [DOI] [PMC free article] [PubMed]


