Abstract
Hyperlipidemia and oxidative stress are risk factors for atherosclerosis. In this study, we investigated the hypolipidemic and anti-lipoprotein oxidation activities of polyphenol-rich extracts from almond hulls using Triton WR-1339 and high-fat diet-induced hyperlipemic mice as experimental models. We demonstrated that the almond hull extract significantly reduced total cholesterol, triglycerides and low-density lipoprotein-related plasma cholesterol (LDL-C) in the two experimental models of hyperlipidemia, but significantly increased high-density lipoprotein-related plasma cholesterol (HDL-C). Another beneficial effect of the extract was its ability to reduce the atherogenic index and LDL-C/HDL-C ratio. However, the extract exhibited effective antiradical activity against 2,2-diphenyl-1-picrylhydrazyl and significantly protected lipoprotein-rich plasma from mice against oxidation induced by copper ion. The extract contains 342.63±3.44 mg/g total phenolics, 144.67±6.83 mg/g tannins, and 20.66±0.92 mg/g flavonoids. These finding indicate that almond hulls contain polar products able to lower plasma lipid concentrations and which might be beneficial for the treatment of hyperlipidemia and prevention of atherosclerosis.
Keywords: almond hulls, anti-lipoprotein oxidation, hypolipidemia, mice
INTRODUCTION
The almond tree is one of the most popular nut trees grown under arid conditions worldwide. In Morocco, almond trees are mainly cultured in mountainous regions of the high atlas, middle atlas (Foum El Jemaa, Ouaouizeght, and Azilal), Rif (lmzouren, Beni-Boufrah, and Targuist), and Oriental (Tafoughalt, Rislan, and Laayoune). Rosaceae is a species of almond (Prunus dulcis) widely cultivated in the Rislan region (East of Morocco) because of its resistance and capacity against water shortage and irrigation deficit. Almond production in this region of Morocco generates large amounts of by-products consisting of hull and shell annually, which are burned or used as livestock feed. Thus, these biowastes remain under-exploited, and further data on their biological activities, phytochemical profiles, and extraction methods are required to propose sustainable and competitive exploitation strategies and veritable management that are compatible with current commercial uses (Prgomet et al., 2017).
Many studies have shown that the almond hulls are an important source of natural phenolic compounds and fibre (Wijeratne et al., 2006). Indeed, catechin, protocatechuic acid, benzoic acid, and 4-hydroxy benzoic acid have been identified in almond hulls from different regions in the world (Sang et al., 2002). Such by-product can be exploited as potentially useful sources of natural antioxidants and other compounds (e.g., fibre) have a beneficial impact on lipid metabolism and for preventing atherosclerosis and cardiovascular diseases (Berryman et al., 2011). Atherosclerosis is a chronic pathology directly related to lipid metabolism disorders and oxidative stress (Viktorinova et al., 2016). Indeed, several clinical studies have shown that an increase in low-density lipoprotein-related plasma cholesterol (LDL-C) plays a crucial role in the early development of atherosclerosis, whereas high-density lipoprotein cholesterol (HDL-C) is an antiatherogenic parameter (Deedwania et al., 2016; Lazo-Porras et al., 2016). In addition, plasma triglycerides represent an independent potential risk factor for atherosclerosis, particularly in diabetic patients (Talayero and Sacks, 2011). Furthermore, oxidative stress leading to oxidation of LDL is the starting point for the formation of foam cells, which trigger the atherosclerotic process (Winklhofer-Roob et al., 2017). Thus, management of atheroscle-rosis requires a strategy based on the treatment of hyperlipidemia and oxidative stress (Song and Jiang, 2017).
In the east of Morocco, infused almond hulls were used as a folk medicine to treat hyperlipidemia and prevent atherosclerosis. However, no experimental study was conducted to confirm the pharmacological activity. Therefore, this study was designed to investigate the antihyperlipidemic effect of polyphenol-rich extracts of hulls obtained from almonds (P. dulcis) cultivated in eastern Morocco using two hyperlipidemic models, Triton WR-1339 and high-fat diet-induced hyperlipidemic mice, and with using the hypolipidemic drug Fenofibrate as a comparison. In addition, we studied the anti-radical and anti-plasma lipoprotein oxidation of the extract. The obtained effects, were compared with those of the standard antioxidant substance butylated hydroxyanisol (BHA).
MATERIALS AND METHODS
Preparation of aqueous almond hull extracts (AHE)
Almond (P. dulcis) hulls were collected during the harvest period in the region of Rislane (Tafoughalt, Eastern Morocco) in September 2019. The samples were air-dried and then reduced to a fine powder using an electric mixer. The powder was immediately stored at 4°C until use. To prepare the aqueous hull extract, 20 g of the powder was infused in boiling distilled water for 30 min. The obtained extract was filtered using Whatman paper no. 2 (pore size 8 mm), concentrated in the rotatory evaporator at 50°C under reduced pressure and then dried in a ventilated oven at 40°C for 24 h. The extraction yielded 32% hull powder, calculated according to the following formula:
The extract was recuperated as a yellowish fine powder.
Determination of total polyphenol content of the AHE
The total polyphenol content of the AHE was determined according to the method of Folin-Ciocalteu (Touiss et al., 2019). Samples (0.5 mL) were added to 0.25 mL of Folin reagent and 0.5 mL of 20% sodium carbonate solution. After stirring, the blue color was allowed to develop for 45 min in the dark. The absorbances were recorded using a spectrophotometer at 725 nm against the blank tube in which the sample was replaced by 0.5 mL of distilled water. The polyphenol content was calculated from a calibration curve of catechin and expressed in milligrams of catechin per gram of extract. All measurements were run in triplicate.
Dosage of tannins in the AHE
The tannin content was measured after precipitation by polyvinylpyrrolidone (PVP) (Makkar et al., 1993). Thus, 50 mL of each sample (1 mg/mL) was homogenized with 0.1 g of PVP and stirred for 30 min. The tannin-PVP complex was allowed to form for two hours, and then the solution was centrifuged at 4,000 rpm for 15 min. The non-adsorbed phenolics constituting the supernatant were determined using the Folin-Ciocalteu method, as previously described. The values obtained were subtracted from the level of total polyphenols to obtain the tannin content expressed in milligram of catechin per gram of dry extract. Trials were run in triplicate.
Quantification of flavonoids in the AHE
Flavonoids were measured using aluminum chloride reagent (Touiss et al., 2019). Samples (5 mL) was mixed with 2.5 mL of AlCl3 reagent (consisting of 133 mg and 400 mg of sodium acetate in 100 mL of methanol). After 10 min, the yellow color of the samples was measured at 430 nm against the blank which contained 5 mL of sample and 2.5 mL of methanol. The flavonoid content was determined from a calibration curve of rutin and expressed as milligram of rutin per gram of extract. All dosages were made in triplicate.
Experimental design to studying the effect of the AHE and Fenofibrate in Triton WR-1339-induced hyperlipidemic mice
The experiment was conducted in adult male Albinos mice weighing 28∼30 g that were bred in the animal house of the University Mohammed I, Oujda, Morocco. The animals were maintained at a constant temperature (22±2°C) with a 12 h light/dark cycle and with access to food and water ad libitum. The mice were used according to the internationally accepted standard guidelines for the use of laboratory animals. This project was approved by the local committee for the use laboratory animals, Faculty of Medicine (approval number: 002016). Thirty-two mice were fasted overnight and divided into four groups of 8 mice as follows:
NCG: normolipidemic control group receiving NaCl 9‰ by intraperitoneal injection and gavaged with distilled water
HCG: hyperlipidemic control group receiving the Triton WR-1339 (200 mg/kg in 9‰ NaCl, pH 7.4) by intraperitoneal injection and gavaged with distilled water
ATG: AHE-treated group injected with Triton and gavaged with the extract of almond hulls at a dose of 500 mg/kg
FTG: Fenofibrate treated group injected with Triton and gavaged with Fenofibrate at a dose of 500 mg/kg
At the end of the experiment (10 h), the animals were anesthetized with diethyl ether, and blood was taken from their retro-orbital sinuses using tubes containing trisodium citrate as an anticoagulant. After centrifugation at 2,500 rpm for 10 min, the plasma was recovered for determination of lipid parameters.
Experimental schedule to study the effect of AHE and Fenofibrate in high-fat diet-induced hyperlipidemic mice
The experiment was carried out in mice who were fed a high-fat diet for 6 weeks. The high-fat diet was prepared with 2% cholesterol, 16% beef fat, and 82% standard mice diet (Société SONABETAIL, Oujda, Morocco). At the beginning of the study, thirty two adult male Albinos mice living in the conditions described above were divided into four groups of 8 animals:
NCG: normolipidemic control group fed with a standard diet and gavaged daily with distilled water for 6 weeks
HCG: hyperlipidemic control group fed with a high-fat diet and gavaged with distilled water for 6 weeks
ATG: AHE-treated group fed with a high-fat diet and gavaged with almond hulls extracts at a dose of 500 mg/kg for 6 weeks
FTG: Fenofibrate treated group fed with a high-fat diet and gavaged with Fenofibrate at a dose of 500 mg/kg for 6 weeks.
At the end of the experiment, animals were anesthetized with diethyl ether, and blood was extracted from their retro-orbital sinuses, as described above, for analysis of plasma lipid parameters.
Dosage of plasma lipid parameters
Dosage of total cholesterol (TC): The enzymatic cholesterol assay method involves hydrolyzing cholesterol esters using a cholesterol ester hydrolase. The free formed and pre-existing cholesterol were then oxidized by a cholesterol oxidase to produce hydrogen peroxide (H2O2). H2O2 in the presence of 4-amino-antipyrine and phenol produces a red-colored quinoneimine that absorbs visible light at 510 nm. One milliliter of enzymatic reagent was then added to 10 μL of the plasma (Reactivos GPL Kits, CHEMELIX S.A., Barcelona, Spain). After stirring and incubating for 10 min at 37°C, the absorbance was measured spectrophotometrically at 510 nm against a blank prepared in the same conditions that contained 10 μL distilled water in place of 10 μL plasma. TC concentration was calculated as follows:
Dosage of TG: Plasma TGs were quantified by an enzymatic method using the Reactivos GPL kit (CHEMELIX S.A.). After hydrolyzing TG by lipases, formation of quinoneimine from H2O2, 4-aminophenazone, and 4-chlorophenol by peroxidase was measured spectrophotometrically. One milliliter of assay reagent was then added to 10 μL of the plasma. The mixture was stirred, incubated for 15 min at 37°C, and the absorbance recorded at 520 nm against a blank containing 10 μL of distilled water and 1 mL of an assay reagent. The TG concentration was calculated as follows:
Dosage of HDL-C: HDL-C content was determined after precipitation of other plasma lipoproteins (LDL and very low-density lipoprotein-related plasma cholesterol) by phosphotungstic acid (PTA) in the presence of MgCl2. First, 20 μL of plasma and 10 μL of the PTA/MgCl2 reagent (Sigma diagnostic 352-4, Sigma-Aldrich Co., St. Louis, MO, USA) were mixed in an eppendorf tube, allowed to stand for 10 min and centrifuged at 5,000 rpm for 15 min. The TC content of the supernatant was then assayed following the TC assay protocol described above. The concentration of HDL-C was calculated according to the following formula:
Calculation of LDL-C content: The LDL-C was calculated according to the Friedwald formula (Friedewald et al., 1972) as follows:
Atherogenic index (AI) and LDL-C/HDL-C ratio: The AI and LDL-C/HDL-C ratio were calculated according to formu-las previously described by Harnafi et al. (2013):
Measurement of the antiradical effect of AHE and BHA
The anti-radical effect of AHE and BHA was determined using 2,2-diphenyl-1-picrylhydrazyl (DPPH) assays as previously described (Ramchoun et al., 2015). In its radical form DPPH absorbs visible light at 517 nm. Upon reduction by antioxidants, the absorbance of DPPH decreases. The decrease in the absorption of DPPH is used to determine the anti-radical power of the tested substances. First, 2,495 μL of 0.1 mM DPPH methanolic solution was added to 5 μL AHE at different concentrations (0.5, 10, 25, 50, 100, and 200 μg/mL). After 30 min incubation in the dark, the absorbance was measured at 517 nm. The synthetic antioxidant BHA was assayed as a standard positive control under the same experimental conditions. The ability to trap the DPPH radical was calculated according to the following formula:
The concentration giving 50% inhibition (IC50) values were calculated from the plotted graph depicting the antiradical effect against concentrations of the extract or BHA. All tests were carried out in triplicate.
Evaluation of the protective effect of AHE and BHA against plasma lipoprotein oxidation
Malondialdehydes (MDA), which represent the secondary products of plasma lipoprotein oxidation, were quantified as thiobarbituric acid reactive substances (TBARS), as previously described (Touiss et al., 2019). The lipoprotein-rich plasma used as a substrate for oxidation was taken from mice treated with 400 mg/kg Triton WR-1339 for 10 h. The plasma contains 100±5 mg/dL of LDL-C (determined as previously described). The oxidative process was induced by a copper sulfate solution, according to the scheme described below:
Control: 40 μL lipoprotein-rich plasma was incubated with distilled water only
Oxidized lipoproteins: 40 μL lipoprotein-rich plasma was incubated with 10 μL CuSO4-5H2O (0.3 mg/mL)
Lipoproteins treated with AHE: 40 μL lipoprotein-rich plasma was incubated with 10 μL copper sulfate solution and almond hull extract at different concentra-tions (5, 12.5, 25, 50, 100, and 200 μg/mL)
Lipoproteins treated with BHA: 40 μL lipoprotein-rich plasma was incubated with 10 μL copper sulfate solution and BHA at different concentrations (5, 12.5, 25, 50, 100, and 200 μg/mL)
The preparations were mixed vigorously, incubated for 24 h at 37°C, and 500 μL of 20% trichloroacetic acid and 500 μL of 0.8% thiobarbituric acid were added. The reaction mixture was then heated at 95°C for 30 min. After cooling, the absorbance of the solution was recorded at 532 nm. The quantities of TBARS were calculated and expressed as MDA equivalent determined by a calibration curve. All measurements were carried out in triplicate.
Statistical analysis
The data were analyzed using Student’s t-tests and one-way ANOVA. P-values <0.05 were considered statistically significant. Results were expressed as means±standard error of the means (SEM).
RESULTS
Polyphenol content of almond hull extracts
The contents of different classes of phenolic compounds in the almond hull extracts are summarized in Table 1. Extracts had was a high content total polyphenols, representing 342.63±3.44 mg/g dry mass. Tannins represented the major fraction of the analyzed polyphenols (144.67±6.83 mg/g; i.e., 42% of the total polyphenols). Flavonoids (content of 20.66±0.92 mg/g) represented 6% of the total polyphenols.
Table 1.
Polyphenol content of almond hull extracts
| Content (mg/g) | Percentage | |
|---|---|---|
| Total polyphenols1) | 342.63±3.44 | − |
| Tannins1) | 144.67±6.83 | 42% |
| Flavonoids2) | 20.66±0.92 | 6% |
Values are expressed as mean±SEM (n=3).
1)mg catechin equivalent/g dry extract.
2)mg rutin equivalent/g dry sec.
Induction of hyperlipidemia using Triton WR-1339
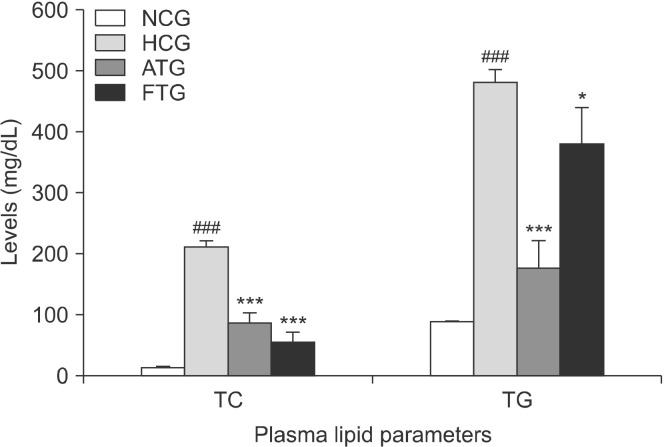
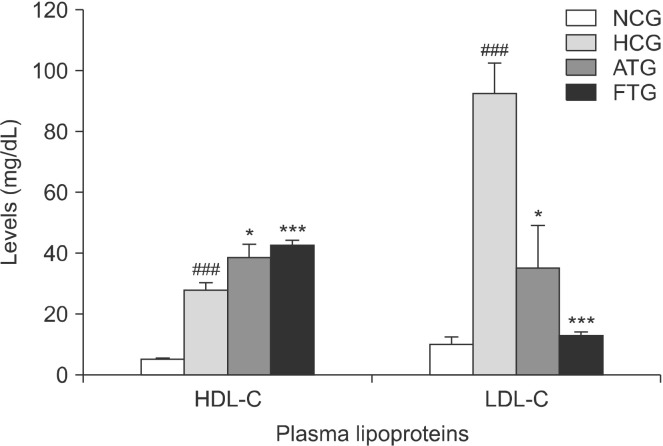
The contents of mouce plasma lipids are reported in Fig. 1. Triton WR-1339 significantly increased the TC and TG contents compared with the normolipidemic control group. Indeed, the plasma TC content was increased 13-fold (P<0.001) and the TG content was increased >4-fold (P<0.001). Furthermore, the LDL-C content was much higher in the Triton WR-1339-treated group (+790%, P<0.001) compared with the normolipidemic control groups. In addition, Triton WR-1339 significantly increased the HDL-C content (+428%, P<0.001) compared with the control group (Fig. 2).
Fig. 1.
Effect of almond hull extracts (AHE) on total plasma cholesterol and triglyceride content in Triton WR-1339-induced hyperlipidemic mice. Values are expressed as mean±SEM (n=8). ###P<0.001 (NCG vs. HCG) and *P<0.05 and ***P<0.001 (HCG vs. ATG and HCG vs. FTG). TC, total cholesterol; TG, triglycer-ides; NCG, normolipidemic control group; HCG, hyperlipidemic control group; ATG, AHE-treated group; FTG, Fenofibrate treated group.
Fig. 2.
Effect of almond hull extracts (AHE) on plasma high density lipoprotein-cholesterol (HDL-C) and low density lipoprotein-cholesterol (LDL-C) levels in Triton WR-1339-induced hyperlip-idemic mice. Values are expressed as mean±SEM (n=8). ###P<0.001 (NCG vs. HCG) and *P<0.05 and ***P<0.001 (HCG vs. ATG and HCG vs. FTG). NCG, normolipidemic control group; HCG, hyperlipidemic control group; ATG, AHE-treated group; FTG, Fenofibrate treated group.
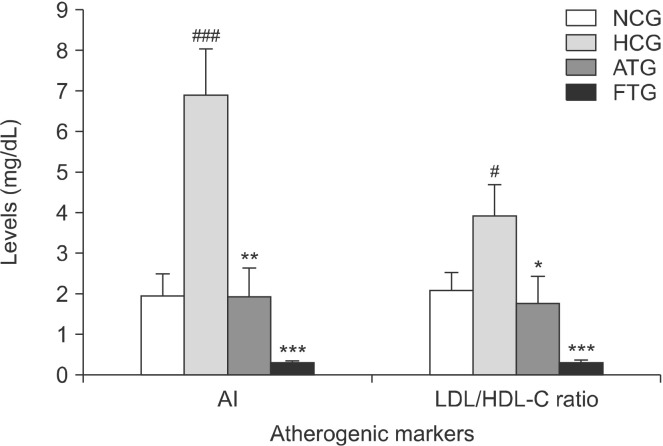
The AI and LDL-C/HDL-C ratio were significantly affected in the hyperlipidemic group. Indeed, the AI was statistically increased by 250% (P<0.001) and the LDL-C/HDL-C ratio by 86% (P<0.05) in the hyperlipidemic group compared with the normolipidemic control group (Fig. 3).
Fig. 3.
Effect of almond hull extracts on atherogenic markers in Triton WR-1339-induced hyperlipidemic mice. Values are expressed as mean±SEM (n=8). #P<0.05 and ###P<0.001 (NCG vs. HCG) and *P<0.05, **P<0.01, and ***P<0.001 (HCG vs. ATG and HCG vs. FTG). AI, atherogenic index; LDL-C/HDL-C, ratio of low-density lipoprotein-cholesterol to high-density lipoprotein-cholesterol; NCG, normolipidemic control group; HCG, hyperlipidemic control group; ATG, AHE-treated group; FTG, Fenofibrate treated group.
Effect of almond hull extract and Fenofibrate on plasma lipid parameters in Triton WR-1339-induced hyperlipidemic mice
Ten hours after administration of almond hull extracts to the Triton WR-1339-treated mice, plasma TC and TG were decreased by 57% (P<0.001) and 63% (P<0.001), respectively (Fig. 1). LDL-C content was reduced by 61% (P<0.05) and HDL-cholesterol content was increased by 39% (P<0.05) (Fig. 2). Furthermore, the almond hull extract significantly decreased the AI (−72%, P<0.01) and LDL-C/HDL-C ratio (−55%, P<0.05) (Fig. 3).
Fenofibrate is a lipid-lowering drug used as a positive control. Fenofibrate reduces total plasma cholesterol levels by 73% (P<0.001) and TGs by 31% (P<0.05) (Fig. 1). Furthermore, treatment of mice with Fenofibrate significantly decreases LDL-C by 86% (P<0.001) and increases HDL-C by 52% (P<0.001) (Fig. 2). In addition, Fenofibrate lowered the AI by 95% (P<0.001) and the LDL-C/HDL-C ratio by 91% (P<0.001) (Fig. 3). This indicates that Fenofibrate is relatively more effective than the almond hull extract for influencing the plasma lipid profile in Triton WR-1339-treated mice.
Induction of hyperlipidemia by the high-fat diet in mice
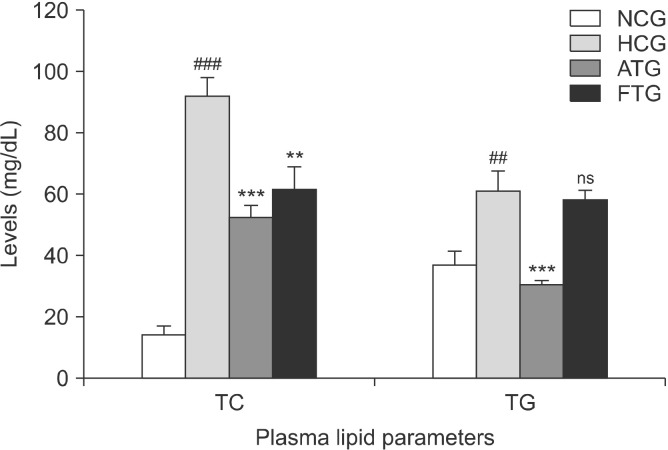
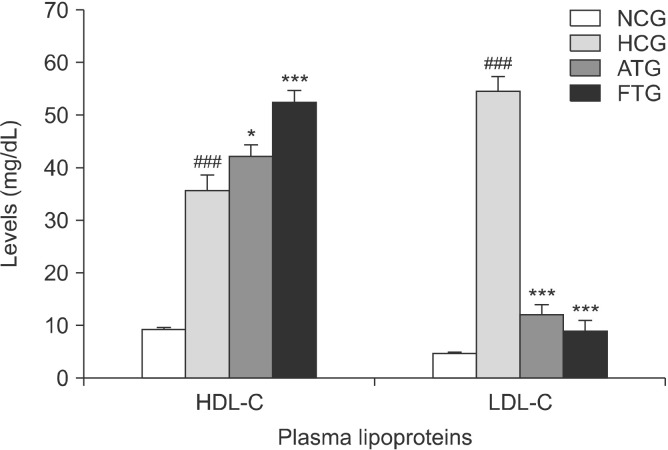
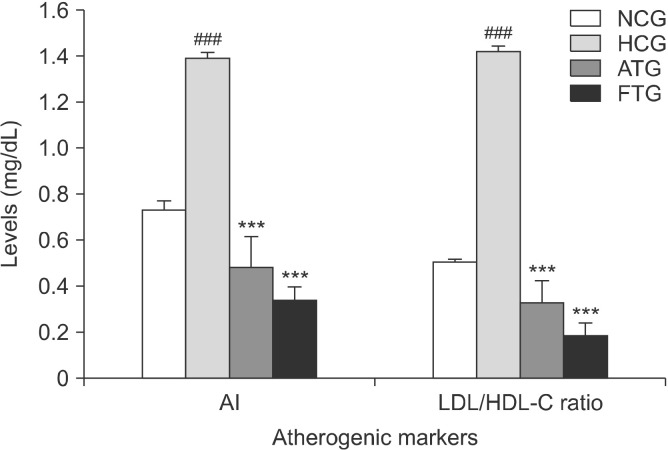
Hyperlipidemic mice exhibited significant increases in their plasma lipid profile in response to the high-fat diet than normal control mice (Fig. 4). Indeed, after 6 weeks of treatment, TC was increased by 544% (P<0.001) and the TG content by 64% (P<0.01). However, the high-fat diet resulted in >10-fold increase in LDL-C (P<0.001) and 278% increase in HDL-C (P<0.001) (Fig. 5). In addion, the high-fat diet significantly increased the AI (+ 89%, P<0.001) and LDL-C/HDL-C ratio (+180%, P< 0.001) (Fig. 6).
Fig. 4.
Effect of almond hull extracts on plasma total cholesterol (TC) and triglyceride (TG) levels in high-fat diet-induced hyperlipidemic mice. Values are expressed as mean±SEM (n=8). ##P< 0.01 and ###P<0.001 (NCG vs. HCG) and **P<0.01 and ***P< 0.001 (HCG vs. ATG and HCG vs. FTG). NCG, normolipidemic control group; HCG, hyperlipidemic control group; ATG, AHE-treated group; FTG, Fenofibrate treated group; ns, not significant.
Fig. 5.
Effect of almond hull extracts on plasma high density lipoprotein-cholesterol (HDL-C) and low density lipoprotein-cholesterol (LDL-C) levels in high-fat diet-induced hyperlipidemic mice. Values are expressed as mean±SEM (n=8). ###P<0.001 (NCG vs. HCG) and *P<0.05 and ***P<0.001 (HCG vs. ATG and HCG vs. FTG). NCG, normolipidemic control group; HCG, hyperlipidemic control group; ATG, AHE-treated group; FTG, Fenofibrate treated group.
Fig. 6.
Effect of almond hull extracts on atherogenic markers in high-fat diet-induced hyperlipidemic mice. Values are expressed as mean±SEM (n=8). ###P<0.001 (NCG vs. HCG) and ***P<0.001 (HCG vs. ATG and HCG vs. FTG). AI, atherogenic index; LDL-C/HDL-C ratio, ratio of low-density lipoprotein-cholesterol to high-density lipoprotein-cholesterol; NCG, normolipidemic control group; HCG, hyperlipidemic control group; ATG, AHE-treated group; FTG, Fenofibrate treated group.
Antihyperlipidemic activity of almond hull extract and Fenofibrate in high-fat diet-induced hyperlipidemic mice
Following 6 weeks of treatment, almond hull extract significantly reduced the elevated levels of plasma lipid in hyperlipidemic mice compared with hyperlipidemic controls (Fig. 4∼6). Indeed, the high-fat diet together with the almond extract significantly decreased the plasma TC content (−42%, P<0.001) and the LDL fraction (−77%, P<0.001). However, the HDL-C content was increased by 18% (P<0.05) and TG content decreased by 49% (P< 0.001). In addition, almond hull extract significantly decreased the AI by 65% (P<0.001) and the LDL-C/HDL-C ratio by 77% (P<0.001).
In this study, Fenofibrate significantly reduced the total plasma cholesterol content by 32% (P<0.01). However, it did not significantly reduce the TG content (−5%, P>0.05) (Fig. 4). Furthermore, Fenofibrate decreased LDL-cholesterol by 83% (P<0.001) and increased HDL-cholesterol by 47% (P<0.001) (Fig. 5). In addition, Fenofibrate reduced the AI by 75% (P<0.001) and the LDL-C/HDL-C ratio by 87% (P<0.001) (Fig. 6).
Radical scavenging activity of AHE and BHA
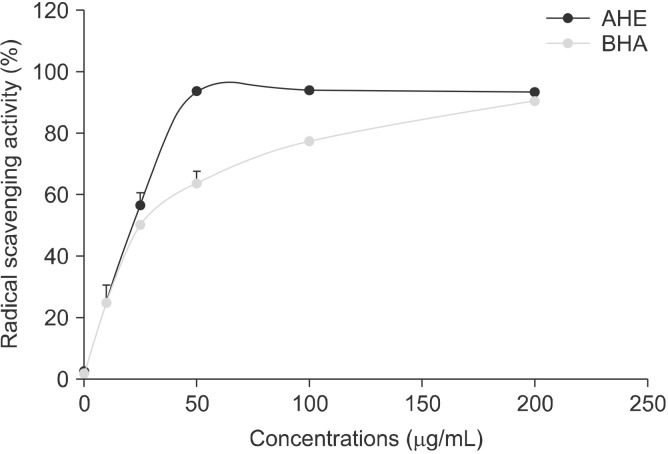
The radical scavenging activities of AHE and BHA were summarized in Fig. 7. The extract scavenged DPPH radicals in a dose-dependent manner (percent inhibition: 2%, 25%, 57%, 93%, and 94% at doses of 0.5, 10, 25, 50, 100, and 200 μg/mL, respectively). BHA is a standard antioxidant that scavenges free radicals by 2%, 25%, 50%, 63%, 77%, and 90% at the concentrations described above. Through comparing the IC50 of tested compounds, we concluded that AHE was more efficient than BHA against DPPH radicals (IC50=18.81±0.55 μg/mL vs. IC50 =21.03±0.11 μg/mL, respectively) (P<0.01).
Fig. 7.
Free radical scavenging activity of almond hull extracts and BHA. AHE, almond hull extract; BHA, butylated hydroxyanisol.
Effect of AHE and BHA on mouse plasma lipoprotein oxidation
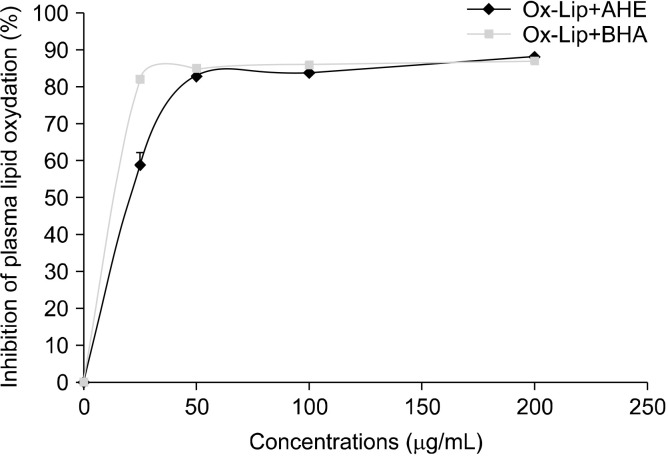
Oxidation of plasma lipoproteins induced by copper in the presence or absence of AHE and BHA was measured by assaying MDA at 532 nm. We showed that copper induces a significant increase in plasma lipoprotein oxidation at 37°C compared with controls (+850%, P<0.001). However, treatment of plasma lipoproteins with copper and different concentrations of AHE and BHA induced significant and dose-dependent decreases in MDA levels (Fig. 8). Indeed, AHE decreased plasma lipoprotein oxidation by 59% (P<0.001), 83% (P<0.001), 84% (P< 0.001) and 88% (P<0.001) at doses of 25, 50, 100, and 200 μg/mL, respectively.
Fig. 8.
Effect of almond hull extracts and BHA on plasma lipoprotein oxidation. Ox-Lip, oxidized lipoproteins; AHE, almond hull extract; BHA, butylated hydroxyanisol.
Furthermore, BHA inhibited plasma lipoprotein oxidation by 82% (P<0.001), 85% (P<0.001), 86% (P<0.001), and 87% (P<0.001), respectively at doses of 25, 50, 100, and 200 μg/mL. After calculating IC50, we concluded that AHE (IC50=13.81±0.57 μg/mL) was relatively less efficient than BHA (IC50=8.01±0.05 μg/mL) (P<0.001) at inhibiting plasma lipoprotein oxidation.
DISCUSSION
Involvement of lipoprotein metabolism disorders and oxidative stress in the onset of cardiovascular diseases, including atherosclerosis, diabetes, and hypertension, is well documented (Viktorinova et al., 2016; Hadjiphilippou and Ray, 2018). Hypercholesterolemia, hypertriglyceridemia, and LDL oxidation are the major risk factors correlated with the development of atherosclerosis and related cardiovascular complications (Hadjiphilippou and Ray, 2018).
Triton WR-1339 is a non-ionic detergent that has been widely used to decrease the activity of lipoprotein lipase (LPL) and block the uptake of plasma lipoproteins by peripheral tissues. We exploited this property to produce animal models of acute hyperlipidemia for the preliminary and rapid screening of lipid-lowering agents and to study lipid metabolism (Surya et al., 2017). Moreover, consumption of a high-fat diet was responsible for development of hyperlipidemia in different laboratory animals, thereby providing suitable models for studying hypolipidemic and anti-hyperlipidemic substances (Chu et al., 2019). In this study, we used two animal models to explore the hypolipidemic activity of almond hull extracts in comparison to the Fenofibrate.
We demonstrated that consumption of almond hull extract by Triton WR-1339-treated mice significantly decreased total plasma cholesterol and TG levels. Furthermore, the reduction of TC was accompanied by a significant drop in the LDL fraction of “bad cholesterol”, which constitutes the main modifiable risk factor for cardiovascular diseases and the therapeutic target of several lipid-lowering drugs. Thus, we suggest that the effect of almond extract arises from redistribution of LDL-C from plasma to the liver and extrahepatic tissues via the LDL receptors. Furthermore, to maintain normal lipid metabolism and prevent cholesterol deposition in the vessel endothelium, excess cholesterol in peripheral tissues must be returned to the liver via the HDL (i.e. “good cholesterol”). Indeed, the hypocholesterolemic effect of almond hull extracts was accompanied by a significant increase in HDL-C levels, leading to elimination of cholesterol as bile acids. This effect is consistent with several previous studies examining the hypolipidemic activity of natural products and extracts (Sidorova et al., 2017).
TGs play a key role in maintaining lipid metabolism homeostasis and are positively correlated with the incidence of cardiovascular diseases (Maki et al., 2016). In this study, we showed that almond hull extracts significantly reduced plasma TG levels in Triton WR 1339-induced hyperlipidemic mice. This suggests that the extracts could restore catabolism of TGs by activating LPL deactivated by Triton WR-1339, which is directly involved in the hydrolysis of TG-rich plasma lipoproteins in peripheral tissues.
In addition, we demonstrated that almond hull extracts exerted the same effect on plasma lipid profiles (TC, LDL-C, HDL-C, and TG) in high-fat diet-induced hyperlipidemic mice. However, since the high-fat diet was given with the almond hull extract from the beginning of the experiment, this effect may be a result of hypolipedemic (effect on receptors and enzymes) or antihyperlipidemic effects. Indeed, it is recently reported that the almond hull contains a high amount of catechin which is able to form complexes with lipids and lipolytic enzymes, thereby interfering with lipid emulsification, hydrolysis, micellar solubilisation, and subsequent uptake (Koo and Noh, 2007).
Furthermore, administration of almond hull extracts to the two hyperlipidemic mice models resulted in decreases in AI, which are closely linked to the lipid-lowering effect. In addition, lower LDL-C/HDL-C ratios help prevent atherogenesis since they are positively correlated with the incidence of atheromatous diseases. The almond hull extracts significantly decreased the LDL-C/HDL-C ratio in hyperlipidemic mice, demonstrating its beneficial effect on lipid metabolism and for preventing atheromatous diseases. These findings are consistent with several previous studies examining the beneficial effects of natural substances on lipid metabolism and cardiovascular disease prevention (Rony et al., 2014; Surya et al., 2017).
In addition, we compared the lipid-lowering effect of almond hull extracts to that of the hypolipidemic drug Fenofibrate in high-fat diet and Triton WR 1339-induced hyperlipidemic mice. We showed that the effect of the extract was relatively comparable to that of Fenofibrate, although Fenofibrate had a greater effect on the AI and LDL-C/HDL-C ratio.
In addition to hyperlipidemia, free radicals cause oxidative modification of plasma lipoproteins, thus contributing to initiation of the atherosclerotic process (Salvayre et al., 2016). Therefore, inhibition of LDL oxidation via antioxidant treatments is a major strategy for preventing atherosclerosis (Kong et al., 2014). In this regard, natural antioxidants are very effective as free radical scavengers and offer protection against lipoprotein oxidation and progression of atherosclerosis (Plaza et al., 2016). Our study demonstrated that almond hull extracts neutralize DPPH radicals and significantly prevent oxidation of plasma lipoproteins in a dose-dependent manner. The observed activity may be attributed to phenolic compounds within the extract that act through scavenging free radicals to stop the chain reaction of lipid oxidation (Harnafi and Amrani, 2007), chelating pro-oxidant metal ions that promote production of free radicals, and preserving the activity of paraoxonase associated with HDL, thus preventing LDL oxidation (Harnafi and Amrani, 2007).
Our results demonstrate that the bioactive compounds contained in almond hull extracts are polar because they are more soluble in water. This is consistent with previous reports showing that several water-soluble plant extracts can modulate lipid metabolism and prevent atherogenesis. Indeed, tannins and flavonoids, a heterogeneous group of plant polyphenols, present different pharmacological activities, including lipid-lowering activity and antiatherogenesis (Zou et al., 2012). By quantifying polyphenols, we demonstrate tannins and flavonoids are the major phenolic compounds in almond hull extracts. Therefore, these substances could be the majorly responsible for the observed lipid-lowering and antioxidant activities.
Based on the results of this study, sweet almond hulls can be considered an important source of bioactive compounds for treatment of hyperlipidemia and prevention of lipoprotein oxidation, which leads to development of atherosclerosis. Thus, almond hull extracts used in traditional medicine could be of value due to the lipid-lowering and antiatherogenic effects of their active compounds.
ACKNOWLEDGEMENTS
This work was supported by CNRST, ANPMA, and UMP. The authors would like to thank El Mostapha BEDRAOUI for helping in animal care.
Footnotes
AUTHOR DISCLOSURE STATEMENT
The authors declare no conflict of interest.
References
- Berryman CE, Preston AG, Karmally W, Deckelbaum RJ, Kris-Etherton PM. Effects of almond consumption on the reduction of LDL-cholesterol: a discussion of potential mechanisms and future research directions. Nutr Rev. 2011;69:171–185. doi: 10.1111/j.1753-4887.2011.00383.x. [DOI] [PubMed] [Google Scholar]
- Chu L, Yang L, Lin L, Wei J, Wang N, Xu M, et al. Chemical composition, antioxidant activities of polysaccharide from pine needle (Pinus massoniana) and hypolipidemic effect in high-fat diet-induced mice. Int J Biol Macromol. 2019;125:445–452. doi: 10.1016/j.ijbiomac.2018.12.082. [DOI] [PubMed] [Google Scholar]
- Deedwania PC, Pedersen TR, DeMicco DA, Breazna A, Betteridge DJ, Hitman GA, et al. Differing predictive relationships between baseline LDL-C, systolic blood pressure, and cardiovascular outcomes. Int J Cardiol. 2016;222:548–556. doi: 10.1016/j.ijcard.2016.07.201. [DOI] [PubMed] [Google Scholar]
- Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. doi: 10.1093/clinchem/18.6.499. [DOI] [PubMed] [Google Scholar]
- Hadjiphilippou S, Ray KK. Lipids and lipoproteins in risk prediction. Cardiol Clin. 2018;36:213–220. doi: 10.1016/j.ccl.2017.12.002. [DOI] [PubMed] [Google Scholar]
- Harnafi H, Amrani S. Flavonoids as potent phytochemicals in cardiovascular diseases prevention. Pharmacog Rev. 2007;1:193– 202. [Google Scholar]
- Harnafi H, Ramchoun M, Tits M, Wauters JN, Frederich M, Angenot L, et al. Phenolic acid-rich extract of sweet basil restores cholesterol and triglycerides metabolism in high fat dietfed mice: a comparison with fenofibrate. Biomed Prev Nutr. 2013;3:393–397. doi: 10.1016/j.bionut.2013.03.005. [DOI] [Google Scholar]
- Kong KW, Mat-Junit S, Ismail A, Aminudin N, Abdul-Aziz A. Polyphenols in Barringtonia racemosa and their protection against oxidation of LDL, serum and haemoglobin. Food Chem. 2014;146:85–93. doi: 10.1016/j.foodchem.2013.09.012. [DOI] [PubMed] [Google Scholar]
- Koo SI, Noh SK. Green tea as inhibitor of the intestinal absorption of lipids: potential mechanism for its lipid-lowering effect. J Nutr Biochem. 2007;18:179–183. doi: 10.1016/j.jnutbio.2006.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazo-Porras M, Bernabe-Ortiz A, Málaga G, Gilman RH, Acuña-Villaorduña A, Cardenas-Montero D, et al. Low HDL cholesterol as a cardiovascular risk factor in rural, urban, and rural-urban migrants: PERU MIGRANT cohort study. Atherosclerosis. 2016;246:36–43. doi: 10.1016/j.atherosclerosis.2015.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maki KC, Guyton JR, Orringer CE, Hamilton-Craig I, Alexander DD, Davidson MH. Triglyceride-lowering therapies reduce cardiovascular disease event risk in subjects with hypertriglyceridemia. J Clin Lipidol. 2016;10:905–914. doi: 10.1016/j.jacl.2016.03.008. [DOI] [PubMed] [Google Scholar]
- Makkar HPS, Blümmel M, Borowy NK, Becker K. Gravimetric determination of tannins and their correlations with chemical and protein precipitation methods. J Sci Food Agric. 1993;61:161– 165. doi: 10.1002/jsfa.2740610205. [DOI] [Google Scholar]
- Plaza M, Batista ÂG, Cazarin CB, Sandahl M, Turner C, Östman E, et al. Characterization of antioxidant polyphenols from Myrciaria jaboticaba peel and their effects on glucose metabolism and antioxidant status: a pilot clinical study. Food Chem. 2016;211:185–197. doi: 10.1016/j.foodchem.2016.04.142. [DOI] [PubMed] [Google Scholar]
- Prgomet I, Gonçalves B, Domínguez-Perles R, Pascual-Seva N, Barros AIRNA. Valorization challenges to almond residues: phytochemical composition and functional application. Molecules. 2017;22:1774. doi: 10.3390/molecules. https://doi.org/10.3390/molecules22101774 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramchoun M, Sellam K, Harnafi H, Alem C, Benlyas M, Khallouki F, et al. Investigation of antioxidant and antihemolytic properties of Thymus satureioides collected from Tafilalet Region, South-East of Morocco. Asian Pac J Trop Biomed. 2015;5:93– 100. doi: 10.1016/S2221-1691(15)30151-9. [DOI] [Google Scholar]
- Rony KA, Ajith TA, Nima N, Janardhanan KK. Hypolipidemic activity of Phellinus rimosus against Triton WR-1339 and high cholesterol diet induced hyperlipidemic rats. Environ Toxicol Pharmacol. 2014;37:482–492. doi: 10.1016/j.etap.2014.01.004. [DOI] [PubMed] [Google Scholar]
- Salvayre R, Negre-Salvayre A, Camaré C. Oxidative theory of atherosclerosis and antioxidants. Biochimie. 2016;125:281–296. doi: 10.1016/j.biochi.2015.12.014. [DOI] [PubMed] [Google Scholar]
- Sang S, Lapsley K, Rosen RT, Ho CT. New prenylated benzoic acid and other constituents from almond hulls (Prunus amygdalus Batsch) J Agric Food Chem. 2002;50:607–609. doi: 10.1021/jf0110194. [DOI] [PubMed] [Google Scholar]
- Sidorova Y, Shipelin V, Mazo V, Zorin S, Petrov N, Kochetkova A. Hypoglycemic and hypolipidemic effect of Vaccinium myrtillus L. leaf and Phaseolus vulgaris L. seed coat extracts in diabetic rats. Nutrition. 2017;41:107–112. doi: 10.1016/j.nut.2017.04.010. [DOI] [PubMed] [Google Scholar]
- Song DX, Jiang JG. Hypolipidemic components from medicine food homology species used in China: pharmacological and health effects. Arch Med Res. 2017;48:569–581. doi: 10.1016/j.arcmed.2018.01.004. [DOI] [PubMed] [Google Scholar]
- Surya S, Kumar RA, Carla B, Sunil C. Antihyperlipidemic effect of Ficus dalhousiae miq. stem bark on Triton WR-1339 and high fat diet-induced hyperlipidemic rats. Bull Fac Pharm Cairo Univ. 2017;55:73–77. doi: 10.1016/j.bfopcu.2016.10.003. [DOI] [Google Scholar]
- Talayero BG, Sacks FM. The role of triglycerides in atherosclerosis. Curr Cardiol Rep. 2011;13:544–552. doi: 10.1007/s11886-011-0220-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touiss I, Harnafi M, Khatib S, Bekkouch O, Ouguerram K, Amrani S, et al. Rosmarinic acid-rich extract from Ocimum basilicum L. decreases hyperlipidemia in high fat diet-induced hyperlipidemic mice and prevents plasma lipid oxidation. Physiol Pharmacol. 2019;23:197–207. [Google Scholar]
- Viktorinova A, Svitekova K, Stecova A, Krizkod M. Relationship between selected oxidative stress markers and lipid risk factors for cardiovascular disease in middle-aged adults and its possible clinical relevance. Clin Biochem. 2016;49:868–872. doi: 10.1016/j.clinbiochem.2016.05.024. [DOI] [PubMed] [Google Scholar]
- Wijeratne SS, Abou-Zaid MM, Shahidi F. Antioxidant polyphenols in almond and its coproducts. J Agric Food Chem. 2006;54:312–318. doi: 10.1021/jf051692j. [DOI] [PubMed] [Google Scholar]
- Winklhofer-Roob BM, Faustmann G, Roob JM. Low-density lipoprotein oxidation biomarkers in human health and disease and effects of bioactive compounds. Free Radic Biol Med. 2017;111:38–86. doi: 10.1016/j.freeradbiomed.2017.04.345. [DOI] [PubMed] [Google Scholar]
- Zou B, Li CM, Chen JY, Dong XQ, Zhang Y, Du J. High molecular weight persimmon tannin is a potent hypolipidemic in high-cholesterol diet fed rats. Food Res Int. 2012;48:970–977. doi: 10.1016/j.foodres.2012.05.024. [DOI] [Google Scholar]