Abstract
T regulatory cells (Tregs) play a critical role in controlling the immune response, often limiting pathogen-specific cells in order to curb immune-mediated damage. Studies in human infants have reported an increased representation of Tregs in these individuals. However, how these cells differ from those in adults at various sites and how they respond to activation signals is relatively unknown. Here we used a newborn nonhuman primate (NHP) model to assess Treg populations present at multiple sites with regard to frequency and phenotype in comparison to those present in adult animals. We found that FoxP3+ cells were more highly represented in the T cell compartment of newborn NHP for all sites examined, i.e. the spleen, lung, and circulation. In the spleen and circulation, newborn-derived Tregs expressed significantly higher levels of FoxP3 and CD25 compared to adults, consistent with an effector phenotype. Strikingly, the phenotype of Tregs in the lungs of adult and infant animals was relatively similar, with both adult and newborn Tregs exhibiting a more uniform PD-1+CD39+ phenotype. Finally, in vitro, newborn Tregs exhibited an increased requirement for TCR engagement for survival. Further, these cells upregulated CD39 more robustly than their adult counterpart. Together, these data provide new insights into the quantity of Tregs in newborns, their activation state, and their potential to respond to activation signals.
INTRODUCTION
T regulatory cells (Tregs) are critical mediators of immune homeostasis, impacting the response to both pathogens and self antigens. The absence of this population can lead to lethal autoimmune disease or increased damage to host tissue following infection. The latter is likely attributable to a Treg imposed reduction in the number of virus-specific T cells generated following infection that has been reported in a number of models (e.g. (1, 2)). Thus, proper regulation of this population is critical in allowing an appropriately robust response that promotes pathogen clearance while at the same time limiting damage to the host.
Tregs can be divided into two major populations, thymus-derived Tregs and peripherally induced Tregs. Thymus-derived Tregs leave the thymus and enter the periphery with suppressive activity while peripherally induced Tregs acquire this activity during their differentiation following activation (3). Tregs are commonly identified by expression of the master regulator FoxP3 (4, 5). Tregs express an array of cell surface molecules that have been associated with their suppressive activity, i.e. CTLA-4, PD-1, ICOS, GITR (6–13). A number of studies have demonstrated a correlation between the level of these markers and suppressive activity (9, 14, 15). For example, ICOS ligation results in increased transcription of IL-4, IL-10, and TGF-β (9, 14). CTLA4 inhibits via an alternative approach, depleting CD80 and CD86 on APC through trans-endocytosis (15), thereby decreasing CD28 ligand availability which deprives T cells of optimal levels of this critical activation signal.
Other mechanisms by which Tregs negatively regulate the immune response include IL-2 consumption, direct cell killing, generation of adenosine, and induced expression of indoleamine 2,3-dioxygenase (IDO) in DC (3). IDO in the environment results in depletion of amino acids which are required for T cell function and survival. Adenosine production by Tregs is the result of metabolism of ATP by the ectoenzymes CD39 and CD73 (3, 11). CD39+ Tregs isolated from human blood demonstrate increased suppressive capacity compared to their CD39- counterpart (13). Adenosine produced by Tregs binds to the A2A receptor on effector T cells, resulting in inhibition of TCR signaling (16).
The immune response of the infant is often decreased and/or altered compared to adults encountering a similar immune challenge. Human infants have been reported to have a higher representation of Tregs in circulation (17–23). This may be the result of the enhanced propensity for cells to differentiate into Tregs in these individuals (23–26). The increase in Tregs has been postulated to contribute to the difficulty in eliciting immune responses in newborns. While higher Treg numbers can suppress pathogen-specific responses, the benefit is evident in the need to maintain tolerance to maternal alloantigens (27) as well as limiting inflammation during the establishment of the microbiome following birth (28).
While the increase in Tregs has been documented, our understanding of the distribution and function of these cells in newborns is limited. This is in part due to the challenges associated with studying these cells in human infants, e.g. their relatively low frequency compared to other immune populations coupled with the small amount of blood that can be obtained. Thus, our understanding of Tregs in human infants has come largely through the study of cord blood (CB) cells. CB Tregs exhibit potent suppressive activity (29, 30), suggesting these cells are likely to be relevant contributors to the reduced responses in newborns following infection or vaccination. A caution with these data comes from the recent report of stark differences in the immune system of 1 week old human infants compared to that present in cord blood (31). Thus, it is unclear how CB Tregs reflect the phenotype, function, and distribution of these cells in newborns. Given the limited information available for Tregs in the newborn, we have used an NHP model to gain insights into the distribution, phenotype, and activation requirements of this critical immunoregulatory population. These studies provide significant new insights into Tregs in the newborn.
MATERIALS AND METHODS
Animal Approval
Newborn and adult African green monkeys were obtained from the breeding colony at Wake Forest School of Medicine. Animals are group housed in pens with daily outdoor access. All animal protocols were approved by the Institutional Animal Care and Use Committee at Wake Forest School of Medicine. The WFSM animal care and use protocol adhered to the US Animal Welfare Act and Regulations.
Analysis of Tregs in Naïve AGM Tissues
Spleens and PBMC were isolated from five newborn AGM (4–5 days of age) and five adult AGM (9.6-12.8 years of age) at necropsy. Lung samples from four adult and newborn were available for these analyses. Single-cell suspensions were prepared from mechanically dispersed spleen and lung tissue or by density gradient separation for blood. Cells from all samples were frozen for future study. Splenocytes, PBMC and lung samples were thawed by placement in a 37°C water bath for 10 minutes followed by washing with warm media (RPMI, 10% FBS, 100U/ml-100μg/ml Pen/Strep, 2mM L-glutamine, 1% NEAA, 1mM Sodium Pyruvate, 10mM HEPES, 50μM β-ME). For flow cytometric analysis, cells were first stained with Zombie Violet (BioLegend, San Diego, CA) to exclude dead cells. Cells were then stained with phycoerythrin-Vio770-conjugated CD3 (Clone 10D12; Miltenyi Biotec, Bergisch Gladbach, Germany), allophycocyanin-conjugated CD279 (PD-1) (Clone eBioJ105; eBioscience, ThermoFisher, Waltham, MA), Brilliant Violet 510-conjugated CD278 (ICOS) (Clone C398.4A; BioLegend), phycoerythrin-conjugated CD25 (Clone CD25-4E3; eBioscience, ThermoFisher) and Brilliant Violet 711-conjugated CD39 (Clone A1; BioLegend). For FoxP3 staining, cells were fixed and permeabilized with the FoxP3 Staining Buffer set (eBioscience) followed by addition of Alexa Fluor 488-conjugated FoxP3 (Clone 206D; BioLegend). Samples were acquired on a BD LSRFortessa X-20 and analyzed with BD Diva Software (BD Immunocytometry Systems, San Jose, CA).
Analysis of in vitro stimulated cells
Splenocytes, 5 x 105 per well, were cultured in media containing RPMI, 10% FBS, 100U/ml-100μg/ml Pen/Strep, 2mM L-glutamine, 1% NEAA, 1mM Sodium Pyruvate, 10mM HEPES, and 50μM β-ME. These cultures were plated in a 48-well plate that was previously coated overnight at 4°C with anti-CD3 (Clone FN18; NHP Resources, location) at 1.0, 0.1 and 0.01μg/ml. Anti-CD28 (Clone CD28.2, NHP Resources), at 5μg/ml, and recombinant human IL-2 (carrier-free) (BioLegend), at 20U/ml, were added to each well. Duplicate cultures were set up for each condition and the plate was incubated at 37°C and 5% CO2 for 4 days. The first set of cultures were harvested two days post stimulation and the second at four days post stimulation. Cells were harvested, counted and stained with Zombie Violet (BioLegend, San Diego, CA) to exclude dead cells. Cells were then stained with phycoerythrin-Vio770-conjugated CD3 (Clone 10D12; Miltenyi Biotec, Bergisch Gladbach, Germany), allophycocyanin-conjugated CD279 (PD-1) (Clone eBioJ105; eBioscience, ThermoFisher, Waltham, MA), Brilliant Violet 510-conjugated CD278 (ICOS) (Clone C398.4A; BioLegend), phycoerythrin-conjugated CD25 (Clone CD25-4E3; eBioscience, ThermoFisher) and Brilliant Violet 711-conjugated CD39 (Clone A1; BioLegend). For FoxP3 staining, cells were fixed and permeabilized with the FoxP3 Staining Buffer set (eBioscience) followed by the addition of Alexa Fluor 488-conjugated FoxP3 (Clone 206D; BioLegend). Samples were acquired on a BD LSR Fortessa X-20 and analyzed with BD Diva Software (BD Immunocytometry Systems, San Jose, CA).
Statistical analysis
Significance was determined by a t-test or one-way ANOVA analysis as appropriate, using GraphPad Prism Software (GraphPad, San Diego, CA).
RESULTS
Newborn AGM have a significantly higher representation of Tregs in the spleen compared to adult animals.
Splenocytes from five newborn (age 4-5 days) or five adult (9.6-12.8 years) African green monkeys (AGM) were isolated and the presence of Tregs identified by staining with anti-CD3 and anti-FoxP3 antibody (Fig. 1A). Comparative ages of AGM to humans is roughly 1:4, so newborns approximate a 3-4 week old infant and adult animals individuals who are 38-51 years of age. For identification of Tregs, we assessed FoxP3+CD25+ cells within the CD3+ population. As would be predicted, the CD3+FoxP3+CD25+ cells were nearly all CD8 negative (Fig. 1A). Gating of Tregs directly from CD3+ cells was chosen to allow detection of all Tregs based on the reported downregulation of CD4 on AGM T cells that can occur following activation (32). This approach is supported by our analysis of CD4 on the Tregs. Both newborn and adults animals had CD4+ as well as CD4- populations within the CD3+FoxP3+CD25+ cells. However, the ratio of CD4-expressing to non-expressing Tregs was significantly higher in newborns (Fig. 1B). This is consistent with reduced immune challenge experienced in newborns.
Fig. 1. Tregs are more highly represented in the spleens of newborn NHP and exhibit higher levels of FoxP3 and CD25.

Splenocytes isolated from five newborn (4-5 days of age) and adult (9.6-12.8 years of age) AGM were evaluated for the presence of Tregs. (A) Splenocytes were initially gated on Zombie excluding (live) CD3+ cells (first column of panels). Tregs within this population were identified by co-staining for FoxP3 and CD25 (second column). We evaluated expression of CD8 on the FoXP3+CD25+ cells, showing the vast majority do not express CD8 (third column). In B, the expression of CD4 on Tregs was assessed. On average, infants had a higher representation of CD4+ cells within the FoXP3+CD25+ population. Data shown are the average of the ratio of CD4-expressing to non-expressing cells. Averaged data (n=5) for the percentage of cells that are positive for CD25 and FoxP3 within the CD3+ population and the number per 5x105 splenocytes are shown in C and D, respectively. The level of CD25 (E) and FoxP3 (F) on the FoxP3+ cells from the five newborn and adult AGM was measured. Data from individuals animals are indicated; the line represents the average±SEM. Significance was determined using a Student’s t-test. *p<0.05, **p<0.005, ***p<0.0001.
Having established our strategy for identifying Tregs we evaluated their representation within the T cell compartment of newborn and adult animals. Splenocytes from newborn animals had a significant increase in the percentage of CD3+ T cells that were FoxP3+ (Fig. 1C). This resulted in an increase in the total number of cells in newborns that were FoxP3+ (data shown are the number per 5x105 splenocytes given the differences in total splenocytes in newborns and adults (Fig. 1D)). CD25 is often used in conjunction with FoxP3 as a marker of Tregs. FoxP3+ from newborn and adult animals co-expressed CD25; however, the expression was significantly higher on newborn Tregs compared to those in adults, suggesting these cells may be more activated (Fig. 1E).
The level of FoxP3 expressed by newborn splenic Tregs is higher than adult Tregs.
The level of FoxP3 has been correlated with the suppressive activity of Tregs (33, 34). As was the case for CD25, we found that on average, newborn splenic Tregs had significantly more (1.7-fold) FoxP3 than their adult counterpart (Fig. 1F). The higher level of FoxP3, similar to the increased CD25, suggested these cells may be more highly activated and/or have more recently encountered activation stimuli.
A similar percentage of splenic Tregs from newborn and adult AGM express PD-1.
PD-1 expression has been shown to regulate Treg proliferation and survival (10, 35). Analysis of this marker revealed a similar percentage of PD-1+ Tregs in newborn and adult splenic Tregs (representative data are shown in Fig. 2A and averaged data in 2B). Unlike what we observed for CD25 and FoxP3, the level of PD-1 on the positive cells was not increased but instead trended towards reduced expression although this was not significant (Fig. 2C). Emerging data suggest high levels of PD-1 may negatively regulate the expansion of Tregs (36, 37) and thus this trend towards lower levels in newborns may reflect a greater proliferative potential of cells which could contribute to the higher number observed in the spleen.
Fig. 2. A higher percentage of newborn splenic Tregs express ICOS compared to adults.
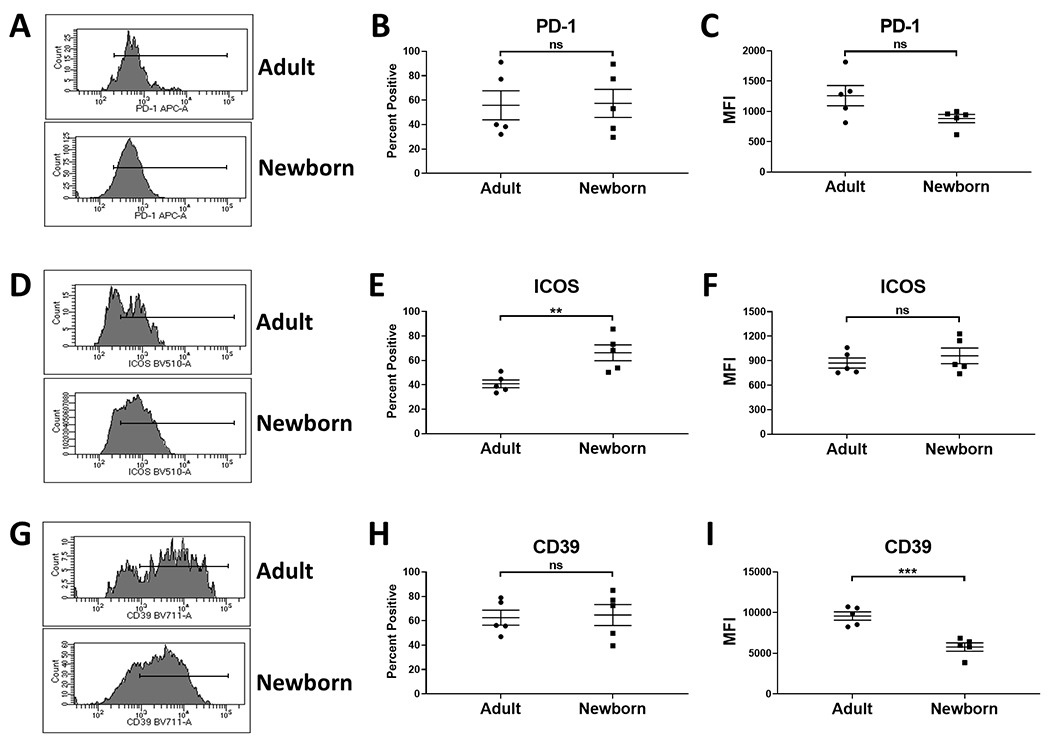
CD3+FoxP3+ splenocytes from newborn (4-5 days of age) and adult (9.6-12.8 years of age) (n=5) were evaluated for the expression of PD-1, ICOS and CD39. Representative flow plots are shown in A, D, and G. Plots represent the expression within the CD3+FoxP3+ population. The marker indicates the positively staining cells. The average percent of cells positive for each marker is shown in B, E, and H. The average MFI for expression on the positively staining cells is shown in C, F, and I. Symbols represent individual animals; the line represents the average±SEM. Significance was determined using a Student’s t-test. **p<0.005, ***p<0.001. ns=not significant.
A higher percentage of newborn splenic Tregs express ICOS.
The increased expression of FoxP3 in newborn Tregs led us to evaluate ICOS given the report of an association between ICOS signaling and FoxP3 level (9). ICOS was expressed on a significantly higher percentage of Tregs from newborns compared to adult animals (representative data are shown in Fig. 2D and averaged data in 2E). Although the an increased percentage of cells expressed ICOS in newborns, there was no difference in the level of expression of this marker on Tregs from adults vs. newborns (Fig. 2F).
We next measured CD39 given its role in generating the immunosuppressive factor adenosine (11). Newborn and adult animals did not differ in the percentage of Tregs that express CD39 (representative data are shown in Fig. 2G and averaged data in 2H) . However, interestingly, the level of CD39 expressed on newborn cells was significantly lower than on Tregs from adult animals (Fig. 2I).
Analysis of Tregs in the lung.
We next asked whether these differences were generally apparent in newborn Tregs. We first evaluated cells in the lungs. Lung tissue from four adults and four newborn was available for study. As in the spleen, FoxP3+ cells were more highly represented in the CD3+ population of the lung (Fig. 3A), demonstrating the increased representation of Tregs extended to the lung. Expression of FoxP3 in cells from the lungs of newborns was not significantly different than those in adults (Fig. 3B). As in the spleen, we evaluated the expression of CD25, PD-1, ICOS, and CD39. Tregs from the lungs of newborns and adults were not significantly different in their expression of these markers (Fig. 3C–F). We note that there was a trend towards a higher level of FoxP3 expression and the percent of ICOS+ cells in newborns. As we were only able to evaluate four newborns here, a limitation of this analysis is the possibility that some differences were missed that would be apparent with a larger sample size.
Fig. 3. Newborns have higher representation of Tregs in the lung compared to adults, but their phenotype is similar in the two age groups.
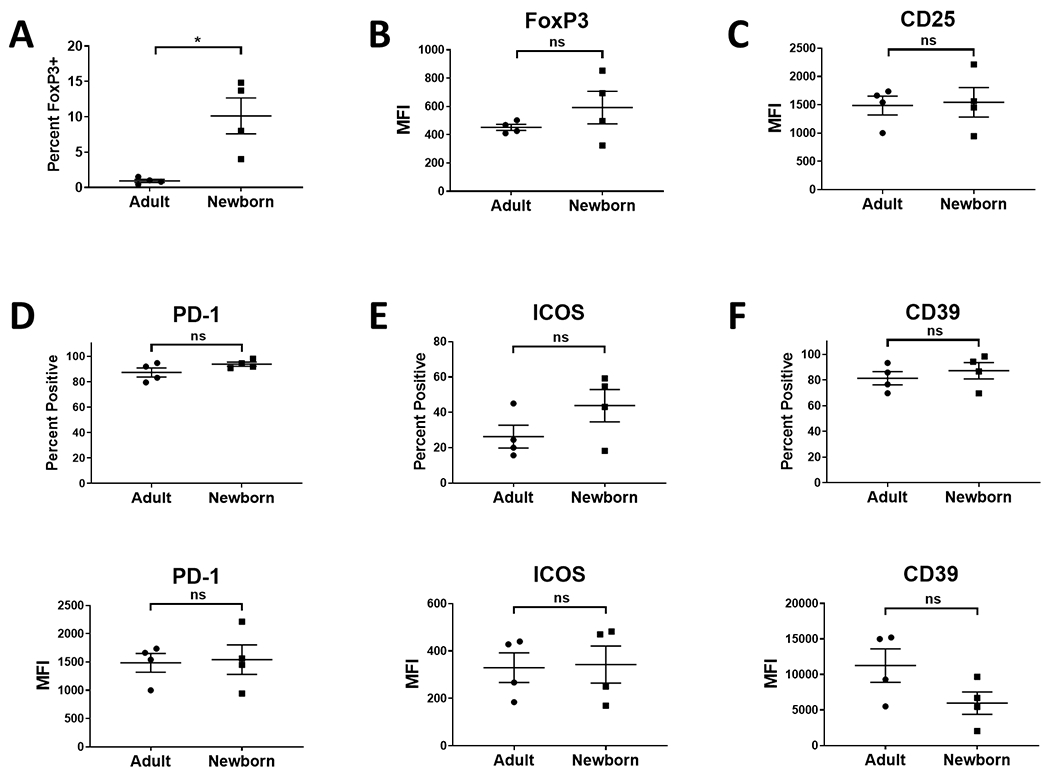
Lung cells isolated from four newborn (4-5 days of age) and four adult (9.6-12.8 years of age) AGM were evaluated for the presence of Tregs. Cells were initially gated on Zombie excluding (live) CD3+ cells. Tregs within this population were identified by co-staining for FoxP3 and CD25. Data for the percentage of CD3+ cells expressing FoxP3 are shown in A. The level of FoxP3 (B) and CD25 (C) on FoxP3+ cells is shown. The percentage of cells expressing the indicated molecule (top panels) and the level expressed by these cells (bottom panel) for PD-1 (D), ICOS (E), and CD39 (F) was also evaluated. Data from four adults is shown in D-F as one animal had a very low percentage of FoxP3+ cells that did not allow confident analysis of subsets. Individuals animals are indicated; the line represents the average±SEM. Significance was determined using a Student’s t-test. **p<0.005. ns=not significant.
While the phenotype of the Tregs did not differ between the newborns and adults, they were distinct from what we found in the spleen. In general, Tregs in the lung displayed a more activated phenotype with high PD-1 and CD39 expression. Together these data show that the basal state of Tregs present in the lungs have a distinct phenotype compared to those in the spleen. This suggests either preferential migration into the lung of cells with this phenotype or regulation following entry into the tissue. It also shows that newborn and adult Tregs have the potential to reach a similar phenotypic activation state.
The phenotype of circulating Tregs is distinct from those in the spleen and lung.
Lastly, we evaluated the Tregs present in circulation. This is of practical importance given that in humans PBMC are the predominant source of cells used to assess immune status and are often viewed as a surrogate for the cells in other tissues. Once again, we observed significantly more Tregs in the newborn versus adult animals and that these cells expressed a higher amount of FoxP3 (Fig. 4A and B). As in the spleen, CD25 expression on these cells was significantly increased (Fig. 4C). Evaluation of the functional markers revealed an increased percentage of Tregs that were positive for each of the regulatory molecules PD-1, ICOS, and CD39 in newborns. Tregs from newborns also had a higher level of PD-1 on a per cell basis, while having similar levels of ICOS and CD39 (Fig. 4D–F).
Fig. 4. Newborns have higher representation of Tregs in circulation compared to adults and a higher percentage display markers associated with suppressive activity.
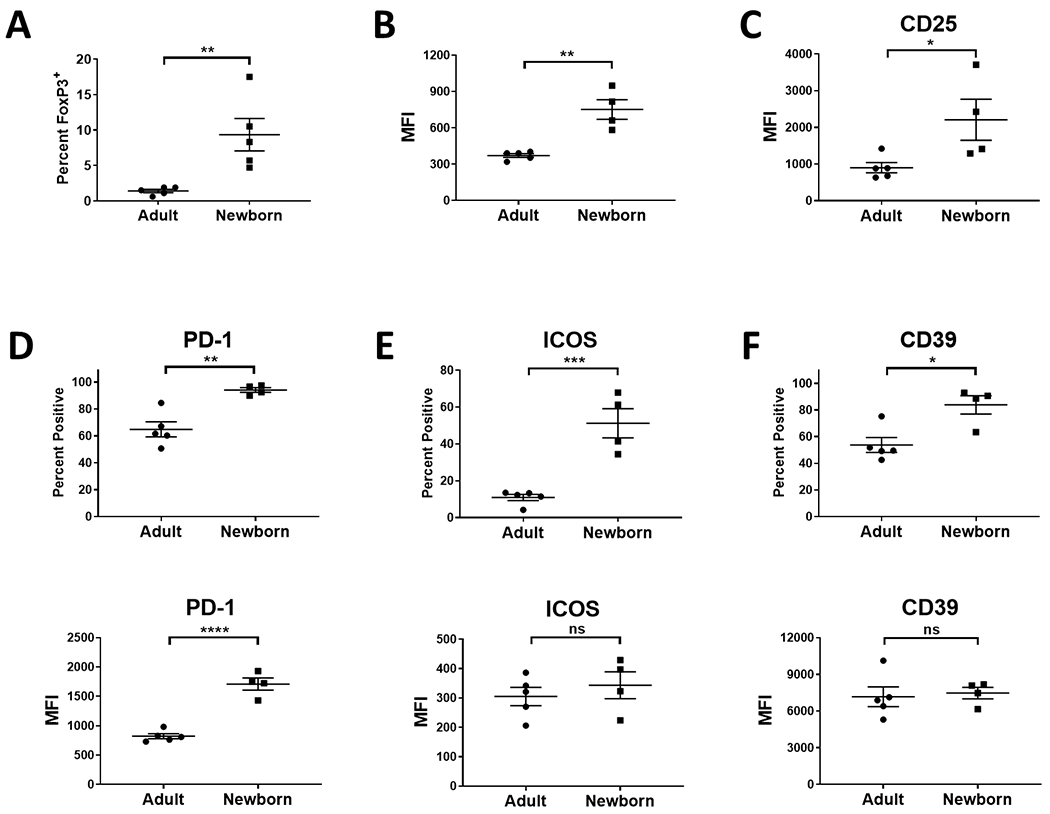
PBMC from four newborn (4-5 days of age) and five adult (9.6-12.8 years of age) AGM were evaluated for the presence of Tregs. Data for the percentage of CD3+ cells expressing FoxP3 are shown in A and the level of FoxP3 and CD25 on FoxP3+ cells in B and C, respectively. The percentage of cells expressing the indicated molecule and the level of PD-1 (D), CD38 (E), and ICOS (F) expressed is shown. Individuals animals are indicated; the line represents the average±SEM. Significance was determined using a Student’s t-test. *p<0.05,**p<0.005,***p<0.001, ****p<0.0001. ns=not significant.
Tregs from newborns have an increased requirement for TCR engagement for survival.
To gain insights into the survival signals required for Tregs from newborn and adult animals, splenocytes were stimulated in vitro with titrated concentrations of immobilized anti-CD3 (1, 0.1, and 0.01 μg/ml) together with anti-CD28 (5 μg/ml). The number of Tregs at the initiation of the culture and at d2 and d4 are shown in Fig. 5. Adult cells showed little change in number regardless of the stimulation received. In contrast, in the absence of strong TCR engagement (1 μg/ml anti-CD3) the number of Tregs in cultures of newborn cells dropped significantly at days 2 and 4 (Fig. 5A). To complement these studies, we evaluated the cultures for the presence of nonviable cells by staining with the fixable Zombie viability dye. We saw an increase in Foxp3+ cells that stained positive for the dye selectively in newborns following stimulation with the lower amounts (0.1 and 0.01 μg/ml) of immobilized anti-CD3 antibody (Fig. 5B). Together, these data support an increased need for potent TCR engagement for survival of Tregs from newborns.
Fig. 5. Tregs from newborns have a greater dependence on TCR engagement for survival.
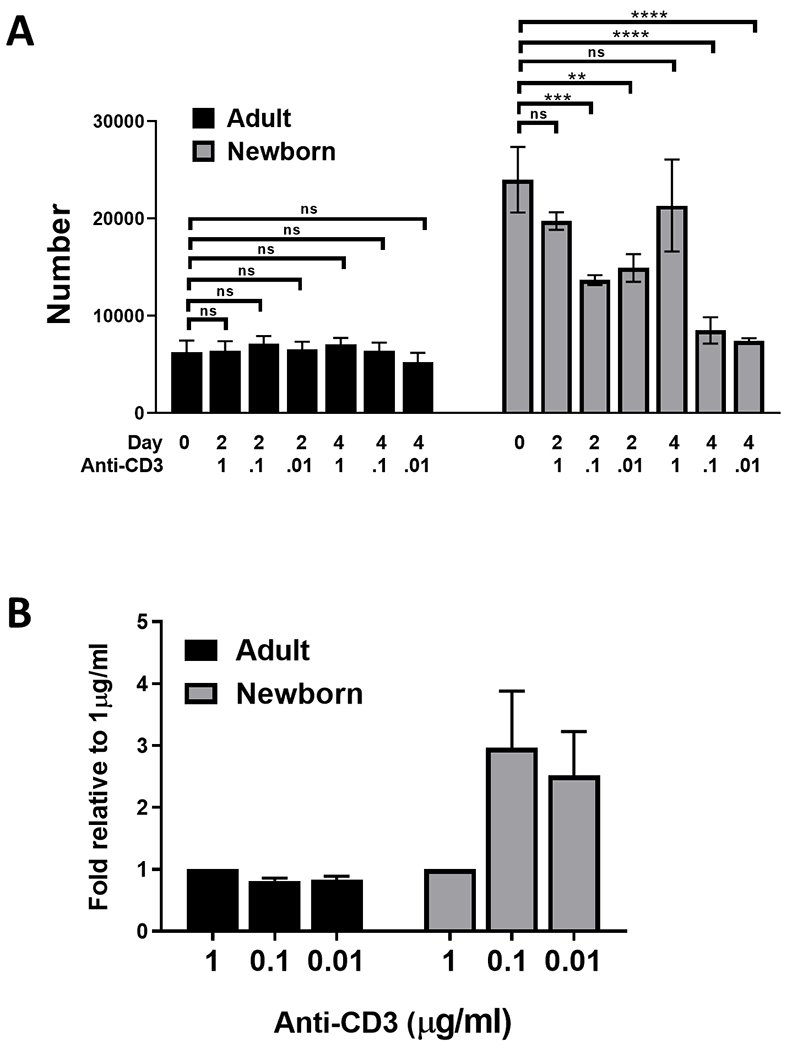
Splenocytes from newborn (4-5 days of age) and adult (9.6-12.8 years of age) were cultured in the presence of titrated amounts of immobilized anti-CD3 antibody together with 5 μg/ml anti-CD28 for 48 or 96 hours. Day 0 data are replotted here for ease of comparison. Viable Tregs in the culture at each timepoint were identified as CD3+FoxP3+ and Zombie™ fixable viability dye excluding. (A) Number of viable Tregs at each timepoint. Significant changes versus culture input (d0) were determined using a one-way ANOVA. (B) The percent of ZombieTM+CD3+FoxP3+ cells in the non-debris FSC/SSC gate at each timepoint was calculated. The fold-increase in the percentage of non-viable cells at 96 hours compared to stimulation with 1 μg/ml condition is shown. Data represent the average±SEM. **p<0.005,***p<0.001, ****p<0.0001. ns=not significant.
Newborn Tregs show more robust increases in the level of ICOS following stimulation than their adult counterpart.
Our finding that the Tregs in the spleens of newborns had a higher percentage of ICOS+ cells led us to assess how the representation of this subpopulation changes following stimulation. These baseline data are replotted in Fig. 6 for ready comparison. Cultures stimulated as above were assessed for ICOS expression on Tregs at d2 and d4 of culture. The percentage of Tregs expressing ICOS did not change significantly in either adult or newborn Treg populations regardless of anti-CD3 level (Fig. 6A, upper panel). However, similar to the increase observed at d0, there was a significant increase in the percent ICOS-expressing cells in newborns on d2 (p=0.04) and d4 (p=0.005) when cells were stimulated with 1 μg/ml anti-CD3. For these analyses, an adult and newborn animal were analyzed in parallel in each experiment. To directly assess Treg populations present in the d4 cultures of cells from newborns versus adults, we calculated the ratio, here the percentage of ICOS+ cells, present in adult vs. newborn animals for each experiment. On average, there was a higher percentage of cells that were ICOS+ in newborns, similar to what was found at the start of culture (SF1).
Fig. 6. Tregs from newborns exhibit more robust increases in CD39 and ICOS following stimulation compared to Tregs from adults.

Splenocytes from newborns (4-5 days of age) and adults (9.6-12.8 years of age) were cultured in the presence of titrated amounts of immobilized anti-CD3 antibody together with 5 μg/ml anti-CD28 for 48 or 96 hours. Day 0 data are replotted here for ease of comparison. Viable Tregs in the culture at each timepoint were identified as CD3+FoxP3+ and Zombie™ fixable viability dye excluding. The percentage of cells expressing each marker and the average expression (MFI) of these markers on the positive population is shown (A) ICOS, (B) CD39, and (C) PD-1. Values represent the average±SEM. Significance was determined using a one-way ANOVA. *p<0.05,**p<0.005,***p<0.001, ****p<0.0001. ns=not significant.
In contrast we observed significant changes in the level of expression of this marker within the ICOS+ subset (Fig. 6A, lower panel). Compared to the d0 cells, a significant increase in ICOS level was observed for both newborn and adult cells at both days and with all three anti-CD3 concentrations. Not surprisingly, the level was highest following stimulation with the 1 μg/ml condition. The increase following stimulation resulted in a significantly higher level of ICOS in newborn versus adult Tregs on d2 (p=0.006) as well as d4 (p=0.002) of culture. These data suggest the ICOS level in both newborn and adult NHP Tregs is calibrated by the strength of TCR engagement and that newborns upregulate ICOS to a greater extent than adults following TCR engagement. Comparison of the relative expression in newborns versus adults at d4 showed that on average, the expression was higher on newborn Tregs (SF1).
Tregs from newborns exhibit more robust upregulation of CD39 following stimulation compared to their adult counterpart.
Our initial analyses of cells directly ex vivo showed a similar percentage of CD39+ cells in the two age groups, with a reduction in the level of CD39 on Tregs from newborns. As above, these baseline data are replotted in Fig. 6B for comparison. Following stimulation, adult Tregs showed no significant change in the percentage of cells expressing CD39 or the level of CD39 expressed by the positive cells (Fig. 6B). In contrast, at d4 there was an increase in CD39+ cells in the newborn Treg population as well as a significant increase in CD39 expression (Fig. 6B). In fact the level of CD39 at d4 following stimulation with the 1 μg/ml condition was significantly higher (1.5-fold, p=0.007) than that on adult Tregs (Fig. 6B and SF1). This is a striking finding given the significantly lower level of expression present on these cells at d0. Taking into account the function of this molecule, this result suggests newborn Tregs may have the potential for higher suppressive activity following activation. Finally, we evaluated PD-1 expression following in vitro stimulation. No significant changes in PD-1 were observed at any timepoint (Fig. 6C). As would be expected based on this finding, similar to what was observed at d0, on average newborns had a reduced level of expression of PD-1 relative to adults on d4 of culture (SF1).
DISCUSSION
Here we investigated Treg populations in newborn NHP to gain a fuller understanding of the baseline (in the absence of immune challenge) distribution, frequency, and phenotype of this immunoregulatory population as well as the response to activation. At present, our knowledge of these cells in newborns is relatively limited, derived predominantly from analysis of human cord blood cells or newborn mice. The NHP model used here is arguably the most representative of human newborns and in addition allows for assessment of Treg populations at multiple sites. Our studies show that FoxP3+ Tregs are consistently more highly represented in newborns compared to adults. An increased frequency of Tregs was observed in all sites evaluated-spleen, circulation, and lung. However, beyond this we found alterations in Treg populations between adult and newborn animals that were dependent on their location. Tregs in the spleen and circulation, but not lungs, of newborns expressed significantly higher levels of FoxP3 compared to their adult counterpart. Further, Tregs in the circulation of newborns were also much more likely to express PD-1, ICOS, and CD39 than their adult counterpart. The increases in FoxP3 and markers associated with inhibitory activity are consistent with the potential for enhanced suppressive activity of Tregs from newborns compared to adults (3, 33, 34).
The increased representation of Tregs in newborn NHP is consistent with what has been reported for humans (17–23). As with human, it is challenging to know how changes in the absolute number of T cells in newborns versus adults impacts the Treg frequency differences that we observed. However, we favor a model wherein the differences in production and differentiation of these cells are significant contributors. The enhanced propensity for newborn T cells to differentiate into Tregs has been reported (25) and it is thus likely that this is a contributor to the increased FoxP3+ cell frequency. In addition, in humans, the decline in Treg frequency begins prior to the time at which the decline occurs for naïve T cells (38). This suggests that changes in absolute number as cells transition from naïve to memory does not fully account for the observed decrease in frequency. Instead we propose this finding is consistent with changes in their production in the thymus. In this regard, peripheral Tregs have been reported to migrate back to the thymus where they suppress production of Tregs, but not conventional T cells (39), suggesting active regulation of their generation via a feedback loop.
A striking observation was the high representation of Tregs within the lungs of newborns. Tregs are known to populate the lungs at steady state following generation in the thymus, with additional cells recruited as a result of inflammation (for review see (40)). A number of studies support the importance of these cells in establishing tolerance to inhaled antigens (41, 42). Specifically FoxP3+ Tregs have been shown to mitigate Th2-mediated lung inflammation in several murine models of tolerance (43, 44). It is tempting to speculate that these cells are recruited to the lungs of newborns to assist in the establishment of tolerance given the constant exposure of this tissue to foreign antigens within the first few weeks of life.
Although Tregs in the lungs of newborns were significantly more plentiful and expressed higher levels of FoxP3, newborn and adult cells were relatively similar in expression of CD39, ICOS, and PD-1. This was the result of an increased percentage of Tregs that expressed CD39, ICOS and PD-1 in adults compared to Tregs in circulation or the spleen. The increased expression of PD-1 on lung versus splenic Tregs in adults is in agreement with a study evaluating these populations in a mouse model (45). The other two markers assessed here, ICOS and CD39, were not evaluated. However, in a separate study, analysis of adult murine Tregs revealed increased ICOS on lung versus splenic cells (7), consistent with our findings. In general, it was notable that this more activated phenotype was not restricted to the lung of newborns as it was present in PBMC and splenic Tregs. Why this is the case is not known, but could reflect generalized higher levels of inflammation present in newborns as a result of exposure to novel environmental entities following birth.
Our data show that CD39 is robustly upregulated on Tregs of newborns following TCR engagement, reaching levels that are 1.5-fold higher than adult-derived Tregs. This was striking given the significantly lower expression of this marker on a per cell basis in newborn Tregs at baseline, a finding noted in analyses performed on human cord blood Tregs (46). Our data show that CD39 is highly responsive to TCR engagement on newborn Tregs. Analysis of Tregs isolated from patients with rheumatoid arthritis revealed a correlation between CD39 levels and suppressive activity (47). This is not surprising given its function as an ectoenzyme that converts proinflammatory extracellular ATP to the immunosuppressive molecule adenosine (11). We propose that the higher CD39 induced following activation of newborn Tregs would allow for more robust generation of suppressive mediators.
ICOS likewise reached higher densities in newborns following activation in spite of starting out at similar levels in the two groups. A recent study showed that the presence of ICOS was directly correlated with FoxP3 demethylation and expression in murine Tregs (48). Thus, the increased frequency of cells expressing ICOS at baseline identified in our study may contribute to the higher FoxP3 expression we observed in newborn Tregs. The expression of ICOS by Tregs is also correlated with increased suppressive activity (49) as well as resistance to cell death (8). With regard to the latter, our results are somewhat unexpected in that the survival of Tregs from newborns is impaired in the absence of strong TCR engagement compared to adult-derived Tregs in spite of having a higher percentage of cells that express this marker. This finding is consistent with differential regulation of survival in Tregs from newborns versus adults, likely reflecting developmental changes in this population. CD28 has been previously reported as an important signal for Treg survival (50). As high level CD28 ligand (anti-CD28 agonist antibody) was present in our cultures, our data suggest that CD28 engagement alone is insufficient for survival of newborn Tregs. In humans, ICOS negative Tregs were strongly stimulated through anti-CD28, while ICOS-expressing Tregs proliferated more strongly to ICOS engagement (51). Thus it is possible that the higher abundance of ICOS- Tregs in adults allowed for increased utilization of anti-CD28 in the culture and thus better survival. Interestingly, as is the case for other T cell populations, Treg survival is dependent on tonic TCR signals (52, 53). The increased strength of TCR engagement required by newborn Tregs may be the result of impaired TCR signaling that has been reported in these T cells (54–56).
How might the differences in this immunoregulatory population impact the ability of the newborn to respond to infection? It seems likely that the increased representation of Tregs is a contributor to the lower T cell response that has been reported in newborn mice following virus infection (57, 58). In support of this, depletion of Tregs in this model resulted in an increased CD4+ and CD8+ T cell response following HSV infection (24). Interestingly, there are instances, where even in adults, the presence of Tregs is deleterious for optimal pathogen clearance, as was reported for Helicobacter pylori infection (59).
The increase in Tregs in the lungs of newborns is particularly intriguing in light of our previous findings showing that newborn AGM have highly reduced inducible BALT (iBALT) following influenza virus infection (60). This is accompanied by a lower antibody response in the respiratory tract. (60). Given the increased representation of Tregs and their high expression of markers associated with suppressive function, it is tempting to speculate that the presence of these cells contributes to the reduced immune response in the respiratory tract. In a weanling mouse model, high numbers of Tregs were found to inhibit iBALT formation (61). Interestingly, there is evidence that Tregs in the lung draining lymph node are also important contributors to the Treg-mediated negative regulation of iBALT formation (62). While we have not evaluated lymph nodes here, we would predict an increase in Tregs similar to what we observed in the spleen and circulation and as we previously found in the lung-draining lymph node following influenza virus infection of newborn AGM (60).
The significantly higher level of CD39 expression present on newborn compared to adult splenic Tregs following stimulation with anti-CD3 was striking given the considerably lower level present on newborn Tregs ex vivo. In addition to the higher level achieved in response to stimulation, a higher percentage of newborn Tregs in circulation were positive for CD39. This molecule is a critical contributor to the production of the immunosuppressive mediator adenosine through its role in conversion of ATP to AMP (63). It is tempting to postulate that the higher or more frequent expression of this molecule may contribute to the increased adenosine reported in the plasma of human infants (64).
In summary, we present here the first analysis of FoxP3+ Tregs in multiple sites in newborn versus adult NHP. Based on what is known, our data support the use of NHP as a model reflective of human newborns, as we find an increased representation of Tregs in newborn versus adult animals as has been reported for humans (17–23). Here we also show that the expression of inhibitory markers can vary across tissues and with age. This is a caution for studies of human peripheral blood cells, which are often used as a surrogate for what is present in the body. Finally, our data also demonstrate differences in survival and upregulation of molecules involved in suppression by Tregs. These data significantly advance our understanding of the regulation of splenic, circulating and tissue-resident newborn Tregs.
Supplementary Material
Key Points:
FoxP3+ cells are more highly represented in newborn spleen, lung, and circulation.
Newborn Tregs express higher levels of FoxP3 and CD25 compared to adults.
Newborn and adult Tregs differ in their survival and response to TCR engagement.
ACKNOWLEDGEMENTS
We thank members of the Alexander-Miller laboratory for helpful suggestions and review of the manuscript. We are appreciative of the staff at the Clarkson Primate Facility for care of the animals used in this study.
This work was supported by National Institutes of Health grants R01AI098339 (to M.A.A.-M.). The Vervet Research Colony is supported in part by P40 OD010965 (to J.R.K.). We acknowledge services provided by the Cell and Viral Vector Core, Synthetic Chemistry Core and Flow Cytometry Core Laboratories of the Wake Forest Comprehensive Cancer Center, supported in part by NCI P30 CA121291.
LITERATURE CITED
- 1.Chappert P, Leboeuf M, Rameau P, Lalfer M, Desbois S, Liblau RS, Danos O, Davoust JM, and Gross DA. 2010. Antigen-specific Treg impair CD8+ T-cell priming by blocking early T-cell expansion. Eur. J. Immunol. 40: 339–350. [DOI] [PubMed] [Google Scholar]
- 2.Haeryfar SM, DiPaolo RJ, Tscharke DC, Bennink JR, and Yewdell JW. 2005. Regulatory T cells suppress CD8+ T cell responses induced by direct priming and cross-priming and moderate immunodominance disparities. J. Immunol. 174: 3344–3351. [DOI] [PubMed] [Google Scholar]
- 3.Josefowicz SZ, Lu LF, and Rudensky AY. 2012. Regulatory T cells: mechanisms of differentiation and function. Annu. Rev. Immunol. 30: 531–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fontenot JD, Gavin MA, and Rudensky AY. 2003. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat. Immunol. 4: 330–336. [DOI] [PubMed] [Google Scholar]
- 5.Hori S, Nomura T, and Sakaguchi S. 2003. Control of regulatory T cell development by the transcription factor Foxp3. Science 299: 1057–1061. [DOI] [PubMed] [Google Scholar]
- 6.Chen Y, Shen S, Gorentla BK, Gao J, and Zhong XP. 2012. Murine regulatory T cells contain hyperproliferative and death-prone subsets with differential ICOS expression. J. Immunol. 188: 1698–1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Busse M, Krech M, Meyer-Bahlburg A, Hennig C, and Hansen G. 2012. ICOS mediates the generation and function of CD4+CD25+Foxp3+ regulatory T cells conveying respiratory tolerance. J. Immunol. 189: 1975–1982. [DOI] [PubMed] [Google Scholar]
- 8.Redpath SA, van der Werf N, Cervera AM, MacDonald AS, Gray D, Maizels RM, and Taylor MD. 2013. ICOS controls Foxp3+ regulatory T-cell expansion, maintenance and IL-10 production during helminth infection. Eur. J. Immunol. 43: 705–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zheng J, Chan PL, Liu Y, Qin G, Xiang Z, Lam KT, Lewis DB, Lau YL, and Tu W. 2013. ICOS regulates the generation and function of human CD4+ Treg in a CTLA-4 dependent manner. PLoS One 8: e82203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gianchecchi E, and Fierabracci A. 2018. Inhibitory receptors and pathways of lymphocytes: The role of PD-1 in Treg development and their involvement in autoimmunity onset and cancer progression. Front. Immunol. 9: 2374-2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Borsellino G, Kleinewietfeld M, Di Mitri D, Sternjak A, Diamantini A, Giometto R, Hopner S, Centonze D, Bernardi G, Dell'Acqua ML, Rossini PM, Battistini L, Rotzschke O, and Falk K. 2007. Expression of ectonucleotidase CD39 by Foxp3+ Treg cells: hydrolysis of extracellular ATP and immune suppression. Blood 110: 1225–1232. [DOI] [PubMed] [Google Scholar]
- 12.Wong FS, and Dayan CM. 2008. Regulatory T cells in autoimmune endocrine diseases. Trends Endocrinol Metab 19: 292–299. [DOI] [PubMed] [Google Scholar]
- 13.Ahlmanner F, Sundstrom P, Akeus P, Eklof J, Borjesson L, Gustavsson B, Lindskog EB, Raghavan S, and Quiding-Jarbrink M. 2018. CD39+ regulatory T cells accumulate in colon adenocarcinomas and display markers of increased suppressive function. Oncotarget 9: 36993–37007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen Q, Mo L, Cai X, Wei L, Xie Z, Li H, Li J, and Hu Z. 2018. ICOS signal facilitates Foxp3 transcription to favor suppressive function of regulatory T cells. Int. J. Med. Sci. 15: 666–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qureshi OS, Zheng Y, Nakamura K, Attridge K, Manzotti C, Schmidt EM, Baker J, Jeffery LE, Kaur S, Briggs Z, Hou TZ, Futter CE, Anderson G, Walker LS, and Sansom DM. 2011. Trans-endocytosis of CD80 and CD86: a molecular basis for the cell-extrinsic function of CTLA-4. Science 332: 600–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang S, Apasov S, Koshiba M, and Sitkovsky M. 1997. Role of A2a extracellular adenosine receptor-mediated signaling in adenosine-mediated inhibition of T-cell activation and expansion. Blood 90: 1600–1610. [PubMed] [Google Scholar]
- 17.Michaelsson J, Mold JE, McCune JM, and Nixon DF. 2006. Regulation of T cell responses in the developing human fetus. J. Immunol. 176: 5741–5748. [DOI] [PubMed] [Google Scholar]
- 18.Nettenstrom L, Alderson K, Raschke EE, Evans MD, Sondel PM, Olek S, and Seroogy CM. 2013. An optimized multi-parameter flow cytometry protocol for human T regulatory cell analysis on fresh and viably frozen cells, correlation with epigenetic analysis, and comparison of cord and adult blood. J. Immunol. Methods 387: 81–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Renno C, Nadaf MI, Zago CA, Carneiro-Sampaio M, and Palmeira P. 2016. Healthy preterm newborns show an increased frequency of CD4+ CD25high CD127low FOXP3+ regulatory T cells with a naive phenotype and high expression of gut-homing receptors. Scand. J. Immunol. 83: 445–455. [DOI] [PubMed] [Google Scholar]
- 20.Grindebacke H, Stenstad H, Quiding-Jarbrink M, Waldenstrom J, Adlerberth I, Wold AE, and Rudin A. 2009. Dynamic development of homing receptor expression and memory cell differentiation of infant CD4+CD25high regulatory T cells. J. Immunol. 183: 4360–4370. [DOI] [PubMed] [Google Scholar]
- 21.van Gent R, van Tilburg CM, Nibbelke EE, Otto SA, Gaiser JF, Janssens-Korpela PL, Sanders EA, Borghans JA, Wulffraat NM, Bierings MB, Bloem AC, and Tesselaar K. 2009. Refined characterization and reference values of the pediatric T- and B-cell compartments. Clin. Immunol. 133: 95–107. [DOI] [PubMed] [Google Scholar]
- 22.Prabhu SB, Rathore DK, Nair D, Chaudhary A, Raza S, Kanodia P, Sopory S, George A, Rath S, Bal V, Tripathi R, Ramji S, Batra A, Aggarwal KC, Chellani HK, Arya S, Agarwal N, Mehta U, Natchu UC, Wadhwa N, and Bhatnagar S. 2016. Comparison of human neonatal and adult blood leukocyte subset composition phenotypes. PLoS One 11: e0162242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hayakawa S, Ohno N, Okada S, and Kobayashi M. 2017. Significant augmentation of regulatory T cell numbers occurs during the early neonatal period. Clin Exp Immunol 190: 268–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fernandez MA, Puttur FK, Wang YM, Howden W, Alexander SI, and Jones CA. 2008. T regulatory cells contribute to the attenuated primary CD8+ and CD4+ T cell responses to herpes simplex virus type 2 in neonatal mice. J. Immunol. 180: 1556–1564. [DOI] [PubMed] [Google Scholar]
- 25.Wang G, Miyahara Y, Guo Z, Khattar M, Stepkowski SM, and Chen W. 2010. "Default" generation of neonatal regulatory T cells. J. Immunol. 185: 71–78. [DOI] [PubMed] [Google Scholar]
- 26.Ndure J, and Flanagan KL. 2014. Targeting regulatory T cells to improve vaccine immunogenicity in early life. Front. Microbiol. 5: 477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mold JE, Michaelsson J, Burt TD, Muench MO, Beckerman KP, Busch MP, Lee TH, Nixon DF, and McCune JM. 2008. Maternal alloantigens promote the development of tolerogenic fetal regulatory T cells in utero. Science 322: 1562–1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kamada N, and Nunez G. 2013. Role of the gut microbiota in the development and function of lymphoid cells. J. Immunol. 190: 1389–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wing K, Larsson P, Sandstrom K, Lundin SB, Suri-Payer E, and Rudin A. 2005. CD4+ CD25+ FOXP3+ regulatory T cells from human thymus and cord blood suppress antigen-specific T cell responses. Immunology 115: 516–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Godfrey WR, Spoden DJ, Ge YG, Baker SR, Liu B, Levine BL, June CH, Blazar BR, and Porter SB. 2005. Cord blood CD4+CD25+-derived T regulatory cell lines express FoxP3 protein and manifest potent suppressor function. Blood 105: 750–758. [DOI] [PubMed] [Google Scholar]
- 31.Olin A, Henckel E, Chen Y, Lakshmikanth T, Pou C, Mikes J, Gustafsson A, Bernhardsson AK, Zhang C, Bohlin K, and Brodin P. 2018. Stereotypic immune system development in newborn children. Cell 174: 1277-1292 e1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Beaumier CM, Harris LD, Goldstein S, Klatt NR, Whitted S, McGinty J, Apetrei C, Pandrea I, Hirsch VM, and Brenchley JM. 2009. CD4 downregulation by memory CD4+ T cells in vivo renders African green monkeys resistant to progressive SIVagm infection. Nat. Med. 15: 879–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chauhan SK, Saban DR, Lee HK, and Dana R. 2009. Levels of Foxp3 in regulatory T cells reflect their functional status in transplantation. J. Immunol. 182: 148–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thiruppathi M, Rowin J, Ganesh B, Sheng JR, Prabhakar BS, and Meriggioli MN. 2012. Impaired regulatory function in circulating CD4+CD25highCD127low/- T cells in patients with myasthenia gravis. Clin. Immunol. 145: 209–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Asano T, Meguri Y, Yoshioka T, Kishi Y, Iwamoto M, Nakamura M, Sando Y, Yagita H, Koreth J, Kim HT, Alyea EP, Armand P, Cutler CS, Ho VT, Antin JH, Soiffer RJ, Maeda Y, Tanimoto M, Ritz J, and Matsuoka KI. 2017. PD-1 modulates regulatory T-cell homeostasis during low-dose interleukin-2 therapy. Blood 129: 2186–2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wong M, La Cava A, and Hahn BH. 2013. Blockade of programmed death-1 in young (New Zealand Black x New Zealand White)F1 mice promotes the suppressive capacity of CD4+ regulatory T cells protecting from lupus-like disease. J. Immunol. 190: 5402–5410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kamada T, Togashi Y, Tay C, Ha D, Sasaki A, Nakamura Y, Sato E, Fukuoka S, Tada Y, Tanaka A, Morikawa H, Kawazoe A, Kinoshita T, Shitara K, Sakaguchi S, and Nishikawa H. 2019. PD-1+ regulatory T cells amplified by PD-1 blockade promote hyperprogression of cancer. Proc. Natl. Acad. Sci. U. S. A. 116: 9999–10008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thome JJ, Bickham KL, Ohmura Y, Kubota M, Matsuoka N, Gordon C, Granot T, Griesemer A, Lerner H, Kato T, and Farber DL. 2016. Early-life compartmentalization of human T cell differentiation and regulatory function in mucosal and lymphoid tissues. Nat. Med. 22: 72–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thiault N, Darrigues J, Adoue V, Gros M, Binet B, Perals C, Leobon B, Fazilleau N, Joffre OP, Robey EA, van Meerwijk JP, and Romagnoli P. 2015. Peripheral regulatory T lymphocytes recirculating to the thymus suppress the development of their precursors. Nat. Immunol. 16: 628–634. [DOI] [PubMed] [Google Scholar]
- 40.Mailloux AW, and Young MR. 2010. Regulatory T-cell trafficking: from thymic development to tumor-induced immune suppression. Crit. Rev. Immunol. 30: 435–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Soroosh P, Doherty TA, Duan W, Mehta AK, Choi H, Adams YF, Mikulski Z, Khorram N, Rosenthal P, Broide DH, and Croft M. 2013. Lung-resident tissue macrophages generate Foxp3+ regulatory T cells and promote airway tolerance. J. Exp. Med. 210: 775–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Duan W, and Croft M. 2014. Control of regulatory T cells and airway tolerance by lung macrophages and dendritic cells. Ann. Am. Thorac. Soc. 11 Suppl 5: S306–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Duan W, So T, and Croft M. 2008. Antagonism of airway tolerance by endotoxin/lipopolysaccharide through promoting OX40L and suppressing antigen-specific Foxp3+ T regulatory cells. J. Immunol. 181: 8650–8659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Josefowicz SZ, Niec RE, Kim HY, Treuting P, Chinen T, Zheng Y, Umetsu DT, and Rudensky AY. 2012. Extrathymically generated regulatory T cells control mucosal TH2 inflammation. Nature 482: 395–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Arpaia N, Green JA, Moltedo B, Arvey A, Hemmers S, Yuan S, Treuting PM, and Rudensky AY. 2015. A distinct function of regulatory T cells in tissue protection. Cell 162: 1078–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zahran AM, Saad K, Abdel-Raheem YF, Elsayh KI, El-Houfey AA, Aboul-Khair MD, and Alblihed MA. 2019. Characterization of regulatory T Cells in preterm and term infants. Arch. Immunol. Ther. Exp. (Warsz.) 67: 49–54. [DOI] [PubMed] [Google Scholar]
- 47.Peres RS, Liew FY, Talbot J, Carregaro V, Oliveira RD, Almeida SL, Franca RF, Donate PB, Pinto LG, Ferreira FI, Costa DL, Demarque DP, Gouvea DR, Lopes NP, Queiroz RH, Silva JS, Figueiredo F, Alves-Filho JC, Cunha TM, Ferreira SH, Louzada-Junior P, and Cunha FQ. 2015. Low expression of CD39 on regulatory T cells as a biomarker for resistance to methotrexate therapy in rheumatoid arthritis. Proc. Natl. Acad. Sci. U. S. A. 112: 2509–2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Landuyt AE, Klocke BJ, Colvin TB, Schoeb TR, and Maynard CL. 2019. Cutting Edge: ICOS-deficient regulatory T cells display normal induction of Il10 but readily downregulate expression of Foxp3. J. Immunol. 202: 1039–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kornete M, Sgouroudis E, and Piccirillo CA. 2012. ICOS-dependent homeostasis and function of Foxp3+ regulatory T cells in islets of nonobese diabetic mice. J. Immunol. 188: 1064–1074. [DOI] [PubMed] [Google Scholar]
- 50.Myers DR, Zikherman J, and Roose JP. 2017. Tonic signals: Why do lymphocytes bother? Trends Immunol. 38: 844–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mohr A, Malhotra R, Mayer G, Gorochov G, and Miyara M. 2018. Human FOXP3+ T regulatory cell heterogeneity. Clin Transl Immunology 7: e1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Levine AG, Arvey A, Jin W, and Rudensky AY. 2014. Continuous requirement for the TCR in regulatory T cell function. Nat. Immunol. 15: 1070–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vahl JC, Drees C, Heger K, Heink S, Fischer JC, Nedjic J, Ohkura N, Morikawa H, Poeck H, Schallenberg S, Riess D, Hein MY, Buch T, Polic B, Schonle A, Zeiser R, Schmitt-Graff A, Kretschmer K, Klein L, Korn T, Sakaguchi S, and Schmidt-Supprian M. 2014. Continuous T cell receptor signals maintain a functional regulatory T cell pool. Immunity 41: 722–736. [DOI] [PubMed] [Google Scholar]
- 54.Miscia S, Di Baldassarre A, Sabatino G, Bonvini E, Rana RA, Vitale M, Di Valerio V, and Manzoli FA. 1999. Inefficient phospholipase C activation and reduced Lck expression characterize the signaling defect of umbilical cord T lymphocytes. J. Immunol. 163: 2416–2424. [PubMed] [Google Scholar]
- 55.Palin AC, Ramachandran V, Acharya S, and Lewis DB. 2013. Human neonatal naive CD4+ T cells have enhanced activation-dependent signaling regulated by the microRNA miR-181a. J. Immunol. 190: 2682–2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Basha S, Surendran N, and Pichichero M. 2014. Immune responses in neonates. Expert Rev. Clin. Immunol. 10: 1171–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.You D, Ripple M, Balakrishna S, Troxclair D, Sandquist D, Ding L, Ahlert TA, and Cormier SA. 2008. Inchoate CD8+ T cell responses in neonatal mice permit influenza-induced persistent pulmonary dysfunction. J. Immunol. 181: 3486–3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Evans IA, and Jones CA. 2005. HSV induces an early primary Th1 CD4 T cell response in neonatal mice, but reduced CTL activity at the time of the peak adult response. Eur. J. Immunol. 35: 1454–1462. [DOI] [PubMed] [Google Scholar]
- 59.Kao JY, Zhang M, Miller MJ, Mills JC, Wang B, Liu M, Eaton KA, Zou W, Berndt BE, Cole TS, Takeuchi T, Owyang SY, and Luther J. 2010. Helicobacter pylori immune escape is mediated by dendritic cell-induced Treg skewing and Th17 suppression in mice. Gastroenterology 138: 1046–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Holbrook BC, Hayward SL, Blevins LK, Kock N, Aycock T, Parks GD, and Alexander-Miller MA. 2015. Nonhuman primate infants have an impaired respiratory but not systemic IgG antibody response following influenza virus infection. Virology 476: 124–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Foo SY, Zhang V, Lalwani A, Lynch JP, Zhuang A, Lam CE, Foster PS, King C, Steptoe RJ, Mazzone SB, Sly PD, and Phipps S. 2015. Regulatory T cells prevent inducible BALT formation by dampening neutrophilic inflammation. J. Immunol. 194: 4567–4576. [DOI] [PubMed] [Google Scholar]
- 62.Foo SY, and Phipps S. 2010. Regulation of inducible BALT formation and contribution to immunity and pathology. Mucosal Immunol. 3: 537–544. [DOI] [PubMed] [Google Scholar]
- 63.Zhao H, Bo C, Kang Y, and Li H. 2017. What else can CD39 tell us? Front. Immunol. 8: 727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Levy O, Coughlin M, Cronstein BN, Roy RM, Desai A, and Wessels MR. 2006. The adenosine system selectively inhibits TLR-mediated TNF-alpha production in the human newborn. J. Immunol. 177: 1956–1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


