Abstract
Objective:
Recent animal work and limited clinical data have suggested that laryngospasm may be involved in the cardiorespiratory collapse seen in sudden unexpected death in epilepsy (SUDEP). In previous work we demonstrated in an animal model of seizures that laryngospasm and sudden death were always preceded by acid reflux into the esophagus. Here, we expand on that work by testing several techniques to prevent the acid reflux or the subsequent laryngospasm.
Methods:
In urethane anesthetized Long Evans rats we used systemic kainic acid to acutely induce seizure activity. We recorded pH in the esophagus, respiration, electrocorticography activity, and measured the liquid volume in the stomach postmortem. We performed three interventions to attempt to prevent acid reflux or laryngospasm and gain insights into mechanisms: fasting animals for 12 hours, severing the gastric nerve, and electrical stimulation of either the gastric nerve or the recurrent laryngeal nerve.
Results:
Seizing animals had significantly more liquid in their stomach. Severing the gastric nerve and fasting animals significantly reduced stomach liquid volume, subsequent acid reflux, and sudden death. Laryngeal nerve stimulation can reverse laryngospasm on demand. Seizing animals are more susceptible to death from stomach-acid induced laryngospasm than non-seizing animals are to artificial-acid induced laryngospasm.
Significance:
These results provide insight into the mechanism of acid production and sudden obstructive apnea in this model. These techniques may have clinical relevance if this model is shown to be similar to human SUDEP.
Keywords: SUDEP, kainic acid, Gastroesophageal reflux, laryngeal chemoreflex, pH
1. INTRODUCTION
Sudden unexpected death in epilepsy (SUDEP) is a complication of epilepsy which is defined as a “sudden, unexpected, witnessed or unwitnessed, nontraumatic and nondrowning death in patients with epilepsy, with or without evidence for a seizure and excluding documented status epilepticus, in which postmortem examination does not reveal a toxicologic or anatomic cause of death” (Nashef et al., 2011). Approximately 4,000 Americans die from SUDEP every year (Berg, 2001; Thurman David et al., 2014). SUDEP is varied in its presentation and is difficult to study because it often occurs unobserved (Lamberts Robert et al., 2011; Langan et al., 2000; Nobili et al., 2011; Ryvlin et al., 2013). Clinical data suggests that SUDEP is a cardiorespiratory collapse that occurs shortly after a seizure (Ryvlin et al., 2013). Recent, limited work has suggested that SUDEP may also occur without a directly preceding seizure (Lhatoo et al., 2016). SUDEP is more common in patients with drug resistant epilepsy who experience generalized tonic-clonic seizures (GTCS) (Devinsky, 2011; Devinsky et al., 2016). SUDEP is more likely to occur at night, and when the patient is in the prone position (Lamberts Robert et al., 2011; Langan et al., 2000; Nobili et al., 2011; Ryvlin et al., 2013). Clinical data suggests that SUDEP may be prevented with prompt emergency care (Ryvlin et al., 2013). Patients with epilepsy have well documented impairments in autonomic function during, immediately after, and chronically in between seizures (Devinsky, 2004; Stewart, 2019; Wannamaker, 1985). Many researchers have suggested and provided evidence that autonomic nervous system (ANS) dysfunction may be related to SUDEP (Devinsky, 2004; Druschky et al., 2001; Lee and Devinsky, 2005; Manolis et al., 2019; Naggar et al., 2014; Sakamoto et al., 2008; Stewart, 2019; Vega, 2018; Vincenzi, 2019; Wannamaker, 1985). Situations which can strongly co-activate both sympathetic and parasympathetic branches of the ANS can cause possibly fatal arrhythmias, especially in epilepsy (Mameli et al., 2001; Paton et al., 2005). Recent work has proposed the mammalian diving reflex, which strong co-activates both ANS pathways, as a possible cause of SUDEP, and it has been a suspected cause of other types of sudden death for many years (Davies et al., 1988; Michael Panneton, 2013; Mooney et al., 2019; Scholander et al., 1962; Vega, 2018; Vincenzi, 2019). Like the mammalian diving reflex, the laryngeal chemoreflex (LCR) can strongly coactivate both ANS pathways, can cause laryngospasm, and has been implicated in other types of sudden death (Davies et al., 1988; Goding, 1998; Heman-Ackah et al., 2009; Marchal et al., 1982; Nakase et al., 2016; Thach, 1997). Recent animal work and limited clinical data have proposed laryngospasm as a contributing or even causative factor to the cardiorespiratory collapse seen in SUDEP (Lacuey et al., 2018; Nakase et al., 2016; Stewart et al., 2017; Tavee and Morris, 2008). Laryngospasm is a reflexive spasmic closure of the larynx, most commonly caused in humans by acid reflux into the larynx, a primary trigger of the laryngeal chemoreflex (Heman-Ackah et al., 2009; Loughlin Christopher et al., 2009). The primary cause of acid reflux and laryngospasm in humans is gastroesophageal reflux disease (GERD), which shares similar risk factors with SUDEP and has a statistically significant comorbidity with epilepsy (Postma and Halum, 2006; Selassie, 2015; Vela et al., 2013).
In our previous work we demonstrated in a seizure model in rats that animals will die suddenly of obstructive apnea (presumably laryngospasm) after acid refluxes into the esophagus, and obstructive apnea and death can be prevented by blocking acid movement with a balloon catheter (Budde et al., 2018). This result is especially noteworthy as rats are typically unable to vomit (Horn et al., 2013). Previously, we proposed that malfunctioning vagal tracts might cause increased stomach acid production, relaxation of the esophageal sphincters, or both, which contribute to acid reflux and death (Budde et al., 2018). In this paper we expand upon our previous work by presenting three intervention techniques to prevent the acid reflux and death. These three intervention strategies: gastric vagotomy, fasting, and nerve stimulation provide insight into the mechanism behind acid overproduction, reflux, and death.
2. METHODS
2.1. Animal procedures
All animal work was approved by the Institutional Animal Care and Use Committee and the Purdue Animal Care and Use Committee. Female Long Evans rats (235g – 331g, Envigo, Indianapolis, IN) were housed in pairs in a 12 h / 12 h light dark cycle with ad lib access to food and water, except when fasted prior to an experiment. We anesthetized animals with urethane (1.5 g/kg i.p., Sigma Aldrich, St. Louis, MO).
2.1.1. Physiological measures
We collected electrocorticography (ECoG) data from over the dorsal hippocampus by placing bone screws at bregma AP − 2.5mm, lateral − 2.0 mm (measurement electrode), and bregma AP + 0.5mm, lateral − 2.0 mm (reference electrode). We placed pH electrodes at the bottom of the esophagus by placing the electrodes inside a modified feeding tube (Instech Laboratories), pushing the feeding tube and electrodes to the lower esophageal sphincter, retracting approximately 3 mm, and removing the tube. We placed two pH electrodes - one at the bottom of the esophagus and one 1 cm rostral, to measure the progress of acid over time. We taped the pH electrodes outside the mouth and measured the distance from the mouth throughout the experiment to ensure the electrodes did not significantly migrate and were not swallowed by the animal. We placed a reference silver/silver-chloride electrode in the subcutaneous space on the right dorsal of the animal, just caudal to the shoulder, and periodically injected saline to keep the electrode moist. We measured respiration via a thermocouple placed near or just inside the nares, similar to (Marks et al., 1995). We removed excess mucous (if present) by wiping the nose with a cotton swab. Animals were grounded from a power supply through a subcutaneous hypodermic needle. We collected baseline physiological measures for at least 5 min before seizure induction.
2.1.2. Fasting
Some animals had food and water withheld for 12 h prior to the start of the experiment. These animals were given an additional 1mL of saline subcutaneously at anesthesia induction to restore them to normal hydration.
2.1.3. Gastric vagotomy
In some animals we severed the gastric nerve. We shaved the ventral aspect of the rat from the caudal edge of the ribcage to approximately 1 cm rostral of the rat’s knees. With the animal supine, we made an incision approximately 0.5 cm left of midline, starting in line with the apex of the xiphoid process and extending approximately 3 cm caudally. This incision went through both the skin and abdominal wall. The right medial lobe, right lateral lobe, and quadrate lobe of the liver were reflected rostrally, and the hepatogastric ligament was cut. We then also rostrally reflected the preventricular part of the papillary process, allowing the esophagus to be visualized. We used the left vagus nerve, visible on the ventral side of the esophagus, as a landmark, and followed it rostrally until the bifurcation with the hepatic branch was visible. The gastric branch (2 – 3 mm caudal to the hepatic branch) was then lifted from the esophagus with a microdissection hook and severed using an electric cautery.
2.1.4. Acute induction of laryngospasm
In some animals we induced acute laryngospasm by placing acid directly on the larynx. These animals also received butorphanol (0.5–2 mg/kg s.c.) in addition to urethane anesthesia. We created an acidic biological buffer using Tris titrated with HCl to a pH of 1.6 to approximate stomach acid. Using a 2.7mm, 30 degree Dyonics 4130 video laryngoscope and a custom 3D printed cannula inspired by Mor et al. (Mor et al., 2014), we were able to visualize the larynx and record using a GoPro Hero5 Black, modified by Back-Bone. Using a catheter, we injected 0.1 mL of the simulated acid onto the larynx to immediately trigger laryngospasm. To accommodate the laryngoscope, we secured these animals supine in a stereotaxic frame. We show this experimental setup in Figure S2.
2.1.5. Nerve stimulation
2.1.5.1. Laryngeal nerve stimulation
In some animals we stimulated the recurrent laryngeal nerves (RLNs). Using blunt dissection techniques, we separated the trachea and RLNs from surrounding structures, and placed the cuff electrode around entire trachea just caudal to the cricoid cartilage. In some experiments we severed the laryngeal nerves on one or both sides to confirm that our stimulation was affecting the nerves directly, not the surrounding muscles. We applied an alternating-phase stimulation of 500 μA with a pulse width of 100 μs and a pulse repeat time of 500 μs, which was sufficient to hold the larynx open (see Section 3.7 for how this stimulation waveform was determined). During these experiments we kept the incision moist with saline and covered it with cling wrap to prevent it from drying out. Successful stimulation of the nerve was obvious from laryngoscope video.
2.1.5.2. Gastric nerve stimulation
We applied electrical stimulation to the gastric nerve to block signaling to achieve a similar effect as severing the nerve with less damage. We used two cuff electrodes and exposed and cuffed the left cervical vagus nerve and the gastric branch of the left vagus nerve. To verify that our electrical blocking was successful we evoked compound nerve action potentials (CNAPs) at the cervical cuff and then blocked them at the gastric cuff. We evoked CNAPs with an alternating-phase waveform of 60 – 150 μA, a pulse width of 250 μs, and a pulse repeat time of 50 ms. We then blocked CNAPs with a continuous square wave between 70% – 110% the amplitude of the stimulation waveform and a pulse width of 250 – 125 μs (a frequency of 2 kHz to 4 kHz) (Crosby et al., 2017; Kilgore and Bhadra, 2014). The last 20% of each blocking square pulse was forced to ground to prevent a buildup of charge on the DC blocking capacitor, maintaining charge balance.
2.1.6. Seizure induction
Kainic acid was injected (10 mg/kg i.p.) to induce seizures, while control animals received an equivalent volume of saline. The KA dose was reduced to 80% for fasted animals, as we observed that they exhibited signs of KA overdosing which include, as also observed by Nakase et al. (Nakase et al., 2016), respiration significantly slower than baseline and severe gradual drop in blood oxygen saturation, which we approximated in our experiments by observing severe cyanosis at the hind- and fore-paws. ECoG analysis in the supplement (section S2, Figure S1) suggests this lower dose resulted in similar seizure development to non-fasted animals at the standard dose.
2.1.7. Endpoints and stomach analysis
Data was collected for at least two hours. We added an additional 30 minutes of observation if there was a strong reflux of acid (pH < 3 on both pH electrodes), or if the animal displayed signs of respiratory distress and appeared to be close to death. Animals were then euthanized with phenytoin/pentobarbital (45 mg / 351 mg) and the stomach was promptly removed. We observed stomach size, pressure, and color, and we removed the stomach contents into a conical tube. All stomach content samples were frozen shortly after collection. We thawed the samples in a water bath at room temperature for approximately three hours before centrifuging them − 2500 rpm for 5 min, followed by 3000 rpm for 5 min – to separate the majority of liquid and solid contents. We weighed the full samples, discarded the liquid contents, and left samples in a lab hood for three days to fully evaporate remaining liquid. We then weighed the samples again to determine the total solid and liquid mass in the stomachs. We qualitatively observed gas quantity in the stomachs prior to removal, but do not have a quantitative measure of stomach gas volume.
2.2. Recording
All physiological measures were recorded using a custom telemeter described in (Pederson et al., 2019). ECoG was collected at 1.25 kHz, gain = 1000, passband = 3.6 – 1500 Hz. pH was collected at 1.25 kHz, gain = 2, passband < 0.3 Hz. Respiration was collected at 1.25 kHz, gain = 3000, passband = 0.5 – 370 Hz. For nerve stimulation and recording we used a National Instruments USB-6343, stimulating and recording at 200 kHz. Data was processed through a p511 pre-amplifier from Grass Technologies with gain = 1000, passband = 0.3 – 1000 Hz. We summed and averaged waveforms in a strategy similar to Ward et al. (Ward et al., 2015). We collected and averaged 10 – 20 full periods (1 – 2 s) of stimulation and/or blocking. We then split the resulting full stimulation period in half, separating the positive and negative pulses, and added them, cancelling much of the stimulation artefact without affecting the CNAP. We still experienced some noise from blocking, so we took the last blocking period of the resulting waveform (500 μs out of 50 ms), inverted this section, and added it to each of the previous blocking periods. This strategy helped eliminate a large amount of the leftover blocking artifact without affecting the CNAP signal. This strategy assumes there is no CNAP in the final 500 μs of the pulse half period, but we always found this to be true. Of note to others in the field, we observed that, after the output of the p511 pre-amplifier is saturated, the recovery can look identical to a CNAP, so we exercised caution to keep the signal well below rails.
2.3. Seizure analysis
As suggested by others (Finnerty and Jefferys, 2000; Lévesque and Avoli, 2013; Lévesque et al., 2009), we defined seizure activity as an increase in signal power over 30 Hz, as there is the potential for slow drifts in signal amplitude caused by urethane anesthesia (Clement et al., 2008; Suzuki and Smith, 1988). We performed an FFT analysis of each ECoG signal, however in all cases we found that the increase in signal amplitude was due to an increase in signal power over 30 Hz, not due to slow wave changes characteristic of urethane. Nakase et al. in similar work defined seizure activity as an increase in signal amplitude, and we believe our data are comparable, as we did not observe the characteristic slow wave signals from urethane, so we show data in amplitude for simplicity. Our previous work (Budde et al., 2018), Nakase et al., and several other publications from the group (Nakase et al., 2016; Saito et al., 2006; Sakamoto et al., 2008) present a more detailed characterization of the EEG/ECoG waveform. Our results are consistent with this previous work, so we do not include this analysis for brevity.
2.4. Material construction
Additional construction information for the pH electrodes and the nerve cuff electrodes are in the online supplement.
2.5. Experimental groups
We report on a total of 38 animals across three intervention strategies, including controls. Experimental groups are summarized in Table 1. Group 1: we studied the effect of gastric vagotomy in 19 animals, 14/19 of which had kainic acid induced seizures during recordings, 7/14 of which also had the gastric nerve severed, and 1/14 had electrical blocking of nerve conduction. Group 2: we studied the effect of low initial stomach volume in 18 animals, 13/18 of which had kainic acid induced seizures during data collection, 7/13 of which were also fasted prior to the experiment. Note that 6 animals are counted in both Group 1 and Group 2 - these animals had kainic acid induced seizures with no intervention performed. Group 3: we studied the effect of laryngeal nerve stimulation in 7 animals, which all received laryngeal stimulation with induced laryngospasm and without seizure.
Table 1:
Experimental group overview.
| Group # | 1 | 2 | 3 |
| Gastric vagotomy | Severed | No procedure | No procedure |
| Nerve stimulation | Gastric (n=1) | No procedure | Laryngeal |
| Diet | Ad lib | 12 h fast | Ad lib |
| n | 19 | 18 | 7 |
KA = kainic acid. Ad lib = unrestricted access to food and water. 12 h fast = food and water restriction for 12 hours before start of surgery, additional subcutaneous saline given prior to surgical procedures to restore to normal hydration. Shaded boxes indicate the characteristic change for each group.
2.6. Statistics
All statistical tests were done in R software. For quantitative data, normality was tested with the Shapiro-Wilk test, homoscedasticity was tested with Bartlett’s test, and comparisons were done with the student’s t-test. For categorical data, groups were compared with Barnard’s exact test (Barnard, 1945). All tests are two-tailed and α = 0.05. Other data are reported as mean ± standard deviation unless otherwise noted.
3. RESULTS
3.1. Seizure development and presentation
As previously reported, we observed that animals can have varying responses to KA-induced seizures. Due to the suppression of motor activity from anesthesia, seizure activity is gauged by ECoG recording, physical observations, and respiratory patterns, instead of motor activity or the Racine scale (Finnerty and Jefferys, 2000; Lüttjohann et al., 2009). For all animals (21/21) seizure activity began with a period of extended status usually lasting at least 10 – 15 min. This period generally began 10 – 45 min after the initial injection of KA. During this period the animal’s respiration slowed, and there were not obvious visual manifestations of seizure, and ECoG was larger than baseline, with additional high frequency components. Following this initial period of status animals experienced a period of continuous seizure activity that typically lasts several hours before declining, but due to our short experimental window the seizure activity appears constant throughout the experiment. As the animals are anesthetized, there are no significant motor movements, but based on extensive experiments on awake animals, this dose would produce GTCS similar to a patient with temporal lobe epilepsy (Lévesque and Avoli, 2013). These seizure responses are described in more detail in our previous work and the Stewart papers (Budde et al., 2018; Nakase et al., 2016; Saito et al., 2006; Sakamoto et al., 2008). After the initial period of status, 19/21 seizing animals displayed a marked increase in respiratory rate with a characteristic respiratory pattern that includes rapid shallow gasping with occasional augmented breaths. Respiratory rate is very high, normally over 300 breaths per minute, with an augmented breath approximately every 7 breaths. Animals with this seizure response may also experience transient apneas with no chest movement. Brain activity was larger than baseline (3.9 ± 2.9 dB) and remained elevated until the end of the experiment. These animals displayed visual signs of seizure during this continuous seizure activity, including exophthalmos, whisker twitching, lifting of the head (which we now suspect to be air swallowing), a rapidly increased respiration rate, occasionally transient apneas with no diaphragmatic movements (seemingly central) and rarely minor limb or tail movements. After status, 2/21 seizing animals experienced a different seizure response marked by respiratory depression and cyanosis, in which we observed severe change in color of the hind- and forepaws. These two animals displayed a gradual reduction in respiratory rate and steady or declining brain activity until death (−1.4 ± 2.7 dB), and none of the visual signs of seizure just described. These observations consistent with a less common response to KA that has been observed before (Budde et al., 2018; Nakase et al., 2016), but has not been extensively studied. ECoG analysis shows that the fasted animals with an 80% KA dose had similar seizure development, magnitude, and presentation as non-fasted animals with the standard dose. This analysis is included in the supplement (Section S2, Figure S1).
3.2. Deaths
Six of 21 animals died from seizures before the end of the experiment. Four of these animals had the more common seizure response. These four animals all received no intervention and had experienced acid reflux prior to death. All four animals displayed high respiratory rates (>300 / min), larger-than-baseline ECoG (6.4 ± 2.4 dB), and visual signs of seizure, until experiencing an abrupt terminal obstructive apnea, with a flat respiratory signal but obvious movements in the abdomen suggesting respiratory effort. Respiratory effort persisted until death, which occurred within 2 minutes of apnea onset. All of these observations are consistent with a fatal laryngospasm caused by acid reflux. Two of the animals that died from their seizures had the less common seizure response. Both of these animals were fasted before the experiment. These animals displayed respiratory rates which gradually slowed from baseline until death (<15 / min). The animals displayed marked cyanosis indicated by blue hind- and forepaws, and one experienced acid reflux prior to death. The two animals had similar or less than baseline ECoG until death (−1.4 ± 2.7 dB). Due to the marked differences in animal presentation both before and during death, we categorize these deaths separately in following statistics. The differences in ECoG activity for these two types of deaths can be seen in Figure 1.
Figure 1:
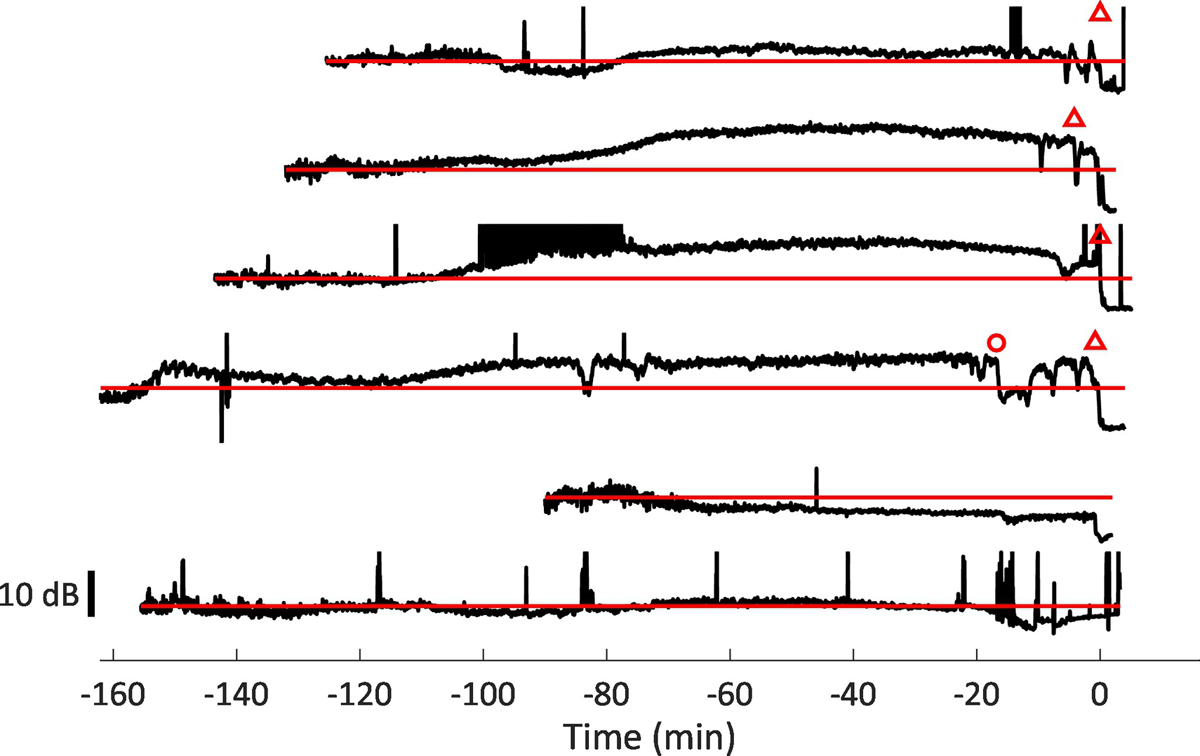
Electrocorticography (ECoG) recordings from all animals (n = 6) with kainic acid seizures that died during seizure. Open triangles denote onset of obstructive apnea. Open circle denotes an extended period of multiple central apneas which was followed by a period of ECoG depression. ECoG is presented as the RMS of the signal, in 3 second bins. Horizontal red lines (grey in print version) denote baseline ECoG, which is collected in the first 5 min of each experiment, before seizures are induced. Each trace represents a separate experiment, and includes all data, including baseline for the first 5 min, until data recording stopped after death. Times are normalized so that death occurs at 0 min for each trace, and kainic acid was injected after the first five minutes of data shown. The top four traces represent animals which experienced sudden obstructive apnea and death. The bottom two traces represent animals which experienced gradual respiratory depression and hypoxia. Animals that suddenly died have ECoG signals that are much larger than baseline until obstructive apnea and death. Animals that experience respiratory depression have ECoG signals that gradually decline until death.
3.3. Effects of interventions on deaths during seizure
Four of 6 seizing animals with no intervention experienced sudden fatal obstructive apnea, and 2/6 survived. Two of 7 seizing animals with the fasting intervention experienced the slow, gradual respiratory depression until death, and 5/7 survived. Therefore, the fasting intervention significantly reduced the incidence of sudden fatal obstructive apnea (4/6 vs 0/7, p = 0.012), but does not significantly increase the incidence of survival (4/6 vs 2/7 p = 0.27). All 7 seizing animals with gastric vagotomy survived. Therefore, gastric vagotomy significantly reduced the rate of sudden obstructive death and increased survival (4/6 vs 0/7, p = 0.012). These outcomes are summarized in Figure 2. All 14 animals with nerve stimulation survived, but procedural differences make statistical comparisons inappropriate, and therefore they are excluded from Figure 2. All 10 non-seizing control animals survived.
Figure 2:
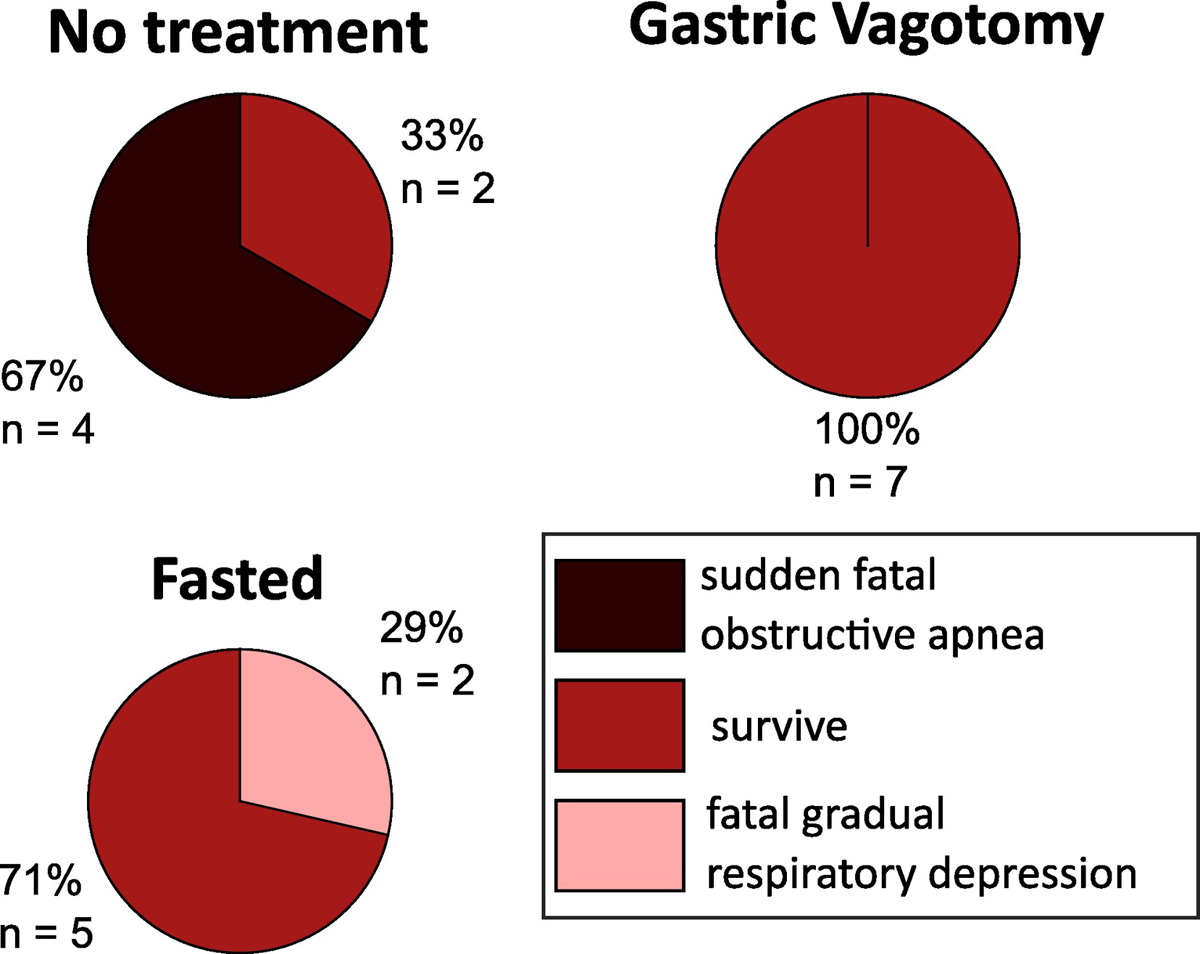
Outcomes for seizure experiments. These charts show the results for seizing animals with no intervention (n = 6), the gastric nerve severed (n = 7), or fasted before the experiment (n = 7). Sudden fatal obstructive apnea = animals experienced acid reflux, followed by sudden obstructive apnea and death, consistent with laryngospasm. Survive = animals survived seizures until euthanasia. Respiratory depression = animals experienced gradual respiratory depression and hypoxia until death.
3.4. Acid reflux
Acid reflux was characterized by pH < 3 on both electrodes. pH change speed, lag between electrodes, and time from reflux until death are all consistent with our previous findings in (Budde et al., 2018). We observed acid reflux in 9/21 seizing animals − 6/6 animals without intervention, 1/7 animals with gastric vagotomy, and 2/7 animals that were fasted. Both gastric vagotomy and fasting significantly reduced incidence of acid reflux (p = 0.001, p = 0.008, respectively). No acid reflux was observed in non-seizing animals.
3.5. Stomach contents
Stomach contents were collected for all animals with seizures (n = 21), and for their non-seizing controls (n = 10). We analyzed the volume of liquid in the stomach, which we normalized to animal weight, and report as a percentage. These data are summarized in Figure 3. Both gastric vagotomy and fasting significantly reduced the amount of liquid in the stomach in seizing animals (p = 0.015, n = 13, p = 0.002, n = 13, respectively), but they were not significantly different from one another (p = 0.25, n = 14). The interventions reduced some, but not all of the acid produced during seizure, as seizing animals with gastric vagotomy or fasting had significantly more liquid compared to non-seizing animals with the same intervention (p = 0.034, n = 12, p = 0.015, n = 12, respectively). These differences are likely greater than the data suggests, as all animals with seizure and without intervention experienced acid reflux, and the amount of liquid we recovered does not include the contents of the esophagus or liquid that exited the mouth.
Figure 3:
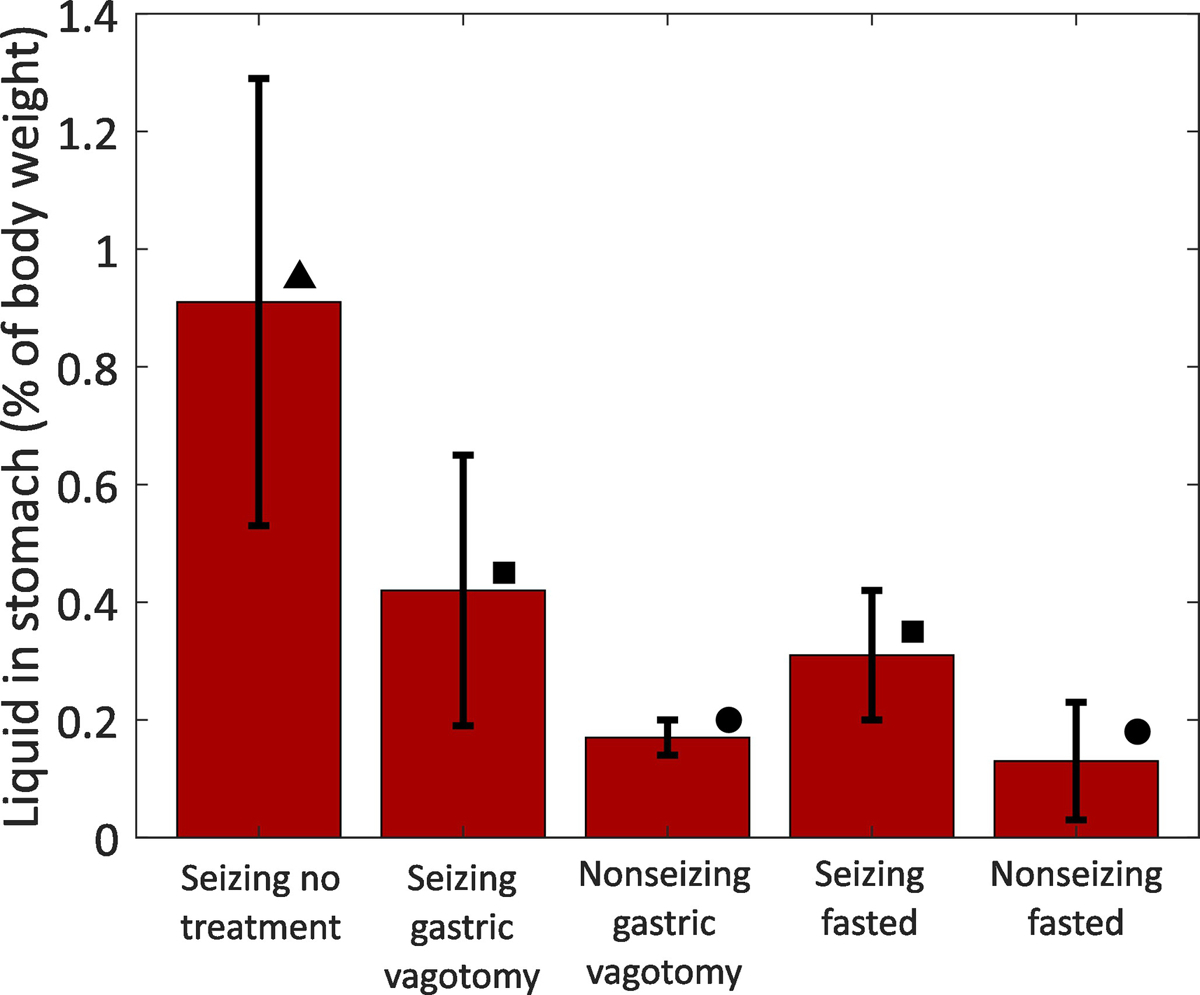
Comparison of liquid volume in animal stomachs. The data show the liquid volume in the stomachs of animals as a percentage of total body weight. Error bars show mean ± standard deviation. Each shape (triangle, square, circle) denotes a group that is statistically significantly different from the other groups. Interventions reduced some, but not all of seizure-induced acid production. Group numbers: Seizing no treatment n = 6; Seizing gastric vagotomy n = 7; Nonseizing gastric vagotomy n = 5; Seizing fasted n = 7; Nonseizing fasted n = 5.
We observed that the stomachs of seizing animals often had a large amount of air and had higher internal pressure than the stomachs of non-seizing animals. Examples are shown in Figure S3. We also observed large quantities of air in the stomach of fasted seizing animals, with almost no solid mass in their stomachs. We observed no air in the stomachs of non-seizing animals. In our previous work we observed that seizing animals will often lift their head during seizures, looking like a hiccup or belch. We now hypothesize that this behavior is aerophagia (air swallowing), which would explain the presence of air.
3.6. Respiratory effort following laryngospasm in seizing and non-seizing animals
We placed acid onto the larynx of a non-seizing animal to observe a normal reflex response. We repeated the experiment 5 times. In each repetition the animal initially experienced strong laryngospasm for a period of 28 – 40 seconds. During strong laryngospasm the animal could make infrequent breaths, but the larynx was completely closed for most of this period. The animal made obvious movement in the neck and abdomen, suggesting respiratory effort. Thermocouple data during this time confirms that there was only airflow during the infrequent breaths. Strong laryngospasm would spontaneously end, and the animal would begin a period of labored breathing. Movements in the abdomen were exaggerated compared to normal respiration, and respiration noise was more noticeable. There was an increase in fluid around the larynx, and it appears that the labored breathing was an attempt to clear this fluid from the larynx. Over several minutes, the amount of the fluid around the larynx decreased, and respiration speed and abdominal movement returned to normal. In the first trial, following strong laryngospasm, the glottis blocked view of the laryngoscope, and the animal began breathing through its nose, so we do not have all respiratory data for this trial, as the thermocouple was placed in the cannula near the mouth. We note that animals normally breathe through their nose, but in the presence of the laryngoscope they will preferentially breathe through their mouth. In every trial the animal was able to recover from laryngospasm on its own and return to normal breathing after several minutes. At no point did these laryngospasms appear to be life threatening. Over approximately 75 minutes the animal experienced 5 obstructive apneas and survived, in stark contrast to the seizing animals who experience obstructive apnea during seizure. Seizing animals that experience sudden obstructive apnea display respiratory effort for approximately 15 seconds before terminal apnea. A comparison of the respiratory patterns and effort following obstructive apnea of both KA and non-KA animals can be seen in Video 1. Portions of Video 1 are shown in Figure 4.
Figure 4:
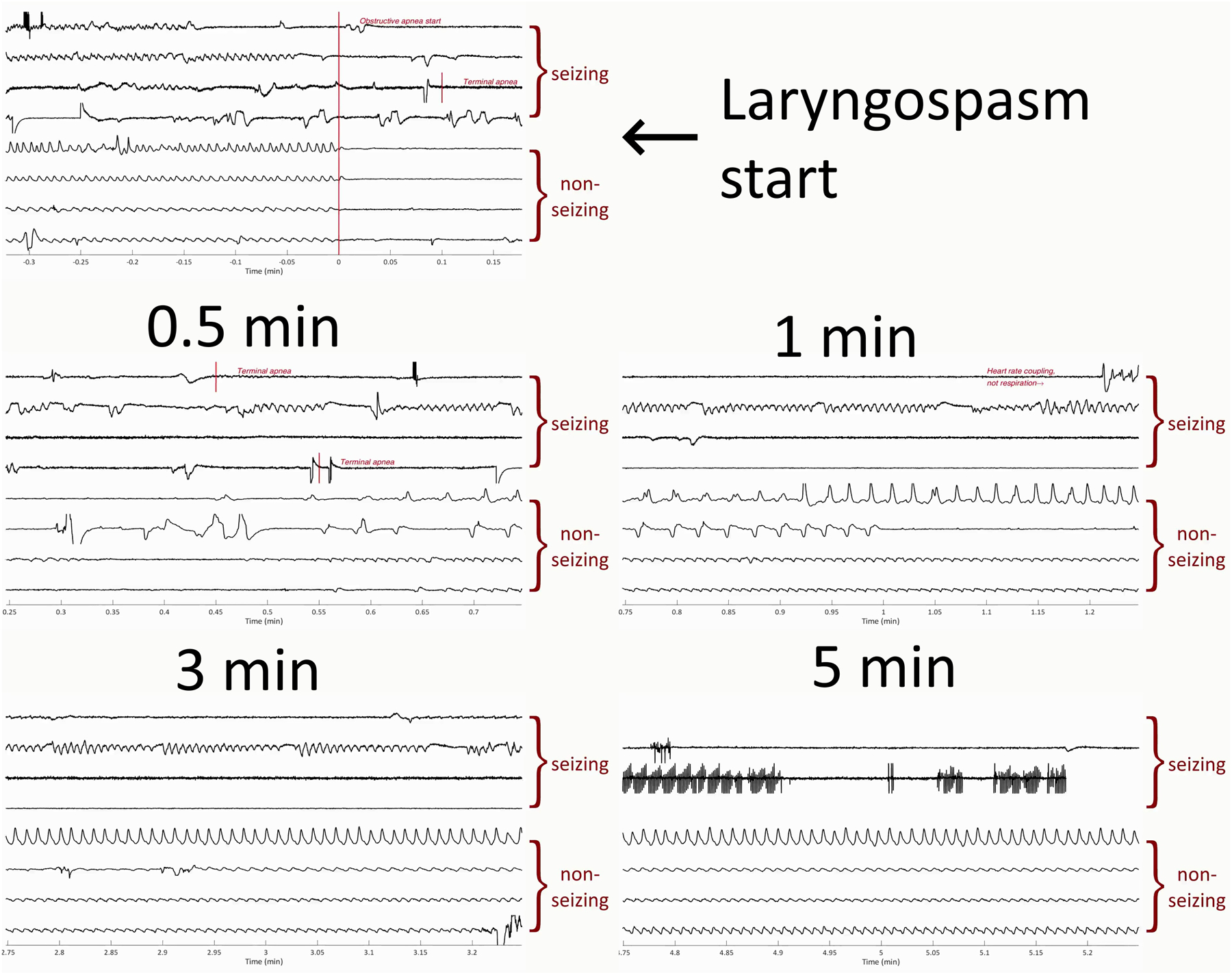
Data from Video 1, showing laryngospasm respiratory response for seizing and non-seizing animals. We encourage readers to view Video 1 for a more complete presentation of the data. All traces show thermocouple data measuring respiration, where amplitudes are normalized and of arbitrary units. The top four traces of each picture are from animals who had seizures induced with kainic acid, no intervention, and experienced acid reflux, obstructive apnea, and death (marked “seizing”). The bottom four traces in each picture are from a non-seizing animal in which we applied artificial acid to the larynx to induce laryngospasm. Times are normalized by the start of laryngospasm. The traces show that seizing animals quickly experience terminal apnea after laryngospasm, while non-seizing animals will recover.
3.7. Laryngeal nerve stimulation
During normal respiration we observed that the larynx is almost entirely open during inhalation and is semi-open during exhalation. We applied waveforms of varying amplitude and found that we could keep the larynx in the semi-open position indefinitely by applying the established waveform (500 μA with a pulse width of 100 μs and a pulse repeat time of 500 μs), and that increasing amplitude did not further open the larynx. When held in this semi-open position, the animal was able to breathe normally. These results can be seen in Figure 5. The respiratory data did not significantly change, even when the larynx is held open by stimulation, suggesting that the stimulation could be sufficient to enable respiration during laryngospasm. After determining this waveform, we applied acid to induce laryngospasm which immediately closed the larynx. When we applied the stimulation, the larynx could be held in the semi-open position, even in the presence of acid, for as long as the stimulation was maintained. During this time there was airflow through the larynx. If we stopped the stimulation before the animal fully recovered (i.e. before the 28 s period of complete closure observed previously), then it would again experience a strong laryngospasm. These results can be best seen in Video 2. Portions of Video 2 can be seen in Figure S4. We measured the distance between vocal folds in this Figure and found the larynx to be 100% open normal respiration, 23% open after laryngospasm but before stimulation, 59% open during laryngospasm and while stimulation was on, and 25% open during laryngospasm but after stimulation had been stopped. We repeated acid applications several times for each animal, but after 5 or more acid injections the respiration rate began to slow significantly, and, in some cases, we also observed that the respiration sounded wet and ragged. We terminated the experiment at this point, but in two cases animals did not recover and died. We believe that inspiration of the acid, mucous, and/or saliva into the trachea may have caused respiratory distress. For three experiments we damaged one or both RLNs, as there was laryngeal paralysis on the damaged side, even during normal respiration. In 3/3 cases electrical stimulation of these damaged nerves yielded no effect. These experiments suggest that our stimulation was acting on the RLNs directly, not the surrounding muscles. These experiments suggest that laryngeal stimulation can open the larynx in the event of laryngospasm, but that survival is still dependent on the amount of acidic fluid inspired.
Figure 5:
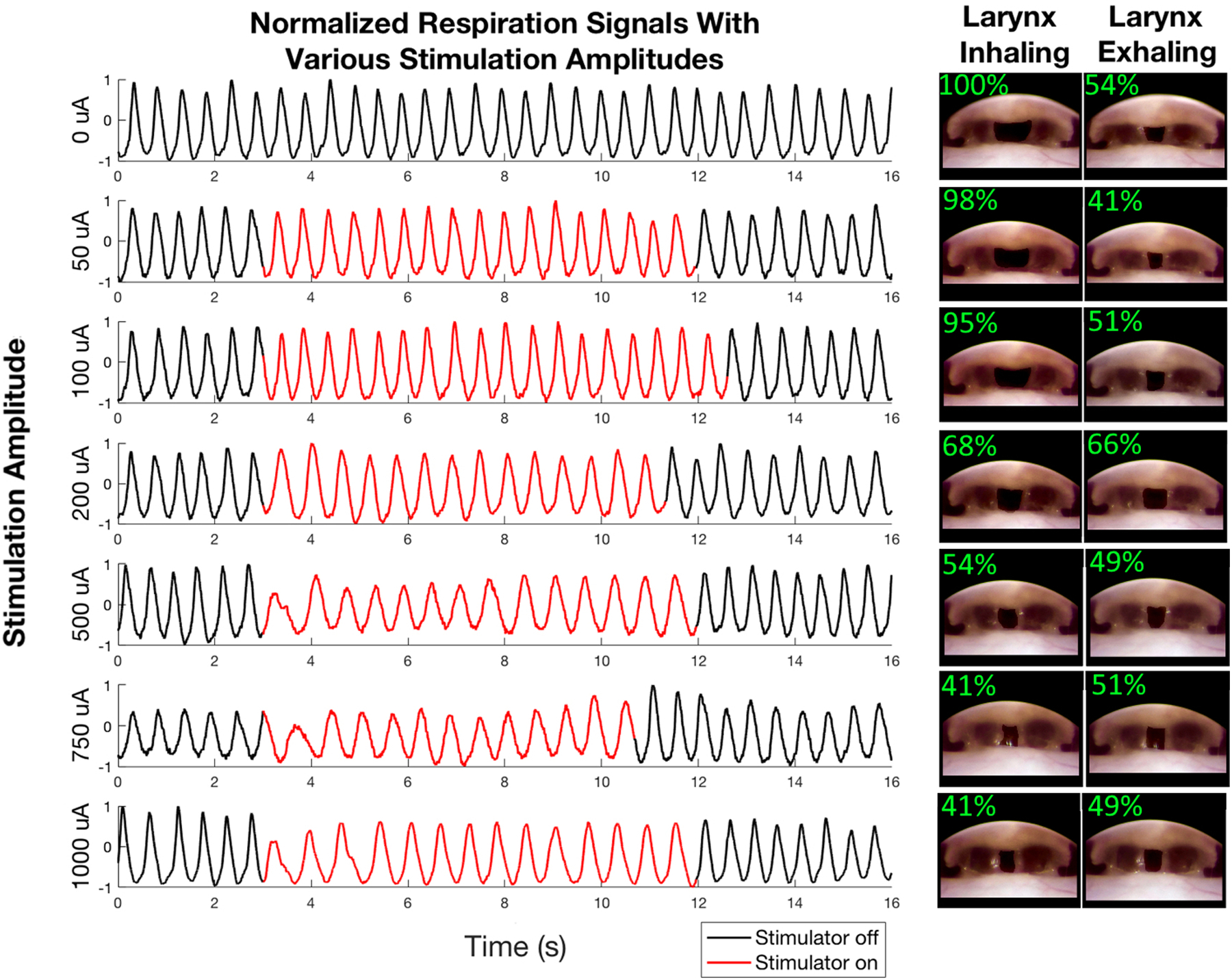
Effects of laryngeal nerve stimulation. We stimulated both of the recurrent laryngeal nerves with biphasic waveform with the pulse width = 100 μs, pulse repeat time = 500 μs, and variable amplitude. The traces show the normalized respiration signal before, during, and after stimulation. We applied stimulation amplitudes of 0, 50, 100, 200, 500, 750, and 1000 μA as indicated to the left of each respiration trace. The images on the right of the Figure depict the larynx during inhalation and exhalation while stimulating with each amplitude. In each image the region between the vocal folds (i.e. the region in which the trachea is visible past the larynx) has been artificially darkened to make it easier to see the degree to which the larynx is open. The top left of each inset figure shows the extent to which the larynx is open.
3.8. Gastric nerve stimulation to block signaling
In one animal we blocked signaling on the gastric nerve during seizure, to approximate the effect of gastric vagotomy with a reversible method. When we applied the stimulation waveform only, we were able to record the evoked CNAP on the recording cuff. This response showed a clear stimulation threshold, suggesting it is a real CNAP and not a stimulation artifact. The distance between the stimulation and recording cuff was approximately 7 cm, and the response was recorded approximately 25 ms after stimulation, so the velocity of this response was approximately 2.8 m/s, suggesting a B fiber (typically 3.0 m/s or more) (Mirza et al., 2018; Qing et al., 2018; Ward et al., 2015). We applied both the stimulation waveform and the blocking waveform simultaneously and did not observe the CNAP response. This response also showed a clear threshold, suggesting that the blocking effect was due to physiological effects, and not masking by extra noise or artifact. We show a representative sample of CNAP evocation and blocking in Figure 6. After verifying that we could block CNAPs, we applied blocking continuously for 5 min to check for immediate nerve damage, which we did not observe. We then tried to apply the blocking waveform for two hours - the duration of our other experiments - to approximate the effect of gastric vagotomy. After this time, we could still evoke and block CNAPs, and the animal was euthanized. This animal did not have acid reflux, displayed common signs of seizure, and its stomach had a large amount of liquid (1% of total body mass).
Figure 6:
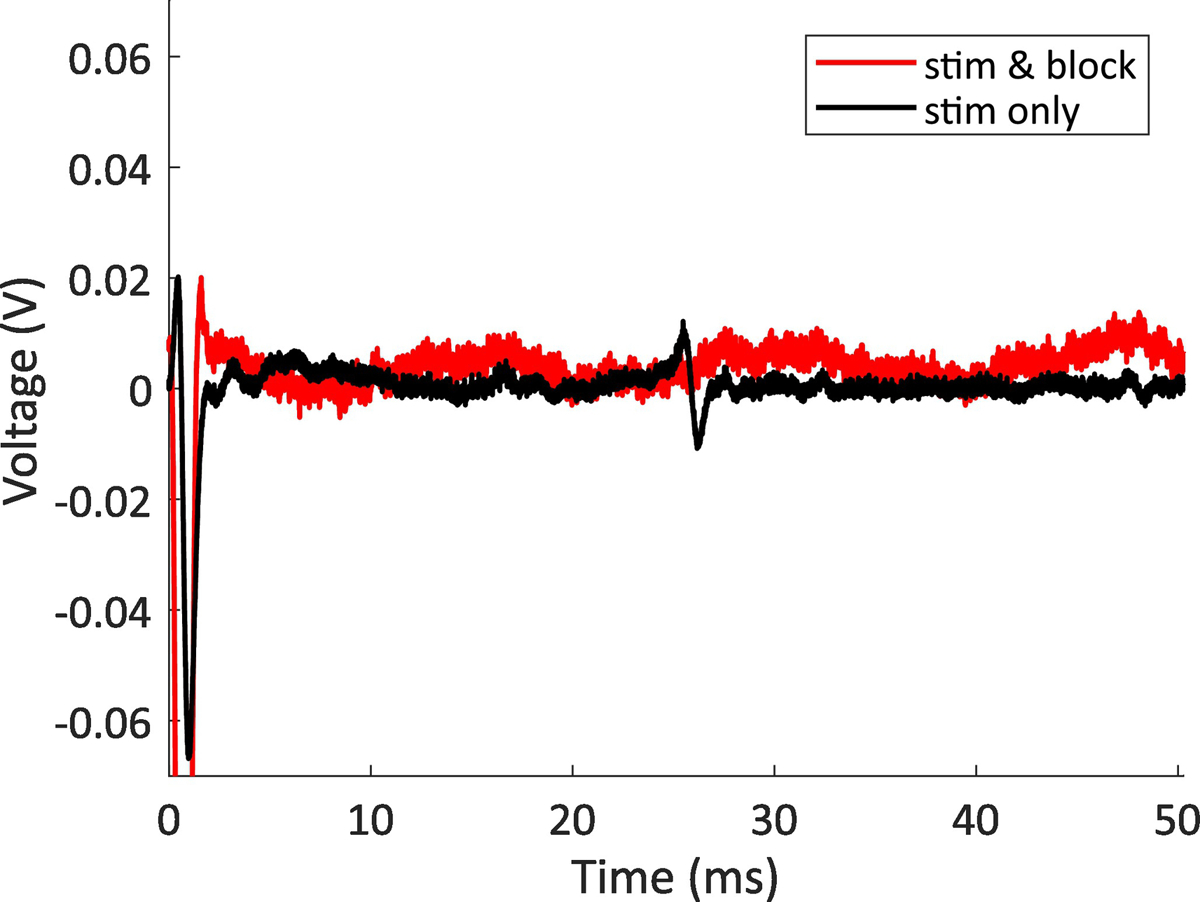
CNAP blocking example. The data show two stimulation parameters, one with the stimulation waveform applied to the cervical vagus, and one with both the stimulation waveform applied to the cervical vagus and the blocking waveform applied to the gastric branch of the vagus. The nerve response is at approximately 25 ms, so for our interelectrode spacing of 7 cm, the response has a conduction velocity of 2.8 m/s, placing it within the normal range for a B fiber (typically 3.0 m/s or more)(Mirza et al., 2018; Qing et al., 2018; Ward et al., 2015). This sample is representative of the results seen in all animals when only applying blocking stimulation for 5 min or less.
4. DISCUSSION
In this paper we explore three interventions to prevent acid reflux, obstructive apnea, and death during seizure in a rat model. Some methods were able to reduce or eliminate sudden death, and all provided additional insight to the mechanism of this type of sudden death. Here we discuss these insights, as well as the potential clinical translation of each technique, assuming these mechanisms are eventually shown to be relevant to human epilepsy.
4.1. Gastric vagotomy
Severing the gastric nerve significantly reduced acid reflux, sudden death, and the amount of liquid in the stomach. This was the most effective technique, as it had the best survival. These results help address some limitations of the model. One limitation of the acute systemic KA model is that the drug is administered systemically, so it is possible that some of the response that we see is not the direct result of seizure activity. In our previous work, we speculated that it is possible that KA may act directly on the stomach, but these experiments suggest otherwise. Our results suggest that signaling along this nerve is vital to the acid reflux / sudden death mechanism in this model. It is still possible that a systemic effect of KA modifies gastric nerve signaling - for example, by acting directly on the brainstem (White et al., 1991; Yang et al., 1999) - but these experiments help support the theory that these mechanisms are seizure-driven. Gastric vagotomies are already performed in patients with GI disorders, such as ulcers or frequent acid reflux (Schirmer, 1989), so this intervention may be translatable to human patients. Patients with drug resistant epilepsy and frequent GTCS have a 35% lifetime risk of SUDEP (Buchhalter et al., 2013; Thurman David et al., 2014), so a gastric vagotomy may be a reasonable preventative step. Gastric nerve blocking with electrical stimulation may be a reversible alternative. Pelot and Grill have recently proposed additional blocking techniques on the vagus (Pelot and Grill, 2020).
4.2. Fasting
Fasting animals significantly reduced acid reflux and sudden fatal obstructive apnea. These animals required a lower dose of KA to develop similar physical and brain signal responses as unfasted animals. Other researchers have found that fasting reduces animal mass, but not brain volume, possibly explaining this result (Jensen et al., 2013). One method of stomach acid production is triggered by distension of the stomach wall (Debas and Carvajal, 1994). Seizure activity may initiate stomach acid production, which is exacerbated by aerophagia, and result in a positive feedback loop in which stomach distension triggers even more acid production. Negative feedback loops to prevent excess stomach acid production (Itoh et al., 1975; Johnson, 1984), may malfunction or be overwhelmed. These processes may exacerbate reflux into the esophagus. Fasting may reduce the starting volume in the stomach such that this process is sufficiently delayed so that it does not occur during our experimental window, or, in a clinical setting, before a patient recovers from a seizure. It is likely that fasting only delays the mechanism we have observed, but a delay may still have clinical relevance. We speculate that if we performed the opposite of these experiments - started with animals with a full stomach - then perhaps we would see an accelerated version of the mechanism. Fasting is the most accessible potential translational intervention. If effective, fasting before bed, which is when most SUDEP cases occur, could help reduce mortality.
4.3. Laryngeal nerve stimulation
These experiments demonstrated that it is possible to hold the larynx semi-open during acid-induced laryngospasm but raise questions as to how valuable this intervention might be. The translational ability of this therapy will hinge on the potential volume of acid inspiration and the damage that this inspiration may cause. Acid inspiration, even if it causes lasting damage, is certainly preferable to sudden death. Many patients with drug resistant epilepsy have vagal nerve stimulation (VNS) devices. As the recurrent laryngeal nerves are branches of the vagus nerve, these devices may be able to activate the same response we target in this study with only minor modifications. Therefore, this therapy, if effective, could reach many patients with minimal additional costs.
4.3.1. Laryngeal nerve stimulation outside epilepsy
Laryngeal stimulation may have clinical relevance outside of epilepsy. Some children with chronic aerodigestive disorders will experience laryngospasm as an acute crisis event (Gaston, 2018). In these cases, laryngospasm is often caused not by a low pH, but by mechanical stimulation of the larynx due to eating or drinking (Halstead, 1999), often in children born premature (Gaston, 2018) with aerodigestive orders such as laryngomalacia, spasmodic croup, or subglottic stenosis (Gaston, 2018; Halstead, 1999; Orenstein, 2001). In these cases, laryngeal stimulation could open the larynx without fear of acid causing permanent damage to the lungs.
4.3.2. Difference in laryngospasm presentation in seizing and non-seizing animals
We observed a noticeable difference in laryngospasm response between seizing and non-seizing animals. Seizing animals that experienced obstructive apnea never recovered, and the time from obstructive apnea onset to terminal apnea was within 5 minutes for all cases. Conversely, the non-seizing animal was able to survive 5 obstructive apneas over more than one hour (with time for recovery in between). These data suggest that seizing animals may die “too quickly” from obstructive apnea, which may imply an additional mechanism that follows laryngospasm. Further work is necessary to explore the differences between the life-threatening laryngospasms seen in seizing animals and the non-life-threatening laryngospasms seen in non-seizing animals.
These differences could support a theory in part proposed to us by Dr. Karl G. Rosén. Dr. Rosén studied infant swimming, the laryngeal chemoreflex, and the breath holding reflex, and suggested they might hold some relevance to our work. The laryngeal chemoreflex (LCR) is present in many young mammals, including humans, and causes central apnea, bradycardia, laryngeal closure, and hypotension in response to laryngeal stimulation (Davies et al., 1988; Heman-Ackah et al., 2009; Marchal et al., 1982). In many cases the response is mild and recoverable, but, in some cases, it is severe and fatal (Goding, 1998; Heman-Ackah et al., 2009). The reflex may be caused by water, acid, or mechanical stimulation, including laryngospasm (Heman-Ackah et al., 2009). The LCR is only evident early in life, often disappearing within the first year for humans, when it is replaced by diving and choking reflexes and fear response (McGraw, 1939). Several authors have suggested the LCR is relevant to sudden infant death syndrome (Donnelly et al., 2016; Heman-Ackah et al., 2009; Marchal et al., 1982; Scadding et al., 2014; Van Der Velde et al., 2003; Xia et al., 2013). Limited evidence has suggested that the apnea seen in the LCR is related to medullary respiratory centers in the brainstem (Donnelly et al., 2016; Van Der Velde et al., 2003). There is some evidence that the LCR response is more severe if animals are already hypoxic (Xia et al., 2013). It is therefore possible, if this reflex is still present in adult animals, that an obstructive apnea could trigger a subsequent central apnea. This central apnea may be severe enough to be fatal, and the severity of the reflex may be exacerbated by hypoxia from previous ictal apneas. The LCR may explain why seizing animals appear to be more susceptible to death from obstructive apnea and show respiratory effort for a shorter duration than non-seizing animals. The LCR is similar to the mammalian diving reflex, another recent focus of study in SUDEP (Mooney et al., 2019; Vega, 2018; Vincenzi, 2019)
4.4. Proposed mechanism
Our data support the following hypothesis: that seizure activity sends malfunctioning signals via the gastric branch of the vagus nerve to the stomach. These signals, through some combination of gastric branch signaling and possibly other acid production pathways, result in an increase in fluid volume in the stomach. This increase in fluid volume, possibly aggravated by aerophagia, results in acid reflux into the esophagus. Acid moves up to the larynx, where it ultimately triggers laryngospasm and obstructive apnea. Obstructive apnea may itself be sufficient for death, or obstructive apnea may activate pathways of the LCR, which may contribute to ictal hypoxia and post-ictal brainstem depression to trigger terminal central apnea and asystole. Further work is needed to verify this hypothesis.
4.5. Limitations and future work
This work is limited by a small sample size (though results are statistically significant), lack of electrocardiography and blood pressure recording, and potential systemic effects of kainic acid. Only female animals were used, so results may be sex-dependent. ECoG was sampled below the bandpass, introducing the possibility for aliasing, however we have no evidence that this occurred. Insights into electrical blocking are limited by the extended seizure duration of this model. Comparisons between laryngospasm response from seizing and non-seizing animals are limited by differences in the stimulating acid (stomach acid vs artificial acid) and by the animal position (prone vs supine). Our hypothesis about the LCR is limited as we do not know to what extent the reflex is present in anesthetized, seizing adult animals. We still do not know if seizure associated acid reflux is present in humans. It is still too early to recommend any of these interventions for human patients. In future work we hope to address some of these concerns by exploring this mechanism in chronic models of epilepsy. We also plan to evaluate the therapeutic effect of common acid reflux treatments like antacids and proton pump inhibitors.
4.6. Translational outlook
Acid reflux during seizure must still be verified in human patients. We hope in future work to incorporate esophageal pH recording, a common, safe procedure, with routine monitoring in an epilepsy monitoring unit (EMU) to determine if there is a correlation between patient seizures and acid reflux. If EMU monitoring establishes a relationship between seizure activity and acid reflux then common steps to prevent acid reflux, like proton pump inhibitors, could be tested in human patients in the EMU. Continued EMU monitoring may establish a relationship between acid reflux, seizure, and a case of SUDEP or near-SUDEP, which may justify preventative treatment of acid-reflux for high risk patients.
4.7. Implications for SIDS
The LCR has been proposed to be relevant to SIDS since the 1970’s. SIDS risk decreases sharply around the same time that the LCR is replaced with the breath holding reflex (Heman-Ackah et al., 2009). SIDS and SUDEP share some risk factors like increased prevalence at night and in the prone position, so it is possible that there is a shared mechanism (Devinsky et al., 2016; Heman-Ackah et al., 2009; Kinney and Thach, 2009). If this is eventually proven, then some of these treatment strategies may hold relevance for SIDS.
5. CONCLUSIONS
We expand upon our previous work in this model and provide new insights on potential mechanisms and prevention of acid reflux induced laryngospasm during seizure. We speculate as to a unified SUDEP mechanism to which laryngospasm may contribute. Our work may have some relevance to human SUDEP.
Supplementary Material
Video 1: Laryngospasm respiration See attached laryngospasm respiration video
Video Caption: Video showing thermocouple respiration traces for several animals. The top four traces, labelled “KA”, are from animals in which seizures were induced with kainic acid. In these traces, animals, who had previously had acid reflux into the esophagus, experienced sudden obstructive apnea and death. The bottom four traces, labelled “Control”, are subsequent experiments from a single non-seizing animal in which we induced laryngospasm by placing an artificial acid on the larynx. The animal recovered to baseline between experiments, and the traces are presented in this order (i.e. we induced laryngospasm to obtain data shown in trace #5. The animal then recovered, and we induced laryngospasm again to obtain the data shown in trace #6). Times are normalized so that laryngospasm begins at 0 min for all traces. For animals that died from the laryngospasm, the last observable breath is marked as “Terminal apnea.” Perturbations in the signal after this time are not animal respiration, and may be the heart rate coupling onto respiration, movement as we moved the animal to remove the stomach after death, or noise from recording open air. Y-axis units are arbitrary. This respiration data was down-sampled by 8x to accommodate the smooth plotting of so much data (functional sample frequency = 156 Hz).
Video 2: Larynx stim video See attached larynx stimulation video
Video Caption: Video showing laryngeal stimulation opening the larynx during laryngospasm. In this video we placed 0.1 mL of simulated stomach acid on the larynx of a non-seizing animal to induce laryngospasm, closing the larynx immediately. We then apply electrical stimulation, which opens the larynx immediately. When electrical stimulation is stopped, the larynx closes immediately again. This stimulation is repeated until the animal begins to recover from laryngospasm.
Highlights:
Animals in the acute KA model have more liquid in their stomach
The gastric nerve is necessary for acid overproduction, reflux, and sudden death
Seizing animals die more easily from laryngospasm than non-seizing animals
6. ACKNOWLEDGEMENTS
We would like to thank Dr Rosén for introducing us to the breath holding reflex and laryngeal chemoreflex. We would also like to thank Dr. Ben Gaston and his team at Rainbow Babies Children’s Hospital for meeting with us to discuss potential mechanisms and clinical translation.
Finally, we would like to thank Dr. Thelma Lovick for her continued advice and support. This research was supported by NIH SPARC (award number OD023847).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure of conflicts of interest
The authors have no conflicts of interest to report.
9 References
- Barnard GA, 1945. A New Test for 2 × 2 Tables. Nature 156, 177. [Google Scholar]
- Berg A, 2001. Mortality in Epilepsy. Epilepsy Currents 1, 28–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchhalter J, So EL, Hesdorffer DC, Berg AT, Kanner AM, Mintzer S, Hirsch LJ, Nashef L, Donner EJ, Bateman LM, Tomson T, Devinsky O, Pollanen M, Goldman A, Richerson G, Faingold CL, Buchanan GF, Panelli R, Noebels J, Vatta M, 2013. Partners Against Mortality in Epilepsy Conference Summary. Epilepsy Currents 13, 5–21. [Google Scholar]
- Budde RB, Arafat MA, Pederson DJ, Lovick TA, Jefferys JGR, Irazoqui PP, 2018. Acid reflux induced laryngospasm as a potential mechanism of sudden death in epilepsy. Epilepsy Research 148, 23–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clement EA, Richard A, Thwaites M, Ailon J, Peters S, Dickson CT, 2008. Cyclic and Sleep-Like Spontaneous Alternations of Brain State Under Urethane Anaesthesia. PLoS ONE 3, e2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosby ND, Janik JJ, Grill WM, 2017. Modulation of activity and conduction in single dorsal column axons by kilohertz-frequency spinal cord stimulation. Journal of Neurophysiology 117, 136–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies AM, Koenig JS, Thach BT, 1988. Upper airway chemoreflex responses to saline and water in preterm infants. Journal of Applied Physiology 64, 1412–1420. [DOI] [PubMed] [Google Scholar]
- Debas HT, Carvajal SH, 1994. Vagal regulation of acid secretion and gastrin release. The Yale Journal of Biology and Medicine 67, 145–151. [PMC free article] [PubMed] [Google Scholar]
- Devinsky O, 2004. Effects of Seizures on Autonomic and Cardiovascular Function. Epilepsy Currents 4, 43–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devinsky O, 2011. Sudden, Unexpected Death in Epilepsy. New England Journal of Medicine 365, 1801–1811. [DOI] [PubMed] [Google Scholar]
- Devinsky O, Hesdorffer DC, Thurman DJ, Lhatoo S, Richerson G, 2016. Sudden unexpected death in epilepsy: epidemiology, mechanisms, and prevention. The Lancet Neurology 15, 1075–1088. [DOI] [PubMed] [Google Scholar]
- Donnelly WT, Bartlett D, Leiter JC, 2016. Serotonin in the solitary tract nucleus shortens the laryngeal chemoreflex in anaesthetized neonatal rats. 101, 946–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Druschky A, Hilz MJ, Hopp P, Platsch G, Radespiel-Tröger M, Druschky K, Kuwert T, Stefan H, Neundörfer B, 2001. Interictal cardiac autonomic dysfunction in temporal lobe epilepsy demonstrated by [123I]metaiodobenzylguanidine-SPECT. Brain 124, 2372–2382. [DOI] [PubMed] [Google Scholar]
- Finnerty GT, Jefferys JGR, 2000. 9–16 Hz Oscillation Precedes Secondary Generalization of Seizures in the Rat Tetanus Toxin Model of Epilepsy. Journal of Neurophysiology 83, 2217–2226. [DOI] [PubMed] [Google Scholar]
- Gaston B, 2018. Personal communication with the authors.
- Goding GS, 1998. Correlation of Laryngeal Chemoreflex Severity With Laryngeal Muscle Response. 108, 863–872. [DOI] [PubMed] [Google Scholar]
- Halstead LA, 1999. Role of gastroesophageal reflux in pediatric upper airway disorders☆☆☆. 120, 208–214. [DOI] [PubMed] [Google Scholar]
- Heman-Ackah YD, Pernell KJ, Goding GS Jr, 2009. The laryngeal chemoreflex: An evaluation of the normoxic response. The Laryngoscope 119, 370–379. [DOI] [PubMed] [Google Scholar]
- Horn CC, Kimball BA, Wang H, Kaus J, Dienel S, Nagy A, Gathright GR, Yates BJ, Andrews PLR, 2013. Why Can’t Rodents Vomit? A Comparative Behavioral, Anatomical, and Physiological Study. PLoS ONE 8, e60537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh Z, Takeuchi S, Aizawa I, Honda R, 1975. NEGATIVE FEEDBACK MECHANISM OF GASTRIC-ACID SECRETION - SIGNIFICANCE OF ACID IN GASTRIC-JUICE IN MAN AND DOG. Surgery 77, 648–660. [PubMed] [Google Scholar]
- Jensen T, Kiersgaard M, Sørensen D, Mikkelsen L, 2013. Fasting of mice: a review. Laboratory Animals 47, 225–240. [DOI] [PubMed] [Google Scholar]
- Johnson LR, 1984. EFFECTS OF SOMATOSTATIN AND ACID ON INHIBITION OF GASTRIN-RELEASE IN NEWBORN RATS. Endocrinology 114, 743–746. [DOI] [PubMed] [Google Scholar]
- Kilgore KL, Bhadra N, 2014. Reversible Nerve Conduction Block Using Kilohertz Frequency Alternating Current. Neuromodulation 17, 242–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinney HC, Thach BT, 2009. The Sudden Infant Death Syndrome. New England Journal of Medicine 361, 795–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacuey N, Vilella L, Hampson JP, Sahadevan J, Lhatoo SD, 2018. Ictal laryngospasm monitored by video-EEG and polygraphy: a potential SUDEP mechanism, Epileptic Disorders. [DOI] [PubMed]
- Lamberts Robert J, Thijs Roland D, Laffan A, Langan Y, Sander Josemir W, 2011. Sudden unexpected death in epilepsy: People with nocturnal seizures may be at highest risk. Epilepsia 53, 253–257. [DOI] [PubMed] [Google Scholar]
- Langan Y, Nashef L, Sander JWAS, 2000. Sudden unexpected death in epilepsy: a series of witnessed deaths. Journal of Neurology, Neurosurgery & Psychiatry 68, 211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Devinsky O, 2005. The role of autonomic dysfunction in sudden unexplained death in epilepsy patients. Reviews in Neurological Diseases 2, 61–69. [PubMed] [Google Scholar]
- Lhatoo SD, Nei M, Raghavan M, Sperling M, Zonjy B, Lacuey N, Devinsky O, 2016. Non-seizure SUDEP: Sudden Unexpected Death in Epilepsy without preceding epileptic seizures. Epilepsia 57, 1161–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loughlin Christopher J, Koufman James A, Averill David B, Cummins Michelle M, Kim YJ, Little John P, Miller Inglis J, Meredith JW, 2009. Acid‐Induced Laryngospasm in a Canine Model. The Laryngoscope 106, 1506–1509. [DOI] [PubMed] [Google Scholar]
- Lévesque M, Avoli M, 2013. The kainic acid model of temporal lobe epilepsy. Neuroscience and biobehavioral reviews 37, 2887–2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lévesque M, Langlois JMP, Lema P, Courtemanche R, Bilodeau G-A, Carmant L, 2009. Synchronized gamma oscillations (30–50 Hz) in the amygdalo-hippocampal network in relation with seizure propagation and severity. Neurobiology of Disease 35, 209–218. [DOI] [PubMed] [Google Scholar]
- Lüttjohann A, Fabene PF, Van Luijtelaar G, 2009. A revised Racine’s scale for PTZ-induced seizures in rats. Physiology & Behavior 98, 579–586. [DOI] [PubMed] [Google Scholar]
- Mameli O, Caria MA, Melis F, Severino C, Tavera C, Mameli P, Mameli S, 2001. Autonomic nervous system activity and life threatening arrhythmias in experimental epilepsy. Seizure 10, 269–278. [DOI] [PubMed] [Google Scholar]
- Manolis TA, Manolis AA, Melita H, Manolis AS, 2019. Sudden unexpected death in epilepsy: The neuro-cardio-respiratory connection. Seizure - European Journal of Epilepsy 64, 65–73. [DOI] [PubMed] [Google Scholar]
- Marchal F, Corke BC, Sundell H, 1982. REFLEX APNEA FROM LARYNGEAL CHEMO-STIMULATION IN THE SLEEPING PREMATURE NEWBORN LAMB. Pediatric Research 16, 621–627. [DOI] [PubMed] [Google Scholar]
- Marks M, South M, Carter B, 1995. Measurement of respiratory rate and timing using a nasal thermocouple. Journal of Clinical Monitoring 11, 159–164. [DOI] [PubMed] [Google Scholar]
- McGraw MB, 1939. Swimming behavior of the human infant. Journal of Pediatrics 15, 485–490. [Google Scholar]
- Michael Panneton W, 2013. The Mammalian Diving Response: An Enigmatic Reflex to Preserve Life? Physiology 28, 284–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirza KB, Alenda A, Eftekhar A, Grossman N, Nikolic K, Bloom SR, Toumazou C, 2018. Influence of Cholecystokinin-8 on Compound Nerve Action Potentials from Ventral Gastric Vagus in Rats. International Journal of Neural Systems 28, 1850006. [DOI] [PubMed] [Google Scholar]
- Mooney S, Chin B, Villiere S, Nakase K, Kollmar R, Kim S, Sundaram K, Silverman JB, Lazar J, Stewart M, 2019. Diving responses elicited by nasopharyngeal irrigation mimic seizure-associated central apneic episodes in a rat model. Neurobiology of Disease 124, 408–415. [DOI] [PubMed] [Google Scholar]
- Mor N, Naggar I, Das O, Nakase K, Silverman JB, Sundaram K, Stewart M, Kollmar R, 2014. Quantitative Video Laryngoscopy to Monitor Recovery from Recurrent Laryngeal Nerve Injury in the Rat. Otolaryngology-Head and Neck Surgery 150, 824–826. [DOI] [PubMed] [Google Scholar]
- Naggar I, Lazar J, Kamran H, Orman R, Stewart M, 2014. Relation of autonomic and cardiac abnormalities to ventricular fibrillation in a rat model of epilepsy. Epilepsy Research 108, 44–56. [DOI] [PubMed] [Google Scholar]
- Nakase K, Kollmar R, Lazar J, Arjomandi H, Sundaram K, Silverman J, Orman R, Weedon J, Stefanov D, Savoca E, Tordjman L, Stiles K, Ihsan M, Nunez A, Guzman L, Stewart M, 2016. Laryngospasm, central and obstructive apnea during seizures: Defining pathophysiology for sudden death in a rat model. Epilepsy Research 128, 126–139. [DOI] [PubMed] [Google Scholar]
- Nashef L, So EL, Ryvlin P, Tomson T, 2011. Unifying the definitions of sudden unexpected death in epilepsy. Epilepsia 53, 227–233. [DOI] [PubMed] [Google Scholar]
- Nobili L, Proserpio P, Rubboli G, Montano N, Didato G, Tassinari CA, 2011. Sudden unexpected death in epilepsy (SUDEP) and sleep. Sleep Medicine Reviews 15, 237–246. [DOI] [PubMed] [Google Scholar]
- Orenstein SR, 2001. An overview of reflux-associated disorders in infants: apnea, laryngospasm, and aspiration. The American Journal of Medicine 111, 60–63. [DOI] [PubMed] [Google Scholar]
- Paton JFR, Boscan P, Pickering AE, Nalivaiko E, 2005. The yin and yang of cardiac autonomic control: vago-sympathetic interactions revisited. Brain Research. Brain Research Reviews 49, 555–565. [DOI] [PubMed] [Google Scholar]
- Pederson DJ, Irazoqui PP, Quinkert CJ, Arafat MA, Somann JP, Williams JD, Bercich RA, Wang Z, Albors GO, Jefferys JGR, 2019. The Bionode. ACM Transactions on Embedded Computing Systems 18, 1–20. [Google Scholar]
- Pelot NA, Grill WM, 2020. In vivo quantification of excitation and kilohertz frequency block of the rat vagus nerve. Journal of Neural Engineering 17, 026005. [DOI] [PubMed] [Google Scholar]
- Postma GN, Halum SL, 2006. Laryngeal and pharyngeal complications of gastroesophageal reflux disease. GI Motility Online.
- Qing KY, Wasilczuk KM, Ward MP, Phillips EH, Vlachos PP, Goergen CJ, Irazoqui PP, 2018. B fibers are the best predictors of cardiac activity during Vagus nerve stimulation. Bioelectronic Medicine 4, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryvlin P, Nashef L, Lhatoo SD, Bateman LM, Bird J, Bleasel A, Boon P, Crespel A, Dworetzky BA, Høgenhaven H, Lerche H, Maillard L, Malter MP, Marchal C, Murthy JMK, Nitsche M, Pataraia E, Rabben T, Rheims S, Sadzot B, Schulze-Bonhage A, Seyal M, So EL, Spitz M, Szucs A, Tan M, Tao JX, Tomson T, 2013. Incidence and mechanisms of cardiorespiratory arrests in epilepsy monitoring units (MORTEMUS): a retrospective study. The Lancet Neurology 12, 966–977. [DOI] [PubMed] [Google Scholar]
- Saito T, Sakamoto K, Koizumi K, Stewart M, 2006. Repeatable focal seizure suppression: A rat preparation to study consequences of seizure activity based on urethane anesthesia and reversible carotid artery occlusion. Journal of Neuroscience Methods 155, 241–250. [DOI] [PubMed] [Google Scholar]
- Sakamoto K, Saito T, Orman R, Koizumi K, Lazar J, Salciccioli L, Stewart M, 2008. Autonomic consequences of kainic acid-induced limbic cortical seizures in rats: Peripheral autonomic nerve activity, acute cardiovascular changes, and death. Epilepsia 49, 982–996. [DOI] [PubMed] [Google Scholar]
- Scadding GK, Brock C, Chouiali F, Hamid Q, 2014. Laryngeal Inflammation in the Sudden Infant Death Syndrome. Current Pediatric Reviews 10, 309–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schirmer BD, 1989. CURRENT STATUS OF PROXIMAL GASTRIC-VAGOTOMY. Annals of Surgery 209, 131–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholander PF, Hammel HT, LeMessurier H, Hemmingsen E, Garey W, 1962. Circulatory adjustment in pearl divers. Journal of Applied Physiology 17, 184–190. [DOI] [PubMed] [Google Scholar]
- Selassie A, 2015. Risk Factors of Epilepsy Outcomes: Comorbidities in Population with Epilepsy in South Carolina.
- Stewart M, 2019. Progress in defining autonomic consequences of seizure activity including sudden death. Clinical Autonomic Research 29, 135–136. [DOI] [PubMed] [Google Scholar]
- Stewart M, Kollmar R, Nakase K, Silverman J, Sundaram K, Orman R, Lazar J, 2017. Obstructive apnea due to laryngospasm links ictal to postictal events in SUDEP cases and offers practical biomarkers for review of past cases and prevention of new ones. Epilepsia 58, e87–e90. [DOI] [PubMed] [Google Scholar]
- Suzuki SS, Smith GK, 1988. Spontaneous EEG spikes in the normal hippocampus. V. Effects of ether, urethane, pentobarbital, atropine, diazepam and bicuculline. Electroencephalography and Clinical Neurophysiology 70, 84–95. [DOI] [PubMed] [Google Scholar]
- Tavee J, Morris HI, 2008. Severe postictal laryngospasm as a potential mechanism for sudden unexpected death in epilepsy: a near-miss in an EMU. Epilepsia 49, 2113–2117. [DOI] [PubMed] [Google Scholar]
- Thach BT, 1997. Reflux associated apnea in infants: evidence for a laryngeal chemoreflex. The American Journal of Medicine 103, 120S–124S. [DOI] [PubMed] [Google Scholar]
- Thurman David J, Hesdorffer Dale C, French Jacqueline A, 2014. Sudden unexpected death in epilepsy: Assessing the public health burden. Epilepsia 55, 1479–1485. [DOI] [PubMed] [Google Scholar]
- Van Der Velde L, Curran AK, Filiano JJ, Darnall RA, Bartlett D, Leiter JC, 2003. Prolongation of the laryngeal chemoreflex after inhibition of the rostral ventral medulla in piglets: a role in SIDS? Journal of Applied Physiology 94, 1883–1895. [DOI] [PubMed] [Google Scholar]
- Vega JL, 2018. Ictal Mammalian Dive Response: A Likely Cause of Sudden Unexpected Death in Epilepsy. Frontiers in neurology 9, 677–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vela MF, Richter JE, Pandolfino JE, 2013. Laryngopharyngeal Reflux, Practical Manual of Gastroesophageal Reflux Disease, 1st ed. John Wiley & Sons, Ltd, pp. 154–160. [Google Scholar]
- Vincenzi FF, 2019. Sudden Unexpected Death and the Mammalian Dive Response: Catastrophic Failure of a Complex Tightly Coupled System. Frontiers in Physiology 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wannamaker BB, 1985. Autonomic nervous system and epilepsy. Epilepsia 26 Suppl 1, S31–39. [DOI] [PubMed] [Google Scholar]
- Ward MP, Qing KY, Otto KJ, Worth RM, John SWM, Irazoqui PP, 2015. A Flexible Platform for Biofeedback-Driven Control and Personalization of Electrical Nerve Stimulation Therapy. Ieee Transactions on Neural Systems and Rehabilitation Engineering 23, 475–484. [DOI] [PubMed] [Google Scholar]
- White RL, Rossiter CD, Hornby PJ, Harmon JW, Kasbekar DK, Gillis RA, 1991. Excitation of neurons in the medullary raphe increases gastric acid and pepsin production in cats. American Journal of Physiology-Gastrointestinal and Liver Physiology 260, G91–G96. [DOI] [PubMed] [Google Scholar]
- Xia L, Leiter JC, Bartlett D, 2013. Laryngeal reflex apnea in neonates: Effects of CO2 and the complex influence of hypoxia. Respiratory Physiology & Neurobiology 186, 109–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Yuan PQ, Wang L, Taché Y, 1999. Activation of the parapyramidal region in the ventral medulla stimulates gastric acid secretion through vagal pathways in rats. 95, 773–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video 1: Laryngospasm respiration See attached laryngospasm respiration video
Video Caption: Video showing thermocouple respiration traces for several animals. The top four traces, labelled “KA”, are from animals in which seizures were induced with kainic acid. In these traces, animals, who had previously had acid reflux into the esophagus, experienced sudden obstructive apnea and death. The bottom four traces, labelled “Control”, are subsequent experiments from a single non-seizing animal in which we induced laryngospasm by placing an artificial acid on the larynx. The animal recovered to baseline between experiments, and the traces are presented in this order (i.e. we induced laryngospasm to obtain data shown in trace #5. The animal then recovered, and we induced laryngospasm again to obtain the data shown in trace #6). Times are normalized so that laryngospasm begins at 0 min for all traces. For animals that died from the laryngospasm, the last observable breath is marked as “Terminal apnea.” Perturbations in the signal after this time are not animal respiration, and may be the heart rate coupling onto respiration, movement as we moved the animal to remove the stomach after death, or noise from recording open air. Y-axis units are arbitrary. This respiration data was down-sampled by 8x to accommodate the smooth plotting of so much data (functional sample frequency = 156 Hz).
Video 2: Larynx stim video See attached larynx stimulation video
Video Caption: Video showing laryngeal stimulation opening the larynx during laryngospasm. In this video we placed 0.1 mL of simulated stomach acid on the larynx of a non-seizing animal to induce laryngospasm, closing the larynx immediately. We then apply electrical stimulation, which opens the larynx immediately. When electrical stimulation is stopped, the larynx closes immediately again. This stimulation is repeated until the animal begins to recover from laryngospasm.


