SUMMARY
Cardiac disease remains the leading causes of morbidity and mortality worldwide. The β1-adrenergic receptor (β1-AR) is a major regulator of cardiac functions and is down-regulated in the majority of heart failure cases. A key physiological process is the activation of heterotrimeric G-protein Gs by β1-ARs, leading to increased heart rate and contractility. Here we use cryo-electron microscopy and functional studies to investigate the molecular mechanism by which β1-AR activates Gs. We find that the tilting of α5-helix breaks an ionic lock between the sidechain of His373 in the C-terminal α5-helix and the backbone carbonyl of Arg38 in the N-terminal αN-helix of Gαs. Together with the disruption of another interacting network involving Gln59 in the α1-helix, Ala352 in the β6-α5 loop, and Thr355 in the α5-helix, these conformational changes might lead to the deformation of the GDP-binding pocket. Our data provide molecular insights into the activation of G-proteins by G-protein-coupled receptors.
Graphical Abstract
eTOC Blurb:
Su et al. report the cryo-EM structure of the complex of isoproterenol-bound β1-adrenergic receptor and heterotrimeric Gs-protein. The structural and functional studies reveal insights into the activation of Gs by β1-adrenergic receptor. This work advances our understanding of the control of heart rate and contractility by the nervous system and hormones.
INTRODUCTION
A structurally diverse repertoire of ligands elicit their physiological functions by activating G-protein-coupled receptors (GPCRs) (Fredriksson et al., 2003; Rosenbaum et al., 2009; Sakmar, 2002; Strange, 2008; Weis and Kobilka, 2018). GPCRs comprise a large and diverse superfamily of transmembrane proteins, and family members have been identified in organisms as evolutionarily distant as yeast and human. Critically, GPCRs constitute the protein class that has been most successfully targeted by drugs, and accordingly are the focus of intense mechanistic study (Strange, 2008). Canonically, GPCRs signal directly to heterotrimeric G-proteins which in turn relay the signals to downstream pathways (Bourne et al., 1990; Gilman, 1987; Simon et al., 1991). These G-proteins are composed of Gα, Gβ, and Gγ subunits, with the Gβ and Gγ subunits tightly associating such that they can be regarded as one functional unit (Gβγ). G-proteins function as molecular binary switches with their biological activity determined by the bound nucleotide (Lappano and Maggiolini, 2012; Oldham and Hamm, 2008; Sprang, 1997). Activated GPCRs function as a guanine-nucleotide exchange factor (GEF), promoting the release of GDP bound on the Gα subunit of G-proteins and creating the thermally labile, transition state of Gα without a bound nucleotide (Bourne, 1997). The subsequent binding of GTP leads to the dissociation of the Gα subunit from the Gβγ dimer resulting in two functional subunits (Gα and Gβγ). Both the Gα and Gβγ subunits signal to various cellular pathways. Based on the sequence and functional homologies, G-protein heterotrimers are categorized into four families: Gs, Gi, Gq, and G12/13 (Simon et al., 1991). The molecular mechanisms by which GPCRs activate these G-proteins are incompletely understood.
The β1-adrenergic receptor (β1-AR) is a member of the GPCR family. In the adult human heart, β1-AR is the predominantly expressed β-AR isoform (70~85%) (Benovic, 2002; Post et al., 1999). The receptor binds and is activated by the catecholamines, norepinephrine and epinephrine, which triggers Gs-protein activation and increased cardiac cAMP levels. These molecular events manifest physiologically as increased heart rate, increased conduction, reduced refractoriness within the atrioventricular node, increased contractility and increased cardiac output (Lohse et al., 2003). Down-regulation of β1-ARs has been described in most cases of heart failure which is one of the main causes of mortality in the developed world (Lohse et al., 2003). Inhibitors of β-ARs (beta-blockers) are used to treat high blood pressure and heart failure, to manage abnormal heart rhythms, and to protect against myocardial infarction (Frishman, 2008). The molecular mechanism by which β1-AR catalyzes the guanine-nucleotide exchange on Gs, thus activating Gs, is not completely clear. Here we use cryo-electron microscopy and functional studies to investigate the activation of Gs by β1-AR. We find that, during its activation by isoproterenol-bound β1-AR, the α-helical domain of Gs rotates away from its Ras-like domain. The rotational opening of the α-helical domain is by ~96° and the distance between mass centers is ~38 Å. These rotation angle and translational distance are different from those observed in the crystal structure of the BI-167107 (a high affinity agonist)-bound β2-AR–Gs complex. This α-helical domain rotation, together with the structural rearrangements (including the tilting) in the C-terminal α5-helix and the GDP-binding pocket, result in the GDP release. These results provide structural insights into the activation of Gs by β1-AR.
RESULTS AND DISCUSSION
Molecular recognition of Gs by β1-AR
To understand the molecular mechanism by which β1-AR activates Gs, we first investigated how β1-AR recognizes Gs during the activation process. We solved the cryo-EM structure of the complex of isoproterenol-bound β1-AR and Gs, at an overall resolution of 2.6 Å (Figure 1, Figures S1 and S2, Table 1). As revealed by this structure, β1-AR recognizes both Gαs (1049 Å2 buried area) and Gβ (153 Å2 buried area), yielding a large interaction surface area (1202 Å2) (Figures 1 and 2, Figure S3). On β1-AR, the interacting elements involve transmembrane domain (TM) 3, TM5, TM6, and intracellular loop (ICL) 2 (Figure 2 A-D, Figure S4). On Gαs, the N-terminal αN-helix and its structurally adjacent regions (the αN-β1 loop and the β2-β3 loop), as well as the C-terminal α5-helix are interacting with β1-AR (Figure 2 A-D, Figure S4).
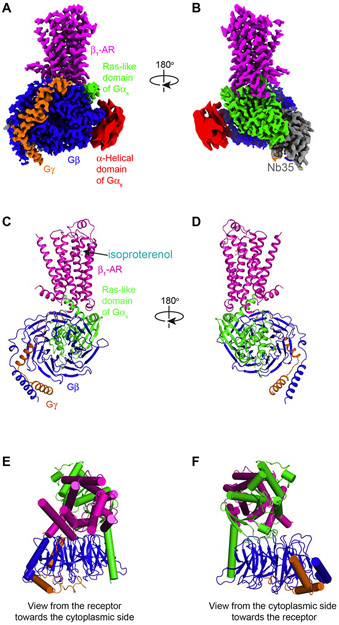
Figure 1. Overall architecture of the structure of the isoproterenol–β1-AR–Gs complex.
(A,B) Orthogonal views of the cryo-EM density map of the isoproterenol–β1-AR–Gs complex colored by subunits (β1-AR in magenta, Ras-like GTPase domain of Gαs in green, α-helical domain of Gαs in red (low-pass filtered to 6 Å for better presentation), Gβ in blue, Gγ in orange, and Nb35 in gray). (C,D) Cartoon diagrams of the isoproterenol–β1-AR–Gs complex are shown without Nb35 and the α-helical domain of Gαs. Color schemes are the same as in A and B. (E,F) Extracellular and cytoplasmic views of the isoproterenol–β1-AR–Gs complex.
Table 1.
Cryo-EM data collection, refinement and validation statistics
| Data collection and processing | |
|---|---|
| Magnification | 22,500 |
| Voltage (kV) | 300 |
| Electron exposure (e-/Å2) | 46 |
| Defocus range (μm) | −1.0 to −2.3 |
| Pixel size (Å) | 1.064 |
| Symmetry imposed | C1 |
| Initial particle images (no.) | 1,498,944 |
| Final particle images (no.) | 452,312 |
| Map resolution (Å) (Full /G-protein | |
| focus /β1-AR focus) | 2.58/2.58/2.56 |
| FSC threshold | 0.143 |
| Refinement | |
| Model resolution (Å) | 2.32/2.68 |
| FSC threshold | 0.143/0.50 |
| Map sharpening B factor (Å2) | −80 |
| Model composition | |
| Non-hydrogen atoms | 7902 |
| Protein residues | 1004 |
| Ligands | 1 |
| B factors (Å2) | 54.0 |
| R.m.s. deviations | |
| Bond lengths (Å) | 0.002 |
| Bond angles (°) | 0.455 |
| Validation | |
| MolProbity score | 1.27 |
| Clashscore | 2.81 |
| Poor rotamers (%) | 1.78 |
| Ramachandran plot | |
| Favored (%) | 97.96 |
| Allowed (%) | 2.04 |
| Disallowed (%) | 0.00 |
Figure 2. Molecular recognition of Gs by β1-AR.
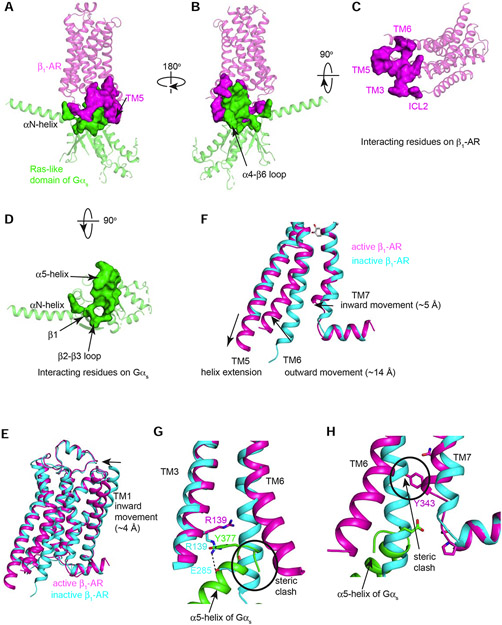
(A-D) Different views of the interaction surface areas between β1-AR (in magenta) and the Ras-like GTPase domain of Gαs (in green) are shown. (E-H) Comparison of the conformations of β1-AR in the active state (magenta) as seen in the β1-AR–Gs complex and with that of β1-AR in the inactive state (PDB: 4GPO) (cyan). (E) The overall alignment of the inactive β1-AR and the active β1-AR. (F) Major conformational changes in TM5, TM6 and TM7. (G and H) Conformational changes in the conserved D(E)RY motif on TM3 (G) and the conserved NPxxY motif on TM7 (H).
We first explored how β1-AR undergoes structural changes to accommodate Gs binding, and then the structural changes on Gs upon β1-AR interaction and during its activation process. Since the Gs interacting surface is on the cytoplasmic side of β1-AR we focused on the structural changes of β1-AR on its cytoplasmic side upon Gs binding (Figure 2 E-H). In the β1-AR–Gs complex with the full agonist isoproterenol, β1-AR adopts an active state conformation (Figure 2E). For comparative analysis between active and inactive state models, we used our new cryo-EM active state structure, and our previously reported β1-AR inactive state structure (PDB: 4GPO) (Huang et al., 2013) (Figure 2 E-H). We note that this inactive state structure is globally similar to other reported structures resolved in the same functional state (Warne et al., 2008). Comparison of the models reveals salient conformational differences (Figure 2 E-H). The overall root-mean-square deviation between the structures of β1-AR in the active and inactive states is ~3 Å over 276 Cα atoms. The largest structural changes upon Gs engagement occur in the cytoplasmic side of β1-AR, with an outward rotation of TM6 by ~14 Å (measured at the Cα of Glu285), a helix extension in TM5, and an inward ~5 Å movement of TM7 (measured at the Cα of Tyr343) (Figure 2F).
Gs-bound active β1-AR undergoes critical conformational changes in the conserved D(E)RY motif on TM3 and the conserved NPxxY motif on TM7 to recognize Gs (Figure 2 G and H). In the inactive β1-AR structure, Arg139 within the D(E)RY motif forms a salt bridge (or the ionic lock) with Glu285 (on TM6) (Figure 2G). This salt bridge stabilizes the inactive state of family A GPCRs, although it is absent in the structure of the inactive state of β2-AR (likely due to the high basal activity of β2-AR) (Cherezov et al., 2007). In the active state of β1-AR, this ionic lock is broken, and the C-terminal end of α5-helix of Gαs occupies the space originally occupied by Glu285 in the inactive state (Figure 2G). The new position for Glu285 in the active state is ~14 Å outwards (Figure 2G). Arg139 (in TM3) now forms a packing interaction with Tyr377 in the α5-helix of Gαs (Figure 2G). Furthermore, TM7 rotates around the conserved motif NPxxY (Figure 2H). This moves Tyr343 toward the position that was occupied by TM6 in the inactive structure (Figure 2H). Also, the last turn of the helix in TM7 in the inactive structure unravels in the active β1-AR, and TM7 has a small inward movement (Figure 2H). Therefore, β1-AR recognizes Gs by forming extensive interactions with Gs. Reciprocally, Gs binding stabilizes the structural changes in the active β1-AR by moving into places originally occupied by the inactive β1-AR.
Structural rearrangements of the C-terminal α5-helix of Gαs.
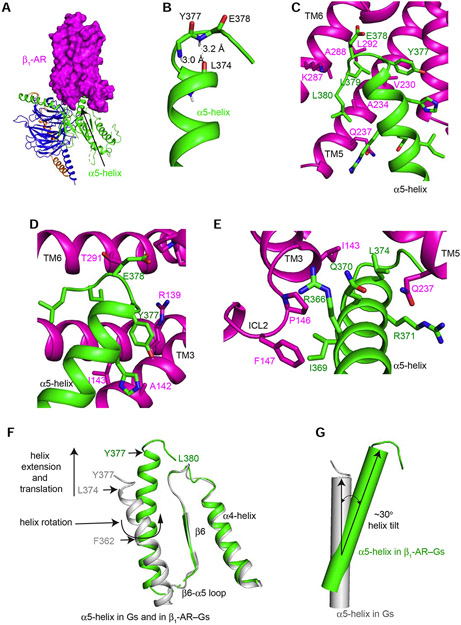
After examining how β1-AR undergoes structural changes to recognize Gs during Gs activation by β1-AR, we next explored the structural changes on Gs after its interaction with β1-AR. Both the N-terminal αN-helix and the C-terminal α5-helix of Gαs are critically involved in interacting with β1-AR (Figures 1 and 2). We examined the C-terminal α5-helix first since it contributes most of the interacting buried surface, suggesting that interactions in this region provide the major binding energy for the formation of the complex (Figure 3A). β1-AR primarily uses the surface formed by TM3, TM5, TM6, and ICL2 to interact with Gs (Figure 3A). This surface resembles a saddle that cradles the C-terminal α5-helix of the Ras-like domain of Gαs (Figure 3A). The C-terminal tail of the α5-helix is critical for GPCR–G-protein coupling specificity, and replacement of the last four amino acid residues of Gαq by Gαi enabled Gq to couple to an otherwise Gi-coupled GPCR (Conklin and Bourne, 1993; Conklin et al., 1993). The last four amino acids (Tyr377 to Leu380) of Gαs form a C-terminal αL capping motif which stabilizes helix ends, prevents helix fraying and imposing a substantial restriction on the set of allowed conformations (Aurora and Rose, 1998; Aurora et al., 1994)(Figure 3B). This capping motif interacts extensively with the cytoplasmic ends of TM3 and TM6 (Figure 3 C-E).
Figure 3. Structural rearrangements of the α5-helix of Gαs upon binding of β1-AR.
(A) β1-AR uses its cytoplasmic surface like a saddle to cradle the C-terminal α5-helix of the Ras-like domain of Gαs. (B) The last 4 amino acids (Tyr377 to Leu380) of α5-helix form a C-terminal αL capping motif with intra-chain interactions. (C and D) Interactions between β1-AR and the C-terminal tail loop of the α5-helix of Gαs. (E) Interactions between the middle of the α5-helix of Gαs and β1-AR. (F) Structural comparison of the α5-helix of Gαs from the β1-AR–Gs complex (colored in green) and from Gαs-GTPγS (colored in gray). (G) Tilting of the α5-helix of Gαs from Gαs-GTPγS (colored in gray) to the β1-AR–Gs complex (colored in green).
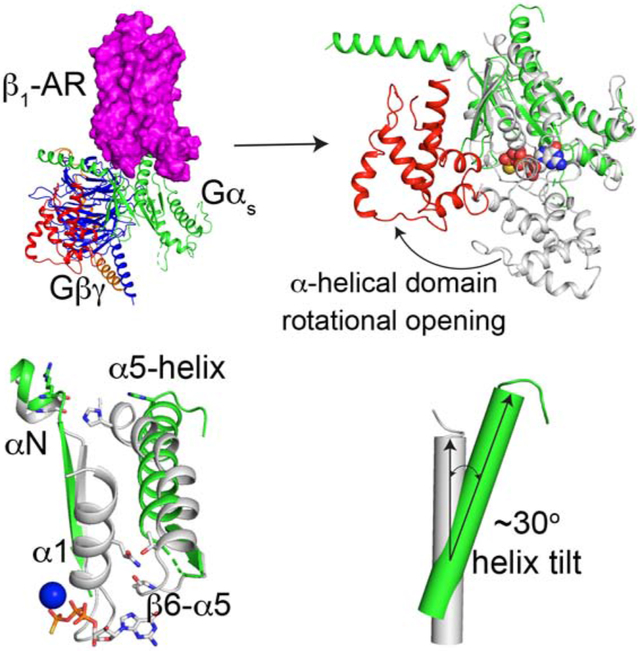
The comparison of the α5-helix in the complex of β1-AR–Gs and in the Gαs alone (Gαs:GTPγS) structure determined by X-ray crystallography (PDB: 1AZT) reveals significant structural rearrangements of the α5-helix during Gs activation by β1-AR (Figure 3 F and G). In Gαs alone, the last 3 amino acid residues (E378 to L380) of the C-terminal capping motif of α5-helix were disordered and unresolved in the structure (Figure 3F). In the β1-AR–Gs complex, residues L374 to Q376 form a helix extension and interact extensively with β1-AR (Figure 3 C-E). These additional helix extension and translation extend α5-helix by ~6 Å. Furthermore, α5-helix undergoes a rotation around Phe362 (Figure 3F). In addition, there is a tilting by ~30° of the α5-helix from its position in Gαs alone to the position in the β1-AR–Gs complex (Figure 3 F and G). The helix tilting, together with the helix extension and rotation, might provide α5-helix as a molecular force buffer transducing β1-AR signal to the GDP/GTP-binding pocket. Computational simulations indicate that α5-helix conformation changes are mainly associated with GDP release (Dror et al., 2015). Hence the structural rearrangements of the C-terminal α5-helix of Gαs upon β1-AR binding are critical to the guanine-nucleotide exchange on Gs.
Rotational opening of α-helical domain of Gs
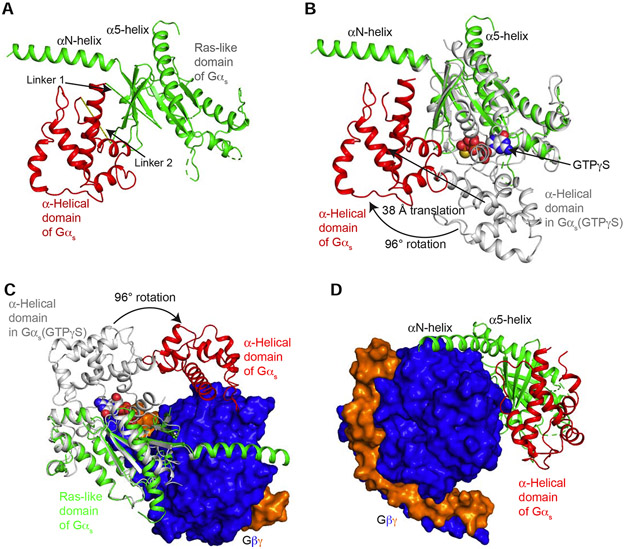
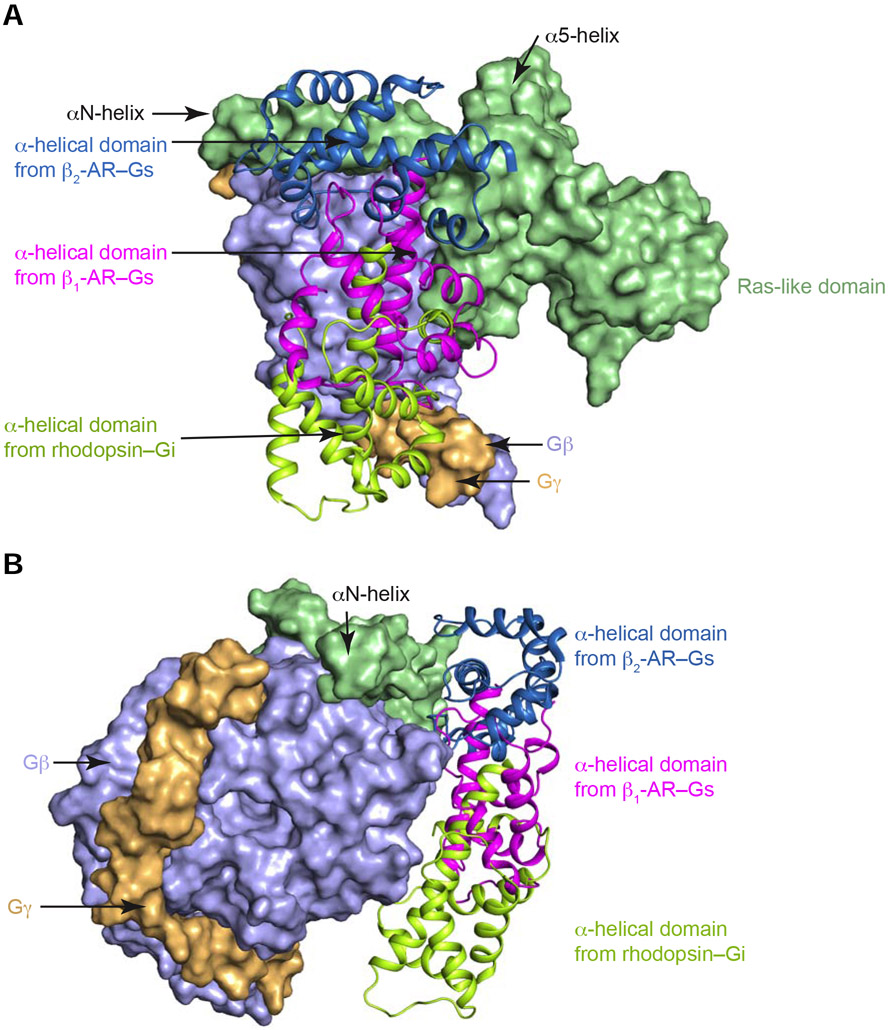
Gα subunits consist of two domains: a Ras-like GTPase domain and an α-helical domain (Dohlman and Jones, 2012; Sprang et al., 2007) (Figure 4 A and B). These two domains are connected by Linkers 1 and 2. Between these two domains lies a deep cleft within which GDP or GTP is tightly bound. The nucleotide is essentially occluded from the bulk solvent (Coleman et al., 1994; Noel et al., 1993). Comparing the conformation of Gαs in our β1-AR–Gs complex structure with the crystal structure of Gαs alone (PDB: 1AZT) (Sunahara et al., 1997) (Figure 4B), the principal change is a large rotation of the α-helical domain by ~96° (Figure 4, A and B). The distance between mass centers is ~38 Å (Figure 4B). The maximum rotation was limited by the presence of Gβγ, and the rotated α-helical domain was in contact with Gβγ and could not rotate any further (Figure 4 C and D). Hence, the observed location of the α-helical domain in the β1-AR–Gs complex likely represents the fully open state (Figure 4, C and D). From all the reported structures of the complexes of GPCR–G-protein trimers, only two structures include the α-helical domains of Gα subunits (Draper-Joyce et al., 2018; Kang et al., 2018; Koehl et al., 2018; Liang et al., 2018; Liang et al., 2017; Rasmussen et al., 2011; Zhang et al., 2017). The positions of the α-helical domains in these two structures are different from that observed in the β1-AR–Gs structure reported here (Figure 5). A comparison between the isoproterenol-bound β1-AR–Gs and the crystal structure of BI-167107-bound β2-AR–Gs (PDB: 3SN6) reveals that, in β2-AR–Gs, the α-helical domain appears to rotate farther towards the receptor and the membrane (Figure 5 A and B). This difference might be due to the crystal lattice contact in the crystal structure of β2-AR–Gs (Hilger et al., 2018). In the constitutively active rhodopsin–Gi structure, the α-helical domain is also in a different position from the α-helical domain in β1-AR–Gs; this might be due to the different G-proteins used (Gs vs. Gi) or the utilization of an antibody to bind and stabilize the α-helical domain and Gβ simultaneously in the rhodopsin–Gi structure (Kang et al., 2018) (Figure 5 A and B). It is worth noting that the relatively weak density for the α-helical domain in the EM map suggests the dynamic nature of the α-helical domain. Hence, the structural data point to the rotational opening of the α-helical domain during G-protein activation that creates an egress route for GDP.
Figure 4. Rotational opening of the α-helical domain of Gαs during its activation by β1-AR.
(A) Structure of Gαs in the complex of β1-AR–Gs shows the open rotation of the α-helical domain from the Ras-like domain. (B) Comparison of the structures of Gαs in the complex of β1-AR–Gs (in green and red) and in the Gαs:GTPγS crystal structure (in gray). (C) View from the receptor towards the cytoplasmic end shows the rotation of the α-helical domain from the position in the Gαs:GTPγS crystal structure (in gray) to the location in the β1-AR–Gs complex (in red). (D) View from Gβγ towards the Ras-like domain shows the position of the α-helical domain relative to Gβ.
Figure 5. Comparison of the locations of the α-helical domains from the structures of the complexes of BI-167107-activated β2-AR–Gs (in skyblue), isoproterenol-activated β1-AR–Gs (in magenta), and constitutively active rhodopsin–Gi (in limon).
The Ras-like domains (in light green), Gβ (in light blue) and Gγ (in light orange) from the three complexes were superimposed and presented in surface diagram. A and B show different views.
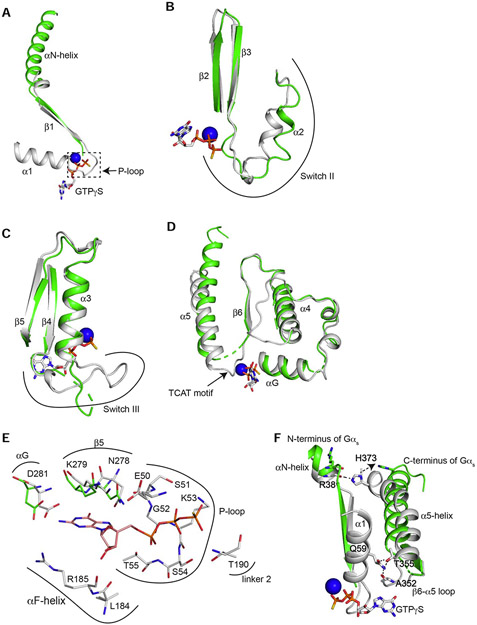
Deformation of the GDP-binding pocket
In the β1-AR–Gs complex, the GDP/GTP binding pocket is conformationally deformed, and the pocket is empty without GDP. Relative to the Ras-like domain in the structure of Gαs:GTPγS, most of the conformational changes of the Ras-like domain in the complex of β1-AR–Gs occur surrounding the GDP/GTP-binding pocket, while leaving the remainder of the Ras-like domain largely unperturbed (Figure 6 A-E). The modified regions include the P-loop (the β1-α1 loop, involved in binding of the diphosphate of the guanine nucleotide) (Figure 6 A), the TCAT motif (the β6-α5 loop, involved in the coordination of the purine ring of the bound nucleotide) (Figure 6D), and the Switch II and III regions (Figure 6 B and C). The cryo-EM map density for these modified regions is poor, indicating dynamic conformations. The binding between the β-phosphate of GDP and the P-loop is critical since GMP binds much weaker (~106-fold lower affinity) than GDP (John et al., 1990). In fact, GEFs for Ras-superfamily of GTPases promote GDP release by disrupting the interaction between the β-phosphate of GDP and the P-loop (Bos et al., 2007). In Gαs:GTPγS, residues from the P-loop (including Glu50, Ser51, Gly52, Lys53, and Ser54) interact with the β-phosphate (Figure 6E). In the complex of β1-AR–Gs, this region is disordered (Figure 6A). The disruption of this P-loop would markedly reduce the GDP binding. Hence β1-AR likely promotes GDP release by disrupting the interaction between the β-phosphate of GDP and the P-loop. Furthermore, Thr190 in Linker 2 is involved in binding of the γ-phosphate of GTP (Figure 6E). In the β1-AR–Gs structure, Linker 2 is disordered (Figure 4A). Additionally, Leu184 and Arg185 in αF-helix (part of the α-helical domain) interact with the pentose sugar in Gαs:GTPγS, but move away as part of the α-helical domain during the rotational opening in the β1-AR–Gs complex (Figure 6E, Figure 4A). Linker 2 and αF-helix are essential for GDP/GTP binding, and are also required to stabilize the GTP binding after GDP/GTP exchange, and to coordinate the γ-phosphate binding (Lambright et al., 1996; Wall et al., 1995). Therefore, the conformation of the GDP-binding pocket in β1-AR–Gs complex is modified with the effect of weakening the interaction between GDP and Gαs. This provides a structural basis for the release of GDP.
Figure 6. Conformational changes of the GDP/GTP-binding pocket after β1-AR interaction.
(A) Comparison of the β1 strand, α1-helix and the β1-α1 loop of the Ras-like domains from β1-AR–Gs (in green) and from Gαs:GTPγS (in gray) when the Ras-like domains are superimposed. (B) Comparison of Switch II region from β1-AR–Gs and from Gαs:GTPγS. (C) Comparison of Switch III region from β1-AR–Gs and from Gαs:GTPγS. (D) Comparison of the regions from αG to α5-helix from β1-AR–Gs and from Gαs:GTPγS. (E) Comparison of all GTP-interacting residues of the Ras-like domains from β1-AR–Gs and from Gαs:GTPγS. (F) Disruptions of intramolecular interactions of Gαs during Gs activation by β1-AR. An ionic lock between the sidechain of His373 in the α5-helix and the backbone carbonyl of Arg38 in the αN-helix is broken. An interacting network involving the sidechain of Gln59 in the α1-helix, the backbone carbonyl of Ala352 in the β6-α5 loop, and the sidechain of Thr355 in the α5-helix is disrupted.
Moreover, the interacting network between the N-terminal part of Gαs and the C-terminal part of Gαs observed in the structure of Gαs:GTPγS is broken in the structure of the β1-AR–Gs complex (Figure 6F). In the Gαs:GTPγS, there is an ionic lock between the sidechain of His373 in the α5-helix and the backbone carbonyl of Arg38 in the αN-helix (Figure 6F). This contact would tighten together the N- and C-terminal ends of Gαs. In the complex of β1-AR–Gs, the tilting and translation of the α5-helix move His373 away and break this ionic lock (Figure 6F). Moreover, there is another interacting network involving Gln59 in the α1-helix in the structure of Gαs alone (Figure 6F). The sidechain of Gln59 forms a hydrogen bond with the backbone carbonyl of Ala352 in the β6-α5 loop, as well as interacts with the sidechain of Thr355 in the α5-helix (Figure 6F). In the complex of β1-AR–Gs, this contacting network is broken, leading to the disordering of the α1-helix, the P-loop and the TCAT motif (Figure 6F). As these regions contribute to the majority of the binding of GDP (Figure 6 A, D and E) , the disruption of these regions would certainly lead to GDP release.
Functional studies of the activation of Gs by β1-AR
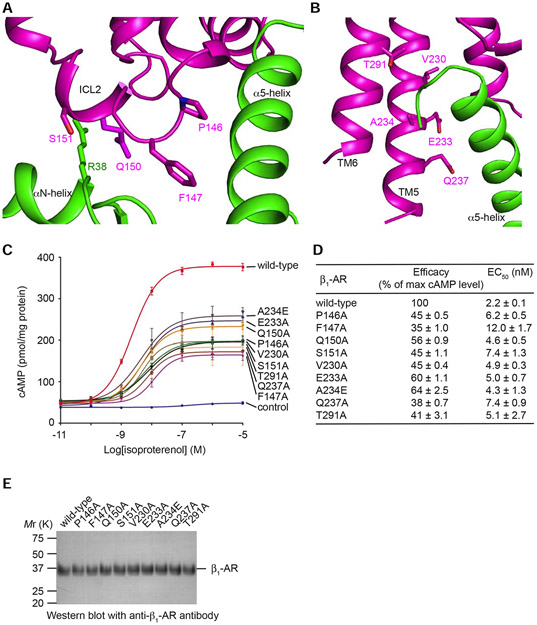
From the structure of β1-AR–Gs complex, there are two principle routes from β1-AR to the GDP/GTP-binding pocket (Figures 1 and 2). One route is through the C-terminal α5-helix of Gαs and the β6–α5 loop which engages the guanine ring (Figure 6D). The role of α5-helix in G-protein activation by GPCRs has been well documented (Hilger et al., 2018). The second route is through ICL2 of β1-AR which interacts with the N-terminal αN-helix (Figure 7A). This signal is transmitted through β1 to the P-loop which coordinates the β-phosphate of the guanine nucleotide (Figure 6A). From the β1-AR–Gs structure, Arg38 in the N-terminal αN-helix of Gαs interacts with Gln150 in ICL2 of β1-AR (Figure 7A). This interaction stabilizes a helical conformation of ICL2, which positions Pro146 and Phe147 in ICL2 to form hydrophobic interactions with Ile369, Arg366, and Phe362 in α5-helix, Val203 in the β2-β3 loop, and His41 in the αN-β1 loop of Gαs (Figure S4). For Gαi1, the αN-β1 loop was shown to be critical for the nucleotide-exchange catalysis (Herrmann et al., 2006). We have performed functional studies of residues on β1-AR that interact with Gαs based on our cryo-EM structure of the complex of β1-AR and Gs. As shown in Figure 7 A and B, residues Pro146, Phe147, and Gln150 in ICL2 of β1-AR are involved in interacting with Gαs. Val230, Glu233, and Gln237 in TM5, as well as Thr291 in TM6 interact with the α5-helix of Gαs. We mutated these residues to Ala, and the functions of these β1-AR mutants were examined by their ability to activate Gs in cells as quantified by the cellular cAMP production (Figure 7, C-E). Comparing with wild-type β1-AR, these β1-AR mutants had reduced efficacy in activating Gs (Figure 7 C and D). These functional studies support our structural data, and confirm the importance of the β1-AR–Gs interactions (revealed by the structural study) in the activation of Gs by β1-AR.
Figure 7. Functional studies of specific interacting residues in Gs activation by β1-AR.
(A and B) Locations of the mutated residues in ICL2 and in the TM5-ICL3-TM6 region of β1-AR. (C) Dose-response data from cells expressing different β1-ARs after stimulation with isoproterenol. Data are represented as mean ± SD of three experiments. (D) Summary of the efficacy (the maximum cAMP level of a mutant receptor / the maximum cAMP level of the wild-type receptor) and EC50 values based on the cAMP assay data shown in C. Data are represented as mean ± SD of three experiments. The analysis was done using the log(agonist) vs. response function of Prism 8 (GraphPad). (E) Western blots of same amounts of total proteins from membrane preparations of cells transfected with wild-type and mutant β1-ARs with anti-β1-AR antibody show similar expression levels of the receptor proteins.
CONCLUSION
We have investigated the structural basis for the activation of Gs by β1-AR. The cryo-EM structure of the complex of β1-AR–Gs reveals the conformation of the active state of β1-AR, the molecular recognition of Gs by the active β1-AR, the direct interaction between ICL2 of β1-AR and the N-terminal αN-helix of Gαs, and the structural changes of Gs upon the coupling to β1-AR. The principal mechanism for β1-AR as a GEF for Gs is to deform the GDP/GTP-binding pocket and to accelerate GDP release from Gs. β1-AR induces a tilting of the α5-helix, the break of the ionic lock between His373 in the α5-helix and Arg38 in the αN-helix, the disruption of the interacting networks involving Gln59 in the α1-helix, Ala352 in the β6-α5 loop, and Thr355 in the α5-helix, the rotational opening of the α-helical domain from the Ras-like domain, and the deformation of the GDP/GTP-binding pocket. All these conformational changes lead to the GDP release. It is worth noting that the Ras-like domain of Gαs, purified as an isolated recombinant protein, had been shown to be able to bind to GDP and GTP, and to stimulate the activity of adenylyl cyclase which could be further enhanced by the addition of the separately purified recombinant α-helical domain of Gαs (Markby et al., 1993). Indeed, in the β1-AR–Gs structure, even when the Ras-like and α-helical domains are separated, some of the residues involved in GDP/GTP binding do not change their relative locations (Figure 6E), thus providing the possibility of a subsequent binding of GTP (albeit weakly). This initial weak binding of GTP is likely strengthened by the subsequent re-closing of the α-helical domain. Reciprocally, GTP binding promotes the association of the Ras-like domain and the α-helical domain, and the α-helical domain accelerates GTP hydrolysis, thus completing one cycle of the guanine nucleotide-exchange on Gαs. Altogether, our studies advance the understanding of Gs activation by β1-AR, and the activation of G-proteins by GPCRs in general.
Limitations:
As shown in the local resolution map (Figure S2), the complex of isoproterenol–β1-AR–Gs was well-resolved in most regions. However, similar to many other cryo-EM density maps, some regions of the map, including the α-helical domain of Gαs, are weaker than other regions. This weak density reflects the highly dynamic nature of the α-helical domain in the nucleotide-free state. To interpret this density, the isoproterenol–β1-AR–Gs density map was low-pass filtered to 6 Å and then the α-helical domain from the X-ray crystal structure of β2-AR–Gs (PDB 3SN6) was manually docked and rigid-body refined. While the density for the α-helical domain is weaker and the high-resolution features are blurred out due to its increased disorder, it is not absent and its position is clearly resolved in the low-pass filtered map. Without high-resolution features, we are limited in modeling its structure to rigid-body fitting into the low-pass filtered map. Therefore, we only used the information about the relative position of the α-helical domain of Gαs in the complex in this paper and future studies will be required to understand the dynamics of the α-helical domain of Gα.
STAR★Methods
RESOURCE AVAILABILITY
LEAD CONTACT
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Xin-Yun Huang (xyhuang@med.cornell.edu).
MATERIALS AVAILABILITY
All unique reagents generated in this study will be made available on request by the Lead Contact with a completed Materials Transfer Agreement (MTA).
DATA AND CODE AVAILABILITY
The cryo-EM reconstructions of the isoproterenol–β1-AR–Gs complex have been deposited in the Electron Microscopy Data Bank (EMDB) under ID codes EMDB: EMD-22357. The corresponding atomic model has been deposited in the Protein Data Bank (PDB) under ID codes PDB: 7JJO.
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Turkey β1-AR, bovine Gβ1 and bovine Gγ2(C68S) were expressed in Sf9 insect cells (Expression Systems) infected with recombinant baculovirus. Bovine Gαs and Nb35 were expressed in E. coli strain BL21(DE3) (New England Biolabs). CHO-K1 cells overexpressing wild-type and mutant β1-ARs were used in cAMP functional assays.
METHODS DETAILS
Expression and purification of β1-AR, Gαs, Gβ1, Gγ2 and Nb35
β1-AR protein was purified as described previously (Huang et al., 2013). The turkey β1-AR construct β1-AR(H12) used in this study was similar to the functional β1-AR(H0) construct described previously with some modifications (Huang et al., 2013). A signal peptide, FLAG tag, PreScission protease cleavage site and T4 lysozyme were fused to the N-terminus with a doublealanine linker, and another PreScission protease cleavage site and His6 tag were added to the C-terminus. β1-AR was expressed and purified from Sf9 insect cells grown in ESF 921 protein-free medium (Expression Systems). Cells were grown to 2 to 3 million cells per ml before 200 ml of baculoviruses were added for infection. 48 hrs later, cells were harvested by centrifugation, flash frozen in liquid nitrogen and stored at −80 °C until use. For membrane preparation, cell pellets were lysed by sonication in a buffer containing 20 mM Tris, pH 8, 1 mM EDTA and protease inhibitor cocktail (Roche) and washed once more using the same buffer. Purified membranes were resuspended in 20 mM Tris, pH 8, 0.2 mM EDTA, and protease inhibitor cocktail and flash frozen in liquid nitrogen and stored at −80 °C. For protein purification, membrane preparations were first thawed in 20 mM Tris, pH 8, 350 mM NaCl, and protease inhibitor cocktail. 1 mM isoproterenol (Sigma) was then added and the mixture was stirred for 1 hr at 4 °C and the membranes were then solubilized in 20 mM Tris, pH 8, 350 mM NaCl, 1% n-Dodecyl-β-D-Maltopyranoside (DDM, Anatrace), 1 mM isoproterenol and protease inhibitor cocktail for 1 hr at 4°C. The DDM concentration was then reduced to 0.5% by adding equal volume of 20 mM Tris, pH 8, 350 mM NaCl, and 1 mM isoproterenol and the mixture was stirred for another 1 hr at 4°C. The preparation was clarified by ultracentrifugation at 142,000 g for 30 min at 8°C. The supernatant was then incubated with Ni-NTA resin (Qiagen) with stirring at 4 °C with 8 mM imidazole. After 4 hrs, the resin was collected by centrifugation and washed three times with 20 mM Tris, pH 8, 500 mM NaCl, 0.02% DDM, 1 mM isoproterenol, and 8 mM imidazole and one time with 20 mM Tris, pH 8, 100 mM NaCl, 0.02% DDM, 1 mM isoproterenol, and 8 mM imidazole. β1-AR was then eluted from the resin with 20 mM Tris, pH 8, 100 mM NaCl, 0.02% DDM, 1 mM isoproterenol, and 120 mM imidazole. The elution was concentrated and further purified by size-exclusion chromatography using a Superdex 200 Increase 10/300 column (GE Healthcare) pre-equilibrated with 20 mM Tris, pH 8, 100 mM NaCl, 0.02% Lauryl Maltose Neopentyl Glycol (LMNG, Anatrace), 1 mM isoproterenol. Purified β1-AR was concentrated to 4 mg/ml and either used immediately for complex assembly or flash frozen in liquid nitrogen and stored at −80 °C.
The recombinant wild-type bovine Gαs was purified from E. coli strain BL21(DE3) (New England Biolabs) (Huang et al., 2015). This Gαs construct had an N-terminal GST tag that was removable through a PreScission protease cleavage site. Cells were grown in 2×YT medium (MP Biomedicals) at 37 °C until OD600 reached 0.6. Protein expression was then induced by 75 μM IPTG (GoldBio) and continued for 16 hrs at 16 °C. Cells were harvested by centrifugation, flash frozen in liquid nitrogen and stored at −80 °C. For protein purification, cell pellets were thawed in a lysis buffer containing 20 mM HEPES, pH 7, 150 mM NaCl, 10% glycerol, 5 mM β-mercaptoethanol, 2 mM MgCl2, 1 mM EDTA, 10 μM GDP (Sigma), 0.1 mg/ml lysozyme, 0.2 mM PMSF and protease inhibitor cocktail, and further lysed by sonication. Cell debris was removed by centrifugation at 20,000 g for 40 min 4 °C. Supernatant was then collected and incubated with Glutathione resin (Pierce) with stirring for 1 hr at 4 °C. Resin was then washed four times with 20 mM HEPES, pH 7, 150 mM NaCl, 10% glycerol, 5 mM β-mercaptoethanol, 2 mM MgCl2, 1 mM EDTA, and 10 μM GDP. To remove the GST tag, PreScission protease was added to the beads at 1:10 (w:w) protease: GST-Gαs ratio and the mixture was rocked overnight at 4 °C with 2 mM DTT. Untagged Gαs was concentrated and further purified by size-exclusion chromatography using a Superdex 200 Increase 10/300 column pre-equilibrated with 20 mM HEPES, pH 7, 150 mM NaCl, 10% glycerol, 5 mM β-mercaptoethanol, 1 mM MgCl2, 1 mM EDTA, 20 μM GDP. Purified Gαs was concentrated to 6 mg/ml, flash frozen in liquid nitrogen and stored at −80 °C.
Bovine Gβ1 and bovine His6-tagged soluble Gγ2(C68S) were co-expressed and purified from Sf9 insect cells. 25 ml of each baculovirus were co-infected into Sf9 cells when the insect cell culture reached a cell density at 3 million cells per ml. 48 hrs post infection, cells were harvested by centrifugation, flash frozen in liquid nitrogen and stored at −80 °C. Cell pellets were thawed in 25 mM HEPES pH 7, 150 mM NaCl, 10% glycerol, 2 mM β-mercaptoethanol, 5 mM MgCl2 and protease inhibitor cocktail. Cells were lysed by sonication and cell debris were removed by centrifugation at 142,000 g for 30 min. Supernatant was collected and incubated with Ni-NTA resin with stirring for 1.5 hrs at 4 °C. Resin was then washed three times with 25 mM HEPES pH 7, 150 mM NaCl, 10% glycerol, 2 mM β-mercaptoethanol, and 25 mM imidazole, and Gβ1γ2 was eluted as a complex with 25 mM HEPES pH 7, 150 mM NaCl, 10% glycerol, 2 mM β-mercaptoethanol, and 250 mM imidazole. Eluted protein was concentrated and further purified using a Superdex 200 Increase 10/300 column pre-equilibrated with 25 mM HEPES pH 7, 150 mM NaCl, and 2 mM β-mercaptoethanol. Purified Gβ1γ2 protein was concentrated to 8 mg/ml, flash frozen in liquid nitrogen and stored at −80 °C.
Nb35-His6 was expressed in the periplasm of E. coli strain BL21(DE3). Cells were grown in LB medium (MP Biomedicals) at 37 °C until OD600 reached 0.6. Protein expression was then induced by 75 μM IPTG and Nb35 was further expressed for 18 hrs at 16 °C. Cells were then harvested, flash frozen in liquid nitrogen and stored at −80 °C. For protein purification, cells were lysed by sonication in a lysis buffer containing 20 mM HEPES pH 7, 100 mM NaCl, 5 mM MgCl2, 0.1 mM lysozyme, and protease inhibitor cocktail. After removal of the cell debris by centrifugation at 20,000 g for 30 min, supernatant was collected and incubated with Ni-NTA resin with stirring for 1.5 hrs at 4 °C. Resin was then washed three times with 20 mM HEPES pH 7, 100 mM NaCl, and 25 mM imidazole. Nb35 was eluted with 20 mM HEPES pH 7, 100 mM NaCl, and 250 mM imidazole. Eluted Nb35 protein was dialyzed against 1 L of 20 mM HEPES pH 7, 100 mM NaCl overnight at 4 °C. Dialyzed protein was concentrated to 3 mg/ml, flash frozen in liquid nitrogen and stored in −80 °C.
Protein complex assembly and purification
To assemble the β1-AR-Gs-Nb35 complex, Gαs, Gβ1γ2 and Nb35 were mixed at 1:1:1.5 molar ratios in the presence of 2 mM MgCl2. The mixture was incubated for 30 min at room temperature and then mixed with β1-AR at 1.2:1 ratio. The mixture was diluted with 160 μl buffer containing 10 mM HEPES pH 7, 100 mM NaCl, 0.1 mM TCEP, 0.02% LMNG, 1 mM isoproterenol, and 2 mM MgCl2 to bring the volume to 600 μl. This mixture was incubated for another 30 min at room temperature before 0.4 U Apyrase (Sigma) was added. After additional 30 min room temperature incubation with Apyrase, the mixture was centrifuged at 16,000 g for 10 min to remove any precipitants. The supernatant was then loaded onto a Superdex 200 Increase 10/300 column pre-equilibrated with 10 mM HEPES pH 7, 100 mM NaCl, 0.1 mM TCEP, 0.02% LMNG and 40 uM isoproterenol. The elution fractions from a single peak containing pure β1-AR-Gs-Nb35 complex was concentrated to ~1.5 mg/ml and used directly for making cryo-EM grids.
Cryo-EM data collection
4 μl of protein complex was applied to a glow-discharged 400 mesh gold Quantifoil R1.2/1.3 holey carbon grids (Quantifoil Micro Tools), and subsequently vitrified using Vitrobot Mark IV (Thermo Fisher Scientific/FEI). Images were collected at liquid nitrogen temperature on a Titan Krios electron microscope (Thermo Fisher Scientific/FEI) operated at 300 kV accelerating voltage, at a nominal magnification of 22,500× using a Gatan K3 direct electron detector (Gatan, Inc.), corresponding to a super-resolution pixel size of 0.532 Å/pixel at the detector. In total, 5633 micrographs with defocus values in the range of −1.0 μm to −2.3 μm were recorded. Images were recorded as 40 dose-fractionated frames with a total accumulated dose of 46 e−/Å2.
Image processing, 3D reconstructions, modeling and refinement
Super-resolution movies were aligned and two-times Fourier cropped using MotionCorr2 1.2.1 (Zheng et al., 2017) resulting in a final pixel size of 1.064 Å/pixel. Relion 3.0b2 (Zivanov et al., 2018) Laplacian-of-Gaussian picking with minimum and maximum dimensions of 76 Å and 119 Å was used to heavily over-pick at a rate of approximately 2300 particles per micrograph. False positives were excluded from the particle stack of 13 million particles through multiple rounds of heterogeneous classification using Fourier cropped particles in CryoSparc v2.12.4 (Punjani et al., 2017) (Figure S2). 2D classification was applied to confirm that the excluded particles corresponded to false positives, free receptors or free G-protein heterotrimers. Iterative classification resulted in a stack of intact complexes was 1.5 million particles. Starting from this point, multiple classification strategies in both Relion 3.0b2 and CryoSparc v2.12.4 were used to confirm that there was only one major conformation present in each data set. Further classification converged to a final high resolution stack of 452,312 particles that was then subjected to Local CTF Refinement procedures in CryoSparc v2.12.4 followed by Bayesian Polishing in Relion 3.0b2, and finally Global CTF Refinement in CryoSparc v2.12.4 to improve higher order aberrations (Figure S2). Final high-resolution reconstructions were subjected to Local Refinement with Non-Uniform Refinement in CryoSparc v2.12.4 for β1-AR and G-proteins independently. The Local Refinement maps showed significantly improved features over the consensus maps, both with resolutions at or below 2.6 Å (Figure S2). The resulting maps were super-sampled in Coot v0.8.9.2 (Emsley and Cowtan, 2004) to 0.532 Å per pixel with a 512 voxel box. The initial models of β1-AR and Gβ1γ2 were derived from the crystal structures of inactive β1-AR (PDB ID: 4GPO) and Gαq-GRK2-Gβ1γ2 complex (PDB ID: 2BCJ), respectively.
Gαs and Nb35 were derived from the crystal structures of β2-AR–Gs complex (PDB ID: 3SN6). The models were manually rebuilt into the focus-refined density maps and refined in real space using Phenix v1.17.1-3660 (Adams et al., 2010). The density of the α-helical domain of Gαs was poor; the α-helical domain from PDB 3SN6 was manually docked into the density and rigid-body fit in COOT. Once refinement converged, enabling a final combined map was derived from the model and the two super-sampled local refinement maps using the Combine Focused Maps feature in Phenix v1.17.1-3660. Since all local and consensus refinements gave gold-standard FSC values of 2.6 Å, we approximate the resolution of this combined consensus map to be 2.6 Å as well (Figure S2). A final round of real space refinement in Phenix v1.17.1-3660 against the combined map yielded the final model.
cAMP assay
CHO-K1 (ATCC) cells were plated onto six-well plates and treated with 1 mM IBMX (Cayman) for 30 min. After washing twice with HEM buffer (20 mM HEPES, pH 7.4, 135 mM NaCl, 4.7 mM KCl, 1.2 mM MgSO4, 2.5 mM NaHCO3, 0.1 mM Ro-20-1724 (Sigma), 0.5 U/ml adenosine deaminase (Roche), and 1 mM IBMX), cells were treated with different concentrations of isoproterenol in HEM buffer for 5 min. After two more washes with HEM buffer, cells were harvested in 0.5% Triton X-100 (Sigma) containing 1 mM IBMX. The amount of cAMP was measured with the Direct Cyclic AMP Enzyme Immunoassay kit (Enzo Life Sciences).
Quantification and Statistical Analysis
In Figure 7C, the cAMP assays were repeated three times, and the data are represented as mean ± SD of the three independent experiments. In Figure 7D, the analysis was done using the log(agonist) vs. response function of Prism 8 (GraphPad) as indicated in the figure legend. Cryo-EM data collection and refinement statistics are listed in Table 1.
Supplementary Material
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Bacterial and Virus Strains | ||
| E. coli strain BL21(DE3) | New England Biolabs | Cat# C2527H |
| Recombinant baculovirus for Gβ1 | Huang et al., 2015 | N/A |
| Recombinant baculovirus for Gγ2(C68S) | Huang et al., 2015 | N/A |
| Chemicals, Peptides, and Recombinant Proteins | ||
| ESF 921 protein-free medium | Expression Systems | Cat# 96-001-01 |
| Protease inhibitor cocktail | Roche | Cat# 5892791001 |
| Isoproterenol | Sigma | Cat# I6504 |
| DDM | Anatrace | Cat# D310S |
| LMNG | Anatrace | Cat# NG310 |
| 2×YT medium | MP Biomedicals | Cat# 113012042 |
| IPTG | GoldBio | Car# I2481C |
| GDP | Sigma | Cat# G7127 |
| PreScission protease | Prepared In-House | N/A |
| LB medium | MP Biomedicals | Cat# 113002042 |
| Apyrase | Sigma | Cat# A6237 |
| IBMX | Cayman | Cat# 13347 |
| Ro-20-1724 | Sigma | Cat# B8279 |
| Adenosine deaminase | Roche | Cat# 10102105001 |
| Triton X-100 | Sigma | Cat# X100 |
| Critical Commercial Assays | ||
| Direct Cyclic AMP Enzyme Immunoassay kit | Enzo Life Sciences | Cat# ADI-900-066 |
| Deposited Data | ||
| β1-AR-Gs-Nb35 coordinates | This paper | PDB: 7JJO |
| β1-AR-Gs-Nb35 EM map | This paper | EMDB: EMD-22357 |
| Experimental Models: Cell Lines | ||
| Insect cell line Sf9 | Expression Systems | Cat# 94-001S |
| CHO-K1 | ATCC | Cat# CCL-61 |
| Recombinant DNA | ||
| pVL1391-β1-AR (H12) | This paper | N/A |
| pGEX-6P-Gαs | Huang et al., 2015 | N/A |
| pET-26b-Nb35 | This paper | N/A |
| pcDNA3.1-β1-AR (H0) | Huang et al., 2013 | N/A |
| pcDNA3.1-β1-AR (H0) (P146A) | This paper | N/A |
| pcDNA3.1-β1-AR (H0) (F147A) | This paper | N/A |
| pcDNA3.1-β1-AR (H0) (Q150A) | This paper | N/A |
| pcDNA3.1-β1-AR (H0) (S151A) | This paper | N/A |
| pcDNA3.1-β1-AR (H0) (V230A) | This paper | N/A |
| pcDNA3.1-β1-AR (H0) (E233A) | This paper | N/A |
| pcDNA3.1-β1-AR (H0) (A234E) | This paper | N/A |
| pcDNA3.1-β1-AR (H0) (Q237A) | This paper | N/A |
| pcDNA3.1-β1-AR (H0) (T291A) | This paper | N/A |
| Software and Algorithms | ||
| MotionCorr2 1.2.1 | Zheng et al., 2017 | https://msg.ucsf.edu/software |
| Relion 3.0b2 | Zivanov et al., 2018 | https://www3.mrc-lmb.cam.ac.uk/relion/index.php/Download_%26_install |
| CryoSparc v2.12.4 | Punjani et al., 2017 | https://cryosparc.com/ |
| Coot v0.8.9.2 | Emsley and Cowtan, 2004 | https://www2.mrc-lmb.cam.ac.uk/personal/pemsley/coot/ |
| Phenix v1.17.1-3660 | Adams et al., 2010 | https://www.phenix-online.org/ |
| Other | ||
| Ni-NTA resin | Qiagen | Cat# 30210 |
| Superdex 200 Increase 10/300 column | GE Healthcare | Cat# 28990944 |
| Glutathione resin | Pierce | Cat# 16100 |
| 400 mesh gold Quantifoil R1.2/1.3 holey carbon grids | Quantifoil Micro Tools | Cat# Q4100AR1.3 |
Highlights:
Cryo-EM structure of β1-adrenergic receptor and Gs at 2.6 Å resolution
Network of interactions within Gαs are disrupted by β1-AR
Rotational opening of the α-helical domain of Gαs during its activation
Functional studies of critical residues on β1-AR involved in the activation of Gs
ACKNOWLEDGMENTS:
We thank members of our research groups for helpful discussion and comments on the manuscript. This work was supported by an NIH grant HL130478 (X.Y.H.), the Josie Robertson Investigators Program (R.K.H.), the Searle Scholars Program (R.K.H), the Canada Excellence Research Chairs program (O.P.E.), and CIHR grant 159464 (O.P.E.). The Simons Electron Microscopy Center and the National Resource for Automated Molecular Microscopy located at the New York Structural Biology Center is supported by grants from the NIH National Institute of General Medical Sciences (GM103310), NYSTAR, and the Simons Foundation (SF349247).
Footnotes
DECLARATION OF INTERESTS: The authors declare no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Adams PD, Afonine PV, Bunkoczi G, Chen VB, Davis IW, Echols N, Headd JJ, Hung LW, Kapral GJ, Grosse-Kunstleve RW, et al. (2010). PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr D Biol Crystallogr 66, 213–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aurora R, and Rose GD (1998). Helix capping. Protein science : a publication of the Protein Society 7, 21–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aurora R, Srinivasan R, and Rose GD (1994). Rules for alpha-helix termination by glycine. Science 264, 1126–1130. [DOI] [PubMed] [Google Scholar]
- Benovic JL (2002). Novel beta2-adrenergic receptor signaling pathways. J Allergy Clin Immunol 110, S229–235. [DOI] [PubMed] [Google Scholar]
- Bos JL, Rehmann H, and Wittinghofer A (2007). GEFs and GAPs: critical elements in the control of small G proteins. Cell 129, 865–877. [DOI] [PubMed] [Google Scholar]
- Bourne HR (1997). How receptors talk to trimeric G proteins. Curr Opin Cell Biol 9, 134–142. [DOI] [PubMed] [Google Scholar]
- Bourne HR, Sanders DA, and McCormick F (1990). The GTPase superfamily: a conserved switch for diverse cell functions. Nature 348, 125–132. [DOI] [PubMed] [Google Scholar]
- Cherezov V, Rosenbaum DM, Hanson MA, Rasmussen SG, Thian FS, Kobilka TS, Choi HJ, Kuhn P, Weis WI, Kobilka BK, et al. (2007). High-resolution crystal structure of an engineered human beta2-adrenergic G protein-coupled receptor. Science 318, 1258–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman DE, Berghuis AM, Lee E, Linder ME, Gilman AG, and Sprang SR (1994). Structures of active conformations of Gi alpha 1 and the mechanism of GTP hydrolysis. Science 265, 1405–1412. [DOI] [PubMed] [Google Scholar]
- Conklin BR, and Bourne HR (1993). Structural elements of G alpha subunits that interact with G beta gamma, receptors, and effectors. Cell 73, 631–641. [DOI] [PubMed] [Google Scholar]
- Conklin BR, Farfel Z, Lustig KD, Julius D, and Bourne HR (1993). Substitution of three amino acids switches receptor specificity of Gq alpha to that of Gi alpha. Nature 363, 274–276. [DOI] [PubMed] [Google Scholar]
- Dohlman HG, and Jones JC (2012). Signal activation and inactivation by the Galpha helical domain: a long-neglected partner in G protein signaling. Sci Signal 5, re2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draper-Joyce CJ, Khoshouei M, Thal DM, Liang YL, Nguyen ATN, Furness SGB, Venugopal H, Baltos JA, Plitzko JM, Danev R, et al. (2018). Structure of the adenosinebound human adenosine A1 receptor-Gi complex. Nature 558, 559–563. [DOI] [PubMed] [Google Scholar]
- Dror RO, Mildorf TJ, Hilger D, Manglik A, Borhani DW, Arlow DH, Philippsen A, Villanueva N, Yang Z, Lerch MT, et al. (2015). SIGNAL TRANSDUCTION. Structural basis for nucleotide exchange in heterotrimeric G proteins. Science 348, 1361–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emsley P, and Cowtan K (2004). Coot: model-building tools for molecular graphics. Acta Crystallogr D Biol Crystallogr 60, 2126–2132. [DOI] [PubMed] [Google Scholar]
- Fredriksson R, Lagerstrom MC, Lundin LG, and Schioth HB (2003). The G-protein-coupled receptors in the human genome form five main families. Phylogenetic analysis, paralogon groups, and fingerprints. Mol Pharmacol 63, 1256–1272. [DOI] [PubMed] [Google Scholar]
- Frishman WH (2008). beta-Adrenergic blockers: a 50-year historical perspective. Am J Ther 15, 565–576. [DOI] [PubMed] [Google Scholar]
- Gilman AG (1987). G proteins: transducers of receptor-generated signals. Annu Rev Biochem 56, 615–649. [DOI] [PubMed] [Google Scholar]
- Herrmann R, Heck M, Henklein P, Hofmann KP, and Ernst OP (2006). Signal transfer from GPCRs to G proteins: role of the G alpha N-terminal region in rhodopsin-transducin coupling. J Biol Chem 281, 30234–30241. [DOI] [PubMed] [Google Scholar]
- Hilger D, Masureel M, and Kobilka BK (2018). Structure and dynamics of GPCR signaling complexes. Nat Struct Mol Biol 25, 4–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Chen S, Zhang JJ, and Huang XY (2013). Crystal structure of oligomeric beta1-adrenergic G protein-coupled receptors in ligand-free basal state. Nat Struct Mol Biol 20, 419–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Sun Y, Zhang JJ, and Huang XY (2015). Pivotal role of extended linker 2 in the activation of Galpha by G protein-coupled receptor. J Biol Chem 290, 272–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John J, Sohmen R, Feuerstein J, Linke R, Wittinghofer A, and Goody RS (1990). Kinetics of interaction of nucleotides with nucleotide-free H-ras p21. Biochemistry 29, 6058–6065. [DOI] [PubMed] [Google Scholar]
- Kang Y, Kuybeda O, de Waal PW, Mukherjee S, Van Eps N, Dutka P, Zhou XE, Bartesaghi A, Erramilli S, Morizumi T, et al. (2018). Cryo-EM structure of human rhodopsin bound to an inhibitory G protein. Nature 558, 553–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koehl A, Hu H, Maeda S, Zhang Y, Qu Q, Paggi JM, Latorraca NR, Hilger D, Dawson R, Matile H, et al. (2018). Structure of the micro-opioid receptor-Gi protein complex. Nature 558, 547–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambright DG, Sondek J, Bohm A, Skiba NP, Hamm HE, and Sigler PB (1996). The 2.0 A crystal structure of a heterotrimeric G protein. Nature 379, 311–319. [DOI] [PubMed] [Google Scholar]
- Lappano R, and Maggiolini M (2012). GPCRs and cancer. Acta Pharmacol Sin 33, 351–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang YL, Khoshouei M, Glukhova A, Furness SGB, Zhao P, Clydesdale L, Koole C, Truong TT, Thal DM, Lei S, et al. (2018). Phase-plate cryo-EM structure of a biased agonist-bound human GLP-1 receptor-Gs complex. Nature 555, 121–125. [DOI] [PubMed] [Google Scholar]
- Liang YL, Khoshouei M, Radjainia M, Zhang Y, Glukhova A, Tarrasch J, Thal DM, Furness SGB, Christopoulos G, Coudrat T, et al. (2017). Phase-plate cryo-EM structure of a class B GPCR-G-protein complex. Nature 546, 118–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohse MJ, Engelhardt S, and Eschenhagen T (2003). What is the role of beta-adrenergic signaling in heart failure? Circ Res 93, 896–906. [DOI] [PubMed] [Google Scholar]
- Markby DW, Onrust R, and Bourne HR (1993). Separate GTP binding and GTPase activating domains of a G alpha subunit. Science 262, 1895–1901. [DOI] [PubMed] [Google Scholar]
- Noel JP, Hamm HE, and Sigler PB (1993). The 2.2 A crystal structure of transducin-alpha complexed with GTP gamma S. Nature 366, 654–663. [DOI] [PubMed] [Google Scholar]
- Oldham WM, and Hamm HE (2008). Heterotrimeric G protein activation by G-protein-coupled receptors. Nat Rev Mol Cell Biol 9, 60–71. [DOI] [PubMed] [Google Scholar]
- Post SR, Hammond HK, and Insel PA (1999). Beta-adrenergic receptors and receptor signaling in heart failure. Annu Rev Pharmacol Toxicol 39, 343–360. [DOI] [PubMed] [Google Scholar]
- Punjani A, Rubinstein JL, Fleet DJ, and Brubaker MA (2017). cryoSPARC: algorithms for rapid unsupervised cryo-EM structure determination. Nat Methods 14, 290–296. [DOI] [PubMed] [Google Scholar]
- Rasmussen SG, DeVree BT, Zou Y, Kruse AC, Chung KY, Kobilka TS, Thian FS, Chae PS, Pardon E, Calinski D, et al. (2011). Crystal structure of the beta2 adrenergic receptor-Gs protein complex. Nature 477, 549–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum DM, Rasmussen SG, and Kobilka BK (2009). The structure and function of G-protein-coupled receptors. Nature 459, 356–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakmar TP (2002). Structure of rhodopsin and the superfamily of seven-helical receptors: the same and not the same. Curr Opin Cell Biol 14, 189–195. [DOI] [PubMed] [Google Scholar]
- Simon MI, Strathmann MP, and Gautam N (1991). Diversity of G proteins in signal transduction. Science 252, 802–808. [DOI] [PubMed] [Google Scholar]
- Sprang SR (1997). G protein mechanisms: insights from structural analysis. Annu Rev Biochem 66, 639–678. [DOI] [PubMed] [Google Scholar]
- Sprang SR, Chen Z, and Du X (2007). Structural basis of effector regulation and signal termination in heterotrimeric Galpha proteins. Adv Protein Chem 74, 1–65. [DOI] [PubMed] [Google Scholar]
- Strange PG (2008). Signaling mechanisms of GPCR ligands. Curr Opin Drug Discov Devel 11, 196–202. [PubMed] [Google Scholar]
- Sunahara RK, Tesmer JJ, Gilman AG, and Sprang SR (1997). Crystal structure of the adenylyl cyclase activator Gsalpha [see comments]. Science 278, 1943–1947. [DOI] [PubMed] [Google Scholar]
- Wall MA, Coleman DE, Lee E, Iniguez-Lluhi JA, Posner BA, Gilman AG, and Sprang SR (1995). The structure of the G protein heterotrimer Gi alpha 1 beta 1 gamma 2. Cell 83, 1047–1058. [DOI] [PubMed] [Google Scholar]
- Warne T, Serrano-Vega MJ, Baker JG, Moukhametzianov R, Edwards PC, Henderson R, Leslie AG, Tate CG, and Schertler GF (2008). Structure of a beta1-adrenergic G-protein-coupled receptor. Nature 454, 486–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weis WI, and Kobilka BK (2018). The Molecular Basis of G Protein-Coupled Receptor Activation. Annu Rev Biochem 87, 897–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Sun B, Feng D, Hu H, Chu M, Qu Q, Tarrasch JT, Li S, Sun Kobilka T, Kobilka BK, et al. (2017). Cryo-EM structure of the activated GLP-1 receptor in complex with a G protein. Nature 546, 248–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng SQ, Palovcak E, Armache JP, Verba KA, Cheng Y, and Agard DA (2017). MotionCor2: anisotropic correction of beam-induced motion for improved cryo-electron microscopy. Nat Methods 14, 331–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zivanov J, Nakane T, Forsberg BO, Kimanius D, Hagen WJ, Lindahl E, and Scheres SH (2018). New tools for automated high-resolution cryo-EM structure determination in RELION-3. Elife 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The cryo-EM reconstructions of the isoproterenol–β1-AR–Gs complex have been deposited in the Electron Microscopy Data Bank (EMDB) under ID codes EMDB: EMD-22357. The corresponding atomic model has been deposited in the Protein Data Bank (PDB) under ID codes PDB: 7JJO.